Abstract
Background
Long-term suppression of SARS-CoV-2 transmission will involve strategies that recognize the heterogeneous capacity of communities to undertake public health recommendations. We highlight the epidemiological impact of barriers to adoption and the potential role of community-led coordination of support for cases and high-risk contacts in urban slums.
Methods
A compartmental model representing transmission of SARS-CoV-2 in urban poor versus less socioeconomically vulnerable subpopulations was developed for Montserrado County, Liberia. Adoption of home-isolation behavior was assumed to be related to the proportion of each subpopulation residing in housing units with multiple rooms and with access to sanitation, water, and food. We evaluated the potential impact of increasing the maximum attainable proportion of adoption among urban poor following the scheduled lifting of the state of emergency.
Results
Without intervention, the model estimated higher overall infection burden but fewer severe cases among urban poor versus the less socioeconomically vulnerable population. With self-isolation by mildly symptomatic individuals, median reductions in cumulative infections, severe cases, and maximum daily incidence were 7.6% (IQR: 2.2%−20.9%), 7.0% (2.0%−18.5%), and 9.9% (2.5%−31.4%), respectively, in the urban poor subpopulation and 16.8% (5.5%−29.3%), 15.0% (5.0%−26.4%), and 28.1% (9.3%−47.8%) in the less socioeconomically vulnerable population. An increase in the maximum attainable percentage of behavior adoption by the urban slum subpopulation was associated with median reductions of 19.2% (10.1%−34.0%), 21.1% (13.3%−34.2%), and 26.0% (11.5%−48.9%) relative to the status quo scenario.
Conclusions
Post-lockdown recommendations that prioritize home-isolation by confirmed cases are limited by resource constraints. Investing in community-based initiatives that coordinate support for self-identified cases and their contacts could more effectively suppress COVID-19 in settings with socioeconomic vulnerabilities.
Key Words: Health behavior, Social ecology, SARS-CoV-2, Liberia, Community engagement, Urban slum communities
1. Background
During the ongoing novel coronavirus (COVID-19) pandemic, tradeoffs among the feasibility, ethics, and effectiveness of mandatory social distancing in the form of large-scale lockdowns have been debated globally (Lashitew, 2020, Partnership for Evidence- Based Response to COVID-19 (PERC), 2020, Kelley et al., 2020, Massinga Loembé et al., 2020). Social distancing measures create socioeconomic constraints for societies and individuals (Contagion or starvation, 2020). In turn, the ability to effectively undertake such measures is dependent on access to essential resources, such as food, sanitation facilities, and physical space. In the context of low and middle-income countries (LMICs) where relatively less COVID-19 morbidity and mortality is being reported (Skrip et al., 2021), the consideration of more targeted rather than sweeping approaches to mitigation could limit the socioeconomic impact of the pandemic on development efforts.
To reduce the socioeconomic consequences of large-scale responses to the COVID-19 pandemic, several LMICs have developed context-specific adaptations of standard approaches to control (African Adaptations to the COVID-19 Response – Africa Center, 2020), from harnessing the benefits of coordinated public-private partnerships (South African Solidarity Response Fund and Uniting The Nation, 2020) to promoting food delivery as a means of reducing congestion in marketplaces to conducting online funerals (Adamu, 2020). In parallel, as part of COVID-19-specific surveillance, LMICs are trying new approaches for passive and active case detection (Drew et al., 2020, Liberia: Public Health Institute, 2020, Rosman, 2020). For the latter, rapid assessment systems (RAS) have been introduced as a means for self-determination of risk and then communication of guidance so that individuals can seek care and undertake personal protective measures to prevent transmission (Liberia: Public Health Institute, 2020, mz-info[at]ux[dot]co[dot]mz U co, 2020). With increasing specificity and sensitivity of symptom-based case definitions for COVID-19 (Menni et al., 2020, Menni et al., 2020), such tools hold potential for efficiently signaling deployment of testing and contact tracing teams, as well as of resources to facilitate participation in public health recommendations.
Notification via RAS would allow for deployment of goods to enable adoption of home quarantine (among high-risk contacts) or isolation (for confirmed, mildly symptomatic or presymptomatic cases). For instance, provision of curtains, masks, food, water, and chamber toilets to people identified as high-risk contacts through RAS alerts could support individuals in socioeconomically vulnerable populations to participate in recommendations for home-based social distancing and care. Independent efforts are being organized to support vulnerable populations during periods of lockdown, with public and private organizations undertaking distribution of basic goods at different scales (e.g. Administrator (2020; Jerving, 2020; Associated Press, 2020; Fox, 2020),). A more coordinated approach across organizations and response pillars—such as through triggering of resource deployment through a centralized RAS or other alert system—to identify, inform, and support presumptive cases in adopting transmission-reducing behaviors could provide one strategy contributing to long-term suppression of the epidemic curves in LMIC contexts without requiring potentially detrimental, population-level lockdowns.
As a long-term strategy that would persist after lifting large-scale lockdowns or states of emergency, such coordination and resources could be managed in partnership with communities. Community-driven surveillance efforts were effective in identifying Ebola transmission clusters during the outbreak in West Africa (Fallah et al., 2017, Wolfe et al., 2017). Additionally, local action had far-reaching and sustained implications for behavior change (Skrip et al., 2020, Bedson et al., 2019). With rising stigma (Camille Malplat With, 2020) against COVID-19 survivors and even against those who are taking precautions, such as wearing face masks, local ownership of response efforts will be critical for effective scale-up. Community-based initiatives, with support from the national response, are more likely to reduce disparities in detection and resource allocation across socioeconomically diverse subpopulations by addressing barriers such as local distrust of authorities (van de Vijver et al., 2015). They could also lead to innovative, context-specific solutions to supplement symptom-based surveillance. Surveillance particularly in urban slum settings with high transmission potential yet significantly younger populations than less socioeconomically vulnerable settings (Bennett et al., 2016, Riley et al., 2007).
Here we present a dynamic transmission modeling framework, parameterized for the SSA context, to evaluate longer-term strategies for case detection and intervention after the lifting of lockdowns. We account for the heterogeneous impact of contact tracing and home isolation strategies across settings with varying capacity to implement the strategies. In particular, we account for household-level access to the food, water, sanitation, and required space needed for home isolation. We also account for variation in detection rates due to differential proportions of symptomatic cases with severe disease across urban subpopulations. To the best of our knowledge, this is the first model to quantify how disparate behavior adoption due to urban poverty leads to disparate gains from response measures during outbreaks. While parameterized with COVID-19-specific epidemiological and response data, the model incorporates data collected through response efforts during the Ebola outbreak in Liberia, and the proposed strategies reflect lessons learned from efforts during the Ebola outbreak aimed at facilitating mitigation efforts across socioeconomically diverse settings.
2. Methods
2.1. Model overview
A compartmental model was developed to investigate the impact of interventions addressing barriers to adoption of home-based quarantine and isolation in urban slum settings ( Fig. 1). The model was implemented as a series of ordinary differential equations with stochasticity coming from parameter values randomly sampled from published distributions to account for uncertainty (Tables 1–2). Model equations are provided in the Supplementary Material.
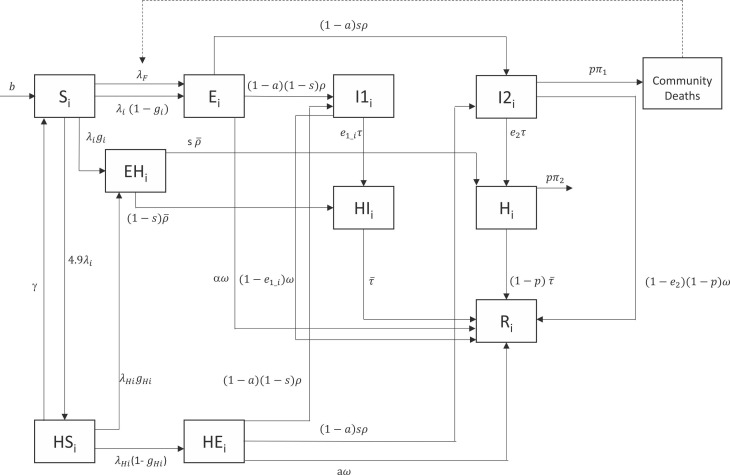
Fig. 1.
Compartmental diagram of dynamic transmission model (single subpopulation stratum, labeled subscript i). In each subpopulation (urban poor or less socioeconomically disadvantaged), susceptible individuals (S) may become infected (E) and remain asymptomatic, develop mild symptoms (I1), or develop severe symptoms (I2). Infected individuals who recover (R) may first undertake home isolation (HI) or hospitalization (H). Severely ill individuals who do not seek care may recover or die in the community. As a result of contact tracing efforts, contacts of newly infected individuals may remove themselves from the community via quarantine (EH) during the incubation period, before continuing to isolate or seek treatment if they develop symptoms. Household members (HS) of newly infected cases are expected to be at higher risk and become infected (HE) at a higher rate than community contacts.
The base SEIR transmission model was stratified into two subpopulations to represent separate dynamics for (1) the subpopulation composed of urban poor (i.e., residents of slum communities) and (2) the subpopulation composed of residents from less socioeconomically vulnerable communities. We considered informal settlements in Liberia (i.e., West Point, New Kru Town, Clara Town, Logan Town, Jallah, Slipway, and Peace Island, Figure S1) as constituting the urban slum subpopulation based on the definition outlined by Monrovia City Corporation’s Slum Initiative (Cole, 2008) and previous work on urban poor communities in Monrovia (Fallah et al., 2015). These communities are characterized by overcrowding, availability of but limited accessibility to electricity and water, limited sanitation, and poor housing; using data from the most recent census and correcting for underrepresentation (Cole, 2008, Liberia Institute of Statistics and Geo-Information Services, 2008), our urban poor subpopulation constituted approximately 20% of the county’s population (Supplementary Material Methods). Drawing from data and observations during the Ebola outbreak in Liberia, cases living in slum communities were expected to have greater numbers of contacts, higher transmission rates due to nature of contacts amidst overcrowding, and to seek care after longer delays than those in less socioeconomically vulnerable settings (Tables 1–2) (Fallah et al., 2015). In addition, based on gender-specific trends in aging, out-migration, and mortality in urban slum communities (Bennett et al., 2016), differential detection and proportions of symptomatic cases with severe disease were assumed between the subpopulations, as described below. Slum communities in Liberia are also characterized by a strong network of informal leaders who held roles in supervising active case finding efforts, conducted by peer-nominated community members and in coordination with national surveillance efforts, during the Ebola outbreak (Fallah et al., 2016). This network was reactivated during the COVID-19 outbreak.
Initial cases were modeled as importations into the less socioeconomically vulnerable subpopulation. For each time step from the first known introduction on March 16 until the airport was closed on April 10, infection importation occurred with a randomly drawn probability to account for possible infections from air travel passengers after discharge from institutional quarantine. Introduction into the seven urban slum communities was assumed to occur after some delay and via either contact between the two subpopulations or, after initial seeding, between urban slum communities within the subpopulation (More details in the Supplementary Material Methods), until all communities had cases and local transmission at the subpopulation-level dominated.
Within each subpopulation, those who are susceptible and not household members of an existing case were infected according to the force of infection, :
| (1) |
where is the rate of infection upon contact with an infectious case from subpopulation i and is the number of contacts per person. Contact probabilities () within and between SES subpopulations were derived from empirical contact matrices reported for Montserrado County in August 2014 (Fallah et al., 2015). Specifically, the contact matrices reflect contacts between Ebola cases and contacts towards the beginning of the outbreak in Monrovia. For each iteration of the model, the number of contacts () per susceptible individual in subpopulation i was drawn from distributions derived from contact tracing data collected during the Ebola outbreak in Liberia. The final term of the force of infection equation reflects the prevalence of infection in subpopulation , after accounting for the relative infectiousness () of asymptomatic to mildly symptomatic (i.e., is 1/1.6 times as infectious as He et al., (2020)) and the relative infectiousness () of severely symptomatic to mildly symptomatic (, 1.1 times as infectious as Liu et al., (2020)) individuals.
Household members of new infections, move to a separate susceptible compartment with a higher rate of infection derived from a study of household contacts of cases in China (, Supplementary Material Table 1) (Bi et al.,, Papenburg et al., 2010). We assumed a Poisson distribution of individuals per household, such that households of new infections were represented as the average number of individuals per household in Liberia (4.9 individuals (Household, 2020)) times the number of new infections in the community (Supplementary Material Methods). It has been found that the average household size tends to consistently be about 5 people across both formal and informal settings within Monrovia. Differences across settings more often tends to be observed in terms of the number of rooms occupied by households, with most low income areas having single-room dwellings (United Nations Human Settlements Programme UN-Habitat, 2014).
A percentage of all infections remain asymptomatic and the rest develop symptoms at a rate reflecting the incubation period. For the latter, we considered two scenarios. Based on evidence available from high transmission settings, we investigated one assumption that 81% of cases with any symptoms in the less socioeconomically vulnerable subpopulation remained mildly symptomatic () (CDC COVID-19 Response Team, 2020, Wu and McGoogan, 2020), while 19% were assumed to develop severe symptoms (). In addition, to account for differing age structures and underlying comorbidities in West Africa, we ran the model to reflect the scenario with a lower percentage, s = 15%, of symptomatic individuals assumed to develop severe symptoms (Skrip and Hagedorn, 2020). As the latter assumption better reflected the observation of less symptomicity in SSA than in countries across Europe, Asia, and the Americas, we present the results of the analyses based on s = 15% in the main text and include the findings from the assumption of = 19% in the Supplementary Material. Those cases with severe symptoms had a rate of disease-associated death (). Mild cases and severe cases who survive the virus recover at a rate . Recovered individuals remained immune, in the absence of clear evidence about duration of protection (Kirkcaldy et al., 2020).
For the urban slum subpopulation, we assumed that the percentage of symptomatic cases with severe symptoms would be lower. The average age of slum-dwelling adults is lower than that of individuals in less socioeconomically disadvantaged populations. This is, in part, due to trends in out-migration among older adults, particularly females in the 50 + age range, from slum-dwelling populations in sub-Saharan Africa (SSA) (Bennett et al., 2016). Given the age-dependent gender ratio for severe COVID-19 disease (Qian et al., 2020, Sobotka et al., 2020), the concentration of the population in younger age groups and higher proportion female among old age groups could lead to significant undetected, and therefore unsupported, asymptomatic or mildly symptomatic disease in slum communities. Accordingly, to account for these population dynamics in the urban poor subpopulation, we adjusted by a factor equal to the ratio of crude death rates among 50 + y.o. individuals in urban slums versus the entire population, as determined by a study in Nairobi (i.e., ).
The transmission model was structured to reflect response efforts being undertaken in Liberia to date. The impact of contact tracing on transmission was modeled by having a subset of new infections immediately removed from the infectious population; that is, a percentage () of pre-symptomatic individuals would undertake quarantine and remain isolated through recovery or death, either at home or in a hospital depending on severity of symptoms, due to contact tracing. The impact of contact tracing was assumed to be time-varying and account for both the effectiveness of intervention in accessing contacts that ultimately became cases as well as the level of distrust or denial in the population around COVID-19 which was assumed to affect willingness to isolate among those traced (More details in Supplementary Material). Additionally, among cases who develop severe symptoms but were not listed as contacts, a time-varying percentage (), assumed to be a maximum 30% based on observations in care-seeking during the Ebola outbreak, undergo isolation in a hospital at a rate () or may remain in the community. While high infection rates among healthcare workers in Liberia is indicative that nosocomial transmission is occurring, we made a simplifying assumption that hospitalization removed individuals from the community-level infectious population; home-isolation reduced the infectiousness of individuals by 80% to reflect estimates for the effectiveness of mask-wearing (Kai et al., 2020). Rates of isolation () were drawn from subpopulation-specific distributions reflecting days to care-seeking during the Ebola outbreak (Fallah et al., 2015) (and consistent with observations for care-seeking after onset of COVID-19 in China (Li et al., 2020)) and percentages of isolation adoption () were subpopulation-specific based on access to resources to do so.
It is expected that realities around overcrowding and shared facilities may inhibit the ability to adopt home isolation recommendations with fidelity, regardless of willingness to do so. Therefore, the percentage of cases adopting home-isolation behavior was assumed to be related to the proportion of the subpopulation residing in housing units with multiple rooms, access to sanitation facilities, and access to basic goods like water and food (Supplementary Material Table 2). These factors would differentially affect the two subpopulations in the model. Data on needs around water, food, and toilet access in informal urban settlements versus less socioeconomically vulnerable urban communities were derived from surveys conducted in Kenya (Kimani-Murage et al., 2014, African Population and Health Research Center (APHRC), 2014). In the less socioeconomically vulnerable urban communities, adoption of home quarantine or isolation would be undertaken by the percentage of respondents from Liberia who had indicated that it was very easy, easy, or neither easy nor difficult to adhere to social distancing on a crowdsourcing survey (COVID-19 - Social Distancing, 2020), as respondents were assumed to be more likely representative of this subpopulation than the urban poor subpopulation.
A separate compartment for community deaths (i.e., severely sick cases who did not seek care and died at home) was used to incorporate transmission clusters due to funerals, as has been observed for SARS-CoV-2 due to the aggregation of high numbers of attendees (Ghinai et al., 2020, Curran et al., 2016). A number of contacts was drawn for each community death based on the number of individuals in attendance at unsafe burials (i.e., traditional burials occurring after recommendations around safe burials were in place) reported in West Africa during the Ebola outbreak and were infected at a rate equal to for representing the prevalence of infection at each time for subpopulation . The distribution of traditional burial attendees for community deaths reflects data from after policies were put into effect for safe burials and therefore were expected to reflect the present case in Liberia despite policies limiting funeral attendance to 10 individuals. The Liberian government also instituted policies to reduce numbers of attendees at religious gatherings, with public support from religious leaders and the Council of Churches (Liberia, 2020), and at sporting events. We assumed that institution-level, organized events would have greater enforcement of lockdown-related policies and did not explicitly include those in the model.
2.2. Model fitting
An expression for was derived using the next generation matrix approach (NGM) (Diekmann et al., 2010) assuming introduction and initial spread in the less socioeconomically vulnerable subpopulation was calibrated to achieve an average subpopulation-level of 2.4 (SD: 0.2). A likelihood-based approach was used to compare model output across sets of parameters, including the transmission parameter , calibrated using NGM to cumulative case data from Montserrado County, Liberia (Supplementary Material Methods). Lastly, Bayesian melding (Poole and Raftery, 2000) was implemented to probabilistically sample 1000 parameter sets according to the weight of their normalized negative log likelihood values for subsequent model projections to evaluate intervention scenarios.
2.3. Intervention scenarios
For all intervention scenarios, we ran the status quo model until the end of the state of emergency and partial lockdown slated for July 21 (after a 30-day extension was declared by the President on June 22 (Liberia extends COVID-19, 2020)), or 128 days post-introduction into Liberia, and then introduced the intervention with scale-up for one month (according to a step function with a uniform percentage change every 5 days), followed by maximum coverage for the subsequent three weeks. The status quo scenario was based around care-seeking by severely symptomatic individuals or isolation of high-risk contacts due to top-down initiatives through contact tracing. We evaluated the difference in cumulative incidence and maximum daily incidence over a 180-day (approximately, six months from introduction) period between each curve from the status quo scenario and the curve with corresponding parameter set for each intervention scenario.
Under the intervention scenario of Self-identification without support, we investigated how community-level efforts with self-identification, such as through RAS, by individuals not on contact tracing lists and with mild symptoms would lead to greater and more proactive coverage of self-isolation by community members. Home isolation by mildly symptomatic individuals would be limited by the subpopulation-specific maximum attainable percentage based on resource constraints (Table 2).
Under the intervention scenario of Self-identification with community-driven support, we modeled coordinated efforts to provide food, water, chamber toilets, masks and other materials necessary for undertaking home-based quarantine or isolation. This was incorporated as an increase in the percentage of adoption () in the urban poor subpopulation. We assumed that the provision of these materials was coordinated through community engagement efforts that were also associated with improved overall adoption of public health recommendations (Skrip et al., 2020). This was modeled across varying reductions in the rates of isolation and treatment-seeking and in the number of contacts attending funerals. Specifically, we explored the role of support in reducing the time (in days) to isolation and the number of funeral attendees each by 25%, 50%, and 75%.
Thus, without support, the maximum attainable percentage of the urban poor subpopulation adopting home isolation or quarantine was determined by the percentage of the subpopulation having resources to do so, namely, access to sanitation, food, water, and physical space needed to safely and effectively do so. With support, the percentage adoption was modeled to reflect equal adoption across the entire population. That is, the percentage of adoption () in the urban poor subpopulation is increased to equal the maximal attainable percentage of adoption in the less socioeconomically disadvantaged population (Table 2). With support and community engagement, there would be additional gains due to further behavior change in the form of quicker care-seeking and more observance of restrictions around public gatherings, namely funerals.
3. Results
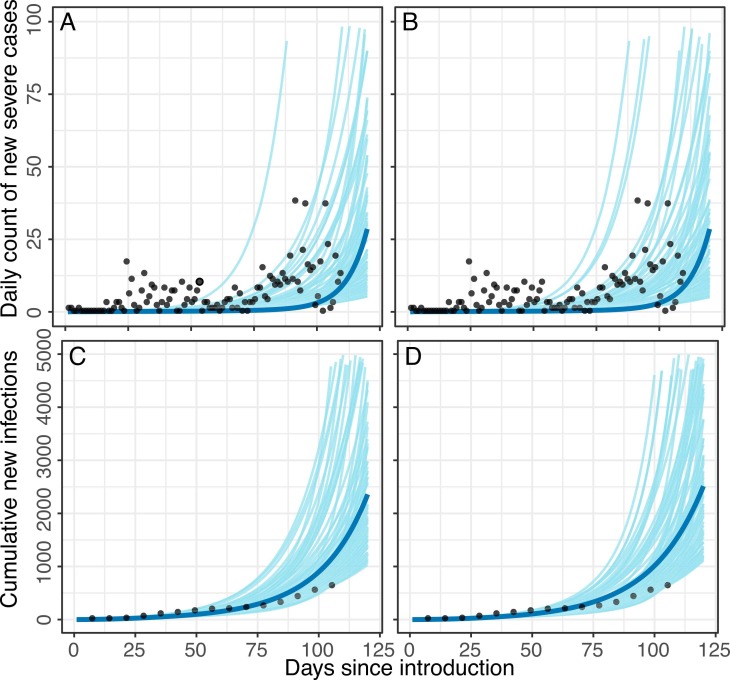
Given uncertainty in actual infection rates due to likely underreporting, a range of probable epidemic curves, accounting for social distancing (i.e., partial lockdown and school closures) and contact tracing measures in place, were considered across intervention scenarios (Fig. 1). Accounting for differential transmission probabilities, contact numbers, mechanisms and timing of infection introduction, and proportion of severe cases, differently shaped and timed epidemic curves were observed for the two subpopulations ( Fig. 2). Here we present the results for the assumption of 15% severity among symptomatic cases in the less socioeconomically disadvantaged population; results for the assumption of 19% symptom severity are reflected in the Figures and Supplementary Material.
Fig. 2.
Modeled daily incidence of severe cases and cumulative infection counts in Montserrado County for assumptions of (A,C) 19% severity among symptomatic cases and (B,D) 15% severity among symptomatic cases. The blue line represents the median of all curves selected via the Bayesian melding approach, conducted separately for each assumption of severity percentage. Gray curves represent 100 random draws from parameter sets selected via Bayesian melding. Black dots represent data points (daily reported cases or cumulative reported cases at weekly intervals) per reports of confirmed cases from the National Public Health Institute of Liberia.
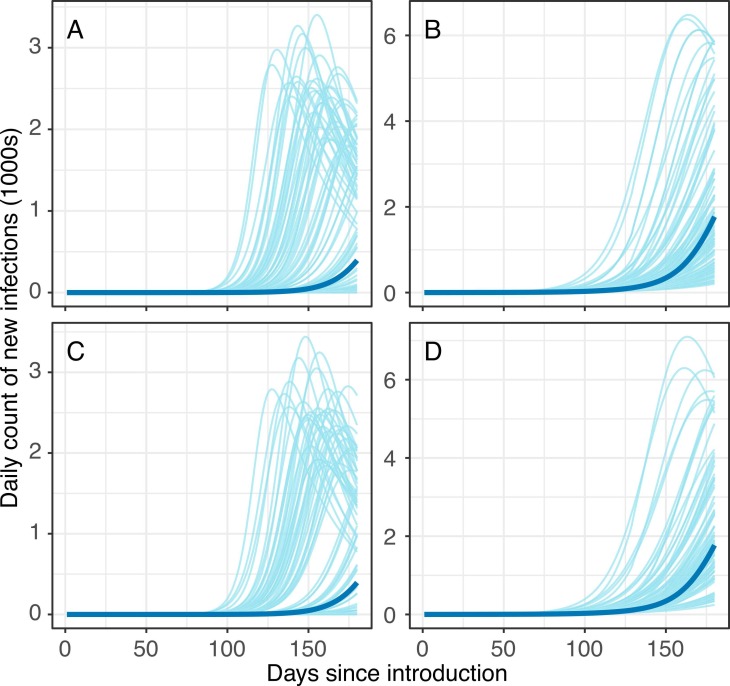
At 150-days post-introduction, the model estimated a median cumulative 6996 infections (IQR: 114–90,324) and a median maximum daily incidence of 27 per 10,000 people (IQR: <1–121) in the subpopulation of urban poor; a median cumulative 37,304 infections (IQR: 19,259–64,318) and median maximum daily incidence of 18 per 10,000 people (IQR: 9–30) were estimated for the less socioeconomically vulnerable subpopulation -- i.e., a 50% difference in incidence across subpopulations ( Fig. 3).
Fig. 3.
Pre-intervention epidemic curves in the (A, C) urban poor subpopulation and (B, D) less socioeconomically vulnerable subpopulation. Gray lines represent 100 random draws from the curves that have 150-day cumulative infection counts in the interquartile range. Blue lines represent curves that are associated with median 150-day cumulative infection counts. Top row panels (A-B) reflect results from assumption of 19% severity among symptomatic cases and bottom row panels (C-D) reflect results from assumption of 15% severity among symptomatic cases.
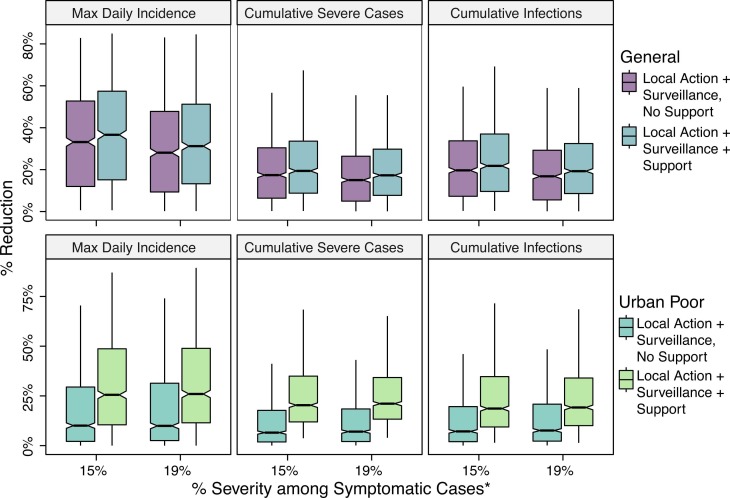
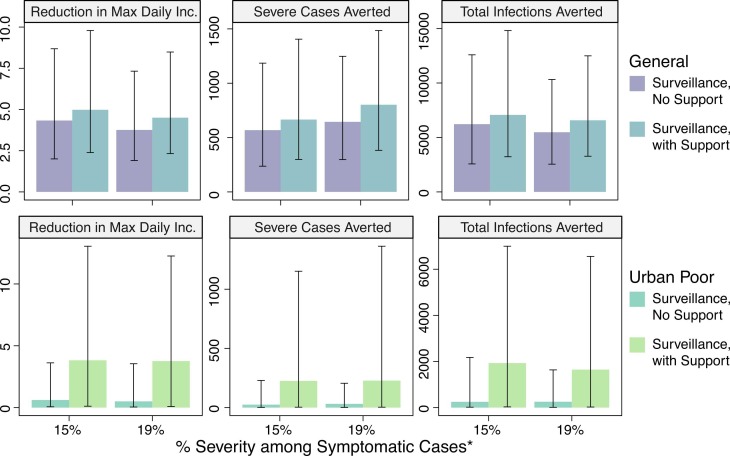
We evaluated reductions in incidence due to the introduction of home isolation of mild cases, such as due to self-protective measures in response to feedback from use of RAS or local action by families and communities in reaction to symptoms ( Fig. 4, Fig. 5). This was introduced after the lockdown ended (July 21) and was implemented under the assumptions that severely sick individuals continue to seek treatment in a hospital at rates consistent with those prior to the end of the lockdown and that contact tracing efficiency and effectiveness would likewise remain consistent. When taking into account differential access to resources, we found that the inability to implement social distancing recommendations meant the policies had limited impact for urban slum communities compared to other communities. In general, burden averted in the less socioeconomically vulnerable subpopulation was as much as three times greater than that in the urban poor subpopulation. Introduction of home isolation of mild cases without support was associated with 7.2% (2.0%−19.6%), 6.5% (IQR: 1.8%−17.7%), and 10.1% (IQR: 2.1%−29.5%) reductions in total infections, severe cases, and maximum daily incidence in the urban poor subpopulation and 19.7% (IQR: 7.3%−33.8%), 17.4% (IQR: 6.4%−30.4%), and 33.2% (IQR: 12.0%−52.7%) reductions in the less socioeconomically vulnerable subpopulation, relative to the status quo scenario. All results are included in Tables S1–S2 ( Fig. 6).
Fig. 4.
Percentage change in cumulative infections, cumulative severe cases, and maximum daily incidence (peak of the curve) with post-lockdown introduction of a symptom-based self-identification intervention, relative to continuation of status quo interventions alone. Subpopulation-specific impact of interventions with and without community-coordinated support efforts are presented. *Results are presented for different assumptions around relative proportions of severe versus mild symptomatic cases in the less socioeconomically vulnerable subpopulation (labeled “General population”).
Fig. 5.
Median change in cumulative infections, cumulative severe cases, and maximum daily incidence (peak of the curve) with post-lockdown introduction of a symptom-based self-identification intervention, relative to continuation of status quo interventions alone. Subpopulation-specific impact of interventions with and without community-coordinated support efforts are presented. *Results are presented for different assumptions around relative proportions of severe versus mild symptomatic cases in the less socioeconomically vulnerable subpopulation (labeled “General population”). Error bars represent interquartile range.
Fig. 6.
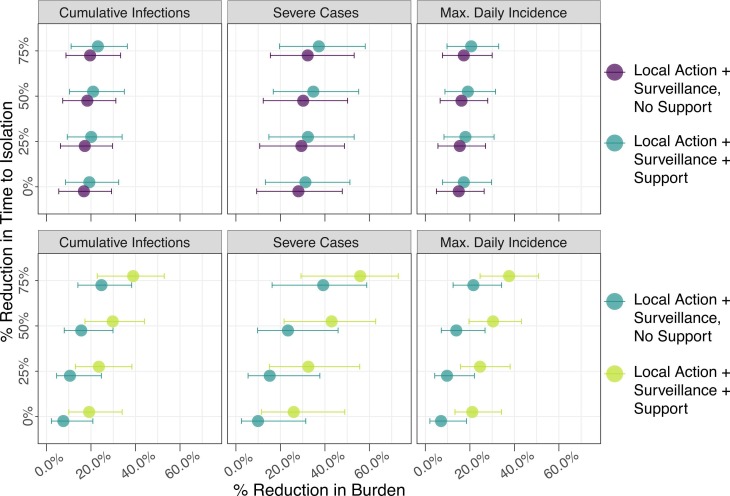
Percentage reduction in infections, cases of severe disease, and maximum daily incidence in the less socioeconomically vulnerable subpopulation (top panel, blue color) and urban poor subpopulation (bottom panel, green color) with introduction of efforts to suppress COVID-19 transmission. Interventions including contact tracing and surveillance efforts were evaluated across simultaneous reductions in time to isolation. Results reflect the assumption of 19% symptom severity.
For the urban poor subpopulation, the most significant increase in impact was observed with the introduction of support, by which the proportion of the subpopulation with necessary resources to safely and effectively quarantine or self-isolate was increased to that of the less socioeconomically vulnerable subpopulation. That is, the disparity in intervention impact was considerably reduced across SES groups. Median reductions in cumulative infections, severe cases, and maximum daily incidence were 18.6% (IQR: 9.4%−34.7%), 20.3% (IQR: 11.9%−35.0%), and 25.5% (10.5%−48.7%), respectively, relative to the status quo scenario (Fig. 4). This corresponds to a median 1933 (IQR: 30–6998) infections averted and 226 (IQR: 3–1152) severe cases averted. Additional impact was observed with simultaneous reductions in time to isolation and/or number of funeral contacts across all symptomatic cases (Fig. 5). However, even with support, 75% reduction in time to isolation, and 75% reduction in funeral contacts, transmission in the urban poor population was sustained at low levels. Nearly 60% of infections from the unmitigated scenario would still be expected in this scenario at 180-day post introduction.
For the less socioeconomically vulnerable subpopulation, the provision of support for the urban poor subpopulation was associated with further transmission reduction, although the added benefit of surveillance alone, relative to local action only, was more drastic than that of surveillance and support, relative to surveillance alone. Median percent reductions in cumulative infections, severe cases, and maximum daily incidence were 21.8% (IQR: 9.6%−37.0%), 19.4% (IQR: 8.8%−33.6%), and 36.6% (IQR: 15.1%−57.4%), respectively, relative to the status quo. These reductions correspond to averting 6575 (IQR: 3275–12,494) infections and 802 (IQR: 382–1485) severe cases.
For the urban poor subpopulation, the median cumulative number of mild cases over the 180-day period of the outbreak was estimated to be 1776 (IQR: 38–42,798) in the scenario by which support was provided. Given the assumption that the maximum attainable percentage of cases that can undertake home isolation is , this translates into 1204 (IQR: 26–29,013) total support packages. Across the outbreak period, the median maximum daily number of support packages needed under this scenario was 44 (IQR: 1–765).
4. Discussion
We developed a stratified compartmental modeling framework to consider adoption of home isolation and quarantine across socioeconomically diverse urban subpopulations. We assumed later viral introduction into the urban poor subpopulation and less severity of disease due to sociodemographic characteristics. The model also reflected assumptions around differential probability of transmission, contact patterns, and behaviors between socioeconomic subpopulations. Our findings suggest the potential for a more severe epidemic peak in the urban poor communities relative to less socioeconomically disadvantaged subpopulations in the absence of alternative, post-lockdown strategies. Currently observed effectiveness and efficiency of contact tracing would be insufficient for surveillance alone to contain the outbreak. Our results highlight how reducing heterogeneity in behavior adoption by supporting high-risk contacts and mildly symptomatic or asymptomatic individuals in urban poor areas to undertake recommendations could lead to nearly a 20% reduction in both infections and severe cases in that subpopulation, relative to the status quo approach. This is compared to less than 10% reductions in the absence of support. Additional gains due to quicker care-seeking would be expected with well-coordinated support efforts that built trust through community-led response.
We have incorporated model adaptations to reflect the Liberia-specific response and the context of West Africa in general, including potentially lower percentage of disease severity among cases, the role of funerals as high-transmission events due to close congregation of individuals and resistance to social distancing at such events, and the impact of constraints around food, water, physical space, and sanitation on adoption of social distancing interventions. Such demographic-based, behavior-based, and response-based model adaptations collectively have implications for more accurately capturing the overall transmission dynamics in settings such as urban slum communities in Liberia. For instance, assumptions around uniform behavior adoption or around symptom severity at the population-level would not account for the underlying heterogeneity that could sustain transmission, detected and/or undetected, despite policies based on evidence-based recommendations. Notably, as our model accounts for, the younger populations of slum communities (Bennett et al., 2016) mean there are fewer people at risk of severe COVID-19 disease (Qian et al., 2020, Sobotka et al., 2020). Low counts of clinically severe infections suggest that fewer people will seek treatment for severe disease (Skrip et al., 2020), undergo diagnosis, and thereby trigger response efforts such as contact tracing and quarantine; furthermore, slum community dwellers experience higher overall disease burden while also having less access to healthcare relative to people residing in areas of higher SES (Riley et al., 2007). As a result, there is likely significant undetected, and therefore unsupported, asymptomatic or mildly symptomatic disease in slum communities.
Previous work has investigated how the sociodemographic characteristics of urban slum communities influence disease transmission dynamics (Adiga et al., 2018). These were implemented to consider the impact of community-initiated, coordinated efforts across response pillars for identifying and supporting cases and their high-risk contacts in order to suppress the epidemic curve without population-level lockdown. The adaptations reflected in this framework have relevance for ongoing transmission in settings with overcrowding and resource constraints—such as with the recent introduction of SARS-CoV-2 into UN camps in South Sudan (Confirmed COVID-19 Cases Found, 2020), and ongoing reports of outbreaks in prisons (Wallace et al., 2020), homeless shelters, and under-resourced aged care homes (Martin, 2020). They also are pertinent to settings with socioeconomic disparity, with the goal of promoting consideration of approaches that engender local agency and facilitate adoption of transmission-reducing practices in complex settings.
We evaluated both surveillance strategies, with and without support, across different changes in time to isolation and numbers of attendees at funerals for community deaths. It is expected that such behavioral changes would be more likely with a coordinated, community-led effort in which people felt supported. Community-based initiatives that contributed to surveillance have precedent in controlling infectious disease outbreaks (Bedson et al., 2019, Fallah et al., 2016) and will be essential to longer term efforts during the COVID-19 pandemic, particularly as funds for government-led, large-scale programs may not sustain top-driven efforts at contact tracing. In the context of COVID-19, community engagement in slum communities would entail (i) well-designed and supported community-led surveillance that is integrated into the overall response; (ii) development and monitoring of feedback mechanisms (e.g., goods distribution, symptom monitoring, testing) that encourage and reinforce self-isolation of mildly symptomatic, symptomatic and presymptomatic cases; and (iii) development and dissemination of messaging in collaboration with communities that addresses the complexities of these strategies (i.e. why a presymptomatic person needs to self-isolate). Such a strategy could begin to address undetected transmission in marginalized urban communities and across the overall population and could reduce the need for prolonged states of emergency or other top-down approaches with social and economic consequences.
National coordinated resource allocation in HMICs and LMICs has primarily focused on distribution of testing materials, procurement of ventilators, and maintenance of isolation centers. While such efforts are critical for improving detection of cases and reducing mortality among severe cases, they tend to overlook strategies for mildly symptomatic, asymptomatic, and presymptomatic cases that could reduce overall transmission and protect individuals at highest risk of severe disease. This may be due to assumptions that people will (and have the resources needed to) undertake home-based self-care, wash hands, and wear masks, or due to policies to isolate all positive cases in facilities. Our findings indicated that symptomatic surveillance alone has limited impact on reducing overall transmission, particularly in the absence of support to individuals in home isolation. However, the use of self-reported symptoms to trigger responsive, community-engaged efforts that facilitate home isolation and other protective measures could offer a scalable, lower cost policy which can be embedded within a larger, multi-pronged response strategy that does not depend on mass lockdowns. It is important to emphasize that, despite challenges, innovations for reducing transmission in complex settings exist (e.g. Shryock, 2020; Fracassa, 2020) and evaluation of their individual or combined potential is important. Such innovations go beyond the standard non-pharmaceutical interventions being modeled (Modeling COVID-19 Transmission in Africa - Center for Disease Dynamics, Economics & Policy (CDDEP), 2020, van Zandvoort et al., 2020) for HMIC and LMIC settings and may warrant investments, such as in sustainable infrastructure and development projects, with longer term impacts (Corburn et al., 2020).
Like all studies, ours is not without limitations. The current model draws on information from varied sources around behavior change during past outbreaks and conditions in LMIC urban settings outside of Liberia. We recognize that the clinical presentation of SARS-CoV-2 infection may result in different behavior with and between subpopulations than Ebola virus disease. The model is therefore intended to offer a framework that can be used to explore country-specific policies with locally available data, or proxy information in the absence of data, on heterogeneous socioeconomic and demographic characteristics of urban populations and COVID-19-specific dynamics. As such, data-driven applications of the framework fit to subpopulation-specific and age-stratified data on infection rates, the ratio of mildly to severely symptomatic cases, and adoption of home-isolation behaviors in urban settings will be critical next steps. Such data will also allow for the model to directly or indirectly account for other barriers to adoption, including fear of stigma, constraints around surveillance efforts, low overall rates of testing due to capacity constraints at the national level, and differences in testing between subpopulations. Recognizing the complexity and interconnectedness of factors contributing to challenges around health access and outcomes in slum communities, COVID-19-specific surveys on barriers to adoption of public health recommendations would improve the coarse assumptions made in the present model. Thus, as a potential future step, it would be important for survey research to better understand and quantify what factors are most significant sources of unwillingness or inability to comply with outbreak response efforts. This would allow for sensitivity analyses within similar types of modeling to investigate on what socioeconomic, political, or other dimension to intervene. Furthermore, we fit the model to data from the first 105 days post-introduction in Liberia, and as has been experienced in countries globally, low testing rates and data unavailability lead to uncertainty around the extent of the epidemic. For instance, the country has been implementing school closures and a partial lockdown, which are affecting contact patterns in currently unmeasured ways and which may be suppressing the curve more than our status quo results suggest.
To mitigate burden in the urban slum subpopulation and overall, rapid and effective intervention among high risk contacts is important; it is expected that, per our model, that delays in introducing additional interventions post-lockdown could result in the numbers of contacts requiring more resource-intensive support-based surveillance efforts overwhelming even locally based efforts. It is therefore important to highlight the potential of low-cost and long-term innovations that will support individuals and communities to undertake transmission-reducing behaviors and actively participate in the greater response. While organizations are independently providing food, water, and other items to address basic needs, a coordinated effort that directs resources based on well-designed and supported community-led epidemiological surveillance findings could more effectively overcome barriers to adoption of public health recommendations, particularly in urban slum communities. Importantly, investing in community-driven initiatives that extend impact from resource allocation to heightened sense of agency, such as that which would encourage more and quicker treatment seeking and adoption of safe burial practices, could lead to significant additional reductions in case counts and deaths. Integration of such efforts into a national mitigation strategy could lead to greater reductions in infection rates and numbers of severe cases than less community-engaged efforts.
CRediT authorship contribution statement
Laura A. Skrip, Mosoka P. Fallah: Conceptualization. Laura A. Skrip: Data curation. Formal analysis, Methodology, Validation. N/A Funding acquisition. Laura A. Skrip, Mosoka P. Fallah, Jamie Bedson, Laurent Hébert-Dufresne, Benjamin M. Althouse: Investigation. Benjamin M. Althouse: Project administration, Supervision. Laura A. Skrip, Mosoka P. Fallah, Jamie Bedson, Laurent Hébert-Dufresne, Benjamin M. Althouse: Resources. Laura A. Skrip, Benjamin M. Althouse: Software, Visualization. Laura A. Skrip, Mosoka P. Fallah, Benjamin M. Althouse: Roles/Writing − original draft. Laura A. Skrip, Mosoka P. Fallah, Jamie Bedson, Laurent Hébert-Dufresne, Benjamin M. Althouse: Writing.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.epidem.2021.100529.
Appendix A. Supplementary material
Supplementary material.
.
Supplementary material.
.
References
- Adamu, Z., 2020, Ghana’s lavish funerals can last up to seven days. Now, a centuries-old tradition has gone online. CNN. 8 Apr 2020. Available: 〈https://www.cnn.com/2020/04/08/world/africa/ghana-burial-traditions-intl/index.html〉 (accessed 13 May 2020).
- Adiga A., Chu S., Eubank S., Kuhlman C.J., Lewis B., Marathe A., et al. Disparities in spread and control of influenza in slums of Delhi: findings from an agent-based modelling study. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-017353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Administrator, 2020, Material support to disadvantaged families. [cited 10 May 2020]. Available: 〈http://www.shabait.com/news/local-news/30540-material-support-to-disadvantaged-families〉.
- African Adaptations to the COVID-19 Response – Africa Center, In: Africa Center for Strategic Studies [Internet]. [cited 7 May 2020]. Available: 〈https://africacenter.org/spotlight/african-adaptations-to-the-covid-19-response/〉.
- African Population and Health Research Center (APHRC), 2014, Population and Health Dynamics in Nairobi’s Informal Settlements: Report of the Nairobi Cross-sectional Slums Survey (NCSS) 2012. APHRC.
- Associated Press, 2020, Stampede in Kenya as Slum Residents Surge for Food Aid. [cited 6 Aug 2020]. Available: 〈https://www.voanews.com/science-health/coronavirus-outbreak/stampede-kenya-slum-residents-surge-food-aid〉.
- Bedson J., Jalloh M.F., Pedi D., Bah S.M., Owen K., Oniba A., et al. Community Engagement during outbreak response: standards, approaches, and lessons from the 2014-2016 Ebola outbreak in Sierra Leone. bioRxiv. 2019 doi: 10.1101/661959. [DOI] [Google Scholar]
- Bennett R., Chepngeno-Langat G., Evandrou M., Falkingham J. Gender differentials and old age survival in the Nairobi slums, Kenya. Soc Sci Med. 2016;163:107–116. doi: 10.1016/j.socscimed.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Bi, Q., Wu, Y., Mei, S., Ye, C., Zou, X., Zhang, Z., et al., Epidemiology and Transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. doi:10.1101/2020.03.03.20028423. [DOI] [PMC free article] [PubMed]
- Camille Malplat With. Virus Stigma Weighs Heavily In Sub-Saharan Africa. Barrons Online. 20 May 2020. Available: 〈https://www.barrons.com/news/virus-stigma-weighs-heavily-in-sub-saharan-africa-01589942705〉 (accessed 20 May 2020).
- CDC COVID-19 Response Team Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, H., 2008, Monrovia City Corporation’s Slum Initiative: Preparation of Monrovia Slum Upgrading Initiative. Cities Alliance.
- Confirmed COVID-19 Cases Found in Crowded U.N.-Run Civilian Protection Camp in South Sudan. Time. Originally published: May 12, 2020. Available: 〈https://time.com/5835684/coronavirus-un-camp-south-sudan/〉 (accessed 13 May 2020).
- Contagion or starvation, the dilemma facing informal workers during the COVID-19 pandemic, 2020. [cited 7 May 2020]. Available: 〈https://www.ilo.org/global/about-the-ilo/newsroom/news/WCMS_744005/lang--en/index.htm〉.
- Corburn J., Vlahov D., Mberu B., Riley L., Caiaffa W.T., Rashid S.F., et al. Slum Health: Arresting COVID-19 and Improving Well-Being in Urban Informal Settlements. J Urban Health. 2020;97:348–357. doi: 10.1007/s11524-020-00438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 - Social Distancing, [cited 24 May 2020]. Available: 〈https://www.premise.com/covid19/socialdistancing/〉.
- Curran K.G., Gibson J.J., Marke D., Caulker V., Bomeh J., Redd J.T., et al. Cluster of Ebola Virus Disease Linked to a Single Funeral - Moyamba District, Sierra Leone, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:202–205. doi: 10.15585/mmwr.mm6508a2. [DOI] [PubMed] [Google Scholar]
- Diekmann O., Heesterbeek J.A.P., Roberts M.G. The construction of next-generation matrices for compartmental epidemic models. J R Soc Interface. 2010;7:873–885. doi: 10.1098/rsif.2009.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew D.A., Nguyen L.H., Steves C.J., Menni C., Freydin M., Varsavsky T., et al. Rapid implementation of mobile technology for real-time epidemiology of COVID-19. Science. 2020 doi: 10.1126/science.abc0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallah M., Dahn B., Nyenswah T.G., Massaquoi M., Skrip L.A., Yamin D., et al. Interrupting Ebola Transmission in Liberia Through Community-Based Initiatives. Ann Intern Med. 2016;164:367–369. doi: 10.7326/M15-1464. [DOI] [PubMed] [Google Scholar]
- Fallah M.P., Skrip L.A., Gertler S., Yamin D., Galvani A.P. Quantifying poverty as a driver of ebola transmission. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0004260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallah M.P., Skrip L.A., Raftery P., Kullie M., Borbor W., Laney A.S., et al. Bolstering community cooperation in ebola resurgence protocols: combining field blood draw and point-of-care diagnosis. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M., 2020, Under Lockdown, Food And Sanitation Products Are Desperately Needed In The Slums Of India. Forbes Magazine. 16 Apr 2020. Available: 〈https://www.forbes.com/sites/meimeifox/2020/04/16/under-lockdown-food-and-sanitation-products-are-desperately-needed-in-the-slums-of-india/〉 (accessed 6 Aug 2020).
- Fracassa, D., 2020, SF to open tent camp on old McDonald’s site near Golden Gate Park. In: SFChronicle.com [Internet]. San Francisco Chronicle; 16 May 2020 [cited 23 May 2020]. Available: 〈https://www.sfchronicle.com/bayarea/article/SF-to-open-sanctioned-tent-camp-on-old-15273859.php〉.
- Ghinai I., Woods S., Ritger K.A., McPherson T.D., Black S.R., Sparrow L., et al. Community Transmission of SARS-CoV-2 at Two Family Gatherings - Chicago, Illinois, February-March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:446–450. doi: 10.15585/mmwr.mm6915e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D., Zhao S., Lin Q., Zhuang Z., Cao P., Wang M.H., et al. The relative transmissibility of asymptomatic COVID-19 infections among close contacts. Int J Infect Dis. 2020;94:145–147. doi: 10.1016/j.ijid.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Household, [cited 6 Jul 2020]. Available: 〈https://population.un.org/Household/index.html#/countries/430〉.
- Jerving, S., 2020, Cash transfers lead the social assistance response to COVID-19. Devex; 2020. Available: 〈https://www.devex.com/news/cash-transfers-lead-the-social-assistance-response-to-covid-19–96949〉.
- Kai, D., Goldstein, G.-P., Morgunov, A., Nangalia, V., Rotkirch, A., 2020, Universal Masking is Urgent in the COVID-19 Pandemic: SEIR and Agent Based Models, Empirical Validation, Policy Recommendations. arXiv [physics.soc-ph]. Available: 〈http://arxiv.org/abs/2004.13553〉.
- Kelley M., Ferrand R.A., Muraya K., Chigudu S., Molyneux S., Pai M., et al. An appeal for practical social justice in the COVID-19 global response in low-income and middle-income countries. Lancet Glob Health. 2020;8:e888–e889. doi: 10.1016/S2214-109X(20)30249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimani-Murage E.W., Schofield L., Wekesah F., Mohamed S., Mberu B., Ettarh R., et al. Vulnerability to food insecurity in urban slums: experiences from Nairobi, Kenya. J Urban Health. 2014;91:1098–1113. doi: 10.1007/s11524-014-9894-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkcaldy R.D., King B.A., Brooks J.T. COVID-19 and Postinfection Immunity: Limited Evidence, Many Remaining Questions. JAMA. 2020 doi: 10.1001/jama.2020.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashitew, A., 2020, Social distancing unlikely to hold up in Africa without a safety net for microentrepreneurs. In: Brookings [Internet]. Brookings; 9 Apr 2020 [cited 12 May 2020]. Available: 〈https://www.brookings.edu/blog/africa-in-focus/2020/04/09/social-distancing-unlikely-to-hold-up-in-africa-without-a-safety-net-for-microentrepreneurs/〉.
- Liberia extends COVID-19 state of emergency as cases rise “exponentially.” Reuters. 22 Jun 2020. Available: 〈https://www.reuters.com/article/us-health-coronavirus-liberia-idUSKBN23T2RU〉 (accessed 25 Jul 2020).
- Liberia Institute of Statistics and Geo-Information Services, 2014, Population by County, District, Clan and Sex, Liberia, 2008, LISGIS.
- Liberia: Public Health Institute, 2020, Health Ministry Launch COVID-19 Rapid Self-Assessment Tool - FrontPageAfrica. In: FrontPageAfrica [Internet]. 14 Apr 2020 [cited 13 May 2020]. Available: 〈https://frontpageafricaonline.com/news/liberia-public-health-institute-health-ministry-launch-covid-19-rapid-self-assessment-tool/〉.
- Liberia : Council of Churches Announces Suspension of Sunday Service in Wake of Covid-19 Pandemic. 28 Mar 2020 [cited 7 Aug 2020]. Available: 〈https://frontpageafricaonline.com/county-news/liberia-council-of-churches-announces-suspension-of-sunday-service-in-wake-of-covid-19-pandemic/〉.
- Liu Y., Yan L.-M., Wan L., Xiang T.-X., Le A., Liu J.-M., et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, L., 2020, Coronavirus: How an aged care crisis seized “ill-prepared” Australia. BBC. 5 Aug 2020. Available: 〈https://www.bbc.com/news/world-australia-53633356〉. Accessed 7 Aug 2020.
- Massinga Loembé M., Tshangela A., Salyer S.J., Varma J.K., Ouma A.E.O., Nkengasong J.N. COVID-19 in Africa: the spread and response. Nat Med. 2020;26:999–1003. doi: 10.1038/s41591-020-0961-x. [DOI] [PubMed] [Google Scholar]
- Menni C., Sudre C.H., Steves C.J., Ourselin S., Spector T.D. Quantifying additional COVID-19 symptoms will save lives. Lancet. 2020;395:e107–e108. doi: 10.1016/S0140-6736(20)31281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menni C., Valdes A.M., Freidin M.B., Sudre C.H., Nguyen L.H., Drew D.A., et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020 doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modeling COVID-19 Transmission in Africa - Center for Disease Dynamics, Economics & Policy (CDDEP), 2020, In: Center for Disease Dynamics, Economics & Policy (CDDEP) [Internet]. [cited 26 Jul 2020]. Available: 〈https://cddep.org/covid-19/covid-19-in-africa/modeling-covid-19-transmission-in-africa/〉.
- mz-info[at]ux[dot]co[dot]mz U co . riscocovid19.misau.gov.mz. [cited 13 May 2020]. Available: 〈https://riscocovid19.misau.gov.mz/〉.
- Papenburg J., Baz M., Hamelin M.-È., Rhéaume C., Carbonneau J., Ouakki M., et al. Household transmission of the 2009 pandemic A/H1N1 influenza virus: elevated laboratory-confirmed secondary attack rates and evidence of asymptomatic infections. Clin Infect Dis. 2010;51:1033–1041. doi: 10.1086/656582. [DOI] [PubMed] [Google Scholar]
- Partnership for Evidence- Based Response to COVID-19 (PERC), Responding to COVID-19 in Africa: Using Data to Find a Balance. PERC; 2020 May. Available: 〈https://preventepidemics.org/coronavirus/perc/〉.
- Poole D., Raftery A.E. Inference for deterministic simulation models: the bayesian melding approach. J Am Stat Assoc. 2000;95:1244–1255. doi: 10.1080/01621459.2000.10474324. [DOI] [Google Scholar]
- Qian J., Zhao L., Ye R.-Z., Li X.-J., Liu Y.-L. Age-dependent gender differences of COVID-19 in mainland China: comparative study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley L.W., Ko A.I., Unger A., Reis M.G. Slum health: diseases of neglected populations. BMC Int Health Hum Rights. 2007;7:2. doi: 10.1186/1472-698X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosman, R., 2020, Senegal: 10-minute coronavirus test may be on its way - for $1. In: Al Jazeera [Internet]. [cited 13 May 2020]. Available: 〈https://www.aljazeera.com/news/2020/03/senegal-10-minute-coronavirus-test-1–200327053901231.html〉.
- Shryock, R., 2020, Senegal Pledges A Bed For Every Coronavirus Patient — And Their Contacts, Too. NPR. 17 May 2020. Available: 〈https://www.npr.org/sections/goatsandsoda/2020/05/17/856016429/senegal-pledges-a-bed-for-every-coronavirus-patient-and-their-contacts-too〉 (accessed 28 May 2020).
- Skrip L.A., Bedson J., Abramowitz S., Jalloh M.B., Bah S., Jalloh M.F., et al. Unmet needs and behaviour during the Ebola response in Sierra Leone: a retrospective, mixed-methods analysis of community feedback from the Social Mobilization Action Consortium. Lancet Planetary Health. 2020;4:e74–e85. doi: 10.1016/S2542-5196(20)30008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrip, L., Derra, K., Kaboré, M., Noori, N., Sondo, K.A., Gansané, A., et al., 2020, Clinical Management and Mortality among COVID-19 Cases in Sub-Saharan Africa: A retrospective study from Burkina Faso and simulated case data analysis. medRxiv. Available: 〈https://www.medrxiv.org/content/10.1101/2020.06.04.20119784v1.abstract〉. [DOI] [PMC free article] [PubMed]
- Skrip, L., Hagedorn, B., 2020, Estimated Country-Specific Rates of Hospitalized Care for Detected COVID Infections. Institute for Disease Modeling; May. Available: 〈https://covid.idmod.org/#/ResearchandReports〉.
- Skrip L.A., Selvaraj P., Hagedorn B., Ouédraogo A.L., Noori N., Orcutt A., et al. Seeding COVID-19 across Sub-Saharan Africa: An Analysis of Reported Importation Events across 49 Countries. Am J Trop Med Hyg. 2021 doi: 10.4269/ajtmh.20-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobotka T., Brzozowska Z., Muttarak R., Zeman K., di Lego V. Age, gender and COVID-19 infections. Public and Global Health. medRxiv. 2020 doi: 10.37473/dac/10.1101/2020.05.24.20111765. [DOI] [Google Scholar]
- South African Solidarity Response Fund | Uniting The Nation, [cited 8 May 2020]. Available: 〈https://www.solidarityfund.co.za/〉.
- United Nations Human Settlements Programme (UN-Habitat), 2014, Liberia Housing Profile.
- van de Vijver S., Oti S., Oduor C., Ezeh A., Lange J., Agyemang C., et al. Challenges of health programmes in slums. Lancet. 2015;386:2114–2116. doi: 10.1016/S0140-6736(15)00385-2. [DOI] [PubMed] [Google Scholar]
- Wallace M., Hagan L., Curran K.G., Williams S.P., Handanagic S., Bjork A., et al. COVID-19 in correctional and detention facilities - United States, February-April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:587–590. doi: 10.15585/mmwr.mm6919e1. [DOI] [PubMed] [Google Scholar]
- Wolfe C.M., Hamblion E.L., Schulte J., Williams P., Koryon A., Enders J., et al. Ebola virus disease contact tracing activities, lessons learned and best practices during the Duport Road outbreak in Monrovia, Liberia, November 2015. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- van Zandvoort, K., Jarvis, C.I., Pearson, C., Davies, N.G., 2020, CMMID COVID-19 working group , Russell TW , et al. Response strategies for COVID-19 epidemics in African settings: a mathematical modelling study. Epidemiology. medRxiv. doi:10.1101/2020.04.27.20081711. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Supplementary material.