Abstract
Objective To investigate sex- and gender-based differences linked to SARS-COV-2 infection and to explore the role of hormonal therapy (HT) in females.
Study design Data from the self-administered, cross-sectional, web-based EPICOVID19 survey of 198,822 adults living in Italy who completed an online questionnaire during the first wave of the epidemic in Italy (April-May 2020) were analyzed.
Main outcomes measures Multivariate binary logistic and multinomial regression models were respectively used to estimate the odds ratios (ORs) with 95% confidence intervals (CIs) for positive nasopharyngeal swab (NPS) test results and severe SARS-CoV-2 infection.
Results The data from 6,873 participants (mean age 47.9 ± 14.1 years, 65.8% females) who had a known result from an NPS test were analyzed. According to the multivariate analysis, females had lower odds of a positive result from the NPS test (aOR 0.75, 95%CI 0.66–0.85) and of having a severe infection (aOR 0.46, 95%CI 0.37–0.57) than did their male counterparts. These differences were greater with decreasing age in both sexes. In addition, females aged ≥60 years receiving HT (N = 2,153, 47.6%) had a 46% lower probability of having a positive NPS test (aOR 0.54, 95%CI 0.36–0.80) than their same-aged peers who had never used HT; there were no differences in the younger age groups with respect to HT status.
Conclusion Female sex was associated with an age-dependent lower risk of having a severe SARS-CoV-2 infection than their male counterparts. Age seemed to modify the relationship between HT status and infection: while the two were not related among younger participants, it was negative in the older ones. Future prospective studies are needed to elucidate the potential protective role sex hormones may play.
Trial registration ClinicalTrials.gov NCT04471701.
Keywords: SARS-CoV-2, COVID-19, Sex, Gender, Hormone therapy, Nasopharyngeal swab testing, Infection severity, Web-based survey, Cross-sectional design
Abbreviations: coronavirus disease, COVID-19; severe acute respiratory syndrome coronavirus-2, SARS-CoV-2; angiotensin-converting enzyme 2, ACE 2; Italian national epidemiological survey on COVID-19, EPICOVID19; nasopharyngeal swab, NPS; EU general data protection regulation, EU-GDPR; hormonal therapy, HT; standard deviation, SD; odds ratio, OR; 95% confidence interval, 95%CI
Introduction
Since its onset, the coronavirus disease (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has been characterized by marked sex differences [1]. Although epidemiological evidence collected early on indicated that males have a higher infection rate than females [2], [3], [4], [5], sex-disaggregated data collected by the Global Health 50/50 research initiative showed that there was no substantial difference in the male:female ratio for SARS-CoV-2 [6]. Some studies have however shown that COVID-19 male patients in the most affected age group (60 years old and older) have a higher risk of requiring intensive care treatment, worse outcomes, and mortality with respect to their female counterparts [7,8]. This sex disparity is not entirely surprising since other studies have already demonstrated that males of all age groups are more susceptible than females to other respiratory tract infections (e.g. severe acute respiratory syndrome [SARS] and the Middle East respiratory syndrome [MERS]) [9,10].
Although the mechanisms underlying these differences are not fully understood, it is probable that they involve an interplay between social, behavioral, and biological factors. Gender-related factors, including socioeconomic status, lifestyles (e.g. smoking habit and alcohol drinking), personal hygiene patterns (e.g. handwashing), healthcare-seeking behavior and access to medical assistance, which in turn can affect the risk of developing diseases, may only partially explain sex differences linked to the SARS-CoV-2 infection [11,12].
From a biological standpoint, it is known that females present an enhanced immune reactivity that makes them both more vulnerable to developing autoimmune diseases as well as more predisposed to mounting an effective immunity to viral infection [13]. Genetics and sex hormones may influence both the expression of viral receptors and the differential regulation of immune responses [13]. Indeed, since they seem to be able to modulate the immune and inflammatory responses and the expression of the Angiotensin-converting enzyme (ACE)-2 gene, which binds the SARS-CoV-2 viral spike protein [14], female hormones may have a protective effect against COVID-19 disease [15]. Conversely, androgens may predispose males to a more severe COVID-19 progression [16]. To the best of our knowledge, only a few studies have investigated the sex- and gender differences linked to the SARS-CoV-2 infection [2,3,5] and the potential therapeutic role of sex hormones [17], [18], [19], [20].
Considering the health system burden due to medical care and disabling sequelae of COVID-19 [21], understanding the extent to which sex hormones underlie the sex and gender differences in the severity of the coronavirus disease could have important clinical and public health implications. Data collected by the large web-based Italian National Epidemiological Survey on COVID-19 (EPICOVID19) during the first early wave of the pandemic were thus analyzed with the intent to: (i) examine if, after adjusting for social, clinical, and behavioral factors, males have a higher probability of having a positive SARS-CoV-2 test result and of developing a severe infection compared to females; and (ii) evaluate the role of hormonal therapy (HT) usage in the female participants.
2. Material and methods
2.1. Study design, setting and population
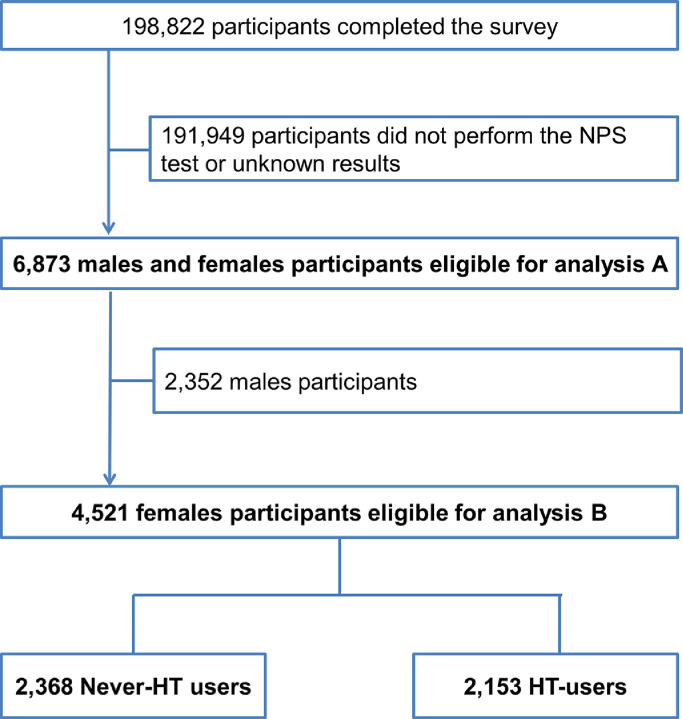
The EPICOVID19 survey consists of a self-selected convenience sample of 198,822 males and females aged 18–100 living in Italy during the first lockdown who filled in a web-based questionnaire between April and May 2020. The study's methodology has been described in detail elsewhere [22]. The inclusion criteria were: age ≥18 years; access to devices connected to internet; and providing online consent to participate in the study. Out of the 198,822 participants who filled out the web questionnaire, two subsets were identified for the purposes of the current study: 1) Sample A = the male and female participants who underwent a nasopharyngeal swab (NPS) test with a known result (N = 6,873); 2) Sample B = the female participants who underwent a NPS test with a known result (N = 4,521) (Fig. 1 ).
Fig. 1.
Flow-chart of the study samples A and B.
2.2. Data collection and exposures definition
As described elsewhere, all the participants of the EPICOVID19 survey were asked to complete an anonymous 38-item questionnaire [22]. The questionnaire was designed to gain information about the participant's life and in particular about his/her age, sex, educational level (illiterate or primary school, middle or high school, and university or postgraduate degree), and employment status (employed, student, unemployed, retired, or other). The questionnaire also asked: if the participant was a healthcare professional, about the participant's residence area (in the northern, central, southern or island regions of Italy), if the participant was living with other individuals at high risk of infection, had self-reported diseases, was taking any medicines, had a flu vaccination during the autumn of 2019 and/or an anti-pneumococcal vaccination over the last 12 months. Other items enquired about how much physical activity the participant were engaged in (never or less than 10 min/week, 10 min to two hours and half/week, and more than two hours and half/week), if the participant had a smoking habit (classified as a non-, former, or current smoker status), had any contacts with confirmed/suspected COVID-19 cases, his/her self-perceived health status (bad, adequate, or good), if she/he had exhibited healthcare-seeking behavior (had contacted an emergency number or his/her general practitioner), had any self-reported SARS-CoV-2 related symptoms, pneumonia, had been hospitalized for confirmed/suspected COVID-19, or had performed a NPS test result (positive vs negative) (Annex 1).
2.3. Exposure to hormonal therapy
Some items specifically addressing the female participants inquired if they were currently taking or had in the past (for more or less than 5 years) taken any form of HT, including hormonal contraceptives and/or hormone replacement therapy. The responses were divided into three groups: (1) never-HT users (reference group) vs - HT users; (2) past HT users vs current HT users; (3) participants who used HT less than 5 years vs participants who used HT more than 5 years.
2.4. Main outcomes
The study's primary outcome measures were: the results of NPS molecular testing and SARS-CoV-2 infection severity identified by combining information on the NPS test and responses regarding symptoms and hospitalization for COVID-19. Each participant was classified as having: (i) a negative NPS test; (ii) an asymptomatic or mild infection (a positive NPS test without symptoms or with at least one COVID-19-like symptom); or, (iii) severe infection (positive NPS test with pneumonia and/or hospitalization for COVID-19) .
2.5. Statistical analysis
Age as a continuous variable was summarized in the descriptive analyses as means and standard deviation (SD); the categorical variables were presented as counts and percentages. The participants’ characteristics according to sex (Sample A) and HT usage (Sample B) were compared using the t-test for age and the chi-squared test for all other categorical variables. Multivariate logistic and multinomial regression models were fitted to assess the relationships between sex (Sample A) and HT usage (Sample B) with positive versus negative NPS test results and the infection severity, respectively. Odds ratio (OR) and 95% confidence interval (95% CI) were estimated. The potential confounders of the two models were selected on the basis of theoretical knowledge and empirical criteria (P-value<0.05 in univariate analysis). Interaction terms were included in the model to investigate if age was a moderator of the effects of sex or HT usage on the NPS test and infection severity. The Wald tests were used to assess the age × sex (and HT interactions). When heterogeneity was present, stratum-specific estimates were evaluated. Healthcare professionals were stratified in a sensitivity analysis to exclude any bias due to the selective inclusion of this category in the survey. In oder to evaluate the effect of HT usage among post-menopausal females, a supplementary analysis focusing only on females aged 50 years or older has been performed. Sample size calculation is available as Supplementary material (S1). All statistical analyses were performed using Stata 15.0 version (StataCorp LP, College Station, Texas, USA) and a two-sided P-value <0.05 was considered statistically significant.
3. Results
3.1. Characteristics of the study samples
The distributions of the participants’ characteristics according to sex (Sample A) and by HT usage (Sample B) are shown in Table 1 . The mean age of the sample population that underwent NPS testing was 47.9 ± 14.1 years; 65.8% were females, 24.8% had a positive result and 7.1% developed a severe disease (sample A). With respect to the males, the females were significantly younger and were more likely to have self-reported headaches, heart palpitations, gastrointestinal disturbances, conjunctivitis, and sore throat/rhinorrhoea. The females had a lower rate of NPS positive test results, hospitalizations, and severe COVID-19 infection compared with males. Nearly half (47.6%) of the females in Sample B were HT users. The never-HT users were older, had more comorbidities, and were more likely to have a positive NPS test result with respect to their HT user counterparts.
Table 1.
Socio-demographic and behavioral characteristics of the study participants according to sex and HT usage in Sample A (N = 6873) and Sample B (females, N = 4521).
| Sample A (N = 6,873) | Sample B (N = 4,521) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Overall N = 6,873 | Males N = 2,352 (34.2%) | Females N = 4,521 (65.8%) | P-value | Never-HT users N = 2,368 (52.4%) | HT users N = 2,153 (47.6%) | P-value | |||||
| Socio-demographic a | ||||||||||||
| Age, years (mean, SD) | 47.9 | 14.1 | 49.5 | 14.1 | 47.1 | 14.1 | < 0.001 | 49.9 | 15.3 | 44.1 | 11.9 | < 0.001 |
| Age classes | < 0.001 | < 0.001 | ||||||||||
| < 50 | 3,693 | 53.7 | 1,127 | 47.9 | 2,566 | 56.8 | 1,190 | 50.2 | 1,376 | 63.9 | ||
| 50–59 | 1,856 | 27.0 | 596 | 25.3 | 1,260 | 27.9 | 689 | 29.1 | 571 | 26.5 | ||
| ≥60 | 1,324 | 19.3 | 629 | 26.7 | 695 | 15.4 | 489 | 20.7 | 206 | 9.6 | ||
| Low educational level | 530 | 7.7 | 151 | 6.4 | 379 | 8.4 | 0.015 | 291 | 12.3 | 88 | 4.1 | <0.001 |
| Retired | 466 | 6.8 | 215 | 9.1 | 251 | 5.6 | 0.001 | 200 | 8.5 | 51 | 2.4 | <0.001 |
| Healthcare professionals | 3,474 | 50.6 | 987 | 42.0 | 2,487 | 55.0 | < 0.001 | 1,243 | 52.5 | 1,244 | 57.8 | < 0.001 |
| Northern area of residence | 5,169 | 75.2 | 1,722 | 73.2 | 3,447 | 76.2 | < 0.001 | 31,802 | 76.1 | 1,645 | 76.4 | 0.904 |
| Personal characteristics | ||||||||||||
| Sedentary habits | 2,094 | 30.5 | 610 | 25.9 | 1,484 | 32.8 | < 0.001 | 887 | 37.5 | 597 | 27.7 | < 0.001 |
| Current smokers | 1,068 | 15.5 | 346 | 14.7 | 722 | 17.9 | < 0.001 | 349 | 14.7 | 373 | 17.3 | <0.001 |
| Good self-perceived health status | 5,518 | 80.3 | 1941 | 82.5 | 3,577 | 79.1 | < 0.001 | 1,802 | 76.1 | 1,775 | 82.4 | < 0.001 |
| Co-habitants at risk° | 1,377 | 20.0 | 402 | 17.1 | 975 | 21.6 | < 0.001 | 598 | 25.3 | 377 | 17.5 | < 0.001 |
| Contact with COVID-19 cases*tb1fn2 | 4,874 | 70.9 | 1,606 | 68.3 | 3,268 | 72.3 | 0.001 | 1,729 | 73.0 | 1,539 | 71.5 | 0.250 |
| Healthcare seeking□ | 2,977 | 43.3 | 1,042 | 44.3 | 1,935 | 42.8 | 0.233 | 1,027 | 43.4 | 908 | 42.2 | 0.417 |
| Medical conditions | ||||||||||||
| Lung diseases | 553 | 8.1 | 206 | 8.8 | 347 | 7.7 | 0.117 | 197 | 8.3 | 150 | 7.0 | 0.088 |
| Heart diseases and/or use of drugs | 306 | 4.5 | 129 | 5.5 | 177 | 3.9 | 0.003 | 217 | 9.2 | 80 | 3.7 | <0.001 |
| Hypertension and/or use of drugs | 1,202 | 17.5 | 587 | 25.0 | 615 | 13.6 | < 0.001 | 402 | 17.0 | 213 | 9.9 | < 0.001 |
| Oncological diseases | 2.22 | 3.2 | 79 | 3.4 | 143 | 3.2 | 0.663 | 77 | 3.3 | 66 | 3.1 | 0.721 |
| Liver diseases | 61 | 0.9 | 24 | 1.0 | 37 | 0.8 | 0.397 | 31 | 1.3 | 6 | 0.3 | <0.001 |
| Renal diseases | 78 | 1.1 | 36 | 1.5 | 42 | 0.9 | 0.025 | 31 | 1.3 | 11 | 0.5 | 0.005 |
| Metabolic diseases and/or use of drugs | 375 | 5.5 | 135 | 5.7 | 240 | 5.3 | 0.455 | 271 | 11.4 | 152 | 7.1 | <0.001 |
| Depression/anxiety and/or use of drugs | 801 | 11.7 | 220 | 9.4 | 581 | 12.9 | < 0.001 | 376 | 15.9 | 205 | 9.5 | < 0.001 |
| Immune system diseases | 670 | 9.8 | 109 | 4.6 | 561 | 12.4 | < 0.001 | 305 | 12.9 | 256 | 11.9 | 0.313 |
| Flu shot vaccination | 2,313 | 33.7 | 876 | 37.2 | 1,437 | 31.8 | < 0.001 | 773 | 32.6 | 664 | 30.8 | 0.194 |
| Anti-pneumococcal vaccination | 331 | 4.8 | 143 | 6.1 | 188 | 4.2 | < 0.001 | 117 | 4.9 | 71 | 3.3 | 0.006 |
| Oncological drugs | 72 | 1.1 | 23 | 1.0 | 49 | 1.1 | 0.682 | 17 | 0.7 | 32 | 1.5 | 0.013 |
| Corticosteroids | 160 | 2.3 | 59 | 2.5 | 101 | 2.2 | 0.474 | 50 | 2.1 | 51 | 2.4 | 0.559 |
| Thyroid drugs | 568 | 8.3 | 75 | 3.2 | 493 | 10.9 | < 0.001 | 268 | 11.3 | 225 | 10.5 | 0.350 |
| Anti-inflammatory drugs | 433 | 6.3 | 111 | 4.7 | 322 | 7.1 | < 0.001 | 179 | 7.6 | 143 | 6.6 | 0.231 |
| COVID-19 related variables | ||||||||||||
| No symptoms | 1,792 | 26.1 | 620 | 26.4 | 1,172 | 25.9 | 0.695 | 651 | 27.5 | 521 | 24.2 | 0.012 |
| Fever | 1,898 | 27.6 | 762 | 32.4 | 1,136 | 25.1 | < 0.001 | 599 | 25.3 | 537 | 24.9 | 0.784 |
| Headache | 2,562 | 37.3 | 715 | 30.4 | 1,847 | 40.9 | < 0.001 | 894 | 37.8 | 953 | 44.3 | <0.001 |
| Muscle/bone pain | 2,381 | 34.6 | 792 | 33.7 | 1,589 | 35.2 | 0.223 | 797 | 33.7 | 792 | 36.8 | 0.028 |
| Olfactory and taste disorders | 1,450 | 21.1 | 497 | 21.1 | 953 | 21.1 | 0.960 | 475 | 20.1 | 478 | 22.2 | 0.078 |
| Shortness of breath | 1,036 | 15.1 | 373 | 15.9 | 663 | 14.7 | 0.189 | 319 | 13.5 | 344 | 16.0 | 0.017 |
| Chest pain | 965 | 14.0 | 308 | 13.1 | 657 | 14.5 | 0.104 | 309 | 13.1 | 348 | 16.2 | 0.003 |
| Heart palpitations | 876 | 12.8 | 205 | 8.7 | 671 | 14.8 | < 0.001 | 316 | 13.3 | 355 | 16.5 | 0.003 |
| Gastrointestinal disturbances | 1,929 | 28.1 | 588 | 25.0 | 1,341 | 29.7 | < 0.001 | 643 | 27.2 | 698 | 32.4 | <0.001 |
| Conjunctivitis | 821 | 12.0 | 254 | 10.8 | 567 | 12.5 | 0.035 | 300 | 12.7 | 267 | 12.4 | 0.786 |
| Sore throat/rhinorrhoea | 2,531 | 36.8 | 753 | 32.0 | 1778 | 39.3 | < 0.001 | 886 | 37.4 | 892 | 41.4 | 0.006 |
| Cough | 2,371 | 34.5 | 839 | 35.7 | 1532 | 33.9 | 0.140 | 753 | 31.8 | 779 | 36.2 | 0.002 |
| Pneumonia | 557 | 8.1 | 289 | 12.3 | 268 | 5.9 | < 0.001 | 137 | 5.8 | 131 | 6.1 | 0.671 |
| Hospitalized for COVID-19 | 528 | 7.7 | 287 | 12.2 | 241 | 5.3 | < 0.001 | 138 | 5.8 | 103 | 4.8 | 0.119 |
| NPS test positive result | 1,702 | 24.8 | 677 | 28.8 | 1025 | 22.7 | < 0.001 | 595 | 25.1 | 430 | 20.0 | < 0.001 |
| Infection severity§ | < 0.001 | < 0.001 | ||||||||||
| Negative NPS test | 5,171 | 75.2 | 1675 | 71.2 | 3,496 | 77.3 | 1773 | 74.9 | 1,723 | 80.0 | ||
| Asymptomatic or mild | 1,214 | 17.7 | 222 | 4.9 | 803 | 17.8 | 471 | 19.9 | 332 | 15.4 | ||
| Severe | 488 | 7.1 | 266 | 11.3 | 222 | 4.9 | 124 | 5.2 | 98 | 4.6 | ||
Older people or anyone with immunocompromising or chronic disease conditions.
Suspected/confirmed.
Contact the emergency number or the general practitioner.
Asymptomatic or mild infection (positive NPS test without symptoms or with at least one COVID-19 like symptom) and severe infection (positive NPS test with pneumonia and/or hospitalization for COVID-19).
3.2. Association analyses
Table 2 shows the logistic regression results considering a positive NPS test as the outcome for the whole sample (Sample A). With respect to their male counterparts, being female was inversely associated with the odds of having a positive NPS test (OR 0.75, 95%CI 0.66–0.85). Since a significant interaction between sex and age was found, six levels of indicator variables of males/females aged ≥60, males/females 50–59, and males/females <50 were created. With respect to the males aged ≥60, a pattern across age classes was noted in both sexes; the males younger than 50, the females between 50 and 59, and the females older than 50 showed a significantly lower probability of testing positive by 39%, 44%, and 52%, respectively. Table 3 reports the association between sex and the probability of getting a severe infection. Overall, females had a lower probability of having a severe infection (aOR 0.46, 95%CI 0.37–0.57) with respect to their male counterparts. Considering the older males with no infection as the reference group, data analysis uncovered that the males aged over 50, the females 60 and older, the females between 50 and 59, and the females younger than 50 had, respectively, a significant risk reduction of 66%, 42%, 67%, and 85%.
Table 2.
Odds ratios of positive NPS molecular test according to sex and age groups in Sample A (N = 6873).
| Total | Negative | Positive | Adjusted | ||||
| Sample A° | N = 6,873 | N = 5,171 | %=75.2 | N = 1,702 | %=24.8 | aOR (95% CI) | |
| Males | 2352 | 34.2 | 1675 | 32.4 | 677 | 39.8 | 1 (ref.) |
| Females | 4,521 | 65.8 | 3,496 | 67.6 | 1,025 | 60.2 | 0.75 (0.66–0.85) |
| SEX X AGE | |||||||
| Males aged≥60 | 629 | 9,3 | 391 | 7.6 | 238 | 14.0 | 1 (ref.) |
| Males aged 50–59 | 596 | 8,8 | 411 | 8.0 | 185 | 10.9 | 0.87 (0.66–1.14) |
| Males aged <50 | 1,127 | 16,6 | 873 | 16.9 | 254 | 14.9 | 0.61 (0.47–0.79) |
| Females aged≥60 | 695 | 10,2 | 428 | 8.3 | 267 | 15.7 | 0.86 (0.67–1.11) |
| Females aged 50–59 | 1,260 | 18,6 | 977 | 18.9 | 283 | 16.6 | 0.56 (0.44–0.72) |
| Females aged <50 | 2,566 | 37,8 | 2091 | 40.4 | 475 | 27.9 | 0.48 (0.38–0.61) |
Males reference category.
Model adjusted for age°, education, employment status, area of residence, healthcare professionals, physical activity, smoking status, living with at risk co-habitants, contact with COVID-19 cases, heart diseases, hypertension, depression, renal diseases, immune system disorders, flu and anti-pneumococcal vaccine, thyroid drugs, anti-inflammatory drugs, and self-perceived health status.
Table 3.
Odds ratios of infection severity according to sex and age groups in Sample A (N = 6873).
| Sample A° | Negative NPS test N = 5,171 (75.2) | Asymptomatic or mild infection N = 1,214 (17.7) | Severe infection N = 488 (7.1) | ||||
|---|---|---|---|---|---|---|---|
| N (%) | OR | (95% CI) | N (%) | OR | (95% CI) | N (%) | |
| Males | 1,675 (34.4) |
1 (ref.) |
– | 411 (33.9) |
1 (ref.) |
– | 266 (54.5) |
| Females | 3,496 (67.6) | 0.92 | 0.79–1.06 | 803 (66.1) | 0.46 | 0.37–0.57 | 222 (45.5) |
| SEX X AGE | |||||||
| Males aged≥60 |
391 (7.6) |
1 (ref.) |
120 (9.9) |
1 (ref.) |
118 (24.2) | ||
| Males aged 50–59 | 411 (8.0) | 0.96 | 0.69–1.34 | 103 (8.5) | 0.74 | 0.51–1.08 | 82 (16.8) |
| Males aged <50 | 873 (16.9) | 0.85 | 0.63–1.16 | 188 (15.5) | 0.34 | 0.23–0.50 | 66 (13.5) |
| Females aged≥60 | 428 (8.3) | 1.15 | 0.87–1.53 | 187 (15.4) | 0.58 | 0.41–0.82 | 80 (16.4) |
| Females aged 50–59 | 977 (18.9) | 0.77 | 0.57–1.04 | 208 (17.3) | 0.33 | 0.22–0.48 | 75 (15.4) |
| Females aged <50 | 2,091 (40.4) | 0.77 | 0.58–1.02 | 408 (33.6) | 0.15 | 0.10–0.22 | 67 (13.7) |
Males and negative NPS test as reference category. Asymptomatic or mild infection (positive NPS test without symptoms or with at least one COVID-19 like symptom) and severe infection (positive NPS test with pneumonia and/or hospitalization for COVID-19).
Model adjusted for age°, education, employment status, area of residence, healthcare professionals, physical activity, smoking status, living with at risk co-habitants, contact with COVID-19 cases, heart diseases, hypertension, depression, renal diseases, immune system disorders, flu and anti-pneumococcal vaccine, thyroid drugs, anti-inflammatory drugs, and self-perceived health status.
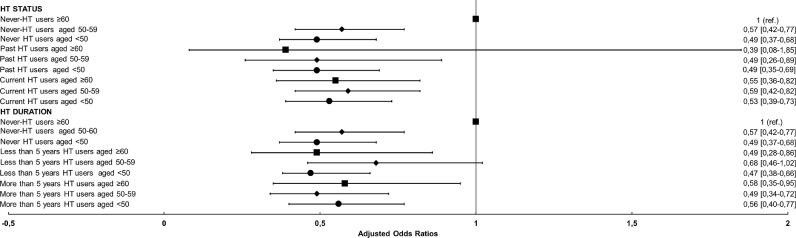
Tables 4 and 5 show that the aORs of having a positive NPS test and of developing a severe infection in the Sample B population significantly decrease with decreasing age. Although no association between HT usage and SARS-CoV-2 infection was found, a statistically significant interaction was observed between age and HT (P-value <0.001). When age class was combined with HT, it was found that, with respect to the never-HT users who were 60 or older, the HT users of the same age class had a 46% reduced odds of receiving a positive result (aOR 0.54). Similar results were found for the younger age classes, irrespective of HT usage. With respect to the never-HT users who were 60 or older, the HT users of the same age class had a lower probability of having an asymptomatic or mild infection (aOR 0.37, 95%CI 0.22–0.61); both the never- and the HT users in the younger age groups (50–59 and <50 years) showed a lower odds of severity and there was no association with severe infection severity. A similar pattern of reduced association in the females who were 60 and over was observed when the HT status and duration were considered (Fig. 2 and Supplementary S23). The associations between the female sex and a positive NPS test result and severity were less pronounced in the healthcare professionals (Supplementary S4). Supplementary material S5–S8 shows the associations between HT usage in females older than 50 years (post-menopausal). With respect to the never-HT users the association between HT users and NPS status and infection severity, even considering the HT status and duration, persisted although the effect was in part attenuated. This is partly due to the differences that the oldest age classes may have when compared to the youngest one, especially with respect to SARS-CoV-2 infection.
Table 4.
Adjusted odds ratios of positive NPS test according to age and HT usage in Sample B (females, N = 4521).
| Model B | Total N = 4,521 | Negative N = 3,496%=77.3 | Positive N = 1,025%=22.7 | Adjusted OR (95%CI) | |||
|---|---|---|---|---|---|---|---|
| AGE | |||||||
| Females aged≥60 | 267 | 15,4 | 428 | 12.2 | 267 | 26.1 | 1 (ref) |
| Females aged 50–60 | 283 | 27,9 | 977 | 28.0 | 283 | 27.6 | 0.69 (0.53–0.90) |
| Females aged <50 | 475 | 56,8 | 2091 | 59.8 | 475 | 46.3 | 0.62 (0.48–0.80) |
| HORMONAL THERAPY° | N | % | N | % | |||
| Never-HT users | 595 | 52,4 | 1773 | 50.7 | 595 | 58.1 | 1 (ref) |
| HT users | 430 | 47,6 | 1723 | 49.3 | 430 | 42.0 | 0.95 (0.81–1.10) |
| AGE X HORMONAL THERAPY | |||||||
| Females never-HT users aged≥60 | 219 | 10,8 | 270 | 7.7 | 219 | 21.4 | 1 (ref) |
| Females HT users aged ≥60 | 48 | 4,6 | 158 | 4.5 | 48 | 4.7 | 0.54 (0.36–0.80) |
| Females never-HT users aged 50–60 | 159 | 15,2 | 530 | 15.2 | 159 | 15.5 | 0.57 (0.42–0.77) |
| Females HT users aged 50–60 | 124 | 12,6 | 447 | 12.8 | 124 | 12.1 | 0.57 (0.41–0.79) |
| Females never-HT users aged <50 | 217 | 26,3 | 973 | 27.8 | 217 | 21.2 | 0.50 (0.37–0.67) |
| Females HT users aged <50 | 258 | 30,4 | 1118 | 32.0 | 258 | 25.2 | 0.52 (0.38–0.69) |
Never-HT users as reference category.
Model adjusted for age°, education, employment status, area of residence, healthcare professionals, physical activity, smoking status, living with at risk co-habitants, contact with COVID-19 cases, heart diseases, depression, liver and metabolic diseases, flu and anti-pneumococcal vaccine, anti-inflammatory and oncological drugs.
Table 5.
Adjusted odds ratios of infection severity according to age and HT usage in Sample B (females, N = 4521).
| Negative NPS test N = 3,496 (77.3) | Asymptomatic or mild infection N = 803 (17.8) | Severe infection N = 222 (4.9) | |||||
|---|---|---|---|---|---|---|---|
| N (%) | OR | (95% CI) | N (%) | OR | (95% CI) | N (%) | |
| AGE | |||||||
| Females aged≥60 | 428 (12.2) | 1 (ref.) | – | 187 (23.3) | 1 (ref) | – | 80 (36.0) |
| Females aged 50–60 | 977 (28.0) | 0.75 | 0.56–1.01 | 208 (25.9) | 0.53 | 0.35–0.82 | 75 (33.8) |
| Females aged <50 | 2,091 (59.8) | 0.80 | 0.60–1.06 | 408 (50.8) | 0.24 | 0.16–0.38 | 67 (30.2) |
| HORMONAL THERAPY° | |||||||
| Never-HT users | 1,773 (50.7) | 1 (ref.) | – | 471 (58.7) | 1 (ref) | – | 124 (55.9) |
| HT users | 1,723 (49.3) | 0.89 | 0.76–1.06 | 332 (41.3) | 1.19 | 0.88–1.62 | 98 (44.1) |
| AGE X HORMONAL THERAPY | |||||||
| Females never-HT users aged≥60 | 270 (7.7) | 1 (ref.) | 163 (20.3) | 1 (ref.) | 56 (25.2) | ||
| Females HT users aged ≥60 | 158 (4.5) | 0.37 | 0.22–0.61 | 24 (3.0) | 1.04 | 0.58–1.86 | 24 (10.8) |
| Females never-HT users aged 50–60 | 530 (15.2) | 0.59 | 0.42–0.84 | 121 (15.1) | 0.47 | 0.28–0.81 | 38 (17.1) |
| Females HT users aged 50–60 | 447 (12.8) | 0.55 | 0.38–0.79 | 87 (10.8) | 0.63 | 0.36–1.09 | 37 (16.7) |
| Females never-HT users aged <50 | 973 (27.8) | 0.59 | 0.43–0.82 | 187 (23.3) | 0.24 | 0.14–0.43 | 30 (13.5) |
| Females HT users aged <50 | 1,118 (32.0) | 0.61 | 0.44–0.85 | 221 (27.5) | 0.25 | 0.15–0.44 | 37 (16.7) |
Negative NPS test and never-HT users as reference category. Asymptomatic or mild infection (positive NPS test without symptoms or with at least one COVID-19 like symptom) and severe infection (positive NPS test with pneumonia and/or hospitalization for COVID-19).
Model adjusted for age°, education, employment status, area of residence, healthcare professionals, physical activity, smoking status, living area, living with at risk co-habitants, contact with COVID-19 cases, heart diseases, depression, liver and metabolic diseases, flu and anti-pneumococcal vaccine, anti-inflammatory and oncological drugs.
Fig. 2.
Model adjusted for age, education, employment status, area of residence, healthcare professionals, physical activity, smoking status, living area, living with at risk co-habitants, contact with COVID-19 cases, heart diseases, depression, liver and metabolic diseases, flu and anti-pneumococcal vaccine, anti-inflammatory and oncological drugs.
4. Discussion
4.1. Main findings
The data were collected from a large web-based survey of an adult population during the first wave of the COVID-19 pandemic in Italy. After adjusting for several socio-demographic, clinical, and behavioral factors, data analysis showed that the female sex was associated with a 25% lower probability of a positive NPS test result and a 46% lower risk of developing a severe infection; the strength of these associations increased as age decreased. These findings are in line with other epidemiological data gathered from the Italian population performed between March and August 2020 showing that males were more likely than females to test positive [2,3,5] and to manifest severe disease leading to an increased risk of COVID-19-related hospitalization, intensive care unit admission, and death [2,5,8]. A systematic review and meta-analysis of 57 studies performed between December 2019 and April 2020 uncovered a pooled prevalence of COVID-19 confirmed cases in males and females of 55% and 45%, respectively [4]; these findings were similar to those reported by the Italian National Institute of Health (ISS) at the end of March 2020 [23]. The Global 50/50 initiative [6] and some reviews and meta-analyses focusing on COVID-19 cases worldwide published after that date, however, reported no difference in the male:female ratio of individuals infected with SARS-CoV-2, although the former face a higher risk of hospitalization, intensive care unit admission, and death with respect to their female counterparts [8,24,25]. A seroprevalence systematic review and meta-analysis likewise reported no substantial sex difference in individuals infected with SARS-CoV-2 [26]. Our cross-sectional data were collected during the early stages of the epidemic in Italy (April 2020) when only individuals experiencing symptoms severe enough to require medical attention underwent NPS testing. The fact that there were more males with positive NPS results may have distorted our results just as the fact there were more female than male participants that underwent the NPS tests possibly because of the high percentage of female healthcare professionals workers.
The male participants of our survey were older and more frequently reported comorbidities and severe COVID-19 symptoms such as fever and pneumonia leading to worse disease progression. The females instead were more likely to be paucisymptomatic, presenting with atypical symptoms characterized by sore throat/rhinorrhea, gastrointestinal disturbances, headache, conjunctivitis, and palpitations that were associated with less severe outcomes, as previously documented [27]. As the age-dependent sex disparities in our population persisted after controlling for comorbidities and high-risk behaviors, they seemed to pointing in the direction of possible biological-related explanations. Sex differences in immune responses throughout the lifecourse are influenced by both age and reproductive status. In fact, it has been reported that females tend to be less susceptible to infections than their male counterparts, and it has been hypothesized that sex steroids contribute to the differential regulation of immune responses between sexes [13]. Both oestrogens and progesterone act by suppressing the production of proinflammatory cytokines associated with the COVID-19 cytokine storm and by enhancing the anti-inflammatory cytokines [28]. Furthermore, it has been reported that ACE2, which is located on the X chromosome, was significantly downregulated after binding viral Spike protein with consequent reduced ACE2 expression in the lung resulting in severe acute respiratory failure [29]. High estrogen concentrations might up-regulate ACE2 expression leading to an over-expression of ACE2 in females protecting them against viral entry. Low oestrogens levels in males may instead contribute to higher disease susceptibility and death rates [30]. Androgens could also promote the transcription of the TMPRSS2 gene facilitating viral entry into the cells [16] and decreasing the antibody response to viral infections [13]. Interestingly, patients with prostate cancer undergoing treatment with androgen deprivation therapy were less likely to develop severe COVID-19 with respect to their non-treated counterparts. Moreover, the low levels of androgens in females may reduce TMPRSS2 expression, further protecting them against the SARS-CoV-2 infection [31].
Our analysis also uncovered that the older females (with natural estrogen deficiency) who were currently receiving or had received HT in the past had a significantly lower odds of getting the infection with respect to their same age counterparts who had never used HT (aOR 0.54). The odds was similar for younger females irrespective of HT use, possibly because of their higher endogenous estrogen levels. These associations seemed to persist independently of the current or past usage of HT or its duration. As females age, the hormone's potential protective effect is attenuated because menopause causes a drastic decline in the natural estrogen levels and affects B and T cells causing post-menopausal women to be more prone to chronic and infectious diseases. In addition, post-menopausal females are reported to have higher levels of pro-inflammatory cytokines that seem to be reduced by HT [32].
Our preliminary data support these hypotheses and agree with the findings of other studies. A study based on an analysis of electronic health records from a large international COVID-19 patient cohort (N = 68,466) recently reported that although estradiol treatment in pre-menopausal females had no effect, fatality risk for post-menopausal females receiving estradiol therapy was reduced by more than 50% [20]. Menopause was found to be an independent risk factor for COVID-19 in a cross-sectional study investigating 1902 COVID-19 female patients, while anti-Mullerian hormone and estradiol appeared to be potential protective factors [18]. A study investigating 152,637 female users of a COVID Symptom Tracker Application in the UK reported that postmenopausal femlaes had a higher rate of predicted COVID while pre-menopausal females taking the contraceptive pill had a significantly lower rate of predicted COVID-19 and hospitalizations with respect to their post-menopausal counterparts [17]. The authors of a study carried out in South Korea did not find any association between females who had been taking HT over the past year and morbidity and clinical outcomes of COVID-19 [19]. Although it is impossible to exclude that post-menopausal females are more susceptible to SARS-COV-2 infection due to age-related factors and comorbidities rather than to lower estrogen levels, our data suggest that HT may play a protective role against COVID-19 in older females. At present, several clinical trials investigating the potential effect of hormonal therapies (i.e. the selective estrogen receptor modulator, raloxifene) on the infection are currently underway [33].
4.2. Limitations and strengths
These findings need to be interpreted cautiously since the sample was self-selected and cannot be considered entirely representative of the Italian population. The study's generalizability is also limited because restricted to relatively young, female, highly educated, healthcare workers, health-conscious individuals, while a low percentage was made up of participants severely ill with COVID-19 . Furthermore, this study suffers from sampling bias because of the exclusion of individuals who were hospitalized for severe infection or have died because of COVID-19. Other investigators have pointed out that a non-random sampling based on the availability of testing may have led to restrict the analysis to individuals who have been tested for active SARS-CoV 2-infection with severe symptoms [34]. The cross-sectional nature of the study did not enable cause-and-effect inference. The study may be subjected to the “healthy women effect”, females taking HT were more educated, had higher socioeconomic status, a healthier lifestyle, and a lower risk of CVD and metabolic diseases, all factors known to be associated with COVID-19, than their non-HT counterpart, although the models have been adjusted for these variables.
The study may also be prone to a recall bias because data regarding homone-related exposure were self-reported and the questions regarding HT were non-standardized therefore, misunderstanding and internal errors due to the lack of qualified assessment might have affected the study results. It was moreover impossible to analyze oral contraceptive use and hormone replacement therapy separately; in addition, no specific drug type description was collected. Lastly, although we controlled for several potential confounders, other conditions not considered may have affected our results, therefore unmeasured or residual confounders cannot completely ruled out. The study does nevertheless boast a large sample, and its data, which was collected directly from the general population, reflected the geographical distribution of SARS-CoV-2 infection in the country over the observation period [22]. In addition, information on the length of time in years that hormonal therapies were being or had been taken made it possible to perform a time-response analysis. To our knowledge, EPICOVID19 is the largest web survey performed in Italy during the first wave of the pandemic; it collected exhaustive information on socio-demographic, medical, behavioral, and environmental factors, which made it possible, to control for many potential confounding factors.
5. Conclusion
These preliminary findings based on data from an Italian adult population collected during the first wave of the pandemic uncovered a sex bias for the SARS-CoV-2 infection rate favoring females. Females over 60 years who were receiving or had received hormonal therapy had a lower probability of having a positive NPS test compared with non-user same-age counterparts. Future prospective studies are warranted to explore how hormonal therapy can affect infection vulnerability and long term COVID19-related clinical outcomes.
Contributors
Federica Prinelli was project administrator and contributed to the study concept and design, performed the statistical analysis, and drafted the original manuscript.
Caterina Trevisan contributed to the study concept and design and to the preparation of the manuscript.
Marianna Noale contributed to the study concept and design and to the preparation of the manuscript.
Michela Franchini contributed to the editing and revision of the manuscript and to the interpretation of the results.
Andrea Giacomelli contributed to the editing and revision of the manuscript and to the interpretation of the results.
Liliana Cori contributed to the editing and revision of the manuscript and to the interpretation of the results.
Nithiya Jesuthasan contributed to the editing and revision of the manuscript and to the interpretation of the results.
Raffaele Antonelli Incalzi contributed to the editing and revision of the manuscript and to the interpretation of the results.
Stefania Maggi contributed to the study concept and design and to the preparation of the manuscript, and supervised the study.
Fulvio Adorni was project administrator and contributed to the study concept and design and to the preparation of the manuscript, and supervised the study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
The Ethics Committee of the Istituto Nazionale per le Malattie Infettive IRCCS Lazzaro Spallanzani (protocol No. 70, 12/4/2020) approved the EPICOVID19 study protocol. The participants were requested to give their informed consent when they first accessed the web-based platform. Participation was voluntary, and no compensation was expected for respondents. The planning, conduct, and reporting of the study were in line with the Declaration of Helsinki, as revised in 2013. All data were handled and stored following the EU General Data Protection Regulation (EU-GDPR) 2016/679, and data transfer was safeguarded by encrypting and decrypting data and password protection. The study has been registered in ClinicalTrials.Gov (NCT04471701).
Provenance and peer review
This article was not commissioned and was externally peer reviewed.
Research data (data sharing and collaboration)
There are no linked research data sets for this paper. Data will be made available on reasonable request to the corresponding author.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
The authors would like to thank all the participants who took part in this study and made it possible and all the collaborators of the EPICOVID19 Working Group°. The authors would like to thank Linda Inverso for editing the English version of the manuscript.
Member of the EPICOVID19 Working Group° (in alphabetical order)
Adorni Fulvio, National Research Council, Institute of Biomedical Technologies, Epidemiology Unit, Via Fratelli Cervi 93, 20090 Segrate (MI), Italy. fulvio.adorni@itb.cnr.it
Andreoni Massimo, Infectious Diseases Clinic, Department of System Medicine, Tor Vergata University of Rome, 00133 Rome, Italy. andreoni@uniroma2.it
Antonelli Incalzi Raffaele, Unit of Geriatrics, Department of Medicine, Biomedical Campus of Rome, via Alvaro del Portillo, 21, 00128 Rome, Italy. r.antonelli@unicampus.it
Bastiani Luca, National Research Council, Institute of Clinical Physiology, Via G. Moruzzi 1, 56124 Pisa (PI), Italy. luca.bastiani@ifc.cnr.it
Bianchi Fabrizio, National Research Council, Institute of Clinical Physiology, Via G. Moruzzi 1, 56124 Pisa (PI), Italy. fabriepi@ifc.cnr.it
Di Bari Mauro, Geriatric Intensive Care Medicine, University of Florence and Azienda Ospedaliero-Universitaria Careggi, Viale Peraccini 18, 50139 Florence, Italy. mauro.dibari@unifi.it
Fortunato Loredana, National Research Council, Institute of Clinical Physiology, Via G. Moruzzi 1, 56124 Pisa (PI), Italy. loredana.fortunato@ifc.cnr.it
Galli Massimo, Infectious Diseases Unit, Department of Biomedical and Clinical Sciences L. Sacco, Università di Milano, ASST Fatebenefratelli Sacco, 20157 Milan, Italy. massimo.galli@unimi.it
Giacomelli Andrea, Infectious Diseases Unit, Department of Biomedical and Clinical Sciences L. Sacco, Università di Milano, ASST Fatebenefratelli Sacco, 20157 Milan, Italy. andrea.giacomelli@unimi.it
Jesuthasan Nithiya, National Research Council, Institute of Biomedical Technologies, Epidemiology Unit, Via Fratelli Cervi 93, 20090 Segrate (MI), Italy. nithiya.jesuthasan@itb.cnr.it
Maggi Stefania, National Research Council-Neuroscience Institute, Aging Branch, Via Vincenzo Maria Gallucci 16, 35128 Padova, Italy. stefania.maggi@in.cnr.it
Mastroianni Claudio, Public Health and Infectious Disease Department, “Sapienza” University, Piazzale Aldo Moro 1, 00185, Rome, Italy. claudio.mastroianni@uniroma1.it
Molinaro Sabrina, National Research Council, Institute of Clinical Physiology, Via G. Moruzzi 1, 56124 Pisa (PI), Italy. sabrina.molinaro@ifc.cnr.it
Noale Marianna, National Research Council-Neuroscience Institute, Aging Branch, Via Vincenzo Maria Gallucci 16, 35128 Padova, Italy. marianna.noale@in.cnr.it
Pagani Gabriele, Infectious Diseases Unit, Department of Biomedical and Clinical Sciences L. Sacco, Università di Milano, ASST Fatebenefratelli Sacco, 20157 Milan, Italy. gabriele.pagani@unimi.it
Pedone Claudio, Unit of Geriatrics, Department of Medicine, Biomedical Campus of Rome, via Alvaro del Portillo, 21, 00128 Rome, Italy. claudio.pedone@gmail.com
Pettenati Carla, National Research Council, Institute of Biomedical Technologies, Via Fratelli Cervi 93, 20090 Segrate (MI), Italy. cpettenati@me.com
Prinelli Federica, National Research Council, Institute of Biomedical Technologies, Epidemiology Unit, Via Fratelli Cervi 93, 20090 Segrate (MI), Italy. federica.prinelli@itb.cnr.it
Rusconi Stefano, Infectious Diseases Unit, Department of Biomedical and Clinical Sciences L. Sacco, Università di Milano, ASST Fatebenefratelli Sacco, 20157 Milan, Italy. stefano.rusconi@unimi.it
Sojic Aleksandra, National Research Council, Institute of Biomedical Technologies, Epidemiology Unit, Via Fratelli Cervi 93, 20090 Segrate (MI), Italy. aleksandra.sojic@itb.cnr.it
Tavio Marcello, Division of Infectious Diseases, Azienda Ospedaliero Universitaria Ospedali Riuniti, Via Conca 71, Torrette, Ancona, Italy. marcello.tavio@ospedaliriuniti.marche.it
Trevisan Caterina, Geriatric Unit, Department of Medicine (DIMED), University of Padova, Via
Giustiniani 2, 35128 Padova, Italy; National Research Council-Neuroscience Institute, Aging Branch, Via Vincenzo Maria Gallucci 16, 35128 Padova, Italy. caterina.trevisan.5@studenti.unipd.it
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.maturitas.2021.11.015.
Appendix. Supplementary materials
References
- 1.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stall N.M., Wu W., Lapointe-Shaw L., Fisman D.N., Giannakeas V., Hillmer M.P., Rochon P.A. Sex- and age-specific differences in COVID19 testing, cases, and outcomes: a population-wide study in Ontario, Canada. J. Am. Geriatr. Soc. 2020;68(10):2188–2191. doi: 10.1111/jgs.16761. OctEpub 2020 Aug 15. PMID: 32743827. [DOI] [PubMed] [Google Scholar]
- 3.Zhao S., Cao P., Chong M.K.C., Gao D., Lou Y., Ran J., Wang K., Wang W., Yang L., He D., Wang M.H. COVID-19 and gender-specific difference: analysis of public surveillance data in Hong Kong and Shenzhen, China, from January 10 to February 15, 2020. Infect. Control Hosp. Epidemiol. 2020;41:1–2. doi: 10.1017/ice.2020.64. JunPMID: 32146921; PMCID: PMC7113032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abate B.B., Kassie A.M., Kassaw M.W., Aragie T.G., Masresha S.A. Sex difference in coronavirus disease (COVID-19): a systematic review and meta-analysis. BMJ Open. 2020;10(10) doi: 10.1136/bmjopen-2020-040129. Oct 6PMID: 33028563; PMCID: PMC7539579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vahidy F.S., Pan A.P., Ahnstedt H., Munshi Y., Choi H.A., Tiruneh Y., Nasir K., Kash B.A., Andrieni J.D., McCullough L.D. Sex differences in susceptibility, severity, and outcomes of coronavirus disease 2019: cross-sectional analysis from a diverse US metropolitan area. PLoS One. 2021;16(1) doi: 10.1371/journal.pone.0245556. Jan 13PMID: 33439908; PMCID: PMC7806140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The sex, gender and COVID-19 project, Men, sex gender and Covid-19. https://globalhealth5050.org/the-sex-gender-and-covid-19-project/men-sex-gender-and-covid-19/.
- 7.Gebhard C., Regitz-Zagrosek V., Neuhauser H.K., Morgan R., Klein S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex Differ. 2020;11(1):29. doi: 10.1186/s13293-020-00304-9. May 25PMID: 32450906; PMCID: PMC7247289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peckham H., de Gruijter N.M., Raine C., et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Channappanavar R., Fett C., Mack M., Ten Eyck P.P., Meyerholz D.K., Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J. Immunol. 2017;198(10):4046–4053. doi: 10.4049/jimmunol.1601896. May 15Epub 2017 Apr 3. PMID: 28373583; PMCID: PMC5450662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alghamdi I.G., Hussain I.I., Almalki S.S., Alghamdi M.S., Alghamdi M.M., El-Sheemy M.A. The pattern of middle east respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int. J. Gen. Med. 2014;7:417–423. doi: 10.2147/ijgm.s67061. Aug 20PMID: 25187734PMCID: PMC4149400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galasso V., Pons V., Profeta P., Becher M., Brouard S., Foucault M. Gender differences in COVID-19 attitudes and behavior: panel evidence from eight countries. Proc. Natl. Acad. Sci. U. S. A. 2020;117(44):27285–27291. doi: 10.1073/pnas.2012520117. Nov 3Epub 2020 Oct 15. PMID: 33060298; PMCID: PMC7959517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai H. Sex difference and smoking predisposition in patients with Covid-19. Lancet Respir. Med. 2020;8(4):e20. doi: 10.1016/S2213-2600(20)30117-X. AprEpub 2020 Mar 11. Erratum in: Lancet Respir Med. 2020 Apr;8(4):e26. PMID: 32171067; PMCID: PMC7103991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. OctEpub 2016 Aug 22. PMID: 27546235. [DOI] [PubMed] [Google Scholar]
- 14.Bukowska A., Spiller L., Wolke C., Lendeckel U., Weinert S., Hoffmann J., Bornfleth P., Kutschka I., Gardemann A., Isermann B., Goette A. Protective regulation of the ACE2/ACE gene expression by estrogen in human atrial tissue from elderly men. Exp. Biol. Med. (Maywood) 2017;242(14):1412–1423. doi: 10.1177/1535370217718808. AugEpub 2017 Jun 29. PMID: 28661206; PMCID: PMC5544171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinna G. Sex and COVID-19: a protective role for reproductive steroids. Trends Endocrinol. Metab. 2021;32(1):3–6. doi: 10.1016/j.tem.2020.11.004. JanEpub 2020 Nov 9. PMID: 33229187; PMCID: PMC7649655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giagulli V.A., Guastamacchia E., Magrone T., Jirillo E., Lisco G., De Pergola G., Triggiani V. Worse progression of COVID-19 in men: is testosterone a key factor? Andrology. 2021;9(1):53–64. doi: 10.1111/andr.12836. JanEpub 2020 Jun 28. PMID: 32524732; PMCID: PMC7307026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.R. Costeira, K.A. Lee, B. Murray, C. Christiansen et al., Estrogen and COVID-19 symptoms: associations in women from the COVID symptom study. medRxiv preprint doi: 10.1101/2020.07.30.20164921. [DOI] [PMC free article] [PubMed]
- 18.Ding T., Zhang J., Wang T., Cui P., Chen Z., Jiang J., Zhou Su, Dai J., Wang Bo, Yuan S., Ma W., Ma L., Rong Y., Chang J., Miao X., Ma X., Wang S. Potential influence of menstrual status and sex hormones on female severe acute respiratory syndrome coronavirus 2 infection: a cross-sectional multicenter study in Wuhan, China. Clin. Infect. Dis. 2020:ciaa1022. doi: 10.1093/cid/ciaa1022. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J.H., Kim Y.C., Cho S.H., Lee J., You S.C., Song Y.G., Won Y.B., Choi Y.S., Park Y.S. Effect of sex hormones on coronavirus disease 2019: an analysis of 5,061 laboratory-confirmed cases in South Korea. Menopause. 2020;27(12):1376–1381. doi: 10.1097/GME.0000000000001657. DecPMID: 33003134; PMCID: PMC7709921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seeland U., Coluzzi F., Simmaco M., Mura C., Bourne P.E., Heiland M., Preissner R., Preissner S. Evidence for treatment with estradiol for women with SARS-CoV-2 infection. BMC Med. 2020;18(1):369. doi: 10.1186/s12916-020-01851-z. Nov 25PMID: 33234138; PMCID: PMC7685778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Sir A, Andrenelli E, Negrini F, Lazzarini SG, Patrini M, Ceravolo MG, International Multiproessional Steering Committee of Cochranef Rehabilitation REH-COVER action. Rehabilitation and COVID-19: the Cochrane Rehabilitation 2020 rapid living systematic review. Eur J Phys Rehabil Med. 2020 doi: 10.23736/S1973-9087.20.06614-9. [DOI] [PubMed] [Google Scholar]
- 22.Adorni F., Prinelli F., Bianchi F., Giacomelli A., Pagani G., Bernacchia D., Rusconi S., Maggi S., Trevisan C., Noale M., Molinaro S., Bastiani L., Fortunato L., Jesuthasan N., Sojic A., Pettenati C., Tavio M., Andreoni M., Mastroianni C., Antonelli Incalzi R., Galli M. Self-reported symptoms of SARS-CoV-2 infection in a nonhospitalized population in Italy: cross-sectional study of the EPICOVID19 web-based survey. JMIR Public Health Surveill. 2020;6(3):e21866. doi: 10.2196/21866. Sep 18PMID: 32650305PMCID: 7505691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Istituto Superiore di Sanità. Epidemia Covid-19. Aggiornamento Nazionale. 2020. Disponibile all'indirizzo: https://www.epicentro.iss.it/coronavirus/bollettino/Infografica_31marzo%20ITA.pdf.
- 24.Pérez-López F.R., Tajada M., Savirón-Cornudella R., Sánchez-Prieto M., Chedraui P., Terán E. Coronavirus disease 2019 and gender-related mortality in European countries: a meta-analysis. Maturitas. 2020;141:59–62. doi: 10.1016/j.maturitas.2020.06.017. NovEpub 2020 Jun 23PMID: 33036704; PMCID: PMC7309755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rozenberga S., Vandrommea J., Martin C. Are we equal in adversity? Does Covid-19 affect women and men differently? Maturitas. 2020;138:62–68. doi: 10.1016/j.maturitas.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rostami A., Sepidarkish M., Leeflang Mariska M.G., Riahi S.M., Shiadeh M.N., Esfandyari S., Mokdad A.H., Hotez P.J., Gasser R.B. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin. Microbiol. Infect. 2021;27:331e340. doi: 10.1016/j.cmi.2020.10.020. Epub 2020 Oct 24. PMID: 33228974; PMCID: PMC7584920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lechien J.R., Chiesa-Estomba C.M., Place S., Van Laethem Y., Cabaraux P., Mat Q., Huet K., Plzak J., Horoi M., Hans S., Barillari M.R, Cammaroto G., Fakhry N., Martiny D., Ayad T., Jouffe L., Hopkins C., Saussez S., COVID-19 task force of YO-IFOS Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J. Intern. Med. 2020;288(3):335–344. doi: 10.1111/joim.13089. SepEpub 2020 Jun 17. PMID: 32352202; PMCID: PMC7267446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauvais-Jarvis F., Klein S.L., Levin E.R. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020;161(9):bqaa127. doi: 10.1210/endocr/bqaa127. SepPMID: 32730568; PMCID: PMC7438701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanff T.C., Harhay M.O., Brown T.S., Cohen J.B., Mohareb A.M. Is there an association between COVID-19 mortality and the renin-angiotensin system? A call for epidemiologic investigations. Clin. Infect. Dis. 2020;71(15):870–874. doi: 10.1093/cid/ciaa329. Jul 28PMID: 32215613; PMCID: PMC7184340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foresta C., Rocca M.S., Di Nisio A. Gender susceptibility to COVID-19: a review of the putative role of sex hormones and X chromosome. J. Endocrinol. Invest. 2021;44(5):951–956. doi: 10.1007/s40618-020-01383-6. MayEpub 2020 Sep 16. PMID: 32936429; PMCID: PMC7492232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montopoli M., Zumerle S., Vettor R., Rugge M., Zorzi M., Catapano C.V., Carbone G.M., Cavalli A., Pagano F., Ragazzi E., Prayer-Galetti T., Alimonti A. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (n= 4532) Ann. Oncol. 2020;31(8):1040–1045. doi: 10.1016/j.annonc.2020.04.479. AugEpub 2020 May 6. PMID: 32387456; PMCID: PMC7202813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Straub R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007;28(5):521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 33.Hong S., Chang J., Jeong K., Lee W. Raloxifene as a treatment option for viral infections. J. Microbiol. 2021;59(2):124–131. doi: 10.1007/s12275-021-0617-7. FebEpub 2021 Feb 1. PMID: 33527314; PMCID: PMC7849956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffith G.J., Morris T.T., Tudball M.J., et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat. Commun. 2020;11:5749. doi: 10.1038/s41467-020-19478-2. PMID: 33184277; PMCID: PMC7665028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.