Abstract
Background
Patients with aggressive lymphomas are at higher risk for venous thromboembolism (VTE). ThroLy is a risk assessment model (RAM) derived to predict the occurrence of VTE in various types of lymphomas. In this study, we assess the clinical application of ThroLy RAM in a unified group of patients with diffuse large B-cell lymphoma (DLBCL).
Methods
Hospital databases were searched for patients with DLBCL and radiologically-confirmed VTE. Items in the ThroLy RAM, including prior VTE, reduced mobility, obesity, extranodal disease, mediastinal involvement, neutropenia and hemoglobin < 10.0 g/dL, were retrospectively reviewed.
Results
A total of 524 patients, median age 49 (range: 18-90) years were included. Patients had high disease burden; 57.3% with stage III/IV and 34.0% with bulky disease. All were treated on unified guidelines; 63 (12.0%) had primary refractory disease. Venous thromboembolic events were reported in 71 (13.5%) patients. Among 121 patients with high (> 3) ThroLy score, 22.3% developed VTE compared to 8.4% and 12.4% in those with low and intermediate risk scores, respectively (P = .014). Simplifying the ThroLy model into two risk groups; high-risk (score ≥ 3) and low risk (score < 3) can still segregate patients; VTE developed in 44 (17.2%) high-risk patients (n = 256) compared to 27 (10.1%) in the low-risk group (n = 268), P = .038. Neutropenia, a component of the ThroLy, was encountered in only 14 (2.7%) patients.
Conclusions
ThroLy RAM can identify patients with DLBCL at high risk for VTE. Model can be modified by dividing patients into two, rather than three risk groups, and further simplified by omitting neutropenia.
Keywords: lymphoma, NHL, diffuse large B-cell lymphoma, DLBCL, venous thromboembolism, VTE, ThroLy, risk assessment model
Introduction
Non-Hodgkin's Lymphomas (NHL) represent over 90% of all lymphomas and are relatively common tumors. According to the 2020 Globocan report, over 500 000 cases are diagnosed annually.1 The NHL entail considerable heterogeneity with regard to morphological, clinical and molecular characteristics.2 Diffuse Large B Cell Lymphoma (DLBCL) is an aggressive lymphoma and accounts for 25% of all NHL encountered among adults worldwide.3
It is well established that patients with cancer are at higher risk for venous thromboembolism (VTE).4,5 The risk of VTE depends on the type and stage of cancer, treatment, comorbid features, and patients’ demographics. 6 When encountered in cancer patients, VTE may result in significant delays in diagnostic and therapeutic interventions, including needed biopsies, surgical resection of a tumor or delivering chemotherapy. Additionally, it may lead to chronic complications,7,8 impact negatively on quality of patients’ life and increase mortality. 9
Patients with NHL undergoing active chemotherapy are known to be at even higher risk of developing VTE.10,11 Several studies have demonstrated a higher risk of VTE with the addition of doxorubicin to the cyclophosphamide-vincristine-prednisone regimen (CHOP vs CVP) in patients with lymphoma.12
Venous thromboembolism prophylaxis is almost routinely practiced for cancer patients admitted for surgical interventions or with acute medical illness.13 However, many researchers raised concerns about rising incidence of VTE among cancer patients treated with chemotherapy in ambulatory setting where VTE prophylaxis is not routinely offered.14–16
Several risk assessments models (RAM) where derived addressing cancer patients as a group, while others addressed particular cancer sites.17–19 The multivariable model of Thrombosis Lymphoma (ThroLy) RAM was developed and validated by Antic et al.20 In this model, multiple clinical variables were grouped into different risk levels. These variables include previous venous and/or arterial thromboembolic events, mediastinal involvement, obesity with body mass index (BMI) > 30 kg/m2, hemoglobin (Hb) level < 100g/L, extranodal localization, reduced mobility, and development of neutropenia. Based on the added scores, patients were then divided into 3 risk categories; low risk (0-1), intermediate risk (2-3), high risk (> 3), Table 1.20
Table 1.
ThroLy Risk Assessment Model.
| Patients’ characteristics | Assigned score |
|---|---|
| Previous venous thromboembolic event | 2 |
| Reduced mobility | 1 |
| Previous acute myocardial infarction or stroke | 2 |
| Body Mass Index (BMI > 30) | 2 |
| Extranodal localization | 1 |
| Mediastinal involvement | 2 |
| Neutropenia | 1 |
| Hemoglobin (< 100 g/L) | 1 |
Total score: add all assigned scores.
Low risk (0-1), Intermediate risk (2-3), High risk (> 3).
In this study, we assess the clinical application of the original and a suggested simplified version of the ThroLy RAM in patients with DLBCL, as a unified group of NHL undergoing chemotherapy in ambulatory setting.
Patients and Methods
Patients with pathologically-confirmed diagnosis of DLBCL were identified and data was extracted retrospectively from hospital databases and electronic medical records. All patients were diagnosed, treated and followed up at our institution. VTE cases were radiologically- confirmed; Deep vein thrombosis (DVT) was diagnosed by doppler ultrasound and computed tomography (CT) angiography was used to diagnose pulmonary embolism (PE) for symptomatic cases; routine screening for VTE was not done.
Items in the ThroLy RAM were reviewed, including previous VTE, reduced mobility, obesity, extranodal disease, mediastinal involvement, low neutrophil counts (ANC < 1 × 109/L) and Hb < 100.0 g/dL.
Thrombosis was considered chemotherapy-related if diagnosed any time after the first dose and up to 4 weeks after the last. Major bleeding, as a complication of anticoagulation, was defined as fatal bleeding and/or symptomatic bleeding in a critical organ and/or bleeding causing a fall in hemoglobin level of ≥ 2.0 g/dL, or leading to transfusion of two or more units of red blood cells. Venous thromboembolism was considered ambulatory, if the patients was not admitted to the hospital during the 30 days prior to VTE diagnosis.
Other clinical and laboratory data related to VTE, including patients’ age, smoking history, Eastern Cooperative Oncology Group performance status (ECOG PS), personal history of VTE, lactate dehydrogenase levels (LDH), response to initial treatment and salvage chemotherapy were also collected.
The study was approved by our Institutional Review Board (IRB) and was carried in accordance with the international guidelines for retrospective studies.
Statistical Analysis
Descriptive data of the patients’ baseline demographical and clinical characteristics were obtained. Patients’ data have been tabulated and described by ranges, medians, and percentages (%). Continuous variables with normal distribution were presented as median (range), and categorical variables were expressed as numbers (percentages).
Risk scores for ThroLy RAM have been tabulated and compared using chi square test. Univariate logistic regression was used to evaluate potential risk factors that may influence VTE. A multivariate analysis was performed with variables found to be significant in the univariate analysis. The odds ratio (OR) for each independent variable was determined with a confidence interval (CI) of 95%. A P-value < .05 was considered as statistically significant.
Results
Patients’ Characteristics
Between 2006 and 2019, a total of 524 patients were diagnosed with DLBCL. Median age was 49 (range: 18-90) years, and 52.3% were males. Stage III and IV disease, at diagnosis, was seen in 57.3% of the patients. Moreover, bulky disease (tumor size more than 10 cm) and mediastinal involvement were present in 178 (34.0%) and 207 (39.5%) patients, respectively. At time of diagnosis, 85 (16.2%) patients had an ECOG PS of ≥ 2. Almost 70% of the study group received R-CHOP regimen (Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone) as an initial treatment and 402 (76.7%) patients had complete response (CR). Patients’ clinical characteristics are summarized in Table 2.
Table 2.
Patients Characteristics (n = 524).
| Number | Percentage | ||
|---|---|---|---|
| Age (Years) | Median: 49 | ||
| Range: 18 to 90 | |||
| Gender | Female | 250 | 47.7 |
| Male | 274 | 52.3 | |
| Disease stage | I-II | 224 | 42.7 |
| III- IV | 300 | 57.3 | |
| Smoking | Active smoker | 139 | 26.5 |
| Prior-smoker | 65 | 12.4 | |
| Never smoked | 314 | 60.0 | |
| Unknown | 6 | 1.1 | |
| BMI (kg/m2) | ≤ 30 | 358 | 68.3 |
| > 30 | 161 | 30.7 | |
| LDH | Low | 226 | 43.1 |
| High | 291 | 55.5 | |
| ECOG Performance Status (PS) | 0 to 1 | 439 | 83.8 |
| 2 | 43 | 8.2 | |
| 3 to 4 | 42 | 8.0 | |
| Hemoglobin (g/L) | < 100 | 57 | 10.9 |
| ≥ 100 | 466 | 88.9 | |
| Response to Treatment | Complete Response (CR) | 402 | 76.7 |
| Partial Response (PR) | 38 | 7.3 | |
| Disease Progression (DP) | 63 | 12.0 | |
| NA | 12 | 2.3 | |
| History of VTE | 45 | 8.6 | |
| Neutropenia (ANC< 1000) | 14 | 2.7 | |
| Extranodal localization | 350 | 66.8 | |
| Mediastinal involvement | 207 | 39.5 | |
| Bulky Disease | 178 | 34.0 | |
| Salvage chemotherapy | 86 | 16.4 | |
BMI: Body Mass Index; LDH: Lactic Dehydrogenase; ECOG: Eastern Cooperative Oncology Group; NA: Not available; VTE: Venous Thromboembolism; ANC: Absolute Neutrophil Count.
Venous Thromboembolism
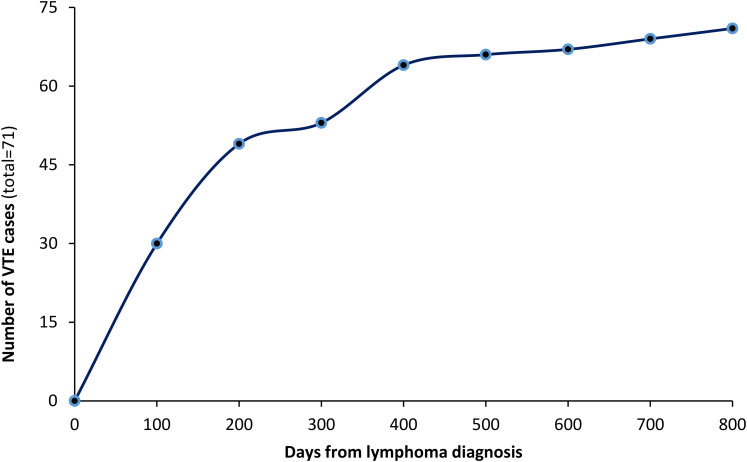
Of all patients included, VTE were reported in 71 (13.5%). The median time from DLBCL diagnosis to VTE was 4.0 months (range: 0-23), Figure 1. All patients had active disease at time of VTE diagnosis, with more than half (56.3%) experienced the VTE while on chemotherapy and 37 (52.1%) patients were ambulatory. Most common site of VTE was the lower extremities (n = 26, 37%), followed by upper extremities (n = 20, 28.2%), while PE alone or with DVT, was diagnosed in 22 (31.0%) patients, Table 3. Patients were treated with anticoagulation, mostly with low molecular weight heparin (LMWH) for a median duration of 6 (range: 1-12) months.
Figure 1.
Cumulative VTE cases from date of diagnosis.
Table 3.
Characteristics of Patients with Venous Thromboembolism (VTE).
| Characteristics | Number (%) |
|---|---|
| Site of thrombosis | |
| Upper extremity | 20 (28.2) |
| Lower extremity | 26 (36.6) |
| Pulmonary embolism | 22 (31.0) |
| Other | 22 (31.0) |
| Time of VTE occurrence | |
| Before chemotherapy | 23 (32.4) |
| During initial chemotherapy | 40 (56.3) |
| After chemotherapy | 8 (11.3) |
| Admission within 30 days | |
| Yes | 34 (47.9) |
| No | 37 (52.1) |
| Disease status | |
| Active disease | 66 (93.0) |
| Disease free | 5 (7.0) |
Analysis of Risk Factors of VTE
In Univariate analysis, bulky disease (odd ratio OR = 1.56, 95% CI, .99-2.44; P = .049), history of prior VTE (OR = 2.06, 95% CI, 1.00-4.26; P = .046), reduced mobility (ECOG PS 2-4), (OR = 2.23, 95% CI, 1.29-3.85; P = .003), and low hemoglobin at baseline (OR = 2.95, 95% CI, 1.59-5.43; P < .001) were found to be significantly associated with the occurrence of VTE. However, in multivariate analysis, only low hemoglobin levels (OR = 2.79, 95% CI, 1.42-5.49; P = .003), and bulky diseases (OR = 2.22, 95% CI, 1.31-3.75; P = .003) were associated with significantly higher risk of VTE, Table 4.
Table 4.
Analysis of Independent Risk Factors (Multivariate Analysis).
| Variables | Odd Ratio (OR) | 95% Confidence Interval (CI) | P-Value |
|---|---|---|---|
| Hemoglobin < 100 g/L | 2.798 | 1.423, 5.498 | .003 |
| History of VTE | 1.659 | .716, 3.847 | .237 |
| Reduced mobility (ECOG 2-4) | 1.888 | .989, 3.606 | .054 |
| High ThroLy score | 1.487 | .799, 2.769 | .211 |
| Bulky disease | 2.217 | 1.309, 3.753 | .003 |
ECOG: Eastern Cooperative Oncology Group; VTE: Venous Thromboembolism.
Risk Assessment Model (RAM)
According to the ThroLy risk assessment model, 154 (29.4%) patients were considered low risk, 249 (47.5%) patients had intermediate risk and 121 (23.1%) patients were high risk for VTE development. Patients with high risk ThroLy score had significantly higher rates of VTE occurrence (n = 27, 22.3%) compared to low risk and intermediate risk (8.4% and 12.4%), respectively, P = .014, Table 5.
Table 5.
Venous Thromboembolism (VTE) Rates in Throly Risk Groups.
| Risk Score and Index | Parameter | Patients (n = 524) | VTE (n = 71) | p-value | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| ThroLy score | Low risk (0-1) | 154 | 29.4 | 13 | 8.4 | .014 |
| Intermediate risk (2-3) | 249 | 47.5 | 31 | 12.4 | ||
| High risk (> 3) | 121 | 23.1 | 27 | 22.3 | ||
| Modified ThroLy | Low risk (< 3) | 268 | 51.1 | 27 | 10.1 | .038 |
| High risk (≥ 3) | 256 | 48.9 | 44 | 17.2 | ||
| Khorana | Intermediate risk (1-2) | 428 | 81.7 | 51 | 11.9 | .080 |
| High risk (≥ 3) | 90 | 17.2 | 18 | 20.0 | ||
Simplifying the ThroLy RAM by grouping the patients into two risk groups; high-risk (score ≥ 3) and low risk (score < 3) can still segregate patients into different risk levels; VTE developed in 44 (17.2%) high-risk patients (n = 256) compared to 27 (10.1%) in the low-risk group (n = 268), P = .038 (Table 5).
Discussion
Patients with lymphoma are at high risk for VTE; more while having active disease and on chemotherapy.21–23 In our study, at least half of lymphoma patients had the VTE while ambulatory around the time of VTE diagnosis. Obviously, none of such patients were on VTE prophylaxis.
Few risk assessment models were developed to highlight the importance of clinical characteristics as risk factors for VTE among ambulatory cancer patients, with a sole purpose of preventing the occurrence of thrombosis and its recurrent episodes, as well as reduce the risk of fatal PE.11
The original Khorana risk assessment model was designed to assess the risk of VTE among ambulatory patients undergoing chemotherapy for various primary cancer sites, including lymphoma.18 In addition to primary cancer site and BMI, the score utilized prechemotherapy blood counts (Leukocyte count > 11X109/L, platelet count ≥ 350 × 109 and Hb < 10 gm/dL) and patients were grouped into three risk levels. When applying the Khorana RAM, patients with lymphoma can be grouped into either intermediate risk (score 1-2) or high risk (score ≥ 3) groups. As such, the model failed to differentiate between risk of VTE in both groups; events developed in 20.0% among 90 patients with high-risk score compared to 11.9% among 428 with intermediate score, P = .08 (Table 5). At least another previously published study reached similar conclusion.24
The original ThroLy RAM enrolled a diverse group of lymphoma patients, including those with low-grade lymphomas that might be at lower risk for VTE. Our VTE rates of 13.5% is significantly higher than a rate of 5.3% in the derivation cohort and 5.8% in the validation cohort as originally reported by Antic, et al.20 The inclusion of only DLBCL, an aggressive lymphoma, in our cohort might explain this difference; almost one in four patients (22.3%) considered as “high-risk” utilizing the ThroLy model, had VTE. This group represent 23.1% of patients enrolled in our study.
Neutropenia (ANC < 1000), a component of the ThroLy, was encountered in only 14 (2.7%) patients and as such, might be removed from the RAM, at least when applied on patients with aggressive lymphomas, like DLBCL.
Both ThroLy and the “modified ThroLy” RAM demonstrate more accuracy in predicting thrombosis in patients with DLBCL than the Khorana RAM. Many of variables in the ThroLy score, like extranodal localization and mediastinal involvement, are special to lymphoma patients.
Notably, multivariate analysis showed that Hb < 100 g/L, and presence of bulky disease had 2.79- and 2.22-fold increase in risk of VTE, respectively.
To our knowledge, only one study attempted to validate the ThroLy RAM. In this study, investigators retrospectively evaluated the association of ThroLy with VTE in 428 patients with DLBCL (n = 241) or Hodgkin lymphoma (n = 187) undergoing first-line chemotherapy in ambulatory setting. Among the whole group, 64 (15.0%) patients developed VTE. Patients with DLBCL were more often classified in the high-risk ThroLy group, and had more VTE events than Hodgkin's lymphoma patients. 25
In a search for an easier and applicable model, we recently attempted to apply the International Prognostic Index (IPI) to identify group of patients with DLBCL at higher risk for VTE. In our study 56 (15.0%) of 373 patients included had radiologically-confirmed VTE; rates were significantly higher in patients with “high and high-intermediate” scores compared to patients with “low and low-intermediate” scores; 19.8% versus 9.7%, respectively (P = .020).26
Our study is not without limitations; patients were treated in a single institution over a relatively long-time span, and data were collected retrospectively. However, we believe that the aggressive nature of DLBCL with its higher risk of VTE, deserve further attention and larger prospective studies to better understand and deal with their VTE risk.
Conclusions
Patients with diffuse large B-cell lymphoma are at high risk of VTE. Many clinical and pathological features may increase this risk. In this subset of patients with aggressive NHL (DLBCL), ThroLy RAM was successful in identifying patients at higher risk for VTE. More studies are needed to further refine and simplify this risk assessment model to enhance its discriminative power and enhance its clinical utilization.
Footnotes
Author Contribution: Hikmat Abdel-Razeq: Conceptualization, analysis, writing final draft. Mohammad Ma’koseh: Investigation, analysis and writing–review and editing. Asem Mansour: Analysis and writing–review and editing. Rayan Bater: Analysis and writing-review and editing. Rula Amarin: Investigation and writing–review and editing. Alaa Abufara: Investigation and writing–review and editing. Khalid Halahleh: Investigation, analysis and writing–review and editing. Mohammad Manassra: Investigation and writing–review and editing. Mohammad Alrwashdeh: Investigation and writing–review and editing. Mohammad Almomani: Investigation and writing–review and editing. Mais Zmaily: Investigation and writing–review and editing.
Data Availability: Data associated with this manuscript will be made available through reasonable requests addressed to corresponding author.
Ethical Approval Statement: The study was approved by the Institutional Review Board (IRB) at King Hussein Cancer Center and was carried in accordance with the international guidelines for retrospective studies.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iDs: Hikmat Abdel-Razeq https://orcid.org/0000-0003-2833-6051
Asem Mansour https://orcid.org/0000-0003-3443-5873
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. [DOI] [PubMed] [Google Scholar]
- 2.Ekström-Smedby K. Epidemiology and etiology of non-Hodgkin lymphoma-a review. Acta Oncol (Madr). 2006;45(3):258-271. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith A, Howell D, Patmore R, Jack A, Roman E. Incidence of haematological malignancy by sub-type: a report from the haematological malignancy research network. Br J Cancer. 2011;105(11):1684-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnellan E, Khorana AA. Cancer and venous thromboembolic disease: a review. Oncologist. 2017;22(2):199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim AS, Khorana AA, McCrae KR. Mechanisms and biomarkers of cancer-associated thrombosis. Transl Res. 2020;225:33-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klok FA, Couturaud F, Delcroix M, Humbert M. Diagnosis of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Eur Respir J. 2020;55(6):2000189. [DOI] [PubMed] [Google Scholar]
- 8.McCabe C, Dimopoulos K, Pitcher A, et al. Chronic thromboembolic disease following pulmonary embolism: time for a fresh look at old clot. Eur Respir J. 2020;55(4):1901934. [DOI] [PubMed] [Google Scholar]
- 9.Rabinovich A, Kahn SR. How I treat the postthrombotic syndrome. Blood. 2018;131(20):2215-2222. [DOI] [PubMed] [Google Scholar]
- 10.Lyman GH, Carrier M, Ay C, et al. American Society of hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Advances. 2021;5(4):927-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanfilippo KM, Wang TF, Gage BF, Luo S, Riedell P, Carson KR. Incidence of venous thromboembolism in patients with non-Hodgkin lymphoma. Thromb Res. 2016;143:86-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyman GH, Culakova E, Poniewierski MS, Kuderer NM. Morbidity, mortality and costs associated with venous thromboembolism in hospitalized patients with cancer. Thromb Res. 2018;164:S112-S118. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Razeq H, Mansour A. Venous thromboembolism prophylaxis for ambulatory cancer patients, can we do better? J Thromb Thrombolysis. 2017;44(3):399-405. [DOI] [PubMed] [Google Scholar]
- 15.Khorana AA, DeSancho MT, Liebman H, Rosovsky R, Connors JM, Zwicker J. Prediction and prevention of cancer-associated thromboembolism. Oncologist. 2020;26(1);e2-e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khorana AA, Cohen AT, Carrier M, et al. Prevention of venous thromboembolism in ambulatory patients with cancer. ESMO Open. 2020;5(6):e000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerotziafas GT, Taher A, Abdel-Razeq H, et al. A predictive score for thrombosis associated with breast, colorectal, lung, or ovarian cancer: the prospective COMPASS-cancer-associated thrombosis study. Oncologist. 2017;22(10):1222-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712-1723. [DOI] [PubMed] [Google Scholar]
- 19.Moik F, Ay C, Pabinger I. Risk prediction for cancer-associated thrombosis in ambulatory patients with cancer: past, present and future. Thromb Res. 2020:191(Suppl1):S3-11. [DOI] [PubMed] [Google Scholar]
- 20.Antic D, Milic N, Nikolovski S, et al. Development and validation of multivariable predictive model for thromboembolic events in lymphoma patients. Am J Hematol. 2016;91(10):1014-1019. [DOI] [PubMed] [Google Scholar]
- 21.Dharmavaram G, Cao S, Sundaram S, et al. Aggressive lymphoma subtype is a risk factor for venous thrombosis. Development of lymphoma - specific venous thrombosis prediction models. Am J Hematol. 2020;95(8):918-926. [DOI] [PubMed] [Google Scholar]
- 22.Borg IH, Bendtsen MD, Bøgsted M, Madsen J, Severinsen MT. Incidence of venous thromboembolism in patients with diffuse large B-cell lymphoma. Leuk Lymphoma. 2016;57(12):2771-2776. [DOI] [PubMed] [Google Scholar]
- 23.Lim SH, Woo S-Y, Kim S, Ko YH, Kim WS, Kim SJ. Cross-sectional study of patients with diffuse large B-cell lymphoma: assessing the effect of host status, tumor burden, and inflammatory activity on venous thromboembolism. Cancer Res Treat. 2016;48(1):312-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rupa-Matysek J, Gil L, Kaźmierczak M, Barańska M, Komarnicki M. Prediction of venous thromboembolism in newly diagnosed patients treated for lymphoid malignancies: validation of the khorana risk score. Medical Oncology. 2017;35(1)5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rupa-Matysek J, Brzeźniakiewicz-Janus K, Gil L, Krasiński Z, Komarnicki M. Evaluation of the ThroLy score for the prediction of venous thromboembolism in newly diagnosed patients treated for lymphoid malignancies in clinical practice. Cancer Med. 2018;7(7):2868-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdel-Razeq H, Ma'koseh M, Abdel-Razeq R, et al. The application of the lymphoma international prognostic index to predict venous thromboembolic events in diffuse large B-cell lymphoma patients. Front Oncol. 2021 May 28;11(677776). [DOI] [PMC free article] [PubMed] [Google Scholar]