Abstract
Background
We aimed to examine the immunogenicity pattern induced by the inactivated SARS-CoV-2 vaccine CoronaVac (Sinovac Life Sciences, Beijing, China) in SARS-CoV-2 seropositive patients with autoimmune rheumatic diseases compared with seropositive controls, seronegative patients with autoimmune rheumatic diseases, and seronegative controls.
Methods
CoronavRheum is an ongoing, prospective, controlled, phase 4 study, in which patients aged 18 years or older with autoimmune rheumatic diseases, and healthy controls were recruited from a single site (Rheumatology Division of Hospital das Clínicas, Faculdade de Medicina da Universidade de São Paulo) in São Paulo, Brazil Participants were vaccinated with two doses of CoronaVac (intramuscular injection, 3 μg in 0·5 mL of β-propiolactone inactivated SARS-CoV-2) on day 0 and on day 28. Blood samples were taken pre-vaccination on day 0, day 28, and also on day 69. For this subgroup analysis, participants were defined as being SARS-CoV-2 seropositive or seronegative prevaccination via anti-SARS-CoV-2 spike (S)1 or S2 IgG (cutoff of 15·0 arbitrary units [AU] per mL) or neutralising antibody titres (cutoff of ≥30%) and were matched for age and sex, via convenience sampling, in a 1:3:1:1 ratio (seropositive patients to seronegative patients to seropositive controls to seronegative controls). The primary outcomes were rates of anti-SARS-CoV-2 S1 and S2 IgG seropositivity and SARS-CoV-2 neutralising antibody positivity at day 28 and day 69 and immunogenicity dynamics assessed by geometric mean titres (GMTs) of IgG and median neutralising activity in seropositive patients with autoimmune rheumatic diseases compared with seronegative patients and seropositive and seronegative controls. We assessed safety in all participants randomly selected for this subgroup analysis. This study is registered with ClinicalTrials.gov, NCT04754698, and is ongoing for long-term immunogenicity evaluation.
Findings
Between Feb 4 and Feb 8, 2021, 1418 patients and 542 controls were recruited, of whom 1685 received two vaccinations (1193 patients and 492 controls). After random sampling, our immunogenicity analysis population comprised 942 participants, of whom 157 were SARS-CoV-2 seropositive patients with autoimmune rheumatic diseases, 157 were seropositive controls, 471 were seronegative patients, and 157 were seronegative controls; the median age was 48 years (IQR 38–56) and 594 (63%) were female and 348 (37%) were male. For seropositive patients and controls, an increase in anti-SARS-CoV-2 S1 and S2 IgG titres (seropositive patients GMT 52·3 [95% CI 42·9–63·9] at day 0 vs 128·9 [105·6–157·4] at day 28; seropositive controls 53·3 [45·4–62·5] at day 0 vs 202·0 [174·8–233·4] at day 28) and neutralising antibody activity (seropositive patients 59% [IQR 39–83] at day 0 vs 82% [54–96] at day 28; seropositive controls 58% [41–79] at day 0 vs 92% [79–96] at day 28), was observed from day 0 to day 28, without further increases from day 28 to day 69 (at day 69 seropositive patients' GMT was 137·1 [116·2–161·9] and neutralising antibody activity was 79% [57–94]); and seropositive controls' GMT was 188·6 [167·4–212·6] and neutralising antibody activity was 92% [75–96]). By contrast, for seronegative patients and controls, the second dose was required for maximum response at day 69, which was lower in seronegative patients than in seronegative controls. GMTs in seronegative patients were 2·3 (95% CI 2·2–2·3) at day 0, 5·7 (5·1–6·4) at day 28, and 29·6 (26·4–33·3) at day 69, and in seronegative controls were 2·3 (2·1–2·5) at day 0, 10·6 (8·7–13·1) at day 28, and 71·7 (63·5–81·0) at day 69; neutralising antibody activity in seronegative patients was 15% (IQR 15–15) on day 0, 15% (15–15) at day 28, and 39% (15–65) at day 69, and in seronegative controls was 15% (15–15) at day 0, 24% (15–37) at day 28, and 61% (37–79) at day 69. Neither seronegative patients nor seronegative controls reached the GMT or antibody activity levels of seropositive patients at day 69.
Interpretation
By contrast with seronegative patients with autoimmune rheumatic diseases, seropositive patients have a robust response after a single dose of CoronaVac. Our findings raise the possibility that the reduced immunogenicity observed in seronegative patients might not be the optimum response potential to SARS-CoV-2 vaccination, and therefore emphasise the importance of at least a single booster vaccination in these patients.
Funding
Fundação de Amparo à Pesquisa do Estado de São Paulo, Conselho Nacional de Desenvolvimento Científico e Tecnológico, and B3-Bolsa de Valores do Brasil.
Translation
For the Portuguese translation of the abstract see Supplementary Materials section.
Introduction
In June, 2021, WHO recommended the emergency use of the inactivated SARS-CoV-2 vaccine, CoronaVac (Sinovac Life Sciences, Beijing, China),1 which has shown a high level of protection against COVID-19-related hospitalisation and death in the Chilean population.2 As of Aug 1, 2021, only a quarter of the Brazilian population had received two doses of a SARS-CoV-2 vaccine and CoronaVac accounted for approximately 38% of all SARS-CoV-2 vaccines administered in Brazil.3
Previously, we have shown a seroconversion rate of 70·4% with two doses of CoronaVac in adults with autoimmune rheumatic diseases without previous SARS-CoV-2 infection, compared with 95·5% in controls, with a diminished frequency of COVID-19 incident cases after vaccination.4
New SARS-CoV-2 variants are emerging and vaccine supply is still restricted worldwide. Therefore, improving strategies to maximise vaccine coverage and enhance immunogenicity is crucial, especially in immunosuppressed populations. A few recent reports, including some preprints, have shown that antibody responses to the first dose of mRNA-based SARS-CoV-2 vaccines in people with previous laboratory-confirmed SARS-CoV-2 infection were similar to or exceeded those found in individuals without previous infection after the second dose,5, 6, 7, 8, 9, 10 raising the possibility of allocating vaccine to other at-risk groups.
However, data are scarce on immune responses to SARS-CoV-2 vaccines in the context of previous SARS-CoV-2 infection in patients with autoimmune rheumatic diseases; a population known to have reduced virus clearance and to be prone to genomic evolution.11 It is crucial to investigate whether immunogenicity of previous SARS-CoV-2 infection in this population might surpass that of patients without previous SARS-CoV-2 infection who have received two doses, or if humoral response will be limited by an intrinsic defect of these patients' immune system or immunosuppressive treatment, as previously described.12, 13 A study in patients with autoimmune diseases showed that a single dose of mRNA-based or adenovirus-based SARS-CoV-2 vaccine in those with previous SARS-CoV-2 infection could elicit antibody responses similar to two vaccine doses in patients without previous infection, with seroconversion in the vast majority of patients on any immunosuppressive treatment.14 However, the small sample size of the seropositive group, heterogeneous schedules for blood collection, and the absence of serial samples hampered a definitive conclusion on the kinetics of humoral response.14 Understanding antibody kinetics is even more relevant in the context of the approval of a third SARS-CoV-2 vaccine dose for immunocompromised individuals in some countries.15
Research in context.
Evidence before this study
Pre-existing immunity for COVID-19 affects vaccine response and might allow a change in the current vaccination guidelines, allowing for increased vaccine availability. We searched PubMed for publications between Dec 1, 2020, and Aug 27, 2021, for studies published in English on COVID-19 vaccines in patients with autoimmune rheumatic disease, using the terms “seropositive” AND (“vaccination” OR “vaccine”) AND (“COVID-19” OR “SARS-CoV-2”) AND (“autoimmune” OR “rheumatic”). Few reports suggested that one dose of mRNA-based SARS-CoV-2 vaccine could elicit a large antibody response in SARS-CoV-2 seropositive individuals, with no further increase in antibody response after the second dose. However, we found no studies with data for inactivated SARS-CoV-2 vaccines and little information on patients with autoimmune rheumatic diseases, in whom immunogenicity is known to be reduced. Moreover, only few studies have focused on immunological analysis of neutralising antibodies, which are relevant in immune protection against SARS-CoV-2 infection.
Added value of this study
This study provides the first evidence that previous exposure to SARS-CoV-2, independent of symptoms, in patients with autoimmune rheumatic diseases results in distinct dynamics of antibody response (measured via anti-SARS-CoV-2 spike antibody titres and neutralising antibody activity) to an inactivated SARS-CoV-2 vaccine (CoronaVac; Sinovac Life Sciences, Beijing, China) compared with patients without previous exposure. Our study expands on previous reports in healthy individuals and a small sample of seropositive patients with autoimmune rheumatic diseases immunised with mRNA-based or adenovirus-based SARS-CoV-2 vaccines, in that seropositive patients showed a robust boost in antibody response after the first dose of inactivated vaccine, independent of their underlying disease or treatment. No further increase in response was observed between the first and second dose, and the antibody response remained up to 6 weeks after the second dose.
Implications of all the available evidence
The CoronaVac vaccine presents distinct kinetics of immune response in seropositive patients with autoimmune rheumatic diseases compared to seronegative patients. Our finding raises the possibility that the reduced immunogenicity observed in seronegative patients might not represent the optimum response potential and suggest that these patients might benefit from booster doses.
To add to this knowledge, we assessed the dynamics of antibody production induced by the inactivated CoronaVac vaccine in patients with autoimmune rheumatic disease who were SARS-CoV-2 seropositive and those who were SARS-CoV-2 seronegative compared with SARS-CoV-2 seropositive and seronegative controls.
Methods
Study design and participants
This is a retrospective subgroup analysis of a large ongoing prospective, controlled, phase 4 study (CoronavRheum) of immunogenicity and safety of two doses of the inactivated SARS-CoV-2 vaccine CoronaVac in patients with autoimmune rheumatic diseases4 being conducted in a single site (Rheumatology Division of Hospital das Clínicas, Faculdade de Medicina da Universidade de São Paulo) in São Paulo, Brazil, to assess the dynamics of response to this SARS-CoV-2 inactivated vaccine in patients with autoimmune rheumatic diseases who are seropositive for SARS-CoV-2-specific antibodies at baseline compared with those who are seronegative at baseline and with controls.
For the main trial, patients with autoimmune rheumatic diseases from our outpatient rheumatology clinics in São Paulo, Brazil, were consecutively invited to participate in the study if they were aged 18 years or older and if they fulfilled the classification criteria for one of the following autoimmune rheumatic diseases: rheumatoid arthritis, systemic lupus erythematosus, spondiloarthritis, vasculitis, primary Sjogren's syndrome, systemic sclerosis, systemic autoimmune myopathies, and primary antiphospholipid syndrome. Additionally, hospital services workers, health professionals, and hospital administrative service employees or their relatives without autoimmune rheumatic disease and not taking immunosuppressive therapy were recruited to comprise the healthy control group. Exclusion criteria were in accordance to our previous report.4 Key exclusion criteria were history of anaphylactic response to vaccine components, acute febrile illness or symptoms compatible with COVID-19 at vaccination, decompensated heart failure (class III or IV), demyelinating disease, previous vaccination with any SARS-CoV-2 vaccine, history of live virus vaccine up to 4 weeks before enrolment, receipt of inactivated virus vaccine up to 2 weeks before enrolment, patients who were being treated in hospital for any reason, and not providing consent to participate.
The study protocol was approved by the National and Institutional Ethical Committee (CAAE: 42566621.0.0000.0068) and written informed consent was obtained from all participants.
Procedures
The CoronaVac COVID-19 vaccine (batch number 20200412, Sinovac Life Sciences, Beijing, China) used in this study was supplied by the Instituto Butantan (São Paulo, Brazil). Patients and controls were vaccinated in a two-dose schedule, via intramuscular injection with 3 μg of vaccine in 0·5 mL of β-propiolactone inactivated SARS-CoV-2. The first dose and blood collection were done for most participants on Feb 9–10, 2021 (day 0), the second dose with blood collection was done on March 9–10, 2021 (day 28), and the last blood collection was done on April 19, 2021 (day 69) at the hospital convention center. For this subgroup analysis, incident COVID-19 cases were assessed from day 0 to day 79.
Laboratory tests were done at the central laboratory division of Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (supervised by AJSD and LS). Human IgG antibodies against the SARS-CoV-2 spike (S) 1 and S2 proteins were measured using a chemiluminescent immunoassay (Indirect ELISA, LIAISON SARS-CoV-2 S1/S2 IgG, DiaSorin, Italy). The lower limit of quantification of the assay was 3·8 UA/mL and seropositivity was defined as anti-SARS-CoV-2 (S1/S2) IgG of more than 15·0 UA/mL. For titres below the limit of quantification, a value of 1·9 UA/mL was assigned.
A SARS-CoV-2 neutralising antibody assay was done using the cPass SARS-CoV-2 neutralisation antibodies detection kit (GenScript, Piscataway, NJ, USA). Results are expressed as positive or negative neutralising antibodies according to the manufacturer recommended cutoff of percentage signal inhibition (≥30% inhibition).16 Medians and IQRs of the percentage of neutralising activity were calculated at all timepoints (at day 0, day 28 and day 69), attributing the value of 15% (half of positive inhibition cutoff) to undetectable levels (<30%).
The study was monitored by independent vaccine experts, who comprised the Data Safety Monitoring Board. Local and systemic vaccine-related adverse effects were carefully reviewed with each participant at in-person visits on day 28 and day 69, as previously reported.2 Vaccine adverse effect severity was ranked according to WHO definitions.17 24 h access to the medical team was available to all participants, including telephone contacts, email, and WhatsApp messages for safety support, from day 0 until day 69.
All participants completed a standardised questionnaire to assess their history of SARS-CoV-2 infection at baseline (appendix 2 p 8). Reports of any previous positive RT-PCR test were requested. Social risk factors associated with increased risk of exposure to SARS-CoV-2 were also registered by all participants. Incident cases were defined as new cases of symptomatic SARS-CoV-2 infection, confirmed with RT-PCR between day 0 and day 79.4 All positive samples tested at our site were further characterised for variants of concern at the same hospital. RNA was extracted using QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) according to manufacturer's instructions, as previously described.18
For this subgroup analysis, seronegative and seropositive patients with autoimmune rheumatic disease and seropositive and seronegative controls were selected from the main cohort. Patients with pre-vaccination positive COVID-19 serology (ie, anti-S1 or S2 IgG or neutralising antibodies) were classified as being seropositive patients or controls and those with pre-vaccination negative COVID-19 serology were classified as seronegative patients or controls.
Outcomes
The primary outcomes were rates of anti-SARS-CoV-2 S1 and S2 IgG seropositivity and SARS-CoV-2 neutralising antibody positivity at day 28 and day 69 and immunogenicity dynamics were assessed by median neutralising activity (ie, activity of neutralising antibodies) and by geometric mean titres (GMTs) of anti-SARS-CoV-2 S1 and S2 IgG and median neutralising antibody activity in SARS-CoV-2 seropositive patients with autoimmune rheumatic diseases compared with seronegative patients and seropositive and seronegative controls.
Secondary outcomes were the influence of previous (ie, prevaccination) symptomatic versus asymptomatic SARS-CoV-2 infection ascertained by RT-PCR or rapid antigen test on vaccine-induced antibody response, antibody dynamics in patients who had symptomatic SARS-CoV-2 infection within the past 3 months (inclusive) versus more than 3 months previously, and vaccine safety.
Exploratory outcomes were prevalence of RT-PCR positive test results among participants (ie, COVID-19 incident cases), analysis of variants of concern, and analysis of infection severity and of social risk factors associated with exposure to SARS-CoV-2.
We did post-hoc analyses of demographic and disease-specific factors associated with anti-SARS-CoV-2 S1 and S2 IgG seropositivity and neutralising antibody positivity at day 28 in seropositive patients, and comparison of vaccine-induced anti-SARS-CoV-2 antibody seropositivity between previously asymptomatic patients and seronegative patients.
Statistical analysis
All treatment groups in this subgroup analysis were selected via convenience sampling from the large phase 4 prospective cohort CoronavRheum.4 Seronegative and seropositive patients with autoimmune rheumatic disease and seropositive and seronegative controls were selected from the main cohort, in a 1:3:1:1 ratio, matched for age (up to 5 years difference) and sex using an in-house program run on Excel (Microsoft 2018) for random selection of individuals in each category.
We present categorical variables as n (%), continuous variables as median (IQR), and anti-SARS-CoV-2 S1 and S2 IgG serology titres as geometric means (95% CI). We did statistical comparisons between groups using the χ2 test or Fisher's exact test for categorical variables and Student's t test or the Mann-Whitney U test for continuous variables. We transformed anti-SARS-CoV-2 S1 and S2 IgG titre data in natural logarithm(ln) before analysis, and we describe the values of ln(IgG) titres and neutralising antibodies according to groups (seropositive and seronegative patients with autoimmune rheumatic diseases and seropositive and seronegative controls) and at each assessment timepoint (day 0, day 28, and day 69). We compared ln-transformed anti-SARS-CoV-2 S1 or S2 IgG titres and neutralising antibody activity between groups and between timepoints (day 0, day 28, and day 69) using generalised estimating equations with normal marginal distribution (for IgG titres) and gamma distribution (for neutralising antibodies) and identified binding function assuming first order autoregressive correlation matrix between timepoints. We did Bonferroni multiple comparisons to identify differences between groups and timepoints.
The primary outcomes and post-hoc analysis of factors associated with anti-SARS-CoV-2 S1 and S2 IgG seropositivity and neutralising antibody positivity at day 28 were assessed in all participants who were selected as part of random sampling. Secondary outcomes were assessed in all participants who received vaccine, before random sapling. We assessed incident case surveillance in all participants of CoronavaRheum of data cutoff (April 29, 2021) from day 0 to day 79. Participants with RT-PCR-confirmed previous SARS-CoV-2 infection between day 0 and day 69 were excluded from the immunogenicity analyses, but were included in incident case surveillance (from day 0 to day 79).
We assessed vaccine safety among all the participants who were randomly selected for this subgroup analysis. We did this by analysing reports of any vaccine side-effect and the reviewing the standardised diary completed by the participants, including local and systemic manifestations. Vaccine-related adverse effects were carefully reviewed with each participant at in-person visits on day 28 and day 69.
We did all analyses using the IBM-SPSS for Windows (version 22.0) and we made graphs of mean profiles and SEs using the Microsoft-Excel 2010 software. The tests were performed with a significance level of 5%. This study is registered with ClinicalTrials.gov, NCT04754698
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Instituto Butantan supplied the study product and had no other role in the trial.
Results
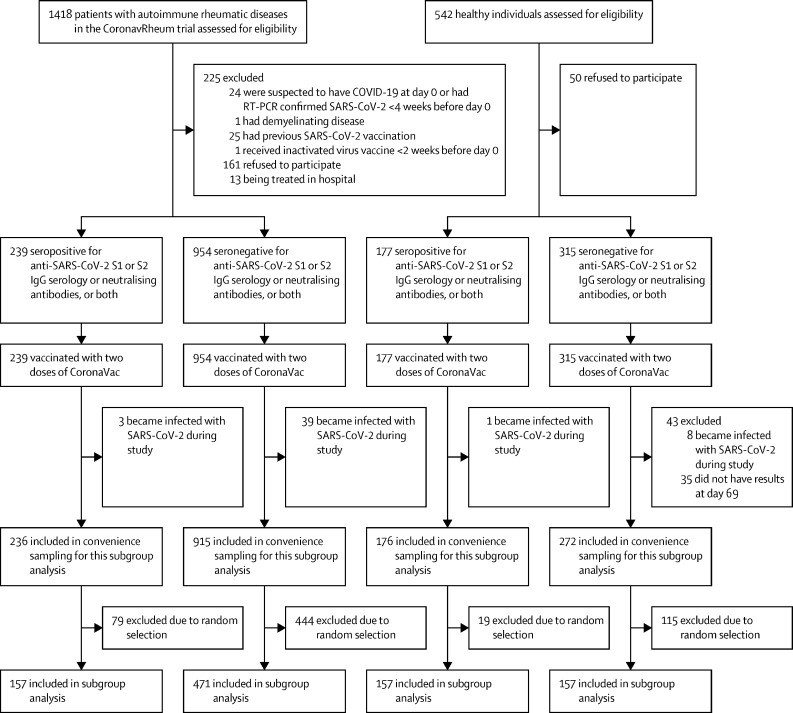
Between Feb 4 and Feb 8, 2021, 1418 patients and 542 controls were recruited to CoronavRheum, of whom 1193 patients and 492 controls attended three study visits that occurred on Feb 9–10, 2021 (day 0), on March 9–10, 2021 (day 28), and on April 19, 2021 (day 69), and received two doses of inactivated SARS-CoV-2 vaccine on days 0 and 28. Of the 1685 participants who received both doses of CoronaVac, 86 were excluded from further analyses because they became infected with SARS-CoV-2 during the study or did not have available data for analysis (figure 1 ). After applying the exclusion criteria and random sampling, the final study groups for this immunogenicity analysis comprised 942 participants, of whom 157 were SARS-CoV-2 seropositive patients with autoimmune rheumatic diseases, 157 were seropositive controls, 471 were seronegative patients with autoimmune rheumatic diseases, and 157 were seronegative controls (figure 1).
Figure 1.
Study profile
S=spike.
In the analysable population, the median age was 48 years (IQR 38–56) and 594 (63%) were female and 348 (37%) were male. Participant groups were comparable with regards to baseline age, sex, and ethnicity distribution (table 1 ) . A shorter disease duration was observed in SARS-CoV-2 seropositive patients with autoimmune rheumatic disease than in seronegative patients (p=0·011; table 1). Disease and treatment distributions were similar between seropositive and seronegative patients (table 1).
Table 1.
Baseline demographic and clinical characteristics of SARS-CoV-2 seropositive and seronegative patients with autoimmune rheumatic diseases and seropositive and seronegative controls
| SARS-CoV-2 seropositive patients with autoimmune rheumatic diseases (n=157) | SARS-CoV-2 seronegative patients with autoimmune rheumatic diseases (n=471) | SARS-CoV-2 seropositive controls (n=157) | SARS-CoV-2 seronegative controls (n=157) | p value | |||
|---|---|---|---|---|---|---|---|
| Demographic data | |||||||
| Age, years | |||||||
| Median | 48 (38–57) | 48 (38–56) | 48 (36–56) | 48 (38–57) | 0·98 | ||
| >65 | 4 (3%) | 12 (3%) | 4 (3%) | 7 (4%) | >0·999 | ||
| At diagnosis | 33 (22–43) | 30 (22–40) | .. | .. | 0·11 | ||
| Disease duration, years | 12 (7–19) | 14 (8–22) | .. | .. | 0·011 | ||
| Sex | .. | .. | .. | .. | >0·999 | ||
| Female | 99 (63%) | 297 (63%) | 99 (63%) | 99 (63%) | .. | ||
| Male | 58 (37%) | 174 (37%) | 58 (37%) | 58 (37%) | .. | ||
| Race | .. | .. | .. | .. | 0·12 | ||
| White | 78 (50%) | 234 (50%) | 58 (37%) | 76 (48%) | .. | ||
| African-Latin American | 76 (48%) | 226 (48%) | 95 (61%) | 74 (47%) | .. | ||
| Asian | 1 (1%) | 7 (1%) | 4 (3%) | 4 (3%) | .. | ||
| Indigenous Brazilian | 2 (1%) | 4 (1%) | 0 | 3 (2%) | .. | ||
| Clinical data | |||||||
| Autoimmune rheumatic disease | |||||||
| Rheumatoid arthritis | 39 (25%) | 125 (27%) | .. | .. | 0·68 | ||
| Axial spondyloarthritis | 32 (20%) | 80 (17%) | .. | .. | 0·34 | ||
| Psoriatic arthritis | 16 (10%) | 56 (12%) | .. | .. | 0·56 | ||
| Systemic lupus erythematosus | 37 (24%) | 115 (24%) | .. | .. | 0·83 | ||
| Systemic vasculitis | 10 (6%) | 32 (7%) | .. | .. | 0·85 | ||
| Systemic autoimmune myopathy | 6 (4%) | 20 (4%) | .. | .. | >0·999 | ||
| Systemic sclerosis | 7 (4%) | 13 (3%) | .. | .. | 0·29 | ||
| Primary Sjögren's syndrome | 6 (4%) | 16 (3%) | .. | .. | 0·80 | ||
| Primary antiphospholipid syndrome | 4 (3%) | 13 (3%) | .. | .. | >0·999 | ||
| Current therapies | |||||||
| Hydroxychloroquine | 44 (28%) | 127 (27%) | .. | .. | 0·80 | ||
| Sulfasalazine | 20 (13%) | 45 (10%) | .. | .. | 0·26 | ||
| Prednisone | 47 (30%) | 182 (39%) | .. | .. | 0·050 | ||
| Dose, mg per day | 6 (5–10) | 5 (5–10) | .. | .. | 0·21 | ||
| Immunosuppressive drugs | 94 (60%)* | 296 (63%) | .. | .. | 0·51 | ||
| Methotrexate | 44 (28%) | 135 (29%) | .. | .. | 0·88 | ||
| Leflunomide | 18 (11%) | 57 (12%) | .. | .. | 0·83 | ||
| Mycophenolate mofetil | 16 (10%) | 55 (12%) | .. | .. | 0·61 | ||
| Azathioprine | 15 (10%) | 49 (10%) | .. | .. | 0·76 | ||
| Other† | 8 (5%) | 19 (4%) | .. | .. | 0·57 | ||
| Biologic agent | 53 (34%) | 174 (37%) | .. | .. | 0·47 | ||
| TNF inhibitor | 27 (17%) | 81 (17%) | .. | .. | >0·999 | ||
| Abatacept | 5 (3%) | 20 (4%) | .. | .. | 0·56 | ||
| Secukinumab | 11 (7%) | 21 (4%) | .. | .. | 0·21 | ||
| Other‡ | 10 (6%) | 49 (10%) | .. | .. | 0·13 | ||
Data are n (%) or median (IQR). p values are calculated using data across all groups where possible, and only between the seropositive and seronegative patients for rheumatic disease characteristics. Categorical variables were compared between groups using the χ2 test or Fisher's exact test and all continuous variables were compared using the Mann-Whitney U.
Sums to more than the patient numbers provided because seven patients were taking more than one immunosuppresive drug.
Cyclophosphamide, cyclosporin, tacrolimus, and tofacitinib.
Tocilizumab, rituximab, belimumab, and ustekinumab.
A high proportion of seropositive patients and controls had anti-SARS-CoV-2 S1 or S2 IgG seropositivity at day 28 (149 [95%] of 157 vs 155 [99%] of 157; p=0·10) and these proportions remained high at day 69 (154 [98%] vs 157 [100%]; p=0·25) with comparable seropositivity rates at both timepoints (table 2 ). In the seropositive patient and control groups we also observed high proportions of participants with neutralising antibody positivity at day 28 (138 [88%] vs 151 [96%]; p=0·0067), which was sustained at day 69 (141 [90%] vs 155 [99%]; p=0·0005); although, a lower proportion of patients were neutralising antibody positive than controls.
Table 2.
Anti-SARS-CoV-2 S1 or S2 IgG and neutralising antibody seropositivity rates at baseline and after the first (day 28) and second (day 69) doses of CoronaVac vaccination
|
Anti-SARS-CoV-2 S1 or S2 IgG seropositivity |
Neutralising antibody positivity |
||||||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 28 | Day 69 | Day 0 | Day 28 | Day 69 | ||
| SARS-CoV-2 seropositive patients with autoimmune rheumatic diseases (n=157) | 140 (89%) | 149 (95%) | 154 (98%) | 135 (86%) | 138 (88%) | 141 (90%) | |
| SARS-CoV-2 seropositive controls (n=157) | 149 (95%) | 155 (99%) | 157 (100%) | 140 (89%) | 151 (96%) | 155 (99%) | |
| SARS-CoV-2 seronegative patients with autoimmune rheumatic diseases (n=471) | 0 | 99 (21%) | 353 (75%) | 0 | 108 (23%) | 289 (61%) | |
| SARS-CoV-2 seronegative controls (n=157) | 0 | 57 (36%) | 150 (96%) | 0 | 56 (36%) | 128 (82%) | |
| p value | |||||||
| Seropositive patients vs seropositive controls | 0·061 | 0·10 | 0·25 | 0·39 | 0·0067 | 0·0005 | |
| Seropositive patients vs seronegative patients | <0·0001 | <0·0001 | <0·0001 | <0·0001 | <0·0001 | <0·0001 | |
| Seropositive patients vs seronegative controls | <0·0001 | <0·0001 | 0·34 | <0·0001 | <0·0001 | 0·036 | |
| Seronegative patients vs seronegative controls | >0·999 | <0·0001 | <0·0001 | >0·999 | 0·0016 | <0·0001 | |
Data are n (%). Positivity for anti-SARS-CoV-2 S1 or S2 IgG was defined as post-vaccination titre of ≥15 AU/mL. Positivity for neutralising antibodies was defined as a neutralising activity ≥30%. Frequencies of seropositivity were compared using the χ2 test.
A distinct pattern was detected for seronegative patients with autoimmune rheumatic diseases, with a low proportion of patients having anti-SARS-CoV-2 S1 or S2 IgG seropositivity (99 [21%] of 471) and neutralising antibody positivity (108 [23%]) at day 28, and the second dose was required to obtain moderate proportions with anti-SARS-CoV-2 S1 or S2 IgG seropositivity (353 [75%]) and neutralising antibody positivity (289 [61%]) at day 69. Likewise, seronegative controls also needed two doses to reach a moderate response at day 69 (proportion with IgG seropositivity was 57 [36%] of 157 at day 28 and 150 [96%] at day 69; neutralising antibody positivity was 56 [36%] at day 28 and 128 [82%] at day 69; table 2). The proportion of seronegative patients who had a response was significantly lower than among seropositive patients at day 28 (p<0·0001) and day 69 (p<0·0001). Also, the proportion of seronegative controls with IgG seropositivity and neutralising antibody positivity was lower than among seropositive patients at day 28 (p<0·0001) but not at day 69 (p=0·34), and the proportion who had neutralising antibody positivity was lower at day 28 (p<0·0001) and day 69 (p=0·036; table 2).
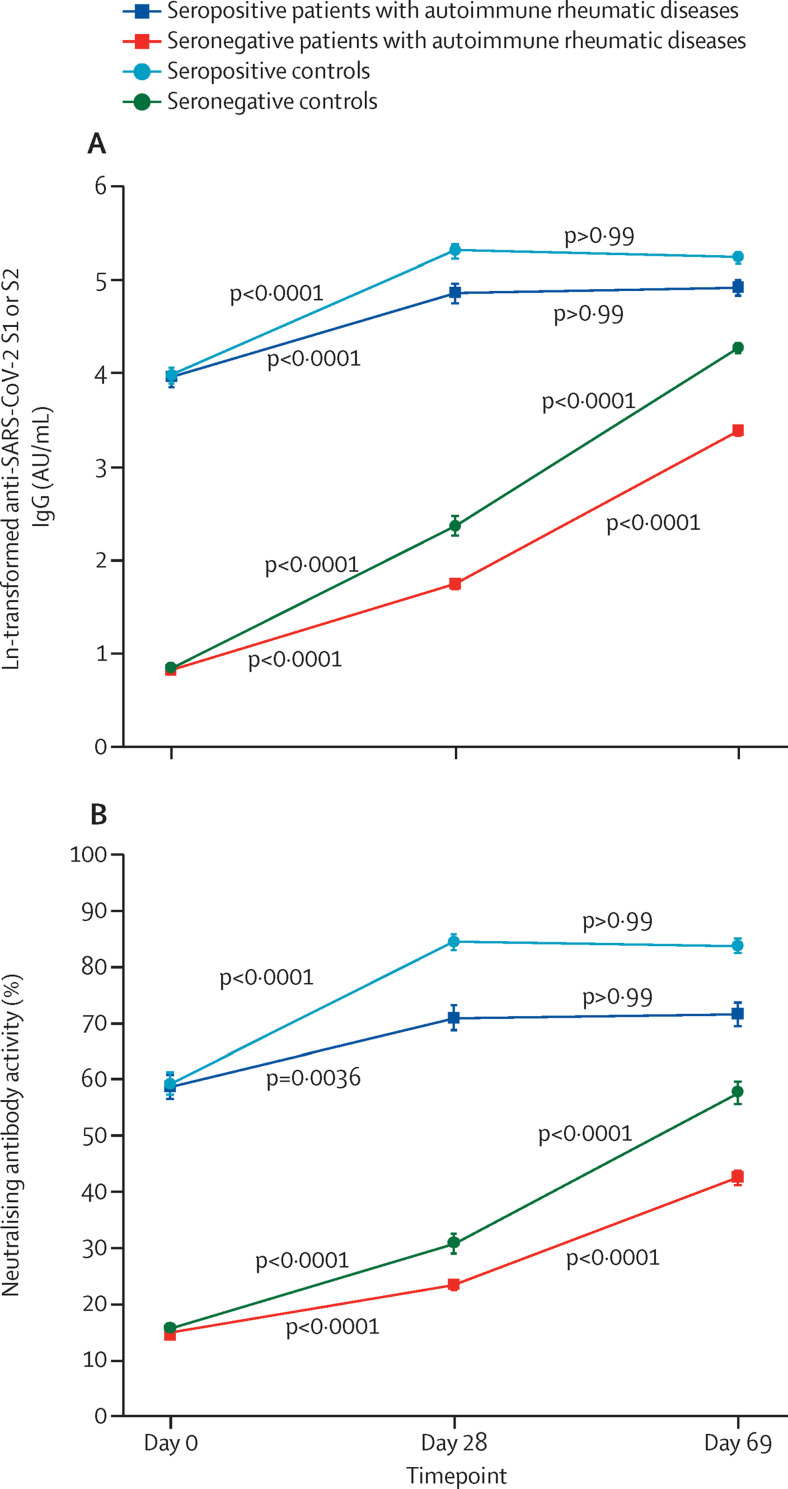
Seropositive patients and controls had similar vaccine-induced antibody dynamics, with substantial increases from day 0 to day 28 and no further increase from day 28 to day 69 (table 3 , figure 2 ; appendix 2 pp 2–3).
Table 3.
Geometric mean titres of anti-SARS-CoV-2 S1 or S2 IgG and median percentage of neutralising activity and before (day 0) and after the first (day 28) and second (day 69) doses of CoronaVac vaccination
|
Anti-SARS-CoV-2 IgG S1 or S2 IgG GMT, AU/mL (95% CI) |
Median neutralising activity of neutralising antibodies, % (IQR) |
||||||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 28 | Day 69 | Day 0 | Day 28 | Day 69 | ||
| SARS-CoV-2 seropositive patients with autoimmune rheumatic diseases (n=157) | 52·3 (42·9–63·9) | 128·9 (105·6–157·4) | 137·1 (116·2–161·9) | 59 (39–83) | 82 (54–96) | 79 (57–94) | |
| SARS-CoV-2 seropositive controls (n=157) | 53·3 (45·4–62·5) | 202·0 (174·8–233·4) | 188·6 (167·4–212·6) | 58 (41–79) | 92 (79–96) | 92 (75–96) | |
| SARS-CoV-2 seronegative patients with autoimmune rheumatic diseases (n=471) | 2·3 (2·2–2·3) | 5·7 (5·1–6·4) | 29·6 (26·4–33·3) | 15 (15–15) | 15 (15–15) | 39 (15–65) | |
| SARS-CoV-2 seronegative controls (n=157) | 2·3 (2·1–2·5) | 10·6 (8·7–13·1) | 71·7 (63·5–81·0) | 15 (15–15) | 24 (15–37) | 61 (37–79) | |
| p value | |||||||
| Seropositive patients vs seropositive controls | >0·999 | 0·0080 | 0·41 | >0·999 | 0·119 | 0·300 | |
| Seropositive patients vs seronegative patients | <0·0001 | <0·0001 | <0·0001 | <0·0001 | <0·0001 | <0·0001 | |
| Seropositive patients vs seronegative controls | <0·0001 | <0·0001 | <0·0001 | <0·0001 | <0·0001 | 0·010 | |
| Seronegative patients vs seronegative controls | >0·999 | <0·0001 | <0·0001 | >0·999 | <0·0001 | <0·0001 | |
Proportion of neutralising activity of neutralising antibodies are expressed as median (IQR) and anti-SARS-CoV-2 S1 or S2 IgG antibody titres are expressed as GMTs with 95% CIs. The minimum possible value for neutralising activity is 15% (attributed for values of <30%). AU=arbitrary units. GMT=geometric mean titre.
Figure 2.
Anti-SARS-CoV-2 S1 or S2 IgG GMTs (A) and neutralising antibody activity (B) before (day 0) and after the first (day 28) and second (day 69) doses of CoronaVac
Datapoints are mean values, with error bars showing SD. The minimum possible value for anti-SARS-CoV-2 S1 or S2 IgG is 0·64 (ln 1·9, the value attributed IgG titres of ≤3·8 AU/mL) and for neutralising activity is 15% (attributed for values of <30%). Data are also shown after Bonferroni's multiple comparison in the appendix (pp 2–3). Tests were always two-sided. AU=arbitrary units. GMT=geometric mean titre. S=spike.
We observed changes from day 0 to day 28 in seronegative patients for anti-SARS-CoV-2 S1 or S2 IgG GMTs (from 2·3 arbitrary units [AU]/mL [95% CI 2·2–2·3] to 5·7 [5·1–6·4]; table 3, figure 2 [data presented as ln(IgG)]) and for neutralising antibody activity (15% [IQR 15–15] to 15% [15–15]; table 3; appendix 2 pp 2–3). A substantial increase was seen in anti-SARS-CoV-2 S1 or S2 IgG GMTs from day 28 to day 69 for seronegative patients (from 5·7AU/mL [95% CI 5·1–5·4] to 29·6 AU/mL [26·4–33·3]). A similar increase was observed for neutralising antibody activity from day 28 to day 69 (15% [IQR 15–15] to 39% [15–65]; table 3; appendix 2 pp 1–2). Seronegative controls had a similar pattern, with minor increases after the first dose and substantial increases after the second dose for both anti-SARS-CoV-2 S1 or S2 IgG GMTs and neutralising antibody activity (table 3; appendix 2 pp 1–2). Significantly lower proportions of seronegative patients had IgG seropositivity and neutralising antibody positivity at day 28 and day 69 than did seronegative controls (table 2).
In line with these findings, when the groups were compared at different timepoints, seropositive patients and controls had similar IgG titres at day 0 (p>0·999) and day 69 (p=0·41) but titres were higher in seropositive controls at day 28 (p=0·0080; table 3). For neutralising antibody activity, the values were similar at day 0 (p>0·999), day 28 (p=0·119), and day 69 (p=0·300; table 3). By contrast, seropositive patients had significantly higher values than seronegative patients at all timepoints for IgG GMTs and neutralising antibody activity (table 3). Seropositive patients also had significantly higher IgG GMTs and neutralising antibody activity than did seronegative controls at all timepoints (table 3; appendix 2 pp 1–2).
In a post-hoc analysis, we found no significant associations between demographic data and specific autoimmune rheumatic diseases and therapies and anti-SARS-CoV-2 S1 or S2 IgG seropositivity and neutralising antibody positivity in the seropositive patient group at day 28 (appendix 2 p 3).
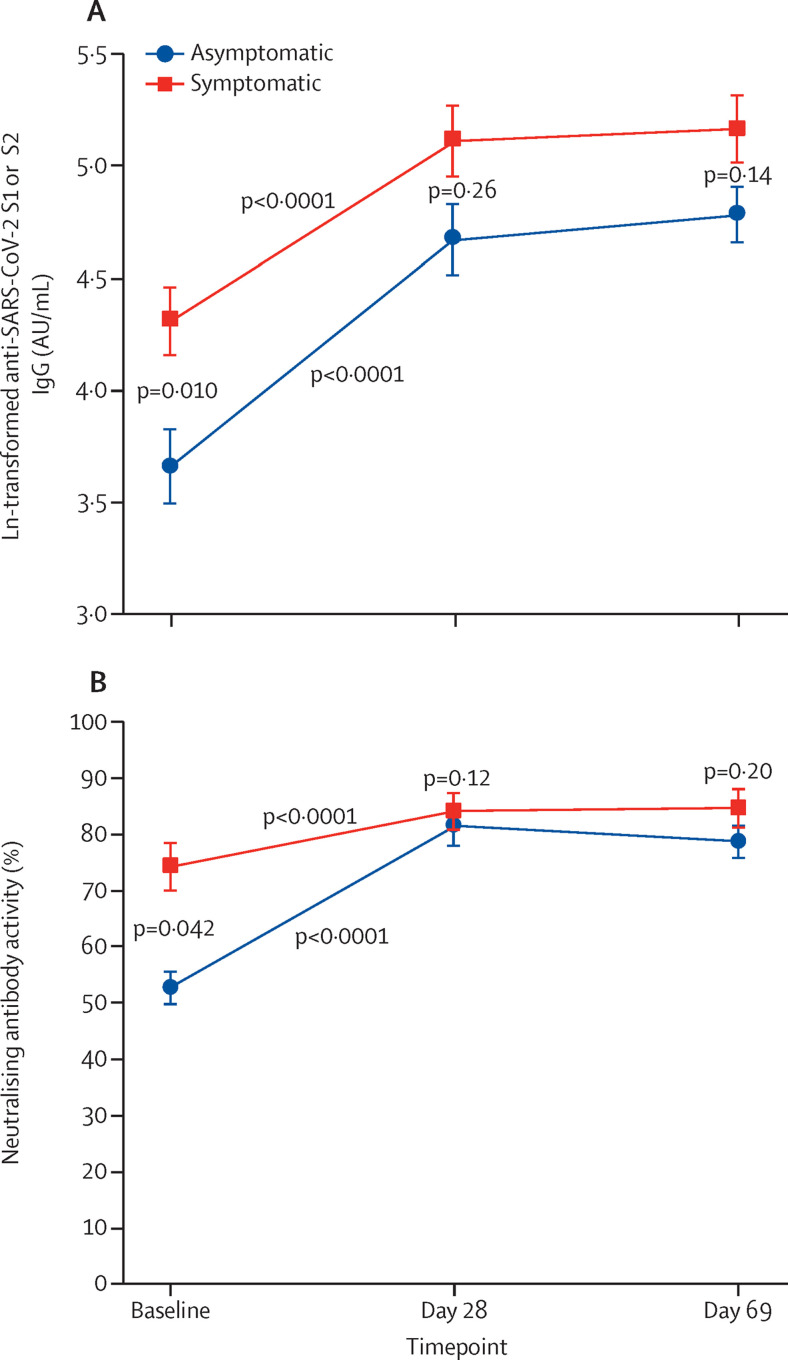
We assessed the effect of previous symptomatic versus asymptomatic SARS-CoV-2 infection on vaccine-induced response. Of 157 seropositive patients with autoimmune rheumatic diseases, 43 had no confirmation of previous acute infection by RT-PCR or rapid antigen test and therefore they were excluded from this analysis. The remaining 114 patients with a previous symptomatic RT-PCR or rapid antigen test confirmed COVID-19 were included. 41 (36%) of 114 had a previous symptomatic infection and 73 (64%) had a previous asymptomatic infection. We found significantly higher levels anti-SARS-CoV-2 S1 or S2 IgG GMTs on day 0 in the symptomatic group than in the asymptomatic group (75·1 AU/mL [95% CI 55·4–101·8] vs 39·0 AU/mL [28·0–54·3]; p=0·010) and thereafter similar levels after each vaccine dose (figure 3A ). Neutralising antibody activity responses showed the same pattern, with higher day 0 neutralising activity in the previously symptomatic group than in the previously asymptomatic group (74% [IQR 47–88] vs 53% [37–75]; p=0·042) but similar levels at day 28 (p=0·12) and day 69 (p=0·20; figure 3B). At day 69, the comparison of previously asymptomatic patients with seronegative patients revealed significantly higher IgG seropositivity (71 [97%] of 73 vs 353 [75%] of 471; p<0·0001) and neutralising antibody positivity (66 [90%] vs 289 [61%]; p<0·0001) in previously asymptomatic seropositive patients than in seronegative patients (post hoc). IgG and neutralising antibodies positivities were also higher in previously asymptomatic seropositive patients than in seronegative patients at day 0 (p<0·0001) and at day 28 (p<0·0001; data not shown).
Figure 3.
Anti-SARS-CoV-2 S1 or S2 IgG GMTs (A) and neutralising antibody activity (B) before (day 0) and after the first (day 28) and second (day 69) doses of CoronaVac in seropositive patients with autoimmune rheumatic diseases who had symptomatic infection (n=41) versus asymptomatic infection (n=73)
Datapoints are means with error bars showing SDs. The minimum possible value for anti-SARS-CoV-2 S1 or S2 IgG is 0·64 (ln 1·9, the value attributed IgG titres of ≤3·8 AU/mL) and for neutralising activity is 15% (attributed for values of <30%). AU=arbitrary units. GMT=geometric mean titre. S=spike.
The median of elapsed time after SARS-CoV-2 infection in symptomatic patients was 81 days (IQR 8–395) before vaccination. Antibody dynamics in patients with symptomatic infection less than or equal to 3 months (n=21) and more than 3 months (n=20) before vaccination were similar for IgG GMTs and neutralising antibody activity, with a significant increase from day 0 to day 28 (≤3 months only for IgG [p=0·038]; >3 months both IgG [p<0·0001] and neutralising antibodies [p=0·0040]) with no further increase from day 28 to day 69 (≤3 months: IgG p=0·92 and neutralising antibodies p=0·64; >3 months: IgG p=0·55 and neutralising antibodies p=0·49; data not shown).
The inactivated SARS-CoV-2 vaccine CoronaVac was well tolerated, with only mild adverse events reported (appendix 2 pp 5–6). Most adverse events were reported at higher frequencies among seropositive patients than among seronegative patients and seropositive and seronegative controls, particularly abdominal pain (p=0·026) and tremor (p=0·0040) after the first vaccine dose. After the second dose, vaccine injection erythema (p=0·022) and induration (p=0·023) were also more frequently reported by seropositive patients than the other groups. (appendix 2 p 5–6). Among all participants in CoronavRheum as of data cutoff (April 29, 2021), incident cases of SARS-CoV-2 infection confirmed with RT-PCR from day 0 to day 79 were less often observed in seropositive patients than in seronegative patients (three [1%] of 239 vs 39 [4%] 954; p=0·031). Eight cases of SARS-CoV-2 infection were reported between day 38 (10 days after complete vaccination) and day 79 (seven among seronegative patients with autoimmune rheumatic diseases and one in a seropositive patient). Regarding infection severity among these cases, seronegative and seropositive patients had a similar frequency of hospital admissions for COVID-19 (one [33%] of three vs five [13%] 39; p=0·378) and mechanical ventilation (one [33%] vs zero; p=0·071). SARS-CoV-2 genotyping could not be done for all symptomatic participants because 24 participants could not attend our centre for testing and instead had a PCR test for suspected SARS-CoV-2 infection at an external site. Among the 18 samples analysed for variants of concern, 16 (89%) had the gamma (P.1) variant, one (6%) had the alpha (B.1.1.7) variant, and one (6%) had a distinct variant.
Further analysis of incident RT-PCR-confirmed COVID-19 cases in seronegative patients with and without seroconversion after full vaccination (from 10 days after vaccine second dose to day 79) showed no difference between both groups (six [1%] of 707 vs one [<1%] of 247; p=0·68).
In the convenience sampled population, the analysis of social risk factors associated with exposure to SARS-CoV-2 showed that suspected COVID-19 contact in close relatives was significantly higher among seropositive patients (70 [45%] of 157) than among seronegative patients (92 [20%] of 471; p<0·0001) and seronegative controls (33 [21%] of 157; p<0·0001), but similar to among seropositive controls (57 [36%] of 157; p=0·035; appendix 2 p 7). Adherence to social quarantine was lower in seropositive controls (25 [16%]) and seronegative controls (35 [22%]) than among seropositive patients (98 [62%]), whereas use of public transportation was less frequent in patients (86 [55%] of seropositive patients and 221 [47%] of seronegative patients) than among controls (130 [83%] of seropositive controls and 121 [77%] seronegative controls; appendix 2 p 7).
Discussion
Here we provide the first evidence that previous exposure to SARS-CoV-2, with or without symptoms, results in distinct dynamics of antibody response in a large population of seropositive and seronegative patients with autoimmune rheumatic diseases and controls immunised with an inactivated SARS-CoV-2 vaccine, CoronaVac. Seropositive patients developed a robust response that plateaued between the first and second dose, whereas seronegative patients had moderate antibody production only after two doses of vaccine.
The criterion of positive pre-vaccination immune response that we used, which was independent of symptoms or RT-PCR positivity, offered a broader definition of SARS-CoV-2-exposure.19 In fact, serological detection is a more precise estimation of previous SARS-CoV-2 infection because asymptomatic infection can account for 40–50% of cases.20
Our findings support those of a previous small study in seropositive patients with autoimmune rheumatic diseases showing that mRNA-based and adenovirus-based SARS-CoV-2 vaccines induced high and similar IgG responses, with a substantial increase after the first dose, and no further increase after a second dose.14 We found here that, in a larger population, the same response occurred with an inactivated vaccine in an immunosuppressed population. The possible underlying mechanism for this robust response is related to pre-existing memory B cells, because recurrent exposure is known to recall responses to a greater extent than the primary response.6 In line with these findings, previous reports on an mRNA-based SARS-CoV-2 vaccine have already found that one dose of vaccine was sufficient to increase both cellular and humoral immune responses in healthy individuals who have recovered from COVID-19.5, 7, 21, 22
Although patients with autoimmune rheumatic diseases have reduced vaccine immunogenicity, not only to SARS-CoV-2 infection1, 4 but also to other vaccines (eg, for H1N1 influenza),23 our study provides convincing evidence that patients who have been exposed to SARS-CoV-2 respond adequately to an inactivated SARS-CoV-2 vaccine independent of intrinsic immunological defects or therapy. This finding is of great relevance for individuals who are immunocompromised because the presence of anti-SARS-CoV-2 S1 or S2 antibodies after infection was associated with a considerable reduction of the risk of COVID-19 in health-care workers.24
Supporting this result, we observed the same kinetics for neutralising antibody activity in seropositive patients and controls, with a peak reached after the first dose in both groups without further increase after the second dose, and with both groups achieving levels of approximately 70–80%. This immune response in seropositive patients with autoimmune rheumatic disease contrasts with the lower neutralising antibody activity observed in seronegative patients after two doses of same the vaccine4 and it was also higher than in the seronegative controls. This observation is relevant because of the reported correlation between serum neutralising antibody titres and protection from SARS-CoV-2 infection in human and animal models.25 Notably, the mRNA-based vaccine BNT162b2 (BioNTech–Pfizer) elicited an increase in anti-SARS-CoV-2 S1 and S2 antibody response after two doses in seropositive healthy individuals (20 times higher than in seronegative individuals)5 compared with what we observed after vaccination with CoronaVac after two doses; an approximately five times higher antibody response in seropositive patients and controls than in seronegative patients.
Previous studies in patients with autoimmune rheumatic diseases have shown effects of immunosuppressive therapy on antibody production after inactivated virus-based, mRNA-based, and adenovirus-vector-based SARS-CoV-2 vaccinations.4, 14, 26, 27 Mycophenolate mofetil, methotrexate, rituximab, and TNF inhibitors had a negative effect on anti-SARS-CoV-2 antibody responses, especially in seronegative populations of patients.4, 26, 27 By contrast, immunosuppression might be less relevant in seropositive patients, because we observed no detrimental effect on humoral response with these drugs, although we cannot draw any definitive conclusions because of the small sample of patients who were seronegative at day 28. The longer disease duration in our population of seronegative patients than in our seropositive patient population is probably not clinically important for immunogenicity, because age remained balanced between the groups.
Neutralising antibody activity before vaccination was higher in seropositive patients with RT-PCR-confirmed or serology-confirmed previous infection who were symptomatic than in those who were asymptomatic, in accordance with previous reports that neutralising antibody activity correlates positively with disease severity.28 However, after the first dose of vaccine, both groups reached a similar peak without further increase after the second dose, suggesting that for seropositive patients, a single dose of vaccine results in a boost to the maximum level of response with CoronaVac, independent of the underlying immunosuppressive condition. However, other investigators have reported that asymptomatic or oligosymptomatic individuals who have been exposed to SARS-CoV-2 but are otherwise healthy had a different response after an mRNA-based vaccine (BNT162b2), with lower antibody responses after two doses than symptomatic individuals.5
In line with previous studies that included healthy individuals,9, 10 we found that seropositive patients with autoimmune rheumatic diseases had more vaccine-related adverse events than did seronegative patients, which could be related to exacerbated immunity after vaccination, although more data are needed to define the underlying mechanism.8, 19 Ebinger and colleagues9 found that previously infected individuals had adverse post-vaccine symptoms more frequently than did individuals who had not been previously infected.
The main strength of our study was its prospective design, with all participants receiving vaccine within 2 days at one site, which enabled an adequate comparison of the kinetics of humoral response between study groups. Moreover, the inclusion of study groups balanced for sex and age, and similar groups of patients with autoimmune rheumatic diseases with regards to the diverse diagnoses allowed a more precise assessment of the specific effect of previous exposure to SARS-CoV-2 on the humoral response pattern in the different groups. SARS-CoV-2 vaccine responses might be affected by the presence of immune-mediated inflammatory diseases, age, and sex.26 Treatment was also similar in the patient groups, which is relevant because glucocorticoids, immunosuppressives, and biological therapies have been reported to impair SARS-CoV-2 vaccine immunogenicity.4, 27 Additionally, few studies on pre-vaccination SARS-CoV-2-exposed individuals have focused on the detailed immunological analysis of neutralising antibodies;6, 7 the leading candidate for a surrogate marker of protection.29 Notably, the ELISA kit we used to detect neutralising antibodies does not completely replace the gold standard live-virus neutralisation assay, but a comparison between the two tests revealed 98·2% sensitivity and 69·5% specificity.30
Our study limitations include the paucity of assessment of memory B-cell and T-cell responses, which is relevant to assess the recall of antibody response.6 Also, we have not assessed the effect of CoronaVac on disease activity, but previous large studies in patients with autoimmune rheumatic diseases reported that disease remains stable after SARS-CoV-2 vaccination.31 The absence of mRNA vaccination as a comparator is another limitation.
In summary, we found that SARS-CoV-2-exposed patients with autoimmune rheumatic diseases have a robust response that plateaus between the first and second dose of CoronaVac, independent of disease or therapy. Our finding raises the possibility that the reduced immunogenicity observed in seronegative patients might not represent the optimum response potential after a first SARS-CoV-2 vaccination, and therefore emphasises the importance of at least a second dose of vaccine in these patients. Future studies are urgently needed to assess whether a third dose of vaccine would be of additional value regarding clinical protection against COVID-19.
Data sharing
Anonymised participant-level data will be made available on request directed to the corresponding author. Proposals will be reviewed by the Hospital das Clinicas da Universidade de São Paulo review board and, after approval, data can be shared via email in line with the policy and procedures available online. If access to clinical and serological results are requested, approval will be needed from the Hospital das Clinicas da Universidade de São Paulo review board and the National Research Ethics Council and a Material Transfer Agreement in place.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We thank Antonio José Rodrigues Pereira, Solange Fusco, and Priscila Tagliaferro Rojo for the organisation of the infrastructure for blood sample collection and vaccination stations. We also thank the Registry Division, Security Division, IT Division, Pharmacy Division of Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, and all the volunteers participating in the study. This study was sponsored by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (number 2015/03756-4 to NEA, SGP, CAS, and EB; number 2019/17272-0 to LVKK; number 2017/14352-7 to TP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (305242/2019-9 to EB, 304984/2020-5 to CAS, 305556/2017-7 to RMRP, and 303379/2018-9 to SKS), and B3-Bolsa de Valores do Brasil. Instituto Butantan supplied the vaccine and had no other role in the trial.
Contributors
NEA, LVKK, SGP, ACM-R, EFNY, CGSS, TP, PRM, EGK, CAS, and EB conceived of and designed the study, participated in data collection and analysis, supervised clinical data management, wrote the manuscript, and revised the manuscript. NEA, LVKK, SGP, ACM-R, EFNY, CGSS, TP, CAS, EB, RF, SKS, PDS-B, DCOA, RMRP, LPCS, JMLV, and FW collected epidemiological and clinical data and assisted with the identification of SARS-CoV-2 infection and follow-up of patients. NEA, LVKK, and ACM-R verified the data and had access to raw data. NEA, LVKK, SGP, ACM-R, EFNY, CGSS, CAS, and EB had final responsibility for the decision to submit for publication. AMCS organised and supervised the vaccination protocol, ECS did the SARS-CoV-2 genotyping of positive RT-PCR samples. AJSD and LA supervised the processing of serum samples, SARS-CoV-2 specific antibody ELISAs and neutralisation assays, and SARS-CoV-2 RT-PCRs. All authors helped to edit the manuscript.
Supplementary Materials
References
- 1.WHO WHO validates Sinovac COVID-19 vaccine for emergency use and issues interim policy recommendations. Geneva: World Health Organization, June 1, 2021. https://www.who.int/news/item/01-06-2021-who-validates-sinovac-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations
- 2.Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385:875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brazilian Ministry of Health COVID-19 vaccination applied doses. https://covid.saude.gov.br/ (in Portuguese).
- 4.Medeiros-Ribeiro AC, Aikawa NE, Saad CGS, et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med. 2021;27:1744–1751. doi: 10.1038/s41591-021-01469-5. [DOI] [PubMed] [Google Scholar]
- 5.Levi R, Azzolini E, Pozzi C, et al. One dose of SARS-CoV-2 vaccine exponentially increases antibodies in individuals who have recovered from symptomatic COVID-19. J Clin Invest. 2021;131:e149154. doi: 10.1172/JCI149154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goel RR, Apostolidis SA, Painter MM, et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6:eabi6950. doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzoni A, Di Lauria N, Maggi L, et al. First-dose mRNA vaccination is sufficient to reactivate immunological memory to SARS-CoV-2 in subjects who have recovered from COVID-19. J Clin Invest. 2021;131:e149150. doi: 10.1172/JCI149150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley T, Grundberg E, CODIEFY study team. Selvarangan R. Antibody responses boosted in seropositive healthcare workers after single dose of SARS-CoV-2 mRNA vaccine. medRxiv. 2021 doi: 10.1101/2021.02.03.21251078. published online Feb 5. (preprint). [DOI] [Google Scholar]
- 9.Ebinger JE, Fert-Bober J, Printsev I, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021;27:981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krammer F, Srivastava K, Alshammary H, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med. 2020;383:2291–2293. doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grainger R, Machado PM, Robinson PC. Novel coronavirus disease-2019 (COVID-19) in people with rheumatic disease: epidemiology and outcomes. Best Pract Res Clin Rheumatol. 2021;35:101657. doi: 10.1016/j.berh.2020.101657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boekel L, Steenhuis M, Hooijberg F, et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol. 2021;3:e778–e788. doi: 10.1016/S2665-9913(21)00222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration Coronavirus (COVID-19) update: FDA authorizes additional vaccine dose for certain immunocompromised individuals. Aug 12, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised
- 16.Taylor SC, Hurst B, Charlton CL, et al. A new SARS-CoV-2 dual-purpose serology test: highly accurate infection tracing and neutralizing antibody response detection. J Clin Microbiol. 2021;59:e2438–e2440. doi: 10.1128/JCM.02438-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO World alliance for patient safety: WHO draft guidelines for adverse event reporting and learning systems: from information to action. Geneva: World Health Organization, 2005. https://apps.who.int/iris/handle/10665/69797
- 18.Vogels CBF, Breban MI, Ott IM, et al. Multiplex qPCR discriminates variants of concern to enhance global surveillance of SARS-CoV-2. PLoS Biol. 2021;19:e3001236. doi: 10.1371/journal.pbio.3001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai CC, Wang JH, Hsueh PR. Population-based seroprevalence surveys of anti-SARS-CoV-2 antibody: an up-to-date review. Int J Infect Dis. 2020;101:314–322. doi: 10.1016/j.ijid.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection : a narrative review. Ann Intern Med. 2020;173:362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gazit S, Shlezinger R, Perez G, et al. Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: reinfections versus breakthrough infections. medRxiv. 2021 doi: 10.1101/2021.08.24.21262415. published online Aug 25. (preprint). [DOI] [Google Scholar]
- 22.Anichini G, Terrosi C, Gandolfo C, et al. SARS-CoV-2 antibody response in persons with past natural infection. N Engl J Med. 2021;385:90–92. doi: 10.1056/NEJMc2103825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saad CGS, Borba EF, Aikawa NE, et al. Immunogenicity and safety of the 2009 non-adjuvanted influenza A/H1N1 vaccine in a large cohort of autoimmune rheumatic diseases. Ann Rheum Dis. 2011;70:1068–1073. doi: 10.1136/ard.2011.150250. [DOI] [PubMed] [Google Scholar]
- 24.Lumley SF, O'Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Addetia A, Crawford KHD, Dingens A, et al. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol. 2020;58:e02107–e02120. doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon D, Tascilar K, Fagni F, et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann Rheum Dis. 2021;80:1312–1316. doi: 10.1136/annrheumdis-2021-220461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyarsky BJ, Ruddy JA, Connolly CM, et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80:1098–1099. doi: 10.1136/annrheumdis-2021-220289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chvatal-Medina M, Mendez-Cortina Y, Patiño PJ, Velilla PA, Rugeles MT. Antibody responses in COVID-19: a review. Front Immunol. 2021 doi: 10.3389/fimmu.2021.633184. published online April 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 30.Müller K, Girl P, von Buttlar H, Dobler G, Wölfel R. Comparison of two commercial surrogate ELISAs to detect a neutralising antibody response to SARS-CoV-2. J Virol Methods. 2021;292:114122. doi: 10.1016/j.jviromet.2021.114122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymised participant-level data will be made available on request directed to the corresponding author. Proposals will be reviewed by the Hospital das Clinicas da Universidade de São Paulo review board and, after approval, data can be shared via email in line with the policy and procedures available online. If access to clinical and serological results are requested, approval will be needed from the Hospital das Clinicas da Universidade de São Paulo review board and the National Research Ethics Council and a Material Transfer Agreement in place.