Abstract
Background and Objective
To evaluate phenotypic and genetic relationships between migraine and lipoprotein subfractions.
Methods
We evaluated phenotypic associations between migraine and 19 lipoprotein subfraction measures in the Women's Genome Health Study (n = 22,788). We then investigated genetic relationships between these traits using summary statistics from the International Headache Genetics Consortium for migraine (ncase = 54,552, ncontrol = 297,970) and combined summary data for lipoprotein subfractions (n up to 47,713).
Results
There was a significant phenotypic association (odds ratio 1.27 [95% confidence interval 1.12–1.44]) and a significant genetic correlation at 0.18 (p = 0.001) between migraine and triglyceride-rich lipoproteins (TRLPs) concentration but not for low-density lipoprotein or high-density lipoprotein subfractions. Mendelian randomization (MR) estimates were largely null, implying that pleiotropy rather than causality underlies the genetic correlation between migraine and lipoprotein subfractions. Pleiotropy was further supported in cross-trait meta-analysis, revealing significant shared signals at 4 loci (chr2p21 harboring THADA, chr5q13.3 harboring HMGCR, chr6q22.31 harboring HEY2, and chr7q11.23 harboring MLXIPL) between migraine and lipoprotein subfractions. Three of these loci were replicated for migraine (p < 0.05) in a smaller sample from the UK Biobank. The shared signal at chr5q13.3 colocalized with expression of HMGCR, ANKDD1B, and COL4A3BP in multiple tissues.
Conclusions
The study supports the association between certain lipoprotein subfractions, especially for TRLP, and migraine in populations of European ancestry. The corresponding shared genetic components may help identify potential targets for future migraine therapeutics.
Classification of Evidence
This study provides Class I evidence that migraine is significantly associated with some lipoprotein subfractions.
Migraine, especially migraine with aura (MA), has been associated with increased risk of ischemic stroke and cardiovascular disease (CVD),1-3 motivating researchers to investigate its potential links to traditional CVD risk factors. Accumulating evidence supports an unfavorable lipid profile among individuals with migraine including relatively elevated triglycerides, total cholesterol, and low-density lipoprotein (LDL), and relatively lower high-density lipoprotein (HDL) compared with healthy controls.4-7 However, these associations are not wholly consistent and even absent in some studies,8 and the mechanisms underlying such potential associations remain unknown.
Beyond assessment of conventional lipids, lipoprotein subfractions (including particle concentration and size) for LDL, HDL, and triglyceride-rich lipoproteins (TRLPs; also known as very low-density lipoproteins) can be determined by nuclear magnetic resonance (NMR) spectroscopy,9,10 providing an additional dimension for analysis of lipid associations with migraine. One recent large-scale epidemiologic study examined associations of lipoprotein subfractions and apolipoproteins with migraine in 8 Dutch cohorts, concluding that alterations in HDL metabolism may be related with migraine status, but finding no associations for LDL particles or TRLP after correcting for multiple testing, possibly due to limited power.7 However, this study did not explore potential differences among active migraine, prior migraine, MA, and migraine without aura (MO), and did not control for potential confounding by menopausal status, postmenopausal hormone therapy usage, smoking, and alcohol consumption.
To avoid some of the biases that may arise in observational epidemiology studies and to further understand the association between migraine and lipoprotein subfractions, genetic approaches can be used to corroborate phenotypic correlation and distinguish between causal compared with shared biological mechanisms underlying phenotypic correlations. We therefore evaluated phenotypic relationships between migraine and lipoprotein subfractions using data from the Women's Genome Health Study (WGHS)11-13 as well as genetic relationships using large-scale genome-wide association study (GWAS) summary statistics for migraine (any migraine, MA, and MO) from the International Headache Genetics Consortium (IHGC)14 and for lipoprotein subfractions by combining data from the WGHS12 and publicly available data generated from 14 cohorts of European ancestry.15 By leveraging genetic methods as applied to lipoprotein fractions, we aimed to explore the biological mechanisms in the observed association between clinical lipid measures and migraine, which may also provide insight into the relationship between migraine and CVD.
Methods
We investigated phenotypical and genotypical associations between migraine, including migraine subtypes, and lipoprotein subfractions. This study provides Class I evidence that migraine is significantly associated with some lipoprotein subfractions.
Study Population
We used data from the Women's Health Study (WHS), for which the design, methods, and main findings have been described in detail previously.11-13 Briefly, a total of 39,876 female health professionals aged 45 years or older and free from CVD, cancer, or other major illnesses at baseline during 1992–1995 were randomly assigned in a balanced 2 × 2 design to low-dose aspirin (100 mg on alternate days) or placebo, or low-dose vitamin E (600 IU on alternate days) or placebo. Baseline information was collected by a mailed questionnaire interrogating demographic information, migraine status, aura status, menopause status, postmenopausal hormone therapy usage, lifestyle habits (such as smoking and drinking), and cardiovascular risk factors. In total, 28,345 female participants provided blood samples prior to randomization, which were collected in tubes containing EDTA and stored at −170°C until measurement of lipoprotein fractions occurred. The WGHS is a nested subset of WHS participants for whole genome genetic analysis and corresponds to women with available DNA from the baseline blood sample. Within the WGHS, after excluding participants with missing information on migraine, lipoprotein fractions, and non-European ancestry (n = 1,490), a total of 25,863 women remained for phenotypic analysis. For genome-wide association analysis in the WGHS on lipoprotein fractions, there were 22,788 women of European ancestry as determined by genetic analysis using whole genome genetic data.12 All participants provided written informed consent and the study protocols were approved by the Partners Institutional Review Board.
Ascertainment of Migraine and Migraine Subtypes
Information on migraine status was collected via the baseline questionnaire: “Have you ever had migraine headaches?” and “In the past year, have you had migraine headaches?” Based on their responses to these questions, participants were categorized into “any migraine” vs “no migraine history.” Those reporting “any migraine” were further classified as “active migraine” (i.e., those reporting migraine in the past year) and “prior migraine” (i.e., those who reported ever having had a migraine but not in the past year). Participants who reported active migraine were asked additional details about their migraine attacks, including “aura or any indication a migraine is coming,” and responses were used to classify individuals with active migraine into “active migraine with aura” and “active migraine without aura.” Migraine diagnosis in the WHS for MO based on formal criteria from the International Classification of Headache Disorders, second edition (ICHD-II) showed excellent agreement with self-reported migraine.16 We acknowledge that migraine ascertainment by self-report may allow some misclassification, especially with respect to aura status. In the supplement, we show that the variant rs11031122 at the MPPED2 gene, which was found to be selectively associated with MA compared to MO in the IHGC GWAS,14 is similarly selective for MA compared with MO in the WGHS.
Measurement of Lipoprotein Subfractions
In the WGHS, lipoprotein subfraction particles (concentrations) for TRLP (total TRLP particles, very large TRLP [VL-TRLP], large TRLP [L-TRLP], medium TRLP [M-TRLP], small TRLP [S-TRLP], and very small TRLP [VS-TRLP] particles), LDL (total LDL particles [LDLP], large LDL [L-LDLP], medium LDL [M-LDLP], and small LDL [S-LDLP] particles), and HDL (total HDL particles [HDLP], large HDL [L-HDLP], medium HDL [M-HDLP], and small HDL [S-HDLP] particles) were measured by targeted NMR spectroscopy.10,17 NMR spectroscopy measures were performed using the H-NMR (400 MHz) LipoProfile-IV (LipoScience [now LabCorp]) platform, and mean particle size for TRLP (abbreviated as TRLPZ), LDLP (LDLZ), and HDLP (HDLZ) were derived from the primary measures for subfraction particles. Apolipoproteins B100 (ApoB) and A1 (ApoA1) were also available and quantified using turbidimetric assays (DiaSorin).18
Phenotypic Analysis
The 19 lipoprotein biomarkers were not normally distributed (eTable 1, data available from Dryad: doi.org/10.5061/dryad.tht76hdxv), and were therefore divided into quintiles based on the distribution among women not taking postmenopausal hormone therapy at blood draw as specified by guidelines for lipid standardization from the Department of Health and Human Services.6 For each lipoprotein measure, logistic regression models were used to compute odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for active migraine, MA, MO, and prior migraine within each quintile compared to the lowest quintile as the reference group. Tests for linear trends were performed using the median value of the lipoprotein subfractions within each quintile. All models were adjusted for age (continuous), body mass index, menopause status, smoking status (current smoking or not), drinking status (rarely or never, 1–3 drinks/month, 1–6 drinks/week, and ≥1 drinks/day), physical exercise (rarely or never, <1 time/week, 1–3 times/week, and ≥4 times/week), fasting status, and postmenopausal hormone therapy usage. Two-sided p < 0.05/19 was considered statistically significant.
GWAS for Lipoprotein Subfractions in WGHS
We first performed GWASs for 19 lipoprotein subfractions for up to 22,788 female participants in WGHS with European ancestry. As previously described, genotyping in the WGHS was performed with Human-Hap300 Duo + (Illumina) using the Infinium II protocol with quality control as described.12 Imputation was conducted based on 1000G phase 1, version 3 reference panel,12,19 resulting in a total of 8,794,756 single nucleotide polymorphisms (SNPs) with imputation quality score >0.3 for inclusion in GWAS analyses. All lipoprotein subfractions were first adjusted for age and top 10 principal components from genomic data and the resulting residuals were transformed to normal distribution by inverse rank-based normal transformation. Genome-wide associations with these residuals were evaluated by linear regressions with additive encoding of imputed genotype dose as implemented in the ProbABEL package.20
GWAS Summary Statistics for Migraine and Lipoprotein Subfractions
We used the most recent GWAS summary-level data from the IHGC for migraine (any migraine and 2 subtypes of migraine: MA and MO).14 Originally, the migraine summary statistics contained 59,674 cases and 316,078 controls from 22 cohorts including the WGHS. To avoid overlapping samples, we removed the WGHS (ncases = 5,122, ncontrols = 18,108) contribution from the IHGC migraine GWAS summary statistics, resulting in 54,552 cases and 297,970 controls for migraine GWAS summary statistics. All samples contributing to the IHGC migraine GWAS had European ancestry as verified by genetic analysis.
To increase power for genetic analysis of lipoproteins, we also used publicly available genome-wide association summary statistics for NMR-derived lipoprotein measures among a total of 24,925 men and women from 14 cohorts with European ancestry.15 The summary statistics were derived by meta-analysis across cohort specific analyses that incorporated suitable corrections for any cohort-specific sub-European population substructure. These data overlapped with 13 lipoprotein fraction and lipoprotein traits in WGHS (VL-TRLP, L-TRLP, M-TRLP, S-TRLP, VS-TRLP, L-LDLP, M-LDLP, S-LDLP, L-HDLP, M-HDLP, S-HDLP, ApoB, and ApoA1). We combined GWAS summary statistics for lipoprotein fractions from the WGHS (n = 22,788) with those from the 14 cohorts using fixed-effects meta-analysis weighted by the inverse variances to obtain a combined effect size, standard error, and p value at each marker21 (total n = 47,713). Analysis of the remaining 6 lipoprotein fractions used data solely from WGHS.
Genetic Correlation Analysis
To evaluate genetic correlation between migraine and each lipoprotein subfraction, we conducted linkage disequilibrium score regression (LDSC) using precomputed linkage disequilibrium scores derived from ∼1.2 million common and well-imputed SNPs in European populations as represented in the Hapmap3 reference panel excluding the human leukocyte antigen region.22 Furthermore, we tested for enrichment of genetic correlation according to functional properties of the genome using partitioned LDSC applied to 11 annotations (DNase I hypersensitivity sites [DHS], fetal DHS, DNaseI digital genomic footprinting [DGF] region, histone marks [H3K4me1, H3K4me3, H3K9ac, and H3K27ac], intron, Super Enhancer, transcription factor binding sites [TFBS], and transcribed region).23 Two-sided false discovery rate–corrected p value (pFDR) <0.05 was considered significant.
Cross-Trait Meta-analysis Between Migraine and Lipoprotein Subfractions
We conducted pairwise cross-trait meta-analysis using cross-phenotype association (CPASSOC)24 through the statistic SHet that implements a sample-size weighted, fixed effect meta-analysis of the association statistics from the individual traits. In these analyses, we used total sample size from the combined summary statistics file for lipoprotein subfractions and an average effective sample size for migraine as weights.21 The advantage of this approach rather than deriving weights from the inverse variance is that it ensures that the traits are on the same scale, as variance is dependent on the scale of measurement. Significant shared signals were defined as loci reaching genome-wide significance in joint analysis (p < 5 × 10−8) and also met a significance threshold (here p < 10−3) separately for individual traits. Replication of these overlapped loci from CPASSOC was performed using logistic regression to test the association with migraine at the lead SNP of each identified locus in an independent data set of European ancestry verified by genetic analysis from the UK Biobank (data field: 20002) with adjustment for age, a quadratic term for age, sex, genotyping array, and the first 20 ancestry principal components in participants of European ancestry (ncases/ncontrols = 13,465/445,790).
Colocalization
We performed colocalization analysis of genetic associations with lipoprotein subfractions and migraine at the shared loci from cross-trait meta-analysis using the R package coloc,25 extracting variants within 500 kb of the index SNP at each shared locus and calculating the posterior probability of colocalization (i.e., posterior probability H3 [PPH3]: colocalized with different causal variants within locus; posterior probability H4 [PPH4]: colocalized at the same causal variants within locus). For loci with evidence of moderate to strong colocalization (H3 or H4 >0.4), we further conducted colocalization analysis between cross-trait meta-analysis signals and GTEx eQTLs from 48 GTEx tissues (version 7) to determine if the shared loci were also related to gene expression. Candidate loci were considered as co-localized with gene expression with PPH4 > 0.5.25
Mendelian Randomization Analysis
To examine evidence for potential causal relationships between migraine and lipoprotein subfractions, we conducted instrumental variable analysis using bidirectional mendelian randomization (MR) implemented in generalized summary data–based MR (GSMR).26 GSMR applies strict criteria to select independent SNP instruments and extends conventional MR by accounting for the sampling variance in the genetic effects on both exposure (bzx) and outcome (bzy) in estimating the instrumental effect. Furthermore, as pleiotropy is an important confounder that could bias the estimates and possibly result in an inflated test statistic in MR analysis, we used heterogeneity criteria in HEIDI (heterogeneity in dependent instruments, pHEIDI < 0.01) in the GSMR package to exclude pleiotropic SNPs from the analysis. We also conducted sensitivity analyses using conventional inverse-variance weighted (IVW) MR, weighted median, simple median, and MR-Egger (Egger regression) implemented in the R package TwoSampleMR27 and MR-PRESSO.28 As migraine is a binary variable, we scaled the reverse causal (i.e., of migraine on lipoprotein subfractions) estimates to represent the average change in the standardized lipoprotein subfraction per doubling (2-fold increase) in the odds of migraine by multiplying the reverse causal estimate by 0.693 (loge2).29
Data Availability
GWAS summary statistics described above are available from cited study authors or from the public domain as indicated. The WGHS sample is not publicly available because access is restricted by the institutional review board but further information about the data is available from the corresponding author upon reasonable request.
Standard Protocol Approvals, Registrations, and Patient Consents
Analysis in the WGHS was performed with written informed consent from study participants and was approved by the institutional review board of Brigham and Women's Hospital. For GWAS summary statistics from meta-analysis, all participants who contributed to cohort-level summary statistics constituting the meta-analyses provided written informed consent and each of the cohort protocols was approved by a local institutional review board.
Results
Phenotypic Association Between Migraine and Lipoprotein Subfractions
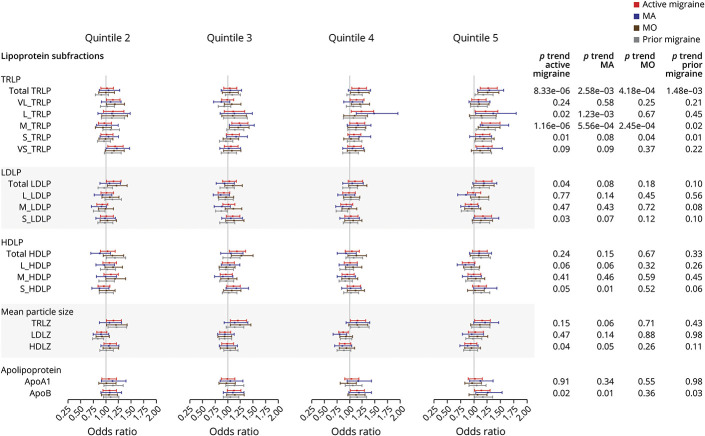
The baseline characteristics of the participants included in phenotypic analysis are presented in eTable 1, data available from Dryad: doi.org/10.5061/dryad.tht76hdxv. A total of 25,863 female participants were included in the final analysis: 3,336 (12.89%) were individuals with active migraine and 2,375 (9.18%) were individuals with prior migraine. The phenotypic associations between lipoprotein subfractions and migraine (active migraine, MA, MO, and prior migraine) are summarized in Figure 1. For active migraine, the multivariable-adjusted OR (95% CI) among the highest compared with lowest quintile was 1.27 (95% CI 1.12–1.44; p for trend: 8.33 × 10−06) for total TRLP and 1.31 (95% CI 1.15–1.49; p for trend: 1.16 × 10−06) for M-TRLP, both significant after correction for multiple testing (p < 0.05/19) (see also eTable 2, data available from Dryad: doi.org/10.5061/dryad.tht76hdxv). There were additional nominal associations (p < 0.05) with active migraine for L-TRLP, S-TRLP, total LDLP, S-LDLP, S-HDLP, HDLZ, and ApoB. For prior migraine, there were nominal associations for total TRLP (p for trend: 1.48 × 10−03), M-TRLP (p for trend: 0.02), S-TRLP (p for trend: 0.01), and ApoB (p for trend: 0.03) (see also eTable 3, data available from Dryad: doi.org/10.5061/dryad.tht76hdxv).
Figure 1. Phenotypic Association Between Lipoprotein Subfractions and Migraine (Active Migraine, Migraine With Aura, Migraine Without Aura, and Prior Migraine) in the Women's Genome Health Study.
Models adjusted for age, body mass index, menopause status, smoking status, drinking status, physical exercise, fasting status, and postmenopausal hormone therapy usage. The odds ratio and 95% confidence intervals for quintiles 2–5 compared to quintile 1 of each lipoprotein subfraction are shown. ApoA1 = apolipoprotein A1; ApoB = apolipoprotein B100.; HDLP = high-density lipoprotein particles; HDLZ = high-density lipoprotein mean particle size; L-HDLP = large high-density lipoprotein particles; L-LDLP = large low-density lipoprotein particles; L-TRLP = large triglyceride-rich lipoprotein particles; LDLP = low-density lipoprotein particles; LDLZ = low-density lipoprotein mean particle size; M-HDLP = medium high-density lipoprotein particles; M-LDLP = medium low-density lipoprotein particles; M-TRLP = medium triglyceride-rich lipoprotein particles; MA = migraine with aura; MO = migraine without aura; S-HDLP = small high-density lipoprotein particles; S-LDLP = small low-density lipoprotein particles; S-TRLP = small triglyceride-rich lipoprotein particles; TRLP = triglyceride-rich lipoprotein particles; TRLZ = triglyceride-rich lipoprotein mean particle size; VL-TRLP = very large triglyceride-rich lipoprotein particles; VS-TRLP = very small triglyceride-rich lipoprotein particles.
Among the migraine subtypes, total TRLP and M-TRLP were significantly related to both MA (odds ratio [OR] for the highest quintile: 1.29 [95% CI 1.07–1.56], p for trend = 2.58 × 10−03 and 1.36 [95% CI 1.12–1.65], p for trend = 5.56 × 10−04, respectively) and MO (OR for the highest quintile: 1.26 [95% CI 1.08–1.47], p for trend: 4.18 × 10−04 and 1.27 [95% CI 1.08–1.49], p for trend = 2.45 × 10−04, respectively) while L-TRLP was only related to MA (OR 1.36 [95% CI 1.03–1.79]; p for trend: 1.23 × 10−03) after correcting for multiple testing (see eTables 4 and 5, data available from Dryad: doi.org/10.5061/dryad.tht76hdxv). In addition, S-HDLP, HDLZ, and ApoB were nominally related to MA but not MO, while S-TRLP was nominally related to MO but not to MA.
Genetic Correlation Between Migraine and Lipoprotein Subfractions
We next evaluated genetic correlations between lipoprotein subfraction measures and migraine using GWAS summary statistics that were derived from individuals of European ancestry14 (Methods, Figure 2, and eTable 6, data available from Dryad: doi.org/10.5061/dryad.tht76hdxv). Any migraine exhibited significant genetic correlation (rg = 0.18, p = 0.001) with total TRLP after correcting for multiple testing (p < 0.05/19), and had nominally significant (p < 0.05) correlations with M-TRLP (rg = 0.15), S-LDLP (rg = 0.16), S-HDLP (rg = 0.20), LDLZ (rg = −0.15), and HDLZ (rg = −0.19). Extending analysis to migraine subtypes, there were only nominally significant genetic correlations of MA with ApoA1 (rg = 0.26), and of MO with total TRLP (rg = 0.23) and LDLZ (rg = −0.31). Sensitivity analysis of the genetic correlation including only the WGHS for lipoprotein subfractions showed similar patterns and magnitudes (eFigure 1, data available from Dryad: doi.org/10.5061/dryad.tht76hdxv).
Figure 2. Whole Genome Genetic Correlations Between Lipoprotein Subfraction Measures and Migraine (Any Migraine, Migraine With Aura, and Migraine Without Aura) Using Linkage Disequilibrium Score Regression.
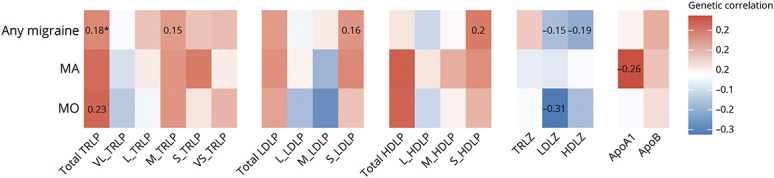
Colors represent the magnitude of genetic correlation between lipoprotein subfraction measures and migraine using linkage disequilibrium score regression: red for positive genetic correlation and blue for negative genetic correlation. Numbers represent the genetic correlation at nominal significance level (p < 0.05). *Significant genetic correlation after controlling for multiple testing (p < 0.05/19). ApoA1 = apolipoprotein A1; ApoB = apolipoprotein B100.; HDLP = high-density lipoprotein particles; HDLZ = high-density lipoprotein mean particle size; L-HDLP = large high-density lipoprotein particles; L-LDLP = large low-density lipoprotein particles; L-TRLP = large triglyceride-rich lipoprotein particles; LDLP = low-density lipoprotein particles; LDLZ = low-density lipoprotein mean particle size; M-HDLP = medium high-density lipoprotein particles; M-LDLP = medium low-density lipoprotein particles; M-TRLP = medium triglyceride-rich lipoprotein particles; MA = migraine with aura; MO = migraine without aura; S-HDLP = small high-density lipoprotein particles; S-LDLP = small low-density lipoprotein particles; S-TRLP = small triglyceride-rich lipoprotein particles; TRLP = triglyceride-rich lipoprotein particles; TRLZ = triglyceride-rich lipoprotein mean particle size; VL-TRLP = very large triglyceride-rich lipoprotein particles; VS-TRLP = very small triglyceride-rich lipoprotein particles.
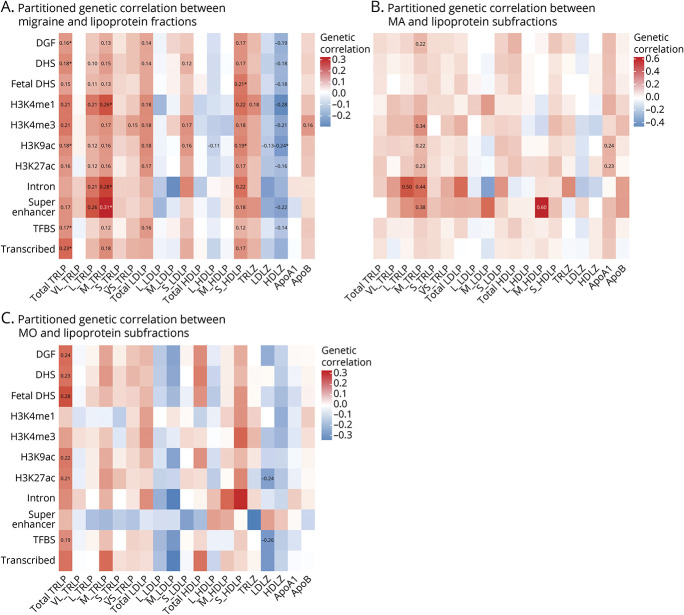
To detect specific biological properties of the genome with potential disproportionate contributions to shared heritability, we partitioned the genetic correlations by 11 functional annotations (Figure 3, see legend for definitions). Consistent with the overall genetic correlations, total TRLP also showed significant partitioned genetic correlation with any migraine at annotations for DGF (rg = 0.16, p = 0.003), DHS (rg = 0.18, p = 0.001), H3K9ac (rg = 0.18, p = 0.001), TFBS (rg = 0.17, p = 0.002), and transcribed regions (rg = 0.23, p = 0.001) after controlling multiple comparisons (all pFDR < 0.05). Although M-TRLP was only nominally correlated with any migraine in whole-genome analysis, it was significantly correlated with any migraine for 3 annotations: H3K4me1 (rg = 0.26, p = 0.002), intron (rg = 0.28, p = 0.001), and super enhancers (rg = 0.31, p = 0.001) after controlling multiple comparisons (all PFDR < 0.05). Among migraine subtypes, there were nominally significant partitioned genetic correlations at 1 or more annotations for L-TRLP, M-TRLP, M-HDLP, and ApoA1 with MA, and for total TRLP and LDLZ with MO. Partitioned genetic correlation between MA and lipoprotein subfractions was notably strong at introns and super enhancers (rg ranges from 0.38 to 0.6).
Figure 3. Partitioned Genetic Correlations Between Lipoprotein Subfraction Measures and Migraine (Any Migraine, Migraine With Aura, and Migraine Without Aura) According to 11 Functional Categories Using Linkage Disequilibrium Score Regression.
Colors represent the magnitude of genetic correlation between lipoprotein subfraction measures and migraine using linkage disequilibrium score regression: red for positive genetic correlation and blue for negative genetic correlation. Numbers represent the genetic correlation at nominal significance level (p < 0.05). *Significant genetic correlation after controlling for multiple testing (p < 0.05/[19 × 11]). ApoA1 = apolipoprotein A1; ApoB = apolipoprotein B100; HDLP = high-density lipoprotein particles; HDLZ = high-density lipoprotein mean particle size; L-HDLP = large high-density lipoprotein particles; L-LDLP = large low-density lipoprotein particles; L-TRLP = large triglyceride-rich lipoprotein particles; LDLP = low-density lipoprotein particles; LDLZ = low-density lipoprotein mean particle size; M-HDLP = medium high-density lipoprotein particles; M-LDLP = medium low-density lipoprotein particles; M-TRLP = medium triglyceride-rich lipoprotein particles; MA = migraine with aura; MO = migraine without aura; S-HDLP = small high-density lipoprotein particles; S-LDLP = small low-density lipoprotein particles; S-TRLP = small triglyceride-rich lipoprotein particles; TRLP = triglyceride-rich lipoprotein particles; TRLZ = triglyceride-rich lipoprotein mean particle size; VL-TRLP = very large triglyceride-rich lipoprotein particles; VS-TRLP = very small triglyceride-rich lipoprotein particles.
Loci Shared by Migraine and Lipoprotein Subfractions
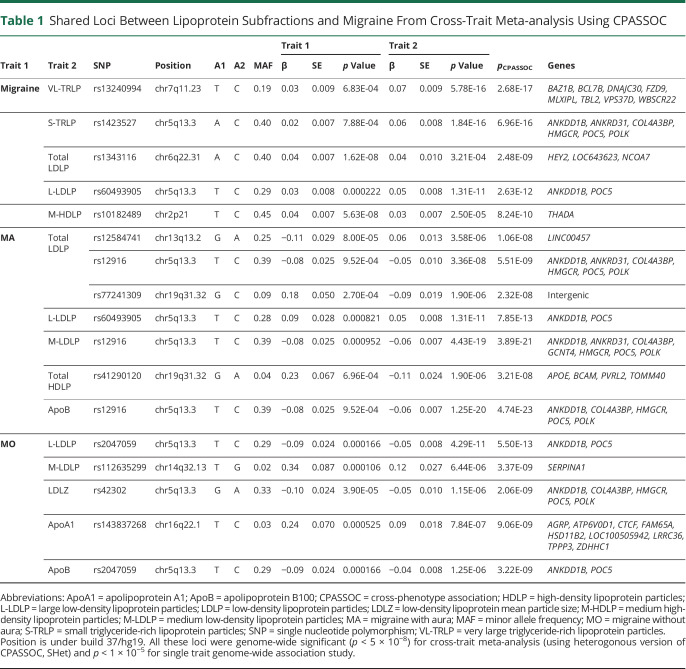
To identify individual SNPs that are associated with both migraine and one or more lipoprotein subfractions, we conducted cross-trait meta-analysis and colocalization analysis (Table 1 and eTable 7, data available from Dryad: doi.org/10.5061/dryad.tht76hdxv). There were shared SNP associations with genome-wide significance (p < 5 × 10−8) for at least one of the lipoprotein subfractions with any migraine at 4 loci (chr2p21, chr5q13.3, chr6q22.31, and chr7q11.23), with MA at 3 loci (chr5q13.3, chr13q13.2, and chr19q31.32), and with MO at 3 loci (chr5q13.3, chr14q32.13, and chr16q22.1). Locus chr5q13.3 was the most consistent shared signal observed for lipoprotein subfractions with migraine and its tested subtypes (MA and MO).
Table 1.
Shared Loci Between Lipoprotein Subfractions and Migraine From Cross-Trait Meta-analysis Using CPASSOC
To determine whether these loci contained the same causal variant, we further evaluated statistical colocalization of the shared association signals. This revealed evidence for the same underlying signal (PPH4 >0.5, eTable 7, data available from Dryad: doi.org/10.5061/dryad.tht76hdxv) for any migraine with M-HDLP at chr2p21 (THADA) for lead SNP rs10182489 (pmeta = 8.24 × 10−10), and with total LDLP at chr6q22.31 (HEY2) for lead SNP rs1343116 (pmeta = 2.48 × 10−09). Colocalization also supported a shared causal variant with MA and total LDLP at chr13q13.2 (intergenic/LINC00457) for lead SNP rs12584741 (pmeta = 1.06 × 10−08) as well as with MO and LDLZ at chr5q13.3 (harboring COL4A3BP, HMGCR, and ANKDD1B) for lead SNP rs42302 (pmeta = 2.06 × 10−09) and with M-LDLP at chr14q32.13 (SERPINA1) for lead SNP rs112635299 (pmeta = 3.37 × 10−09).
Additional shared associations between any migraine and VL-TRLP at chr7q11.23 (harboring MLXIPL) for lead SNP rs13240994 (pmeta = 2.68 × 10−17) and between MA and total HDLP at chr19q31.32 (APOE region) for lead SNP rs41290120 (pmeta = 3.21 × 10−08) had marginal support by colocalization (PPH4 = 0.43 and 0.46, respectively). Rs13240994 is proximal to a migraine locus reported previously by Pickrell et al.30 (lead SNP: rs202203062, p = 2.50 × 10−08) but was not identified as a genome-wide significant migraine locus in the IHGC migraine GWAS. By contrast, colocalization analysis of the joint associations at chr5q13.3 of L-LDLP with either any migraine or MO suggested that a different causal variant may drive the migraine and lipoprotein signals (PPH3 > 0.5 and PPH4 < 0.1).
To identify likely candidate genes at the colocalized loci, we next considered colocalization of the novel migraine association with GTEx eQTLs across 48 tissues (eTables 8–20, data available from Dryad: doi.org/10.5061/dryad.tht76hdxv). These additional analyses further supported shared effects on migraine and gene expression involving the MLXIPL, COL4A3BP, HMGCR, and ANKDD1B genes in multiple tissues from circulation, digestive, and nervous systems, especially esophagus mucosa, tibial nerve, and artery (PPH4 > 0.5, eTables 8–20, data available from Dryad: doi.org/10.5061/dryad.tht76hdxv). However, due to insufficient number of samples for liver in GTEx, the analysis was not able to address whether gene expression at these loci in that tissue, perhaps the most relevant for circulating lipoproteins, was consistent with the colocalized, cross-trait association signals.
We next tested for independent replication of the novel migraine loci in the UK Biobank. Lead SNPs at chr5q13.3 and chr14q32.13 were significantly associated after correcting for multiple testing (p < 0.05/14, eTable 21, data available from Dryad: doi.org/10.5061/dryad.tht76hdxv), and lead SNPs at loci chr6q22.31 and chr7q11.23 were nominally significant while the rest were not replicated. Notably, the UK Biobank replication dataset was limited by lower power due to smaller number of migraine cases (ncases = 13,465), and limited information on migraine subtypes for most migraine cases (504 MA cases [ICD10 code G43.1] and 71 MO cases [ICD10 code G43.0]) for genetic analysis.
Mendelian Randomization Analysis
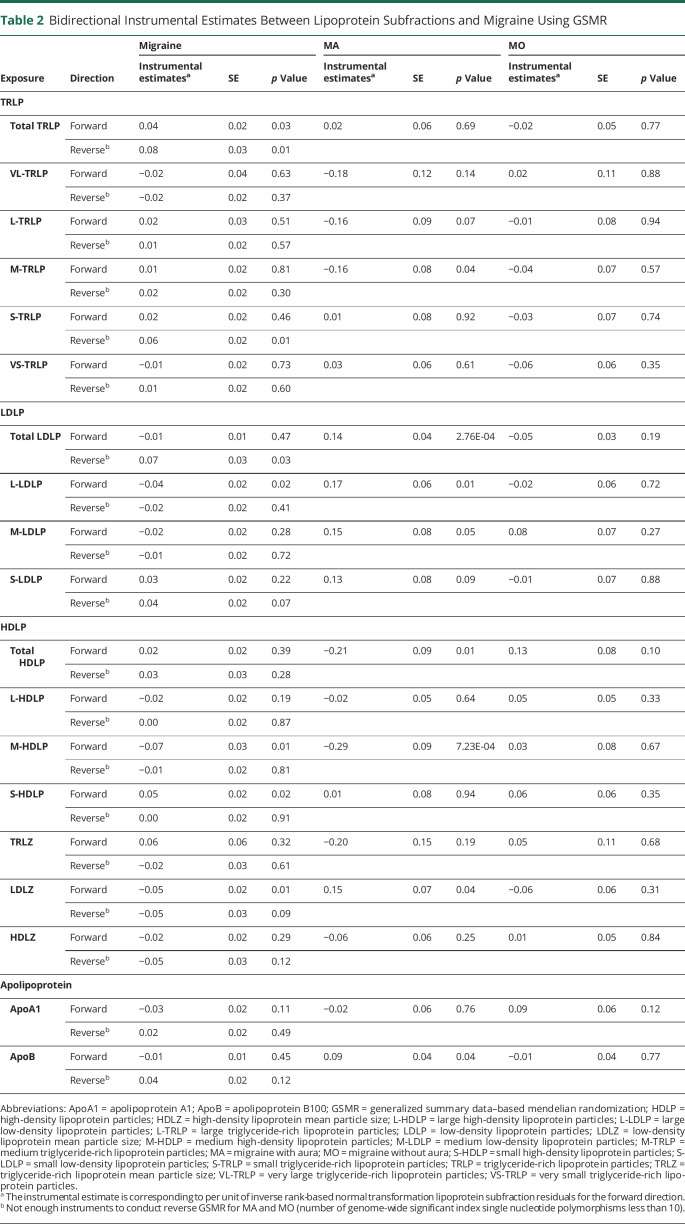
Finally, we used bidirectional MR instrumental analysis to assess evidence of potential causality (as opposed to shared biology) in the relationship between migraine and any of the lipoprotein subfractions (Table 2). For any migraine, there were no significant instrumental effects from the lipoprotein subfractions or vice versa after correcting for multiple comparisons (p < 0.05/19). Nominally significant instrumental effects on any migraine were observed for total TRLP (β [log(OR)/unit of INT lipoprotein residuals] = 0.04, p = 0.03), L-LDLP (β = −0.04, p = 0.02), M-HDLP (β = −0.07, p = 0.01), S-HDLP (β = 0.05, p = 0.02), and LDLZ (β = −0.05, p = 0.01) on any migraine, and for any migraine on total TRLP (β = 0.08, p = 0.01), S-TRLP (β = 0.06, p = 0.01), and total LDLP (β = 0.07, p = 0.03). Similarly, there were no significant instrumental effects for MO (all p > 0.05). However, instruments for total LDLP (β = 0.14, p = 2.76 × 10−04) and M-HDLP (β = −0.29, p = 7.23 × 10−04) were significant for effects on MA. Sensitivity analyses using IVW, MR-Egger, simple median, weighted median, and MR-PRESSO showed no significant instrumental effects for any lipoprotein subfraction for any of the migraine outcomes except for total LDLP on MA using the weighted median approach (β = 0.17, p = 1.00 × 10−03) (eTables 22–24, data available from Dryad: doi.org/10.5061/dryad.tht76hdxv). Therefore, MR analysis exhibited limited evidence (no significant causal estimates after controlling for multiple testing) that the lipoprotein subfractions were causally related with migraine.
Table 2.
Bidirectional Instrumental Estimates Between Lipoprotein Subfractions and Migraine Using GSMR
Discussion
In both phenotypic and genetic analyses, associations with migraine were consistently more evident for TRLP subfractions than for LDL or HDL. This suggests biological links between TRLP metabolism and migraine, which supports previous reports of correlations between triglycerides and migraine.5,31 These phenotypic and genetic analyses were independent, and therefore complementary, and the genetic analysis is expected to be minimally susceptible to residual, environmental, and unmeasured confounding.32 However, MR effects were not robust, suggesting that pleiotropic effects rather than causality relationships may explain associations between lipoprotein metabolism and migraine.
Cross-trait and colocalization analysis pointed to shared biological mechanisms between migraine and the lipoprotein subfractions. The most consistent shared signal across multiple lipoprotein subfractions, which mapped to chr5q13.3, was colocalized at HMGCR in tissues from circulation and musculoskeletal systems, at ANKDD1B in tissues from digestive and nervous systems, and at COL4A3BP in tissues from circulation, digestive, and nervous systems. HMGCR, encoding 3-hydroxy-3-methylglutaryl–coenzyme A reductase, is the target of statins.33 This may support a potential effect of statin therapy on migraine that warrants future investigation34 and may also provide insights into the underlying physiology of the relationship between migraine and CVD. At the same locus, ANKDD1B was recently identified as a gene potentially driving a shared genetic component between migraine and major depressive disorder.35 Finally, also at this locus, COL4A3BP encodes collagen type IV α-3-binding protein, also known as ceramide transfer protein (CERT), which moves ceramide, a potential migraine biomarker,36,37 from the endoplasmic reticulum to the Golgi apparatus in a nonvesicular manner.38 The colocalization revealed additional genes potentially relevant to migraine. For example, the colocalization at HEY2 recapitulates a known genome-wide significant migraine locus implicating embryonic cardiovascular development and neurogenesis in migraine etiology.14 THADA was identified as a regulator of the balance between energy consumption and energy storage, and knockout of THADA in animal models induced obesity, less production of heat, and more sensitivity to the cold compared to controls.39 The shared locus at chr19q31.32 implicates a long intergenic non–protein coding RNA (LINC00457) and APOE, a key driver of dementia risk and lipid metabolism.40 Finally, shared genetics implicated SERPINA1, which has been associated with cluster headache and stroke.41,42
Lipoprotein subfractions, reflecting differences in size, density, lipid composition, and function, may be more closely related to the biology of lipid metabolism than conventional lipid measures and have provided novel information about atherogenesis.43 Similarly, studying lipoprotein subfractions may provide important insights into the mechanisms of the association between conventional lipids and migraine in observational studies. While prior work suggested an overall genetic correlation between migraine and triglyceride levels,44 the current study helps focus the lipid associations with migraine to TRLP subcategories. This finding is consistent with the phenotypic association reported by Goulart et al.,45 who found positive associations between migraine and TRLP cholesterol and their cholesterol-rich remnants among 3,155 participants. In contrast, although a previous study reported associations between HDL metabolism and migraine,7 we observed a nominally significant association only with S-HDLP. Further studies are needed to determine the potential role of HDL particles in migraine pathology.
The strength of our study includes its large sample size, detailed information on migraine subtypes (MA and MO), consideration of important confounders including menopause status and postmenopausal hormone therapy in the phenotypic analysis, and the consistency of phenotypic and genetic relationships. We also acknowledge some limitations. First, we were restricted to female individuals in phenotypic analyses and the results for male individuals may differ. However, the genome-wide summary statistics used in the genetic analyses included both male and female individuals and were concordant with the phenotypic analysis among women only. Moreover, the populations included in this study were restricted to European ancestry as verified by genetics, and findings may not be generalizable to populations of other ancestries. Second, although we observed no consistent instrumental effects using MR, power may have been limited to detect weak associations of the exposures, that is, the lipoprotein subfractions, due to smaller sample size. GWAS in larger samples with lipoprotein subfraction measures may be needed for definitive conclusions from this analytic approach. Third, in spite of support for shared associations between migraine and some lipoproteins from gene expression in several tissues, we were not able to extend this analysis to liver, the primary organ for lipoprotein metabolism, due to limited sample size of liver tissue in the GTEx resource. Finally, although we observed excellent agreement between self-reported migraine and MO as classified by the ICHD-II diagnostic criteria, the potential for misclassification of migraine and aura status remains. Replication, particularly of the phenotypic analysis, remains warranted.
Our results provided evidence of associations between lipid metabolism and migraine, specifically TRLP. Genetic analyses suggested a lack of causal effects between lipoprotein subfractions and migraine but revealed shared genetic components that could further our understanding of the biological mechanisms underlying both lipid metabolism and migraine pathology. These insights may provide direction for identification of potential therapeutic targets for migraine treatment.
Acknowledgment
This research was conducted using the UK Biobank Resource under Application Number 29273. The authors thank the participants and researchers from the WGHS, UK Biobank, and International Headache Genetics Consortium (IHGC Consortium members listed in eAppendix 2 on Dryad: datadryad.org/stash/share/TGVW3XBROJS31-3zj0EjYG7QnizaYTW3vG2-dozaVCs) who contributed or collected data and the research participants and employees of 23 and Me for making this work possible. The NMR measurements were provided to the study by LabCorp, Inc.
Glossary
- ApoA1
apolipoprotein A1
- ApoB
apolipoprotein B100
- CI
confidence interval
- CPASSOC
cross-phenotype association
- CVD
cardiovascular disease
- DGF
DNaseI digital genomic footprinting
- DHS
DNase I hypersensitivity sites
- GSMR
generalized summary data–based Mendelian randomization
- GWAS
genome-wide association study
- HDL
high-density lipoprotein
- HDLP
total high-density lipoprotein particles
- ICHD-II
International Classification of Headache Disorders, second edition
- IHGC
International Headache Genetics Consortium
- IVW
inverse-variance weighted
- L-HDLP
large high-density lipoprotein particles
- L-LDLP
large low-density lipoprotein particles
- L-TRLP
large triglyceride-rich lipoprotein particles
- LDL
low-density lipoprotein
- LDLP
total low-density lipoprotein particles
- LDSC
linkage disequilibrium score regression
- M-HDLP
medium high-density lipoprotein HDL particles
- M-LDLP
medium low-density lipoprotein particles
- M-TRLP
medium triglyceride-rich lipoprotein particles
- MA
migraine with aura
- MO
migraine without aura
- MR
mendelian randomization
- NMR
nuclear magnetic resonance
- OR
odds ratio
- S-HDLP
small high-density lipoprotein particles
- S-LDLP
small low-density lipoprotein particles
- S-TRLP
small triglyceride-rich lipoprotein particles
- SNP
single nucleotide polymorphism
- TFBS
transcription factor binding sites
- TRLP
triglyceride-rich lipoprotein
- VL-TRLP
very large triglyceride-rich lipoprotein particles
- VS-TRLP
very small triglyceride-rich lipoprotein particles
- WGHS
Women's Genome Health Study
- WHS
Women's Health Study
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
D.I. Chasman is funded by the US NIH and US National Institute of Neurologic Disorders and Stroke (R21NS09296 and R21NS104398). P.M. Rist is funded by K01 HL128791. S. Mora was funded by R01 HL134811, HL 117861, R01 DK112940, and K24 HL136852. The WGHS is supported by the National Heart, Lung, and Blood Institute (HL043851 and HL080467) and the National Cancer Institute (CA047988 and UM1CA182913) with funding for genotyping provided by Amgen.
Disclosure
S. Mora has served as a consultant to Quest Diagnostics and Pfizer for work unrelated to this manuscript and is a coinventor on a patent regarding a glycosylation biomarker from NMR spectroscopy and risk of colorectal cancer. T. Kurth reports personal fees from Eli Lilly, Newsenselab, Total, and the BMJ outside the submitted work. P. Gormley is a former full-time employee of Merck & Co., Inc., outside the submitted work. P.M. Rist reports grants from National Heart, Lung, and Blood Institute during the conduct of the study; grants from Biogen, Inc.; and personal fees from American Heart Association outside the submitted work. P.M. Ridker reports grants from Kowa, NHLBI, Novartis, and Amarin and personal fees from Inflazome, CorVidia, Flame, Jannsen, sIRNomics, Omeicos, Novo Nordisc, Civi Biopharm, Agepha, Uppton, and AstraZeneca outside the submitted work. The other authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Kurth T, Schürks M, Logroscino G, Gaziano JM, Buring JE. Migraine, vascular risk, and cardiovascular events in women: prospective cohort study. BMJ. 2008;337:a636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurth T, Winter AC, Eliassen AH, et al. Migraine and risk of cardiovascular disease in women: prospective cohort study. BMJ. 2016;353:i2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adelborg K, Szépligeti SK, Holland-Bill L, et al. Migraine and risk of cardiovascular diseases: Danish population based matched cohort study. BMJ. 2018;360:k96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scher AI, Terwindt GM, Picavet HS, Verschuren WM, Ferrari MD, Launer LJ. Cardiovascular risk factors and migraine: the GEM population-based study. Neurology. 2005;64(4):614-620. [DOI] [PubMed] [Google Scholar]
- 5.Rist PM, Tzourio C, Kurth T. Associations between lipid levels and migraine: cross-sectional analysis in the Epidemiology of Vascular Ageing Study. Cephalalgia. 2011;31(14):1459-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurth T, Ridker PM, Buring JE. Migraine and biomarkers of cardiovascular disease in women. Cephalalgia. 2008;28(1):49-56. [DOI] [PubMed] [Google Scholar]
- 7.Onderwater GLJ, Ligthart L, Bot M, et al. Large-scale plasma metabolome analysis reveals alterations in HDL metabolism in migraine. Neurology. 2019;92(16):e1899–e1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tietjen GE, Khubchandani J. Vascular biomarkers in migraine. Cephalalgia. 2015;35(1 suppl):95-117. [DOI] [PubMed] [Google Scholar]
- 9.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26(4):847-870. [DOI] [PubMed] [Google Scholar]
- 10.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119(7):931-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294(1):47-55. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Chasman DI, Zee RY, et al. Rationale, design, and methodology of the Women's Genome Health Study: a genome-wide association study of more than 25,000 initially healthy American women. Clin Chem. 2008;54(2):249-255. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293-1304. [DOI] [PubMed] [Google Scholar]
- 14.Gormley P, Anttila V, Winsvold BS, et al. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat Genet. 2016;48(8):856-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kettunen J, Demirkan A, Würtz P, et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat Commun. 2016;7:11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schürks M, Buring JE, Kurth T. Agreement of self-reported migraine with ICHD-II criteria in the Women's Health Study. Cephalalgia. 2009;29(10):1086-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad S, Moorthy MV, Demler OV, et al. Assessment of risk factors and biomarkers associated with risk of cardiovascular disease among women consuming a Mediterranean diet. JAMA Netw Open. 2018;1:e185708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116(19):2110-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chasman DI, Paré G, Mora S, et al. Forty-three loci associated with plasma lipoprotein size, concentration, and cholesterol content in genome-wide analysis. Plos Genet. 2009;5(11):e1000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aulchenko YS, Struchalin MV, van Duijn CM. ProbABEL package for genome-wide association analysis of imputed data. BMC Bioinformatics. 2010;11:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bulik-Sullivan BK, Loh PR, Finucane HK, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47(3):291-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finucane HK, Bulik-Sullivan B, Gusev A, et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat Genet. 2015;47(11):1228-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu X, Feng T, Tayo BO, et al. Meta-analysis of correlated traits via summary statistics from GWASs with an application in hypertension. Am J Hum Genet. 2015;96(1):21-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giambartolomei C, Vukcevic D, Schadt EE, et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. Plos Genet. 2014;10:e1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Z, Zheng Z, Zhang F, et al. Causal associations between risk factors and common diseases inferred from GWAS summary data. Nat Commun. 2018;9(1):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgess S, Labrecque JA. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol. 2018;33(10):947-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickrell JK, Berisa T, Liu JZ, Ségurel L, Tung JY, Hinds DA. Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet. 2016;48(7):709-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gelaye B, Larrabure-Torrealva GT, Qiu C, et al. Fasting lipid and lipoproteins concentrations in pregnant women with a history of migraine. Headache. 2015;55(5):646-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sodini SM, Kemper KE, Wray NR, Trzaskowski M. Comparison of genotypic and phenotypic correlations: Cheverud's conjecture in humans. Genetics. 2018;209(3):941-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ference BA, Robinson JG, Brook RD, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375(22):2144-2153. [DOI] [PubMed] [Google Scholar]
- 34.Buettner C, Nir RR, Bertisch SM, et al. Simvastatin and vitamin D for migraine prevention: a randomized, controlled trial. Ann Neurol. 2015;78(6):970-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y, Zhao H, Boomsma DI, et al. Molecular genetic overlap between migraine and major depressive disorder. Eur J Hum Genet. 2018;26(8):1202-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren C, Liu J, Zhou J, et al. Lipidomic analysis of serum samples from migraine patients. Lipids Health Dis. 2018;17(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peterlin BL, Mielke MM, Dickens AM, et al. Interictal, circulating sphingolipids in women with episodic migraine: a case-control study. Neurology. 2015;85(14):1214-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugiki T, Egawa D, Kumagai K, et al. Phosphoinositide binding by the PH domain in ceramide transfer protein (CERT) is inhibited by hyperphosphorylation of an adjacent serine-repeat motif. J Biol Chem. 2018;293(28):11206-11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moraru A, Cakan-Akdogan G, Strassburger K, et al. THADA regulates the organismal balance between energy storage and heat production. Dev Cell. 2017;41(1):450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forero DA, López-León S, González-Giraldo Y, et al. APOE gene and neuropsychiatric disorders and endophenotypes: a comprehensive review. Am J Med Genet B Neuropsychiatr Genet. 2018;177(2):126-142. [DOI] [PubMed] [Google Scholar]
- 41.Summ O, Gregor N, Marziniak M, Gralow I, Husstedt IW, Evers S. Cluster headache and alpha 1-antitrypsin deficiency. Cephalalgia. 2010;30(1):113-117. [DOI] [PubMed] [Google Scholar]
- 42.Malik R, Dau T, Gonik M, et al. Common coding variant in SERPINA1 increases the risk for large artery stroke. Proc Natl Acad Sci USA. 2017;114(14):3613-3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu CG, Zhang Y, Xu RX, et al. Circulating non-HDL-C levels were more relevant to atherogenic lipoprotein subfractions compared with LDL-C in patients with stable coronary artery disease. J Clin Lipidol. 2015;9:794-800. [DOI] [PubMed] [Google Scholar]
- 44.Siewert KM, Klarin D, Damrauer SM, et al. Cross-trait analyses with migraine reveal widespread pleiotropy and suggest a vascular component to migraine headache. Int J Epidemiol 2020;49(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goulart AC, Lotufo PA, Santos IS, et al. The relationship between migraine and lipid sub-fractions among individuals without cardiovascular disease: a cross-sectional evaluation in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Cephalalgia. 2018;38(3):528-542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
GWAS summary statistics described above are available from cited study authors or from the public domain as indicated. The WGHS sample is not publicly available because access is restricted by the institutional review board but further information about the data is available from the corresponding author upon reasonable request.