Abstract
Introduction
Endothelial damage and thrombosis caused by COVID-19 may imperil cardiovascular health. More than a year since the WHO declared COVID-19 pandemic, information on its effects beyond the acute phase is lacking. We investigate endothelial dysfunction, coagulation and inflammation, 3 months post-COVID-19.
Materials and methods
A cohort study was conducted including 203 patients with prior COVID-19. Macrovascular dysfunction was assessed by measuring the carotid artery diameter in response to hand immersion in ice-water. A historic cohort of 312 subjects served as controls. Propensity score matching corrected for baseline differences. Plasma concentrations of endothelin-1 were measured in patients post-COVID-19, during the acute phase, and in matched controls. Coagulation enzyme:inhibitor complexes and inflammatory cytokines were studied.
Results and conclusions
The prevalence of macrovascular dysfunction did not differ between the COVID-19 (18.6%) and the historic cohort (22.5%, RD −4%, 95%CI: −15–7, p = 0.49). Endothelin-1 levels were significantly higher in acute COVID-19 (1.67 ± 0.64 pg/mL) as compared to controls (1.24 ± 0.37, p < 0.001), and further elevated 3 months post-COVID-19 (2.74 ± 1.81, p < 0.001). Thrombin:antithrombin(AT) was high in 48.3%. Markers of contact activation were increased in 16–30%. FVIIa:AT (35%) and Von Willebrand Factor:antigen (80.8%) were elevated. Inflammatory cytokine levels were high in a majority: interleukin(IL)-18 (73.9%), IL-6 (47.7%), and IL-1ra (48.9%). At 3 months after acute COVID-19 there was no indication of macrovascular dysfunction; there was evidence, however, of sustained endothelial cell involvement, coagulation activity and inflammation. Our data highlight the importance of further studies on SARS-CoV-2 related vascular inflammation and thrombosis, as well as longer follow-up in recovered patients.
Abbreviations: α1AT, alpha 1 antitrypsin; C1inh, C1 esterase inhibitor; CAR, Carotid Artery Reactivity; CI, Confidence Interval; COVID-19, Coronavirus Disease 2019; ELISA, Enzyme-Linked Immunosorbent Assay; ET-1, Endothelin-1; F, Factor; IL, Interleukin; NLRP3, Nod-like receptor family pyrin domain containing 3; PCR, Polymerase Chain Reaction; PKa, Kallikrein; PSM, Propensity Score Matching; RD, Risk Difference; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; SD, Standard Deviation; SMD, Standardized Mean Differences; TAT, Thrombin:Antithrombin; VWF:Ag, Von Willebrand Factor:Antigen; WHO, World Health Organization
Keywords: SARS-CoV-2, COVID-19, Endothelial cells, Blood coagulation, Inflammation
1. Introduction
Coronavirus disease 2019 (COVID-19) results from infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), preferentially affecting the upper airways and pulmonary system [1]. Depending on the severity of infection, dissemination towards multiple other organs occurs and systemic COVID-19 is associated with a high incidence of thromboembolic complications and risk of multi-organ failure [2], [3], [4]. Endothelial vascular injury and thrombo-inflammation are emerging key factors in COVID-19 pathophysiology [5], [6], [7], [8]. Pronounced endothelial cell damage was found in COVID-19 autopsy studies [5], and markers of endothelial cell activation are significantly increased in severe COVID-19 [9] and associated with organ damage, including liver, heart and brain [10]. This endothelial cell activation and dysfunction may be aggravated by the interplay of thrombo-inflammatory mediators and cells, including neutrophils that trigger contact and complement activation, perturbing the barrier function and contributing to thrombosis and cardiovascular disease [11].
Severe COVID-19 occurs more often in subjects with established cardiovascular disease or in those with cardiovascular risk factors [4], [12]. Notably, all factors associated with worse disease outcome, such as cardiovascular disease, higher age, obesity, hypertension and diabetes, also predispose to endothelial dysfunction [13], [14], [15], [16], [17]. In different cohorts of cardiovascular patients without COVID-19, the presence of endothelial dysfunction is strongly related to the occurrence of cardiovascular events, including stroke, myocardial infarction and limb events [18], [19], [20].
Although it has been more than a year since the WHO declared COVID-19 a global pandemic, there is a paucity of information related to the effects of COVID-19 on the cardiovascular system beyond the acute phase [21]. Moreover, the SARS-Cov-2 pandemic represents a tremendous health problem including the frequent occurrence of often unexplained prolonged complaints and morbidity long after the acute infection (so-called “long COVID” [22]). We hypothesize that sustained inflammation, coagulation activation and endothelial cell activation may ultimately lead to macrovascular dysfunction with an increased risk for developing cardiovascular complications later on [11].
Therefore, we firstly investigated whether macrovascular dysfunction could be observed 3 months after recovery from acute COVID-19. Furthermore, we determined whether signs of endothelial cell activation [9], [23], [24], [25], coagulation system activation [26], and circulating inflammatory cytokines [9], [27], [28], as observed during the acute phase, are present 3 months after recovery from acute COVID-19 symptoms.
2. Materials and methods
2.1. Study design
COVAS was a cross-sectional observational cohort study, initiated at Radboudumc (Nijmegen, the Netherlands) and conducted at Bernhoven hospital (Uden, the Netherlands). The study was approved by the regional ethics committee Arnhem-Nijmegen (reference number NL74101.091.20), and local approval has been obtained of the local directory boards. This study was conducted in accordance with the latest revision of the Declaration of Helsinki. Data available on request from the authors.
2.2. Participants
Patients who had experienced SARS-CoV-2 infection, confirmed by polymerase chain reaction on nasopharyngeal swab, sputum or bronchoalveolar lavage, were recruited. Patients had to be aged 16 years or older and recovered from acute COVID-19 symptoms for at least 6 and no more than 20 weeks. Exclusion criteria were recent (<3 months) episode of angina pectoris, myocardial infarction, stroke or heart failure, and abnormalities of the upper extremities restricting cold pressor testing. Abnormalities of the upper extremities included severe bilateral Raynaud syndrome, scleroderma, complex regional pain syndrome, or presence of arteriovenous fistula or open wounds. Written informed consent was obtained from all participants.
2.3. Procedures
Data about cardiovascular risk factors, comorbidities, medication use, and severity of acute COVID-19 infection were retrieved from electronic patient files. Case report forms were used to obtain information about lifestyle and COVID-19 symptoms.
2.3.1. Measurement of macrovascular dysfunction: carotid artery reactivity test
Eligible patients visited the hospital once. During the visit, the carotid artery reactivity (CAR) test [29], [30], [31] was performed. The CAR test is a simple, non-invasive procedure to examine macrovascular function by measuring the carotid artery diameter in reaction to a cold pressor test (sympathetic stimulus) [29], [30], [31]. Participants rest in the supine position with the neck extended. The left carotid artery is visualized using L12–4 MHz linear array probe of Philips Lumify, ultrasound device. The carotid artery diameter is measured with custom-designed edge-detection and wall-tracking software during baseline (30 s) and during hand-immersion in icy water of 4 °C (sympathetic stimulus) for 3 min.
A historic control cohort was created from all studies initiated by Radboudumc that completed data collection before November 2019, i.e. before the pandemic and therefore not affected by COVID-19. In these studies, the CAR test had been used to determine macrovascular function and data on risk factors for endothelial dysfunction were collected. Prespecified risk factors for endothelial dysfunction were age [13], sex [32], body mass index (BMI) [14], smoking status [33], atherosclerotic cardiovascular disease [15], hypertension [16], hyperlipidaemia [34] and diabetes mellitus [17]. Subjects were either healthy volunteers, patients with cardiovascular risk factors or patients with symptomatic cardiovascular disease. Incomplete case files were excluded from final analyses. Propensity score matching was used to select individuals, based on baseline characteristics and cardiovascular risk profile, to match those with COVID-19.
2.3.2. Measurement of endothelin-1, coagulation and inflammatory cytokines
Whole blood of recovered COVID-19 patients was collected by venipuncture in Lithium-Heparin (Vacuette) tubes. Platelet poor plasma was prepared by centrifuging whole blood at 2500g for 10 min followed by a second centrifugation step at 2500g for 20 min, both at room temperature. Subsequently, platelet poor plasma was snap frozen and stored at −80 °C until use. Plasma concentrations of endothelin-1 (ET-1), interleukin (IL)-18, IL-6, and IL-1ra were quantified using commercial ELISA kits (R&D, Minneapolis, Minnesota. Catalogue numbers DET100, DL180, D6050 and DRA00B, respectively). Activated coagulation factors in complex with their natural inhibitors, including thrombin:antithrombin (TAT), factor(F)IXa:AT, FVIIa:AT, FXIa:AT, FXIa:alpha-1-antitrypsin (α1AT), FXIa:C1-esterase-inhibitor (C1inh) and kallikrein:C1inh (PKa:C1Inh), as well as von Willebrand factor antigen (VWF:Ag) levels, were quantified by in-house developed enzyme-linked immunosorbent assay (ELISA) methods as described previously [35]. The use of lithium-heparin plasma for the mentioned biomarkers was validated by comparing citrate with lithium-heparin plasma from 38 healthy volunteers. ET-1 was also measured in samples of patients who consented with blood plasma preservation during the acute phase of COVID-19 (subjects of the ongoing BioMarCo-19 study, (M de Groot, unpublished data, 2021, https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001325-31/NL) and in a matched control group of patients without a history of COVID-19 infection. Plasma concentrations below the lower detection limit were set on the lowest detectable value.
2.4. Endpoints
The primary endpoint was the prevalence of macrovascular dysfunction, defined as a constrictive response to the CAR test in the COVAS cohort compared to the historic control cohort [29]. The CAR response was either a dilatory or a constrictive reaction, dichotomized by an area under the curve of at least zero or below zero, respectively. The secondary endpoint was the plasma concentration of ET-1 in the COVAS cohort compared to the acute phase and matched controls.
2.5. Statistical analysis
This study was designed to detect a 2.5-fold excess risk of macrovascular dysfunction in the COVAS cohort compared to the matched historic control cohort. With a power of 0.8 and an alpha of 5%, 97 patients with COVID-19 infection were required. Assuming an acceptable match rate of 50%, the final sample size was set on 200 participants.
For the CAR test, propensity score matching (PSM) was applied to correct for systematic differences in baseline characteristics that could influence endothelial function (i.e. age, sex, BMI, smoking status, atherosclerotic cardiovascular disease, hypertension, hyperlipidemia and diabetes) between post-COVID-19 patients and historic controls. The propensity score is defined as the chance to be in the COVAS cohort, based on baseline characteristics. Propensity score was estimated using logistic regression analysis with the group (COVAS or historic control) as dependent variable in relation to baseline characteristics. The balance in covariates was evaluated using standardized mean differences (SMD), where a SMD of 0.1 indicates a negligible correlation [36]. After PSM, univariable logistic regression analysis was performed to estimate the difference in CAR response and value between the historic control cohort and the COVAS cohort.
ET-1 levels of the COVAS cohort were compared to matched controls using the Mann-Whitney U test. Sub-analyses were performed on patients with preserved blood plasma during the acute phase of infection to compare ET-1 concentrations of the acute phase with ET-1 concentrations post-COVID-19.
Blood plasma markers of coagulation activation and inflammatory cytokines were presented as mean ± standard deviation (SD) and the proportion of patients with concentrations above normal range. Normal ranges of in-house developed ELISA methods were defined as above normal mean + 1 SD (based on previous validation studies [35]). The Mann-Whitney U test was used to compare post-COVID-19 values of patients that stayed home during the acute phase of infection to patients that needed hospitalization.
Correlations between endothelial dysfunction, markers of coagulation and inflammatory cytokines were explored using Spearman's rank correlation coefficient.
Analyses were performed using R 4.0.3 and IBM SPSS Statistics 25. p values below 0.05 were considered significant.
3. Results
3.1. Post-COVID-19 patients
In total, 787 patients were diagnosed with COVID-19 between February 1st and June 1st 2020 within the Bernhoven hospital, of which 571 patients survived (72.5%). Ultimately, 203 eligible patients gave written informed consent.
3.2. Historic controls
For the control cohort, 330 participants of 5 different studies underwent the CAR test before November 2019 and were previously assessed for the prespecified risk factors for endothelial dysfunction. Complete case files were obtained of 312 participants (Fig. 1 ).
Fig. 1.
Flow-chart showing the composition of the post-COVID-19 cohort and the historic control cohort
Single column fitting image.
3.3. Patient characteristics and infection course
The mean age at COVID-19 diagnosis was 62.7 years and 63.5% of the patients were male (Table 1 ). The most common comorbidities were hypertension (43.3%), hyperlipidemia (26.6%) and coronary artery disease (17.7%). Thrombocytopenia (<150 ∗ 10^9/l) was present in 16.6% of the patients. Four patients had asymptomatic disease and were diagnosed with COVID-19 during a visit to the emergency ward for other medical reasons. For patients with symptomatic COVID-19, the median duration of COVID-19 related symptoms was 18 days (range 1–80 days). Fatigue, dyspnea and fever above 39 degrees were the most frequently experienced symptoms, 94.1%, 77.3% and 70.9%, respectively. Hospitalization was needed for 130 (64.0%) patients and 28 (21.5%) of these patients were transferred to the intensive care unit.
Table 1.
Baseline characteristics and COVID-19 infection course of patients included in the COVAS cohort and of all patients that were diagnosed with COVID-19 between February 1st and June 1st 2020 within the Bernhoven hospital.
| COVAS selection (n = 203) |
COVID-19 cohort (n = 787) |
||||
|---|---|---|---|---|---|
| All (n = 787) | Non-survivors (n = 217) |
Survivors (n = 570) |
|||
| Patient characteristics | Age, mean (SD) | 62.7 (12.4) | 70.0 (14.2) | 78.4 (8.8) | 66.8 (14.5) |
| Male sex, no (%) | 129 (63.5) | 470 (59.7) | 148 (68.2) | 322 (56.5) | |
| BMI, mean (SD) | 28.3 (4.3) | 28.52 (5.2) | 28.35 (5.0) | 28.58 (5.2) | |
| History of smoking, no (%) | 137 (67.5) | 371 (56.3) | 86 (53.8) | 285 (57.1) | |
| Missing 128 | Missing 57 | Missing 71 | |||
| Comorbidities | Missing | 12 (1.5) | 7 (3.2) | 5 (0.9) | |
| Hypertension, no (%) | 88 (43.3) | 377 (48.6) | 121 (57.6) | 256 (45.3) | |
| Hyperlipidaemia, no (%) | 54 (26.6) | 149 (19.2) | 44 (21.0) | 105 (18.6) | |
| Coronary artery disease, No (%) | 36 (17.7) | 147 (19.0) | 55 (26.2) | 92 (16.3) | |
| Stroke/TIA, no (%) | 23 (11.3) | 111 (14.3) | 43 (20.5) | 68 (12.0) | |
| Peripheral arterial disease, no (%) | 6 (3.0) | 70 (9.0) | 33 (15.7) | 37 (6.5) | |
| Diabetes mellitus, no (%) | 32 (15.8) | 183 (23.6) | 58 (27.6) | 125 (22.1) | |
| Disease severity | Days of illness, median [range] | 18 [1–80] | |||
| Hospital care (n = 121) | 130 (64.0) | 516 (65.6) | 176 (81.1) | 340 (59.6) | |
| Days, median [range] | 7 [1–61] | 11 (0−103) | 8 (0–60) | 12 (0–103) | |
| Intensive care (n = 27) | 28 (13.8) | 117 (14.9) | 41 (18.9) | 76 (13.4) | |
| Days, median [range] | 16 [1–42] | 19 (0–82) | 15 (0–56) | 21 (1–82) | |
| Days recovered, median [range] | 114 [50–157] | ||||
SD = standard deviation, TIA = transient ischemic attack.
3.4. Recovery from COVID-19 infection does not result in macrovascular dysfunction
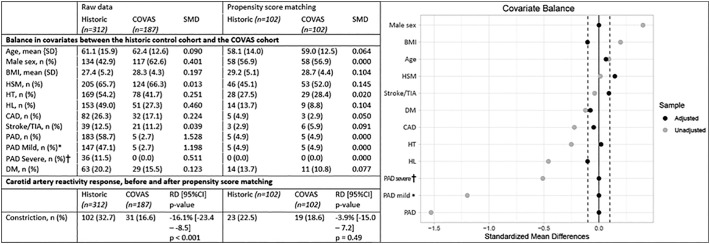
The prevalence of the prespecified risk factors for endothelial dysfunction in the historic control cohort and the COVAS cohort are shown in Fig. 2 . Besides age, history of smoking and history of stroke, all risk factors substantially differed between the historic control cohort and the COVAS cohort, with the most noticeable differences seen in the rate of male participants (42.9% vs 62.6%), the prevalence of hyperlipidemia (49.0% vs 27.3%) and the prevalence of peripheral arterial disease (58.7% vs 2.7%). Macrovascular dysfunction, as measured by carotid artery reactivity testing, was more prevalent in the historic control cohort compared to the COVAS cohort, 32.7% vs 16.6% (RD −16.1%, 95%CI: −23.4 to −8.5, p < 0.001). PSM was used to account for differences between the historic control cohort and the COVAS cohort and resulted in a mean SMD below 0.1 for most variables and below 0.15 for all variables. After PSM, the prevalence of macrovascular dysfunction was not different between the cohorts, 22.5% vs 18.6% (RD −4%, 95%CI: −15 to 7, p = 0.49), respectively (Fig. 2).
Fig. 2.
Carotid artery reactivity response, based on propensity score matching of covariates in the historic control cohort and the COVAS cohort.
SMD = standard mean difference, SD = standard deviation, HSM = history of smoking, HT = hypertension, HL = hyperlipidaemia, CAD = coronary artery disease, TIA = transient ischaemic attack, DM = diabetes mellitus, RD = risk difference, 95%CI = 95% confidence interval.
* - Rutherford stage I–III.
† - Rutherford stage IV–VI.
2 - column fitting image.
3.5. Elevated levels of endothelin-1 during the acute phase of COVID-19 and even higher levels in recovered patients
Concentrations of ET-1 were significantly higher in the COVAS cohort as compared to matched controls (2.52 ± 1.50 vs 1.24 ± 0.37 pg/mL, p < 0.001. A selection of 36 patients had consented with blood plasma preservation during the acute phase of COVID-19. Levels of ET-1 of those 36 patients were significantly higher during acute phase COVID-19 (1.67 ± 0.64 pg/mL) as compared to controls (1.24 ± 0.37 pg/mL, p < 0.001), and were further elevated 3 months post-COVID-19 (2.74 ± 1.81 pg/mL, p < 0.001, Fig. 3 ).
Fig. 3.

Elevated levels of endothelin-1 during the acute phase of COVID-19 and further elevated in recovered patients (n = 36) when compared to controls (n = 56); Mann-Whitney U test.
Single column fitting image.
3.6. Markers of coagulation and inflammatory cytokines are elevated in recovered COVID-19 patients
In vivo coagulation activity was measured in the post-COVID-19 cohort and increased coagulation activity was observed in patients 6–20 weeks after recovery of acute COVID-19 (Table 2 ). TAT and FIXa:AT complexes, reflecting a prothrombotic state, were elevated in 48.3% and 29.6% of the patients, respectively. FVIIa:AT, a marker of the extrinsic pathway, was increased in a third of the patients (35%), while markers of contact activation were elevated in a minority of the patients (PKa:C1inh (16.3%), FXIa:AT (16.3%), FXIa:α1AT (20.7%), and FXIa:C1inh (17.7%)). VWF:Ag was predominantly elevated in 80.8% of the post-COVID-19 patients. Inflammatory cytokine levels were determined. IL-18 levels were high in a majority of patients (73.9%), but IL-6 and IL-1ra were also frequently elevated (47.7% vs 48.9%, respectively).
Table 2.
Sustained inflammation, coagulation activation and elevated endothelin-1 levels.
| Normal range | All (n = 203) |
Home (n = 73) |
Hospital (n = 130) |
Home vs hospital |
||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | High, % | Mean ± SD | High, % | Mean ± SD | High, % | MWU | ||
| Markers of endothelial dysfunction | ||||||||
| CAR (n = 187) | ≥0% | 3.5 ± 4.8 | 16.6 | 3.8 ± 5.2 | 19.4 | 3.4 ± 5.3 | 15.0 | p = 0.334 |
| ET-1 (n = 203) | 0.87–1.61 pg/mL⁎ | 2.52 ± 1.50 | 64.5 | 2.25 ± 1.17 | 61.6 | 2.67 ± 1.64 | 66.2 | p = 0.171 |
| Coagulation factor:inhibitor complexes (n = 203) | ||||||||
| TAT | ≤ 4.0 μg/L | 4.8 ± 4.0 | 48.3 | 6.0 ± 6.2 | 63.0 | 4.2 ± 1.5 | 40.0 | p = 0.002 |
| FXIa:AT | 7.0–12.5 pg/mL⁎ | 25.0 ± 85.5 | 16.3 | 20.4 ± 57.3 | 16.4 | 27.6 ± 98.0 | 16.2 | p = 0.983 |
| FXIa:α1AT | 78.6–120.1 pg/mL⁎ | 206.6 ± 698.0 | 20.7 | 210.8 ± 855.6 | 19.2 | 204.3 ± 595.3 | 21.5 | p = 0.768 |
| FXIa:C1inh | 176.7–396.7 pg/mL⁎ | 633.0 ± 2102.5 | 17.7 | 516.1 ± 1664.1 | 20.5 | 698.7 ± 2316.1 | 16.2 | p = 0.773 |
| FIXa:AT | 187.3–265.9 pg/mL⁎ | 259.1 ± 87.9 | 29.6 | 270.8 ± 126.3 | 34.2 | 252.5 ± 55.4 | 26.9 | p = 0.745 |
| FVIIa:AT | 237.7–374.6 pg/mL⁎ | 463.7 ± 743.1 | 35.0 | 519.2 ± 1157.6 | 37.0 | 432.6 ± 337.8 | 33.8 | p = 0.999 |
| Pka:C1inh | 1.2–2.2 ng/mL⁎ | 17.7 ± 93.1 | 16.3 | 19.7 ± 98.7 | 17.8 | 16.6 ± 90.2 | 15.4 | p = 0.994 |
| VWF:Ag | ≤160% | 267 ± 133 | 80.8 | 289 ± 151 | 78.1 | 254 ± 120 | 82.3 | p = 0.097 |
| Inflammatory cytokines (n = 176) | ||||||||
| IL-18 | 37–215 pg/mL | 306.5 ± 128.2 | 73.9 | 281.7 ± 124.4 | 66.1 | 319.9 ± 128.7 | 78.1 | p = 0.026 |
| IL-6 | ≤ 1.8 pg/mL | 3.1 ± 7.7 | 47.7 | 3.7 ± 12.6 | 41.9 | 2.8 ± 2.7 | 50.9 | p = 0.033 |
| IL-1ra | 100–400 pg/mL | 491.3 ± 364.3 | 48.9 | 489.8 ± 475.5 | 48.8 | 492.1 ± 288.9 | 49.1 | p = 0.194 |
SD = standard deviation, MWU = Mann-Whitney U test.
Normal mean ± SD.
3.7. Poor correlations between coagulation and inflammation markers in patients recovered from COVID-19 infection
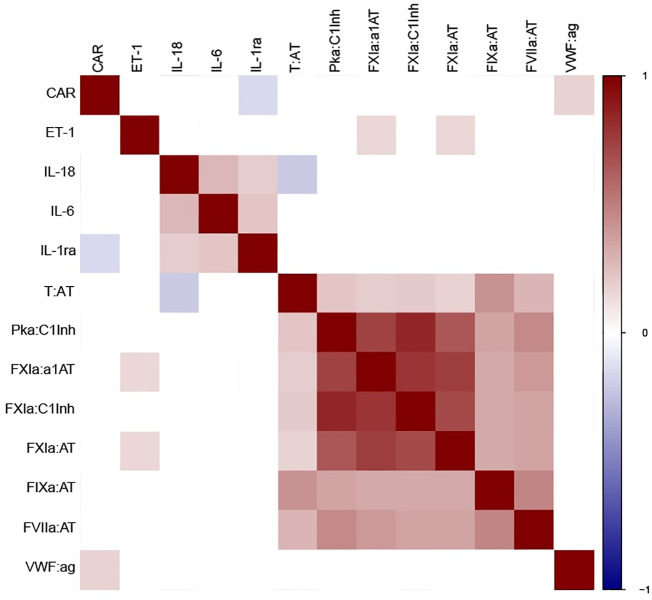
A heatmap of correlations between markers of endothelial dysfunction, markers of coagulation and inflammatory cytokines is presented in Fig. 4 . Macrovascular dysfunction as represented by a negative CAR, was correlated with lower levels of VWF:Ag (correlation coefficient (r) = 0.172, 95%CI 0.034–0.303, p = 0.019), and higher levels of IL-1ra (r = −0.150, 95%CI −0.283 to −0.012, p = 0.047). An association exists between ET-1 and contact activation markers FXIa:AT (r = 0.155, 95%CI 0.017–0.287, p = 0.027) and FXIa:α1AT (r = 0.163, 95%CI 0.025–0.295, p = 0.020).
Fig. 4.
Correlation heatmap showing rho correlations between factors involved with endothelial dysfunction, inflammation and coagulation. Cell colors indicate Spearman's rank correlations from blue (negative) to red (positive), where only p values lower than 0.05 are colored. Elevated levels of endothelin-1 during the acute phase of COVID-19 and even higher levels in recovered patients.
1.5 - column fitting image. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Strong correlations exist between the coagulation enzyme:inhibitor complexes themselves, especially the complexes related to the contact pathway (FXIa:AT, FXIa:α1AT, FXIa:C1inh, PKa:C1inh). Markers of a prothrombotic state (TAT and FIXa:AT), as well as FVIIa:AT, show moderate correlations between themselves and with the complexes related to the contact pathway. Moderate correlations were established between the inflammatory cytokines. There was a negative correlation between T:AT and Il-18 (correlation coefficient (r) = −0.204, 95%CI −0.334 to −0.067, p = 0.006), but not between any of the other coagulation markers and inflammatory cytokines.
4. Discussion
This study is, to our best knowledge, the first to investigate macrovascular dysfunction, coagulation activation and vascular inflammation in patients that have recovered from previous COVID-19 infection. Our data demonstrate that, 3 months after COVID-19 infection, patients manifest with sustained inflammation, coagulation activation and elevated endothelin-1 levels. These changes, however, do not coincide with macrovascular dysfunction. While previous studies on the effects of COVID-19 on the cardiovascular system mainly focused on the immediate (i.e. days following infection) cardiovascular complications [3], [37], [38], our data are amongst the first to reveal potentially detrimental lasting effects beyond the acute phase. In line with our findings, Fien et al. reported sustained prothrombotic changes based on enhanced thrombin-generating capacity and decreased plasma fibrinolytic potential in 52 patients with a resolved COVID-19 infection, 4 months after hospital discharge [39]. However few groups have endeavored to study sustained elevations in inflammation following recovery from acute COVID infection.
Acute COVID-19 is associated with a remarkably high incidence of thrombotic complications, which can be explained by the unique and complex interplay between SARS-CoV-2, pneumocytes, endothelial cells, the local and systemic inflammatory response, and the coagulation system. Although clinical recovery is paralleled by normalization of C-reactive protein (CRP) and D-dimer levels, we demonstrate that 3 months after acute COVID-19, low grade activation of coagulation, inflammation and signs of endothelial dysfunction are still present. COVID-19 infection is strongly associated with thrombus formation, both in venous and arterial vasculature [3], [37], [38], and coagulation factors in COVID-19-associated coagulopathy show a strong correlation with disease severity [40]. Different mechanisms have been proposed, including neutrophil and complement activation, vascular damage, and tissue factor expression. Recently, our group demonstrated that neutrophils and contact activation of coagulation are potential drivers of COVID-19 [23]. Both the intrinsic and extrinsic pathways of coagulation, do not only amplify fibrin generation, but also link to inflammation. Protease Activated Receptor (PAR) 1 is a high-affinity thrombin receptor which is highly expressed on platelets as well as on endothelial cells [41]. Thrombin-induced endothelial PAR1 activation, leads to regulation of vascular tone, permeability, and signals for endothelial adhesion molecules (vascular cell adhesion molecule-1, intercellular adhesion molecule-1, and E-selectin) [42], [43]. FXa is an important agonist for PAR2 receptors, which is present on monocytes, macrophages and Kupffer cells [44]. Through PAR2 activation, FXa contributes to the production of inflammatory cytokines [45]. Therefore, activation of the coagulation system could lead to inflammation mediated through PAR-receptor activation on endothelial and immune cells.
The COVAS cohort showed indicators of a prothrombotic state, as demonstrated by elevated TAT, in half of the patients, and signs of continued inflammation in the majority of the patients, approximately 3 months after resolution of acute COVID-19 symptoms. Acute COVID-19 infection is associated with an exaggerated inflammatory response, where cases of deadly cytokine storm are common in severe infections, often leading to multiorgan failure and death. In the acute state, levels of IL-1 family of cytokines and IL-6 have been reported to be elevated [9], [24], [25]. Interestingly, we found that IL-18 levels remained elevated in the vast majority of recovered patients, with IL-6 and IL-1ra also remaining elevated in half of the individuals. Heightened IL-18 and IL-6 levels are seen in states of vascular inflammation and have been associated with worse outcome in cardiovascular disease [46], [47]. Our data, therefore, suggest that there may be a state of chronic low-grade inflammation in the arteries of acute COVID-19 survivors. The activation and secretion of the IL-1 family of cytokines is mediated through the formation and activity of the Nod-like receptor family pyrin domain containing 3 (NLRP3) inflammasome, which is similarly enhanced in states of chronic inflammation [45], [48]. Elevated TAT is also associated with worse outcome for coronary artery disease [48], suggesting a link with vascular inflammation as well.
Widespread endothelial involvement in acute COVID-19 and in COVID-19-associated coagulopathy has been extensively suggested [11], [49]. Varga et al [27] and Rovas et al [28] provided evidence that COVID-19 could directly infect the endothelial cell and cause severe alterations of the microcirculation, accumulation of inflammatory cells and diffuse endothelial inflammation in patients suffering from COVID-19. Decreased endothelium-dependent vasodilator responses and increased serum cytokines and chemokines involved in the regulation of vascular function, indicating endothelial dysfunction, were found in hospitalized patients with COVID-19 [50]. We add the novel insight that, also 3 months following COVID-19 infection, markers of endothelial activation remained elevated in the greater share of recovered patients. This persisting endothelial activation could reflect disruption of vascular integrity which may lead to the sustained exposure of the thrombogenic basement membrane and the activation of the intrinsic coagulation pathway. This contact pathway could contribute to PAR2 activation [44], and lead to the secretion of IL-1 family cytokines by monocytes [45], and eventually to vascular inflammation with macrovascular dysfunction, subsequently contributing to accelerating cardiovascular disease progression [46], [47].
The proposed causes of the global health care problem termed ‘long COVID’, are thought to derive from virus-specific pathophysiological changes, inflammatory damage, and sequelae of critical illnesses (i.e. microvascular damage, immobility and metabolic alterations) [22]. Our data suggest not only an indication of chronic inflammation, but also potential microvascular damage, reflected by persistent endothelial activation, in the majority of acute COVID-19 survivors (Table 2). Remarkably, endothelial activation was present to a similar degree in patients that experienced critical illness and patients that remained at home during the acute phase of infection. An important question remains whether the observed sustained inflammation and endothelial activation relate to long COVID complaints and morbidity.
Macrovascular dysfunction, represented by carotid artery vasoconstriction upon sympathetic stimulation, was successfully determined in 187 patients of the COVAS cohort and in 330 historic controls. To correct for systematic differences in known risk factors for macrovascular dysfunction, propensity score matching was used. Although a perfect balance could not be achieved for three of the variables (BMI, history of smoking and hyperlipidemia), most SMDs could be reduced to below 0.1 and all SMDs were below 0.15, indicating minimal difference in baseline characteristics after correction. After PSM, the COVAS study found no signs of COVID-19-induced macrovascular dysfunction 6–20 weeks after recovery of acute COVID-19. Our study, therefore, suggests that macrovascular dysfunction is not yet present 3 months following COVID-19. As changes of the larger arteries may not occur within 3 months, a longer follow-up may be required to detect COVID-19-induced macrovascular dysfunction. It has been demonstrated previously that an elevated risk of cardiovascular disease persists during 10 years following hospitalization for pneumonia [51].
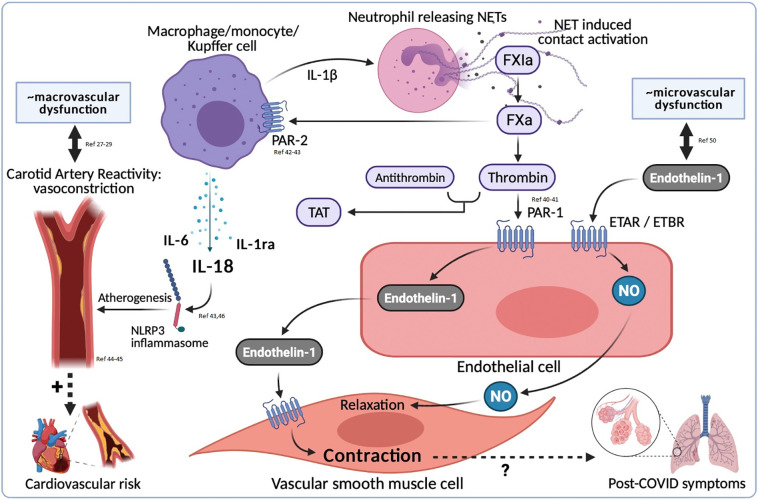
Despite explicit elevations in both coagulation enzyme:inhibitor complexes and inflammatory cytokines, no correlation was established between the two, suggesting a dissociation between thrombotic and inflammatory states in COVID-19, or at some point during recovery. An association exists between different contact activation markers and ET-1, supporting the hypothesis of endothelial cell activation, likely partially mediated through PAR1 stimulation. ET-1, as an indicator of microvascular dysfunction [52], was not related to macrovascular dysfunction. Since matched controls clearly confirmed a connection between high levels of ET-1 and past COVID-19, while macrovascular dysfunction was not present 3 months post-COVID-19, this is as expected. A moderate positive correlation exists between macrovascular dysfunction and IL-1ra, which could be explained by the role of IL-1 family cytokines in arterial/vascular inflammation (Fig. 5 ).
Fig. 5.
Reflection on elevated endothelin-1 levels, coagulation activation and sustained inflammation, in the medium to long term post-COVID-19.
NET = Neutrophil Extracellular Traps, ETAR = Endothelin Type A Receptor, ETBR = Endothelin Type B Receptor, NO = nitric oxide.
1.5 - column fitting image.
This study investigated endothelial dysfunction, coagulation activation and vascular inflammation in patients recovered from COVID-19 infection. The availability of a large historic control cohort with known risk factors for macrovascular dysfunction enabled an appropriate comparison between patients recovered from acute COVID-19 and patients that had unquestionably not experienced COVID-19. CAR measurements may vary over time and may depend on the observer, which could unfortunately not be controlled for in the present study. Semi-automated edge-detection and wall-tracking software was used to reduce observer variation.
5. Conclusions
Based on this unique design, we found evidence of sustained endothelial cell activation, coagulation activation and inflammation. However, these changes did not coincide with macrovascular dysfunction, 3 months after acute COVID-19. Our data highlight the importance of further studies on SARS-CoV-2 related coagulation activation and vascular inflammation, with longer follow-up periods in recovered patients, with the newly raised hypothesis of IL-1 family cytokines, IL-18 in particular, induced arterial inflammation. Sustained endothelial activation as reflected by high ET-1 levels could be maintained by contact pathway activation, possibly induced through PAR1 stimulation. Future studies should pursue if high ET-1 levels as a marker of microvascular dysfunction play a role in long-term COVID complications.
Sources of funding
This work was supported by a grant from The Netherlands Organization for Health Research and Development [grant number: 10430042010044].
CRediT authorship contribution statement
LW, JL, DHJT, GH, ASP and MCW contributed to the concept of the study. LW, LG, DHJT, GH, ASP and MCW contributed to the trial design. LW, MN, HC, HMHS, LG, NAFJ, DHJT and ASP contributed to the data collection. LW, MN, LG, DHJT, GH and MCW contributed to the data analysis. All authors contributed to the interpretation of the data. LHW and MCW wrote the first draft of the manuscript. All authors read, revised and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank its collaborators Romy Kuiper, Nina Kooijman and Ilse Rijken, who provided assistance and support for the practical execution of this study; Jelle van de Waterbeemd, Kim Albers, Helga Dijkstra and Aline de Nooijer, for providing assistance in the analyses; and Bregje Raap-van Sleuwen for research coordination and assistance.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Wei Y., Wu W., Zie Z., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang J., Jiang R., Gao Z., Jin Q., Wang J., Cao B., Xia J. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A., Jain S.S., Burkhoff D., Kumaraiah D., Rabbani L., Schwarts A., Uriel N. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020 Mar 19;141(20):1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 3.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020 Jun;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardon S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., the Nortwell COVID-19 Research Consortium, Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., Cookingham J., Coppa K., Diefenbach M.A., Dominello A.J., Huer-Hefele J., Falzon L., Gitlin J., Hajizadeh N., Harvin T.G., Hirschwerk D.A., Lim E.J., Kozel Z.M., Marrast L.M., Mogavero J.N., Osorio G.A., Qiu M., Zanos T.P. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020 May 26;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020 Jul 9;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leentjens J., van Haaps T.F., Wessels P.F., Schutgens R.E.G., Middeldorp S. COVID-19-associated coagulopathy and antithrombotic agents-lessons after 1 year. Lancet Haematol. 2021 Jul;8(7):e524–e533. doi: 10.1016/S2352-3026(21)00105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed S., Zimba O., Gasparyan A.Y. Thrombosis in coronavirus disease 2019 (COVID-19) through the prism of Virchow's triad. Clin. Rheumatol. 2020 Sep;39(9):2529–2543. doi: 10.1007/s10067-020-05275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kipshidze N., Dangas G., White C.J., Kipshidze N., Siddiqui F., Lattimer C.R., Carter C.A., Fareed J. Viral coagulopathy in patients with COVID-19: treatment and care. Clin. Appl. Thromb. Hemost. 2020;26 doi: 10.1177/1076029620936776. 1076029620936776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dupont A., Rauch A., Staessens S., Moussa M., Rosa M., Corseaux D., Jeanpierre E., Goutay J., Caplan M., Varlet P., Lefevre G., Lassalle F., Bauters A., Faure K., Lampert M., Duhamel A., Labreuche J., Garrigue D., De Meyer S.F., Staels B., Vincent F., Rousse N., Kipnis E., Lenting P., Poissy J., Susen S. Lille Covid research network (LICORNE). Vascular endothelial damage in the pathogenesis of organ injury in severe COVID-19. Arterioscler. Thromb. Vasc. Biol. 2021 May 5;41(5):1760–1773. doi: 10.1161/ATVBAHA.120.315595. [DOI] [PubMed] [Google Scholar]
- 10.Mueller C., Giannitsis E., Jaffe A.S., Huber K., Mair J., Cullen L., Hammarsten O., Mills N.L., Möckel M., Krychtiuk K., Thygesen K., Lindahl B., ESC Study Group on Biomarkers in Cardiology of the Acute Cardiovascular Care Association Cardiovascular biomarkers in patients with COVID-19. Eur. Heart J. Acute Cardiovasc. Care. 2021 May 11;10(3):310–319. doi: 10.1093/ehjacc/zuab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonaventura A., Vecchié A., Dagna L., Martinod K., Dixon D.L., Van Tassel B.W., Dentali F., Montecucco F., Massberg S., Levi M., Abbate A. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021 May;21(5):319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 Apr 30;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Celermajer D.S., Sorensen K.E., Spiegelhalter D.J., Georgakopoulos D., Robinson J., Deanfield J.E. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J. Am. Coll. Cardiol. 1994 Aug;24(2):471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 14.Van der Heijden D.J., van Leeuwen M.A.H., Janssens G.N., Lenzen M.J., van de Ven P.M., Eringa E.C., van Royen N. Body mass index is associated with microvascular endothelial dysfunction in patients with treated metabolic risk factors and suspected coronary artery disease. J. Am. Heart Assoc. 2017 Sep 14;6(9) doi: 10.1161/JAHA.117.006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lerman A., Zeiher A.M. Endothelial function: cardiac events. Circulation. 2005 Jan 25;111(3):363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 16.Panza J.A., Quyyumi A.A., Brush J.E., Jr., Epstein S.E. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N. Engl. J. Med. 1990 Jul 5;323(1):22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 17.Mäkimattila S., Virkamäki A., Groop P.H., Cockcroft J., Utriainen T., Fagerudd J., Yki-Järvinen H. Chronic hyperglycemia impairs endothelial function and insulin sensitivity via different mechanisms in insulin-dependent diabetes mellitus. Circulation. 1996 Sep 5;94(6):1276–1282. doi: 10.1161/01.cir.94.6.1276. [DOI] [PubMed] [Google Scholar]
- 18.Bonetti P.O., Lerman L.O., Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003 Feb 1;23(2):168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 19.Schächinger C., Britten M.B., Zeiher A.M. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000 Apr 25;101(16):1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 20.Perticone F., Ceravolo R., Pujia A., Ventura G., Jacopino S., Scozzafava A., Ferraro A., Chello M., Mastroroberto P., Verdecchia P., Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001 Jul 10;104(2):191–196. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- 21.Maleszewski J.J., Young P.M., Ackerman M.J., Halushka M.K. Urgent need for studies of the late effects of SARS-CoV-2 on the cardiovascular system. Circulation. 2021 Mar 30;143(13):1271–1273. doi: 10.1161/CIRCULATIONAHA.120.051362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G.F., Bernstein E.J., Mohan S., Beckley A.A., Seres D.S., Choueiri T.K., Uriel N., Ausiello J.C., Accili D., Freedberg D.E., Baldwin M., Schwartz A., Brodie D., Garcia C.K., Elkind M.S.V., Connors J.M., Bilezikian J.P., Landry D.W., Wan E.Y. Post-acute COVID-19 syndrome. Nat. Med. 2021 Apr;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guervilly C., Burtey S., Sabatier F., Cauchois R., Lano G., Abdili E., Daviet F., Arnaud L., Brunet P., Hraiech S., Jourde-Chiche N., Koubi M., Lacroix R., Pietri L., Berda Y., Robert T., Degioanni C., Velier M., Papazian L., Kaplanski G., Dignat-George F. Circulating endothelial cells as a marker of endothelial injury in severe COVID-19. J. Infect. Dis. 2020 Nov 9;222(11):1789–1793. doi: 10.1093/infdis/jiaa528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 May 2;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rovas A., Osiaevi I., Buscher K., Sackarnd J., Tepasse P.R., Fobker M., Kühn J., Braune S., Göbel U., Thölking G., Gröschel A., Pavenstädt H., Vink H., Kümpers P. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis. 2020 Oct;14:1–13. doi: 10.1007/s10456-020-09753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Busch M.H., Timmermans S.A.M.E.G., Nagy M., Visser M., Huckriede J., Aendekerk J.P., de Vries F., Potjewijd J., Jallah B., Ysermans R., Oude Lashof A.M.L., Breedveld P.H., van de Poll M.C.G., van de Horst I.C.C., van Bussel B.C.T., Theunissen R.O.M.F.I.H., Spronk H.M.H., Damoiseaux J.G.M.C., ten Cate H., Nicolaes G.A.F., Reutelingsperger C.P., van Paassen P. Neutrophils and contact activation of coagulation as potential drivers of COVID-19. Circulation. 2020 Nov;142(18):1787–1790. doi: 10.1161/CIRCULATIONAHA.120.050656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckley L.F., Wohlford G.F., Ting C., Alahmed A., van Tassell B.W., Abbate A., Devlin J.W., Libby P. Role for anti-cytokine therapies in severe coronavirus disease 2019. Crit. Care Explor. 2020 Aug 10;2(8):20178. doi: 10.1097/CCE.0000000000000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vecchié A., Bonaventura A., Toldo S., Dagna L., Dinarello C.A., Abbate A. IL-18 and infections: is there a role for targeted therapies? J. Cell. Physiol. 2021 Mar;236(3):1638–1657. doi: 10.1002/jcp.30008. [DOI] [PubMed] [Google Scholar]
- 29.Van Mil A.C.C.M., Pouwels S., Wilbrink J., Warlé M.C., Thijssen D.H.J. Carotid artery reactivity predicts events in peripheral arterial disease patients. Ann. Surg. 2019 Apr;269(4):767–773. doi: 10.1097/SLA.0000000000002558. [DOI] [PubMed] [Google Scholar]
- 30.van Mil A.C.C.M., Hartman Y., van Oorschot F., Heemels A., Bax N., Dawson E.A., Hopkins N., Hopman M.T.E., Green D.J., Oxborough D.L., Thijssen D.H.J. Correlation of carotid artery reactivity with cardiovascular risk factors and coronary artery vasodilator responses in asymptomatic, healthy volunteers. J. Hypertens. 2017 May;35(5):1026–1034. doi: 10.1097/HJH.0000000000001274. [DOI] [PubMed] [Google Scholar]
- 31.Van Mil A.C.C.M., Tymko M.M., Kerstens T.P., Stembridge M., Green D.J., Ainslie P.N., Thijssen D.H.J. Similarity between carotid and coronary artery responses to sympathetic stimulation and the role of α 1-receptors in humans. J. Appl. Physiol. 2018 Aug 1;125(2):409–418. doi: 10.1152/japplphysiol.00386.2017. (1985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanhewicz A.E., Wenner M.M., Stachenfeld N.S. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am. J. Physiol. Heart Circ. Physiol. 2018 Dec 1;315(6):H1569–H1588. doi: 10.1152/ajpheart.00396.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Münzel T., Hahad O., Kuntic M., Keaney J.F., Deanfield J.E., Daiber A. Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur. Heart J. 2020 Nov 1;41(41):4057–4070. doi: 10.1093/eurheartj/ehaa460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casino P.R., Kilcoyne C.M., Quyyumi A.A., Hoeg J.M., Panza J.A. The role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation. 1993 Dec;88(6):2541–2547. doi: 10.1161/01.cir.88.6.2541. [DOI] [PubMed] [Google Scholar]
- 35.Govers-Riemslag J.W.P., Smid M., Cooper J.A., Bauer K.A., Rosenberg R.D., Hack C.E., Hamulyak K., Spronk H.M.H., Miller G.J., ten Cate H. The plasma kallikrein-kinin system and risk of cardiovascular disease in men. J. Thromb. Haemost. 2007 Sep;5(9):1896–1903. doi: 10.1111/j.1538-7836.2007.02687.x. [DOI] [PubMed] [Google Scholar]
- 36.Austin P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009 Nov 10;28(25):3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020 Jul;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology. 2020 Sep;296(3):E186–E188. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Von Meijenfeldt F.A., Havervall S., Adelmeijer J., Lundström A., Magnusson M., Mackman N., Thalin C., Lisman T. Sustained prothrombotic changes in COVID-19 patients 4 months after hospital discharge. Blood Adv. 2021 Feb 9;5(3):756–759. doi: 10.1182/bloodadvances.2020003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iba T., Levy J.H., Levi M., Thachil J. Coagulopathy in COVID-19. J. Thromb. Haemost. 2020 Sep;18(9):2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Brien P.J., Molino M., Kahn M., Brass L.F. Protease activated receptors: theme and variations. Oncogene. 2001 Mar;20(13):1570–1581. doi: 10.1038/sj.onc.1204194. [DOI] [PubMed] [Google Scholar]
- 42.Hamilton J.R., Cocks T.M. Heterogeneous mechanisms of endothelium-dependent relaxation for thrombin and peptide activators of protease-activated receptor-1 in porcine isolated coronary artery. Br. J. Pharmacol. 2000 May;130(1):181–188. doi: 10.1038/sj.bjp.0703146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bae J.S., Kim Y.U., Park M.K., Rezaie A.R. Concentration dependent dual effect of thrombin in endothelial cells via Par-1 and Pi3 kinase. J. Cell. Physiol. 2009 Jun;219(3):744–751. doi: 10.1002/jcp.21718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borisoff J.L., Spronk H.M., ten Cate H. The hemostatic system as a modulator of atherosclerosis. N. Engl. J. Med. 2011;364:1746–1760. doi: 10.1056/NEJMra1011670. [DOI] [PubMed] [Google Scholar]
- 45.Zuo P., Zuo Z., Wang X., Chen L., Zheng Y., Ma G., Zhou Q. Factor xa induces pro-inflammatory cytokine expression in RAW 264.7 macrophages via protease-activated receptor-2 activation. Am. J. Transl. Res. 2015 Nov;7(11):2326–2334. [PMC free article] [PubMed] [Google Scholar]
- 46.Ridker P.M., MacFadyen J.G., Thuren T., Libby P. Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1β inhibition with canakinumab: further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis. Eur. Heart J. 2020 Jun 14;41(23):2153–2163. doi: 10.1093/eurheartj/ehz542. [DOI] [PubMed] [Google Scholar]
- 47.Jefferis J.J.M.H., Papacosta O., Owen C.G., Wannamethee S.G., Humphries S.E., Woodward M., Lennon L.T., Thomson A., Welsh P., Rumley A., Lowe G.D.O., Whincup P.H. Interleukin 18 and coronary heart disease: prospective study and systematic review. Atherosclerosis. 2011 Jul;217(1):227–233. doi: 10.1016/j.atherosclerosis.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ten Cate H., Hemker H.C. Thrombin generation and atherothrombosis: what does the evidence indicate? J. Am. Heart Assoc. 2016 Aug;8l5(8) doi: 10.1161/JAHA.116.003553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nägele M.P., Haubner B., Tanner F.C., Ruschitzka F., Flammer A.J. Endothelial dysfunction in COVID-19: current findings and therapeutic implications. Atherosclerosis. 2020 Dec;314:58–62. doi: 10.1016/j.atherosclerosis.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabioni L., De Lorenzo A., Lamas C., Mucillo F., Castro-Faria-Neto H.C., Estato V., Tibirica E. Systemic microvascular endothelial dysfunction and disease severity in COVID-19 patients: evaluation by laser doppler perfusion monitoring and cytokine/chemokine analysis. Microvasc. Res. 2021 Mar;134 doi: 10.1016/j.mvr.2020.104119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corrales-Medina V.F., Alvarez K.N., Weissfeld L.A., Angus D.C., Chirinos J.A., Chang C.C.H., Newman A., Loehr L., Folsom A.R., Elkind M.S., Lyles M.F., Kronmal R.A., Yende S. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015 Jan 20;313(3):264–274. doi: 10.1001/jama.2014.18229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lerman A., Edwards B.S., Hallett J.W., Heublein D.M., Sandberg S.M., Burnett J.C., Jr. Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N. Engl. J. Med. 1991 Oct 3;325(14):997–1001. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]