Abstract
Objective:
To explore the interaction between chronic bronchitis and blood cadmium on the prevalence of myocardial infarction.
Methods:
We used weighted US-NHANES data. Multivariate survey logistic regression was used to examine the associations between myocardial infarction, cadmium concentration and chronic bronchitis. Adjusted odds ratios, 95% confidence intervals were computed.
Results:
There was a significant interaction (OR=1.33, CI=[1.01, 1.74]) between chronic bronchitis and blood cadmium level on the presence of myocardial infarction. For 1 μg/L increase in cadmium level, people with chronic bronchitis had 1.65 (1.24*1.33) times the odds of having myocardial infarction, while those without chronic bronchitis would be only 1.24 times as likely having the outcome (OR=1.24, CI=[1.05, 1.46]).
Conclusion:
Findings highlights the role of chronic bronchitis on the relationship between blood cadmium concentration and myocardial infarction. Prospective cohort designs are needed to confirm these findings.
Keywords: myocardial infarction, blood, cadmium, chronic bronchitis, interaction, NHANES, cardiovascular disease, heavy metal
Introduction
There has been strong evidence that cadmium is a risk factor of cardiovascular disease (CVD), including myocardial infarction, stroke, peripheral arterial disease and heart failure [1:6]. As a ubiquitous heavy metal, cadmium is mainly distributed through smoking, diet and industrial emission. The relationship between cadmium and CVD persists even after the adjustment of smoking status and dose[6,7]. The mechanisms for this association remains uncharted, although some investigators allude to a possible role for increased risk of atherosclerosis and hypertension seen in persons with cadmium exposure [4,8,9]. And the association may be affected by gender [4,10].
Cadmium can also lead to chronic bronchitis, one of two common forms of chronic obstructive pulmonary disease (COPD). Chronic bronchitis is commonly defined as “chronic cough and sputum production for at least 3 months per year for two consecutive years” [11]. Elevated blood or urinary cadmium level is associated with reduced pulmonary function [12:15]. A study indicated that there was increased mortality rate of bronchitis or emphysema among people with high level of cadmium exposure [16] while another study showed that the risk of bronchitis was significantly associated with the intensity of cadmium exposure but the mortality rate was not [17].
It is believed that patients with COPD are more likely to have CVD due to common risk factors such as smoking [18:20]. And airflow limitation can serve as an independent risk factor for CVD [21:23]. Chronic bronchitis is also associated with increased mortality from cardiovascular diseases [24,25]. Patients with chronic bronchitis has significantly higher plasma fibrinogen level, which is a signal for higher coronary heart disease risk, after adjustment of major CVD risk factors [26,27].
We are interested in the role of chronic bronchitis on the relationship between cadmium and myocardial infarction. We hypothesized that there is an interaction between chronic bronchitis and blood cadmium level on the history of myocardial infarction.
Method
This study used the National Health and Nutrition Examination Survey (NHANES) data from 2005 to 2016. NHANES is a national cross-sectional survey of the US population which is assessing health and nutritional status of USA population since 1980s. NHANES demographic data, Medical Conditions data, containing history of myocardial infarction and chronic bronchitis, and blood metal data set containing cadmium level information were used. There were a total of 58,660 participants in the data set. A total of 33,924 participants who were both interviewed and examined in the Mobile Examination Center and were older than 18 years when taking the survey were included in our study. There were 1,053 missing data for history of myocardial infarction and 1,072 missing data for chronic bronchitis.
Measurement
Measurement of diseases
The outcome of interest was the history of myocardial infarction and was determined with the survey question “Has a doctor or other health professional ever told you that you had a heart attack (also called myocardial infarction)?” from the Medical Conditions data set. And the mediator chronic bronchitis was determined using the “Has a doctor or other health professional ever told you that you had chronic bronchitis?” question from the same data set.
The variable history of hypertension, weak kidney function and diabetes from the Medical Condition dataset were selected as co-variates because these are important risk factors for myocardial infarction. These variables were self-reported in the NHANES survey with the question “Have you ever been told by a doctor or other health professional that you had hypertension/weak or failing kidneys/diabetes?”
Measurement of blood Cadmium level
Blood cadmium level (μg/L) was obtained from the blood metal data set called “Blood Lead, Cadmium, Total Mercury, Selenium and Manganese”. It was measured in whole blood specimens using mass spectrometry with dilution sample preparation step at the National Center for Environmental Health (NCEH). Quality assurance and quality control (QA/QC) protocols were developed and laboratory team performances were monitored.
Measurement of other covariates
Co-variates including age, gender, race, body mass index (BMI), education, household income, physical activity and marital status were taken from the demographic data set. Cut-offs were selected based on previous study findings. Physical activity was measured with MET score. Less than 500 MET-minutes activity per week was coded as insufficient physical activity. Smoking status was obtained from Cigarette Use dataset with question “Smoked at least 100 cigarettes in life?” and “Do you now smoke cigarettes?”, and was recoded as never-smoker, ex-smoker, occasional smoker and everyday smoker. Alcohol use was defined as moderate, medium, and heavy consumption according to the Dietary Guidelines for Americans 2015–2020 by the U.S. Department of Health and Human Services and U.S. Department of Agriculture.
Analysis
All the analysis were conducted with SAS statistical software, version 9.4. Chi-square test was used to test the association between the history of myocardial infarction and categorical covariates. T-test was used for testing equality of means in continuous variables between groups with myocardial infarction and without. Multivariate logistic analysis was performed to examine if there were significant interactions between blood cadmium level and chronic bronchitis on the history of myocardial infarction. Survey logistic procedure was used to control for the complex, multistage survey design. In the process, we used weight variable “WTMEC2YR”, strata variable “SDMVSTRA” and cluster variable “SDMVPSU”, from the “Demographics Dataset” in NHANES. These three variables were included in the multivariate models using survey logistic procedure. Adjusted Odds Ratio, 95% Confidence Intervals and p-values were obtained from the multiple logistic regression analysis
Results
Among the 33,924 adult participants, 1,388 (4.22%) had myocardial infarction. And 1,842 (5.61%) had chronic bronchitis. The prevalence of myocardial infarction among patient of chronic bronchitis was 10.18%, compared to 3.86% among adults without chronic bronchitis.
Table 1 demonstrates the distribution of demographic characteristics comparing the self-reported myocardial infarction and no myocardial infarction groups. Higher incidence of myocardial infarction was seen in groups older than 60 years, men, non-Hispanic whites, and in person with BMI>30, less than high school education, income<$20,000, insufficient physical activity and comorbidities like hypertension, diabetes and weak kidney function. Smokers (including ex-smokers and current smokers) and heavy drinkers are more likely to have myocardial infarction.
Table 1:
Baseline characteristics from NHANES 2005–2016, of adults more than 18 year old
| Characterstics | Categories | Myocardial infarction | ||
|---|---|---|---|---|
| No (n=32871) | Yes (n=1388) | |||
| Mean (SD) | Mean (SD) | P-value for T-test | ||
| Blood Cadmium Level | Mean | 0.50 (0.01) | 0.70 (0.03) | <0.0001 |
| Column % | Column % | P-value for Chi-square test | ||
| Chronic Bronchitis | No | 94.75% | 86.47% | <0.0001 |
| Yes | 5.25% | 13.53% | ||
| Age | 18–39 | 35.84% | 3.31% | <0.0001 |
| 40–59 | 33.23% | 21.11% | ||
| 60–74 | 21.19% | 40.99% | ||
| >=75 | 9.74% | 34.58% | ||
| Sex | Male | 47.60% | 65.78% | <0.0001 |
| Female | 52.40% | 34.22% | ||
| Race | Non-Hispanic White | 42.18% | 57.71% | <0.0001 |
| Mexican American | 16.25% | 9.80% | ||
| Non-Hispanic Black | 21.65% | 18.73% | ||
| Other Hispanic | 9.66% | 7.78% | ||
| Other Race | 10.26% | 5.98% | ||
| Body Mass Index, BMI | <18.5 | 1.65% | 1.72% | <0.0001 |
| 18.5–24.9 | 28.04% | 20.79% | ||
| 25–29.9 | 33.07% | 31.86% | ||
| 30+ | 37.23% | 45.62% | ||
| Education | <High School | 25.62% | 36.97% | <0.0001 |
| High School /GED | 22.67% | 25.78% | ||
| >High School | 51.71% | 37.26% | ||
| Household Income | <USD 20,000 | 21.61% | 38.80% | <0.0001 |
| >=USD 20,000 | 78.39% | 61.20% | ||
| Marital Status | Married | 51.63% | 51.30% | <0.0001 |
| Widowed | 7.68% | 20.75% | ||
| Divorced | 10.55% | 13.83% | ||
| Separated | 3.38% | 3.03% | ||
| Never Married | 18.65% | 7.06% | ||
| Living with partner | 8.11% | 4.03% | ||
| Physical Activity | Not sufficient | 48.42% | 63.98% | <0.0001 |
| Sufficient | 51.58% | 36.02% | ||
| Diabetes | No | 86.40% | 61.82% | <0.0001 |
| Borderline | 2.06% | 3.96% | ||
| Yes | 11.54% | 34.22% | ||
| Weak kidney function | No | 97.38% | 87.86% | <0.0001 |
| Yes | 2.62% | 12.14% | ||
| Hypertension | No | 66.20% | 26.10% | <0.0001 |
| Yes | 33.80% | 73.90% | ||
| Smoking Status | Non-smoker | 56.13% | 33.31% | <0.0001 |
| Former smoker | 23.11% | 42.97% | ||
| Sometimes | 3.90% | 2.60% | ||
| Everyday | 16.86 | 21.12% | ||
| Alcohol Consumption | Moderate | 68.44% | 84.44% | <0.0001 |
| Medium | 20.16% | 9.51% | ||
| Heavy | 11.40% | 6.05% | ||
The myocardial infarction group had significantly higher mean blood cadmium level (0.70 μg/L, ± 0.03) than the healthy group (0.50 μg/L, ± 0.01) (p-value <0.0001).
Univariate surveylogistic analysis is presented in Table 2. Blood cadmium level was significantly associated with the history of myocardial infarction. For every 1 μg/L increase in cadmium concentration there was a 39% increase in the odds of having myocardial infarction (OR=1.39, CI=[1.30, 1.49]). Participants with chronic bronchitis were almost 3-fold the odds of having a myocardial infarction than those without (OR=2.81, CI=[2.33, 3.37]). Age, gender, race, BMI, education, household income, physical activity, some of the marital status, hypertension, weak kidney function, diabetes, alcohol consumption level and smoking status were also significantly associated with myocardial infarction, and thus were adjusted in the multivariate model.
Table 2:
Univariate logistic model of myocardial infarction with demographics from NHANES 2005–2016, of adults more than 18 years old
| Crude Odds Ratio (95% CI) | ||
|---|---|---|
| Cadmium | Mean | 1.39(1.30, 1.49)* |
| Bronchitis | No | Reference |
| Yes | 2.81(2.33, 3.37)* | |
| Age | 18–39 | Reference |
| 40–59 | 7.54(5.24, 10.83)* | |
| 60–74 | 24.94(17.56, 35.40)* | |
| >=75 | 43.18(30.24, 61.65)* | |
| Gender | Male | Reference |
| Female | 0.56(0.49, 0.64)* | |
| Race | Non-Hispanic White | Reference |
| Mexican American | 0.42(0.34, 0.52)* | |
| Non-Hispanic Black | 0.74(0.63, 0.87)* | |
| Other Hispanic | 0.54(0.42, 0.71)* | |
| Other Race | 0.75(0.54, 1.03) | |
| BMI | 18.5–24.9 | Reference |
| <18.5 | 1.73(0.93, 3.21) | |
| 25–29.9 | 1.42(1.17, 1.71)* | |
| 30+ | 1.97(1.67, 2.32)* | |
| Education | <High School | Reference |
| High School /GED | 0.78(0.65, 0.93)* | |
| >High School | 0.43(0.37, 0.51)* | |
| Household Income | <20,000 | Reference |
| >=20,000 | 0.41(0.34, 0.48)* | |
| Marital Status | Married | Reference |
| Widowed | 3.45(2.96, 4.03)* | |
| Divorced | 1.23(1.02, 1.50)* | |
| Separated | 0.99(0.63, 1.56) | |
| Never Married | 0.36(0.28, 0.48)* | |
| Living with partner | 0.67(0.46, 0.97)* | |
| Physical Activity | No | Reference |
| Moderate | 0.54(0.46, 0.63)* | |
| Weak Kidney Function | No | Reference |
| Yes | 5.33(4.38, 6.47)* | |
| Hypertension | No | Reference |
| Yes | 5.7(4.95, 6.56)* | |
| Diabetes | No | Reference |
| Borderline | 2.92(2.02, 4.22)* | |
| Yes | 5.06(4.30, 5.96)* | |
| Smoke | No | Reference |
| Former | 3.05(2.57, 3.61)* | |
| Sometimes | 1.43(0.88, 2.33) | |
| Everyday | 2.14(1.75, 2.60)* | |
| Alcohol | Light | Reference |
| Moderate | 0.34(0.27, 0.44)* | |
| Heavy | 0.39(0.30, 0.52)* |
Table 3 shows the results of the multivariate analysis. The interaction between chronic bronchitis and blood cadmium level on the history of myocardial infarction was observed (OR=1.33, CI=[1.01, 1.74]). For 1 μg/L increase in cadmium level, people with chronic bronchitis had 1.65 (1.24*1.33) times the odds of having myocardial infarction compared to the original cadmium level, while those without chronic bronchitis would be only 1.24 times as likely having the outcome (OR=1.24, CI=[1.05, 1.46]). Table 4 is provided to better illustrate the interaction. The odds ratio of MI for groups with and without chronic bronchitis are different at different cadmium levels. The higher cadmium level indicates higher odds ratio, meaning that people with chronic bronchitis are more sensitive to the effect of cadmium.
Table 3:
Adjusted Odds Ratio and 95% Confidence Intervals for Multivariate logistic models of Myocardial infarction from NHANES 2005–2016, of adults more than 18 years old
| Myocardial Infarction | |||
|---|---|---|---|
| Adjusted Odds Ratio (95% CI) | p-value | ||
| Cadmium | Mean | 1.24(1.05, 1.46) | 0.015 |
| Bronchitis | No | Reference | |
| Yes | 1.40(0.98, 1.99) | 0.069 | |
| Cadmium*Bronchitis | 1.33(1.01, 1.74) | 0.046 | |
| Age | 18–39 | Reference | |
| 40–59 | 4.63(2.88, 7.46) | <.0001 | |
| 60–74 | 10.66(6.62, 17.17) | <.0001 | |
| >=75 | 14.82(8.9, 24.66) | <.0001 | |
| Gender | Male | Reference | |
| Female | 0.39(0.31, 0.48) | <.0001 | |
| Race | Non-Hispanic White | Reference | |
| Mexican American | 0.58(0.42, 0.79) | 0.001 | |
| Non-Hispanic Black | 0.67(0.52, 0.85) | 0.002 | |
| Other Hispanic | 0.69(0.50, 0.94) | 0.018 | |
| Other Race | 0.96(0.65, 1.41) | 0.822 | |
| BMI | 18.5–24.9 | Reference | |
| <18.5 | 0.83(0.36, 1.91) | 0.664 | |
| 25–29.9 | 1.03(0.79, 1.34) | 0.818 | |
| 30+ | 1.44(1.13, 1.84) | 0.004 | |
| Education | <High School | Reference | |
| High School /GED | 1.16(0.87, 1.56) | 0.304 | |
| >High School | 0.87(0.7, 1.07) | 0.181 | |
| Household Income | <20,000 | Reference | |
| >=20,000 | 0.52(0.41, 0.67) | <.0001 | |
| Marital Status | Married | Reference | |
| Widowed | 1.22(0.93, 1.61) | 0.152 | |
| Divorced | 0.96(0.65, 1.4) | 0.819 | |
| Separated | 1.1(0.44, 2.75) | 0.837 | |
| Never Married | 0.91(0.53, 1.56) | 0.730 | |
| Living with partner | 2.02(1.13, 3.58) | 0.018 | |
| Physical Activity | No | Reference | |
| Moderate | 0.83(0.66, 1.05) | 0.124 | |
| Weak Kidney Function | No | Reference | |
| Yes | 2.09(1.54, 2.85) | <.0001 | |
| Hypertension | No | Reference | |
| Yes | 2.34(1.87, 2.93) | <.0001 | |
| Smoke | No | Reference | |
| Former | 1.51(1.17, 1.96) | 0.002 | |
| Sometimes | 2.24(1.06, 4.75) | 0.036 | |
| Everyday | 1.62(1.10, 2.38) | 0.015 | |
| Diabetes | No | Reference | |
| Borderline | 1.23(0.69, 2.18) | 0.474 | |
| Yes | 1.87(1.45, 2.42) | <.0001 | |
| Alcohol | Light | Reference | |
| Moderate | 0.54(0.37, 0.78) | 0.002 | |
| Heavy | 0.70(0.45, 1.09) | 0.109 | |
Table 4:
Odds Ratio of myocardial infarction for people with/without chronic bronchitis at different cadmium levels, NHANES 2005–2016, of adults more than 18 years old
| Odds of MI | ||||
|---|---|---|---|---|
| Cadmium=0μg/L | Cadmium=1μg/L | Cadmium=2μg/L | ||
| Chronic Bronchitis | No | Ref | Ref | Ref |
| Yes | 1.4 | 1.86 | 2.48 | |
After controlling for the interaction effects, we observed the presence of racial disparity in the prevalence of myocardial infarction. Compared to non-Hispanic white, Mexican American (OR=0.58, CI=[0.42, 0.79]), Non-Hispanic Black (OR=0.67, CI=[0.52, 0.85]) and Other Hispanic (OR=0.69, CI=[0.50, 0.94]) have lower odd of disease. Higher age indicates significant higher prevalence of myocardial infarction. Female (OR=0.37, CI=[0.31, 0.48]), BMI>30 (OR=1.44, CI=[1.13, 1.84]), higher household income (OR=0.52, CI=[0.41, 0.67]), comorbidities including hypertension (OR=2.34, CI=[1.87, 2.93]), weak kidney function (OR=2.09, CI=[1.54, 2.85]) and diabetes (OR=1.87, CI=[1.45, 2.42]), smoking status (Former: OR=1.51, CI=[1.17, 1.96]; Occasional: OR=2.24, CI=[1.06, 4.75]; Everyday: OR=1.62, CI=[1.10, 2.38]) and moderate alcohol consumption (OR=0.54, CI=[0.37, 0.78]) were associated with myocardial infarction.
We also did the same analysis process for stroke and didn’t see the significant interactions between cadmium level and chronic bronchitis.
Discussion
To our knowledge, this is the first study to investigate interaction between chronic bronchitis and cadmium on the history of myocardial infarction. We found that participants with a history of chronic bronchitis had almost 3-fold odds of reporting a history of myocardial infarction than those without chronic bronchitis (OR=2.81, CI=[2.33, 3.37]). The finding is consistent with other studies of the relationship between chronic bronchitis and coronary heart disease [26:28]. We also found that every 1 μg/L increase in cadmium concentration corresponded to a 39% increase in the likelihood of having a myocardial infarction (OR=1.39, CI=[1.30, 1.49]). The potential mechanism by which cadmium exerts its effect on cardiovascular disease could be via its association with dyslipidemia, hypertension, oxidative stress and some other vascular diseases [29,30].
We found that people with a history of chronic bronchitis were more sensitive to the change of blood cadmium levels on the likelihood of having myocardial infarction compared to non-bronchitis participants (OR=1.33, CI=[1.01, 1.74]). With 1 unit increase in blood cadmium concentration, risk for myocardial infarction increased by 24% for people without chronic bronchitis and 65% for those with chronic bronchitis. This information will be useful for clinicians and policymakers as it pertains to medical surveillance of employees with occupational exposure to cadmium.
Racial disparity was noticed in our result. The prevalence of myocardial infarction is significantly higher among non-Hispanic White than other racial groups including non-Hispanic Black, other Hispanics and Mexican Americans. It is consistent with previous studies [31, 32]. Some studies found that the racial differences between black and white people in incidence rates of myocardial infarction is small and insignificant [32,33]. In our study, the disparity remains after adjustment of all covariates mentioned above. The reason of this racial disparity is still unclear.
Smoking is significantly related to the higher odds of history of myocardial infarction after adjustment of cadmium, which is believed to be a mediator of tobacco-related cardiovascular disease. The effect is significant even for the former smokers. But it is possible that some smokers quit smoking after been diagnosed with myocardial infarction, or they already smoked too much before quitting. Lifetime cumulative smoking amount could be considered in the future studies.
It is a limitation of our study as cross-sectional studies cannot lead to causal inferences. Thus, prospective studies are needed to confirm the findings. Another limitation of this study is the possible misclassification that could arise from self-report, as most of the data were self-reported. The strengths of the study include the large sample size, the population-based design and rigorous protocols associated with the NHANES.
In summary, our study demonstrated the existence of the interaction between chronic bronchitis and blood cadmium level on the prevalence of myocardial infarction. Medical surveillance for cadmium exposure need to take into account the history of chronic bronchitis. Future studies to elucidate the mechanism behind this interaction are needed.
Figure 1:
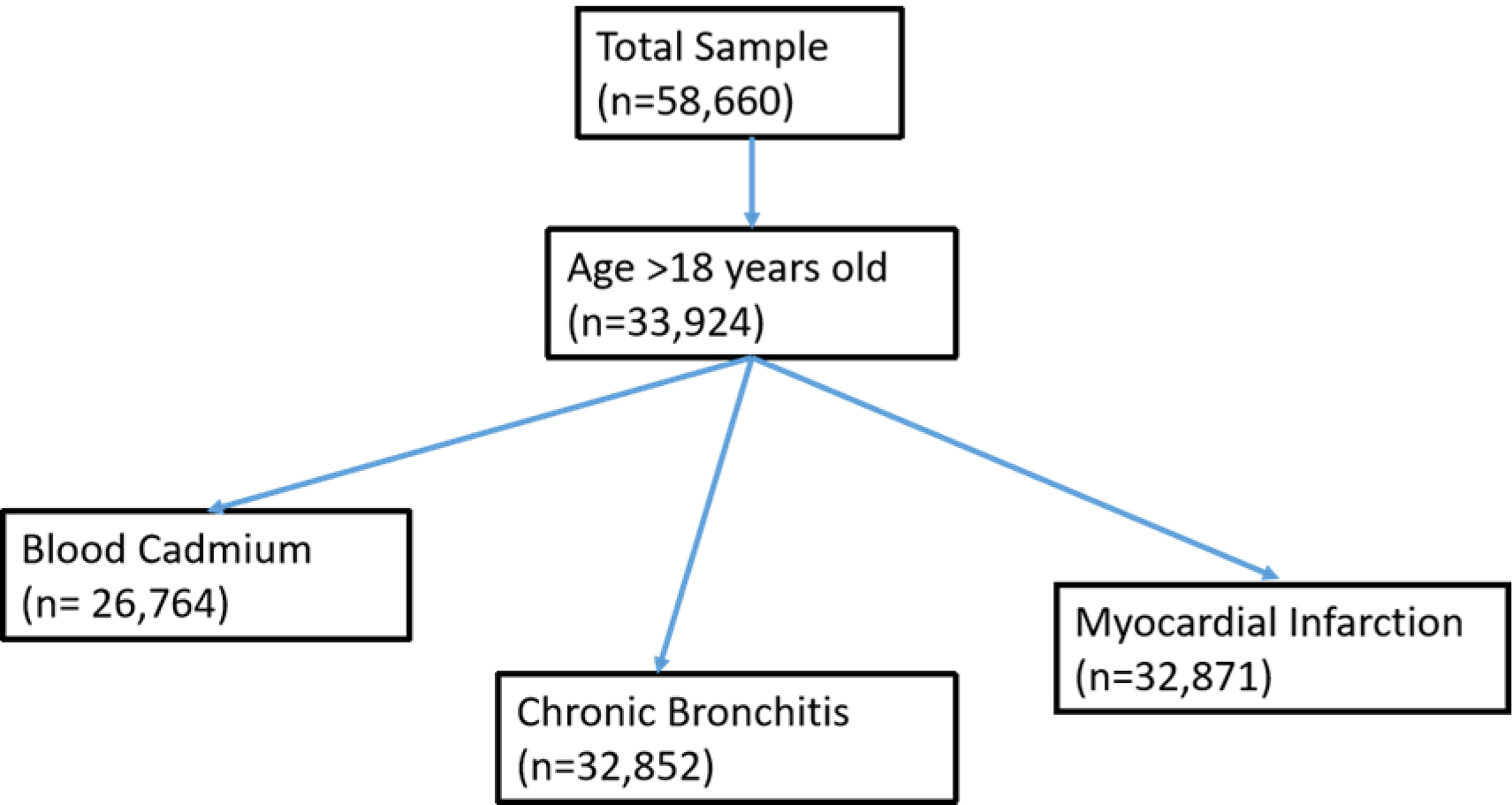
Sample selection flow chart
Funding:
This work is supported in part by The National Heart Lung and Blood Institute[Grant number: K01 HL146944]
Footnotes
Conflict of Interest: None declared
References
- 1-.Agarwal S, Zaman T, Tuzcu EM, & Kapadia SR (2011). Heavy metals and cardiovascular disease: Results from the national health and nutrition examination survey (NHANES) 1999–2006. Angiology, 62(5), 422–429. 10.1177/0003319710395562 [DOI] [PubMed] [Google Scholar]
- 2-.Barregard L, Sallsten G, Fagerberg B, Borné Y, Persson M, Hedblad B, & Engström G (2016). Blood cadmium levels and incident cardiovascular events during follow-up in a population-based cohort of swedish adults: The malmö diet and cancer study. Environmental Health Perspectives, 124(5), 594–600. 10.1289/ehp.1509735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3-.Everett CJ, & Frithsen IL (2008). Association of urinary cadmium and myocardial infarction. Environmental Research, 106(2), 284–286. 10.1016/j.envres.2007.10.009 [DOI] [PubMed] [Google Scholar]
- 4-.Lee M, Park SK, Hu H, & Lee S (2011). Cadmium exposure and cardiovascular disease in the 2005 korea national health and nutrition examination survey. Environmental Research, 111(1), 171–176. 10.1016/j.envres.2010.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5-.Peters JL, Perlstein TS, Perry MJ, McNeely E, & Weuve J (2010a). Cadmium exposure in association with history of stroke and heart failure. Environmental Research, 110(2), 199–206. 10.1016/j.envres.2009.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6-.Tellez-Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W, … Navas-Acien A (2013). Cadmium exposure and incident cardiovascular disease. Epidemiology, 24(3), 421. 10.1097/EDE.0b013e31828b0631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7-.Hecht EM, Arheart KL, Lee DJ, Hennekens CH, & Hlaing WM (2016). Interrelation of cadmium, smoking, and cardiovascular disease (from the national health and nutrition examination survey). The American Journal of Cardiology, 118(2), 204–209. 10.1016/j.amjcard.2016.04.038 [DOI] [PubMed] [Google Scholar]
- 8-.Houtman JP (1993). Prolonged low-level cadmium intake and atherosclerosis. The Science of the Total Environment, 138(1–3), 31–36. [DOI] [PubMed] [Google Scholar]
- 9-.Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, & Guallar E (2008). Cadmium exposure and hypertension in the 1999–2004 national health and nutrition examination survey (NHANES). Environmental Health Perspectives, 116(1), 51–56. 10.1289/ehp.10764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10-.Menke A, Muntner P, Silbergeld EK, Platz EA, & Guallar E (2009). Cadmium levels in urine and mortality among U.S. adults. Environmental Health Perspectives, 117(2), 190–196. 10.1289/ehp.11236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11-.Pauwels RA, Buist AS, Calverley PMA, Jenkins CR, & Hurd SS (2005). Global initiative for chronic obstructive lung disease strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: An Asia–Pacific perspective. Respirology, 10(1), 9–17. 10.1111/j.1440-1843.2005.00692.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12-.Lampe BJ, Park SK, Robins T, Mukherjee B, Litonjua AA, Amarasiriwardena C, … Hu H (2008). Association between 24-hour urinary cadmium and pulmonary function among community-exposed men: The VA normative aging study. Environmental Health Perspectives, 116(9), 1226–1230. 10.1289/ehp.11265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13-.Mannino DM, Holguin F, Greves HM, Savage-Brown A, Stock AL, & Jones RL (2004). Urinary cadmium levels predict lower lung function in current and former smokers: Data from the third national health and nutrition examination survey. Thorax, 59(3), 194–198. 10.1136/thorax.2003.012054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14-.Oh C, Oh I, Lee J, Park YH, Choe B, Yoon T, & Choi J (2014). Blood cadmium levels are associated with a decline in lung function in males. Environmental Research, 132, 119–125. 10.1016/j.envres.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 15-.Rokadia HK, & Agarwal S (2013). Serum heavy metals and obstructive lung disease: Results from the national health and nutrition examination survey. Chest, 143(2), 388–397. S0012–3692(13)60086–0 [pii] [DOI] [PubMed] [Google Scholar]
- 16-.Armstrong BG, & Kazantzis G (1985). Prostatic cancer and chronic respiratory and renal disease in british cadmium workers: A case control study. British Journal of Industrial Medicine, 42(8), 540–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17-.Kazantzis G, Lam TH, & Sullivan KR (1988). Mortality of cadmium-exposed workers. A five-year update. Scandinavian Journal of Work, Environment & Health, 14(4), 220–223. [DOI] [PubMed] [Google Scholar]
- 18-.Baty F, Putora PM, Isenring B, Blum T, & Brutsche M (2013). Comorbidities and burden of COPD: A population based case-control study. PloS One, 8(5), e63285. 10.1371/journal.pone.0063285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19-.Chen C, Li Q, Nie X, Han B, Chen Y, Xia F, … Lu Y, (2017). Association of lead exposure with cardiovascular risk factors and diseases in chinese adults. Environmental Science and Pollution Research International, 10.1007/s11356-017-9884-6 [DOI] [PubMed] [Google Scholar]
- 20-.Curkendall SM, deLuise C, Jones JK, Lanes S, Stang MR, Goehring E, & She D (2006). Cardiovascular disease in patients with chronic obstructive pulmonary disease. Annals of Epidemiology, 16(1), 63–70. 10.1016/j.annepidem.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 21-.de Lucas-Ramos P, Izquierdo-Alonso JL, Rodriguez-Gonzalez Moro JM, Frances JF, Lozano PV, & Bellón-Cano JM (2012). Chronic obstructive pulmonary disease as a cardiovascular risk factor. results of a case-control study (CONSISTE study). International Journal of Chronic Obstructive Pulmonary Disease, 7, 679–686. 10.2147/COPD.S36222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22-.Feng W, Huang X, Zhang C, Liu C, Cui X, Zhou Y, … Wu T, (2015). The dose-response association of urinary metals with altered pulmonary function and risks of restrictive and obstructive lung diseases: A population-based study in china. BMJ Open, 5(5), 007643. 10.1136/bmjopen-2015-007643 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23-.MACLAY JD, McALLISTER DA, & MacNEE W (2007). Cardiovascular risk in chronic obstructive pulmonary disease. Respirology, 12(5), 634–641. 10.1111/j.1440-1843.2007.01136.x [DOI] [PubMed] [Google Scholar]
- 24-.Pelkonen MK, Notkola IK, Laatikainen TK, & Jousilahti P (2017). Chronic bronchitis in relation to hospitalization and mortality over three decades. Respiratory Medicine, 123, 87–93. 10.1016/j.rmed.2016.12.018 [DOI] [PubMed] [Google Scholar]
- 25-.Pelkonen M, Notkola I, Nissinen A, Tukiainen H, & Koskela H (2006). Thirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: A follow-up in middle-aged rural men. Chest, 130(4), 1129–1137. 10.1378/chest.130.4.1129 [DOI] [PubMed] [Google Scholar]
- 26-.Jousilahti P, Salomaa V, Rasi V, & Vahtera E (1999). Symptoms of chronic bronchitis, haemostatic factors, and coronary heart disease risk. Atherosclerosis, 142(2), 403–407. [DOI] [PubMed] [Google Scholar]
- 27-.Jousilahti P, Vartiainen E, Tuomilehto J, & Puska P (1996). Symptoms of chronic bronchitis and the risk of coronary disease. Lancet (London, England), 348(9027), 567–572. 10.1016/S0140-6736(96)02374-4 [DOI] [PubMed] [Google Scholar]
- 28-.Peters JL, Perlstein TS, Perry MJ, McNeely E, & Weuve J (2010b). Cadmium exposure in association with history of stroke and heart failure. Environmental Research, 110(2), 199–206. 10.1016/j.envres.2009.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29-.Eum K, Lee M, & Paek D (2008). Cadmium in blood and hypertension. The Science of the Total Environment, 407(1), 147–153. 10.1016/j.scitotenv.2008.08.037 [DOI] [PubMed] [Google Scholar]
- 30-.Houston MC (2007). The role of mercury and cadmium heavy metals in vascular disease, hypertension, coronary heart disease, and myocardial infarction. Alternative Therapies in Health and Medicine, 13(2), 128. [PubMed] [Google Scholar]
- 31-.Mensah George A, Mokdad Ali H, Ford Earl S, Greenlund Kurt J, & Croft Janet B (2005). State of disparities in cardiovascular health in the United States. Circulation, 111(10), 1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04 [DOI] [PubMed] [Google Scholar]
- 32-.Chi GC, Kanter MH, Li BH, Qian L, Reading SR, Harrison TN, Jacobsen SJ, Scott RD, Cavendish JJ, Lawrence JM, Tartof SY, & Reynolds K (2020). Trends in Acute Myocardial Infarction by Race and Ethnicity. Journal of the American Heart Association, 9(5), e013542. 10.1161/JAHA.119.013542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33-.Jones DW, Chambless LE, Folsom AR, et al. Risk Factors for Coronary Heart Disease in African Americans: The Atherosclerosis Risk in Communities Study, 1987–1997. Arch Intern Med. 2002;162(22):2565–2571. doi: 10.1001/archinte.162.22.2565 [DOI] [PubMed] [Google Scholar]


