Abstract
Using a high-copy-number suppressor screen to obtain clues about the role of the yeast RNA polymerase II subunit RPB4 in transcription, we identified three suppressors of the temperature sensitivity resulting from deletion of the RPB4 gene (ΔRPB4). One suppressor is Sro9p, a protein related to La protein, another is the nucleosporin Nsp1p, and the third is the RNA polymerase II subunit RPB7. Suppression by RPB7 was anticipated since its interaction with RPB4 is well established both in vitro and in vivo. We examined the effect of overexpression of each suppressor gene on transcription. Interestingly, suppression of the temperature-sensitive phenotype correlates with the correction of a characteristic transcription defect of this mutant: each suppressor restored the level of promoter-specific, basal transcription to wild-type levels. Examination of the effects of the suppressors on other in vivo transcription aberrations in ΔRPB4 cells revealed significant amelioration of defects in certain inducible genes in Sro9p and RPB7, but not in Nsp1p, suppressor cells. Analysis of mRNA levels demonstrated that overexpression of each of the three suppressors minimally doubled the mRNA levels during stationary phase. However, the elevated mRNA levels in Sro9p suppressor cells appear to result from a combination of enhanced transcription and message stability. Taken together, these results demonstrate that these three proteins influence transcription and implicate Sro9p in both transcription and posttranscription events.
The eukaryotic RNA polymerase II holoenzyme is a highly conserved, multiprotein complex that plays an essential role in transcription (20, 41). The RNA polymerase II component of this holoenzyme in yeast Saccharomyces cerevisiae comprises 12 subunits (designated RPB1 to RPB12) that have a range of functions, many of which are still poorly defined (21, 64, 69). The RPB1, RPB2, and RPB3 genes are relatives of the bacterial β′, β, and α subunits, respectively, based on strong sequence similarity (RPB1 and RPB2 with β′ and β) or modest sequence similarity coupled with functional similarities (RPB3 and α) (28, 30, 39, 60).
RPB4 is a 221-amino-acid, 25-kDa protein that is one of two nonessential subunits of RNA polymerase II. S. cerevisiae cells lacking RPB4 (ΔRPB4 cells) grow at moderate temperatures but not above 32°C or below 12°C (65), while RPB4 in Schizosaccharomyces pombe is essential for growth (even at moderate temperatures [53]). RPB4 has a functional relationship with another small (171-amino-acid, 19-kDa) essential subunit, RPB7. This relationship was first suggested by biochemical data, later substantiated by in vitro and in vivo interaction data, and recently was corroborated by overexpression experiments.
Upon purification of RNA polymerase II, it was observed that both RPB4 and RPB7 dissociate from the other 10 subunits under mild denaturing conditions, during native gel electrophoresis, and upon DEAE-Sephadex anion exchange chromatography (6, 9, 51). The two subunits likely form a stable subcomplex upon dissociation from RNA polymerase II, since several reports have confirmed their association. Interaction-trap experiments demonstrated contact between the two subunits in vivo, in addition to a stable but lower affinity interaction between human RPB7 and the yeast counterpart of RPB4 (26, 27). Biochemical experiments with Arabidopsis subunits demonstrated reciprocal interactions between RPB4 and RPB7 (31), and affinity chromatography was enlisted to confirm that RPB4 associates with RPB7 in S. pombe (53). The association of RPB7 with RNA polymerase II is thought to be dependent upon its interaction with RPB4, since RNA polymerase II immunoprecipitated or biochemically purified from ΔRPB4 cells also appears to lack RPB7 (9, 29). However, these results were inconsistent with the fact that RPB7, but not RPB4, is essential for growth. We proposed that RPB7 must either have an essential function independent of its association with RNA polymerase II or be associated with RNA polymerase at nondetectable levels that are above the threshold required for growth of ΔRPB4 cells (36). Recent experiments support the latter scenario, since this work and that of others (33, 56) demonstrate that overexpression of RPB7 suppresses the temperature-sensitive defect of ΔRPB4 cells. Multicopy suppression by RPB7 appears to result from the increased association of RPB7 molecules with RNA polymerase II (56).
The RPB4 and RPB7 subunits are represented at substoichiometric levels (∼0.5 molecules per RNA polymerase) (29), resulting in a heterogeneous mix of wild-type and Δ4/7 enzyme upon purification. Therefore, ΔRPB4 S. cerevisiae cells were the source of RNA polymerase II for most structural studies (3–5, 8, 13). However, a relatively low-resolution structure of the entire 12-subunit enzyme has been recently reported, and comparison to the Δ4/7 enzyme revealed differences in conformation (1, 24). The wild-type enzyme favored the closed conformation while the ΔRPB4 enzyme favored the open conformation, suggesting that the presence of RPB4 (and RPB7) stabilizes the initiation complex. This structural data is consistent with previously published work demonstrating an appreciable decrease in levels of basal transcription by ΔRPB4 cell extracts in vitro (9). Edwards et al. (9) suggested that the growth defects seen with ΔRPB4 cells at permissive temperature may be due to low overall transcription initiation efficiency—a phenotype consistent with the new structural information suggesting that the enzyme exists in a less stable, more open conformation at the promoter. Previously, the lethality seen with ΔRPB4 cells at temperature extremes appeared to result from transcription initiation inefficiency compounded by the inability to mount a normal stress response, since transcription of crucial stress response genes is low or absent upon heat shock (2, 56). However, a recent report has suggested that the general activity of RNA polymerase II is abrogated after the switch to the 37°C nonpermissive temperature, since transcription of three genes (DED1, ACT1, and STE2) is arrested (33). Therefore, heat shock genes are not the only genes whose expression is down after temperature shift; all RNA polymerase II gene expression is projected to cease upon temperature shift.
In this work, three diverse proteins—an RNA polymerase II subunit, a nucleoporin, and an RNA binding protein—are unified by their ability to independently function as high-copy suppressors of the temperature-sensitive phenotype of ΔRPB4 cells. Overexpression of either protein appears to suppress by increasing RNA polymerase II transcription at certain genes, or, in the case of Sro9p, suppression appears to result from a combination of augmented RNA polymerase II transcription and increased mRNA stability.
MATERIALS AND METHODS
Suppressor isolation and characterization.
Yeast strains and plasmids used in this study are listed in Table 1. The high-copy-number genomic library in the vector YEp24 (kindly provided by the G. Fink laboratory) was transformed into the ΔRPB4 strain (WY-4) using electroporation. The library originated from D. Botstein and M. Carlson, has an average insert size of 12.0 kb, and was prepared by ligation of Sau3A partially digested S. cerevisiae genomic DNA to the BamHI site of YEp24.
TABLE 1.
Yeast strains and plasmids used in this study
| Strain or plasmid | Genotype or description |
|---|---|
| Strains | |
| N114 | MATα his3Δ200 leu2-3 leu2-112 ura3-52 |
| WY-4 | MATα RPB4Δ1::HIS3 his3Δ200 leu2-3 leu2-112 ura3-52 |
| WY-197 | MATα RPB4Δ1::HIS3 his3Δ200 leu2-3 leu2-112 ura3-52 pNW247 |
| WY-198 | MATα RPB4Δ1::HIS3 his3Δ200 leu2-3 leu2-112 ura3-52 pNW251 |
| WY-199 | MATα RPB4Δ1::HIS3 his3Δ200 leu2-3 leu2-112 ura3-52 pRP721 |
| WY-200 | MATα RPB4Δ1::HIS3 his3Δ200 leu2-3 leu2-112 ura3-52 pNW249 |
| Plasmids | |
| pNW247 | 1.8-kb EcoRI/XhoI SRO9-containing fragment in YEplac181 |
| pNW248 | SRO9-coding region with PCR-constructed EcoRI ends in pGEX-4T-1 |
| pNW249 | pNW247 lacking region encoding amino acids 297–338 of SRO9 |
| pNW250 | Nsp1p-coding region with PCR-constructed BamHI ends in pGEX-2TK |
| pNW251 | 3.5-kb NSP1-containing BamHI fragment in YEplac181 |
| pRP716 | RPB7-coding region with PCR-constructed BamHI (5′)-EcoRI (3′) ends in pGEX-2T |
| pRP721 | 1.5-kb RPB7-containing PCR fragment with ∼700-bp 5′ (BamHI added) and ∼300-bp 3′ (SalI added) DNA in YEplac181 |
| pRP731 | YEplac181 containing BamHI/SalI fragment encoding RPB7 lacking amino acids 1–12 |
| pRP732 | YEplac181 containing BamHI/SalI fragment encoding RPB7 lacking amino acids 1–22 |
| pRP733 | YEplac181 containing BamHI/SalI fragment encoding RPB7 lacking amino acids 1–33 |
| pRP734 | YEplac181 containing BamHI/SalI fragment encoding RPB7 lacking amino acids 1–49 |
| pRP735 | YEplac181 containing BamHI/SalI fragment encoding RPB7 lacking amino acids 157–171 |
| pRP736 | YEplac181 containing BamHI/SalI fragment encoding RPB7 lacking amino acids 143–171 |
| pRP737 | YEplac181 containing BamHI/SalI fragment encoding RPB7 lacking amino acids 125–171 |
| pRP738 | YEplac181 containing BamHI/SalI fragment encoding RPB7 lacking amino acids 109–171 |
Approximately 50,000 transformants were tested for better growth than ΔRPB4 at 24°C, and those also able to grow at 34°C were pursued in detail. Once plasmid-dependent suppression was established, the identity of the insert was determined by sequence analysis using primers to the 5′ and 3′ ends only (if the DNA fragment was identifiable based on S. cerevisiae genome sequence available at the time of suppressor identification), or the entire length of the suppressing insert was sequenced to define the length and number of open reading frames in each insert. All possible open reading frames and corresponding promoter regions within each suppressing fragment were ligated to the high-copy vector YEplac181, transformed into ΔRPB4 cells, and tested for suppression. Only the open reading frames derived from separate suppressing fragments encoding RPB7, Sro9p, and Nsp1p (pRP721 [52], pNW247, and pNW251, respectively) supported growth at 34°C. Overall, six plasmids containing overlapping fragments of SRO9, one plasmid containing NSP1, and one plasmid containing RPB7 were identified in this suppressor screen. In addition, five suppressing plasmids with overlapping inserts containing RPB4 were identified.
Construction of plasmids.
To create pNW248, oligonucleotides were used to amplify the SRO9 open reading frame by PCR from the pNW247 template and add EcoRI sites to both the 5′ and 3′ ends. The resulting fragment was digested with EcoRI and ligated to the EcoRI sites of pGEX4T-1, and clones in the correct orientation were selected by restriction digestion. To construct pNW250, oligonucleotides were used to amplify the NSP1 open reading frame and add BamHI sites to both ends using pNW251 as a template. Clones in the correct orientation were selected by restriction digestion. The integrity of the PCR products and orientation of the inserts in pNW248 and pNW250 were confirmed by DNA sequence analysis. We used a two-step PCR method (22) to construct pNW249, the plasmid expressing the SRO9 gene lacking the sequences encoding 46 amino acids (293 to 338). The PCR fragment containing the SRO9-1 mutant gene was then inserted into vector Yeplac181 at the XbaI/EcoRI sites, and the DNA sequence was verified. The eight RPB7 truncation mutants (pRP731 to pRP738) were created by two-step PCR (22) using pRP721 as a template and primers complementary to YEplac181 sequences flanking the pRP721 insert. The PCR products were cut with BamHI/SalI and ligated to the BamHI/SalI sites of YEplac181.
Transcription extract preparation.
Whole-cell extracts were prepared from isogenic wild type, ΔRPB4 cells, and the ΔRPB4 suppressor cells according to published procedures (63). Yeast cells were grown in yeast-peptone-dextrose (YPD) (wild type, ΔRPB4) or synthetic complete (SC)-Leu medium (ΔRPB4 suppressor cells) to an optical density at 600 nm (OD600) between 3 and 7. The final extract was dialyzed until the conductivity of a 1:200 dilution was below 100 μS/cm. The protein concentration was measured, and the extract was stored in aliquots at −70°C.
In vitro transcription assays.
Transcription reactions were based on published procedures (32). The template plasmid used for the transcription reactions, pGAL4CG−, contains a single GAL4 binding site and a CYC1 TATA element controlling the expression of two predominant transcripts (∼350 and 370 nucleotides) from a G-less cassette. Omission of GTP during the reaction and subsequent treatment with RNase T1 significantly reduces the background of nonspecific transcription. Transcription reactions contained 300 μg of extract and 200 ng of template. Experiments with added recombinant proteins contained 20 or 50 ng of added protein per reaction.
Preparation of recombinant suppressor proteins.
The glutathione S-transferase (GST) fusion plasmids containing the suppressor genes were transformed into Escherichia coli BL21 cells. For induction, 100-ml cultures were grown at 37°C to an OD600 of 0.5 to 0.8. Induction of GST fusion protein expression in BL21 cells was initiated by the addition of 100 mM isopropyl-β-d-thiogalactopyranoside to a final concentration of 0.1 mM. Cells were grown for 3 h following induction, harvested, and resuspended in 5 ml of TSE (50 mM Tris [pH 8.0], 1 mM EDTA, and 100 mM NaCl). After four rounds of sonication (20 pulses each), 10% Triton X-100 was added to a final concentration of 1%. The resulting cell lysate was centrifuged for 10 min at 15,000 × g, and the supernatant was recovered and incubated with prewashed (TSE containing 10% Triton X-100) glutathione-Sepharose 4B beads (Amersham Pharmacia Biotech) at 4°C for 1 h. To obtain the GST fusion proteins, the glutathione beads bound with GST fusion proteins were washed three times with TSE containing 10% Triton X-100 and were eluted with 200 μl of freshly prepared 50 mM Tris [pH 8.0] containing 5 mM reduced glutathione. Glycerol was added to a final concentration of 15%, and the proteins were stored at −70°C.
Analysis of heat shock and inducible gene expression.
For INO1, cells were grown at 27°C to mid-log phase in minimal medium containing 400 μM inositol. Cells were collected by centrifugation, washed, and then grown for 10 h in minimal medium containing 10 μM inositol. For analysis of GAL1 expression, cells were grown to mid-log phase at 27°C in SC medium containing 2% raffinose and were collected by centrifugation, washed, and then grown for 3 h at 27°C in SC medium containing 5% galactose. For PHO5, cells were grown at 27°C in either low-phosphate YPD (activating conditions) or high-phosphate YPD (nonactivating conditions). In low- and high-phosphate YPD, potassium phosphate is added to a final concentration of 0.1 and 7.5 mM, respectively. Both low- and high-phosphate cultures were started at an OD600 of ∼0.01 and were harvested at an OD600 of ∼0.5.
For analysis of SSA1, SSA3, and HSP26 induction, yeast cells were grown in SC-Leu at 27°C to log phase and were subjected to the standard yeast heat shock temperature of 39°C (42) by placing the culture in a water bath shaker. Cells were grown in large flasks with relatively small volumes of media to enable rapid equilibration of temperature after shift. Cell samples were removed for RNA preparation 0, 15, and 105 min after heat shock.
Total RNA was prepared (55), and 15 μg was loaded into each lane of the formaldehyde agarose gels. After transfer to nitrocellulose, membranes were hybridized with radioactively labeled DNA and band intensities were quantified using a phosphorimager. The plasmid names and fragment sizes used as gene-specific probes were as follows: INO1 (pN333), 0.9-kb HindIII/ClaI; GAL1 (pGAL1-GAL10), 2.1-kb EcoRI; PHO5 (pN973), 625-bp BamHI/SalI; and U3(pJD161), 0.5-kb BamHI/HpaI. The DNA fragments used to visualize the three suppressor transcripts (see Fig. 1B) and SSA1, SSA3, and Hsp26 (see Fig. 4) were prepared by PCR using oligonucleotide pairs within each coding region.
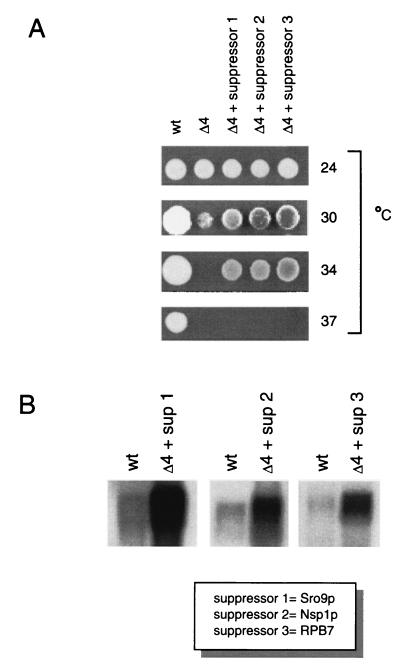
FIG. 1.
Suppression of the conditional lethal phenotype of ΔRPB4 by three high-copy-number suppressors. (A) YPD plates were spotted with wild-type (wt), isogenic mutant ΔRPB4 (▵4), and suppressed cells (▵4 + suppressor 1, 2, or 3) followed by incubation at the temperatures indicated. (B) All three suppressor genes are transcribed at high levels in suppressed cells. RNA was prepared from the strains indicated and hybridized to radioactively labeled DNA corresponding to the coding region of each suppressor. Equivalent levels of total RNA were loaded in each lane.
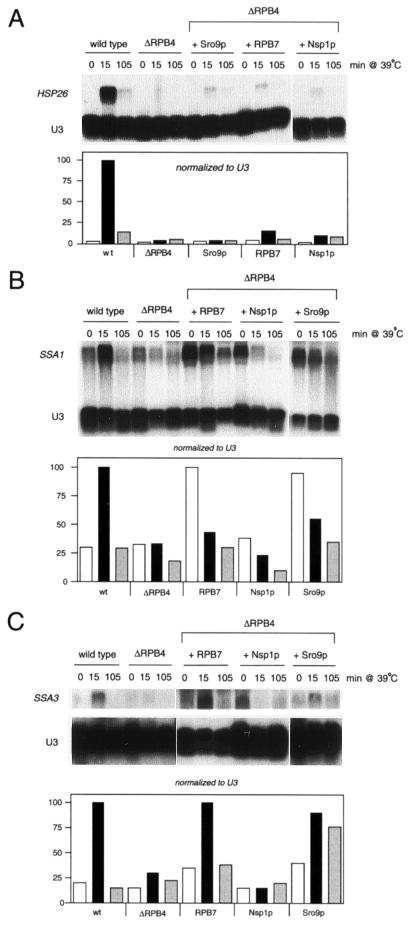
FIG. 4.
Suppression by RPB7 and Sro9p alters expression of certain heat shock genes. Total RNA was prepared from cells exposed to the indicated conditions and was hybridized to HSP26, SSA1, or SSA3 DNA. U3 (RNA polymerase II transcribed snRNA) was included as a loading control, test transcripts were normalized to U3, and activation levels were graphed relative to the activated wild-type control.
Analysis of total mRNA levels.
The samples used to prepare total RNA from two log-phase and two stationary-phase time points were selected from samples of cells grown in SC-Leu (wild-type cells were transformed with YEplac181) that were harvested at regular intervals during a complete 27°C growth cycle, starting with cells diluted to an OD600 of ∼0.01 and ending at late log phase. Growth curves were plotted for each strain, and total RNA was prepared from cell samples corresponding to the four specific phases of growth: early log phase, late log phase, early stationary phase, and late stationary phase. Forty micrograms of total RNA per sample was immobilized with nitrocellulose, and mRNA levels were determined after hybridization with an excess of radioactively labeled (poly)dT oligonucleotide followed by quantification using a phosphorimager.
For the mRNA stability experiments, cells were harvested from 500-ml cultures grown to an OD600 of 0.6 to 0.7 at 27°C. The 500-ml culture was split into two 250-ml portions and collected by centrifugation, and the pellets were resuspended in 20 ml of fresh medium with or without 20 μg of thiolutin/ml (from a 2-mg/ml stock). The cultures were returned to a 27°C shaker, and RNA was prepared from 2-ml aliquots collected after 3, 5, 8, 10, 20, 30, 40, 50, or 60 min. Forty micrograms of RNA from each sample was immobilized to nitrocellulose, hybridized with an excess of radioactively labeled (poly)dT oligonucleotide, and quantified using a phosphorimager.
RESULTS
Overexpression of three proteins with apparently unrelated functions suppresses the ΔRPB4 temperature-sensitive phenotype.
We used a high-copy-number suppressor screen to obtain insight into the role of the RNA polymerase II subunit RPB4 in transcription. Since the multicopy suppressors were selected in an ΔRPB4 mutant background, we wanted to determine if we could identify functional homologs (although no other yeast proteins have sequences similar to RPB4) or proteins that bypass the requirement of RPB4 at nonpermissive temperatures. Deletion of the RPB4 subunit causes lethality at 12°C and over 32°C in our genetic background (65). A high-copy genomic library was transformed into the ΔRPB4 strain, and ∼50,000 transformants were screened for growth faster than that of ΔRPB4 cells at the permissive temperature (24°C). The resulting colonies were then screened for growth at the restrictive high temperature of 34°C. This approach was implemented as an alternative to direct selection of colonies appearing at 34°C, since only plasmids containing RPB4 were isolated as suppressors by the direct method. Plasmid-based suppressors that grew better than ΔRPB4 at 24°C and then were able to grow at 34°C fell into three classes (excluding RPB4) based on their overlapping insert sequences. Subcloning of individual open reading frames within the smallest suppressing insert from each class revealed three different genes. None of the other open reading frames within the original inserts containing one of these three suppressors were able to suppress the temperature-sensitive phenotype.
To investigate the resulting growth phenotypes of the three suppressors relative to ΔRPB4 and wild-type cells, we spotted equal numbers of cells onto YPD plates and incubated them at 24, 30, 34, and 37°C (Fig. 1A). None of the suppressors restored growth to wild-type levels at any temperature, nor did they support growth at the highest nonpermissive temperature of 37°C. We also confirmed that each suppressor strain was transcribing high levels of the respective suppressor mRNA (Fig. 1B).
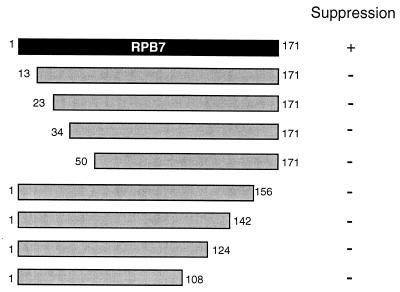
Suppressor 1 encoded Sro9p, a relative of the La protein (67) that has recently been shown to bind to RNA and associate with polyribosomes (57). Suppressor 2 encoded the nucleoporin Nsp1p (23), a component of the nuclear pore complex that mediates nuclear import (11, 54). Suppressor 3 encoded RNA polymerase II subunit RPB7. In order to pinpoint regions of RPB7 important for suppression, we prepared strains expressing either of eight truncated forms of RPB7 (Fig. 2). Surprisingly, we found that none of the strains expressing truncated RPB7 were able to suppress the ΔRPB4 34°C growth defect. (Since the RPB7 protein—like most holoenzyme components—is present at relatively low levels, we were unable to detect it in wild-type cells by Western blotting. Therefore, we could not verify the presence of stable protein products in cells expressing each of the RPB7 truncation plasmids.) Isolation of suppressors of the ΔRPB4 high-temperature growth defect demonstrated that the essential function of RPB4 at restrictive temperatures can be partially compensated for by overexpression of other genes with seemingly diverse roles.
FIG. 2.
The carboxy and amino termini of RPB7 appear to be indispensable for suppression. Full-length RPB7 is at the top, and truncated versions are shown below. Sizes are approximate. Suppression was defined as growth at 34°C.
ΔRPB4 suppressors can alleviate characteristic in vitro transcription defects.
Previous studies revealed that ΔRPB4 cells were defective in promoter-specific transcription initiation in vitro but not in activation by GAL4-VP16 (9). We wanted to determine if suppression of the conditional lethal phenotype of ΔRPB4 by the three suppressors correlated with improvement or correction of this transcription defect. Therefore, we measured the effect of the suppressor genes on transcription in vitro by using two approaches. First, we tried to reconstitute the effect of the suppressor on transcription by adding recombinant suppressor proteins to ΔRPB4 whole-cell extracts before the transcription reaction was initiated. Second, we prepared whole-cell extracts from each suppressor strain grown at 34°C and used these to drive the transcription reaction.
Recombinant Sro9p, Nsp1p, and RPB7 GST fusion proteins were purified and added to the transcription reaction containing ΔRPB4 extract (Fig. 3A). The template used for in vitro transcription has a GAL4 binding site to test for activated transcription, the yeast CYC1 promoter region, and a G-less cassette. Since it is known that extracts from ΔRPB4 cells respond normally to activation by GAL4-VP16 (9), we assessed suppressor effects only on basal transcription. The amount of the two transcripts that initiate within the G-less cassette is a measure of the efficiency of basal transcription. In agreement with previously published work (9), the ΔRPB4 whole-cell extract supported only low levels of basal transcription. Interestingly, the addition of either Sro9p or RPB7 recombinant protein to the transcription reaction resulted in the synthesis of more transcript, while the addition of Nsp1p did not significantly affect transcription levels (Fig. 3A). Addition of either RPB7 or Sro9p did not elevate transcription to wild-type levels, consistent with most published add-back experiments. Typically, the addition of recombinant protein increases transcription to only 40 to 50% of wild-type levels, and reconstitution to wild-type levels is rare. These experiments suggest that the mechanism of suppression by Nsp1p is distinct from that of RPB7 and Sro9p.
FIG. 3.
Correction of the ΔRPB4 in vitro transcription defect by the Sro9p, Nsp1p, and RPB7 suppressors. (A) Some GST-fused suppressor proteins can ameliorate the ΔRPB4 transcription defect in vitro. Twenty and 50 ng of recombinant GST fusion proteins were added as indicated to in vitro transcription reactions using ΔRPB4 whole-cell extracts. Consistent results were obtained in three independent experiments. (B) GST fusion proteins have no stimulating effect on in vitro transcription with wild-type extracts. Fifty nanograms of the respective recombinant proteins used in panel A was added to transcription reactions as indicated. (C) Whole-cell extracts were prepared from the strains indicated and were used in the same in vitro transcription assay used in panels A and B. Consistent results were obtained in three independent experiments.
To determine if the increase in transcription caused by the addition of recombinant suppressor protein is specific for the ΔRPB4 mutants, we added recombinant protein to the wild-type extract prior to transcription. Amounts corresponding to the highest concentrations used in Fig. 3A (50 ng) did not alter transcription levels (Fig. 3B). These results suggest that the suppressors function by specifically correcting the transcription defect resulting from the loss of the RPB4 subunit.
In the second approach, we performed the transcription reactions with extracts prepared from cells overexpressing the individual suppressor genes. Since recombinant add-back experiments often do not reconstitute wild-type levels of transcription, this in vivo expression experiment should more clearly demonstrate that the suppressors support an increase in the level of basal transcription. Extracts from all three suppressors now supported nearly wild-type levels of transcription (Fig. 3C), representing a five- to sevenfold increase in transcription compared to transcription supported by ΔRPB4 extracts. These results support a mechanism of suppression involving alleviation of transcription insufficiencies experienced by cells at the permissive temperature.
Sro9p and RPB7 multicopy suppressors enhance expression of multiple heat shock genes.
If the suppressors increase transcription levels in vitro, they would be predicted to lessen the severity of some other transcription defects in ΔRPB4 cells in vivo. One documented transcription abnormality is the failure to induce expression of the two heat shock genes SSA3 and HSP26 or the ubiquitin gene UBI4 (involved in the stress response) (2, 56). We also detected a defect in another heat shock gene, SSA1, in ΔRPB4 cells (Fig. 4). In fact, Maillet et al. (33) recently demonstrated that ΔRPB4 cells were unable to fully induce over 50 heat shock proteins, likely due to inactivation of RNA polymerase II at the heat shock temperature. We tested the effects of each suppressor on expression of HSP26, SSA1, and SSA3 after heat shock at 39°C (the temperature required for consistent induction of the full complement of heat shock genes [42]). Upon normalization to the RNA loading control, we found that only the Sro9p and RPB7 suppressors enhanced induction of some or all of these genes (Fig. 4), while Nsp1p had a marginal effect (HSP26) or no effect (SSA1 and SSA3). Curiously, Sro9p and RPB7 overexpression appears to result in significant induction of the SSA1 gene even under non-heat shock conditions (27°C).
Nuclear localization of the zinc finger protein Msn2p—a key regulator of stress-responsive gene expression in yeast—is regulated by stress. Msn2p, and its partially redundant relative Msn4p, accumulate in the nucleus under stress conditions (16). Since we identified a component of the nuclear import complex in our suppressor screen, we tested if Msn2p overexpression could also function as a suppressor. We found that Msn2p, like Nsp1p, could suppress ΔRPB4 temperature sensitivity at 34°C but not at higher temperatures (data not shown). Therefore, in ΔRPB4 cells it is possible that overexpression of Nsp1p now allows Msn2p and Msn4p to gain access to the nucleus at levels that enable cells to mount enough of a stress response to support growth during moderate temperature stress at 34°C.
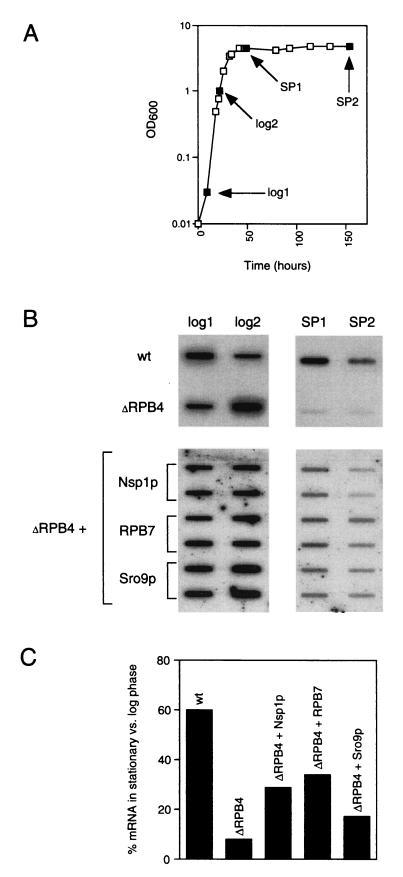
Expression of each multicopy suppressor increases in vivo mRNA levels during stationary phase.
ΔRPB4 cells grown at permissive temperature are also known to have decreased mRNA levels in stationary phase relative to wild type (2). Although it is normal to see less mRNA at stationary phase (mRNA levels at stationary phase in wild-type cells are ∼60% of log phase mRNA levels), stationary phase mRNA levels in ΔRPB4 cells are down even further (to 8% of log phase levels). We measured the amount of mRNA [i.e., the amount of poly(A) RNA in a 40-μg sample of total RNA] in samples harvested from suppressor and wild-type cells at log phase and stationary phase (Fig. 5A and B). We averaged the amount of mRNA in the two samples taken from log phase and averaged the amount of mRNA in the two samples taken from stationary phase and then determined the percentage of mRNA at stationary phase compared to log phase (represented in Fig. 5C). Again, in wild-type cells, the amount of mRNA at stationary phase is 60% of that in log phase; in ΔRPB4 cells, the amount of mRNA at stationary phase is 8% of that in log phase. Multicopy expression of each suppressor resulted in at least a twofold and up to a fourfold increase in the abundance of mRNA at stationary phase (34% for RPB7, 29% for Nsp1p, and 17% for Sro9p). These results suggest that each of the three suppressors may support the synthesis of other essential genes or facilitate more efficient transcription at all promoters, bringing the levels of gene expression above a critical level needed to support growth under these conditions.
FIG. 5.
Suppression results in an increase in mRNA levels present at stationary phase. (A) Full-growth profiles with accompanying cell samples were prepared for each strain grown under identical growth conditions, and total RNA was prepared from only those samples corresponding to the four phases of growth shown. log, logarithmic phase; SP, stationary phase. (B) Forty micrograms of total RNA from each sample was immobilized to nitrocellulose and hybridized with an excess of radioactively labeled (poly)dT oligonucleotide. (C) Poly(A) RNA in panel B was quantified, and the means of the two (for wt and ΔRPB4) or four (for each suppressor sample) SP samples, divided by the means of the two (for wt and ΔRPB4) or four (for each suppressor sample) log samples in each strain, are represented as percentages. The graph shows the resulting ratio of SP/log mRNA (wt, 60%; ΔRPB4, 8%; ΔRPB4+Nsp1p, 29%; ΔRPB4+RPB7, 34%; ΔRPB4+Sro9p, 17%).
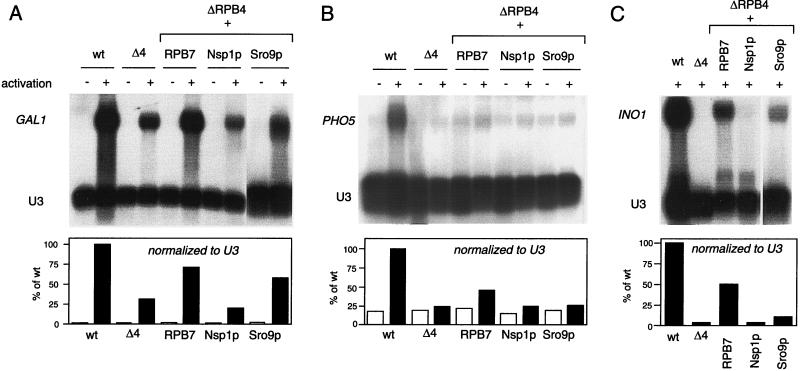
Some suppressors lessen the severity of activation defects for certain inducible genes.
Although promoter-dependent transcription with ΔRPB4 extracts is responsive to activation by GAL4-VP16 (9), this assay serves as a rough measure of overall activator responsiveness and does not necessarily predict the effects at specific promoters in vivo. Therefore, we looked for gene-specific activation abnormalities in ΔRPB4 cells at permissive temperature by measuring the levels of three well characterized inducible genes before and after induction (Fig. 6). ΔRPB4 cells were defective in activation at all three genes tested, with induced message levels 4, 30, and 25% of those of their wild-type isogenic counterparts for INO1, GAL1, and PHO5, respectively. Interestingly, we also found a complete loss of GAL1 gene activation (no detectable message levels upon induction) in ΔRPB4 cells at the nonpermissive temperature of 37°C (data not shown). Therefore, the use of inducible GAL fusion plasmids at the nonpermissive temperature is not a reliable method to assess transcription in ΔRPB4 cells. This observation is consistent with data suggesting that transcription by RNA polymerase II is rapidly shut off upon shifting ΔRPB4 cells to 37°C (33).
FIG. 6.
Some suppressors correct activation defects at inducible genes. (A and B) Northern blot analysis of GAL1 and PHO5 transcripts before (−) and after (+) activation. (C) Northern blot analysis of INO1 transcripts under activating conditions only (no INO1 transcripts are present before activation). All RNA was prepared from cells grown at the permissive temperature (27°C). U3 (RNA polymerase II transcribed snRNA) was included as a loading control, test transcripts were normalized to U3, and activation levels were graphed relative to the activated wild-type control.
Once we demonstrated the existence of activation abnormalities at these genes, we tested if the suppressors lessened their severity at permissive temperatures (Fig. 6). Activation in RPB7 suppressor cells was substantially improved at all three genes: a 13-fold increase for INO1, 2.4-fold increase for GAL1, and 1.8-fold increase for PHO5. Sro9p suppressor cells showed a significant increase in GAL1 expression (1.8-fold higher than ΔRPB4) but had only a marginal effect on induction of PHO5 and INO1. Nsp1p suppressor cells displayed no significant increase in induction of these three genes. We discovered that the genes encoding Nsp1p and Sro9p were not significantly overexpressed under the conditions of severe nutrient deprivation required for induction of INO1 (data not shown). Therefore, the moderate enhancement of INO1 induction in Sro9p suppressor cells would likely improve under bona fide high-copy-number conditions. The interpretation of the GAL1 and PHO5 data remains unchanged, since all three suppressors were expressed at high levels under inducing conditions. Overall, these experiments indicate that the RPB7 and Sro9p suppressors also can affect transcription by influencing induction of certain genes.
We verified that the level of Gal4 protein was uniform and was at wild-type levels in all strains (data not shown). Therefore, the defect seen at the GAL1 promoter was not attributable to an indirect effect resulting from deficient transcription of the GAL4 gene. Since we do not have antibody to the gene-specific activators that regulate PHO5 and INO1 (PHO4 and INO2, respectively), we cannot exclude the possibility that the activation defects result from diminished activator levels. However, since we were interested in testing whether the suppressors ameliorate any transcription abnormality, and not necessarily activation per se, monitoring the final outcome is more relevant than pinpointing the step in the pathway to activation.
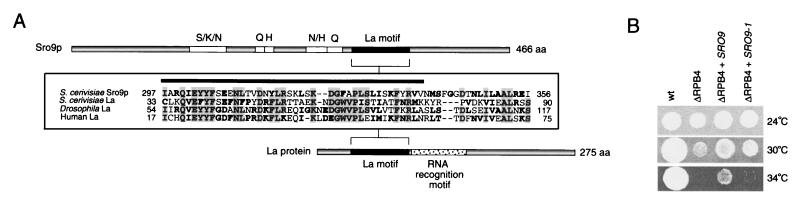
The domain conserved between Sro9p and the La protein is essential for suppression.
An approximately 60-amino-acid portion of Sro9p has significant sequence similarity to a highly conserved motif present in La proteins (Fig. 7A). The overall similarity between the S. cerevisiae La protein (designated Lhp1p) and Sro9p is limited to ∼20% of the total length of Lhp1p (67). La protein was originally identified as an autoimmune antigen in rheumatic disease patients. La protein is a phosphoprotein (12) that binds to all nascent RNA polymerase III transcripts (49, 50) at their 3′-end UUUOH termination sequence (58). It is also associated with a multitude of other functions: it is required for maturation of pre-tRNAs (68), it is involved in RNA polymerase III initiation (34) and termination (17), it enhances viral RNA translation (38, 59), and it stabilizes newly synthesized U6 RNA (43) and even stabilizes histone mRNA from degradation (37). Therefore, La protein is implicated in both transcription and posttranscription events.
FIG. 7.
A region of Sro9p related to La protein is essential for suppression of lethality. (A) Feature comparison of S. cerevisiae Sro9p and La protein drawn to scale. Amino acid stretches containing at least 50% of S/K/N, Q, H, N/H, or Q residues are shown. Similarity between Sro9p and the La motif is demonstrated with representative species; more extensive alignments have been reported earlier (57, 67). Identical amino acids are shaded and in boldface, and conserved amino acids (a score of 1 or greater using the Blosum 62 matrix) are in boldface. The amino acids absent in the truncation mutant SRO9-1 are indicated below the line. (B) Comparison of growth phenotypes of ΔRPB4 cells overexpressing SRO9 or mutant SRO9-1. Equivalent amounts of cells were spotted onto YPD plates and grown at the temperatures indicated.
The conserved domain between the Sro9p and La protein—referred to as the La motif—corresponds to a region of La protein that does not directly interact with RNA but is essential for RNA binding activity (short deletions in this region influence RNA binding) (14, 48). We tested if the same region is essential for suppression of ΔRPB4 growth defects. We constructed a ΔRPB4 yeast strain containing a multicopy plasmid expressing the truncated version of Sro9p in this conserved region (lacking 46 amino acids, residues 293 to 338) and tested for growth at a range of temperatures. Overexpression of mutant Sro9p could not suppress the lethality of ΔRPB4 mutant cells at 12 and 34°C (Fig. 7B and data not shown). Although we were not able to entirely eliminate the possibility of low expression or altered stability for the truncation mutant, normal levels of the identical truncated protein were obtained in another strain background (25). These results indicate that this short, conserved region of Sro9p plays an essential role in suppression. Since we have demonstrated that suppression of lethality at 34°C by Sro9p is associated with amelioration or correction of certain transcription aberrations, it is likely that this region also contributes to the transcription effects linked to suppression.
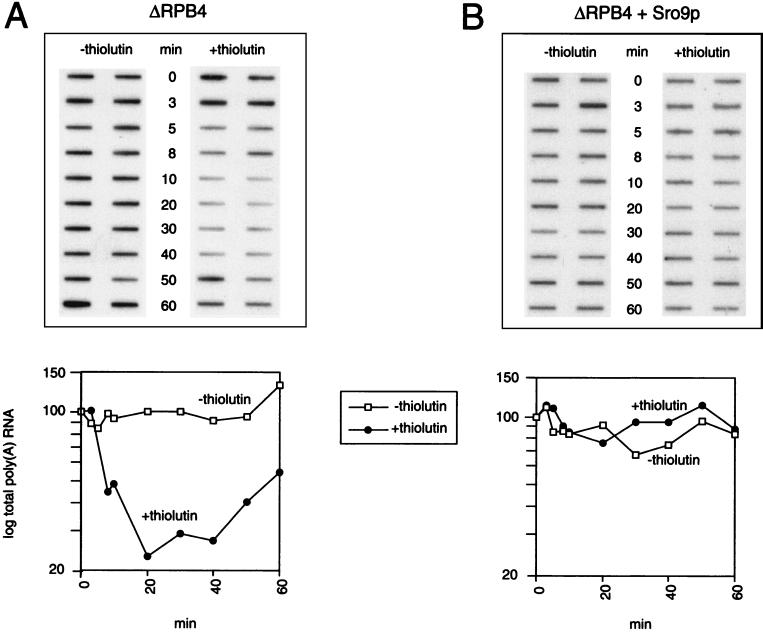
Overexpression of Sro9p increases mRNA stability.
Sro9p is related to La protein, and La protein increases the stability of histone mRNA in vitro (37). To determine whether the increase in mRNA levels seen with the suppressor Sro9p could be partially attributed to an increase in mRNA stability—in addition to an increase in transcription of certain genes noted—we measured mRNA stability in the ΔRPB4 mutant and Sro9p suppressor strains (Fig. 8). We prepared RNA from samples harvested from equivalent log phase cultures with or without the addition of the fast-acting transcription inhibitor thiolutin. Thiolutin is an antifungal agent that inhibits transcription by RNA polymerases I, II, and III in vitro and in vivo (46). Therefore, one can analyze total mRNA decay in samples harvested in the presence of the drug (when no new synthesis occurs) relative to equivalent samples harvested from cells in the absence of the drug (when mRNA levels typically increase). Sro9p suppressor cells showed significantly less decay in mRNA after thiolutin treatment relative to the ΔRPB4 mutant (Fig. 8A and B), suggesting that overexpression of Sro9p increases mRNA stability in vivo. These results lead us to propose a mechanism of suppression by Sro9p that involves both mRNA transcription and mRNA stability, and they suggest a new role for Sro9p in these two processes.
FIG. 8.
Increased mRNA stability in Sro9p suppressor cells. ΔRPB4 cells (A) or ΔRPB4 + Sro9p cells (B) were grown at permissive temperature (27°C) to mid-log phase; cells were harvested in two equal portions, and the pellets were resuspended in fresh medium with (+thiolutin) or without (−thiolutin) 20 μg of thiolutin/ml. The cultures were returned to a 27°C shaker, and RNA was prepared from aliquots collected after 3, 5, 8, 10, 20, 30, 40, 50, or 60 min. Forty micrograms of total RNA from each sample was immobilized to nitrocellulose and was hybridized with an excess of radioactively labeled (poly)dT oligonucleotide; poly(A) RNA was quantified using a phosphorimager. The values shown on each curve were normalized to their respective 0-min control and converted to a percentage. The effectiveness of RNA polymerase II transcription inhibition by thiolutin consistently diminished with time, as noted by an increase in mRNA synthesis in both thiolutin-treated strains after approximately 20 min.
DISCUSSION
Employing a high-copy suppressor screen with the ΔRPB4 mutant, we identified three proteins that suppress its temperature-sensitive phenotype. Suppression in each case is associated with enhancement of transcription in vitro (all three suppressors completely correct the pronounced in vitro basal transcription defect characteristic of the ΔRPB4 strain) and in vivo (all three have increased total mRNA levels, and RPB7 and Sro9p also correct transcription defects at several inducible genes). Interestingly, the increase in mRNA levels noted in Sro9p suppressed strains appears to result from a combination of enhanced transcription of certain RNA polymerase II genes and increased mRNA stability.
Sro9p is a 466-amino-acid, 52-kDa protein with unusual stretches of amino acids rich in histidine, glutamine, and asparagine residues (Fig. 7A) that is not essential for yeast cell growth (25). It has been isolated as a high-copy suppressor of phenotypes associated with a variety of mutations in unrelated processes: (i) the secretory pathway (the sec7-1 secretory pathway mutant and deletion mutant of the gene encoding the transport GTPase Ypt6p [61]), (ii) organization of the actin cytoskeleton (deletion of the gene encoding the GTPase Rho3p, partial deletion of the tropomyosin gene, and the actin point mutant act1-1 [25]), and (iii) pre-mRNA splicing (57).
Because Sro9p was first recognized as a relative of La protein (68), examination of its role in S. cerevisiae cells initially focused on a hallmark function attributed to La protein—RNA binding. In vitro studies revealed that Sro9p, like La protein, binds to RNA (57). Since the only region with significant similarity in these two proteins is the La motif (Fig. 7A), it is assumed that this region is essential for RNA binding even though it does not contain a known canonical RNA recognition motif (57).
Sobel and Wolin (57) recently demonstrated that Sro9p is primarily cytoplasmic and preferentially associates with translating ribosomes. Strains lacking Sro9p are viable but also exhibit reduced sensitivity to some translation inhibitors, suggesting that Sro9p somehow influences translation (57). mRNA translation and decay are thought to be connected, since each requires polysome-associated mRNA. Therefore, the authors proposed that Sro9p may function in some aspect of mRNA stability and decay. Our data support a role for Sro9p in mRNA stability. Total mRNA levels in thiolutin-treated Sro9p suppressor cells were relatively stable compared to those in thiolutin-treated ΔRPB4 cells. In addition, total mRNA levels were stabilized in Sro9p suppressor cells relative to ΔRPB4 cells after a shift to the nonpermissive temperature of 38°C (data not shown), when RNA polymerase activity is defective, and presumably mRNA synthesis is shut down (33). We have also demonstrated that Sro9p influences transcription of certain RNA polymerase II inducible genes. Sro9p, like La protein, appears to contribute to a panoply of cellular processes, including transcription initiation, translation, and mRNA stability. However, the extent of the functional parallels between Sro9p and La protein is not yet known. The diverse portfolio of functions linked to Sro9p is consistent with its ability to suppress a wide variety of mutant phenotypes.
In contrast to RPB7 and Sro9p, Nsp1p suppression appears to be indirect, since the addition of recombinant Nsp1p to transcription reactions does not affect transcription (Fig. 3). Nsp1p is a large (823-amino-acid, 86.5-kDa) protein that is one of the many nucleoporins comprising the ∼120-mDa nuclear pore complex (NPC) in eukaryotic cells (11, 54). The NPC mediates bidirectional protein transport between the cytoplasm and nucleus (35, 47). Synthetic lethal screens revealed that Nsp1p interacts with several nucleoporins, most of which are known to play a role in mRNA export (7, 10). Biochemical experiments indicate that Nsp1p can also be isolated in two distinct subcomplexes. One subcomplex—designated the Nsp1p complex—contains Nsp1p, Nup49p, Nup57p, and Nic96p (18). The second subcomplex comprises Nsp1p and Nup82p (19).
Nsp1p has been proposed to play a direct role in protein import from the cytoplasm to the nucleus (11). More specifically, electron microscopic localization of Nsp1p within the NPC revealed that it resides in three distinct subcomplexes in the NPC. Interestingly, each of the three subcomplexes is located in a region where cargo or transport ligands can be arrested (15, 44, 45). Therefore, Fahrenkrog et al. (11) suggested that Nsp1p may interact with cargo in transit through the NPC, or, alternatively, Nsp1p may bind ligands or factors involved in transport through the NPC.
If Nsp1p is directly involved in nuclear import and export, then suppression by Nsp1p overexpression may be a consequence of altered NPC function and perturbation of normal nuclear transport. Therefore, Nsp1p overexpression may (i) enable enhanced import of compensating subunits (e.g., RPB7), transcription factors (e.g., Msn2p), or activators into the nucleus; (ii) curtail the import of repressing proteins to the nucleus or facilitate their export; or (iii) mediate enhanced export of mRNAs encoding proteins that facilitate enhanced transcription. All three approaches could result in an increase in mRNA levels at or above the level required for viability at 34°C, and all are also consistent with the results shown in Fig. 3 (demonstrating reconstitution of normal in vitro transcription using extracts prepared from Nsp1p suppressed cells but not with ΔRPB4 extracts containing an excess of recombinant Nsp1p).
Curiously, another component of the nuclear transport machinery—the importin (karyopherin) designated Kap114—has been isolated as a high-copy-number suppressor of a temperature-sensitive TATA-binding protein (TBP) mutant (40). TBP and Kap114 interact and Kap114 appears to mediate TBP import into the nucleus (40, 47). As another example of NPC transport proteins identified in genetic screens with mutants in transcription complex components, the importin-α Srp1p was first identified as a suppressor of a temperature-sensitive mutation in the largest subunit of S. cerevisiae RNA polymerase I (66). Finally, an interaction between the nucleoporin Nup57p (a component of the Nsp1p complex) and TAF17 (a histone-like TBP-associated factor) was uncovered by two-hybrid analysis (62). Therefore our data, combined with reports identifying direct and/or genetic interactions between other NPC proteins and transcription machinery components, suggest a role for Nsp1p in the transport of proteins influencing transcription by RNA polymerase II.
Using a genetic approach, we uncovered unexpected roles for two disparate proteins in transcription. Now that some of their functions are revealed, we can build upon this information to obtain a clearer picture of their precise roles in transcription, nuclear transport, mRNA decay, and other cellular processes.
ACKNOWLEDGMENTS
We thank Carlos Gonzalez and Stewart Peltz for plasmids and the generous gift of thiolutin, Michael Hampsey and Danny Reinberg for helpful discussions, the members of the Hampsey laboratory, especially Wei-Hua Wu and Zu-Wen Sun, for providing advice and reagents, and Keith McKune for technical support. We obtained a generous gift of RPB7 antibody from Andre Sentenac and Michel Riva. Strains and plasmids were kindly provided by D. Gross, P. Silver, the J. Dinman laboratory, and the Hampsey laboratory.
This work was funded by grant GM 55736 from the National Institutes of Health to N.A.W.
REFERENCES
- 1.Asturias F J, Meredith G D, Poglitsch C L, Kornberg R D. Two conformations of RNA polymerase II revealed by electron crystallography. J Mol Biol. 1997;272:536–540. doi: 10.1006/jmbi.1997.1273. [DOI] [PubMed] [Google Scholar]
- 2.Choder M, Young R A. A portion of RNA polymerase II molecules has a component essential for stress responses and stress survival. Mol Cell Biol. 1993;13:6984–6991. doi: 10.1128/mcb.13.11.6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darst S A, Edwards A M, Kubalek E W, Kornberg R D. Three-dimensional structure of yeast RNA polymerase II at 16 A resolution. Cell. 1991;66:121–128. doi: 10.1016/0092-8674(91)90144-n. [DOI] [PubMed] [Google Scholar]
- 4.Darst S A, Kubalek E W, Edwards A M, Kornberg R D. Two-dimensional and epitaxial crystallization of a mutant form of yeast RNA polymerase II. J Mol Biol. 1991;221:347–357. doi: 10.1016/0022-2836(91)80223-h. [DOI] [PubMed] [Google Scholar]
- 5.Darst S A, Kubalek E W, Kornberg R D. Three-dimensional structure of Escherichia coli RNA polymerase holoenzyme determined by electron crystallography. Nature. 1989;340:730–732. doi: 10.1038/340730a0. [DOI] [PubMed] [Google Scholar]
- 6.Dezelee S, Wyers F, Sentenac A, Fromageot P. Two forms of RNA polymerase B in yeast. Proteolytic conversion in vitro of enzyme BI into BII. Eur J Biochem. 1976;65:543–552. doi: 10.1111/j.1432-1033.1976.tb10372.x. [DOI] [PubMed] [Google Scholar]
- 7.Doye V, Hurt E. From nucleoporins to nuclear pore complexes. Curr Opin Cell Biol. 1997;9:401–411. doi: 10.1016/s0955-0674(97)80014-2. [DOI] [PubMed] [Google Scholar]
- 8.Edwards A M, Darst S A, Feaver W J, Thompson N E, Burgess R R, Kornberg R D. Purification and lipid-layer crystallization of yeast RNA polymerase II. Proc Natl Acad Sci USA. 1990;87:2122–2126. doi: 10.1073/pnas.87.6.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards A M, Kane C M, Young R A, Kornberg R D. Two dissociable subunits of yeast RNA polymerase II stimulate the initiation of transcription at a promoter in vitro. J Biol Chem. 1991;266:71–75. [PubMed] [Google Scholar]
- 10.Fabre E, Hurt E. Yeast genetics to dissect the nuclear pore complex and nucleocytoplasmic trafficking. Annu Rev Genet. 1997;31:277–313. doi: 10.1146/annurev.genet.31.1.277. [DOI] [PubMed] [Google Scholar]
- 11.Fahrenkrog B, Hurt E C, Aebi U, Pante N. Molecular architecture of the yeast nuclear pore complex: localization of Nsp1p subcomplexes. J Cell Biol. 1998;143:577–588. doi: 10.1083/jcb.143.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan H, Sakulich A L, Goodier J L, Zhang X, Qin J, Maraia R J. Phosphorylation of the human La antigen on serine 366 can regulate recycling of RNA polymerase III transcription complexes. Cell. 1997;88:707–715. doi: 10.1016/s0092-8674(00)81913-3. [DOI] [PubMed] [Google Scholar]
- 13.Fu J, Gnatt A L, Bushnell D A, Jensen G J, Thompson N E, Burgess R R, David P R, Kornberg R D. Yeast RNA polymerase II at 5 A resolution. Cell. 1999;98:799–810. doi: 10.1016/s0092-8674(00)81514-7. [DOI] [PubMed] [Google Scholar]
- 14.Goodier J L, Fan H, Maraia R J. A carboxy-terminal basic region controls RNA polymerase III transcription factor activity of human La protein. Mol Cell Biol. 1997;17:5823–5832. doi: 10.1128/mcb.17.10.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorlich D, Mattaj I W. Nucleocytoplasmic transport. Science. 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- 16.Gorner W, Durchschlag E, Martinez-Pastor M T, Estruch F, Ammerer G, Hamilton B, Ruis H, Schuller C. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 1998;12:586–597. doi: 10.1101/gad.12.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottlieb E, Steitz J A. Function of the mammalian La protein: evidence for its action in transcription termination by RNA polymerase III. EMBO J. 1989;8:851–861. doi: 10.1002/j.1460-2075.1989.tb03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grandi P, Doye V, Hurt E C. Purification of NSP1 reveals complex formation with ‘GLFG’ nucleoporins and a novel nuclear pore protein NIC96. EMBO J. 1993;12:3061–3071. doi: 10.1002/j.1460-2075.1993.tb05975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grandi P, Emig S, Weise C, Hucho F, Pohl T, Hurt E C. A novel nuclear pore protein Nup82p which specifically binds to a fraction of Nsp1p. J Cell Biol. 1995;130:1263–1273. doi: 10.1083/jcb.130.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenblatt J. RNA polymerase II holoenzyme and transcriptional regulation. Curr Opin Cell Biol. 1997;9:310–319. doi: 10.1016/s0955-0674(97)80002-6. [DOI] [PubMed] [Google Scholar]
- 21.Hampsey M. Molecular genetics of the RNA polymerase II general transcriptional machinery. Microbiol Mol Biol Rev. 1998;62:465–503. doi: 10.1128/mmbr.62.2.465-503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 23.Hurt E C. A novel nucleoskeletal-like protein located at the nuclear periphery is required for the life cycle of Saccharomyces cerevisiae. EMBO J. 1988;7:4323–4334. doi: 10.1002/j.1460-2075.1988.tb03331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen G J, Meredith G, Bushnell D A, Kornberg R D. Structure of wild-type yeast RNA polymerase II and location of Rpb4 and Rpb7. EMBO J. 1998;17:2353–2358. doi: 10.1093/emboj/17.8.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kagami M, Toh-e A, Matsui Y. SRO9, a multicopy suppressor of the bud growth defect in the Saccharomyces cerevisiae rho3-deficient cells, shows strong genetic interactions with tropomyosin genes, suggesting its role in organization of the actin cytoskeleton. Genetics. 1997;147:1003–1016. doi: 10.1093/genetics/147.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khazak V, Estojak J, Cho H, Majors J, Sonoda G, Testa J R, Golemis E A. Analysis of the interaction of the novel RNA polymerase II (pol II) subunit hsRPB4 with its partner hsRPB7 and with pol II. Mol Cell Biol. 1998;18:1935–1945. doi: 10.1128/mcb.18.4.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khazak V, Sadhale P P, Woychik N A, Brent R, Golemis E A. Human RNA polymerase II subunit hsRPB7 functions in yeast and influences stress survival and cell morphology. Mol Biol Cell. 1995;6:759–775. doi: 10.1091/mbc.6.7.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimura M, Ishiguro A, Ishihama A. RNA polymerase II subunits 2, 3, and 11 form a core subassembly with DNA binding activity. J Biol Chem. 1997;272:25851–25855. doi: 10.1074/jbc.272.41.25851. [DOI] [PubMed] [Google Scholar]
- 29.Kolodziej P A, Woychik N, Liao S M, Young R A. RNA polymerase II subunit composition, stoichiometry, and phosphorylation. Mol Cell Biol. 1990;10:1915–1920. doi: 10.1128/mcb.10.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolodziej P A, Young R A. Mutations in the three largest subunits of yeast RNA polymerase II that affect enzyme assembly. Mol Cell Biol. 1991;11:4669–4678. doi: 10.1128/mcb.11.9.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larkin R M, Guilfoyle T J. Two small subunits in Arabidopsis RNA polymerase II are related to yeast RPB4 and RPB7 and interact with one another. J Biol Chem. 1998;273:5631–5637. doi: 10.1074/jbc.273.10.5631. [DOI] [PubMed] [Google Scholar]
- 32.Liao S M, Taylor I C, Kingston R E, Young R A. RNA polymerase II carboxy-terminal domain contributes to the response to multiple acidic activators in vitro. Genes Dev. 1991;5:2431–2440. doi: 10.1101/gad.5.12b.2431. [DOI] [PubMed] [Google Scholar]
- 33.Maillet I, Buhler J M, Sentenac A, Labarre J. Rpb4p is necessary for RNA polymerase II activity at high temperature. J Biol Chem. 1999;274:22586–22590. doi: 10.1074/jbc.274.32.22586. [DOI] [PubMed] [Google Scholar]
- 34.Maraia R J. Transcription termination factor La is also an initiation factor for RNA polymerase III. Proc Natl Acad Sci USA. 1996;93:3383–3387. doi: 10.1073/pnas.93.8.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattaj I W, Englmeier L. Nucleocytoplasmic transport: the soluble phase. Annu Rev Biochem. 1998;67:265–306. doi: 10.1146/annurev.biochem.67.1.265. [DOI] [PubMed] [Google Scholar]
- 36.McKune K, Richards K L, Edwards A M, Young R A, Woychik N A. RPB7, one of two dissociable subunits of yeast RNA polymerase II, is essential for cell viability. Yeast. 1993;9:295–299. doi: 10.1002/yea.320090309. [DOI] [PubMed] [Google Scholar]
- 37.McLaren R S, Caruccio N, Ross J. Human La protein: a stabilizer of histone mRNA. Mol Cell Biol. 1997;17:3028–3036. doi: 10.1128/mcb.17.6.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meerovitch K, Svitkin Y V, Lee H S, Lejbkowicz F, Kenan D J, Chan E K, Agol V I, Keene J D, Sonenberg N. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J Virol. 1993;67:3798–3807. doi: 10.1128/jvi.67.7.3798-3807.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitobe J, Mitsuzawa H, Yasui K, Ishihama A. Isolation and characterization of temperature-sensitive mutations in the gene (rpb3) for subunit 3 of RNA polymerase II in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1999;262:73–84. doi: 10.1007/s004380051061. [DOI] [PubMed] [Google Scholar]
- 40.Morehouse H, Buratowski R M, Silver P A, Buratowski S. The importin/karyopherin Kap114 mediates the nuclear import of TATA-binding protein. Proc Natl Acad Sci USA. 1999;96:12542–12547. doi: 10.1073/pnas.96.22.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myer V E, Young R A. RNA polymerase II holoenzymes and subcomplexes. J Biol Chem. 1998;273:27757–27760. doi: 10.1074/jbc.273.43.27757. [DOI] [PubMed] [Google Scholar]
- 42.Nicolet C M, Craig E A. Inducing and assaying heat-shock response in Saccharomyces cerevisiae. Methods Enzymol. 1991;194:710–717. doi: 10.1016/0076-6879(91)94052-e. [DOI] [PubMed] [Google Scholar]
- 43.Pannone B K, Xue D, Wolin S L. A role for the yeast La protein in U6 snRNP assembly: evidence that the La protein is a molecular chaperone for RNA polymerase III transcripts. EMBO J. 1998;17:7442–7453. doi: 10.1093/emboj/17.24.7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pante N, Aebi U. Exploring nuclear pore complex structure and function in molecular detail. J Cell Sci Suppl. 1995;19:1–11. doi: 10.1242/jcs.1995.supplement_19.1. [DOI] [PubMed] [Google Scholar]
- 45.Pante N, Aebi U. Sequential binding of import ligands to distinct nucleopore regions during their nuclear import. Science. 1996;273:1729–1732. doi: 10.1126/science.273.5282.1729. [DOI] [PubMed] [Google Scholar]
- 46.Parker R, Herrick D, Peltz S W, Jacobson A. Measurement of mRNA decay rates in Saccharomyces cerevisiae. Methods Enzymol. 1991;194:415–423. doi: 10.1016/0076-6879(91)94032-8. [DOI] [PubMed] [Google Scholar]
- 47.Pemberton L F, Blobel G, Rosenblum J S. Transport routes through the nuclear pore complex. Curr Opin Cell Biol. 1998;10:392–399. doi: 10.1016/s0955-0674(98)80016-1. [DOI] [PubMed] [Google Scholar]
- 48.Pruijn G J, Slobbe R L, van Venrooij W J. Analysis of protein-RNA interactions within Ro ribonucleoprotein complexes. Nucleic Acids Res. 1991;19:5173–5180. doi: 10.1093/nar/19.19.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rinke J, Steitz J A. Association of the lupus antigen La with a subset of U6 snRNA molecules. Nucleic Acids Res. 1985;13:2617–2629. doi: 10.1093/nar/13.7.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rinke J, Steitz J A. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1982;29:149–159. doi: 10.1016/0092-8674(82)90099-x. [DOI] [PubMed] [Google Scholar]
- 51.Ruet A, Sentenac A, Fromageot P, Winsor B, Lacroute F. A mutation of the B220 subunit gene affects the structural and functional properties of yeast RNA polymerase B in vitro. J Biol Chem. 1980;255:6450–6455. [PubMed] [Google Scholar]
- 52.Sadhale P P, Woychik N A. C25, an essential RNA polymerase III subunit related to the RNA polymerase II subunit RPB7. Mol Cell Biol. 1994;14:6164–6170. doi: 10.1128/mcb.14.9.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakurai H, Mitsuzawa H, Kimura M, Ishihama A. The Rpb4 subunit of fission yeast Schizosaccharomyces pombe RNA polymerase II is essential for cell viability and similar in structure to the corresponding subunits of higher eukaryotes. Mol Cell Biol. 1999;19:7511–7518. doi: 10.1128/mcb.19.11.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schlaich N L, Haner M, Lustig A, Aebi U, Hurt E C. In vitro reconstitution of a heterotrimeric nucleoporin complex consisting of recombinant Nsp1p, Nup49p, and Nup57p. Mol Biol Cell. 1997;8:33–46. doi: 10.1091/mbc.8.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmitt M E, Brown T A, Trumpower B L. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 1990;18:3091–3092. doi: 10.1093/nar/18.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sheffer A, Varon M, Choder M. Rpb7 can interact with RNA polymerase II and support transcription during some stresses independently of Rpb4. Mol Cell Biol. 1999;19:2672–2680. doi: 10.1128/mcb.19.4.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sobel S G, Wolin S L. Two yeast La motif-containing proteins are RNA-binding proteins that associate with polyribosomes. Mol Biol Cell. 1999;10:3849–3862. doi: 10.1091/mbc.10.11.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stefano J E. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3′ termini of RNA polymerase III transcripts. Cell. 1984;36:145–154. doi: 10.1016/0092-8674(84)90083-7. [DOI] [PubMed] [Google Scholar]
- 59.Svitkin Y V, Pause A, Sonenberg N. La autoantigen alleviates translational repression by the 5′ leader sequence of the human immunodeficiency virus type 1 mRNA. J Virol. 1994;68:7001–7007. doi: 10.1128/jvi.68.11.7001-7007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan Q, Linask K L, Ebright R H, Woychik N A. Activation mutants in yeast RNA polymerase II subunit RPB3 provide evidence for a structurally conserved surface required for activation in eukaryotes and bacteria. Genes Dev. 2000;14:339–348. [PMC free article] [PubMed] [Google Scholar]
- 61.Tsukada M, Gallwitz D. Isolation and characterization of SYS genes from yeast, multicopy suppressors of the functional loss of the transport GTPase Ypt6p. J Cell Sci. 1996;109:2471–2481. doi: 10.1242/jcs.109.10.2471. [DOI] [PubMed] [Google Scholar]
- 62.Uetz P, Giot L, Cagney G, Mansfield T A, Judson R S, Knight J R, Lockshon D, Narayan V, Srinivasan M, Pochart P, Qureshi-Emili A, Li Y, Godwin B, Conover D, Kalbfleisch T, Vijayadamodar G, Yang M, Johnston M, Fields S, Rothberg J M. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature. 2000;403:623–627. doi: 10.1038/35001009. [DOI] [PubMed] [Google Scholar]
- 63.Wootner M, Wade P A, Bonner J, Jaehning J A. Transcriptional activation in an improved whole-cell extract from Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:4555–4560. doi: 10.1128/mcb.11.9.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woychik N A. Fractions to functions: RNA polymerase II thirty years later. Cold Spring Harbor Symp Quant Biol. 1998;LXIII:311–317. doi: 10.1101/sqb.1998.63.311. [DOI] [PubMed] [Google Scholar]
- 65.Woychik N A, Young R A. RNA polymerase II subunit RPB4 is essential for high- and low-temperature yeast cell growth. Mol Cell Biol. 1989;9:2854–2859. doi: 10.1128/mcb.9.7.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yano R, Oakes M, Yamaghishi M, Dodd J A, Nomura M. Cloning and characterization of SRP1, a suppressor of temperature-sensitive RNA polymerase I mutations, in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:5640–5651. doi: 10.1128/mcb.12.12.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoo C J, Wolin S L. La proteins from Drosophila melanogaster and Saccharomyces cerevisiae: a yeast homolog of the La autoantigen is dispensable for growth. Mol Cell Biol. 1994;14:5412–5424. doi: 10.1128/mcb.14.8.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoo C J, Wolin S L. The yeast La protein is required for the 3′ endonucleolytic cleavage that matures tRNA precursors. Cell. 1997;89:393–402. doi: 10.1016/s0092-8674(00)80220-2. [DOI] [PubMed] [Google Scholar]
- 69.Young R A. RNA polymerase II. Annu Rev Biochem. 1991;60:689–715. doi: 10.1146/annurev.bi.60.070191.003353. [DOI] [PubMed] [Google Scholar]