Abstract
Background
Patients often report that living with a condition such as tinnitus can be debilitating, worrying, and frustrating. Efficient ways to foster management strategies for individuals with tinnitus and promoting tinnitus self-efficacy are needed. Internet-based cognitive behavioral therapy (ICBT) for tinnitus shows promise as an evidence-based intervention in Europe, but is not available in the United States. The aim of this pilot study was to evaluate the feasibility of an ICBT intervention for tinnitus in the United States.
Method
This study reports the Phase 1 trial intended to support implementation of a larger randomized clinical trial (RCT) comparing ICBT to a weekly monitoring group. As a pilot study, a single-group pretest–posttest design was used to determine outcome potential, recruitment strategy, retention, and adherence rates of ICBT for tinnitus. The primary outcome was a change in tinnitus distress. Secondary outcome measures included measures of anxiety, depression, insomnia, tinnitus cognitions, hearing-related difficulties, and quality of life.
Results
Of the 42 screened participants, nine did not meet the inclusion criteria and six withdrew. There were 27 participants who completed the intervention, with a mean age of 55.48 (± 9.9) years.
Feasibility was established, as a large pretest–posttest effect size of d = 1.6 was found for tinnitus severity. Large pretest–posttest effect sizes were also found for tinnitus cognitions and hearing-related effects, and a medium effect was found for insomnia and quality of life. Treatment adherence varied with a retention rate of 85% (n = 23) at post-intervention assessment and 67% (n = 18) for the follow-up assessment.
Conclusions
This pilot study supported the feasibility of ICBT for tinnitus in the United States. Ways of improving intervention retention and recruitment rates need to be explored in future ICBT studies. Protocol refinements that were identified will be implemented prior to further RCTs to investigate the efficacy of ICBT for tinnitus in the United States.
Supplemental Material
Although some health-related conditions may not be life-threatening, their effects may produce durable life-changing and debilitating experiences for patients. One such symptom is tinnitus, in which individuals hear sounds in their ears or head that do not originate from the environment. Various conditions are associated with developing tinnitus, including ear disorders (Kostev et al., 2019), exposure to loud noise, presence of a hearing loss, and increasing age (Kim et al., 2015). Tinnitus is highly prevalent, with an estimated 10%–15% of the adult population reporting hearing tinnitus (McCormack et al., 2016). Reactions to tinnitus can greatly vary between individuals (Beukes, Manchaiah, et al., 2020). Although tinnitus is not bothersome for the majority of individuals, there are millions of individuals who find it distressing, resulting in activity limitations and participation restrictions (Manchaiah et al., 2018). For those with chronic distressing tinnitus, various management strategies can address quality-of-life issues, thereby facilitating coping with tinnitus effects, and fostering individuals' habituation to the tinnitus sensation. Audiologists often employ strategies that include directed counseling, sound enrichment, and when indicated by hearing loss, the fitting of hearing aids (Zenner et al., 2017). In addition to these strategies, however, the strongest evidence-based approach found helpful for addressing negative reactions and behaviors toward tinnitus is a psychological approach known as cognitive behavioral therapy (CBT). Numerous clinical trials and systematic reviews have indicated the efficacy of CBT in tinnitus management (see systematic reviews by Fuller et al., 2020, Landry et al., 2020, and Supplementary Material S1). CBT is recommended in most practice guidelines including those provided by the American Academy of Otolaryngology–Head and Neck Surgery (Fuller et al., 2017; Tunkel et al., 2014). Despite these recommendations, CBT is seldom provided to those with tinnitus in the United States and the world at large. For instance, a large-scale epidemiological study (n = 75,764) in the United States showed that CBT was discussed with only 0.2% of patients, whereas the use of medication, for which supporting evidence was weakest, was discussed with 46% of patients (Bhatt et al., 2016). Several barriers limit accessible CBT interventions for tinnitus. These include medicolegal obstacles, such as psychologists not being allowed to practice across states. Boundaries between disciplines may reduce the number of clinicians willing to employ strategies for which they are not licensed, few psychologists routinely provide CBT for patients with bothersome tinnitus, and indeed, a limited number of audiologists routinely deliver tinnitus services (Planey, 2019) despite the great need among the clinical population. Although audiologists are generally involved in the management of tinnitus, their primary training is not the use of psychological interventions, and this lack is expressed in the United States and most other countries. Although additional training may be obtained, the required resources are not always available to clinical audiologists. Nevertheless, audiologists routinely rely upon tenets of CBT in their counseling when fitting hearing aids, offering falls prevention strategies, and when working with families of patients who receive cochlear implants. Many audiologists focus tinnitus management around sound enrichment and information counseling approaches (Henry et al., 2019) despite familiarity with potentially helpful elements of CBT.
Due to the efficacy and effectiveness of CBT for tinnitus, there is growing interest among practitioners specializing in the care of patients with bothersome tinnitus to increase access to CBT using creative approaches. One such approach is the development of an Internet-based CBT (ICBT) intervention for tinnitus (Andersson et al., 2002). ICBT was used in Europe, and its efficacy was demonstrated in nine randomized clinical trials (RCTs) indicating a moderate effect size for both tinnitus distress and insomnia and improvements for anxiety, depression, and quality of life (Beukes et al., 2019). Unfortunately, ICBT for tinnitus is not yet routinely offered in the United States. The availability of an additional self-help tinnitus intervention, such as ICBT, could improve the accessibility of tinnitus care. Prior to identifying if ICBT may be a suitable approach for a U.S. population, its feasibility first needs to be established. Health care and medicolegal practices differ in the United States to Europe, where psychologists are, for instance, not allowed to practice across states. Feasibility for a U.S. population cannot be assumed, as ICBT would be an unfamiliar treatment approach. Most tinnitus therapies provided in the United States, such as tinnitus retraining therapy (TRT; Jastreboff & Jastreboff, 2000) and Progressive Tinnitus Management (Henry et al., 2010), are generally provided in an in-person format, although Henry, Piskosz, et al. (2019) recently published a trial delivering Progressive Tinnitus Management via telephone presentation. Acceptance of an Internet-based format is thus not known within the United States. It is also not known whether those undertaking such an intervention would engage sufficiently or whether they would engage at all with any self-help intervention. It is furthermore not known if a psychological approach will be accepted by audiologists, as the emphasis of most audiologic tinnitus management programs is on sound therapy and fitting devices (Henry, Thielman, et al., 2019; Tyler et al., 2020).
To identify the feasibility of ICBT in the United States, a pilot study was undertaken prior to implementing a larger RCT (Leon et al., 2012). In addition, we also evaluated outcomes of ICBT. The aim of this study was to run a small-scale pilot study to investigate the feasibility of a full-scale RCT in the U.S. population. The research questions were:
Do the outcomes obtained from ICBT for tinnitus indicate that a full-scaled study should be conducted?
Is the protocol feasible in a U.S. population in terms of recruitment potential, retention rates, intervention compliance, and engagement?
Method
Study Design
This study provided the Phase 1 trial of a larger RCT. Phase 1 trials are intended to focus on establishing safety of trial, adverse effects, and information on outcomes by involving small numbers or participants (Mahan, 2014). A single-group pretest–posttest design was used to determine the feasibility of ICBT in the United States and identify any adverse effects. On recommendation from the funding body, this was to be an initial small-scale study (n = 30) without a control group to test the protocol prior to allocating resources to a larger scale study (n = 150). Phase 1 trials are an important initial part of clinical trial designs for complex interventions (Campbell et al., 2000). Ethical approval was obtained from the institutional review board at Lamar University, Beaumont, TX, United States (IRB-FY17–209). To ensure that best practice was followed, the Transparent Reporting of Evaluations with Nonrandomized Designs checklist (Des Jarlais et al., 2004) was used to report this trial. An independent data monitoring committee monitored the running of the trial.
Study Population
To comply with the U.S. government's health promotion initiative requiring health care be linguistically and culturally accessible (U.S Department of Health and Human Services, 2010), all the study materials were made available in both English and Spanish (Beukes et al., 2019; Manchaiah, Muñoz, et al., 2020). A range of strategies were used to disseminate information, including social media, flyers, e-mails, forums, and newsletters, which were distributed to local communities and put up in clinic waiting rooms. Professionals such as audiologists and otolaryngologists serving those with tinnitus in southeast Texas were also notified about the study. Those interested were directed to the study website where they could read more about the study and register interest in partaking in the study. Study eligibility was determined as follows:
Inclusion criteria:
adults, aged 18 years and over, living in Texas in the United States;
the ability to read and type in English or Spanish;
access to a computer, the Internet, and the ability to e-mail;
experiencing tinnitus for a minimum period of 3 months;
a tinnitus severity score of 25 or greater on the Tinnitus Functional Index (TFI) indicating the need for an intervention; and
any configuration of hearing levels (normal or any degree of hearing loss) and any use of hearing devices (using or not using hearing aids).
Exclusion criteria:
indication of significant depression (≥ 15) on the Patient Health Questionnaire (PHQ-9);
Indications of self-harm thoughts or intent, answering affirmingly on Question 10 of the PHQ-9 (Spitzer et al., 1999) completed during the screening procedure;
reporting any major medical or psychiatric conditions;
reporting pulsatile, objective, or unilateral tinnitus, which has not been investigated medically or tinnitus still under medical investigation; and
undergoing any tinnitus therapy concurrent with participation in this study.
Eligibility was determined by an initial assessment as follows:
An online screening questionnaire, which included demographic information, health- and mental health–related questions, and standardized outcome measures as shown in Table 1.
A telephone interview during which the researcher rechecked eligibility, and provided the opportunity for potential participants to ask any questions related to the study. The study procedures were explained, and motivational interviewing was done to encourage participants to commit and engage in the intervention.
Any participants with a score of 15 or more on the PHQ-9 or indicated self-harm on Question 10 received a phone consultation from a clinical psychologist on the research team. This call ensured that they were under care elsewhere or necessary resources and/or referral were provided.
Table 1.
Study outcome measures used pre-intervention, post-intervention, and at 2-month follow-up.
| Outcome measures | Internal consistency | Range of scores | Scores interpretation | Assessment time frame |
|---|---|---|---|---|
| Tinnitus Functional Index (TFI; Meikle et al., 2012) | .8 | 0–100 | > 25 = mild 26–50 = significant 50+ = severe |
Pre, post, and follow-up |
| Generalized Anxiety Disorder (GAD-7; Spitzer et al., 2006) | .89 | 0–21 | 0–4 = minimal anxiety 5–9 = mild anxiety 10–14 = moderate anxiety 15–21 = severe anxiety |
Pre, post, and follow-up |
| Patient Health Questionnaire (PHQ-9; Spitzer et al., 1999) | .83 | 0–27 | 5–9 = mild depression 10–14 = moderate 15–19 = moderately severe 20–18 = severe depression |
Pre, post, and follow-up |
| Insomnia Severity Index (ISI; Bastien et al., 2001) | .74 | 0–28 | 0–7 = not clinically significant 8–14 = subthreshold insomnia 15–21 = clinical insomnia (moderate severity) 22–28 = clinical insomnia (severe degree) |
Pre, post, and follow-up |
| Tinnitus Cognitions Questionnaire (TCQ; Wilson & Henry, 1998) | .91 | 0–104 | Higher scores indicate a greater tendency to engage in negative cognitions in response to tinnitus | Pre, post, and follow-up |
| EQ-5D-5L (Herdman et al., 2011) | .7–.85 | 0–15 | Measures 5 dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression | Pre, post, and follow-up |
| EQ-5D-5L Visual Analogue Scale (VAS; Herdman et al., 2011) | .7–.85 | 0–100 | VAS for overall health. Higher scores indicated improved health | Pre, post, and follow-up |
| Tinnitus and Hearing Survey (THS; Henry et al., 2015) | .86–.94 | Subscale for tinnitus: 0–16 Hearing: 0–16; sound tolerance: 0–8 |
Pre, post, and follow-up | |
| Weekly monitoring | ||||
| Tinnitus Handicap Inventory–Screening (THI-S; Newman et al., 2008) | .93 | 0–40 | > 6 tinnitus handicap | Weekly while undertaking the 8-week intervention |
| Tinnitus Qualities Questionnaire (TQQ; Beukes, Andersson, Manchaiah, & Kaldo, 2021) | Not assessed | 0–100 | Designed to determine whether tinnitus qualities such as loudness, pitch, the number of tones heard, and so forth improve while undertaking an intervention. Higher scores indicate more bothersome aspects of tinnitus are present. | Weekly while undertaking the 8-week intervention |
| Intervention satisfaction (Beukes et al., 2016) |
Not assessed | 0–75 | Higher scores indicate more intervention satisfaction | Post-intervention |
Intervention
The ICBT intervention content was based on a CBT self-help program originally developed in Swedish (Andersson & Kaldo, 2004) and translated into German (Weise et al., 2016) and English (Abbott et al., 2009). The intervention was then adapted into an 8-week interactive e-learning version suitable for a U.K. population (Beukes et al., 2016). For the purposes of this pilot investigation, the program employed additional linguistic and cultural adaptations to ensure suitability for a U.S. population (Beukes et al., 2019). These adaptations included ensuring accessibility of the intervention, such as confirming readability at below the recommended sixth-grade level. The ICBT platform was enhanced further with the addition of a module on mindfulness and adding videos for all modules discussing techniques. As reported herein, the ICBT program employed 22 modules with worksheets and quizzes as outlined in Beukes, Andersson, Manchaiah, and Kaldo (2021).
The intervention platform was housed in the United States to comply with mandated data protection regulations. Prior to this feasibility trial, acceptability and functionality of this intervention for a U.S. population were ensured; details regarding related features and functionality of the intervention were reported previously (Manchaiah, Muñoz, et al., 2020).
Audiologist Guidance
Guidance was provided to support individuals who participated in the intervention. The study design included monitoring progress, monitoring weekly scores, providing feedback on worksheets completed, outlining the content of new modules, and answering questions. Participants who enrolled, but displayed minimal activity on the platform, were contacted using an encrypted two-way messaging system within the ePlatform to encourage engagement and discuss possible barriers. Although psychologists have traditionally guided CBT interventions, tinnitus management is generally delivered by audiologists (Henry, Thielman, et al., 2019). To maintain consistency with the standard clinical approach to tinnitus management, an experienced audiologist provided patient support. This approach was shown to be feasible in previous trials in the United Kingdom (Beukes, Andersson, Allen, et al., 2018; Beukes, Baguley, Allen, et al., 2018). If required, further support was available from a specialist tinnitus audiologist or a licensed CBT therapist.
Outcome Measures
Primary Outcome Measure
Tinnitus severity as measured by the TFI (Meikle et al., 2012) was selected as the primary measure to determine the outcome of ICBT in a pilot U.S. population. The TFI was selected over other tinnitus questionnaires as it was specifically developed to measure tinnitus severity, assess responsiveness to treatment, and for the purpose of comparing results with similar trials in the United Kingdom (Beukes et al., 2017). It has been translated into more than 15 languages and been validated for numerous populations including Chinese, Dutch, Swedish, and German (Henry et al., 2016).
Secondary Outcome Measures
Secondary outcomes included measures of anxiety, depression, insomnia, tinnitus cognitions, hearing-related difficulties, and health-related quality of life, as shown in Table 1. To reduce the number of questionnaires and questions to be answered, the Tinnitus and Hearing Survey, a 10-item questionnaire (Henry et al., 2015), was used to identify participant perceptions of hearing disability and hyperacusis. The section on tinnitus also served as a secondary tinnitus measure. The (EuroQol measure EQ-5D-5L; Herdman et al., 2011) was selected to measure health-related quality of life. All questionnaires were used with the required permissions, and agreements were set up for those that are not freely available to use. For Spanish speakers, validated Spanish-translated versions were used. When these were unavailable, the investigators developed validated translations (Manchaiah, Vlaescu, et al., 2020).
Weekly Monitoring During the Intervention
Throughout the program, participants were monitored weekly by means of the Tinnitus Handicap Inventory, Screening version (THI-S). The THI-S is a 10-item questionnaire, and scores are comparable (r = .9) with the full version of the THI (Newman et al., 2008). The weekly score was used to identify the possibility of an adverse event. If scores increased by more than 10 points between two consecutive weeks, then the change was considered an adverse event. Those indicating adverse effects were contacted to address the identified problems. Participants were also monitored by a newly developed Tinnitus Qualities Questionnaire (TQQ; Beukes, Andersson, Manchaiah, & Kaldo, 2021). The TQQ measures psychoacoustic tinnitus qualities such as pitch, loudness, and the number of tones heard.
Intervention Variables
Intervention adherence was assessed by determining retention rates and questionnaire completion rates. Intervention engagement was assessed by the number of logins, the number of modules read, and the number of messages sent during the intervention. Intervention satisfaction was measured by collecting participants' views regarding the presentation, content, usability, and information in the intervention using a 0- to 5-point Likert scale with a maximum score of 75 points. Messages written and free text responses in the outcome questionnaire were used to identify any adverse effects.
Questionnaire Administration
Online questionnaires were used throughout the study. All the measures were completed pre- and post-intervention, and at 2-month follow-up. To maximize retention, three electronic reminders were sent to participants who had not completed questionnaires, on the three consecutive days after the release of the questionnaire. A further reminder was sent out via e-mail and text message. If questionnaires were still not completed, participants were telephoned to encourage questionnaire completion. Participants were also phoned after completing the intervention to discuss the progress they had made and share their questionnaire results.
Data Analysis
Statistical analyses were performed using the SPSS (Version 26.0). The primary study outcomes of interest were retention, feasibility, and effect size at post-intervention. For all analyses, the goal of this pilot was to estimate the pretest–posttest effect size for all primary and secondary outcomes; however, two-sided p values using alpha = .05 were also reported. For some outcome measures, more than 15% of data were missing. To account for missing data from participants not completing the post-intervention or follow-up intervention analysis, an imputation analysis was undertaken. Missing data were handled through multiple imputation using the Markov Chain Monte Carlo approach. In addition, a completers analysis was also performed by analyzing only the completed questionnaire data without imputing missing data. The data that support the findings of this study are openly available in Figshare (http://doi.org/10.6084/m9.figshare.13678711).
Effect Sizes and Statistical Modeling
Effect sizes (Cohen's d; Cohen, 1992) at post-intervention were calculated by dividing the differences in pre- and post-intervention means by the pooled standard deviations. The reliable change index (Jacobson & Truax, 1991) was used as a means of calculating clinical significance for the TFI as the primary outcome. This was calculated using the mean pretest–posttest score difference, the pretreatment standard deviation, and a test–retest reliability coefficient of.78, and as reported in the validation study (Meikle et al., 2012). Finally, linear mixed models with random intercept for patient were used to account for repeated measures and incorporate all available data points in the analysis. The models were used to determine the effect of the pre-intervention scores on follow-up scores. The linear mixed model induces a compound symmetry covariance structure. Bonferroni-corrected pairwise post hoc tests were applied to determine which time points were significantly different, for each variable.
Sample Characteristics
Descriptive statistics including gender, age, tinnitus duration, hearing aid use, and professionals consulted were used to describe the sample. The mean and standard deviation were reported for each outcome measure at each time point. Descriptive statistics were also used to describe intervention adherence and engagement including the number of logins and modules read.
Results
Participant Characteristics
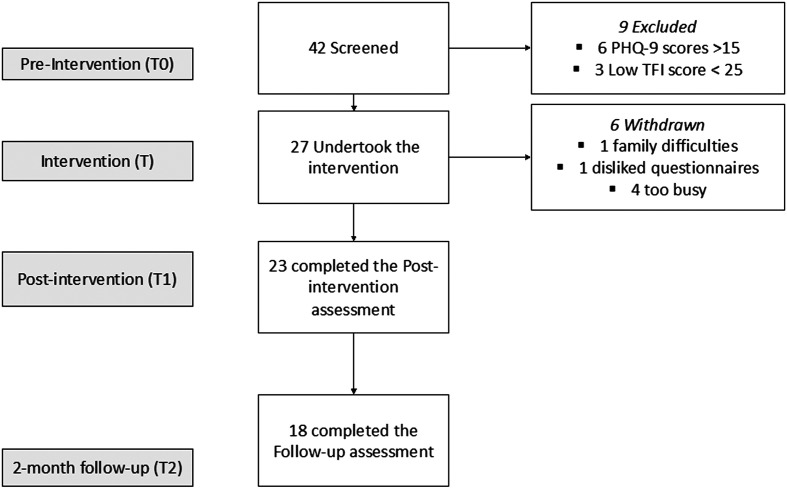
Of the 42 screened participants, nine did not meet the inclusion criteria and six withdrew (see Figure 1). The demographic profile of the remaining 27 participants completing the intervention is shown in Table 2. All participants selected to do the intervention in English, despite ethnicity type.
Figure 1.
Study profile. PHQ-9 = Patient Health Questionnaire; TFI = Tinnitus Functional Index.
Table 2.
Demographic characteristics of the participants (n = 27).
| Demographical information | M (SD) or number (%) |
|---|---|
| Gender | 18 (67%) female 9 (33%) male |
| Average age | 55.48 ± 9.9 years Range: 34–71 years |
| Tinnitus duration | 11.75 ± 13.36 years |
| Ethnicity | Hispanic or Latino: 2 (7%) Not Hispanic or Latino: 25 (93%) |
| Race | White: 26 (96%) More than one race: 1 (4%) |
| All Professionals seen for tinnitus Note: For some individuals more than one professional was seen |
Primary care physician: 19 (70%) ENT physician: 23 (85%) Audiologist: 24 (89%) Neurologist: 3 (11%) None: 2 (7%) |
| Hearing aid use | Bilateral: 7 (26%) Unilateral: 3 (11%) Hearing aids help mask the tinnitus: 4 (40%) Hearing aids don't mask the tinnitus: 6 (60%) |
| Highest educational level | School: 7 (22%) College/vocational training: 10 (31%) Undergraduate degree: 13 (41%) Postgraduate degree: 2 (6%) |
| Employment | Skilled or professional: 21 (78%) Retired: 6 (22%) |
Note. Multiple imputation using the Markov Chain Monte Carlo approach was used for imputation analysis. A decrease in scores indicates improvement for all outcomes except for the EQ-5D overall score, where an increase in scores indicates an improvement.
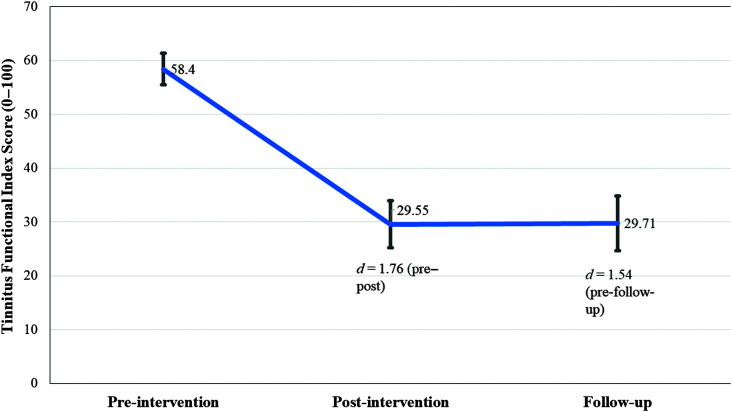
Primary Outcome Result
A significant, large effect size was observed for the change in tinnitus severity post-intervention (see Table 3). This change was maintained at a 2-month follow-up as shown in Figure 2. The reliable change index indicated a pre–post score difference of 19.51 on the TFI would be a clinically significant change. This was obtained by 22/27 participants (81%) using imputation analysis and 16/23 (70%) of the participants using completers' analysis.
Table 3.
Pre-, post-, and follow-up intervention comparisons for the various outcome measures.
| Outcome measure | Pre-intervention M (SD) [range] | Analysis protocol | Post-intervention M (SD) | Follow-up M (SD) | Effect size, Cohen's d [95% confidence intervals for T0–T1] | Linear mixed model, Type III test of fixed effects (using all available data) | T0–T1 pairwise comparison: mean difference (SE), significant* |
T0–T2 pairwise comparison: mean difference (SE), significant* |
T1–T2 pairwise comparison: mean difference (SE), significant* |
|---|---|---|---|---|---|---|---|---|---|
| TFI | 58.4 (15.01) [24–90] |
Completers analysis | 29.98 (20.99) [2–86] |
29.53 (21.55) [3–84] | 1.60 [0.91, 2.23] | F(2, 43) = 34.42, p = .001* | 28.04 (SE = 3.94), p = .001 * | 29.48 (SE = 4.30), p = .001 * | 1.44 (SE = 4.46), p = 1.00 |
| Imputation analysis | 29.55 (19.36) [2–86] | 29.71 (17.49) | 1.76 [1.11, 2.36] | ||||||
| GAD-7 | 7.15 (4.68) [1–17] | Completers analysis | 4.57 (4.02) [0–14] | 4.35 (2.42) [0–9] | 0.58 [−0.02, 1.16] | F(2, 39) = 7.07, p = .002* | 2.74 (SE = 0.82), p = .005 * | 2.68 (SE = 0.91), p = .01* | −.07 (SE = 0.93), p = 1.00 |
| Imputation analysis | 4.69 (3.75) [0–14] | 4.91 (2.43) [0–9] | 0.58 [0.03, 1.12] | ||||||
| PHQ-9 | 6.00 (3.17) [0–12] | Completers analysis | 4.91 (3.94) [0–14] | 4.71 (2.78) [1–9] | 0.30 [−0.28, 0.89] | F(2, 39) = 1.99, p = .15 | N/A N/A |
||
| Imputation analysis | 4.76 (3.71) [0–14] | 4.52 (2.51) [1–9] | 0.36 [−0.18, 0.89] | ||||||
| ISI | 12.67 (6.50) [2–27] | Completers analysis | 8.85 (6.02) [0–20] 7.04 (4.81) |
11.53 (6.43) [1–23] | 0.61 [0.01, 1.19] | F(2, 35) = 7.90, p = .001* | 4.32 (SE = 1.09), p = .001* | 2.43 (SE = 1.16), p = .13 | −1.90 (SE = 1.20), p = .37 |
| Imputation analysis | 8.74 (5.35) [0–20] | 10.69 (5.27) [1–23] | 0.66 [0.10, 1.20] | ||||||
| EQ-5D-5L | 7.33 (1.94) [5–15] | Completers analysis | 6.40 (1.19) [5–9] | 6.53 (1.18) [5–9] | 0.56 [−0.04, 1.14] | F(2, 32) = 6.73, p = .004* | 0.90 (SE = 0.26), p = .005* | .77 (SE = 0.28), p = .03 * | −.13 (SE = 0.30), p = 1.00 |
| Imputation analysis | 6.57 (1.15) [5–9] | 6.42 (1.01) [5–9] | 0.46 [−0.13, 1.04] | ||||||
| EQ-5D-5L VAS | 73.85 (16.03) [9–90] |
Completers analysis | 81.60 (7.50) [70–90] | 80.94 (10.35) [50–90] | 0.59 [−0.01, 1.17] | F(2, 18) = 2.63, p = .10 | N/A | ||
| Imputation analysis | 80.71 (6.96) [70–90] | 81.01 (8.42) [50–90 | 0.56 [0.00, 1.09] | ||||||
| THS: Tinnitus | 7.15 (4.13) [1–6] | Completers analysis | 3.70 (4.47) [0–14] | 3.69 (4.27) [0–16] | 0.81 [0.19, 1.39] | F(2, 36) = 15.17, p = .001* | 3.31 (SE = 0.68), p = .001* | 3.28 (SE = 0.74), p = .001* | −.03 (SE = 0.77), p = 1.0 |
| Imputation analysis | 3.87 (3.91) [0–14] | 3.61 (3.54) [0–16] | 0.82 [0.25, 1.36] | ||||||
| THS: Hearing | 7.04 (4.33) [0–16] | Completers analysis | 4.05 (3.65) [0–12] | 3.69 (3.30) [0–12] | 0.74 [0.13, 1.32] | F(2, 36) = 10.39, p = .001* | 2.9 (SE = 0.76), p = .002* | 3.2 (SE = 0.82), p = .001* | .36 (SE = 0.85), p = 1.0 |
| Imputation analysis | 4.32 (3.2) [0–12] | 3.41 (2.86) [0–12] | 0.71 [0.15, 1.25] | ||||||
| THS: Sound tolerance | 1.33 (1.24) [0–4] | Completers analysis | 0.60 (0.82) [0–3] | 0.81 (0.98) [0–3] | 0.67 [0.07, 1.26] | F(2, 36) = 7.23, p = .002* | 0.76 (SE = 0.21), p = .002* | .48 (SE = 0.23), p = .11 | −.29 (SE = 0.23), p = .66 |
| Imputation analysis | 0.61 (0.76) [0–3] | 0.84 (0.89) [0–3] | 0.70 [0.14, 1.24] | ||||||
| TCQ | 41.7 (11.37) [22–62] | Completers analysis | 29.65 (13.94) [11–57] | 29.19 (13.11) [7–48] | 1.76 [1.06, 2.41] | F(2, 37) = 13.87, p = .001* | 12.00 (SE = 2.63), p = .001 * | 12.27 (SE = 2.86), p = .001* | −.27 (SE = 2.97), p = 1.0 |
| Imputation analysis | 29.47 (12.02) [11–57] | 30.01 (10.37) [7–48) | 1.05 [0.46, 1.60] |
Note. Results from both the completers and imputation analysis are provided for comparison. TFI = Tinnitus Functional Index; GAD-7 = Generalized Anxiety Disorder-7; PHQ-9 = Patient Health Questionnarie-9; ISI = Insomnia Severity Index; EQ-5D = EuroQol measure; VAS = Visual Analogue Scale; THS = Tinnitus Hearing Screener; TCQ = Tinnitus Cognitions Questionnaire; N/A = not applicable.
Significance at p < .05.
Figure 2.
Change in tinnitus distress over time as measured by the Tinnitus Functional Index at baseline, after the intervention, and at 1-year post-intervention.
Secondary Outcome Results
A large effect was found for tinnitus cognitions and medium effect for insomnia, hearing disability, and hyperacusis (see Table 3). Due to excluding participants who presented with significant levels of depression, the pretreatment scores and pre-intervention scores for depression and anxiety were below the level of clinical significance. Posttreatment improvements were not found for depression and were only found for anxiety using the imputation analysis protocol, but not for the completers' analysis. A significant effect for overall global quality of life score was found only for the imputation analysis but not for the quality of life visual analogue scale.
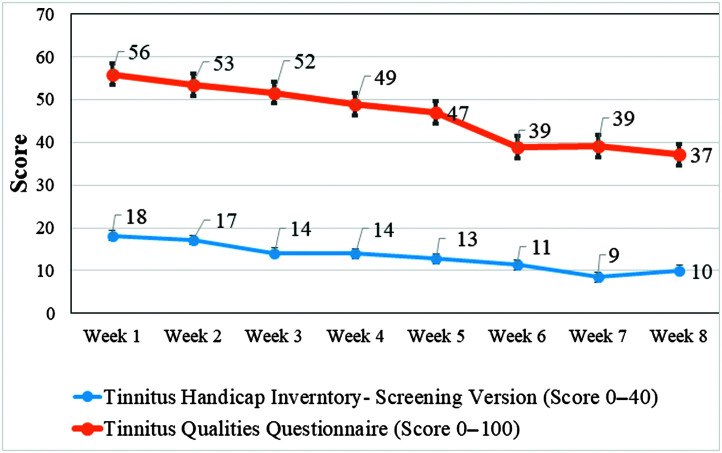
Weekly Monitoring
Overall, there was a reduction in tinnitus severity, THI-S, F(7, 175) = 2.92, p = .02, and tinnitus qualities, TQQ, F(7, 175) = 3.45, p = .002, over the 8-week intervention period using a linear mixed model, as seen in Figure 3. Pairwise comparison of the THI-S scores in Week 1 to subsequent weeks of the intervention displayed significant differences between Week 1 and Weeks 3–8 (p < .01). When comparing the TQQ scores in Week 1 with subsequent weeks of the intervention, there were significant differences between Week 1 and Weeks 4–8 (all ps < .01).
Figure 3.
Change in tinnitus severity and tinnitus qualities during the 8 weeks of the intervention.
Retention, Adherence, and Engagement
The completion rate for the post-intervention was 85%, and for the follow-up outcome measures, 67%. Participant engagement with the intervention was highly variable. During the 8-week intervention, the average number of logins was 20 (SD = 17). An average of 12 (SD = 8) modules were read by participants. During the course of the intervention, participants sent an average of five (SD = 5) messages during the course of the intervention and received an average of 17 messages from the audiologist.
All the participants completed at least the first modules' worksheets. For the initial modules, worksheets were generally completed by 16/27 (59%), and for the last modules, by 10/27 (37%). Engagement thus decreased during the course of the intervention.
Intervention Satisfaction
An average score of 50/75 (67%) was obtained for the post-intervention satisfaction questionnaire with most questions scoring an average of 3–3.5 out of 5 for questions such as suitability of the information, ease of navigation, and benefit of the topics. When answering the open-ended question, participants explained that some of the video captioning was difficult to read and that there were too many worksheets. They felt that more time was needed for the intervention with one participant saying, “I feel the time frame for the study should be longer because the content is excellent but to master the techniques takes longer than the time given.”
Participants also mentioned beneficial aspects of the platform, including the range of techniques provided: “It was helpful learning about a number of techniques to help me cope. If one was difficult it didn't work for me, I could try something else.” Additionally, contents provided on the platform helped participants and that it helped them accept the tinnitus, such as “The most positive aspect of this intervention is that I've ACCEPTED my tinnitus. It isn't a negative and I don't dwell on it. I can comfortably own it, and talk about it with friends. I no longer do I feel that my tinnitus is invasive.” They mentioned finding the materials helpful, for example: “The materials were informative, interesting, well-presented, and easy to consume. There were very clear instructions and tips for practicing the different techniques. I really liked the videos. Examples cited within the text helped me to expand the ways I could apply concepts and techniques to other parts of my life. The writing was factual yet engaging, and easy to apply to my own situation.” The guidance was furthermore beneficial as explained: “It was great to have a contact at any time with the audiologist when needed. The support was understanding, very positive and helpful throughout. It was a great experience.”
Discussion
The primary objective of this pilot study was to investigate the feasibility of a full-scale RCT regarding ICBT for tinnitus in the United States. A pilot study is an essential prerequisite before larger scale RCTs are undertaken (Leon et al., 2012).
The ICBT intervention reduced tinnitus severity significantly when assessed post-intervention and the improvements were maintained at 2 months follow-up. For the current sample, 70% of participants indicated clinically significant changes at post-intervention. Although this outcome may reflect primarily the positive effects of patients receiving tinnitus care, versus no care, the lack of homogeneity in the findings suggests that the notion of providing care, on its own, cannot explain the results. The current results are encouraging and justify further RCTs. Indeed, the findings of this study are in accord with those of the ICBT feasibility trial in the United Kingdom (Beukes et al., 2017).
Tinnitus is often accompanied by various comorbidities, particularly co-occurring mental health conditions. To assess intervention effects on these comorbidities, outcome measures for anxiety, depression, insomnia, hearing-related difficulties, tinnitus cognitions, and health-related quality of life were included. The intervention provided a large effect size related to tinnitus cognitions indicating fewer negative cognitions were associated with tinnitus after completing the intervention. This outcome measure has not been used in previous ICBT trials but was recommended to use for tinnitus therapeutic research (Handscomb et al., 2017). As negative thinking appears to be associated with more problematic tinnitus, intervention reducing such thought patterns are important (Handscomb et al., 2017). Further RCTs are needed to monitor whether and to what degree the ICBT intervention reduces negative tinnitus cognitions.
A medium effect size was found for insomnia, hearing disability, and hyperacusis. This result was encouraging; although significant improvements have been found for insomnia, they have not always been found for hearing disability and hyperacusis in previous trials (e.g., Beukes, Andersson, Allen, et al., 2018; Beukes, Baguley, Allen, et al., 2018). Although the intervention improved some comorbid conditions, effects were not significant for anxiety and depression. The exclusion of individuals with severe mental health conditions likely reduced the opportunity to observe an intervention effect; however, such affected individuals may form an important participant group in subsequent trials.
The intervention was offered through an 8-week period, and from the weekly measures, it appeared as though a 4-week time frame of intervention was sufficient to produce a positive effect, as we previously have reported (Beukes, Baguley, Allen, et al., 2018). These results indicated the feasibility of ICBT in the United States as a suitable intervention. Further RCTs would more conclusively determine the efficacy of this intervention.
The protocol feasibility for ICBT delivered to a U.S. population was investigated during this pilot study. Participant analysis indicated that, although different ethnic groups were recruited, no participants selected to do the intervention in Spanish. They explained that they preferred health-related materials to be in English as they perceived translated versions as less accurate. Further work on effective recruitment strategies to attract Spanish speakers will be needed. Recruitment through word of mouth, building rapport and trust, and personalizing the benefits of participation were suggested to support recruitment of Hispanic and Latino research participants (Sha et al., 2017). Recognizing cultural differences and building trust within Hispanic communities prior to recruitment should be emphasized to support larger trials (Levkoff & Sanchez, 2003).
The overall retention rate of 82% was consistent with that of the previous ICBT for tinnitus studies with rates between 57% and 95% (Beukes et al., 2019). These rates were particularly high for earlier studies (e.g., Abbott et al., 2009; Andersson et al., 2002) and have increased with improvements made in later studies. Those who withdrew in this study indicated the decision was due to time constraints. One person's withdrawal was attributed to the assessment burden of the intervention. Subsequent trials should further highlight the time demands and provide motivational interviewing at the screening stage to encourage intervention engagement and compliance. Completion for the follow-up questionnaire was only 67%, despite numerous reminders. Although more needs to be done to improve these retention rates, the present rates indicate the feasibility of ICBT within the United States, and an effectiveness trial is warranted. Understanding factors contributing to retention in intervention studies is important, and undertaking a process evaluation may be helpful to identify strategies to enhance participation (Beukes, Manchaiah, Baguley, et al., 2018).
Intervention engagement was variable. Despite regular therapeutic encouragement, some participants found it difficult to consistently engage with the intervention. Barriers to engagement included time constraints, family, and work pressures. An unexpected additional barrier was identified: Some participants had previously completed tinnitus retraining therapy, and as part of that protocol, the patients were encouraged to use sound enrichment for at least 8 hr a day. Recall that during the course of the ICBT intervention, participants were asked to not only rely on sound enrichment but also try the other strategies. This approach was very difficult for some participants, who were in the habit of using sound enrichment exclusively, for many years in some cases. Further trials should consider this possible barrier and offer additional instructions for those patients who indicate at intake adherence to a previously recommended sound therapy regimen. As ICBT is largely a self-help therapeutic approach, it is not going to suit all individuals with tinnitus. For some, progress may be more reasonable if patients receive clinical sessions from a professional, either individually or in a group context. Individuals not progressing or engaging should be directed to other forms of care. ICBT may also not be the most appropriate treatment for those with other serious health conditions that may make it difficult to work on an intervention independently. Although ICBT has the potential to reach more individuals, it will not suit everyone, and a range of approaches should be available to these people.
Due to the evidence supporting the use of both ICBT and CBT for tinnitus (Fuller et al., 2020; Landry et al., 2020), further ways of delivering these interventions should be sought. Although formulation driven CBT for specific psychological difficulties or conditions should always be provided by a CBT-licensed psychologist, guided CBT self-help interventions may be assisted by other professionals, and indeed, tenets of CBT are routinely practiced by audiologists with regard to audiological rehabilitation and falls prevention. Previous studies for other health conditions have indicated that the level of qualification and experience of the professional providing guidance does not appear to affect treatment efficacy (Baumeister et al., 2014). Outcomes have, for instance, been comparable using a psychologist versus a technical assistant for depression (Titov et al., 2010), social phobia, (Titov et al., 2009), and anxiety (Robinson et al., 2010). Likewise, no significant difference in outcomes was found when comparing guidance by a psychologist versus a student psychologist for social anxiety (Andersson et al., 2012). Similarly, no difference was found when comparing guidance between psychologists with and without specialist training for anxiety (Johnston et al., 2011). Outcomes have, for instance, been comparable using a psychologist versus a technical assistant for social phobia and depression (Titov et al., 2010). Likewise, favorable outcomes were obtained using an audiologist instead of a psychologist for ICBT for tinnitus in the U.K. population (e.g., Beukes, Andersson, Allen, et al., 2018; Beukes, Baguley, Allen, et al., 2018; Beukes et al., 2019). Equipping audiologists to deliver or guide psychological interventions such as CBT should be prioritized during audiology training programs and continued professional development opportunities. The importance of available remotely accessible tinnitus interventions have been highlighted during the tinnitus pandemic, and ways of delivering these should be sought (Beukes, Baguley, et al., 2020; Beukes, Onozuka, et al., 2021).
Overall intervention satisfaction was lower than that reported for ICBT when presented in the United Kingdom (Beukes, Manchaiah, Baguley, et al., 2018; Beukes, Manchaiah, Davies, et al., 2018). This was surprising as great efforts were made to ensure that the intervention was culturally and linguistically suitable for this population (Beukes, Fagelson, et al., 2020; Manchaiah, Muñoz, et al., 2020). Suggestions made by participants in the free text should be implemented to see if satisfaction can be improved. Public involvement in planning and implementing subsequent research phases should consider the factors important to participants (Staniszewska et al., 2019). Numerous other CBT interventions for tinnitus have been developed (e.g., Schmidt et al., 2018), and increasing evidence for their effects are shown in reducing tinnitus distress and associated problems such as insomnia (e.g., Curtis et al., 2020). Evaluating the components of each to ensure the most suitable intervention is delivered should be investigated with the goal of improving patient outcomes.
Limitations
The results of this study need to be considered in the context of this study. This study represents a pilot investigation to identify the feasibility of ICBT in the United States, and the results were not intended to evaluate the efficacy of ICBT as no control group was included and only a small sample was studied. The placebo effect may be present, which could elevate findings and need to be considered during result interpretation. Although the results were maintained at 2 months post-intervention, further studies are required to assess whether they are maintained long term. These results could be further explored in an RCT.
Conclusions
Tinnitus is a prevalent condition that can be very debilitating. Ways of increasing access to standardized evidence-based interventions for tinnitus are required. Together with the urgent need to improve access to evidence-based tinnitus interventions, the COVID-19 pandemic has highlighted the need for evidence-based teleaudiology approaches to overcome limited in-person contact. Due to the importance of such remote intervention tools being highlighted during the COVID-19 pandemic, some barriers to implementing Internet-interventions may be addressed. ICBT has the potential to reduce the debilitating effects of tinnitus, but is not available in the United States. An ICBT intervention was adapted linguistically and culturally for a U.S. population, but its efficacy in an RCT remains unknown. This pilot study has indicated the feasibility of ICBT for tinnitus in the United States. The results have been encouraging and further RCTs should be undertaken (Beukes, Andersson, Fagelson, & Manchaiah, 2021).
Supplementary Material
Acknowledgments
This work is funded by the National Institute on Deafness and Communication Disorders under Award R21DC017214. We would like to thank webmasters George Vlaescu and Shrinivas Varadaraj for the help in installing the intervention platform and for the technical assistance provided. Our thanks are extended to all the participants who partook in this study and to the organizations that helped with recruitment. We also thank Stacia DeSantis for statistical assistance.
Funding Statement
This work is funded by the National Institute on Deafness and Communication Disorders under Award R21DC017214.
References
- Abbott, J. M. , Kaldo, V. , Klein, B. , Austin, D. , Hamilton, C. , Piterman, L. , & Andersson, G. (2009). A cluster randomised trial of an Internet-based intervention program for tinnitus distress in an industrial setting. Cognitive Behaviour Therapy, 38(3), 162–173. https://doi.org/10.1080/16506070902763174 [DOI] [PubMed] [Google Scholar]
- Andersson, G. , Carlbring, P. , & Furmark, T. (2012). Therapist experience and knowledge acquisition in internet-delivered CBT for social anxiety disorder: A randomized controlled trial. PLOS ONE, 7(5), e37411. https://doi.org/10.1371/journal.pone.0037411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, G. , & Kaldo, V. (2004). Internet-based cognitive behavioral therapy for tinnitus. Journal of Clinical Psychology, 60(2), 171–178. https://doi.org/10.1002/jclp.10243 [DOI] [PubMed] [Google Scholar]
- Andersson, G. , Strömgren, T. , Ström, L. , & Lyttkens, L. (2002). Randomized controlled trial of Internet-based cognitive behavior therapy for distress associated with tinnitus. Psychosomatic Medicine, 64(5), 810–816. https://doi.org/10.1097/01.PSY.0000031577.42041.F8 [DOI] [PubMed] [Google Scholar]
- Bastien, C. H. , Vallières, A. , & Morin, C. M. (2001). Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Medicine, 2(4), 297–307. https://doi.org/10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- Baumeister, H. , Reichler, L. , Munzinger, M. , & Lin, J. (2014). The impact of guidance on Internet-based mental health interventions—A systematic review. Internet Interventions, 1(4), 205–215. https://doi.org/10.1016/j.invent.2014.08.003 [Google Scholar]
- Beukes, E. W. , Allen, P. M. , Manchaiah, V. , Baguley, D. M. , & Andersson, G. (2017). Internet-based intervention for tinnitus: Outcome of a single-group open trial. Journal of the American Academy of Audiology, 28(4), 340–351. https://doi.org/10.3766/jaaa.16055 [DOI] [PubMed] [Google Scholar]
- Beukes, E. W. , Andersson, G. , Allen, P. M. , Manchaiah, V. , & Baguley, D. M. (2018). Effectiveness of guided Internet-based cognitive behavioral therapy vs face-to-face clinical care for treatment of tinnitus: A randomized clinical trial. JAMA Otolaryngology–Head & Neck Surgery, 144(12), 1126–1133. https://doi.org/10.1001/jamaoto.2018.2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukes, E. , Andersson, G. , Fagelson, M. A. , & Manchaiah, V. (2021). Internet-based audiologist-guided Internet-based cognitive behavioral therapy for tinnitus in the United States: A randomized controlled trial. Journal of Medical Internet Research Preprints. https://doi.org/10.2196/preprints.27584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukes, E. , Andersson, G. , Manchaiah, V. , & Kaldo, V. (2021). Cognitive behavioral therapy for tinnitus. Plural. [Google Scholar]
- Beukes, E. W. , Baguley, D. M. , Allen, P. M. , Manchaiah, V. , & Andersson, G. (2018). Audiologist-guided Internet-based cognitive behavior therapy for adults with tinnitus in the United Kingdom: A randomized controlled trial. Ear and Hearing, 39(3), 423–433. https://doi.org/10.1097/AUD.0000000000000505 [DOI] [PubMed] [Google Scholar]
- Beukes, E. W. , Baguley, D. M. , Jacquemin, L. , Lourenco, M. P. C. G. , Allen, P. M. , Onozuka, J. , Stockdale, D. , Kaldo, V. , Andersson, G. , & Manchaiah, V. (2020). Changes in tinnitus experiences during the COVID-19 pandemic. Frontiers in Public Health, 8, 592878. https://doi.org/10.3389/fpubh.2020.592878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukes, E. W. , Fagelson, M. , Aronson, E. P. , Munoz, M. F. , Andersson, G. , & Manchaiah, V. (2020). Readability following cultural and linguistic adaptations of an Internet-based intervention for tinnitus for use in the United States. American Journal of Audiology, 29(2), 97–109. https://doi.org/10.1044/2019_AJA-19-00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukes, E. W. , Manchaiah, V. , Allen, P. M. , Andersson, G. , & Baguley, D. M. (2020). Exploring tinnitus heterogeneity. Elsevier.https://doi.org/10.1016/bs.pbr.2020.05.022 [DOI] [PubMed] [Google Scholar]
- Beukes, E. W. , Manchaiah, V. , Allen, P. M. , Baguley, D. M. , & Andersson, G. (2019). Internet-based interventions for adults with hearing loss, tinnitus, and vestibular disorders: A systematic review and meta-analysis. Trends in Hearing, 23, 233121651985174. https://doi.org/10.1177/2331216519851749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukes, E. W. , Manchaiah, V. , Baguley, D. M. , Allen, P. M. , & Andersson, G. (2018). Process evaluation of Internet-based cognitive behavioural therapy for adults with tinnitus in the context of a randomised control trial. International Journal of Audiology, 57(2), 98–109. https://doi.org/10.1080/14992027.2017.1384858 [DOI] [PubMed] [Google Scholar]
- Beukes, E. W. , Manchaiah, V. , Davies, A. S. A. , Allen, P. M. , Baguley, D. M. , & Andersson, G. (2018). Participants' experiences of an Internet-based cognitive behavioural therapy intervention for tinnitus. International Journal of Audiology, 57(12), 947–954. https://doi.org/10.1080/14992027.2018.1514538 [DOI] [PubMed] [Google Scholar]
- Beukes, E. W. , Onozuka, J. , Brazell, T. P. , & Manchaiah, V. (2021). Coping with tinnitus during the COVID-19 pandemic. American Journal of Audiology, 30(2), 385–393. https://doi.org/10.1044/2021_AJA-20-00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukes, E. W. , Vlaescu, G. , Manchaiah, V. , Baguley, D. M. , Allen, P. M. , Kaldo, V. , & Andersson, G. (2016). Development and technical functionality of an Internet-based intervention for tinnitus in the UK. Internet Interventions, 6, 6–15. https://doi.org/10.1016/j.invent.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt, J. M. , Lin, H. W. , & Bhattacharya, N. (2016). Prevalence, severity, exposures, and treatment patterns of tinnitus in the united states. JAMA Otolaryngology–Head & Neck Surgery, 142(10), 959–965. https://doi.org/10.1001/jamaoto.2016.1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, M. , Fitzpatrick, R. , Haines, A. , Kinmonth, A. L. , Sandercock, P. , Spiegelhalter, D. , & Tyrer, P. (2000). Framework for design and evaluation of complex interventions to improve health. BMJ, 321(7262), 694–696. https://doi.org/10.1136/bmj.321.7262.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. (1992). A power primer. Psychological Bulletin, 112(1), 155–159. https://doi.org/10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- Curtis, F. , Laparidou, D. , Bridle, C. , Law, G. R. , Durrant, S. , Rodriguez, A. , Pierzycki, R. H. , & Siriwardena, A. N. (2020). Effects of cognitive behavioural therapy on insomnia in adults with tinnitus: Systematic review and meta-analysis of randomised controlled trials. Sleep Medicine Reviews, 56, 101405. https://doi.org/10.1016/j.smrv.2020.101405 [DOI] [PubMed] [Google Scholar]
- Des Jarlais, D. C. , Lyles, C. , Crepaz, N. , & TREND Group. (2004). Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: The TREND statement. American Journal of Public Health, 94(3), 361–366. https://doi.org/10.2105/AJPH.94.3.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller, T. , Cima, R. , Langguth, B. , Mazurek, B. , Vlaeyen, J. W. , & Hoare, D. J. (2020). Cognitive behavioural therapy for tinnitus. Cochrane Database of Systematic Reviews, 2020(1), Article CD012614. https://doi.org/10.1002/14651858.CD012614.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller, T. E. , Haider, H. F. , Kikidis, D. , Lapira, A. , Mazurek, B. , Norena, A. , Rabau, S. , Lardinois, R. , Cederroth, C. R. , Edvall, N. K. , Brueggemann, P. G. , Rosing, S. N. , Kapandais, A. , Lungaard, D. , Hoare, D. J. , & Cima, R. F. F. (2017). Different teams, same conclusions? A systematic review of existing clinical guidelines for the assessment and treatment of tinnitus in adults. Frontiers in Psychology, 8, 206. https://doi.org/10.3389/fpsyg.2017.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handscomb, L. E. , Hall, D. A. , Shorter, G. W. , & Hoare, D. J. (2017). Positive and negative thinking in tinnitus: Factor structure of the tinnitus cognitions questionnaire. Ear and Hearing, 38(1), 126–132. https://doi.org/10.1097/AUD.0000000000000365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, J. A. , Griest, S. , Thielman, E. , McMillan, G. , Kaelin, C. , & Carlson, K. F. (2016). Tinnitus functional index: Development, validation, outcomes research, and clinical application. Hearing Research, 334, 58–64. https://doi.org/10.1016/j.heares.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Henry, J. A. , Griest, S. , Zaugg, T. L. , Thielman, E. , Kaelin, C. , Galvez, G. , & Carlson, K. F. (2015). Tinnitus and hearing survey: A screening tool to differentiate bothersome tinnitus from hearing difficulties. American Journal of Audiology, 24(1), 66–77. https://doi.org/10.1044/2014_AJA-14-0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, J. , Piskosz, M. , Norena, A. , & Fournier, P. (2019). Audiologists and tinnitus. American Journal of Audiology, 28(4), 1059–1064. https://doi.org/10.1044/2019_AJA-19-0070 [DOI] [PubMed] [Google Scholar]
- Henry, J. A. , Thielman, E. J. , Zaugg, T. L. , Kaelin, C. , McMillan, G. P. , Schmidt, C. J. , Myers, P. J. , & Carlson, K. F. (2019). Telephone-based progressive tinnitus management for persons with and without traumatic brain injury: A randomized controlled trial. Ear and Hearing, 40(2), 227–242. https://doi.org/10.1097/AUD.0000000000000609 [DOI] [PubMed] [Google Scholar]
- Henry, J. A. , Zaugg, T. L. , Myers, P. J. , & Kendall, C. J. (2010). Progressive tinnitus management: Clinical handbook for audiologists (pp. 59–97). VA Employee Education System. [Google Scholar]
- Herdman, M. , Gudex, C. , Lloyd, A. , Janssen, M. F. , Kind, P. , Parkin, D. , Bonsel, G. , & Badia, X. (2011). Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Quality of Life Research, 20(7), 1727–1736. https://doi.org/10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson, N. S. , & Truax, P. (1991). Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology, 59(1), 12–19. https://doi.org/10.1037//0022-006X.59.1.12 [DOI] [PubMed] [Google Scholar]
- Jastreboff, P. J. , & Jastreboff, M. M. (2000). Tinnitus Retraining Therapy (TRT) as a method for treatment of tinnitus and hyperacusis patients. Journal of the American Academy of Audiology, 11(3), 162–177. [PubMed] [Google Scholar]
- Johnston, L. , Titov, N. , Andrews, G. , Spence, J. , & Dear, B. F. (2011). A RCT of a transdiagnostic Internet-delivered treatment for three anxiety disorders: Examination of support roles and disorder-specific outcomes. PLOS ONE, 6(11), e28079. https://doi.org/10.1371/journal.pone.0028079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H.-J. , Lee, H.-J. , An, S.-Y. , Sim, S. , Park, B. , Kim, S. W. , Lee, J. S. , Hong, S. K. , & Choi, H. G. (2015). Analysis of the prevalence and associated risk factors of tinnitus in adults. PLOS ONE, 10(5), e0127578. https://doi.org/10.1371/journal.pone.0127578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostev, K. , Alymova, S. , Kössl, M. , & Jacob, L. (2019). Risk factors for tinnitus in 37,692 patients followed in general practices in Germany. Otology & Neurotology, 40(4), 436–440. https://doi.org/10.1097/MAO.0000000000002161 [DOI] [PubMed] [Google Scholar]
- Landry, E. C. , Sandoval, X. C. R. , Simeone, C. N. , Tidball, G. , Lea, J. , & Westerberg, B. D. (2020). Systematic review and network meta-analysis of cognitive and/or behavioral therapies (CBT) for tinnitus. Otology & Neurotology, 41(2), 153–166. https://doi.org/10.1097/MAO.0000000000002472 [DOI] [PubMed] [Google Scholar]
- Leon, A. C. , Davis, L. L. , & Kraemer, H. C. (2012). The role and interpretation of pilot studies in clinical research. Journal of Psychiatric Research, 45(5), 626–629. https://doi.org/10.1016/j.jpsychires.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkoff, S. , & Sanchez, H. (2003). Lessons learned about minority recruitment and retention from the centers on minority aging and health promotion. The Gerontologist, 43(1), 18–26. https://doi.org/10.1093/geront/43.1.18 [DOI] [PubMed] [Google Scholar]
- Mahan, V. L. (2014). Clinical trial phases. International Journal of Clinical Medicine, 5(21), 1374–1383. https://doi.org/10.4236/ijcm.2014.521175 [Google Scholar]
- Manchaiah, V. , Beukes, E. W. , Granberg, S. , Durisala, N. , Baguley, D. M. , Allen, P. M. , & Andersson, G. (2018). Problems and life effects experienced by tinnitus research study volunteers: An exploratory study using the ICF classification. Journal of the American Academy of Audiology, 29(10), 936–947. https://doi.org/10.3766/jaaa.17094 [DOI] [PubMed] [Google Scholar]
- Manchaiah, V. , Muñoz, M. F. , Hatfield, E. , Fagelson, M. A. , Parks Aronson, E. , Andersson, G. , & Beukes, E. W. (2020). Translation and adaptation of three English tinnitus patient-reported outcome measures to Spanish. International Journal of Audiology, 59(7), 513–518. https://doi.org/10.1080/14992027.2020.1717006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchaiah, V. , Vlaescu, G. , Varadaraj, S. , Aronson, E. P. , Fagelson, M. A. , Munoz, M. F. , Andersson, G. , & Beukes, E. W. (2020). Features, functionality, and acceptability of Internet-based cognitive behavioral therapy for tinnitus in the United States. American Journal of Audiology, 29(3), 476–490. https://doi.org/10.1044/2020_AJA-20-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack, A. , Edmondson-Jones, M. , Somerset, S. , & Hall, D. (2016). A systematic review of the reporting of tinnitus prevalence and severity. Hearing Research, 337, 70–79. https://doi.org/10.1016/j.heares.2016.05.009 [DOI] [PubMed] [Google Scholar]
- Meikle, M. B. , Henry, J. A. , Griest, S. E. , Stewart, B. J. , Abrams, H. B. , McArdle, R. , Myers, P. J. , Newman, C. W. , Sandridge, S. , Turk, D. C. , Folmer, R. L. , Frederick, E. J. , House, J. W. , Jacobson, G. P. , Kinney, S. E. , Martin, W. H. , Nagler, S. M. , Reich, G. E. , Searchfield, G. , … Vernon, J. A. (2012). The Tinnitus Functional Index: Development of a new clinical measure for chronic, intrusive tinnitus. Ear and Hearing, 33, 153–176. https://doi.org/10.1097/AUD.0b013e31822f67c0 [DOI] [PubMed] [Google Scholar]
- Newman, C. W. , Sandridge, S. A. , & Bolek, L. (2008). Development and psychometric adequacy of the screening version of the Tinnitus Handicap Inventory. Otology & Neurotology, 29(3), 276–281. https://doi.org/10.1097/MAO.0b013e31816569c4 [DOI] [PubMed] [Google Scholar]
- Planey, A. M. (2019). Audiologist availability and supply in the United States: A multi-scale spatial and political economic analysis. Social Science & Medicine, 222(2), 216–224. https://doi.org/10.1016/j.socscimed.2019.01.015 [DOI] [PubMed] [Google Scholar]
- Robinson, E. , Titov, N. , Andrews, G. , McIntyre, K. , Schwencke, G. , & Solley, K. (2010). Internet treatment for generalized anxiety disorder: A randomized controlled trial comparing clinician vs. technician assistance. PLOS ONE, 5(6), e10942. https://doi.org/10.1371/journal.pone.0010942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, C. J. , Kerns, R. D. , Finkel, S. , Michaelides, E. , & Henry, J. A. (2018). Cognitive behavioral therapy for veterans with tinnitus. Federal Practitioner, 35(8), 36–46. [PMC free article] [PubMed] [Google Scholar]
- Sha, M. , McAvinchey, G. , Quiroz, R. , & Moncada, J. (2017). Successful techniques to recruit Hispanic and Latino research participants. Survey Practice, 10(3), 1–9. https://doi.org/10.29115/SP-2017-0014 [Google Scholar]
- Spitzer, R. L. , Kroenke, K. , Williams, J. B. W. , & the Patient Health Questionnaire Primary Care Study Group. (1999). Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. JAMA, 282(18), 1737–1744. https://doi.org/10.1001/jama.282.18.1737 [DOI] [PubMed] [Google Scholar]
- Spitzer, R. L. , Kroenke, K. , Williams, J. B. W. , & Löwe, B. (2006). A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine, 166(10), 1092–1097. https://doi.org/10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- Staniszewska, S. , Brett, J. , Simera, I. , Seers, K. , Mockford, C. , Goodlad, S. , Altman, D. G. , Moher, D. , Barber, R. , Denegri, S. , Entwistle, A. , Littlejohns, P. , Morris, C. , Suleman, R. , Thomas, V. , & Tysall, C. (2019). GRIPP2 reporting checklists: Tools to improve reporting of patient and public involvement in research. Research Involvement and Engagement, 3(1), 13. https://doi.org/10.1186/s40900-017-0062-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titov, N. , Andrews, G. , Davies, M. , McIntyre, K. , Robinson, E. , & Solley, K. (2010). Internet treatment for depression: A randomized controlled trial comparing clinician vs. technician assistance. PLOS ONE, 5(6), Article e10939. https://doi.org/10.1371/journal.pone.0010939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titov, N. , Andrews, G. , Schwencke, G. , Solley, K. , Johnston, L. , & Robinson, E. (2009). An RCT comparing effect of two types of support on severity of symptoms for people completing Internet-based cognitive behaviour therapy for social phobia. Australian and New Zealand Journal of Psychiatry, 43(10), 920–926. https://doi.org/10.1080/00048670903179228 [Google Scholar]
- Tunkel, D. E. , Bauer, C. A. , Sun, G. H. , Rosenfeld, R. M. , Chandrasekhar, S. S. , Cunningham, E. R., Jr. , Archer, S. M. , Blakley, B. W. , Carter, J. M. , Granieri, E. C. , Henry, J. A. , Hollingsworth, D. , Khan, F. A. , Mitchell, S. , Monfared, A. , Newman, C. W. , Omole, F. S. , Phillips, C. D. , Robinson, S. K. , … Whamond, E. J. (2014). Clinical practice guideline: Tinnitus. Otolaryngology—Head & Neck Surgery, 151(Suppl. 2), S1–S40. https://doi.org/10.1177/0194599814545325 [DOI] [PubMed] [Google Scholar]
- Tyler, R. S. , Perreau, A. , Powers, T. , Watts, A. , Owen, R. , Ji, H. , & Mancini, P. C. (2020). Tinnitus sound therapy trial shows effectiveness for those with tinnitus. Journal of the American Academy of Audiology, 31(1), 006–016. https://doi.org/10.3766/jaaa.18027 [DOI] [PubMed] [Google Scholar]
- U.S Department of Health and Human Services. (2010). Healthy people. U.S. Government Printing Office. https://www.cdc.gov/nchs/healthy_people/hp2010.htm [Google Scholar]
- Weise, C. , Kleinstäuber, M. , & Andersson, G. (2016). Internet-delivered cognitive-behavior therapy for tinnitus: A randomized controlled trial. Psychosomatic Medicine, 78(4), 501–510. https://doi.org/10.1097/PSY.0000000000000310 [DOI] [PubMed] [Google Scholar]
- Wilson, P. , & Henry, J. (1998). Tinnitus Cognitions Questionnaire: Development and psychometric properties of a measure of dysfunctional cognitions associated with tinnitus. The International Tinnitus Journal, 4(1), 23–30. [PubMed] [Google Scholar]
- Zenner, H.-P. , Delb, W. , Kröner-Herwig, B. , Jäger, B. , Peroz, I. , Hesse, G. , Mazurek, B. , Goebel, G. , Gerloff, C. , Trollmann, R. , Biesinger, E. , Seidler, H. , & Langguth, B. (2017). A multidisciplinary systematic review of the treatment for chronic idiopathic tinnitus. European Archives of Oto-Rhino-Laryngology, 274, 2079–2091. https://doi.org/10.1007/s00405-016-4401-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.