Abstract
Objectives
The purpose of this study was to investigate the impact of different types of maskers on speech understanding as a function of cognitive status in older adults. The hypothesis tested was that individuals with a diagnosis of mild cognitive impairment (MCI) or mild dementia would perform like their age- and hearing status–matched control counterparts in modulated noise but would perform more poorly in the presence of competing speech.
Design
Participants (n = 39; age range: 55–77 years old) performed a speech-in-noise task and completed two cognitive screening tests and a measure of working memory. Sentences were presented in the presence of two types of maskers (i.e., speech envelope–modulated noise and two-talker, same-sex competing speech). Two analyses were undertaken: (a) a between-groups comparison of individuals diagnosed with MCI/dementia, individuals who failed both cognitive screeners (possible MCI), and age- and hearing status–matched neurologically healthy control individuals and (b) a mixed-model analysis of variance of speech perception performance as a function of working memory capacity.
Results
The between-groups comparison yielded significant group differences for speech understanding in both masking conditions, with the MCI/dementia group performing more poorly than the neurologically healthy controls and possible MCI groups. A single measure of working memory (Size Comparison Span [SICSPAN]) was correlated with performance on the speech perception task in the competing speech conditions.
Conclusions
Adults with a diagnosis of MCI or mild dementia performed more poorly on a speech perception task than their age- and hearing status–matched control counterparts in the presence of both maskers, with larger group mean differences when the target speech was presented in a two-talker masker. This suggests increased difficulty understanding speech in the presence of distracting backgrounds for people with MCI/dementia. Future studies should consider how to target this potentially vulnerable population as they may be experiencing increased difficulty communicating in challenging environments.
There is an ever-growing body of literature to support the claim that older adults have more difficulty than younger adults understanding speech in complex, noisy backgrounds (Committee on Hearing, Bioacoustics, and Biomechanics, 1988; Helfer & Freyman, 2008; Humes, 1996, 2013; Humes & Dubno, 2010; Humes et al., 2012). There are multiple contributing factors that are difficult to parse, including audibility, auditory processing of acoustic features, and cognitive processing required for speech understanding. In much of the speech-in-noise literature related to aging, different contributing factors to speech understanding are controlled as best as possible to attempt to isolate the components of hearing and cognition that may contribute to speech understanding (Humes et al., 2012; Pichora-Fuller & Souza, 2003; Schneider et al., 2005). For example, failing a cognitive screening measure is a typical exclusion factor in studies of speech understanding abilities as a function of age (Goossens et al., 2017; Gordon-Salant & Cole, 2016; Helfer & Freyman, 2014; Sommers & Phelps, 2016; Vaughan et al., 2008). However, given the associations between age-related hearing loss and cognitive decline, it is imperative to understand more about the relative contributions of each of these factors to age-related changes in speech understanding. This study sought to build on the speech-in-noise literature by recruiting adults with diagnoses of mild cognitive impairment (MCI) or mild dementia.
The process of diagnosis for MCI or dementia is complex and not based on a single score or cognitive domain. Current diagnostic criteria for MCI include the following: (a) subjective concern, (b) impaired performance in one or more cognitive domains (e.g., memory, attention, executive function, language, and visuospatial skills), (c) reduced independence in functional abilities, and (d) not demented (Smith & Bondi, 2013). Determination of impaired cognitive performance is made using a comprehensive battery of neuropsychological tests. The other three characteristics that comprise a diagnosis of MCI are based on careful interviews with the person and close family members to establish what that person’s normal, baseline cognitive function had been and whether a change in function more pronounced than that expected with normal aging has occurred. An important characteristic of any MCI or dementia diagnosis is that the person’s current cognitive function reflects a change from their baseline performance. In light of this process, simply categorizing individuals as “normal” and “probable MCI” on the basis of a screening measure is likely insufficient to learn about the speech understanding abilities of individuals with an MCI or dementia diagnosis.
Effects of Age on Speech Understanding
When older adults have normal or near-normal audiometric thresholds, they often perform similarly to younger adults for speech materials presented in quiet and in steady-state speech-shaped noise (Buss et al., 2019; Goossens et al., 2017; Grose et al., 2009; Rajan & Cainer, 2008). However, the introduction of complexities to the background noise can result in group differences in performance. For example, when comparing speech understanding in the presence of steady-state and modulated broadband noise, both younger and older adults improve when given the opportunity to glimpse speech information during the dips in the noise; however, younger adults improve more (i.e., exhibit more release from masking; Grose et al., 2009; Mamo, Grose, & Buss, 2019). Studies that have tried to isolate the auditory processing that contributes to this reduced benefit from fluctuations in the background masker typically argue that reduced auditory temporal processing plays a role and that it is an age effect that happens independently of changes in auditory thresholds (Dubno et al., 2002, 2003; Gifford et al., 2007; Grose et al., 2009; Strouse et al., 1998).
Another method for adding complexity to the background noise is to present the target in the presence of maskers that consist of understandable speech. Steady-state speech-shaped noise and most modulated noise or speech babble paradigms introduce energetic masking that overlaps in terms of frequency content with the target signal. On the other hand, competing speech can add excess masking due to the intelligibility of the speech content in the masker on top of the energetic masking inherent in the overlapping spectra. Informational masking paradigms not only require precise temporal processing to benefit from the masker fluctuations but also may carry a high cognitive burden to selectively attend to the target in the presence of distracting background talkers. Our research group and others have compared performance on speech understanding in the presence of background talkers across the adult age spectrum (Helfer & Freyman, 2008, 2014; Helfer et al., 2017). In general, older adults perform more poorly than younger adults when the background speech is intelligible and thus causes high confusability between the target and background speech. One interesting finding that highlights the age-related difficulties in speech maskers is that, in a study of spatial release from masking, older adults demonstrated less benefit when the speech maskers were moved to a side location and the target remained in the front (Helfer et al., 2010). This suggests that the informational masking caused by speech maskers carries an element of confusability (e.g., determining which speaker is the target compared to the maskers) and distractibility (e.g., maintaining selective attention on the target in the presence of a distractor). The younger adults seemed better able to manage the issue of distractibility when the issue of confusability was reduced (i.e., the target and the masker were no longer colocated). The older adults, however, remained sufficiently distracted by the competing intelligible masker so as not to benefit significantly from the spatial release from masking. This pattern of results suggests that beyond the issues of audibility and temporal processing of the complex signal, the cognitive challenge associated with understanding speech in the presence of speech maskers produces poorer performance for older adults compared to that of younger adults. Further evidence of an age-related effect of increased cognitive processing in competing speech was observed in a study by Helfer and Freyman (2008). Results of that study indicated that, after adjusting for baseline performance, the largest difference in speech understanding between groups of older and younger listeners occurred when the target and masking speech signals were from opposite-sex talkers (Helfer & Freyman, 2008). While speech perception in both groups of participants was disrupted by the presence of same-sex targets and maskers, younger adults were generally unaffected by the presence of the opposite-sex speech masker; however, this condition was challenging for most of the older participants.
While the studies discussed thus far have attempted to minimize the audibility differences between the younger and older listeners, some degree of decreased audibility is typically present for the older listeners, especially at higher frequencies. Elevated thresholds, even mild elevations and/or elevations limited to high frequency thresholds, affect speech-in-noise abilities (Humes, 1996; Pichora-Fuller & Souza, 2003). Multiple approaches have been undertaken to address this concern, such as using high-pass masking noise with all listeners (Souza, 2000; Souza & Turner, 1994), presenting stimuli with equivalent sensation levels (Lu et al., 2016; Pichora-Fuller et al., 1995), and applying prescriptive gain for each listener to minimize audibility differences (Humes, 2007; Humes et al., 2013). Nevertheless, the contributions of elevated thresholds, changes in auditory processing, and changes in cognitive abilities can never be perfectly isolated. That said, to better understand the cognitive processing impacts on speech perception and aging, we need to be able to explain variance in performance beyond that which would be predicted based on hearing thresholds.
Effects of Age-Related Cognitive Decline on Speech Understanding
An important question of interest in the aging auditory research relates to how changes in cognitive processing abilities affect speech understanding in background noise. These studies will often use a single cognitive metric (e.g., working memory) or a small battery of cognitive measures to test across multiple domains. The findings from two systematic reviews suggest that results with regard to the cognitive factors contributing to speech-in-noise performance are relatively inconsistent (Akeroyd, 2008; Dryden et al., 2017). The inconsistencies are due to a variety of factors, including the difficulty of the speech task employed, type(s) of background noise used, sample type (e.g., clinic-based or community), and sample size. Dryden et al. (2017) performed meta-analyses across 25 studies that measured speech-in-noise performance and cognitive abilities in adults. By pooling the findings across studies (allowing for sample sizes ranging from 150 to 1,026, depending on the measures included), the following processes had significant associations with speech-in-noise performance: inhibitory control, working memory, episodic memory, and processing speed. Overall, these findings are based on neurologically healthy adults, ranging in age from 18 to 85 years, with varying degrees of sufficient audibility.
In this study, we focus on a working memory metric as a cognitive measure hypothesized to contribute to variance in performance in speech understanding, particularly in the complex, speech-masker conditions. Importantly, in a scoping review of the literature, Akeroyd (2008) suggested that, after audibility, working memory was the most consistent cognitive predictor of performance. Furthermore, the review found that as the difficulty of the task increased, the effect of working memory became more pronounced; for example, there was a greater percentage of variance explained by cognitive tests (e.g., visual letter monitoring and digit monitoring) at lower signal-to-noise ratio (SNR) test conditions (Gatehouse et al., 2003).
There has been less research on the contribution of cognitive processes to speech-in-noise understanding in persons with known age-related cognitive decline or dysfunction. There is some information in the literature regarding the auditory processing abilities of people with MCI and/or early-stage dementia. Previous studies that have investigated auditory processing deficits in adults with Alzheimer’s disease (AD) and/or MCI report normal performance on speech measures in the presence of speech-weighted noise and declines in dichotic listening tasks as compared to neurologically healthy older adults (Gates et al., 2008; Idrizbegovic et al., 2011). Some studies have explored measures of central auditory processing as early biomarkers of dementia-related cognitive changes and have found that performance on the Dichotic Sentence Identification (DSI) task has a strong relationship with future AD status (Gates et al., 2011; Tuwaig et al., 2017). One hypothesis suggests that the complex attentional and behavioral processing necessary to extract auditory signals in the presence of competing signals is more sensitive to early neurological changes such that poor performance on these measures emerges earlier than poor performance on standard cognitive measures (Gates et al., 2011).
The purpose of the current investigation was to compare older adults with a clinical diagnosis of MCI or mild dementia to neurologically healthy, age- and hearing status–matched adults on measures of speech-in-noise performance. The experiment tested speech understanding in the presence of fluctuating noise and speech maskers as a function of cognitive status. There was no a priori reason to expect that individuals with MCI/early-stage dementia would have reduced ability to use spectrotemporal glimpses present in the fluctuating speech-shaped noise. However, cognitive decline might be expected to lead to a decreased ability to ignore a masker that consists of understandable speech due to greater than expected difficulties in memory, attention, executive function, and/or language skills (as per the diagnostic characteristics of MCI/dementia). Therefore, the hypothesis was that both groups of older adults would perform similarly in quiet and in the presence of fluctuating speech-shaped noise, but the group with MCI/dementia would perform more poorly than the cognitively healthy controls group in the presence of the two-talker competing speech. In addition to the between-groups analyses, the full sample was analyzed using a mixed-model analysis to determine the contributions of audibility and working memory to variance in performance. The hypothesis was that working memory would contribute significantly to variance in speech understanding in the presence of competing speech, especially in more adverse SNR conditions.
Materials and Method
Study Population
Two groups of older adults participated: (a) eight adults (age range: 63–77 years; M = 70.8, SD = 4.8) with a diagnosis of MCI or mild dementia and (b) 31 adults (age range: 55–77 years; M = 66.4, SD = 6.2) with no history of cognitive impairment. The MCI or dementia diagnosis was confirmed through documentation from each participant’s health care provider. Participants without a diagnosis of MCI/dementia were recruited with the aim of matching each participant with MCI/dementia to a control participant in terms of age (± 2 years) and hearing status (± 5 dB HL based on a four-frequency pure-tone average (PTA; [octave frequencies of 0.5–4 kHz] in the better-hearing ear). Of the 31 adults who volunteered as “neurologically healthy control participants,” 10 passed two cognitive screeners, 11 failed one cognitive screener, and 10 failed two cognitive screeners (see Cognitive Measures section in the subsequent pages). No one was excluded from the study on the basis of their hearing or their cognitive screening measures.
Hearing Testing
All listeners completed a brief case history over the phone prior to coming to the laboratory to rule out a history of ear disease or congenital hearing loss. Further screening at the time of the experiment included otoscopy to confirm clear and healthy outer ears, tympanometry to rule out middle ear dysfunction, and an air-conduction pure-tone audiogram. The tympanogram was measured using a GSI-39 auto tympanometer, and standard type A responses were deemed to reflect normal middle ear function. Hearing threshold testing was performed using a modified Hughson–Westlake approach in a single-walled sound-attenuated booth using calibrated DD450 RadioEar headphones and a GSI Pello audiometer. Testing was completed by trained research assistants. No one was excluded on the basis of their audiologic assessment, and the hearing losses measured by air-conduction audiometry in this sample are typical of age-related hearing loss (see Participant Characteristics subsection in Results section in the subsequent pages).
Participants also completed an abbreviated set of questions from the Speech, Spatial and Qualities of Hearing Scale (SSQ) related to speech understanding in noisy backgrounds (Gatehouse & Noble, 2004). Seven questions were included in the baseline measures for all participants. Participants indicated their responses using a Likert scale from 0 to 10 with lower scores suggesting more perceived difficulty in noisy or distracting backgrounds. An average score for the seven questions was calculated for each participant.
Typical age-related hearing loss was permitted as long as the four-frequency PTA was ≤ 45 dB HL in the better-hearing ear to ensure sufficient audibility of the target speech material. Other exclusion criteria included history of other neurological issues (e.g., major stroke and Parkinson’s) and being a nonnative English speaker. All participants signed a consent form approved by the University of Massachusetts Amherst Institutional Review Board and were paid for their participation. All participants with a diagnosis of MCI or dementia also completed a capacity-to-consent assessment.
Cognitive Measures
All participants completed the Memory Orientation Screening Test (MOST) as a cognitive screening measure to ensure that the controls were cognitively normal and to have a single metric by which to compare the MCI/dementia participants who may have been diagnosed by different providers. The MOST is an iPad-administered cognitive screener that has a dementia cutoff score of 18 out of 29 points (Clionsky & Clionsky, 2010, 2014). It also categorizes possible MCI, depending on age and years of education, for scores between 19 and 23 points. In addition, most participants (n = 36) also completed a Montreal Cognitive Assessment (MoCA) screener (Nasreddine et al., 2005) to compare the consistency of the two measures in their identification of potential cognitive impairment. Ten of the 31 participants without a diagnosis of cognitive impairment did not score in the normal range on either cognitive screening measure; these participants were categorized as “possible MCI” in the group comparison analyses. To be included in the “neurologically healthy control” group (n = 10), a passing score was required on both screening measures. 1 In addition, all participants completed a working memory task (Size Comparison Span [SICSPAN]; Sörqvist et al., 2010). The SICSPAN is a nonauditory task that asks participants to remember a list of words that are presented on the computer screen between a series of yes/no questions to which they must respond. There are 10 trials, and the number of questions to respond to and the number of words to remember in each trial range from 2 to 9. At the end of each trial, the participant must state aloud what words they remember (from those that were shown on the screen between yes/no questions), and the maximum score is 40 correct responses.
Stimuli
The target and masking sentences used in this study were from the TVM-Colors corpus, which was developed with the purpose of testing speech perception in the presence of competing speech maskers (Helfer et al., 2016). Importantly, the sentences in this corpus are brief and have simple syntactic structure to minimize the complexity of the cognitive processing required; however, they do not have semantic meaning, which increases the difficulty of the task. An example of a target sentence is as follows: “Michael found the blue towel and the evil rule here.” with key words used for scoring underlined. Sentences were presented in quiet and in the presence of two masking conditions: (a) speech-envelope-modulated (SEM) noise and (b) two-talker, same-sex competing speech. The SEM noise was created by filtering wideband noise with the envelope of a sample of two-talker speech utterances. All testing was done in the sound field with target speech at 0° azimuth and competing signals presented from each side at ±60° azimuth. When the masker was speech, one competing sentence was presented from the right loudspeaker and the other from the left loudspeaker. The competing speech sentences were looped such that each of the masker sentences began at a random point in the sentence and then the beginning of the sentence was appended to the end. The speakers were located 1.3 m from the seated listener. The target sentence was presented at a fixed conversational level (65 dBA), and three SNR conditions were tested: −4, 0, and +4 dB SNRs. The SNRs were chosen based on previous research showing that these SNRs lead to an appropriate range of performance levels in cognitively healthy older adults.
Procedure
All testing was performed in an IAC sound-treated room. Seven conditions (quiet + two masker types × three SNRs) were presented in randomized blocks with 19 sentences per block. Prior to beginning the first condition, the listener heard four sentences in the presence of the two-talker masker at +10 dB SNR for practice. The first three sentences in each block were treated as practice trials and were excluded from scoring in order for the listener to adjust to the new presentation condition. 2 Scoring was based on five key words per sentence and was calculated as percent correct out of 80 key words per condition. Researchers scored the stimuli off-line using audio-recorded responses.
The listener was told to repeat aloud whatever they heard from the loudspeaker directly in front of them. They were oriented to the fact that the sentence structure was always “<Name> found the <color> <noun> and the <adjective> <noun> here.” They were also told that the name would always be either Michael, Victor, or Theo. The listeners were encouraged to guess whenever possible. When the listener finished stating what they heard, they pushed a button to advance to the next sentence. There was a brief break between each block, and listeners were offered the chance to take a break and leave the sound booth after every other block or per request.
Results
Results will be presented in two stages. The first analysis (n = 28) compared the performance of three age- and hearing status matched groups based on cognitive status. The second analysis considered the data from all listeners (n = 39) and used a measure of working memory as a continuous variable to explain variance in speech perception performance. Analyses were performed in STATA 16.1 (StataCorp, LLC) and R Version 3.6.1. The descriptive statistics were completed using STATA, and the analyses of variance (ANOVAs) for the experimental conditions were completed in R using the lme4 package for linear mixed-effects models (Bates et al., 2015).
Participant Characteristics
The full sample included 39 adults aged 55–77 years old (M age = 67.3, SD = 6.2). The overall range of hearing thresholds based on a four-frequency (0.5–4 kHz) better-hearing ear PTA was −1.25 to 42.5 dB HL (M = 14.8, SD = 10.6). The range of scores on the SSQ was 1–9.3 (M = 6.5, SD = 1.8). The SSQ scores and PTA were negatively correlated, r(37) = −.36, p = .02. The range of scores on the cognitive screener tests was 15–27 points out of 29 on the MOST (M = 22.6, SD = 2.9) and 16–30 out of 30 on the MoCA (n = 36; M = 25.8, SD = 3.4). The scores on the MOST and the MoCA were positively correlated, r(34) = .39, p = .02. Finally, the range of scores on the SICSPAN working memory task was 9–37 out of 40 (M = 24.8, SD = 7.1), and the SICSPAN scores were positively correlated with performance on both cognitive screeners, r MOST(37) = .50, p = .001 and r MoCA(34) = .38, p = .02.
Between-Groups Comparisons for Speech Perception
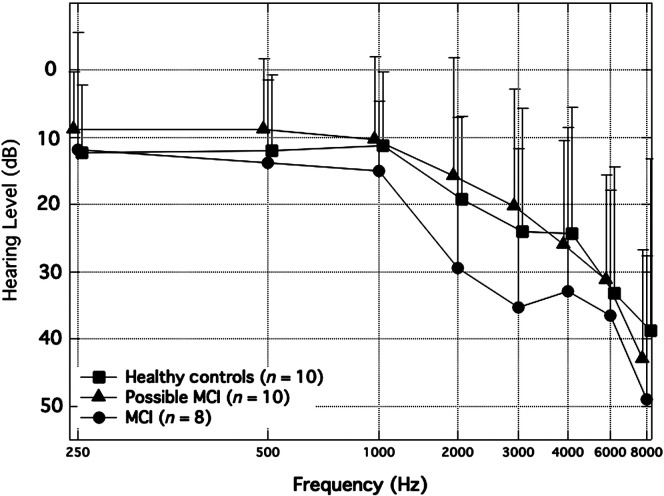
Participants were split into three groups for this analysis: healthy controls (n = 10), possible MCI (n = 10; individuals who failed two cognitive screeners), and MCI/dementia (n = 8; individuals who had a clinician diagnosis of MCI or mild dementia). 3 Group mean audiograms are shown in Figure 1. Group comparisons of demographic characteristics (see Table 1) were analyzed using an ANOVA with the Scheffe test for multiple comparisons. There were no differences between groups for age, F(2, 25) = 1.00, p = .38; PTA, F(2, 25) = 1.20, p = .32; or mean SSQ score, F(2, 25) = 1.99, p = .16. In addition, an ANOVA including hearing thresholds at all test frequencies showed significant main effects of group, F(2, 23) = 4.64, p = .01, and test frequency, F(7, 23) = 11.72, p < .001, and no Group × Frequency interaction, F(14, 23) = 0.27, p = 1.00. Tukey's honestly significant difference (HSD) pairwise comparisons of the main effect of group show significant differences between the MCI/dementia and possible MCI groups for hearing thresholds (mean difference = 8.45, HSD test = 4.39, p < .05) and no group differences between the MCI/dementia and healthy controls groups (mean difference = 4.52, HSD test = 2.35, p > .05) or between the healthy controls and possible MCI groups (mean difference = 3.94, HSD test = 2.05, p > .05). In addition, the expected pattern of worse hearing thresholds with an increase in the test frequency is consistent with typical age-related hearing loss present in the sample. Finally, there were no group differences in the SICSPAN scores, F(2, 25) = 2.64, p = .09, with the MCI/dementia group showing a trend toward lower scores (mean difference MCI/dementia – healthy controls = −7.8, p = .09).
Figure 1.
Averaged audiograms are shown for each participant group: healthy controls, possible mild cognitive impairment (MCI), and MCI/dementia. Thresholds are collapsed across both ears. Error bars represent 1 SD. Datapoints are offset to visualize error bars for all three groups.
Table 1.
Participant demographics and performance on the speech perception task.
| Variable | Total (n = 39) | Healthy controls (n = 10) | Possible MCI (n = 10) | MCI or mild dementia (n = 8) |
|---|---|---|---|---|
| Age | 67.3 (6.2) | 66.6 (7.3) | 67.6 (6.4) | 70.8 (4.8) |
| PTA | 14.8 (10.6) | 15.9 (12.0) | 11.9 (9.4) | 20.3 (13.2) |
| SSQ | 6.5 (1.8) | 7.3 (1.4) | 6.1 (2.5) | 5.5 (1.7) |
| SICSPAN | 24.8 (7.1) | 28.3 (6.6) | 25.1 (7.0) | 20.5 (8.1) |
| MOST | 22.6 (2.9) | 25.1 (1.3) | 21.6 (1.6) * | 20.3 (4.6) ** |
| MoCAa | 25.8 (3.4) | 27.9 (1.4) | 23.3 (2.7) ** | 24.8 (4.4) |
|
Condition |
Performance on speech perception task |
|||
| Quiet | 91.4 (10.6) | 94.9 (6.6) | 94.0 (5.3) | 80 (17.6) * |
| SEM noise | ** | |||
| −4 dB SNR | 62.8 (16.9) | 68.3 (18.7) | 61.6 (14.1) | 53.0 (23.1) |
| 0 dB SNR | 79.1 (15.0) | 84.3 (14.5) | 79.0 (11.9) | 64.5 (18.6) |
| +4 dB SNR | 84.8 (13.2) | 86.3 (11.0) | 85.0 (8.9) | 73.3 (19.7) |
| Speech masker | *** | |||
| −4 dB SNR | 56.7 (22.4) | 63.3 (23.5) | 58.5 (18.5) | 35.6 (23.9) |
| 0 dB SNR | 69.4 (20.1) | 76.4 (19.1) | 71.3 (15.9) | 47.5 (22.4) |
| +4 dB SNR | 80.3 (17.0) | 85.3 (9.1) | 84.4 (16.7) | 59.4 (18.8) |
Note. Data are presented as mean (SD). For all measures except PTA, higher scores mean better performance. Significant differences are between the group indicated with the asterisk and the other bolded group(s). For the noise conditions, the MCI/dementia group had lower mean performance than the other two groups, and there was no Group × Noise Type × SNR interaction. MCI = mild cognitive impairment; PTA = pure-tone average threshold for octave frequencies of 500–4000 Hz in the better-hearing ear; SSQ = Speech, Spatial and Qualities of Hearing Scale, i.e., questionnaire (max score = 10); SICSPAN = Size Comparison Span, i.e., working memory task (max score = 40); MOST = Memory Orientation Screening Test, i.e., cognitive screener (max score = 29); MoCA = Montreal Cognitive Assessment, i.e., cognitive screener (max score = 30); SNR = signal-to-noise ratio.
MoCA data missing for three MCI/dementia participants.
p < .05.
p < .01.
p < .001.
In the quiet speech perception condition, using an ANOVA with the Scheffe test for multiple comparisons, there was a significant group difference, F(2, 25) = 5.34, p = .01, with the MCI/dementia group’s mean score significantly lower than that of the healthy controls group (mean difference = −14.9, p = .02) and of the possible MCI group (mean difference = −14, p = .03).
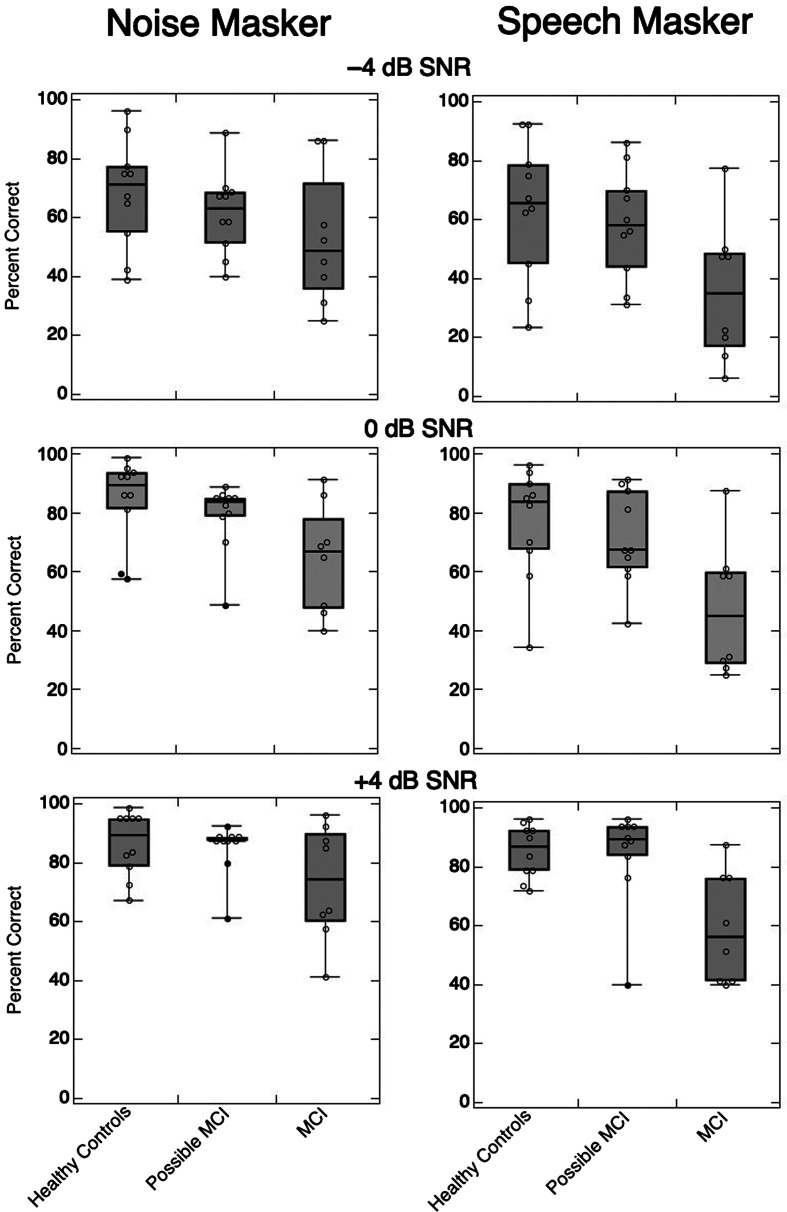
Results for all experimental conditions are displayed in Figure 2. Experimental conditions were analyzed using a split-plot ANOVA with three groups (healthy controls, possible MCI, and MCI) as the between-subjects factor and two noise types (SEM and two-talker masker) and three SNR conditions (+4, 0, and −4 dB SNRs) as the within-subject factors. Main effects of group, F(2, 25) = 4.8, p = .02; noise type, F(1, 125) = 35.6, p < .001; and SNR, F(2, 125) = 89.2, p < .001, were all significant. Observation of the main effects in the data (see Figure 2) show that the MCI/dementia group performed more poorly than the other two groups, participants across all groups performed more poorly in the two-talker masker than in the SEM noise, and lower SNR presentations resulted in poorer performance. Although there was a significant interaction of Group × Noise Type, F(2, 125) = 7.8, p < .001, none of the other interactions were significant (Group × SNR, F(4, 125) = 0.6, p = .6; Noise Type × SNR, F(2, 125) = 1.4, p = .2; and Group × Noise Type × SNR, F(4, 125) = 0.1, p = 1.0).
Figure 2.
Box and whisker plots for all speech-in-noise conditions per experimental group: healthy controls, possible mild cognitive impairment (MCI), and MCI/dementia. Max and min of the boxes represent the 75th and 25th quartiles, respectively; the midline of the box is the median. The max and min caps on the whiskers represent the max and min datapoints. Each individual datapoint is shown as an unfilled circle; filled circles represent outlier data (per Tukey’s method).
Based on the data pattern observed (see Figure 2) and the hypothesis that the MCI/dementia group would perform more poorly in the speech masker but not in the noise masker, post hoc comparisons were strategically undertaken. Specifically, the Scheffe contrast allows comparison of specific group means without undertaking an exhaustive pairwise comparison of all means (Scheffe, 1953). Post hoc analysis of group and noise types showed that the MCI/dementia group mean was significantly lower than the other two groups’ means in both the two-talker masker (Scheffe contrast = 25.71, F_Scheffe(1, 125) = 48.29, p < .001) and the SEM noise (Scheffe contrast = 13.84, F_Scheffe(1, 125) = 13.99, p < .01).
Given the interaction between group and noise type, a reasonable extension of the main hypothesis might be that the possible MCI group showed a pattern of performing similarly to the healthy controls group in the SEM noise but had poorer performance than expected in the two-talker masker. To test this comparison, another Scheffe contrast was undertaken comparing only the possible MCI group to the healthy controls group in the two different masker types. Neither of these contrasts was significant to the α = .05 level. Therefore, the possible MCI group did not differ significantly from the healthy controls group for either noise type.
Cognition Status as a Continuous Variable
A mixed-model ANOVA was undertaken with the full sample (n = 39) to examine the contributions of the independent variables of age, hearing thresholds, and cognitive function to variance in the dependent variable of speech perception performance. The analysis presented here used SICSPAN as the cognitive measure of interest because that test reflects a specific cognitive domain that has been found to be a significant contributor to performance on speech-in-noise tasks in previous literature. Furthermore, since age was not a significant contributor to variance in any experimental condition, it was removed from the model. With hearing threshold (PTA) and working memory capacity (SICSPAN) in the model, there was no effect of either variable for performance in quiet, F(2, 36) = 1.91, p = .16.
For the masker conditions, a mixed-model ANOVA analyzed performance as a function of noise type (SEM noise and two-talker masker), SNR (+4, 0, −4 dB SNRs), PTA, and SICSPAN scores for the full sample. The results showed the main effects of noise type, F(1, 190) = 36.0, p < .001; SNR, F(2, 190) = 139.6, p < .001; and PTA, F(1, 36) = 26.5, p < .001. Calculation of partial eta-squared values for the main effects shows that SNR explained the most variance in performance (12.5%) whereas noise type and PTA explained 1.6% and 1.2% of the variance in performance, respectively.
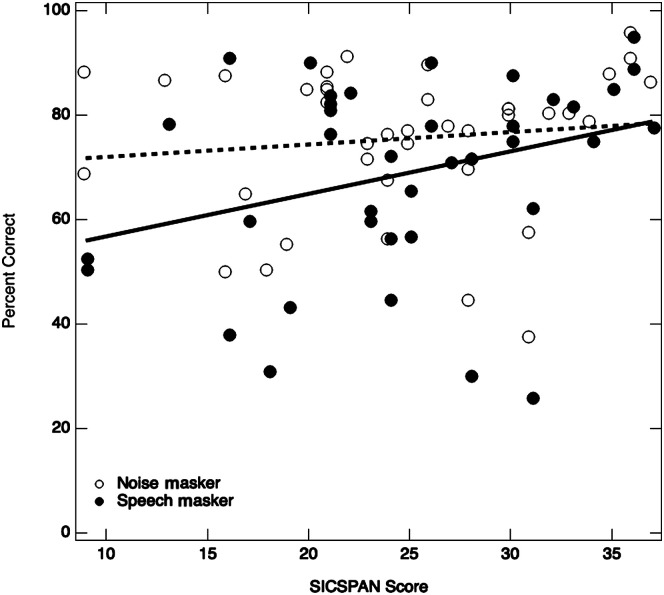
There was no observed main effect of working memory, F(1, 36) = 3.7, p = .06. A follow-up analysis examined the subset correlations of SICSPAN (working memory score) and speech performance in SEM noise, as well as SICSPAN and speech performance in the two-talker masker. Figure 3 displays an averaged value for each listener in the noise masker and in the speech masker (i.e., averaged across the three SNR conditions) as a function of SICSPAN score. There was no significant correlation between SICSPAN and performance in SEM noise, r(37) = .1, p = .46. There was moderate correlation between SICSPAN and performance in the two-talker masker, r(37) = .3, p = .05. Correlation lines are fit to the data in Figure 3. Upon further examination of the correlations at each SNR level for each noise type, only the −4 dB SNR condition for the two-talker masker showed a significant, moderate correlation, r(37) = .4, p = .03.
Figure 3.
Scatter plot data for all participants (n = 39) as a function of Size Comparison Span (SICSPAN) score. The averaged performance across all signal-to-noise ratio conditions is plotted for each participant in each noise type. The open circles show percent correct in the noise masker, and the solid circles show percent correct in the speech masker. The dotted line reflects the correlation between performance in the noise masker and SICSPAN score, and the solid line reflects the correlation between performance in the speech masker and SICSPAN score.
Discussion
The results presented here are consistent with the hypothesis that participants with a diagnosis of MCI or mild dementia would fare more poorly than their age- and hearing status–matched counterparts on measures of speech perception in the presence of background talkers. We also observed poorer performance in the MCI/dementia group in the SEM noise as compared to both the healthy controls group and the possible MCI group. Interestingly, the possible MCI group, an intermediate group of listeners who failed to pass either cognitive screener, did not differ in their speech performance from the healthy controls group that passed both cognitive screeners. In addition, for the full-sample mixed-model analysis with working memory (SICSPAN) as the cognitive domain of interest, audibility (i.e., better-ear PTA) was significant for all conditions (except in quiet) whereas working memory was only significant in the subset correlation between SICSPAN and speech performance in the two-talker masker, specifically in the hardest SNR condition.
Speech Understanding With MCI or Mild Dementia
While working memory capacity serves as a useful, single measure related to speech understanding in complex backgrounds, one’s cognitive function is a much more complex tapestry of multiple domains. In order to consider cognitive function as a complex interplay across multiple domains, we recruited participants who had received a diagnosis of MCI or mild dementia after undergoing a comprehensive diagnostic clinical battery. Consistent with our hypothesis, the MCI/dementia group performed more poorly in the competing speech background; however, in a departure from the hypothesis, they also performed more poorly in the SEM noise condition. It is noteworthy that the group mean differences between the MCI/dementia group, and the healthy controls and possible MCI groups were larger in the two-talker masker (27.5% and 23.9%, respectively) than in the SEM noise (16% and 11.6%, respectively). Another unexpected finding was that the MCI/dementia group achieved a mean performance of 80% for five key words per sentence in quiet (range: 50.0%–97.5%). This is likely due to limited memory for immediate recall of five words, with two of eight participants in this category having particularly low performance (50% and 59%). It should be noted that both of those poor performers in quiet had normal hearing thresholds with PTAs of 6.25 dB HL and 25 dB HL.
As we recruited age- and hearing status–matched healthy controls, we encountered numerous participants testing into a category of “typically associated with MCI” per the results of the MOST cognitive screener. In order to better understand this “gray zone” of performance for people who came to the research laboratory as healthy volunteers, we implemented a protocol of testing them with the MoCA as well because it has been used in many auditory studies (Dupuis et al., 2015; Edwards et al., 2017; Fausto et al., 2018; Goossens et al., 2017). Some participants were tested with the MoCA on a different day than completing the study; otherwise, the MoCA was administered at the end of the test session, whereas the MOST was completed relatively early in the session (after the screening audiogram). There were 11 participants who failed one but not both cognitive screeners; eight participants failed the MOST (with scores ranging from 19 to 23 out of 29), and three failed the MoCA (with scores ranging from 20 to 24 out of 30). It is beyond the scope of this study to try to interpret the varied performances on the two cognitive screeners, but it does raise important questions regarding the utility of completing a single cognitive screener as part of the audiologic evaluation, which has been suggested (Raymond et al., 2020; Shen et al., 2016). Perhaps a better approach to obtaining cognitive information that will be useful to the audiologist and patient as they endeavor to go through the rehabilitative process would be to use a domain-specific task that relates to speech-in-noise abilities, such as a working memory task, or to include a more cognitively taxing speech perception measure, such as an informational masking task. Typical speech-in-noise tests in the clinic are first and foremost predicted by audibility, but the presence of competing speech requires more cognitive processing.
Importantly, the group of possible MCI participants (based on failing both cognitive screeners) did not perform significantly differently than the healthy controls group. That said, their mean performance generally fell between the other two experimental groups in the two more difficult competing speech conditions (−4 and 0 dB SNRs). In addition, the possible MCI group performed significantly better than the MCI/dementia group in the SEM noise and the two-talker masker. This pattern continues to support the idea that providing persons with hearing loss a cognitively difficult speech understanding task, rather than a cognitive screener, will yield a more beneficial assessment of how their cognitive function may be expected to affect their speech communication.
All eight participants with a diagnosis of MCI/dementia had been assigned an age- and hearing status–matched participant. Taking a closer look at pairings demonstrates the types of patterns observed for the cognitively impaired participants. Table 2 shows exemplars of these three patterns. The first comparison reflects the hypothesized performance. The person with mild dementia scored nearly perfectly in quiet, achieved 52%–80% correct in the SEM noise, and achieved 13%–76% correct in competing speech (depending on the SNR condition). On the other hand, the matched control’s scores range from 77% to 95% in SEM noise and from 67% to 92% in competing speech. As such, the person with mild dementia performs more poorly across all SNRs and never returns to higher than 90% performance as the control participant does in the easiest SNR conditions for both maskers. The second pair demonstrates a pattern of responses in which the person with mild dementia was indeed greatly impacted by the competing speech masker (20%–41% correct performance), but somewhat unexpectedly, their best performance (in quiet) was only 58.75% correct. This was not expected based on their hearing thresholds (i.e., slight hearing loss), and their matched control achieved 100% correct in quiet and performance ranging from 75% to 95% across the competing speech conditions. Finally, we observe a matched pair with mildly elevated hearing thresholds. The MCI and matched control participants match reasonably well in quiet (77.5% vs. 82.8% correct, respectively), both reflecting the impact of elevated thresholds on the speech task. Unlike other control participants, the participant with mild hearing loss never approaches ceiling performance, even in the advantageous SNR conditions. That said, the MCI participant shows performance that is depressed beyond what would be expected based on age and audiogram to a certain degree in the SEM noise (31%–41%) and to a greater extent in the competing speech masker (6%–41%). Across all eight matched pairs, three MCI participants fell into the expected pattern, two performed worse than expected (even in quiet), and two showed increased effects of elevated thresholds on their speech performance in the masking conditions. One MCI participant performed nearly identically to their healthy control match in the competing speech conditions (79%–89% vs. 78%–88%).
Table 2.
Exemplar matched pairs (age- and hearing status–matched).
| Variable | Most expected pattern |
Reduced performance in quiet |
Mild hearing loss |
|||
|---|---|---|---|---|---|---|
| Cog impaired | Control | Cog impaired | Control | Cog impaired | Control | |
| Age | 69 | 67 | 63 | 63 | 75 | 74 |
| PTA | 6.25 | 8.75 | 25 | 27.5 | 33.5 | 38.75 |
| Education | Post-grad | College grad | Post-grad | Post-grad | Associate deg | Post-grad |
| MOST | 15 | 24 | 16 | 26 | 18 | 26 |
| SICSPAN | 24 | 32 | 18 | 30 | 31 | 19 |
| Diagnosis | Mild dementia/AD | — | Mild dementia | — | MCI | — |
|
Condition |
Speech performance (%) |
Speech performance (%) |
Speech performance (%) |
|||
| Quiet | 97.5 | 100 | 58.75 | 100 | 77.5 | 82.81 |
| SEM noise | ||||||
| −4 dB SNR | 52.5 | 77.5 | 45 | 75 | 31.25 | 39.06 |
| 0 dB SNR | 68.75 | 92.5 | 48.75 | 93.75 | 40 | 59.38 |
| +4 dB SNR | 85 | 95 | 57.5 | 95 | 41.25 | 67.19 |
| Speech masker | ||||||
| −4 dB SNR | 13.75 | 67.5 | 20 | 75 | 6.25 | 23.44 |
| 0 dB SNR | 61.25 | 86.25 | 31.25 | 85 | 30 | 34.38 |
| +4 dB SNR | 76.25 | 92.5 | 41.25 | 95 | 41.25 | 71.88 |
Note. Em dashes indicate no diagnosis. PTA = pure-tone average; MOST = Memory Orientation Screening; SICSPAN = Size Comparison Span; AD = Alzheimer’s disease; MCI = mild cognitive impairment; SEM = speech-envelope-modulated; SNR = signal-to-noise ratio.
The pattern of results in the current study is consistent with the literature on speech understanding in adults with cognitive impairment, which consistently finds poorer performance for speech-on-speech tasks as a function of cognitive status. Idrizbegovic et al. (2011) found equivalent performance between groups with AD, MCI, and subjective memory complaints (i.e., no diagnosis of memory impairment) for words in quiet and speech-weighted noise, but they found decreased performance for those with AD on a dichotic digits test. In addition, performance on the Synthetic Sentence Identification With Ipsilateral Competing Message (SSI-ICM) Test has been associated with attentional control and is decreased in persons with AD (Gates et al., 2011; Tuwaig et al., 2017). The SSI-ICM Test asks a listener to identify a target while an intelligible masker is presented to the same ear over headphones, which is similar to the binaural, sound-field competing speech task in the current study. In a prospective cohort of 274 participants who were dementia-free at baseline, the DSI task was highly predictive of a future dementia diagnosis for persons with a score of < 50% at baseline (Gates et al., 2011). The DSI task uses the same sentences as the SSI but presents one sentence to each ear and asks the listener to choose both sentences heard from a list of options. Gates et al. (2011) suggest that performance on dichotic speech tests may be a useful early indicator of AD.
Our current results are notably different from those reported in a recent study that has found group differences among healthy and probable MCI volunteers for speech understanding in the presence of competing speech (Edwards et al., 2017). They used the MoCA to separate the sample into probable MCI (≤ 25) or without MCI (> 25) groups to investigate measures of speech understanding (SSI-ICM and DSI) and auditory temporal processing (within-channel and between-channels gap detection and time-compressed speech). Edwards et al. found differences in both speech tasks and for within-channel gap detection. In contrast, none of our speech perception conditions elicited a group difference between the healthy controls and possible MCI groups, as defined by failing two cognitive screeners. When we compared our participants using the same grouping criterion as Edwards et al.’s (MoCA ≤ 25; n = 14 and MoCA > 25; n = 22), we only observe a group difference in the speech-in-quiet condition, which is likely due to the poor performance of two of our MCI/dementia participants as noted previously. These differences between studies could be due to differences in the experimental tasks, smaller sample size in the current study, and/or the fact that our sample was younger and had better hearing thresholds than the sample in Edwards et al.’s study.
Working Memory and Informational Masking
Working memory capacity has been associated with performance on speech perception tasks in complex, noisy backgrounds. This article provides an informative demonstration of the impact of working memory by including participants with varying degrees of cognitive function and by testing listeners in maskers that either do or do not produce informational masking. Many studies of the impact of cognition on speech understanding include only cognitively healthy volunteers; however, in our current sample of 39 participants, eight had a diagnosis of MCI or mild dementia and another 21 failed at least one brief cognitive screener.
Consistent with the conventional wisdom, PTA was highly associated with variance in performance across all the speech-in-noise conditions. In contrast, working memory capacity only trended toward significance in its contribution to variance in the presence of competing speech. This finding differs somewhat from the literature that supports working memory as a predictor of speech-in-noise performance. For example, a study with hearing aid users reported a significant partial correlation with working memory (Reading Span Test) after controlling for age and hearing thresholds in the presence of speech-shaped noise (Lunner, 2003). This could be due to different test paradigms (i.e., fixed SNRs in the current study vs. adaptive noise levels to yield 40% correct; use of a visual rather than an auditory measure of working memory), better hearing in the current sample, and/or the smaller sample size in the current study. In a recent study of older adults who were not hearing aid users, Heinrich and Knight (2016) have found significant correlations between speech understanding and performance on the Reading Span Test as well; although similar to the current data, the correlation was only significant when the listening situation became sufficiently difficult (i.e., low SNR). In the current study, the contribution of working memory capacity was significantly correlated with performance in the presence of the more difficult competing speech masker at the lowest SNR, whereas not significant for any of the SEM noise conditions.
Notably, there are multiple other cognitive domains that have been shown to contribute to speech understanding. Some examples, while not an exhaustive list, include the domains of language (e.g., word fluency and naming tests), memory (e.g., delayed recall and learning or logical memory tests), and executive function (e.g., processing speed and attention tasks; Mamo, Reed, et al., 2019). Working memory (which falls into the category of executive function) might be particularly relevant to competing speech backgrounds due to the challenge of selectively attending to the target in the presence of relevant distractions (i.e., other talkers). Rönnberg et al. (2013), in the Ease of Language Understanding model, argue that working memory capacity allows us to store relevant information, inhibit distractions, and selectively attend to a conversation. Furthermore, when the incoming target speech is degraded due to either age-related hearing loss or other auditory processing declines, the listener must rely more heavily on their working memory capacity. As such, when the listening task becomes sufficiently difficult (e.g., poor SNR and/or competing speech backgrounds), differences in working memory capacity among listeners lead to differences in speech understanding performance.
Clinical Implications
As demonstrated in this study and in the literature, persons with MCI or mild dementia may be having increased speech understanding difficulties in complex backgrounds—beyond that predicted by the audiogram. Our typical diagnostic procedures would not identify these challenges because conventional clinical speech perception measures use single words in quiet and sentences in multitalker babble, which yields primarily energetic masking. In order to provide more nuanced aural rehabilitation counseling and person-centered care, it may be worth including a more cognitively taxing speech perception task such as a competing speech paradigm. Notably, these already exist for use in the diagnostic battery for auditory processing evaluations such as DSI or SSI-ICM (Fifer et al., 1983; Humes et al., 2012; Speaks & Jerger, 1965).
It is also worth noting that many patients will not know and/or will not report their cognitive impairments. In addition, when persons are in the early stages of cognitive dysfunction, it is not typically apparent in a structured environment like an audiological evaluation because the impairment has more to do with functional changes in their everyday life. As such, without taxing their cognitive processing during the evaluation, providers will not recognize the need for more accommodations for many of these patients. Furthermore, a brief cognitive screener is unlikely to tell the provider what they need to know to customize their aural rehabilitation plan; however, considering a particular domain score like working memory might better help us predict performance. Multiple meta-analyses have found working memory to explain variance in speech-in-noise performance in older adults (after audibility; Dryden et al., 2017; Füllgrabe & Rosen, 2016), and future research should consider the usefulness of such measures, specifically in a clinical population.
Limitations
The small sample size in the current study is a limitation of these findings. It was difficult to recruit volunteers with a known diagnosis of MCI. The stigma and anxiety associated with the diagnosis is pervasive. However, continued efforts to engage with this clinical group is important as demonstrated by the increased speech understanding difficulties measured in this sample. Dementia care experts advocate for holistic approaches to optimizing health for persons with dementia (Austrom et al., 2018), and addressing hearing loss and communication support early in the disease process is likely to be beneficial.
Conclusions
In conclusion, older adults with a diagnosis of MCI or mild dementia performed more poorly than their cognitively healthy counterparts on a speech perception task in the presence of either modulated noise or competing speech. This pattern holds when the MCI/dementia group is compared to a group of age- and hearing-matched controls who passed two brief cognitive screeners as well as when compared to age- and hearing-matched controls who failed two brief cognitive screeners. Moreover, speech perception performance was correlated with working memory capacity in the presence of competing speech but not in the presence of modulated noise.
Beyond the need to improve our knowledge base related to the effects of cognitive decline on speech understanding, we need to learn more about what people with cognitive decline need to optimize communication. For example, persons with MCI or mild dementia might benefit from hearing and communication intervention being part of a holistic care plan upon diagnosis of cognitive impairment. Moreover, aural rehabilitation should be customized to address when one’s speech understanding is affected more by cognitive decline than by audibility problems. Future research should test unique rehabilitation protocols to match the needs of older adults experiencing hearing loss and cognitive impairment.
Acknowledgments
Research reported in this publication was supported by the National Institute on Deafness and Other Communication Disorders under Awards K23DC016855 and R01DC012057. The authors gratefully acknowledge Daniel Barch for statistical consulting support. They would like to thank Richard Freyman, Michael Rogers, Julia Read, Julia Serra, and Kara Wheeler for their assistance with the experimental procedures and data collected in this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding Statement
Research reported in this publication was supported by the National Institute on Deafness and Other Communication Disorders under Awards K23DC016855 and R01DC012057.
Footnotes
For the group comparison analyses, 11 participants were excluded due to the fact that they passed one but not both cognitive screeners. These participants were included in the analysis related to working memory.
The first three participants did not have practice sentences in each block.
As noted above, there were 11 participants who were excluded from the group analysis because they failed one but not both cognitive screeners, which made it difficult to determine if they should be in the healthy controls group or the possible MCI group.
References
- Akeroyd, M. A. (2008). Are individual differences in speech reception related to individual differences in cognitive ability? A survey of twenty experimental studies with normal and hearing-impaired adults. International Journal of Audiology, 47(Suppl. 2), S53–S71. https://doi.org/10.1080/14992020802301142 [DOI] [PubMed] [Google Scholar]
- Austrom, M. G. , Boustani, M. , & LaMantia, M. A. (2018). Ongoing medical management to maximize health and well-being for persons living with dementia. The Gerontologist, 58(Suppl. 1), S48–S57. https://doi.org/10.1093/geront/gnx147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. https://doi.org/10.18637/jss.v067.i01 [Google Scholar]
- Buss, E. , Hodge, S. E. , Calandruccio, L. , Leibold, L. J. , & Grose, J. H. (2019). Masked sentence recognition in children, young adults, and older adults: Age-dependent effects of semantic context and masker type. Ear and Hearing, 40(5), 1117–1126. https://doi.org/10.1097/aud.0000000000000692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clionsky, M. I. , & Clionsky, E. (2010). Development and validation of the Memory Orientation Screening Test (MOST): A better screening test for dementia. American Journal of Alzheimer’s Disease and Other Dementias, 25(8), 650–656. https://doi.org/10.1177/1533317510386216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clionsky, M. I. , & Clionsky, E. (2014). Psychometric equivalence of a paper-based and computerized (iPad) version of the Memory Orientation Screening Test (MOST®). The Clinical Neuropsychologist, 28(5), 747–755. https://doi.org/10.1080/13854046.2014.913686 [DOI] [PubMed] [Google Scholar]
- Committee on Hearing, Bioacoustics, and Biomechanics (CHABA). (1988). Speech understanding and aging. The Journal of the Acoustical Society of America, 83, 859–895. https://doi.org/10.1121/1.395965 [PubMed] [Google Scholar]
- Dryden, A. , Allen, H. A. , Henshaw, H. , & Heinrich, A. (2017). The association between cognitive performance and speech-in-noise perception for adult listeners: A systematic literature review and meta-analysis. Trends in Hearing, 21, 1–21. https://doi.org/10.1177/2331216517744675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubno, J. R. , Horwitz, A. R. , & Ahlstrom, J. B. (2002). Benefit of modulated maskers for speech recognition by younger and older adults with normal hearing. The Journal of the Acoustical Society of America, 111(6), 2897–2907. https://doi.org/10.1121/1.1480421 [DOI] [PubMed] [Google Scholar]
- Dubno, J. R. , Horwitz, A. R. , & Ahlstrom, J. B. (2003). Recovery from prior stimulation: Masking of speech by interrupted noise for younger and older adults with normal hearing. The Journal of the Acoustical Society of America, 113(4, Pt. 1), 2084–2094. https://doi.org/10.1121/1.1555611 [DOI] [PubMed] [Google Scholar]
- Dupuis, K. , Pichora-Fuller, M. K. , Chasteen, A. L. , Marchuk, V. , Singh, G. , & Smith, S. L. (2015). Effects of hearing and vision impairments on the Montreal Cognitive Assessment. Neuropsychology, Development, and Cognition Section B, Aging, Neuropsychology and Cognition, 22(4), 413–437. https://doi.org/10.1080/13825585.2014.968084 [DOI] [PubMed] [Google Scholar]
- Edwards, J. D. , Lister, J. J. , Elias, M. N. , Tetlow, A. M. , Sardina, A. L. , Sadeq, N. A. , Brandino, A. D. , & Harrison Bush, A. L. (2017). Auditory processing of older adults with probable mild cognitive impairment. Journal of Speech, Language, and Hearing Research, 60(5), 1427–1435. https://doi.org/10.1044/2016_JSLHR-H-16-0066 [DOI] [PubMed] [Google Scholar]
- Fausto, B. A. , Badana, A. N. S. , Arnold, M. L. , Lister, J. J. , & Edwards, J. D. (2018). Comparison of subjective and objective measures of hearing, auditory processing, and cognition among older adults with and without mild cognitive impairment. Journal of Speech, Language, and Hearing Research, 61(4), 945–956. https://doi.org/10.1044/2017_JSLHR-H-17-0263 [DOI] [PubMed] [Google Scholar]
- Fifer, R. C. , Jerger, J. F. , Berlin, C. I. , Tobey, E. A. , & Campbell, J. C. (1983). Development of a Dichotic Sentence Identification test for hearing-impaired adults. Ear and Hearing, 4(6), 300–305. https://doi.org/10.1097/00003446-198311000-00007 [DOI] [PubMed] [Google Scholar]
- Füllgrabe, C. , & Rosen, S. (2016). On the (un)importance of working memory in speech-in-noise processing for listeners with normal hearing thresholds. Frontiers in Psychology, 7, 1268. https://doi.org/10.3389/fpsyg.2016.01268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatehouse, S. , Naylor, G. , & Elberling, C. (2003). Benefits from hearing aids in relation to the interaction between the user and the environment. International Journal of Audiology, 42(Suppl. 1), 77–85. https://doi.org/10.3109/14992020309074627 [DOI] [PubMed] [Google Scholar]
- Gatehouse, S. , & Noble, W. (2004). The Speech, Spatial and Qualities of Hearing Scale (SSQ). International Journal of Audiology, 43(2), 85–99. https://doi.org/10.1080/14992020400050014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates, G. A. , Anderson, M. L. , Feeney, M. P. , McCurry, S. M. , & Larson, E. B. (2008). Central auditory dysfunction in older persons with memory impairment or Alzheimer dementia. Archives of Otolaryngology—Head & Neck Surgery, 134(7), 771–777. https://doi.org/10.1001/archotol.134.7.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates, G. A. , Anderson, M. L. , McCurry, S. M. , Feeney, M. P. , & Larson, E. B. (2011). Central auditory dysfunction as a harbinger of Alzheimer dementia. Archives of Otolaryngology—Head & Neck Surgery, 137(4), 390–395. https://doi.org/10.1001/archoto.2011.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford, R. H. , Bacon, S. P. , & Williams, E. J. (2007). An examination of speech recognition in a modulated background and of forward masking in younger and older listeners. Journal of Speech, Language, and Hearing Research, 50(4), 857–864. https://doi.org/10.1044/1092-4388(2007/060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens, T. , Vercammen, C. , Wouters, J. , & van Wieringen, A. (2017). Masked speech perception across the adult lifespan: Impact of age and hearing impairment. Hearing Research, 344, 109–124. https://doi.org/10.1016/j.heares.2016.11.004 [DOI] [PubMed] [Google Scholar]
- Gordon-Salant, S. , & Cole, S. S. (2016). Effects of age and working memory capacity on speech recognition performance in noise among listeners with normal hearing. Ear and Hearing, 37(5), 593–602. https://doi.org/10.1097/aud.0000000000000316 [DOI] [PubMed] [Google Scholar]
- Grose, J. H. , Mamo, S. K. , & Hall, J. W., III. (2009). Age effects in temporal envelope processing: Speech unmasking and auditory steady state responses. Ear and Hearing, 30(5), 568–575. https://doi.org/10.1097/AUD.0b013e3181ac128f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich, A. , & Knight, S. (2016). The contribution of auditory and cognitive factors to intelligibility of words and sentences in noise. Advances in Experimental Medicine and Biology, 894, 37–45. https://doi.org/10.1007/978-3-319-25474-6_5 [DOI] [PubMed] [Google Scholar]
- Helfer, K. S. , Chevalier, J. , & Freyman, R. L. (2010). Aging, spatial cues, and single- versus dual-task performance in competing speech perception. The Journal of the Acoustical Society of America, 128(6), 3625–3633. https://doi.org/10.1121/1.3502462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer, K. S. , & Freyman, R. L. (2008). Aging and speech-on-speech masking. Ear and Hearing, 29(1), 87–98. https://doi.org/10.1097/AUD.0b013e31815d638b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer, K. S. , & Freyman, R. L. (2014). Stimulus and listener factors affecting age-related changes in competing speech perception. The Journal of the Acoustical Society of America, 136(2), 748–759. https://doi.org/10.1121/1.4887463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer, K. S. , Merchant, G. R. , & Freyman, R. L. (2016). Aging and the effect of target-masker alignment. The Journal of the Acoustical Society of America, 140(5), 3844–3853. https://doi.org/10.1121/1.4967297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer, K. S. , Merchant, G. R. , & Wasiuk, P. A. (2017). Age-related changes in objective and subjective speech perception in complex listening environments. Journal of Speech, Language, and Hearing Research, 60(10), 3009–3018. https://doi.org/10.1044/2017_JSLHR-H-17-0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes, L. E. (1996). Speech understanding in the elderly. Journal of the American Academy of Audiology, 7(3), 161–167. [PubMed] [Google Scholar]
- Humes, L. E. (2007). The contributions of audibility and cognitive factors to the benefit provided by amplified speech to older adults. Journal of the American Academy of Audiology, 18(7), 590–603. https://doi.org/10.3766/jaaa.18.7.6 [DOI] [PubMed] [Google Scholar]
- Humes, L. E. (2013). Understanding the speech-understanding problems of older adults. American Journal of Audiology, 22(2), 303–305. https://doi.org/10.1044/1059-0889(2013/12-0066) [DOI] [PubMed] [Google Scholar]
- Humes, L. E. , & Dubno, J. R. (2010). Factors affecting speech understanding in older adults. In Gordon-Salant S., Frisina R. D., Popper A. N., & Fay R. R. (Eds.), The aging auditory system (pp. 211–258). Springer. https://doi.org/10.1007/978-1-4419-0993-0_8 [Google Scholar]
- Humes, L. E. , Dubno, J. R. , Gordon-Salant, S. , Lister, J. J. , Cacace, A. T. , Cruickshanks, K. J. , Gates, G. A. , Wilson, R. H. , & Wingfield, A. (2012). Central presbycusis: A review and evaluation of the evidence. Journal of the American Academy of Audiology, 23(8), 635–666. https://doi.org/10.3766/jaaa.23.8.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes, L. E. , Kidd, G. R. , & Lentz, J. J. (2013). Auditory and cognitive factors underlying individual differences in aided speech-understanding among older adults. Frontiers in Systems Neuroscience, 7, 55. https://doi.org/10.3389/fnsys.2013.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrizbegovic, E. , Hederstierna, C. , Dahlquist, M. , Kämpfe Nordström, C. , Jelic, V. , & Rosenhall, U. (2011). Central auditory function in early Alzheimer's disease and in mild cognitive impairment. Age and Ageing, 40(2), 249–254. https://doi.org/10.1093/ageing/afq168 [DOI] [PubMed] [Google Scholar]
- Lu, Z. , Daneman, M. , & Schneider, B. A. (2016). Does increasing the intelligibility of a competing sound source interfere more with speech comprehension in older adults than it does in younger adults. Attention, Perception, & Psychophysics, 78(8), 2655–2677. https://doi.org/10.3758/s13414-016-1193-5 [DOI] [PubMed] [Google Scholar]
- Lunner, T. (2003). Cognitive function in relation to hearing aid use. International Journal of Audiology, 42(Suppl. 1), S49–S58. https://doi.org/10.3109/14992020309074624 [DOI] [PubMed] [Google Scholar]
- Mamo, S. K. , Grose, J. H. , & Buss, E. (2019). Perceptual sensitivity to, and electrophysiological encoding of, a complex periodic signal: Effects of age. International Journal of Audiology, 58(7), 441–449. https://doi.org/10.1080/14992027.2019.1587179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo, S. K. , Reed, N. S. , Sharrett, A. R. , Albert, M. S. , Coresh, J. , Mosley, T. H. , Knopman, D. , Lin, F. R. , & Deal, J. A. (2019). Relationship between domain-specific cognitive function and speech-in-noise performance in older adults: The atherosclerosis risk in communities hearing pilot study. American Journal of Audiology, 28(4), 1006–1014. https://doi.org/10.1044/2019_aja-19-00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine, Z. S. , Phillips, N. A. , Bédirian, V. , Charbonneau, S. , Whitehead, V. , Collin, I. , Cummings, J. L. , & Chertkow, H. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller, M. K. , Schneider, B. A. , & Daneman, M. (1995). How young and old adults listen to and remember speech in noise. The Journal of the Acoustical Society of America, 97(1), 593–608. https://doi.org/10.1121/1.412282 [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller, M. K. , & Souza, P. E. (2003). Effects of aging on auditory processing of speech. International Journal of Audiology, 42(Suppl. 2), 2S11–2S16. https://doi.org/10.3109/14992020309074638 [PubMed] [Google Scholar]
- Rajan, R. , & Cainer, K. E. (2008). Ageing without hearing loss or cognitive impairment causes a decrease in speech intelligibility only in informational maskers. Neuroscience, 154(2), 784–795. https://doi.org/10.1016/j.neuroscience.2008.03.067 [DOI] [PubMed] [Google Scholar]
- Raymond, M. J. , Lee, A. C. , Schader, L. M. , Moore, R. H. , Raol, N. R. , & Vivas, E. X. (2020). Practices and perceptions of cognitive assessment for adults with age-related hearing loss. Laryngoscope Investigative Otolaryngology, 5(1), 137–144. https://doi.org/10.1002/lio2.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnberg, J. , Lunner, T. , Zekveld, A. , Sörqvist, P. , Danielsson, H. , Lyxell, B. , Dahlström, O. , Signoret, C. , Stenfelt, S. , Pichora-Fuller, M. K. , & Rudner, M. (2013). The Ease of Language Understanding (ELU) model: Theoretical, empirical, and clinical advances. Frontiers in Systems Neuroscience, 7, 31. https://doi.org/10.3389/fnsys.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffe, H. (1953). A method for judging all contrasts in the analysis of variance. Biometrika, 40(1–2), 87–104. https://doi.org/10.2307/2333100 [Google Scholar]
- Schneider, B. A. , Daneman, M. , & Murphy, D. R. (2005). Speech comprehension difficulties in older adults: Cognitive slowing or age-related changes in hearing. Psychology and Aging, 20(2), 261–271. https://doi.org/10.1037/0882-7974.20.2.261 [DOI] [PubMed] [Google Scholar]
- Shen, J. , Anderson, M. C. , Arehart, K. H. , & Souza, P. E. (2016). Using cognitive screening tests in audiology. American Journal of Audiology, 25(4), 319–331. https://doi.org/10.1044/2016_AJA-16-0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G. E. , & Bondi, M. W. (2013). Mild cognitive impairment and dementia : Definitions, diagnosis, and treatment [Electronic Resource] . Oxford University Press. http://silk.library.umass.edu/login?url=https://search.ebscohost.com/login.aspx?direct=true&db=cat06087a&AN=umass.017492181&site=eds-live&scope=site, https://ebookcentral.proquest.com/lib/uma/detail.action?docID=1480990 [Google Scholar]
- Sommers, M. S. , & Phelps, D. (2016). Listening effort in younger and older adults: A comparison of auditory-only and auditory-visual presentations. Ear and Hearing, 37(Suppl. 1), 62S, 68S. https://doi.org/10.1097/aud.0000000000000322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza, P. E. (2000). Older listeners' use of temporal cues altered by compression amplification. Journal of Speech, Language, and Hearing Research, 43(3), 661–674. https://doi.org/10.1044/jslhr.4303.661 [DOI] [PubMed] [Google Scholar]
- Souza, P. E. , & Turner, C. W. (1994). Masking of speech in young and elderly listeners with hearing loss. Journal of Speech and Hearing Research, 37(3), 655–661. https://doi.org/10.1044/jshr.3703.655 [DOI] [PubMed] [Google Scholar]
- Sörqvist, P. , Ljungberg, J. K. , & Ljung, R. (2010). A sub-process view of working memory capacity: Evidence from effects of speech on prose memory. Memory, 18(3), 310–326. https://doi.org/10.1080/09658211003601530 [DOI] [PubMed] [Google Scholar]
- Speaks, C. , & Jerger, J. (1965). Method for measurement of speech identification. Journal of Speech and Hearing Research, 8(2), 185–194. https://doi.org/10.1044/jshr.0802.185 [Google Scholar]
- Strouse, A. , Ashmead, D. H. , Ohde, R. N. , & Grantham, D. W. (1998). Temporal processing in the aging auditory system. The Journal of the Acoustical Society of America, 104(4), 2385–2399. https://doi.org/10.1121/1.423748 [DOI] [PubMed] [Google Scholar]
- Tuwaig, M. , Savard, M. , Jutras, B. , Poirier, J. , Collins, D. L. , Rosa-Neto, P. , Fontaine, D. , Breitner, J. C. S. , & PREVENT-AD Research Group. (2017). Deficit in central auditory processing as a biomarker of pre-clinical Alzheimer's disease. Journal of Alzheimer's Disease, 60(4), 1589–1600. https://doi.org/10.3233/JAD-170545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan, N. , Storzbach, D. , & Furukawa, I. (2008). Investigation of potential cognitive tests for use with older adults in audiology clinics. Journal of the American Academy of Audiology, 19(7), 533–541. https://doi.org/10.3766/jaaa.19.7.2 [DOI] [PubMed] [Google Scholar]