Abstract
Background:
Biliary tract cancers (BTCs) represent a heterogeneous group of aggressive solid tumors with limited therapeutic options, and include gallbladder cancer (GBC), ampulla of Vater cancer (AVC), intrahepatic cholangiocarcinoma (iCCA) and extrahepatic cholangiocarcinoma (eCCA).
Methods & Results:
In the current review, we will discuss recent results of clinical trials testing targeted therapies in BRAF-mutant BTCs, with a particular focus on the recently published Phase II ROAR trial and ongoing active and recruiting clinical trials.
Conclusions:
Although the extended use of molecular profiling has paved the way toward a new era in BTC management, targeted therapies are limited to iCCA so far, and the prognosis of patients with metastatic disease has substantially not changed in the last decade. In this discouraging scenario, BRAF inhibition is currently emerging as a novel treatment option in patients harboring BRAF mutations.
Keywords: BRAF, dabrafenib, trametinib, biliary tract cancer, cholangiocarcinoma
Introduction
Biliary tract cancers (BTCs) include a heterogeneous group of aggressive malignancies arising in the bile duct system, accounting for approximately the 3% of all gastrointestinal tumors and representing the second most frequent type of primary liver cancer after hepatocellular carcinoma (HCC). 1,2 BTCs comprise gallbladder cancer (GBC), ampulla of Vater cancer (AVC), and cholangiocarcinoma (CCA), which is further subdivided into intrahepatic (iCCA) and extrahepatic cholangiocarcinoma (eCCA) (Figure 1) 3 ; eCCA, occurring outside the liver parenchyma, is further classified into perihilar (pCCA) and distal cholangiocarcinoma (dCCA). 4,5 Despite the remarkable differences in incidence among distinct geographic areas, BTC rates are rising in the vast majority of western countries, mainly as a consequence of improved diagnostic techniques and the growing incidence of iCCAs. 6,7
Figure 1.
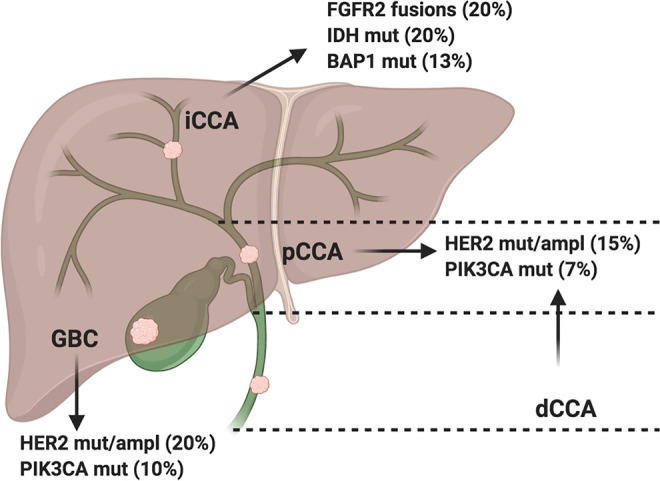
Schematic figure reporting anatomical subgroups of biliary tract cancer and commonly occurring gene aberrations; ampl: amplifications; BAP1: BRCA1 associated protein-1 (ubiquitin carboxy-terminal hydrolase); dCCA: distal cholangiocarcinoma; eCCA: extrahepatic cholangiocarcinoma; FGFR2: Fibroblast Growth Factor Receptor 2; GBC: gallbladder cancer; HER2: Human Epidermal growth factor Receptor 2; iCCA: intrahepatic cholangiocarcinoma; IDH: isocitrate dehydrogenase 1; mut: mutations; pCCA: perihilar cholangiocarcinoma; PIK3CA: Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha.
Radical surgical resection remains the mainstay of cure, but unfortunately, since BTCs are frequently asymptomatic in early stages and given the lack of specific screening programs, most of patients are diagnosed with locally advanced / unresectable or metastatic disease. 8 -10 In addition, a relevant percentage of patients considered to have localized, resectable disease at diagnosis is subsequently found to be unresectable during exploratory laparotomy. 11 Moreover, recurrence rates remain high even after radical surgery, with 5-year overall survival (OS) rate of less than 10% for all patients. 12,13
Recent controversial results of the randomized phase III BILCAP trial support the use of adjuvant capecitabine, on the basis of an OS benefit in the experimental arm compared to observation alone (53 months versus 36 months, respectively, Hazard Ratio [HR] 0.75, 95% CI 0.58-0.97; P = 0.0028 in the prespecified per-protocol analysis). 14 In BTC patients with advanced disease, first-line systemic chemotherapy represents the current standard of care, following the landmark ABC-02 trial comparing the cisplatin-gemcitabine (CisGem) doublet to gemcitabine monotherapy. 15 According to the results of this phase III trial on 410 BTCs, CisGem reported a statistically significant OS benefit compared to gemcitabine (11.7 months versus 8.1 months, HR 0.64, 95% CI 0.52-0.80; P < 0.001) in the overall population as well as in distinct anatomical subgroups. These results have been confirmed by the Japanese BT22 trial, with a median OS of 11.2 months achieved in the reference doublet arm compared to 7.7 months in patients receiving gemcitabine monotherapy. 16
As regards second-line setting, the fast deterioration of patients’ performance status following disease progression often limits the possibility of active treatment. Moreover, second-line systemic chemotherapy (including doublet chemotherapy with capecitabine and irinotecan, 5-fluorouracil monotherapy, etc.) has historically shown limited benefit, as suggested by a systematic literature review reporting a mean progression-free survival (PFS) of 3.2 months, a mean OS of 7.2 months and a response rate of 7.7%. 9 More recently, a phase II randomized trial observed a 9-month PFS benefit in BTC patients receiving second-line XELIRI over irinotecan monotherapy (60.9% versus 32.0%, p = 0.045), with a tolerable safety profile. 8 However, in patients failing first-line reference doublet, there is now evidence that chemotherapy with modified FOLFOX (mFOLFOX) could provide substantial improvement in 6-month and 12-month survival rate compared to active symptom control (ASC), following recent results of the ABC-06 trial. 17 Moreover, this phase III randomized trial has suggested a statistically significant OS benefit in patients treated with mFOLFOX (5-fluorouracil and oxaliplatin) plus ASC versus ASC alone (6.2 months versus 5.3 months, HR 0.69, 95% CI 0.50-0.97; P = 0.031). Nonetheless, the overall prognosis of BTC patients with metastatic disease remains poor, with a median OS of less than a year, something which urgently calls for novel, more effective therapeutic options. 18,19
In the last decade, the advent of genomic sequencing has led to a better understanding of the complex molecular landscape of BTC, revealing that each anatomical subgroup displays distinct mutational features and potentially actionable targets. 20 -22 Interestingly, recent genomic sequencing data have suggested that approximately half of BTC patients harbor at least 1 driver mutation, paving the way toward a number of trials assessing targeted treatments in biomarker-enriched populations. In fact, novel agents are emerging in BTCs, with isocitrate dehydrogenase (IDH), fibroblast growth factor receptor (FGFR) and epidermal growth factor receptor 2 (HER2), representing promising targets. 23 -26 Among genetic aberrations described in BTC, BRAF mutations have been reported in around the 5-7% of BTCs, mainly in the subcohort with iCCA. 27,28 Of note, recent years have seen a growing interest toward targeting BRAF in BTCs, as witnessed by the recently published ROAR trial which evaluated the dual inhibition of dabrafenib (BRAF inhibitor [BRAFi]) plus trametinib (MEK inhibitor [MEKi]) in BTC patients harboring the BRAFV600E mutation. 29
In the current review, we aim to provide an overview of recent advancements and emerging therapeutic options in BRAF-mutant metastatic BTC, especially focusing on recently published data and ongoing trials evaluating this novel therapeutic approach in BTCs.
The Genomic Landscape of Biliary Tract Cancer
In recent years, the extensive use of tumor genomic profiling has led to the identification of important features of BTCs, revealing the presence of remarkable differences among distinct subtypes. 30 -32 Firstly, the pivotal study by Nakamura and colleagues analyzed 260 BTC Japanese patients (including 231 CCAs and 29 GBCs), by performing whole-exome and transcriptome sequencing 33 ; interestingly, the authors identified a number of potentially targetable genetic aberrations, including FGFR2, ATP1B-PRKACA and ATP1B-PRKACB gene fusions. In addition, several clusters of missense mutations were detected, including IDH1, NRAS, GNAS, KRAS, ERBB2, and PIK3CA, which were more common in pCCA and dCCA compared with iCCA. 33 Javle and colleagues—using the FoundationOne platform—systematically investigated the correlation between genomic mutations and clinical features in a large cohort of BTC patients including 412 iCCAs, 85 GBCs and 57 eCCAs. 34 According to the results of this landmark study, different gene aberrations were observed depending on BTC anatomical subtype, with a predominance of TP53 aberrations and FGFR mutations in iCCAs and KRAS and ERBB2 aberrations in eCCAs and GBCs, respectively. 34 Moreover, IDH mutations and FGFR2 gene fusions were mainly limited to intrahepatic forms, also appearing to be mutually exclusive; in addition, a positive correlation with survival was highlighted in iCCA patients harboring FGFR genetic aberrations while TP53 and KRAS mutation carriers presented lower survival. 34
More recently, a prospective study using the MSK-IMPACT platform confirmed these findings analyzing tumor samples from 195 BTC patients, observing remarkable heterogeneity among different anatomical subgroups and describing the preponderance of IDH1, BAP1, TP53 mutations and FGFR2 gene fusions in iCCAs. 35 In addition, a recent report by Jusakul and colleagues shed further light on BTC landscape, leading to the identification of 4 distinct molecular subtypes by performing integrative clustering analysis of genomic and clinical data in a large cohort of almost 500 patients. 36 Among the clusters suggested by this international report, Cluster 1 and Cluster 2 tumors mainly included fluke-positive malignancies harboring ERBB2 amplifications, TP53 and ARID1A gene alterations and CpG hypermethylation. In addition, these 2 clusters were associated with worse prognosis and a more aggressive behavior. 36 Conversely, Cluster 3 and Cluster 4 BTCs were not associated to fluke infection, presenting high PD-L1 and PD-1 expression and IDH and BAP1 mutations. 36 Moreover, the specific group of Cluster 4 tumors mainly included iCCA patients with FGFR alterations, which were associated with better survival compared to Cluster 1 and 2.
The identification of distinct molecular subtypes has represented a historical step forward in the comprehension of BTC features. 37 -39 Importantly, about half of BTC patients harbor potentially targetable genetic mutations, and thus, a wide number of potential targets such as IDH mutations and FGFR2 gene fusions are currently under evaluation, with a view to provide novel and more effective treatment options. 40 -44 In particular, IDH mutations alter the physiological catalytic activity of isocitrate dehydrogenase 1 and 2, hesitating in the formation of the new metabolite 2-hydroxyglutarate (2-HG), which has an oncogenic role. 21 The recent phase III ClarIDHy trial has randomized 185 IDH-mutant BTC patients whose disease progressed on standard of care chemotherapy to the IDH1 inhibitor ivosidenib or placebo. 25 Of note, the primary endpoint of the study was met, with a median PFS of 2.7 versus 1.4 months for patients treated with ivosidenib and with placebo, respectively (HR 0.37, 95% CI 0.25-0.54; P < 0.001). 25 Additionally, the intention-to-treat analysis observed a median OS of 10.8 months in the ivosidenib group versus 9.7 months in the placebo arm. 25 Another important target is represented by FGFR2 gene fusions, with several trials which have reported interesting results in this setting. 27 Firstly, a phase II study evaluating the FGFR inhibitor infigratinib has shown an overall response rate (ORR) of 18.8% and a disease control rate (DCR) of 83.3% 28 ; similarly, the FGFR inhibitor derazantinib has obtained an ORR and a DCR of 20.7% and 82.8%, respectively, in a phase II trial. 32 More recently, the FGFR1, FGFR2 and FGFR3 inhibitor pemigatinib has been tested in the FIGHT-202 trial, reporting a remarkable 35.5% of ORR, a median PFS of 6.9 months and a median duration of response of 7.5 months, with these results leading to the US FDA approval of pemigatinib. 23,43 Moreover, several other targets are currently under exploration, including mutations in DNA damage repair (DDR) genes, NTRK gene fusions and PIK3CA mutations. 18 -20,22
However, as observed in other malignancies treated with targeted treatments, acquired resistance represents an important issue in BTC management limiting the durability of response and liquid biopsy has the potential to play an important role in this setting. 26,41,42 For example, although a wide number of FGFR inhibitors have shown promising antitumor activity in iCCA, several reports have observed the onset of specific resistance mechanisms to FGFR inhibition. A pivotal study by Goyal and colleagues reported acquired resistance to FGFR inhibitors in 3 FGFR-fusion positive iCCAs receiving infigratinib. 41 Of note, the authors performed biopsy sample and sequencing of cell-free DNA (cfDNA) at baseline and following progressive disease, observing secondary FGFR mutations. In a subsequent study, the third-generation, irreversible FGFR inhibitor TAS-120 (or futibatinib) was able to overcome these resistance mutations, reporting activity against multiple mutations conferring resistance to derazantinib. 42 Thus, strategic sequencing of FGFR inhibition with serial liquid biopsies and circulating tumor DNA (ctDNA) could prolong the duration of response from FGFR inhibitors, orienting the therapeutic management of these patients. In fact, a key role for liquid biopsy and cfDNA/ctDNA analysis could be represented by monitoring response to targeted treatments, by tracking the emergence of resistance—and thus, translating previous evidence observed in other malignancies such as non-small cell lung cancer and colorectal cancer in BTC management.
BRAF Mutations in Biliary Tract Cancer
The RAS-RAF-MEK-ERK, or mitogen-activated protein kinase (MAPK), pathway is involved in crucial processes of cell proliferation and survival. 45,46 Since BRAF is a member of these kinases, BRAF mutations—which have been found in several malignancies, including melanoma, non-small cell lung cancer and colorectal cancer—constitutively activate this pathway. 47 -50 To date, more than 50 BRAF mutations have been described, the most common of which is the V600E point mutation. 51
BRAF mutations are rare in BTCs, occurring almost exclusively in iCCAs and presenting an overall prevalence ranging between 5% and 7%. 52 A study on 926 Chinese patients with hepatobiliary malignancies (469 iCCAs, 203 eCCAs, 195 GBCs and 59 hepatocholangiocarcinomas) has been presented at the ASCO Virtual 2020 Meeting 53 ; of note, BRAF activating mutations were observed in the 5.5% of patients, with BRAF V600E mutations detected in the 1.5% of iCCAs and the 0.5% of GBCs. Additionally, no BRAF V600E mutations were highlighted in the other cohorts. 53 Interestingly, BTCs harboring BRAFV600E mutations have been described as a unique molecular and clinical subtype of biliary tumors.
Although early studies, including a German report on 69 CCA patients, suggested no correlation between survival and BRAF mutations, BRAFV600E-mutated BTCs have been more recently associated with higher TNM stage, resistance to systemic chemotherapy, aggressive clinical course and worse survival. 54,55 In particular, a landmark study by Robertson and colleagues identified the presence of BRAF mutations in the 7.4% of patients by using immunohistochemistry, with overall survival resulting 37.3 months in wild-type patients and 13.5 months in BRAF-mutated subjects. 55
The possibility to target BRAF mutations in BTCs was firstly explored in a Phase II basket trial evaluating the BRAFi vemurafenib as monotherapy in pretreated patients with metastatic disease (Figure 2). 56 According to the results of this trial, vemurafenib monotherapy yielded limited responses, with only 1 out of 8 patients achieving partial response (PR) and an ORR of 12%. 56
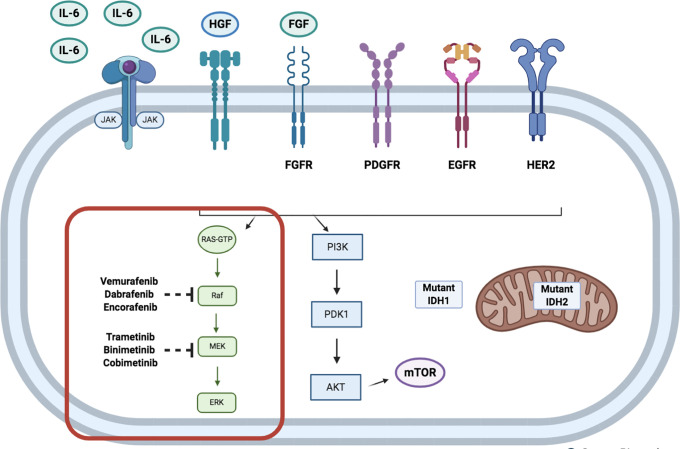
Figure 2.
Schematic representation of signaling pathways in biliary tract cancer, with a particular focus on the mitogen-activated protein kinase (MAPK) / extracellular signal-regulated kinase (ERK), or RAS/RAF/MEK/ERK pathway, with BRAF and MEK inhibitors.
An alternative strategy used to target MAPK has seen the evaluation of MEKi in solid tumors, including BTC. 57 The MEKi selumetinib has been tested as monotherapy in 25 metastatic CCAs in a multi-institutional Phase II trial in which the 39% of subjects had previously received at least 1 prior systemic chemotherapy. 58 Of note, no BRAF V600E mutations were found in enrolled patients. Three PRs and 17 SDs were observed, with median PFS and OS of 3.7 months and 9.7 months, respectively. Mild toxicities were detected, with rash (90%) and xerostomia (54%) reported as the most frequent; only 1 patient experienced grade 4 toxicity (fatigue). Trametinib, a highly selective MEKi, has been evaluated as second-line therapy in advanced CCA patients with disease progression after prior CisGem in the SWOG S1310 trial. 59 Unfortunately, the trial was stopped prematurely following the discouraging lack of responses detected. 59 Similarly, the selective MEKi binimetinib has reported disappointing responses in advanced BTC, as monotherapy or in combination with cytotoxic chemotherapy. 60 The overall limited activity of MEK inhibition has been further highlighted in other recent trials, including a recent phase I/II study evaluating binimetinib in combination with CisGem in chemotherapy-naïve BTC patients. 61 According to the results of this study, the triplet did not show an improvement in terms of response rate and PFS at 6 months. Similarly, a phase II, single-arm trial investigating the efficacy and safety of trametinib in Japanese patients with previously treated advanced BTC observed no benefit in terms of 12-week non-progressive disease (PD) rate. 62 Although the primary endpoint of this trial was not met, signals of activity were detected, as witnessed by the prolonged PFS in a patient harboring specific mutations—including synonymous NF1 exon 12 splice variant and a loss-of-function variant in ARID1A.
As evidenced in other malignancies (e.g. malignant melanoma) even after initial response to BRAFi monotherapy, the onset of acquired resistance represents a major obstacle in BRAF targeted treatments. 63,64 On the basis of preclinical and clinical data which have evidenced that the dual inhibition of BRAF and MEK could play a synergistic effect—thus delaying the emergence of resistance—BRAFi and MEKi combinations have been evaluated in BRAF-mutated BTCs. Sporadic case reports and case series regarding BRAFV600E-mutant BTC patients receiving dabrafenib plus trametinib combination have reported remarkable results, with cases of complete response to BRAFi plus MEKi. 65,66 In particular, Kocsis and colleagues reported the case of a 59-year-old BRAF V600E-mutated eCCA patient reporting complete response after dabrafenib plus trametinib combination treatment. 65 Of note, the included patient—whose disease progressed after first-line chemotherapy with cisplatin plus gemcitabine doublet—was firstly treated with dabrafenib orally 150 mg twice daily with subsequent addition of trametinib 2 mg once a day. Similarly, Lavingia reported 2 cases of BRAF V600E-mutated iCCA treated with dabrafenib plus trametinib, reporting one complete response and a partial response, confirming the promising activity of dual BRAF and MEK targeting in these patients. 66
Interestingly, Subbiah and colleagues recently published the results of the BTC subgroup of the ROAR study, a basket trial assessing dabrafenib plus trametinib combination in different cohorts of solid tumors harboring BRAF V600E mutation. 29 In this open-label, Phase II, non-randomized trial, 43 BTC patients with metastatic disease have been treated with dabrafenib 150 mg twice daily plus oral trametinib 2 mg once daily as second- or later-line treatment. 29 39 out of 43 patients were affected by iCCA, representing the 91% of the entire population. According to the results of this trial, dabrafenib plus trametinib combination yielded an overall response rate of 51% (95% CI 36-67, 22 of 43 patients), with median PFS and median OS of 9.0 months (95% CI 5.0-10.0) and 14.0 months (95% CI 10.0-33.0), respectively. The results of this study are particularly relevant if we consider the patient population of this subcohort, affected by metastatic and highly pretreated BRAFV600E-mutated BTCs. In addition, the BRAFi plus MEKi combination reported a manageable safety profile, with increased γ-glutamyltransferase observed as the most common grade 3 or worse adverse event in 5 out of 43 patients (12%) and no treatment-related deaths. 29 The clinical benefit highlighted in the BTC subcohort with the dabrafenib plus trametinib combination represents an important step forward in the management of this group of malignancies, and routine testing for BRAF V600E mutation should be carefully considered in all BTC patients—especially in iCCAs, where this mutation is relatively more frequent.
Ongoing Trials
In this changing landscape, several Phase I and II basket trials are evaluating the role of BRAFi in BRAF-mutant solid tumors, including advanced BTC (Table 1). The BRAF V600 inhibitor ABM-1310 is being assessed in a Phase I trial (NCT04190628) enrolling advanced or metastatic BRAF V600-mutated solid tumors such as melanoma, glioblastoma, colorectal cancer, NSCLC, thyroid cancer, ovarian cancer and CCA. The primary endpoint of this trial is the determination of the maximum tolerated dose (MTD), with safety and ORR also assessed as secondary endpoints. This study has a planned enrollment of 27 patients with an estimated primary completion date in December 2021. Regarding second-generation inhibitors, the BRAFi BGB-3245 is under investigation in patients with advanced solid tumors—including BTCs—harboring BRAF mutations in a Phase I trial (NCT04249843). With a planned recruitment of 69 patients, this study has MTD and safety as co-primary endpoints.
Table 1.
Ongoing Trials Evaluating BRAF Targeted Therapies in Advanced Biliary Tract Cancer Registered on https://ClinicalTrials.gov (September 2020).a
| NCT name | Phase | Setting | Arm A | Arm B | Compounds description | Estimated enrollment | Primary outcomes |
|---|---|---|---|---|---|---|---|
| NCT04190628 | 1 | Second- or later-line; advanced BRAF-mutant solid tumors,
including BTC |
ABM-1310 | ABM-1310: BRAF inhibitor | 27 | MTD / RP2D | |
| NCT04249843 | 1 | Second- or later-line; advanced BRAF-mutant solid tumors,
including BTC |
BGB-3245 | BGB-3245: BRAF inhibitor | 69 | DLT MTD / RP2D |
|
| NCT03839342 | 2 | Second- or later-line; advanced BRAF-mutant solid tumors,
including BTC |
Binimetinib + encorafenib | Binimetinib: MEK inhibitor Encorafenib: BRAF inhibitor |
26 | ORR | |
| NCT01989585 | 1/2 | Second- or later-line; advanced BRAF-mutant solid tumors,
including BTC |
Dabrafenib + trametinib | Dabrafenib + trametinib + navitoclax | Dabrafenib: BRAF inhibitor Trametinib: MEK inhibitor Navitoclax: Bcl-2 inhibitor |
75 | MTD CR rate |
|
NCT04418167 |
1 | Second- or later-line; advanced solid tumors, including BTC with
MAPK pathways mutations |
JSI-1187 | JSI-1187 + dabrafenib | JSI-1187: ERK inhibitor Dabrafenib: BRAF inhibitor |
124 | AEs |
|
NCT03272464 |
1 | Second- or later-line; advanced BRAF-mutant solid tumors,
including BTC |
Dabrafenib + trametinib + itacitinib | Dabrafenib: BRAF inhibitor Trametinib: MEK inhibitor Itacitinib: JAK1 inhibitor |
38 | MTD |
a Abbreviations: AEs: adverse events; BTC: biliary tract cancer; CR: complete response; DLTs: dose-limiting toxicities; MTD: maximum tolerated dose; JAK1: Janus-associated kinase 1; MEK: mitogen-activated protein kinase; ORR: overall response rate; RP2D: recommended phase 2 dose.
Regarding the dual inhibition of BRAF and MEK, the Phase II BEAVER trial (NCT03839342) is exploring the role of binimetinib plus encorafenib combination in advanced solid tumors harboring non-V600E BRAF mutations. ORR represents the primary endpoint of this basket trial. The dual inhibition of BRAF and MEK is also under investigation in a Phase I/II trial evaluating dabrafenib plus trametinib in combination with the Bcl-2 inhibitor navitoclax (NCT01989585). This trial is currently enrolling patients with BRAF-mutant advanced malignancies, with a view to primarily determine the MTD, toxicity and safety profile of this triplet. Other approaches under investigation involve the combination of the selective ERK1/2 inhibitor JSI-1187 with BRAFi. In particular, a Phase 1 trial is investigating the JSI-1187 as monotherapy or in combination with dabrafenib in advanced solid tumors (including BTCs) harboring MAPK pathway mutations or BRAF V600E mutations (NCT04418167). Primary endpoint of this study is the incidence of treatment emergent adverse events while co-secondary endpoints are ORR, duration of response, time to response, disease control rate, PFS and OS. The novel and selective Janus-associated kinase 1 (JAK1) inhibitor itacitinib (INCB039110) is being tested in a Phase I trial evaluating the combination of itacitinib plus dabrafenib plus trametinib in BRAF-mutant melanoma and other solid tumors (NCT03272464). This study has a planned enrollment of 38 patients with an estimated primary completion date in September 2023.
Conclusions
Recent years have witnessed a new era in BTC management, and previous treatment paradigms are quickly evolving. 67,68 However, the prognosis of this heterogeneous group of malignancies remains poor, with limited treatment options currently available. 69 -71 In this scenario, novel treatments are under investigation, with BRAF mutations having the potential to become important therapeutic targets in the near future, moving toward a more personalized approach in these aggressive malignancies.
Footnotes
Authors’ Note: Alessandro Rizzo and Alessandro Di Federico are equally contributing first authors. Our study did not require an ethical board approval because it did not contain human or animal trials.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Alessandro Rizzo, MD  https://orcid.org/0000-0002-5257-8678
https://orcid.org/0000-0002-5257-8678
References
- 1. Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383(9935):2168–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rizzo A, Ricci AD, Tober N, et al. Second-line treatment in advanced biliary tract cancer: today and tomorrow. Anticancer Res. 2020;40(6):3013–3030. [DOI] [PubMed] [Google Scholar]
- 4. Chun YS, Javle M. Systemic and adjuvant therapies for intrahepatic cholangiocarcinoma. Cancer Control. 2017;24(3):1073274817729241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mertens JC, Rizvi S, Gores GJ. Targeting cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis. 2018;1864(4 pt B):1454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Forner A, Vidili G, Rengo M, et al. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019;39(suppl 1):98–107. [DOI] [PubMed] [Google Scholar]
- 7. Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the US: intrahepatic disease on the rise. Oncologist. 2016;21:594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng Y, Tu X, Zhao P, et al. A randomised phase II study of second-line XELIRI regimen versus irinotecan monotherapy in advanced biliary tract cancer patients progressed on gemcitabine and cisplatin. Br J Cancer. 2018;119(3):291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lamarca A, Hubner RA, David Ryder W, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. 2014;25(12):2328–2338. [DOI] [PubMed] [Google Scholar]
- 10. Brandi G, Rizzo A, Dall’Olio FG, et al. Percutaneous radiofrequency ablation in intrahepatic cholangiocarcinoma: a retrospective single-center experience. Int J Hyperthermia. 2020;37(1):479–485. [DOI] [PubMed] [Google Scholar]
- 11. Bridgewater JA, Goodman KA, Kalyan A, Mulcahy MF. Biliary tract cancer: epidemiology, radiotherapy, and molecular profiling. Am Soc Clin Oncol Educ Book. 2016;35:e194–203. [DOI] [PubMed] [Google Scholar]
- 12. Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557–588. doi:10.1038/s41575-020-0310-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kelley RK, Bridgewater J, Gores GJ, Zhu AX. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72(2):353–363. [DOI] [PubMed] [Google Scholar]
- 14. Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663–673. [DOI] [PubMed] [Google Scholar]
- 15. Valle J, Wasan H, Palmer DH, et al. ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. [DOI] [PubMed] [Google Scholar]
- 16. Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103(4):469-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lamarca A, Palmer DH, Wasan HS, et al. ABC-06. A randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin/5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced/metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisgEM) chemotherapy. J Clin Oncol. 2019. 37abstr 4003. [Google Scholar]
- 18. Sirica AE, Gores GJ, Groopman JD, et al. Intrahepatic cholangiocarcinoma: continuing challenges and translational advances. Hepatology. 2019;69(4):1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Massa A, Varamo C, Vita F, et al. Evolution of the experimental models of cholangiocarcinoma. Cancers. 2020;12:2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mizrahi JD, Shroff RT. New treatment options for advanced biliary tract cancer. Curr Treat Options Oncol. 2020;21(8):63. [DOI] [PubMed] [Google Scholar]
- 21. Tariq NU, McNamara MG, Valle JW. Biliary tract cancers: current knowledge, clinical candidates and future challenges. Cancer Manag Res. 2019;11:2623–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ricci AD, Rizzo A, Brandi G. The DNA damage repair (DDR) pathway in biliary tract cancer (BTC): a new Pandora’s box? ESMO Open. 2020;5:e001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020. pii: S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Galdy S, Lamarca A, McNamara MG, et al. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: a potential therapeutic target? Cancer Metastasis Rev. 2017;36(1):141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(6):796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rizzo A, Ricci AD, Tavolari S, Brandi G. Circulating tumor DNA in biliary tract cancer: current evidence and future perspectives. Cancer Genomics Proteomics. 2020;17(5):441–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lamarca A, Barriuso J, McNamara MG, Valle JW. Molecular targeted therapies: ready for “prime time” in biliary tract cancer. J Hepatol. 2020;73(1):170–185. [DOI] [PubMed] [Google Scholar]
- 28. Valle JW, Lamarca A, Goyal L, Barriuso J, Zhu AX. New horizons for precision medicine in biliary tract cancers. Cancer Discov. 2017;7(9):943–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Subbiah V, Lassen U, Élez E, et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020;21:1234–1243. [DOI] [PubMed] [Google Scholar]
- 30. Jain A, Kwong LN, Javle M. Genomic profiling of biliary tract cancers and implications for clinical practice. Curr Treat Options Oncol. 2016;17(11):58. [DOI] [PubMed] [Google Scholar]
- 31. Zhao DY, Lim KH. Current biologics for treatment of biliary tract cancer. J Gastrointest Oncol. 2017;8(3):430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mazzaferro V, El-Rayes BF, Droz Dit Busset M, et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br J Cancer. 2019;120(2):165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003–1010. [DOI] [PubMed] [Google Scholar]
- 34. Javle M, Bekaii-Saab T, Jain A, et al. Biliary cancer: utility of next-generation sequencing for clinical management. Cancer. 2016;122(24):3838–3847. [DOI] [PubMed] [Google Scholar]
- 35. Lowery MA, Ptashkin R, Jordan E, et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin Cancer Res. 2018;24(17):4154–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jusakul A, Cutcutache I, Yong CH, et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017;7(10):1116–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ma B, Meng H, Tian Y, et al. Distinct clinical and prognostic implication of IDH1/2 mutation and other most frequent mutations in large duct and small duct subtypes of intrahepatic cholangiocarcinoma. BMC Cancer. 2020;20(1):318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ong CK, Subimerb C, Pairojkul C, et al. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat Genet. 2012;44(6):690–693. [DOI] [PubMed] [Google Scholar]
- 39. Zou S, Li J, Zhou H, et al. Mutational landscape of intrahepatic cholangiocarcinoma. Nat Commun. 2014;5:5696. [DOI] [PubMed] [Google Scholar]
- 40. Cao J, Hu J, Liu S, et al. Intrahepatic cholangiocarcinoma: genomic heterogeneity between eastern and western patients. JCO Precis Oncol. 2020;4:557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goyal L, Saha SK, Liu LY, et al. Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion-positive cholangiocarcinoma. Cancer Discov. 2017;7(3):252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goyal L, Shi L, Liu LY, et al. TAS-120 overcomes resistance to ATP-competitive FGFR inhibitors in patients with FGFR2 fusion-positive intrahepatic cholangiocarcinoma. Cancer Discov. 2019;9(8):1064–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hoy SM. Pemigatinib: first approval. Drugs. 2020;80(9):923–929. [DOI] [PubMed] [Google Scholar]
- 44. Javle M, Churi C, Kang HC, et al. HER2/neu-directed therapy for biliary tract cancer. J Hematol Oncol. 2015;8:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schaider H, Sturm RA. The evolving universe of BRAF mutations in melanoma. Br J Dermatol. 2017;177(4):893. [DOI] [PubMed] [Google Scholar]
- 46. Iyer P, Chen MH, Goyal L, Denlinger CS. Targets for therapy in biliary tract cancers: the new horizon of personalized medicine. Chin Clin Oncol. 2020;9(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rose AAN. Encorafenib and binimetinib for the treatment of BRAF V600E/K-mutated melanoma. Drugs Today (Barc). 2019;55(4):247–264. [DOI] [PubMed] [Google Scholar]
- 48. Lamberti G, Andrini E, Sisi M, et al. Beyond EGFR, ALK and ROS1: current evidence and future perspectives on newly targetable oncogenic drivers in lung adenocarcinoma. Crit Rev Oncol Hematol. 2020;156:103119. doi:10.1016/j.critrevonc.2020.103119 [DOI] [PubMed] [Google Scholar]
- 49. Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 2016;17(7):984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tol J, Nagtegaal ID, Punt CJA. BRAF mutation in metastatic colorectal cancer. N Engl J Med. 2009;361(1):98–99. [DOI] [PubMed] [Google Scholar]
- 51. Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366(8):707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chakrabarti S, Kamgar M, Mahipal A. Targeted therapies in advanced biliary tract cancer: an evolving paradigm. Cancers (Basel). 2020;12(8):2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li W, Cui Y, Yin F, et al. BRAF mutation in Chinese biliary tract cancer patients. J Clin Oncol. 2020;38(15_suppl). e16678–e16678. [Google Scholar]
- 54. Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52(5):706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Robertson S, Hyder O, Dodson R, et al. The frequency of KRAS and BRAF mutations in intrahepatic cholangiocarcinomas and their correlation with clinical outcome. Hum Pathol. 2013;44(12):2768–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373(8):726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kim C, Giaccone G. MEK inhibitors under development for treatment of non-small-cell lung cancer. Expert Opin Investig Drugs. 2018;27(1):17–30. [DOI] [PubMed] [Google Scholar]
- 58. Bekaii-Saab T, Phelps MA, Li X, et al. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol. 2011;29(17):2357–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim RD, McDonough SL, El-Khoueiry AB, et al. SWOG S1310: randomized phase II trial of single agent MEK inhibitor trametinib vs. 5-fluorouracil or capecitabine in refractory advanced biliary cancer. J Clin Oncol. 2017;35(15):4016–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Finn RS, Ahn DH, Javle MM, et al. Phase 1b investigation of the MEK inhibitor binimetinib in patients with advanced or metastatic biliary tract cancer. Invest New Drugs. 2018;36(6):1037–1043. [DOI] [PubMed] [Google Scholar]
- 61. Lowery MA, Bradley M, Chou JF, et al. Binimetinib plus gemcitabine and cisplatin phase I/II trial in patients with advanced biliary cancers. Clin Cancer Res. 2019;25(3):937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ikeda M, Ioka T, Fukutomi A, et al. Efficacy and safety of trametinib in Japanese patients with advanced biliary tract cancers refractory to gemcitabine. Cancer Sci. 2018;109(1):215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hall RD, Kudchadkar RR. BRAF mutations: signaling, epidemiology, and clinical experience in multiple malignancies. Cancer Control. 2014;21(3):221–230. [DOI] [PubMed] [Google Scholar]
- 64. Kudchadkar RR, Gonzalez R, Lewis K. New targeted therapies in melanoma. Cancer Control. 2013;20(4):282–288. [DOI] [PubMed] [Google Scholar]
- 65. Kocsis J, Árokszállási A, András C, et al. Combined dabrafenib and trametinib treatment in a case of chemotherapy-refractory extrahepatic BRAF V600E mutant cholangiocarcinoma: dramatic clinical and radiological response with a confusing synchronic new liver lesion. J Gastrointest Oncol. 2017;8(2):E32–E38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lavingia V, Fakih M. Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma-2 case reports and a brief review. J Gastrointest Oncol. 2016;7(6):E98–E102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ricci AD, Rizzo A, Brandi G. Immunotherapy in biliary tract cancer: worthy of a second look. Cancer Control. 2020;27(3):1073274820948047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Morizane C, Ueno M, Ikeda M, Okusaka T, Ishii H, Furuse J. New developments in systemic therapy for advanced biliary tract cancer. Jpn J Clin Oncol. 2018;48(8):703–711. [DOI] [PubMed] [Google Scholar]
- 69. Oneda E, Abu Hilal M, Zaniboni A. Biliary tract cancer: current medical treatment strategies. Cancers (Basel). 2020;12(5):1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Malenica I, Donadon M, Lleo A. Molecular and immunological characterization of biliary tract cancers: a paradigm shift towards a personalized medicine. Cancers. 2020;12(8):2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rizzo A, Ricci AD, Brandi G. Futibatinib, an investigational agent for the treatment of intrahepatic cholangiocarcinoma: evidence to date and future perspectives. Expert Opin Investig Drugs. 2020:1–8. [DOI] [PubMed] [Google Scholar]