Abstract
Objectives
Single-agent heat shock protein 90 (HSP90) inhibition has demonstrated activity in oncogene-driven non-small cell and small cell lung cancers. SNX-5422 is an oral HSP90 inhibitor with increased activity in vitro with the addition of carboplatin and paclitaxel. Therefore, we conducted a phase 1, open-label, multicenter study to evaluate SNX-5422, carboplatin and paclitaxel followed by SNX-5422 maintenance in patients with advanced lung cancers.
Materials and methods
In part 1 (3+3 dose escalation), SNX-5422 (50/75/100-mg/m2) was dosed every other day (qod) for 21 days (28-day cycle) for ≤ 4 cycles; carboplatin (AUC 5)-paclitaxel (175 mg/m2) was administered once every 3 weeks for ≤ 6 courses. In part 2 (maintenance), subjects who achieved at least stable disease in part 1 received 100 mg/m2 SNX-5422 monotherapy qod for 21 days (28-day cycle).
Results
Twenty-three patients with advanced non-small cell lung cancer (NSCLC, n=20) and small cell lung cancer (SCLC, n=3) were enrolled. The median age was 60 years and 61% (n=14/23) had ≥1 prior treatment regimens. The maximum tolerated dose of SNX-5422 was 100 mg/m2 qod in combination with carboplatin-paclitaxel. The most common treatment-related grade 3/4 adverse events (part 1/part 2) were diarrhea (26%/15%) and nausea (9%/0%). In response-evaluable patients with NSCLC, 33% (6/18) had a partial response, 55% (10/18) stable disease, and 11% (2/18) progressive disease. Patients who remained on single-agent SNX-5422 maintenance therapy ≥ 2 months (n=9) had cancers enriched for oncogenic drivers (n=3 KRAS mutation, n=1 EGFR exon 20 mutation, n=1 HER2 mutation, and n=1 RET fusion).
Conclusions
The triplet combination of SNX-5422, carboplatin and paclitaxel followed by maintenance SNX-5422 therapy was well-tolerated and showed anti-tumor activity. Cancers for which disease control on single-agent SNX-5422 maintenance was observed were enriched for oncogene-driven NSCLCs.
Keywords: SNX-5422, HSP90 inhibitor, NSCLC, EGFR wild-type, platinum therapy
1. Introduction
Targeted therapies are current care standards for various oncogene-driven lung cancers. However, chemotherapy is still an important therapeutic option because resistance to targeted therapy eventually develops. Attempts to improve on the outcomes achieved with chemotherapy alone have led to the development of combination therapies that include a chemotherapy backbone.
Heat shock proteins (HSP) are a family of molecular chaperones that protect proteins from degradation and regulate cell proliferation and differentiation [1]. HSP90 is one of most abundant proteins in cells and is the best-studied of the stress proteins [2]. HSP90 corrects the folding and maintains the stability of oncogenic proteins, including HER2, BRAF, and MET [3–5]. HSP90 inhibition can also decrease KRAS signaling by suppressing MAPK or AKT signaling [6]. Thus, HSP90 inhibitors are potential anticancer therapeutics.
SNX-5422 is an oral indazol-4-one derivative prodrug of SNX-2112, a highly selective inhibitor of HSP90 [7]. In head and neck squamous cancer cell lines, SNX-2112 showed combinatorial activity with carboplatin and paclitaxel [8]. SNX-5422 inhibits oncogenic signaling pathways in vitro [8], is well-tolerated, and has shown activity in clinical trials for patients with solid tumors, including lung cancer [9]. In this exploratory trial, we investigated the safety and efficacy of SNX-5422 in combination with carboplatin and paclitaxel in patients with advanced lung cancers.
2. MATERIALS AND METHODS
2.1. Study design and participants
This was a phase 1, multicenter, open-label, dose finding (3+3 dose escalation) and dose expansion trial of triplet SNX-5422, carboplatin and paclitaxel therapy followed by maintenance SNX-5422 monotherapy in patients with advanced lung cancers. The study protocol was conducted in accordance with the International Conference on Harmonization Good Clinical Practice Guideline and the Declaration of Helsinki and was approved by local Institutional Review Boards (IRB). All participants provided written informed consent prior to enrollment. Eligible patients had pathologically confirmed lung cancers and had adequate organ function. There were no limitations on prior lines of anti-tumor therapy for metastatic disease. Patients with significant glaucoma, retinitis pigmentosa, or macular degeneration were excluded given reports of ocular toxicity (vision darkening that was alleviated with every other day dosing) in a prior trial [9]. This clinical trial was registered on clinicaltrials.gov (NCT01892046).
Patients with small cell lung cancer (SCLC) were originally included based on the drug’s preclinical activity [10]. Three patients with SCLC were enrolled before the protocol was amended and limited to patients with non-small cell lung cancers (NSCLCs: adenocarcinomas or squamous cell carcinomas) due to lack of activity of the triplet in SCLCs in this study. These three patients were included in the safety population.
All lung adenocarcinoma specimens were tested for EGFR mutation. At the discretion of the investigators, additional testing for other oncogenes included polymerase chain reaction for ERBB2 exon 20 (Applied Biosystems), break apart fluorescence in-situ hybridization (FISH) for ALK/ROS1/RET fusions (VYSIS or MetaSystems Group, Inc.), or Sequenom mass-spectrometry genotyping for BRAF, HER2, and KRAS (Sequenom). Molecular testing was performed locally in a Clinical Laboratory Improvement Amendments (CLIA) compliant setting.
2.2. Dosing
Dosing occurred in two phases: part 1 consisted of combination treatment with SNX-5422, carboplatin, and paclitaxel, and part 2 consisted of maintenance therapy with SNX-5422 alone. A treatment cycle was defined as 28 days. SNX-5422 was dosed orally every other day (qod) for 21 days (11 doses) followed by a 7-day drug-free period. Carboplatin and paclitaxel were administered once every three weeks (on a non-SNX-5422 dosing day) for 4 courses during cycles 1–3. After the treatment of 12 subjects, two additional optional courses of carboplatin and paclitaxel were allowed (for a total of 6 courses) at the investigators’ discretion. Only two patients in this study received more than four courses of chemotherapy. SNX-5422 dose levels were 50 mg/m2, 75 mg/m2 and a maximum of 100 mg/m2, the previously established single-agent maximum tolerated dose (MTD) for SNX-5422 alone [9]. Paclitaxel was dosed at 175 mg/m2 and carboplatin was given at AUC 5 mg/mL/min (not to exceed 750 mg). Only subjects for whom therapy achieved at least stable disease in part 1 could continue SNX-5422 maintenance monotherapy in part 2.
2.3. Endpoints and Assessments
The primary objective of the study was to determine the MTD of SNX-5422 when given with carboplatin and paclitaxel. Dose-limiting toxicities (DLTs) that informed selection of the MTD were assessed in cycle 1. Secondary objectives included objective response and safety using Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Toxicity was assessed at each visit starting with screening until the final study visit and scans were performed. Ophthalmologic exams were performed at screening, cycle 1 day 21, and at the final visit. Scans were performed prior to dosing, at the end of maintenance cycle 2, and every 12 weeks thereafter. Response was assessed as per the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1.
2.4. Statistics
Time on therapy was measured from the start of chemotherapy and/or SNX-5422 to the time of treatment discontinuation for any reason. Progression-free survival (PFS) was measured from the start of treatment to time of progression. Descriptive statistics were used to summarize subject characteristics, safety, and tumor response data. Mann-Whitney test was used to compare overall response rates (ORR) between groups. The p-value for PFS was determined using the log-rank test. Pharmacokinetic parameters were determined from plasma concentration-time data using non-compartmental analysis methods (NCA Model 200–202, Phoenix® WinNonlin®, version 7.0; Pharsight Corp., Mountain View, California). Statistical analyses were conducted with the SAS® software package v9.2 or higher.
3. RESULTS
3.1. Patient characteristics
The baseline characteristics of 23 patients enrolled to the study are summarized in Table 1. Characteristics by dose cohort are detailed in Supplementary Table 1. The median age was 60 years. Patients were predominantly male (61%) and Caucasian (74%) with adenocarcinomas (74%) pre-treated with one or more prior systemic therapy regimens (61%). Seven out of ten patients with adenocarcinomas that had additional molecular testing harbored the following drivers: EGFR exon 20 insertion (n=1), BRAF mutation (n=1), HER2 mutation (n=1), KRAS mutation (n=3), and RET fusion (n=1). The first subject was enrolled in August 2013 and the last patient was enrolled November 2016.
Table 1.
Baseline Characteristics
| Characteristic | Total N=23 |
|---|---|
|
| |
| Age in years, median (range) | 60 (42–72) |
| Sex, n (%) | |
| Male | 14 (61) |
| Female | 9 (39) |
| Race, n (%) | |
| Caucasian | 17 (74) |
| Black | 5 (22) |
| Asian | 1 (4) |
| Ethnicity, n (%) | |
| Not Hispanic or Latino | 22 (96) |
| Hispanic or Latino | 1 (4) |
| Histology, n (%) | |
| Adenocarcinoma | 17 (74) |
| Squamous Cell Carcinoma | 3 (13) |
| SCLC1 | 3 (13) |
| Prior Regimens2, n (%) | |
| 0 | 9 (39) |
| ≥1 | 14 (61) |
| Canonical Drivers in NSCLC, n (%), N=20 | |
| Broad oncogene testing not performed3 | 11 (55) |
| No oncogene detected | 2 (10) |
| KRAS mutation | 3 (15) |
| HER2 mutation | 1 (5) |
| KIF5B-RET fusion | 1 (5) |
| EGFR exon 20 insertion | 1 (5) |
| BRAF mutation | 1 (5) |
Subjects enrolled prior to protocol amendment limiting subject population to NSCLC.
Prior systemic anticancer therapy, including adjuvant therapy, but not including radiation therapy.
Only EGFR sensitizing mutation testing performed.
3.2. Dose Determination
There were no DLTs in the 50 mg/m2 (n=3) and 75 mg/m2 (n=4) dose cohorts. One of six patients in the 100 mg/m2 cohort, the highest dose level, had a DLT, establishing 100 mg/m2 of SNX-5422 in combination with carboplatin (AUC 5) and paclitaxel (175 mg/m2) as the MTD. The SNX-5422 dose and schedule in this combination were the same as the MTD of the drug as a single-agent [9]. Carboplatin plus paclitaxel was given for a median of 4 courses (range, 1 to 6 courses). Patients were treated with SNX-5422 for a median of 20 weeks (range, 1 to 63 weeks). In patients with NSCLC, treatment was discontinued for disease progression (n=16), an adverse event (AE; n=2), or voluntary withdrawal (n=2).
Of patients in the 100 mg/m2 cohort, one patient in the initial cohort of 6 had a DLT of grade 3 elevated AST and ALT at the end of cycle 1, but was able to resume at a reduced dose of 75 mg/m2. The patient eventually stopped treatment after disease progression in cycle 3. The cohort was later expanded to include 10 more patients. Of these patients, one had a DLT of diarrhea, leading to study drug discontinuation before completing cycle 1, and one patient experienced grade 3 nausea and vomiting and was taken off study in cycle 2.
3.3. Pharmacokinetics
Oral administration of SNX-5422 resulted in rapid conversion to the active metabolite, SNX-2112. Samples collected after dosing showed no measurable concentrations of SNX-5422. Peak concentrations of SNX-2112 were observed between 1.0 and 4.0 hours after dosing (Supplementary Table 2). SNX-2112 exhibited a relatively short terminal half-life in plasma, with a mean t1/2 of approximately 8 hours. Maximum plasma concentration of SNX-2112 increased in approximate proportion to dose across SNX-5422 doses of 50, 75, and 100 mg/m2, while the AUC0–24 values for SNX-2112 increased in a slightly greater than proportional manner relative to increasing SNX-5422 doses.
3.4. Overall Safety
Safety was evaluable in 23 patients who received at least one dose of study medication. One patient treated with study drug was included in the safety population who was later withdrawn for not meeting eligibility criteria on further review. Most patients (96%; n=22/23) experienced at least one treatment-related AE (Table 2). Most treatment-related AEs were grade 1/2 in severity. The most common treatment-related AEs of any grade were diarrhea (n=16, 70%), nausea (n=11, 48%), fatigue (n=9, 39%), alopecia (n=6, 26%), and vomiting (n=6, 26%). Forty-eight percent (n=11) of patients experienced a grade 3 or higher treatment-related AE, which included diarrhea (n=6, 26%), nausea (n=2, 9%), and neutropenia (n=2, 9%).
Table 2.
Treatment-Related Adverse Events Reported in ≥10% of All Subjects
| Adverse Event | Combination Treatment (SNX-5422 + Carboplatin/Paclitaxel) | ||||
| All (N=23) | |||||
| Any Grade n (%) | Grade 3/4 n (%) | ||||
| Any | 22 (96) | 11 (48) | |||
| Diarrhea | 16 (70) | 6 (26) | |||
| Nausea | 11 (48) | 2 (9) | |||
| Fatigue | 9 (39) | 0 | |||
| Alopecia | 6 (26) | 0 | |||
| Vomiting | 6 (26) | 1 (4) | |||
| Thrombocytopenia | 4 (17) | 0 | |||
| Neutropenia | 3 (13) | 2 (9) | |||
| ALT increased | 3 (13) | 1 (4) | |||
| ALP increased | 3 (13) | 0 | |||
| Neuropathy* | 4 (17) | 1 (4) | |||
| SNX-5422 Maintenance Monotherapy (N=13) | |||||
| Grade 1 n (%) | Grade 2 n (%) | Grade 3 n (%) | Grade 4 n (%) | All Grade n (%) | |
| Any | 1 (8) | 4 (31) | 2 (15) | 0 | 7 (54) |
| Diarrhea | 0 | 1 (8) | 2 (15) | 0 | 3 (23) |
| Nausea | 2 (15) | 0 | 0 | 0 | 2 (15) |
| Fatigue | 1 (8) | 1 (8) | 0 | 0 | 2 (15) |
| Decreased appetite | 1 (8) | 1 (8) | 0 | 0 | 2 (15) |
includes peripheral neuropathy and peripheral sensory neuropathy; one patient with adenocarcinoma was treated with SNX-5422 and included in the safety population, but was later found to not meet eligibility criteria and was withdrawn.
Despite HSP90 inhibition being associated with retinal changes in preclinical models and visual darkening in a prior trial, no substantial ophthalmological events were observed [9]. In the 50 mg/m2 SNX-5422 cohort, one patient developed photophobia along with nausea and vomiting during combination therapy; this was low-grade toxicity that didn’t require treatment discontinuation. In the 75 mg/m2 and 100 mg/m2 SNX-5422 cohorts, ocular toxicity was not observed during monotherapy.
Twelve patients with NSCLCs went on to receive SNX-5422 monotherapy in the maintenance phase. Monotherapy maintenance with SNX-5422 was tolerable; the median time on SNX-5422 maintenance was 11 weeks (range, 0 to 53 weeks). The most commonly reported treatment-related AEs were diarrhea (23%), fatigue (15%), nausea (15%), and decreased appetite (15%).
A total of 12/23 subjects (52%) had AEs leading to dose interruption, including two subjects with DLTs, as previously described. Two unrelated deaths were reported either during the study or within 30 days of study discontinuation. The deaths were due to disease progression (n=1) and pneumonia (n=1).
3.5. Anti-tumor Activity
Tumor response was evaluable for 18 of 20 patients with NSCLCs. The ORR was 33% (n=6/18, 95% CI 12% to 51%). There were no complete responses (0%), six had a partial response (33%), ten had stable disease (56%, one had an unconfirmed partial response), and two had primary progressive disease (11%). The best overall percent change from baseline in target lesion measurement for the NSCLC population is shown in Figure 1. The median time to progression (Kaplan-Meier estimate) was 7 months (95% CI: 3 to 7 months; Supplementary Figure 1). Prior systemic therapy did not appear to influence outcomes. The ORR and PFS for patients in the treatment naïve group was 33% (n=3/9) and 7.2 months, respectively, whereas ORR and PFS for patients in the previously treated group was 33% (n=3/9; p = 1.0) and 7.5 months (p = 0.8), respectively.
Fig. 1. Maximal response to therapy by mutation status in patients with NSCLC.
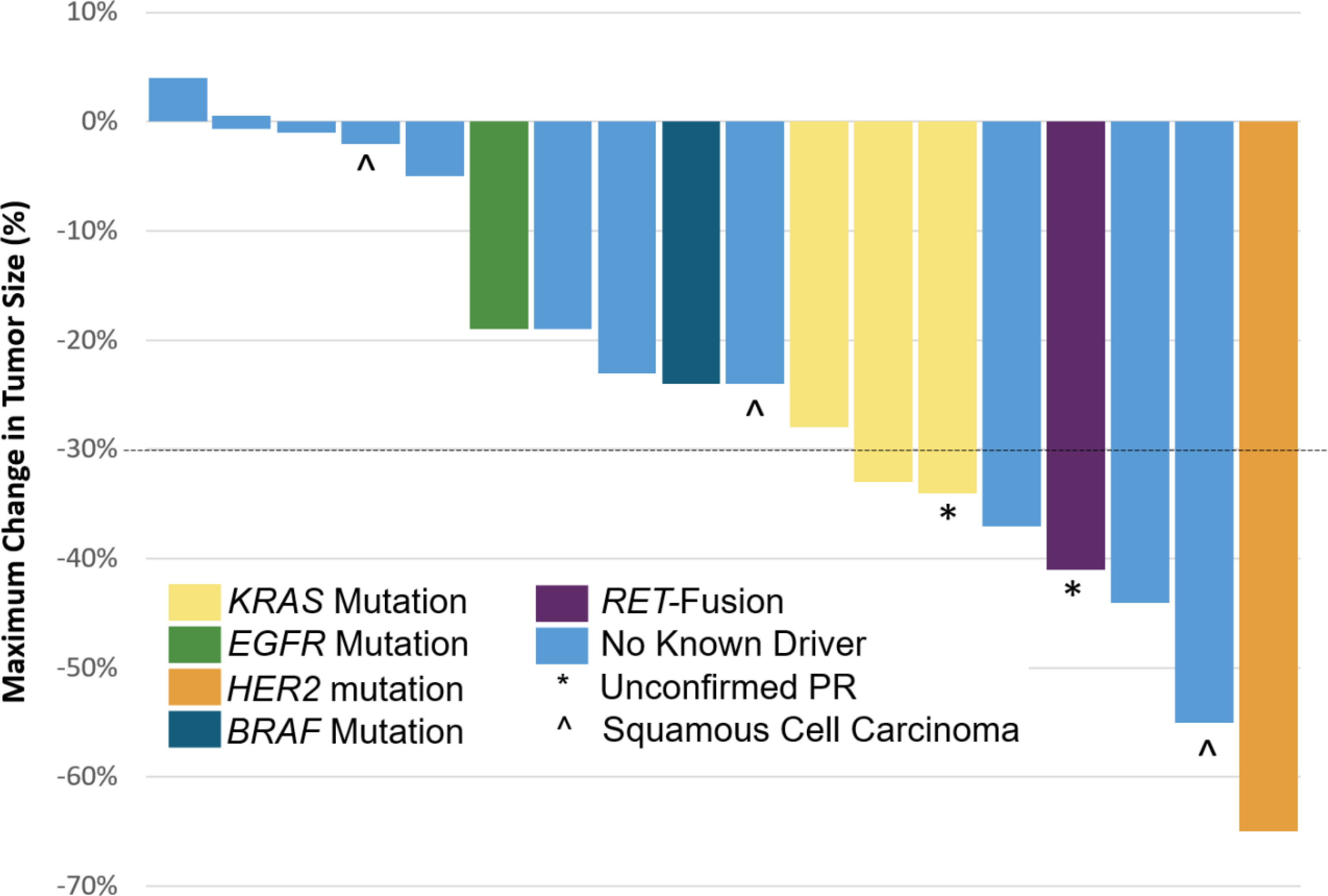
Waterfall plot showing the maximal change in tumor size. The color of the bar represents the mutation type as shown. Unconfirmed partial responses and patients with squamous cell carcinoma are also indicated. A dashed black horizontal line is shown at −30%, the threshold for a partial response.
Time on therapy is shown in Figure 2. Benefit to this combination appeared to be enriched in NSCLCs with oncogenic-drivers. Six of the seven patients (88%) with oncogene-driven NSCLC went onto maintenance therapy. Furthermore, the overall response was 43% (n=3/7) in this group. One patient with HER2-mutant lung cancer for whom a partial response (−65%) was achieved remained on SNX-5422 maintenance for 12 months. One of three patients with KRAS-mutant and another patient with a KIF5B-RET fusion NSCLCs had partial responses.
Fig. 2. Duration of therapy by mutation status in patients with NSCLC.
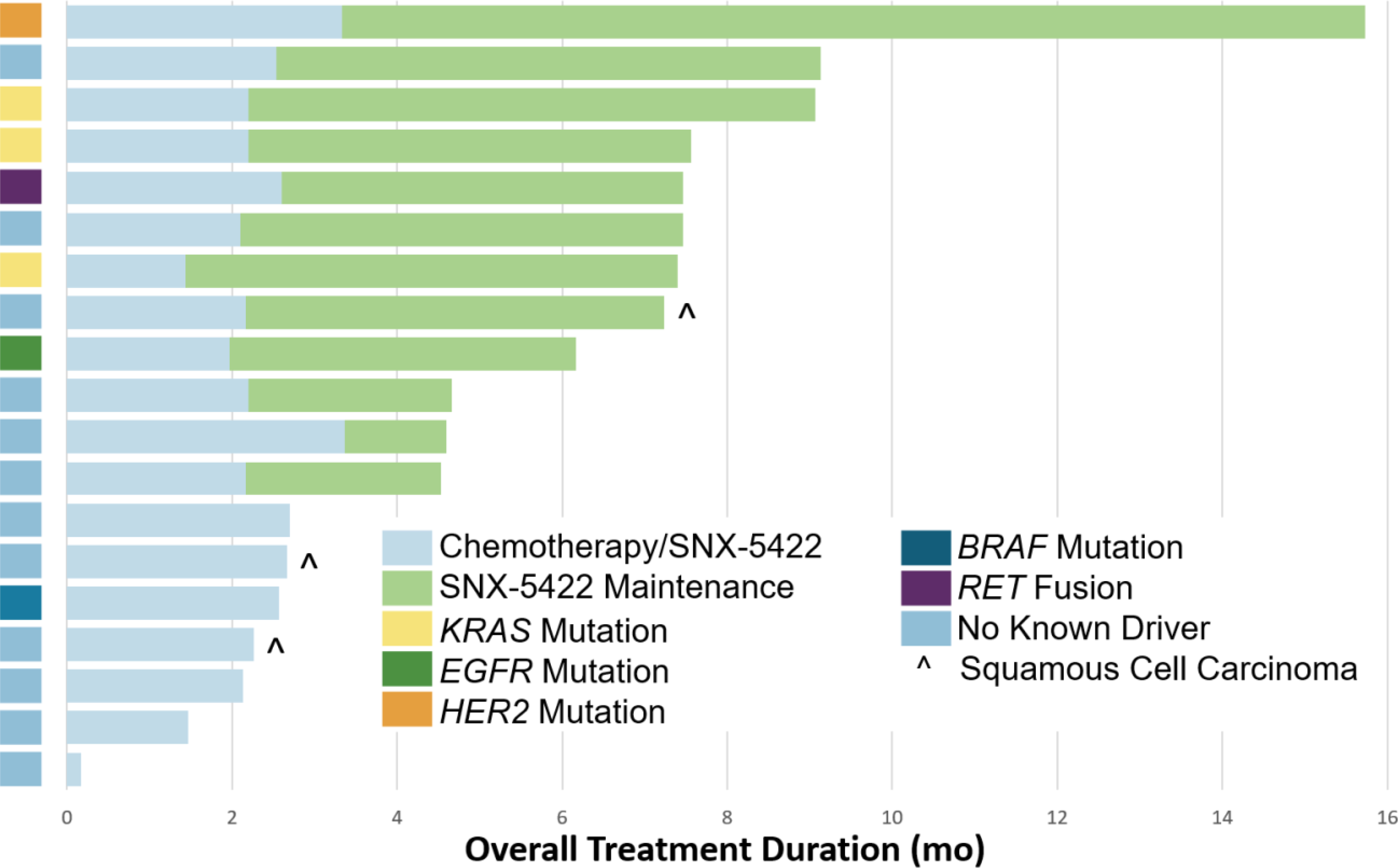
The time on part 1 (chemotherapy plus SNX-5422) and part 2 (SNX-5422 maintenance therapy) of treatment is indicated by the bar color. The bar on the far left indicates the mutation status of each tumor by row.
No activity was seen in the SCLC cohort (n=3). Two patients had stable disease and one had primary disease progression.
4. DISCUSSION
In this prospective, multicenter phase 1 study, the combination of the HSP90 inhibitor, SNX-5422, with carboplatin and paclitaxel was both safe and active in subjects with advanced lung cancers. The overall AE profile of the triplet was consistent with that seen with carboplatin and paclitaxel alone, and the persistent gastrointestinal treatment-related AEs in the monotherapy phase highlight the consequences of single-agent HSP90 use. Diarrhea was the most common treatment-related AE with SNX-5422 monotherapy, consistent with the results of a previous phase 1 trial of the drug [9]. Careful monitoring for diarrhea and supportive care optimization should be considered for future HSP90 inhibitor trials.
In this trial, the activity of the triplet and subsequent maintenance therapy with HSP90 inhibition alone appeared to cluster in oncogene-driven lung cancers, consistent with the role that HSP90 plays as a chaperone of these mutant oncoproteins. Acknowledging that the observed activity cannot be substantially disentangled from the effects of the chemotherapy backbone, target lesion shrinkage was pronounced in HER2-mutant, RET-rearranged and KRAS-mutant lung cancers. Furthermore, disease control on single-agent HSP90 inhibitor maintenance therapy was enriched in lesions with HER2, RET, KRAS, and EGFR-mutations, with durations of approximately 4–12 months.
The patient with a HER2-mutant NSCLC had a partial response and derived benefit from SNX-5422 maintenance for ~12 months before progression. One patient with KRAS-mutant NSCLC had a partial response and was on SNX-5422 maintenance for ~7 months prior to progression. Another patient with KRAS-mutant NSCLC had an unconfirmed response and was on SNX-5422 maintenance for ~5 months. These results are in line with pre-clinical models where HSP90 inhibition promotes the degradation of HER2 receptors and suppresses RAS pathway signaling, leading to tumor shrinkage [6]. Further exploration of HSP90 inhibition as monotherapy or in combination with other treatments may benefit from oncogenic driver enrichment.
An additional limitation of this study was the lack of a control group, which did not allow for a direct comparison against standard-of-care platinum doublet therapy. Furthermore, contemporary platinum doublet inclusive lung cancer therapy has since migrated to include antiangiogenic therapy and/or immunotherapy for most patients. A third major limitation was the lack of uniform biomarker testing, recognizing that comprehensive sequencing had not been more widely adopted in routine practice when this trial began in 2013. No activity was seen in SCLCs; our ability to adequately test activity in this cancer type was limited due to the small cohort and exclusion of accrual of this subtype shortly after trial initiation.
In conclusion, this prospective, multicenter study demonstrated the tolerability of the HSP90 inhibitor SNX-5422 with and without chemotherapy. While this particular combination will not be further pursued, the data generated from this trial suggests that future HSP90 trial designs should be based on a strong biologic rationale for combination therapy, include contemporary standards of care when appropriate, and consider enrichment for oncogene-driven tumors.
Supplementary Material
Highlights.
The combination of the HSP90 inhibitor SNX-5422 with carboplatin and paclitaxel is safe and active in patients with advanced lung cancer.
The preliminary efficacy of this combination is encouraging in an enriched subpopulation of lung cancers with oncogenic drivers.
Future development of current and next-generation HSP90 inhibitors should consider the inclusion of oncogene-driven lung cancers.
Acknowledgements
The authors thank the participating patients and their families. Medical writing support was provided by Lorraine R. Baer, PharmD (Baer PharMed Consulting, Ltd.), and was funded by Esanex Inc.
Funding:
This work was supported by Esanex, Inc. The sponsor had a role in the study design, in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. This study was partially supported by a National Cancer Institute Cancer Center grant to Memorial Sloan Kettering Cancer Center (P30 CA008748).
Conflict of Interest Statement
RG reports funding from Esanex and honoraria from Pfizer.
GG was an employee of Esanex during the design and all aspects of data collection of this study; has stock with Esanex.
EOO was an employee of Esanex during the design and all aspects of data collection of this study; has stock with Esanex.
S.V.L. declares Advisory Board / Consultant: Amgen, AstraZeneca, Bayer, Beigene, Blueprint, Bristol-Myers Squibb, Daiichi Sankyo, Eisai, Elevation Oncology, Genentech/Roche, Guardant Health, Janssen, Jazz Pharmaceuticals, Lilly, Merck/MSD, Novartis, Regeneron, Sanofi, Takeda, Turning Point Therapeutics. Research grant (to institution): Alkermes, Bayer, Blueprint, Bristol-Myers Squibb, Elevation Oncology, Genentech, Lilly, Merck, Merus, Pfizer, Rain Therapeutics, RAPT, Turning Point Therapeutics.
C.H. has served as a speaker for AstraZeneca and Daiichi-Sankyo and has acted as a consultant for JHU partners in the metastatic breast cancer program.
M.G.K. receives personal fees from Novartis, Sanofi-Genzyme, AstraZeneca, Pfizer, Janssen, and Daiichi-Sankyo; received honoraria for participation in educational programs from WebMD, OncLive, Physicians Education Resources, Prime Oncology, Intellisphere, Creative Educational Concepts, Peerview, i3 Health, Paradigm Medical Communications, AXIS, Carvive Systems, and AstraZeneca; received travel support from AstraZeneca, Pfizer, and Genentech; received editorial support from Hoffman La-Roche. Memorial Sloan Kettering has received research funding from The National Cancer Institute (USA), The Lung Cancer Research Foundation and Genentech Roche for research conducted by Dr. Kris.
A.D. declares: Honoraria/Advisory Boards: Ignyta/Genentech/Roche, Loxo/Bayer/Lilly, Takeda/Ariad/Millenium, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Helsinn, Beigene, BergenBio, Hengrui Therapeutics, Exelixis, Tyra Biosciences, Verastem, MORE Health, Abbvie, 14ner/Elevation Oncology, Remedica Ltd., ArcherDX, Monopteros, Novartis, EMD Serono, Melendi, Liberum, Repare RX; Associated Research Paid To Institution: Pfizer, Exelixis, GlaxoSmithKline, Teva, Taiho, PharmaMar; Royalties: Wolters Kluwer; Other: Merck, Puma, Merus, Boehringer Ingelheim; CME Honoraria: Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, Research to Practice, Axis, Peerview Institute, Paradigm Medical Communications, WebMD, MJH Life Sciences.
All other authors have no Conflict of Interest to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Chatterjee S, Burns TF, Targeting Heat Shock Proteins in Cancer: A Promising Therapeutic Approach, International Journal of Molecular Sciences 18(9) (2017) 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Finka A, Goloubinoff P, Proteomic data from human cell cultures refine mechanisms of chaperone-mediated protein homeostasis, Cell Stress and Chaperones 18(5) (2013) 591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang H, Burrows F, Targeting multiple signal transduction pathways through inhibition of Hsp90, Journal of Molecular Medicine 82(8) (2004). [DOI] [PubMed] [Google Scholar]
- [4].Liu H, Lu J, Hua Y, Zhang P, Liang Z, Ruan L, Lian C, Shi H, Chen K, Tu Z, Targeting heat-shock protein 90 with ganetespib for molecularly targeted therapy of gastric cancer, Cell Death & Disease 6(1) (2015) e1595–e1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lee H, Saini N, Parris A, Zhao M, Yang X, Ganetespib induces G2/M cell cycle arrest and apoptosis in gastric cancer cells through targeting of receptor tyrosine kinase signaling, International Journal of Oncology (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lin SF, Lin JD, Hsueh C, Chou TC, Yeh CN, Chen MH, Wong RJ, Efficacy of an HSP90 inhibitor, ganetespib, in preclinical thyroid cancer models, Oncotarget 8(25) (2017) 41294–41304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Huang KH, Veal JM, Fadden RP, Rice JW, Eaves J, Strachan JP, Barabasz AF, Foley BE, Barta TE, Ma W, Silinski MA, Hu M, Partridge JM, Scott A, DuBois LG, Freed T, Steed PM, Ommen AJ, Smith ED, Hughes PF, Woodward AR, Hanson GJ, McCall WS, Markworth CJ, Hinkley L, Jenks M, Geng L, Lewis M, Otto J, Pronk B, Verleysen K, Hall SE, Discovery of novel 2-aminobenzamide inhibitors of heat shock protein 90 as potent, selective and orally active antitumor agents, J Med Chem 52(14) (2009) 4288–305. [DOI] [PubMed] [Google Scholar]
- [8].Friedman JA, Wise SC, Hu M, Gouveia C, Vander Broek R, Freudlsperger C, Kannabiran VR, Arun P, Mitchell JB, Chen Z, Van Waes C, HSP90 Inhibitor SNX5422/2112 Targets the Dysregulated Signal and Transcription Factor Network and Malignant Phenotype of Head and Neck Squamous Cell Carcinoma, Transl Oncol 6(4) (2013) 429–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Infante JR, Weiss GJ, Jones S, Tibes R, Bauer TM, Bendell JC, Hinson JM Jr., Von Hoff DD, Burris HA 3rd, Orlemans EO, Ramanathan RK, Phase I dose-escalation studies of SNX-5422, an orally bioavailable heat shock protein 90 inhibitor, in patients with refractory solid tumours, Eur J Cancer 50(17) (2014) 2897–904. [DOI] [PubMed] [Google Scholar]
- [10].Mayer P, Harjung A, Breinig M, Fischer L, Ehemann V, Malz M, Scherübl H, Britsch S, Werner J, Kern MA, Bläker H, Schirmacher P, Bergmann F, Expression and therapeutic relevance of heat-shock protein 90 in pancreatic endocrine tumors, Endocrine-Related Cancer 19(3) (2012) 217–232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


