Abstract
Purpose
Hyperacusis is a complex and poorly understood auditory disorder characterized by decreased tolerance to sound at levels that would not trouble most individuals. Recently, it has been suggested that individuals who experience otalgia in response to everyday sounds (termed pain hyperacusis) may differ clinically from those whose primary symptom is the perception of everyday sounds as excessively loud (termed loudness hyperacusis). Despite this theoretical distinction, there have been no empirical studies directly comparing these two populations of hyperacusis patients.
Method
Using data from a multinational patient registry (the Coordination of Rare Diseases at Sanford Registry), we examined self-reported demographics, symptoms, comorbidity, and response to treatment in a sample of 243 adults with hyperacusis, 152 of whom were classified as having pain hyperacusis based on reported symptoms. Bayesian statistical tests were used to investigate both the presence and absence of group differences between patients with loudness and pain hyperacusis.
Results
Individuals with pain hyperacusis presented with a more severe clinical phenotype, reporting a higher frequency of temporary symptom exacerbations (i.e., “setbacks”), less perceived symptom improvement over time, more severe comorbid headache disorders, and reduced benefit from sound therapy. However, the two hypothesized hyperacusis subtypes exhibited more similarities than differences, with the majority of symptoms and comorbidities being equally prevalent across groups. Multiple comorbidities were commonly observed, including tinnitus, primary headache disorders, psychiatric disorders, and functional somatic syndromes. Intolerance of sensory stimuli in other modalities was also frequently reported.
Conclusion
Although this study provides little evidence that loudness and pain hyperacusis are pathophysiologically distinct conditions, our findings indicate that a pain-predominant phenotype may be a meaningful prognostic marker in patients with hyperacusis.
Hyperacusis is a hearing disorder characterized by decreased tolerance to sound at levels that would not trouble most people (Fackrell et al., 2019). Individuals experiencing hyperacusis report that everyday sounds are perceived as excessively loud, intense, frightening, overwhelming, or physically painful, and can trigger additional physical symptoms, leading to significant distress and functional impairment (Ke et al., 2020; Tyler et al., 2014). This condition is distinct from misophonia (an aversive reaction to specific “trigger” sounds characterized by anger, extreme annoyance, and disgust) and phonophobia (a persistent, abnormal, and unwarranted fear of certain sounds), although these other forms of decreased sound tolerance can co-occur with hyperacusis (Jastreboff & Jastreboff, 2015). Although prevalence estimates of hyperacusis are varied and highly method dependent, an estimated 5.9% of U.S. adults report problems tolerating everyday sounds based on data from the 2014 National Health Interview Survey (Zelaya et al., 2015), and self-reported sound intolerance was endorsed by 9% of adults in a large Swedish study (Paulin et al., 2016). Hyperacusis is also associated with a range of other disorders, including tinnitus, hearing impairment, traumatic brain injury, migraine, anxiety disorders, mood disorders, autism spectrum disorder, and functional somatic syndromes such as fibromyalgia and irritable bowel syndrome (Assi et al., 2018; Cederroth et al., 2020; Paulin et al., 2016; Sheldrake et al., 2015; Williams, He, et al., 2021; Williams, Suzman, & Woynaroski, 2021). At this time, research on hyperacusis is still in its infancy; the etiology, pathology, and natural history of this condition remain poorly understood (Baguley & Hoare, 2018; Pienkowski et al., 2014; Tyler et al., 2014), and no evidence-based recommendations currently exist to guide its diagnosis or treatment.
One recent approach to the study of hyperacusis has been dividing the condition into subtypes based on the specific aversive reactions to sounds that patients experience. In their seminal review, Tyler et al. (2014) proposed that hyperacusis could be separated into four subtypes: loudness hyperacusis, pain hyperacusis, annoyance hyperacusis, and fear hyperacusis, occurring either separately or in combination. Though many researchers consider misophonia (i.e., Tyler's “annoyance hyperacusis”) and phonophobia (i.e., Tyler's “fear hyperacusis”) to be separate conditions from hyperacusis (Fackrell et al., 2019; Fagelson & Baguley, 2018; Jastreboff & Jastreboff, 2015), the more narrowly defined hyperacusis can still be subdivided into loudness and pain subtypes. For individuals with loudness hyperacusis, the threshold for loudness discomfort is reduced, and sounds of moderate intensity are judged to be very loud (Phillips & Carr, 1998). These symptoms are associated with disturbances of loudness perception, as evidenced by excessive growth of perceived loudness as a function of sound intensity (Brandy & Lynn, 1995; Hébert et al., 2013; Noreña & Chéry-Croze, 2007). Pain hyperacusis is a less well-understood form of the condition in which an individual perceives physical pain in the ear when exposed to certain sounds at levels far below those needed to cause pain in a typical listener (i.e., approximately 120 dB SPL). The character of the pain is variable across individuals (e.g., dull ache, burning, sharp, stabbing, or throbbing), and patients often report experiencing transient symptom exacerbations in response to certain sounds, colloquially referred to as “setbacks” (Pollard, 2019). Although the pathophysiology of pain hyperacusis is still poorly understood, recent discoveries have implicated the population of Type II cochlear afferent neurons in the perception of noxious or painful auditory stimuli (Flores et al., 2015; Liu et al., 2015; Wu et al., 2018). Although speculative, researchers have hypothesized that increased sensitivity or inappropriate activation of this population of Type II afferents may mediate the symptoms of pain hyperacusis (Auerbach, 2019; Noreña et al., 2018). To date, there has been very little empirical research characterizing the clinical phenotype of pain hyperacusis, and it remains unclear whether pain hyperacusis can be meaningfully distinguished from loudness hyperacusis in terms of clinical severity, natural history, associated symptoms, comorbidity, or response to treatment.
Patient self-report is increasingly recognized as an important modality for assessing the impact of disease states and generating novel questions for investigation (Acquadro et al., 2003; Weldring & Smith, 2013). These data are particularly informative when investigating disorders such as hyperacusis that are predominantly characterized by subjective symptoms, and large-scale patient surveys have provided useful insights into several similar conditions (Bennett et al., 2007; Chu et al., 2018; Kanazawa et al., 2016; Rouw & Erfanian, 2018). Leveraging an existing database of patient survey responses (the Coordination of Rare Diseases at Sanford [CoRDS] Registry; Trudeau, 2013), the current study was designed to provide an in-depth clinical description of the hyperacusis phenotype, with the specific goal of examining meaningful differences between loudness and pain subtypes of the condition. The major aims of this study were to compare individuals with loudness and pain hyperacusis with respect to (a) demographics, background, and onset of hyperacusis; (b) quality of life (QoL) and functional impairment; (c) specific symptoms attributed to hyperacusis; (d) clinical course and symptom fluctuation (including the presence and severity of “setbacks”); (e) comorbid conditions; (f) comorbid nonauditory sensory symptoms; and (g) perceived benefit of interventions such as medication and sound therapy.
Method
Study Sample and Data Source
Data for the current study were drawn from a multinational hyperacusis patient registry (hereafter the “CoRDS Registry”) established in 2015 by the nonprofit organization Hyperacusis Research Limited, Inc. in partnership with Sanford Research (Pollard, 2019; Trudeau, 2013). Anonymized data from this repository are available from Sanford Research upon request (see Availability of Data and Materials section for additional details). For the current study, data were obtained from the CoRDS Registry in October of 2020. The study protocol for the secondary analysis of these data was approved by the institutional review board of Vanderbilt University Medical Center.
At the time of data access, a total of 456 individuals had provided data for this registry, including both adult patients with self-reported hyperacusis and parents or caregivers of minors with hyperacusis. For the current study, we limited our scope to self-reporting adults, given the subjective nature of hyperacusis symptoms and the relatively small number of minors in the sample (n = 18 [3.9%]). Included patients were required to (a) self-report a diagnosis (including self-diagnosis) of hyperacusis in response to the question “Please list all rare disease diagnoses,” (b) report symptoms consistent with either loudness or pain hyperacusis (i.e., everyday sounds are perceived as overly loud and/or cause physical pain), and (c) provide an answer to the question, “In the past twelve (12) months, approximately how often has the Participant experienced pain in one or both ears?” (response options: “Never,” “Once a month,” “2–3 times per month,” “Once a week,” “Every day,” and “Continuously”), which we used to characterize individuals into the categories of loudness and pain hyperacusis along with free-text reports of hyperacusis symptomatology. A total of 249 adults answered the question regarding pain frequency, and of these, an additional six prospective participants were eliminated from the study due to either not reporting a diagnosis of hyperacusis or reporting no symptoms consistent with loudness or pain hyperacusis (i.e., some patients recorded only strong emotional reactions to sound [i.e., misophonia] or reactive tinnitus without endorsing either pain in the ears or the perception of everyday sounds as excessively loud). Thus, the final sample of participants analyzed in the current study comprised 243 unique individuals with hyperacusis.
Survey Questions
Participants completed two questionnaires, including a 79-item background survey completed by patients in all CoRDS registries and a 90-item hyperacusis-specific survey designed for this particular registry (full questionnaires available from CoRDS on request). Analyzed questions from the background survey included (a) demographic variables (age, sex, race, ethnicity, country of residence), (b) rare and non–rare disease diagnoses (free text), (c) symptoms attributed to hyperacusis (free text), (d) and five items assessing various facets of QoL (1. “In general, would the participant say his/her health is…?”; 2. “Does the participant's health now limit him/her in doing vigorous activities?”; 3. “How much did pain interfere with the participant's enjoyment of life?”; 4. “How often does the participant feel tired?”; 5. “The participant feels depressed….”). Participants responded to these QoL items using a 5-point Likert scale, with the first question rated from “Excellent” to “Poor” and the other four rated from Never to Always. Item scores were coded such that higher scores indicated higher QoL (i.e., a response of “Always” was scored as “1” and a response of “Never” was scored as “5”).
The hyperacusis-specific questionnaire was composed of four sections: (a) background and onset of hyperacusis (e.g., duration of hyperacusis, laterality, symptom change over time, hyperacusis risk factors), (b) symptoms (e.g., perception of everyday sounds as unbearably loud, pain character and frequency, associated symptoms such as pressure and ear fullness, setback frequency and duration, and degree of functional impairment from hyperacusis), (c) comorbid conditions (e.g., presence and characteristics of tinnitus, headache frequency and severity, additional medical conditions, presence and severity of sensory intolerance in other modalities), and (d) hyperacusis management and treatment (e.g., response to sound therapy, use of ear protection, perceived benefit from medications). Specific outcomes analyzed in the current study and the survey questions used to assess them are detailed in Table 1.
Table 1.
Outcomes of interest derived from closed-ended questions on the hyperacusis-specific CoRDS survey.
| Outcome | Data type | Source question | Response options | n Pain | n Loudness |
|---|---|---|---|---|---|
| Duration of hyperacusis | Ordinal | How long has the participant had hyperacusis? | Less than 1 year; 1–3 years; 4–6 years; 7–10 years; 11–14 years; 15+ years | 144 | 87 |
| Sudden onset | Binary (yes/no) | How did the onset of the condition start? | Suddenly = yes Gradually; uncertain = no |
143 | 85 |
| Bilateral symptoms | Binary (yes/no) | Which ear is affected by hyperacusis? | Both = yes; Left; right = no |
149 | 90 |
| Symmetric symptoms | Binary (yes/no) | Are both ears equally affected? | Yes; no | 150 | 89 |
| Professional diagnosis | Binary (yes/no) | How was the participant initially diagnosed? | Audiologist; ENT; medical doctor = yes Self; other = no |
141 | 86 |
| Pre-existing hearing loss | Binary (yes/no) | Does the participant have pre-existing hearing loss? | Yes; no | 152 | 88 |
| Wears hearing aids | Binary (yes/no) | Does the participant wear hearing aids? | Both ears; left; right; = yes No |
140 | 83 |
| Head injury | Binary (yes/no) | Has the participant ever had a serious head injury (from car accident, fall, etc.)? | Yes; no | 147 | 88 |
| Loud noise exposure | Binary (yes/no) | Does the participant have a history of loud noise exposures? | Yes No; uncertain = no |
150 | 89 |
| Traumatic noise/blast exposure | Binary (yes/no) | Has the participant had traumatic impulse noise exposures (blasts, gun fire, etc.)? | Yes; no | 151 | 90 |
| Delayed pain onset | Binary (yes/no) | When the participant experienced ear pain as a result of an event, how long after the event did the pain begin? | After a few hours; the next day; a few days later; weeks later = yes Immediately = No |
131 | 57 |
| Duration of pain | Ordinal | When the participant experiences ear pain from environmental sounds, how long does the pain last? | Less than 1 hr; 1–4 hr; 5–24 hr; several days; several weeks; several months; more than several months | 128 | 56 |
| Functional impairment: occupational | Ordinal | Rate the impact of the participant's hyperacusis on the participant's education/career. | 0–10 (10 indicates the participant's condition prevents them from going to school or working) | 142 | 88 |
| Functional impairment: domestic | Ordinal | Rate the impact of the participant's hyperacusis on the participant's home life. | 0–10 (10 indicates the participant's condition ruined their home life) | 147 | 89 |
| Functional impairment: social | Ordinal | Rate the impact of the participant's hyperacusis on the participant's social life. | 0–10 (10 indicates the participant's condition ruined their social life) | 145 | 89 |
| Symptom change over time | Categorical | Has the severity of the participant's hyperacusis changed over time? | Better; worse; fluctuated; same | 147 | 87 |
| Setback severity | Ordinal | When the participant experiences a setback, how does the participant's condition compare to earlier periods? | Small setback; moderately worse; worse than it ever was previously | 121 | 69 |
| Setback recovery time | Ordinal | How long does it take for the participant to recover from a setback? | Several days; several weeks; months; years | 119 | 70 |
| Recovery worsened by sounds | Binary (yes/no) | Does the participant's recovery take longer if they are exposed to a louder noise? | Yes; no | 120 | 73 |
| Tinnitus impairs sleep | Binary (yes/no) | Does the participant have difficulty sleeping due to their tinnitus? | Yes; no | 126 | 78 |
| Tinnitus impairs concentration | Binary (yes/no) | Does the participant have difficulty concentrating while reading in a quiet room due to their tinnitus? | Yes; no | 125 | 78 |
| Reactive tinnitus | Binary (yes/no) | Do exposure sounds raise the volume of the participant's tinnitus? | Yes; no | 122 | 77 |
| Reactive tinnitus duration | Ordinal | If the volume level of the participant's tinnitus increases, how long does the increase in volume last? | Minutes; hours; days; weeks; permanently | 117 | 68 |
| Headache frequency | Ordinal | How often does the participant experience headaches? | Never; rarely; monthly; weekly; daily | 143 | 88 |
| Headache severity | Ordinal | How severe are the participant's headaches? (10 being the most severe) | 1–10 | 124 | 70 |
| Headache worsened by sounds | Binary (yes/no) | Does the participant experience headaches more frequently after they have been exposed to loud noises? | Yes; no | 122 | 71 |
| Vertigo daily/weekly | Binary (yes/no) | How often does the participant experience balance problems (vertigo)? | Never; rarely; monthly = no Weekly; daily = yes |
144 | 86 |
| Vertigo severity | Ordinal | Rate the severity of the participant's balance issues (10 being the most severe). | 1–10 | 90 | 58 |
| Vertigo worsened by sound | Binary (yes/no) | Does the participant experience increased/more frequent balance issues after they are exposed to loud noises? | Yes; no | 91 | 57 |
| Photophobia daily/weekly | Ordinal | How often do bright lights bother the participant? | Never; rarely; monthly = no Weekly; daily = yes |
141 | 83 |
| Photophobia severity | Ordinal | How severely do bright lights bother the participant? (10 being the most severe) | 1–10 | 104 | 61 |
| Osmophobia present | Binary (yes/no) | Is the participant bothered by strong smells? | Yes; no | 140 | 81 |
| Tactile intolerance present | Binary (yes/no) | Is the participant bothered by touch? | Yes; no | 143 | 85 |
| Duration of sound therapy | Ordinal | How long has the participant followed/utilized sound therapy protocol? | Less than 1 year; 1–2 years; 3–4 years; 5–6 years; 7–8 years; 8+ years | 88 | 48 |
| Outcome of sound therapy | Ordinal | How much improvement has the participant experienced from following/utilizing sound therapy protocol? | Worsened hyperacusis; worsened tinnitus = worsened symptoms No improvement; minor improvement; some improvement; significant improvement; hyperacusis is almost eliminated |
91 | 49 |
Note. Additional outcomes examined were (a) derived from CoRDS background survey, (b) derived from free-text fields, or (c) derived from a combination of free-text and multiple-choice fields. Number of nonmissing responses determined both by voluntary skipping (e.g., providing no answer) and survey logic (e.g., severity of headache not rated by individuals not endorsing headaches); n Pain/n Loudness = number of patients in pain/loudness hyperacusis group providing nonmissing response to question. CoRDS = Coordination of Rare Diseases at Sanford.
Classification of Loudness and Pain Hyperacusis
As part of the hyperacusis-specific survey, participants answered the following question: “In the past twelve (12) months, approximately how often has the Participant experienced pain in one or both ears?” Notably, only 9.5% of our sample (n = 23) responded “Never” to this question, indicating that the majority of hyperacusis patients do endorse noise-induced pain at least some of the time. However, the frequency of pain episodes in the sample varied widely, with 24.7% of participants (n = 60) endorsing pain once per week or less, 45.7% (n = 111) endorsing pain daily, and 20.2% (n = 49) endorsing continuous pain in their ears. Thus, for an individual to be classified as having pain hyperacusis, we required that they report ear pain as occurring “Every day” or “Continuously” (n = 160 [65.8%]). Individuals reporting pain weekly or less were classified as having loudness hyperacusis. Participants who reported pain were also surveyed regarding the character of their pain (with response options of “Dull ache,” “Burning pain,” “Throbbing pain,” “Sharp pain,” “Stabbing pain,” and “Other”), and free-text explanations of those who marked the “Other” response were examined to determine whether the sensation being described was clearly indicative of pain. In cases where the free text unambiguously clarified that the sensation was not pain (e.g., the responses “a nervous panicky reaction” and “A buzzy distortion”), participants were removed from the pain hyperacusis group and classified as having solely loudness hyperacusis. This process resulted in eight individuals being reclassified as having loudness hyperacusis rather than pain hyperacusis. Thus, using this method, 152 individuals in our sample (62.6%) were classified as having pain hyperacusis, while 91 (37.4%) were classified as having loudness hyperacusis. Notably, those classified as having pain hyperacusis frequently reported excessive loudness perception, with over 90% of participants in the pain hyperacusis group reporting that they find everyday sounds to be “unbearably loud,” as evidenced by affirmative responses to the question, “Are everyday sounds unbearably loud to the Participant?”
Coding of Free-Text Responses
In addition to the multiple-choice questions in the background and hyperacusis-specific surveys, participants answered several open-ended questions to qualitatively describe their symptoms (e.g., “Please list symptoms of rare disease diagnosis” and “List any other primary medical conditions (disease/condition, autoimmune disorder, cancer, etc.) that affect the participant”). Open-ended questions were also included to elaborate on responses of “Other” provided by participants to questions like “What other types of physical sensations does the participant experience along with hyperacusis?” and “When the participant experiences ear pain from environmental sounds, what type of pain do they experience?”
In order to more fully characterize the clinical phenotypes of participants in this registry, we examined each participant's free-text responses, classifying them according to the additional sound-evoked symptoms they reported (e.g., ear fullness, jaw pain, hearing changes). These responses were categorized into binary variables that indicated whether a specific symptom was mentioned by a given patient. A similar procedure was undertaken for the questions regarding additional medical comorbidities and specific medications that patients found helpful for their hyperacusis symptoms. As the presence of certain comorbidities (e.g., temporomandibular joint disorders [TMJDs] and tinnitus) could be indicated in a multiple-choice question or a free-text field, we coded a given condition as present if it was endorsed by a patient in either location. Binary variables were created for any symptom category, comorbid condition, or medication class that was reported by 5% or more of the sample (n ≥ 12; see below paragraph for a full list). Notably, as only 84 patients provided a response to the question regarding medication benefit, we created categories for medication classes perceived as beneficial by eight or more individuals (i.e., approximately 10% of the individuals who responded to that question).
In the domain of symptoms, we categorized pain character into three nonexclusive categories based on the latent structure of the Short-form McGill Pain Questionnaire (Dworkin et al., 2009): (a) continuous pain (“throbbing,” “aching,” “gnawing,” “heavy,” or “tender”); (b) intermittent pain (“stabbing,” “sharp,” “shooting,” “splitting,” “electric-shock,” and “piercing”); and (c) neuropathic-like pain (“hot/burning,” “cold/freezing,” “pain caused by light touch”; note that, while paresthesias are also included in this section of the Short-form McGill Pain Questionnaire, we classified such sensations as associated symptoms rather than pain per se). Participants who described multiple types of pain in their descriptions were classified in multiple categories. For participants who described pain but did not respond to the multiple-choice item describing pain character or provide any descriptors, we considered responses to the pain character categories to be missing. With regard to ancillary symptoms, the following were reported frequently enough to warrant categorization: pain in face, jaw, and neck regions; ear fullness; ear pressure; scalp or head pressure; flu-like symptoms (e.g., fatigue, malaise); fluttering/middle ear myoclonus; hearing changes (e.g., distortion, saturation, autophony, temporary hearing loss); sounds such as clicking or popping; and paresthesia (defined broadly, including numbness, tingling, heat, cold, and vibrotactile sensations). Comorbidities were classified into the following categories: tinnitus, hearing loss, migraine, phonophobia (i.e., a specific phobia of sound, including pronounced sound-induced anxiety reactions), tension headache, chronic daily headache of any type, TMJD, bruxism, osteoarthritis, any anxiety disorder (including posttraumatic stress disorder, obsessive–compulsive disorder, and phonophobia), any unipolar depressive disorder, any psychiatric disorder (including misophonia; Jager et al., 2020; Schröder et al., 2013), and any functional somatic syndrome (defined as the following diagnoses: fibromyalgia, myalgic encephalomyelitis/chronic fatigue syndrome, chronic daily headache, irritable bowel syndrome, TMJD, multiple chemical sensitivity, functional neurological [conversion] disorder, interstitial cystitis/bladder pain syndrome, and whiplash-associated disorders). Lastly, we created binary variables to represent perceived benefit from three classes of medications: benzodiazepines, opioids, and gabapentinoids.
Statistical Analyses
In order to determine which aspects of the hyperacusis phenotype differed meaningfully between individuals classified as having loudness and pain hyperacusis, we compared values of each outcome of interest between the two groups in a Bayesian framework. When the outcome of interest was categorical (e.g., the presence or absence of tinnitus), we examined group differences using a Bayesian analogue of the Pearson chi-squared test (Gûnel & Dickey, 1974; Jamil et al., 2017). When the outcome of interest was a continuous variable (e.g., age), we examined mean differences using a Bayesian analogue of the Welch (unequal-variances) t test (Kruschke, 2013). Lastly, when the outcome of interest was ordinal (e.g., a Likert scale item), group differences were assessed using a Bayesian ordered probit regression model (Bürkner & Vuorre, 2019; Liddell & Kruschke, 2018), which assumes that a normally distributed continuous latent variable underlies each ordinal dependent variable. Additional details on the specifics of each model are presented later in this section.
Conducting group comparisons in a Bayesian hypothesis testing framework allowed us to quantify evidence both for or against a given null hypothesis using Bayes factors (Kass & Raftery, 1995; Ly et al., 2020; Wagenmakers et al., 2018). When considering an arbitrary null hypothesis (H 0) and a mutually exclusive alternative hypothesis (H 1), the Bayes factor (BF 10) is defined as the ratio of how likely the data are under H 1 divided by how likely the data are under H 0. Values of BF 10 greater than 3 are typically considered to provide substantial evidence for H 1 over H 0, and values of BF 10 less than 0.333 are typically considered to provide substantial evidence for H 0 over H 1 (Wagenmakers et al., 2011). Values of BF 10 between 0.333 and 3 are typically considered inconclusive, providing only “anecdotal” evidence for either H 0 or H 1. Thus, for every group comparison, results could be interpreted as (a) providing significant support for a true group difference on the outcome variable (BF 10 > 3), (b) providing significant support for the absence of a group difference on the outcome variable (BF 10 < 0.333), or (c) providing inconclusive evidence for or against a group difference on the outcome variable. With the use of Bayesian statistics, we are therefore able to characterize significant differences between groups as well as significant similarities, allowing for a more nuanced comparison of loudness and pain hyperacusis relative to more conventional, frequentist analytic approaches.
All statistical computations were performed in R (R Core Team, 2020). 1 Categorical variables were compared between groups using default Gûnel–Dickey Bayes factors for contingency tables (as implemented in the BayesFactor R package; Morey & Rouder, 2018) based on the independent multinomial sampling scheme (Gûnel & Dickey, 1974; Jamil et al., 2017). In this test, the number of individuals in each clinical group (pain hyperacusis and loudness hyperacusis) is treated as fixed, and cell counts are multinomially distributed within each row of the contingency Table. A Dirichlet prior with parameters a 1, …,a k = 1 is placed on the parameters of each multinomial distribution, and the analytically derived Bayes factor provides evidence for or against the null hypothesis of equivalent distributions between groups.
For 2 × 2 contingency tables, the Bayes factor for the independent multinomial sampling plan reduces to a test for the equality of two proportions (i.e., the odds ratio [OR] of endorsement is equal to 1). Thus, when groups were being compared on binary variables, we additionally calculated the OR along with its 95% highest-density credible interval (CrI) using 15,000 Monte Carlo samples from the joint posterior distribution of the model parameters. For comparison with a frequentist hypothesis test, group differences were deemed “statistically significant” when the full 95% CrI excluded OR = 1. Notably, as the null hypothesis of complete equality between groups is always false at the population level (Cohen, 1994), we further sought to test whether the OR was greater than an interval null hypothesis representing effects that are thought to be too small to be practically meaningful (Kirk, 1996). This interval, termed the region of practical equivalence (ROPE; Kruschke & Liddell, 2018), was defined as the interval OR = [0.905, 1.105] (i.e., values in which log(OR) is between −0.1 and 0.1). In order to test the null hypothesis that the population effect lies within the ROPE (i.e., the difference in proportions in the population is too small to be of practical importance), we calculated the ROPE Bayes factor (BF ROPE; Makowski et al., 2019), defined as the odds of the prior OR distribution falling within the ROPE divided by the odds of the posterior OR distribution falling within the ROPE. Like other Bayes factors, BF ROPE allows for the quantification of evidence for or against the interval null hypothesis and can be interpreted in the same manner as BF 10 (with H 1 being that the true parameter value falls outside of the ROPE). When BF ROPE provided substantial support in favor of the parameter value falling within the ROPE, we deemed the parameters “practically equivalent.” BF ROPE values were calculated using the R package bayestestR (Makowski et al., 2019).
When comparing continuous variables between groups, we utilized a Bayesian t test similar to that proposed by Kruschke (2013), albeit without data-dependent prior distributions. Dependent variables were standardized (M = 0, SD = 1) and fit to an unequal-variances t test model, with a Normal (0, 1) prior on regression coefficients (i.e., the intercept term and mean difference between groups), a Normal (0, 1) prior on log-transformed standard deviation parameters for each group, and a Gamma (2, 0.1) prior on ν, the degrees of freedom of the t-distribution. Model parameters were estimated via Markov chain Monte Carlo (MCMC) as implemented in the R package brms (Bürkner, 2017, 2018). Posterior distributions of the parameters were based on 40,000 postwarmup MCMC draws from five separate Markov chains. The primary parameter of interest was the standardized mean difference between groups (i.e., Cohen's d), which we summarized using the posterior median and 95% CrI. Group differences were deemed “statistically significant” when the full 95% CrI excluded zero. Tests of the point null hypothesis were also supplemented with a Bayes factor (BF 10) calculated using the Savage–Dickey Density Ratio (Dickey & Lientz, 1970; Wagenmakers et al., 2010). In order to determine whether d values were large enough to be practically meaningful, we further examined BF ROPE values to test the interval null hypothesis that the population value of d lies within the interval [−0.1, 0.1].
For ordinal variables, we fit an unequal-variances ordered probit regression model (Bürkner & Vuorre, 2019; Liddell & Kruschke, 2018) to the data, which assumes that the ordinal dependent variable represents a normally distributed latent variable. In these models, the standard deviation of the reference group (loudness hyperacusis) was set to 1 in order to set the scale of the latent variable. The other parameters estimated in the model were similar to those in the Bayesian t test (e.g., mean difference, standard deviation for pain group, Cohen's d effect size), with the addition of multiple intercept parameters needed to model the category thresholds of the dependent variable. For these models, we placed a Normal (0, 1) prior on the mean difference between groups, a Normal (0, 1) prior on the log-transformed standard deviation, and a Student-t 3(0, 2.5) prior on each of the threshold values. Model parameters were estimated in brms using the same MCMC procedure described for the continuous models. Again, d was the primary parameter of interest, and its value was tested against the point null by examining whether 0 fell within the 95% CrI (supplemented by BF 10 calculated using the Savage–Dickey method). Furthermore, practical significance was determined by examining BF ROPE values based on the interval null hypothesis that the population value of d lies within [−0.1, 0.1].
Results
Availability of Data and Material
Data from the current study are available from the Coordination of Rare Diseases at Sanford (CoRDS) Registry, organized by Sanford Research. Interested parties can apply to access the data at https://research.sanfordhealth.org/rare-disease-registry. Data generated by the authors of this study from the original CoRDS data (e.g., binary codes for qualitative responses) can be requested from the corresponding author if written permission for the author to share those data is obtained from Sanford Research.
Demographics, Background, and Onset of Hyperacusis
The study sample was composed of 243 patients with self-reported hyperacusis (152 pain hyperacusis, 91 loudness hyperacusis per our operational definitions) between the ages of 18 and 85 years (M ± SD age = 45.65 ± 15.75 years; 49.8% female). The majority of participants were located within the United States or Canada (n = 160 [66.9%]), and 95% of the sample identified as White. Most participants (72.6%) had been formally diagnosed with hyperacusis by a professional (audiologist: 26.9%, otolaryngologist: 33.5%, other physician: 10.1%, other medical professional: 2.2%). More detailed demographic information for the full sample and loudness/pain subsamples can be found in Table 2.
Table 2.
Participant demographics and clinical characteristics by hyperacusis subtype.
| Demographics and clinical characteristics | n Pain/n Loudness | Pain hyperacusis | Loudness hyperacusis | Full sample |
|---|---|---|---|---|
| Age (years) | 150/89 | 43.69 (15.58) | 48.96 (15.56) | 45.65 (15.75) |
| Sex | 145/87 | |||
| Male | 79 (54.5%) | 32 (36.8%) | 111 (47.8%) | |
| Female | 66 (45.5%) | 55 (63.2%) | 121 (52.2%) | |
| Location | 150/89 | |||
| United States/Canada | 102 (68.0%) | 58 (65.2%) | 160 (66.9%) | |
| Europe | 38 (25.3%) | 26 (29.%) | 64 (26.8%) | |
| Australia/New Zealand | 3 (2.0%) | 4 (4.5%) | 7 (2.9%) | |
| Other | 7 (4.7%) | 1 (1.1%) | 8 (3.3%) | |
| Professional diagnosis | 141/86 | 103 (73.0%) | 62 (72.0%) | 165 (72.7%) |
| Duration of hyperacusis | 144/87 | |||
| Less than 1 year | 28 (19.4%) | 15 (17.2%) | 43 (18.6%) | |
| 1–3 years | 35 (24.3%) | 16 (18.4%) | 51 (22.1%) | |
| 4–6 years | 28 (19.4%) | 13 (14.9%) | 41 (17.7%) | |
| 7–10 years | 16 (11.1%) | 18 (20.7%) | 34 (14.7%) | |
| 11–14 years | 9 (6.2%) | 7 (8.0%) | 16 (6.9%) | |
| 15 years or more | 28 (19.4%) | 18 (19.4%) | 46 (19.9%) | |
| Symptom trajectory | 147/87 | |||
| Improved | 11 (7.5%) | 18 (20.7%) | 29 (12.4%) | |
| Same | 20 (13.6%) | 9 (13.6%) | 29 (12.4%) | |
| Worsened | 75 (51.0%) | 30 (34.5%) | 105 (44.9%) | |
| Fluctuated | 41 (27.9%) | 30 (34.5%) | 71 (30.3%) | |
| Symptom laterality | 149/90 | |||
| Bilateral | 125 (83.9%) | 71 (78.9%) | 196 (82.0%) | |
| Unilateral – left | 11 (7.4%) | 11 (12.2%) | 22 (9.2%) | |
| Unilateral – right | 13 (8.7%) | 8 (8.9%) | 21 (8.8%) | |
| Hearing loss | 152/88 | 73 (48.0%) | 42 (47.7%) | 115 (47.9%) |
| Tinnitus | 152/91 | 129 (84.9%) | 79 (86.8%) | 200 (85.6%) |
| Functional impairment (0–10) | ||||
| Career | 142/88 | 7.67 (2.89) | 5.59 (3.40) | 6.87 (3.25) |
| Domestic | 147/89 | 7.15 (2.58) | 5.75 (2.79) | 6.62 (2.74) |
| Social | 145/89 | 8.52 (1.82) | 7.12 (2.73) | 7.99 (2.31) |
Note. Values for continuous variables (including 0–10 rating scales for impairment) are presented as mean (SD), while values for categorical/ordinal variables are presented as n (%); n Pain/n Loudness = number of patients in pain/loudness hyperacusis group providing nonmissing response to question.
Comparing demographics between the two groups, we found that patients with pain hyperacusis were on average 5.32 (95% CrI [1.16, 9.45]) years younger than patients with loudness hyperacusis (d = −0.345 [−0.615, −0.073], BF 10 = 6.82, BF ROPE = 4.58). Despite the age difference between patients with loudness and pain hyperacusis, the two groups did not meaningfully differ in duration of hyperacusis symptoms (d = −0.144 [−0.424, 0.122], BF 10 = 0.345, BF ROPE = 0.254). Sex ratio also differed between the groups, with approximate parity in the pain hyperacusis group and a predominance of women in the loudness hyperacusis group (pain: 54.5% male, loudness: 36.8% male; OR = 2.037 [1.193, 3.52], BF 10 = 5.07, BF ROPE = 2.97). Participants in each group were similarly likely to be located in the United States (pain: 64.0%, loudness: 61.8%; OR = 1.105 [0.639, 1.911], BF 10 = 0.170, BF ROPE = 0.095) and had approximately the same proportion of individuals with professionally diagnosed hyperacusis (pain: 73.0%, loudness: 72.0%; OR = 1.056 [0.587, 1.955], BF 10 = 0.154, BF ROPE = 0.104).
Considering the laterality of hyperacusis, the majority of patients in both groups experienced symptoms bilaterally (pain: 83.9%, loudness: 78.9%; OR = 1.393 [0.723, 2.739], BF 10 = 0.207, BF ROPE = 0.205). However, fewer patients indicated that both ears were affected equally, with practically equivalent proportions of individuals in each group endorsing asymmetric symptoms (pain: 48.0%, loudness: 51.6%; OR = 0.865 [0.525, 1.487], BF 10 = 0.193, BF ROPE = 0.103). Data were not reported regarding the laterality of asymmetric symptoms, although monaural hyperacusis was reported with approximately equal frequencies in either ear (right: n = 21, left: n = 22). With regard to common hyperacusis risk factors, the pain hyperacusis group reported significantly increased rates of loud noise exposure (pain: 52.6%, loudness: 39.3%; OR = 1.699 [1.022, 2.948]), although Bayes factors were inconclusive regarding the practical significance of this effect (BF 10 = 1.20, BF ROPE = 0.757). Conversely, practically equivalent proportions of individuals in each group reported a history of exposure to traumatic impulse noise exposures such as blasts and gun fire (pain: 36.4%, loudness: 31.1%; OR = 1.259 [0.743, 2.226], BF 10 = 0.221, BF ROPE = 0.138) and serious head injuries (pain: 25.2%, loudness: 20.5%; OR = 1.285 [0.681, 2.421], BF 10 = 0.196, BF ROPE = 0.155). A diagnosis of hearing loss preceded the hyperacusis diagnosis in approximately 20% of cases across groups (pain: 20.3%, loudness: 21.6%; OR = 0.918 [0.484, 1.745], BF 10 = 0.139, BF ROPE = 0.115), and a relatively small proportion of individuals in each group reported wearing hearing aids at the time of the survey (pain: 9.3%, loudness: 7.2%; OR = 1.255 [0.460, 3.295], BF 10 = 0.108, BF ROPE = 0.207). All but two participants reporting hearing aid use (both in the pain group) indicated that they wore hearing aids binaurally.
QoL and Functional Impairment
Confirmatory factor analysis of the five QoL items indicated that they did not form a unidimensional composite (Comparative Fit Index [CFIcML] = 0.913, Tucker-Lewis Index [TLIcML] = 0.826, Root Mean Square Error of Approximation [RMSEAcML] = 0.161; Savalei, 2020). Thus, scores were compared between groups on an item-by-item basis using ordered probit models. Comparing QoL Item 1 (general self-perceived health) between groups, patients with pain hyperacusis group reported numerically lower scores than patients with loudness hyperacusis, although scores were practically equivalent between groups (d = −0.132 [−0.415, 0.156], BF 10 = 0.317, BF ROPE = 0.231). Similarly, inconclusive results were observed regarding QoL Item 2 (vigorous activity limitation; d = −0.174 [−0.463, 0.114], BF 10 = 0.420, BF ROPE = 0.329). Perhaps unsurprisingly, large group differences were observed in QoL Item 3 (pain interference with enjoyment of life), with the pain hyperacusis group reporting much lower QoL due to pain interference (d = −1.104 [−1.440, −0.772], BF 10 = 6.25 × 105, BF ROPE = 4.37 × 105). Numerically lower scores in the pain group were also demonstrated on QoL Item 4 (tiredness), although results were inconclusive regarding whether a significant group difference was present (d = −0.205 [−0.509, 0.097], BF 10 = 0.553, BF ROPE = 0.455). On the final QoL item (depression), persons classified as having pain hyperacusis again reported lower numerical scores, but the ROPE Bayes factor indicated practical equivalence between groups (d = −0.175 [−0.462, 0.118], BF 10 = 0.417, BF ROPE = 0.329).
Three questions on the hyperacusis-specific survey asked respondents to rate the impact of hyperacusis on their occupational, domestic, and social functioning on an 11-point scale from 0 (no impact) to 10 (completely ruined by hyperacusis). Comparing these measures between groups, individuals with pain hyperacusis reported substantially more impairment in the occupational domain (d = 0.701 [0.425, 0.987], BF 10 = 2.94 × 103, BF ROPE = 1.93 × 103), as well as moderately more impairment in domestic functioning (d = 0.527 [0.261, 0.791], BF 10 = 377, BF ROPE = 161) and social functioning (d = 0.541 [0.247, 0.840], BF 10 = 111, BF ROPE = 70.1).
Symptoms Attributed to Hyperacusis
Pain in the “intermittent” category (e.g., “stabbing,” “sharp”) was by far the most commonly endorsed in both groups (pain: 63.8%, loudness: 29.3%), with individuals in the pain hyperacusis group substantially more likely to endorse this symptom (OR = 4.177 [2.328, 7.437], 4.82 × 104, BF ROPE = 1.26 × 103). Pain of the “continuous” type (e.g., “throbbing,” “dull ache”) was less frequent in both groups (pain: 36.2%, loudness: 19.5%) and similarly more prevalent in patients with pain hyperacusis (OR = 2.272 [1.219, 4.409], BF 10 = 5.07, BF ROPE = 3.84). However, this pattern was not replicated for the category of “neuropathic-like” pain, the prevalence of which was practically equivalent across the groups (pain: 25.9%, loudness: 18.6%; OR = 1.360 [0.661, 2.759], BF 10 = 0.197 BF ROPE = 0.188). Although the majority of individuals in both groups reported that sound-induced pain occurred immediately after hearing the sound, a sizable minority reported experiencing pain hours to days after the inciting event (pain: 15.2%, loudness [excluding individuals who reported no pain]: 36.2%; OR = 0.321 [0.153, 0.682], BF 10 = 12.5, BF ROPE = 15.1). Moreover, the two groups differed minimally in the duration of sound-induced pain (d = 0.188 [−0.151, 0.522], BF 10 = 0.443, BF ROPE = 0.362).
With regard to sound-evoked symptoms, a practically equivalent proportion of participants in each group endorsed scalp pressure, flu-like symptoms, fluttering/middle ear myoclonus, hearing changes, sounds such as clicking or popping, and paresthesia (all BF 10 < 0.33; see Table 3). Individuals with pain hyperacusis were significantly more likely to endorse facial pain (pain: 36.9%, loudness: 23.5%; OR = 1.868 [1.027, 3.446], BF 10 = 1.40, BF ROPE = 1.04), ear fullness (pain: 66.2%, loudness: 51.7%; OR = 1.824 [1.070, 3.131], BF 10 = 1.79, BF ROPE = 1.18), and ear pressure (pain: 64.0%, loudness: 47.7%; OR = 1.933 [1.151, 3.313], BF 10 = 3.26, BF ROPE = 1.95) than those with loudness hyperacusis, although Bayes factors were inconclusive regarding the practical significance of these differences. Numerically higher proportions of pain hyperacusis patients also endorsed both jaw pain (pain: 34.0%, loudness: 22.4%; OR = 1.760 [0.950, 3.246], BF 10 = 0.875, BF ROPE = 0.671) and neck pain (pain: 34.8%, loudness: 24.7%; OR = 1.601 [0.888, 2.952], BF 10 = 0.542, BF ROPE = 0.420), with Bayes factors providing inconclusive evidence regarding group differences in symptom endorsement.
Table 3.
Sound-evoked symptom frequencies by hyperacusis subtype.
| Symptom | n Pain/n Loudness | Pain hyperacusis | Loudness hyperacusis | OR (95% CrI) | BF 10 | BF ROPE |
|---|---|---|---|---|---|---|
| Ear pain: intermittent | 141/82 | 90 (63.8%) | 24 (29.3%) | 4.177 [2.328, 7.437] | 4.82 × 10 4 | 1.26 × 10 4 |
| Ear pain: continuous | 141/82 | 51 (36.2%) | 16 (19.5%) | 2.272 [1.219, 4.409] | 5.07 | 3.84 |
| Ear pain: neuropathic-like | 141/82 | 29 (20.6%) | 13 (15.7%) | 1.360 [0.661, 2.759] | 0.197 | 0.188 |
| Pain in face | 141/85 | 52 (36.9%) | 20 (23.5%) | 1.868 [1.027, 3.446] | 1.40 | 1.04 |
| Pain in jaw | 141/85 | 48 (31.6%) | 19 (20.9%) | 1.760 [0.950, 3.246] | 0.875 | 0.671 |
| Pain in neck | 141/85 | 49 (34.8%) | 21 (24.7%) | 1.601 [0.888, 2.952] | 0.542 | 0.420 |
| Ear fullness | 148/87 | 98 (66.2%) | 45 (51.7%) | 1.824 [1.07, 3.131] | 1.79 | 1.18 |
| Ear pressure | 150/88 | 96 (64.0%) | 42 (47.7%) | 1.933 [1.151, 3.313] | 3.26 | 1.95 |
| Scalp pressure | 141/85 | 45 (31.9%) | 26 (30.6%) | 1.056 [0.598, 1.902] | 0.161 | 0.098 |
| Flu-like symptoms | 141/86 | 29 (20.6%) | 13 (15.1%) | 1.417 [0.689, 2.871] | 0.218 | 0.212 |
| Flutter/middle ear myoclonus | 152/91 | 12 (7.9%) | 4 (4.4%) | 1.69 [0.608, 5.704] | 0.136 | 0.330 |
| Hearing changes/distortion | 152/91 | 7 (4.6%) | 7 (7.7%) | 0.579 [0.202, 1.634] | 0.130 | 0.364 |
| Sounds (clicking, popping, etc.) | 152/91 | 19 (12.5%) | 14 (15.4%) | 0.773 [0.374, 1.63] | 0.140 | 0.175 |
| Paresthesia | 152/91 | 9 (5.9%) | 4 (4.4%) | 1.251 [0.427, 4.317] | 0.083 | 0.236 |
| Reactive tinnitus | 122/77 | 99 (81.1%) | 60 (77.9%) | 1.217 [0.623, 2.498] | 0.170 | 0.149 |
| Headache exacerbation | 122/71 | 79 (74.8%) | 43 (60.6%) | 1.204 [0.669, 2.221] | 0.212 | 0.134 |
| Vertigo exacerbation | 91/57 | 46 (50.5%) | 57 (40.4%) | 1.509 [0.772, 2.917] | 0.428 | 0.264 |
Note. Odds ratio (OR) and 95% credible interval (CrI) calculated using 15,000 samples from joint posterior distribution of category proportions. Credible intervals that exclude OR = 1 are presented in bold. Bayes factor (BF) values greater than 3 (providing substantial evidence for a group difference) are bolded, whereas BF values less than 1/3 (providing substantial evidence against a group difference) are italicized. n Pain/n Loudness = number of patients in pain/loudness hyperacusis group providing codable/nonmissing responses; BF 10 = Bayes factor testing comparing proportions between the two groups; BF ROPE = Bayes factor versus the interval null hypothesis log(OR) = [−0.1, 0.1], that is, the region of practical equivalence to 0 (ROPE).
Clinical Course and Symptom Fluctuations
The onset of hyperacusis symptoms was described as sudden in 49.0% of the pain hyperacusis group and 49.4% of the loudness hyperacusis group, with the difference between the groups being practically insignificant (OR = 0.982 [0.583, 1.689], BF 10 = 0.170, BF ROPE = 0.089). When describing the natural history of hyperacusis symptoms, participants categorized their clinical course as Better, Worse, Same, or Fluctuated. Examining the full 2 × 4 contingency table (see Table 2), there was substantial evidence for a difference in symptom trajectories between groups (BF 10 = 3.86). Examining each category separately, the pain group was substantially less likely to endorse symptoms getting better over time (pain: 7.5%, loudness: 20.7%; OR = 0.320 [0.142, 0.697], BF ROPE = 11.7) and significantly more likely to endorse symptoms worsening over time (pain: 51.0%, loudness: 34.5%; OR = 1.96 [1.148, 3.392], BF ROPE = 2.22), although the ROPE Bayes factor provided inconclusive evidence that the latter difference was large enough to be of practical significance. Notably, a similar proportion of individuals in each group indicated that their symptoms had stayed the same over time (pain: 13.6%, loudness: 10.3%; OR = 1.316 [0.581, 3.007], BF ROPE = 0.187) or fluctuated between better and worse (pain: 27.9%, loudness: 34.5%; OR = 0.734 [0.423, 1.306], BF ROPE = 0.189).
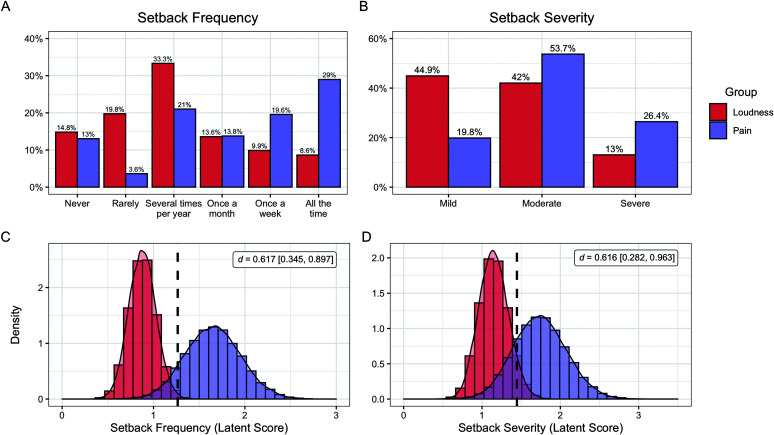
The majority of individuals in both groups reported experiencing “setbacks” (defined as specific events that noticeably worsened symptoms), with only 14.8% of the loudness group and 13.0% of the pain group stating they had never experienced temporary symptom exacerbations (see Figure 1). Setbacks were most commonly attributed to moderate or loud noise exposure, with equivalent proportions of both groups identifying environmental noises as the cause (pain: 77.1%, loudness: 70.8%; OR = 1.388 [0.729, 2.763], BF 10 = 0.259, BF ROPE = 0.204). A minority of patients attributed their setbacks to stress, exhaustion, or fatigue, with inconclusive evidence for differences between groups (pain: 11.0%, loudness: 19.4%; OR = 0.513 [0.232, 1.167], BF 10 = 0.469, BF ROPE = 0.631). Notably, individuals in the pain group reported that they experienced setbacks significantly more often than individuals in the loudness group (d = 0.617 [0.345, 0.897], BF 10 = 672, BF ROPE = 435). While 62.3% of individuals with pain hyperacusis reported experiencing setbacks once per month or more, only 32.1% of individuals in the loudness group reported having setbacks this frequently (OR = 3.442 [1.939, 6.099], BF ROPE = 233). Among the patients who reported experiencing setbacks, those with pain hyperacusis also reported greater worsening of symptoms during setbacks than those with loudness hyperacusis (d = 0.616 [0.282, 0.963]), BF 10 = 137, BF ROPE = 98.9). However, there was inconclusive evidence regarding whether the two groups differed in terms of the amount of time needed to recover from a setback (d = 0.252 [−0.138, 0.668], BF 10 = 0.613, BF ROPE = 0.544), with the majority of patients (56.1%) reporting that these setbacks typically last several days. Patients frequently perceived the need to avoid loud noises during a setback, with the majority of individuals in both groups responding affirmatively to the question, “Does the Participant's recovery take longer if they are exposed to a louder noise?” (pain: 91.5%, loudness: 84.7%; OR = 1.922 [0.799, 4.803], BF 10 = 0.331, BF ROPE = 0.517).
Figure 1.
Comparison of setback frequency and severity between patients with loudness and pain hyperacusis. Percentages of individuals in each group endorsing each response on the Coordination of Rare Diseases at Sanford survey regarding (A) setback frequency and (B) setback severity. Ordered probit models were used to compare latent mean scores between groups, and the posterior distributions of latent mean scores are depicted for (C) setback frequency and (B) setback severity. Vertical dotted lines indicate the grand mean score of the sample on each variable.
Comorbid Conditions
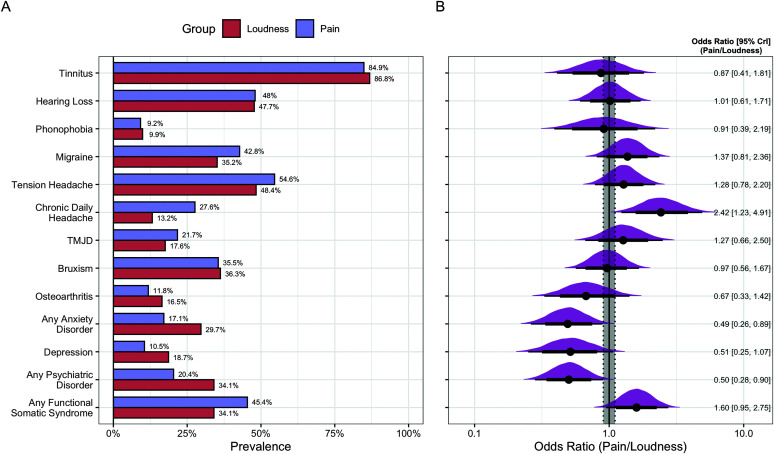
As expected, there was high comorbidity between hyperacusis, tinnitus, and hearing loss in our sample, with approximately 85% of patients in each group reporting tinnitus (pain: 84.9%, loudness: 86.8%; OR = 0.870 [0.410, 1.813], BF 10 = 0.125, BF ROPE = 0.143) and nearly half of patients reporting some form of hearing loss (pain: 48.0%, loudness: 47.7%; OR = 1.012 [0.607, 1.713], BF 10 = 0.166, BF ROPE = 0.085). Of the patients who endorsed tinnitus, the proportion whose tinnitus interfered with sleep was substantially smaller and practically equivalent between groups (pain: 39.0%, loudness: 50.0%; OR = 0.728 [0.43, 11.334], BF 10 = 0.327, BF ROPE = 0.195). A similar proportion of individuals indicated that their tinnitus causes difficulty concentrating while reading in a quiet room, with no practically meaningful group differences in endorsement (pain: 60.0%, loudness: 56.4%; OR = 1.166 [0.665, 2.086], BF 10 = 0.200, BF ROPE = 0.113). The majority of patients with comorbid tinnitus also noted that their tinnitus increased in volume with exposure to sounds (pain: 81.1%, loudness: 77.9%; OR = 1.217 [0.623, 2.498], BF 10 = 0.170, BF ROPE = 0.149). Within the group of patients with reactive tinnitus, there was no difference between patients with loudness hyperacusis and pain hyperacusis in regard to the duration of tinnitus increases after hearing a loud sound (d = 0.130 [−0.180, 0.448], BF 10 = 0.312, BF ROPE = 0.229), with the most common response (40%) being that tinnitus exacerbations lasted several hours.
Headache disorders were also prevalent in our sample, with equivalent proportions of individuals in each group reporting both migraines (pain: 42.8%, loudness: 35.2%; OR = 1.370 [0.810, 2.357], BF 10 = 0.316, BF ROPE = 0.197) and tension headaches (pain: 54.6%, loudness: 48.4%; OR = 1.280 [0.784, 2.203], BF 10 = 0.256, BF ROPE = 0.145). However, chronic daily headaches of any type were experienced significantly more frequently by patients with pain hyperacusis (pain: 29.4%, loudness: 13.6%; OR = 2.534 [1.281, 5.149], BF 10 = 6.71, BF ROPE = 6.21). Headaches occurred frequently in both groups of patients, with individuals in the pain hyperacusis group experiencing headaches slightly more often than individuals in the loudness hyperacusis group (d = 0.361 [0.086, 0.627], BF 10 = 5.86, BF ROPE = 4.17). Average headache severity, rated on a 1–10 scale, was also higher in the pain group as compared to the loudness group (pain: M = 6.19, loudness: M = 5.20; d = 0.466 [0.175, 0.758], BF 10 = 29.0, BF ROPE = 18.7). The majority of patients in both groups who experienced headaches also endorsed an increased frequency of headaches after being exposed to loud noises (pain: 64.8%, loudness: 60.6%; OR = 1.204 [0.669, 2.221], BF 10 = 0.212, BF ROPE = 0.134).
Additional comorbidities reported by over 5% of the sample included TMJD, bruxism, osteoarthritis, phonophobia, anxiety disorders, depressive disorders, psychiatric disorders in general, and functional somatic syndromes (see Figure 2). Although not reaching the 5% threshold, it is notable that 10 individuals (4.1%; eight in pain group, two in loudness group) reported experiencing misophonia comorbid with their hyperacusis. Phonophobia, including both self-reported phonophobia diagnoses and descriptions of pathological anxiety/panic responses evoked by sounds, was present in just under 10% of the sample and endorsed to an equivalent degree across groups (pain: 9.2%, loudness: 9.9%; OR = 0.911 [0.391, 2.195], BF 10 = 0.099, BF ROPE = 0.165). TMJD was reported by 20% of the sample, with practically equivalent endorsement across groups (pain: 21.7%, loudness: 17.6%; OR = 1.271 [0.665, 2.498], BF 10 = 0.175, BF ROPE = 0.153). Bruxism was also relatively common, occurring in 35.8% of the sample and similarly across groups (pain: 35.5%, loudness: 36.3%; OR = 0.965 [0.565, 1.667], BF 10 = 0.159, BF ROPE = 0.090). Anxiety disorders (inclusive of phonophobia) were present in 21.8% of the sample, with a significantly higher rate of endorsement in the loudness group but inconclusive evidence for a practically significant difference (pain: 17.1%, loudness: 29.7%; OR = 0.492 [0.262, 0.893], BF 10 = 1.80, BF ROPE = 1.73). Similar patterns were also observed for the categories of depressive disorders (pain: 10.5%, loudness: 18.7%; OR = 0.515 [0.251, 1.071], BF 10 = 0.553, BF ROPE = 0.789) and any psychiatric disorder (pain: 20.4%, loudness: 34.1%; OR = 0.501 [0.281, 0.901], BF 10 = 2.24, BF ROPE = 1.85), although the group difference in depression prevalence did not reach statistical significance (i.e., the 95% CrI included OR = 1). Consistent with previous studies (Paulin et al., 2016), we found a high prevalence of functional somatic syndromes in individuals with hyperacusis, with the pain hyperacusis group numerically more likely to report one of these conditions (pain: 45.5%, loudness: 34.1%; OR = 1.597 [0.948, 2.749], BF 10 = 0.724, BF ROPE = 0.465). Notably, when excluding chronic daily headache from the functional somatic syndrome category, nearly equal proportions of each group endorsed a functional somatic syndrome diagnosis (pain: 27.0%, loudness: 26.4%; OR = 1.023 [0.571, 1.828], BF 10 = 0.146, BF ROPE = 0.098).
Figure 2.
Comparison of comorbid conditions and nonauditory sensory complaints between patients with loudness and pain hyperacusis. (A) Percentages of participants with loudness and pain hyperacusis who reported a certain diagnosis/symptom. (B) Posterior densities of each odds ratio (OR) comparing respondents with pain hyperacusis to those with loudness hyperacusis. OR values are presented with their 95% CrIs. Thick and thin horizontal lines indicate bounds of the 80% and 95% CrIs, respectively. The gray shaded area indicates the bounds of the interval null hypothesis (i.e., the region of practical equivalence OR = [0.905, 1.105]). CrI = credible interval; TMJD = temporomandibular joint disorder.
Comorbid Nonauditory Sensory Symptoms
The surveys also included several questions regarding sensory symptoms in the vestibular, visual, olfactory, and somatosensory modalities. Vertigo was a commonly reported symptom, occurring daily or weekly in 27.8% of the sample (pain: 29.2%, loudness: 25.6%; OR = 1.184 [0.653, 2.139], BF 10 = 0.178, BF ROPE = 0.121). Among the patients reporting vertigo, neither the severity of vertigo symptoms (d = 0.058 [−0.273, 0.394], BF 10 = 0.260, BF ROPE = 0.176) nor the frequency of vertigo attacks differed between loudness and pain groups (d = −0.099 [−0.382, 0.192], BF 10 = 0.268, BF ROPE = 0.181). Additionally, approximately half of patients experiencing vertigo reported an increase in vertigo attack frequency or severity after exposure to loud noises (pain: 50.5%, loudness: 40.4%; OR = 1.509 [0.772, 2.917], BF 10 = 0.428, BF ROPE = 0.264). Discomfort due to bright lights (i.e., photophobia) was also common, occurring daily or weekly in approximately 40% of patients (pain: 39.7%, loudness: 39.8%; OR = 0.994 [0.581, 1.756], BF 10 = 0.168, BF ROPE = 0.093). Of the patients reporting photophobia, no differences were found between loudness and pain groups in terms of photophobia severity (d = 0.016 [−0.286, 0.322], BF 10 = 0.217, BF ROPE = 0.135) or frequency (d = 0.039 [−0.248, 0.328], BF 10 = 0.219, BF ROPE = 0.133). A sizable proportion of individuals in each group also endorsed being bothered by strong smells (i.e., osmophobia; pain: 33.6%, loudness: 38.3%; OR = 0.813 [0.463, 1.432], BF 10 = 0.213, BF ROPE = 0.131) and being bothered by touch (pain: 22.4%, loudness: 23.5%; OR = 0.929 [0.492, 1.747], BF 10 = 0.146, BF ROPE = 0.111).
As an exploratory analysis, we also examined the rates at which the various nonauditory sensory symptoms co-occurred, additionally investigating the relationships of these symptoms with psychiatric and functional somatic syndrome diagnoses. Notably, disturbances in nonauditory sensory modalities co-occurred at rates substantially higher than chance. Of the individuals who reported daily or weekly photophobia, 62.7% endorsed osmophobia (vs. 16% of those without photophobia; OR = 8.475 [4.508, 16.472]), 35.4% endorsed tactile intolerance (vs. 16.3% of those without photophobia; OR = 2.765 [1.496, 5.44]), and 43.7% endorsed daily or weekly episodes of vertigo (vs. 16.4% of those without photophobia; OR = 3.871 [2.127, 7.386]). Similarly, individuals who endorsed osmophobia reported higher rates of both bothersome tactile experiences (OR = 3.554 [1.884, 7.045]) and daily/weekly vertigo (OR = 3.425 [1.861, 6.501]), and individuals who endorsed tactile intolerance had higher rates of daily/weekly vertigo (OR = 4.274 [2.274, 8.561]). Exploratory analyses also revealed that symptoms in nonauditory sensory modalities were associated with a functional somatic syndrome diagnosis (photophobia: OR = 2.212 [1.288, 3.865]; tactile intolerance: OR = 2.719 [1.300, 5.936]; vertigo: OR = 2.755 [1.542, 5.017]), with the exception of osmophobia, the prevalence of which was equivalent between groups with and without functional somatic syndrome diagnoses (OR = 1.403 [0.801, 2.463], BF 10 = 0.327, BF ROPE = 0.213). Conversely, the diagnosis of a psychiatric disorder was unrelated to daily/weekly photophobia (OR = 1.128 [0.613, 2.072], BF 10 = 0.202, BF ROPE = 0.118) or daily/ weekly vertigo (OR = 1.207 [0.638, 2.285], BF 10 = 0.196, BF ROPE = 0.139), although osmophobia was more likely to occur in individuals with psychiatric diagnoses (OR = 2.807 [1.537, 5.228], BF 10 = 44.2, BF ROPE = 19.7). Patients with psychiatric comorbidities were also numerically more likely to report tactile intolerance, although evidence for this association was inconclusive (OR = 1.896 [0.989, 3.617], BF 10 = 0.927, BF ROPE = 0.873).
Perceived Response to Hyperacusis Treatments
Of our sample, over 50% of each group had attempted to treat their hyperacusis with sound therapy, including both self-directed protocols and those prescribed by professionals (pain: 59.9%, loudness: 53.8%; OR = 1.273 [0.763, 2.173], BF 10 = 0.248, BF ROPE = 0.141). The most common form of sound therapy was self-administered pink noise (n = 85), followed by self-administered white noise (n = 84), structured interventions that included counseling (n = 58), tinnitus retraining therapy (n = 55), “Other” (n = 48), hearing-aid sound generators (n = 26), and the Neuromonics protocol (n = 3). Participants reported engaging in sound therapy for a median of 1–2 years, with the loudness hyperacusis group reporting a moderately longer duration of sound therapy compared to the pain hyperacusis group (d = −0.525 [−0.908, −0.137], BF 10 = 8.42, BF ROPE = 7.55). Notably, the loudness group also reported more perceived benefit from sound therapy than the pain group (d = −0.425 [−0.806, −0.047], BF 10 = 3.12, BF ROPE = 2.84). Individuals in the loudness group were substantially more likely to report that sound therapy resulted in “significant improvement” or “hyperacusis [being] almost eliminated” (pain: 4.4%, loudness: 22.4%; OR = 0.176 [0.054, 0.557]). However, the proportions of patients reporting (a) no change in symptoms with sound therapy (pain: 38.5%, loudness: 32.7%; OR = 1.274 [0.618, 2.605], BF ROPE = 0.162) or (b) worsening tinnitus/hyperacusis (pain: 27.5%, loudness: 18.4%; OR = 1.616 [0.702, 3.801], BF ROPE = 0.313) did not differ meaningfully between the two groups.
The three medication classes reported as beneficial by eight or more individuals were benzodiazepines (n = 30), opioids (n = 9), and gabapentinoids (n = 10). Benzodiazepines were reported as beneficial for hyperacusis symptoms by equivalent proportions of patients in each group (pain: 11.8%, loudness: 13.2%; OR = 0.871 [0.400, 1.870], BF 10 = 0.115, BF ROPE = 0.150). In contrast, all individuals reporting symptom reduction from opioids or gabapentinoids were in the pain hyperacusis group, likely due to the specific effects of these medications on nociceptive processes.
Discussion
Using a multinational patient registry, the current study investigated how patients with loudness and pain hyperacusis differed with respect to demographics, background/onset characteristics, symptoms, comorbidities, and response to treatment. On the whole, the two hyperacusis subgroups were more alike than they were different, yielding equivalent results on the majority of tested variables. However, the group differences that were detected suggest that pain hyperacusis is likely a more severe phenotype than loudness hyperacusis, associated with more frequent and severe setbacks, less improvement over time, a higher burden of comorbid headache disorders, reduced benefit from sound therapy, and more overall functional impairment. Notably, the distinction between loudness and pain hyperacusis was somewhat blurred in this cohort, as over 90% of patients with pain hyperacusis perceived everyday sounds as unbearably loud, and over 60% of our “loudness hyperacusis” group experienced sound-induced pain at least monthly. Thus, while our study does not indicate that pain and loudness hyperacusis are pathophysiologically distinct conditions, it does suggest that a pain-predominant phenotype could serve as a prognostic marker within the hyperacusis population.
Demographically, the loudness hyperacusis group was slightly older and more likely to be female, but the two groups were generally well matched on most other background variables. However, reported symptom trajectories differed substantially between the two groups, with the pain hyperacusis group more likely to report worsening over time and less likely to report improvement. Individuals with pain hyperacusis also reported higher scores on all three functional impairment items when compared with the loudness hyperacusis group. Group differences in QoL items (other than the pain interference item) were small and largely not significant, but the directions of these effects were all consistent with slightly decreased QoL in pain hyperacusis. Larger studies that implement more reliable measures of health-related QoL may permit more precise characterization of differences between these subsets of the hyperacusis population.
Specific symptoms of hyperacusis were typically reported to a similar degree between the two groups, although several symptoms such as facial pain, ear pressure, and ear fullness were reported slightly more often by patients with pain versus loudness hyperacusis. However, large group differences were found in the domain of setbacks, with the pain hyperacusis group reporting significantly more frequent and severe symptom exacerbations than the loudness hyperacusis group. As the majority of patients in our sample experienced setbacks at least monthly, our study supports the claims of patient advocates who argue that setbacks should be considered a core feature of hyperacusis deserving of additional clinical and research attention (Pollard, 2019). Future research on this symptom cluster should consider how to best quantify the frequency, severity, and duration of setbacks, as such a measure would likely be extremely useful when evaluating the safety and efficacy of clinical interventions. Additional research is also necessary to characterize the specific events that trigger setbacks, determine whether setbacks are associated with measurable changes in auditory physiology, and investigate the factors that enhance or inhibit recovery from setbacks, such as sound exposure/avoidance and medication use.
Comorbidities were frequent in this sample of hyperacusis patients. As expected, tinnitus was the most frequently reported condition, occurring in 85.6% of our sample (Anari et al., 1999; Sheldrake et al., 2015). Approximately 80% of individuals with tinnitus also reported that their tinnitus increased in volume with sound exposure, consistent with previous reports (Schecklmann et al., 2014). Additional commonly reported comorbidities included primary headache disorders, psychiatric disorders, and functional somatic syndromes, all of which have been associated with hyperacusis in prior studies (Cederroth et al., 2020; Goebel & Floezinger, 2008; Jüris et al., 2013; Paulin et al., 2016; Schecklmann et al., 2014). Most co-occurring conditions were equally prevalent in the two groups, although individuals with pain hyperacusis were more likely to report chronic daily headaches. Individuals with loudness hyperacusis were somewhat more likely to report comorbid psychiatric disorders, particularly anxiety disorders, although data were inconclusive regarding the practical significance of this group difference. As temporal trends in the onset of hyperacusis and these comorbid disorders were not assessed in the CoRDS survey, we were unable to determine whether any of these conditions could be considered risk markers for developing hyperacusis (though see Goebel & Floezinger, 2008). Additional research is necessary to better understand the ways in which comorbid disorders confer hyperacusis risk and whether hyperacusis itself is a risk factor for developing certain comorbidities.
Individuals with both loudness and pain hyperacusis further reported fairly high rates of nonauditory sensory complaints, including photophobia, osmophobia, tactile intolerance, and vertigo attacks, consistent with previous qualitative findings (Ke et al., 2020). Though the severity and frequency of nonauditory sensory complaints did not differ between patients with loudness and pain hyperacusis, the presence of one nonauditory sensory symptom was highly predictive of additional symptoms in other modalities. Moreover, the presence of a functional somatic syndrome predicted symptoms of photophobia, tactile intolerance, and vertigo, potentially indicating that these processes may arise from the pathologic process of central sensitization thought to underlie functional somatic syndromes (den Boer et al., 2019; La Touche et al., 2018; Suhnan et al., 2017; Yunus, 2015). With the exception of osmophobia, sensory abnormalities were also not related to the presence of psychiatric disorders, indicating that the occurrence and co-occurrence of sensory symptoms cannot be easily attributed to the presence of anxiety or trait negative affectivity. Further research is warranted to better understand the overlap between hyperacusis, other sensory complaints, and functional somatic syndromes, and subsequent studies should seek to determine whether central sensitization delineates a clinically meaningful hyperacusis subgroup.
Lastly, we investigated relations between hyperacusis subtypes and response to treatments, including sound therapy and medications. The majority of patients in our sample had utilized some form of sound therapy to treat hyperacusis, although self-reported treatment outcomes were modest. One notable finding was that individuals with loudness hyperacusis were more likely to report substantial improvement with sound therapy than individuals with pain hyperacusis, although approximately one third of pain hyperacusis patients reported at least minor improvement. Additionally, a substantial minority of patients (27.4% in the pain group, 18.4% in the loudness group) reported symptomatic worsening of either hyperacusis or tinnitus due to sound therapy, indicating that these treatments may be harmful to a subset of the population. The field of hyperacusis research would benefit substantially from research designed to elucidate factors that predict differential response to sound therapy in hyperacusis patients.
Few medications were reported by multiple patients to be beneficial for symptoms, and only benzodiazepines, opioids, and gabapentinoids were reported by at least 10% of respondents. Gabapentinoids and opioids were both only reported as beneficial in patients with pain hyperacusis, indicating that certain medications targeting nociceptive symptoms may be specifically useful for individuals with pain hyperacusis but not loudness hyperacusis. Notably, benzodiazepines were the medication class with the most perceived benefit by patients across hyperacusis groups. This finding is consistent with the hypothesis that hyperacusis is caused by decreased inhibition in the central auditory system (Auerbach et al., 2014; Sheppard et al., 2020) and indicates that benzodiazepines and other GABAergic medications may reduce hyperacusis symptoms by counteracting maladaptive auditory gain within the central nervous system. Benzodiazepines have also been found to be beneficial in reducing tinnitus in some cases, although evidence for this indication is mixed and may not be sufficient to outweigh the potential harms of these medications (Jufas & Wood, 2015). However, given the substantial disability associated with cases of severe hyperacusis and the lack of established pharmacological treatments, we believe that additional research should further explore the potential benefits of benzodiazepines in the context of controlled clinical trials. Specific unanswered questions include (a) whether benzodiazepines provide long-term symptom reduction with repeated dosing, (b) whether a short-term course of benzodiazepines will improve recovery from a setback, and (c) whether acute doses of benzodiazepines objectively alter psychoacoustic correlates of hyperacusis such as loudness discomfort levels and loudness growth functions. In the absence of high-quality randomized controlled trials supporting the use of benzodiazepines in this population, we strongly caution against the routine prescribing of benzodiazepines for patients with hyperacusis, although judicious use of these medications (with frequent re-assessment of benefits and harms) may be warranted in some cases. Given the promising open-label results seen with safer medications such as nortriptyline and topiramate (Abouzari et al., 2020), clinicians should consider prescribing benzodiazepines only in cases that fail to respond to more conservative pharmacotherapies and/or sound therapy.
Despite the rich information gleaned from this investigation, our study is not without limitations. Most notably, all information was self-reported by patients in the CoRDS Registry and was unable to be empirically verified. Moreover, participants in this registry were self-selected and quite possibly not representative of the broader hyperacusis population. In particular, it may be the case that individuals with pain hyperacusis were overrepresented in this sample, as noise-induced pain is a particularly strong focus of Hyperacusis Research Ltd., the organization sponsoring the CoRDS hyperacusis survey. Given that many individuals in this registry were recruited from social media hyperacusis support groups, it is also possible that individuals in our sample differed from the population of hyperacusis patients in terms of chronicity of symptoms, comorbid conditions, illness severity, or sociodemographic factors (Sautier et al., 2014). Another limitation concerns our operationalization of pain hyperacusis. As no clinical or research criteria have been established to parse hyperacusis into loudness and pain subtypes, we chose the arbitrary criterion of pain experienced daily based on (a) preliminary results from this sample (Pollard, 2019) and (b) the notion that pain caused by “everyday sounds” would result in pain experienced at least daily. However, it may potentially be the case that individuals who report no sound-induced pain whatsoever differ systematically from those who report sound-induced pain in any capacity, and additional research will be needed to determine whether such subgroups provide additional prognostic information. A third limitation is the lack of data in the CoRDS registry on the perceived efficacy of cognitive-behavioral therapy (CBT; Aazh et al., 2019), which has demonstrated some efficacy in reducing hyperacusis symptoms and improving loudness discomfort levels in a single randomized controlled trial (Jüris et al., 2014). Future research investigating the utility of CBT as a potential treatment for hyperacusis should specifically investigate the degree to which response to CBT may differ between individuals with and without pain hyperacusis. Notably, as CBT has demonstrated efficacy in treating other forms of chronic pain with diverse etiologies (Hoffman et al., 2007; Morley et al., 1999; Pike et al., 2016), the adaptation of existing CBT protocols to pain hyperacusis remains a potentially fruitful area of future research. Lastly, the information in the CoRDS Registry was gathered using an ad hoc survey measure rather than previously validated questionnaires. As a result, it is difficult to compare this sample of patients to other groups in terms of hyperacusis or tinnitus severity, comorbid anxiety/depression symptoms, QoL, and other constructs of interest. Future survey studies in this population should additionally include validated measures of sound tolerance symptoms (Fackrell & Hoare, 2018; Greenberg & Carlos, 2018) and other patient-reported outcomes (Cella et al., 2007). Despite the limitations of the CoRDS Registry as a source of information on the phenotype of hyperacusis, we believe that the information gathered from CoRDS and other patient surveys has the potential to identify important aspects of the hyperacusis experience from the perspective of patients and to guide clinicians and researchers in providing higher quality care to this particular patient population.
Author Contributions
Zachary J. Williams: Conceptualization (Lead), Data curation (Lead), Formal analysis (Lead), Investigation (Lead), Methodology (Lead), Visualization (Lead), Writing – original draft (Lead), Writing – review & editing (Lead). Evan Suzman: Conceptualization (Supporting), Methodology (Supporting), Visualization (Supporting), Writing – original draft (Supporting), Writing – review & editing (Equal). Tiffany G. Woynaroski: Conceptualization (Supporting), Formal analysis (Supporting), Resources (Lead), Supervision (Lead), Writing – review & editing (Equal).
Acknowledgments
This work was supported by National Institute on Deafness and Other Communication Disorders Grant F30-DC019510 (awarded to Z. J. W.), National Institute of General Medical Sciences Grant T32-GM007347 (awarded to Z. J. W.), and the Nancy Lurie Marks family foundation (awarded to Z. J. W./T. G. W.). The authors would like to acknowledge Hyperacusis Research, Ltd. and Sanford Research for developing and maintaining the CoRDS hyperacusis registry, as well as Alyssa Mendel of Sanford Research for assisting in data curation and management. The authors would also like to thank Maura Black, David Treworgy, and other hyperacusis patient-advocates for their hard work in recruiting individuals experiencing hyperacusis to register with CoRDS.
Funding Statement
This work was supported by National Institute on Deafness and Other Communication Disorders Grant F30-DC019510 (awarded to Z. J. W.), National Institute of General Medical Sciences Grant T32-GM007347 (awarded to Z. J. W.), and the Nancy Lurie Marks family foundation (awarded to Z. J. W./T. G. W.).
Footnote
Custom R code to perform statistical analyses can be found on the ResearchGate profile of the corresponding author (https://www.researchgate.net/profile/Zachary_Williams19/publications). The remainder of research materials can be obtained from the corresponding author upon request.
References
- Aazh, H. , Landgrebe, M. , Danesh, A. A. , & Moore, B. C. J. (2019). Cognitive behavioral therapy for alleviating the distress caused by tinnitus, hyperacusis and misophonia: Current perspectives. Psychology Research and Behavior Management, 12, 991–1002. https://doi.org/10.2147/PRBM.S179138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abouzari, M. , Tan, D. , Sarna, B. , Ghavami, Y. , Goshtasbi, K. , Parker, E. M. , Lin, H. W. , & Djalilian, H. R. (2020). Efficacy of multi-modal migraine prophylaxis therapy on hyperacusis patients. Annals of Otology, Rhinology & Laryngology, 129(5), 421–427. https://doi.org/10.1177/0003489419892997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acquadro, C. , Berzon, R. , Dubois, D. , Leidy, N. K. , Marquis, P. , Revicki, D. , & Rothman, M. (2003). Incorporating the patient's perspective into drug development and communication: An ad hoc task force report of the patient-reported outcomes (PRO) harmonization group meeting at the food and drug administration. Value in Health, 6(5), 522–531. https://doi.org/10.1046/j.1524-4733.2003.65309.x [DOI] [PubMed] [Google Scholar]
- Anari, M. , Axelsson, A. , Eliasson, A. , & Magnusson, L. (1999). Hypersensitivity to sound: Questionnaire data, audiometry and classification. Scandinavian Audiology, 28(4), 219–230. https://doi.org/10.1080/010503999424653 [DOI] [PubMed] [Google Scholar]
- Assi, H. , Moore, R. D. , Ellemberg, D. , & Hébert, S. (2018). Sensitivity to sounds in sport-related concussed athletes: A new clinical presentation of hyperacusis. Scientific Reports, 8(1), 9921. https://doi.org/10.1038/s41598-018-28312-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach, B. D. (2019, January, 1). Physiological mechanisms of hyperacusis: An update. ENT & Audiology News, 27(6). https://www.entandaudiologynews.com/features/audiology-features/post/physiological-mechanisms-of-hyperacusis-an-update [Google Scholar]
- Auerbach, B. D. , Rodrigues, P. V. , & Salvi, R. J. (2014). Central gain control in tinnitus and hyperacusis. Frontiers in Neurology, 5(6), 206. https://doi.org/10.3389/fneur.2014.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguley, D. M. , & Hoare, D. J. (2018). Hyperacusis: Major research questions. HNO, 66(5), 358–363. https://doi.org/10.1007/s00106-017-0464-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, R. M. , Jones, J. , Turk, D. C. , Russell, I. J. , & Matallana, L. (2007). An internet survey of 2,596 people with fibromyalgia. BMC Musculoskeletal Disorders, 8(1), 27. https://doi.org/10.1186/1471-2474-8-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandy, W. T. , & Lynn, J. M. (1995). Audiologic findings in hyperacusic and nonhyperacusic subjects. American Journal of Audiology, 4(1), 46–51. https://doi.org/10.1044/1059-0889.0401.46 [Google Scholar]
- Bürkner, P.-C. (2017). brms: An R package for Bayesian multilevel models using Stan. Journal of Statistical Software, 80(1). https://doi.org/10.18637/jss.v080.i01 [Google Scholar]
- Bürkner, P.-C. (2018). Advanced Bayesian multilevel modeling with the R package brms. The R Journal, 10(1), 395–411. https://doi.org/10.32614/RJ-2018-017 [Google Scholar]
- Bürkner, P.-C. , & Vuorre, M. (2019). Ordinal regression models in psychology: A tutorial. Advances in Methods and Practices in Psychological Science, 2(1), 77–101. https://doi.org/10.1177/2515245918823199 [Google Scholar]
- Cederroth, C. R. , Lugo, A. , Edvall, N. K. , Lazar, A. , Lopez-Escamez, J.-A. , Bulla, J. , Uhlen, I. , Hoare, D. J. , Baguley, D. M. , Canlon, B. , & Gallus, S. (2020). Association between hyperacusis and tinnitus. Journal of Clinical Medicine, 9(8), 2412. https://doi.org/10.3390/jcm9082412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella, D. , Yount, S. , Rothrock, N. , Gershon, R. , Cook, K. , Reeve, B. , Ader, D. , Fries, J. F. , Bruce, B. , Rose, M. , & PROMIS Cooperative Group. (2007). The patient-reported outcomes measurement information system (PROMIS): Progress of an NIH roadmap cooperative group during its first two years. Medical Care, 45(5 Suppl. 1), S3–S11. https://doi.org/10.1097/01.mlr.0000258615.42478.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, L. , Valencia, I. J. , Garvert, D. W. , & Montoya, J. G. (2018). Deconstructing post-exertional malaise in myalgic encephalomyelitis/ chronic fatigue syndrome: A patient-centered, cross-sectional survey. PLOS ONE, 13(6), Article e0197811. https://doi.org/10.1371/journal.pone.0197811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. (1994). The earth is round (p < .05). American Psychologist, 49(12), 997–1003. https://doi.org/10.1037//0003-066x.49.12.997 [Google Scholar]
- den Boer, C. , Dries, L. , Terluin, B. , van der Wouden, J. C. , Blankenstein, A. H. , van Wilgen, C. P. , Lucassen, P. , & van der Horst, H. E. (2019). Central sensitization in chronic pain and medically unexplained symptom research: A systematic review of definitions, operationalizations and measurement instruments. Journal of Psychosomatic Research, 117, 32–40. https://doi.org/10.1016/j.jpsychores.2018.12.010 [DOI] [PubMed] [Google Scholar]
- Dickey, J. M. , & Lientz, B. P. (1970). The weighted likelihood ratio, sharp hypotheses about chances, the order of a Markov chain. The Annals of Mathematical Statistics, 41(1), 214–226. https://doi.org/10.1214/aoms/1177697203 [Google Scholar]
- Dworkin, R. H. , Turk, D. C. , Revicki, D. A. , Harding, G. , Coyne, K. S. , Peirce-Sandner, S. , Bhagwat, D. , Everton, D. , Burke, L. B. , Cowan, P. , Farrar, J. T. , Hertz, S. , Max, M. B. , Rappaport, B. A. , & Melzack, R. (2009). Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). PAIN®, 144(1), 35–42. https://doi.org/10.1016/j.pain.2009.02.007 [DOI] [PubMed] [Google Scholar]
- Fackrell, K. , & Hoare, D. J. (2018). Scales and questionnaires for decreased sound tolerance. In Fagelson M. A. & Baguley D. M. (Eds.), Hyperacusis and disorders of sound intolerance (pp. 43–58). Plural. [Google Scholar]
- Fackrell, K. , Stratmann, L. , Kennedy, V. , MacDonald, C. , Hodgson, H. , Wray, N. , Farrell, C. , Meadows, M. , Sheldrake, J. , Byrom, P. , Baguley, D. M. , Kentish, R. , Chapman, S. , Marriage, J. , Phillips, J. , Pollard, T. , Henshaw, H. , Gronlund, T. A. , & Hoare, D. J. (2019). Identifying and prioritising unanswered research questions for people with hyperacusis: James Lind Alliance Hyperacusis Priority Setting Partnership. BMJ Open, 9(11), Article e032178. https://doi.org/10.1136/bmjopen-2019-032178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagelson, M. A. , & Baguley, D. M. (2018). Disorders of sound tolerance: History and terminology. In Fagelson M. A. & Baguley D. M. (Eds.), Hyperacusis and disorders of sound intolerance: Clinical and research perspectives (pp. 3–14). Plural. [Google Scholar]
- Flores, E. N. , Duggan, A. , Madathany, T. , Hogan, A. K. , Márquez, F. G. , Kumar, G. , Seal, R. P. , Edwards, R. H. , Liberman, M. C. , & García-Añoveros, J. (2015). A non-canonical pathway from cochlea to brain signals tissue-damaging noise. Current Biology, 25(5), 606–612. https://doi.org/10.1016/j.cub.2015.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel, G. , & Floezinger, U. (2008). Pilot study to evaluate psychiatric co-morbidity in tinnitus patients with and without hyperacusis. Audiological Medicine, 6(1), 78–84. https://doi.org/10.1080/16513860801959100 [Google Scholar]
- Greenberg, B. , & Carlos, M. (2018). Psychometric properties and factor structure of a new scale to measure hyperacusis: Introducing the inventory of hyperacusis symptoms. Ear and Hearing, 39(5), 1025–1034. https://doi.org/10.1097/AUD.0000000000000583 [DOI] [PubMed] [Google Scholar]
- Gûnel, E. , & Dickey, J. (1974). Bayes factors for independence in contingency tables. Biometrika, 61(3), 545–557. https://doi.org/10.1093/biomet/61.3.545 [Google Scholar]
- Hébert, S. , Fournier, P. , & Noreña, A. (2013). The auditory sensitivity is increased in tinnitus ears. Journal of Neuroscience, 33(6), 2356–2364. https://doi.org/10.1523/JNEUROSCI.3461-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, B. M. , Papas, R. K. , Chatkoff, D. K. , & Kerns, R. D. (2007). Meta-analysis of psychological interventions for chronic low back pain. Health Psychology, 26(1), 1–9. https://doi.org/10.1037/0278-6133.26.1.1 [DOI] [PubMed] [Google Scholar]
- Jager, I. , de Koning, P. , Bost, T. , Denys, D. , & Vulink, N. (2020). Misophonia: Phenomenology, comorbidity and demographics in a large sample. PLOS ONE, 15(4), Article e0231390. https://doi.org/10.1371/journal.pone.0231390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamil, T. , Ly, A. , Morey, R. D. , Love, J. , Marsman, M. , & Wagenmakers, E.-J. (2017). Default “Gunel and Dickey” Bayes factors for contingency tables. Behavior Research Methods, 49(2), 638–652. https://doi.org/10.3758/s13428-016-0739-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff, P. J. , & Jastreboff, M. M. (2015). Decreased sound tolerance: Hyperacusis, misophonia, diplacousis, and polyacousis. In Aminoff M. J., Boller F., & Swaab D. F. (Eds.), Handbook of clinical neurology (Vol. 129, pp. 375–387). Elsevier. https://doi.org/10.1016/B978-0-444-62630-1.00021-4 [DOI] [PubMed] [Google Scholar]
- Jufas, N. E. , & Wood, R. (2015). The use of benzodiazepines for tinnitus: Systematic review. The Journal of Laryngology & Otology, 129(S3), S14–S22. https://doi.org/10.1017/S0022215115000808 [DOI] [PubMed] [Google Scholar]
- Jüris, L. , Andersson, G. , Larsen, H. C. , & Ekselius, L. (2013). Psychiatric comorbidity and personality traits in patients with hyperacusis. International Journal of Audiology, 52(4), 230–235. https://doi.org/10.3109/14992027.2012.743043 [DOI] [PubMed] [Google Scholar]
- Jüris, L. , Andersson, G. , Larsen, H. C. , & Ekselius, L. (2014). Cognitive behaviour therapy for hyperacusis: A randomized controlled trial. Behaviour Research and Therapy, 54, 30–37. https://doi.org/10.1016/j.brat.2014.01.001 [DOI] [PubMed] [Google Scholar]
- Kanazawa, M. , Miwa, H. , Nakagawa, A. , Kosako, M. , Akiho, H. , & Fukudo, S. (2016). Abdominal bloating is the most bothersome symptom in irritable bowel syndrome with constipation (IBS-C): A large population-based Internet survey in Japan. BioPsychoSocial Medicine, 10(1), 19. https://doi.org/10.1186/s13030-016-0070-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass, R. E. , & Raftery, A. E. (1995). Bayes factors. Journal of the American Statistical Association, 90(430), 773–795. https://doi.org/10.1080/01621459.1995.10476572 [Google Scholar]
- Ke, J. , Du, Y. , Tyler, R. S. , Perreau, A. , & Mancini, P. C. (2020). Complaints of people with hyperacusis. Journal of the American Academy of Audiology, 31(8), 553–558. https://doi.org/10.1055/s-0040-1709447 [DOI] [PubMed] [Google Scholar]
- Kirk, R. E. (1996). Practical significance: A concept whose time has come. Educational and Psychological Measurement, 56(5), 746–759. https://doi.org/10.1177/0013164496056005002 [Google Scholar]
- Kruschke, J. K. (2013). Bayesian estimation supersedes the t test. Journal of Experimental Psychology: General, 142(2), 573–603. https://doi.org/10.1037/a0029146 [DOI] [PubMed] [Google Scholar]
- Kruschke, J. K. , & Liddell, T. M. (2018). The Bayesian new statistics: Hypothesis testing, estimation, meta-analysis, and power analysis from a Bayesian perspective. Psychonomic Bulletin & Review, 25(1), 178–206. https://doi.org/10.3758/s13423-016-1221-4 [DOI] [PubMed] [Google Scholar]
- La Touche, R. , Paris-Alemany, A. , Hidalgo-Pérez, A. , López-de-Uralde-Villanueva, I. , Angulo-Diaz-Parreño, S. , & Muñoz-García, D. (2018). Evidence for central sensitization in patients with temporomandibular disorders: A systematic review and meta-analysis of observational studies. Pain Practice, 18(3), 388–409. https://doi.org/10.1111/papr.12604 [DOI] [PubMed] [Google Scholar]
- Liddell, T. M. , & Kruschke, J. K. (2018). Analyzing ordinal data with metric models: What could possibly go wrong? Journal of Experimental Social Psychology, 79, 328–348. https://doi.org/10.1016/j.jesp.2018.08.009 [Google Scholar]
- Liu, C. , Glowatzki, E. , & Fuchs, P. A. (2015). Unmyelinated type II afferent neurons report cochlear damage. Proceedings of the National Academy of Sciences, 112(47), 14723–14727. https://doi.org/10.1073/pnas.1515228112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly, A. , Stefan, A. , van Doorn, J. , Dablander, F. , van den Bergh, D. , Sarafoglou, A. , Kucharský, S. , Derks, K. , Gronau, Q. F. , Raj, A. , Boehm, U. , van Kesteren, E.-J. , Hinne, M. , Matzke, D. , Marsman, M. , & Wagenmakers, E.-J. (2020). The Bayesian methodology of Sir Harold Jeffreys as a practical alternative to the p value hypothesis test. Computational Brain & Behavior, 3(2), 153–161. https://doi.org/10.1007/s42113-019-00070-x [Google Scholar]
- Makowski, D. , Ben-Shachar, M. S. , Chen, S. H. A. , & Lüdecke, D. (2019). Indices of effect existence and significance in the Bayesian framework. Frontiers in Psychology, 10. https://doi.org/10.3389/fpsyg.2019.02767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski, D. , Ben-Shachar, M. S. , & Lüdecke, D. (2019). BayestestR: Describing effects and their uncertainty, existence and significance within the Bayesian framework. Journal of Open Source Software, 4(40), 1541. https://doi.org/10.21105/joss.01541 [Google Scholar]
- Morey, R. D. , & Rouder, J. N. (2018). BayesFactor: Computation of bayes factors for common designs. https://CRAN.R-project.org/package=BayesFactor
- Morley, S. , Eccleston, C. , & Williams, A. (1999). Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain, 80(1), 1–13. https://doi.org/10.1016/S0304-3959(98)00255-3 [DOI] [PubMed] [Google Scholar]
- Noreña, A. J. , & Chéry-Croze, S. (2007). Enriched acoustic environment rescales auditory sensitivity. NeuroReport, 18(12), 1251–1255. https://doi.org/10.1097/wnr.0b013e3282202c35 [DOI] [PubMed] [Google Scholar]
- Noreña, A. J. , Fournier, P. , Londero, A. , Ponsot, D. , & Charpentier, N. (2018). An integrative model accounting for the symptom cluster triggered after an acoustic shock. Trends in Hearing, 22, 2331216518801725. https://doi.org/10.1177/2331216518801725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulin, J. , Andersson, L. , & Nordin, S. (2016). Characteristics of hyperacusis in the general population. Noise & Health, 18(83), 178–184. https://doi.org/10.4103/1463-1741.189244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, D. P. , & Carr, M. M. (1998). Disturbances of loudness perception. Journal of the American Academy of Audiology, 9(5), 371–379. [PubMed] [Google Scholar]
- Pienkowski, M. , Tyler, R. S. , Roncancio, E. R. , Jun, H. J. , Brozoski, T. , Dauman, N. , Coelho, C. B. , Andersson, G. , Keiner, A. J. , Cacace, A. T. , Martin, N. , & Moore, B. C. J. (2014). A review of hyperacusis and future directions: Part II. Measurement, mechanisms, and treatment. American Journal of Audiology, 23(4), 420–436. https://doi.org/10.1044/2014_AJA-13-0037 [DOI] [PubMed] [Google Scholar]
- Pike, A. , Hearn, L. , & de C Williams, A. C. (2016). Effectiveness of psychological interventions for chronic pain on health care use and work absence: Systematic review and meta-analysis. Pain, 157(4), 777–785. https://doi.org/10.1097/j.pain.0000000000000434 [DOI] [PubMed] [Google Scholar]
- Pollard, B. (2019). Clinical advancements for managing hyperacusis with pain. The Hearing Journal, 72(10), 10. https://doi.org/10.1097/01.HJ.0000602900.16223.0e [Google Scholar]
- R Core Team. (2020). R: A language and environment for statistical computing (4.0.2) [Computer software] . R Foundation for Statistical Computing. https://www.R-project.org/ [Google Scholar]
- Rouw, R. , & Erfanian, M. (2018). A large-scale study of misophonia. Journal of Clinical Psychology, 74(3), 453–479. https://doi.org/10.1002/jclp.22500 [DOI] [PubMed] [Google Scholar]
- Sautier, L. , Mehnert, A. , Höcker, A. , & Schilling, G. (2014). Participation in patient support groups among cancer survivors: Do psychosocial and medical factors have an impact? European Journal of Cancer Care, 23(1), 140–148. https://doi.org/10.1111/ecc.12122 [DOI] [PubMed] [Google Scholar]
- Savalei, V. (2020). Improving fit indices in structural equation modeling with categorical data. Multivariate Behavioral Research. https://doi.org/10.1080/00273171.2020.1717922 [DOI] [PubMed] [Google Scholar]
- Schecklmann, M. , Landgrebe, M. , Langguth, B. , & the TRI Database Study Group. (2014). Phenotypic characteristics of hyperacusis in tinnitus. PLOS ONE, 9(1), Article e86944. https://doi.org/10.1371/journal.pone.0086944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder, A. E. , Vulink, N. , & Denys, D. (2013). Misophonia: Diagnostic criteria for a new psychiatric disorder. PLOS ONE, 8(1), Article e54706. https://doi.org/10.1371/journal.pone.0054706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrake, J. , Diehl, P. U. , & Schaette, R. (2015). Audiometric characteristics of hyperacusis patients. Frontiers in Neurology, 6(1), 105. https://doi.org/10.3389/fneur.2015.00105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard, A. , Stocking, C. , Ralli, M. , & Salvi, R. (2020). A review of auditory gain, low-level noise and sound therapy for tinnitus and hyperacusis. International Journal of Audiology, 59(1), 5–15. https://doi.org/10.1080/14992027.2019.1660812 [DOI] [PubMed] [Google Scholar]
- Suhnan, A. P. , Finch, P. M. , & Drummond, P. D. (2017). Hyperacusis in chronic pain: Neural interactions between the auditory and nociceptive systems. International Journal of Audiology, 56(11), 801–809. https://doi.org/10.1080/14992027.2017.1346303 [DOI] [PubMed] [Google Scholar]
- Trudeau, S. (2013). CoRDS registry: An HIT case study concerning setup and maintenance of a disease registry. In Sarnikar S., Bennett D., & Gaynor M. (Eds.), Cases on Healthcare Information Technology for Patient Care Management (pp. 197–207). IGI Global. https://doi.org/10.4018/978-1-4666-2671-3 [Google Scholar]
- Tyler, R. S. , Pienkowski, M. , Roncancio, E. R. , Jun, H. J. , Brozoski, T. , Dauman, N. , Coelho, C. B. , Andersson, G. , Keiner, A. J. , Cacace, A. T. , Martin, N. , & Moore, B. C. J. (2014). A review of hyperacusis and future directions: Part I. Definitions and manifestations. American Journal of Audiology, 23(4), 402–419. https://doi.org/10.1044/2014_AJA-14-0010 [DOI] [PubMed] [Google Scholar]
- Wagenmakers, E.-J. , Lodewyckx, T. , Kuriyal, H. , & Grasman, R. (2010). Bayesian hypothesis testing for psychologists: A tutorial on the Savage–Dickey method. Cognitive Psychology, 60(3), 158–189. https://doi.org/10.1016/j.cogpsych.2009.12.001 [DOI] [PubMed] [Google Scholar]
- Wagenmakers, E.-J. , Marsman, M. , Jamil, T. , Ly, A. , Verhagen, J. , Love, J. , Selker, R. , Gronau, Q. F. , Šmíra, M. , Epskamp, S. , Matzke, D. , Rouder, J. N. , & Morey, R. D. (2018). Bayesian inference for psychology. Part I: Theoretical advantages and practical ramifications. Psychonomic Bulletin & Review, 25(1), 35–57. https://doi.org/10.3758/s13423-017-1343-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenmakers, E.-J. , Wetzels, R. , Borsboom, D. , & van der Maas, H. L. J. (2011). Why psychologists must change the way they analyze their data: The case of psi: Comment on Bem (2011). Journal of Personality and Social Psychology, 100(3), 426–432. https://doi.org/10.1037/a0022790 [DOI] [PubMed] [Google Scholar]
- Weldring, T. , & Smith, S. M. S. (2013). Article commentary: Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Services Insights, 6, HSI.S11093. https://doi.org/10.4137/HSI.S11093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, Z. J. , He, J. L. , Cascio, C. J. , & Woynaroski, T. G. (2021). A review of decreased sound tolerance in autism: Definitions, phenomenology, and potential mechanisms. Neuroscience & Biobehavioral Reviews, 121, 1–17. https://doi.org/10.1016/j.neubiorev.2020.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, Z. J. , Suzman, E. , & Woynaroski, T. G. (2021). Prevalence of decreased sound tolerance (hyperacusis) in individuals with autism spectrum disorder: A meta-analysis. Ear and Hearing. Advance online publication. https://doi.org/10.1097/AUD.0000000000001005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. S. , Vyas, P. , Glowatzki, E. , & Fuchs, P. A. (2018). Oppos1ing expression gradients of calcitonin-related polypeptide alpha (Calca/Cgrpα) and tyrosine hydroxylase (Th) in type II afferent neurons of the mouse cochlea. Journal of Comparative Neurology, 526(3), 425–438. https://doi.org/10.1002/cne.24341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunus, M. B. (2015). Editorial review (Thematic issue: An update on central sensitivity syndromes and the issues of nosology and psychobiology). Current Rheumatology Reviews, 11(2), 70–85. https://doi.org/10.2174/157339711102150702112236 [DOI] [PubMed] [Google Scholar]
- Zelaya, C. E. , Lucas, J. W. , & Hoffman, H. J. (2015). Percentage of adults with selected hearing problems, by type of problem and age group—National Health Interview Survey, United States, 2014. Morbidity and Mortality Weekly Report, 64(37), 1058. https://doi.org/10.15585/mmwr.mm6437a8 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from the current study are available from the Coordination of Rare Diseases at Sanford (CoRDS) Registry, organized by Sanford Research. Interested parties can apply to access the data at https://research.sanfordhealth.org/rare-disease-registry. Data generated by the authors of this study from the original CoRDS data (e.g., binary codes for qualitative responses) can be requested from the corresponding author if written permission for the author to share those data is obtained from Sanford Research.