Abstract
Background:
Ghrelin may influence several alcohol-related behaviors in animals and humans by modulating central and/or peripheral biological pathways. The aim of this exploratory analysis was to investigate associations between ghrelin administration and the human circulating metabolome during alcohol exposure in non-treatment seeking, heavy drinking individuals with alcohol use disorder (AUD).
Methods:
We used serum samples from a randomized, crossover, double-blind, placebo-controlled human laboratory study with intravenous (IV) ghrelin or placebo infusion in two experiments. A loading dose (3 μg/kg) was followed by continuous infusion (16.9 ng/kg/min) of acyl-ghrelin or placebo during each session. The first experiment included an IV alcohol self-administration (IV-ASA) session, and the second experiment included an IV alcohol clamp (IV-AC) session, both under the counterbalanced infusion of ghrelin or placebo. Serum metabolite profiles were analyzed from repeated blood samples collected during each session.
Results:
In both experiments, ghrelin infusion was associated with altered serum metabolite profile, specifically with significantly increased levels of cortisol (IV-ASA q-value = 0.0003, and IV-AC q < 0.0001), corticosterone (IV-ASA q = 0.0202, and IV-AC q <0.0001), and glycochenodeoxycholic acid (IV-ASA q = 0.0375, and IV-AC q = 0.0013). Furthermore, in the IV-ASA experiment, increased levels of cortisone (q = 0.0352) and fatty acids 18:1 (q = 0.0406) and 18:3 (q = 0.0320) were associated with ghrelin infusion. Moreover, in the IV-AC experiment, levels of glycocholic acid (q < 0.0001) and phenylalanine (q = 0.0458) were significantly increased in relation to ghrelin infusion.
Conclusion:
IV ghrelin infusion, combined with IV alcohol administration, was associated with changes in the circulating metabolite levels of, among others, corticosteroids and glycine-conjugated bile acids. More studies are required to understand the role that metabolome changes may play in the complex interaction between ghrelin and alcohol.
Keywords: Ghrelin, Alcohol Use Disorder, Metabolomics, Corticosteroids, Bile acids
Introduction
Ghrelin is a 28-amino acid residue peptide, produced by endocrine cells mainly localized in the stomach, which circulates in an acylated form (acyl ghrelin, the active form) and a des-acylated form (des-acyl ghrelin). Originally identified as a hormone that regulates growth hormone release, ghrelin has been established as an orexigenic hormone with several effects on appetite, food intake, and metabolism. In addition, ghrelin has been shown to be important in food-related reward, influencing the hedonic control of feeding behavior (Abizaid et al., 2006). Central ghrelin signaling can influence several brain regions involved in reward processing, including the ventral tegmental area and the nucleus accumbens (Guan et al., 1997; Malik et al., 2008; Naleid et al., 2005).
Ghrelin also seems to play a role in regulating alcohol consumption and in the neurobiology of alcohol use disorder (AUD) (for reviews, see: Farokhnia et al., 2019; Morris et al., 2018; Zallar et al., 2017). Circulating ghrelin concentrations are positively correlated with alcohol craving and responses to alcohol-related cues (Addolorato et al., 2006; Koopmann et al., 2012; Leggio et al., 2012; Ralevski et al., 2017). Furthermore, intravenous ghrelin administration increases alcohol craving and self-administration in heavy drinking individuals with AUD (Farokhnia et al., 2018; Leggio et al., 2014). Preliminary evidence also suggests that ghrelin receptor blockade is safe when co-administered with alcohol and might decrease alcohol cue-induced craving in heavy drinking individuals (Lee et al., 2020).
Alcohol use is associated with changes in the human metabolome, such as levels of amino acids (e.g., decreased glutamine and asparagine), steroid hormones (e.g., increased cortisol), neurotransmitters (e.g., decreased serotonin, increased glutamate), lipids (e.g., increased fatty acids [FA], like palmitoleic acid, docosapentaenoic acid, FA 16:1, and FA 22:5) and microbiota-associated metabolites (e.g., increased lactate and decreased 3-indolepropionic acid) (Heikkinen et al., 2019; Irwin et al., 2018; Jaremek et al., 2013; Kärkkäinen et al., 2021, 2020; Lehikoinen et al., 2018; Mostafa et al., 2016; Voutilainen and Kärkkäinen, 2019; Würtz et al., 2016). Furthermore, some of these metabolites, such as microbiota-associated metabolites, have been shown to influence ghrelin-mediated signaling (Leeuwendaal et al., 2021; Torres-Fuentes et al., 2019). Moreover, ghrelin has been shown to modulate metabolic processes and alter the metabolome (Barazzoni et al., 2005; Gortan Cappellari and Barazzoni, 2019; Goshadrou et al., 2018). To our knowledge, no studies have investigated possible effects of ghrelin on the human metabolite profile in the context of alcohol use.
To this end, we investigated the relationship between ghrelin administration and the human circulating metabolome during alcohol exposure by using a non-targeted metabolomics approach. This methodology allowed us to perform an exploratory analysis of human serum samples collected during a placebo-controlled, human laboratory study with intravenous (IV) ghrelin infusion in heavy drinking individuals with AUD, who underwent two alcohol administration experiments: 1) IV alcohol self-administration (IV-ASA), and 2) IV alcohol clamp (IV-AC). We used samples from individuals with AUD, because of the relationship between ghrelin and different alcohol-related outcomes (for reviews, see: Farokhnia et al., 2019; Zallar et al., 2017).
Materials and methods
Human laboratory experiments
This study was an exploratory analysis of serum samples collected during a clinical study conducted at the National Institutes of Health (NIH) Clinical Center in Bethesda, Maryland, USA (ClinicalTrials.gov - NCT01779024). Details of the parent study have been previously described (Farokhnia et al., 2018). In brief, this was a randomized, crossover, double-blind, placebo-controlled human laboratory study with IV ghrelin or placebo infusion. Participants were non-treatment seeking heavy alcohol drinkers aged 21–65 years. Heavy drinking was defined as drinking above 15 or 20 standard drinks per week for women and men, respectively. Participant demographics are shown in Table 1 and detailed information about the inclusion and exclusion criteria are described in the Supplementary Methods. Although not an eligibility criterion, all participants had a Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR) based diagnosis of current alcohol dependence.
Table 1:
Demographic characteristics of the participants
| Variable | IV-ASA Experiment (n = 17) |
IV-AC Experiment (n = 10) |
|---|---|---|
| Sex, n (%) | ||
| Male | 12 (70.59) | 8 (80) |
| Female | 5 (29.41) | 2 (20) |
|
| ||
| Age, M (SD) | 41.01 (10.98) | 39.24 (12.33) |
|
| ||
| Race, n (%) | ||
| White | 2 (11.76) | 1 (10) |
| Black/African American | 14 (82.35) | 1 (5.88) |
| More than one race | 8 (80) | 1 (10) |
|
| ||
| Years of education, M (SD) | 13.35 (1.69) | 13.30 (1.70) |
|
| ||
| Cigarette smoker, n (%) | 11 (64.70) | 8 (80) |
|
| ||
| Age at first drink, M (SD) | 16.13 (2.10) | 16.25 (1.58) |
|
| ||
| AUDIT total score, M (SD) | 20.71 (8.55) | 23.38 (7.90) |
|
| ||
| Alcohol TLFB (90 days), M (SD) | ||
| Average drinks per drinking days | 8.04 (6.08) | 9.78 (7.29 |
| Number of heavy drinking days | 45.18 (27.49) | 46.50 (29.04) |
Abbreviations: AUDIT=Alcohol Use Disorders Identification Test; TLFB = Timeline Followback; M=Mean; SD=Standard Deviation.
All participants provided written informed consent prior to participation in the study. The study protocol was approved by the NIH Addictions Institutional Review Board and reviewed by the Food and Drug Administration. Alcohol administrations were performed consistent with the National Institute on Alcohol Abuse and Alcoholism (NIAAA) Council Guidelines on Alcohol Administration (https://www.niaaa.nih.gov/Resources/ResearchResources/job22.htm).
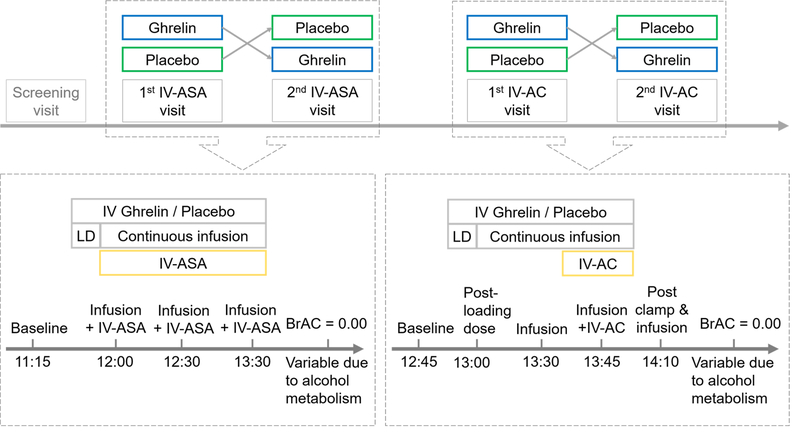
The study consisted of two experiments: IV-ASA and IV-AC (Figure 1). In both experiments, a 10-minute loading dose of IV acyl-ghrelin (3 μg/kg), or placebo, was administered first. This loading dose was followed by a continuous infusion of acyl-ghrelin (16.9 ng/kg/min), or placebo, throughout the session. Participants underwent up to four inpatient visits; they were admitted the evening before each experimental session and were discharged the day after. Participants ate standardized meals during the visits (Farokhnia et al., 2018). Serum metabolite profiles were measured from repeated blood samples collected during each session, as shown in Figure 1.
Figure 1: Study flow of intravenous alcohol self-administration and intravenous alcohol clamp experiments.
Simplified schematic view of the study showing the crossover design and the serum sampling time-points used in the metabolomics analysis. A minimum of a 3-day washout period occurred between each session. During each session, after sampling for pre-drug baseline, a 10-minute loading dose of intravenous acyl-ghrelin (3 μg/kg) or placebo was administered. This dose was followed by a continuous infusion of acyl-ghrelin (16.9 ng/kg/min) or placebo. Abbreviations: BrAC=Breath Alcohol Concentration; IV=Intravenous; IV-ASA=IV Alcohol Self-Administration; IV-A=IV Alcohol Clamp; LD=Loading Dose.
During the IV-ASA session, participants self-administered alcohol infusions via the Computer-Alcohol Infusion System (CAIS) (Plawecki et al., 2013; Zimmermann et al., 2013). Participants needed to press a button in a progressive ratio manner to get alcohol infusions. Each infusion increased the breath alcohol concentration (BrAC) by 7.5 mg% over 2.5 minutes, followed by a fall of 0.5 mg%/min until the next infusion. Maximum BrAC was set to 120 mg% for safety reasons. As reported in the parent study, the primary results of this experiment were that IV ghrelin significantly increased the total amount of alcohol self-administration when compared to placebo (Farokhnia et al., 2018).
During the IV-AC experiment, a predetermined dose of IV alcohol infusion was first administered to increase the participant’s BrAC linearly to 80 mg% within 20 minutes, then the BrAC was maintained at this level for 15 minutes. The neuroimaging results from this experiment showed that IV ghrelin increased alcohol-related brain activity in the amygdala and modulated food-related signal in the medial orbitofrontal cortex and nucleus accumbens (Farokhnia et al., 2018).
Non-targeted liquid chromatography mass spectrometry metabolomics
Serum metabolite profiles were analyzed from repeated serum samples collected during IV-ASA (number of participants = 17) and IV-AC (number of participants = 10). The workflow of the non-targeted liquid chromatography mass spectrometry (LC-MS) metabolomics analysis has been described in detail elsewhere (Klåvus et al., 2020). Briefly, the analysis order of the serum samples was randomized within subject and within visit: first, the analysis order of the subjects was randomized; then, within each subject, the analysis order of visits was randomized; last, within each visit, the analysis order of the samples from individual time points was randomized. This type of randomization aimed to render samples within subject as comparable as possible, while still eliminating a possible bias caused by the analysis order of the samples. Serum samples from one time point (collected at 13:00, not shown in Figure 1) during the continuous infusion + IV-ASA phase of the IV-ASA experiment were left out of the analysis to enable analysis of all samples in one batch.
Serum samples were thawed on ice and then 100 μL of each sample was mixed by pipette with 400 μL of acetonitrile (LC-MS grade) in the well of a Captiva non-drip filter plate (Agilent Technologies). The filter plate was centrifuged at 700 relative centrifugal force (rcf) for 5 minutes at 4°C. Then, the protein-free filtrate was collected on a 96-well polypropylene plate. A pooled quality control (QC) sample was prepared and injected after every 12 analytical samples.
For the metabolomics analysis, we utilized ultrahigh performance liquid chromatography (UPLC) coupled with Thermo Q Exactive™ Hybrid Quadrupole-Orbitrap Mass Spectrometer (Thermo Scientific, United States). For more lipophilic compounds, we used reverse phase (RP) chromatography column: Zorbax Eclipse XDB-C18, particle size 1.8 μm, 2.1 × 100 mm (Agilent Technologies, United States) and both positive and negative ionization. For more hydrophilic compounds, we used hydrophilic interaction (HILIC) chromatography column: Acquity UPLC BEH Amide, 100 mm × 2.1 mm, 1.7 μm (Waters Corporation, United States) and both positive and negative ionization. The chromatography parameters were as follows: for RP chromatography, the column oven temperature was set to 50°C, and the flow rate to 0.4 mL/min gradient elution with water (eluent A) and methanol (eluent B) both containing 0.1% (v/v) of formic acid. Gradient profile for RP separations was: 0–10 min: 2% B → 100% B; 10–14.5 min: 100% B; 14.5–14.51 min: 100% B → 2% B; 14.51–16.5 min: 2% B; for HILIC, the column oven temperature was set to 45°C, flow rate 0.6 mL/min, gradient elution with 50% v/v ACN in water (eluent A) and 90% v/v ACN in water (eluent B), both containing 20 mM ammonium formate (pH 3). The gradient profile for HILIC separations was: 0–2.5 min: 100% B, 2.5–10 min: 100% B → 0% B; 10–10.01 min: 0% B → 100% B; 10.01–12.5 min: 100% B.
We used the open-source software MS-DIAL version 4.00 (Tsugawa et al., 2015) for peak picking and molecular feature alignment. The parameters used for peak picking were mass range 120–1,600 Da, MS1 tolerance 0.005 Da, MSMS tolerance 0.01 Da, minimum peak width 8, minimum peak height 50,000 amplitude, and mass slice width 0.1 Da. The parameters used for feature alignment were retention time tolerance 0.1 min, MS1 tolerance 0.005 Da, detected in at least 50% of the samples in one group, and gap filling by compulsion.
The preprocessing procedure has been described in more detail elsewhere (Klåvus et al., 2020). In brief, the molecular features were corrected for the drift pattern caused by the LC-MS procedures using regularized cubic spline regression, fit separately for each feature on the QC samples. The smoothing parameter was chosen from an interval between 0.5 and 1.5, using leave-one-out cross validation to prevent overfitting. After the drift correction, feature quality was assessed, and low-quality features were flagged. Features were kept if their relative standard deviation (RSD)* was below 20% and their D-ratio was below 40%. In addition, features with classic RSD, RSD* and basic D-ratio all below 10% were kept. This additional condition prevents the flagging of features with very low values in all but a few samples. These features tend to have a very high value of D-ratio*, since the median absolute deviation of the biological samples is not affected by the large concentration in a handful of samples, causing the D-ratio* to overestimate the significance of random errors in measurements of QC samples (Broadhurst et al., 2018). Thus, other quality metrics were applied with a conservative limit of 0.1 to ensure that only good quality features were kept. Missing values were imputed using random forest imputation. The imputation was performed in two phases: first, only good quality features were imputed, to prevent the flagged features from affecting the imputation. Next, flagged features were imputed. QC samples were removed prior to imputation to prevent their effect on the imputation.
Metabolite identification focused on molecular features with raw p-values <0.05 in linear mixed-effect models explained in the statistical analysis section in either of the experiments. Metabolites were identified using MS-DIAL version 4.00 (Tsugawa et al., 2015). Metabolite identifications were ranked to levels 1–3 according to the community guidelines (Sumner et al., 2007). Metabolites in level 1 were matched against mass, retention time, and tandem mass spectrometry (MS/MS) spectra of fragmented ions from the in-house library of chemical standards built using the same instrument and experimental conditions. Level 2 included metabolites with matching exact mass and MS/MS spectra from public libraries (METLIN, lipidome atlas in MS-DIAL, and Human Metabolome DataBase were used (Guijas et al., 2018; Tsugawa et al., 2020; Wishart et al., 2018). In level 3, only the chemical group of the compound – not the exact compound – could be identified.
Total lipid extraction analysis of phospholipids
We used a targeted mass spectrometry method to measure concentrations of phospholipids from serum samples collected during the IV-AC experiment at three time points (baseline [12:45], post-loading dose [13:00], and IV-ghrelin/placebo infusion [13:30]; see Figure 1). Details of the analysis are described in the Supplementary Methods. In brief, phospholipids were extracted from serum samples, by first mixing them with 498 μL of chloroform/methanol (1:1 v/v) and 2 μL of internal standard mix (10:0/10:0 glycerophosphocholine (PC) and 10:0/10:0 glycerophoshoethanolamine (PE) at 2.5 mg/mL). Next, 75 μL of water were mixed to the sample, followed by centrifugation and collection of the lower organic phase of the mixture, which was evaporated to dryness, resuspended with 100 μL methanol/chloroform (95:5 v/v). 5 μL of the phospholipid extract was then diluted with 95 μL of 10 mM ammonium acetate in methanol. The phospholipids were measured in positive ion mode on an Oribtrap Velos (Thermo Fisher, United States) with a heated electrospray ionization (HESI) ion source coupled with an auto sampler (Ultimate 3000 HPLC, Thermo Fisher). Intensities of lipid species (36 PC, 15 PE and 11 sphingomyelin (SM)) were normalized with the intensity of the internal standard.
Statistical analysis
The goal of the statistical analysis was to find differential features between the IV ghrelin and placebo conditions. Analyses were performed separately for samples from the two experiments (IV-ASA and IV-AC). For each feature, measurements of a single visit were summarized by computing the area under the ion abundance vs. time-point curve (AUC). All time points analyzed were included in the calculation of AUC. This resulted in one AUC value per subject per visit. Differential features were then identified with linear mixed-effects models, where the AUC values of a feature were used as the dependent variable, drug condition (IV ghrelin or placebo) as a fixed effect, and subject identifier as a random effect. Linear mixed-effects models were used to account for the dependence between multiple visits by the same subject. The models were fit separately for each feature, and p-values were adjusted for multiple testing using the Benjamini-Hochberg false discovery rate (FDR) method (q-value). Molecular features with a FDR corrected q-value < 0.05 were considered significantly different between the IV ghrelin and placebo conditions. Molecular features with a raw p-value < 0.05 but an FDR corrected q-value ≥ 0.05, were considered to be trends. In addition to the AUC analysis, the differences between the IV ghrelin and placebo conditions were analyzed at each time-point. The analysis was conducted using previously described linear mixed-effects models, but with the feature level in the specified time-point as the dependent variable. Each of the time-points was analyzed separately. The data from the total lipid extraction analysis of phospholipids was analyzed similarly to the metabolomics data, using linear mixed-effects models with each time point analyzed separately. The linear mixed-effects models were fit using R packages lme4 (version 1.1) and lmerTest (version 3.1.2) in R version 3.6.1 (Bates et al., 2015; Kuznetsova et al., 2017).
Results
A total of 295 individual metabolites were identified (identification level 1 and, for some metabolites, level 2), consisting of 15 steroids and steroid derivatives, 20 fatty acids and conjugates, 82 glycerophospholipids, 49 amino acids and amino acid metabolites, 21 acylcarnitines, and 117 other metabolites. Identified metabolites with a raw p-value < 0.05 in either of the experiments are shown in Supplementary Table 1. We used four different analytical modes to measure a large spectrum of different metabolites. However, because of redundancy in the non-targeted metabolomics data, some of the metabolites were measured in more than one analytical modes. Metabolites were selected for Supplementary Table 1 based on the best representative molecular feature for each identified metabolite with p-value < 0.05 in at least one of the experiments. All measured molecular features are reported in Supplementary Table 2.
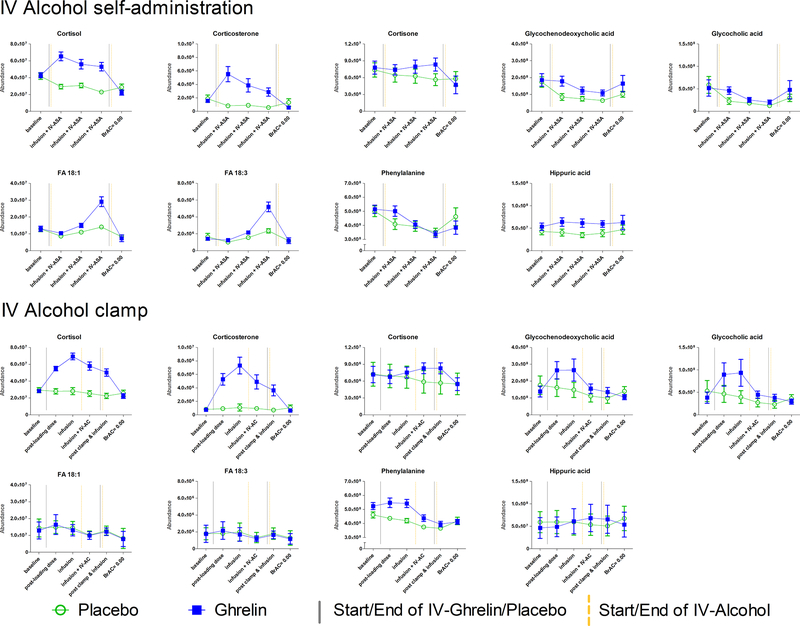
Ghrelin infusion was associated with changes in steroid metabolism in both the IV-ASA and IV-AC experiments. In both experiments, ghrelin infusion was associated with an increase in levels of the steroid hormones corticosterone and cortisol, while levels of these steroids stayed at the baseline level under the placebo condition even with alcohol exposure (Figure 2). There was also a significant increase in cortisone levels in the IV-ASA experiment under IV ghrelin, compared to placebo (Supplementary Table 1).
Figure 2: Intravenous ghrelin administration alters the circulating metabolite profile.
Serum metabolite profiles were analyzed from repeated serum samples collected during each of the four sessions (see Figure 1). When comparing area under the curve values using linear mixed-effect models, significant differences between ghrelin and placebo infusions were seen in both the intravenous (IV) alcohol self-administration (ASA) experiment and the IV alcohol clamp (AC) experiment in levels of cortisol (FRD corrected q-value = 0.0003, and <0.0001, respectively), corticosterone (q = 0.0202, and <0.0001, respectively), and glycochenodeoxycholic acid (q = 0.0375, and 0.0013, respectively). Furthermore, during the IV-ASA experiment, significant differences between ghrelin and placebo infusions were seen also in the levels of cortisone (q = 0.0352), fatty acids (FA) 18:1 (q = 0.0406) and FA 18:3 (q = 0.0320), and hippuric acid (q = 0.0112). However, these metabolites were not significantly altered in the IV-AC experiment (cortisone q = 0.5237, FA 18:1 q = 0.9698, FA 18:3 q = 0.9843, and hippuric acid q = 0.9886). Moreover, in the IV-AC experiment, significant differences between the ghrelin and placebo infusions were seen in levels of glycocholic acid (q < 0.0001), and phenylalanine (q = 0.0458). However, these metabolites were not significantly altered in the IV-ASA experiment (glycocholic acid q = 0.3197, and phenylalanine q = 0.9259). Mean ion abundance and standard error of mean are shown for each measurement point. Abbreviations: BrAC=Breath Alcohol Concentration; infusion=continuous infusion of ghrelin or placebo; IV=Intravenous; IV-ASA=IV Alcohol Self-Administration; IV-A=IV Alcohol Clamp; LD=Loading Dose.
Furthermore, under IV ghrelin vs. placebo, glycochenodeoxycholic acid AUCs were significantly increased in both experiments, and glycocholic acid AUCs were increased in the IV-AC experiment. A trend of increased levels of other bile acids and bile salts, as well as a trend of decreased levels of cholesterol and other cholesterol metabolites was observed under IV ghrelin vs. placebo (Supplementary Table 1).
Ghrelin infusion was also associated with significantly increased fatty acids (FA) 18:1 and 18:3 AUCs in the IV-ASA experiment (Figure 2). Furthermore, there were trends of increased levels of other fatty acids and trends of decreased levels of lysophospholipids with the same fatty acid side chains in response to IV ghrelin, compared to placebo, in the IV-ASA experiment (Supplementary Table 1).
In the IV-ASA experiment, we also observed a significant association between IV ghrelin administration and increased hippuric acid AUC (Figure 2). In the IV-AC experiment, ghrelin infusion was significantly associated with increased phenylalanine AUC.
As regards some metabolites, such as glycolithocholic acid, it should be noted that the significant difference between IV ghrelin and placebo was due to a significant difference observed at baseline and was therefore considered not related to the ghrelin infusion (Supplementary Table 1).
Finally, no significant differences were found between the IV ghrelin and placebo conditions in the total lipid extraction analysis of phospholipids conducted at three time-points (baseline [12:45], post-loading dose [13:00], and IV-ghrelin/placebo infusion [13:30]) from the IV-AC experiment. Results of the total lipid extraction analysis are shown in Supplementary Table 3.
Discussion
In the present placebo-controlled study, we found that IV ghrelin infusion, combined with alcohol, led to increased levels of corticosteroids and glycine-conjugated bile acids.
We have previously shown increased cortisol levels following ghrelin infusion using chemiluminescence immunoassays in blood samples from the same study, where we analyzed a small number of stress- and feeding-related peripheral hormones that were selected a priori (Farokhnia et al., 2021). In the present study, we employed a hypothesis-free exploratory approach via non-targeted LC-MS metabolomics. The results confirm the robust effect of IV ghrelin in increasing cortisol and, in general, corticosteroids in this sample of heavy drinking individuals with AUD.
The observed effect of IV ghrelin infusion in increasing peripheral corticosteroids levels aligns with, and expands, previous literature showing that ghrelin administration increases cortisol levels in rodents (Asakawa et al., 2001) and in humans (Farokhnia et al., 2021; Haass-Koffler et al., 2019). The association between corticosteroids and ghrelin seems to be bidirectional, since acute stress increases ghrelin levels, whereas chronic stress blunts this response (Fahrngruber-Velasquez et al., 2021). Notably, in a previous study with a different cohort of heavy drinking individuals with alcohol dependence, we reported that IV ghrelin per se (i.e., in the absence of alcohol co-administration) led to an increase in cortisol levels, an observation that supports the role of ghrelin in HPA axis activity in individuals with AUD (Haass-Koffler et al., 2019). Blunted cortisol responses to alcohol have been reported in heavy drinking individuals in comparison to light drinking individuals, where acute alcohol exposure increased cortisol levels (Adinoff et al., 2003; Blaine et al., 2019; Blaine and Sinha, 2017; King et al., 2011, 2002; Mennella et al., 2005; Roche et al., 2014; Zhang et al., 2020). Consistent with these studies, we did not observe an increase in cortisol levels after alcohol administration in either of the experiments under the placebo condition. In contrast, a clear increase in both cortisol and corticosterone levels was seen in response to ghrelin infusion. Overall, the connection between ghrelin and corticosteroids seems to be one of the biological links potentially underlying how ghrelin influences alcohol drinking behavior (Morris et al., 2018).
IV ghrelin also led to an increase in the peripheral levels of glycine-conjugated bile acids, namely glycocholic acid and glycochenodeoxycholic acid. We also observed trends towards increased levels of other unconjugated and taurine-conjugated bile acids indicating that IV ghrelin led to an overall increase in serum levels of bile acids (Supplementary Table 1). Since ghrelin influences appetite, gastrointestinal tract motility, and gastric emptying (Levin et al., 2006; Tack et al., 2006), it seems plausible that IV ghrelin infusion could increase secretion of bile acids in an indirect manner. However, previous human studies with food intake have reported negative correlations between ghrelin and bile acids levels (Roberts et al., 2011). Glycine (and taurine) conjugation of bile acids is performed by two enzymes: the microsomal enzyme cholyl-CoA synthetase (EC 6.2.1.7) converts bile acid into acyl-CoA thioester; subsequently, the cytosolic enzyme bile acid-CoA:amino acid N-acyltransferase (EC 2.3.1.65) transfers the bile acid moiety to either glycine or taurine (Killenberg, 1978; Killenberg and Jordan, 1978). Ghrelin infusion may alter this process - a hypothesis in need of testing. On the other hand, even though bile acids influence many other appetite-related hormones, they do not seem to influence ghrelin levels, at least in rats (Kuhre et al., 2018). Furthermore, bile acids themselves are capable of influencing metabolic processes, and possibly behavior, through the farnesoid X receptor and Takeda G protein-coupled receptor 5, which are also found in the brain, and by inducing release of glucagon-like peptide 1 and fibroblast growth factor 19 (Huang et al., 2016; Keitel et al., 2010; Maruyama et al., 2002; Mertens et al., 2017). Given that increased bile acid levels have been associated with fatty liver disease, while reduced bile acid levels seem to have a protective effect against fatty liver disease (Bajaj, 2019; Clifford et al., 2021; Jung et al., 2021), future work is needed to shed light on the role of ghrelin-related changes in bile acids, if any, in different alcohol-related outcomes including alcohol-associated liver disease.
In the present study, ghrelin infusion was associated with a significant increase in levels of FA 18:1 and FA 18:3 in the IV-ASA experiment. Similar effects were also seen in other fatty acids, but they did not reach statistical significance after FDR correction for multiple testing (Supplementary Table 1). Furthermore, trends toward decreased levels of lysophospholipids with similar fatty acid side chains, were observed as a result of ghrelin infusion in the IV-ASA experiment (Supplementary table 1). This effect could be associated with an increase in lipolysis, which ghrelin can induce on its own, and/or indirectly by increasing growth hormone levels (Kopchick et al., 2020; Theander-Carrillo et al., 2006; Vestergaard et al., 2008). Acute and chronic alcohol consumption have been associated with increased circulating levels of fatty acids (Frayn et al., 1990; Kärkkäinen et al., 2020; Steiner and Lang, 2017). Additionally, increased levels of fatty acids, especially 18-carbon fatty acyls such as FA 18:1 and FA 18:3, have been linked to alcohol-associated liver disease (Bradford et al., 2008; Clugston et al., 2017, 2011; Jang et al., 2012; Loftus et al., 2011). Therefore, since ghrelin administration increased the amount of alcohol self-administered (Farokhnia et al., 2018), the increase in fatty acid levels during the IV-ASA experiment could be due to a synergistic effect of the combined alcohol and ghrelin infusions.
Limitations of this study include the small sample size, which limits the statistical power of the study and may have led us to miss some ghrelin-associated changes that have a small effect size. For example, several bile acids and fatty acids showed trends towards increased AUC levels, but did not reach statistical significance after correction for multiple testing. Furthermore, the present study was an exploratory analysis of samples from an existing clinical study whose main outcomes and results were reported elsewhere (Farokhnia et al., 2018). Therefore, the study design was geared towards research questions of the parent study and did not enable us to separate the effect of IV ghrelin or IV alcohol alone from the combined administration of IV ghrelin and IV alcohol. In addition, as stated above, IV ghrelin administration led to increased IV alcohol self-administration in the IV-ASA experiment, which could influence the results of the IV-ASA experiment (Farokhnia et al., 2018). Some of the differences in the results between the IV-ASA and IV-AC experiments, for example in FA 18:1 and FA 18:3 levels, could be due to relatively short IV alcohol exposure in the IV-AC experiment, compared to the IV-ASA experiment. Another reason may be related to the fact that there was an actual alcohol-free time point in the IV-AC, but not in the IV-ASA experiment (Figure 1). Some results, such as increased level of phenylalanine and hippuric acid, also failed to replicate between the two experiments, without a clear indication to why, reducing the reliability of those observations (Figure 2). Moreover, since we used LC-MS based metabolomics, we were not able to detect potentially interesting volatile metabolites, such as short-chain fatty acids, which would need a gas chromatography mass spectrometry method to be analyzed. Lastly, since steroid hormones do not ionize well, we were only able to measure the most abundant steroid hormones with the present untargeted method and a more sensitive targeted approach might be needed to unravel all the effects that ghrelin has on the steroid system.
In conclusion, the present analysis from a double-blind, placebo-controlled human laboratory study in heavy drinking individuals with AUD showed that IV ghrelin infusion, combined with IV alcohol, resulted in changes in the circulating metabolome, as well as chiefly increased levels of corticosteroids and glycine-conjugated bile acids. Future studies are needed to understand the potential causal link between these changes and the effects of ghrelin manipulations on alcohol-related outcomes.
Supplementary Material
Acknowledgements
We thank Miia Reponen for technical assistance with the mass spectrometry analyses. The authors also want to thank Biocenter Finland and Biocenter Kuopio for supporting the core LC-MS laboratory facility.
We thank the clinical and research staff involved in patient care, safety and clinical monitoring, data collection/analysis, and technical support in the joint NIDA/NIAAA Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, in the NIAAA clinical program of the Division of Intramural Clinical and Biological Research (DICBR), at the NIH Clinical Center (Departments of Nursing, Nutrition, and Pharmacy). We would also like to thank Dr. Vijay Ramchandani (Section on Human Psychopharmacology, NIAAA DICBR) and Dr. Reza Momenan (Clinical NeuroImaging Research Core, NIAAA DICBR) for their support in the execution of the parent study. The authors would also like to express their gratitude to the participants who took part in this study. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
The mass spectrometry work conducted at the University of East Finland was supported by the Finnish Foundation for Alcohol Research (OK). The mass spectrometry conducted at the National Institute on Drug Abuse Intramural Research Program (NIDA IRP) was supported by the NIDA IRP Translational Analytical Core. The parent clinical study was supported by National Institutes of Health (NIH) intramural funding ZIA-DA000635 and ZIA-AA000218 (PI: Lorenzo Leggio), jointly funded by the NIDA IRP and the Division of Intramural Clinical and Biological Research (DICBR) of the National Institute on Alcohol Abuse and Alcoholism (NIAAA). The development of the Computerized Alcohol Infusion System (CAIS) software was supported by Dr. Vijay Ramchandani’s Section on Human Psychopharmacology in the NIAAA DICBR and by the NIAAA-funded Indiana Alcohol Research Center (AA007611).
Footnotes
Conflicts of interest
OK and AK are co-owners of Afekta Technologies Ltd., a company providing metabolomics analysis services. The other authors do not report any conflict of interest relevant to this work.
References
- Abizaid A, Liu Z-W, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschöp MH, Gao X-B, Horvath TL (2006) Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest 116:3229–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addolorato G, Capristo E, Leggio L, Ferrulli A, Abenavoli L, Malandrino N, Farnetti S, Domenicali M, D’Angelo C, Vonghia L, Mirijello A, Cardone S, Gasbarrini G (2006) Relationship between ghrelin levels, alcohol craving, and nutritional status in current alcoholic patients. Alcohol Clin Exp Res 30:1933–1937. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Ruether K, Krebaum S, Iranmanesh A, Williams MJ (2003) Increased salivary cortisol concentrations during chronic alcohol intoxication in a naturalistic clinical sample of men. Alcohol Clin Exp Res 27:1420–1427. [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Fujimiya M, Katsuura G, Makino S, Fujino MA, Kasuga M (2001) A Role of Ghrelin in Neuroendocrine and Behavioral Responses to Stress in Mice. Neuroendocrinology 74:143–147. [DOI] [PubMed] [Google Scholar]
- Bajaj JS (2019) Alcohol, liver disease and the gut microbiota. Nat Rev Gastroenterol Hepatol 16:235–246. [DOI] [PubMed] [Google Scholar]
- Barazzoni R, Bosutti A, Stebel M, Cattin MR, Roder E, Visintin L, Cattin L, Biolo G, Zanetti M, Guarnieri G (2005) Ghrelin regulates mitochondrial-lipid metabolism gene expression and tissue fat distribution in liver and skeletal muscle. Am J Physiol Endocrinol Metab 288:E228–235. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S (2015) Fitting Linear Mixed-Effects Models Using lme4. J Stat Soft 67. [Google Scholar]
- Blaine SK, Nautiyal N, Hart R, Guarnaccia JB, Sinha R (2019) Craving, cortisol and behavioral alcohol motivation responses to stress and alcohol cue contexts and discrete cues in binge and non-binge drinkers. Addict Biol 24:1096–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Sinha R (2017) Alcohol, stress, and glucocorticoids: From risk to dependence and relapse in alcohol use disorders. Neuropharmacology 122:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford BU, O’Connell TM, Han J, Kosyk O, Shymonyak S, Ross PK, Winnike J, Kono H, Rusyn I (2008) Metabolomic profiling of a modified alcohol liquid diet model for liver injury in the mouse uncovers new markers of disease. Toxicology and Applied Pharmacology 232:236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadhurst D, Goodacre R, Reinke SN, Kuligowski J, Wilson ID, Lewis MR, Dunn WB (2018) Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 14:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford BL, Sedgeman LR, Williams KJ, Morand P, Cheng A, Jarrett KE, Chan AP, Brearley-Sholto MC, Wahlström A, Ashby JW, Barshop W, Wohlschlegel J, Calkin AC, Liu Y, Thorell A, Meikle PJ, Drew BG, Mack JJ, Marschall H-U, Tarling EJ, Edwards PA, de Aguiar Vallim TQ (2021) FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab 33:1671–1684.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clugston RD, Gao MA, Blaner WS (2017) The Hepatic Lipidome: A Gateway to Understanding the Pathogenes is of Alcohol-Induced Fatty Liver. CMP 10:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clugston RD, Jiang H, Lee MX, Piantedosi R, Yuen JJ, Ramakrishnan R, Lewis MJ, Gottesman ME, Huang L-S, Goldberg IJ, Berk PD, Blaner WS (2011) Altered hepatic lipid metabolism in C57BL/6 mice fed alcohol: a targeted lipidomic and gene expression study. J Lipid Res 52:2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrngruber-Velasquez C, Duszka K, König J (2021) The Impact of Chronic Stress and Eating Concern on Acylated Ghrelin Following Acute Psychological Stress in Healthy Men. Stresses 1:16–29. [Google Scholar]
- Farokhnia M, Abshire KM, Hammer A, Deschaine SL, Saravanakumar A, Cobbina E, You Z-B, Haass-Koffler CL, Lee MR, Akhlaghi F, Leggio L (2021) Neuroendocrine response to exogenous ghrelin administration, combined with alcohol, in heavy-drinking individuals: Findings from a randomized, double-blind, placebo-controlled human laboratory study. Int J Neuropsychopharmacol pyab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhnia M, Faulkner ML, Piacentino D, Lee MR, Leggio L (2019) Ghrelin: From a gut hormone to a potential therapeutic target for alcohol use disorder. Physiol Behav 204:49–57. [DOI] [PubMed] [Google Scholar]
- Farokhnia M, Grodin EN, Lee MR, Oot EN, Blackburn AN, Stangl BL, Schwandt ML, Farinelli LA, Momenan R, Ramchandani VA, Leggio L (2018) Exogenous ghrelin administration increases alcohol self-administration and modulates brain functional activity in heavy-drinking alcohol-dependent individuals. Mol Psychiatry 23:2029–2038. [DOI] [PubMed] [Google Scholar]
- Frayn KN, Coppack SW, Walsh PE, Butterworth HC, Humphreys SM, Pedrosa HC (1990) Metabolic responses of forearm and adipose tissues to acute ethanol ingestion. Metabolism 39:958–966. [DOI] [PubMed] [Google Scholar]
- Gortan Cappellari G, Barazzoni R (2019) Ghrelin forms in the modulation of energy balance and metabolism. Eat Weight Disord 24:997–1013. [DOI] [PubMed] [Google Scholar]
- Goshadrou F, Arefi Oskouie A, Eslami M, Nobakht Mothlagh Ghoochani BF (2018) Effect of ghrelin on serum metabolites in Alzheimer’s disease model rats; a metabolomics studies based on 1H-NMR technique. Iran J Basic Med Sci 21:1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD (1997) Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res 48:23–29. [DOI] [PubMed] [Google Scholar]
- Guijas C, Montenegro-Burke JR, Domingo-Almenara X, Palermo A, Warth B, Hermann G, Koellensperger G, Huan T, Uritboonthai W, Aisporna AE, Wolan DW, Spilker ME, Benton HP, Siuzdak G (2018) METLIN: A Technology Platform for Identifying Knowns and Unknowns. Anal Chem 90:3156–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler CL, Long VM, Farokhnia M, Magill M, Kenna GA, Swift RM, Leggio L (2019) Intravenous administration of ghrelin increases serum cortisol and aldosterone concentrations in heavy-drinking alcohol-dependent individuals: Results from a double-blind, placebo-controlled human laboratory study. Neuropharmacology 158:107711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen N, Kärkkäinen O, Laukkanen E, Kekkonen V, Kaarre O, Kivimäki P, Könönen M, Velagapudi V, Nandania J, Lehto SM, Niskanen E, Vanninen R, Tolmunen T (2019) Changes in the serum metabolite profile correlate with decreased brain gray matter volume in moderate-to-heavy drinking young adults. Alcohol 75:89–97. [DOI] [PubMed] [Google Scholar]
- Huang C, Wang J, Hu W, Wang C, Lu X, Tong L, Wu F, Zhang W (2016) Identification of functional farnesoid X receptors in brain neurons. FEBS Lett 590:3233–3242. [DOI] [PubMed] [Google Scholar]
- Irwin C, van Reenen M, Mason S, Mienie LJ, Wevers RA, Westerhuis JA, Reinecke CJ (2018) The 1H-NMR-based metabolite profile of acute alcohol consumption: A metabolomics intervention study. PLoS ONE 13:e0196850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Z-H, Chung H-C, Ahn YG, Kwon Y-K, Kim J-S, Ryu J-H, Ryu DH, Kim C-H, Hwang G-S (2012) Metabolic profiling of an alcoholic fatty liver in zebrafish (Danio rerio). Mol BioSyst 8:2001. [DOI] [PubMed] [Google Scholar]
- Jaremek M, Yu Z, Mangino M, Mittelstrass K, Prehn C, Singmann P, Xu T, Dahmen N, Weinberger KM, Suhre K, Peters A, Döring A, Hauner H, Adamski J, Illig T, Spector TD, Wang-Sattler R (2013) Alcohol-induced metabolomic differences in humans. Transl Psychiatry 3:e276–e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Koo BK, Jang SY, Kim D-I, Lee H, Lee DH, Joo SK, Jung YJ, Park JH, Yoo T, Choi M, Lee MK, Kang SW, Chang MS, Kim W, Hwang G-S, Innovative Target Exploration of NAFLD (ITEN) consortium (2021) Association between circulating bile acid alterations and nonalcoholic steatohepatitis independent of obesity and diabetes mellitus. Liver Int. [DOI] [PubMed] [Google Scholar]
- Kärkkäinen O, Klåvus A, Voutilainen A, Virtanen J, Lehtonen M, Auriola S, Kauhanen J, Rysä J (2020) Changes in circulating metabolome precede alcohol-related diseases in middle-aged men: a prospective population-based study with a 30-year follow-up. Alcohol Clin Exp Res 44:2457–2467. [DOI] [PubMed] [Google Scholar]
- Kärkkäinen O, Kokla M, Lehtonen M, Auriola S, Martiskainen M, Tiihonen J, Karhunen PJ, Hanhineva K, Kok E (2021) Changes in the metabolic profile of human male postmortem frontal cortex and cerebrospinal fluid samples associated with heavy alcohol use. Addict Biol e13035. [DOI] [PubMed] [Google Scholar]
- Keitel V, Görg B, Bidmon HJ, Zemtsova I, Spomer L, Zilles K, Häussinger D (2010) The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia 58:1794–1805. [DOI] [PubMed] [Google Scholar]
- Killenberg PG (1978) Measurement and subcellular distribution of choloyl-CoA synthetase and bile acid-CoA:amino acid N-acyltransferase activities in rat liver. J Lipid Res 19:24–31. [PubMed] [Google Scholar]
- Killenberg PG, Jordan JT (1978) Purification and characterization of bile acid-CoA:amino acid N-acyltransferase from rat liver. J Biol Chem 253:1005–1010. [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D (2011) Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry 68:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A (2002) Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res 26:827–835. [PubMed] [Google Scholar]
- Klåvus A, Kokla M, Noerman S, Koistinen VM, Tuomainen M, Zarei I, Meuronen T, Häkkinen MR, Rummukainen S, Farizah Babu A, Sallinen T, Kärkkäinen O, Paananen J, Broadhurst D, Brunius C, Hanhineva K (2020) “notame”: Workflow for Non-Targeted LC–MS Metabolic Profiling. Metabolites 10:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmann A, von der Goltz C, Grosshans M, Dinter C, Vitale M, Wiedemann K, Kiefer F (2012) The association of the appetitive peptide acetylated ghrelin with alcohol craving in early abstinent alcohol dependent individuals. Psychoneuroendocrinology 37:980–986. [DOI] [PubMed] [Google Scholar]
- Kopchick JJ, Berryman DE, Puri V, Lee KY, Jorgensen JOL (2020) The effects of growth hormone on adipose tissue: old observations, new mechanisms. Nat Rev Endocrinol 16:135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhre RE, Wewer Albrechtsen NJ, Larsen O, Jepsen SL, Balk-Møller E, Andersen DB, Deacon CF, Schoonjans K, Reimann F, Gribble FM, Albrechtsen R, Hartmann B, Rosenkilde MM, Holst JJ (2018) Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Mol Metab 11:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest Package: Tests in Linear Mixed Effects Models. J Stat Soft 82. [Google Scholar]
- Lee MR, Tapocik JD, Ghareeb M, Schwandt ML, Dias AA, Le AN, Cobbina E, Farinelli LA, Bouhlal S, Farokhnia M, Heilig M, Akhlaghi F, Leggio L (2020) The novel ghrelin receptor inverse agonist PF-5190457 administered with alcohol: preclinical safety experiments and a phase 1b human laboratory study. Mol Psychiatry 25:461–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeuwendaal NK, Cryan JF, Schellekens H (2021) Gut peptides and the microbiome: focus on ghrelin. Curr Opin Endocrinol Diabetes Obes 28:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Cardone S, Nesci A, Miceli A, Malandrino N, Capristo E, Canestrelli B, Monteleone P, Kenna GA, Swift RM, Addolorato G (2012) Ghrelin system in alcohol-dependent subjects: role of plasma ghrelin levels in alcohol drinking and craving. Addict Biol 17:452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Zywiak WH, Fricchione SR, Edwards SM, de la Monte SM, Swift RM, Kenna GA (2014) Intravenous ghrelin administration increases alcohol craving in alcohol-dependent heavy drinkers: a preliminary investigation. Biol Psychiatry 76:734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehikoinen AI, Kärkkäinen OK, Lehtonen MAS, Auriola SOK, Hanhineva KJ, Heinonen ST (2018) Alcohol and substance use are associated with altered metabolome in the first trimester serum samples of pregnant mothers. European Journal of Obstetrics & Gynecology and Reproductive Biology 223:79–84. [DOI] [PubMed] [Google Scholar]
- Levin F, Edholm T, Schmidt PT, Grybäck P, Jacobsson H, Degerblad M, Höybye C, Holst JJ, Rehfeld JF, Hellström PM, Näslund E (2006) Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J Clin Endocrinol Metab 91:3296–3302. [DOI] [PubMed] [Google Scholar]
- Loftus N, Barnes A, Ashton S, Michopoulos F, Theodoridis G, Wilson I, Ji C, Kaplowitz N (2011) Metabonomic Investigation of Liver Profiles of Nonpolar Metabolites Obtained from Alcohol-Dosed Rats and Mice Using High Mass Accuracy MS n Analysis. J Proteome Res 10:705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, McGlone F, Bedrossian D, Dagher A (2008) Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab 7:400–409. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Nakamura T, Itadani H, Tanaka K (2002) Identification of membrane-type receptor for bile acids (M-BAR). Biochemical and Biophysical Research Communications 298:714–719. [DOI] [PubMed] [Google Scholar]
- Mennella JA, Pepino MY, Teff KL (2005) Acute alcohol consumption disrupts the hormonal milieu of lactating women. J Clin Endocrinol Metab 90:1979–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens KL, Kalsbeek A, Soeters MR, Eggink HM (2017) Bile Acid Signaling Pathways from the Enterohepatic Circulation to the Central Nervous System. Front Neurosci 11:617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris LS, Voon V, Leggio L (2018) Stress, Motivation, and the Gut-Brain Axis: A Focus on the Ghrelin System and Alcohol Use Disorder. Alcohol Clin Exp Res 42:1378–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa H, Amin AM, Teh C-H, Murugaiyah V, Arif NH, Ibrahim B (2016) Metabolic phenotyping of urine for discriminating alcohol-dependent from social drinkers and alcohol-naive subjects. Drug Alcohol Depend 169:80–84. [DOI] [PubMed] [Google Scholar]
- Naleid AM, Grace MK, Cummings DE, Levine AS (2005) Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides 26:2274–2279. [DOI] [PubMed] [Google Scholar]
- Plawecki MH, Wetherill L, Vitvitskiy V, Kosobud A, Zimmermann US, Edenberg HJ, O’Connor S (2013) Voluntary intravenous self-administration of alcohol detects an interaction between GABAergic manipulation and GABRG1 polymorphism genotype: a pilot study. Alcohol Clin Exp Res 37 Suppl 1:E152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevski E, Horvath TL, Shanabrough M, Hayden R, Newcomb J, Petrakis I (2017) Ghrelin is Supressed by Intravenous Alcohol and is Related to Stimulant and Sedative Effects of Alcohol. Alcohol Alcohol 52:431–438. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Glicksman C, Alaghband-Zadeh J, Sherwood RA, Akuji N, le Roux CW (2011) The relationship between postprandial bile acid concentration, GLP-1, PYY and ghrelin. Clin Endocrinol (Oxf) 74:67–72. [DOI] [PubMed] [Google Scholar]
- Roche DJO, Palmeri MD, King AC (2014) Acute alcohol response phenotype in heavy social drinkers is robust and reproducible. Alcohol Clin Exp Res 38:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JL, Lang CH (2017) Alcohol, Adipose Tissue and Lipid Dysregulation. Biomolecules 7:16. [Google Scholar]
- Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, Fan TW-M, Fiehn O, Goodacre R, Griffin JL, Hankemeier T, Hardy N, Harnly J, Higashi R, Kopka J, Lane AN, Lindon JC, Marriott P, Nicholls AW, Reily MD, Thaden JJ, Viant MR (2007) Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 3:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack J, Depoortere I, Bisschops R, Delporte C, Coulie B, Meulemans A, Janssens J, Peeters T (2006) Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut 55:327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theander-Carrillo C, Wiedmer P, Cettour-Rose P, Nogueiras R, Perez-Tilve D, Pfluger P, Castaneda TR, Muzzin P, Schürmann A, Szanto I, Tschöp MH, Rohner-Jeanrenaud F (2006) Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest 116:1983–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Fuentes C, Golubeva AV, Zhdanov AV, Wallace S, Arboleya S, Papkovsky DB, El Aidy S, Ross P, Roy BL, Stanton C, Dinan TG, Cryan JF, Schellekens H (2019) Short-chain fatty acids and microbiota metabolites attenuate ghrelin receptor signaling. FASEB J 33:13546–13559. [DOI] [PubMed] [Google Scholar]
- Tsugawa H, Cajka T, Kind T, Ma Y, Higgins B, Ikeda K, Kanazawa M, VanderGheynst J, Fiehn O, Arita M (2015) MS-DIAL: data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat Methods 12:523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugawa H, Ikeda K, Takahashi M, Satoh A, Mori Y, Uchino H, Okahashi N, Yamada Y, Tada I, Bonini P, Higashi Y, Okazaki Y, Zhou Z, Zhu Z-J, Koelmel J, Cajka T, Fiehn O, Saito K, Arita M, Arita M (2020) A lipidome atlas in MS-DIAL 4. Nat Biotechnol 38:1159–1163. [DOI] [PubMed] [Google Scholar]
- Vestergaard ET, Gormsen LC, Jessen N, Lund S, Hansen TK, Moller N, Jorgensen JOL (2008) Ghrelin infusion in humans induces acute insulin resistance and lipolysis independent of growth hormone signaling. Diabetes 57:3205–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutilainen T, Kärkkäinen O (2019) Changes in the Human Metabolome Associated With Alcohol Use: A Review. Alcohol and Alcoholism 54:225–234. [DOI] [PubMed] [Google Scholar]
- Wishart DS, Feunang YD, Marcu A, Guo AC, Liang K, Vázquez-Fresno R, Sajed T, Johnson D, Li C, Karu N, Sayeeda Z, Lo E, Assempour N, Berjanskii M, Singhal S, Arndt D, Liang Y, Badran H, Grant J, Serra-Cayuela A, Liu Y, Mandal R, Neveu V, Pon A, Knox C, Wilson M, Manach C, Scalbert A (2018) HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res 46:D608–D617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würtz P, Cook S, Wang Q, Tiainen M, Tynkkynen T, Kangas AJ, Soininen P, Laitinen J, Viikari J, Kähönen M, Lehtimäki T, Perola M, Blankenberg S, Zeller T, Männistö S, Salomaa V, Järvelin M-R, Raitakari OT, Ala-Korpela M, Leon DA (2016) Metabolic profiling of alcohol consumption in 9778 young adults. Int J Epidemiol 45:1493–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallar LJ, Farokhnia M, Tunstall BJ, Vendruscolo LF, Leggio L (2017) The Role of the Ghrelin System in Drug Addiction. Int Rev Neurobiol 136:89–119. [DOI] [PubMed] [Google Scholar]
- Zhang A, Price JL, Leonard D, North CS, Suris A, Javors MA, Adinoff B (2020) Alcohol Use Disorder Masks the Effects of Childhood Adversity, Lifetime Trauma, and Chronic Stress on Hypothalamic-Pituitary-Adrenal Axis Reactivity. Alcohol Clin Exp Res 44:1192–1203. [DOI] [PubMed] [Google Scholar]
- Zimmermann US, O’Connor S, Ramchandani VA (2013) Modeling alcohol self-administration in the human laboratory. Curr Top Behav Neurosci 13:315–353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.