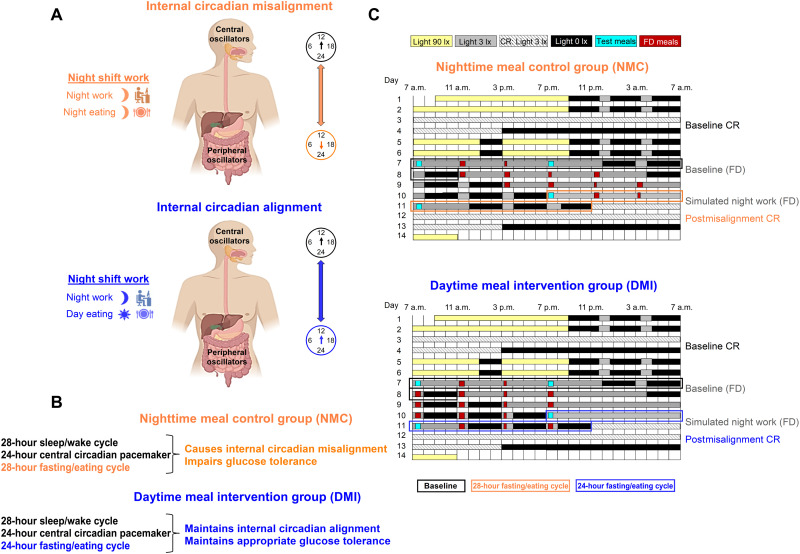
Appropriate meal timing, despite mistimed sleep, maintains internal circadian alignment and prevents glucose intolerance.
Abstract
Night work increases diabetes risk. Misalignment between the central circadian “clock” and daily behaviors, typical in night workers, impairs glucose tolerance, likely due to internal misalignment between central and peripheral circadian rhythms. Whether appropriate circadian alignment of eating can prevent internal circadian misalignment and glucose intolerance is unknown. In a 14-day circadian paradigm, we assessed glycemic control during simulated night work with either nighttime or daytime eating. Assessment of central (body temperature) and peripheral (glucose and insulin) endogenous circadian rhythms happened during constant routine protocols before and after simulated night work. Nighttime eating led to misalignment between central and peripheral (glucose) endogenous circadian rhythms and impaired glucose tolerance, whereas restricting meals to daytime prevented it. These findings offer a behavioral approach to preventing glucose intolerance in shift workers.
INTRODUCTION
Night shift work is highly prevalent in industrialized countries (1, 2) and is a risk factor for prediabetes and type 2 diabetes mellitus (T2DM) (3–6). This increased risk is not fully explained by differences in lifestyle, family history, and/or socioeconomic status, raising the question of which other mechanisms are involved (7, 8). Controlled human experimental studies show that misalignment between the central circadian “clock” and daily behaviors, typical in night workers, impairs glucose tolerance (9–13). Furthermore, eating during the nighttime impairs glucose tolerance (14), whereas not eating at night does not impair glucose tolerance under simulated night work conditions (15). These findings indicate adverse glycemic effects of mistimed food intake. Animal work indicates that restricting food intake to the active phase in rodents can prevent adverse metabolic effects of simulated shift work (16–19). Together, these findings raise the question of whether appropriate meal timing can prevent the adverse effects of circadian misalignment during night work in humans. Animal work suggests that the adverse metabolic consequences of circadian misalignment are due to a state of internal circadian misalignment between tissue “clocks” synchronized by the central circadian pacemaker versus those synchronized by the fasting/eating cycle (20). Consequently, metabolic organs would receive “mixed messages” from different Zeitgebers (time cues) that regulate their function, with subsequent temporal disruption of anabolic and catabolic processes, resulting in suboptimal metabolism (21). While animal work convincingly shows internal circadian misalignment (22, 23), in humans, there is limited evidence for it (24, 25). Furthermore, there are no established interventions to prevent internal circadian misalignment in humans.
We investigated whether humans exhibit internal circadian misalignment and impaired glucose tolerance during simulated night work, when they eat during the nighttime, and if so, whether daytime eating can prevent it (Fig. 1, A and B). Healthy young participants [12 men and 7 women; age, 26.5 ± 4.1 years; body mass index (BMI), 22.7 ± 2.1 kg/m2; hemoglobin A1c (HbA1c) range, 4.9 to 5.4%] (fig. S1 and table S1) underwent a 14-day stringently-controlled circadian laboratory protocol (Fig. 1C). First, to assess endogenous circadian rhythms without masking effects from behaviors or environmental factors (26), participants completed a baseline constant routine (CR) protocol under constant behavioral and environmental conditions (CR; 32-hour sustained wakefulness, semirecumbent posture, dim light intensity [~3 lx], and hourly isocaloric snacks (26)). CR protocols were used in this laboratory study, as they allow disentangling the relative contribution of the endogenous circadian system from the acute influences of e.g., sleep/wake, fasting/eating, rest/activity, and dark/light transitions (26). Subsequently, to simulate night work, participants underwent a forced desynchrony (FD) protocol in dim light [~3 lx] [four 28-hour “days,” which is outside the range of circadian entrainment (27)]. By the fourth FD “day” (simulated night work), participants were 12 hours out of sync when compared to the first FD “day” (simulated day work; baseline). In the Nighttime meal control (NMC) group (n = 10), participants underwent a typical 28-hour FD protocol with all behaviors kept on a 28-hour cycle, including the fasting/eating cycle. Because of that, meals occurred at fixed times relative to scheduled wake time with participants consuming food during both the daytime and nighttime, a typical behavior among shift workers. This approach has been found to cimpair glucose tolerance (13). In the Daytime meal intervention (DMI) group (n = 9), participants underwent a modified 28-hour FD protocol with all behaviors (sleep/wake, rest/activity, supine/upright posture, dark/light, etc.) scheduled on a 28-hour cycle, except for the fasting/eating cycle, which was on a 24-hour cycle. Therefore, participants consumed meals only during the daytime. At the end of each FD, all participants had a 40-hour postmisalignment CR. The latter was used to assess the aftereffect of the simulated night work on central circadian rhythms [i.e., core body temperature (CBT), which is under tight control of the central circadian pacemaker (28)], and on peripheral endogenous circadian rhythms (through hourly blood samplings of glucose and insulin).
Fig. 1. Conceptual scheme for meal timing effects in shift work settings, study aims, and experimental design.
(A) Top: Night work, whereby meals typically occur at night, is hypothesized to misalign central and peripheral circadian oscillators. Bottom: Night work, whereby meals occur during the day, is aimed to align central and peripheral circadian oscillators (internal circadian alignment). (B) In this study, we tested whether endogenous circadian rhythms of metabolic markers (glucose and insulin) can be entrained to an FD 28-hour sleep/wake and 28-hour fasting/eating cycles, resulting in a misalignment between the central circadian pacemaker and metabolic organs, and in impaired glucose tolerance. We tested whether restricting food intake to the daytime (on a 24-hour cycle) during a 28-hour sleep/wake cycle maintains internal circadian alignment and appropriate glucose tolerance. (C) The 14-day laboratory study design presented as relative clock time (for a participant whose habitual wake-up schedule was 7 a.m.). Participants were randomized to the Nighttime meal control (NMC) group or the Daytime meal intervention (DMI) group (see Methods for details on the study design). Meals consumed during the FD protocol (including the identical test meals) are included in the scheme (see text for detailed timings). Isocaloric snacks were consumed hourly during the CR protocols.
RESULTS
Effects of meal timing intervention on endogenous circadian rhythms of CBT and energy expenditure after simulated night work
CBT
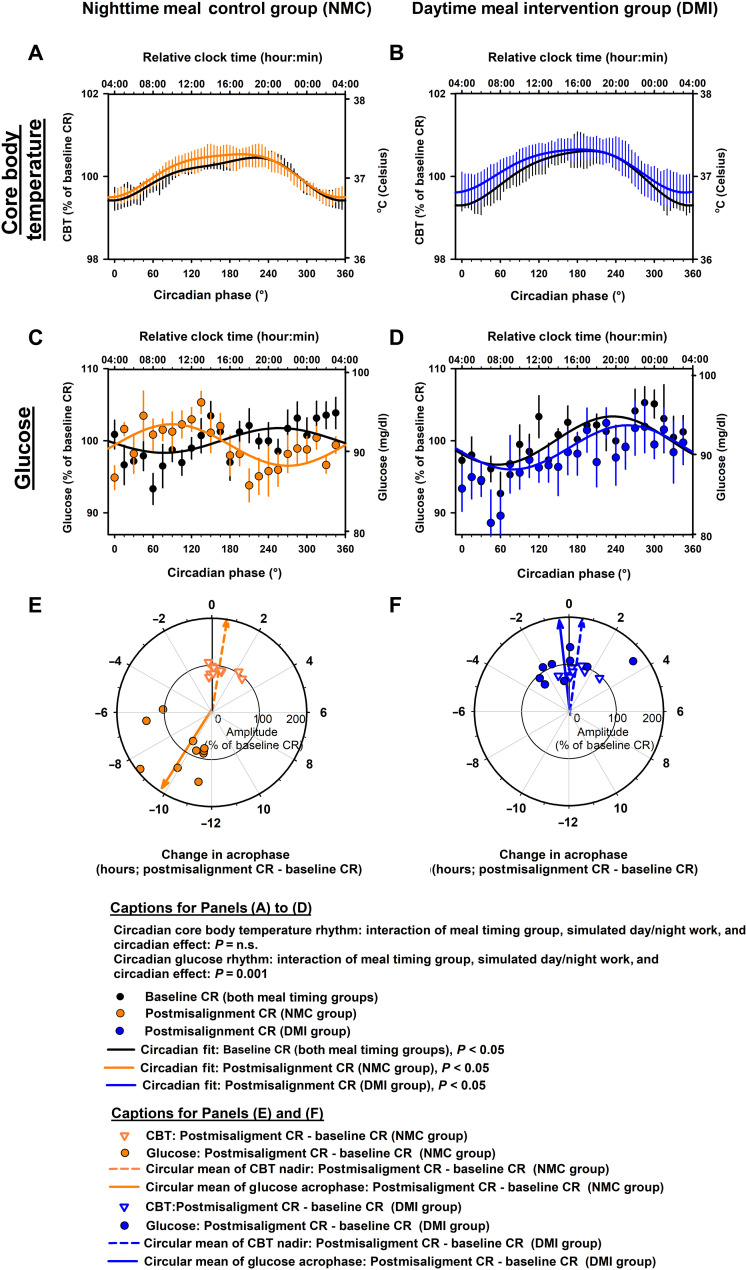
The meal timing intervention did not significantly modify the impact of simulated night work on the endogenous circadian CBT rhythms [cosinor mixed-model analyses; interaction of meal timing group, simulated day/night work, and circadian effect: pFDR = not significant (n.s.); Fig. 2, A and B]. Accordingly, simulated night work did not significantly affect the endogenous circadian CBT rhythms, as compared to baseline, in the NMC group (Tukey’s post hoc test adjusted for multiple comparisons, P = n.s.; Fig. 2A) or in the DMI group (Tukey’s post hoc test adjusted for multiple comparisons, P = n.s.; Fig. 2B). Furthermore, we also tested whether the meal timing intervention affected the change in amplitude and phase of the endogenous circadian CBT rhythms from baseline to simulated night work. The change from baseline to simulated night work in the amplitude of the endogenous circadian CBT rhythms did not significantly differ between groups [95% confidence interval (CI): NMC group, −0.3 to 0.2% (−0.1° to 0.2°C); DMI group, −0.2 to 0.1% (−0.1° to 0.2°C); two-sided, unpaired t test, P = n.s.; Fig. 2, E and F]. Likewise, the change from baseline to simulated night work in the phase of the endogenous circadian CBT rhythms did not significantly differ between groups (mean direction ± circular variance: NMC group, 0.63 ± 0.03 hours; DMI group, 0.57 ± 0.03 hours; Watson-Williams F test, P = n.s.; Fig. 2, E and F).
Fig. 2. Effects of meal timing intervention on central and peripheral circadian rhythms after simulated night work.
(A and B) The meal timing intervention did not significantly modify the impact of simulated night work on the endogenous circadian CBT rhythms. Accordingly, simulated night work did not significantly affect the endogenous circadian CBT rhythms, as compared to baseline, in the NMC group (A) or in the DMI group (B). (C and D) The meal timing intervention significantly modified the impact of simulated night work on the endogenous circadian glucose rhythms. Accordingly, simulated night work significantly affected the endogenous circadian glucose rhythms, as compared to baseline, in the NMC group (C), but not in the DMI group (D). (E and F) The change from baseline to simulated night work in the phase of the endogenous circadian CBT rhythms did not significantly differ between groups [inverted triangles in (E) and (F)]. In contrast, the change from baseline to simulated night work in the phase of the circadian glucose rhythms significantly differed between groups [circles in (E) and (F)]. In the NMC group, the phase shift of the endogenous circadian glucose rhythms closely matched the 12-hour shift of the sleep/wake cycle induced by the 28-hour FD protocol (which was not observed in the DMI group). Data in (A) to (D) were grouped into 15°-circadian windows (~1-hour resolution) with SEM error bars and the top x axes were scaled to the approximate group-averaged time of the CBT minimum for reference (i.e., relative clock time). Data in (A) to (D) correspond to the average (mean ± SEM) across participants per simulated day/night work condition and per meal timing group (n = 10 in the NMC group and n = 9 in the DMI group). Individual (symbols) and group-averaged (arrows) data are presented in (E) to (F).
Energy expenditure
The meal timing intervention did not significantly modify the impact of simulated night work on the endogenous circadian resting energy expenditure and respiratory quotient rhythms (cosinor mixed-model analyses; interaction of meal timing group, simulated day/night work, and circadian effect: pFDR = n.s.; fig. S2). Accordingly, simulated night work did not significantly affect the endogenous circadian resting energy expenditure and respiratory quotient rhythms, as compared to baseline, in the NMC group (Tukey’s post hoc test adjusted for multiple comparisons, P = n.s.; fig. S2, A and C) or in the DMI group (Tukey’s post hoc test adjusted for multiple comparisons, P = n.s.; fig. S2, B and D).
Effects of meal timing intervention on endogenous circadian rhythms of glucose and insulin after simulated night work
Glucose
The meal timing intervention significantly modified the impact of simulated night work on the endogenous circadian glucose rhythms (cosinor mixed-model analyses; interaction of meal timing group, simulated day/night work, and circadian effect: pFDR = 0.001; Fig. 2, C and D). Accordingly, simulated night work significantly affected the endogenous circadian glucose rhythms, as compared to baseline, in the NMC group (Tukey’s post hoc test adjusted for multiple comparisons, P = 0.003; Fig. 2C), but not in the DMI group (Tukey’s post hoc test adjusted for multiple comparisons, P = n.s.; Fig. 2D). Furthermore, we also tested whether the meal timing intervention affected the change in amplitude and phase of the endogenous circadian glucose rhythms from baseline to simulated night work. The change from baseline to simulated night work in the amplitude of the endogenous circadian glucose rhythms did not significantly differ between groups [95% CI: NMC group, −0.7 to 1.3% (−0.6 to 1.2 mg/dl); DMI group, −0.9 to 1.7% (−0.8 to 1.6 mg/dl); two-sided, unpaired t test, P = n.s.; Fig. 2, E and F]. In contrast, the change from baseline to simulated night work in the phase of the endogenous circadian glucose rhythms significantly differed between groups (mean direction ± circular variance: NMC group, 9.81 ± 0.11 hours; DMI group, 0.57 ± 0.11 hours; Watson-Williams F test, P < 0.001; Fig. 2, E and F).
Insulin
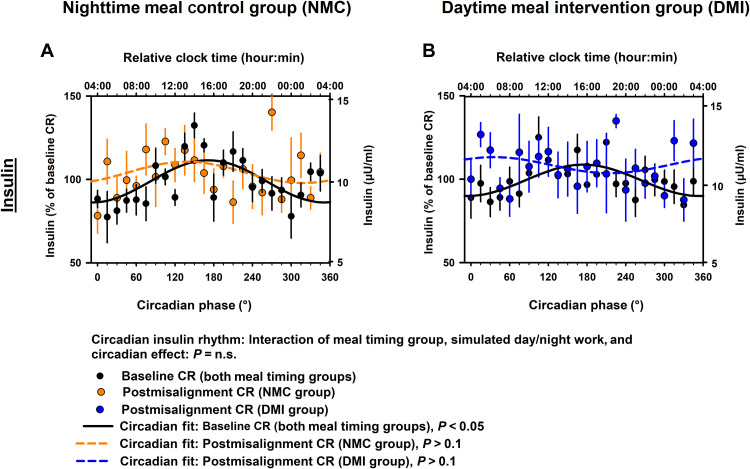
The meal timing intervention did not significantly modify the impact of simulated night work on the endogenous circadian insulin rhythms (cosinor mixed-model analyses; interaction of meal timing group, simulated day/night work, and circadian effect: pFDR = n.s.; Fig. 3, A and B). Accordingly, simulated night work did not significantly affect the endogenous circadian insulin rhythms, as compared to baseline, in the NMC group (Tukey’s post hoc test adjusted for multiple comparisons, P = n.s.; Fig. 3A) or in the DMI group (Tukey’s post hoc test adjusted for multiple comparisons, P = n.s.; Fig. 3B).
Fig. 3. Effects of meal timing intervention on endogenous circadian insulin rhythms after simulated night work.
(A and B) The meal timing intervention did not significantly modify the impact of simulated night work on the endogenous circadian insulin rhythms. [NMC group (A), DMI group (B)]. Bottom x axis: Data grouped into 15°-circadian windows (~1-hour resolution) with SEM error bars. We scaled top x axes to the time of the CBT minimum. Data in (A) and (B) correspond to the average (mean ± SEM) across participants per simulated day/night work condition and per meal timing group (n = 10 in the NMC group and n = 9 in the DMI group).
Restricting meals to the daytime mitigates impaired glucose tolerance in simulated night work
To determine the impact of internal circadian alignment/misalignment on glucose tolerance, we assessed the latter during the first and fourth “days” of 28-hour FD protocol (i.e., during simulated day and night work, respectively). We assessed the 3-hour postprandial profiles of glucose and postprandial early-phase insulin (as an estimate of β cell function) and late-phase insulin (as an estimate of insulin sensitivity) following the breakfast and dinner test meals (“breakfast” defined here as the first meal after extended fast within each fasting/eating cycle and “dinner” as the last meal within each fasting/eating cycle). During baseline [i.e. simulated day work (first FD “day”)], no significant differences were observed between groups for glucose and insulin levels immediately before test meals or for postprandial glucose and early- and late-phase insulin profiles (all pFDR = n.s).
Effects of meal timing intervention on the 3-hour postprandial glucose profile after breakfast and dinner test meals
Breakfast test meal
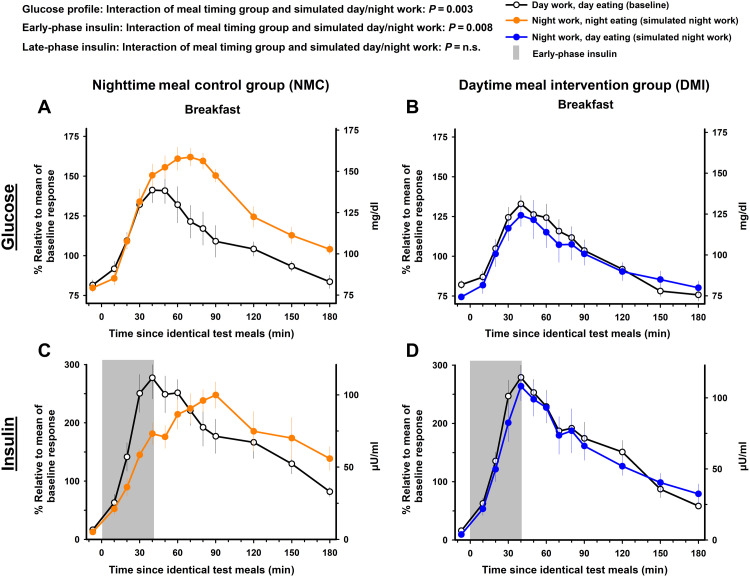
The meal timing intervention significantly modified the impact of simulated night work on postprandial the 3-hour glucose profile after the breakfast test meal (mixed-model analyses of variance; interaction of meal timing group and simulated day/night work: pFDR = 0.003). In the NMC group, the 3-hour postprandial glucose profile during the breakfast test meal under simulated night work significantly increased by 19.4% [95% CI, 4.7 to 34.2% (18.4 mg/dl; 95% CI, 4.8 to 31.8 mg/dl); Tukey’s post hoc test adjusted for multiple comparisons, P = 0.002; Fig. 4A], as compared to baseline. Conversely, in the DMI group, no significant changes occurred relative to baseline [95% CI, −13.9 to 10.1% (−15.6 to 12.8 mg/dl); Tukey’s post hoc test adjusted for multiple comparisons, P = n.s.; Fig. 4B].
Fig. 4. Effects of meal timing intervention on glucose tolerance after the breakfast test meal.
The meal timing intervention significantly modified the impact of simulated night work on the 3-hour postprandial glucose and early-phase insulin profiles after the breakfast test meal. Simulated night work in the NMC group adversely influenced the 3-hour postprandial glucose profile (A) and early-phase insulin (C) (gray bar) after the breakfast test meal. In contrast, no such effects occurred in the DMI group (B and D). See Materials and Methods for details on the fasting duration before each breakfast test meal. Data correspond to the mean ± SEM across participants per simulated day/night work condition and per meal timing group (n = 10 in the NMC group and n = 9 in the DMI group).
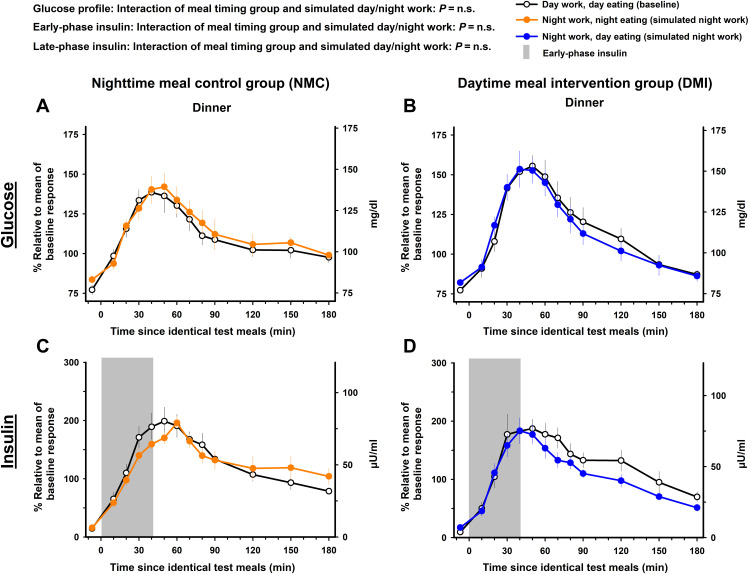
Dinner test meal
The meal timing intervention did not significantly modify the impact of simulated night work on the 3-hour postprandial glucose profile after the dinner test meal (mixed-model analyses of variance; interaction of meal timing group and simulated day/night work: pFDR = n.s.). Accordingly, simulated night work did not significantly affect the 3-hour postprandial glucose profile during the dinner test meal, as compared to baseline, in the NMC group [95% CI, −9.7 to 14.3% (−9.1 to 16.2 mg/dl); Tukey’s post hoc test adjusted for multiple comparisons, P = n.s.; Fig. 5A] or in the DMI group [95% CI, −12.5 to 6.4% (−11.1 to 6.3 mg/dl); Tukey’s post hoc test adjusted for multiple comparisons, P = n.s.; Fig. 5B].
Fig. 5. Effects of meal timing intervention on glucose tolerance after the dinner test meal.
The meal timing intervention did not significantly modify the impact of simulated night work on the 3-hour postprandial glucose and the early- and late-phase insulin profiles after the dinner test meal. Simulated night work in both the NMC group (A and C) and the DMI group (B and D) did not affect the 3-hour postprandial glucose and the early- and late-phase insulin profiles after the dinner test meal (see Materials and Methods for details on the fasting duration before each dinner test meal). Data correspond to the mean ± SEM across participants per simulated day/night work condition and per meal timing group (n = 10 in the NMC group and n = 9 in the DMI group).
Effects of meal timing intervention on the 3-hour postprandial early-phase insulin after breakfast and dinner test meals
Breakfast test meal
The meal timing intervention significantly modified the impact of simulated night work on postprandial early-phase insulin after the breakfast test meal (mixed-model analyses of variance; interaction of meal timing group and simulated day/night work: pFDR = 0.008). In the NMC group, simulated night work decreased postprandial early-phase insulin after the breakfast test meal, changing it by −52.9% [95% CI, −98.6 to −7.1% (−23.5 μU/ml; 95% CI, −42.8 to −4.2 μU/ml); Tukey’s post hoc test adjusted for multiple comparisons, P = 0.01; gray bar in Fig. 4C], as compared to baseline. In contrast, it was not significantly affected in the DMI group [95% CI, −39.8 to 3.4% (−24.1 to 1.3 μU/ml); Tukey’s post hoc test adjusted for multiple comparisons, P = n.s.; gray bar in Fig. 4D].
Dinner test meal
The meal timing intervention did not significantly modify the impact of simulated night work on postprandial early-phase insulin after the dinner test meal (mixed-model analyses of variance; interaction of meal timing group and simulated day/night work: pFDR = n.s.). Accordingly, simulated night work did not significantly affect postprandial early-phase insulin after the dinner test meal, as compared to baseline, in the NMC group [95% CI, −25.6 to 1.2% (−10.7 to 0.2 μU/ml); Tukey’s post hoc test adjusted for multiple comparisons, P = n.s.; gray bar in Fig. 5C] or in the DMI group [95% CI, −23.3 to 17.7% (−7.3 to 6.9 μU/ml); Tukey’s post hoc test adjusted for multiple comparisons, P = n.s.; gray bar in Fig. 5D].
Effects of meal timing intervention on the 3-hour postprandial late-phase insulin after breakfast and dinner test meals
Breakfast test meal
The meal timing intervention did not significantly modify the impact of simulated night work on postprandial late-phase insulin after the breakfast test meal (mixed-model analyses of variance; interaction of meal timing group and simulated day/night work: pFDR = n.s.). Simulated night work did not significantly affect postprandial late-phase insulin profile after the breakfast test meal, as compared to baseline, in the NMC group [95% CI, −21.2 to 53.6% (−8 to 18.1 μU/ml); Tukey’s post hoc test adjusted for multiple comparisons, P = n.s.; Fig. 4C] or in the DMI group [95% CI, −34.6 to 46.5% (−21.4 to 22.7 μU/ml); Tukey’s post hoc test adjusted for multiple comparisons, P = n.s.; Fig. 4D].
Dinner test meal
The meal timing intervention did not significantly modify the impact of simulated night work on postprandial late-phase insulin after the dinner test meal (mixed-model analyses of variance; interaction of meal timing group and simulated day/night work: pFDR = n.s.). Simulated night work did not significantly affect postprandial late-phase insulin profile after the dinner test meal, as compared to baseline, in the NMC group [95% CI, −23.7 to 29.1% (−10.5 to 13.2 μU/ml); Tukey’s post hoc test adjusted for multiple comparisons, P = n.s.; Fig. 5C] or in the DMI group [95% CI, −40.1 to 4.9% (−15.4 to 2.3 μU/ml); Tukey’s post hoc test adjusted for multiple comparisons, P = n.s.; Fig. 5D].
Effects of prior sleep on glucose tolerance between the meal timing groups
Sleep can influence glucose tolerance and increase diabetes risk (29); hence, we tested the potential effects of prior sleep on glucose tolerance. Sleep structure before baseline and simulated night work did not significantly differ between the meal timing groups (table S2). Furthermore, sleep structure (i.e., wake between lights off and lights on, non-rapid eye movement (NREM) sleep stages 1 to 3, REM sleep, sleep efficiency, and total sleep time) did not significantly modify the reported meal timing effects on postprandial glucose and insulin profiles (pFDR = n.s).
Effects of meal timing intervention on 28-hour profiles of glucose, insulin, CBT, and cortisol
Glucose
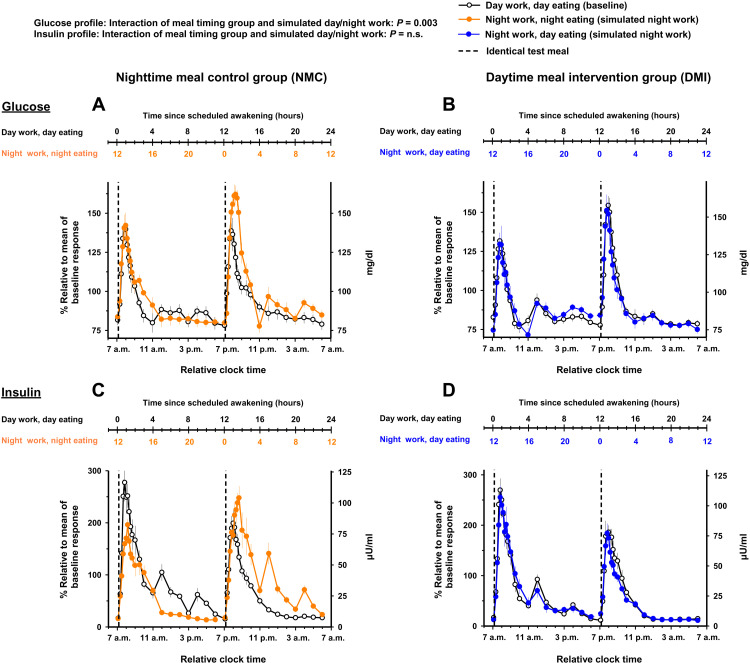
The meal timing intervention significantly modified the impact of simulated night work on the 28-hour glucose profile (mixed-model analyses of variance; interaction of meal timing group and simulated day/night work: pFDR = 0.003). In the NMC group, simulated night work increased the average glucose profile by 6.4% relative to baseline [95% CI, 2.7 to 10% (6.3 mg/dl; 95% CI, 3.3 to 9.7 mg/dl); Tukey’s post hoc test adjusted for multiple comparisons, P = 0.003; Fig. 6A and fig. S7A]. Conversely in the DMI group, no significant effects were observed as compared to baseline [95% CI, −1.7 to 4.2% (−0.2 to 4.1 mg/dl); Tukey’s post hoc test adjusted for multiple comparisons, P = n.s.; Fig. 6B and fig. S7B].
Fig. 6. Effects of meal timing intervention on the time course of glucose and insulin.
The meal timing intervention significantly modified the impact of simulated night work on the average values of glucose but not on insulin. Accordingly, simulated night work in the NMC group adversely influenced glucose profile, with overall higher concentrations (A), but not the overall insulin concentrations (C). In contrast, simulated night work in the DMI group did not adversely affect glucose (B) or insulin profiles (D). Data are shown on a 24-hour scale to highlight comparisons between baseline and simulated night work conditions matched by time of day (relative clock time, 7 a.m. as habitual wake time). Data correspond to the average (mean ± SEM) across participants per simulated day/night work condition and per meal timing group (n = 10 in the NMC group and n = 9 in the DMI group).
Insulin
The meal timing intervention did not significantly modify the impact of simulated night work on the 28-hour insulin profile (mixed-model analyses of variance; interaction of meal timing group and simulated day/night work: pFDR = n.s.). Simulated night work did not significantly affect the average insulin profiles, relative to baseline, in the NMC group [95% CI, −8.5 to 0.4% (−6 to 0.1 μU/ml); Tukey’s post hoc test adjusted for multiple comparisons, P = n.s.; Fig. 6C and fig. S7C] or in the DMI group [95% CI, −9.4 to 4.1% (−6.3 to 2 μU/ml); Tukey’s post hoc test adjusted for multiple comparisons, P = n.s.; Fig. 6D and fig. S7D].
CBT and cortisol
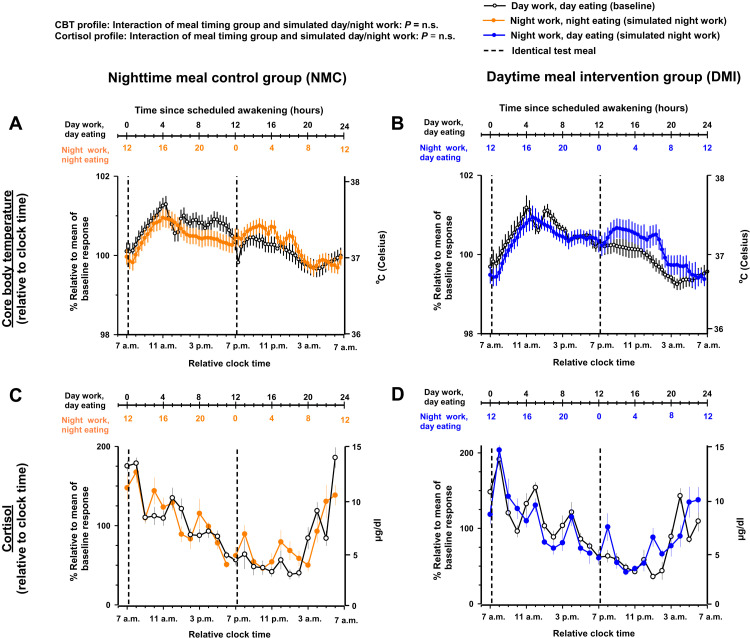
The meal timing intervention did not significantly modify the impact of simulated night work on the 28-hour profiles of CBT and cortisol (mixed-model analyses of variance; interaction of meal timing group and simulated day/night work: all pFDR = n.s.). Accordingly, simulated night work did not significantly affect the CBT and cortisol profiles, as compared to baseline, in either group (Tukey’s post hoc test adjusted for multiple comparisons, all P = n.s.; Fig. 7 and fig. S8). As expected for outputs under strong central circadian control (27), their profiles followed the 24-hour clock time more closely than the behavioral sleep/wake and fasting/eating cycles.
Fig. 7. Effects of meal timing intervention on the time course of CBT and cortisol.
The meal timing intervention did not significantly modify the impact of simulated night work on the average values of CBT and cortisol. Accordingly, simulated night work did not significantly impact the overall levels of CBT (A and B) and cortisol (C and D), as compared to baseline, in either group. Results are shown relative to 24-hour clock time, as a proxy for circadian phase. As expected for outputs under strong central circadian control, CBT and cortisol profiles closely followed the 24-hour clock time. Data correspond to the average (mean ± SEM) across participants per simulated day/night work condition and per meal timing group (n = 10 in the NMC group and n = 9 in the DMI group).
Association of internal circadian misalignment with glucose intolerance during simulated night work
Last, we assessed whether there was an association between the magnitudes of internal circadian misalignment with that of impaired glucose tolerance during circadian misalignment. Accordingly, the degree of internal circadian misalignment was positively associated with impaired glucose tolerance during simulated night work (r = 0.86, P < 0.001; fig. S9).
DISCUSSION
We found evidence for human internal circadian misalignment and impaired glucose tolerance during simulated night work. Our data indicate that a DMI, with meals allocated to the habitual daytime rather than to the nighttime, can maintain internal circadian alignment and prevent the adverse effects of simulated night shift work on glucose tolerance and pancreatic β cell function.
Human internal circadian misalignment and the role of appropriate meal timing
Our findings in the NMC group (during postmisalignment CR) indicate a state of marked internal circadian misalignment, whereby the phase shift of the endogenous circadian glucose rhythms closely matched the 12-hour shift of the sleep/wake cycle induced by the FD protocol. Conversely, in the DMI group, in which food was constrained to the daytime, the endogenous circadian glucose rhythms remained virtually identical to that under baseline despite the simulated night work condition. Moreover, the endogenous circadian glucose rhythms remained phase-locked to the endogenous circadian CBT rhythms, thereby preserving internal circadian alignment. These findings suggest that food timing synchronizes peripheral metabolic circadian rhythms, but not central circadian rhythms, possibly resulting in an uncoupling between peripheral and central circadian rhythms (22). The circadian system needs to continuously adapt to and synchronize with environmental, behavioral, and physiological signals to organize different cellular oscillators and combine tissue subnetworks into a coherent network to orchestrate metabolic function (30). Food intake is a powerful zeitgeber for peripheral circadian oscillators such that that inverting the time of feeding in mouse models can affect the phase of circadian gene expression in peripheral cell types by up to 12 hours, whereas no such changes occur in the suprachiasmatic nucleus (22). Peripheral oscillators regulate the response to nutrient challenges through cellular transcription factors that modulate gene expression with regulatory roles in nutrient transport, uptake, utilization, and storage (31). When the fasting/eating cycle is in alignment with the central circadian pacemaker, the circadian timing system initiates nutrient-sensing pathways to maintain nutrient homeostasis (31). However, if the fasting/eating cycle is out of sync with the central circadian pacemaker (32), then this can disrupt the internal alignment among peripheral oscillators and metabolite rhythms (24, 33). Nutrient-responsive pathways may provide feedback to circadian oscillators to “phase shift” in anticipation to the fasting/eating cycle, which may involve metabolic and genomic reprogramming (34). Consequently, this disordered temporal compartmentalization might hypothetically render humans incapable of appropriately responding to metabolic challenges. The resultant internal circadian misalignment might underlie the adverse glucoregulatory consequences of shift work. Our findings suggest that the marked differences in the endogenous circadian glucose rhythms between meal timing groups (i.e., inverted circadian phase between groups) are unlikely due to differences in the endogenous circadian insulin rhythms that were abolished after simulated night work in both groups. Other factors, including insulin sensitivity (12) or endogenous glucose production (35), which is produced in the liver and is exquisitely sensitive to food as a zeitgeber (22, 36), might play a role in the meal timing intervention effects on endogenous circadian glucose rhythms.
In the DMI group, we show that humans maintain internal circadian alignment when the fasting/eating cycle is aligned with the central circadian pacemaker, irrespective of exposure to circadian misalignment of the sleep/wake cycle. Animal work indicates that restricting food intake to their habitual active phase ameliorates weight gain and metabolic dysfunction (19, 21). Because food-elicited signals can entrain peripheral oscillations (22, 32), food timing may be a determining factor for the loss of balance at the circadian and metabolic levels resulting in internal circadian misalignment, as well as a powerful means to ameliorate it (see Fig. 8 for a conceptual framework). Collectively, these results might explain why shift workers have increased metabolic risk and highlight the importance of effective behavioral interventions, e.g., meal timing to mitigate internal circadian misalignment and glucose intolerance during shift work schedules.
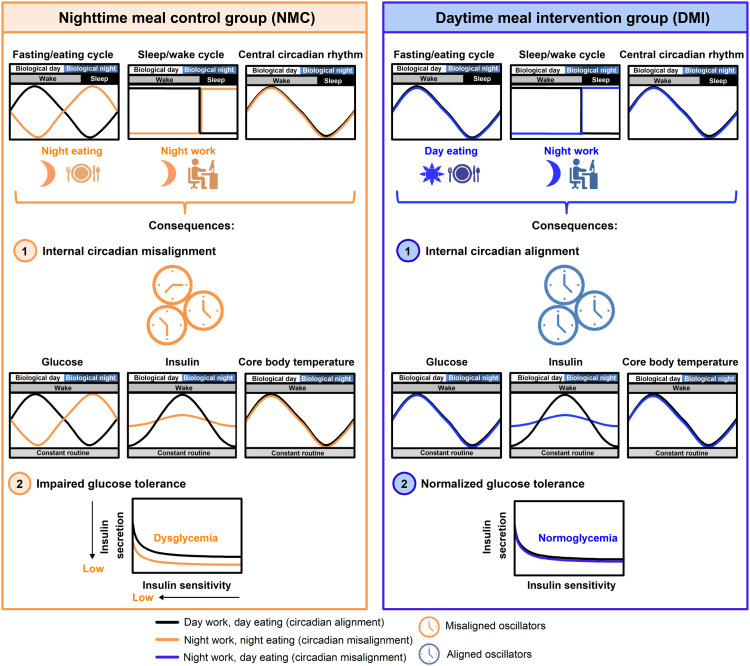
Fig. 8. Conceptual scheme for meal timing effects on internal circadian alignment and glucose tolerance.
In the NMC group (left), there is a misalignment of the fasting/eating cycle (i.e., night eating) and the sleep/wake cycle (i.e., night work) with the central circadian rhythm. This scenario results in (1) internal circadian misalignment across circadian oscillators (illustrated by hypothesized out-of-phase clocks) and with the misalignment of circadian peripheral rhythms (e.g., endogenous circadian glucose and insulin rhythms) relative to the central circadian rhythm (e.g., endogenous circadian CBT rhythm). It also results in (2) impaired glucose tolerance, predicted to occur because of decreased insulin release and insulin sensitivity, thereby causing dysglycemia. In contrast, the DMI group (right) maintains the alignment of the fasting/eating cycle (i.e., day eating) with the central circadian rhythm, despite the misalignment of the sleep/wake cycle (i.e., night work). Consequently, this leads to (1) internal circadian alignment and (2) normalized glucose tolerance. This state of normoglycemia may prevent glucose intolerance in individuals experiencing circadian rhythm disruption, as in the case of night workers.
Meal timing as a behavioral intervention against impaired glucose tolerance
In the NMC group, simulated night work increased the postprandial glucose profile consistent with previous human laboratory studies that have shown impaired glucose tolerance due to circadian misalignment (11, 13). Furthermore, the decreased postprandial early-phase insulin response to a glycemic challenge was consistent with the concept that circadian misalignment may induce pancreatic beta cell dysfunction (37). Simulated night work did not affect late-phase insulin, which is in contrast to a previous reported ~15% increase due to circadian misalignment (11), probably because of differences in study design and/or statistical power. These reported effects likely depend on the interplay of the circadian timing of meals, circadian alignment/misalignment, and fasting duration. We showed that the 3-hour postprandial glucose levels increased only after the breakfast test meal during the simulated night work in the NMC group (Fig. 4 and fig. S4). In contrast, no statistical differences occurred after the dinner test meal during the simulated night work in the NMC group (Fig. 5 and fig. S5). While these findings may seem unexpected at first glance, this is predicted because of the combined effects of circadian misalignment and circadian phase (10–12). These studies showed that glucose tolerance was impaired by circadian misalignment per se. Moreover, these same studies also showed that glucose tolerance was impaired during the circadian evening as compared to the circadian morning. Therefore, the glucose intolerance observed after the breakfast test meal during the simulated night work compared to baseline in the NMC group was expected given the combined effects of circadian misalignment and the change from the circadian morning (e.g., at ~7 a.m.) to the circadian evening (e.g., at ~7 p.m.), with both effects decreasing glucose tolerance. Conversely, glucose tolerance assessed after dinner test meal during simulated night work as compared to baseline in the NMC group was affected by the opposing effects of circadian misalignment (decreasing glucose tolerance) and the change from the circadian evening (e.g., at ~7 p.m.) to the circadian morning (e.g., at ~7 a.m.) (increasing glucose tolerance). The net effect would result in similar glucose excursions assessed after the dinner test meal during both simulated day work and night work conditions. Notably, a similar finding was observed in a previous laboratory study using a 28-hour FD protocol as in the current study (13) in which glucose tolerance was markedly impaired when breakfast was misaligned but changed minimally when dinner was misaligned. Consistently, the inversion of the sleep/wake cycle in our current NMC protocol resulted in a very large impairment only for breakfast, whereas a very small difference occurred for dinner. Furthermore, to isolate the effect of circadian misalignment while accounting for that of circadian phase, we averaged the glucose and insulin levels after the breakfast and dinner during the simulated night versus simulated day shift conditions (figs. S3 and S6). By averaging the glucose and insulin levels during, e.g., ~7 a.m. and ~7 p.m., thus accounting for circadian phase effects, circadian misalignment per se was shown to clearly impair glucose tolerance in the NMC group. In contrast, glucose tolerance remained virtually identical for the average of the breakfast and dinner test meals under the simulated night versus simulated day shift conditions in the DMI group. Collectively, what this unveiled was that circadian misalignment of both the sleep/wake and fasting/eating cycles combined relative to the central circadian clock resulted in glucose intolerance in the NMC group. Conversely, by avoiding the circadian misalignment of the fasting/eating cycle despite the misalignment of the sleep/wake cycle, glucose intolerance was prevented in the DMI group. Together, these observations indicate that circadian misalignment of the fasting/eating cycle with the central circadian clock, and not circadian misalignment of the sleep/wake cycle with the central clock, primarily underlies the adverse effect of circadian misalignment on glucose tolerance.
Animal work shows that the timing of food intake can prevent detrimental metabolic effects of circadian disruption (16, 17). A human laboratory study previously showed that eating at night can increase postprandial glucose area under the curve (AUC) following meal tolerance tests, compared to not eating at night, after 4 days of simulated night work (15). In a similar vein, we show that daytime meal timing avoids the adverse effects of stimulated night work on glucose tolerance. Our study is therefore an important step, as it demonstrates that a behavioral intervention can mitigate adverse glucoregulatory effects induced by a mistimed sleep/wake schedule in humans. There were no changes in postprandial glucose and insulin profiles between simulated night work and day work for in the DMI group. Critically, the laboratory design of both groups was identical (i.e., caloric and macronutrient intake, physical activity, posture, scheduled sleep duration, and lighting conditions) except for the timing of meals. The meal timing effects on glucose tolerance are unlikely driven by group differences before the laboratory protocol since screening comprehensive metabolic panel, thyroid-stimulating hormone (TSH), complete blood count (CBC), and HbA1c were within typical range for all participants, and participant demographics and study-related characteristics did not differ between groups (table S1). Moreover, sleep structure before the baseline and simulated night work conditions did not differ between groups (table S2), and it did not statistically modify the reported meal timing effects on glucose tolerance. In our trial, we enrolled participants who were healthy, without sleep disorders, other comorbidities, or medication use. Thus, participants were potentially less susceptible to the effects of the laboratory protocol on sleep duration and quality. We scheduled one-third of the time (to match 8-hour sleep opportunity during a regular 24-hour day) as protected sleep, in a private, quiet, dark, and temperature-controlled environment, thus conducive to sleep. Moreover, the study included multiple adaptation days to accommodate to the laboratory protocol before the FD protocol. Last, we scheduled the baseline and the simulated night work conditions to occur under an FD protocol, not under a total sleep deprivation or a sleep restriction paradigm. Total sleep time and N3 sleep in both meal timing groups were substantially more than in (similarly short-term) sleep restriction paradigms that negatively affect glucose tolerance (38, 39). Collectively, these factors are likely reasons why the subtle sleep structure differences did not modify the reported meal timing effects on glucose tolerance.
Differences in fasting duration before the test meals are unlikely to have mediated the effects of meal timing on glucose tolerance. In both NMC and DMI groups, fasting duration before the breakfast test meal during baseline was ~12 hours. In the NMC and DMI groups, fasting duration before the breakfast test meal during the simulated night work was respectively ~16 and ~12 hours. This duration is in accordance with the recommended fasting duration for clinical glucose tolerance tests by the American Diabetes Association, which recommends overnight fasting for at least 8 hours, with varying durations of 8 to16 hours (40, 41). Currently, most laboratory studies have compared overnight fasting (~12 hours) with more extreme and prolonged fasting durations of e.g., 3 days (42). A laboratory study comparing a 12-hour fast with a 36-hour fast found no statistical difference in glucose AUC after identical test meals (43). Hence, we expect that the subtle differences in fasting duration in our laboratory protocol have a negligible effect on glucose tolerance. The meal timing effects were also not likely driven by changes in CBT (Fig. 7, A and B, and fig. S8, A and B) or cortisol during the simulated night work (Fig. 7, C and D, and fig. S8, C and D), both of which under strong central circadian control (27), and not statistically different between groups. The preserved glycemic control in the DMI group during simulated night work was likely due, at least in part, to the maintenance of normal pancreatic β cell function, as no impairments occurred for the postprandial early-phase insulin response. Consequently, this suggests improved pancreatic β cell function with important ramifications for glucose tolerance (44). This daytime eating pattern may allow optimizing metabolic function by timing food intake to the acrophase of endogenous metabolic rhythms (31), which may consolidate circadian rhythmicity in gene expression and circadian activation of numerous metabolic pathways (45). By rendering the fasting/eating cycle and the central circadian pacemaker aligned, this may ultimately improve whole-body insulin sensitivity and glycemic control with a possible beneficial effect on metabolic health outcomes in shift work conditions.
Night shift workers often reschedule their meal intake to the nighttime, as they are awake during those hours (46, 47). Exposure to circadian misalignment may also impair glucose tolerance and insulin sensitivity in real-life shift workers (10), raising the possibility that long-term exposure to circadian misalignment contributes to the increased risk of prediabetes or T2DM in this population. In this context, our findings may help in the development of evidence-based circadian strategies (e.g., timing of eating) to prevent glucose intolerance in individuals experiencing circadian rhythm disruption. The effects of a daytime eating pattern on glucose tolerance likely extend to most types of shift workers. Only under exceptional situations, e.g., oilrigs and Arctic stations, the central circadian pacemaker can show an adaptation to night work (48, 49). Moreover, less than a quarter of night workers show sufficient adjustment to derive any benefit (50–52). As permanent or rotating night shifts are unlikely to result in adequate circadian adjustment in most individuals, the alignment of fasting/eating cycle to the central circadian pacemaker may prevent glucose intolerance in most shift workers. Future translational studies with individuals undergoing real-life shift work schedules (e.g., permanent, rotating or irregular night shifts, morning shifts, and evening shifts) are required to establish if our reported beneficial effects on glucose tolerance (as well as other health and performance outcomes) apply to this vulnerable population.
MATERIALS AND METHODS
Trial procedures and participants
The protocol was approved by the Partners HealthCare’s institutional review board (IRB) and performed in accordance with the principles of the Declaration of Helsinki, and participants provided written informed consent. IRB guidelines were followed with human individuals. Laboratory protocols were conducted at the Center for Clinical Investigation at Brigham and Women’s Hospital, Boston, United States, between 19 March 2015 and 29 August 2018. The study ended when the laboratory protocol for the final randomized participant was completed.
Participants admitted to laboratory protocols were free from medical conditions and medical suitability using clinical history, biochemical and toxicology blood and urine screenings, and physical and psychological exams. Biochemical blood panels at screening included a comprehensive metabolic panel, TSH, CBC, and HbA1c, all of which had to be within typical range for study inclusion. Participants were not taking medications (excepting oral contraceptives), caffeine, smoking, or using recreational drugs (verified with urine toxicological panel). After screening, participants were randomly assigned to one of two meal timing groups: NMC group, which included simulated day work with day eating (baseline) followed by simulated night work with night eating, typical in night shift workers; and DMI group, which included simulated day work with day eating (baseline) followed by simulated night work with day eating (see the “Study design” section for details of the laboratory protocol). Participants were randomized using minimization (Minim.exe, MS-DOS free access program for randomizing participants into the arms of a clinical trial) to minimize imbalance between meal timing groups. Minimization was performed—in decreasing sequence of importance—by sex, BMI, and age (these factors were dichotomized, i.e., woman or man, 18.5 to 24.9 kg/m2 or 25 to 29.9 kg/m2, and 18 to 26 years or 27 to 35 years, respectively). While participants were informed that they might be sleeping and eating at different times of day or night, they were unaware that there were different meal timing groups. Therefore, our study comprises a randomized, parallel, controlled, single-blinded trial from a single center. We randomized 24 participants to the laboratory protocols. Four participants discontinued because of mild adverse events unrelated to the intervention. One participant reported headache before the simulated night work in the DMI group, two had loose stools before the simulated night work (one in the NMC and one in the DMI group), and one could not consume all provided meals during simulated day work (baseline) in the NMC group. No data are available for these participants, as they did not complete the entire study. We removed these four participants from the minimization model (and from the randomization) after each discontinuation and the minimization otherwise continued. Therefore, 20 participants [mean age, 26.6 years (SD, 4.2 years; range, 18 to 35 years); eight women; BMI range, 18.5 to 29.9 kg/m2; HbA1c range, 4.9 to 5.4%] were randomized to our study: Ten were allocated to the NMC group and 10 to the DMI group (fig. S1). Four women underwent the laboratory protocol on menstrual cycle days 1 to 5 (two women per meal timing group) and four during days 14 to 19 (two women per meal timing group).
The final study sample included 10 participants for the NMC group [mean age, 27 years (SD, 4.4 years); four women; BMI, 22.5 kg/m2 (SD, 3.5)] and 9 participants for the DMI group [mean age, 26.2 years (SD, 4.1 years); three women; BMI, 23 kg/m2 (SD, 3.1)]. We excluded data from one participant in the DMI group due to their inability to consume all meals during the simulated night work. No statistical differences in participant demographics and study-related characteristics (including age, sex, BMI, and HbA1c values) occurred between groups (table S1). No important harms or serious adverse effects occurred in either meal timing groups.
Study design
Before the laboratory protocol, participants maintained a fixed, self-selected habitual bedtime with 8-hour time in bed per day for 2 weeks. We verified compliance using ambulatory actigraphy (Actiwatch, Respironics), sleep logs, and time-stamped voicemails. During the 3 days before the laboratory study, participants received all meals from the metabolic kitchen to meet dietary requirements (Harris-Benedict formula with an activity factor of 1.4) and controlled macronutrient distribution (45 to 50% carbohydrate, 15 to 20% protein, and 30 to 35% fat, with 150 meq of Na+ (±20%) and 100 meq of K+ (±20%), which matched the subsequent laboratory diet.
During the laboratory study, participants remained in individual suites in an environment free of time cues. Throughout the study, when participants were not involved in a study task, they could undertake leisure activities, such as reading, writing, watching movies, crafts, etc. We monitored each participant’s activity for compliance by means of closed circuit TV and wrist-worn actigraphy. During the CR protocol (see below), a study staff member remained with the participant in the room to ensure they remained awake throughout scheduled wakefulness. Participants did not nap or perform exercise during the study. Each suite had a porthole for 24-hour blood sample collection without disturbing the participant’s sleep.
Days 1 and 2 comprised the laboratory adaptation days, and on days 3 and 4, there was a baseline CR (Fig. 1C) that allowed for a circadian baseline assessment on metabolic markers and CBT. The CR protocol enables the assessment of endogenous circadian rhythms because it minimizes the influences of behavioral and environmental factors by maintaining constant wakefulness, constant semirecumbent posture, markedly limited physical activity, dim light conditions, and evenly distributing isocaloric snacks (26). During baseline CR, participants spent 32-hour continuously awake in a constant semirecumbent body posture, without physical exertion, in dim light (~3 lx in the horizontal angle of gaze) and eating hourly isocaloric CR snacks (see also the “Diet” section). Following baseline CR, participants had a 12-hour sleep opportunity to recover. On days 5 and 6, participants had further recovery from the baseline CR. On day 7, participants underwent a 28-hour FD protocol to induce circadian misalignment with 28-hour sleep/wake cycles under dim light (~3 lx), to which the central circadian pacemaker in humans cannot entrain (27). We used a 28-hour FD protocol to assess the impact of circadian misalignment on metabolic function (see also the “Diet” section) (13). During each 28-hour cycle, the ratio of scheduled wakefulness (18 hours:40 min) and sleep (9 hours:20 min) was maintained at 2:1, to match the self-selected 8-hour habitual time in bed per 24 hours. The participant’s sleep episodes were split into three identical blocks, each separated by 1 hour of scheduled wakefulness in dim light (~3 lx) while remaining at rest in a semirecumbent posture in bed. This allowed the participants to consume food during the circadian day when otherwise they would be sleeping. Participants woke during each sleep episode irrespective of meal consumption to ensure that both study groups had three equal sleep blocks during the FD protocol. On the first 28-hour sleep/wake cycle, participants had normal circadian alignment (waking up at their habitual wake-time, e.g., 7 a.m.; baseline). In contrast, on the fourth sleep/wake cycle, participants were 12-hour misaligned as compared to the fourth cycle (wake up at, e.g. 7 p.m.; simulated night work) in both meal timing groups. The NMC group (Fig. 1C, top) is a traditional 28-hour FD protocol with all behaviors, including the fasting-feeding cycle, maintained on a 28-hour cycle. Because of that, three meals and a snack were scheduled at fixed times relative to scheduled wake time (at 0 hours:10 min, 4 hours:10 min, 8 hours:10 min, and 12 hours:10 min since scheduled awakening, during baseline and simulated night work). Thus, each meal was shifted to 4 hours later each cycle in alignment with the sleep/wake cycle. Participants thus consumed food during both the circadian day and night, which is a typical behavior of night shift workers. In contrast, the DMI group (Fig. 1C, bottom) is a nontraditional 28-hour FD protocol, with all behaviors identically scheduled on a 28-hour cycle, except for the fasting/feeding cycle (maintained on a 24-hour cycle). Accordingly, participants had standardized meals at 0 hours:10 min, 4 hours:10 min, 8 hours:10 min, and 12 hours:10 min since scheduled awakening, during baseline, whereas meals occurred at 0 hours:10 min, 12 hours:10 min, 16 hours:10 min, and 20 hours:10 min since scheduled awakening, during the simulated night work. This meal timing approach allowed alignment of the fasting/eating cycle to the ~24-hour central circadian cycle and ensured meal consumption during the circadian day at the same clock time during each FD cycle. Subsequent to the 4 “days” of a 28-hour FD protocol, participants underwent a postmisalignment CR (days 11/12/13) that allowed assessing the aftereffect of circadian misalignment on endogenous central and peripheral circadian rhythms. During the postmisalignment CR, participants spent 40-hour under the same experimental conditions described for the baseline CR. Thereafter, participants were scheduled to 12-hour sleep opportunity to allow them to recover partially from the postmisalignment CR protocol and then were discharged from the study.
Diet
CR protocol
Participants received an isocaloric diet (i.e., CR snacks) that was calculated according to the Harris-Benedict equation with an activity factor of 1.2 (as participants had decreased activity). The diet consisted of 45 to 50% carbohydrate, 15 to 20% protein, 30 to 35% fat, with 150 meq of Na+ (±20%), 100 meq of K+ (±20%), and at least 2.5 liters of water per 24 hours. CR snacks comprised two alternating CR options (e.g., CR snack A, then CR snack B, then CR snack A, and so forth) based on a food preference form for each participant’s two CR preselected CR snack choices (two of six snack choices with different ingredients but same macronutrient composition). CR snacks were calculated with the same two snack options and same caloric level throughout the both CR protocols per participant. Participants had 10 to 15 min to consume the CR snacks and were instructed to consume all food provided (verified by checking their food trays). During the CR protocol, actual energy consumption in the NMC group was 99.9% (SEM, 0.01) and 99.9% (SEM, 0.04%) during baseline CR and postmisalignment CR, respectively. In the DMI group, it was 98.6% (SEM, 1.9%) and 99.9% (SEM, 0.01%) during baseline CR and postmisalignment CR, respectively.
FD protocol
Participants received meals (breakfast, lunch, snack, and dinner) that were standardized across days based on a food preference form for each participant. Meals were calculated according to a 28-hour day for the NMC group and to a 24-hour day for the DMI group during the four days in the FD protocol. Diet was calculated according to the Harris-Benedict equation with an activity factor of 1.4 and consisted of 45 to 50% carbohydrate, 15 to 20% protein, 30 to 35% fat, with 150 meq of Na+ (±20%), 100 meq of K+ (±20%), and at least 2.5 liters of water per 24 hours. The energy content of the meals (percentage of total day’s calorie intake) was as follows: breakfast, 33.3% (±35 kcal); lunch, 23.4% (±20 kcal); snack, 10% (±10 kcal); dinner, 33.3% (±35 kcal). Breakfast and dinner test meals (used for glucose tolerance assessment; see the subsection below) were preselected (one of two test meal choices based on a food preference form for each participant). Test meals included a dextrose solution of glucose (0.45 g/kg) [provided as glucose drink (Glucola) with a glycemic index of 60 to 65]. For the test meals, participants chose one of the following: (i) Glucola, a bagel with butter, cereal with milk and sugar, egg, and peanuts; or (ii) Glucola, a bagel with butter, cereal with milk and sugar, turkey sausage, and almonds. Test meals were consumed within 20 min. Glucola was consumed within the first 1 min, and other food items were consumed subsequently in the order listed (from high to low glycemic index foods). The food items and their sequence of consumption was thus identical during test meals within each participant. These breakfast and dinner test meals were preceded by isocaloric “premeals.” The dinner before the both breakfast test meals was identical within each participant. The lunch and snack before both dinner test meals were also identical within each participant. Participants were instructed to consume all food provided (verified by checking their food trays). During the FD protocol, actual energy consumption (expressed as a percentage of planned caloric intake) in the NMC group was 99.8% (SEM, 0.3) and 99.7% (SEM, 0.9%) during baseline and simulated night work, respectively. In the DMI group, it was 99.8% (SEM, 0.4%) and 99.2% (SEM, 1.6%) during baseline and simulated night work, respectively.
During the other segments of the laboratory protocol, participants received an isocaloric diet, calculated according to the Harris-Benedict equation with an activity factor of 1.4. The diet had the same macronutrient composition as for the CR and FD segments, which was 45 to 50% carbohydrate, 15 to 20% protein, 30 to 35% fat, with 150 meq of Na+ (±20%), 100 meq of K+ (±20%), and at least 2.5 liter of water per 24 hours. Participants were instructed to consume all food provided (verified by checking their food trays).
Glucose tolerance assessment
To assess glucose tolerance, four identical test meals per participant were strictly timed at 0 hours:10 min and 12 hours:10 min since scheduled wakefulness (thus 12 hours apart) on the days of baseline and simulated night work (during the FD protocol). Test meals occurred once during the morning (e.g., 7:10 a.m., for a participant with a habitual wake time of 7 a.m.) and once during the evening (e.g., 7:10 p.m., for a participant with a habitual wake time of 7 a.m.) per baseline and per simulated night work. In the NMC and DMI groups, fasting duration before the first test meal (breakfast) during baseline was ~12 and ~ 4 hours before the second test meal (dinner). In the NMC group, fasting duration before the first test meal (breakfast) during the simulated night work was ~16 and ~ 4 hours before the second test meal (dinner). In the DMI group, fasting duration before the second test meal (breakfast) during the simulated night work was ~12 and ~ 4 hours before the first test meal (dinner).
Venous blood collection and processing
On admission to the laboratory, an 18-gauge intravenous catheter was inserted into the participant’s forearm. The catheter was connected to a triple-stopcock manifold (Cobe Laboratories Inc., Lakewood, CO) via an intravenous loop with a 12-foot small-lumen extension cable (Sorex Pharmaceuticals, Salt Lake City, UT) through which blood sampling could continue in the next room without disturbing sleep. Between samples, infusion of a solution of 0.45% saline with 5000 IU/liter heparin at one drop every 5 to 10 s maintained patency. Blood was transferred to 5-cm3 vacutainer tubes and centrifuged at 4°C, pipetted into polystyrene tubes, and frozen at −80°C until analysis. Participant’s hematocrit and hemoglobin were measured on each CR day and on FD day 1 and day 4 (when blood measurements took place) to assess whether levels remained within normal range.
Outcome measures
During the CR protocols, one primary outcome was central circadian measurement (circadian phase), which was determined from CBT during the CR protocols. We used nonorthogonal cosinor analyses for each participant’s CBT data to estimate circadian phase. Fitted circadian CBT minimum was the reference phase marker of 0° for each individual, and data were then assigned a specific circadian phase (0° to 359°). Peripheral metabolic circadian rhythms were determined from plasma glucose and insulin measurements (primary outcomes), which were obtained through hourly blood samples during the CR protocols. Given that participants received hourly isocaloric snacks throughout the CRs, glucose and insulin levels hence correspond to a mixed pre- and postprandial states.
Energy expenditure (i.e., resting energy expenditure and respiratory exchange ratio) was an exploratory outcome and an additional circadian measure during the CR. We assessed energy expenditure using a validated indirect calorimeter with ventilated hood (VMAX Encore 29 N, Carefusion, San Diego, CA) over 20-min stable resting windows every 2 hours during the CR protocols, thus a total of ~36 times per participant.
During the FD protocol, the primary outcome measures were the 3-hour postprandial glucose profile, postprandial early-phase insulin profile (first 40 min after test meals, during which time glucose levels rose similarly between conditions and protocols, allowing estimation of β cell function), and postprandial late-phase insulin profile (beyond 40 min until 3 hours after test meals). These primary outcomes are well-established indices of glucose control, and circadian misalignment adversely affects those (11). We report time series analyses of glucose and early-phase and late-phase insulin profiles, as it captures the rapid increases and decreases in glucose and insulin profiles following exposure to a glycemic challenge. Measurements of plasma glucose and insulin happened immediately before each of the four test meals (fasting blood was drawn ~7 min before the test meals), and thereafter every 10 min for 90 min and then every 30 min for another 90 min. From 3 hours after the start of the test meals, sample collection frequency was every 60 min. Assessments of glucose and insulin occurred only during baseline and simulated night work. CBT and plasma cortisol were measured as exploratory outcomes to verify that participants were under circadian misalignment during simulated night work in both meal timing groups, as CBT and cortisol are under strong circadian control (28). Continuous CBT measurements occurred throughout the laboratory protocol. Participants used a flexible rectal temperature sensor (Yellow Springs Instrument Company, OH, USA), and they used it continuously throughout the laboratory protocol (except for during showers and bowel movements) for CBT measurement. CBT was assessed every 1 min across the baseline and simulated night work, and plasma cortisol was assessed every 60 min across the baseline and simulated night work. Furthermore, we assessed the influence of prior sleep on glucose control before the baseline and simulated night. Sleep was measured by electroencephalography (EEG), electrooculography (EOG), and submental electromyogram using American Academy of Sleep Medicine recommended EEG derivations (C3/4, F3/4, O1/2, referenced to M2/1) (Vitaport recorder, TEMEC, Netherlands). Sleep structure included wake during sleep, NREM sleep stages 1 to 3, REM sleep, sleep efficiency, and sleep onset latency.
Statistical analysis
We performed statistical analyses using SAS version 9.4 (SAS Institute, Cary, NC, USA), NCSS 2020, v20.0.3 (for the circular data analyses) and SigmaPlot version 14.0 for the linear regressions and illustrations. The sample size was derived from the difference in the effect of misalignment on glucose tolerance (3-hour postprandial glucose profiles) between the meal timing groups. To determine a large effect size (d = 1.5) with ~80% power, eight participants per group were required (total sample = 16). We increased the number of participants per group to 10 to mitigate potential data losses. We compared participants’ characteristics with Yates’s chi-square tests or t tests for independent groups, and their demographics and study-related characteristics did not statistically differ between the meal timing groups (table S1). Moreover, we did not test for sex differences and for differences in menstrual phase in the effects of the intervention because of limited sample size.
For the CR data, we normalized CBT, glucose, and insulin data using an average of each participant’s levels measured throughout the baseline CR to minimize interindividual differences in baseline temperature and glycemic control. Notably, parallel study designs in particular require data normalization. The first 5 hours after starting the CRs were excluded from analysis, as is standard, to allow for stabilization of circadian rhythms. The effects of the circadian cycle and circadian alignment condition were assessed by cosinor analyses using mixed model analyses of variance (PROC MIXED, SAS), which were applied to the CBT, glucose, and insulin data (primary outcomes), and to the energy expenditure data (exploratory outcomes). These cosinor mixed-models included circadian effect (a fundamental circadian component of ∼24 hours), time since scheduled waketime (hours into the CR protocol), simulated day/night work (baseline CR versus postmisalignment CR), and the interaction of meal timing group, simulated day/night work, and circadian effect (interaction effect reported in Figs. 2 and 3 and fig. S2). For the CBT data, a main effect harmonic (a component of ∼12 hours) was included. Participant was included as a random factor. We used cosinor analyses to identify statistically significant endogenous circadian rhythms for the primary and exploratory outcomes (CBT: fitted nadir; glucose, insulin, and energy expenditure: fitted peak). Post hoc comparisons used the Tukey’s test to adjust for multiple testing. Comparison of the amplitude of the endogenous circadian CBT and glucose rhythms was performed with two-sided, unpaired t tests for the meal timing group effect. Comparisons of the phase of the endogenous circadian CBT and glucose rhythms were performed using circular statistical data analyses. Accordingly, we tested equal directions (i.e., changes in phase) using the Watson-Williams F test (53), which assumes a Von Mises data distribution with equal κ > 1. We show the mean direction (θ) and circular variance (designed to assess and compare the variation in the data, where 1 = high data dispersion, 0 = little dispersion) for the polar plots (Fig. 2, E and F), which depict changes in phase of the endogenous circadian CBT and glucose rhythms from baseline CR to postmisalignment CR. Last, we applied linear regression models to test the association of internal circadian misalignment with glucose tolerance during simulated night work (fig. S9).
For the FD data, all primary and exploratory outcomes were normalized using an average of each participant’s levels measured throughout the baseline day to minimize any effect of interindividual differences in baseline e.g., glycemic control. This normalization is needed when using a parallel study design. Mixed-model analyses of variance (PROC MIXED, SAS) included three main factors: (i) meal timing group (NMC group versus DMI group); (ii) simulated day/night work [baseline (simulated day work) versus simulated night work]; (iii) time (time after start of test meals for 3-hour postprandial profiles). This statistical model was used for the breakfast and dinner test meals separately. The interaction of meal timing group and simulated day/night work was used to identify whether the meal timing intervention significantly modified the impact of simulated night work on the 3-hour postprandial glucose and postprandial early- and late-phase insulin profiles after the breakfast and the dinner test meals separately (reported, respectively, in Figs. 4 and 5). Post hoc comparisons used the Tukey’s test to adjust for multiple testing. In addition, we averaged baseline postprandial glucose and insulin responses to the two test meals (breakfast and dinner) into one profile per primary outcome variable. Averaging of test meals that were exactly 12 hours apart (see Fig. 1C) reflects the overall glucose tolerance that varies throughout a given day, while simultaneously matching for circadian phase and duration since scheduled wakefulness when comparing the baseline with the simulated night work. Mixed-model analyses of variance (PROC MIXED, SAS) included three main factors: (i) meal timing group (NMC group versus DMI group); (ii) simulated day/night work [baseline (simulated day work) versus simulated night work]; and (iii) time (time after start of test meals for 3-hour postprandial profiles). The interaction of meal timing group and simulated day/night work was used to identify whether the meal timing intervention significantly modified the impact of simulated night work on the 3-hour postprandial glucose and postprandial early- and late-phase insulin profiles after the test meals (reported in fig. S3). In addition, a second statistical model was performed using the abovementioned mixed-model analyses, where the main factor time corresponded to hours into each simulated day and night shift cycles (reported in Figs. 6 and 7 and figs. S7 and S8). Post hoc comparisons used the Tukey’s test to adjust for multiple testing. We performed exploratory analyses on the 3-hour postprandial glucose and insulin AUC, which was determined from start of the test meal until 180 min after it and results are presented in the Supplementary Materials. AUC was calculated using the trapezoidal method. The significance of any effects of the meal timing intervention on the change in 3-hour postprandial glucose and insulin AUC from baseline to simulated night work was determined using two-sided, unpaired t test comparisons for each test meal separately (figs. S4 and S5). Moreover, in exploratory analyses to isolate the effects of circadian misalignment independent of circadian phase and fasting duration, we determined the effects of the meal timing intervention on the change in 3-hour postprandial glucose and insulin AUC from baseline to simulated night work using two-sided, unpaired t test comparisons, on the average of both test meals (fig. S6).
Because sleep can influence glucose tolerance, we assessed sleep structure characteristics before the baseline and simulated night work conditions in both meal timing groups using mixed-model analyses of variance with main factors meal timing group and simulated day/night work, as well as their interaction (table S2). Moreover, we tested whether sleep structure before the baseline and simulated night work conditions in both meal timing groups affected the reported effects. We thus performed mixed-model analyses of variance including sleep structure (i.e., wake between lights off and lights on, NREM sleep stages 1 to 3, REM sleep, sleep efficiency, and total sleep time) as covariates. Last, we performed a linear regression model to assess whether there was an association between the magnitudes of internal circadian misalignment (change from baseline CR to postmisalignment CR) with impaired glucose tolerance during circadian misalignment (change from baseline to simulated night work). Participant was included as a random factor. Post hoc comparisons were derived using the Tukey-Kramer test. Missing data were not included in the analyses (0.79 and 1.6% of observations for the 3-hour postprandial glucose and insulin profiles, respectively). To control overall type I error in null hypothesis testing when conducting multiple comparisons, P values from the mixed-model analysis for the primary outcomes of the CR and FD protocols were adjusted using false discovery rates (pFDR) (PROC MULTTEST, SAS). P values for primary outcomes of the CR and FD protocols correspond to pFDR. Unless specified, data correspond to the mean and SEM. Significance for all statistical tests was set as P < 0.05.
Acknowledgments
We thank the research volunteers and Center for Clinical Investigation nursing, as well as B. J. Lockyer for the polysomnography assessments and technical staff.
Funding: This study was funded by grant numbers NIH R01HL118601 (ClinicalTrials.gov number: NCT02291952), 1UL1TR001102, and 1UL1TR002541-01. F.A.J.L.S. was supported by NIH R01HL118601, R01DK099512, R01DK102696, R01DK105072, and R01HL140574. S.L.C. is supported by the Alexander Von Humboldt Foundation. J.Q. was supported by American Diabetes Association #1-17-PDF-103 and is supported by NIH K99HL148500. G.K.A. was supported by K24HL103845. M.G. was supported by The Spanish Government of Investigation, Development and Innovation (SAF2017-84135-R), the Autonomous Community of the Region of Murcia through the Seneca Foundation (20795/PI/18), and NIDDK R01DK105072. S.A.S. was supported by R01HL125893, R01HL142064, R01HL125893-03S1, R01HL140577, and the Oregon Institute of Occupational Health Sciences (ORS 656.630). C.A.C. was supported in part by NIH grant R01HL118601.
Author contributions: Conceptualization: F.A.J.L.S. Funding acquisition: F.A.J.L.S. Investigation: S.L.C., J.Q., N.V., C.J.M., A.N., H.N., N.R., S.W.H., L.K., K.K.-M., and S.S. Visualization: S.L.C., H.N., N.R., S.W.H., L.K., K.K.-M., and S.S. Project administration: S.L.C., J.Q., N.V., C.J.M., and A.N. Supervision: F.A.J.L.S. Data curation: F.A.J.L.S. Formal analysis: S.L.C. and W.W. Software: W.W. Validation: F.A.J.L.S. Writing—review and editing: S.L.C., J.Q., N.V., C.J.M., A.N., H.N., N.R., S.W.H., L.K., K.K.-M., S.S., W.W., D.A., C.A.C., S.A.S., G.K.A., M.G., and F.A.J.L.S.
Competing interests: C.J.M. is an employee of and holds stock/stock options in Biogen. This employment is not related to the current work. F.A.J.L.S. has received lecture fees from Bayer HealthCare, Sentara HealthCare, Philips, Vanda Pharmaceuticals, and Pfizer Pharmaceuticals. G.K.A. received consulting fees from Pfizer Pharmaceuticals. C.A.C. reports grants/contracts to the Brigham and Women’s Hospital from Dayzz Live Well, Delta Airlines, Jazz Pharma, Puget Sound Pilots, and Regeneron Pharmaceuticals/Sanofi; research/education gifts through Brigham and Women’s Hospital from Arbor Pharmaceuticals, Avadel Pharmaceuticals, Bryte, Alexandra Drane, DR Capital Ltd, Eisai, Harmony Biosciences, Jazz Pharmaceuticals, Johnson & Johnson, Mary Ann & Stanley Snider via Combined Jewish Philanthropies, NeuroCare, Inc., Optum, Philips Respironics, Regeneron, Regional Home Care, ResMed, San Francisco Bar Pilots, Sanofi, Schneider, Simmons, Sleep Cycle, Sleep Number, Sysco, Teva Pharmaceuticals, and Vanda Pharmaceuticals; has an equity interest in Vanda Pharmaceuticals; receives royalties from Philips Respironics; and is the incumbent of an endowed professorship given to Harvard by Cephalon. C.A.C. is/was a paid consultant, speaker and/or advisor for: Institute of Digital Media and Child Development, Klarman Family Foundation, National Council for Mental Wellbeing, National Sleep Foundation, Physician’s Seal, SRS Foundation, Tencent, Teva Pharma Australia, With Deep, and Vanda Pharmaceuticals; is/was an expert witness in legal cases, including those involving Advanced Power Technologies, Aegis Chemical Solutions, Amtrak, Casper Sleep Inc, C&J Energy Services, Catapult Energy Services Group, Covenant Testing Technologies, Dallas Police Association, Enterprise Rent-A-Car, Eagle Transport Group/Steel Warehouse Inc, FedEx, Greyhound, PAR Electrical Contractors, Product & Logistics Services LLC, Puckett EMS, Puget Sound Pilots, Union Pacific Railroad, UPS, and Vanda Pharmaceuticals; and received travel support from Aspen Brain Institute, Bloomage International, Dr. Stanley Ho Medical Development Foundation, and the German National Academy of Sciences. F.A.J.L.S., G.K.A., and C.A.C. interests were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. F.A.J.L.S., G.K.A., and C.A.C. consultancies/lectures are not related to the current work. The other authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. As per the NIH Policy on Data Sharing, we will make the datasets available to other investigators following publication of the final study results. These datasets will not contain identifying information per the regulations outlined in HIPPA. Per standard Partners HealthCare System policies, we will require from any investigator or entity requesting the data a data-sharing agreement that provides for (i) a commitment to using the data only for research purposes and not to identify any individual participant, (ii) a commitment to securing the data using appropriate computer technology, and (iii) a commitment to destroying or returning the data after analyses are completed.
Supplementary Materials
This PDF file includes:
Supplementary Results
Figs. S1 to S9
Tables S1 to S2
REFERENCES AND NOTES
- 1.NHIS, 2010 National Health Interview Survey. Public-use data file and documentation (2010); ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHIS/2010/samadult_freq.pdf [accessed 19 February 2020].
- 2.A. Parent-Thirion, I. Biletta, J. Cabrita, O. Vargas Llave, G. Vermeylen, A. Wilczyńska, M. Wilkens, Sixth European Working Conditions Survey—Overview report (2019); www.eurofound.europa.eu/publications/report/2016/working-conditions/sixth-european-working-conditions-survey-overview-report [accessed 19 February 2020].
- 3.Kroenke C. H., Spiegelman D., Manson J., Schernhammer E. S., Colditz G. A., Kawachi I., Work characteristics and incidence of type 2 diabetes in women. Am. J. Epidemiol. 165, 175–183 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Manodpitipong A., Saetung S., Nimitphong H., Siwasaranond N., Wongphan T., Sornsiriwong C., Luckanajantachote P., Mangjit P., Keesukphan P., Crowley S. J., Hood M. M., Reutrakul S., Night-shift work is associated with poorer glycaemic control in patients with type 2 diabetes. J. Sleep Res. 26, 764–772 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Vetter C., Dashti H. S., Lane J. M., Anderson S. G., Schernhammer E. S., Rutter M. K., Saxena R., Scheer F. A. J. L., Night shift work, genetic risk, and type 2 diabetes in the UK biobank. Diabetes Care 41, 762–769 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vetter C., Devore E. E., Ramin C. A., Speizer F. E., Willett W. C., Schernhammer E. S., Mismatch of sleep and work timing and risk of type 2 diabetes. Diabetes Care 38, 1707–1713 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shan Z., Li Y., Zong G., Guo Y., Li J., Manson J. A. E., Hu F. B., Willett W. C., Schernhammer E. S., Bhupathiraju S. N., Rotating night shift work and adherence to unhealthy lifestyle in predicting risk of type 2 diabetes: Results from two large US cohorts of female nurses. BMJ 363, k4641 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vetter C., Scheer F. A. J. L., A healthy lifestyle - Reducing T2DM risk in shift workers? Nat. Rev. Endocrinol. 15, 194–196 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leproult R., Holmback U., Van Cauter E., Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 63, 1860–1869 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris C. J., Purvis T. E., Mistretta J., Scheer F. A. J. L., Effects of the internal circadian system and circadian misalignment on glucose tolerance in chronic shift workers. J. Clin. Endocrinol. Metab. 101, 1066–1074 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris C. J., Yang J. N., Garcia J. I., Myers S., Bozzi I., Wang W., Buxton O. M., Shea S. A., Scheer F. A. J. L., Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. U.S.A. 112, E2225–E2234 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian J., Dalla Man C., Morris C. J., Cobelli C., Scheer F. A. J. L., Differential effects of the circadian system and circadian misalignment on insulin sensitivity and insulin secretion in humans. Diabetes Obes. Metab. 20, 2481–2485 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheer F. A., Hilton M. F., Mantzoros C. S., Shea S. A., Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. U.S.A. 106, 4453–4458 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Minguez J., Saxena R., Bandin C., Scheer F. A., Garaulet M., Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: A randomized, cross-over study. Clin. Nutr. 37, 1133–1140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant C. L., Coates A. M., Dorrian J., Kennaway D. J., Wittert G. A., Heilbronn L. K., Pajcin M., Della Vedova C., Gupta C. C., Banks S., Timing of food intake during simulated night shift impacts glucose metabolism: A controlled study. Chronobiol. Int. 34, 1003–1013 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Hatori M., Vollmers C., Zarrinpar A., DiTacchio L., Bushong E. A., Gill S., Leblanc M., Chaix A., Joens M., Fitzpatrick J. A. J., Ellisman M. H., Panda S., Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 15, 848–860 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salgado-Delgado R., Angeles-Castellanos M., Saderi N., Buijs R. M., Escobar C., Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology 151, 1019–1029 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Arble D. M., Bass J., Laposky A. D., Vitaterna M. H., Turek F. W., Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 17, 2100–2102 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fonken L. K., Workman J. L., Walton J. C., Weil Z. M., Morris J. S., Haim A., Nelson R. J., Light at night increases body mass by shifting the time of food intake. Proc. Natl. Acad. Sci. U.S.A. 107, 18664–18669 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalsbeek A., Scheer F. A., Perreau-Lenz S., la Fleur S. E., Yi C. X., Fliers E., Buijs R. M., Circadian disruption and SCN control of energy metabolism. FEBS Lett. 585, 1412–1426 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salgado-Delgado R., Angeles-Castellanos M., Buijs M. R., Escobar C., Internal desynchronization in a model of night-work by forced activity in rats. Neuroscience 154, 922–931 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Damiola F., le Minh N., Preitner N., Kornmann B., Fleury-Olela F., Schibler U., Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 14, 2950–2961 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escobar C., Cailotto C., Angeles-Castellanos M., Delgado R. S., Buijs R. M., Peripheral oscillators: The driving force for food-anticipatory activity. Eur. J. Neurosci. 30, 1665–1675 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Skene D. J., Skornyakov E., Chowdhury N. R., Gajula R. P., Middleton B., Satterfield B. C., Porter K. I., van Dongen H. P. A., Gaddameedhi S., Separation of circadian- and behavior-driven metabolite rhythms in humans provides a window on peripheral oscillators and metabolism. Proc. Natl. Acad. Sci. U.S.A. 115, 7825–7830 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wehrens S. M. T., Christou S., Isherwood C., Middleton B., Gibbs M. A., Archer S. N., Skene D. J., Johnston J. D., Meal timing regulates the human circadian system. Curr. Biol. 27, 1768–1775.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duffy J. F., Dijk D. J., Getting through to circadian oscillators: Why use constant routines? J. Biol. Rhythm. 17, 4–13 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Czeisler C. A., Duffy J. F., Shanahan T. L., Brown E. N., Mitchell J. F., Rimmer D. W., Ronda J. M., Silva E. J., Allan J. S., Emens J. S., Dijk D. J., Kronauer R. E., Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 284, 2177–2181 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Krauchi K., How is the circadian rhythm of core body temperature regulated? Clin. Auton. Res. 12, 147–149 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Anothaisintawee T., Reutrakul S., Van Cauter E., Thakkinstian A., Sleep disturbances compared to traditional risk factors for diabetes development: Systematic review and meta-analysis. Sleep Med. Rev. 30, 11–24 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Albrecht U., Timing to perfection: The biology of central and peripheral circadian clocks. Neuron 74, 246–260 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Hawley J. A., Sassone-Corsi P., Zierath J. R., Chrono-nutrition for the prevention and treatment of obesity and type 2 diabetes: From mice to men. Diabetologia 63, 2253–2259 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Crosby P., Hamnett R., Putker M., Hoyle N. P., Reed M., Karam C. J., Maywood E. S., Stangherlin A., Chesham J. E., Hayter E. A., Rosenbrier-Ribeiro L., Newham P., Clevers H., Bechtold D. A., O’Neill J. S., Insulin/IGF-1 drives PERIOD synthesis to entrain circadian rhythms with feeding time. Cell 177, 896–909.e20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.West A. C., Bechtold D. A., The cost of circadian desynchrony: Evidence, insights and open questions. BioEssays 37, 777–788 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eckel-Mahan K. L., Patel V. R., de Mateo S., Orozco-Solis R., Ceglia N. J., Sahar S., Dilag-Penilla S. A., Dyar K. A., Baldi P., Sassone-Corsi P., Reprogramming of the circadian clock by nutritional challenge. Cell 155, 1464–1478 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foppen E., Tan A. A., Ackermans M. T., Fliers E., Kalsbeek A., Suprachiasmatic nucleus neuropeptides and their control of endogenous glucose production. J. Neuroendocrinol. 28, (2016). [DOI] [PubMed] [Google Scholar]
- 36.Guan D., Xiong Y., Trinh T. M., Xiao Y., Hu W., Jiang C., Dierickx P., Jang C., Rabinowitz J. D., Lazar M. A., The hepatocyte clock and feeding control chronophysiology of multiple liver cell types. Science 369, 1388–1394 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buxton O. M., Cain S. W., O’Connor S. P., Porter J. H., Duffy J. F., Wang W., Czeisler C. A., Shea S. A., Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med. 4, 129ra143 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spiegel K., Knutson K., Leproult R., Tasali E., Van Cauter E., Sleep loss: A novel risk factor for insulin resistance and Type 2 diabetes. J. Appl. Physiol. 99, 2008–2019 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Tasali E., Leproult R., Ehrmann D. A., Van Cauter E., Slow-wave sleep and the risk of type 2 diabetes in humans. Proc. Natl. Acad. Sci. U.S.A. 105, 1044–1049 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American Diabetes Association , 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2020. Diabetes Care 43, S14–S31 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Bogdanet D., O’Shea P., Lyons C., Shafat A., Dunne F., The oral glucose tolerance test—Is it time for a change?—A literature review with an emphasis on pregnancy. J. Clin. Med. 9, 3451 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horton T. J., Hill J. O., Prolonged fasting significantly changes nutrient oxidation and glucose tolerance after a normal mixed meal. J. Appl. Physiol. 90, 155–163 (2001). [DOI] [PubMed] [Google Scholar]
- 43.Jørgensen S. W., Hjort L., Gillberg L., Justesen L., Madsbad S., Brøns C., Vaag A. A., Impact of prolonged fasting on insulin secretion, insulin action, and hepatic versus whole body insulin secretion disposition indices in healthy young males. Am. J. Physiol. Endocrinol. Metab. 320, E281–E290 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bergman R. N., Finegood D. T., Kahn S. E., The evolution of beta-cell dysfunction and insulin resistance in type 2 diabetes. Eur. J. Clin. Investig. 32( Suppl 3), 35–45 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Asher G., Sassone-Corsi P., Time for food: The intimate interplay between nutrition, metabolism, and the circadian clock. Cell 161, 84–92 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Lowden A., Moreno C., Holmback U., Lennernas M., Tucker P., Eating and shift work—Effects on habits, metabolism and performance. Scand. J. Work Environ. Health 36, 150–162 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Kosmadopoulos A., Kervezee L., Boudreau P., Gonzales-Aste F., Vujovic N., Scheer F. A. J. L., Boivin D. B., Effects of shift work on the eating behavior of police officers on patrol. Nutrients 12, 999 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bjorvatn B., Stangenes K., Øyane N., Forberg K., Lowden A., Holsten F., Åkerstedt T., Subjective and objective measures of adaptation and readaptation to night work on an oil rig in the North Sea. Sleep 29, 821–829 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Harris A., Waage S., Ursin H., Hansen Å. M., Bjorvatn B., Eriksen H. R., Cortisol, reaction time test and health among offshore shift workers. Psychoneuroendocrinology 35, 1339–1347 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Folkard S., Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm. Chronobiol. Int. 25, 215–224 (2008). [DOI] [PubMed] [Google Scholar]
- 51.Dumont M., Benhaberou-Brun D., Paquet J., Profile of 24-h light exposure and circadian phase of melatonin secretion in night workers. J. Biol. Rhythm. 16, 502–511 (2001). [DOI] [PubMed] [Google Scholar]
- 52.Garde A. H., Begtrup L., Bjorvatn B., Bonde J. P., Hansen J., Hansen Å. M., Härmä M., Jensen M. A., Kecklund G., Kolstad H. A., Larsen A. D., Lie J. A., Moreno C. R. C., Nabe-Nielsen K., Sallinen M., How to schedule night shift work in order to reduce health and safety risks. Scand. J. Work Environ. Health 46, 557–569 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.K. V. Mardia, P. E. Jupp, Directional Statistics (Wiley Series in Probability and Statistics, 1999). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Results
Figs. S1 to S9
Tables S1 to S2