Abstract
Esophageal squamous cell carcinoma (ESCC) is the sixth leading cause of death due to cancer, indicating that finding new therapeutic targets or approaches for ESCC treatment is imperative. Transient Receptor Potential cation channel subfamily M, member 2 (TRPM2) is a calcium-permeable, nonselective cation channel that responds to reactive oxygen species (ROS), which are found in the tumor microenvironment and are important regulators of tumorigenesis, cell proliferation, apoptosis, and the therapeutic response. Here, we used immunohistochemical analysis of tumor tissue derived from patients with ESCC to find that the TRPM2 channel protein expression level was increased in tumor tissue compared with adjacent normal tissue. Intracellular calcium concentration measurements, western blotting, and ROS and cell viability assays were used with a human ESCC cell line (TE-1 cells) to find that TRPM2 participated in the ROS hydrogen peroxide-induced increase in intracellular calcium. This increased calcium inhibited cell proliferation and enhanced apoptosis. Pretreatment of cells with the anticancer agent 5-fluorouracil (5-FU) significantly increased ROS production, which potentiated TRPM2-mediated calcium signaling, decreased cell proliferation, and increased apoptosis in TE-1 cells, suggesting that the therapeutic effect of 5-FU in ESCC cells may be mediated by the TRPM2 channel-mediated calcium influx. These findings offer a potential treatment target and provide mechanistic insight into the therapeutic effects of 5-FU in patients with ESCC.
Keywords: esophageal squamous cell carcinoma, TRPM2 channels, reactive oxygen species, 5-fluorouracil, proliferation, apoptosis
Introduction
Esophageal cancer is one of the most common malignant neoplasms in the digestive tract. According to the global Cancer Statistics Report of 2018, the number of new cases of esophageal cancer worldwide that year was approximately 572 000, and the number of deaths due to this cancer was 509 000, ranking seventh in malignant tumor incidence and the sixth leading cause of death due to cancer. 1 Esophageal cancer can be separated by histological type into squamous cell carcinoma, adenocarcinoma, and other subtypes. Esophageal squamous cell carcinoma (ESCC) accounts for approximately 90% of all esophageal cancers, and the incidence shows an alarming increasing trend in Asia.2,3 Despite systemic treatment, including surgery, chemotherapy, molecular-targeting therapy, and immunotherapy, saving many lives, mortality is still high.4,5 Therefore, it is imperative to develop new therapeutic targets and treatment approaches for ESCC.
Transient Receptor Potential cation channel subfamily M, member 2 (TRPM2), a calcium (Ca2+)-permeable, nonselective cation channel, was first identified in 1998. TRPM2 has numerous functions in a variety of cell types and has been shown to be involved in neurological disorders, insulin release, cell motility, and cell death.6–10 The TRPM2 channel is activated by intracellular adenosine diphosphate ribose, hydrogen peroxide (H2O2), intracellular Ca2+ concentration ([Ca2+]i), and nicotinic acid adenine dinucleotide phosphate, whereas it is inhibited by the nucleotide adenosine monophosphate and by cellular acidification.11–14 Because the TRPM2 channel responds to reactive oxygen species (ROS), it is involved in many oxidative stress-related pathological processes. For example, cancer cells have high levels of ROS that induce tumorigenesis. Increased ROS might behave as the TRPM2 channel to facilitate cancer therapy.10,15 Recent studies have shown that 5-fluorouracil (5-FU) and cyclophosphamide may activate the TRPM2 channel in colon or breast cancer cells to enhance cell apoptosis. 15 However, the functional role of the TRPM2 channel in ESCC remains unknown.
In the present study, we used tumor tissue samples derived from patients with ESCC as well as the human ESCC cell line (TE-1) to perform immunohistochemistry, (Ca2+)i measurements, western blotting, ROS assays, and cell viability assays to investigate the expression of the TRPM2 channel protein in ESCC and to assess the role of the TRPM2 channel in H2O2- and 5-FU–induced ESCC proliferation and apoptosis.
Materials and Methods
Reagents and Antibodies
We used the following reagents and antibodies: RPMI-1640 medium and fetal bovine serum (Biological Industries); anti-TRPM2 antibody (#HPA035260, Sigma); anticleaved caspase-3 antibody (#AF7022, Affinity Biosciences); anti-β-tubulin antibody (#AF7011, Affinity Biosciences); horseradish peroxidase (HRP)-conjugated antirabbit (#S0001, Affinity Biosciences); electrochemiluminescence (ECL) detection system (#WP20005, Thermo Fisher Scientific, Pittsburgh); Cell Counting Kit-8 (CCK-8) (#A311-01, Vazyme Biotech); ROS assay kit (#S0033S, Beyotime Biotech); N-(p-amylcinnamoyl) anthranilic acid (ACA) (#A8486-5MG, Sigma); H2O2 (#HX0636, Sigma); and 5-FU (#F6627-1G, Sigma). Specific small interfering (si)RNAs for human TRPM2 (5′-GUCUCGGACAUCACUAUCUTT-3′, 5′-GGGAAGAUGUCUCAGCAGACG-3′) 16 and scrambled siRNAs (5′-ACGCGUAACGCGGGAAUUU-3′, 5′-UUCUCCGAACGUGUCACGUTT-3′) were designed and synthesized by Biomics Company.
Cell Culture
The human ESCC TE-1 cells were obtained from the Cell Bank of the Chinese Academy of Sciences, 17 and cultured in RPMI-1640 medium, which was supplemented with 10% fetal bovine serum and antibiotics (100 μg/mL penicillin and 100 U/mL streptomycin) at 37°C in an atmosphere containing 5% CO2. Specific siRNAs against human TRPM2 or scrambled control siRNA were transiently transfected into cells using Lipofectamine 2000 reagent (#11668019, Thermo Fisher Scientific, Pittsburgh) according to the manufacturer's protocol. TE-1 cells were cultured for the following experiments 48 h after siRNA transfection.
Immunohistochemistry
We obtained ESCC tumor tissues from 20 patients during clinical surgery. Written informed consent was obtained from each participating patient before the specimens were collected. All experiments were approval by the Ethics Committee of Anhui Medical University (5101128). The procedures were performed consistent with the Declaration of Helsinki and Good Clinical Practice. 18 Briefly, the tumor and adjacent tissues were dissected and fixed with 4% paraformaldehyde at 4°C. After the specimens were embedded in paraffin, they were sliced into 5-μm-thick sections. Following deparaffinization and rehydration, heat-mediated antigen retrieval was performed with citrate buffer in a microwave oven. The specimens were then incubated in H2O2 (3%) for 10 min to extinguish endogenous peroxidase activity. The specimens were incubated with an anti-TRPM2 antibody (1:100) at 4°C overnight and then incubated with an antirabbit secondary antibody. Following with HRP and then with 3,3′-diaminobenzidine tetrahydrochloride, the sections were counterstained with hematoxylin. For the negative control group, the primary antibody was omitted. Images of specimens were captured using a light microscope (Olympus BX53, Camera: Olympus DP74) and assessed using integrated optical density analysis.
(Ca2+)i Measurement
The (Ca2+)i was measured according to the method we previously used. 19 Briefly, TE-1 cells were incubated with 10 μmol/L Fluo-8/AM and 0.02% pluronic F-127 in a normal physiological saline solution (in mmol/L: 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 glucose, 5 Hepes, pH 7.4) at 37°C for 1 h in a dark incubator. The fluorescence signal was recorded using fluorescence microscopy (Nikon T200, Camera: SensiCam, Software: MetaFluor), with excitation and emission wavelengths of 488 nm and 515 nm, respectively. The (Ca2+)i is shown as the ratio of the fluorescence intensity before (F0) and after (F1) the application of H2O2. The fluorescence ratio data in each experiment are the mean values from approximately 40 cells.
Western Blotting
Western blotting was performed as in our previous study. 20 In brief, the TE-1 cells were lysed using a detergent extraction buffer that consisted of 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM Na2EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulphate, sodium orthovanadate, sodium fluoride and leupeptin. Extracted proteins (30 μg) were loaded onto a 10% gel for sodium dodecyl sulphate–polyacrylamide gel electrophoresis. After electrophoretic separation, the proteins were transferred to a polyvinylidene difluoride membrane. Membranes containing proteins were blocked and then incubated with primary antibody (1:200) overnight at 4°C. The next day, following incubation with HRP-conjugated secondary antibody, the immunosignals were detected using an ECL detection system. The optical densities of each protein were analysed using ImageJ software (National Institutes of Health). β-tubulin was used as a loading control.
CCK-8 Assay
The TE-1 cells were cultured to the logarithmic growth phase and then digested with trypsin. The cells were seeded into a 96-well plate with a density of 5 to 10 × 104/well. Before an experiment, a serum-free culture medium was added to synchronize cell growth. When cells were 80% confluent, they were divided into different groups, and each group contained five repeats. The cells were treated with H2O2 or 5-FU and cultured for 24 h. The next day, the supernatant from each well was discarded, and 10 μL of CCK-8 reagent was added to each well. The results were analyzed by a plate reader.
ROS Assay
The TE-1 cells were cultured and seeded as described in the directions for the CCK-8 assay. The cell-permeable fluorogenic dye 2′7′-dichlorofluorescin diacetate (DCFH-DA) was dissolved in a serum-free medium (10 μmol/L). 21 The cells were then suspended at a density of 1 × 107/mL to 2 × 108/mL in a serum-free medium containing DCFH-DA (10 μmol/L) and incubated at 37°C for 20 min. The cells and DCFH-DA were mixed by pipetting the solution in and out of the pipette tip three to five times. The cells were collected and washed with a serum-free medium three times to fully remove DCFH-DA. The fluorescence intensity of each well was examined with a fluorescence plate reader at excitation and emission wavelengths of 488 nm and 515 nm, respectively.
Statistical Analysis
All data are expressed as the mean ± SEM. The statistical significance was determined using two-tailed Mann-Whitney tests. Differences between groups were considered statistically significant at a value of P < .05. For the (Ca2+)i measurement experiments, n represents the number of total experiments.
Results
TRPM2 Channel Expression in ESCC
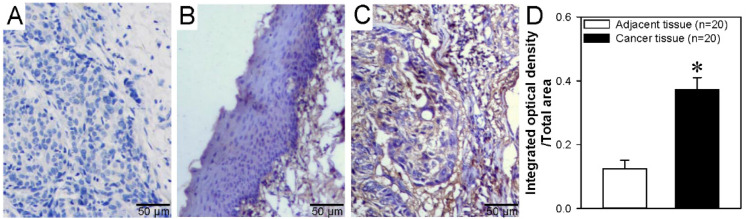
Recent studies have shown that the TRPM2 channel protein is expressed in cancer cells in breast, gastric, lung, pancreatic, prostate, and tongue squamous cell carcinoma. 22 However, the TRPM2 channel expression level in ESCC is unknown. Our immunohistochemistry results examining tumor tissue from patients with ESCC showed that the TRPM2 channel expression level is significantly increased in ESCC tissue compared with that in normal adjacent tissue (Figure 1). These results indicated that TRPM2 channel expression is increased in ESCC.
Figure 1.
TRPM2 protein expression levels in human ESCC and adjacent tissues. (A-C) Representative images of negative control (A), normal adjacent tissue (B), and ESCC tissue (C) showing TRPM2 protein expression levels via immunohistochemical analysis. Blue represents cell nuclei; brown, TRPM2 protein. (D) Summary data showing the ratio of integrated optical density/total area of TRPM2 immunostaining in ESCC tumor and adjacent tissues. In the negative control, the primary antibody was omitted. Microscopy objective: Olympus UPlanXApo. Magnification: 40×/0.95. Data are shown as the mean ± SEM; n = 20. *P < .05 versus adjacent tissue.
Abbreviations: TRPM2, transient receptor potential cation channel subfamily M, member 2; ESCC, esophageal squamous cell carcinoma.
Role of the TRPM2 Channel in H2O2-Induced Ca2+ Influx, Proliferation, and Apoptosis in TE-1 Cells
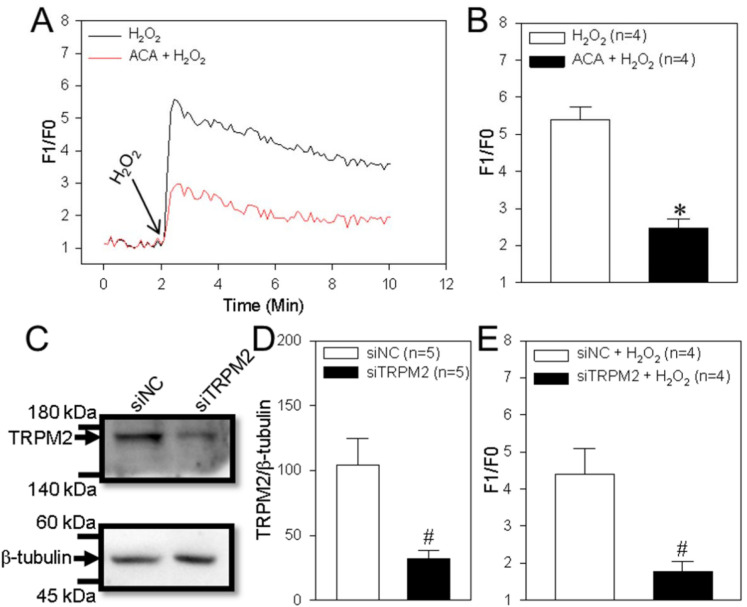
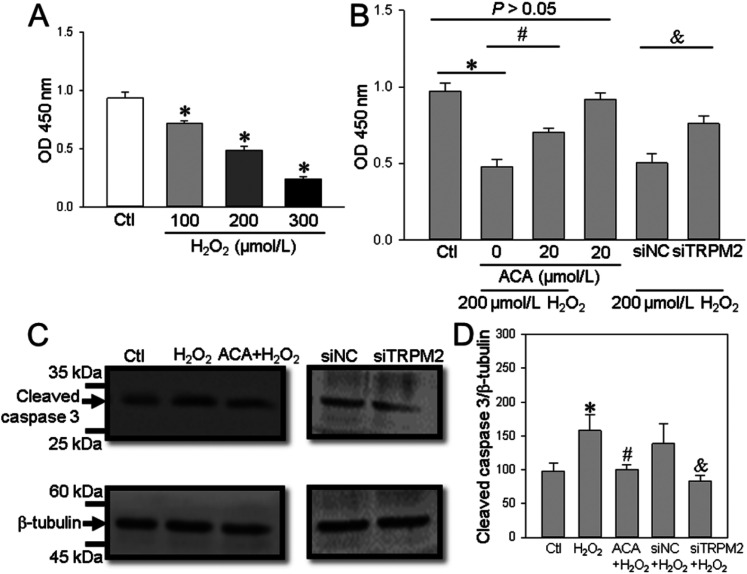
The TRPM2 channel is activated by H2O2 and is capable of mediating Ca2+ influx to trigger Ca2+ signaling, which is involved in cell proliferation and apoptosis. 23 Thus, we next assessed TRPM2 channel function in the human ESCC cell line TE-1. Our data indicated that treatment of TE-1 cells with H2O2 (200 μM) induced a marked transient increase in (Ca2+)i and that this effect was significantly decreased by ACA (a TRPM2 channel inhibitor) or TRPM2 siRNA (Figure 2A-E). Using in vitro functional assays, we found that H2O2 significantly suppressed TE-1 cell viability (as detected using the CCK-8 assay) in a concentration-dependent manner (Figure 3A) and increased cleaved caspase-3 expression levels. These effects were significantly inhibited by ACA or TRPM2 siRNA (Figure 3B-D). These results suggested that TRPM2-mediated Ca2+ signaling induced by H2O2 decreased ESCC proliferation and enhanced apoptosis.
Figure 2.
H2O2-induced intracellular Ca2+ increase in TE-1 cells. (A and B) Representative traces (A) and summary data (B) showing that hydrogen peroxide (H2O2; 200 μM) induces a transient increase in the intracellular Ca2+ concentration in a human ESCC TE-1 cells and the effect of a TRPM2 channel inhibitor (ACA, 20 μM) on these cells. Data are shown as the mean ± SEM; n = 4. *P < .05 versus H2O2. (C-E) Representative western blot images (C) and summary data (D and E) showing the effect of TRPM2 siRNA (siTRPM2) and control siRNA (siNC) on the protein expression of TRPM2 (D), and intracellular Ca2+ concentration change (E) in a human ESCC cell line (TE-1 cells). β-tubulin is used as a loading control. Data are shown as the mean ± SEM; n = 4-5. #P < .05 versus siNC or siNC + H2O2.
Abbreviations: TRPM2, transient receptor potential cation channel subfamily M, member 2; ESCC, esophageal squamous cell carcinoma; ACA, anthranilic acid; TE-1 cells, cell line.
Figure 3.
H2O2-induced cell proliferation and apoptosis in TE-1 cells. (A and B) Summary data showing the effect of H2O2 on TE-1 cell proliferation in a concentration-dependent manner (A), and the effect of ACA alone or with H2O2 and TRPM2 siRNA (siTRPM2) or control siRNA (siNC) on cell proliferation (B) as detected by a CC assay and expressed as OD. Data are shown as the mean ± SEM; n = 5. *P < .05 versus Ctl; #P < .05 versus 0 μM ACA; &P < .05 versus siNC; top thin line, P > .05 versus Ctl. (C and D) Representative western blot images (C) and summary data (D) showing the effect of H2O2 (200 μM) in the presence or absence of ACA, or in the presence of TRPM2 siRNA (siTRPM2) or control siRNA (siNC) on the protein expression of cleaved caspase-3. β-tubulin is used as a loading control. Data are shown as the mean ± SEM; *P < .05 versus Ctl; #P < .05 versus H2O2; &P < .05 versus siNC + H2O2.
Abbreviations: TRPM2, transient receptor potential cation channel subfamily M, member 2; TE-1 cells, cell line; ACA, anthranilic acid; OD, optical density; Ctl, control.
Role of 5-FU on ROS Production and the TRPM2 Channel in 5-FU–Potentiated Ca2+ Influx, Proliferation, and Apoptosis in TE-1 Cells
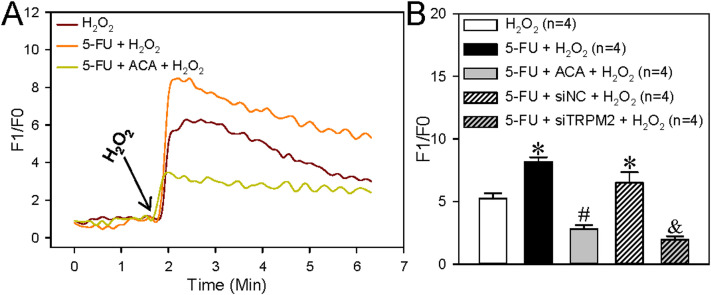
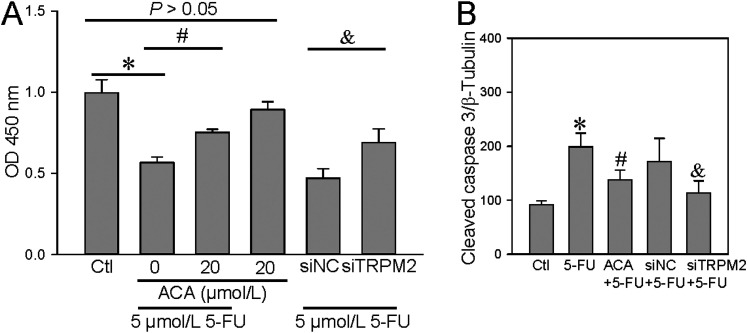
The anticancer drug 5-FU can enhance TRPM2-mediated Ca2+ influx to suppress cell viability and to increase ROS production and cell apoptosis in breast and colon cancer cells. 15 Thus, we next examined the effect of 5-FU on ROS production, cell proliferation, and apoptosis in the human ESCC TE-1 cell line. We found that 5-FU treatment (5 μM, incubation for 24 h) significantly increased the H2O2-induced transient increase in (Ca2+)i (Figure 4) and ROS production (Figure 5) in ESCC, and that these effects were also inhibited by ACA or TRPM2 siRNA. In addition, 5-FU treatment significantly suppressed TE-1 cell viability but increased cleaved caspase-3 expression in these cells; these effects were also inhibited by ACA or TRPM2 siRNA (Figure 6). Taken together, these data suggested that 5-FU treatment significantly increased TRPM2-mediated Ca2+ signaling evoked by H2O2 and its effect on cell proliferation and apoptosis in ESCC.
Figure 4.
Effect of 5-FU on H2O2-induced intracellular Ca2+ increase in TE-1 cells. (A and B) Representative traces (A) and summary data (B) showing that the transient increase in intracellular Ca2+ concentration induced by treatment with hydrogen peroxide (H2O2) (200 μM) is increased by 5-FU treatment (5 μM, incubation for 24 h; 5-FU + H2O2), or suppressed by TRPM2 siRNA (siTRPM2). The TRPM2 channel inhibitor ACA (20 μM, 5-FU + ACA + H2O2) blocks these effects. Data are shown as the mean ± SEM; n = 4. *P < .05 versus H2O2; #P < .05 versus 5-FU + H2O2; &P < .05 versus 5-FU + siRNA control (siNC) + H2O2.
Abbreviations: TRPM2, transient receptor potential cation channel subfamily M, member 2; ACA, anthranilic acid; 5-FU, 5-fluorouracil.
Figure 5.
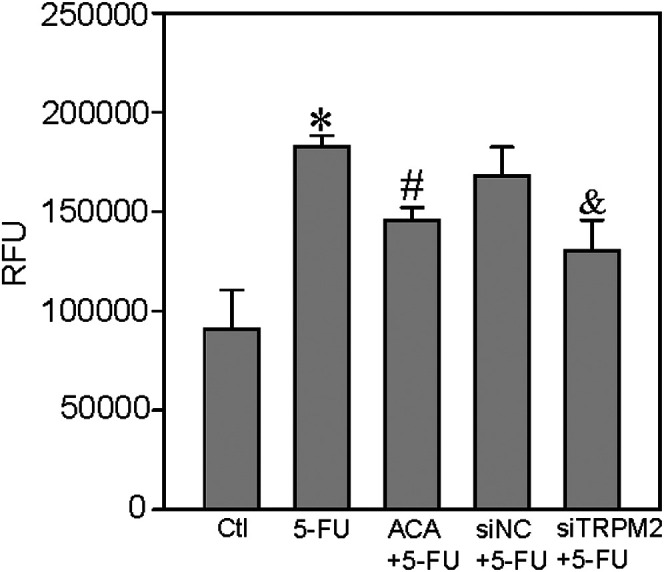
5-FU–induced ROS production in TE-1 cells. ROS was assessed by the fluorogenic dye DCFH-DA. The ordinate represents relative fluorescence units (RFU). Summary data show that 5-fluorouracil (5-FU, 5 μM; 24-h incubation) induces ROS production and that this effect is inhibited by the TRPM2 channel inhibitor ACA (20 μM of ACA + 5 µM of 5-FU) or TRPM2 siRNA (siTRPM2). Data are shown as the mean ± SEM; n = 6. *P < .05 versus control (Ctl); #P < .05 versus 5-FU; &P < .05 versus siRNA control (siNC) + 5-FU.
Abbreviations: TRPM2, transient receptor potential cation channel subfamily M, member 2; ACA, anthranilic acid; 5-FU, 5-fluorouracil; ROS, reactive oxygen species; DCFH-DA, dichlorofluorescin diacetate.
Figure 6.
Effect of 5-FU on the TRPM2-modulated TE-1 cell proliferation and apoptosis. (A) Summary data showing that 5-fluorouracil (5-FU, 5 μM; 24-h incubation) suppresses TE-1 cell proliferation and showing the effect of a TRPM2 channel inhibitor (ACA, 20 μM) and TRPM2 siRNA (siTRPM2). Cell proliferation is detected by a CCK-8 assay and expressed as OD. Data are shown as the mean ± SEM; n = 5. *P < .05 versus control (Ctl); #P < .05 versus 0 μM ACA; &P < .05 versus siRNA control (siNC); P > .05 versus Ctl. (B) Summary data showing the effect of 5-FU (5 μM, incubation for 24 h) on the protein expression level of cleaved caspase-3 and the effect of ACA and siTRPM2. β-tubulin is used as a loading control. Data are shown as the mean ± SEM; n = 6. *P < .05 versus Ctl; #P < .05 versus 5-FU; &P < .05 versus siNC + 5-FU.
Abbreviations: TRPM2, transient receptor potential cation channel subfamily M, member 2; ACA, anthranilic acid; 5-FU, 5-fluorouracil; TE-1 cells, cell line; CCK-8, Cell Counting Kit-8; OD, optical density.
Discussion
To find new treatment approaches and therapeutic targets are important directions in the study of ESCC. In the present study, we found that: (1) the level of TRPM2 channel protein expression was increased in ESCC tumor tissue compared with that in normal adjacent tissue; (2) the TRPM2 channel was involved in the H2O2-induced transient increase in (Ca2+)i to regulate cell proliferation and apoptosis in ESCC; (3) the addition of 5-FU to the human ESCC TE-1 cell line not only significantly increased ROS production but also potentiated TRPM2-mediated Ca2+ signaling and its effect on cell proliferation and apoptosis. These results provide insights into the functional role of the TRPM2 channel in ESCC and suggest that it may be a potential target in ESCC chemotherapy.
Tumor microenvironments affect cancer development as well as therapeutic resistance and clinical outcome.24,25 ROS located in the tumor microenvironment is an important regulator of tumorigenesis, cell proliferation, apoptosis, and therapeutic response. 25 A target of ROS, the TRPM2 channel is a Ca2+-permeable nonselective cation channel that mediates Ca2+ influx and affects numerous biological processes, such as cell proliferation, apoptosis, and inflammation. 23 Therefore, clarifying the function of the TRPM2 channel in cancer cells and its effect on cellular behaviors are important. In the present study, we found that the TRPM2 channel protein was expressed in ESCC tumor tissue and mediated Ca2+ influx evoked by the ROS H2O2 in TE-1 cells. In addition, TRPM2-mediated Ca2+ signaling suppressed cell proliferation but enhanced cell apoptosis in an ESCC cell line. These findings suggest that TRPM2 channel may be a new therapeutic target and have potential functions in ESCC treatment. In future, drug screen for activating TRPM2 channel may be useful to develop new drugs to treat ESCC.
The cytotoxic agent 5-FU is a widely used anticancer drug in clinical treatment, and in ESCC, 5-FU improves the response rate, progression-free survival, and overall survival among patients with ESCC. 5 It has been reported that 5-FU decreases the activity of superoxide dismutase and glutathione peroxidase but enhances catalase activity and superoxide anion levels in myocardial tissue and myoblasts.15,26,27 A recent study has also shown that 5-FU increases ROS production and potentiates the cumene hydroperoxide–evoked (Ca2+)i increase, cell viability suppression, and apoptosis by enhancing the expression of TRPM2 channels in breast cancer or colon cancer cells. 15 In our study, we also found that 5-FU treatment significantly increased ROS production in the human ESCC TE-1 cell line. Furthermore, functional in vitro experiments showed that 5-FU markedly increased TRPM2-mediated Ca2+ signaling and its effect on cell proliferation and apoptosis in this cell line. Therefore, our results indicate that 5-FU–induced cell toxicity may be mediated, at least in part, by increasing ROS production to potentiate the opening of TRPM2 channels and the ensuing Ca2+ signaling. Moreover, TRPM2 channel agonist may be used in combination with 5-FU in the future for the treatment of ESCC. In this drug combination, the dose of 5-FU can be decreased and the side effect of 5-FU may be reduced.
In summary, our results indicated that TRPM2-mediated Ca2+ signaling suppressed cell proliferation but increased cell apoptosis, which was involved in the 5-FU–induced cell toxicity in ESCC. These findings shed new light on the functional role of TRPM2-induced cell death in ESCC, provide a new potential therapeutic target for the treatment of ESCC, and provide mechanistic insight into 5-FU–induced cell toxicity.
Acknowledgments
We only used one cell line TE-1 in our study because TE-1 is a well-used cell line, and expresses TRPM2 protein enough to test our hypothesis.
Abbreviation
- ECL
electrochemiluminescence.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The current work was supported by grants from the Natural Science Foundation of Anhui Province (Grant Nos. 1808085MH251 and 1808085MH252).
ORCID iD: Kaile Wu https://orcid.org/0000-0002-6578-5000
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2018;68(6):394‐424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381‐387. doi: 10.1136/gutjnl-2014-308124. [DOI] [PubMed] [Google Scholar]
- 3.Ohashi S, Miyamoto S, Kikuchi O, Goto T, Amanuma Y, Muto M. Recent advances from basic and clinical studies of esophageal squamous cell carcinoma. Gastroenterology. 2015;149(7):1700‐1715. doi: 10.1053/j.gastro.2015.08.054. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359‐E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 5.Hirano H, Kato K. Systemic treatment of advanced esophageal squamous cell carcinoma: chemotherapy, molecular-targeting therapy and immunotherapy. Jpn J Clin Oncol. 2019;49(5):412‐420. doi: 10.1093/jjco/hyz034. [DOI] [PubMed] [Google Scholar]
- 6.Nagamine K, Kudoh J, Minoshima S, et al. Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain. Genomics. 1998;54(1):124‐131. doi: 10.1006/geno.1998.5551. [DOI] [PubMed] [Google Scholar]
- 7.Grubisha O, Rafty LA, Takanishi CL, et al. Metabolite of SIR2 reaction modulates TRPM2 ion channel. J Biol Chem. 2006;281(20):14057‐14065. doi: 10.1074/jbc.M513741200. [DOI] [PubMed] [Google Scholar]
- 8.Uchida K, Dezaki K, Damdindorj B, et al. Lack of TRPM2 impaired insulin secretion and glucose metabolisms in mice. Diabetes. 2011;60(1):119‐126. doi: 10.2337/db10-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belrose JC, Jackson MF. TRPM2: a candidate therapeutic target for treating neurological diseases. Acta Pharmacol Sin. 2018;39(5):722‐732. doi: 10.1038/aps.2018.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guler Y, Ovey IS. LLLT Enhance cyclophosphamide induced TRPM2 channel activation in human colon cancer cells. Bratisl Lek Listy. 2020;1(5):334‐338. doi: 10.4149/BLL_2020_053. [DOI] [PubMed] [Google Scholar]
- 11.Kolisek M, Beck A, Fleig A, Penner R. Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol Cell. 2005;18(1):61‐69. doi: 10.1016/j.molcel.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 12.Lange I, Penner R, Fleig A, Beck A. Synergistic regulation of endogenous TRPM2 channels by adenine dinucleotides in primary human neutrophils. Cell Calcium. 2008;44(6):604‐615. doi: 10.1016/j.ceca.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csanady L, Torocsik B. Four Ca2+ ions activate TRPM2 channels by binding in deep crevices near the pore but intracellularly of the gate. J Gen Physiol. 2009;133(2):189‐203. doi: 10.1085/jgp.200810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang W, Zou J, Xia R, et al. State-dependent inhibition of TRPM2 channel by acidic pH. J Biol Chem. 2010;285(40):30411‐8. doi: 10.1074/jbc.M110.139774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guler Y, Ovey IS. Synergic and comparative effect of 5-fluorouracil and leucoverin on breast and colon cancer cells through TRPM2 channels. Bratisl Lek Listy. 2018;119(11):692‐700. doi: 10.4149/BLL_2018_124. [DOI] [PubMed] [Google Scholar]
- 16.Zheng QX, Tan QF, Ren YP, et al. Hyperosmotic stress-induced TRPM2 channel activation stimulates NLRP3 inflammasome activity in primary human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2018;59(8):3259‐3268. doi: 10.1167/iovs.18-23965. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Lan L, Niu L, et al. Oxidative stress regulates cellular bioenergetics in esophageal squamous cell carcinoma cell. Biosci Rep. 2017;37(6):1-13. 10.1042/BSR20171006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu K, Shen B, Jiang F, et al. TRPP2 enhances metastasis by regulating epithelial-mesenchymal transition in laryngeal squamous cell carcinoma. Cell Physiol Biochem: Int J Exp Cell Physiol, Biochem, Pharmacol. 2016;39(6):2203‐2215. doi: 10.1159/000447914. [DOI] [PubMed] [Google Scholar]
- 19.Kwan HY, Shen B, Ma X, et al. TRPC1 associates with BKCa channel to form a signal complex in vascular smooth muscle cells (vol 104, pg 670, 2009). Circ Res. 2009;105(6):E6. doi: 10.1161/RES.0b013e3181bc389d. [DOI] [PubMed] [Google Scholar]
- 20.Zhao R, Zhou MY, Li J, et al. Increased TRPP2 expression in vascular smooth muscle cells from high-salt intake hypertensive rats: the crucial role in vascular dysfunction. Mol Nutr Food Res. 2015;59(2):365‐372. doi: 10.1002/mnfr.201400465. [DOI] [PubMed] [Google Scholar]
- 21.Rhee SG, Chang TS, Jeong W, Kang D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol Cells. 2010;29(6):539‐549. doi: 10.1007/s10059-010-0082-3. [DOI] [PubMed] [Google Scholar]
- 22.Miller BA. TRPM2 in cancer. Cell Calcium. 2019;80:8‐17. doi: 10.1016/j.ceca.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sumoza-Toledo A, Penner R. TRPM2: a multifunctional ion channel for calcium signalling. J Physiol. 2011;589(Pt 7):1515‐1525. doi: 10.1113/jphysiol.2010.201855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61‐68. doi: 10.1016/j.canlet.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 25.Weinberg F, Ramnath N, Nagrath D. Reactive oxygen species in the tumor microenvironment: an overview. Cancers. 2019;11(8):1-20. doi: 10.3390/cancers11081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durak I, Karaayvaz M, Kavutcu M, et al. Reduced antioxidant defense capacity in myocardial tissue from Guinea pigs treated with 5-fluorouracil. J Toxicol Environ Health Part A. 2000;59(7):585‐589. doi: 10.1080/009841000156709. [DOI] [PubMed] [Google Scholar]
- 27.Lamberti M, Porto S, Marra M, et al. 5-Fluorouracil Induces apoptosis in rat cardiocytes through intracellular oxidative stress. J Exp Clin Cancer Res: CR. 2012;31:60. doi: 10.1186/1756-9966-31-60. [DOI] [PMC free article] [PubMed] [Google Scholar]