Abstract
Objective: We tried to find the relationship between statin and diabetes retinopathy (DR) in patients with type 2 diabetes mellitus (T2DM). Methods: We searched the databases of PubMed, EMBASE, and the Cochrane Library for eligible studies reporting on the relationships between statin use and DR, from inception to September 25, 2020. The terms searched including Diabetes Mellitus, Type 2, Hydroxymethylglutaryl-CoA Reductase Inhibitors, and Diabetic Retinopathy. We expressed the results as the odds ratios (ORs) with 95% confidence intervals (CIs) which were calculated using a random-effects model. Results: A total of 6 eligible studies, including 43 826 patients, were included in the meta-analysis. The meta-analysis showed that statin was not associated with elevated risk of DR [OR = 0.96 (95% CI: 0.80-1.16), P = .68]. Similarly, no differences were found between statin and placebo in participants ≥500 [OR = 0.98 (95% CI: 0.80-1.21)] or participants <500 [OR = 0.90 (95% CI: 0.49-1.66)]. Further, we conducted a meta-analysis to study the effect of statin therapy on DR in people with type 2 diabetes according to age and found that statin use was associated with a decreased risk of DR in patients with type 2 diabetes 40 years of age or older [OR = 0.87 (95% CI: 0.82-0.92)]. Conclusion: Our meta-analysis revealed that statin was not associated with elevated risk of DR in patients with T2DM. Moreover, statin use was associated with a lower incidence of DR in patients with type 2 diabetes 40 years of age or older.
Keywords: diabetes retinopathy, statin, type 2 diabetes mellitus
Introduction
In recent years, the prevalence of diabetes mellitus (DM) is high in the world. The most common is type 2 diabetes mellitus (T2DM), which usually occurs in adults. Patients with T2DM are at high risk for macrovascular complications and microvascular complications. 1 The vascular complications of T2DM have always aroused the greatest concern. The macrovascular complications mainly include cerebrovascular disease, cardiovascular disease, and peripheral artery disease, with high morbidity and mortality. As one of the serious microvascular complications of T2DM, DR can cause vision loss in diabetic patients. 2 The cholesterol control is beneficial in the prevention of macrovascular events3,4 and the glucose control is beneficial in the prevention of microvascular events. However, the relationship between DR and dyslipidaemia is not consistent.5-8
Statins (inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase) could effectively reduce the level of serum cholesterol. Statin therapy is effective for the primary prevention of vascular events in patients with DM. 9 One study indicated that statin therapy can reduce the rate of major vascular events in diabetic patients without diagnosed occlusive arterial disease. 10 Nowadays, statin has been widely used in the primary and secondary prevention of atherosclerotic cardiovascular disease. 11 However, some studies indicated that statins may increase the prevalence of new-onset DMs.12-16 Moreover, statin use might increase the level of plasma glucose 17 which can elevate the risk of DR in patients with DM. Intensive therapy slows the progression of DR in patients with insulin-dependent DM. 18 Adhyaru et al 19 have demonstrated the benefits and adverse effects of statin therapy.
The relationship between statin use and microvascular disease in diabetic patients is presently unknown.20-22 Moreover, the effect of statin on DR is still debated at present23-27 and has become a concern. Statin use can reduce the risk of DR28-30 and postpone the development of retinopathy.31,32 It is unclear whether statin use is associated with a higher incidence of DR. Therefore, we conducted this meta-analysis to study the relationship between the use of statin and incidence of DR in patients with T2DM.
Methods
The meta-analysis was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 33 and Cochrane's Handbook guidelines. 34
Search Strategy
A literature search was conducted using the databases of PubMed, EMBASE, and the Cochrane Library for eligible studies reporting on the relationships between statin use and DR, from inception to September 25, 2020. We used a combination of MeSH (medical subject headings) and Entry Terms including “Diabetes Mellitus, Type 2,” “Hydroxymethylglutaryl-CoA Reductase Inhibitors,” and “Diabetic Retinopathy.”
Selection of Studies
The included studies should meet the following inclusion criteria: (1) the studies included type 2 diabetes patients, (2) statin was compared with control (Nonstatin Group), (3) the outcomes of studies should include incidence of DR or progression of DR. Exclusion criteria were as follows: (1) the review articles, case reports, and letters; (2) the studies were not published in English; (3) the studies on unidentified type of diabetes; (4) the studies included the patients undergoing vitrectomy; (5) the studies included the patients who received coadministration of statin and other lipid lowering drugs; and (6) animal experimental studies. Two researchers independently did the search and selection.
Data extraction and quality assessment
The information was collected from the selected studies, including design type, sample size, outcomes, and so on. Newcastle–Ottawa Scale (NOS) was used for assessing the quality of studies (case–control, cohort, and cross-sectional studies) in meta-analysis. The 3 categories for case–control studies as follows: selection (① case definition, ② representativeness, ③ control selection, and ④ control definition), comparability and exposure (① ascertainment of exposure, ② same method of ascertainment for cases and controls, and ③ nonresponse rate). The 3 categories for cohort studies include: selection (① representativeness of the exposed cohort, ② selection of the nonexposed cohort, ③ ascertainment of exposure, and ④ demonstration that outcome of interest was not present at start of study), comparability and outcomes (① assessment of outcome, ② follow-up long enough for outcomes to occur, and ③ adequacy of follow up of cohorts). The studies included could be awarded 1 star for each item. A maximum of 9 stars can be given for each study.
Statistical Analyses
Meta-analysis of the included studies was conducted to evaluate the effect of statin on DR in people with T2DM. Revman software version 5 was employed to perform a meta-analysis. We expressed the results as the odds ratio (OR) with 95% confidence intervals (CIs) which was calculated using a random-effects model. The I2 was used to assess the heterogeneity across included studies. The risk of bias was assessed for studies using the NOS. Forest plots were constructed for analysis groups. It was considered as a statistically significant difference when P < .05.
Results
Search Results and Patient Characteristics
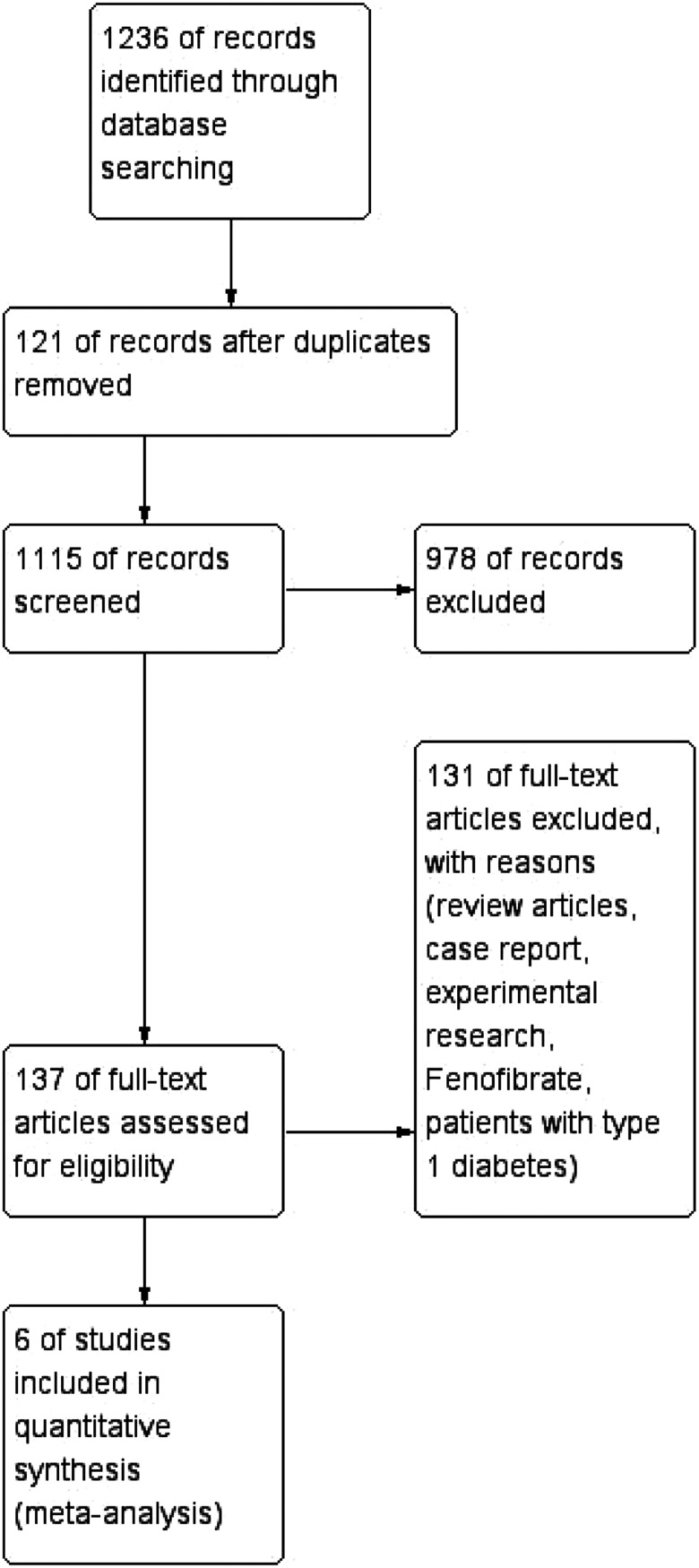
A total of 1236 studies were identified from the initial search, 121 duplicate studies were removed and 1115 studies remained. After reading the titles and abstracts of the 1115 studies, 978 studies were further excluded. The full text of the remaining 137 studies were retrieved after the original screening. Finally, a total of 6 eligible studies, including 43 826 patients, were included in the meta-analysis. The statin group included 22 056 patients. The nonstatin treatment group included 21 770 patients. The study flow diagram of the screening process is shown in Figure 1. The characteristics of studies and the patients are summarized in Table 1. The quality assessment of the included studies is also summarized in Table 1. Three studies provided information on race characteristics. Two cross-sectional studies, 2 case–control studies, and 1 cohort study were included. Five trials investigated the effects of statin on the presence of DR and 1 trial 29 studied the progression of DR.
Figure 1.
Study flow diagram of the included trials.
Table 1.
Baseline Data of the Included Trials in the Meta-Analysis.
| Study, Year (Reference) | Study Design | Statin Group | Nonstatin Group | Mean age (year) | Male sex, No. (%) | Quality Score | Race (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Events, No. | Total patients, No. | Events, No. | Total patients, No. | Statin Group | Nonstatin Group | Statin Group | Nonstatin Group | ||||
| Kang et al 28 | Cohort study | 2004 | 18 947 | 2269 | 18 947 | 61.5 | 61.0 | 8511 | 8517 | 8* | Taiwanese patients (100%) |
| Chung et al 29 | Retrospective study | 16 (23%) | 70 | 7 (18%) | 40 | 58.1 ± 11.6 | 52.3 ± 12.2 | 41 (58.6) | 17 (42.5) | 8* | NR |
| Sacks et al 35 | Case–control Study | 571 | 2451 | 631 | 2434 | NR | NR | NR | NR | 8* | White/European (41%-51%) |
| Choi et al 36 | Cross-sectional study | 23 | 50 | 19 | 46 | NR | NR | NR | NR | 8* | NR |
| Larroumet et al 37 | Cross-sectional design | 124 | 456 | 39 | 224 | NR | NR | NR | NR | 8* | NR |
| Walus-Miarka et al 38 | Case–control study | 25 | 82 | 35 | 79 | NR | NR | NR | NR | 8* | European Caucasians (100%) |
Abbreviation: NR, not reported.
The Effects of Statin on DR
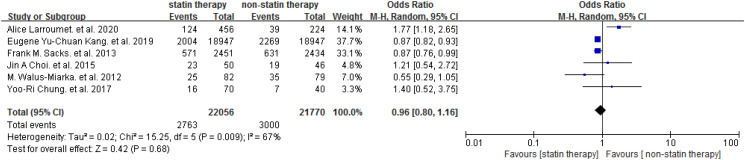
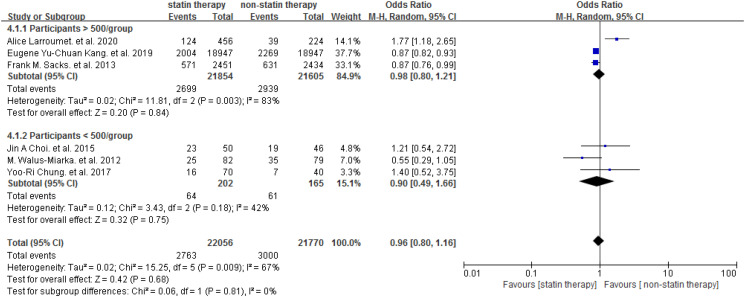
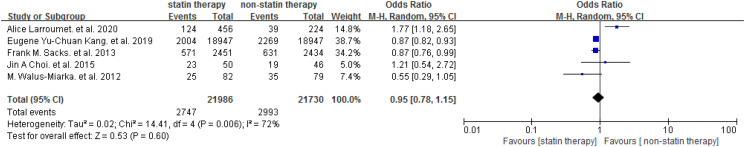
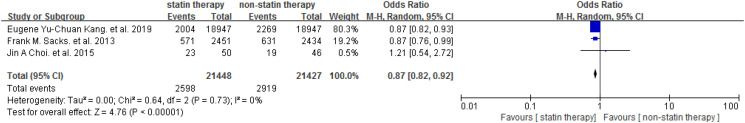
The included trials investigated the effect of statin on DR in people with T2DM. The Meta-analysis showed that statin was not associated with elevated risk of DR [OR = 0.96 (95% CI: 0.80-1.16), P = .68] (Figure 2). Subgroup analysis was performed based on the number of participants (n ≥ 500 or n < 500). Similarly, no difference was found between the studies with different scales (P = .81) (Figure 3), and no differences were found between statin and placebo in participants ≥500 [OR = 0.98 (95% CI: 0.80-1.21)] or participants <500 [OR = 0.90 (95% CI: 0.49-1.66)] (Figure 3). One study focused on the DR progression (≥2 steps of DRSS). 29 We performed a meta-analysis for the other 5 studies to assess the efficiency of statin on the presence of DR and the result did not show a significant association between the statin use and the presence of DR [OR = 0.95 (95% CI: 0.78-1.15)] (Figure 4). Among the included studies, 3 studies involved diabetic patients with age ≥40 years. Further, we conducted a meta-analysis to study the effect of statin therapy on DR in people with type 2 diabetes according to age and found that statin use was associated with a decreased risk of DR in patients with type 2 diabetes 40 years of age or older [OR = 0.87 (95% CI: 0.82-0.92)] (Figure 5).
Figure 2.
Forest plots for the effect of statin therapy on DR in people with T2DM. Abbreviations: DR, diabetes retinopathy; T2DM, type 2 diabetes mellitus.
Figure 3.
Subgroup analyses effects of statin therapy on DR according to the number of participants. Abbreviation: DR, diabetes retinopathy.
Figure 4.
Effect of statin therapy on the presence of DR. Abbreviation: DR, diabetes retinopathy.
Figure 5.
Effect of statin therapy on DR in people with type 2 diabetes 40 years of age or older. Abbreviation: DR, diabetes retinopathy.
Discussion
We made the meta-analysis to estimate the effect of statin on DR and found no significant association between statin therapy and DR in patients with T2DM. In addition, we performed a subgroup analysis according to the number of participants. The analysis found no differences between statin and placebo group too. Besides, we made other subgroups analysis and found that statin use was associated with a decreased risk of DR in patients with T2DM 40 years of age or older. Overall, patients with T2DM who received statin showed no increase in the risk of DR in the statin group compared with the nonstatin group in our study. The results of our study provided additional insights on the safety and limitations of statin therapy.
The effects of statins on DR are uncertain in previous studies. Some studies found that the use of statin was related to a decreased risk of DR. 28 One study reported that dyslipidemia treatment was associated with the prevention of diabetic microvascular disease. 35 The combination treatment of dyslipidemia and glycemic control can reduce the progression of DR. 39 ACCORD-EYE study (Action to Control Cardiovascular Risk in Diabetes-EYE) indicated that Fenofibrate combined with simvastatin in the treatment of DR was more effective compared with simvastatin alone. 40 Sen et al 30 have proposed that simvastatin retards the progression of DR. One cohort study including 37 894 Taiwanese patients indicated that the use of statin was related to a lower risk of DR in individuals with T2DM. 28 Moreover, Nielsen et al 41 found a similar result in statin users too. Nevertheless, the studies investigated the relationship between serum lipids and DR reported conflicting results. 42 Some studies did not support the association between statin and DR.24,29 Statin users had no significant difference in the progression of DR when compared with nonusers (23% vs 18%, P = .506). 29 The statin therapy in the pre-diagnosis of diabetes stage did not increase the risk of microvascular disease. 41 The results of studies before were conflicting. Statin has several nonlipid effects which may contribute to the clinical efficacy and explain the discordant findings in previous studies.
Nowadays, statin was widely used to prevent the macrovascular complications of DM. The underlying disadvantage of statin therapy in patients with DM has attracted more attention. However, there is a lack of studies assessing the effect of statin on DR in patients with T2DM currently. Statin may increase the prevalence of new-onset DMs. 12 The disadvantage of statin may be explained by the complex mechanisms of statin including increased insulin resistance or impaired insulin secretion. 43 The insulin resistance in patients with T2DM can lead to the abnormalities in lipoprotein transport.
DR is one of the microvascular complications of diabetes which can lead to vision loss. 13 Previous study demonstrated that examination of the optic disc in patients with T2DM and albuminuria might be necessary. 36 The mechanisms of microvascular damage that occurs during DM are complex. Many studies have been made to understand the pathophysiology of DR. The pathogenetic events in DR include vascular changes, hypoxia, blood–retina barrier disruption. 44 Ion channels may play a role in the pathophysiology of coronary microvascular dysfunction in patients with DM. 45 Oxidative Stress can affect ion channel function. The exposure to oxidative stress in diabetic patients may promote lipid peroxidation, leading to the determinism of microvascular dysfunction in DM. 46 Rapid decline of HbA1c 37 and changed fibrin-clot properties 38 were associated with DR. Advanced glycosylation end products (AGEs), protein kinase C (PKC) pathways and renin–angiotensin system (RAS) activation might have important roles in the development of DR. 44 Besides the consistent risk factors for DR (hyperglycaemia, hypertension, diabetes duration), 47 dyslipidaemia might be a risk factor. Furthermore, the AGE and protein kinase C (PKC) pathways mentioned above are involved in the lipid levels. 48 Other associated mechanisms contributing to DR include pro-inflammatory cytokine production and vascular endothelial growth factor (VEGF). Statins have anti-inflammatory effects. 49 VEGF plays important role in the development of DR. Statins which might influence the concentration of vitreous VEGF in patients with DR. 50 Our study has several limitations. The main limitation of our meta-analysis is the lack of RCT. The results should be confirmed by high-quality RCT studies in the future. Moreover, some studies had small numbers of participants. In summary, our meta-analysis revealed that statin was not associated with elevated risk of DR in patients with T2DM. Moreover, statin use was associated with a lower incidence of DR in patients with type 2 diabetes 40 years of age or older.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Jun Liu https://orcid.org/0000-0002-3732-6448
Yi-Ping Wu https://orcid.org/0000-0003-4946-0182
Jun-Juan Qi https://orcid.org/0000-0003-1279-6555
Zeng-Ping Yue https://orcid.org/0000-0002-8472-2443
Cheng-Dong Hu https://orcid.org/0000-0002-6982-8143
References
- 1.DeFronzo RA, Ferrannini E, Groop Let al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. doi: 10.1038/nrdp.2015.19. PMID:27189025. [DOI] [PubMed] [Google Scholar]
- 2.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366(13):1227-1239. doi: 10.1056/NEJMra1005073. PMID:22455417. [DOI] [PubMed] [Google Scholar]
- 3.Brown AS. Lipid management in patients with diabetes mellitus. Am J Cardiol. 2005;96(4A):26E-32E. doi: 10.1016/j.amjcard.2005.07.001. PMID:16098840. [DOI] [PubMed] [Google Scholar]
- 4.Thomason MJ, Colhoun HM, Livingstone SJet al. Baseline characteristics in the Collaborative AtoRvastatin Diabetes Study (CARDS) in patients with type 2 diabetes. Diabet Med. 2004;21(8):901-905. doi: 10.1111/j.1464-5491.2004.01401.x. PMID:15270795. [DOI] [PubMed] [Google Scholar]
- 5.Klein R, Sharrett AR, Klein BEet al. et al. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: the atherosclerosis risk in communities study. Ophthalmology. 2002;109(7):1225-1234. doi: 10.1016/s0161-6420(02)01074-6. PMID:12093643. [DOI] [PubMed] [Google Scholar]
- 6.Rema M, Srivastava BK, Anitha B, Deepa R, Mohan V. Association of serum lipids with diabetic retinopathy in urban South Indians—the Chennai urban rural epidemiology study (CURES) Eye study–2. Diabet Med. 2006;23(9):1029-1036. doi: 10.1111/j.1464-5491.2006.01890.x. PMID:16922712. [DOI] [PubMed] [Google Scholar]
- 7.Wong TY, Cheung N, Tay WTet al. et al. Prevalence and risk factors for diabetic retinopathy: the Singapore Malay Eye Study. Ophthalmology. 2008;115(11):1869-1875. doi: 10.1016/j.ophtha.2008.05.014. Epub 2008 Jun 26. PMID:18584872. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Xu L, Jonas JB, You QS, Wang YX, Yang H. Dyslipidemia and eye diseases in the adult Chinese population: the Beijing eye study. PLoS ONE. 2012;7(3):e26871. doi: 10.1371/journal.pone.0026871. Epub 2011 Oct 28. PMID:22128290; PMCID:PMC3419255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, et al. Efficacy of cholesterol-lowering therapy in 18 686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371(9607):117-125. doi: 10.1016/S0140-6736(08)60104-X. PMID:18191683. [DOI] [PubMed] [Google Scholar]
- 10.Collins R, Armitage J, Parish S, Sleigh P, Peto R, Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361(9374):2005-2016. doi: 10.1016/s0140-6736(03)13636-7. PMID:12814710. [DOI] [PubMed] [Google Scholar]
- 11.Stone NJ, Robinson JG, Lichtenstein AHet al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889-2934. doi: 10.1016/j.jacc.2013.11.002. Epub 2013 Nov 12. Erratum in: J Am Coll Cardiol. 2014 Jul 1;63(25 Pt B):3024-3025. Erratum in: J Am Coll Cardiol. 2015 Dec 22;66(24):2812. PMID:24239923. [DOI] [PubMed] [Google Scholar]
- 12.Tangelloju S, Little BB, Esterhay RJ, Brock G, LaJoie S. Statins are associated with new onset type 2 diabetes mellitus (T2DM) in medicare patients ≥65 years. Diabetes Metab Res Rev. 2020;36(6):e3310. doi: 10.1002/dmrr.3310. Epub 2020 Apr 3. PMID:32162770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mansi I, Frei CR, Wang CP, Mortensen EM. Statins and new-onset diabetes mellitus and diabetic complications: a retrospective cohort study of US healthy adults. J Gen Int Med. 2015;30(11):1599-1610. doi: 10.1007/s11606-015-3335-1. Epub 2015 Apr 28. PMID:25917657; PMCID:PMC4617949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Preiss D, Seshasai SR, Welsh Pet al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305(24):2556-2564. doi: 10.1001/jama.2011.860. PMID:21693744. [DOI] [PubMed] [Google Scholar]
- 15.Bell DS, DiNicolantonio JJ, O’Keefe JH. Is statin-induced diabetes clinically relevant? A comprehensive review of the literature. Diabetes Obes Metab. 2014;16(8):689-694. doi: 10.1111/dom.12254. Epub 2014 Jan 20. PMID:24373206. [DOI] [PubMed] [Google Scholar]
- 16.Katsiki N, Athyros VG, Karagiannis A, Mikhailidis DP. The role of statins in the treatment of type 2 diabetes mellitus: an update. Curr Pharm Des. 2014;20(22):3665-3674. doi: 10.2174/13816128113196660673. Erratum in: Curr Pharm Des. 2015;21(24):3565. PMID:24040875. [DOI] [PubMed] [Google Scholar]
- 17.Mehta JL. Statins and altered glucose metabolism: a laboratory curiosity or a new disease? J Am Coll Cardiol. 2010;56(8):680. Author reply 680-1. doi:10.1016/j.jacc.2010.03.062. PMID:20705228. [DOI] [PubMed] [Google Scholar]
- 18.Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin Jet al. et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. doi: 10.1056/NEJM199309303291401. PMID:8366922. [DOI] [PubMed] [Google Scholar]
- 19.Adhyaru BB, Jacobson TA. Safety and efficacy of statin therapy. Nat Rev Cardiol. 2018;15(12):757-769. doi: 10.1038/s41569-018-0098-5. PMID:30375494. [DOI] [PubMed] [Google Scholar]
- 20.Preiss D. Do statins reduce microvascular complications in diabetes? Lancet Diabetes Endocrinol. 2014;2(11):858-859. doi: 10.1016/S2213-8587(14)70177-9. Epub 2014 Sep 9. PMID:25217179. [DOI] [PubMed] [Google Scholar]
- 21.Goldfine AB. Statins: is it really time to reassess benefits and risks? N Engl J Med. 2012;366(19):1752-1755. doi: 10.1056/NEJMp1203020. Epub 2012 Apr 25. PMID:22533536. [DOI] [PubMed] [Google Scholar]
- 22.Lim LS, Wong TY. Lipids and diabetic retinopathy. Expert Opin Biol Ther. 2012;12(1):93-105. doi: 10.1517/14712598.2012.641531. Epub 2011 Nov 28. PMID:22122357. [DOI] [PubMed] [Google Scholar]
- 23.Colhoun HM, Betteridge DJ, Durrington PNet al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative AtoRvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685-696. doi: 10.1016/S0140-6736(04)16895-5. PMID:15325833. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, McGwin G., Jr Association of statin use with the risk of developing diabetic retinopathy. Arch Ophthalmol. 2007;125(8):1096-1099. doi: 10.1001/archopht.125.8.1096. PMID:17698757. [DOI] [PubMed] [Google Scholar]
- 25.Lee JD, Morrissey JR, Mikhailidis DP, Patel V. CARDS on the table: should everybody with type 2 diabetes take a statin? Curr Med Res Opin. 2005;21(3):357-362. doi: 10.1185/030079905X36413. PMID:15811203. [DOI] [PubMed] [Google Scholar]
- 26.Gupta A, Gupta V, Thapar S, Bhansali A. Lipid-lowering drug atorvastatin as an adjunct in the management of diabetic macular edema. Am J Ophthalmol. 2004;137(4):675-682. doi: 10.1016/j.ajo.2003.11.017. PMID:15059707. [DOI] [PubMed] [Google Scholar]
- 27.Belalcazar LM, Raghavan VA, Ballantyne CM. Statin-induced diabetes: will it change clinical practice? Diabetes Care. 2009;32(10):1941-1943. doi: 10.2337/dc09-1277. PMID:19794006; PMCID:PMC2752927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang EY, Chen TH, Garg SJet al. Association of statin therapy with prevention of vision-threatening diabetic retinopathy. JAMA Ophthalmol. 2019;137(4):363-371. doi: 10.1001/jamaophthalmol.2018.6399. PMID:30629109; PMCID:PMC6459113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung YR, Park SW, Choi SYet al. et al. Association of statin use and hypertriglyceridemia with diabetic macular edema in patients with type 2 diabetes and diabetic retinopathy. Cardiovasc Diabetol. 2017;16(1):4. doi: 10.1186/s12933-016-0486-2. PMID:28061854; PMCID:PMC5219811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sen K, Misra A, Kumar A, Pandey RM. Simvastatin retards progression of retinopathy in diabetic patients with hypercholesterolemia. Diabetes Res Clin Pract. 2002;56(1):1-11. doi: 10.1016/s0168-8227(01)00341-2. PMID:11879715. [DOI] [PubMed] [Google Scholar]
- 31.Ludwig S, Shen GX. Statins for diabetic cardiovascular complications. Curr Vasc Pharmacol. 2006;4(3):245-251. doi: 10.2174/157016106777698388. PMID:16842142. [DOI] [PubMed] [Google Scholar]
- 32.Ozkiris A, Erkiliç K, Koç A, Mistik S. Effect of atorvastatin on ocular blood flow velocities in patients with diabetic retinopathy. Br J Ophthalmol. 2007;91(1):69-73. doi: 10.1136/bjo.2006.098285. Epub 2006 Sep 14. PMID:16973667; PMCID:PMC1857585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269, W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 34.Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Wiley; 2011. [Google Scholar]
- 35.Sacks FM, Hermans MP, Fioretto Pet al. Association between plasma triglycerides and high-density lipoprotein cholesterol and microvascular kidney disease and retinopathy in type 2 diabetes mellitus: a global case-control study in 13 countries. Circulation. 2014;129(9):999-1008. doi: 10.1161/CIRCULATIONAHA.113.002529. Epub 2013 Dec 18. PMID:24352521. [DOI] [PubMed] [Google Scholar]
- 36.Choi JA, Ko SH, Park YR, Jee DH, Ko SH, Park CK. Retinal nerve fiber layer loss is associated with urinary albumin excretion in patients with type 2 diabetes. Ophthalmology. 2015;122(5):976-981. doi: 10.1016/j.ophtha.2015.01.001. Epub 2015 Feb 7. PMID:25666831. [DOI] [PubMed] [Google Scholar]
- 37.Larroumet A, Rigo M, Lecocq Met al. Previous dramatic reduction of HbA1c and retinopathy in type 2 diabetes. J Diabetes Complications. 2020;34(7):107604. doi: 10.1016/j.jdiacomp.2020.107604. Epub 2020 Apr 27. PMID:32360194. [DOI] [PubMed] [Google Scholar]
- 38.Walus-Miarka M, Wolkow P, Cyganek K, Mirkiewicz-Sieradzka B, Malecki MT, Undas A. Altered fibrin-clot properties are associated with retinopathy in type 2 diabetes mellitus. Diabetes Metab. 2012;38(5):462-465. doi: 10.1016/j.diabet.2012.03.007. Epub 2012 May 11. PMID:22579719. [DOI] [PubMed] [Google Scholar]
- 39.ACCORD Study Group, ACCORD Eye Study Group , Chew EY, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363(3):233-244. doi: 10.1056/NEJMoa1001288. Epub 2010 Jun 29. Erratum in: N Engl J Med. 2011 Jan 13;364(2):190. Erratum in: N Engl J Med. 2012 Dec 20;367(25):2458. PMID:20587587; PMCID:PMC4026164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hermans MP, Fruchart JC. Reducing vascular events risk in patients with dyslipidaemia: an update for clinicians. Ther Adv Chronic Dis. 2011;2(5):307-323. doi: 10.1177/2040622311413952. PMID:23251757; PMCID:PMC3513890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nielsen SF, Nordestgaard BG. Statin use before diabetes diagnosis and risk of microvascular disease: a nationwide nested matched study. Lancet Diabetes Endocrinol. 2014;2(11):894-900. doi: 10.1016/S2213-8587(14)70173-1. Epub 2014 Sep 9. PMID:25217178. [DOI] [PubMed] [Google Scholar]
- 42.Su DH, Yeo KT. Diabetic retinopathy and serum lipids. Singapore Med J. 2000;41(6):295-297. PMID:11109348. [PubMed] [Google Scholar]
- 43.Robinson JG. Statins and diabetes risk: how real is it and what are the mechanisms? Curr Opin Lipidol. 2015;26(3):228-235. doi: 10.1097/MOL.0000000000000172. PMID:25887679. [DOI] [PubMed] [Google Scholar]
- 44.Wong TY, Cheung CM, Larsen M, Sharma S, Simó R. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012. doi: 10.1038/nrdp.2016.12. PMID:27159554. [DOI] [PubMed] [Google Scholar]
- 45.Severino P, D’Amato A, Netti L, et al. Diabetes mellitus and ischemic heart disease: the role of ion channels. Int J Mol Sci. 2018;19(3):802. doi: 10.3390/ijms19030802. PMID:29534462; PMCID:PMC5877663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Severino P, D’Amato A, Netti L, et al. Myocardial ischemia and diabetes mellitus: role of oxidative stress in the connection between cardiac metabolism and coronary blood flow. J Diabetes Res. 2019;2019:9489826. doi: 10.1155/2019/9489826. PMID:31089475; PMCID:PMC6476021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102(4):527-532. doi: 10.1001/archopht.1984.01040030405011. PMID:6367725. [DOI] [PubMed] [Google Scholar]
- 48.Stitt AW. AGEs and diabetic retinopathy. Invest Ophthalmol Vis Sci. 2010;51(10):4867-4874. doi: 10.1167/iovs.10-5881. PMID:20876889. [DOI] [PubMed] [Google Scholar]
- 49.Agrawal NK, Kant S. Targeting inflammation in diabetes: newer therapeutic options. World J Diabetes. 2014;5(5):697-710. doi: 10.4239/wjd.v5.i5.697. PMID:25317247; PMCID:PMC4138593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liinamaa MJ, Savolainen MJ. High vitreous concentration of vascular endothelial growth factor in diabetic patients with proliferative retinopathy using statins. Ann Med. 2008;40(3):209-214. doi: 10.1080/07853890701749209. PMID:18382886. [DOI] [PubMed] [Google Scholar]