Abstract
Background
Antibody detection of SARS-CoV-2 requires an understanding of its variation, course, and duration.
Methods
Antibody response to SARS-CoV-2 was evaluated over 5–430 days on 828 samples across COVID-19 severity levels, for total antibody (TAb), IgG, IgA, IgM, neutralizing antibody (NAb), antibody avidity, and for receptor-binding-domain (RBD), spike (S), or nucleoprotein (N). Specificity was determined on 676 pre-pandemic samples.
Results
Sensitivity at 30–60 days post symptom onset (pso) for TAb-S/RBD, TAb-N, IgG-S, IgG-N, IgA-S, IgM-RBD, and NAb was 96.6%, 99.5%, 89.7%, 94.3%, 80.9%, 76.9% and 92.8%, respectively. Follow-up 430 days pso revealed: TAb-S/RBD increased slightly (100.0%); TAb-N decreased slightly (97.1%); IgG-S and IgA-S decreased moderately (81.4%, 65.7%); NAb remained positive (94.3%), slightly decreasing in activity after 300 days; there was correlation with IgG-S (Rs = 0.88) and IgA-S (Rs = 0.71); IgG-N decreased significantly from day 120 (15.7%); IgM-RBD dropped after 30–60 days (22.9%). High antibody avidity developed against S/RBD steadily with time in 94.3% of patients after 430 days. This correlated with persistent antibody detection depending on antibody-binding efficiency of the test design. Severe COVID-19 correlated with earlier and higher antibody response, mild COVID-19 was heterogeneous with a wide range of antibody reactivities. Specificity of the tests was ≥99%, except for IgA (96%).
Conclusion
Sensitivity of anti-SARS-CoV-2 assays was determined by test design, target antigen, antibody avidity, and COVID-19 severity. Sustained antibody detection was mainly determined by avidity progression for RBD and S. Testing by TAb and for S/RBD provided the highest sensitivity and longest detection duration of 14 months so far.
Keywords: Persistence SARS-CoV-2 antibodies, Antibody avidity, Sensitivity, Specificity, COVID-19, neutralization
Abbreviations
- Ab
antibody
- AU
arbitrary units
- COVID-19
coronavirus disease 2019
- ELISA
enzyme-linked immunosorbent assay
- CLIA
chemiluminescence immunoassay
- ECLIA
electrochemiluminescence immunoassay
- IgA/IgG/IgM/
immunoglobulin A, G, M
- NAb
neutralizing antibody
- NAT
nucleic acid amplification test
- N
nucleocapsid protein
- pso
post symptom onset
- RBD
receptor-binding-domain
- S
spike protein
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- s/co
sample to cutoff ratio
- sVNT
surrogate virus neutralization test
- TAb
total antibody.
1. Introduction
SARS-CoV-2 infection was declared a pandemic by WHO on March 11th, 2020 [1]. SARS-CoV-2 is a novel human coronavirus that can cause severe respiratory illness. Primary diagnosis is performed in the first 1–2 weeks after the onset of symptoms by direct detection of SARS-CoV-2 by nucleic acid amplification test (NAT) or antigen testing from respiratory secretions of nasal or throat swabs [2, 3]. Specific antibodies to SARS-CoV-2 develop relatively rapidly, with most patients becoming seropositive within 15–21 days [2] of infection, while viral load decreases and patients eventually become virus-negative. Antibody testing can therefore aid diagnosis in the acute phase adjunct to PCR or antigen testing [4], and identify previous SARS-CoV-2 infection. Antibody detection may thus be useful to assess antibody response after infection or vaccination, for serosurveillance studies, and to distinguish vaccine-induced seropositivity from natural infection [4, 5]. However, interpretation of SARS-CoV-2 antibody responses remains challenging because of considerable heterogeneity among individuals [6], and because the results of SARS-CoV-2 antibody assays may vary widely [7]. In addition, it is still unclear how binding antibodies relate to neutralizing antibodies [6, 8, 9]. Besides, there remains the crucial question how long an antibody response persists.
The aim of this study was to characterize the course and duration of antibody responses over more than one year in symptomatic patients covering the range of different COVID-19 symptom severity levels and by using 12 anti-SARS-CoV-2 tests encompassing various test designs, detected antibody types, target antigens, and by evaluating test sensitivity, antibody titers, neutralizing activity and antibody avidity.
2. Material and methods
Twelve anti-SARS-CoV-2 tests were used as described in Table 1 . The tests cover a range of antibody (Ab) type detections (total-Ab (TAb), IgG, IgA, IgM), SARS-CoV-2 target antigens (nucleocapsid protein (N), receptor binding domain (RBD), spike protein (S)), test principles (sandwich, indirect, competitive), result interpretation (qualitative, quantitative), detection of neutralizing antibodies and determination of antibody avidity.
Table 1.
Sensitivity of SARS-CoV-2 antibody tests over time (30–430 days pso).
| Manufacturer | Wantai | Siemens | Roche | Roche | Euroimmun | Diasorin | Euroimmun | Abbott | Euroimmun | Wantai | Genscript | Mikrogen GmbH | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test name | Wantai SARS-CoV-2 Ab ELISA | Advia Centaur COV2T | Elecsys Anti-SARS-CoV-2 S | Elecsys Anti-SARS-CoV-2 | Anti-SARS-CoV-2 ELISA (IgG) | Liaison SARS-CoV-2 S1/S2 IgG | Anti-SARS-CoV-2-NCP-ELISA (IgG) | Architect SARS-CoV-2 IgG | Anti-SARS-CoV-2 ELISA (IgA) | Wantai SARS-CoV-2 IgM ELISA | SARS-CoV-2 sVNT | recomLine SARS-CoV-2 IgG [Avidity] | |||
| Test format | ELISA | CLIA | ECLIA | ECLIA | ELISA | CLIA | ELISA | CLIA | ELISA | ELISA | ELISA | Immunoblot + avidity | |||
| Test design | Sw | Sw | Sw | Sw | indirect | indirect | indirect | indirect | indirect | indirect | competitive | indirect + Ab elution | |||
| Antibody-type detected | TAb | TAb | TAb | TAb | IgG | IgG | IgG | IgG | IgA | IgM | NAb | IgG | |||
| Target antigen | RBD | RBD | RBD | N | S1 | S1/S2 | N | N | S1 | RBD | RBD | S1 | RBD | N | |
| Test interpretation | qual | qual | quant | qual | qual | quant | qual | qual | qual | qual | qual | supplemental | |||
| Test cutoff (≥) | 1 | 1 | 0.8 | 1 | 1.1 | 15 | 1.1 | 1.4 | 1.1 | 1 | 20% | 60% | |||
| Days pso | N samples | Sensitivity (%) of the tests for mild COVID-19 (severity scores 1-3) | |||||||||||||
| 30 | 25 | 88.0 | 76.0 | 84.0 | 72.0 | 76.0 | 68.0 | 68.0 | 76.0 | 64.0 | 64.0 | 84.0 | 0.0 | 0.0 | 0.0 |
| 60 | 166 | 97.6 | 91.6 | 98.8 | 99.4 | 89.2 | 86.7 | 91.6 | 95.2 | 77.7 | 71.7 | 91.6 | 4.2 | 3.0 | 0.6 |
| 120 | 118 | 100 | 95.8 | 100 | 100 | 87.3 | 90.7 | 84.7 | 85.6 | 67.8 | 57.6 | 92.4 | 13.6 | 14.4 | 3.4 |
| 180 | 144 | 98.6 | 97.2 | 100 | 98.6 | 78.5 | 82.6 | 62.5 | 57.6 | 66.0 | 36.1 | 84.0 | 39.6 | 43.1 | 10.4 |
| 240 | 86 | 100 | 94.2 | 100 | 97.7 | 76.7 | 87.2 | 47.7 | 38.4 | 72.1 | 30.2 | 94.2 | 65.1 | 66.3 | 8.1 |
| 300 | 84 | 98.8 | 96.4 | 98.8 | 96.4 | 78.6 | 86.9 | 33.3 | 23.8 | 73.8 | 28.6 | 96.4 | 73.8 | 71.4 | 10.7 |
| 365 | 87 | 100 | 96.6 | 100 | 92.0 | 71.3 | 78.2 | 23.0 | 17.2 | 66.7 | 24.1 | 89.7 | 75.9 | 74.7 | 18.4 |
| 430 | 31 | 100 | 100 | 100 | 96.8 | 74.2 | 83.9 | 16.1 | 12.9 | 61.3 | 16.1 | 93.5 | 87.1 | 93.5 | 25.8 |
| Sensitivity (%) of the tests for severe COVID-19 (severity scores 4-7) | |||||||||||||||
| 30 | 14 | 100 | 100 | 100 | 100 | 100 | 100 | 100* | 100* | 100* | 100 | 100 | 35.7* | 14.3* | 0.0* |
| 60 | 28 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 90.5* | 100 | 47.6 | 40.5 | 4.8 |
| 120 | 5 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 81.8 | 100 | 60.6 | 60.6 | 12.1 |
| 180 | 18 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 95.7 | 60.9 | 100 | 87.0 | 87.0 | 21.7 |
| 240 | 9 | 100 | 100 | 100 | 100 | 100 | 100 | 96.3 | 96.3 | 100 | 63.0 | 100 | 85.2 | 85.2 | 25.9 |
| 300 | 5 | 100 | 100 | 100 | 100 | 100 | 100 | 78.6 | 71.4 | 100 | 50.0 | 100 | 92.9 | 92.9 | 50.0 |
| 365 | 4 | 100 | 100 | 100 | 100 | 100 | 100 | 77.8 | 66.7 | 100 | 55.6 | 100 | 100 | 100 | 44.4 |
| 430 | 4 | 100 | 100 | 100 | 100 | 100 | 100 | 81.3 | 81.3 | 100 | 65.4 | 100 | 100 | 100 | 12.5 |
| Sensitivity (%) overall | |||||||||||||||
| 30 | 39 | 92.3 | 84.6 | 89.7 | 82.1 | 84.6 | 79.5 | 79.5 | 84.6 | 76.9 | 76.9 | 89.7 | 12.8 | 5.1 | 0.0 |
| 60 | 194 | 97.9 | 92.8 | 99.0 | 99.5 | 90.7 | 88.7 | 92.8 | 95.9 | 80.9 | 73.7 | 92.8 | 11.3 | 10.3 | 1.5 |
| 120 | 123 | 100 | 95.9 | 100 | 100 | 87.8 | 91.1 | 85.4 | 86.2 | 69.1 | 57.7 | 92.7 | 17.1 | 17.9 | 4.9 |
| 180 | 162 | 98.8 | 97.5 | 100 | 98.8 | 80.9 | 84.6 | 66.7 | 62.3 | 69.1 | 38.9 | 85.8 | 44.4 | 47.5 | 11.1 |
| 240 | 95 | 100 | 94.7 | 100 | 97.9 | 78.9 | 88.4 | 51.6 | 43.2 | 74.7 | 33.7 | 94.7 | 67.4 | 68.4 | 11.6 |
| 300 | 89 | 98.9 | 96.6 | 98.9 | 96.6 | 79.8 | 87.6 | 34.8 | 24.7 | 75.3 | 28.1 | 96.6 | 75.3 | 73.0 | 13.5 |
| 365 | 91 | 100 | 96.7 | 100 | 92.3 | 72.5 | 79.1 | 25.3 | 19.8 | 68.1 | 27.5 | 90.1 | 76.9 | 75.8 | 18.7 |
| 430 | 35 | 100 | 100 | 100 | 97.1 | 77.1 | 85.7 | 17.1 | 14.3 | 65.7 | 22.9 | 94.3 | 88.6 | 94.3 | 22.9 |
Samples were collected post symptom onset (pso) for a period of up to 430 days. All individuals tested positive for SARS-CoV-2-NAT and were symptomatic for COVID-19. Patients reported being unvaccinated or samples were collected prior to vaccine availability (12/21/2020). In total 828 samples from 390 patients from three sites were analyzed:
The infectious diseases’ outpatient department of the University of Frankfurt provided 752 serum samples from 365 patients. Patient samples had been prospectively collected since May 11th, 2020, from day 5 − 430 days (median 147 days) pso, and retrospectively categorized at sample time by reviewing individuals’ worst prior disease state, using symptom severity scores of 0–8 according to the contemporary WHO ordinal scale classification [10]. The study was approved by the Frankfurt University Ethics Committee (Votes-No. 11/17 & 20–748), and patients gave written informed consent to be admitted.
Forty-five samples were from Erlangen and Nürnberg (Germany) from two family clusters with total 14 COVID-19 patients. Serum collection was eight days pso for one patient and 42, 171, 311, 407 days pso in the other patients. Thirty-one samples of 11 patients were from the medical service of the Paul-Ehrlich-Institute collected 5 − 392 days pso. Patients of these latter two populations had mild COVID-19 symptoms (scores 1–2 [10]). Ethics committee approval was obtained from the Hessische Landesärztekammer (60314 Frankfurt am Main, Germany, ballot no. 2020–1664-evBO), including written informed consent from patients participating in the study.
Testing was carried out according to manufacturers' instructions at the IVD Testing Laboratory at the Paul-Ehrlich-Institute. Sensitivity of anti-SARS-CoV-2 detection was calculated by standard formula, relative to the reported positive first diagnosis of SARS-CoV-2 infection by NAT. Antibody quantification was performed with the Liaison SARS-CoV-2 S1/S2 IgG test (Diasorin SpA, 13040 Saluggia, Italy), calibrated in arbitrary units (AU/mL) and a measuring range of 15–400 AU/ml. The surrogate virus neutralization test (sVNT), (Genscript USA Inc., NJ 08854) detecting ACE-2 blocking Ab was used to refer to neutralizing antibodies (NAb) based on its correlation to virus neutralization test [11]. An inhibition rate of ≥20% was the cutoff for positive neutralization. All samples were subjected to avidity testing by recomLine SARS-CoV-2 avidity reagent (Mikrogen GmbH, 82061 Neuried, Germany) and quantified using BLOTrix Reader BTR48 and recomScan 3.4 test strip analysis software (Mikrogen GmbH). High avidity was defined as antibody binding capacity of ≥60%. Correlation between antibody quantification, avidity, neutralization and COVID-19 symptom severity were calculated using Spearman rank analysis [12]. Relative antibody binding efficiency (affinity) between different tests was determined by endpoint titration: samples were serially diluted (4-fold) up to the intercept with the assay's cutoff. The titer was calculated by linear interpolation, with a higher titer representing a higher relative affinity of the respective test. Specificity of the tests was determined by standard formula and 95% confidence intervals (Clopper-Pearson for binomial distributed data), using pre-pandemic samples 576 plasma blood donation retention samples of 2014 obtained from the Red Cross Blood Donor Service NSTOB (Springe, Germany) and 100 serum samples from US blood donation units purchased from Trina Bioreactives (Nänikon, Switzerland) in 2011.
3. Results
3.1. Presentation of cases and correlation with COVID-19 symptom severity
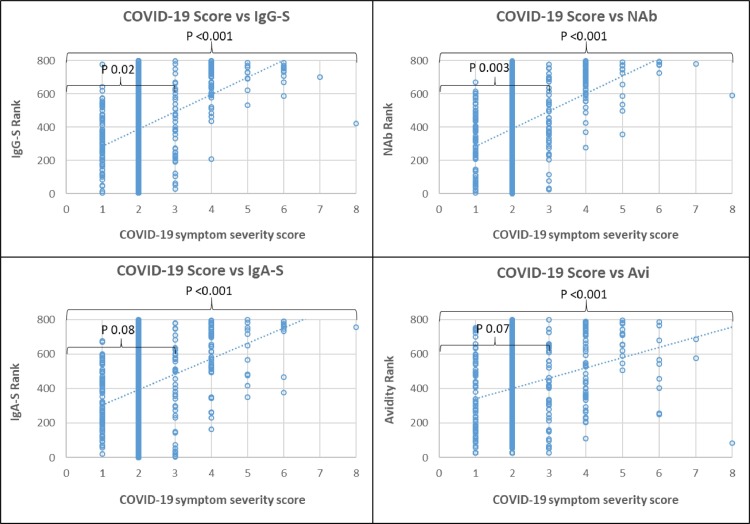
The majority of subjects (84.2%) had mild ambulatory-treated COVID-19 symptoms (scores 1–2), 5.3% moderate hospitalized without respiratory therapy (score 3), 7.0% moderate hospitalized and respiratory therapy (score 4), the remaining 3.5% had severe COVID-19 (score > 5) requiring ventilation and intensive treatment. Symptom severity correlated with antibody reactivity (Fig. 1 ). Severe COVID-19 symptom levels (>3) showed higher reactivity in all antibody tests compared to mild COVID-19 symptoms (p < 0.001). However, within mild COVID-19 (scores 1–3), there were large differences in antibody reactivity among individual patients and lower significance with COVID-19 severity (p 0.02 – 0.08). Therefore, COVID-19 severity was grouped into scores 1–3 for mild COVID-19 and scores 4–8 for severe COVID-19, and compared as shown in Table 1 and Fig. 2 .
Fig. 1.
Spearman rank correlation of COVID-19 severity scores with SARS-CoV-2 antibody response. Footnotes: Spearman rank correlation of COVID-19 severity (x) with (y) IgG-S titer, neutralizing antibodies (NAb), IgA-S titer and antibody avidity (Avi). P value for significance of correlation: for all COVID-19 severity scores 0–8 = large bracket; within severity scores 1–3 small bracket.
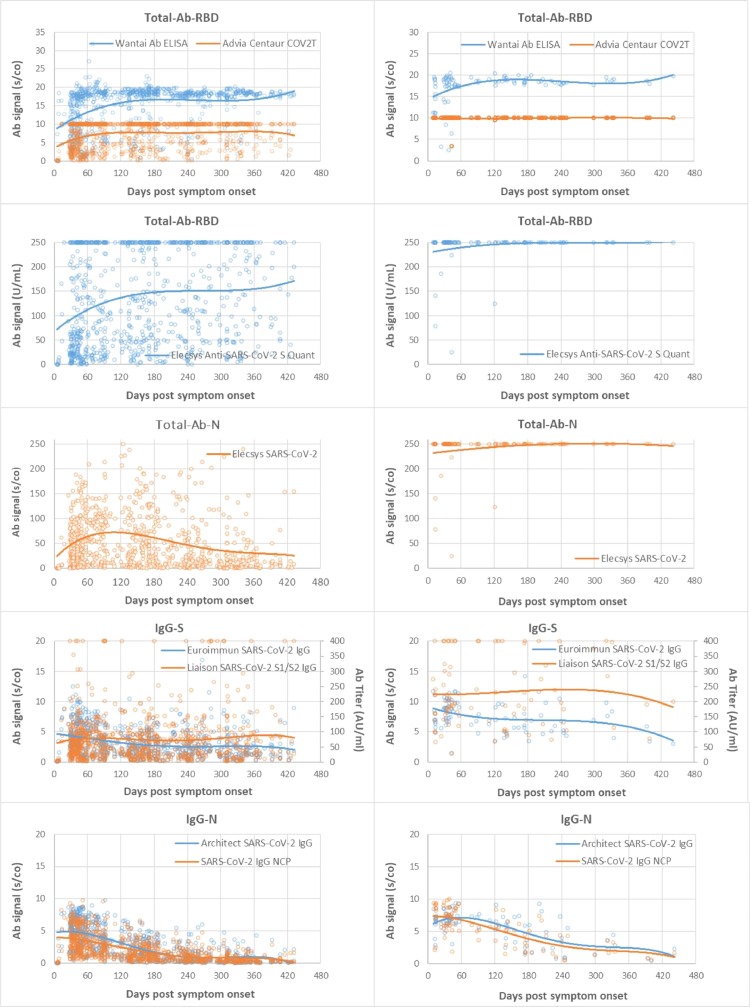
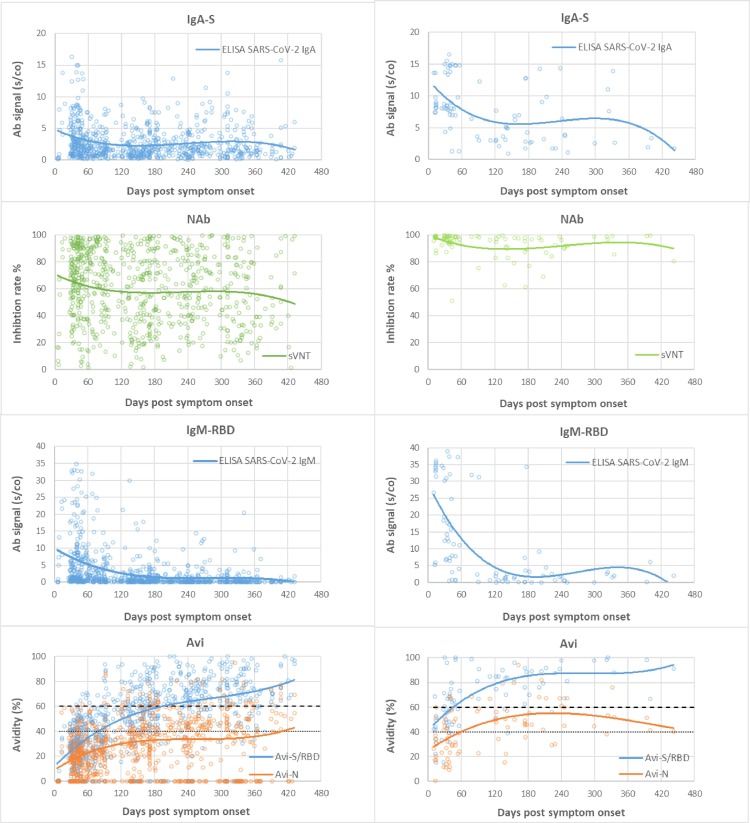
Fig. 2.
Time course and duration of SARS-CoV-2 antibody detection. A: Mild COVID-19, B: Severe COVID-19. Footnotes: Days post symptom onset (pso) of patient sample (x) plotted against test signal (s/co = sample to cutoff ratio) of the respective tests (y), and (y2) AU/ml in IgG-S for the Liaison test. Group (A) samples of patients with mild COVID-19 and (B) severe COVID-19. Trend lines are polynomial.
3.2. Sensitivity and titers of SARS-CoV-2 antibody tests over time
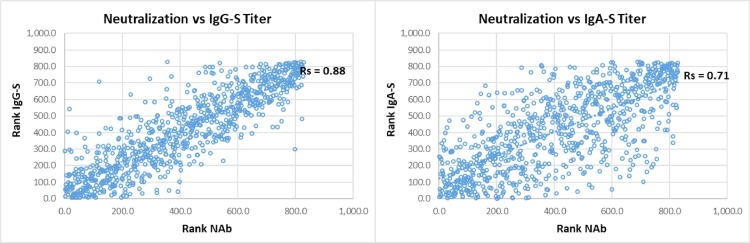
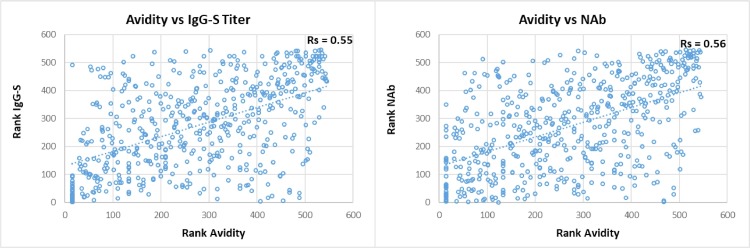
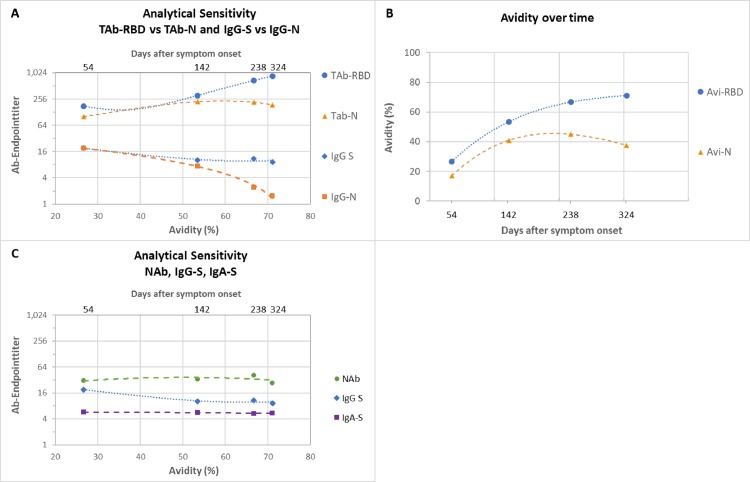
Table 1 summarizes the sensitivity for the different tests over time, grouped into mild and severe COVID-19. Fig. 2 depicts all patient data points for the different antibody tests and the respective time course as trend line. Severe COVID-19 showed a homogeneous picture with high sensitivity and stable antibody titers in almost all tests. Exceptions were IgG-N, which dropped to 66.7–81.3% sensitivity after 240 days pso, and IgM, with an initial drop in sensitivity after 120 days pso, then remaining at 50–65.4% until 430 days pso. In comparison, mild COVID-19 showed later antibody development, 2–3-fold lower titers than in severe COVID-19 and shorter duration of detection. The time course in sensitivity of the tests from 30 to 60 to 430 days pso showed the following pattern. (i) TAb-RBD showed the highest sensitivity of 96.6% to 100% (mean of three TAb-tests) constant over time with stable or slightly increasing antibody titers until day 430 pso. (ii) IgG-S showed 88% to 82.7% sensitivity (mean of two IgG-S-tests) over 300 days pso, then decreasing moderately to 79% by 430 days pso. (iii) IgA showed a similar course as IgG at lower baseline sensitivity of 77.7% by day 60 – 61.3% at 430 days pso. (iv) NAb showed consistently high sensitivity of 91.6% from 60 days pso to 93.5% at 430 days pso, with NAb activity decreasing after 300 days pso, comparable to IgG-S and IgA-S. There was strong correlation between NAb and quantitative IgG-S (Rs = 0.88) and with IgA-S (Rs = 0.71) (Fig. 3 ). (v) IgG-N showed high initial sensitivity of 93.4% (mean of two IgG-N tests) up to 120 days pso and then dropped rapidly to 14.5% by 430 days pso. (vi) TAb-N sensitivity was 99–100% until day 120 pso and decreased only slightly to 96.8% by day 430 pso. TAb-N titers also decreased after 120 days of pso slowly and steadily, with a similar trend as IgG-N. (vii) IgM-RBD dropped rapidly after 30–60 days to low sensitivity of 16.1% at 430 days pso but showing a wider range between individual patients including persistent IgM. (viii) Antibody avidity increased with time, primarily for RBD and S. In severe COVID-19, high avidity (>60%) developed rapidly within 30–60 days pso, whereas in mild COVID-19 avidity progressed slowly reaching high avidity (>60%) on average after 120 days pso. Most patients (93.5%) had high avidity for RBD at 430 days pso. There was significant correlation (p < 0.001) of avidity with IgG-S and NAb (Rs 0.55, 0.56), adjusted for time lag until maturation to high avidity (Fig. 4 ). Increasing avidity suggests enhancement of antibody affinity in the tests, as verified in an antibody titration experiment (Fig. 5 ). Twelve patients of varying COVID-19 severity (ten score 1–3, two score 4), each with four follow-up samples (mean 54, 142, 238, and 324 days pso) and mean avidity for RBD of 26.7, 53.5, 66.7, and 71.1%, respectively, were plotted against the endpoint titers of each sample at the cutoff of the respective assay. TAb-RBD titers (176, 310, 680, and 858) increased (Fig. 5 A) with the increasing avidity to RBD/S (Fig. 5 B), while titers for IgG-S and IgA-S were overall lower (IgG-S: 19, 10, 11, 9; IgA-S: 6, 6, 5, 5) and slightly decreasing (Fig. 5 C). In comparison, TAb-N and IgG-N titers decreased 5- to 6-fold relative to TAb-RBD and IgG-S tests, consistent with decreasing avidity to N. Fig. 5 A also shows that despite equal rates of decline in N-based assays versus S, absolute TAb-N titers were nevertheless 5–120-fold higher than IgG-N. NAb showed a similar titer profile as IgG-S and IgA (Fig. 5 C), but with baseline 2–3 and 5-fold higher titers than IgG-S and IgA-S, respectively.
Fig. 3.
Correlation of neutralizing antibodies vs quantitative IgG-S and vs IgA antibody titers. Footnote: Spearman rank correlation (Rs): neutralizing antibodies (NAb) (x) plotted against quantitative IgG-S titer and IgA titer (y).
Fig. 4.
Correlation of avidity vs quantitative IgG-S titers and vs neutralizing antibodies. Footnote: Spearman rank correlation (Rs): Avidity (x) plotted against quantitative IgG-S titer and NAb activity (y).
Fig. 5.
Endpoint titers in different SARS-CoV-2 antibody test designs depending on antibody avidity and time. Footnotes: Fig. 5A: Endpoint titers (y) of TAb-RBD vs TAb-N and IgG-S vs IgG-N against avidity (x1) and days after symptom onset (x2) of the neat samples. Shown as polynomial trend line order 2. Fig. 5B: Avidity for RBD and N versus time used in Fig. 5A and Fig. 5B. Fig. 5C: Analogous to (A) endpoint titers for NAb, IgG-S and IgA-S. Data table 1 to Fig. 5.
3.3. Test specificity
Specificity (Table 2 ) of the tests was 99.0–100% for IgG, TAb and NAb tests, 98.8% for IgM and 96% for IgA-S, independent of the target antigen, and no detectable cross-reactivity with human endemic coronaviruses by immunoblot (data not shown).
Data table 1.
to Fig 5.
| Avi- | TAb- | TAb-RBD / | IgG-S / | TAb-N / | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days | RBD | Avi-N | RBD | TAb-N | IgG-S | IgG-N | IgA-S | NAb | IgG-S | TAb-N | IgA-S | NAb | IgG-N | IgG-N |
| pso | % | % | Endpoint titera | Ratiob | ||||||||||
| 54 | 26.7 | 17.0 | 176 | 101 | 19 | 19 | 6 | 31 | 9 | 2 | 31 | 6 | 1 | 5 |
| 142 | 53.5 | 40.9 | 310 | 221 | 10 | 7 | 6 | 34 | 30 | 1 | 55 | 9 | 1 | 30 |
| 238 | 66.7 | 45.0 | 680 | 214 | 11 | 2 | 5 | 41 | 63 | 3 | 128 | 17 | 5 | 89 |
| 324 | 71.1 | 37.6 | 858 | 187 | 9 | 2 | 5 | 27 | 93 | 5 | 158 | 32 | 6 | 120 |
Reciprocal value of the highest dilution with a positive result at the assay cutoff.
Quotient endpoint titers of TAb-RBD, IgG-S or TAb-N with the respective test below.
Table 2.
Specificity of SARS-CoV-2 antibody tests.
| Manufacturer | Wantai | Siemens | Roche | Roche | Euroimmun | Diasorin | Euroimmun | Abbott | Euroimmun | Wantai | Genscript |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test name | SARS-CoV-2 Ab ELISA | Advia Centaur COV2T | Elecsys Anti-SARS-CoV-2 S | Elecsys Anti-SARS-CoV-2 | SARS-CoV-2 IgG | Liaison SARS-CoV-2 S1/S2 IgG | SARS-CoV-2 IgG NCP | Architect SARS-CoV-2 IgG | SARS-CoV-2 IgA | SARS-CoV-2 IgM ELISA | SARS-CoV-2 sVNT |
| Antibody type | TAb | TAb | TAb | TAb | IgG | IgG | IgG | IgG | IgA | IgM | NAb |
| Target antigen | RBD | RBD | RBD | N | S1 | S1/S2 | N | N | S | RBD | RBD |
| Test design | Sw | Sw | Sw | Sw | indirect | indirect | indirect | indirect | indirect | indirect | competitive |
| Test cutoff (<) | 1 | 1 | 0.8 | 1 | 1.1 | 15 | 1.1 | 1.4 | 1.1 | 1 | 20% |
| N total negative 1) | 676 | 676 | 676 | 676 | 676 | 676 | 676 | 676 | 676 | 676 | 100 |
| N test negative | 671 | 671 | 676 | 676 | 672 | 671 | 671 | 672 | 624 | 669 | 100 |
| N false-positive | 5 | 5 | 0 | 0 | 4 | 5 | 5 | 4 | 27 | 8 | 0 |
| Specificity (%) | 99.3 | 99.3 | 100 | 100 | 99.4 | 99.3 | 99.3 | 99.4 | 96.0 | 98.8 | 100 |
| 95% confidence interval | 98.3–99.8 | 98.3–99.8 | 99.5–100 | 99.5–100 | 98.5–99.8 | 98.3–99.8 | 98.3–99.8 | 98.5–99.8 | 90.0–94.2 | 97.7–99.5 | 96.4–100 |
4. Discussion
The variation, time course, and duration of antibody responses to SARS-CoV-2 over 14 months after the onset of the pandemic were examined. Criteria were test sensitivity, antibody titers of the tests over time in relation to the severity of COVID-19 symptoms, target antigens of the tests, types of antibodies detected, association with NAb and antibody avidity. Symptom distribution of COVID-19 patients was representative for the overall distribution with mild symptomatic COVID-19 representing the majority of cases [13, 14]. COVID-19 severity is known to correlate with antibody levels and persistence [15], [16], [17], [18]. In this study, the impact of COVID-19 symptom severity was differentiated between two groups, which were not limited to outpatient/hospitalized treatment but also by need for respiratory therapy, i.e. scores 1–3 versus scores 4–7 (requiring oxygen therapy or ventilation) [19, 20]. The picture within the mild COVID-19 group was heterogeneous with a wide range of antibody reactivities partly in overlap with severe COVID-19, as previously reported [18, 21], and a corresponding variability between the different tests. Nevertheless, the results demonstrate a distinct test-specific pattern over time, allowing differences between tests to be classified. In summary, sensitivity and duration of antibody detection was dependent on test design and progression to high avidity. The sandwich design of TAb assays, and RBD/S-based assays, resulted in higher sensitivity over time based on increasing avidity for S/RBD. N-based assays were less sensitive than the corresponding S-based assays consistent with decreasing avidity for N. (i) TAb-S/RBD tests thus showed the highest and longest-lasting sensitivity, with stable antibody titers throughout the 14-month observation period and no predictable detection endpoint. (ii) The sensitivities of IgG-S/RBD and IgA-S were stable until 300 days pso followed by moderate decrease of antibody titers and in sensitivity of about 10–16% until 430 days pso. (iii) N-based assays showed an early decline in antibody titers starting from day 120 pso. Although sensitivity (test-positivity) of TAb-N decreased only slightly by day 430 pso, due to its sandwich design, declining antibody titers suggest a further decrease in sensitivity. For IgG-N, the decline was much faster after 120 days pso with short-lived sensitivity becoming insignificant after 240–300 days pso. (iv) IgM-RBD sensitivity was essentially confined to the early phase (30–60 days pso), then declined rapidly, although not consistently, since some individuals showed persistent IgM. Therefore, IgM does not appear to be suitable for accurate timing of infection. (v) NAb, targeting RBD, showed consistently high or slightly increased base sensitivity (>20% inhibition) of 90–94% during 430 days pso, indicating more sustained duration of neutralization than published [18, 21]. Neutralization potency correlated strongly over time with quantitative IgG-S titers (Rs 0.88) [6, 18, 23] and with IgA-S (Rs 0.71) involved in mucosal immunity [22]. (vi) Antibody avidity developed mainly for RBD and S and increased slowly over time, from about 120 days pso to high avidity (>60%), remaining stable or increasing further up to 430 days pso. Most patients attained high avidity, 76% at one year and 94% after 430 days pso. Considering the time lag of avidity maturation, there was positive correlation with IgG-S antibody titers and neutralizing activity (Rs 0.55, 0.56). In contrast to the immunogenic RBD and S, [24], avidity against N was low, with 20–26% of patients showing high avidity.
Increasing avidity reflects affinity maturation of antibodies, mediation of cross-reactivity, and formation of long-lived plasma cells that can secrete antibodies in absence of antigen [9, [24], [25], [26], [27], [28], [29]]. Mature antibodies formed after acute infection may have up to 100-fold higher affinity [30, 31]. On the other hand, the test design determines the extent to which a test benefits from higher affinity. This was confirmed by evaluation of the endpoint titers, reflecting the relative affinity and concentration of antibody in the sample. The sandwich test design of TAb-RBD assays exploited high avidity most effectively with 5-fold higher titers at high versus low avidity, and 9- to 93-fold or 31- to 158-fold higher titers than the corresponding IgG-S or IgA-S test in the indirect assay format. In contrast, N-based TAb and IgG endpoint titers decreased 5- to 6-fold over time relative to S-based assays, in line with decreasing avidity to N. Nevertheless, TAb-N due to its sandwich design [32], provided 5–120-fold higher baseline titers than indirect IgG-N (as the ratio TAb-S/RBD to IgG-S), explaining its high test-positivity in clinical samples and making TAb-N suitable for long-term detection of anti-N. The sVNT assay for NAb, detecting TAb using a competitive test design, showed a base sensitivity between TAb sandwich and indirect IgG-S and IgA-S assays, with a time course similar to the latter.
Sensitivity of anti-SARS-CoV-2 tests in this study compared with high specificity of 99% or greater (except IgA) in 676 pre-pandemic blood donation samples, indicating reliable positive predictive value.
A limitation of the study is that no asymptomatic individuals were included. Nevertheless, the results of this study with the mildly symptomatic patients suggest a basically similar picture in asymptomatic individuals.
In conclusion, antibody detection showed a distinct pattern dependent on the antibody type detected, the target antigen, antibody avidity level, the test design, and severity of COVID-19. Duration of detection was mainly driven by avidity progression for S/RBD and its exploitation by the respective test design. TAb assays based on S/RBD showed high sensitivity and persistent antibody detection for more than 14 months with consistent antibody titers and no predictable end of detection so far.
5. Funding statement
The study was supported by the German Federal Ministry of Health.
Declaration of Competing Interest
The authors declare no conflict of interests.
Acknowledgements
We sincerely thank Prof. Franklin Kiesewetter and Dr. Maria Vogelsang for providing the samples from Erlangen and Nürnberg. We are also very grateful to Dr. Jork for providing the blood donation retention samples. We would like to thank the staff of the IVD Testing Laboratory at the Paul-Ehrlich-Institute for excellent technical assistance.
References
- 1.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wölfel R., Corman V.M., Guggemos W., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 3.Zheng S., Fan J., Yu F., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y.V., Wiencek J., Meng Q.H., et al. AACC practical recommendations for implementing and interpreting SARS-CoV-2 emergency use authorization and laboratory-developed test serologic testing in clinical laboratories. Clin. Chem. 2021;67:1188–1200. doi: 10.1093/clinchem/hvab051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deeks J.J., Dinnes J., Takwoingi Y., et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020;6 doi: 10.1002/14651858.CD013652. CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Post N., Eddy D., Huntley C., et al. Antibody response to SARS-CoV-2 infection in humans: a systematic review. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0244126. e0244126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitman J.D., Hiatt J., Mowery C.T., et al. Evaluation of SARS-CoV-2 serology assays reveals a range of test performance. Nat. Biotechnol. 2020;38:1174–1183. doi: 10.1038/s41587-020-0659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang A.T., Garcia-Carreras B., Hitchings M.D.T., et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat. Commun. 2020;11:4704. doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer G. The variability of the serological response to SARS-corona virus-2: potential resolution of ambiguity through determination of avidity (functional affinity) J. Med. Virol. 2021;93:311–322. doi: 10.1002/jmv.26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. COVID-19 therapeutic trial synopsis. (2020). Available at: https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf.
- 11.Tan C.W., Chia W.N., Qin X., et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat. Biotechnol. 2020;38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 12.Wessa P. Spearman rank correlation (v1.0.3) in free statistics software (v1.2.1). Office for Research Development and Education, 2017.
- 13.Schilling J., Lehfeld A.-.S., Schumacher D., et al. Disease severity of the first COVID-19 wave in Germany using reporting data from the national notification system. Robert Koch-Institut, 2021. [DOI] [PMC free article] [PubMed]
- 14.Oran D.P., Topol E.J. The proportion of SARS-CoV-2 infections that are asymptomatic: a systematic review. Ann. Intern. Med. 2021;174:655–662. doi: 10.7326/M20-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henss L., Scholz T., von Rhein C, et al. Analysis of humoral immune responses in patients with severe acute respiratory syndrome coronavirus 2 infection. J. Infect. Dis. 2021;223:56–61. doi: 10.1093/infdis/jiaa680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rijkers G., Murk J.-.L., Wintermans B., et al. Differences in antibody kinetics and functionality between severe and mild severe acute respiratory syndrome coronavirus 2 infections. J. Infect. Dis. 2020;222:1265–1269. doi: 10.1093/infdis/jiaa463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X., Pan Z., Yue S., et al. Disease severity dictates SARS-CoV-2-specific neutralizing antibody responses in COVID-19. Signal Transduct Target Ther. 2020;5:180. doi: 10.1038/s41392-020-00301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peluso M.J., Takahashi S., Hakim J., et al. SARS-CoV-2 antibody magnitude and detectability are driven by disease severity, timing, and assay. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abh3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He X., Cheng X., Feng X., Wan H., Chen S., Xiong M. Clinical symptom differences between mild and severe COVID-19 patients in China: a meta-analysis. Front. Public Health. 2020;8 doi: 10.3389/fpubh.2020.561264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 21.Chia W.N., Zhu F., Ong S.W.X., et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2:e240–e249. doi: 10.1016/S2666-5247(21)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterlin D., Mathian A., Miyara M., et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Infantino M., Manfredi M., Grossi V., et al. Closing the serological gap in the diagnostic testing for COVID-19: the value of anti-SARS-CoV-2 IgA antibodies. J. Med. Virol. 2021;93:1436–1442. doi: 10.1002/jmv.26422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephens D.S., McElrath M.J. COVID-19 and the path to immunity. JAMA. 2020;324:1279–1281. doi: 10.1001/jama.2020.16656. [DOI] [PubMed] [Google Scholar]
- 25.Turner J.S., Kim W., Kalaidina E., et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. 2021;595:421–425. doi: 10.1038/s41586-021-03647-4. [DOI] [PubMed] [Google Scholar]
- 26.Cohen K.W., Linderman S.L., Moodie Z., et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep. Med. 2021;2 doi: 10.1016/j.xcrm.2021.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H., Wu N.C., Yuan M., et al. Cross-neutralization of a SARS-CoV-2 antibody to a functionally conserved site is mediated by avidity. Immunity. 2020;53 doi: 10.1016/j.immuni.2020.10.023. 1272-1280.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siggins M.K., Thwaites R.S., Openshaw P.J.M. Durability of immunity to SARS-CoV-2 and other respiratory viruses. Trends Microbiol. 2021;29:648–662. doi: 10.1016/j.tim.2021.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dan J.M., Mateus J., Kato Y., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021:371. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foote J., Eisen H.N. Kinetic and affinity limits on antibodies produced during immune responses. Proc. Natl. Acad. Sci. U S A. 1995;92:1254–1256. doi: 10.1073/pnas.92.5.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manivel V., Sahoo N.C., Salunke D.M., Rao K.V. Maturation of an antibody response is governed by modulations in flexibility of the antigen-combining site. Immunity. 2000;13:611–620. doi: 10.1016/s1074-7613(00)00061-3. [DOI] [PubMed] [Google Scholar]
- 32.Porstmann T., Kiessig S.T. Enzyme immunoassay techniques an overview. J. Immunol. Methods. 1992;150:5–21. doi: 10.1016/0022-1759(92)90061-w. [DOI] [PubMed] [Google Scholar]