Abstract
Stress is a major risk factor for neurodevelopmental and neuropsychiatric disorders, with the capacity to impact susceptibility to disease as well as long-term neurobiological and behavioral outcomes. Parvalbumin (PV) interneurons, the most prominent subtype of GABAergic interneurons in the cortex, are uniquely responsive to stress due to their protracted development throughout the highly plastic neonatal period and into puberty and adolescence. Additionally, PV+ interneurons appear to respond to stress in a sex-specific manner. This review aims to discuss existing preclinical studies that support our overall hypothesis that the sex-and age-specific impacts of stress on PV+ interneurons contribute to differences in individual vulnerability to stress across the lifespan, particularly in regard to sex differences in the diagnostic rate of neurodevelopmental and neuropsychiatric diseases in clinical populations. We also emphasize the importance of studying sex as a biological variable to fully understand the mechanistic and behavioral differences between males and females in models of neuropsychiatric disease.
Keywords: parvalbumin, stress, sex differences, lifespan, estrogen, plasticity
Introduction
Stress is a common and unavoidable aspect of life as well as a major risk factor for numerous neurodevelopmental and neuropsychiatric diseases, including major depressive disorder (MDD) (Xie et al., 2017; Belleau et al., 2018; Slavich and Sacher 2019), bipolar disorder (Ellicott et al., 1990; Kapczinkski et al., 2008; Davis et al., 2017), posttraumatic stress disorder (PTSD) (Davidson and Baum 1986; Ottenweller et al., 1989; Musazzi et al., 2018), anxiety disorders (Baranyi et al., 2005; Bondi et al., 2008; Smoller 2016), autism spectrum disorder (ASD) (Ronald et al., 2011; Manzari et al., 2019), and schizophrenia (Calabrese et al., 2009; Howes et al., 2017; Gomes et al., 2019). Although all individuals experience stress throughout the lifespan, stress only triggers pathological symptoms in a small fraction of the population, suggesting individual differences in vulnerability to stress.
There are many proposed neurobiological mechanisms and neural circuits implicated in vulnerability and resilience to stress, including differential activity within the hypothalamic-pituitary-adrenal (HPA) axis (Froger et al., 2004; Bangasser et al., 2010; Wood et al., 2010), altered cortical connectivity (Diorio et al., 1993; McEwen 2001; Bergström et al., 2008; Taliaz et al., 2010), degree of prefrontal cortex (PFC) activity and control of the amygdala (McEwen and Morrison 2013; Kumar et al., 2014), dysregulation within the reward pathway (Krishnan et al., 2007), serotonin transporter (5HTTLPR) polymorphism (Caspi et al., 2003; Hariri and Holmes 2006; Uher and McGuffin 2008; Way and Taylor 2010), and GABAergic dysfunction (Herman et al., 2004; Luscher et al., 2010; Guillox et al., 2012; Shepard et al., 2016; Zhu et al., 2017; Duman et al., 2019). Additionally, females have been shown to be differentially vulnerable to stress throughout stages of the menstrual cycle, suggesting a role played by ovarian steroid hormones on brain circuits involved in stress response (e.g., PFC and amygdala; Ossewaarde et al., 2010).
These neurobiological differences in stress vulnerability and resilience often come from complex interactions between genes, the environment, and stress, beginning as early as the prenatal period. Maternal stress, including infection or sickness during pregnancy as well as psychosocial stressors like bereavement stress, has been associated with changes in emotionality and a risk for developing ASD and schizophrenia in offspring (van Os and Selten, 1998; Darnaudery and Maccari, 2008; Mueller and Bale 2008; Bale 2011; Howerton and Bale, 2012). Paternal stress has also been shown to play a role in modulating epigenetic vulnerability to stress in offspring (Rodgers et al., 2013; Rogers and Bale, 2015). Like prenatal factors, early life experiences from birth throughout adolescence play a role in shaping individual vulnerability to stress later in life (Benjet et al., 2010; Lindert et al., 2014; Allen and Dwivedi, 2020; Lippard and Nemeroff, 2020). The multitude of biological and environmental influences and their complex interactions on specific vulnerability to stress makes determining discrete effects on the role of stress on development of neurodevelopmental and neuropsychiatric diseases uniquely challenging.
Both clinical and preclinical models are used to study how brain regions respond to acute and chronic stressors, as well as the resulting behavioral effects of these stressors. Clinical studies in populations exposed to major stressors such as natural disasters or chronic illness are perfectly positioned to provide evidence of the relationship between adverse events and behavioral pathologies in humans. However, to gain a mechanistic understanding of the effects of stress on the brain (at different levels of analysis) and their consequences on behaviors, preclinical models are more appropriate. Animal-based paradigms have several advantages, including the study of gene-environment interactions and control over length and intensity of stressor, type of stressor, and time of stress in relation to the lifespan (i.e., prenatal stress, early life stress, adulthood stress) (Nestler et al., 2002; Burrows et al., 2011). For instance, the effects of prenatal stress on neurodevelopmental outcomes in offspring are highly dependent on the type of stress and the exact gestational period at which the stress is applied (Howerton and Bale, 2012). In addition, animal models allow for the controlled inclusion of sex as a biological variable, which is of particular importance when considering the clinical implications of neuropsychiatric disease.
Neurodevelopmental and neuropsychiatric diseases are not equally diagnosed among men and women. Men are more likely to be diagnosed with neurodevelopmental disorders, including ASD (Baron-Cohen et al., 2011; McCarthy and Wright 2017), schizophrenia (Tamminga 1997; Cyr et al., 2002; Grossman et al., 2008), and attention deficit hyperactivity disorder (ADHD) (Pauls 1991; Andersen and Teicher 2000; Bálint et al., 2008), whereas women are more likely to be diagnosed with affective disorders including MDD (Weissman et al., 1996; Altemus 2006; Rubinow and Schmidt 2018), anxiety (Hankin 2009; Altemus et al., 2014) and PTSD (Kessler et al., 1995; Christiansen and Berke, 2020). Evidence supports the idea that sex-specific vulnerabilities to neuropsychiatric and neurodevelopmental disorders are, at least in part, driven by differences at the cellular, molecular, and histological levels. We also cannot ignore societal reasons, such as a lack of understanding of divergent symptomology in diseases such as ASD leading to underdiagnosis and later age of in females, in facilitating some sex differences in these disorders (Lundstrom et al., 2019; Hull et al., 2020; Ferri et al., 2018). In spite of this, sex differences in neurodevelopmental and neurobiological disorders are receiving more attention now than ever before, and research to established mechanisms underlying sex differences in these disorders has identified numerous factors that may contribute to sex-specific pathologies.
The GABAergic system is of particular interest when studying the neurobiology of neurodevelopmental and neuropsychiatric diseases. GABA is the primary inhibitory neurotransmitter in the central nervous system and plays a critical role in modulating the activity of numerous other neurotransmitter systems in the brain, notably its excitatory glutamatergic counterpoint. GABAergic circuit development begins mid-gestation and continues until the end of adolescence, playing a role in the maturation of cortical and subcortical regions. Disruption of GABAergic circuitry can therefore have potent effects on brain development and behavior. Importantly, clinical and preclinical studies have identified sex differences in the GABAergic system under both basal and pathological conditions. In the rat hypothalamus, glutamic acid decarboxylase (GAD), the rate-limiting enzyme in the GABA synthesis pathway, was expressed 2-fold greater in males than females, suggesting that males have a higher rate of GABA turnover (Searles et al., 2000). Stress also differentially affects the GABAergic systems in male and female mice. At baseline, male mice have a greater number of GABAA receptors compared to females in all cortical regions. Following two days of acute swim stress in adulthood, the number of GABAA receptors decreases in males but increases in females in the frontal cortex, cingulate cortex, and dentate gyrus (DG) (Skilbeck et al., 2008). Interestingly, reduced GABA concentration in the superior temporal sulcus in humans is associated with ASD in females, but not males (Kirkovski et al., 2018) and disruption of GABAergic neurotransmission and interneurons have been associated with numerous neurodevelopmental and neuropsychiatric diseases, including ASD, schizophrenia, MDD, and anxiety (Dhossche et al., 2002; Fatemi et al., 2007; Coghlan et al., 2012; Garbutt et al., 1983; Gonzalez-Burgos et al., 2010; Lewis et al., 1999; Mohler 2012; Petty 1995).
GABAergic circuitry consists of multiple subtypes of GABAergic interneurons which are distinct based on their morphological, molecular, and electrophysiological properties. Nearly all GABAergic interneurons express one of three unique markers, allowing them to be sorted into three broad classes: somatostatin (SST), 5HT3AR, or parvalbumin (PV). SST+ interneurons make up approximately 30% of all cortical interneurons and control spiking input to pyramidal neurons by targeting dendrites (Tremblay et al., 2016). Disruption within SST+ interneurons have been associated with several stress-related pathologies, particularly MDD (Fogaca & Duman, 2019; McKleeven et al, 2019). Decreases in the number of SST+ interneurons in the cortex have been also associated with both schizophrenia and Alzheimer’s disease (AD) (Morris et al., 2008; Perez et al., 2019). 5HT3AR+ interneurons are a heterogenous group of interneurons, consisting of many subtypes (including calreticulin, cholecystokinin, calbindin, and vasoactive intestinal peptide-expressing interneurons (VIP)) that all express the 5HT3A and nicotinic receptors. Reductions in VIP+ interneurons are seen in both bipolar disorder and schizophrenia, and increases in calbindin-positive interneurons have also been in schizophrenia (Fung et al., 2014). The most abundant subtype of GABAergic interneuron in the cortex is PV+ interneurons, which are characterized by their fast-spiking properties. PV+ interneurons play a critical role in maintaining the balance between excitatory and inhibitory neurotransmission in the central nervous system (Ferguson & Gao, 2018). Maintenance of the excitatory:inhibitory (E:I) balance is critical for emotional regulation, cognition, and information processing (Lew & Tseng, 2014; Caballero et al., 2021); dysregulations in the E:I balance within cortical and subcortical circuits have been suggested as potential causative mechanisms behind multiple neurodevelopmental and neuropsychiatric disorders (for review, see Nelson & Valakh, 205; Ferguson & Gao, 2018; Page & Coutellier, 2019). PV+ interneurons specifically undergo a protracted development throughout early postnatal life and adolescence and are sensitive to the effects of stress (discussed below), making them a prime candidate for a potential mechanistic role in the pathophysiology of neurodevelopmental and neuropsychiatric disease. Although multiple subpopulations of GABAergic interneurons have been identified in the cortex and sub-cortical regions involved in regulation of complex behaviors, such as the hippocampus and amygdala (for review, see Marín 2012; Fogaca & Duman, 2019; Fee et al., 2017; Yang et al., 2021), this review will address the impacts of stress on PV+ interneurons in preclinical models based on type of stress, biological sex, age at which stress is applied, and brain region.
Here, we gather existing preclinical work looking at the differential impact of stress on PV+ interneurons throughout the lifespan in support of the hypothesis that the age- and sex-specific effects of stress on PV+ neurons play a role in sex-specific differences in vulnerability to stress throughout the lifespan. We will discuss how models of acute and chronic stress performed during the prenatal, juvenile, adolescent, and adult periods lead to contrasting cellular and behavioral phenotypes in male and female subjects. A clear understanding of age- and sex-dependent effects of stress on PV+ interneurons would have broad implications for the interpretation of future studies modeling the role of PV+ interneurons in stress-related affective disorders and neurodevelopmental diseases. Additionally, we discuss the role that estrogen and other gonadal steroid hormones may play in differentiating the impact of stress on PV+ interneurons between males and females in preclinical studies, and how this relates to sex differences seen in clinical populations. We will also emphasize the importance of studying sex as a biological variable to fully understand the mechanistic and behavioral differences between males and females in models of neuropsychiatric disease. This is of utmost importance to fully grasp sex-specific differences in symptom presentation and neural correlates in stress-related pathologies in clinical populations.
1. A role for parvalbumin interneurons in neuropsychiatric and neurodevelopmental disease
Beyond maintenance of the E:I balance, PV+ interneurons have multiple physiological properties that are implicated in disease pathology. PV+ interneurons synapse onto multiple local excitatory pyramidal neurons to regulate their firing activity, allowing for both feedback and feedforward inhibition within circuitry (Packer & Yuste, 2011). This mechanism allows for the creation and maintenance of gamma oscillations (~25–100 Hz) which function as a ‘metronome’ within the cortex, allowing for coordination and synchronization of neuronal firing and play a role in cognition and information processing (Buzsáki & Draguhn, 2004; Gaetz et al., 2011). Disruptions in gamma oscillations have been linked to the cognitive symptoms seen in ASD, schizophrenia and AD; these disruptions may be due to differential activity in PV+ interneurons leading to changes in gamma oscillation patterning (Steullet et al., 2010; Mably & Colgin 2018). Reductions in PV+ interneuron activity lead to reductions in gamma oscillations, while increases in PV+ interneuron activity lead to increases in gamma oscillations. Interestingly, work from Fernando and Mody finds that gamma oscillations are also altered during pregnancy, suggesting that the change in female hormone milieu may modulate PV+ interneurons activity (Ferando & Mody, 2013). Importantly, PV+ interneurons are sensitive to stress, an important risk factor to several neurodevelopmental and neuropsychiatric disorders, and this sensitivity may contribute to emergence of these diseases. Disruption to PV+ interneurons cause many cognitive and behavioral deficits sufficient to model numerous psychiatric diseases, including schizophrenia, ASD, MDD, and anxiety (Dienel & Lewis, 2019; Wohr et al., 2015; Page et al., 2019).
Aside from their sensitivity to stress, another characteristic of PV+ interneurons that is relevant to the etiology of neurodevelopmental and neuropsychiatric disorders is that PV+ interneurons undergo a protracted maturation, continuing to develop into postnatal life and throughout adolescence. Immunohistological staining shows that the number of PV+ interneurons in the PFC steadily increases from juvenility throughout adolescence in rats (Caballero et al., 2014). Additional rodent studies have found that this maturation is mediated by estradiol in females (Wu et al., 2014) and BDNF in males (Du et al., 2018). In non-human primates, the amount of PV protein also significantly increased from adolescence to adulthood, suggesting maturation of inhibitory tone. The number of cells expressing PV, however, were decreased in adulthood compared to juveniles in non-human primates (Fish et al., 2013). This may be indicative of synaptic pruning leading to stronger and more efficient inhibitory synapses as part of overall circuit maturations.
An increase in PV during juvenile and adolescent maturation plays a role in shifting the tone of the PFC through a gain of inhibitory control. The resulting change in the E:I balance is thought to play a role in triggering the onset of a critical period in adolescence (Berger et al, 2013; Hensch et al., 1998) Critical periods of development are defined by specific time windows in which experience and neural circuitry interact to shape development of brain circuitry and behavior (Laresen & Luna, 2018). The role of PV+ interneurons in regulating critical periods has been evidenced in the primary visual cortex, where maturation of PV+ interneurons is necessary for normal development of vision from early life to into adulthood (Chattopadhyaya et al., 2004; Toyoizumi et al., 2013). It has also been suggested that in adolescence, PV+ maturation drives a similar critical period maturation of PFC circuitry and inhibitory behaviors (Caballero & Tseng, 2016; Reynolds et al., 2019). These periods are marked by high level of plasticity at the cellular and circuit levels, emphasizing the importance to study the effects of environmental insults on PV+ interneurons populations and the downstream neural circuits they regulate throughout the lifespan.
PV+ interneurons reach a mature phenotype with the formation of perineuronal nets (PNNs), which are specialized components of extracellular matrix that preferentially surround PV+ interneurons. PNNs contribute to the closure of the critical period by forming a barrier against new synapse formation while increasing stability of existing synapses (Baker et al., 2017). In addition, PNNs increase the excitability of PV+ interneurons and protect them against the effects of oxidative stress (Cabungcal et al., 2013). Interestingly, PNN formation at the end of the critical period is regulated by pubertal onset in females, but not males (Drzewiecki et al., 2020). PNNs are also vulnerable to the effects of stress, disrupting the protective barrier surrounding PV+ interneurons and leading to more instability within the circuitry (Gildawie et al., 2020; Wen et al., 2018). Considering the role PNNs play in numerous behaviors including executive function, memory storage, and behavioral information (Gogolla et al., 2009; Testa et al., 2019), it is highly important to consider how exposure to stress can influence their formation during development.
Finally, PV+ interneuron development is marked by important sex differences that can help understand why specific neuropsychiatric and neurodevelopmental disorders are bias toward one sex or the other. In female mice, expression of PV protein and GAD67 increases throughout adolescence and early adulthood in the hippocampus in a process mediated by estradiol, while no such increase is seen in males (Wu et al., 2014). Maturation of inhibitory transmission in the frontal cortex is also mediated by ovarian hormones in female mice (Piekarski et al., 2017). Interestingly, PV+ interneurons heavily colocalize with estrogen receptor beta (ERβ) (Blurton-Jones & Tuszynski, 2002), suggesting that there may be a direct modulation of PV+ interneuron development by pubertal estrogens. Studies assessing normal development of the PFC in humans also finds a developmental increase in PV mRNA throughout postnatal development and adolescence (Fung et al., 2010), but to our knowledge there is no data showing that this is driven by estradiol. Importantly, a large body of research suggests that PV+ interneurons may be sensitive to stress in a sex-specific manner, an effect that is also modulated by age at the time of stress exposure (Table 1). These findings, which will be discussed in the sections below, support our overall hypothesis that age- and sex-specific effects of stress on PV+ interneurons play a critical role in sex-specific vulnerability to neurodevelopmental and neuropsychiatric disorders.
Table 1.
Chronic stress effects PV+ interneurons and behavior in a sex, age-, and region-dependent manner.
| Period | Stress paradigm | Effect on PV | Brain region | Behavioral phenotype | Sex | Reference |
|---|---|---|---|---|---|---|
| Prenatal | Maternal restraint and light stress | ↓ number of PV+ interneurons (P21) | mPFC, CA1, layer III of S1 | Not assessed | Only assessed males | Uchida et al., 2014 |
| Maternal restraint and light stress | ↓ PV+ interneuron density (P21) | mPFC | Not assessed | Only assessed males | Wang et al., 2018 | |
| Maternal restraint and light stress | ↓ PV+ interneuron density (P21) ↑ PV+ interneuron density (P150) |
mPFC, CA | ↑ anxiety-like behavior | Only assessed males | Lussier & Stevens, 2016 | |
| Maternal restraint stress | ↓ PV/cFos co-labeling | mPFC | ↓ social behavior | Changes in males only | Heslin & Coutellier, 2017 | |
| Maternal social stress | ↓ number of PV+ interneurons (P30) | Hippocampus, BLA | ↑ anxiety-like behavior (males only) | Sex- and region- dependent changes in PV | Scott et al., 2020 | |
| Maternal restraint stress | ↑ PV protein expression | Hippocampus | ↓ novel object recognition | Only assessed males | Shang et al., 2021 | |
| Neonatal | Maternal separation | ↓ PV protein expression ↓ PV+ interneuron density |
OFC | ↓ rule-reversal learning | Changes in females only | Goodwill et al., 2018 |
| Maternal separation | ↓ PV protein expression (P27 in females, P45 in males) | PFC | ↓ social behavior | Females affected in juvenility; males affected in adolescence | Holland et al., 2014 | |
| Maternal separation | ↑ PV+ interneuron density (males only) | BLA | ↓ fear recall | Changes in PV in males only | Nieves et al., 2020 | |
| Maternal separation | ↓ PV+ interneuron intensity ↑ PV/8-oxo-DG co-labeling |
mPFC, BLA, hippocampus | Not assessed | Male and female | Soares et al., 2020 | |
| Maternal separation | ↓ PV protein expression (males only) | mPFC | ↓ cognitive function | Male and female | Grassi-Oliviera et al., 2016 | |
| Limited bedding | ↑ PV/PNN colocalization ↓ activity of PV+ interneurons |
BLA | No behavioral changes | Changes in males only | Guadagno et al., 2020 | |
| Juvenility | Social isolation | ↓ PV+ interneurons ↓ PV/PNN co-labeling |
Hippocampus, temporal cortex | Not assessed | Only assessed males | Ueno et al., 2017 |
| Prepubertal stress | ↑ PV protein expression | Hippocampus | ↓ conditioned fear response | Changes in males only | Brydges et al., 2018 | |
| Social isolation | ↓ PV+ interneuron activation | mPFC | ↓ social behavior | Male and female | Bicks et al., 2020 | |
| Chronic stress | ↓ size of PV+ interneuron soma ↓ PNN intensity |
Hippocampus, dAC, PFC | ↓ social behavior ↓ depressive-like behavior |
Only assessed males | Ueno et al., 2018 | |
| Pubertal and adolescence | Unpredictable chronic mild stress | ↓ number of PV+ interneurons |
mPFC | ↑ anxiety-like behavior ↓ contextual information processing |
Number of PV cells correlates with anxiety-like behavior in females | Page & Coutellier, 2018 |
| Unpredictable chronic mild stress | ↑ dendritic complexity of PV+ interneurons | mPFC | ↑ locomotion | Changes in females only | Bueron-Fernandez et al., 2021 | |
| Social isolation | ↓ PV cell density ↓ PV/PNN formation |
Hippocampus, visual cortex, anterior cingulate cortex | Not assessed | Only assessed males | Ueuno et al., 2017 | |
| “Two-hit” | polyI:C and peripubertal stress | ↓ number of PV+ interneurons | Hippocampus | Not assessed | Only assessed males | Giovanoli et al., 2014 |
| polyI:C and peripubertal stress | ↓ number of PV+ interneurons (females only) | Hippocampus | ↑ locomotion ↓ working memory, social behavior, PPI |
Changes in PV in females only | Monte et al., 2019 | |
| Maternal separation and social isolation | ↓ PV protein expression | PFC, amygdala | ↑ depressive-like behavior | Only assessed females | Lukkes et al., 2018 | |
| Adulthood | Unpredictable chronic mild stress | ↑ PV protein expression ↑ number of PV+ interneurons |
mPFC | ↑ emotionality (females only) | Sex- and region- dependent changes in PV | Shepard et al., 2016 |
| Unpredictable chronic mild stress | ↑ number of PV+ interneurons (females only) | mPFC | ↑ anxiety-like behavior ↑ depressive-like behavior |
More severe behavioral phenotype in females | Shepard & Coutellier, 2017 | |
| Chronic restraint stress | ↑ PV gene expression (females only) | mPFC | ↓ extradimensional set shifting | Changes in PV in females only | Moench et al., 2020 | |
| Unpredictable chronic mild stress | ↑ PV/c-Fos colocalization | mPFC | ↑ anxiety-like behavior (females only) | Established causal relationship between increase in PV+ activity and anxiety-like behavior in females | Page et al., 2019 | |
| Chronic unpredictable stress | Not assessed (chemogenetic inhibition of PV interneurons) | mPFC | ↓ depressive-like behavior | Only assessed males | Fogaca et al., 2020 | |
| Chronic variable stress | Not assessed (chemogenetic inhibition of PV interneurons) | mPFC | ↑ active coping strategy | Only assessed males | Nawreen et al., 2020 | |
| Chronic mild stress | ↓ number of PV+ interneurons | mPFC | ↓ cognitive performance | Only assessed males | Czéh et al., 2018 | |
| Social isolation | ↓ PV+ interneuron density | Hippocampus | Not assessed | Only assessed females | Harte et al., 2007 | |
| Chronic restraint stress | ↓ number of PV+ interneurons | Hippocampus | Not assessed | Only assessed males | Hu et al., 2010 | |
| Social isolation | ↓ PV protein expression (effects seen after chronic isolation only) | Hippocampus | Not assessed | Only assessed males | Filipovic et al., 2013 |
2. Prenatal stress
Prenatal stress is associated with numerous neurodevelopmental and neuropsychiatric disorders including ASD, PTSD, and schizophrenia (Bale 2015; Bale and Epperson, 2015; Rogers and Bale 2015). In humans, the cognitive, emotional, and behavioral effects of prenatal stress seem to be sex specific, with males being more vulnerable to short- and long-term effects of exposure to stress during this critical period than females (Sandman et al., 2013; Bale and Epperson, 2015). In preclinical models, stress during the prenatal period has been associated with changes in several behavioral domains as well as altered HPA axis functionality, changes in cortical thickness, and abnormalities in GABAergic circuitry (for review, see Fine et al., 2014). In support of our hypothesis, aberrations in developmental trajectory, protein quantification, and cell counts of PV+ interneurons have been found following prenatal stress.
Most existing studies assessing PV+ interneurons following prenatal stress use a model of maternal restraint. Unfortunately, few of them directly examine sex differences in offspring following the stressor. Multiple studies use a heterozygous GAD67+/− mouse, a model of Gad1 gene disruption. Gad1 encodes GAD67 or glutamate decarboxylase, which is responsible for synthesizing GABA, and is a common genetic risk factor for schizophrenia (Uchida et al., 2014). Heterozygous (GAD67+/−) male offspring from dams exposed to restraint under bright light over gestational day (GD)15–17.5 have fewer PV+ interneurons within the mPFC, hippocampus, and somatosensory cortex (S1), as well as decreased density of PV+ interneurons expressing GAD67 in all layers of the mPFC and the CA1 region of the hippocampus at post-natal day (P)P21 compared to P21 male mice from non-stressed dams (Uchida et al., 2014, Wang et al., 2018). Male GAD67+/− offspring from dams subjected to 45 minutes of restraint under bright light three times daily from GD12 to birth show a similarly altered developmental trajectory of PV+ interneurons throughout juvenility and adulthood. At P24, PV+ cell number was decreased in the mPFC and hippocampus compared to non-stressed controls. In adult (P150), the number of PV+ interneurons in the mPFC and hippocampus increased above that of controls, suggesting an overcorrection within the circuit (Lussier and Stevens, 2016). These adult mice displayed increased anxiety-like behavior in the elevated plus maze and the open field compared to non-stressed controls, and this behavioral phenotype was associated with increased GAD67 in the hippocampus (Lussier and Stevens, 2016). The aforementioned studies, however, only assessed male offspring.
When offspring of both sexes are assessed following prenatal stress, males show an exacerbated cellular and behavioral phenotype. Npas4 helps regulate the formation of inhibitory synapses onto excitatory cells in the cortex in an activity-dependent manner and has transcriptional control over other essential genes, including BDNF and Erg1 (Lin et al., 2008; Spiegel et al., 2014). In Npas4-deficient offspring of dams exposed to chronic restraint stress from GD7–19, males showed deficits in social behavior as well as reduced PV/cFos co-labeling in the infralimbic cortex compared to controls. Female offspring of the same model did not show any behavioral or histological changes (Heslin and Coutellier, 2017).
Male offspring were also found to be more susceptible to prenatal stress than females in a rat model of maternal social stress, in which an adapted resident-intruder paradigm was applied from GD16–20. In this study, male offspring displayed a reduced number of PV+ interneurons in the PFC, all regions of the hippocampus, and the basolateral amygdala (BLA) compared to controls, whereas female offspring only displayed decreased PV+ interneuron density in the CA1 region of the hippocampus. Males, but not females, displayed anxiety-like behavior. Interestingly, both the behavioral and cellular deficits caused by prenatal stress are recovered by antioxidant treatment in males, without effect in females (Scott et al., 2020). These studies show that the social and affective behaviors and PV+ interneuron populations of males are more sensitive to prenatal stress than females, with effects continuing into adulthood.
However, other findings suggest a more complex relationship between age-specific changes in the PV system and behavioral alterations. A study using a rat model of maternal restraint from GD15–21 found an increase in levels of PV protein in the hippocampus in early life, puberty, and adulthood of male offspring exposed to prenatal stress. Behaviorally, these male offspring had memory deficits in the Morris water maze and novel object recognition tasks during early life and the pubertal time periods, but not in adulthood (Shang et al., 2021). This reveals that long term cellular changes in the hippocampus may not necessitate behavioral changes in adulthood (Shang et al., 2021), potentially through plasticity of other systems.
Going beyond correlational analyses between stress-induced changes within PV circuitries and behavioral endpoints should be the next necessary step to unravel the exact role played by age-specific changes in PV-dependent inhibition and behavioral deficits. Chemogenetic studies, for instance, have been used to modulate PV+ interneuron activity and determine the behavioral impacts. Additionally, mice that are heterozygous for the gene regulating PV protein transcription, Pvalb, provide unique opportunities to study the effects of prenatal stress in a PV knockdown model. Notably, most existing studies examining the role of prenatal stress on PV+ interneurons only utilize male subjects. More studies directly assessing sex as a biological variable in the impact of prenatal stress on PV+ interneurons must be conducted to draw stronger conclusions about sex-specific impacts.
3. Early life stress
Neural development, particularly within the cortex and limbic system, continues at a rapid pace during early postnatal life and throughout adolescence, leaving the brain vulnerable to the effects of stress during this time. The use of preclinical models to study stress during early life are advantageous in that distinct periods of development, including neonatal (P0–21), juvenile (P21–30), and adolescent (P30–60)(Eiland and Romero, 2013) periods, have been mapped and can easily be targeted to determine the specific effects of stress during those specific developmental periods. Additionally, the pubertal period (P28–42), which overlaps with the end of juvenility and the beginning of adolescence, has been implicated in unique developmental outcomes. Brain regions that are known to be stress responsive, including the hippocampus, amygdala, and the PFC, are also increasingly plastic during early life, and it is during this time that the GABAergic system undergoes important maturational events (Elrlich et al, 2013; Wu et al, 2014; Caballero & Tseng, 2016). During early life, the E:I balance is refined within the PFC, controlling the maturation of higher-order cognitive abilities including working memory, impulse control, and decision making (Le Magueresse and Monyer, 2013; Takesian and Hensch, 2013; Caballero et al., 2016). Refinement of E:I balance is largely dependent on maturation of GABAergic interneurons, including PV+ interneurons, in a process that is sex-specific and reliant on numerous endogenous factors, including BDNF in males (Du et al., 2018) and estradiol in females (Wu et al., 2014). Age-specific changes do not limit themselves to local circuits as long-range connections between the PFC and subcortical regions also mature during this time. This includes PFC-BLA and PFC-ventral striatum connections that subserve social, cognitive, and affective functions (Brummelte et al., 2007; Brenhouse and Andersen, 2011; Anastasiades and Carter, 2021).
3.1. Neonatal stress (P0–21)
Exposure to early life stress (ELS) increases risk of developing a neuropsychiatric disorder (Heim & Nemeroff, 2002). In preclinical models, neonatal stress takes place prior to weaning (from P0-P21) and mimics early life neglect through limited bedding or maternal separation paradigms (Plotsky and Meaney, 1993; Rice et al., 2008; George et al., 2010; Walker et al., 2017).
Female rats submitted to maternal separation from P2–20 expressed lower levels of PV protein in the PFC during the juvenile period (P25) compared to controls, which was correlated with deficits in social interactions. In males exposed to maternal separation, deficits in PV protein expression were not seen until adolescence (P40) (Holland et al., 2014). The earlier loss of prefrontal PV protein level in females could be indicative of their increased sensitivity to neonatal stress. This idea is supported by other studies; for instance, females exposed to limited bedding from P4–11 have fewer PV+ interneurons in the olfactory cortex (OFC) in adulthood, an effect not seen in males. This deficit in PV+ interneurons was accompanied by deficits in rule-reversal learning, which could be recapitulated through chemogenetic silencing of PV+ interneurons in the OFC of healthy controls (Goodwill et al., 2018). Other studies, however, find that ELS preferentially effects males. Maternal separation from P2–20 reduces prefrontal PV protein expression at P40 and P55 in male rats only, although behavioral changes in the win-shift task from P40-P55 are seen in both males and females following ELS. Interestingly, this study also found that maternal separation accelerates the onset of puberty in female rats (Grassi-Oliviera et al., 2016). Altogether, these findings might indicate that ELS affects differently the trajectory of PV+ interneurons postnatal development in males and females.
Studies examining PV+ interneurons in the BLA suggest that this region may be particularly susceptible to ELS. A study reports an increase in the number of cells expressing PV in the BLA of male mice at P21 following a limited bedding paradigm (Nieves et al., 2020), and field and whole cell electrophysiological recording in juvenile rats following a limited bedding paradigm from P0–10 revealed reduced activity of PV+ interneurons in the right BLA of males, with no changes in females (Guadagno et al., 2020). However, following maternal separation, both male and female rats were found to have reduced staining intensity of PV+ interneuron at P20, suggesting lower levels of PV protein (Soares et al., 2020). These changes in PV protein expression and PV+ interneuron number following ELS may be driven by oxidative stress. In a rat model of maternal separation from P2–20, increased colocalization of PV and 8-oxo-DG (a marker of oxidative stress) is depicted in the mPFC, the CA1 region of the hippocampus, and the DG at P20 (Soares et al., 2020). Although the effects of ELS on PV+ interneurons are relatively widespread across sex and brain region, it is evident that this period is critical for development of normal circuitries and behavior. Further studies should attempt to dissect the effects of ELS on the developmental trajectory of PV+ interneurons in a sex- and brain region-specific manner through longitudinal investigations to gain a clear understanding of how ELS can impact behaviors on the long-term.
3.2. Juvenile stress (P21–30)
The juvenile phase consists of a short post-weaning period (P21–30) that overlaps with the beginning of puberty before adolescence begins. It appears that the effects of stress during this period differ according to the type of stress and by brain region, and behavioral outcomes are equally variable. Furthermore, various aspects of PV+ interneurons physiology, anatomy, and properties seem to be differently affected by juvenile stress. A short-term pre-pubertal stress paradigm (P25–27) led to increased double-labeling of PV and doublecortin, a marker of newborn neurons, in the adult DG, suggesting that juvenile stress alters adult hippocampal neurogenesis. Changes at the cellular level were only evident in males, despite females displaying impairments in hippocampal-dependent contextual fear conditioning following pre-pubertal stress (Brydges et al., 2018). Interestingly, exposure to chronic stress from P21–30 in male mice reduced depressive-like behaviors in the forced swim test at the end of the juvenile period. These mice also displayed altered social behavior, but did not display changes in anxiety-like behavior compared to non-stressed controls. These behavioral outcomes were accompanied by a reduction in intensity of PNNs in the CA1 region of the hippocampus, the PFC, and the dAC. Additionally, this stress paradigm reduced the area of the soma of PV+ interneurons in the CA3 region of the hippocampus and the dAC (Ueno et al., 2018). These changes in PNNs may suggest that juvenile stress may lead to increased synaptic plasticity, however the long-term effects of this paradigm have not been assessed.
Another paradigm of juvenile stress, juvenile social isolation, leads to altered social behavior in adulthood, even after mice were returned to a group-housed environment. Whole-cell patch clamp recording also revealed that PV+ interneurons of socially isolated male mice failed to follow a normal developmental trajectory in the dorso-medial PFC, leading to decreased activation patterns of PV+ interneurons, decreases in intrinsic excitability, and an altered E:I ratio in adulthood (Bicks et al., 2020). Juvenile social isolation, which can induce oxidative stress, lessens the number of PV+ interneurons in the DG of male mice in adulthood compared to group-housed controls. These socially isolated male mice also had a lower percentage of PV+ interneurons surrounded by PNNs in the CA1 and DG regions of the hippocampus. Additionally, levels of PV protein expression, as measured by intensity of fluorescence, were lower in socially isolated mice in all regions of the hippocampus as well as the temporal cortex (Ueno et al., 2017). Very few studies exist directly examining the effects of stress during the juvenile period, and less is known about the long-term implications of stress during this time. Of particular interest is whether exposure to juvenile stress imparts vulnerability or resilience to future stressors (for review, see Schroeder et al., 2018). While some studies suggest that juvenile stress exposure may lead to resilience in the face of adulthood stress (Ricon et al., 2011; Albrecht et al., 2011), the role of PV+ interneurons in facilitating risk and resilience to future stressors has yet to be determined.
3.3. Pubertal and adolescent stress (P30–60)
The adolescent period, and the coinciding process of puberty, is marked by behavioral changes associated with maturation of the frontal cortex and re-organization of the limbic system. Although these developmental phases overlap, it is important to recognize that puberty is a key phase of development conserved across species, and plays a role in the maturation of numerous brain systems, such as the HPA axis (Schroeder et al., 2018). Together, these developmental phases interact, and increased neural plasticity during this period is influenced by the direct and indirect actions of the influx of gonadal hormones associated with puberty. Adolescence is associated with a second period of synaptogenesis, a process deeply linked with the actions of estradiol (Matsumoto 1991). PV and PNN expression are also regulated by puberty. Both male and female rodents express estrogen receptor β (ER-β) on PV+ interneurons and may be sensitive to the effects of increased circulating estradiol during this period (Blurton-Jones and Tuszynski, 2002; Kritzer 2002). We are unaware, however, of any studies identifying ER-β on PV+ interneurons in humans at this time. In female rats, the density and intensity of PV+ interneurons in the PFC increases from P20–40, but decreases by P70 (Gildawie et al., 2020). Additionally, female rats show an abrupt decrease in PNN number in the mPFC around P35 that is specifically associated with pubertal status and may be associated with closure of critical periods of plasticity and stabilization of PV networks (Takesian and Hench 2013; Drzewiecki et al., 2020; Delevich et al., 2021). Furthermore, an increase in circulating 17β-estradiol is necessary for maturation of inhibitory neurotransmission within the PFC in females, a process which is blocked by ovariectomy (Piekarski et al., 2017).
Evidence indicates that the effects of stress during the adolescent and pubertal periods may be sex specific. Male and female C57BL/6 mice that underwent two weeks of unpredictable chronic mild stress (UCMS) during adolescence from P28–42 displayed sexually dimorphic phenotypes, with only stress-exposed males showing a decrease in the number of PV+ cells in the prelimbic (PrL) PFC, associated with increased adolescent and adult anxiety-like behavior. Interestingly, while females exposed to UCMS displayed increased anxiety-like behavior, UCMS did not affect PV+ interneurons number in the PFC, suggesting that the adolescent stress-induced anxiety in females might be driven by other mechanisms (Page and Coutellier, 2018). This study also reports an inverse correlation between number of PV+ interneurons and levels of anxiety-like behaviors in the open field test, especially in females, a finding that was reported previously in adult female mice (Shepard et al, 2016) and that warrant further investigation to better understand the relationship between number of prefrontal PV+ interneurons and anxiety. This finding, however, suggests a role for prefrontal PV cells in maturation of emotionality, potentially through the mPFC-BLA pathway, which has been shown to mediate stress-induced increase of anxiety (Liu et al., 2020). Interestingly, exposure to ELS has been linked to accelerated maturation of projection neurons that extend from the PFC to the BLA in females only (Nieves et al., 2020). It would be interesting to determine whether adolescent stress similarly affect prefrontal-amygdala circuits.
Other studies support the sex-specific effects of adolescent and pubertal stress in parvalbumin systems. Bueron-Fernandez et al. found that female, but not male, mice exposed to stress during the pubertal period (P28–42) had an increase in dendritic complexity of prefrontal PV+ interneurons (Bueron-Fernandez et al., 2021). On the other hand, male mice submitted to social isolation throughout the duration of juvenility and adolescence (P21-P56) display a lower density of PV+ interneurons in the DG and fewer WFA+ PNNs surrounding PV+ cells in the CA1 region of the hippocampus and the DG compared to controls, however females were not assessed (Ueno et al., 2017).
Unfortunately, studies which include females to examine sex differences in adolescent stress and the role of circulating gonadal steroids on mediating the effects of stress on PV+ cells are scarce, leading to an overall lack of understanding on how stress impacts behavioral and central maturational processes differently in males and females. Additional studies determining the role of stress on mediating molecular and behavioral changes during this time period, when sex differences in rates of diagnoses of affective disorders begin to appear in clinical populations (Bale & Epperson, 2015), are therefore required.
3.4. “Two-hit” models of early life stress
An additional model of ELS assesses how exposure to one stressor early in life impacts the response to a later stressor. These “two-hit” models of stress exposure are stem from the “two-hit” hypothesis of neuropsychiatric disease, which posits that a first “hit” during early brain development increases vulnerability to develop a neuropsychiatric disease while a second “hit” later on in life will trigger the onset of said disorder (Bayer et al, 1999; Hill et al, 2014). Often, preclinical studies modeling schizophrenia or other neurodevelopmental disorders will combine an initial hit of maternal immune activation (MIA) with a second stressor in early life. These models can be applied to other neuropsychiatric disorders including depression and anxiety (Monte et al, 2017; Jaric et al, 2019). In one study, pregnant mouse dams were exposed to polyinosinic:polycytidylic acid (polyI:C), a potassium salt that activates the maternal immune system, at GD9 (equivalent to the middle of the first trimester of gestation). Offspring were then exposed to peripubertal stress from P30–40. The combination of the MIA and peripubertal stress led to fewer PV+ interneurons in the DG of adult offspring, while neither stressor was sufficient on its own to induce changes in the number of PV+ interneurons (sex not stratified) (Giovanoli et al, 2014). In a similar paradigm with polyI:C injection from GD5–7 and peripubertal stress from P40–48, both male and female rats displayed reduced prepulse inhibition (PPI) and social preference in adulthood, but only females had a significant reduction in PV+ interneurons in the PFC and striatum. Both cellular and behavioral measures in males and females were corrected by administration of N-acetylcysteine, an antioxidant and precursor to glutathione, a free radical scavenger implicated in schizophrenia (Monte et al, 2019). Other “two-hit” models combine early maternal separation with social isolation during the juvenile/adolescent period. One study reported that this “two-hit” model leads to fewer PV+ interneurons in the PFC in adulthood in females only, associated with hyperactivity and a decrease in risk-assessment behavior in the elevated zero maze (Gildawie et al., 2021). Another study in female rats found that levels of PV protein were lower in the amygdala and higher in the PFC of females presenting an anhedonic phenotype following the two-hit exposure, compared to resilient, non-anhedonic animals, and to non-stressed controls (Lukkes et al., 2018). These data show that PV+ interneurons in cortical and limbic regions are very plastic during sensitive periods of development, particularly in females, which could be a contributing factor to females increased risk for psychiatric disorders in adulthood. However, further studies are required to investigate the molecular and/or endocrine factors that lead to sex-specific plasticity of PV+ interneurons as a way to gain new insights on the etiology and clinical presentation of neuropsychiatric disorders.
4. Adult stress
Although adulthood is not a critical period of brain development, the adult brain is still sensitive to the effects of stress and is subject to many allostatic-induced plastic mechanisms, including changes in gene expression, HPA axis output, and the effects of steroid hormones (McEwen 2004; Radley et al., 2015). Very few studies exist that directly compare the effects of adult stress on PV+ interneurons in male and female rodents, and the results are highly variable, making it difficult to postulate how the effects of stress in these cells contributes to the sex differences we see in neuropsychiatric disorders in clinical populations. Several studies have found that adult female PV+ interneurons are more susceptible to the effects of chronic stress than males. Female rats maintained in social isolation from weaning and throughout adulthood have decreased density of PV+ interneurons in the DG and CA2/3 region of the hippocampus, accompanied by a decrease in average PPI of the startle reflex (Harte et al., 2007). An initial study using adult C57Bl/6 mice subjected to two or four weeks of UCMS found that female mice had increased PV mRNA as well as an increased number of PV+ interneurons in the PrL PFC and infralimbic (IL) PFC, while males only displayed an increase in PV+ interneurons in the Prl PFC. Behaviorally, males did not show significant changes in overall emotionality. Females, on the other hand, displayed an overall increase in emotionality based on behavior in the open-field and forced-swim tests, which was inversely correlated to level of PV mRNA expression in the PFC (Shepard et al., 2016). In a follow-up study using BALB/c mice, a strain noted for their increased propensity for anxiety, females displayed more severe emotional dysregulations following two weeks of UCMS than males, including increased anxiety- and depressive-like behaviors, as well as deficits in PFC-dependent cognitive tasks; this was associated with an increase in the number of PV+ interneurons in the PFC and an increase in prefrontal PV mRNA. While males showed increased anxiety in the open field, they did not display changes in overall cognitive function or changes in PV expression (Shepard and Coutellier, 2017). Other studies have also found changes in PV+ interneurons in the PFC of adult females following chronic stress. After six days of chronic restraint stress female, but not male, rats displayed increased PV mRNA expression (Moench et al., 2020). After four weeks of UCMS, both male and female mice have more PV+ interneurons expressing cFos within the PFC, suggesting that chronic stress increased PV+ neuron activity. This increase in PV activity was associated with increased anxiety- (but not depressive-) like behavior in female mice only, a relationship that was causally verified by a chemogenetic approach (Page et al., 2019). In support to stress-induced increased activity of prefrontal PV+ interneurons being a driver to increased expression of emotional behaviors, others reported decreased depressive-like behaviors in the novelty suppressed feeding test and/or forced swim test following chemogenetic inhibition of prefrontal PV+ interneurons during three weeks of chronic variable stress in male mice (Fogaca et al., 2020; Nawreen et al., 2020). Together, these studies indicate that plasticity of PV+ interneurons in response to adult stress drives changes in emotional behaviors, with effects on specific sub-domains of affective behaviors being potentially sex-specific.
Other studies support the impact of stress on PV+ interneurons in adult males. Chronic mild stress reduced the number of PV+ interneurons within the mPFC of phenotypically anhedonic male rats (Czéh et al., 2018). Similarly, male rats exposed to chronic restraint stress have fewer PV+ interneurons in all hippocampal subregions (Hu et al., 2010). Interestingly, these effects are only seen following chronic stress. Filipovic et al. investigated the influence of acute versus chronic or combined acute and chronic stress on PV expression in the hippocampus of adult male mice and found that 21 days of chronic social isolation, or its combination with two hours of an acute immobilization stress, decreased the number of PV+ cells in CA1, CA3, and the DG of the hippocampus, while acute stress alone does not lead to any changes in number of PV+ cells (Filipovic et al., 2013). Additionally, acute, but not chronic, inhibition of PV+ interneurons led to a reduction in struggling duration in the tail suspension test, suggestive of a depressive phenotype. This is supported by unpublished data from our lab, which finds that acute activation of PV+ interneurons leads to reduced depressive behavior in males, but not females (Coutellier lab, unpublished). Overall, these data suggest differential effects of acute vs. chronic activity in PV+ interneurons on adult depressive-like behavior in males, a topic that deserve further investigation and should include female subjects.
Altogether, data suggests that susceptibility to the cellular and behavioral effects of stress in females is most pronounced in adolescence and adulthood, while males see greater effects prior to adulthood (Figure 1). This increased vulnerability to changes in adolescence and adulthood in females is an interesting deviation from many hypotheses regarding neuroprotection in females, which assume that stable estrogen levels in adulthood confer endogenous neuroprotective effects and decrease damage against chronic stress, brain injury, and neurodegenerative diseases. This specific topic warrant further investigation.
Figure 1. The impact of stress on parvalbumin interneurons varies in an age- and sex-specific manner.
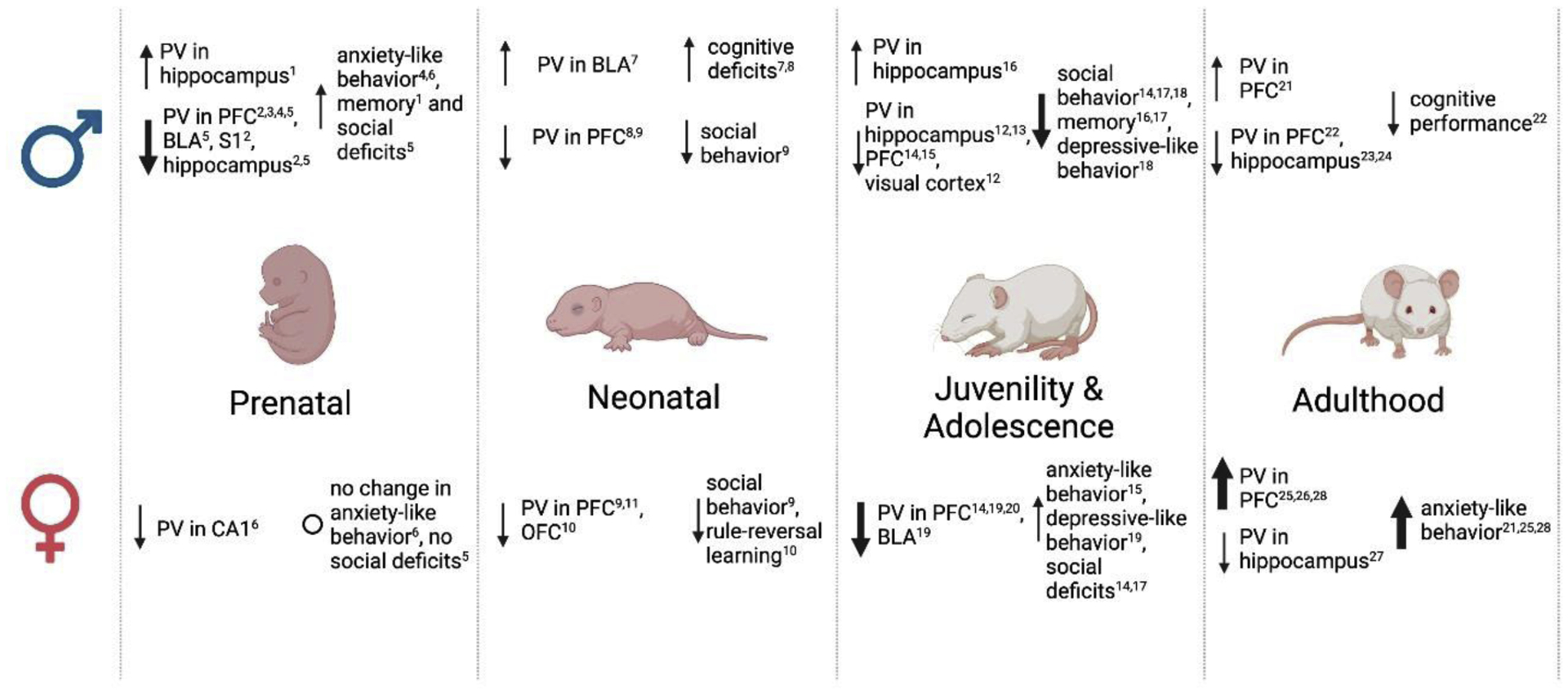
Schematic representation that summarizes existing literature that assesses the influence of stress on parvalbumin (PV) interneurons and behavioral outcomes in preclinical models of neurodevelopmental and neuropsychiatric disease. Findings reveal that males are more heavily impacted by prenatal stress, multiple studies finding decreased measures of PV in several brain regions as well as increased anxiety-like behavior, social deficits, and memory impairments. Females, on the other hand, seem to show greater changes in PV and behavior following stress in the neonatal phase and in adulthood, suggesting an increased vulnerability to stress during these periods. These findings could play a role in the differential rates of diagnosis of neurodevelopmental and neuropsychiatric disorders among men and women in clinical populations. An increased susceptibility to prenatal stress in males leading to more significant losses of parvalbumin may play a role in increased vulnerability to neurodevelopmental disease (e.g., ASD, ADHD), while more susceptibility to postnatal stress in females may correspond with increased diagnostic rates of affective disorders (e.g. MDD, anxiety). These findings have large implications for considering age, sex, and their interaction in future studies addressing the impacts of stress on individual vulnerability to stress. For footnotes, please see Appendix 2.
5. Discussion
5.1. Brain region specificity
Most existing studies assessing the impact of stress on PV+ interneurons focus on three brain regions heavily associated with the stress response: the PFC, the hippocampus, and the amygdala. As discussed, the effects of chronic stress on PV+ interneurons vary heavily within these regions (Figure 2). This variation may be explained, in part, by differences in the basal role of PV+ interneurons in each of these regions. In the PFC, activation of PV+ interneurons has been associated with numerous behavioral effects, including extinction of reward-seeking behavior, reduced helplessness, and increased anxiety-like behaviors in females only (Sparta et al., 2014; Perova et al., 2015; Page et al., 2019). Reducing PV+ interneuron activity in the PFC leads to reductions in extinction learning, increases in coping behaviors, deficits in sensorimotor gating, and increased novelty seeking (Caballero et al., 2020; Nawreen et al., 2020; Brown et al., 2015). Following stress, measures of PV+ interneurons overwhelmingly decrease in the PFC, with the exception of increases in PV+ cell number and PV protein expression in adult females only. Chemogenetic studies find that inhibition of PV+ interneurons reduce depressive-like behaviors following chronic stress exposure in adult males, suggesting that perhaps decreases in PV+ interneuron number in the PFC is a compensatory mechanism against the depressive effects of stress exposure (Fogaca et al., 2020; Nawreen et al., 2020). The increase in PV+ interneuron number and PV protein expression in the PFC in adult females, however, may suggest that PV+ interneurons play a sex-dependent role in the regulation of emotionality in adulthood. This does not take into account, however, increased risk for depression following early life or adolescent stress, which is also associated with decreases in prefrontal PV.
Figure 2. The impact of stress on PV+ interneurons varies based on age, sex, and brain region.
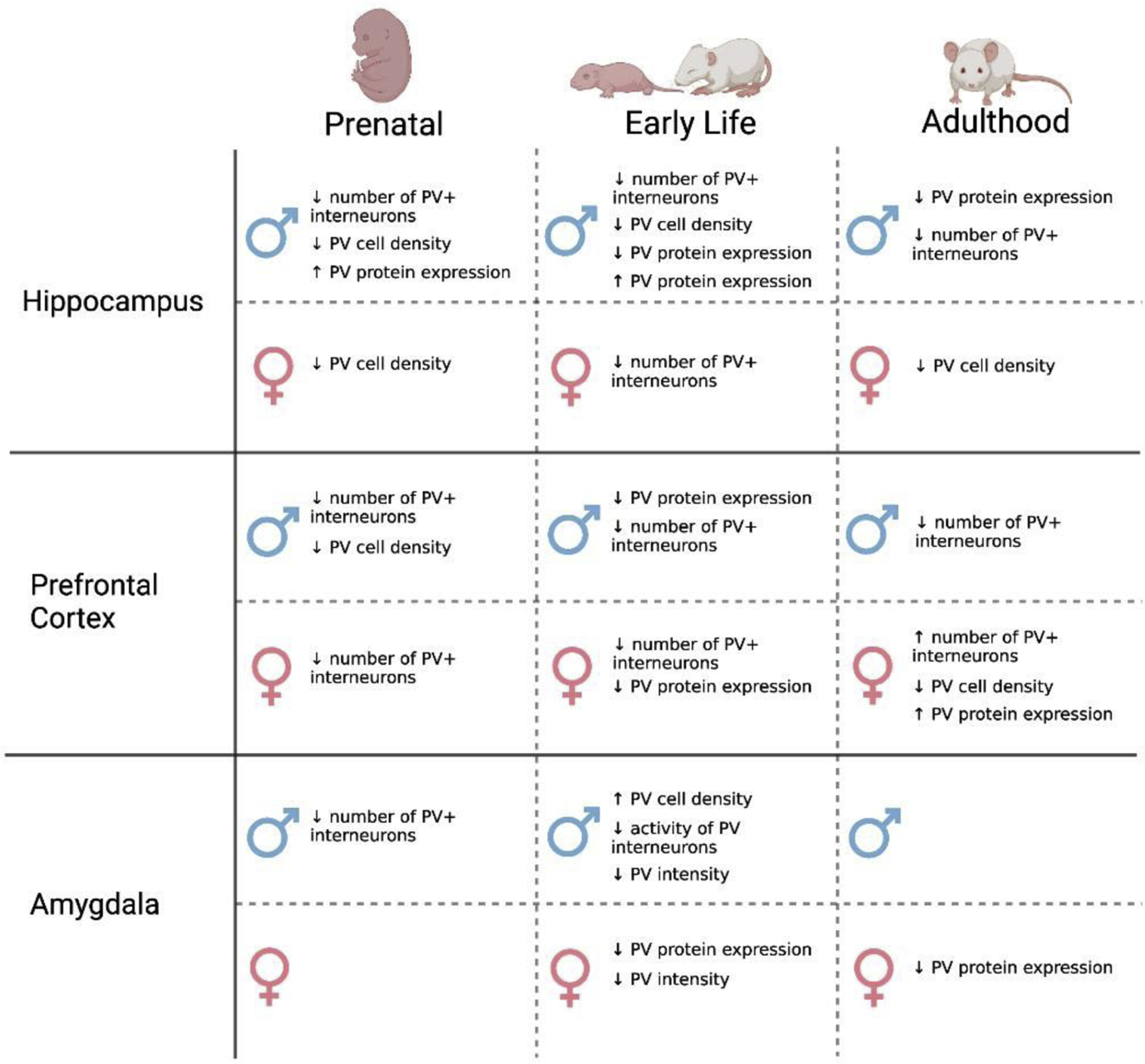
Schematic representation of the results of key studies assessing the influence of stress on PV+ interneurons, organized by brain region. Most studies assessing the influence of stress on PV+ interneurons focus on three brain regions highly integrated with the stress response: the hippocampus, the prefrontal cortex, and the amygdala. Exposure to stress largely decreases measures of PV+ interneurons and protein expression in the hippocampus. In the prefrontal cortex, stress in adult females is associated with an increased number of PV+ interneurons and increased PV protein expression. Less information is available regarding the effects of stress across the lifespan on PV+ interneurons in the amygdala. These differences in PV+ interneurons by sex, brain region, and phase of life contribute to the complex interactions between PV+ interneurons and chronic stress exposure.
PV+ interneurons in the hippocampus are widely associated with maintaining hippocampal oscillations, which play a role in memory consolidations. These oscillations are also suggested to play a role in anxiety and the determination of adverse or safe situations (Khemka et al., 2017). Studies assessing the impact of chronic stress on PV+ interneurons in the hippocampus overwhelmingly find decreases in markers of PV+ cell number and density, although studies examining the prenatal and early life periods in males suggest there may be an increase in PV protein expression following stress at these times. Interestingly, study in aged C57Bl/6 mice found that increased oxidative stress in surgery and associated decreased PV protein expression in the hippocampus led to memory impairment, suggesting a role for these cells in post-operative cognitive decline (Qui et al., 2016). In the amygdala, PV+ interneurons are critical for several aspects of fear and anxiety. Chemogenetic inhibition of PV+ interneurons in the BLA of unstressed mice decreases anxiety-like behavior in the elevated plus maze (Luo et al., 2020), while cFos expression in PV+ interneurons in the BLA is increased following administration of anxiogenic drugs (Hale et al., 2010). Activation of PV+ interneurons in the BLA is essential for retrieval of memory in a conditioned taste aversion paradigm (Yiannakas et al., 2021). As PV+ interneurons have been implicated in numerous functions in stress-related brain regions, it is to be anticipated that any disruption of PV+ interneurons, including following exposure to stress, will lead to abnormal emotional or cognitive functions.
While studying changes in PV+ interneurons at the local circuit level can provide insight on emergence of abnormal behavioral phenotypes, it is important to note that the PFC, hippocampus, and amygdala function in concert with one another, and modulating PV+ interneurons in one region has vast effects on other regions, causing complex systemic changes. Caballero et al. found that RNAi downregulation of PV expression during adolescence is sufficient to disrupt overall GABAergic function in the PFC and disrupt hippocampal-PFC transmission throughout adulthood. This disruption in hippocampal-PFC transmission also impaired downstream hippocampal regulation of the BLA (Caballero et al., 2020). Interestingly, this downregulation of PV expression also impaired regulation of extinction of fear learning, which relies on functional connectivity between the PFC, hippocampus, and BLA. Similarly, inhibition of PV interneurons in the dorso-medial PFC reduces activation of prefrontal projections to the BLA, causing an increase in fear expression. Efforts made to study the intricate relationships between local changes in PV-dependent inhibition and changes in long-range projections should continue. Novel technologies that have been developed to interrogate circuit activity and function in freely behaving animals will contribute to increasing our understanding of the role played by PV+ interneurons in the regulation of complex behaviors.
5.2. Potential mechanisms behind changes in PV+ interneurons
While work has been done to characterize changes in PV+ interneuron circuitry following stress exposure, less is known about the mechanisms behind these changes. One potential mediator of changes in PV+ interneurons following stress exposure, particularly through decreases in cell number and protein expression, is oxidative stress. PV+ interneurons are particularly sensitive to the effects of oxidative stress as they contain a high number of mitochondria in order to maintain their fast-spiking properties (Steullet et al., 2017). Exposure to stress has been shown to mediate oxidative stress, and oxidative stress through activation of NOX2 signaling is a common mechanism in preclinical models of schizophrenia, leading to decreased PV+ interneuron numbers in the cortex (Powell et al., 2012). While PNNs play a role in protecting PV+ interneurons against the effects of oxidative stress, they do not emerge until late adolescence, suggesting that oxidative stress induced by prenatal or ELS may be a mechanism behind PV+ interneuron reduction. PNNs, however, have also been shown to be susceptible to the effects of stress, and their protective properties may decrease following chronic stress exposure during adolescence and adulthood. Therefore, stress-induced reduction in PNNs is another potential mechanism underlying changes in PV+ interneurons following stress exposure. Multiple studies have found evidence of reduced PNN intensity in the PFC and hippocampus following chronic stress in juvenility and adulthood (Ueno et al., 2018; Yu et al., 2020; Folha et al., 2017). PV+ interneuron susceptibility to stress following reductions in PNNs should be more closely analyzed. However, a recent study shows that depleting PNNs surrounding PV+ interneurons increases plasticity in local circuitry and reduces inhibitory output (Testa et al., 2019).
Stress can also lead to reductions in BDNF, or brain-derived neurotrophic factor. BDNF is a prominent neurotrophic factor in the brain and is essential in supporting the growth, differentiation, and survival of neurons in the cortex, hippocampus, and amygdala. BDNF and its receptor, TrkB, play a crucial role in PV+ interneuron development and function. BDNF mediates adolescent development of PV+ interneurons (Huang et al., 1999; Du et al., 2018), while reductions in BDNF impair GABAergic function and delay maturation of PV interneurons (Jiao et al., 2011; Koh et al., 2016). Male mice with a BDNF+/− (heterozygous) genotype do not display increases in PV protein expression that are seen with normal aging (Du et al., 2018). While, both male and female heterozygous mice display significantly decreased BDNF expression compared to controls in adulthood, no age-associated changes in PV protein expression were observed in females. Polymorphisms in the BDNF gene have been associated with increased susceptibility to stress in mice (Hill et al., 2020). Additionally, knocking down TrkB on PV+ interneurons leads to spatial memory deficits in male mice only (Grech et al., 2019), and decreases in BDNF and TrkB have been observed in the PFC and hippocampus of individuals with schizophrenia, which is more common in males than females (Ray et al., 2014). Because expression of both BDNF and TrkB has been shown to decrease following acute or chronic stress exposure throughout the lifespan (Roceri et al., 2002; Marmigére et al., 2003; Bland et al., 2005), these findings suggest that reductions in BDNF following stress exposure may mediate changes in PV+ interneurons and behavior in males, providing a molecular substrate for sex-specific effects of stress on PV circuitries. Notably, the transcription of BDNF mRNA is regulated by estrogen (Solum & Handa, 2002). It has been suggested that the deleterious effects of BDNF knockdown are prevented by estrogen in females. Ovariectomized BDNF+/− female mice display impaired performance on memory assessments, an effect that was prevented by estrogen replacement (Wu et al., 2015). In fact, estrogen has been suggested to play a protective role against schizophrenia in females, as the onset of schizophrenia in females is later than that in males, with a notable increase of diagnoses in women at menopause (Hafner et al., 1993). Women who are diagnosed with schizophrenia prior to menopause, when levels of estrogen are still high, often have fewer negative treatment outcomes and an improved treatment response (Begemann et al., 2012). The role of estrogen as a neurosteroid is extremely complex, and the relationship between estrogen and PV+ interneurons has yet to be fully examined.
5.3. Impacts of estrogen
Beyond their roles as regulators of the neuroendocrine and reproductive systems, sex hormones such as estrogen have critical functions in the central nervous system. Estrogen is a potent neurosteroid that plays a regulatory role in numerous functions including synaptic plasticity, neuroinflammation, intracellular signaling, cognition, memory, and mood. Estrogen has historically been considered a neuroprotective factor in the brain, particularly in the face of ischemia, brain injuries, and neuropsychiatric diseases, particularly schizophrenia (Schroeder et al., 2016). Interactions between estrogens and PV+ interneurons are extremely complex. Estrogens play a role in regulating the BDNF/TrkB signaling cascade and the development of PV+ interneurons in females (Wu et al., 2014). Interestingly, male mice heterozygous for Pvalb have recovery of behavioral phenotypes associated with ASD following estradiol administration (Filice et al., 2018). The role of estrogens on PV+ interneurons in females, however, are less characterized. Studies examining PV+ interneurons following stress suggest that females have an increased susceptibility to changes in PV+ interneuron number or PV protein expression in adulthood, when estrogen levels are at their highest. Supposedly, this high level of estrogen should be protective against stress-induced changes in PV+ interneurons, but many studies report an opposite effect. We hypothesize that estrogen binding to ERβ on PV+ interneurons facilitates maturation in a sex-specific process beginning during puberty. The binding of estrogen to ERβ increases the plasticity of PV+ interneurons in females, making them more responsive to environmental stimuli in adulthood. This change in plasticity does not necessarily result in an increased vulnerability to stress in females, but rather an increased adaptability in the face of positive or negative environments. Environmental enrichment has been shown to have positive effects on PV+ interneurons, ameliorating the cellular behavioral effects of early life stress in males (Sun et al., 2016; Hendershott et al., 2016). Studies specifically assessing outcomes on PV+ interneurons following environmental enrichment in adulthood, and the role of estrogens in mediating this response, have not been performed. More work assessing the role of estrogen binding ERβ on PV+ interneurons during puberty and the effects of estrogen on regulating synaptic plasticity specifically in PV+ interneurons must be conducted in order to determine if the sex-specific effects of stress in adulthood are mediated through these processes.
6. Conclusions
Clinical studies show that men are more likely to be diagnosed with neurodevelopmental disorders and women are more likely to be diagnosed with affective disorders (Altemus 2006; Hanamsagar and Bilbo, 2016; Altemus et al., 2019). The mechanism behind these sex differences, however, remains unclear. Researchers utilize preclinical models of stress and vulnerability to neuropsychiatric diseases to identify potential causes of this dimorphism. As summarized in Figure 1, this review highlighted the hypothesis that the differential effects of stress on PV+ interneurons throughout the lifespan could explain some of the sex differences we see in clinical populations of neuropsychiatric and neurodevelopmental disorders, with stress during the prepubertal period having a greater impact on PV+ expression in males, and females being predominantly impacted by stress following puberty.
Aside from the future directions described throughout this review, a vastly ignored topic is that of the impacts of stress on PV+ interneurons during aging. PV+ interneurons have been implicated in the pathophysiologies of many major neuropsychiatric diseases that persist or even emerge in elderly (e.g., MDD), and evidence exists that PV+ interneurons are also affected by neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease (Satoh et al., 1991; Soós et al., 2004; Reiner et al., 2013). Additionally, the aged population is subject to unique stressors, particularly social isolation, which may be risk factors for these age-related diseases. Changes in ovarian hormones at puberty have been associated with changes in inhibitory neurotransmission (Piekarski et al., 2017), however potential interactions between PV+ interneurons and the ovarian hormone fluctuations associated with menopause are less characterized. To our knowledge no existing studies assess the effects of menopause on PV+ interneurons, despite this periods’ association with increased risk for schizophrenia and MDD in women, for stress is a known risk factor (Bale & Epperson, 2015).Studies must be conducted to keep up with the growing population of individuals who are reaching reproductive senescence and undergoing unique social, environmental, and personal stressors.
Highlights.
Stress is a risk factor for neurodevelopmental and neuropsychiatric disease.
Parvalbumin (PV) interneurons respond to stress in an age- and sex-specific manner.
Preclinical studies find that males show decreased PV+ cells after prenatal stress.
Females show more significant changes in PV+ cells after neonatal and adult stress.
These findings may correspond to sex differences seen in clinical populations.
Acknowledgements
Funding support was provided by the National Institutes of Health (National Institute on Mental Health) grant R21MH119090 (LC).
Appendix 1
- ADHD
attention deficit hyperactivity disorder
- ASD
autism spectrum disorder
- BDNF
brain-derived neurotrophic factor
- BLA
basolateral amygdala
- CA
cornu ammonis
- CIH
chronic intermittent hypoxia
- dAC
dorsal anterior cingulate cortex
- DG
dentate gryus
- ELS
early life stress
- ER-β
estrogen receptor beta
- E:I
excitatory:inhibitory
- GAD
glutamic acid decarboxylase
- GD
gestational day
- GR
glucocorticoid receptor
- HPA
hypothalamic-pituitary-adrenal
- IL
infralimbic
- MDD
major depressive disorder
- PV
parvalbumin
- PNN
perineuronal net
- Poly I:C
polyinosinic:polycytidyic acid
- P
postnatal day
- PTSD
posttraumatic stress disorder
- PFC
prefrontal cortex
- PrL
prelimbic
- PPI
prepulse inhibition
- TrkB
tropomyosin-related kinase B
Appendix 2
Shang et al., 2020
Wang et al., 2018
Heslin & Coutellier, 2017
Page & Coutellier, 2018
Monte et al., 2019
Filipovic et al., 24
Shepard & Coutellier, 2017
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: none.
Figures created in BioRender.
References
- Allen L, & Dwivedi Y (2020). MicroRNA mediators of early life stress vulnerability to depression and suicidal behavior. Molecular psychiatry, 25(2), 308–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altemus M (2006). Sex differences in depression and anxiety disorders: potential biological determinants. Hormones and behavior, 50(4), 534–538. [DOI] [PubMed] [Google Scholar]
- Altemus M, Sarvaiya N, & Epperson CN (2014). Sex differences in anxiety and depression clinical perspectives. Frontiers in neuroendocrinology, 35(3), 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiades PG, & Carter AG (2021). Circuit organization of the rodent medial prefrontal cortex. Trends in Neurosciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, & Teicher MH (2000). Sex differences in dopamine receptors and their relevance to ADHD. Neuroscience & Biobehavioral Reviews, 24(1), 137–141. [DOI] [PubMed] [Google Scholar]
- Baker KD, Gray AR, & Richardson R (2017). The development of perineuronal nets around parvalbumin gabaergic neurons in the medial prefrontal cortex and basolateral amygdala of rats. Behavioral neuroscience, 131(4), 289. [DOI] [PubMed] [Google Scholar]
- Bale TL (2011). Sex differences in prenatal epigenetic programing of stress pathways. Stress, 14(4), 348–356. [DOI] [PubMed] [Google Scholar]
- Bale TL (2015). Epigenetic and transgenerational reprogramming of brain development. Nature Reviews Neuroscience, 16(6), 332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale Tracy L., and Epperson C. Neill. “Sex differences and stress across the lifespan.” Nature neuroscience 18.10 (2015): 1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bálint S, Czobor P, Komlósi S, Meszaros A, Simon V, & Bitter I (2009). Attention deficit hyperactivity disorder (ADHD): gender-and age-related differences in neurocognition. Psychological medicine, 39(8), 1337–1345. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, … & Valentino RJ (2010). Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Molecular psychiatry, 15(9), 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranyi J, Bakos N, & Haller J (2005). Social instability in female rats: the relationship between stress-related and anxiety-like consequences. Physiology & behavior, 84(4), 511–518. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B, & Knickmeyer R (2011). Why are autism spectrum conditions more prevalent in males?. PLoS biology, 9(6), e1001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer TA, Falkai P, & Maier W (1999). Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the” two hit hypothesis.”. Journal of psychiatric research. [DOI] [PubMed] [Google Scholar]
- Begemann MJ, Dekker CF, van Lunenburg M, & Sommer IE (2012). Estrogen augmentation in schizophrenia: a quantitative review of current evidence. Schizophrenia research, 141(2–3), 179–184. [DOI] [PubMed] [Google Scholar]
- Belleau EL, Treadway MT, & Pizzagalli DA (2019). The impact of stress and major depressive disorder on hippocampal and medial prefrontal cortex morphology. Biological psychiatry, 85(6), 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjet C, Borges G, & Medina-Mora ME (2010). Chronic childhood adversity and onset of psychopathology during three life stages: childhood, adolescence and adulthood. Journal of psychiatric research, 44(11), 732–740. [DOI] [PubMed] [Google Scholar]
- Berger JM, Rohn TT, Oxford JT (2013). Autism as the early closure of a neuroplastic critical period normally seen in adolescence. Biological systems, open access. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström A, Jayatissa MN, Mørk A, & Wiborg O (2008). Stress sensitivity and resilience in the chronic mild stress rat model of depression; an in situ hybridization study. Brain research, 1196, 41–52. [DOI] [PubMed] [Google Scholar]
- Bicks LK, Yamamuro K, Flanigan ME, Kim JM, Kato D, Lucas EK, … & Morishita H (2020). Prefrontal parvalbumin interneurons require juvenile social experience to establish adult social behavior. Nature communications, 11(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ST, Schmid MJ, Der-Avakian A, Watkins LR, Spencer RL, & Maier SF (2005). Expression of c-fos and BDNF mRNA in subregions of the prefrontal cortex of male and female rats after acute uncontrollable stress. Brain research, 1051(1–2), 90–99. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M, & Tuszynski MH (2002). Estrogen receptor-beta colocalizes extensively with parvalbumin-labeled inhibitory neurons in the cortex, amygdala, basal forebrain, and hippocampal formation of intact and ovariectomized adult rats. Journal of Comparative Neurology, 452(3), 276–287. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, & Morilak DA (2008). Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology, 33(2), 320–331. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, & Andersen SL (2011). Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neuroscience & Biobehavioral Reviews, 35(8), 1687–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Ramikie TS, Schmidt MJ, Báldi R, Garbett K, Everheart MG, … & Mirnics K (2015). Inhibition of parvalbumin-expressing interneurons results in complex behavioral changes. Molecular psychiatry, 20(12), 1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelte S, Witte V, & Teuchert-Noodt G (2007). Postnatal development of GABA and calbindin cells and fibers in the prefrontal cortex and basolateral amygdala of gerbils (Meriones unguiculatus). International journal of developmental neuroscience, 25(3), 191–200. [DOI] [PubMed] [Google Scholar]
- Brydges NM, Moon A, Rule L, Watkin H, Thomas KL, & Hall J (2018). Sex specific effects of pre-pubertal stress on hippocampal neurogenesis and behaviour. Translational psychiatry, 8(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, & Draguhn A (2004). Neuronal oscillations in cortical networks. Science, 304(5679), 1926–1929. [DOI] [PubMed] [Google Scholar]
- Burrows EL, McOmish CE, & Hannan AJ (2011). Gene–environment interactions and construct validity in preclinical models of psychiatric disorders. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35(6), 1376–1382. [DOI] [PubMed] [Google Scholar]
- Caballero A, Flores-Barrera E, Cass DK, & Tseng KY (2014). Differential regulation of parvalbumin and calretinin interneurons in the prefrontal cortex during adolescence. Brain Structure and Function, 219(1), 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Granberg R, & Tseng KY (2016). Mechanisms contributing to prefrontal cortex maturation during adolescence. Neuroscience & Biobehavioral Reviews, 70, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Flores-Barrera E, Thomases DR, & Tseng KY (2020). Downregulation of parvalbumin expression in the prefrontal cortex during adolescence causes enduring prefrontal disinhibition in adulthood. Neuropsychopharmacology, 45(9), 1527–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Orozco A, & Tseng KY (2021, March). Developmental regulation of excitatory-inhibitory synaptic balance in the prefrontal cortex during adolescence. In Seminars in Cell & Developmental Biology. Academic Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabungcal JH, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK, & Do KQ (2013). Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proceedings of the National Academy of Sciences, 110(22), 9130–9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Giordano J, Crupi R, Di Paola R, Ruggieri M, Bianchini R, … & Calabrese EJ (2017). Hormesis, cellular stress response and neuroinflammation in schizophrenia: early onset versus late onset state. Journal of neuroscience research, 95(5), 1182–1193. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, … & Poulton R (2003). Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science, 301(5631), 386–389. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ (2004). Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. Journal of neuroscience 24(3), 9598–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen DM, & Berke ET (2020). Gender-and sex-based contributors to sex differences in PTSD. Current psychiatry reports, 22(4), 1–9. [DOI] [PubMed] [Google Scholar]
- Coghlan S, Horder J, Inkster B, Mendez MA, Murphy DG, & Nutt DJ (2012). GABA system dysfunction in autism and related disorders: from synapse to symptoms. Neuroscience & Biobehavioral Reviews, 36(9), 2044–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr M, Calon F, Morissette M, & Di Paolo T (2002). Estrogenic modulation of brain activity: implications for schizophrenia and Parkinson’s disease. Journal of psychiatry & neuroscience. [PMC free article] [PubMed] [Google Scholar]
- Czéh B, Vardya I, Varga Z, Febbraro F, Csabai D, Martis LS, … & Wiborg O, (2018). Long-term stress disrupts the structural and functional integrity of GABAergic neuronal networks in the medial prefrontal cortex of rats. Frontiers in cellular neuroscience, 12, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnaudery M, & Maccari S (2008). Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain research reviews, 57(2), 571–585. [DOI] [PubMed] [Google Scholar]
- Davidson LM, & Baum A (1986). Chronic stress and posttraumatic stress disorders. Journal of consulting and clinical psychology, 54(3), 303. [DOI] [PubMed] [Google Scholar]
- Davis MT, Holmes SE, Pietrzak RH, & Esterlis I (2017). Neurobiology of chronic stress-related psychiatric disorders: evidence from molecular imaging studies. Chronic Stress, 1, 2470547017710916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhossche D, Applegate H, Abraham A, Maertens P, Bland L, Bencsath A, & Martinez J (2002). Elevated plasma gamma-aminobutyric acid (GABA) levels in autistic youngsters: stimulus for a GABA hypothesis of autism. Medical Science Monitor, 8(8), PR1–PR6. [PubMed] [Google Scholar]
- Dienel SJ, & Lewis DA (2019). Alterations in cortical interneurons and cognitive function in schizophrenia. Neurobiology of disease, 131, 104208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diorio D, Viau V, & Meaney MJ (1993). The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. Journal of Neuroscience, 13(9), 3839–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzewiecki CM, Willing J, & Juraska JM (2020). Influences of age and pubertal status on number and intensity of perineuronal nets in the rat medial prefrontal cortex. Brain Structure and Function, 225(8), 2495–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Serena K, Hwang WJ, Grech AM, Wu YWC, Schroeder A, & Hill RA (2018). Prefrontal cortical parvalbumin and somatostatin expression and cell density increase during adolescence and are modified by BDNF and sex. Molecular and Cellular Neuroscience, 88, 177–188. [DOI] [PubMed] [Google Scholar]
- Duman RS, Sanacora G, & Krystal JH (2019). Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron, 102(1), 75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich DE, Ryan SJ, Hazra R, Guo JD, & Rainnie DG (2013). Postnatal maturation of GABAergic transmission in the rat basolateral amygdala. Journal of neurophysiology, 110(4), 926–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, & Romeo RD (2013). Stress and the developing adolescent brain. Neuroscience, 249, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellicott A, Hammen C, Gitlin M, Brown G, & Jamison K (2019). Life events and the course of bipolar disorder. In The Science of Mental Health (pp. 78–82). Routledge. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, & Thuras PD (2009). GABA A receptor downregulation in brains of subjects with autism. Journal of autism and developmental disorders, 39(2), 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee C, Banasr M, & Sibille E (2017). Somatostatin-positive gamma-aminobutyric acid interneuron deficits in depression: cortical microcircuit and therapeutic perspectives. Biological psychiatry, 82(8), 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferando I, & Mody I (2015). In vitro gamma oscillations following partial and complete ablation of δ subunit-containing GABAA receptors from parvalbumin interneurons. Neuropharmacology, 88, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri SL, Abel T, & Brodkin ES (2018). Sex differences in autism spectrum disorder: a review. Current psychiatry reports, 20(2), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filice F, Lauber E, Vörckel KJ, Wöhr M, & Schwaller B (2018). 17-β estradiol increases parvalbumin levels in Pvalb heterozygous mice and attenuates behavioral phenotypes with relevance to autism core symptoms. Molecular autism, 9(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipović D, Zlatković J, Gass P, & Inta D (2013). The differential effects of acute vs. chronic stress and their combination on hippocampal parvalbumin and inducible heat shock protein 70 expression. Neuroscience, 236, 47–54. [DOI] [PubMed] [Google Scholar]
- Fine R, Zhang J, & Stevens HE (2014). Prenatal stress and inhibitory neuron systems: implications for neuropsychiatric disorders. Molecular psychiatry, 19(6), 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish KN, Hoftman GD, Sheikh W, Kitchens M, Lewis DA (2013). Parvalbumin-containing chandelier and basket cell boutons have distinct modes of maturation in monkey prefrontal cortex. Journal of Neuroscience, 33(19), 8352–8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogaça MV, & Duman RS (2019). Cortical GABAergic dysfunction in stress and depression: new insights for therapeutic interventions. Frontiers in cellular neuroscience, 13, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogaça MV, Wu M, Li C, Li XY, Picciotto MR, & Duman RS (2020). Inhibition of GABA interneurons in the mPFC is sufficient and necessary for rapid antidepressant responses. Molecular Psychiatry, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froger N, Palazzo E, Boni C, Hanoun N, Saurini F, Joubert C, … & Lanfumey L (2004). Neurochemical and behavioral alterations in glucocorticoid receptor-impaired transgenic mice after chronic mild stress. Journal of Neuroscience, 24(11), 2787–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folha OADAC, Bahia CP, de Aguiar GPS, Herculano AM, Coelho NLG, de Sousa MBC, … & Pereira A (2017). Effect of chronic stress during adolescence in prefrontal cortex structure and function. Behavioural brain research, 326, 44–51. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Fillman SG, Webster MJ, & Weickert CS (2014). Schizophrenia and bipolar disorder show both common and distinct changes in cortical interneuron markers. Schizophrenia research, 155(1–3), 26–30. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, & Weickert CS (2010). Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. American Journal of Psychiatry, 167(12), 1479–1488. [DOI] [PubMed] [Google Scholar]
- Gaetz W, Edgar JC, Wang DJ, & Roberts TP (2011). Relating MEG measured motor cortical oscillations to resting γ-aminobutyric acid (GABA) concentration. Neuroimage, 55(2), 616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt JC, & van Kammen DP (1983). The interaction between GABA and dopamine: implications for schizophrenia. Schizophrenia bulletin, 9(3), 336–353. [DOI] [PubMed] [Google Scholar]
- George ED, Bordner KA, Elwafi HM, & Simen AA (2010). Maternal separation with early weaning: a novel mouse model of early life neglect. BMC neuroscience, 11(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildawie KR, Honeycutt JA, & Brenhouse HC (2020). Region-specific effects of maternal separation on perineuronal net and parvalbumin-expressing interneuron formation in male and female rats. Neuroscience, 428, 23–37. [DOI] [PubMed] [Google Scholar]
- Gildawie KR, Ryll LM, Hexter JC, Peterzell S, Valentine AA, & Brenhouse HC (2021). A two-hit adversity model in developing rats reveals sex-specific impacts on prefrontal cortex structure and behavior. Developmental Cognitive Neuroscience, 48, 100924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanoli S, Weber L, & Meyer U (2014). Single and combined effects of prenatal immune activation and peripubertal stress on parvalbumin and reelin expression in the hippocampal formation. Brain, behavior, and immunity, 40, 48–54. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Lüthi A, & Herry C (2009). Perineuronal nets protect fear memories from erasure. science, 325(5945), 1258–1261. [DOI] [PubMed] [Google Scholar]
- Gomes FV, Zhu X, & Grace AA (2019). Stress during critical periods of development and risk for schizophrenia. Schizophrenia research, 213, 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Hashimoto T, & Lewis DA (2010). Alterations of cortical GABA neurons and network oscillations in schizophrenia. Current psychiatry reports, 12(4), 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwill HL, Manzano-Nieves G, LaChance P, Teramoto S, Lin S, Lopez C, … & Bath KG (2018). Early life stress drives sex-selective impairment in reversal learning by affecting parvalbumin interneurons in orbitofrontal cortex of mice. Cell reports, 25(9), 2299–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Honeycutt JA, Holland FH, Ganguly P, & Brenhouse HC (2016). Cognitive impairment effects of early life stress in adolescents can be predicted with early biomarkers: Impacts of sex, experience, and cytokines. Psychoneuroendocrinology, 71, 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grech AM, Du X, Murray SS, Xiao J, & Hill RA (2019). Sex-specific spatial memory deficits in mice with a conditional TrkB deletion on parvalbumin interneurons. Behavioural brain research, 372, 111984. [DOI] [PubMed] [Google Scholar]
- Grossman LS, Harrow M, Rosen C, Faull R, & Strauss GP (2008). Sex differences in schizophrenia and other psychotic disorders: a 20-year longitudinal study of psychosis and recovery. Comprehensive psychiatry, 49(6), 523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno A, Verlezza S, Long H, Wong TP, & Walker CD (2020). It is all in the right amygdala: increased synaptic plasticity and perineuronal nets in male, but not female, juvenile rat pups after exposure to early-life stress. Journal of Neuroscience, 40(43), 8276–8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, … & Sibille E (2012). Molecular evidence for BDNF-and GABA-related dysfunctions in the amygdala of female subjects with major depression. Molecular psychiatry, 17(11), 1130–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häfner H, Riecher-Rössler A, Der Heiden WA, Maurer K, Fätkenheuer B, & Löffler W (1993). Generating and testing a causal explanation of the gender difference in age at first onset of schizophrenia. Psychological medicine, 23(4), 925–940. [DOI] [PubMed] [Google Scholar]
- Hale MW, Johnson PL, Westerman AM, Abrams JK, Shekhar A, & Lowry CA (2010). Multiple anxiogenic drugs recruit a parvalbumin-containing subpopulation of GABAergic interneurons in the basolateral amygdala. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 34(7), 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamsagar R, & Bilbo SD (2016). Sex differences in neurodevelopmental and neurodegenerative disorders: focus on microglial function and neuroinflammation during development. The Journal of steroid biochemistry and molecular biology, 160, 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL (2009). Development of sex differences in depressive and co-occurring anxious symptoms during adolescence: Descriptive trajectories and potential explanations in a multiwave prospective study. Journal of Clinical Child & Adolescent Psychology, 38(4), 460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, & Holmes A (2006). Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends in cognitive sciences, 10(4), 182–191. [DOI] [PubMed] [Google Scholar]
- Harte MK, Powell SB, Swerdlow NR, Geyer MA, & Reynolds GP (2007). Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. Journal of neural transmission, 114(7), 893–898. [DOI] [PubMed] [Google Scholar]
- Heim C, & Nemeroff CB (2002, April). Neurobiology of early life stress: clinical studies. In Seminars in clinical neuropsychiatry (Vol. 7, No. 2, pp. 147–159). [DOI] [PubMed] [Google Scholar]
- Hendershott TR, Cronin ME, Langella S, McGuinness PS, & Basu AC (2016). Effects of environmental enrichment on anxiety-like behavior, sociability, sensory gating, and spatial learning in male and female C57BL/6J mice. Behavioural brain research, 314, 215–225. [DOI] [PubMed] [Google Scholar]
- Hensch TK, & Fagiolini M (2005). Excitatory–inhibitory balance and critical period plasticity in developing visual cortex. Progress in brain research, 147, 115–124. [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK, & Figueiredo H (2004). Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Annals of the New York Academy of Sciences, 1018(1), 35–45. [DOI] [PubMed] [Google Scholar]
- Heslin K, & Coutellier L (2018). Npas4 deficiency and prenatal stress interact to affect social recognition in mice. Genes, Brain and Behavior, 17(5), e12448. [DOI] [PubMed] [Google Scholar]
- Hill RA, Von Soly SK, Ratnayake U, Klug M, Binder MD, Hannan AJ, & van den Buuse M (2014). Long-term effects of combined neonatal and adolescent stress on brain-derived neurotrophic factor and dopamine receptor expression in the rat forebrain. Biochimica Et Biophysica Acta (BBA)-Molecular Basis of Disease, 1842(11), 2126–2135. [DOI] [PubMed] [Google Scholar]
- Hill RA, Grech AM, Notaras MJ, Sepulveda M, & van den Buuse M (2020). Brain-Derived Neurotrophic Factor Val66Met polymorphism interacts with adolescent stress to alter hippocampal interneuron density and dendritic morphology in mice. Neurobiology of Stress, 13, 100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland FH, Ganguly P, Potter DN, Chartoff EH, & Brenhouse HC (2014). Early life stress disrupts social behavior and prefrontal cortex parvalbumin interneurons at an earlier time-point in females than in males. Neuroscience letters, 566, 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howerton CL, & Bale TL (2012). Prenatal programing: at the intersection of maternal stress and immune activation. Hormones and behavior, 62(3), 237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, McCutcheon R, Owen MJ, & Murray RM (2017). The role of genes, stress, and dopamine in the development of schizophrenia. Biological psychiatry, 81(1), 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Zhang M, Czéh B, Flügge G, & Zhang W (2010). Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology, 35(8), 1693–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, … & Tonegawa S (1999). BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell, 98(6), 739–755. [DOI] [PubMed] [Google Scholar]
- Jaric I, Rocks D, Cham H, Herchek A, & Kundakovic M (2019). Sex and estrous cycle effects on anxiety-and depression-related phenotypes in a two-hit developmental stress model. Frontiers in molecular neuroscience, 12, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Zhang Z, Zhang C, Wang X, Sakata K, Lu B, & Sun QQ (2011). A key mechanism underlying sensory experience-dependent maturation of neocortical GABAergic circuits in vivo. Proceedings of the National Academy of Sciences, 108(29), 12131–12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapczinski F, Vieta E, Andreazza AC, Frey BN, Gomes FA, Tramontina J, … & Post RM (2008). Allostatic load in bipolar disorder: implications for pathophysiology and treatment. Neuroscience & Biobehavioral Reviews, 32(4), 675–692. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, & Nelson CB (1995). Posttraumatic stress disorder in the National Comorbidity Survey. Archives of general psychiatry, 52(12), 1048–1060. [DOI] [PubMed] [Google Scholar]
- Khemka S, Barnes G, Dolan RJ, & Bach DR (2017). Dissecting the function of hippocampal oscillations in a human anxiety model. Journal of Neuroscience, 37(29), 6869–6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkovski M, Suo C, Enticott PG, Yücel M, & Fitzgerald PB (2018). sex-linked differences in gamma-aminobutyric acid (GABA) are related to social functioning in autism spectrum disorder. Psychiatry Research: Neuroimaging, 274, 19–22. [DOI] [PubMed] [Google Scholar]
- Koh DX, & Sng JC (2016). HDAC1 negatively regulates Bdnf and Pvalb required for parvalbumin interneuron maturation in an experience-dependent manner. Journal of neurochemistry, 139(3), 369–380. [DOI] [PubMed] [Google Scholar]
- Krishnan Vaishnav, Han Ming-Hu, Graham Danielle L., Berton Olivier, Renthal William, Russo Scott J., LaPlant Quincey et al. “Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions.” Cell 131, no. 2 (2007): 391–404. [DOI] [PubMed] [Google Scholar]
- Kumar S, Hultman R, Hughes D, Michel N, Katz BM, & Dzirasa K (2014). Prefrontal cortex reactivity underlies trait vulnerability to chronic social defeat stress. Nature communications, 5(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B, & Luna B (2018). Adolescence as a neurobiological critical period for the development of higher-order cognition. Neuroscience & Biobehavioral Reviews, 94, 179–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Magueresse C, & Monyer H (2013). GABAergic interneurons shape the functional maturation of the cortex. Neuron, 77(3), 388–405. [DOI] [PubMed] [Google Scholar]
- Lew SE, & Tseng KY (2014). Dopamine modulation of GABAergic function enables network stability and input selectivity for sustaining working memory in a computational model of the prefrontal cortex. Neuropsychopharmacology, 39(13), 3067–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Pierri JN, Volk DW, Melchitzky DS, & Woo TUW (1999). Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biological psychiatry, 46(5), 616–626. [DOI] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, … & Greenberg ME (2008). Activity-dependent regulation of inhibitory synapse development by Npas4. Nature, 455(7217), 1198–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindert J, von Ehrenstein OS, Grashow R, Gal G, Braehler E, & Weisskopf MG (2014). Sexual and physical abuse in childhood is associated with depression and anxiety over the life course: systematic review and meta-analysis. International journal of public health, 59(2), 359–372. [DOI] [PubMed] [Google Scholar]
- Lippard Elizabeth TC, and Nemeroff Charles B.. “The devastating clinical consequences of child abuse and neglect: increased disease vulnerability and poor treatment response in mood disorders.” American journal of psychiatry 177.1 (2020): 20–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Jiao Z, Yu Y, Zhang C, He X, Li Q, … & Wang H (2018). Programming for increased expression of hippocampal GAD67 mediated the hypersensitivity of the hypothalamic–pituitary–adrenal axis in male offspring rats with prenatal ethanol exposure. Cell death & disease, 9(6), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Meda S, Norman KJ, & Andersen SL (2018). Anhedonic behavior and γ-amino butyric acid during a sensitive period in female rats exposed to early adversity. Journal of psychiatric research, 100, 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundström S, Mårland C, Kuja-Halkola R, Anckarsäter H, Lichtenstein P, Gillberg C, & Nilsson T (2019). Assessing autism in females: The importance of a sex-specific comparison. Psychiatry research, 282, 112566. [DOI] [PubMed] [Google Scholar]
- Luo ZY, Huang L, Lin S, Yin YN, Jie W, Hu NY, … & Gao TM (2020). Erbin in amygdala parvalbumin-positive neurons modulates anxiety-like behaviors. Biological psychiatry, 87(10), 926–936. [DOI] [PubMed] [Google Scholar]
- Luscher B, & Fuchs T (2015). GABAergic control of depression-related brain states. In Advances in pharmacology (Vol. 73, pp. 97–144). Academic Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier SJ, & Stevens HE (2016). Delays in GABAergic interneuron development and behavioral inhibition after prenatal stress. Developmental neurobiology, 76(10), 1078–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mably AJ, & Colgin LL (2018). Gamma oscillations in cognitive disorders. Current opinion in neurobiology, 52, 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzari N, Matvienko-Sikar K, Baldoni F, O’Keeffe GW, & Khashan AS (2019). Prenatal maternal stress and risk of neurodevelopmental disorders in the offspring: a systematic review and meta-analysis. Social psychiatry and psychiatric epidemiology, 54(11), 1299–1309. [DOI] [PubMed] [Google Scholar]
- Marín O (2012). Interneuron dysfunction in psychiatric disorders. Nature Reviews Neuroscience, 13(2), 107–120. [DOI] [PubMed] [Google Scholar]
- Marmigère F, Givalois L, Rage F, Arancibia S, & Tapia-Arancibia L (2003). Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus, 13(5), 646–655. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, & Wright CL (2017). Convergence of sex differences and the neuroimmune system in autism spectrum disorder. Biological psychiatry, 81(5), 402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2001). Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Annals of the New York Academy of Sciences, 933(1), 265–277. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2004). Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Annals of the New York Academy of Sciences, 1032(1), 1–7. [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Morrison JH (2013). The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron, 79(1), 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moench Kelly M., Breach Michaela R., and Wellman Cara L.. “Prior stress followed by a novel stress challenge results in sex-specific deficits in behavioral flexibility and changes in gene expression in rat medial prefrontal cortex.” Hormones and behavior 117 (2020): 104615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhler H (2012). The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology, 62(1), 42–53. [DOI] [PubMed] [Google Scholar]
- Monte AS, Mello BSF, Borella VCM, da Silva Araujo T, da Silva FER, de Sousa FCF, … & Macêdo D (2017). Two-hit model of schizophrenia induced by neonatal immune activation and peripubertal stress in rats: Study of sex differences and brain oxidative alterations. Behavioural brain research, 331, 30–37. [DOI] [PubMed] [Google Scholar]
- Monte AS, da Silva FER, Lima CNDC, Vasconcelos GS, Gomes NS, Miyajima F, … & Macedo DS (2020). Sex influences in the preventive effects of N-acetylcysteine in a two-hit animal model of schizophrenia. Journal of Psychopharmacology, 34(1), 125–136. [DOI] [PubMed] [Google Scholar]
- Morris HM, Hashimoto T, & Lewis DA (2008). Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cerebral Cortex, 18(7), 1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller BR, & Bale TL (2008). Sex-specific programming of offspring emotionality after stress early in pregnancy. Journal of Neuroscience, 28(36), 9055–9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musazzi L, Tornese P, Sala N, & Popoli M (2018). What acute stress protocols can tell us about PTSD and stress-related neuropsychiatric disorders. Frontiers in pharmacology, 9, 758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawreen N, Cotella EM, Morano R, Mahbod P, Dalal KS, Fitzgerald M, … & Herman JP (2020). Chemogenetic Inhibition of Infralimbic Prefrontal Cortex GABAergic Parvalbumin Interneurons Attenuates the Impact of Chronic Stress in Male Mice. Eneuro, 7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, & Manji H (2002). Preclinical models: status of basic research in depression. Biological psychiatry, 52(6), 503–528. [DOI] [PubMed] [Google Scholar]
- Nieves GM, Bravo M, Baskoylu S, & Bath KG (2020). Early life adversity decreases pre-adolescent fear expression by accelerating amygdala PV cell development. Elife, 9, e55263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossewaarde L, Hermans EJ, van Wingen GA, Kooijman SC, Johansson IM, Bäckström T, & Fernández G (2010). Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology, 35(1), 47–55. [DOI] [PubMed] [Google Scholar]
- Ottenweller JE, Natelson BH, Pitman DL, & Drastal SD (1989). Adrenocortical and behavioral responses to repeated stressors: toward an animal model of chronic stress and stress-related mental illness. Biological psychiatry, 26(8), 829–841. [DOI] [PubMed] [Google Scholar]
- Packer AM, & Yuste R (2011). Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition?. Journal of Neuroscience, 31(37), 13260–13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page CE, & Coutellier L (2019). Prefrontal excitatory/inhibitory balance in stress and emotional disorders: Evidence for over-inhibition. Neuroscience & Biobehavioral Reviews, 105, 39–51. [DOI] [PubMed] [Google Scholar]
- Page CE, Shepard R, Heslin K, & Coutellier L (2019). Prefrontal parvalbumin cells are sensitive to stress and mediate anxiety-related behaviors in female mice. Scientific reports, 9(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls DL (1991). Genetic factors in the expression of attention-deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology, 1(5), 353–360. [DOI] [PubMed] [Google Scholar]
- Perez SM, Boley A, & Lodge DJ (2019). Region specific knockdown of Parvalbumin or Somatostatin produces neuronal and behavioral deficits consistent with those observed in schizophrenia. Translational psychiatry, 9(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perova Z, Delevich K, & Li B (2015). Depression of excitatory synapses onto parvalbumin interneurons in the medial prefrontal cortex in susceptibility to stress. Journal of Neuroscience, 35(7), 3201–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty F, Trivedi MH, Fulton M, & Rush AJ (1995). Benzodiazepines as antidepressants: does GABA play a role in depression?. Biological psychiatry, 38(9), 578–591. [DOI] [PubMed] [Google Scholar]
- Piekarski DJ, Boivin JR, & Wilbrecht L (2017). Ovarian hormones organize the maturation of inhibitory neurotransmission in the frontal cortex at puberty onset in female mice. Current Biology, 27(12), 1735–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM, & Meaney MJ (1993). Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Molecular brain research, 18(3), 195–200. [DOI] [PubMed] [Google Scholar]
- Powell SB, Sejnowski TJ, & Behrens MM (2012). Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin-interneuron maturation in rodent models of schizophrenia. Neuropharmacology, 62(3), 1322–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley J, Morilak D, Viau V, & Campeau S (2015). Chronic stress and brain plasticity: mechanisms underlying adaptive and maladaptive changes and implications for stress-related CNS disorders. Neuroscience & Biobehavioral Reviews, 58, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray MT, Weickert CS, & Webster MJ (2014). Decreased BDNF and TrkB mRNA expression in multiple cortical areas of patients with schizophrenia and mood disorders. Translational psychiatry, 4(5), e389–e389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Shelby E, Wang H, DeMarch Z, Deng Y, Guley NH, … & Faull RL (2013). Striatal parvalbuminergic neurons are lost in Huntington’s disease: implications for dystonia. Movement Disorders, 28(12), 1691–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roceri M, Hendriks WJAJ, Racagni G, Ellenbroek BA, & Riva MA (2002). Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Molecular psychiatry, 7(6), 609–616. [DOI] [PubMed] [Google Scholar]
- Rodgers AB, & Bale TL (2015). Germ cell origins of posttraumatic stress disorder risk: the transgenerational impact of parental stress experience. Biological psychiatry, 78(5), 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Bronson SL, Revello S, & Bale TL (2013). Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. Journal of Neuroscience, 33(21), 9003–9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, Pennell CE, & Whitehouse AJ (2011). Prenatal maternal stress associated with ADHD and autistic traits in early childhood. Frontiers in psychology, 1, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow DR, & Schmidt PJ (2018). Is there a role for reproductive steroids in the etiology and treatment of affective disorders?. Dialogues in clinical neuroscience, 20(3), 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Glynn LM, & Davis EP (2013). Is there a viability–vulnerability tradeoff? Sex differences in fetal programming. Journal of psychosomatic research, 75(4), 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh J, Tabira T, Sano M, Nakayama H, & Tateishi J (1991). Parvalbumin-immunoreactive neurons in the human central nervous system are decreased in Alzheimer’s disease. Acta neuropathologica, 81(4), 388–395. [DOI] [PubMed] [Google Scholar]
- Schroeder A, Notaras M, Hill R (2016) Sex-Steroid Hormones & Neuropsychiatric Disorders: Pathophysiology, Symptomology & Treatment. In Costa A & Villalba E(Eds.), Horizons in Neuroscience Research (pp. 157–202). New York: Nova Biomedical Publishing. [Google Scholar]
- Schroeder A, Notaras M, Du X, Hill R (2018) On the developmental timing of stress: delineating sex-specific effects of stress across development on adult behavior. Brain sciences 8(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H, Phillips TJ, Sze Y, Alfieri A, Rogers MF, Volpato V, … & Brunton PJ (2020). Maternal antioxidant treatment prevents the adverse effects of prenatal stress on the offspring’s brain and behavior. Neurobiology of stress, 13, 100281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searles RV, Yoo MJ, He JR, Shen WB, & Selmanoff M (2000). Sex differences in GABA turnover and glutamic acid decarboxylase (GAD65 and GAD67) mRNA in the rat hypothalamus. Brain research, 878(1–2), 11–19. [DOI] [PubMed] [Google Scholar]
- Shang Y, Chen R, Li F, Zhang H, Wang H, & Zhang T (2021). Prenatal stress impairs memory function in the early development of male-offspring associated with the gaba function. Physiology & Behavior, 228, 113184. [DOI] [PubMed] [Google Scholar]
- Shepard R, Page CE, & Coutellier L (2016). Sensitivity of the prefrontal GABAergic system to chronic stress in male and female mice: relevance for sex differences in stress-related disorders. Neuroscience, 332, 1–12. [DOI] [PubMed] [Google Scholar]
- Shepard R, & Coutellier L (2018). Changes in the prefrontal glutamatergic and parvalbumin systems of mice exposed to unpredictable chronic stress. Molecular neurobiology, 55(3), 2591–2602. [DOI] [PubMed] [Google Scholar]
- Skilbeck KJ, Hinton T, & Johnston GAR (2008). Sex-differences and stress: effects on regional high and low affinity [3H] GABA binding. Neurochemistry international, 52(6), 1212–1219. [DOI] [PubMed] [Google Scholar]
- Slavich GM, & Sacher J (2019). Stress, sex hormones, inflammation, and major depressive disorder: Extending Social Signal Transduction Theory of Depression to account for sex differences in mood disorders. Psychopharmacology, 236(10), 3063–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW (2016). The genetics of stress-related disorders: PTSD, depression, and anxiety disorders. Neuropsychopharmacology, 41(1), 297–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Smithuis J, Stamatakis AM, Jennings JH, Kantak PA, Ung RL, & Stuber GD (2014). Inhibition of projections from the basolateral amygdala to the entorhinal cortex disrupts the acquisition of contextual fear. Frontiers in behavioral neuroscience, 8, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel I, Mardinly AR, Gabel HW, Bazinet JE, Couch CH, Tzeng CP, … & Greenberg ME (2014). Npas4 regulates excitatory-inhibitory balance within neural circuits through cell-type-specific gene programs. Cell, 157(5), 1216–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares AR, Gildawie KR, Honeycutt JA, & Brenhouse HC (2020). Region-specific effects of maternal separation on oxidative stress accumulation in parvalbumin neurons of male and female rats. Behavioural brain research, 388, 112658. [DOI] [PubMed] [Google Scholar]
- Solum DT, & Handa RJ (2002). Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. Journal of Neuroscience, 22(7), 2650–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soós J, Engelhardt JI, Siklós L, Havas L, & Majtényi K (2004). The expression of PARP, NF-κB and parvalbumin is increased in Parkinson disease. Neuroreport, 15(11), 1715–1718. [DOI] [PubMed] [Google Scholar]
- Steullet P, Cabungcal JH, Kulak A, Kraftsik R, Chen Y, Dalton TP, … & Do KQ (2010). Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. Journal of Neuroscience, 30(7), 2547–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XR, Zhang H, Zhao HT, Ji MH, Li HH, Wu J, … & Yang JJ (2016). Amelioration of oxidative stress-induced phenotype loss of parvalbumin interneurons might contribute to the beneficial effects of environmental enrichment in a rat model of post-traumatic stress disorder. Behavioural Brain Research, 312, 84–92. [DOI] [PubMed] [Google Scholar]
- Takesian AE, & Hensch TK (2013). Balancing plasticity/stability across brain development. Progress in brain research, 207, 3–34. [DOI] [PubMed] [Google Scholar]
- Taliaz D, Stall N, Dar DE, & Zangen A (2010). Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Molecular psychiatry, 15(1), 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA (1997). Gender and schizophrenia. Journal of Clinical Psychiatry, 58(15), 33–37. [PubMed] [Google Scholar]
- Testa D, Prochiantz A, & Di Nardo AA (2019, May). Perineuronal nets in brain physiology and disease. In Seminars in cell & developmental biology (Vol. 89, pp. 125–135). Academic Press. [DOI] [PubMed] [Google Scholar]
- Tremblay R, Lee S, & Rudy B (2016). GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron, 91(2), 260–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoizumi T, Miyamoto H, Yazaki-Sugiyama Y, Atapour N, Hensch TK, & Miller KD (2013). A theory of the transition to critical period plasticity: inhibition selectively suppresses spontaneous activity. Neuron, 80(1), 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T, Furukawa T, Iwata S, Yanagawa Y, & Fukuda A (2014). Selective loss of parvalbumin-positive GABAergic interneurons in the cerebral cortex of maternally stressed Gad1-heterozygous mouse offspring. Translational psychiatry, 4(3), e371–e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H, Suemitsu S, Murakami S, Kitamura N, Wani K, Okamoto M, … & Ishihara T (2017). Region-specific impairments in parvalbumin interneurons in social isolation-reared mice. Neuroscience, 359, 196–208. [DOI] [PubMed] [Google Scholar]
- Ueno H, Suemitsu S, Murakami S, Kitamura N, Wani K, Matsumoto Y, … & Ishihara T (2018). Juvenile stress induces behavioral change and affects perineuronal net formation in juvenile mice. BMC neuroscience, 19(1), 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, & McGuffin P (2008). The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Molecular psychiatry, 13(2), 131–146. [DOI] [PubMed] [Google Scholar]
- Van Os J, & Selten JP (1998). Prenatal exposure to maternal stress and subsequent schizophrenia: the May 1940 invasion of the Netherlands. The british journal of psychiatry, 172(4), 324–326. [DOI] [PubMed] [Google Scholar]
- Walker CD, Bath KG, Joels M, Korosi A, Larauche M, Lucassen PJ, … & Baram TZ (2017). Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress, 20(5), 421–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way BM, & Taylor SE (2010). The serotonin transporter promoter polymorphism is associated with cortisol response to psychosocial stress. Biological psychiatry, 67(5), 487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, … & Yeh EK (1996). Cross-national epidemiology of major depression and bipolar disorder. Jama, 276(4), 293–299. [PubMed] [Google Scholar]
- Wen TH, Binder DK, Ethell IM, & Razak KA (2018). The perineuronal ‘safety’net? Perineuronal net abnormalities in neurological disorders. Frontiers in molecular neuroscience, 11, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhr M, Orduz D, Gregory P, Moreno H, Khan U, Vörckel KJ, … & Schwaller B (2015). Lack of parvalbumin in mice leads to behavioral deficits relevant to all human autism core symptoms and related neural morphofunctional abnormalities. Translational psychiatry, 5(3), e525–e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, Walker HE, Valentino RJ, & Bhatnagar S (2010). Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology, 151(4), 1795–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YC, Du X, Van den Buuse M, & Hill RA (2014). Sex differences in the adolescent developmental trajectory of parvalbumin interneurons in the hippocampus: a role for estradiol. Psychoneuroendocrinology, 45, 167–178. [DOI] [PubMed] [Google Scholar]
- Wu YWC, Du X, Van Den Buuse M, & Hill RA (2015). Analyzing the influence of BDNF heterozygosity on spatial memory response to 17β-estradiol. Translational psychiatry, 5(1), e498–e498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Chen Y, Ma L, Shen Q, Huang L, Zhao B, … & Fu Z (2017). Major depressive disorder mediates accelerated aging in rats subjected to chronic mild stress. Behavioural brain research, 329, 96–103. [DOI] [PubMed] [Google Scholar]
- Yang J (2016). Nox-2-mediated phenotype loss of hippocampal parvalbumin interneurons might contribute to postoperative cognitive decline in aging mice. Frontiers in aging neuroscience, 8, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yang X, & Tang K (2021). Interneuron development and dysfunction. The FEBS Journal. [DOI] [PubMed] [Google Scholar]
- Yiannakas A, Chandran SK, Kayyal H, Gould N, Khamaisy M, & Rosenblum K (2021). Parvalbumin interneuron inhibition onto anterior insula neurons projecting to the basolateral amygdala drives aversive taste memory retrieval. Current Biology. [DOI] [PubMed] [Google Scholar]
- Yu Z, Chen N, Hu D, Chen W, Yuan Y, Meng S, … & Shi J (2020). Decreased density of perineuronal net in prelimbic cortex is linked to depressive-like behavior in young-aged rats. Frontiers in molecular neuroscience, 13, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Wang G, Ma K, Cui S, & Wang JH (2017). GABAergic neurons in nucleus accumbens are correlated to resilience and vulnerability to chronic stress for major depression. Oncotarget, 8(22), 35933. [DOI] [PMC free article] [PubMed] [Google Scholar]


