Abstract
Chloroplast translation is mediated by nucleus-encoded factors that interact with distinct cis-acting RNA elements. A U-rich sequence within the 5′ untranslated region of the psbD mRNA has previously been shown to be required for its translation in Chlamydomonas reinhardtii. By using UV cross-linking assays, we have identified a 40-kDa RNA binding protein, which binds to the wild-type psbD leader, but is unable to recognize a nonfunctional leader mutant lacking the U-rich motif. RNA binding is restored in a chloroplast cis-acting suppressor. The functions of several site-directed psbD leader mutants were analyzed with transgenic C. reinhardtii chloroplasts and the in vitro RNA binding assay. A clear correlation between photosynthetic activity and the capability to bind RNA by the 40-kDa protein was observed. Furthermore, the data obtained suggest that the poly(U) region serves as a molecular spacer between two previously characterized cis-acting elements, which are involved in RNA stabilization and translation. RNA-protein complex formation depends on the nuclear Nac2 gene product that is part of a protein complex required for the stabilization of the psbD mRNA. The sedimentation properties of the 40-kDa RNA binding protein suggest that it interacts directly with this Nac2 complex and, as a result, links processes of chloroplast RNA metabolism and translation.
Translational regulation has been shown to represent one of the essential control mechanisms for chloroplast gene expression in both green algae and higher plants (for review, see references 10, 17, 20, and 36). The assumed rate-limiting steps of translation initiation are mediated via the 5′ untranslated regions (UTRs) of many, if not all, chloroplast transcripts (22). However, recently obtained evidence suggests that the 3′ UTRs of chloroplast mRNAs may also participate in their own translation (39).
Some of the cis-acting RNA elements required for protein synthesis have been mapped after mutagenesis studies of different 5′ UTRs followed by either analysis of mutant phenotypes after biolistic chloroplast transformation or by in vitro translation, a system developed for tobacco chloroplasts (24). For instance, chloroplast sequence elements resembling prokaryotic Shine-Dalgarno boxes were found to be inessential for translation in some cases (13), whereas a modulative function might be held in others (5, 35, 41). The alteration of translational AUG start codons had variable effects on protein synthesis (7, 8, 35), and the deletion of a putative stem-loop structure within the psbC 5′ UTR (37) affected the function of the nucleus-encoded Tbc1 gene product involved in psbC translation in Chlamydomonas reinhardtii (44). Two short elements (16 and 14 nucleotides [nt] in length) essential for translation were mapped within the petD 5′ UTR, one of which forms a stem-loop structure in vivo (23). In general, it is assumed that these crucial cis-acting elements are required for maintaining secondary RNA structures involved in the translation initiation process (12, 23, 26, 31) and/or serve as target signals for trans-acting translation factors.
Genetic and biochemical evidence for the translational control of chloroplast gene expression by trans-acting, nucleus-encoded factors has been obtained from green algae and from higher plants. Several nuclear mutants have been described that exhibit defects in translation of different chloroplast mRNAs (2, 22, 29, 32), and chloroplast as well as nuclear suppressors of defects in chloroplast translation have been characterized (9, 36, 42, 44). The recently cloned Crp1 locus from maize is required for processing and translation of petA and petD mRNAs. In addition, the Crp1 protein belongs to a novel class of so-called PPR (pentatrico-peptide repeat) proteins (40) and is part of a stromal high-molecular-weight complex, which is not associated with chloroplast polysomes (15).
By using in vitro RNA binding assays, several proteins have been detected that interact with different chloroplast 5′ UTRs (11, 21, 34, 45, 46) and might mediate the translational control mechanism. Recently, two of these factors interacting with the psbA 5′ UTR of C. reinhardtii were identified as a poly(A) binding protein and a protein disulfide isomerase regulating the activity of the former protein in vitro (6, 25, 43).
The chloroplast psbD gene of C. reinhardtii encoding the D2 protein of photosystem II (PS II) is expressed under the control of the nucleus-encoded Nac2 factor, whose principal target site is the 5′ UTR of the psbD mRNA (27, 34). Recent mutational analysis of this 5′ UTR has revealed at least three distinct RNA elements, which are involved in the translational control of psbD gene expression (35). One of these elements codes for the AUG initiation codon, and a second one (PRB1) resembles a bacterial Shine-Dalgarno motif (GGAG) and is located 10 nt upstream of the start codon. In addition, the deletion of a striking U tract, located immediately upstream of the PRB1 element, completely inhibited psbD mRNA translation.
Here, we report on the identification and characterization of a 40-kDa RNA binding protein (RBP40) which interacts specifically with the translational U-rich element. Site-directed mutagenesis of this element helped identify the minimal requirements for binding of RBP40 to the psbD 5′ UTR in vitro, and the simultaneously performed analysis of chloroplast transformants revealed a correlation between binding activities and D2 synthesis in vivo. Furthermore, interaction of RBP40 with the psbD 5′ UTR was found to be dependent on the Nac2 factor, which is required for the stabilization of the psbD mRNA.
MATERIALS AND METHODS
Algal strains, suppressor isolation, and characterization.
The wild-type strain 137c, the mutant strain ΔU (35), and mφ14 (S. Purton, unpublished results) were maintained on Tris-acetate-phosphate (TAP) medium (18) at 25°C. Suppressor suΔU was isolated as follows. A total of 5 × 108 cells were plated on minimal medium selecting for photosynthetic growth (HSM) (37) and kept in the dark for 24 h. Subsequently, plates were irradiated with UV light (7.5 mJ, 254 nm) in a Stratalinker (Stratagene) and kept in the dark for another 24 h to prevent photoreactivation (19). Finally, suppressors were selected in bright light (100 μE m−2 s−1) over a period of up to 6 weeks. To test whether the suppressor mutation resides within the nuclear or chloroplast genome, suΔU (mt+) was genetically crossed (19) to the wild type (mt−). All 4 members out of 20 analyzed tetrads from this cross were able to grow photoautotrophically on minimal medium, indicating a chloroplast localization of the suppressor mutation. For the molecular analysis of psbD 5′ regions, total DNA from C. reinhardtii was isolated with the DNeasy Plant kit (Qiagen). PCR amplification of the psbD 5′ region with oligonucleotides 1365 and 1963 was performed as described previously (35), and, subsequently, PCR fragments were subjected to automated sequencing (MWG Biotech).
Preparation of chloroplast subfractions and sedimentation analysis.
The strains used harbored either the cw15 (wild type) or the cwd (mφ14 and mcos5) mutation, which facilitate chloroplast isolation. Cultures were grown in TAP medium containing 1% sorbitol to a density of 2 × 106 cells/ml. Cells were harvested by centrifugation, and chloroplasts were prepared as described previously (46). Isolated chloroplasts were lysed in hypotonic buffer (10 mM Tricine [pH 7.8], 10 mM EDTA, 5 mM 2-mercaptoethanol), loaded onto a 1.0 M sucrose cushion prepared in hypotonic buffer, and centrifuged at 100,000 × g for 3 h. The stroma fraction, which did not enter the sucrose cushion, was collected and diluted with the same volume of 75% glycerol. Crude thylakoid membranes in the pellet (cT fraction) were resuspended in 2× lysis buffer (20 mM Tricine [pH 7.8], 120 mM KCl, 10 mM 2-mercaptoethanol, 0.4 mM EDTA, 0.2% Triton X-100) and diluted with the same volume of 75% glycerol. For further purification, crude thylakoid membranes were resuspended in hypotonic buffer containing 1.8 M sucrose, overlayered with a 1.3 M sucrose solution (in hypotonic buffer), and centrifuged at 100,000 × g for 3 h. The floated thylakoid membranes were collected from the interphase, diluted with hypotonic buffer, and sedimented by centrifugation at 100,000 × g for 1 h. Finally, thylakoid membranes were resuspended in 2× lysis buffer and diluted with the same volume of 75% glycerol. Chloroplast lysates were prepared by lysis of isolated chloroplasts in 2× lysis buffer and dilution with the same volume of 75% glycerol. All preparations were stored at −20°C for less than 2 weeks before use. Longer storage, extensive dialysis, or quick-freezing of samples in liquid nitrogen lead to the selective loss of some RBP activities. Protein concentrations were determined by using the Bradford assay (Bio-Rad).
For sedimentation analysis, isolated chloroplasts were hypotonically lysed in buffer containing 5 mM ɛ-amino caproic acid, 25 μg of pepstatin A per ml, 10 μg of leupeptin per ml, 1 mM benzamidine HCl, and 1 mM phenylmethylsulfonyl fluoride. After centrifugation for 1 h at 100,000 × g, the supernatant containing only stromal proteins was loaded on a 15 to 35% linear glycerol gradient and centrifuged for 18 h at 180,000 × g in an SW41 rotor (Beckman Instruments, Inc.). The gradient was fractionated in 10 fractions of 1 ml. Fifty microliters of these fractions was used for Western analysis, and 10 μl was used for UV cross-linking experiments.
In vitro synthesis of RNA and UV cross-linking of RNA with proteins.
Templates for the in vitro synthesis of psbD leader RNA probes were generated by PCR amplification from appropriate DNAs by using oligonucleotide 3131, which is complementary to the region downstream of position +1, and oligonucleotide 2126 spanning the 5′ region from position −74, as well as the T7 promoter sequence (34). The template for pBluescript KS RNA synthesis was generated by digesting the pBluescript KS+ vector (Stratagene) with HindIII. In vitro transcription of RNA probes with T7 RNA polymerase (Promega) and UV cross-linking of RNAs with proteins were done essentially as described previously (33). Binding reactions (20 μl) were adjusted to 30 mM Tris HCl (pH 7.0), 50 mM KCl, 5 mM MgCl2, 5 mM 2-mercaptoethanol, 0.5 mM EDTA, 6 μg of protein, and 50 fmol of radiolabeled RNA. For competition experiments, radiolabeled RNA and nonlabeled competitor RNA were mixed prior to addition of proteins. Samples were incubated at room temperature for 5 min in contrast to previous experiments in which samples were left on ice for the same time (34). This alteration significantly increased the number and intensity of detected signals. Afterwards, samples were irradiated with UV light, treated with RNase, and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described previously (33). Quantification of competitor RNA amounts was performed by measuring the incorporation of low levels of radioactivity into transcripts.
Plasmid constructions and chloroplast transformation.
Constructs for chloroplast transformation which contain mutations for the in vivo analysis of the psbD 5′ region were generated by using a PCR-based method exactly as described in reference 35. The oligonucleotides used were mu1-1 (5′-CGTAACGATGAGTTGAGCCGGATCCGGAGATACACGCAATG-3′) and mu1-2 (5′-CATTGCGTGTATCTCCGGATCCGGCTCAACTCATCGTTACG-3′) [mutant suΔU(T→C)], mu2-1 (5′-CGTAACGATGAGTTGAAAAAAATAAAAGGAGATACACGCAATG-3′) and mu2-2 (5′-CATTGCGTGTATCTCCTTTTATTTTTTTCAACTCATCGTTACG-3′) [mutant poly(A)], mu3-1 (5′-CGTAACGATGAGTTGAGAAGGATCCGGAGATACACGCAATG-3′) and mu3-2 (5′-CATTGCGTGTATCTCCGGATCCTTCTCAACTCATCGTTACG-3′) [mutant suΔU(T→A)], U6-1 (5′-CGTAACGATGAGTTGTTTTTTGGAGATACACGCAATG-3′) and U6-2 (5′-CATTGCGTGTATCTCCAAAAAACAACTCATCGTTACG-3′) (mutant U6), U7-1 (5′-CGTAACGATGAGTTGTTTTTTTGGAGATACACGCAATG-3′) and U7-2 (5′-CATTGCGTGTATCTCCAAAAAAACAACTCATCGTTACG-3′) (mutant U7), U8-1 (5′-CGTAACGATGAGTTGTTTTTTTTGGAGATACACGCAATG-3′) and U8-2 (5′-CATTGCGTGTATCTCCAAAAAAAACAACTCATCGTTACG-3′) (mutant U8), and U9-1 (5′-CGTAACGATGAGTTGTTTTTTTTTGGAGATACACGCAATG-3′) and U9-2 (5′-CATTGCGTGTATCTCCAAAAAAAAACAACTCATCGTTACG-3′) (mutant U9). Chloroplasts of mutant ΔU were transformed by using a helium-driven particle gun as described previously (14), and transformants were selected for photoautotrophic growth on HSM minimal plates. RNA secondary structure calculations were performed by using the RNAdraw software (30).
Nac2 antiserum production.
For antiserum production, a 0.9-kb PstI fragment of the Nac2 cDNA (4) was cloned into the PstI site of the pQE31 expression vector (Qiagen) and transformed into Escherichia coli strain JM109. After induction with 1 mM isopropylthio-β-d-galactoside, the overexpressed 40-kDa protein containing an N-terminal His tag was purified on Ni-nitrilotriacetic acid agarose columns (Qiagen). Eluates were dialyzed against 50 mM ammonium carbonate and evaporated. The production of antiserum in rabbits was performed by Eurogentech.
Northern and Western analyses.
Northern and Western analyses were carried out as described previously (35). Signal intensities were quantitated densitometrically by using an ICU-1 unit and the Image Doc/EASY Win2 software from Herolab. Relative amounts of psbD mRNA and D1 were calculated after standardization according to the internal rbcL mRNA- and PsaD-derived signals, respectively.
RESULTS
Analysis of protein binding to the psbD 5′ UTR.
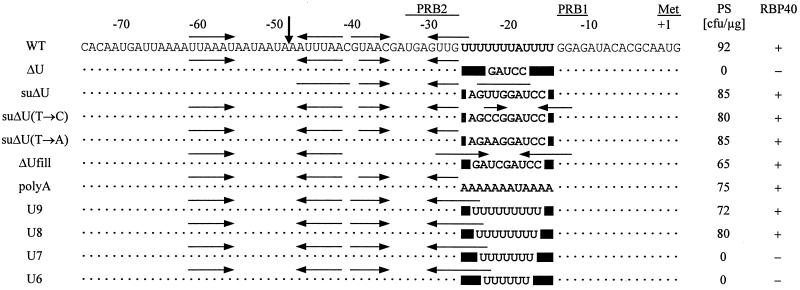
Recent studies have shown that a striking U-rich region within the 5′ UTR of the chloroplast psbD mRNA (positions −25 to −14) (Fig. 1) is required for photoautotrophic growth of C. reinhardtii cells (35). In the chloroplast mutant ΔU, a BamHI restriction site replaced this U tract after site-directed mutagenesis (Fig. 1) and subsequently led to the complete inhibition of D2 synthesis. It was speculated whether the U tract may serve as a recognition site for a translational trans-acting factor (35), similar to the proposed role of an AU-rich element within the psbA 5′ UTR in tobacco, which is required for D1 synthesis in vitro (24).
FIG. 1.
Sequence alignment of the psbD 5′ UTR of the wild type (WT) and different mutants of the poly(U) region. Dots and solid boxes indicate conserved residues and deletions, respectively. The sequence of the poly(U) region is given in boldface. Positions relative to the initiation codon (Met), the PRB1 and PRB2 elements, and the mature 5′ end (vertical arrow) are marked above the alignment, and horizontal arrows represent computer-predicted stem-loop structures. PS, number of photoautotrophically growing chloroplast transformants (CFU per microgram of DNA) of the mutant ΔU. RBP40, RNA binding activity of RBP40 to the corresponding RNA measured by competition experiments shown in Fig. 3 and 5, respectively.
We have isolated a photosynthetic revertant after UV mutagenesis of ΔU cells and their subsequent selection on minimal medium. Further genetic and molecular characterization (see Materials and Methods) of this strain, called suΔU, revealed that the underlying suppressor mutation resides within the chloroplast genome. By sequencing of the psbD 5′ region from suΔU, a 5-bp duplication of the sequence AGUUG immediately upstream of the initial ΔU mutation was detected (Fig. 1). A back-transformation of mutant ΔU cells with a construct harboring the psbD leader region of suΔU showed that the 5-bp insertion is sufficient to restore photosynthetic growth (Fig. 1).
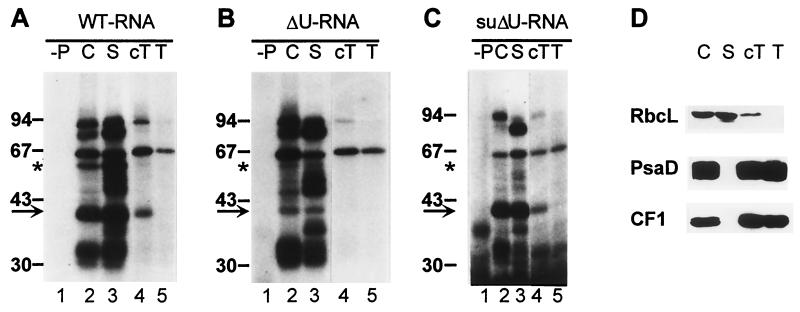
Assuming that the putative interaction with a trans-acting factor is abolished in ΔU, this interaction, should it be crucial, ought to be restored in the suppressor suΔU. Consequently, a comparative analysis of protein binding to the three different 5′ UTR RNAs was carried out in order to find RBPs that follow this particular binding mode. In previous UV cross-linking experiments, the psbD 5′ UTR had been shown to interact with at least two proteins of 47 and 40 kDa (34). In the course of this work, the conditions for the in vitro RNA binding assay were optimized by modifying the procedure described in Materials and Methods. These experimental changes unveiled several RNA binding activities in addition to the described 47- and 40-kDa proteins, when a radioactively labeled RNA probe spanning the psbD 5′ UTR (positions −74 to +1 [Fig. 1]) was analyzed by using wild-type chloroplast lysates in combination with the UV cross-linking technique.
RBPs of 90, 80, 63, 58, 50, 47, 40, and 33 to 30 kDa were radiolabeled with the wild-type psbD leader probe in chloroplast lysates (Fig. 2A, lane 2). When chloroplasts were fractionated further, most of these RBPs were found in the stromal fraction, which also contains the previously described low-density membranes (46). However, in the cT fraction (representing crude thylakoid membranes), RBPs of 90, 63, and 40 kDa were detected (Fig. 2A, lane 4). After purification of these thylakoids by floating in a second sucrose step gradient, only RBP63 and trace amounts of RBP90 were still present (Fig. 2A, lane 5). These data indicate that RBP63 is associated with thylakoid membranes, while RBP40 and RBP90 appear to represent stromal proteins contaminating the cT fraction. This was supported by the finding that the cT fraction still contained a substantial amount of the stromal Rubisco enzyme (Fig. 2D).
FIG. 2.
UV cross-linking analysis of proteins binding to psbD 5′ UTR RNAs from the wild-type (A), mutant ΔU (B), and suppressor suΔU (C). (D) Western control of chloroplast fractionation with antibodies against RbcL, CF1 subunit of the ATP synthase, and PsaD. C, chloroplast lysate; S, stroma fraction; T, floated thylakoid membrane fraction; −P, protein-free control. The arrows point to the 40-kDa signals; the 58-kDa signals are marked by asterisks. The sizes of marker proteins are indicated.
When the same fractions were tested with an RNA probe containing the mutant ΔU leader, two major differences in the RNA binding patterns compared to that of the wild type were observed. First, the labeling of a stromal RBP of 58 kDa was reduced; in addition, the binding signal at 40 kDa could not be detected with the ΔU leader probe in the cT fraction and was found to be drastically reduced in the stromal fraction (Fig. 2B, lanes 3 and 4). The remaining RNA binding activities were not at all or only slightly affected. Thus, RBP58 and RBP40 appeared to represent good candidates for trans-acting factors recognizing the U tract within the psbD leader. Furthermore, at least two different RBPs of 40 kDa seem to exist in C. reinhardtii chloroplasts. One is sensitive to the U tract deletion mutation and partially cofractionates with crude thylakoids, while the other, which is only detectable in the chloroplast and stromal fractions, is not.
When a psbD leader probe from the suppressor suΔU was analyzed, once again a reduced labeling of RBP58 was detected, but strikingly, the binding activity of the U tract-dependent RBP40 was restored (Fig. 2C, lanes 2 and 3). Hence, the activity of RBP40 followed exactly the above-mentioned mode, which was predicted for an essential trans-acting protein recognizing the translational U-rich element of the psbD 5′ UTR. Therefore, we conclude that RBP40 might be an essential factor for psbD mRNA translation.
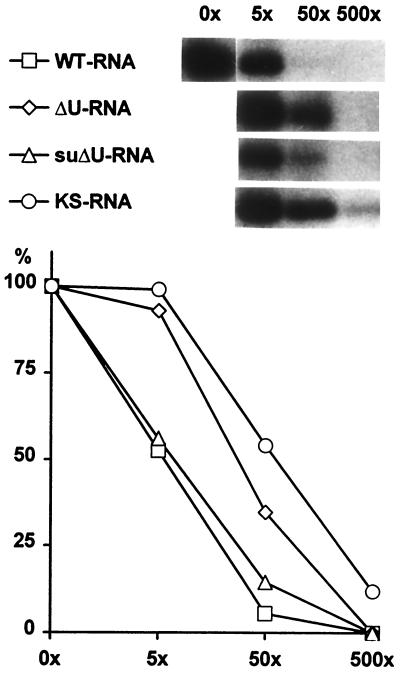
To further confirm the different RNA binding properties of RBP40, competition experiments were performed with radiolabeled wild-type and unlabeled wild-type, ΔU, and suΔU leader RNAs and with in vitro transcripts synthesized from the pBluescript KS+ polylinker region. The cT fraction was used as a protein source because it is devoid of the poly(U)-insensitive 40-kDa RBP. Both the homologous wild-type and the suΔU RNAs efficiently competed with the wild-type probe, while ΔU and KS RNAs had a significantly reduced effect on binding of RBP40 to the psbD leader, confirming their low affinity to RBP40 (Fig. 3).
FIG. 3.
RBP40 binding competition experiments. The cT fractions were incubated with radiolabeled wild-type (WT) psbD 5′ UTR RNA and a 5-, 50-, or 500-fold (5×, 50×, and 500×, respectively) molar excess of the indicated unlabeled competitor RNAs. The diagram displays the intensities of RBP40 signals in relation to that of the RBP40 signal without competitor.
cis-acting determinants for psbD mRNA translation.
One surprising finding was that the psbD 5′ UTR of suΔU enabled nearly wild-type levels of D2 synthesis, although the effective 5-bp duplication (AGUUG) does not restore an obvious U tract around position −20 of the psbD leader, except for two additional U residues (Fig. 1). Three possible models may be considered to explain this effect. (i) The two U residues introduced by the suppressor mutation are sufficient enough to restore sequence-specific binding of RBP40 and thus translational activity. (ii) The suppressor mutation creates a secondary structure element that resembles the poly(U) tract region. (iii) The 5-nt-spanning insertion in suΔU restores the spacing between the PRB1 site involved in translation and the PRB2 site required for stabilization of the psbD mRNA (Fig. 1) (35). To test these models, several site-directed mutations within the psbD leader were created (Fig. 1) and cloned into an appropriate chloroplast transformation vector (see Materials and Methods). These constructs were then used to biolistically transform chloroplasts of the translational mutant ΔU. Subsequent selection on minimal medium revealed whether the different leader versions were able to complement the mutation in ΔU.
To test whether the two additional U residues in suΔU were responsible for the suppression effect, these were changed into A or C residues (Fig. 1) in mutants suΔU(T→A) and suΔU(T→C). Both mutant versions generated photoautotrophically growing transformants with a rate in the range of constructs containing either the wild-type or the suΔU 5′ UTR (Fig. 1). Transformants harboring the suΔU(T→C) 5′ UTR, however, exhibited only a slow growth on minimal plates. Control experiments performed without DNA or with the initial mutant ΔU leader region yielded no transformants (Fig. 1). These data indicated that neither of the U residues present in suΔU is strictly required for psbD mRNA translation, thus suggesting that a sequence-independent determinant is constituted by the poly(U) region of the psbD 5′ UTR. To further confirm this, we exchanged the whole poly(U) tract with its complementary sequence, giving rise to an A-rich element in mutant poly(A), and, indeed, this construct complemented the mutant ΔU (Fig. 1). The predicted secondary structure of the suppressor suΔU (Fig. 1) suggested that the region between PRB1 and PRB2 does not necessarily need to be single stranded in order to be functional. To verify this, a stem-loop structure was introduced into this region. The resulting construct, ΔUfill, complemented ΔU (Fig. 1), indicating that neither the sequence nor the secondary structure alone is essential for psbD mRNA translation. Thus, we concluded that the third proposed model requiring a defined spacing between the cis-acting elements PRB1 and PRB2 should be valid. To map the minimal spacer length requirements, the poly(U) region was shortened in successive stages from 9 to 6 nt, since a spacer of 10 nt is apparently sufficient to drive psbD gene expression in suΔU, while a 5-nt spacer in ΔU is not. Constructs containing either nine or eight U residues (U9 and U8, respectively) (Fig. 1) still complemented ΔU, while constructs U7 and U6 (Fig. 1) produced no photosynthetic clones after chloroplast transformation of ΔU cells. Thus, the minimal spacer length between PRB1 and PRB2 must be 8 nt in order to enable D2 synthesis.
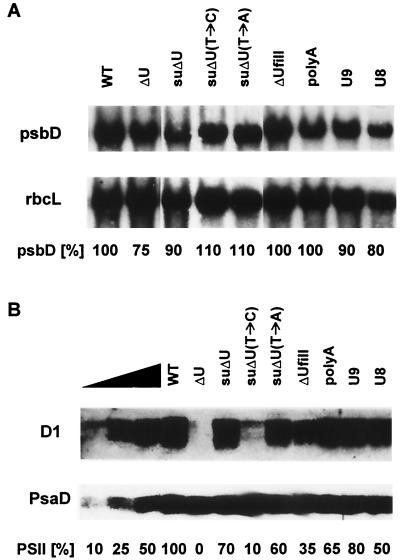
Homoplasmic transformants were then subjected to both Northern and Western analyses to quantify their psbD gene expression. The levels of psbD mRNA were only slightly affected in the different mutants compared to that in the wild type (Fig. 4A), confirming previous data which identified the poly(U) region as an essential translational element (35). The amounts of PS II in the same strains were determined by using an antibody raised against the D1 protein. Because the D1 and D2 proteins accumulate to the same level in mutant cells, amounts of D2 can be indirectly measured by determining the accumulation of D1 (28, 35). As an internal standard, the amount of the psaD gene product was analyzed at the same time (Fig. 4B). Transformants that were able to grow photoautotrophically contained different amounts of PS II. While the mutants U9, suΔU (suppressor), suΔU(T→A), poly(A), and U8 accumulated 80 to 50% of PS II compared to the wild type, a more pronounced reduction of PS II levels to 35% was observed in ΔUfill. Only in suΔU(T→C) was a drastic reduction to 10% of the wild-type PS II level found, consistent with the slow-growth phenotype of this transformant mentioned above.
FIG. 4.
Northern (A) and Western (B) analyses of chloroplast transformants. Total RNAs (20 μg) from the mutants indicated at the top were electrophoretically separated, blotted onto Nylon membranes, and hybridized with either a radiolabeled psbD- or rbcL-specific DNA probe. Total proteins (corresponding to 7 μg of chlorophyll) from the mutants were separated by SDS-PAGE, blotted onto filters, and immunolabeled with antibodies against either D1 or PsaD. The triangle marks a serial dilution of wild-type proteins. The autoradiogram was overexposed to allow detection of low D1 levels down to 10%. High D1 levels were quantitated from a less-exposed autoradiogram.
Binding of RBP40 to mutant 5′ UTRs.
The initial RNA binding experiments suggested that binding of RBP40 to the psbD 5′ UTR is required for D2 synthesis. Hence, the possible interaction of RBP40 with the different mutant psbD leader RNAs was tested by performing competition UV-cross-linking experiments similar to those shown in Fig. 3. All mutant leader versions that enabled photosynthetic growth also competed with the wild-type 5′ UTR, although with different efficiencies. While leader RNAs from the poly(A), suΔU(T→A), U9, and U8 mutants (Fig. 5) exhibited a competition effect in the range of the wild-type and suΔU RNAs (Fig. 3), the binding of RBP40 to mutant ΔUfill and, even more significant, to suΔU(T→C) was reduced (Fig. 5A). These different affinities roughly corresponded to the different levels of restored D2 accumulation (Fig. 4B). Especially in the mutants ΔUfill and suΔU(T→C), the low levels of D2 were accompanied by a corresponding, low-competition effect of these RNAs in the RNA binding assay. Probes from the 5′ UTRs of U7 and U6, which are not sufficient to drive psbD mRNA translation, showed a competition effect in the range of the mutant ΔU RNA and the unrelated KS RNA, indicating that RBP40 cannot efficiently bind to these RNAs containing reduced poly(U) tracts. Taken together, the strong correlation between the ability to mediate psbD mRNA translation and the ability to interact with RBP40 is evident for all different 5′ UTR mutants, suggesting that RBP40 plays an essential role in D2 synthesis.
FIG. 5.
Competition experiments with RBP40 binding to psbD leader mutants. For explanation, see the legend to Fig. 3.
RBP40 binds directly to the poly(U) tract.
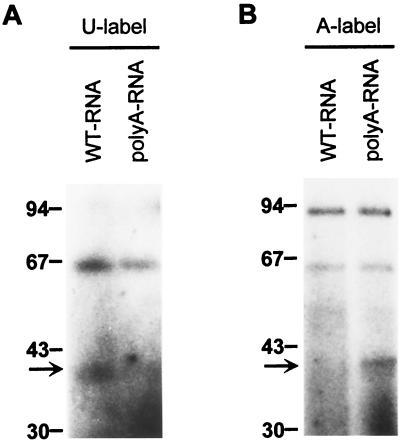
The competition data indicated that the region between PRB1 and PRB2 is required for the binding of RBP40. To test now whether the poly(U) tract itself is bound by RBP40, comparative UV cross-linking experiments with the wild-type RNA and the poly(A) RNA were performed. The poly(A) mutant version supported translation and competed the RBP40 binding activity, although it contains no poly(U) tract. If the RBP40 binding site was the region between PRB1 and PRB2, a poly(A) RNA probe radiolabeled at U residues by in vitro transcription with [α-32P]UTP should not label RBP40 during UV cross-linking. Conversely, detection of the RBP40 signal with this probe would indicate that the binding site was located elsewhere within the leader. As shown in Fig. 6A, the U-labeled poly(A) RNA probe did not detect the 40-kDa signal in cT fractions, suggesting that the poly(U) tract indeed represents the binding region. To further confirm this, both RNA probes were then labeled at their A residues by in vitro transcription with [α-32P]ATP. Now, the opposite result was obtained; i.e., RBP40 was detected with poly(A) RNA, but not with the wild-type RNA probe (Fig. 6B). These results indicated that RBP40 specifically binds to the region between PRB1 and PRB2 independent of its nucleotide sequence. The A-labeled RNA probes led to a significantly enhanced signal at 90 kDa, suggesting that this protein preferentially recognizes A residues. The RBP63 signal was only slightly affected by an A-specific probe labeling.
FIG. 6.
Labeling of RBP40 by the poly(A) RNA probe. UV cross-linking analysis of proteins from the cT fraction was performed by using wild-type (WT) RNA and poly(A) RNA probes, which were radiolabeled at either their U residues (A) or their A residues (B). The arrow marks RBP40. Values to the left are in kilodaltons.
Binding of RBP40 to the psbD leader depends on the RNA stability factor Nac2.
The stability of the psbD mRNA in C. reinhardtii depends on the nuclear Nac2 locus that mediates its function via the psbD 5′ UTR (34). Insertion of a poly(G) sequence into the psbD leader restored RNA stability even in the absence of the Nac2 function. However, accumulating psbD transcripts were not translated, suggesting that Nac2 is also involved in psbD mRNA translation (35). Therefore, we tested whether the binding activity of RBP40 is affected in the nuclear mutant mφ14, which contains a deletion within the Nac2 gene (4). When the cT fractions from wild-type and mφ14 cells were analyzed by UV cross-linking assays with a wild-type psbD leader RNA probe, hardly any binding signal of RBP40 could be observed in mφ14, while the 63-kDa signal was unaffected or even stronger in this mutant (Fig. 7B, lanes 1 and 2). In whole chloroplasts, only the signal of the poly(U)-insensitive 40-kDa RBP was visible in mφ14 (Fig. 7A, lanes 1 and 2; and 2B, lane 2). To verify that the binding of RBP40 is dependent on the Nac2 function, an mφ14 strain (mcos5) was tested, which had been rescued to photoautotrophic growth by transformation with cosmid cosnac5 containing the wild-type Nac2 locus (4). As seen in Fig. 7A and B, lane 3, RBP40 activity was restored in mcos5, indicating that Nac2 is actually required for efficient binding of RBP40 to the psbD leader.
FIG. 7.
RBP40 binding in the nuclear mutant mφ14. Chloroplast lysates (A [6 μg of proteins]) and cT fractions (B [12 μg of proteins]) from the strains indicated at the top were UV cross-linked to radiolabeled psbD leader RNA from the wild-type (WT). RBP40 and RBP58 are marked by arrows and asterisks, respectively. Values to the left are in kilodaltons.
The Nac2 gene has recently been cloned and has been shown to encode a 140-kDa TPR (tetratrico-peptide repeat) protein, which is part of a stromal, RNA-associated, high-molecular-weight complex (4). Thus, it appeared possible that RBP40 represents another subunit of this complex, thereby explaining its strong dependence on the Nac2 function. When stromal chloroplast fractions from C. reinhardtii wild-type cells were analyzed in 15 to 35% glycerol gradients, most of the Nac2 complex was found in fractions 3 to 8, corresponding to a size of 500 to 600 kDa with a peak in fractions 4 and 5 (Fig. 8). This is in agreement with sedimentation data obtained with linear sucrose gradients (4). Correspondingly, RBP40 binding activity was detected in the fractions 3 to 8 only, thus confirming that RBP40 activity depends on the presence of Nac2. The peak fractions, however, were found to be 6 to 8 instead of 4 and 5, as for the Nac2 complex. The identity of the RBP40 signal was confirmed by testing the fractions with a leader RNA probe from ΔU (data not shown). These data suggest that only a subfraction of an Nac2 core complex of ca. 500 kDa might be closely associated with RBP40, and this larger Nac2 core-RBP40 complex could be represented by the material detected in fractions 6 to 8. Alternatively, the Nac2 core complex might interact just transiently with RBP40. Since only the stromal protein fraction (see Materials and Methods) was subjected to this sedimentation analysis, these data also show that RBP40 is located in the chloroplast stroma instead of being associated with the previously described low-density membrane fraction, in which several RNA binding activities appear to be selectively enriched (46).
FIG. 8.
Sedimentation analysis of RBP40. Stromal chloroplast proteins were centrifuged on a 15 to 35% glycerol gradient. Sedimentation of the Nac2 complex and the Rubisco enzyme was followed by Western analysis of fractions with antibodies raised against Nac2 and RbcL. RBP40 was detected after UV cross-linking of fraction proteins with a radiolabeled psbD leader RNA probe from the wild type. Sedimentation of marker proteins (in kilodaltons) is indicated at the top.
DISCUSSION
In this report, the identification and characterization of a stromal 40-kDa RBP (RBP40) are described; this protein interacts specifically with a U-rich region required for 5′ UTR-mediated translation of the psbD mRNA in C. reinhardtii, thereby linking the processes of both RNA stabilization and protein synthesis (35). Previously, we had identified at least two proteins of 47 and 40 kDa which interact with the psbD 5′ UTR in vitro (34). RBP47 bound the RNA in a Nac2-dependent manner (34) (Fig. 7, lane 2), but appeared to recognize sequences upstream of the 5′ processing site at position −47 of the psbD leader (34). Its binding activity was not affected by the ΔU mutation (Fig. 2B), and, hence, it is not likely to be involved in the translational control mechanisms mediated via the U-rich motif around position −20. The precise role of RBP47 still remains to be clarified. In contrast to our recent data, the previously detected binding activity of a 40-kDa protein was not dependent on the Nac2 factor. This apparent discrepancy is most probably due to the fact that at least two different 40-kDa RBPs are present in the C. reinhardtii chloroplast. In the previous work, most likely, only the poly(U) tract-insensitive RBP40 was detected, which binds the psbD 5′ UTR in a Nac2-independent manner (Fig. 7, lane 2). By using our improved preparation procedure for chloroplast proteins, now, the poly(U) tract-sensitive one becomes detectable, which is the one that depends on the presence of Nac2. This idea is supported by the finding that the poly(U) tract binding activity is sensitive toward different previously performed treatments, such as freezing of samples and storage for longer than 2 to 4 weeks (data not shown). Thus, it is likely that this activity escaped detection in the previous work.
The analysis of a cis-acting chloroplast suppressor and several site-directed mutants shows that neither the sequence nor the single-stranded character of the U-rich region is strictly necessary for its function. Instead, it appears that only a minimal spacing of at least 8 nt between the adjacent elements PRB1 and PRB2 is critical for psbD mRNA translation. However, the moderate reduction of PS II in mutant ΔUfill and, especially, the drastic decrease in D2 synthesis in suΔU(T→C) suggest that secondary RNA structures within the region between PRB1 and PRB2 can significantly affect translational efficiencies (Fig. 1 and 4B).
The binding of RBP40 to the various 5′ UTR probes in vitro correlates with their activity in vivo. This suggests that the interaction of the psbD leader with RBP40 is required for translation, although formally it cannot be ruled out that the binding is a consequence rather than a cause of translation. The specificity of this interaction was surprising, since long AU-rich stretches are also present in the upstream part of the psbD leader (positions −70 to −40; Fig. 1). Nevertheless, these are not recognized by RBP40. It is likely that this specificity is mediated by the Nac2 complex, which acts in a gene-specific manner by stabilizing psbD transcripts only (34). RBP40 activity depends on Nac2 function, and the sedimentation data suggest that RBP40 interacts either stably or transiently with an Nac2 core complex, which was recently shown to be associated with RNA (4). Furthermore, besides its role in RNA stabilization, Nac2 function has been shown to be involved in 5′ processing and/or translation of the psbD mRNA (35). The precise target region of the Nac2 complex within the psbD 5′ UTR has not yet been mapped, but indirect evidence suggests that this target is located downstream of the processing site at position −47 (Fig. 1), close to or at the PRB2 site, which is needed for RNA stabilization (35). In view of these data, we propose a model for the posttranscriptional mechanism of psbD gene expression, which involves the binding of the Nac2 complex to the region around the PRB2 site soon after the RNA has left the RNA polymerase. This interaction protects downstream regions against exonucleolytic degradation from the 5′ end (35) and, furthermore, results in the proper positioning of RBP40 on the poly(U) tract region, which has to be at least 8 nt in length. Once this complex is formed on the psbD leader, subsequent steps of translation initiation, e.g., binding of the small ribosomal subunit, are directed by RBP40 and D2 synthesis starts.
The interaction of RBP40 with the psbD leader and its proposed function in translation resemble the properties of the ribosomal protein S1, which has been shown to bind to U tracts located upstream of Shine-Dalgarno elements in E. coli (3). In spinach, the chloroplast S1 protein (CS1) has been reported to have a high affinity to either A- or U-rich sequences (1, 16). While the E. coli S1 protein has a size of 61 kDa, the cloned CS1 gene from spinach encodes a mature protein of 40 kDa. However, in testing RBP40's cross-reaction with a polyclonal antiserum against the E. coli S1 protein, which has been shown to cross-react with spinach CS1 (1), a signal at the 40-kDa protein was not detectable. Instead, a 63-kDa protein was immunolabeled, which probably represents the C. reinhardtii CS1 protein (data not shown). Thus, the immunological data do not support the notion that the 40-kDa protein is the chloroplast S1 homologue of C. reinhardtii. Consequently, only sequencing of the protein or cloning of the gene for RBP40 will provide a conclusive answer to this question.
ACKNOWLEDGMENTS
We thank B. Schwencke and T. Stratmann for excellent technical assistance and U. Kück for providing laboratory space and basic support. Antisera against the D1 protein, the Rubisco holoenzyme from spinach, the chloroplast ATP synthase, PsaD, and the S1 protein from E. coli were kindly provided by A. Trebst, G. Wildner, R. Berzborn, J.-D. Rochaix, and R. Brimacombe, respectively.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft to J.N. (Ni390/2-3).
REFERENCES
- 1.Alexander C, Faber N, Klaff P. Characterization of protein-binding to the spinach chloroplast psbA mRNA 5′ untranslated region. Nucleic Acids Res. 1998;26:2265–2272. doi: 10.1093/nar/26.10.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barkan A, Voelker R, Mendel-Hartvig J, Johnson D, Walker M. Genetic analysis of chloroplast biogenesis in higher plants. Physiol Plant. 1995;93:163–170. [Google Scholar]
- 3.Boni I V, Isaeva D M, Musychenko M L, Tzareva N V. Ribosome-messenger recognition: mRNA target sites for ribosomal protein S1. Nucleic Acids Res. 1991;19:155–162. doi: 10.1093/nar/19.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boudreau E, Nickelsen J, Lemaire S D, Ossenbühl F, Rochaix J-D. The Nac2 gene of Chlamydomonas encodes a chloroplast TPR-like protein involved in psbD mRNA stability. EMBO J. 2000;19:3366–3376. doi: 10.1093/emboj/19.13.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruick R K, Mayfield S P. Processing of the psbA 5′ untranslated region in Chlamydomonas reinhardtii depends upon factors mediating ribosome association. J Cell Biol. 1998;143:1145–1153. doi: 10.1083/jcb.143.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruick R K, Mayfield S P. Light-activated translation of chloroplast mRNAs. Trends Plant Sci. 1999;4:190–195. doi: 10.1016/s1360-1385(99)01402-8. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Kindle K L, Stern D B. Initiation codon mutations in the Chlamydomonas chloroplast petD gene result in temperature-sensitive photosynthetic growth. EMBO J. 1993;12:3627–3635. doi: 10.1002/j.1460-2075.1993.tb06036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Kindle K L, Stern D B. The initiation codon determines the efficiency but not the site of translation initiation in Chlamydomonas chloroplasts. Plant Cell. 1995;7:1295–1305. doi: 10.1105/tpc.7.8.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Simpson C L, Kindle K L, Stern D B. A dominant mutation in the Chlamydomonas reinhardtii nuclear gene SIM30 suppresses translational defects caused by initiation codon mutations in chloroplast genes. Genetics. 1997;145:935–943. doi: 10.1093/genetics/145.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen A, Mayfield S P. Translational regulation of gene expression in plants. Curr Opin Biotechnol. 1997;8:189–194. doi: 10.1016/s0958-1669(97)80101-2. [DOI] [PubMed] [Google Scholar]
- 11.Danon A, Mayfield S P. Light-regulated translational activators: identification of chloroplast gene specific mRNA binding proteins. EMBO J. 1991;10:3993–4001. doi: 10.1002/j.1460-2075.1991.tb04974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fargo D C, Boynton J E, Gillham N W. Mutations altering the predicted secondary structure of a chloroplast 5′ untranslated region affect its physical and biochemical properties as well as its ability to promote translation of reporter mRNAs both in the Chlamydomonas reinhardtii chloroplast and in Escherichia coli. Mol Cell Biol. 1999;19:6980–6990. doi: 10.1128/mcb.19.10.6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fargo D C, Zhang M, Gillham N W, Boynton J E. Shine-Dalgarno-like sequences are not required for translation of chloroplast mRNAs in Chlamydomonas reinhardtii chloroplasts or in Escherichia coli. Mol Gen Genet. 1998;257:271–282. doi: 10.1007/s004380050648. [DOI] [PubMed] [Google Scholar]
- 14.Fischer N, Stampacchia O, Redding K, Rochaix J-D. Selectable marker recycling in the chloroplast. Mol Gen Genet. 1996;251:373–380. doi: 10.1007/BF02172529. [DOI] [PubMed] [Google Scholar]
- 15.Fisk D G, Walker M B, Barkan A. Molecular cloning of the maize gene crp1 reveals similarity between regulators of mitochondrial and chloroplast gene expression. EMBO J. 1999;18:2621–2630. doi: 10.1093/emboj/18.9.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franzetti B, Carol P, Mache R. Characterization and RNA binding properties of a chloroplast S1-like ribosomal protein. J Biol Chem. 1992;267:19075–19081. [PubMed] [Google Scholar]
- 17.Goldschmidt-Clermont M. Coordination of nuclear and chloroplast gene expression in plant cells. Int Rev Cytol. 1998;177:115–180. doi: 10.1016/s0074-7696(08)62232-9. [DOI] [PubMed] [Google Scholar]
- 18.Gorman D S, Levine R P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1965;54:1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris E H. The Chlamydomonas sourcebook. San Diego, Calif: Academic Press; 1989. [Google Scholar]
- 20.Harris E H, Boynton J E, Gillham N W. Chloroplast ribosomes and protein synthesis. Microbiol Rev. 1994;58:700–754. doi: 10.1128/mr.58.4.700-754.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauser C R, Gillham N W, Boynton J E. Translational regulation of chloroplast genes. Proteins binding to the 5′-untranslated regions of chloroplast mRNAs in Chlamydomonas reinhardtii. J Biol Chem. 1996;271:1486–1497. doi: 10.1074/jbc.271.3.1486. [DOI] [PubMed] [Google Scholar]
- 22.Hauser C R, Gillham N W, Boynton J E. Regulation of chloroplast translation. In: Rochaix J-D, Goldschmidt-Clermont M, Merchant S, editors. The molecular biology of chloroplasts and mitochondria in Chlamydomonas. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 197–217. [Google Scholar]
- 23.Higgs D C, Shapiro R S, Kindle K L, Stern D B. Small cis-acting sequences that specify secondary structures in a chloroplast mRNA are essential for RNA stability and translation. Mol Cell Biol. 1999;19:8479–8491. doi: 10.1128/mcb.19.12.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirose T, Sugiura M. Cis-acting elements and trans-acting factors for accurate translation of chloroplast psbA mRNAs: development of an in vitro translation system from tobacco chloroplasts. EMBO J. 1996;15:1687–1695. [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J, Mayfield S P. Protein disulfide isomerase as a regulator of chloroplast translational activation. Science. 1997;278:1954–1957. doi: 10.1126/science.278.5345.1954. [DOI] [PubMed] [Google Scholar]
- 26.Klaff P, Mundt S M, Steger G. Complex formation of the spinach chloroplast psbA mRNA 5′ untranslated region with proteins is dependent on the RNA structure. RNA. 1997;3:1468–1479. [PMC free article] [PubMed] [Google Scholar]
- 27.Kuchka M R, Goldschmidt-Clermont M, van Dillewijn J, Rochaix J-D. Mutation at the Chlamydomonas nuclear Nac2 locus specifically affects stability of the chloroplast psbD transcript encoding polypeptide D2 of PSII. Cell. 1989;58:869–876. doi: 10.1016/0092-8674(89)90939-2. [DOI] [PubMed] [Google Scholar]
- 28.Kuchka M R, Mayfield S P, Rochaix J-D. Nuclear mutations specifically affect the synthesis and/or degradation of the chloroplast-encoded D2 polypeptide of photosystem II in Chlamydomonas reinhardtii. EMBO J. 1988;7:319–324. doi: 10.1002/j.1460-2075.1988.tb02815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leon P, Arroyo A, Mackenzie S. Nuclear control of plastid and mitochondrial development in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:453–480. doi: 10.1146/annurev.arplant.49.1.453. [DOI] [PubMed] [Google Scholar]
- 30.Matzura O, Wennborg A. RNAdraw: an integrated program for RNA secondary structure calculation and analysis under 32-bit Microsoft Windows. CABIOS. 1996;12:247–249. doi: 10.1093/bioinformatics/12.3.247. [DOI] [PubMed] [Google Scholar]
- 31.Mayfield S P, Cohen A, Danon A, Yohn C B. Translation of the psbA mRNA of Chlamydomonas reinhardtii requires a structured element contained within the 5′ untranslated region. J Cell Biol. 1994;127:1537–1545. doi: 10.1083/jcb.127.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meurer J, Berger A, Westhoff P. A nuclear mutant of Arabidopsis with impaired stability on distinct transcripts of the plastid psbB, psbD/psbC, ndhH and ndhC operons. Plant Cell. 1996;8:1193–1207. doi: 10.1105/tpc.8.7.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nickelsen J. RNA stability in chloroplasts. In: Gelvin S B, Schilperoort R A, editors. Plant molecular biology manual. 2nd ed., D6. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 1–18. [Google Scholar]
- 34.Nickelsen J, van Dillewijn J, Rahire M, Rochaix J-D. Determinants for stability of the chloroplast psbD RNA are located within its short leader region in Chlamydomonas reinhardtii. EMBO J. 1994;13:3182–3191. doi: 10.1002/j.1460-2075.1994.tb06617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nickelsen J, Fleischmann M, Boudreau E, Rahire M, Rochaix J-D. Identification of cis-acting RNA leader elements required for chloroplast psbD gene expression in Chlamydomonas reinhardtii. Plant Cell. 1999;11:957–970. doi: 10.1105/tpc.11.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rochaix J-D. Post-transcriptional regulation of chloroplast gene expression in Chlamydomonas reinhardtii. Plant Mol Biol. 1996;32:327–341. doi: 10.1007/BF00039389. [DOI] [PubMed] [Google Scholar]
- 37.Rochaix J-D, Dron M, Rahire M, Malnoe P. Sequence homology between the 32 k Dalton and the D2 chloroplast membrane polypeptides of Chlamydomonas reinhardtii. Plant Mol Biol. 1989;3:363–370. doi: 10.1007/BF00033383. [DOI] [PubMed] [Google Scholar]
- 38.Rochaix J-D, Mayfield S P, Goldschmidt-Clermont M, Erickson J. Molecular biology of Chlamydomonas. In: Shaw C H, editor. Plant molecular biology. A practical approach. Oxford, United Kingdom: IRL Press; 1988. pp. 253–275. [Google Scholar]
- 39.Rott R, Levy H, Drager R G, Stern D B, Schuster G. 3′-Processed mRNA is preferentially translated in Chlamydomonas reinhardtii chloroplasts. Mol Cell Biol. 1998;18:4605–4611. doi: 10.1128/mcb.18.8.4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Small I D, Peeters N. The PPR motif—a TPR-related motif prevalent in plant organellar proteins. Trends Biochem Sci. 2000;25:46–47. doi: 10.1016/s0968-0004(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 41.Staub J M, Maliga P. Translation of psbA mRNA is regulated by light via the 5′ untranslated region in tobacco plastids. Plant J. 1994;6:547–553. doi: 10.1046/j.1365-313x.1994.6040547.x. [DOI] [PubMed] [Google Scholar]
- 42.Wu H Y, Kuchka M R. A nuclear suppressor overcomes defects in the synthesis of the chloroplast psbD gene product caused by mutations in two distinct nuclear genes of Chlamydomonas. Curr Genet. 1995;27:263–269. doi: 10.1007/BF00326159. [DOI] [PubMed] [Google Scholar]
- 43.Yohn C B, Cohen A, Danon A, Mayfield S P. A poly(A) binding protein functions in the chloroplast as a message-specific translation factor. Proc Natl Acad Sci USA. 1998;95:2238–2243. doi: 10.1073/pnas.95.5.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zerges W, Girard-Bascou J, Rochaix J-D. Translation of the chloroblast psbC mRNA is controlled by interactions between its 5′ leader and the nuclear loci TBC1 and TBC3 in Chlamydomonas reinhardtii. Mol Cell Biol. 1997;17:3440–3448. doi: 10.1128/mcb.17.6.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zerges W, Rochaix J-D. The 5′ leader of a chloroplast mRNA mediates the translational requirements for two nucleus-encoded functions in Chlamydomonas reinhardtii. Mol Cell Biol. 1994;14:5268–5277. doi: 10.1128/mcb.14.8.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zerges W, Rochaix J-D. Low density membranes are associated with RNA binding proteins and thylakoids in the chloroplast of Chlamydomonas reinhardtii. J Cell Biol. 1998;140:101–110. doi: 10.1083/jcb.140.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]