Abstract
Objective
To determine whether aldehyde dehydrogenase 1 (ALDH1) immunostaining in axillary lymph node metastases in patients with breast cancer is associated with poor clinical prognosis.
Methods
This retrospective study reviewed data from the medical records of patients with immunohistochemistry-confirmed invasive ductal carcinoma (IDC) and 1–3 metastatic lymph nodes in the ipsilateral axilla between December 2012 and July 2015. The association between ALDH1 immunostaining in axillary lymph node metastases and clinical parameters and prognosis was analysed using χ2-test, Kaplan–Meier survival analysis, univariate and multivariate Cox regression analyses.
Results
A total of 229 patients with IDC were enrolled in the study. The median follow-up was 61 months (range, 20–89 months). Patients with ALDH1-positive axillary lymph node metastases had significantly shorter relapse-free survival and overall survival compared with those with ALDH1-negative axillary lymph node metastases. ALDH1 immunostaining in axillary lymph node metastases was a significant predictor of poor prognosis in univariate and multivariate analyses.
Conclusion
This large study with long-term follow-up suggests that ALDH1 immunostaining in axillary lymph node metastases is an independent predictor of poor prognosis in patients with breast cancer. The clinical relevance of this finding should be confirmed in further well-designed prospective studies.
Keywords: Breast cancer, cancer stem cells, ALDH1, axillary lymph node metastases, prognosis
Introduction
Breast cancer is the most common cancer in women. 1 Research on breast cancer has shown that the prognosis of different types of breast cancer is significantly different.2,3 Cancer cells display abnormal cell proliferation and growth, and a small subpopulation exhibit a stem cell phenotype. 4 Aldehyde dehydrogenase 1 (ALDH1) is an enzyme that is expressed in the liver and involved in retinoic acid biosynthesis.5,6 Aldehyde dehydrogenases play a role in stem cell regulation and possessing cancer-related functions, and regulating multiple pathways implicated in stem cell signalling and carcinogenesis.7–9 Clinical data suggest that ALDH1 is an effective cancer stem cell (CSC) biomarker in breast cancer that is significantly associated with poor prognosis.10–15
Axillary lymph node status is among the most consistent prognostic factors in patients with breast cancer.16–18 Previous studies demonstrated that the ALDH1 level in primary breast tumours was significantly associated with poor prognosis.19,20 A previous study demonstrated that ALDH1 expression in primary breast tumours may be a predictor of poor clinical outcome. 7 However, reports on the prevalence and clinical significance of ALDH1 levels in axillary lymph node metastases are scarce.7,21,22 Recently, CSCs were shown to be responsible for lymph node metastases in supraglottic carcinoma. 23 Therefore, ALDH1 immunostaining in axillary lymph node metastases might have clinical significance for predicting prognosis in patients with breast cancer.
The objective of this current study was to analyse the association between the clinicopathological parameters and ALDH1 levels in lymph node metastases and to investigate whether the presence of ALDH1 in axillary lymph node metastases in patients with breast cancer was associated with clinical prognosis.
Patients and methods
Patients
This retrospective clinical study reviewed the medical records of consecutive patients with histologically-diagnosed invasive ductal carcinoma (IDC) and 1–3 metastatic lymph nodes in the ipsilateral axilla that underwent appropriate surgery in the Department of Breast Surgery, The First Hospital of Jilin University, Changchun, Jilin Province, China between December 2012 and July 2015. The inclusion criteria were as follows: (i) patients without neoadjuvant chemotherapy or neoadjuvant endocrine therapy that underwent total mastectomy or breast conserving surgery; (ii) patients diagnosed with invasive IDC by paraffin section histopathology; (iii) patients with unilateral breast cancer; (iv) patients with tumour stage T1a–T4; (v) patients with lymph node stage N1 (all of them were macro metastases and the metastatic lesion was larger than 2 mm). The exclusion criteria were as follows: (i) breast cancer patients that received neoadjuvant chemotherapy or neoadjuvant endocrine therapy; (ii) patients with bilateral breast cancer; (iii) patients diagnosed with stage IV breast cancer; (iv) patients with incomplete clinicopathological data or incomplete follow-up data. All patients with IDC that met all the inclusion criteria and did not meet any of the exclusion criteria were enrolled in the study.
The clinicopathological and prognostic information of all patients were age, tumour size, lymph node metastasis, tumour stage according to American Joint Committee on Cancer, oestrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER-2), Ki-67, molecular subtypes, operation date, operation method, adjuvant therapy, date of recurrence or metastasis, survival status, time of death and causes of death. Follow-up was from the day of surgery to the last follow-up (30 January 2020) or death. Patients were administered standard anthracycline-based adjuvant chemotherapy according to the pathology results. Patients with ER and/or PR positive breast cancer were administered standard adjuvant endocrine therapy, including tamoxifen or letrozole for 5 years.
This study was approved by the Medical Ethics Committee of the First Hospital of Jilin University, Changchun, Jilin Province, China (no. 20200104113). The study was conducted in compliance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Due to its retrospective design and anonymous characteristics, the requirement for informed consent from the patients was waived.
Immunohistochemical staining
Samples of axillary lymph node metastases were collected from the Paraffin Specimens Library in the Department of Pathology, The First Hospital of Jilin University, Changchun, Jilin Province, China. Histology was confirmed by two experienced pathologists using haematoxylin and eosin stained sections. ALDH1, ER, PR and HER-2 immunostaining in axillary lymph node metastases were evaluated using immunohistochemistry. Formalin-fixed paraffin-embedded specimens were processed for immunohistochemistry. Sections (3 µm) were dewaxed, hydrated, treated with sodium citrate buffer (pH 6.0) in a pressure cooker (50X3-301, inner diameter 22 cm; Midea, Guangdong, China) and cooled at 37 °C for 3 min. Endogenous peroxidase activity was inhibited with 3% H2O2 for 10 min at 37 °C. Non-specific binding was blocked with non-immune serum for 15 min at 37 °C. The primary antibodies included the following: purified mouse antihuman ALDH1-A1 (1:200 dilution; BD Biosciences, San Jose, CA, USA); mouse antihuman ER (1:1000 dilution; ZhongShan Golden Bridge Biotechnology Company, Beijing, China); mouse antihuman PR (1:1000 dilution; ZhongShan Golden Bridge Biotechnology Company); and mouse antihuman HER-2 (1:200 dilution; ZhongShan Golden Bridge Biotechnology Company). After incubation with the primary antibody at 4 °C overnight, the sections were washed three times for 5 min each at room temperature with 0.01 mol/l phosphate-buffered saline (PBS; pH 7.4). Then, the sections were incubated with secondary antibody (Ultra Sensitive TM S-P [Mouse/Rabbit] IHC Kit; MaiXin Biotechnology Company, Fuzhou, China) at 37 °C for 15 min. The sections were then washed three times for 5 min each at room temperature with 0.01 mol/l PBS (pH 7.4). The slides were then incubated with avidin-biotin-peroxidase complex at 37 °C for 15 min. The antigen–antibody reaction was visualized with 3,3′-diaminobenzidine at room temperature in the dark for 5 min and counterstained with haematoxylin. Specificity of staining was confirmed using healthy human liver tissue (ALDH1 positive control), breast cancer tissue (ER, PR and HER-2 positive control) and 0.01 mol/l PBS (pH 7.4) in place of the primary antibody (negative control).
Scoring the levels of immunostaining
Tumour cells with ALDH1 cytoplasmic staining were considered ALDH1 positive. ALDH1 staining was scored as 0, 1, 2 and 3 according to percentage of ALDH1-positive tumour cells. Sections with <5% ALDH1-positive tumour cells were scored 0 (negative). Sections with ≥5% ALDH1-positive tumour cells were scored 1, 2 and 3 (<10% ALDH1-positive tumour cells were scored 1 [weakly positive], <40% ALDH1-positive tumour cells were scored 2 [moderately positive], ≥40% ALDH1-positive tumour cells were scored 3 [strongly positive]). Sections with ≥10% tumour cells with nuclear staining for ER or PR were classified as ER or PR positive. HER-2 expression either with immunohistochemistry 3+ or fluorescence in situ hybridization (FISH) amplified (ratio of HER-2 to CEP17 of ≥2.0 or with a mean HER-2 copy number ≥6) was considered FISH positive. Immunohistochemical staining was evaluated by three independent pathologists that were blinded to the clinical outcomes. When the results of assessment differed, consensus was reached through discussion.
Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 19.0 (IBM Corp., Armonk, NY, USA). Associations between the levels of ALDH1 immunostaining in the axillary lymph node metastases and clinicopathological characteristics were evaluated using χ2-test. Relapse-free survival (RFS) and overall survival (OS) were calculated using the Kaplan–Meier method. RFS was defined as the time from diagnosis to relapse or metastasis. OS was defined as the time from diagnosis to breast cancer-related death. Differences in RFS and OS were evaluated using the log-rank test. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated using univariate and multivariate Cox regression analyses. A P-value <0.05 was considered statistically significant.
Results
This retrospective study included 229 patients (mean age, 46 years; range, 17–77 years) with histologically-confirmed IDC and 1–3 metastatic lymph nodes in the ipsilateral axilla postoperatively. There were no signs of distant metastases in any of the patients. The demographic and clinical characteristics of the patients are summarized in Table 1. There were 118 patients ≤50 years, 48 patients with stage T1 (tumour size ≤2 cm), 147 patients with stage T2 (tumour size >2 cm and ≤5 cm) and 34 patients with stage T3 (tumour size >5 cm). There were 159 ER-positive patients, 158 PR-positive patients, 53 HER-2-positive patients; and three patients lacked ER, PR and HER-2 information. The median follow-up was 61 months (range, 20–89 months). Among the 229 patients, 181 patients were administered standard anthracycline-based adjuvant chemotherapy according to the pathology results; and 159 patients with ER and/or PR positive breast cancer were administered standard adjuvant endocrine therapy, including tamoxifen or letrozole for 5 years. Breast cancer recurred in 80 patients and caused death in 60 patients.
Table 1.
Demographic and clinical characteristics of patients (n = 229) that were enrolled in a study to investigate whether the presence of aldehyde dehydrogenase 1 in axillary lymph node metastases in patients with breast cancer was associated with clinical prognosis.
| Characteristic | Study cohort n = 229 |
|---|---|
| Age | |
| ≤50 years | 118 |
| >50 years | 111 |
| Pathology | |
| Size of tumour ≤2 cm | 48 |
| Size of tumour >2 cm and ≤5 cm | 147 |
| Size of tumour >5 cm | 34 |
| Histology | |
| ER positive | 159 |
| ER negative | 67 |
| PR positive | 158 |
| PR negative | 68 |
| HER-2 positive | 53 |
| HER-2 negative | 173 |
| Lack of ER, PR and HER-2 data | 3 |
| Outcome | |
| Distant metastasis | 80 |
| Death | 60 |
Data presented as n of patients.
ER, oestrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor 2.
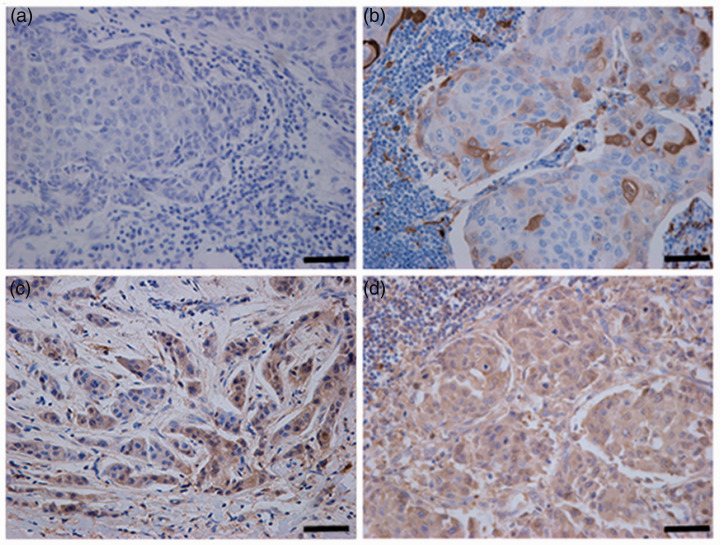
The range of ALDH1 immunostaining in axillary lymph node metastases is shown in Figure 1 as follows: Figure 1A shows an ALDH1-negative axillary lymph node metastasis; Figure 1B shows an ALDH1-weakly positive axillary lymph node metastasis; Figure 1C shows an ALDH1-moderately positive axillary lymph node metastasis; and Figure 1D shows an ALDH1-strongly positive axillary lymph node metastasis. Among the 229 patients, 150 patients (65.5%) had ALDH1-negative axillary lymph node metastases and 79 patients (34.5%) had ADLH1-postive axillary lymph node metastases (i.e. sections with ≥5% ALDH1-positive tumour cells). Among the 79 patients with ALDH1-positive axillary lymph node metastases, 17 (21.5%) had ≥5% ALDH1-positive tumour cells but <10% ALDH1-positive tumour cells, which were scored 1 (weakly positive); 34 (43.0%) had ≥10% ALDH1-positive tumour cells but <40% ALDH1-positive tumour cells, which were scored 2 (moderately positive); and 28 had (35.4%) had ≥40% ALDH1-positive tumour cells, which were scored 3 (strongly positive). The association between the levels of ALDH1 immunostaining in axillary lymph node metastases and clinicopathological characteristics is summarized in Table 2. The levels of ALDH1 immunostaining in axillary lymph node metastases was significantly associated with ER-negativity (P = 0.012). However, there was no association between the levels of ALDH1 immunostaining in axillary lymph node metastases and age, menopausal status, tumour size, PR or HER-2 status.
Figure 1.
Representative photomicrographs showing the levels of aldehyde dehydrogenase 1 (ALDH1) immunostaining in axillary lymph node metastases from patients with breast cancer: (a) negative, score 0; (b) weakly positive, score 1; (c) moderately positive, score 2; (d) strongly positive, score 3. Scale bar 100 μm. The colour version of this figure is available at: http://imr.sagepub.com.
Table 2.
Association between the levels of aldehyde dehydrogenase 1 (ALDH1) immunostaining and clinicopathological characteristics in patients (n = 229) that were enrolled in a study to investigate whether the presence ALDH1 in axillary lymph node metastases in patients with breast cancer was associated with clinical prognosis.
| Characteristic | n | ALDH1 positive n = 79 | ALDH1 negative n = 150 | Statistical analysesa |
|---|---|---|---|---|
| Age, years | ||||
| ≤50 | 118 | 42 | 76 | NS |
| >50 | 111 | 37 | 74 | |
| Menopausal status | ||||
| Premenopausal | 120 | 43 | 77 | NS |
| Postmenopausal | 109 | 36 | 73 | |
| Tumour size, cm | ||||
| ≤2 | 48 | 16 | 32 | NS |
| 2–5 | 147 | 50 | 97 | |
| >5 | 34 | 13 | 21 | |
| ER | ||||
| Positive | 159 | 46 | 113 | P = 0.012 |
| Negative | 67 | 31 | 36 | |
| PR | ||||
| Positive | 158 | 49 | 109 | NS |
| Negative | 68 | 28 | 40 | |
| HER-2 | ||||
| Positive | 53 | 22 | 31 | NS |
| Negative | 173 | 55 | 118 | |
Data presented as n of patients.
aAssociation between the levels of ALDH1 immunostaining in the axillary lymph node metastases and clinicopathological characteristics were evaluated using χ2-test; NS, no significant between-group difference (P ≥ 0.05).
ER, oestrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor 2.
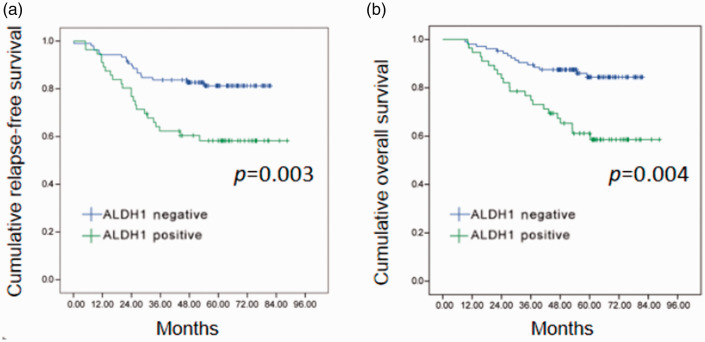
The median follow-up was 61 months (range, 20–89 months). Kaplan–Meier and log-rank test analysis demonstrated that patients with ALDH1-positive axillary lymph node metastases had significantly shorter RFS (P = 0.003) and OS (P = 0.004) compared with those with ALDH1-negative axillary lymph node metastases (Figure 2).
Figure 2.
Relapse-free survival (a) and overall survival (b) in patients (n = 229) with breast cancer stratified according to the levels of aldehyde dehydrogenase 1 (ALDH1) immunostaining in axillary lymph node metastases. The colour version of this figure is available at: http://imr.sagepub.com.
Univariate and multivariate analyses to identify predictors of RFS and OS are summarized in Tables 3 and 4. On univariate analysis, ALDH1 immunostaining (P = 0.005), ER-negativity (P = 0.001), PR-negativity (P = 0.004), HER-2 positivity (P = 0.013) of axillary lymph node metastases and tumour size >2 cm (P = 0.031) were associated with shorter RFS (Table 3). On multivariate analysis, ALDH1 immunostaining (P = 0.034) and ER status (P = 0.009) in axillary lymph node metastases were associated with shorter RFS. On univariate analysis, ALDH1 immunostaining (P = 0.005), ER-negativity (P = 0.003), PR-negativity (P = 0.010) and HER-2 positivity (P = 0.023) in axillary lymph node metastases were associated with OS (Table 4). On multivariate analysis, ALDH1 immunostaining (P = 0.048) and ER-negativity (P = 0.004) in axillary lymph node metastases were the only independent prognostic factors associated with OS. PR status and HER-2 status were no longer independent risk factors for OS.
Table 3.
Univariate and multivariate analyses to identify predictors of relapse-free survival in patients (n = 229) that were enrolled in a study to investigate whether the presence aldehyde dehydrogenase 1 (ALDH1) in axillary lymph node metastases (ALNM) in patients with breast cancer was associated with clinical prognosis.
| Characteristic | Univariate analyses |
Multivariate analyses |
||||
|---|---|---|---|---|---|---|
| OR | P-value | 95% CI | OR | P-value | 95% CI | |
| Age, ≤50 versus >50 years | 0.792 | NS | 0.516, 1.515 | |||
| Menopausal status, pre versus post | 0.869 | NS | 0.475, 1.913 | |||
| Tumour size, ≤2 versus >2 cm | 2.987 | P = 0.031 | 1.264, 6.113 | 2.175 | NS | 0.938, 4.117 |
| ER in ALNM, –/+ | 3.747 | P = 0.001 | 2.236, 6.280 | 3.413 | P = 0.009 | 2.016, 5.117 |
| PR in ALNM, –/+ | 2.994 | P = 0.004 | 1.791, 5.007 | 0.760 | NS | 0.413, 1.445 |
| HER-2 in ALNM, –/+ | 2.002 | P = 0.013 | 1.161, 3.452 | 0.895 | NS | 0.418, 1.957 |
| ALDH1 in ALNM, –/+ | 2.084 | P = 0.005 | 1.255, 3.459 | 1.746 | P = 0.034 | 1.037, 2.939 |
OR, odds ratio; CI, confidence interval; ER, oestrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor 2; NS, no significant association (P ≥ 0.05.
Table 4.
Univariate and multivariate analyses to identify predictors of overall survival in patients (n = 229) that were enrolled in a study to investigate whether the presence aldehyde dehydrogenase 1 (ALDH1) in axillary lymph node metastases (ALNM) in patients with breast cancer was associated with clinical prognosis.
| Characteristic | Univariate analyses |
Multivariate analyses |
||||
|---|---|---|---|---|---|---|
| OR | P-value | 95% CI | OR | P-value | 95% CI | |
| Age, ≤50 versus >50 years | 0.913 | NS | 0.495, 1.670 | |||
| Menopausal status, pre versus post | 0.819 | NS | 0.452, 1.140 | |||
| Tumour size, ≤2 versus >2 cm | 1.575 | NS | 0.073, 4.431 | 1.675 | NS | 0.829, 5.126 |
| ER in ALNM, −/+ | 3.150 | P = 0.003 | 1.624, 4.521 | 3.798 | P = 0.004 | 1.139, 2.037 |
| PR in ALNM, −/+ | 2.710 | P = 0.010 | 2.177, 6.118 | 0.563 | NS | 0.413, 2.445 |
| HER-2 in ALNM, −/+ | 1.879 | P = 0.023 | 1.092, 3.233 | 0.775 | NS | 0.617, 2.101 |
| ALDH1 in ALNM, −/+ | 2.084 | P = 0.005 | 1.255, 3.459 | 1.713 | P = 0.048 | 1.004, 2.925 |
OR, odds ratio; CI, confidence interval; ER, oestrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor 2; NS, no significant association (P ≥ 0.05).
Discussion
This current retrospective study investigated whether ALDH1 immunostaining in axillary lymph node metastases in patients with ALDH1-positive breast cancer was associated with clinical prognosis. The findings showed that patients with ALDH1-positive axillary lymph node metastases had significantly shorter RFS and OS compared with those with ALDH1-negative axillary lymph node metastases. ALDH1 immunostaining in axillary lymph node metastases was a significant predictor of poor prognosis on univariate and multivariate analyses. Aldehyde dehydrogenases play a role in stem cell regulation and possessing cancer-related functions, regulating multiple pathways implicated in stem cell signalling and carcinogenesis.7–9 Clinical data suggest that ALDH1 is an effective CSC biomarker in breast cancer that is significantly associated with poor prognosis.10–15 The above findings combined with these current results suggest that CSCs are present in axillary lymph node metastases in patients with breast cancer and that ALDH1 has the potential to be an important biomarker of survival and prognosis in this patient population.
Metastasis often appears first in the ipsilateral axillary lymph nodes in patients with breast cancer. 24 Previously, the number of metastatic lymph nodes has been significantly associated with a worse prognosis. 25 More recently, attention has focused on using biomarkers to provide important and decisive information about patient outcomes.8,26 Specifically, the presence of ALDH1 in breast cancer detected by immunostaining was correlated with poor prognosis. 7 In triple-negative breast cancer, patients with ALDH1 immunostaining demonstrated shorter RFS and OS compared with patients without ALDH1 immunostaining; and ALDH1 immunostaining was an independent prognostic indicator of RFS and OS.27,28
Reports on the prevalence and clinical significance of ALDH1 immunostaining in axillary lymph node metastases in patients with breast cancer are scarce and the results are conflicting. For example, previous research showed no association between ALDH1 status in lymph node metastases and OS.22,29 Whereas, another study demonstrated shorter RFS and OS in patients with positive ALDH1 immunostaining in primary tumours and in axillary lymph node metastases compared with those whose tissues were ALDH1 negative. 19 ALDH1 immunostaining in axillary lymph node metastases was associated with significantly shorter RFS and OS in a univariate analysis but not in a multivariate analysis. 19 In accordance with these current findings, one study showed that the presence of ALDH1 in axillary lymph node metastases was significantly associated with the disease-free survival in patients with primary breast cancer and 1–3 metastatic lymph nodes. 30
To the best of our knowledge, the current study is the largest one to investigate the prognostic significance of ALDH1 immunostaining in axillary lymph node metastases in patients with ALDH1-positive breast cancer. Notably, follow-up was long term, with a median follow-up of around 5 years. Only patients with IDC and 1–3 metastatic lymph nodes were included to eliminate histology as a confounding factor. These current findings showed a correlation between ALDH1 immunostaining and ER negativity in axillary lymph node metastases with a poor prognosis. Accordingly, clinical studies show that breast cancer patients with negative hormone receptor status have poor prognosis as endocrine chemotherapy strategies are ineffective. 31
This current study had several limitations. First, it was a retrospective analysis and treatment decisions were affected by the pathological results and patient preference rather than randomization. Secondly, more patients and longer follow-up periods should be analysed to identify more significant differences in univariate and multivariate analysis. Thirdly, as the present study was a retrospective study, the histopathological evaluation procedure followed the conventional rules and not a defined study procedure, so there might be selection bias. Larger scale clinical observations and gene expression research are needed to uncover the mechanisms and provide strategies for personalized treatments.
In conclusion, this large study with long-term follow-up suggests that ALDH1 immunostaining in axillary lymph node metastases is an independent predictor of poor prognosis in patients with ALDH1-positive breast cancer. The clinical relevance of this finding should be confirmed in further well-designed prospective studies.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605211047279 for Aldehyde dehydrogenase 1 (ALDH1) immunostaining in axillary lymph node metastases is an independent prognostic factor in ALDH1-positive breast cancer by Xin Guan, Yi Dong, Zhimin Fan, Yue Zhan, Xinpeng Xie, Gege Xu, Yu Zhang, Guoqiang Guo and Aiping Shi in Journal of International Medical Research
Acknowledgements
We are grateful to Dr Yi Li from the Lester and Sue Smith Breast Center, Baylor College of Medicine, Houston, TX, USA for his considerable help. Many thanks go to the Department of Pathology, First Hospital of Jilin University, for tissue processing and pathological diagnosis.
Footnotes
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present study was financially supported by grants from the Bethune Plan B, Jilin, China (no. 2012217), the Department of Finance of Jilin Province (no. JLSWSRCZX2020-0042) and the Department of Science and Technology of Jilin Province (no. 20190701041GH).
ORCID iD: Xin Guan https://orcid.org/0000-0002-2232-0465
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Sørlie T, Perou CM, Tibshiranie R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001; 98: 10869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sørlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A 2003; 100: 8418–8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang F, Xu J, Tang L, et al. Breast cancer stem cell: the roles and therapeutic implications. Cell Mol Life Sci 2017; 74: 951–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duester G. Genetic dissection of retinoid dehydrogenases. Chem Biol Interact 2001; 130-132(1-3): 469–480. [DOI] [PubMed] [Google Scholar]
- 6.Niederreither K, Subbarayan V, Dolle P, et al. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet 1999; 21: 444–448. [DOI] [PubMed] [Google Scholar]
- 7.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007; 1: 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balicki D. Moving forward in human mammary stem cell biology and breast cancer prognostication using ALDH1. Cell Stem Cell 2007; 1: 485–487. [DOI] [PubMed] [Google Scholar]
- 9.Croker AK, Goodale D, Chu J, et al. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J Cell Mol Med 2009; 13: 2236–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xing P, Dong H, Liu Q, et al. ALDH1 expression and vasculogenic mimicry are positively associated with poor prognosis in patients with breast cancer. Cell Physiol Biochem 2018; 49: 961–970. [DOI] [PubMed] [Google Scholar]
- 11.Louhichi T, Ziadi S, Saad H, et al. Clinicopathological significance of cancer stem cell markers CD44 and ALDH1 expression in breast cancer. Breast Cancer 2018; 25: 698–705. [DOI] [PubMed] [Google Scholar]
- 12.Tomita H, Tanaka K, Tanaka T, et al. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget 2016; 7: 11018–11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Ma H, Zhang J, et al. Unraveling the roles of CD44/CD24 and ALDH1 as cancer stem cell markers in tumorigenesis and metastasis. Sci Rep 2017; 7: 13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo AN, Lee HJ, Kim EJ, et al. Expression of breast cancer stem cell markers as predictors of prognosis and response to trastuzumab in HER2-positive breast cancer. Br J Cancer 2016; 114: 1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shima H, Kida K, Adachi S, et al. Lnc RNA H19 is associated with poor prognosis in breast cancer patients and promotes cancer stemness. Breast Cancer Res Treat 2018; 170: 507–516. [DOI] [PubMed] [Google Scholar]
- 16.Qiu PF, Liu JJ, Wang YS, et al. Risk factors for sentinel lymph node metastasis and validation study of the MSKCC nomogram in breast cancer patients. Jpn J Clin Oncol 2012; 42: 1002–1007. [DOI] [PubMed] [Google Scholar]
- 17.Sciubba DM, Goodwin CR, Yurter A, et al. A systematic review of clinical outcomes and prognostic factors for patients undergoing surgery for spinal metastases secondary to breast cancer. Global Spine J 2016; 6: 482–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JH, Jeong H, Choi JW, et al. Liquid biopsy prediction of axillary lymph node metastasis, cancer recurrence, and patient survival in breast cancer: a meta-analysis. Medicine (Baltimore) 2018; 97: e12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito M, Shien T, Omori M, et al. Evaluation of aldehyde dehydrogenase 1 and transcription factors in both primary breast cancer and axillary lymph node metastases as a prognostic factor. Breast Cancer 2016; 23: 437–444. [DOI] [PubMed] [Google Scholar]
- 20.Dong Y, Bi LR, Xu N, et al. The expression of aldehyde dehydrogenase 1 in invasive primary breast tumors and axillary lymph node metastases is associated with poor clinical prognosis. Pathol Res Pract 2013; 209: 555–561. [DOI] [PubMed] [Google Scholar]
- 21.Tanei T, Morimoto K, Shimazu K, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res 2009; 15: 4234–4241. [DOI] [PubMed] [Google Scholar]
- 22.Yoshioka T, Umekita Y, Ohi Y, et al. Aldehyde dehydrogenase 1 expression is a predictor of poor prognosis in node-positive breast cancers: a long-term follow-up study. Histopathology 2011; 58: 608–616. [DOI] [PubMed] [Google Scholar]
- 23.Lu S, Tian J, Lv Z, et al. The probable role of tumor stem cells for lymph node metastasis in supraglottic carcinoma. Pathol Oncol Res 2011; 17: 33–38. [DOI] [PubMed] [Google Scholar]
- 24.Alsaif AA. Sentinel lymph node biopsy in breast cancer. Saudi Med J 2015; 36: 1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakin A andAldemir MN.. Lymph Node Ratio Predicts Long-Term Survival in Lymph Node-Positive Breast Cancer. Eur J Breast Health 2020; 16: 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nogi H, Suzuki M, Kamio M, et al. Impact of CD44+CD24− cells on non-sentinel axillary lymph node metastases in sentinel node-positive breast cancer. Oncol Rep 2011; 25: 1109–1115. [DOI] [PubMed] [Google Scholar]
- 27.O’Conor CJ, Chen T, González I, et al. Cancer stem cells in triple-negative breast cancer: a potential target and prognostic marker. Biomark Med 2018; 12: 813–820. [DOI] [PubMed] [Google Scholar]
- 28.Ma F, Li H, Li Y, et al. Aldehyde dehydrogenase 1 (ALDH1) expression is an independent prognostic factor in triple negative breast cancer (TNBC). Medicine (Baltimore) 2017; 96: e6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morimoto K, Kim SJ, Tanei T, et al. Stem cell marker aldehyde dehydrogenase 1-positive breast cancers are characterized by negative estrogen receptor, positive human epidermal growth factor receptor type 2, and high Ki67 expression. Cancer Sci 2009; 100: 1062–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nogami T, Shien T, Tanaka T, et al. Expression of ALDH1 in axillary lymph node metastases is a prognostic factor of poor clinical outcome in breast cancer patients with 1-3 lymph node metastases. Breast Cancer 2014; 21: 58–65. [DOI] [PubMed] [Google Scholar]
- 31.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 365: 1687–1717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605211047279 for Aldehyde dehydrogenase 1 (ALDH1) immunostaining in axillary lymph node metastases is an independent prognostic factor in ALDH1-positive breast cancer by Xin Guan, Yi Dong, Zhimin Fan, Yue Zhan, Xinpeng Xie, Gege Xu, Yu Zhang, Guoqiang Guo and Aiping Shi in Journal of International Medical Research