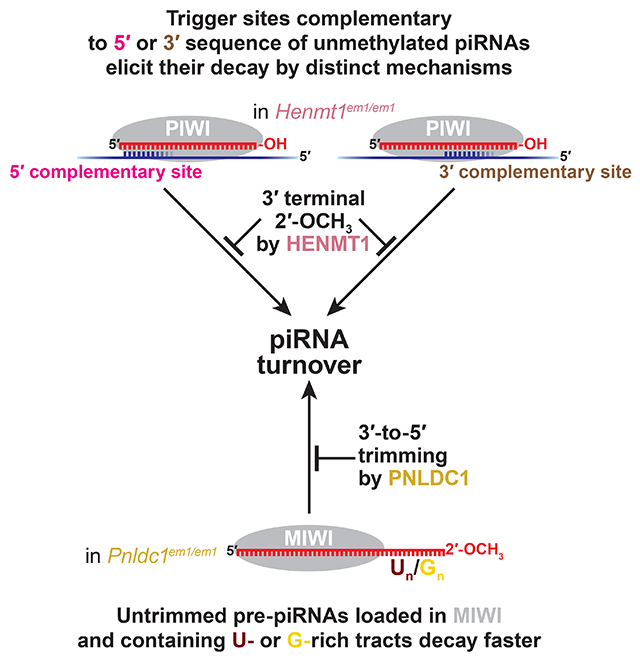
SUMMARY
In animals, piRNAs silence transposons, fight viral infections, and regulate gene expression. piRNA biogenesis concludes with 3′ terminal trimming and 2′-O-methylation. Both trimming and methylation influence piRNA stability. Our biochemical data show that multiple mechanisms destabilize unmethylated mouse piRNAs, depending on whether the piRNA 5′ or 3′ sequence is complementary to a trigger RNA. Unlike target-directed degradation of microRNAs, complementarity-dependent destabilization of piRNAs in mice and flies is blocked by 3′ terminal 2′-O-methylation and does not require base pairing to both the piRNA seed and 3′ sequence. In flies, 2′-O-methylation also protects siRNAs from complementarity-dependent destruction. By contrast, pre-piRNA trimming protects mouse piRNAs from a degradation pathway unaffected by trigger complementarity. In testis lysate and in vivo, internal or 3′-terminal uridine- or guanine-rich tracts accelerate pre-piRNA decay. Loss of both trimming and 2′-O-methylation causes the mouse piRNA pathway to collapse, demonstrating that these modifications collaborate to stabilize piRNAs.
Graphical Abstract
eTOC Blurb
piRNAs are an animal-specific class of small RNAs that repress transposons and regulate genes. piRNA biogenesis concludes with 3′-terminal trimming and 2′-O-methylation. Gainetdinov et al. show that trimming and 2′-O-methylation protect piRNAs from multiple degradation mechanisms triggered by extensively complementary RNAs or sequence motifs in the piRNA.
INTRODUCTION
In animals, three classes of small silencing RNAs direct Argonaute proteins to target RNAs. MicroRNAs (miRNAs) regulate host mRNAs (Bartel, 2018). Small interfering RNAs (siRNAs) target host, transposon, and viral transcripts (Carthew and Sontheimer, 2009). PIWI-interacting RNAs (piRNAs) defend the genome against transposable elements and, in some animals, also regulate gene expression or fight viral infection (Huang et al., 2017; Czech et al., 2018; Yamashiro and Siomi, 2018; Ozata et al., 2019). Although their sequences, lengths, and genomic origins vary, piRNAs guide PIWI proteins, a clade of Argonaute proteins found in nearly all animals, including sponges, cnidarians, arthropods, nematodes and chordates (Aravin et al., 2006; Girard et al., 2006; Lau et al., 2006; Vagin et al., 2006; Grivna et al., 2006; Saito et al., 2006; Houwing et al., 2007; Grimson et al., 2008; Das et al., 2008; Batista et al., 2008; Juliano et al., 2014; Lim et al., 2014b; Lewis et al., 2018).
Unlike miRNA and siRNA biogenesis, piRNA production begins with long single-stranded precursors, which are transcribed by RNA polymerase II from dedicated genomic loci called piRNA clusters (Vagin et al., 2006; Brennecke et al., 2007; Aravin et al., 2006; Girard et al., 2006; Li et al., 2013; Fu et al., 2018b; Özata et al., 2020). PIWI proteins guided by pre-existing piRNAs cleave these precursors, creating 5′ monophosphorylated pre-pre-piRNAs on which piRNA biogenesis initiates (Wang et al., 2014; Han et al., 2015a; Gainetdinov et al., 2018; Izumi et al., 2020). The 5′ monophosphate enables a PIWI protein to bind the pre-pre-piRNA, a process believed to occur in a structure called nuage (Kawaoka et al., 2011; Cora et al., 2014; Wang et al., 2014; Mohn et al., 2015; Han et al., 2015a; Matsumoto et al., 2016; Yamaguchi et al., 2020). After relocalization of the PIWI-bound pre-pre-piRNA to the outer mitochondrial membrane, the endonuclease PLD6 (Zucchini in Drosophila melanogaster) cleaves the pre-pre-piRNA 3′ to the footprint of the PIWI protein, releasing a PIWI-bound pre-piRNA and a new 5′ monophosphorylated pre-pre-piRNA that can bind yet another PIWI protein (Haase et al., 2010; Ipsaro et al., 2012; Nishimasu et al., 2012; Han et al., 2015a; Mohn et al., 2015; Homolka et al., 2015; Gainetdinov et al., 2018; Ge et al., 2019; Munafò et al., 2019; Ishizu et al., 2019; Izumi et al., 2020). Successive cycles of pre-pre-piRNA binding by PIWI proteins and cleavage by PLD6 convert the original piRNA precursor transcript into phased, tail-to-head strings of pre-piRNAs.
In the penultimate step in piRNA biogenesis, the 3′-to-5′ exoribonuclease PNLDC1 in Mus musculus, Trimmer in most arthropods, or PARN-1 in Caenorhabditis elagans establishes the mature length of the piRNA, which reflects the specific PIWI protein to which the piRNA is bound (Tang et al., 2016; Izumi et al., 2016; Zhang et al., 2017; Ding et al., 2017; Nishimura et al., 2018; Gainetdinov et al., 2018). (In flies and likely other members of the Brachycera suborder of Diptera, which lack PNLDC1, the miRNA-trimming exoribonuclease, Nibbler, trims a subset of piRNAs (Han et al., 2011; Liu et al., 2011; Feltzin et al., 2015; Wang et al., 2016; Hayashi et al., 2016; Xie et al., 2020).) piRNA biogenesis concludes when the S-adenosylmethionine-dependent methyltransferase HENMT1 in mice, Hen1 in arthropods, or HENN-1 in worms modifies the 2′ hydroxyl at the 3′ end of the piRNA (Saito et al., 2007; Horwich et al., 2007; Kirino and Mourelatos, 2007; Kamminga et al., 2010; Montgomery et al., 2012; Billi et al., 2012; Kamminga et al., 2012; Lim et al., 2015; Svendsen et al., 2019). Pnldc1−/− mutant mice accumulate untrimmed pre-piRNAs bound to PIWI proteins (Ding et al., 2017; Zhang et al., 2017; Nishimura et al., 2018; Gainetdinov et al., 2018). The abundance of these piRNA intermediates is 50–70% lower than in wild-type (Gainetdinov et al., 2018). Conversely, Henmt1−/− mutant males trim their piRNAs but cannot methylate their 3′ termini. Failure to methylate piRNAs halves the abundance of unmethylated piRNAs (Lim et al., 2015). The molecular defects in these mutants suggest that both trimming and 2′-O-methylation play a role in stabilizing piRNAs.
Dicer enzymes set the mature length of animal miRNAs and siRNAs, and they are typically stable without further modification (Bartel, 2018). However, most arthropod siRNAs are 2′-O-methylated (Pélisson et al., 2007; Lewis et al., 2018; Fu et al., 2018b); in flies, siRNAs are unstable in the absence of 2′-O-methylation (Ameres et al., 2010).
Here, we report that 3′ terminal 2′-O-methylation and 3′-to-5′ trimming protect mouse piRNAs against distinct degradation mechanisms. More than a decade ago, methylation was proposed to protect piRNAs from extensively complementary targets (Ameres et al., 2010). Our genetic and biochemical data show that RNA sequences complementary to either the piRNA 5′ or 3′ portion trigger destruction of unmethylated piRNAs. Biochemical data show that trigger RNAs with complementarity restricted to piRNA 5′ or 3′ sequence invoke distinct degradation mechanisms; 2′-O-methylation protects piRNAs from both pathways, suggesting that the decay of unmethylated piRNAs is distinct from target-directed miRNA degradation (TDMD), which is insensitive to 2′-O-methylation. In vivo in flies, 2′-O-methylation blocks complementarity-dependent destabilization of both piRNAs and siRNAs. Animal miRNAs are unmethylated, and complementarity-dependent destabilization helps explain differences in miRNA decay rates in both mice and flies. By contrast, complementary RNAs do not destabilize untrimmed mouse pre-piRNAs. Instead, both PIWI protein identity and the presence of uridine- or guanine-rich tracts in the untrimmed sequence of a pre-piRNA predict faster decay. In Pnldc1em1/em1; Henmt1em1/em1 double-mutant male mice, which can neither trim nor methylate piRNAs, the piRNA pathway collapses. Thus, piRNA trimming and methylation collaborate to stabilize piRNAs: the double-mutant mice make fivefold fewer piRNAs, and spermatogenesis arrests at the pachytene stage of meiosis I. The loss of piRNAs derepresses piRNA target transcripts. By decreasing the turnover of piRNAs, methylation and trimming maintain the high steady-state level of piRNAs required for successful spermatogenesis.
RESULTS
Pre-piRNA Trimming and 2′-O-methylation Protect Mouse piRNAs From Different Degradation Mechanisms
Do methylation and trimming protect mouse piRNAs from the same or different degradation mechanisms (Figure S1A)? If the same mechanism acts on unmethylated piRNAs and untrimmed pre-piRNAs, then the abundance of an unmethylated but trimmed piRNA in a Henmt1 mutant should correlate with the abundance of the corresponding untrimmed but 2′-O-methylated pre-piRNA in a Pnldc1 mutant. We compared the decrease in abundance of unmethylated piRNAs and the corresponding untrimmed pre-piRNAs by identifying piRNAs in Henmt1em1pdz/em1pdz and pre-piRNAs in Pnldc1em1pdz/em1pdz (henceforth, Henmt1em1/em1 and Pnldc1em1/em1) that began with the same 24-nt sequence (i.e., 5′ prefix). The decrease of piRNAs in Henmt1em1/em1 and of pre-piRNAs in Pnldc1em1/em1 were poorly correlated (Pearson’s ρ = 0.08 ± 0.03, p = 0.01 ± 0.03 for primary spermatocytes; ρ = 0.26 ± 0.07, p = 1 ± 3 × 10− 26 for spermatogonia; Figure 1A). Because overlapping but distinct subsets of piRNAs or pre-piRNAs were lost in each mutant, we conclude that 2′-O-methylation and trimming protect piRNAs from distinct degradation mechanisms.
Figure 1. In mice, 2′-O-methylation Protects piRNAs from Destabilization by Complementary Sites in the Transcriptome.

(A) Change in piRNA abundance in Henmt1em1/em1 and Pnldc1em1/em1 in FACS-purified mouse spermatogonia and primary spermatocytes. A representative experiment is shown, correlation data are the mean ± SD for all possible permutations of biological replicates (C57BL/6, nspermatogonia = 2, np.spermatocytes = 3; Henmt1em1/em1, nspermatogonia = 3, np.spermatocytes = 3; Pnldc1em1/em1, nspermatogonia = 2, np.spermatocytes = 3).
(B) Left, the predicted fraction of different regions of mouse piRNAs bound to complementary sites in transcripts; data are from a representative experiment from FACS-purified mouse primary spermatocytes. Right, the difference between the predicted median fraction bound for stable and unstable unmethylated piRNAs for contiguous complementarity starting at different piRNA nucleotides; data are for all possible permutations of biological replicates of FACS-purified primary spermatocytes (C57BL/6, np.spermatocytes = 3; Henmt1em1/em1, np.spermatocytes = 3). The 95% confidence interval was calculated with 10,000 bootstrapping iterations.
2′-O-methylation Inhibits Complementarity-Dependent Destabilization of Mouse piRNAs
miRNAs with extensively complementary targets are unstable—a phenomenon termed target-directed miRNA degradation (TDMD; Cazalla et al., 2010; Ameres et al., 2010; Baccarini et al., 2011; Libri et al., 2012; Marcinowski et al., 2012; Rüegger and Großhans, 2012; Lee et al., 2013; de la Mata et al., 2015; Marzi et al., 2016; Duffy et al., 2015; Bitetti et al., 2018; Kleaveland et al., 2018; Ghini et al., 2018; Sheu-Gruttadauria et al., 2019; Zhang et al., 2019; Han et al., 2020; Shi et al., 2020). In most animals, miRNAs bear a 3′ terminal 2′-hydroxyl. TDMD is insensitive to 2′-O-methylation, and synthetic 2′-O-methylated miRNAs transfected into cultured animal cells are nonetheless destroyed by TDMD (Han et al., 2020; Shi et al., 2020). Yet all miRNAs in the sea anemone Nematostella vectensis are 2′-O-methylated to some extent, and depletion of the N. vectensis homolog of the methyltransferase HENMT1 reduces miRNA stability (Moran et al., 2014; Modepalli et al., 2018). Like piRNAs, N. vectensis miRNAs often cleave their targets through near-perfect complementarity (Moran et al., 2014).
A role for 2′-O-methylation in protecting piRNAs from destabilization by extensively complementary RNAs was proposed a decade ago (Ameres et al., 2010). Some piRNAs remain stable in Henmt1em1/em1 mutants, while others decrease in abundance compared to wild-type C57BL/6 (Figure 1A). If complementarity-dependent destabilization triggers piRNA degradation in the absence of 2′-O-methylation, unstable piRNAs are expected to have more high-affinity complementary sites in the transcriptome than stable piRNAs. That is, at any moment in time, the likelihood for an unstable piRNA to be bound by a complementary trigger RNA is greater than for a stable piRNA. Fraction bound reflects equilibrium between free piRNA and piRNA bound to sites in the transcriptome. If an unstable piRNA has more complementary sites, then, for an unstable piRNA, the fraction of molecules bound to complementary sites will be higher than for a stable piRNA. The fraction bound, f, takes into account both the abundance of complementary sites and their affinity for a piRNA (Ka, association constant). By definition,
To estimate f for stable and unstable piRNAs, we (1) measured the intracellular concentration of their complementary sites ([complementary sites]total) by sequencing transcripts from FACS-sorted primary mouse germ cells, and (2) approximated the corresponding association constants using the nearest neighbor method (Lorenz et al., 2011; Anzelon et al., 2020; STAR Methods). We defined unstable piRNAs as those whose steady-state abundance in Henmt1em1/em1 was reduced to ≤ 20% of C57BL/6 levels, and stable piRNAs as those whose steady-state abundance remained ≥ 80% of C57BL/6 levels.
For complementary sites beginning at piRNA nucleotides g2, g9, or g13, the predicted median fraction bound for unstable piRNAs was ≥ 0.9, whereas the median fraction bound for stable piRNAs was ≤ 0.2 (Figure 1B). Thus, the predicted fraction bound for unstable piRNAs was significantly higher than for stable piRNAs (Figures 1B and S1B). For example, in primary spermatocytes, for complementary sites starting at nucleotide g2, the difference in predicted median fraction bound between unstable and stable piRNAs was ~0.9 (95% confidence interval [CI]: 0.87–0.92).
The large difference in predicted median fraction bound between unstable and stable piRNAs was specific for Henmt1em1/em1. Without MIWI or the piRNA biogenesis factor TDRKH, piRNA are lost, but are still 2′-O-methylated (Reuter et al., 2011; Saxe et al., 2013). piRNA instability in Miwi−/− and Tdrkh−/− mutants was not associated with complementary sites in the transcriptome: the difference in the predicted median fraction bound for stable and unstable piRNAs was < 0.2 in both Miwi−/− and Tdrkh−/− testes (Figure S1C).
Degradation of unmethylated piRNAs is distinct from TDMD. For miRNAs, TDMD requires both a seed match and extensive pairing to the 3′ miRNA sequence (Cazalla et al., 2010; Ameres et al., 2010; Sheu-Gruttadauria et al., 2019). Our data from Henmt1em1/em1 mice show that unmethylated piRNAs become unstable if they bear sites complementary to either the piRNA 5′ or 3′ sequence. When we confined analyses to sites complementary to the piRNA 5′ sequence and devoid of 3′ supplemental contiguous complementarity, the difference in the predicted median fraction bound between stable and unstable piRNAs was ~0.9 (CI: 0.86–0.92; Figure S1D). Similarly, for sites complementary to the piRNA 3′ sequence and devoid of additional 5′ contiguous pairing, the difference in the predicted median fraction bound between stable and unstable piRNAs was ~0.8 (CI: 0.74–0.84; Figure S1D). We conclude that 3′ terminal 2′-O-methylation protects mouse piRNAs from degradation elicited by RNAs complementary to either 5′ or 3′ piRNA sequences.
Different Mechanisms Destabilize Unmethylated Mouse piRNAs with Complementarity to 5′ or 3′ piRNA Sequences
Testis lysate recapitulates complementarity-dependent degradation of an unmethylated, but not a 2′-O-methyl modified, synthetic 30-nt piRNA bound to affinity purified, recombinant MIWI (piRISC; Arif et al., 2021). In the absence of a complementary trigger, piRNAs bearing either a 3′ terminal 2′-O-methyl or hydroxyl group were stable in Henmt1em1/em1 cytoplasmic testis lysate for up to 20 h (Figure 2A). Addition of a 30-nt, fully 2′O-methylated complementary RNA (cRNA) containing a 7-nt seed match to piRNA nucleotides g2–g8 did not alter the stability of the unmethylated piRNA, suggesting that seed-matched targets do not influence piRNA stability (Figure 2A). (The 2′-O-methyl modification stabilizes the cRNA in testis lysate.) cRNAs bearing ≥ 9-nt matches to the 5′ or 3′ sequence of the MIWI-bound piRNA triggered shortening of an unmodified, but not a 2′-O-methylated, piRNA (Figure 2A). The shortened, 15–29-nt unmethylated piRNA guides were still loaded in MIWI, since they co-precipitated with the protein (Figure S2A). MIWI binds 28–31 -nt piRNAs both in C57BL/6 and in Henmt1em1/em1 primary spermatocytes (Figure S2B), suggesting that, in vivo, either that the shortened piRNA guides are removed from MIWI or that MIWI loaded with shortened piRNAs is destroyed, steps not recapitulated in testis lysate (Figure S2A). We propose that in vivo extensively complementary RNA sites trigger shortening of unmethylated piRNAs and initiate destruction of MIWI piRISC with shortened piRNAs.
Figure 2. Complementary RNAs Destabilize MIWI-loaded, Unmethylated piRNAs In Testis Lysate.

(A) MIWI piRISC, loaded with a synthetic, 5′-32P-radiolabeled, 30-nt piRNA bearing a 2′-O-methyl or hydroxyl 3′ end was incubated in Henmt1em1/em1 testis lysate with 30-nt, fully 2′-O-methylated complementary RNA (cRNA). The mean of the guide abundance is shown (n = 3).
(B) As in (A), a time series is shown for MIWI piRISC incubated in Henmt1em1/em1 testis lysate with 30-nt, fully 2′-O-methylated cRNA.
(C) As in (A), except Henmt1em1/em1 testis lysate was preincubated for 5 min at the indicated temperature before adding MIWI piRISC and cRNA; MIWI piRISC was then incubated in lysate with cRNA for an additional 5 h.
Distinct mechanisms destabilize piRNAs when the trigger matches piRNA 5′ versus 3′ sequences. Before adding MIWI piRISC and cRNA, we preincubated Henmt1−/− testis lysate at different temperatures to determine the heat lability of each pathway. When the testis lysate was heated for 5 min at >45°C, the 2′-hydroxy piRNA was stable in the presence of cRNAs complementary to piRNA nucleotides g13–g30 or g16–g30 (Figures 2C and S3B). By contrast, piRNA shortening triggered by cRNAs complementary to nucleotides g2–g10 or g2–g12 was detectable even when the lysate was preincubated at 55°C (Figures 2C and S3B). In addition to differential temperature sensitivity, the two piRNA degradation pathways had different cRNA length requirements: cRNAs whose length and extent of piRNA complementarity were the same triggered piRNA shortening only when base-pairing with piRNA 3′ sequence but not with the 5′ sequence (Figure S3C). For example, a 13-nt cRNA complementary to g2–g14 failed to trigger piRNA shortening, while a 13-nt cRNA complementary to g18–g30 triggered extensive piRNA shortening (Figure S3C).
Adding a seed match to the cRNA complementary to piRNA nucleotides g13-g30 enhanced piRNA shortening relative to the cRNA bearing only g13–g30 complementarity to the piRNA (Figure 2B). Nonetheless, shortening still depended solely on the activity responsive to 3′ complementary sites: shortening triggered by the g2-g8 + g13–g30 cRNAs was undetectable when the testis lysate was preincubated for 5 min at >45°C (Figure 2C). These data suggest that the addition of a seed match extends the residence time of the cRNA on piRISC but does not trigger piRNA shortening via 5′ complementarity. We conclude that (1) in testis lysate and in vivo (Figure S1D), a seed match is dispensable for piRNA destabilization triggered by sites complementary to the piRNA 3′ region; and (2) distinct mechanisms underlie destabilization of unmethylated piRNAs triggered by RNAs complementary to the 5′ and 3′ sequence of a piRNA.
2′-O-methylation Inhibits Complementarity-Dependent Destabilization of Fly piRNAs
Terminal 2′-O-methylation also protected piRNAs from complementarity-dependent destabilization in D. melanogaster. Compared to w1118 controls, total piRNA abundance decreased ~5-fold in hen1f00810 ovaries, which lack the Hen1 methyltransferase (Horwich et al., 2007), and the majority of unmethylated piRNAs were ≥ 20% of w1118 levels (Figures S4A). As in mice, the predicted fraction of unstable piRNAs bound to complementary long RNAs was greater than that of stable piRNAs: e.g., for complementary sites starting at piRNA nucleotide g14, the difference in the predicted median fraction bound between unstable and stable piRNAs was ~0.75 (95% CI: 0.56–0.79; Figure 3). Unlike mouse piRNAs, fly piRNAs were destabilized only by transcripts with extensive complementarity to the 3′ sequence of the piRNA (complementary sites beginning at nucleotides g9–g14); sites complementary to the piRNA 5′ sequence had little effect on stability (Figure 3).
Figure 3. In Vivo in Flies, 2′-O-methylation Protects piRNAs against Destabilization by Complementary Sites.
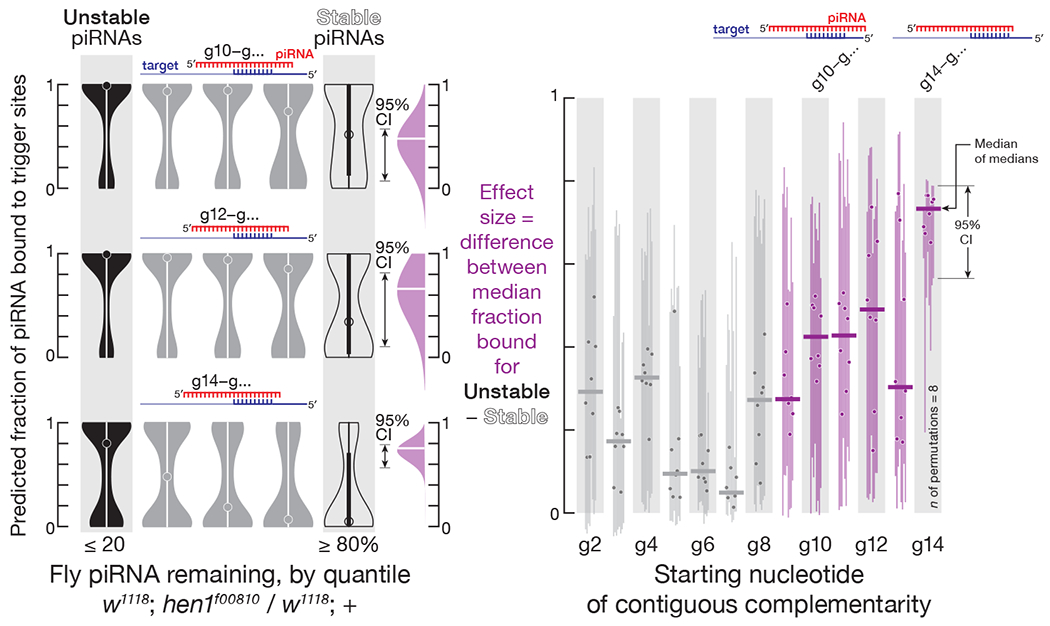
Left, the predicted fraction of fly piRNAs bound to complementary sites in transcripts for different regions of the piRNA; a representative experiment from fly ovaries is shown. Right, the difference in the predicted median fraction bound for stable (≥ 80% of w1118 levels in hen1f00810) and unstable (≤ 20% of w1118 levels in hen1f00810) unmethylated piRNAs for contiguous complementarity starting at different piRNA nucleotides; data are for all possible permutations of biological replicates of fly ovaries (w1118, n = 4; hen1f00810, n = 2). Grey: data for piRNA regions for which the predicted median fraction bound did not decrease monotonically in the direction from unstable to stable piRNAs. The 95% confidence interval for the effect size of median difference was calculated with 10,000 bootstrapping iterations.
The majority of fly piRNAs are derived from transposon sequences. Transposon-derived piRNAs are expected to bear many extensively complementary sites in transposon transcripts and have a high predicted fraction bound to trigger RNAs. Supporting this idea, for piRNAs derived from mRNAs or long non-coding RNAs, the predicted median fraction bound was ~0.45. By contrast, the predicted median fraction bound was ~0.9 for LINE-derived piRNAs and >0.7 for piRNAs corresponding to the LTR-retrotransposon families Copia and Gypsy (Figure S4B).
Our data demonstrate that, as in mice, 3′ terminal 2′-O-methylation protects piRNAs from complementarity-dependent destabilization in flies. While mouse piRNAs are destabilized by complementarity to piRNA 5′ or 3′ sequences, fly piRNAs instability is triggered only by transcripts with matches to piRNA 3′ sequence.
Destabilization of Untrimmed Mouse Pre-piRNAs does not Depend on Complementarity to Trigger RNAs
Untrimmed pre-piRNAs in Pnldc1 mutant mice are 2′-O-methylated (Nishimura et al., 2018), suggesting that a pathway insensitive to 3′ terminal modification degrades pre-piRNAs in the absence of trimming. Our data suggest that complementarity to sites in the transcriptome plays a limited role in untrimmed pre-piRNA instability. We estimated the fraction of stable and unstable pre-piRNAs bound to potential complementary trigger sequences. The difference in the predicted median fraction bound between unstable and stable untrimmed pre-piRNAs in Pnldc1em1/em1 was at least three-fold smaller than the corresponding difference for unstable and stable unmethylated piRNAs in Henmt1em1/em1 (Figure S1C): e.g., in primary spermatocytes for complementarity starting at position g2, the difference in predicted median fraction bound between unstable and stable pre-piRNAs in Pnldc1em1/em1 was ~0.2 versus ~0.9 between unstable and stable unmethylated piRNAs in Henmt1em1/em1 (Figure S1C).
Mouse Testis Lysate Recapitulates Pre-piRNA Trimming
Wild-type testis lysate recapitulated trimming of a 45-nt synthetic pre-piRNA bound to MIWI. After 6 h incubation in C57BL/6 whole testis lysate, a MIWI-bound pre-piRNA was trimmed to 28–38 nt (Figure 4). Silkmoth Trimmer, the ortholog of mouse PNLDC1, activity is enriched in the mitochondrial fraction of BmN4 cell lysate (Izumi et al., 2016). When ectopically co-expressed with TDRKH, mouse PNLDC1 localizes to mitochondria in HeLa cells (Ding et al., 2019). Incubation of MIWI-bound, 45-nt pre-piRNA in mitochondrial extract efficiently trimmed the pre-piRNA to 28 nt (Figure 4), suggesting that PNLDC1 is also associated with mitochondria in mouse germ cells.
Figure 4. Mouse Pre-piRNA Trimming in Testis Lysate.
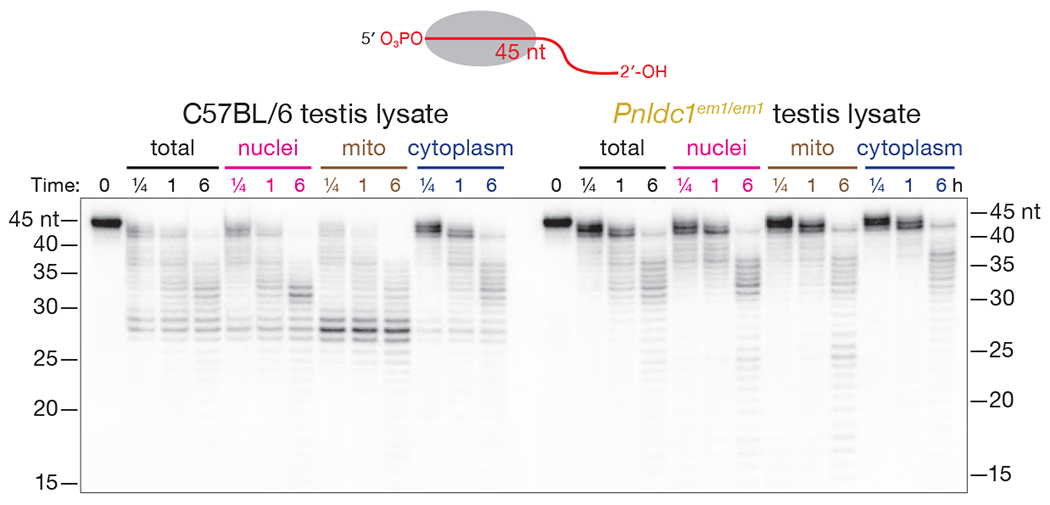
MIWI piRISC loaded with a 2′-hydroxyl, 5′-32P-radiolabeled, synthetic, 45-nt pre-piRNA was incubated in total lysate or subcellular fractions of C57BL/6 or Pnldc1em1/em1 testes.
Oligouridine or Oligoguanine Tracts Promote Pre-piRNA Decay in Testis Lysate
A subset of untrimmed pre-piRNAs become unstable and decrease in abundance in Pnldc1em1/em1, yet other pre-piRNAs are as stable as the corresponding trimmed piRNAs in C57BL/6 testes (Figure 1A). What makes some untrimmed pre-piRNAs unstable? Non-templated 3′-terminal oligouridylation accelerates decay of pre-miRNAs and mRNAs (Rissland and Norbury, 2009; Heo et al., 2009; Thornton et al., 2012; Chang et al., 2013; Ustianenko et al., 2013; Malecki et al., 2013; Faehnle et al., 2014; Lim et al., 2014a; Kim et al., 2015; Reimão-Pinto et al., 2016; Łabno et al., 2016; Ustianenko et al., 2016; Morgan et al., 2017; Faehnle et al., 2017; Chang et al., 2018; Zigáčková and Vaňáčová, 2018; Morgan et al., 2019). Similarly, 3′-terminal oligoadenylation destabilizes miRNAs and some nuclear transcripts (Backes et al., 2012; Vanácová et al., 2005; Wyers et al., 2005; LaCava et al., 2005; Tudek et al., 2018).
To test whether homooligomer sequences promote pre-piRNA destruction, we compared the stability of MIWI-bound, 45-nt pre-piRNAs bearing six 3′ adenines, cytosines, guanines, or uridines. The mean lifetime of MIWI-loaded, synthetic pre-piRNAs with 3′ oligoadenine or oligocytosine were similar to the control pre-piRNA (τcontrol = 600 ± 800 min; τ3′6 = 600 ± 700 min; τ3′C6 = 300 ± 400 min; Figures 5A and S4C). By contrast, pre-piRNAs with 3′-terminal oligouridine or oligoguanine were destroyed faster than the control pre-piRNA (τ3′U6 = 116 ± 6 min, τ3′6 = 60 ± 2 min; Figure 5A). We note that unlike oligouridine, oligoguanine caused a rapid initial pre-piRNA decay followed by a slow phase that reached an asymptote after ~3 h (Figure 5A). These data are consistent with separate mechanisms destroying pre-piRNAs with G- or U-tracts, although it is formally possible that a single pathway destroys both at different rates.
Figure 5. Guanine or Uridine Tracts Destabilize Untrimmed pre-piRNAs in Testis Lysate and In Vivo.

(A) MIWI piRISC loaded with a 2′-O-methyl, 5′-32P-radiolabeled, synthetic, 45-nt pre-piRNA was incubated in Pnldc1em1/em1 testis lysate. A representative experiment is shown, the lifetime is mean ± SD (n = 3).
(B) Frequency of 5mers in all pre-piRNAs or in unstable pre-piRNAs in FACS-purified Pnldc1em1/em1 mouse primary spermatocytes. A representative experiment is shown.
Curiously, internal G- or U-tracts destabilized pre-piRNAs as efficiently as terminal oligomers. MIWI-bound, synthetic pre-piRNAs with 3′ terminal and internal oligouridine tracts were destroyed at similar rates (τu6internal = 56 ± 2 min; Figure 5A). Likewise, pre-piRNAs with 3′ terminal and internal oligoguanine stretches decayed at similar rates (τG6internal = 80 ± 30 min; Figure 5A).
In Vivo, Unstable pre-piRNAs Often Contain U- or G-Tracts
The results of our biochemical experiments in testis lysate were borne out in vivo: unstable pre-piRNAs in Pnldc1em1/em1 mice were enriched for U- or G-tracts (Figure 5B, right). These U- or G-rich sequences were associated with pre-piRNA instability and were especially prominent in the trimmed part of the pre-piRNA, i.e., the sequence that is removed by PNLDC1 to generate a mature piRNA (Figure 5B, left). The instability-associated G- and U-rich sequences do not reflect non-templated nucleotide addition, but rather were part of the original piRNA precursor transcript. Neither positional nucleotide composition (Figure S4D) nor the strength of predicted secondary structures within a pre-piRNA sequence explained pre-piRNA instability (Spearman’s ρ = 0.09 ± 0.02, p = 1 ± 1 × 10−5, npermutations = 9; Figure S4E).
Degradation of Untrimmed Mouse Pre-piRNAs Reflects PIWI Protein Identity
Untrimmed pre-piRNAs bound to MIWI were less stable in vivo than those bound to MILI. To compare the stability of untrimmed pre-piRNAs bound to MILI versus MIWI, we exploited the observation that Argonautes generally are unstable without a small RNA guide (Haase et al., 2010; Zamparini et al., 2011; Derrien et al., 2012; Martinez and Gregory, 2013; Martinez et al., 2013; Smibert et al., 2013; Kobayashi et al., 2019). Because piRNA biogenesis requires binding of PIWI protein to the 5′ end of a pre-pre-piRNA, all pre-piRNAs and piRNAs are anticipated to be bound by PIWI proteins (Gainetdinov et al., 2018). We therefore used the change in the abundance of MIWI and MILI (Figures S5A and S5B) to infer the change in abundance of MIWI- and MILI-bound pre-piRNAs in Pnldc1em1/em1 and piRNAs in Henmt1em1/em1 males. In Pnldc1em1/em1 primary spermatocytes, MIWI abundance was ~30% of C57BL/6, whereas MILI was ~80% of the control level (Gainetdinov et al., 2018). In contrast, the abundance of MIWI and MILI declined by similar extents in Henmt1em1/em1 primary spermatocytes compared to cells from C57BL/6 (~70% for MIWI and ~80% for MILI; Figure S5B).
Irrespective of the PIWI protein to which they are bound, long pre-piRNAs might be inherently unstable, and pre-piRNAs bound to MIWI are, on average, ~3 nt longer than those bound to MILI (Ding et al., 2017; Gainetdinov et al., 2018). Our analyses do not support this explanation: for both MIWI- and MILI-bound pre-piRNAs, length was not correlated with instability in Pnldc1em1/em1 primary spermatocytes (MIWI: Spearman’s ρ = 0.11 ± 0.04, p = 5 ± 7 × 10−30, npermutations = 4; MILI: Spearman’s ρ = − 0.03 ± 0.03, p = 0.4 ± 0.4, npermutations = 4; Figure S5C). We conclude that PIWI protein partner identity, not pre-piRNA length, determines the instability of untrimmed pre-piRNAs.
Decreased piRNA Abundance in Henmt1em1/em1 and Pnldc1em1/em1 Mouse Spermatocytes Reduces Cleavage of Target RNAs
Previous studies suggest that pachytene piRNAs regulate their targets by an siRNA-like cleavage mechanism (Reuter et al., 2011; Zhang et al., 2015; Goh et al., 2015; Wu et al., 2020; Choi et al., 2021). Consistent with this model, target cleavage was reduced for mRNAs whose slicing was directed by unstable, unmethylated piRNAs in Henmt1em1/em1 or unstable, untrimmed pre-piRNAs in Pnldc1em1/em1 mouse primary spermatocytes. We sequenced 5′ monophosphorylated long RNAs from mouse primary spermatocytes to identify putative 3′ cleavage products generated by piRNA-directed, MIWI- or MILI-catalyzed slicing (Figure 6). To restrict the candidates to high-confidence cleavage sites, we required piRNA nucleotides g2–g14 to pair with the site of complementarity such that the cleavage occurred between target nucleotides t10 and t11 (Reuter et al., 2011; Wang et al., 2014; Zhang et al., 2015; Goh et al., 2015; Wu et al., 2020; Figure 6A). We then classified each putative 3′ cleavage product by the stability of its corresponding candidate piRNA in Henmt1em1/em1 or pre-piRNA in Pnldc1em1/em1. In Henmt1em1/em1 primary spermatocytes, the abundance of 3′ cleavage products produced by unstable piRNAs decreased more than those produced by stable piRNAs (two-tailed KS test, p = 2 × 10−4; Figure 6B). Similarly, in Pnldc1em1/em1 primary spermatocytes, the decrease in abundance of 3′ cleavage products generated by unstable pre-piRNAs was greater compared to those generated by stable pre-piRNAs (two-tailed KS test, p = 4 × 10−5; Figure 6B).
Figure 6. In Mice, a Decrease in piRNA Abundance Causes a Corresponding Reduction in Target RNA Cleavage.

(A) Strategy to identify piRNA target RNAs by sequencing 5′ monophosphorylated RNAs.
(B) The change in steady-state abundance of putative 3′ cleavage products produced by piRNA-guided MILI- or MIWI-catalyzed cleavage. Targets were required to pair contiguously with piRNA nucleotides g2–g14. Data are from FACS-purified primary spermatocytes. Only piRNAs whose abundance was ≥ 50 molecules per C57BL/6 primary spermatocyte were analyzed. P values were calculated using a two-tailed KS test.
In Henmt1em1/em1, the decreased abundance of individual unmethylated piRNAs in primary spermatocytes explained the increased steady-state level of 15 different mRNAs (Table S1). Increased target RNA abundance lagged the decrease in unmethylated piRNAs, and was often observed only in secondary spermatocytes or round spermatids (Table S1). This phenomenon was observed previously for mice lacking piRNAs from a piRNA-producing locus on chromosome 6 (Wu et al., 2020).
Similarly, the decreased abundance of untrimmed pre-piRNAs in primary spermatocytes explained the increased steady-state level of 26 mRNAs in Pnldc1em1/em1 mutant primary or secondary spermatocytes or round spermatids (Table S1). Consistent with the finding that different subsets of piRNAs are unstable in Henmt1em1/em1 and Pnldc1em1/em1 testes, just three increased mRNAs were common to the two mutants; these were targeted by piRNAs that were unstable in both Henmt1em1/em1 and Pnldc1em1/em1 germ cells (Table S1).
We conclude that trimming and methylation collaborate to stabilize piRNAs, maintaining the high steady-state levels required to silence piRNA targets.
The Mouse piRNA Pathway Collapses in the Absence of Both Trimming and 2′-O-Methylation
Without HENMT1 and PNLDC1, piRNA abundance in spermatogonia decreased ~fivefold (Figures 7A and 7B), and Henmt1em1/em1; Pnldc1em1/em1 germ cells developed no further than the pachytene stage of meiosis (Figures 7C–7E). By contrast, single mutant Henmt1em1/em1 and Pnldc1em1/em1 spermatogonia had ~2–3-fold fewer piRNAs and arrested after concluding meiosis (Figures 7A–7E). The greater decrease in piRNA abundance in Henmt1em1/em1; Pnldc1em1/em1 double mutants compared to single mutant mice reinforces the idea that distinct sets of piRNAs become unstable in the absence of trimming and methylation.
Figure 7. The piRNA Pathway Collapses in Henmt1em1/em1; Pnldc1em1/em1 Double Mutants.
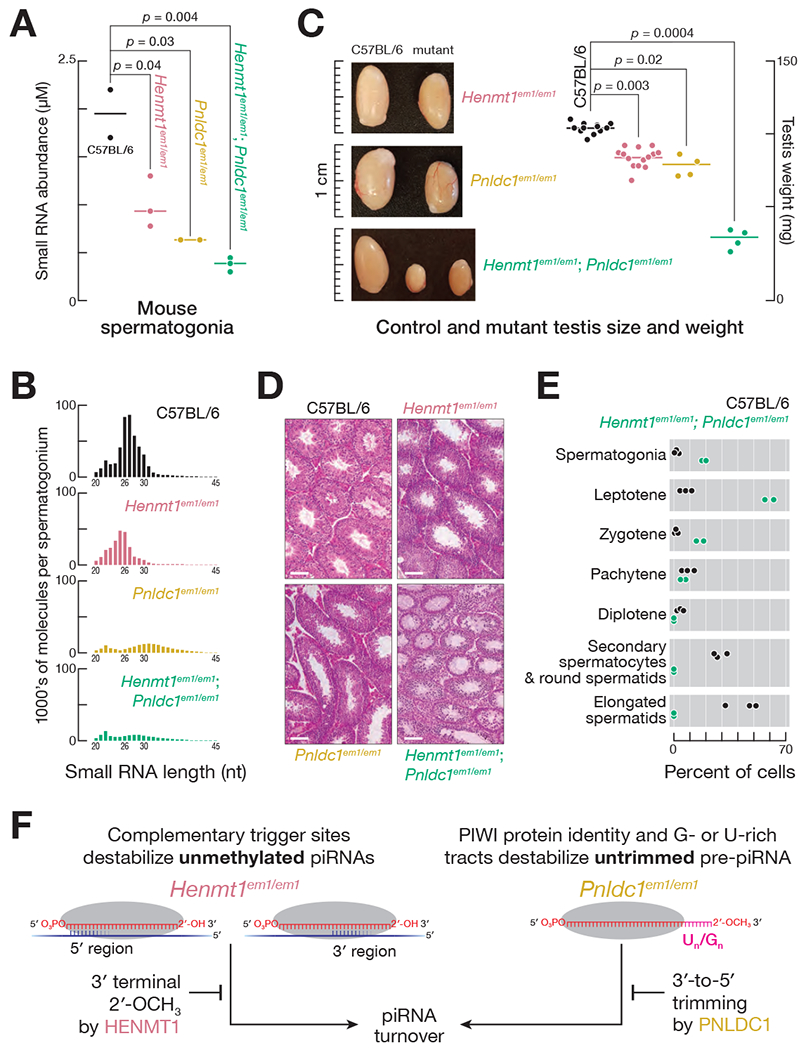
(A) Median (n = 2–3) abundance of mouse pre-pachytene piRNAs and pre-piRNAs in FACS-purified spermatogonia (24–33-nt small RNAs for C57BL/6 and Henmt1em1/em1; 24–45-nt small RNAs for Pnldc1em1/em1 and Pnldc1em1/em1; Henmt1em1/em1). P values were calculated using a two-tailed Mann–Whitney U test.
(B) Mean small RNA length profiles for ≥ 20-nt small RNAs from C57BL/6 (n = 2), Henmt1em1/em1 (n = 3), Pnldc1em1/em1 (n = 2), and Henmt1em1/em1; Pnldc1em1/em1 (n = 3) FACS-purified spermatogonia.
(C) Size and median (n = 4–13) weight of testes from 2–4 month-old C57BL/6, Henmt1em1/em1, Pnldc1em1/em1 or Henmt1em1/em1; Pnldc1em1/em1 mice. P values were calculated using a two-tailed Mann–Whitney U test.
(D) Hematoxylin and eosin staining of sections from 2–4 month-old C57BL/6, Henmt1em1/em1 or Pnldc1em1/em1 single-mutant or Henmt1em1/em1; Pnldc1em1/em1 double-mutant testes.
(E) Germ cell composition of C57BL/6 and Henmt1em1/em1; Pnldc1em1/em1 testes. Each data point corresponds to one animal.
(F) A model for how trimming and 2′-O-methylation collaborate to stabilize mouse piRNAs.
Unlike Henmt1em1/em1 or Pnldc1em1/em1 single mutants, the steady-state abundance of transposon mRNA increased in Henmt1em1/em1; Pnldc1em1/em1 spermatogonia (~2-fold for L1-A, padj = 10−8; ~ 1.7-fold for L1-Gf, padj = 0.045; ~3.6-fold for IAPEY4, padj = 2 × 10−8; Figures S6A and S6B, Table S2). piRNAs bound to MIWI2 direct DNA methylation of transposons in the fetal testis (Aravin et al., 2008; Kuramochi-Miyagawa et al., 2008). We used targeted bisulfite sequencing to measure the extent of DNA methylation of evolutionarily younger IAP LTR elements and LINE subfamilies LI-Gf and L1-A. In both C57BL/6 and Henmt1em1/em1; Pnldc1em1/em1 spermatogonia, the median level of CpG methylation for these elements was ≥ 80% (Figure S6C), suggesting that transposon derepression in the double mutants reflects impaired post-transcriptional transposon silencing rather than loss of MIWI2-directed DNA methylation.
In Henmt1em1/em1; Pnldc1em1/em1 spermatogonia, pre-piRNAs are both unmethylated (data not shown) and untrimmed (Figure 7B). Our data suggest that these pre-piRNAs are degraded both by complementarity-dependent destabilization and by the pathways that destroy untrimmed pre-piRNAs. First, the predicted fraction of pre-piRNA bound to sites in trigger RNAs was higher for unstable pre-piRNAs compared to stable pre-piRNAs (Figure S1C). Second, like unstable pre-piRNAs in single mutant Pnldc1em1/em1 germ cells (Figure 5B), unstable pre-piRNAs were enriched for G- and U-tracts (Figure S6D). We conclude that loss of both 3′-to-5′ trimming and 2′-O-methylation increases the turnover of most pre-piRNAs, causing the piRNA pathway to collapse in Henmt1em1/em1; Pnldc1em1/em1 testes.
DISCUSSION
Pre-piRNA trimming by PNLDC1 and 2′-O-methylation by HENMT1 protect mouse piRNAs from distinct degradation mechanisms (Figure 7F). piRNA 2′-O-methylation inhibits turnover triggered by binding to extensively complementary RNAs. In mammals, the testis has the most complex transcriptome of all tissues (Soumillon et al., 2013). Consequently mouse piRNAs are more likely to encounter a complementary site than somatic small RNAs. Our biochemical experiments show that different mechanisms direct the destruction of unmethylated piRNAs depending on whether the 5′ or 3′ sequence of a piRNA is complementary to the site in the trigger RNA. We currently do not know whether piRNA decay uses endo- or exonucleases, involves piRNA unloading from the PIWI protein, or requires turnover of the PIWI protein. We note that for 3′ complementary sites, longer matches elicit greater destabilization (Figure 2A). Yet all 5′ matches that extend beyond the seed sequence result in similar degree of degradation (Figure 2A). Perhaps, like EfPiwi (Anzelon et al., 2020), a match to piRNA 5′ sequence extending beyond the seed alters the conformation of MIWI, permitting piRNA destabilization.
We find that complementarity-dependent destabilization of piRNAs is conserved in animals as evolutionarily distant as mice and flies, whose last common ancestor existed ~800 million years ago (Kumar et al., 2017). In contrast to studies in S2 cells (Kingston and Bartel, 2021), 2′-O-methylation protects fly siRNAs from complementarity-dependent destabilization in vivo (Figure S7A). These findings together with studies in Cnidaria, Ciliophora, and plants (Park et al., 2002; Chen et al., 2002; Yu et al., 2005; Li et al., 2005; Kurth and Mochizuki, 2009; Moran et al., 2014; Modepalli et al., 2018) reinforce the view that the ancestral function of 2′-O-methylation was to stabilize small silencing RNAs in eukaryotes.
Methylation inhibits complementarity-dependent piRNA destabilization, yet target-directed miRNA degradation (TDMD) is insensitive to 2′-O-methylation (Han et al., 2020; Shi et al., 2020), and miRNAs are unmethylated in most animals. The difference in sensitivity to 2′-O-methylation between TDMD and piRNA turnover suggests distinct molecular mechanisms mediate degradation of these two small RNA classes upon their exposure to complementary RNAs.
To trigger TDMD, a complementary site must base pair with both the seed and miRNA 3′ sequences, but not to ≥1 central nucleotides (Cazalla et al., 2010; Ameres et al., 2010; Sheu-Gruttadauria et al., 2019). TDMD requires the ubiquitin ligase substrate receptor ZSWIM8 (Han et al., 2020; Shi et al., 2020), and in ZSWIM8 knock-out mouse and fly cell lines only a subset of miRNAs with short half-lives are stabilized (Shi et al., 2020). Short-lived miRNAs, whose turnover rate does not change when TDMD is inactivated in ΔZSWIM8 cells, are likely destabilized by a separate, ZSWIM8-independent mechanism. Our analyses of data from mouse and fly cell lines suggest that miRNA decay rates are, in part, determined by long RNAs complementary to the miRNA central region (Figures S7B and S7C). Moreover, mammalian AGO2-loaded miRNAs are unstable in a cell-free system when exposed to RNAs complementary to both the miRNA central and 3′ sequences, and miRNA destabilization was blocked by 3′-terminal 2′-O-methylation (Park et al., 2017).
Degradation of untrimmed pre-piRNAs does not depend on complementarity to long RNAs. Instead, unstable, untrimmed pre-piRNAs are generally loaded into MIWI rather than MILI and contain uridine- or guanine-tracts in the trimmed sequence of the pre-piRNA. In testis lysate, both internal and 3′-terminal oligoguanine or oligouridine destabilizes pre-piRNAs, suggesting that a single exonucleolytic step cannot explain pre-piRNA decay.
Pre-piRNA trimming by PNLDC1 is also required to stabilize mature piRNAs in silkmoth (Izumi et al., 2016; Izumi et al., 2020). PNLDC1 is present in most animals, except fish and dipteran insects, whose pre-piRNAs are just a few nucleotides longer than mature piRNAs (Hayashi et al., 2016; Gainetdinov et al., 2018). In the dipteran insect D. melanogaster, 3′ terminal trimming by the 3′-to-5′ exonuclease Nibbler is required for the biogenesis of piRNAs loaded into the PIWI proteins Ago3 and Aub, which function in the cytoplasm (Hayashi et al., 2016).
Like mouse piRNAs, C. elegans piRNAs (21U-RNAs) rely on the combination of trimming and 2′-O-methylation for their stability: in the absence of both PARN-1 and HENN-1, 21U-RNA abundance declines to a greater degree than in either parn-1 or henn-1 single mutants (Pastore et al., 2021). In mice, the finding that pre-piRNA trimming and 2′-O-methylation act additively to protect different subsets of piRNAs from distinct decay mechanisms offers an explanation for the surprisingly mild phenotype—post-meiotic spermatogenic arrest—of Henmt1em1/em1 and Pnldc1em1/em1 single mouse mutants (Lim et al., 2015; Zhang et al., 2017; Ding et al., 2017; Nishimura et al., 2018). Removing both PNLDC1 and HENMT1 precipitates the collapse of the piRNA pathway and arrests spermatogenesis at the onset of meiosis, as observed for mice deficient for other piRNA biogenesis proteins (Tanaka et al., 2000; Kuramochi-Miyagawa et al., 2004; Carmell et al., 2007; Soper et al., 2008; Ma et al., 2009; Shoji et al., 2009; Yoshimura et al., 2009; Zheng et al., 2010; Frost et al., 2010; Huang et al., 2011; Watanabe et al., 2011). By collaborating, 3′-to-5′ trimming and 2′-O-methylation maintain the high steady-state abundance required for the piRNA pathway function.
Limitations of the Study
In estimating the fraction of piRNAs bound to triggers, only fully complementary matches of different lengths are counted. Because triggers with imperfect pairing to piRNAs are not considered, our approach may underestimate the fraction bound. Our biochemical model also does not account for potential differences in accessibility of complementary triggers due to an RNA-binding protein or a secondary structure occluding a complementary sequence. However, these limitations are unlikely to alter the conclusions of the study, because only the difference between the fraction of stable and unstable piRNAs bound to complementary triggers is used to interpret the data.
STAR METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to, and will be fulfilled by, the Lead Contact, Phillip D. Zamore (phillip.zamore@umassmed.edu), or by completing the request form at https://www.zamorelab.umassmed.edu/reagents.
Materials Availability
Strains generated in this study are available for non-commercial use without restriction upon request or, where indicated, from the Bloomington Drosophila Stock Center (https://bdsc.indiana.edu) or the Jackson Laboratory (https://www.jax.org/jax-mice-and-services/find-and-order-jax-mice).
Data and Code Availability
Sequencing data have been deposited at National Center for Biotechnology Information Sequence Read Archive and are publicly available as of the date of publication using accession number PRJNA660633 (see the key resources table). Original autoradiography, microscopy, and Western blotting images have been deposited at Mendeley and are publicly available as of the date of publication. The DOI is listed in the key resources table.
All published software required to reanalyze the data reported in this paper is described in the Quantification and Statistical Analysis section below.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-SYCP3 | Abcam | Cat# ab15093, RRID:AB_301639 |
| Mouse monoclonal Anti-Histone H2A.X | Millipore | Cat# 05-636, RRID:AB_309864 |
| Donkey anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 594 |
Thermo Fisher Scientific | Cat# A-21203, RRID:AB_2535789 |
| Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 |
Thermo Fisher Scientific | Cat# A-21206, RRID:AB_2535792 |
| Rabbit polyclonal PIWIL2 antibody | Abcam | Cat# ab36764, RRID:AB_777284 |
| Rabbit polyclonal PIWIL1 antibody | Abcam | Cat# ab12337, RRID:AB_470241 |
| Mouse monoclonal Anti-PIWIL1 | Wako | Cat # 017-23451, RRID:AB_2721829 |
| IRDye 680RD Donkey anti-Rabbit IgG (H + L), antibody |
LI-COR Biosciences |
Cat# 926-68073, RRID:AB_1095444 |
| IRDye 800CW Donkey anti-Mouse IgG (H + L), antibody |
LI-COR Biosciences |
Cat# 926-32212, RRID:AB_621847 |
| Deposited Data | ||
| Raw sequencing data from Mus musculus | This paper | NCBI: PRJNA660633 |
| Original autoradiography and Western blotting images | This paper | http://dx.doi.org/10.17632/2X65btsysm.3 |
| Experimental Models: Organisms/Strains | ||
| C57BL/6J mice | Jackson Laboratory | IMSR # JAX:000664, RRID:IMSR_JAX:000664 |
| Pnldc1em1Pdz/em1Pdz mice | (Gainetdinov et al., 2018) | MGI: 6161374 |
| Henmt1em1Pdz/em1Pdz mice | This paper | MGI: 6452642 |
| Tdrkh−/− mice | Jackson Laboratory | MGI: 5521576 |
| Miwi−/− mice | Jackson Laboratory | MGI: 2182488 |
| w[*]; wg[Sp-1]/CyO; P{w[+mC]=UAS-GFP.dsRNA.R}142 flies | Bloomington Drosophila Stock Center | BDSC: 44415; RRID:BDSC_ 44415 |
| y[1] w[*]; wg[Sp-1]/CyO; P{w[+mC]=longGMR-GAL4}3/TM2 flies | Bloomington Drosophila Stock Center | BDSC: 8121; RRID:BDSC_ 8121 |
| PBac{WH}Hen1f00810 flies | (Horwich et al., 2007) | N/A |
| Oligonucleotides | ||
| Small RNA cloning 3′ adapter (AppBA3-UMI, 36-nt custom DNA oligo with 5′ adenylation and 3′ ddC): 5′ - rAppNNNGTCNNNTAGNNNTGGAATTCTCGGGTGCCAAGG/ddC/-3′ | Integrated DNA Technologies | N/A |
| Small RNA cloning 5′ adapter 1 (BA5-UMI-a, 41-nt custom RNA oligos): 5′ - GUUCAGAGUUCUACAGUCCGACGAUCNNNCGANNNUACNNN-3′ | Integrated DNA Technologies | N/A |
| Small RNA cloning 5′ adapter 2 (BA5-UMI-b, 41-nt custom RNA oligos): 5′ - GUUCAGAGUUCUACAGUCCGACGAUCNNNAUCNNNAGUNNN-3′ | Integrated DNA Technologies | N/A |
| RT primer (custom DNA oligo): 5′-CCTTGGCACCCGAGAATTCCA-3′ | Integrated DNA Technologies | N/A |
| PCR forward primer (50-nt custom DNA oligo): 5′-AATGATACGGCGACCACCGAGATCTACACGTTCAGAGTTCTACAGTCCGA-3′ | Integrated DNA Technologies | N/A |
| Multiplexing Adapter 1: 5′-P-GATCGGAAGAGCACACGTCT-3′ |
Integrated DNA Technologies | N/A |
| Multiplexing Adapter 2: 5′-ACACTCTTTCCCTACACGACGCTCTTCCGATCT-3′ |
Integrated DNA Technologies | N/A |
| Multiplexing PCR Primer 1: 5′-AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCT-3′ |
Integrated DNA Technologies | N/A |
| Multiplexing PCR Primer 2 (NNNNNN represents barcode) 5′-CAAGCAGAAGACGGCATACGAGATNNNNNNGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT-3′ |
Integrated DNA Technologies | N/A |
| Software and Algorithms | ||
| Bowtie version v2.2.0 | Langmead, B., and Salzberg, S. L. (2012). Nat Methods 9, 357. | http://bowtie-bio.sourceforge.net/index.shtml |
| Bowtie v1.0.0 | Langmead, B., Trapnell, C., Pop, M., and Salzberg, S. L. (2009). Genome Biol, 10, R25 | http://bowtie-bio.sourceforge.net/index.shtml |
| piPipes | Han, B. W., Wang, W., Zamore, P. D., and Weng, Z. (2015). Bioinformatics 31, 593. | https://github.com/bowhan/piPipes |
| BEDtools version v2.25.0 | Quinlan, A. R., and Hall, I. M. (2010). Bioinformatics 26:841. | http://bedtools.readthedocs.io |
| STAR version v2.3.1 | Dobin, A., Davis, C. A., Schlesinger, F., Drenkow, J., Zaleski, C., Jha, S., Batut, P., Chaisson, M., and Gingeras, T. R. (2013). Bioinformatics 29, 15. | https://github.com/alexdobin/STAR/releases |
| Samtools version v1.0.0 | Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., Marth, G., Abecasis, G., Durbin, R., and 1000, G. P. D. P. S. (2009). Bioinformatics 25, 2078. | http://samtools.sourceforge.net |
| DESeq2 version v1.18.1 | Love, M. I., Huber, W., and Anders, S. (2014). Genome Biol 15, 550. | https://bioconductor.org/packages/release/bioc/html/DESeq2.html |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mouse Strains and Mutants
Mice (wild-type C57BL/6J, IMSR # JAX:000664, RRID:IMSR_JAX:000664; Pnldc1em1pdz/em1pdz mutants, MGI: 6161374; Tdrkh−/− mutants, MGI: 5521576; Miwi−/− mutants, MGI: 2182488) were maintained and sacrificed according to the guidelines of the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School.
Guide RNA (sgRNA: 5′-GGC ATC TCC ACA TCC CAG GTC GG-3′) targeting exon 4 of Henmt1 to generate Henmt1em1pdz/em1pdz (MGI: 6452642) was designed using CRISPR design tool (crispr.mit.edu/). sgRNA was transcribed with T7 RNA Polymerase and then purified by electrophoresis on 10% denaturing polyacrylamide gel. The donor single-stranded oligonucleotide to generate Henmt1em1pdz/em1pdz was ordered from Integrated DNA Technologies. A mix of sgRNA (20 ng/μl), Cas9 mRNA (50 ng/μl, TriLink Biotechnologies, L-7206) and 195-nt, single-stranded oligonucleotide donor (100 ng/μl) were injected together into the pronucleus of one-cell C57BL/6 zygotes in M2 medium (Sigma, M7167). After injection, the zygotes were cultured in EmbryoMax Advanced KSOM Medium (Sigma, MR-106-D) at 37°C under 5% CO2 until the blastocyst stage (3.5 days), then transferred into uterus of pseudopregnant ICR females 2.5 dpc.
To screen for mutant founders, genomic DNA extracted from tail tissues was analyzed by PCR. Primers used for genotyping Henmt1em1pdz/em1pdz were 5′-GTT GCC AAC GCT GTA GCC-3′ and 5′-AAT AAG GGC ACC CTG CAC TA-3′. Mutant sequences were confirmed by Sanger sequencing. The Henmt1em1pdz/em1pdz mutant changes amino acid residues 54–58 from DLGCG to NAVAV, which is predicted to disrupt catalytic activity (Kirino and Mourelatos, 2007) and causes loss of the protein: the Henmt1 mRNA level in Henmt1em1pdz/em1pdz and C57BL/6 are the same, but the HENMT1 protein level in Henmt1em1pdz/em1pdz is ~4% that in C57BL/6.
Drosophila melanogaster Strains and Mutants
Fly stocks were maintained at 25°C. All strains were in the w1118 background. Flies (0–3 days after eclosion) were fed yeast paste for two days before ovaries or heads were dissected and collected in phosphate-buffered fly saline (PBS: 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4) at 4°C. Ovaries or heads were washed once with ice-cold PBS, and then used for subsequent experiments.
METHOD DETAILS
Mouse Phenotypic Analysis
Male mice, (2–8 months old, were housed individually with one 2–4 month-old C57BL/6J female. The presence of a vaginal plug was examined the following morning to confirm insemination. If a plug was observed, the female was separated and monitored for pregnancy. Males mated to females who failed to produce pups within 2 months after a vaginal plug was detected were deemed sterile. The presence of epididymal sperm, testis weight, and testis histology were also scored.
Histology.
Testes were collected from 2–6 month-old mice; fixed overnight in Bouin’s solution; washed three times with 70% (v/v) ethanol; then stored in 70% ethanol. Tissues were embedded in paraffin, cut into 5 pm cross-sections, stained with hematoxylin and counter stained with eosin by the UMass Morphology Core.
FACS Isolation and Immunostaining of Mouse Germ Cells
Testes of 2–5 month-old mice were isolated, decapsulated, and incubated for 15 min at 33°C in 1× Gey’s Balanced Salt Solution (GBSS, Sigma, G9779) containing 0.4 mg/ml collagenase type 4 (Worthington, LS004188) rotating at 150 rpm. Seminiferous tubules were then washed twice with 1× GBSS and incubated for 15 min at 33°C in 1× GBSS with 0.5 mg/ml Trypsin and 1 μg/ml DNase I rotating at 150 rpm. Next, tubules were homogenized by pipetting through a glass Pasteur pipette for 3 min at 4°C. Fetal bovine serum (FBS; 7.5% f.c., v/v) was added to inactivate trypsin, and the cell suspension was then strained through a pre-wetted 70 μm cell strainer (Thermo Fisher, 22363548); cells were collected by centrifugation at 300 × g for 10 min. The supernatant was removed, cells resuspended in 1× GBSS containing 5% (v/v) FBS, 1 μg/ml DNase I, and 5 μg/ml Hoechst 33342 (Thermo Fisher, 62249) and rotated at 150 rpm for 45 min at 33°C. Propidium iodide (0.2 μg/ml, f.c.; Thermo Fisher, P3566) was added, and cells strained through a pre-wetted 40 μm cell strainer (Thermo Fisher, 22363547). Spermatogonia, primary spermatocytes, secondary spermatocytes, round spermatids were purified using a FACSAria II Cell Sorter (BD Biosciences; UMass Medical School FACS Core) as described (Bastos et al., 2005; Gainetdinov et al., 2018). Briefly, the 355-nm laser was used to excite Hoechst 33342; the 488-nm laser was used to excite Propidium iodide and record forward and side scatter. Propidium iodide emission was detected using a 610/20 bandpass filter. Hoechst 33342 emission was recorded using 450/50 and 670/50 band pass filters.
Germ cell stages in the unsorted population and the purity of sorted fractions were assessed by immunostaining aliquots of cells. Cells were incubated for 20 min in 25 mM sucrose and then fixed on a slide with 1% (w/v) paraformaldehyde containing 0.15% (v/v) Triton X-100 for 2 h at room temperature in a humidifying chamber. Slides were washed sequentially for 10 min in: (1) PBS containing 0.4% (v/v) Photo-Flo 200 (Kodak, 1464510); (2) PBS containing 0.1% (v/v) Triton X-100; and (3) PBS containing 0.3% (w/v) BSA, 1% (v/v) donkey serum (Sigma, D9663), and 0.05% (v/v) Triton X-100. After washing, slides were incubated with primary antibodies in PBS containing 3% (w/v) BSA, 10% (v/v) donkey serum, and 0.5% (v/v) Triton X-100 overnight at room temperature in a humidified chamber. Rabbit polyclonal anti-SYCP3 (Abeam, ab15093, RRID:AB_301639, 1:1000 dilution) and mouse monoclonal anti-γH2AX (Millipore, 05-636, RRID:AB_309864, 1:1000 dilution) were used as primary antibodies. Slides were washed again as described and then incubated with secondary donkey anti-mouse IgG (H+L) Alexa Fluor 594 (Thermo Fisher, A-21203, RRID:AB_2535789, 1:2000 dilution) or donkey anti-rabbit IgG (H+L) Alexa Fluor 488 (Thermo Fisher, A-21206, RRID:AB_2535792, 1:2000 dilution) for 1 h at room temperature in a humidified chamber. After incubation, slides were washed three times (10 min each) in PBS containing 0.4% (v/v) Photo-Flo 200 and once for 10 min in 0.4% (v/v) Photo-Flo 200. Finally, slides were dried and mounted in ProLong Gold Antifade Mountant with DAPI (Thermo Fisher, P36931). To assess the purity of sorted fractions, 50–100 cells were staged by DNA, γH2AX, and SYCP3 staining (Bastos et al., 2005). All samples used here met these criteria:
Spermatogonia, ~95–100% pure with ≤ 5% pre-leptotene spermatocytes;
Primary spermatocytes, ~10–15% leptotene/zygotene spermatocytes, ~45–50% pachytene spermatocytes, ~35–40% diplotene spermatocytes;
Secondary spermatocytes, ~100%;
Round spermatids, ~95–100%, ≤ 5% elongated spermatids.
Western Blotting
Cells were homogenized in NP-40 Lysis Buffer (20 mM Tris-HCl, pH 7.5, 2.5 mM MgCl2, 200 mM NaCl, 0.05% (v/v) NP-40, 0.1 mM EDTA, 1 mM 4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride, 0.3 μM Aprotinin, 40 μM Bestatin, 10 μM E-64, 10 μM Leupeptin) and centrifuged at 20,000 × g for 20 min at 4°C. The supernatant was transferred to a new tube, an equal volume of 120 mM Tris-HCl, pH 6.8, 4% (w/v) SDS, 20% (v/v) glycerol, 2.5% (v/v) 2-Mercaptoethanol, 0.2% (w/v) bromophenol blue was added, the sample was incubated at 90°C for 5 min, and then resolved by electrophoresis through a 4–20% gradient polyacrylamide/SDS gel (Bio-Rad Laboratories, 5671085). Next, proteins were transferred to PVDF (Millipore, IPVH00010), the membrane blocked in Blocking Buffer (Rockland Immunochemicals, MB-070) at room temperature for 2 h, and then incubated overnight at 4°C in Blocking Buffer containing primary antibody (anti-mouse PIWIL2/MILI, Abeam, ab36764, RRID:AB_777284, 1:1000 dilution; anti-PIWIL1/MIWI, Abeam, ab12337, RRID:AB_470241, 1:1000 dilution; anti-mouse LINE-1 ORF1p rabbit polyclonal, 1:10000 dilution, generous gift of Alex Bortvin, Martin, 1991; Soper et al., 2008). The membrane was washed three times (30 min each) with Blocking Buffer at room temperature and incubated for 2 h at room temperature with donkey anti-rabbit IRDye 680RD secondary antibody (LI-COR Biosciences, 926-68073, RRID:AB_1095444, diluted 1:20,000) in Blocking Buffer. Finally, the membrane was washed three times (30 min each) with Blocking Buffer at room temperature, and signal detected using an Odyssey Infrared Imaging System. In western-blotting assays with anti-MILI and anti-MIWI antibodies, the relationship between signal and abundance of MILI and MIWI was linear between 1% and 120% of the level in C57BL/6 primary spermatocytes (Figure S5A).
Small RNA Immunoprecipitation
Sorted mouse germ cells were homogenized with NP-40 Lysis Buffer (20 mM Tris-HCl, pH 7.5, 2.5 mM MgCl2, 200 mM NaCl, 0.05% (v/v) NP-40, 0.1 mM EDTA, 1 mM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride, 0.3 μm Aprotinin, 40 μM Bestatin, 10 μM E-64, 10 μM Leupeptin) and then centrifuged at 20,000 × g for 20 min at 4°C, retaining the supernatant. Anti-MIWI (Wako, 017-23451, RRID:AB_2721829, ~5 μg) or anti-MILI (Abeam, ab36764, RRID:AB_777284, ~5 μg) antibodies were incubated with rotation with 30 μl of Protein G Dynabeads (Thermo Fisher, 10003D) in PBS containing 0.02% (v/v) Tween 20 (PBST) for 1 h at 4°C. The bead-antibody complex was washed once with PBST. Freshly prepared germ cell lysate was added to the bead-antibody complex and incubated with rotation overnight at 4°C. The next day, the beads were washed once with NP-40 Lysis Buffer and three times with 0.1 M trisodium citrate (pH 5.0). After washing, RNA was extracted from the beads with Trizol (Thermo Fisher, 15596026) and used for small RNA library preparation.
Small RNA-seq Library Preparation
Total RNA from sorted mouse germ cells was extracted using the mirVana miRNA isolation kit (Thermo Fisher, AM1560). Small RNA libraries were constructed as described (Gainetdinov et al., 2018) with modifications. Briefly, before library preparation, a mix of nine synthetic RNA oligonucleotides (Table S3) was added to each RNA sample to enable absolute quantification of small RNAs (Table S4); median cell volume from Gainetdinov et al., 2018 was used to calculate intracellular concentration. To reduce ligation bias and eliminate PCR duplicates, the 3′ and 5′ adaptors both contained nine random nucleotides at their 5′ and 3′ ends, respectively (see Key Resources Table for adaptor sequences; Fu et al., 2018a); 3′ adaptor ligation reactions contained 20% (w/v) PEG-8000 (f.c.). After 3′ adaptor ligation, RNA was purified by 15% urea polyacrylamide gel electrophoresis (PAGE), selecting for 15–55 nt small RNAs (i.e., 50–90 nt with 3′ adaptor). Small RNA-seq libraries samples were sequenced using a NextSeq 500 (illumina) to obtain 79 nt, single-end reads. Data sets of MILI- and MIWI-bound piRNAs in C57BL/6 and Pnldc1em1pdz/em1pdz were from Gainetdinov et al., 2018.
RNA-seq Library Preparation
Total RNA from sorted germ cells was extracted using the mirVana miRNA isolation kit (Thermo Fisher, AM1560) and used for library preparation as described (Zhang et al., 2012) with modifications, including the addition of the ERCC spike-in mix to enable absolute quantification of RNAs and the use of unique molecular identifiers to eliminate PCR duplicates (Fu et al., 2018a). Briefly, before library preparation, 1 μl of 1:100 diluted ERCC spike-in mix 1 (Thermo Fisher, 4456740, LOT00418382; Table S5A) was added to 0.5–1 μg total RNA. To remove rRNA, the RNA was hybridized in 10 μl to a pool of 186 rRNA antisense oligos (0.05 μM each) in 10 mM Tris-HCl (pH 7.4), 20 mM NaCl by heating the mixture to 95°C, cooling it at ℃ 0.1°C/sec to 22°C, and incubating at 22°C for 5 min. RNase H (10 U; Lucigen, H39500) was added and the mixture incubated at 45°C for 30 min in 20 μl containing 50 mM Tris-HCl (pH 7.4), 100 mM NaCl, and 20 mM MgCl2. The reaction volume was adjusted to 50 μl with 1× TURBO DNase buffer (Thermo Fisher, AM2238) and then incubated with 4 U TURBO DNase (Thermo Fisher, AM2238) for 20 min at 37°C. Next, RNA was purified using RNA Clean 8i Concentrator-5 (Zymo Research, R1016). RNA-seq libraries were sequenced using a NextSeq 500 (Illumina) to obtain 79 + 79 nt, paired-end reads. Sequencing data for C57BL/6 secondary spermatocytes and round spermatids were from Gainetdinov et al., 2018.
Cloning and Sequencing of 5′ Monophosphorylated Long RNAs
Total RNA from sorted mouse germ cells was extracted using mirVana miRNA isolation kit (Thermo Fisher, AM 1560) and used to prepare a library of 5′ monophosphorylated long RNAs as described (Wang et al., 2014). Libraries were sequenced using a NextSeq 500 (Illumina) to obtain 79 + 79 nt, paired-end reads (Table S5B).
DNA Methylation
DNA methylation was assessed using DNA bisulfate sequencing with EZ-DNA Methylation Direct Kit (Zymo Research, D5020). Imprinted locus H19 was used as a methylated DNA positive control. Bisulfate-treated DNA served as the template for one round (for L1-Gf and L1-A LINE elements) or two nested rounds (H19 and IAP LTR elements) of PCR with EpiMark Hot Start Taq DNA Polymerase (NEB, M0490S) using the following protocol: initial denaturation, 95°C for 30 sec; 35 cycles of 95°C for 30 sec, annealed for 60 sec, and extended at 68°C for 30 sec; final extension, 68°C for 5 min. Table S6 provides primer sequences and annealing temperatures. Two pairs of primers specific to thousands of L1-Gf and L1-A genomic copies and a primer pair specific to a single copy of IAP LTR element were used (Kojima-Kita et al., 2016; Nishimura et al., 2018). PCR products were cloned with TOPO TA Cloning Kit (Thermo Fisher, 450641) and Sanger sequenced (GENEWIZ).
Testis Lysate
Dissected animal tissues were homogenized at 4°C in five volumes of 30 mM HEPES-KOH, pH 7.5, 100 mM potassium acetate, 3.5 mM magnesium acetate, 1 mM DTT, 15% (v/v) glycerol in a Dounce homogenizer using 10 strokes of the loose-fitting pestle A, followed by 20 strokes of tight-fitting pestle B to generate crude lysate. S20 was prepared by clarifying the crude lysate at 20,000 × g.
Separation of the crude lysate into nuclear pellet, mitochondrial fraction, and cytoplasm was as described (Wieckowski et al., 2009). Briefly, dissected animal tissues were homogenized at 4°C in two volumes of Mitochondria Resuspending Buffer (MRB: 30 mM HEPES-KOH, pH 7.5, 225 mM D-mannitol, 75 mM sucrose, 0.1 mM EGTA, 0.5 mM DTT, 15% [v/v] glycerol) in a Dounce homogenizer using 10 strokes of the loose-fitting pestle A, followed by 20 strokes of tight-fitting pestle B to generate crude lysate. After an aliquot of crude lysate was centrifuged at 600 × g for 5 min, the nuclear pellet was resuspended in two volumes of MRB, and the supernatant was clarified by additional centrifugation at 600 × g for 5 min. The clarified supernatant was then centrifuged at 7,000 × g for 10 min: the supernatant constituting the cytoplasmic fraction was transferred to a clean tube and crude mitochondrial pellet was resuspended in two volumes of MRB.
Protein concentration was estimated using the BCA assay (Thermo Fisher, 23200). Crude and fractionated testis lysate was flash frozen in liquid nitrogen and stored at − 80°C.
Recombinant MIWI Purification and piRNA Loading
[5′-32P] radiolabeled synthetic piRNA guides were prepared by incubating 200 pmole 5′ phosphorylated RNA (Sigma; Table S7) with 100 pmole [γ-32P] ATP (6000 Ci/mmol; Perkin Elmer, NEG035C005MC) and 50 U T4 polynucleotide kinase (NEB, M0201S) in 10 mM adenosine diphosphate, 70 mM Tris-HCl pH 7.6, 10 mM MgCl2, 5 mM DTT for 1 h at 37°C. Unincorporated [γ -32P] ATP was removed using a G-25 spin column (Cytiva, 27532501), and labeled guide piRNA was purified by electrophoresis through a 15% denaturing polyacrylamide gel.
HEK293T cells expressing SNAP-, 3xFLAG-tagged MIWI were generated as described (Arif et al., 2021). To maximize recombinant MIWI expression, lentiviral transduction of HEK293T cells was performed sequentially three times. HEK293T cells with the highest MIWI expression were selected by FACS using EGFP fluorescence. (A high level of recombinant MIWI production appears to be required to generate a sufficient amount of unloaded protein to allow programming piRISC with synthetic guides.) FACS-sorted HEK293T cells were harvested at ~70% confluency using a TC Cell Scraper (Thermo Fisher, 50809263) into ice-cold PBS and collected by centrifugation at 500 × g. Supernatant was removed, and the pellet was stored at − 80°C until lysed in 10 ml of 30 mM HEPES-KOH, pH 7.5, 100 mM potassium acetate, 3.5 mM magnesium acetate, 1 mM DTT, 0.1% (v/v) Triton X-100, 15% (v/v) glycerol, and 1× protease inhibitor cocktail (1 mM 4-(2-Aminoethyl)benzenesulfonyl fluoride hydrochloride [Sigma; A8456], 0.3 μM Aprotinin, 40 μM betanin hydrochloride, 10 μM E-64 [Sigma; E3132], 10 μM leupeptin hemisulfate) per g frozen cells. Cell lysis was monitored by staining with trypan blue. Crude cytoplasmic lysate was clarified at 20,000 × g, flash frozen in liquid nitrogen and stored at − 80°C. To capture MIWI, clarified lysate was incubated with 20 μl Anti-FLAG M2 paramagnetic beads (Sigma, M8823) per ml lysate for 4 h to overnight rotating at 4°C. To biotinylate MIWI, SNAP-Biotin (1 μM f.c.; NEB, S9110S) was added during the capture step. Beads were washed four times with wash buffer (30 mM HEPES-KOH, pH 7.5, 3.5 mM magnesium acetate, 1 mM DTT) containing 2 M potassium acetate and four times with wash buffer containing 100 mM potassium acetate and 0.01% (v/v) Triton X-100. To assemble MIWI piRISC, beads were resuspended in 30 mM HEPES-KOH, pH 7.5, 3.5 mM magnesium acetate, 100 mM potassium acetate, 1 mM DTT, containing 100 nM radiolabeled, synthetic piRNA guide and incubated with rotation for 30 min at 37°C or room temperature. After five washes in 2 M potassium acetate wash buffer and five washes in 100 mM potassium acetate/0.01 % Triton X-100 wash buffer, MIWI piRISC was eluted from the beads with 200 ng/ul 3XFLAG peptide in 30 mM HEPES-KOH, pH 7.5, 3.5 mM magnesium acetate, 100 mM potassium acetate, 1 mM DTT, 15% (v/v) glycerol for 1 h at room temperature. Eluate containing MIWI piRISC was flash frozen in liquid nitrogen and stored at − 80°C.
Reactions were performed with 1 nM MIWI piRISC (f.c.) and testis extract (3 μg/μl f.c. total protein) in 15 mM HEPES-KOH, pH 7.5, 100 mM potassium acetate, 5 mM magnesium acetate, 5 mM DTT, 1 mM ATP, 25 mM creatine phosphate, 30 μg/ml creatine kinase in 10 μl. Fully 2′-O-methyl modified complementary RNAs (cRNAs, Table S8) were added at 100 nM (f.c.). Reactions were deproteinized by adding 290 μl 50 mM Tris-Cl, pH 7.5, 100 mM NaCl, 25 mM EDTA, 1% (w/v) SDS, containing proteinase K (1 mg/ml f.c.) and incubated at 45°C for 15 min, followed by extraction with phenol:chloroform:isoamyl alcohol (25:24:1, pH 6.7) and precipitation with three volumes of ethanol for 1 h on ice. After centrifugation at 20,000 × g at 4°C for 30 min, the precipitate was washed in 1 ml ice-cold 75% (v/v) ethanol, resuspended in 10 μl of 95% (v/v) formamide, 5 mM EDTA, 0.025% (w/v) bromophenol blue, 0.025% (w/v) xylene cyanol, heated at 95°C for 2 min, and resolved by 15% denaturing PAGE. Gels were dried, exposed to a storage phosphor screen, and imaged on a Typhoon FLA 7000 (GE).
To affinity purify biotin-labeled MIWI, 10 μl streptavidin-coated paramagnetic beads (Thermo Fisher, 65604D) and 80 μl of 30 mM HEPES-KOH, pH 7.5, 3.5 mM magnesium acetate, 100 mM potassium acetate, 1 mM DTT were added to each reaction and incubated with rotation for 1 h at room temperature. Beads were washed twice in 2 M potassium acetate wash buffer and twice in 100 mM potassium acetate/0.01% Triton X-100 wash buffer. Beads were then deproteinized, and RNA extracted as described above.
QUANTIFICATION AND STATISTICAL ANALYSIS
Analysis of Small RNA Data Sets
Sequences were filtered by requiring their Phred quality score to be ≥ 20 for all nucleotides and the 3′ adapter was removed with fastx toolkit (v0.0.14). PCR duplicates were eliminated as described (Fu et al., 2018a). Reads not fully matching the genome were analyzed using the Tailor pipeline (Chou et al., 2015) to account for non-templated tailing of small RNAs. Unambiguously mapping piRNA or pre-piRNA reads were grouped by their 5′, 24-nt prefix, and piRNA prefix groups ≥10 ppm were further analyzed, ensuring that the standard deviation of abundance for a majority of piRNA prefix groups varied by ≤ 20% among the three wild-type replicates. The strength of secondary structure of pre-piRNAs was predicted using RNAfold 2.4.14 (Lorenz et al., 2011). Sequences of synthetic spike-in oligonucleotides (Table S3) were identified allowing no mismatches with using Bowtie (parameter -v 0; v1.0.0; Langmead et al., 2009), and the absolute abundance of small RNAs calculated (Table S4). Publicly available data used in analyses are listed in Table S9.
RNA-seq Analysis
RNA-seq analysis was performed using piPipes for genomic alignment (Han et al., 2015b). Briefly, before starting piPipes, sequences were reformatted to extract unique molecular identifiers (Fu et al., 2018a). The reformatted reads were then aligned to rRNA using Bowtie2 (v2.2.0; Langmead and Salzberg, 2012). Unaligned reads were mapped to mouse genome mm10 using STAR (v2.3.1; Dobin et al., 2013), and PCR duplicates removed (Fu et al., 2018a). Transcript abundance was calculated using StringTie (v1.3.4; Pertea et al., 2016). Differential expression analysis was performed using DESeq2 (v1.18.1; Love et al., 2014). In parallel, reformatted reads were aligned to an index of ERCC spike-in transcripts (Thermo Fisher, 4456740, LOT00418382) using Bowtie (v1.0.0; Langmead et al., 2009), PCR duplicates were removed as described (Fu et al., 2018a), and the absolute quantity of transcripts calculated (Table S5A). Publicly available data used in analyses are listed in Table S9.
Analysis of 5′ Monophosphorylated Long RNA Sequencing Data
Analysis of 5′ monophosphorylated long RNA sequencing data was performed with piPipes (Han et al., 2015b). Briefly, rRNA sequences were removed using Bowtie2 (v2.2.0). Unaligned reads were then mapped to mouse genome mm10 using STAR (v2.3.1) and alignments with soft clipping of ends were removed with SAMtools (v1.0.0; Li et al., 2009).
Calculation of Small RNA Fraction Bound to Long RNA
We considered the simplified reaction:
For a given piRNA binding site i, we considered these molecular species,
[complementary sitei], the concentration of unbound complementary site i, [PIWI:piRNA], the concentration of unbound, piRNA-loaded PIWI protein; and [PIWI:piRNA: complementary sitei], the concentration of the ternary complex of piRNA, PIWI protein bound, and complementary site i,
and defined the following rates,
kon,i, association rate of PIWI:piRNA for complementary sitei,
koff,i, dissociation rate of PIWI:piRNA from complementary site i,
kcleave,i, single-turnover cleavage rate for PIWI:piRNA bound to complementary binding site RNAi.
Equation 1 describes the change of ternary complex concentration with time:
| Equation 1 |
Assuming that the cleavage step is slow (Arif et al., 2021), i.e., kcleave << koff, Equation 1 becomes:
| Equation 2 |
Steady-state can be approximated by assuming equilibrium conditions (i.e., when d[PIWI:piRNA:complementary sitei] dt is 0), allowing Equation 2 to be expressed as:
| Equation 3 |
where Ka represents the association constant of PIWI:piRNA for a complementary site in a trigger RNA.
The fraction bound, f, is given by:
| Equation 4 |
where n is the total number of piRNA binding sites.
Substituting [PIWI:piRNA:complementary sitei] from Equation 3 into Equation 4 yields,
which rearranges to
| Equation 5 |
Considering that
Equation 5 becomes:
| Equation 6 |
Substituting [PIWI:piRNA:complementary site i] from Equation 3 into Equation 6 yields,
which rearranges to,
then,
to yield,
| Equation 7 |
Using the assumptions (1) that the rank order of [complementary site] can be approximated by the rank order of [complementary site]total, and (2) that the rank order of affinities of PIWI:piRNA for complementary site can be approximated using the computationally predicted Gibbs free energy (Δ G0) of base pairing between two RNA strands (Ka = e−ΔG0/RT; Anzelon et al., 2020), equation (7) becomes:
| Equation 8 |
where R = 1.987 cal·K−1·mol−1 and T = 298.15 K for fly heads, 300.15 K for fly S2 cells, 306.15 K for mouse testis (Kandeel and Swerdloff, 1988), and T = 310.15 K for mouse embryonic stem cells (mESCs) and mouse embryonic fibroblasts (MEFs). Predicted ΔG0 was calculated from nearest neighbor values using RNAfold 2.4.14 (Lorenz et al., 2011).
For each starting nucleotide of piRNA-complementary sequence, we identified all 5-to-11-nt contiguously complementary sites in the RNA-seq measured transcriptome. For example, when identifying matches starting at g4 for piRNA 5′-UUG CUA GCU ACG UGU AGU GAG CAC GAC GGA-3′, we identified sites in all transcripts with complementary to:
5′-CUAGC-3′,
5′-CUAGCU-3′,
5′-CUAGCUA-3′,
5′-CUAGCUAC-3′,
5′-CUAGCUACG-3′,
5′-CUAGCUACGU-3′, or
5′-CUAGCUACGUG-3′.
To avoid double-counting, shorter matches contained within longer matches, e.g., a 5-nt and a 6-nt match inside a 7-nt match, were discarded.
Using the mean number of transcript molecules per cell (measured by RNA-seq with ERCC spike-ins; Table S5A) and the median cell volume reported in Gainetdinov et al., 2018, we calculated the intracellular concentration of all 5-to-11-nt matches (i.e., [complementary site5-nt]total, [complementary site6-nt]total), …, [complementary site11-nt]total). We thus estimated the fraction of molecules of piRNA 5′-UUG CUA GCU ACG UGU AGU GAG CAC GAC GGA-3′ whose region starting at g4 was bound to 5-to-11-nt complementary sites in the transcriptome using Equation 8.
To calculate the fraction bound for fly piRNAs (Figure 3), we sequenced long RNAs from ovaries of 3-to-4-day-old w1118 flies (Table S5A). For estimates of the fraction bound for fly GFP-derived siRNAs (Figure S7A), RNA-seq data from FACS-sorted GFP-positive cells from heads of w1118; GMR-Gal4 > {UAS-GFP.n/s}8 flies were used (Potier et al., 2014; Table S9). RNA-seq data from fly ago2 S2 cells (Reichholf et al., 2019; Table S9), and from mESC and MEF cells (Kingston and Bartel, 2019; Table S9) were used to calculate the fraction bound for fly and mouse miRNAs (Figures S7B and S7C). We assumed that the total intracellular concentrations of long transcripts in fly ovaries, fly eyes, S2 cells, mESC or MEF cells are similar to the mean total transcript concentration in mouse spermatogonia or primary spermatocytes (~1,500,000 transcripts per 1000 μm3), which allowed converting the relative transcript abundance to intracellular concentration. For fly piRNAs (Figure 3), the mean piRNA abundance (n = 4 for w1118, n = 2 for hen1f00810; Table S4) was used. For fly siRNAs (Figure S7A), the mean siRNA abundance (n = 4 for w1118, n = 2 for hen1f00810, Table S4) and the mean abundance of mRNAs (n = 2; Table S9) were used to estimate fraction bound. For fly and mouse miRNAs (Figures S7B and S7C), the mean miRNA half-life (n = 2; reported in Kingston and Bartel, 2019 and Reichholf et al., 2019) and the mean (n = 2; Table S9) abundance of mRNAs were used to estimate fraction bound.
Statistical Tests
P values were calculated using two-tailed KS test in Figure 6, and two-tailed Mann–Whitney U test in Figures 7A, 7C, and S1A. Padj values in Figure S6A and Tables S1 and S2 were calculated using Wald test with correction for multiple hypothesis testing using the Benjamini-Hochberg procedure (DESeq2; Love et al., 2014).
Supplementary Material
Table S1. mRNAs whose Derepression in Henmt1em1/em1 and Pnldc1em1/em1 Mice can be Explained by Reduced Cleavage by Destabilized piRNAs, Related to Figure 6.
Table S4. Number of Cells and Amount of Spike-In Mix Used to Prepare Small RNA Sequencing Libraries, Related to Small RNA-seq Library Preparation in STAR Methods.
Table S5. Number of Cells and Amount of ERCC Spike-In Mix 1 Used to Prepare RNA Sequencing Libraries (A) and List of Libraries of 5′ Monophosphorylated Long RNAs (B), Related to RNA-seq Library Preparation in STAR Methods.
Highlights.
2′-O-methylation protects mouse and fly piRNAs from complementarity-dependent decay
Distinct pathways decay mouse piRNAs with triggers complementary to piRNA 5′ or 3′ end
Mouse pre-piRNAs with U- or G-rich tracts decay faster in testis lysate and in vivo
MIWI-bound pre-piRNAs decay faster than MILI-bound pre-piRNAs
ACKNOWLEDGEMENTS
We thank the UMass Flow Cytometry Core Facility for help sorting mouse germ cells; the UMass Transgenic Animal Modeling Core for help generating Pnldc1em1pdz/em1pdz and Henmt1em1pdz/em1pdz mice; members of the Zamore and Mello laboratories for discussions and critical comments on the manuscript; Dimas Echeverria Moreno, Matthew R Hassler, and Jacquelyn Sousa from the Khvorova laboratory for technical assistance; Alex Bortvin for the anti-mouse LINE-1 ORF1p antibody. This work was supported in part by National Institutes of Health grants GM65236 and P01HD078253 to P.D.Z, and 1S10 OD028576 to the UMass Flow Cytometry Core Facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
P.D.Z. is a member of the editorial board of Molecular Cell.
INCLUSION AND DIVERSITY
One or more of the authors of this paper self-identifies as a member of the LGBTQ+ community.
REFERENCES
- Ameres SL, Horwich MD, Hung JH, Xu J, Ghildiyal M, Weng Z, and Zamore PD (2010). Target RNA-directed trimming and tailing of small silencing RNAs. Science 328, 1534–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzelon TA, Chowdhury S, Hughes SM, Xiao Y, Lander GC, and MacRae IJ (2021). Structural basis for piRNA targeting. Nature [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravin A, Gaidatzis D, Pfeffer S, Lagos-Quintana M, Landgraf P, Iovino N, Morris P, Brownstein MJ, Kuramochi-Miyagawa S, Nakano T, Chien M, Russo JJ, Ju J, Sheridan R, Sander C, Zavolan M, and Tuschl T (2006). A novel class of small RNAs bind to MILI protein in mouse testes. Nature 442, 203–207. [DOI] [PubMed] [Google Scholar]
- Aravin AA, Sachidanandam R, Bourc’his D, Schaefer C, Pezic D, Toth KF, Bestor T, and Hannon GJ (2008). A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol Cell 31, 785–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif A, Ozata DM, Andersson C, Izumi N, Tomari Y, and Zamore PD (2021). The tiny, conserved zinc-finger protein GTSF1 helps PIWI proteins achieve their full catalytic potential. bioRxiv [Google Scholar]
- Baccarini A, Chauhan H, Gardner TJ, Jayaprakash AD, Sachidanandam R, and Brown BD (2011). Kinetic analysis reveals the fate of a microRNA following target regulation in mammalian cells. Curr Biol 21, 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backes S, Shapiro JS, Sabin LR, Pham AM, Reyes I, Moss B, Cherry S, and tenOever BR (2012). Degradation of host microRNAs by poxvirus poly(A) polymerase reveals terminal RNA methylation as a protective antiviral mechanism. Cell Host Microbe 12, 200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP (2018). Metazoan MicroRNAs. Cell 173, 20–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos H, Lassalle B, Chicheportiche A, Riou L, Testart J, Allemand I, and Fouchet P (2005). Flow cytometric characterization of viable meiotic and postmeiotic cells by Hoechst 33342 in mouse spermatogenesis. Cytometry A 65, 40–49. [DOI] [PubMed] [Google Scholar]
- Batista PJ, Ruby JG, Claycomb JM, Chiang R, Fahlgren N, Kasschau KD, Chaves DA, Gu W, Vasale JJ, Duan S, Conte D, Luo S, Schroth GP, Carrington JC, Bartel DP, and Mello CC (2008). PRG-1 and 21U-RNAs interact to form the piRNA complex required for fertility in C. elegans. Mol Cell 31, 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billi AC, Alessi AF, Khivansara V, Han T, Freeberg M, Mitani S, and Kim JK (2012). The Caenorhabditis elegans HEN1 ortholog, HENN-1, methylates and stabilizes select subclasses of germline small RNAs. PLoS Genet 8, e1002617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitetti A, Mallory AC, Golini E, Carrieri C, Carreño Gutiérrez H, Perlas E, Pérez-Rico YA, Tocchini-Valentini GP, Enright AJ, Norton WHJ, Mandillo S, O’Carroll D, and Shkumatava A (2018). MicroRNA degradation by a conserved target RNA regulates animal behavior. Nat Struct Mol Biol 25, 244–251. [DOI] [PubMed] [Google Scholar]
- Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, and Hannon GJ (2007). Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 128, 1089–1103. [DOI] [PubMed] [Google Scholar]
- Carmell MA, Girard A, van de Kant HJ, Bourc’his D, Bestor TH, de Rooij DG, and Hannon GJ (2007). MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev Cell 12, 503–514. [DOI] [PubMed] [Google Scholar]
- Carthew RW, and Sontheimer EJ (2009). Origins and Mechanisms of miRNAs and siRNAs. Cell 136, 642–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalla D, Yario T, Steitz JA, and Steitz J (2010). Down-regulation of a host microRNA by a Herpesvirus saimiri noncoding RNA. Science 328, 1563–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Yeo J, Kim JG, Kim H, Lim J, Lee M, Kim HH, Ohk J, Jeon HY, Lee H, Jung H, Kim KW, and Kim VN (2018). Terminal Uridylyltransferases Execute Programmed Clearance of Maternal Transcriptome in Vertebrate Embryos. Mol Cell 70, 72–82.e7. [DOI] [PubMed] [Google Scholar]
- Chang HM, Triboulet R, Thornton JE, and Gregory RI (2013). A role for the Perlman syndrome exonuclease Dis3l2 in the Lin28-let-7 pathway. Nature 497, 244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Liu J, Cheng Y, and Jia D (2002). HEN1 functions pleiotropically in Arabidopsis development and acts in C function in the flower. Development 129, 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Wang Z, and Dean J (2021). Sperm acrosome overgrowth and infertility in mice lacking chromosome 18 pachytene piRNA. PLoS genetics 17, e1009485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou MT, Han BW, Hsiao CP, Zamore PD, Weng Z, and Hung JH (2015). Tailor: a computational framework for detecting non-templated tailing of small silencing RNAs. Nucleic Acids Res 43, e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cora E, Pandey RR, Xiol J, Taylor J, Sachidanandam R, McCarthy AA, and Pillai RS (2014). The MID-PIWI module of Piwi proteins specifies nucleotide- and strand-biases of piRNAs. RNA 20, 773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech B, Munafò M, Ciabrelli F, Eastwood EL, Fabry MH, Kneuss E, and Hannon GJ (2018). piRNA-Guided Genome Defense: From Biogenesis to Silencing. Annu Rev Genet 52, 131–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das PP, Bagijn MP, Goldstein LD, Woolford JR, Lehrbach NJ, Sapetschnig A, Buhecha HR, Gilchrist MJ, Howe KL, Stark R, Matthews N, Berezikov E, Ketting RF, Tavare S, and Miska EA (2008). Piwi and piRNAs act upstream of an endogenous siRNA pathway to suppress Tc3 transposon mobility in the Caenorhabditis elegans germline. Mol Cell 31, 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Mata M, Gaidatzis D, Vitanescu M, Stadler MB, Wentzel C, Scheiffele P, Filipowicz W, and Großhans H (2015). Potent degradation of neuronal miRNAs induced by highly complementary targets. EMBO Rep 16, 500–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien B, Baumberger N, Schepetilnikov M, Viotti C, De Cillia J, Ziegler-Graff V, Isono E, Schumacher K, and Genschik P (2012). Degradation of the antiviral component ARGONAUTE1 by the autophagy pathway. Proc Natl Acad Sci U S A 109, 15942–15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Liu J, Dong K, Melnick AF, Latham KE, and Chen C (2019). Mitochondrial membrane-based initial separation of MIWI and MILI functions during pachytene piRNA biogenesis. Nucleic Acids Res 47, 2594–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Liu J, Dong K, Midic U, Hess RA, Xie H, Demireva EY, and Chen C (2017). PNLDC1 is essential for piRNA 3′ end trimming and transposon silencing during spermatogenesis in mice. Nat Commun 8, 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy EE, Rutenberg-Schoenberg M, Stark CD, Kitchen RR, Gerstein MB, and Simon MD (2015). Tracking Distinct RNA Populations Using Efficient and Reversible Covalent Chemistry. Mol Cell 59, 858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faehnle CR, Walleshauser J, and Joshua-Tor L (2014). Mechanism of Dis3l2 substrate recognition in the Lin28-let-7 pathway. Nature 514, 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faehnle CR, Walleshauser J, and Joshua-Tor L (2017). Multi-domain utilization by TUT4 and TUT7 in control of let-7 biogenesis. Nat Struct Mol Biol 24, 658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltzin VL, Khaladkar M, Abe M, Parisi M, Hendriks GJ, Kim J, and Bonini NM (2015). The exonuclease Nibbler regulates age-associated traits and modulates piRNA length in Drosophila. Aging Cell 14, 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost RJ, Hamra FK, Richardson JA, Qi X, Bassel-Duby R, and Olson EN (2010). MOV10L1 is necessary for protection of spermatocytes against retrotransposons by Piwi-interacting RNAs. Proc Natl Acad Sci U S A 107, 11847–11852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Wu PH, Beane T, Zamore PD, and Weng Z (2018a). Elimination of PCR duplicates in RNA-seq and small RNA-seq using unique molecular identifiers. BMC Genomics 19, 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Yang Y, Zhang H, Farley G, Wang J, Quarles KA, Weng Z, and Zamore PD (2018b). The genome of the Hi5 germ cell line from Trichoplusia ni, an agricultural pest and novel model for small RNA biology. Elife 7, e31628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov I, Colpan C, Arif A, Cecchini K, and Zamore PD (2018). A Single Mechanism of Biogenesis, Initiated and Directed by PIWI Proteins, Explains piRNA Production in Most Animals. Mol Cell 71, 775–790.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge DT, Wang W, Tipping C, Gainetdinov I, Weng Z, and Zamore PD (2019). The RNA-Binding ATPase, Armitage, Couples piRNA Amplification in Nuage to Phased piRNA Production on Mitochondria. Mol Cell 74, 982–995.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghini F, Rubolino C, Climent M, Simeone I, Marzi MJ, and Nicassio F (2018). Endogenous transcripts control miRNA levels and activity in mammalian cells by target-directed miRNA degradation. Nat Commun 9, 3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard A, Sachidanandam R, Hannon GJ, and Carmell MA (2006). A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 442, 199–202. [DOI] [PubMed] [Google Scholar]
- Goh WS, Falciatori I, Tam OH, Burgess R, Meikar O, Kotaja N, Hammell M, and Hannon GJ (2015). piRNA-directed cleavage of meiotic transcripts regulates spermatogenesis. Genes Dev 29, 1032–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimson A, Srivastava M, Fahey B, Woodcroft BJ, Chiang HR, King N, Degnan BM, Rokhsar DS, and Bartel DP (2008). Early origins and evolution of microRNAs and Piwi-interacting RNAs in animals. Nature 455, 1193–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivna ST, Beyret E, Wang Z, and Lin H (2006). A novel class of small RNAs in mouse spermatogenic cells. Genes Dev 20, 1709–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase AD, Fenoglio S, Muerdter F, Guzzardo PM, Czech B, Pappin DJ, Chen C, Gordon A, and Hannon GJ (2010). Probing the initiation and effector phases of the somatic piRNA pathway in Drosophila. Genes Dev 24, 2499–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han BW, Hung JH, Weng Z, Zamore PD, and Ameres SL (2011). The 3′-to-5′ exoribonuclease Nibbler shapes the 3′ ends of microRNAs bound to Drosophila Argonaute1. Curr Biol 21, 1878–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han BW, Wang W, Li C, Weng Z, and Zamore PD (2015a). Noncoding RNA. piRNA-guided transposon cleavage initiates Zucchini-dependent, phased piRNA production. Science 348, 817–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han BW, Wang W, Zamore PD, and Weng Z (2015b). piPipes: a set of pipelines for piRNA and transposon analysis via small RNA-seq, RNA-seq, degradome- and CAGE-seq, ChIP-seq and genomic DNA sequencing. Bioinformatics 31, 593–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, LaVigne CA, Jones BT, Zhang H, Gillett F, and Mendell JT (2020). A ubiquitin ligase mediates target-directed microRNA decay independently of tailing and trimming. Science 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi R, Schnabl J, Handler D, Mohn F, Ameres SL, and Brennecke J (2016). Genetic and mechanistic diversity of piRNA 3′-end formation. Nature 539, 588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo I, Joo C, Kim YK, Ha M, Yoon MJ, Cho J, Yeom KH, Han J, and Kim VN (2009). TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell 138, 696–708. [DOI] [PubMed] [Google Scholar]
- Homolka D, Pandey RR, Goriaux C, Brasset E, Vaury C, Sachidanandam R, Fauvarque MO, and Pillai RS (2015). PIWI Slicing and RNA Elements in Precursors Instruct Directional Primary piRNA Biogenesis. Cell Rep 12, 418–428. [DOI] [PubMed] [Google Scholar]
- Horwich MD, Li C, Matranga C, Vagin V, Farley G, Wang P, and Zamore PD (2007). The Drosophila RNA methyltransferase, DmHen1, modifies germline piRNAs and single-stranded siRNAs in RISC. Curr Biol 17, 1265–1272. [DOI] [PubMed] [Google Scholar]
- Houwing S, Kamminga LM, Berezikov E, Cronembold D, Girard A, van den Elst H, Filippov DV, Blaser H, Raz E, Moens CB, Plasterk RH, Hannon GJ, Draper BW, and Ketting RF (2007). A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in Zebrafish. Cell 129, 69–82. [DOI] [PubMed] [Google Scholar]
- Huang H, Gao Q, Peng X, Choi SY, Sarma K, Ren H, Morris AJ, and Frohman MA (2011). piRNA-associated germline nuage formation and spermatogenesis require MitoPLD profusogenic mitochondrial-surface lipid signaling. Dev Cell 20, 376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Fejes Tóth K, and Aravin AA (2017). piRNA Biogenesis in Drosophila melanogaster. Trends Genet 33, 882–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipsaro JJ, Haase AD, Knott SR, Joshua-Tor L, and Hannon GJ (2012). The structural biochemistry of Zucchini implicates it as a nuclease in piRNA biogenesis. Nature 491, 279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizu H, Kinoshita T, Hirakata S, Komatsuzaki C, and Siomi MC (2019). Distinct and Collaborative Functions of Yb and Armitage in Transposon-Targeting piRNA Biogenesis. Cell Rep 27, 1822–1835.e8. [DOI] [PubMed] [Google Scholar]
- Izumi N, Shoji K, Sakaguchi Y, Honda S, Kirino Y, Suzuki T, Katsuma S, and Tomari Y (2016). Identification and Functional Analysis of the Pre-piRNA 3′ Trimmer in Silkworms. Cell 164, 962–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi N, Shoji K, Suzuki Y, Katsuma S, and Tomari Y (2020). Zucchini consensus motifs determine the mechanism of pre-piRNA production. Nature 578, 311–316. [DOI] [PubMed] [Google Scholar]
- Juliano CE, Reich A, Liu N, Gotzfried J, Zhong M, Uman S, Reenan RA, Wessel GM, Steele RE, and Lin H (2014). PIWI proteins and PIWI-interacting RNAs function in Hydra somatic stem cells. Proceedings of the National Academy of Sciences 111, 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamminga LM, Luteijn MJ, den Breeder MJ, Redl S, Kaaij LJ, Roovers EF, Ladurner P, Berezikov E, and Ketting RF (2010). Hen1 is required for oocyte development and piRNA stability in zebrafish. EMBO J 29, 3688–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamminga LM, van Wolfswinkel JC, Luteijn MJ, Kaaij LJ, Bagijn MP, Sapetschnig A, Miska EA, Berezikov E, and Ketting RF (2012). Differential impact of the HEN1 homolog HENN-1 on 21U and 26G RNAs in the germline of Caenorhabditis elegans. PLoS Genet 8, e1002702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandeel FR, and Swerdloff RS (1988). Role of temperature in regulation of spermatogenesis and the use of heating as a method for contraception. Fertil Steril 49, 1–23. [DOI] [PubMed] [Google Scholar]
- Kawaoka S, Izumi N, Katsuma S, and Tomari Y (2011). 3′ end formation of PIWI-interacting RNAs in vitro. Mol Cell 43, 1015–1022. [DOI] [PubMed] [Google Scholar]
- Kim B, Ha M, Loeff L, Chang H, Simanshu DK, Li S, Fareh M, Patel DJ, Joo C, and Kim VN (2015). TUT7 controls the fate of precursor microRNAs by using three different uridylation mechanisms. EMBO J 34, 1801–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston ER, and Bartel DP (2019). Global analyses of the dynamics of mammalian microRNA metabolism. Genome Res 29, 1777–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston ER, and Bartel DP (2021). Ago2 protects Drosophila siRNAs and microRNAs from target-directed degradation, even in the absence of 2′-O-methylation. RNA [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino Y, and Mourelatos Z (2007). The mouse homolog of HEN1 is a potential methylase for Piwi-interacting RNAs. RNA 13, 1397–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleaveland B, Shi CY, Stefano J, and Bartel DP (2018). A Network of Noncoding Regulatory RNAs Acts in the Mammalian Brain. Cell 174, 350–362.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Shoji K, Kiyokawa K, Negishi L, and Tomari Y (2019). Iruka Eliminates Dysfunctional Argonaute by Selective Ubiquitination of Its Empty State. Mol Cell 73, 119–129.e5. [DOI] [PubMed] [Google Scholar]
- Kojima-Kita K, Kuramochi-Miyagawa S, Nagamori I, Ogonuki N, Ogura A, Hasuwa H, Akazawa T, Inoue N, and Nakano T (2016). MIWI2 as an Effector of DNA Methylation and Gene Silencing in Embryonic Male Germ Cells. Cell Rep 16, 2819–2828. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Suleski M, and Hedges SB (2017). TimeTree: A Resource for Timelines, Timetrees, and Divergence Times. Mol Biol Evol 34, 1812–1819. [DOI] [PubMed] [Google Scholar]
- Kuramochi-Miyagawa S, Kimura T, Ijiri TW, Isobe T, Asada N, Fujita Y, Ikawa M, Iwai N, Okabe M, Deng W, Lin H, Matsuda Y, and Nakano T (2004). Mili, a mammalian member of piwi family gene, is essential for spermatogenesis. Development 131, 839–849. [DOI] [PubMed] [Google Scholar]
- Kuramochi-Miyagawa S, Watanabe T, Gotoh K, Totoki Y, Toyoda A, Ikawa M, Asada N, Kojima K, Yamaguchi Y, Ijiri TW, Hata K, Li E, Matsuda Y, Kimura T, Okabe M, Sakaki Y, Sasaki H, and Nakano T (2008). DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev 22, 908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth HM, and Mochizuki K (2009). 2′-O-methylation stabilizes Piwi-associated small RNAs and ensures DNA elimination in Tetrahymena. RNA 15, 675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łabno A, Warkocki Z, Kuliń ski T, Krawczyk PS, Bijata K, Tomecki R, and Dziembowski A (2016). Perlman syndrome nuclease DIS3L2 controls cytoplasmic non-coding RNAs and provides surveillance pathway for maturing snRNAs. Nucleic Acids Res 44, 10437–10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCava J, Houseley J, Saveanu C, Petfalski E, Thompson E, Jacquier A, and Tollervey D (2005). RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell 121, 713–724. [DOI] [PubMed] [Google Scholar]
- Langmead B, and Salzberg SL (2012). Fast gapped-read alignment with Bowtie 2. Nat Methods 9, 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, and Salzberg SL (2009). Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10, R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau NC, Seto AG, Kim J, Kuramochi-Miyagawa S, Nakano T, Bartel DP, and Kingston RE (2006). Characterization of the piRNA complex from rat testes. Science 313, 363–367. [DOI] [PubMed] [Google Scholar]
- Lee S, Song J, Kim S, Kim J, Hong Y, Kim Y, Kim D, Baek D, and Ahn K (2013). Selective degradation of host MicroRNAs by an intergenic HCMV noncoding RNA accelerates virus production. Cell Host Microbe 13, 678–690. [DOI] [PubMed] [Google Scholar]
- Lewis SH, Quarles KA, Yang Y, Tanguy M, Frézal L, Smith SA, Sharma PP, Cordaux R, Gilbert C, Giraud I, Collins DH, Zamore PD, Miska EA, Sarkies P, and Jiggins FM (2018). Pan-arthropod analysis reveals somatic piRNAs as an ancestral defence against transposable elements. Nat Ecol Evol 2, 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, and 1000, G. P. D. P. S. (2009). The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yang Z, Yu B, Liu J, and Chen X (2005). Methylation protects miRNAs and siRNAs from a 3′-end uridylation activity in Arabidopsis. Curr Biol 15, 1501–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XZ, Roy CK, Dong X, Bolcun-Filas E, Wang J, Han BW, Xu J, Moore MJ, Schimenti JC, Weng Z, and Zamore PD (2013). An ancient transcription factor initiates the burst of piRNA production during early meiosis in mouse testes. Mol Cell 50, 67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libri V, Helwak A, Miesen P, Santhakumar D, Borger JG, Kudla G, Grey F, Tollervey D, and Buck AH (2012). Murine cytomegalovirus encodes a miR-27 inhibitor disguised as a target. Proc Natl Acad Sci U S A 109, 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Ha M, Chang H, Kwon SC, Simanshu DK, Patel DJ, and Kim VN (2014a). Uridylation by TUT4 and TUT7 marks mRNA for degradation. Cell 159, 1365–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim RS, Anand A, Nishimiya-Fujisawa C, Kobayashi S, and Kai T (2014b). Analysis of Hydra PIWI proteins and piRNAs uncover early evolutionary origins of the piRNA pathway. Dev Biol 386, 237–251. [DOI] [PubMed] [Google Scholar]
- Lim SL, Qu ZP, Kortschak RD, Lawrence DM, Geoghegan J, Hempfling AL, Bergmann M, Goodnow CC, Ormandy CJ, Wong L, Mann J, Scott HS, Jamsai D, Adelson DL, and O’Bryan MK (2015). HENMT1 and piRNA Stability Are Required for Adult Male Germ Cell Transposon Repression and to Define the Spermatogenic Program in the Mouse. PLoS Genet 11, e1005620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Abe M, Sabin LR, Hendriks GJ, Naqvi AS, Yu Z, Cherry S, and Bonini NM (2011). The exoribonuclease Nibbler controls 3′ end processing of microRNAs in Drosophila. Curr Biol 21, 1888–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz R, Bernhart SH, Höner Zu Siederdissen C, Tafer H, Flamm C, Stadler PF, and Hofacker IL (2011). ViennaRNA Package 2.0. Algorithms Mol Biol 6, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, and Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Buchold GM, Greenbaum MP, Roy A, Burns KH, Zhu H, Han DY, Harris RA, Coarfa C, Gunaratne PH, Yan W, and Matzuk MM (2009). GASZ is essential for male meiosis and suppression of retrotransposon expression in the male germline. PLoS Genet 5, e1000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecki M, Viegas SC, Carneiro T, Golik P, Dressaire C, Ferreira MG, and Arraiano CM (2013). The exoribonuclease Dis3L2 defines a novel eukaryotic RNA degradation pathway. EMBO J 32, 1842–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinowski L, Tanguy M, Krmpotic A, Rädle B, Lisnić VJ, Tuddenham L, Chane-Woon-Ming B, Ruzsics Z, Erhard F, Benkartek C, Babic M, Zimmer R, Trgovcich J, Koszinowski UH, Jonjic S, Pfeffer S, and Dölken L (2012). Degradation of cellular miR-27 by a novel, highly abundant viral transcript is important for efficient virus replication in vivo. PLoS Pathog 8, e1002510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SL (1991). Ribonucleoprotein particles with LINE-1 RNA in mouse embryonal carcinoma cells. Mol Cell Biol 77, 4804–4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez NJ, Chang HM, Borrajo JR, and Gregory RI (2013). The co-chaperones Fkbp4/5 control Argonaute2 expression and facilitate RISC assembly. RNA 19, 1583–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez NJ, and Gregory RI (2013). Argonaute2 expression is post-transcriptionally coupled to microRNA abundance. RNA 19, 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi MJ, Ghini F, Cerruti B, de Pretis S, Bonetti P, Giacomelli C, Gorski MM, Kress T, Pelizzola M, Muller H, Amati B, and Nicassio F (2016). Degradation dynamics of microRNAs revealed by a novel pulse-chase approach. Genome Res 26, 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto N, Nishimasu H, Sakakibara K, Nishida KM, Hirano T, Ishitani R, Siomi H, Siomi MC, and Nureki O (2016). Crystal Structure of Silkworm PIWI-Clade Argonaute Siwi Bound to piRNA. Cell 167, 484–497.e9. [DOI] [PubMed] [Google Scholar]
- Modepalli V, Fridrich A, Agron M, and Moran Y (2018). The methyltransferase HEN1 is required in Nematostella vectensis tox microRNA and piRNA stability as well as larval metamorphosis. PLoS Genet 14, e1007590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn F, Handler D, and Brennecke J (2015). Noncoding RNA. piRNA-guided slicing specifies transcripts for Zucchini-dependent, phased piRNA biogenesis. Science 348, 812–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery TA, Rim YS, Zhang C, Dowen RH, Phillips CM, Fischer SE, and Ruvkun G (2012). PIWI associated siRNAs and piRNAs specifically require the Caenorhabditis elegans HEN1 ortholog henn-1. PLoS Genet 8, e1002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran Y, Fredman D, Praher D, Li XZ, Wee LM, Rentzsch F, Zamore PD, Technau U, and Seitz H (2014). Cnidarian microRNAs frequently regulate targets by cleavage. Genome Res 24, 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M, Kabayama Y, Much C, Ivanova I, Di Giacomo M, Auchynnikava T, Monahan JM, Vitsios DM, Vasiliauskaite L, Comazzetto S, Rappsilber J, Allshire RC, Porse BT, Enright AJ, and O’Carroll D (2019). A programmed wave of uridylation-primed mRNA degradation is essential for meiotic progression and mammalian spermatogenesis. Cell Res 29, 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M, Much C, DiGiacomo M, Azzi C, Ivanova I, Vitsios DM, Pistolic J, Collier P, Moreira PN, Benes V, Enright AJ, and O’Carroll D (2017). mRNA 3′ uridylation and poly(A) tail length sculpt the mammalian maternal transcriptome. Nature 548, 347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò M, Manelli V, Falconio FA, Sawle A, Kneuss E, Eastwood EL, Seah JWE, Czech B, and Hannon GJ (2019). Daedalus and Gasz recruit Armitage to mitochondria, bringing piRNA precursors to the biogenesis machinery. Genes Dev 33, 844–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimasu H, Ishizu H, Saito K, Fukuhara S, Kamatani MK, Bonnefond L, Matsumoto N, Nishizawa T, Nakanaga K, Aoki J, Ishitani R, Siomi H, Siomi MC, and Nureki O (2012). Structure and function of Zucchini endoribonuclease in piRNA biogenesis. Nature 491, 284–287. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Nagamori I, Nakatani T, Izumi N, Tomari Y, Kuramochi-Miyagawa S, and Nakano T (2018). PNLDC1, mouse pre-piRNA Trimmer, is required for meiotic and post-meiotic male germ cell development. EMBO Rep [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozata DM, Gainetdinov I, Zoch A, O’Carroll D, and Zamore PD (2019). PIWI-interacting RNAs: small RNAs with big functions. Nat Rev Genet 20, 89–108. [DOI] [PubMed] [Google Scholar]
- Özata DM, Yu T, Mou H, Gainetdinov I, Colpan C, Cecchini K, Kaymaz Y, Wu PH, Fan K, Kucukural A, Weng Z, and Zamore PD (2020). Evolutionarily conserved pachytene piRNA loci are highly divergent among modern humans. Nat Ecol Evol 4 156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore B, Hertz HL, Price IF, and Tang W (2021). pre-piRNA trimming and 2′-O-methylation protect piRNAs from 3′ tailing and degradation in C. elegans. Cell Reports 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Shin SY, and Shin C (2017). Non-canonical targets destabilize microRNAs in human Argonautes. Nucleic Acids Res 45, 1569–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W, Li J, Song R, Messing J, and Chen X (2002). CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr Biol 12, 1484–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pélisson A, Sarot E, Payen-Groschêne G, and Bucheton A (2007). A novel repeat-associated small interfering RNA-mediated silencing pathway downregulates complementary sense gypsy transcripts in somatic cells of the Drosophila ovary. J Virol 81, 1951–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea M, Kim D, Pertea GM, Leek JT, and Salzberg SL (2016). Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc 11, 1650–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier D, Davie K, Hulselmans G, Naval Sanchez M, Haagen L, Huynh-Thu VA, Koldere D, Celik A, Geurts P, Christiaens V, and Aerts S (2014). Mapping gene regulatory networks in Drosophila eye development by large-scale transcriptome perturbations and motif inference. Cell Rep 9, 2290–2303. [DOI] [PubMed] [Google Scholar]
- Reichholf B, Herzog VA, Fasching N, Manzenreither RA, Sowemimo I, and Ameres SL (2019). Time-Resolved Small RNA Sequencing Unravels the Molecular Principles of MicroRNA Homeostasis. Mol Cell 75, 756–768.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimão-Pinto MM, Manzenreither RA, Burkard TR, Sledz P, Jinek M, Mechtler K, and Ameres SL (2016). Molecular basis for cytoplasmic RNA surveillance by uridylation-triggered decay in Drosophila. EMBO J 35, 2417–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Berninger P, Chuma S, Shah H, Hosokawa M, Funaya C, Antony C, Sachidanandam R, and Pillai RS (2011). Miwi catalysis is required for piRNA amplification-independent LINE1 transposon silencing. Nature 480, 264–267. [DOI] [PubMed] [Google Scholar]
- Rissland OS, and Norbury CJ (2009). Decapping is preceded by 3′ uridylation in a novel pathway of bulk mRNA turnover. Nat Struct Mol Biol 16, 616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegger S, and Großhans H (2012). MicroRNA turnover: when, how, and why. Trends Biochem Sci 37, 436–446. [DOI] [PubMed] [Google Scholar]
- Saito K, Sakaguchi Y, Suzuki T, Suzuki T, Siomi H, and Siomi MC (2007). Pimet, the Drosophila homolog of HEN1, mediates 2′-O-methylation of Piwi- interacting RNAs at their 3′ ends. Genes Dev 21, 1603–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Nishida KM, Mori T, Kawamura Y, Miyoshi K, Nagami T, Siomi H, and Siomi MC (2006). Specific association of Piwi with rasiRNAs derived from retrotransposon and heterochromatic regions in the Drosophila genome. Genes & development 20, 2214–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe JP, Chen M, Zhao H, and Lin H (2013). Tdrkh is essential for spermatogenesis and participates in primary piRNA biogenesis in the germline. EM BO J 32, 1869–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu-Gruttadauria J, Pawlica P, Klum SM, Wang S, Yario TA, Schirle Oakdale NT, Steitz JA, and MacRae IJ (2019). Structural Basis for Target-Directed MicroRNA Degradation. Mol Cell 75, 1243–1255.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CY, Kingston ER, Kleaveland B, Lin DH, Stubna MW, and Bartel DP (2020). The ZSWIM8 ubiquitin ligase mediates target-directed microRNA degradation. Science 370, eabc9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M, Tanaka T, Hosokawa M, Reuter M, Stark A, Kato Y, Kondoh G, Okawa K, Chujo T, Suzuki T, Hata K, Martin SL, Noce T, Kuramochi-Miyagawa S, Nakano T, Sasaki H, Pillai RS, Nakatsuji N, and Chuma S (2009). The TDRD9-MIWI2 complex is essential for piRNA-mediated retrotransposon silencing in the mouse male germline. Dev Cell 17, 775–787. [DOI] [PubMed] [Google Scholar]
- Smibert P, Yang JS, Azzam G, Liu JL, and Lai EC (2013). Homeostatic control of Argonaute stability by microRNA availability. Nat Struct Mol Biol 20, 789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper SF, van der Heijden GW, Hardiman TC, Goodheart M, Martin SL, de Boer P, and Bortvin A (2008). Mouse maelstrom, a component of nuage, is essential for spermatogenesis and transposon repression in meiosis. Dev Cell 15, 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumillon M, Necsulea A, Weier M, Brawand D, Zhang X, Gu H, Barthès P, Kokkinaki M, Nef S, Gnirke A, Dym M, de Massy B, Mikkelsen TS, and Kaessmann H (2013). Cellular source and mechanisms of high transcriptome complexity in the mammalian testis. Cell Rep 3, 2179–2190. [DOI] [PubMed] [Google Scholar]
- Svendsen JM, Reed KJ, Vijayasarathy T, Montgomery BE, Tucci RM, Brown KC, Marks TN, Nguyen DAH, Phillips CM, and Montgomery TA (2019). henn-1/HEN1 Promotes Germline Immortality in Caenorhabditis elegans. Cell Rep 29, 3187–3199.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka SS, Toyooka Y, Akasu R, Katoh-Fukui Y, Nakahara Y, Suzuki R, Yokoyama M, and Noce T (2000). The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev 14, 841–853. [PMC free article] [PubMed] [Google Scholar]
- Tang W, Tu S, Lee HC, Weng Z, and Mello CC (2016). The RNase PARN-1 Trims piRNA 3′ Ends to Promote Transcriptome Surveillance in C. elegans. Cell 164, 974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JE, Chang HM, Piskounova E, and Gregory RI (2012). Lin28-mediated control of let-7 microRNA expression by alternative TUTases Zcchc11 (TUT4) and Zcchc6 (TUT7). RNA 18, 1875–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudek A, Lloret-Llinares M, and Jensen TH (2018). The multitasking polyA tail: nuclear RNA maturation, degradation and export. Philos Trans R Soc Lond B Biol Sci 373, 20180169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustianenko D, Hrossova D, Potesil D, Chalupnikova K, Hrazdilova K, Pachernik J, Cetkovska K, Uldrijan S, Zdrahal Z, and Vanacova S (2013). Mammalian DIS3L2 exoribonuclease targets the uridylated precursors of let-7 miRNAs. RNA 19, 1632–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustianenko D, Pasulka J, Feketova Z, Bednarik L, Zigackova D, Fortova A, Zavolan M, and Vanacova S (2016). TUT-DIS3L2 is a mammalian surveillance pathway for aberrant structured non-coding RNAs. EMBO J 35, 2179–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagin VV, Sigova A, Li C, Seitz H, Gvozdev V, and Zamore PD (2006). A distinct small RNA pathway silences selfish genetic elements in the germline. Science 313, 320–324. [DOI] [PubMed] [Google Scholar]
- Vanácová S, Wolf J, Martin G, Blank D, Dettwiler S, Friedlein A, Langen H, Keith G, and Keller W (2005). A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS Biol 3, e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ma Z, Niu K, Xiao Y, Wu X, Pan C, Zhao Y, Wang K, Zhang Y, and Liu N (2016). Antagonistic roles of Nibbler and Hen1 in modulating piRNA 3′ ends in Drosophila. Development 143, 530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Yoshikawa M, Han BW, Izumi N, Tomari Y, Weng Z, and Zamore PD (2014). The initial uridine of primary piRNAs does not create the tenth adenine that Is the hallmark of secondary piRNAs. Mol Cell 56, 708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Chuma S, Yamamoto Y, Kuramochi-Miyagawa S, Totoki Y, Toyoda A, Hoki Y, Fujiyama A, Shibata T, Sado T, Noce T, Nakano T, Nakatsuji N, Lin H, and Sasaki H (2011). MITOPLD is a mitochondrial protein essential for nuage formation and piRNA biogenesis in the mouse germline. Dev Cell 20, 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieckowski MR, Giorgi C, Lebiedzinska M, Duszynski J, and Pinton P (2009). Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat Protoc 4, 1582–1590. [DOI] [PubMed] [Google Scholar]
- Wu PH, Fu Y, Cecchini K, Özata DM, Arif A, Yu T, Colpan C, Gainetdinov I, Weng Z, and Zamore PD (2020). The evolutionarily conserved piRNA-producing locus pi6 is required for male mouse fertility. Nat Genet 52, 728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyers F, Rougemaille M, Badis G, Rousselle JC, Dufour ME, Boulay J, Régnault B, Devaux F, Namane A, Séraphin B, Libri D, and Jacquier A (2005). Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 121, 725–737. [DOI] [PubMed] [Google Scholar]
- Xie W, Sowemimo I, Hayashi R, Wang J, Burkard TR, Brennecke J, Ameres SL, and Patel DJ (2020). Structure-function analysis of microRNA 3′-end trimming by Nibbler. Proc Natl Acad Sci U S A 117, 30370–30379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Oe A, Nishida KM, Yamashita K, Kajiya A, Hirano S, Matsumoto N, Dohmae N, Ishitani R, Saito K, Siomi H, Nishimasu H, Siomi MC, and Nureki O (2020). Crystal structure of Drosophila Piwi. Nat Commun 11, 858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro H, and Siomi MC (2018). PIWI-Interacting RNA in Drosophila: Biogenesis, Transposon Regulation, and Beyond. Chem Rev 118, 4404–4421. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Toyoda S, Kuramochi-Miyagawa S, Miyazaki T, Miyazaki S, Tashiro F, Yamato E, Nakano T, and Miyazaki J (2009). Gtsf1/Cue110, a gene encoding a protein with two copies of a CHHC Zn-finger motif, is involved in spermatogenesis and retrotransposon suppression in murine testes. Dev Biol 335, 216–227. [DOI] [PubMed] [Google Scholar]
- Yu B, Yang Z, Li J, Minakhina S, Yang M, Padgett RW, Steward R, and Chen X (2005). Methylation as a crucial step in plant microRNA biogenesis. Science 307, 932–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamparini AL, Davis MY, Malone CD, Vieira E, Zavadil J, Sachidanandam R, Hannon GJ, and Lehmann R (2011). Vreteno, a gonad-specific protein, is essential for germline development and primary piRNA biogenesis in Drosophila. Development 138, 4039–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Kang JY, Gou LT, Wang J, Xue Y, Skogerboe G, Dai P, Huang DW, Chen R, Fu XD, Liu MF, and He S (2015). MIWI and piRNA-mediated cleavage of messenger RNAs in mouse testes. Cell Res 25, 193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Guo R, Cui Y, Zhu Z, Zhang Y, Wu H, Zheng B, Yue Q, Bai S, Zeng W, Guo X, Zhou Z, Shen B, Zheng K, Liu M, Ye L, and Sha J (2017). An essential role for PNLDC1 in piRNA 3′ end trimming and male fertility in mice. Cell Res 27, 1392–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Pi J, Zou D, Wang X, Xu J, Yu S, Zhang T, Li F, Zhang X, Zhao H, Wang F, Wang D, Ma Y, and Yu J (2019). microRNA arm-imbalance in part from complementary targets mediated decay promotes gastric cancer progression. Nat Commun 10, 4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Theurkauf WE, Weng Z, and Zamore PD (2012). Strand-specific libraries for high throughput RNA sequencing (RNA-Seq) prepared without poly(A) selection. Silence 3, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K, Xiol J, Reuter M, Eckardt S, Leu NA, McLaughlin KJ, Stark A, Sachidanandam R, Pillai RS, and Wang PJ (2010). Mouse MOV10L1 associates with Piwi proteins and is an essential component of the Piwi-interacting RNA (piRNA) pathway. Proc Natl Acad Sci U S A 107, 11841–11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigáčková D, and Vaňácová Š (2018). The role of 3′ end uridylation in RNA metabolism and cellular physiology. Philos Trans R Soc Lond B Biol Sci 373, 20180171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. mRNAs whose Derepression in Henmt1em1/em1 and Pnldc1em1/em1 Mice can be Explained by Reduced Cleavage by Destabilized piRNAs, Related to Figure 6.
Table S4. Number of Cells and Amount of Spike-In Mix Used to Prepare Small RNA Sequencing Libraries, Related to Small RNA-seq Library Preparation in STAR Methods.
Table S5. Number of Cells and Amount of ERCC Spike-In Mix 1 Used to Prepare RNA Sequencing Libraries (A) and List of Libraries of 5′ Monophosphorylated Long RNAs (B), Related to RNA-seq Library Preparation in STAR Methods.
Data Availability Statement
Sequencing data have been deposited at National Center for Biotechnology Information Sequence Read Archive and are publicly available as of the date of publication using accession number PRJNA660633 (see the key resources table). Original autoradiography, microscopy, and Western blotting images have been deposited at Mendeley and are publicly available as of the date of publication. The DOI is listed in the key resources table.
All published software required to reanalyze the data reported in this paper is described in the Quantification and Statistical Analysis section below.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-SYCP3 | Abcam | Cat# ab15093, RRID:AB_301639 |
| Mouse monoclonal Anti-Histone H2A.X | Millipore | Cat# 05-636, RRID:AB_309864 |
| Donkey anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 594 |
Thermo Fisher Scientific | Cat# A-21203, RRID:AB_2535789 |
| Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 |
Thermo Fisher Scientific | Cat# A-21206, RRID:AB_2535792 |
| Rabbit polyclonal PIWIL2 antibody | Abcam | Cat# ab36764, RRID:AB_777284 |
| Rabbit polyclonal PIWIL1 antibody | Abcam | Cat# ab12337, RRID:AB_470241 |
| Mouse monoclonal Anti-PIWIL1 | Wako | Cat # 017-23451, RRID:AB_2721829 |
| IRDye 680RD Donkey anti-Rabbit IgG (H + L), antibody |
LI-COR Biosciences |
Cat# 926-68073, RRID:AB_1095444 |
| IRDye 800CW Donkey anti-Mouse IgG (H + L), antibody |
LI-COR Biosciences |
Cat# 926-32212, RRID:AB_621847 |
| Deposited Data | ||
| Raw sequencing data from Mus musculus | This paper | NCBI: PRJNA660633 |
| Original autoradiography and Western blotting images | This paper | http://dx.doi.org/10.17632/2X65btsysm.3 |
| Experimental Models: Organisms/Strains | ||
| C57BL/6J mice | Jackson Laboratory | IMSR # JAX:000664, RRID:IMSR_JAX:000664 |
| Pnldc1em1Pdz/em1Pdz mice | (Gainetdinov et al., 2018) | MGI: 6161374 |
| Henmt1em1Pdz/em1Pdz mice | This paper | MGI: 6452642 |
| Tdrkh−/− mice | Jackson Laboratory | MGI: 5521576 |
| Miwi−/− mice | Jackson Laboratory | MGI: 2182488 |
| w[*]; wg[Sp-1]/CyO; P{w[+mC]=UAS-GFP.dsRNA.R}142 flies | Bloomington Drosophila Stock Center | BDSC: 44415; RRID:BDSC_ 44415 |
| y[1] w[*]; wg[Sp-1]/CyO; P{w[+mC]=longGMR-GAL4}3/TM2 flies | Bloomington Drosophila Stock Center | BDSC: 8121; RRID:BDSC_ 8121 |
| PBac{WH}Hen1f00810 flies | (Horwich et al., 2007) | N/A |
| Oligonucleotides | ||
| Small RNA cloning 3′ adapter (AppBA3-UMI, 36-nt custom DNA oligo with 5′ adenylation and 3′ ddC): 5′ - rAppNNNGTCNNNTAGNNNTGGAATTCTCGGGTGCCAAGG/ddC/-3′ | Integrated DNA Technologies | N/A |
| Small RNA cloning 5′ adapter 1 (BA5-UMI-a, 41-nt custom RNA oligos): 5′ - GUUCAGAGUUCUACAGUCCGACGAUCNNNCGANNNUACNNN-3′ | Integrated DNA Technologies | N/A |
| Small RNA cloning 5′ adapter 2 (BA5-UMI-b, 41-nt custom RNA oligos): 5′ - GUUCAGAGUUCUACAGUCCGACGAUCNNNAUCNNNAGUNNN-3′ | Integrated DNA Technologies | N/A |
| RT primer (custom DNA oligo): 5′-CCTTGGCACCCGAGAATTCCA-3′ | Integrated DNA Technologies | N/A |
| PCR forward primer (50-nt custom DNA oligo): 5′-AATGATACGGCGACCACCGAGATCTACACGTTCAGAGTTCTACAGTCCGA-3′ | Integrated DNA Technologies | N/A |
| Multiplexing Adapter 1: 5′-P-GATCGGAAGAGCACACGTCT-3′ |
Integrated DNA Technologies | N/A |
| Multiplexing Adapter 2: 5′-ACACTCTTTCCCTACACGACGCTCTTCCGATCT-3′ |
Integrated DNA Technologies | N/A |
| Multiplexing PCR Primer 1: 5′-AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCT-3′ |
Integrated DNA Technologies | N/A |
| Multiplexing PCR Primer 2 (NNNNNN represents barcode) 5′-CAAGCAGAAGACGGCATACGAGATNNNNNNGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT-3′ |
Integrated DNA Technologies | N/A |
| Software and Algorithms | ||
| Bowtie version v2.2.0 | Langmead, B., and Salzberg, S. L. (2012). Nat Methods 9, 357. | http://bowtie-bio.sourceforge.net/index.shtml |
| Bowtie v1.0.0 | Langmead, B., Trapnell, C., Pop, M., and Salzberg, S. L. (2009). Genome Biol, 10, R25 | http://bowtie-bio.sourceforge.net/index.shtml |
| piPipes | Han, B. W., Wang, W., Zamore, P. D., and Weng, Z. (2015). Bioinformatics 31, 593. | https://github.com/bowhan/piPipes |
| BEDtools version v2.25.0 | Quinlan, A. R., and Hall, I. M. (2010). Bioinformatics 26:841. | http://bedtools.readthedocs.io |
| STAR version v2.3.1 | Dobin, A., Davis, C. A., Schlesinger, F., Drenkow, J., Zaleski, C., Jha, S., Batut, P., Chaisson, M., and Gingeras, T. R. (2013). Bioinformatics 29, 15. | https://github.com/alexdobin/STAR/releases |
| Samtools version v1.0.0 | Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., Marth, G., Abecasis, G., Durbin, R., and 1000, G. P. D. P. S. (2009). Bioinformatics 25, 2078. | http://samtools.sourceforge.net |
| DESeq2 version v1.18.1 | Love, M. I., Huber, W., and Anders, S. (2014). Genome Biol 15, 550. | https://bioconductor.org/packages/release/bioc/html/DESeq2.html |


