Abstract
Background.
Chronic alcohol consumption is associated with structural brain changes and increased inflammatory signaling throughout the brain and body. Increased inflammation in the brain has been associated with structural brain damage. Recent studies have also shown that neurofilament light polypeptide (NfL) is released into circulation following neuronal damage. NfL has thus been proposed as a biomarker for neurodegenerative diseases but has not been explored in connection with alcohol use disorder. For this secondary data analysis, we proposed a conceptual model linking alcohol consumption, the pro-inflammatory cytokine IL-6, brain structure and NfL in heavy-drinking participants.
Methods.
Of the 182 individuals enrolled in this study, 81 participants had useable gray matter (GM) data and 80 had useable white matter (WM) data. A subset of these had NfL (n = 78) and IL-6 (n = 117) data. GM thickness was extracted from middle frontal brain regions using Freesurfer. Mean WM diffusivity values were extracted from Tract Based Spatial Statistics. NfL and IL-6 were measured from blood. Regression models were used to test individual linkages in the conceptual model. Based on significant regression results, we created a simplified conceptual model which was tested using path analysis.
Results.
In regressions, negative relationships emerged between GM and both drinks per drinking day (DPDD) (p = .018) and NfL (p = .004). A positive relationship emerged between WM diffusivity and DPDD (p =.033). IL-6 was not significantly associated with alcohol use, GM or WM. The final path model demonstrated adequate fit to the data and showed significant, negative associations between DPDD and middle frontal gyrus (MFG) Thickness and between MFG Thickness and NfL, but a non-significant association between DPDD and NfL.
Conclusions.
Data suggest that drinking is associated with lower GM thickness and higher WM diffusivity and that lower GM thickness is associated with higher circulating NfL. This is the first study to demonstrate an association between brain structure and NfL in a sample of heavy drinkers.
Keywords: Alcohol, neuroimaging, inflammation, brain structure, neurofilament light (NfL)
INTRODUCTION
More than half of the adult population of the United States has consumed alcohol in the past month, and while many engage in moderate consumption, hazardous drinking practices are common (Ahrnsbrak et al., 2017). Excessive drinking can result in numerous negative consequences, ranging from those psychosocial in nature to potentially detrimental biological changes. In particular, chronic and heavy drinking has been associated with a number of deleterious neural adaptations. Specifically, heavy alcohol use and alcohol use disorder (AUD) have been associated with reductions in cortical thickness and blood-oxygen-level- dependent (BOLD) response during cognitive tasks in frontal regions such as the superior frontal cortex and gyrus (Tapert et al., 2001; Thayer et al., 2017; van Holst et al., 2012), anterior cingulate cortex (Durazzo et al., 2011; Mashhoon et al., 2014), and rostral anterior cingulate, caudal middle frontal, middle orbitofrontal and lateral orbitofrontal cortices (Durazzo et al., 2011). Alcohol consumption appears to be strongly linked to alterations within middle frontal brain regions (Mechtcheriakov et al., 2007; Nakamura-Palacios et al., 2014; Quaglieri et al., 2020), and heavy drinking has also been consistently associated with compromised white matter (WM) integrity across a number of brain regions (Bagga et al., 2014; Bava et al., 2013; Pfefferbaum and Sullivan, 2005). Specifically, prior work has shown links between alcohol consumption and white matter damage within the body of the corpus callosum, fornix, external capsule, superior longitudinal fasciculus, and cingulate gyrus (Monnig et al., 2015).
In addition, a number of studies support a relationship between chronic alcohol consumption and increased peripheral inflammation (Bishehsari et al., 2017; Crews et al., 2006a; Feldman et al., 2020). For example, a recent systematic review and meta-analysis found there was a significantly greater peripheral cytokine concentration, especially of pro-inflammatory cytokines (i.e., interleukin 6 [IL-6], interleukin 7 [IL-7], interleukin 8 [IL-8], and tumor necrosis factor [TNF-α]), among individuals with alcohol use disorder (AUD) compared to control individuals (Adams et al., 2020). Importantly, chronic alcohol consumption is also thought to be related to neuroinflammation, or inflammation within the brain (Crews et al., 2006). Though direct in vivo assessment of this relationship in humans would require invasive measurement of neural tissue and thus is not feasible at present, preclinical studies provide compelling evidence of a causal relationship between alcohol use and neuroinflammation (e.g.,(Doremus-Fitzwater et al., 2014; Gano et al., 2016). For example, in an experiment using an acute ethanol intoxication paradigm, rats showed increases in IL-6 concentration compared to a vehicle control after daily ethanol exposure in several regions of the brain including the hippocampus and amygdala (Gano et al., 2016). Similarly, another study found that rats exposed to ethanol had increased IL-6 expression post-exposure (i.e., 3 and/or 9 hours after injection) compared to saline-injected controls in the hippocampus, hypothalamus and cerebellum (Doremus-Fitzwater et al., 2014).
Increases in neuroinflammation may have serious ramifications, including potentially impacting neural health over the course of the lifespan. Inflammatory processes are implicated in several neurocognitive diseases (Schain and Kreisl, 2017) and peripheral inflammation is associated with structural brain damage in both cross-sectional and longitudinal studies (e.g., (Bettcher et al., 2014; Marsland et al., 2008; O’Donovan et al., 2020; Walker et al., 2017). For instance, one study found that groups of individuals with moderate levels of inflammation in the blood which increased over the course of the study period (i.e., 18 years) had lower WM volume and compromised WM integrity compared to individuals in the “lower-stable” group, even after controlling for demographic, disease, and lifestyle factors (O’Donovan et al., 2020). Likewise, another study found that an inflammatory marker measured in blood samples was associated with later-life structural WM abnormalities, over and above demographic and disease factors (Walker et al., 2017). Cross sectional research has also found that higher levels of inflammation (e.g., the pro-inflammatory cytokine IL-6) measured from blood samples are associated with smaller hippocampal GM volume (Marsland et al., 2008) and lower corpus callosum WM integrity (Bettcher et al., 2014)).
Damage to the brain (i.e., due to chronic alcohol use, long-term upregulation in inflammatory signaling or other disease-states) can result in an increase in circulating levels of neurofilament light polypeptides (NfL), which are released into the blood and cerebral spinal fluid following neuronal damage (Khalil et al., 2018; Kim et al., 2020). Given that NfL is produced following neuronal cell damage and can be measured fairly easily through peripheral blood samples, it has been proposed as a biomarker for a variety of neurodegenerative diseases. Studies have supported the use of NfL as a biomarker for Huntington’s disease, Parkinson’s disease, frontotemporal dementia and other neurological disorders (Byrne et al., 2017; Hansson et al., 2017; Khalil et al., 2018; Rohrer et al., 2016). NfL may also be a useful measure of neuronal damage in the context of heavy alcohol use or AUD, but no research to date has investigated the relationship between alcohol consumption and circulating NfL measured in blood samples.
In summary, the existing literature supports a model of alcohol-related inflammatory brain damage in which excess drinking causes inflammation. This inflammation is thought to inflict structural damage within the brain (e.g., decreased GM and compromised WM integrity), which may be evidenced by increased circulating NfL. The present study is a secondary analysis of a larger dataset that aims to test these relationships directly. Specifically, we proposed a broad conceptual model, in which we predict that drinks per drinking day (DPDD) will be associated with plasma IL-6, as well as GM and WM, and that IL-6 will be correlated with GM and WM. IL-6 was selected as the candidate cytokine of interest given the consistent links between IL-6 and alcohol consumption in the preclinical (Gano et al., 2016) and human literature (Adams et al., 2020), as well as between IL-6 and both GM (Marsland et al., 2008) and WM (Bettcher et al., 2014). We further hypothesize that GM and WM will be associated with plasma NfL. We first explored individual linkages within this model, and based on significant relationships that emerged, created an updated conceptual model to test using path analysis. As an exploratory analysis, we also tested associations between NfL, IL-6 and DPDD and both WM and GM matter across the whole brain.
MATERIALS AND METHODS
Participants
Participants were recruited from the greater Denver/Boulder metropolitan area via social media postings and mailed flyers and screened over the phone by trained research assistants. Individuals were included if they were between 21–60 years of age, had consumed alcohol within the 10 days prior to screening, were interested in reducing their drinking, and reported drinking >14 drinks per week (female) or >21 drinks per week (male) with at least 2 heavy drinking days (>4 drinks for women; >5 drinks for men) during 4 consecutive weeks within three months of beginning the study (consistent with drinking criteria for project COMBINE (Group, 2003)). Participants were excluded if they were currently undergoing alcohol withdrawal, using medications to treat bipolar or psychotic disorders, endorsed current suicidality, met criteria for psychotic or bipolar disorder or a current major depressive episode, reported using illicit drugs in the 30 days prior to beginning the study, or were pregnant.
Procedure
The present study is a retrospective analysis of data collected as part of a Randomized Clinical Trial (RCT) comparing 8-weeks of Mindfulness-Based Relapse Prevention to 8-weeks of Relapse Prevention therapy for individuals who want to decrease their drinking. Note that the present analyses focus solely on measures collected at baseline (i.e., prior to participants undergoing any therapy intervention). For the RCT, eligible individuals completed a baseline appointment followed by eight weeks of therapy and follow-up visits at 20- and 32- weeks post-therapy. During the baseline session, participants provided informed consent and were administered a Breathalyzer (Intoximeter, Inc., St. Louis, MO) in addition to a urinalysis test. Females were required to take a pregnancy test and screen negative. Participants who had a breath alcohol level above (BAC) 0.00 g/dL and/or who had a positive urine drug screen were unable to continue participation. In the former case, participants could be rescheduled to come back the following day and continue if their BAC was 0.00 g/dL at that time. Participants were also administered the Clinical Institute Withdrawal Assessment; those who scored over an 8 were referred to a doctor for follow up and, if still interested in participation, instructed to reach out to the study coordinator after completing medically supervised detoxification. Participants completed questionnaires measuring health, demographics, history of drinking, smoking and drug use, as well as additional measures not included in the current paper (e.g., neurocognitive measures). During this session, participants additionally completed an MRI scan and had their blood drawn to assess blood levels of cytokines and NFL.
Measures
Demographics Questionnaire.
A demographics questionnaire was administered at baseline to collect information on age, sex, marital status, SES, occupation, income, education, and race.
Substance Use.
The Timeline Follow-Back (TLFB) (Sobell and Sobell, 1992) was used to assess retrospective recall of daily cannabis, tobacco, alcohol and other substance use for the month prior to the baseline appointment. The TLFB has been demonstrated to have good psychometric characteristics and can generate variables that provide a wide range of information about an individual’s drinking (Donohue et al., 2004). The average drinks per drinking day (DPDD) variable was used to quantify alcohol consumption in all analyses. This variable calculates the average number of drinks per drinking day consumed in the month prior to the baseline session. Cannabis and tobacco consumption were quantified by daily endorsement of cannabis use or cigarette use.
Neuroimaging
Structural Scans.
A high-resolution protocol sufficient to permit accurate tissue classification and anatomical parcellation was used. For optimal contrast between GM, WM and cerebral spinal fluid (CSF) at 3T, we used a multi-echo MPRAGE (MEMPR) sequence with the following parameters: TR/TE/TI = 2300/2.74/900 ms, flip angle = 8°, FOV = 256×256 mm, Slab thickness = 176 mm, Matrix = 256×256×176, Voxel size =1×1×1 mm, Number of echos = 4, Pixel bandwidth =650 Hz. During this portion of the scan, participants were asked to lie still for six minutes.
Diffusion Scans.
An echo planar image spin echo acquisition was used to obtain diffusion weighted images with the following parameters: TR/TE = 4000/77.00 ms, flip angle = 84°, FOV = 248×248 mm, Slice thickness = 3 mm, Matrix = 248×248×168 mm, b-value = 2400 s/mm2, Voxel size = 2×2×2 mm, Multiband acceleration factor = 3.
All scans were conducted at the University of Colorado Boulder in the MRI Suite using a 2016 Siemens Magnetom 3T PrismaFit scanner. This system includes a 32-channel coil, multinuclear support and additional software (e.g., BLADE, inline diffusion/BOLD, spectroscopy, cardiac, advanced functional software). Scanner instability and quality control on a phantom were monitored weekly.
Structural Image Preprocessing
Images were converted from DICOM to NIFTI and visually inspected for gross artifacts. Following visual QA checks, automatic cortical and subcortical segmentation and parcellation were performed in Freesurfer’s automated Recon-all pipeline (7.1.0, http://surfer.nmr.mgh.harvard.edu). Intensities attributed to magnetic inhomogeneities were corrected and normalized, images were then skull-stripped and segmented into gray/white matter and cerebrospinal fluid following image intensities and gradients. Triangular tessellation was then applied to the resulting image to provide a smooth representation of the gray/white matter interaction. For more technical details see Dale et al., 1999; Fischl et al., 2002, 1999.
Diffusion Tensor Imaging Preprocessing
To address potential susceptability induced distortions, reversed phase-encode blips were used in acquisition of data, thereby resulting in pairs of images containing bidirectional distortions. A susceptability-induced off-resonance field was then estimated from these images (Andersson et al., 2003) and later implemented in FSL (Smith et al., 2004). A single corrected image was then obtained by combining the two images using FSL’s top-up tool. The data were further corrected by restrospectively estimating eddy currents and participant motion and applying that estimation to the data with FSL’s eddy tool (Andersson and Sotiropoulos, 2016). A custom MATLAB script was then used to estimate and flag bad volumes based on the output from FSL’s eddy tool. A tensor was then calculated using AFNI’s AFNI-Fit tool by assigning a single diffusivity estimation matrix to each voxel. The data were then spatially normalized to the MNI template and smoothed with a Guassian kernel of 1.25mm. TBSS was then used to asess the mean diffusivity in multiple tracts (using the JHU White Matter atlas (Mori et al., 2005)) in relation to DPDD, IL-6 and NFL.
GM Analysis
Automatic cortical and subcortical segmentation and parcellation were performed in Freesurfer (7.1.0, http://surfer.nmr.mgh.harvard.edu). Intensities attributed to magnetic inhomogeneities were corrected and normalized, images were then skull-stripped and segmented into gray/white matter and cerebrospintal fluid following image intensities and gradients. Triangular tessellation was then applied to the resulting image to provide a smooth representation of the gray/white matter interaction. For more technical details see (Dale et al., 1998, Fischl et al., 1998, Fischl et al., 2002). Data were visualized for artifacts caused by motion, signal loss, magnetic inhomogeneity, signal noise and/or pathology. Scans exhibiting these any of these characteristics were excluded from analyses (N=1).
Cortical thickness values for gray matter ROI (see statistical analysis section) were exteracted from freesurfer and imported in SPSS (Version 27) for use in primary analyses. To futher explore associations between IL-6, NfL and DPDD and other regions of the brain that were not included in the ROI, statistical analysis and visualization of the effects of DPDD, Il-6 and NFL on cortical thickness across the whole brain were carried out using Freesurfer’s QDEC group analysis toolbox. The data were smoothed to 10 mm full-width half maximum and fed into Freesurfer’s general linear model program. To address any errors associated with multiple comparisons in the whole brain analysis, a Monte Carlo simulation was also applied. Specifically, this correction reduces the rate of false positives by performing simulations under the null hypothesis to see how frequently the value of a statistic from the “true” analysis is exceeded. This frequency can be interpreted as a p-value. In Freesurfer, the Monte Carlo correction involves three phases: estimation (running the analysis on the data without simulation), simulation (running the simulation with the same parameters) and clustering (identifying spatially related vertices that exceed the threshold to reject the null hypothesis in the simulation, with significance set at p<.05).
Diffusion Tensor Imaging Analysis
FMRIB Software Library’s Tract Based Spatial Statistics program (Smith et al., 2006) was used to identify major white matter tracts, which were registered to a common space. Diffusivity values (mean diffusivity) for white matter tracts of interest (see Statistical Analysis section) were extracted from TBSS and imported into SPSS (Version 27) for regression analyses. Next, distortion and eddy corrected diffusion data were run through FMRIB Software Library’s Tract Based Spatial Statistics program (Smith et al., 2006) to assess associations between IL-6, NFL and DPDD and white matter microstructure across the whole brain. To minimize type I errors due to multiple comparisons, the data were cluster corrected through FSL’s Randomise program and images were thresholded at p = 0.05.
Blood Samples
10 ml of whole blood was drawn to measure protein levels of the proinflammatory cytokine IL-6 as well as protein levels of circulating NfL. The cytokines were assessed in blood plasma using a multiplex assay (Quanterix Corp, Billerica MA). Following procedures from prior cytokine studies (Ostrowski et al., 1999), the log of IL-6 values was calculated for use in all analyses, due to non-normal distribution of IL-6 data. NfL protein levels were measured from blood plasma samples using the UmanDiagnostics NF-Light assay (Quanterix Corp, Billerica MA). NfL and IL-6 are reported in pg/mL. Inter-assay coefficients of variation were 5.86% (n=3 plates) for NfL and 5.88% (n= 10 plates) for IL-6. Intra-assay coefficients of variation were 6.78% (n=78) for NfL and 3.68 (n=117) for IL-6. These numbers are within acceptable ranges (Salimetrics, 2021).
Statistical Analysis Plan
ROI selection
First, to avoid type 1 error and take a more conservative approach to the analysis, GM analyses were constrained to caudal and rostral middle frontal gyrus regions only (ROI referred to as “MFG Thickness”), given associations between heavy drinking and damage to frontal GM (Durazzo et al., 2011; Mashhoon et al., 2014; Tapert et al., 2001; van Holst et al., 2012), implicating middle frontal regions in particular (Mechtcheriakov et al., 2007; Nakamura-Palacios et al., 2014; Quaglieri et al., 2020). For WM analyses, a composite of WM tracts was created based on our prior work demonstrating associations between WM microstructure and clinical characteristics in heavy drinkers (Monnig et al., 2015). This composite was composed of the average of the body of the corpus callosum, fornix, external capsule, superior longitudinal fasciculus, and cingulate gyrus. Specifically, mean diffusivity (MD) was used in this study as the white matter metric of interest, given that alterations in this diffusivity metric have been observed in heavy drinking populations (Bagga et al., 2014; Bava et al., 2013; Pfefferbaum and Sullivan, 2005). Note that higher values for white matter mean diffusivity are generally indicative of greater WM damage.
Whole Brain Exploratory Analysis
After conducting the ROI analyses described above (which were based on a priori hypotheses regarding associations between specific brain regions and our variables of interest), we aimed to take a more global, discovery approach by exploring associations between NfL, IL-6 and DPDD and both WM and GM across the whole brain. These whole brain analyses may have important implications for informing future work in this area. For GM analyses, we used a whole brain approach in Freesurfer (using Monte Carlo correction to correct for multiple comparisons) to identify additional locations of associations in the brain outside the middle frontal gyrus. We also conducted whole brain white matter analyses using TBSS (using cluster correction through FSL’s Randomise program to correct for multiple comparisons), to explore potential associations between variables of interest (IL-6, DPDD and NfL) and additional white matter tracts that were not included in our white matter composite. Age was included as a covariate in all whole brain analyses.
Proposed Conceptual Model
We proposed a broad conceptual model, in which we predicted that drinks per drinking day (DPDD) would be positively associated with plasma IL-6, negatively associated with MFG Thickness and positively associated with WM MD, and that IL-6 would be correlated negatively with MFG thickness and positively with the WM MD. We further hypothesized that plasma NfL would be negatively associated with GM thickness and positively associated with WM MD. We also predicted that cannabis use, BMI and age might be negatively associated with MFG thickness and positively associated with WM MD. Figure 1 shows a model depicting these proposed relationships. Gender, age, cannabis use and BMI were also included as covariates in this model. Because this is a new conceptual model which has not been explored previously, the first goal was to test individual linkages in the framework (via regression analyses described below) to understand which variables should be carried through to a formal test of the model. These individual linkages were first tested using regression models. Note that for all models, the significance level was set at p < 0.05 for all outcomes. In addition, p values above .05 below .1 were considered trends.
Figure 1. Conceptual model for path analysis.
This model shows the hypothesized relationships between alcohol consumption, IL-6, middle frontal gyrus gray matter, white matter mean diffusivity and NfL, including the effects of age, BMI, gender and cannabis use frequency.
Testing Individual Relationships Within Conceptual Model: Regression Analyses
For each relationship of interest within the conceptual model, we ran Ordinary Least Squares (OLS) regressions in SPSS (Version 27, IBM), in which each outcome variable of interest was regressed on the predictor of interest, age, total days of cannabis use in the past month, BMI and gender. Note that the cannabis use covariate was included because nearly half (48.1%) of scanned subjects in this study reported using cannabis in the past month. Total estimated intracranial volume was also included as a covariate in any regression involving MFG Thickness as a predictor or outcome. In total, we conducted 7 regression models testing relationships within the conceptual model: 1) IL-6 regressed on DPDD, 2) MFG Thickness regressed on DPDD, 3) WM MD regressed on DPDD, 4) MFG Thickness regressed on IL-6, 5) WM MD regressed on IL-6, 6) NfL regressed on MFG Thickness, and 7) NfL regressed on WM MD. In all regression models reported below, slope values are reported as standardized regression coefficients (unstandardized betas are included in Table 1).
Table 1.
Regression Results
| Model | Unstandardized B | Std Error | Standardized β | t | p | F | df | p | R 2 | adj R2 |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Criterion variable: IL-6 | ||||||||||
| Overall model | 3.187 | 5,110 | .010 | .127 | .087 | |||||
| Gender | −.213 | .152 | −.142 | −1.401 | .164 | |||||
| Age | .018 | .007 | .249 | 2.729 | .007 | |||||
| BMI | .026 | .015 | .164 | 1.801 | .074 | |||||
| Total Cannabis Days | −.003 | .008 | −.030 | −.333 | .740 | |||||
| Drinks Per Drinking Day | −.002 | .026 | −.008 | −.083 | .934 | |||||
| Criterion variable: NFL | ||||||||||
| Overall model | 6.317 | 5,62 | <.001 | .338 | .284 | |||||
| Gender | 1.311 | .720 | .196 | 1.821 | .073 | |||||
| Age | .115 | .037 | .358 | 3.146 | .003 | |||||
| BMI | −.160 | .074 | −.232 | −2.154 | .035 | |||||
| Total Cannabis Days | −.073 | .045 | −.173 | −1.627 | .109 | |||||
| White Matter Mean Diffusivity | 15342.290 | 10754.148 | .166 | 1.427 | .159 | |||||
| Criterion variable: MFG Thickness | ||||||||||
| Overall model | 4.611 | 6,73 | .001 | .275 | .215 | |||||
| Gender | −.019 | .032 | −.085 | −.601 | .550 | |||||
| Age | −.005 | .001 | .455 | −4.328 | <.001 | |||||
| BMI | −.002 | .002 | −.065 | −.641 | .524 | |||||
| Total Cannabis Days | .003 | .001 | .204 | 2.002 | .049 | |||||
| Est. Total Intracranial Volume | <.001 | <.001 | −.017 | −.136 | .892 | |||||
| Drinks Per Drinking Day | −.010 | .004 | −.287 | −2.416 | .018 | |||||
| Criterion variable: White Matter Mean Diffusivity | ||||||||||
| Overall Model | 3.950 | 5,73 | .003 | .213 | .159 | |||||
| Gender | <.001 | <.001 | .282 | 2.398 | .019 | |||||
| Age | <.001 | <.001 | .400 | 3.669 | <.001 | |||||
| BMI | <.001 | <.001 | −.178 | −1.679 | .097 | |||||
| Total Cannabis Days | <.001 | <.001 | .127 | 1.192 | .237 | |||||
| Drinks Per Drinking Day | <.001 | <.001 | .261 | 2.179 | .033 | |||||
| Criterion variable: MFG Thickness | ||||||||||
| Overall Model | 4.001 | 6,67 | .002 | .264 | .198 | |||||
| Gender | .012 | .028 | .058 | .438 | .663 | |||||
| Age | −.005 | .001 | −.436 | −4.100 | <.001 | |||||
| BMI | −.002 | .003 | −.079 | −.694 | .490 | |||||
| Total Cannabis Days | .003 | .002 | .206 | 1.850 | .069 | |||||
| Est. Total Intracranial Volume | <.001 | <.001 | .037 | .284 | .777 | |||||
| IL-6 | −.017 | .021 | −.098 | −.823 | .414 | |||||
| Criterion variable: NfL | ||||||||||
| Overall Model | 6.041 | 6,63 | <.001 | .365 | .305 | |||||
| Gender | 1.053 | .883 | .151 | 1.193 | .237 | |||||
| Age | .086 | .037 | .255 | 2.323 | .023 | |||||
| BMI | −.237 | .073 | −.330 | −3.235 | .002 | |||||
| Total Cannabis Days | −.019 | .047 | −.042 | −.396 | .694 | |||||
| Estimated Total Intracranial Volume | <.001 | <.001 | −.020 | −.160 | .873 | |||||
| MFG Thickness | −10.896 | 3.606 | −.340 | −3.022 | .004 | |||||
| Criterion variable: White Matter Mean Diffusivity | ||||||||||
| Overall Model | 3.647 | 5,66 | .006 | .216 | .157 | |||||
| Gender | <.001 | <.001 | .164 | 1.467 | .147 | |||||
| Age | <.001 | <.001 | .318 | 2.868 | .006 | |||||
| BMI | <.001 | <.001 | −.231 | −1.944 | .056 | |||||
| Total Cannabis Days | <.001 | <.001 | .169 | 1.457 | .150 | |||||
| IL-6 | <.001 | <.001 | .213 | 1.731 | .088 | |||||
Note. Significant p=values in bold type. MFG=Middle Frontal Gyrus
Final Conceptual Model
Based on any significant results that emerged from our regression analyses, we created an updated conceptual model to test using path analysis, which provides the multivariate and fully-specified test of the entire model. Analyses were conducted via structural path models using the SemTools (Jorgensen, Pornprasertmanit, Schoemann, & Rosseel, 2020) and Lavaan (Yves, 2012) packages in R (R Core Team, 2020). As in the regression models, age, gender, BMI and cannabis use were included as covariates in the path model. They were added as predictors in the path from DPDD to MFG Thickness. Model fit was assessed by traditional model fit indices including the χ2 likelihood ratio test, Root Mean Square Error of Approximation (RMSEA), the 95% confidence interval of the RMSEA (95% CIs), the Standardized Root Mean Square Residual (SRMR), and the Comparative Fit Index (CFI).
Results
Participant Characteristics.
Of the 182 individuals enrolled in the study, a subsample of n =81 were scanned (81 of whom had useable GM data and 80 of whom had useable WM data). Note that the entire sample of 182 was not able to be scanned due to a scanner upgrade that occurred during the middle of the study, as well as ineligibility due to MRI contraindications (e.g., metal in the body, previous head injuries, etc.). In total, 117 individuals had IL-6 data and 78 had NfL data. Of the 81 scanned individuals, 75 had both cytokine and GM data, and 71 had both NfL and GM data. In addition, 69 individuals had both NfL and WM data, and 73 had both cytokine and WM data. Demographics for all scanned individuals (n =81) are presented in Table 2.
Table 2.
Sample Demographics
| Characteristic | Total Scanned Participants (n = 81) % or Mean (SD) |
|---|---|
|
| |
| Age | 43.81 (10.19) |
| Gender (% female) | 38.30% |
| Race | |
| % American Indian/Alaska Native | 3.7% |
| % Asian | 0% |
| % Black | 1.2% |
| % Native Hawaiian or Pacific Islander | 0% |
| % White | 88.9% |
| % Other | 1.2% |
| % Mixed Race | 4.9% |
| Ethnicity | |
| % Hispanic/Latinx | 6.2% |
| %Non-Hispanic/Latinx | 93.8% |
| Baseline AUDIT | 17.02 (5.90) |
| Baseline BDI | 13.37 (7.72) |
| Baseline BAI | 6.89 (6.19) |
| Baseline Total Drinks (TLFB) | 118.85 (79.92) |
| Baseline Total Drinking Days (TLFB) | 21.67 (7.35) |
| Baseline Drinks per Drinking Day (TLFB) | 5.58 (3.05) |
| Baseline Percent Heavy Drinking Days (TLFB) | 57% (37%) |
| Baseline Tobacco Use Days (TLFB) | 5.12 (10.78) |
| Baseline Cannabis Use Days (TLFB) | 5.04 (8.37) |
Note. AUDIT=Alcohol Use Disorders Identification Test, BDI=Beck Depression Inventory-II, BAI=Beck Anxiety Inventory; TLFB=Timeline Followback
Drinking and GM (n=81)
In the model in which MFG Thickness was the criterion and DPDD at baseline was the predictor of interest, predictors accounted for 27.5% of the variance in MFG Thickness. Inspection of individual regression slopes indicated that baseline DPDD was significantly negatively associated with MFG Thickness b = −.287, t(73) = −2.416 p =.018. Total cannabis use days was positively associated with greater MFG Thickness b = .204, t(73) = 2.002, p =.049.
In the exploratory whole brain analysis, the association between DPDD and whole brain GM thickness across the whole brain was tested using a Monte Carlo correction (corrected at p = 0.05, covarying age). DPDD showed a significant negative correlation with the superior frontal, rostral middle frontal and caudal anterior cingulate regions (see Figure 2).
Figure 2. Association between drinks per drinking day (DPDD) and cortical thickness.
Panel A shows a scatterplot of the association between DPDD and cortical thickness (in mm) in our a priori brain region of interest, the middle frontal gyrus. Panel B shows the results of exploratory whole brain analyses. DPDD showed a significant negative correlation (blue) in the superior frontal, rostral middle frontal and caudal anterior cingulate. Results were Monte Carlo corrected and thresholded at p < 0.05
Drinking and WM (n=80)
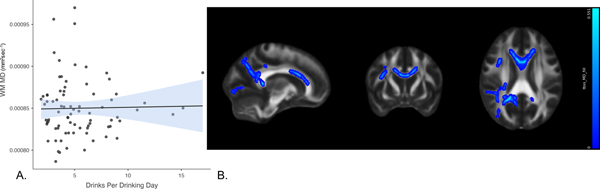
In the model in which WM MD was the criterion and DPDD was the variable of interest, predictors accounted for 21.3% of the variance in diffusivity. Inspection of individual regression slopes indicates that DPDD was positively associated with WM MD, b = .261, t(73) = 2.179, p = 0.033 (note that higher diffusivity suggests WM damage). In the exploratory whole brain analysis, mean diffusivity analyses were conducted in TBSS using DPDD as the predictor, covarying age. Positive associations with MD (Fig 3b) were found in the body and genu of the corpus callosum, superior and anterior corona radiata, and superior longitudinal fasciculus. However, these results did not pass thresholding at p=.05 (see Figure 3).
Figure 3. Association between drinks per drinking day and white matter mean diffusivity.
Panel A shows a scatterplot of the overall association between DPDD and mean diffusivity (in mm2sec−1) in the a priori white matter tracts of interest. Panel B [78, 134, 95] shows the results of exploratory whole brain analyses. DPDD showed positive associations (blue) with mean diffusivity in the body and genu of corpus callosum, superior and anterior corona radiata, and superior longitudinal fasciculus. These images are not thresholded. Significant voxels accentuated for visualization purposes.
IL-6 and DPDD (n =117)
No significant associations emerged between IL-6 and DPDD.
IL-6 and GM (n=75)
In the model in which MFG Thickness was the criterion and IL-6 was the predictor of interest, IL-6 was not significantly associated with MFG Thickness. In the exploratory whole brain analysis, the association between IL-6 and whole brain GM thickness across the whole brain was tested using a Monte Carlo correction (corrected at p = 0.05, covarying age). IL6 showed a negative correlation with the inferior parietal, middle temporal, and supra marginal regions (see Figure 4).
Figure 4. Associations between IL-6 and cortical thickness.
Panel A shows a scatterplot of the association between IL-6 (in pg/mL) and cortical thickness (in mm) in our a priori brain region of interest, the middle frontal gyrus. Panel B shows the results of exploratory whole brain analyses. IL-6 showed a significant negative correlation (blue) in inferior parietal, middle temporal, and supra marginal regions. Results were Monte Carlo corrected and thresholded at p < 0.05.
IL-6 and WM (n=73)
In the model in which WM MD was the criterion and IL-6 was the variable of interest, predictors accounted for 21.6% of the variance in diffusivity. Inspection of individual regression slopes indicates that IL-6 was positively associated with WM MD at trend-level, b = .213, t(66) = 1.731, p = 0.088 (note that higher diffusivity suggests WM damage). In the exploratory whole brain analysis, mean diffusivity analyses were conducted in TBSS using IL-6 as the predictor, covarying age. Positive associations with MD were found in the body, genu and splenium of the corpus callosum, left anterior corona radiata and left posterior limb of the internal capsule. However, these results did not pass thresholding at p=.05 (see Figure 5).
Figure 5. Association between IL-6 and white matter mean diffusivity.
Panel A shows a scatterplot of the overall association between IL-6 (in pg/mL) and mean diffusivity (in mm2sec−1) in the a priori white matter tracts of interest. Panel B [84 112 88] shows the results of exploratory whole brain analyses. IL-6 showed positive associations (blue) with mean diffusivity in the body, genu and splenium of the corpus callosum, left anterior corona radiata and left posterior limb of the internal capsule. These images are not thresholded. Significant voxels accentuated for visualization purposes.
NfL and GM (n =71)
In the model in which NfL was the criterion and MFG Thickness was the predictor of interest, predictors accounted for 36.5% of the variance in NfL, and MFG Thickness was significantly negatively associated with NfL, b = −.340, t(63) = −3.022, p = 0.004. Exploratory whole brain analyses were conducted in freesurfer using a Monte Carlo correction (corrected at p = 0.05, covarying age), with NfL as the predictor. Significant negative associations with NfL emerged in the middle temporal, superior temporal, pars opercularis, rostral middle frontal, caudal middle frontal, precentral, postcentral, superior frontal and precuneus regions (see Figure 6).
Figure 6. Association between NFL and cortical thickness.
Panel A shows a scatterplot of the association between NfL (in pg/mL) and cortical thickness (in mm) in our a priori brain region of interest, the middle frontal gyrus. Panel B shows the results of exploratory whole brain analyses. NfL shows a significant negative correlation (blue) in the middle temporal, superior temporal, pars opercularis, rostral middle frontal, caudal middle frontal, precentral, postcentral, superior frontal and precuneus regions. Results corrected for multiple comparisons with MonteCarlo simulation and thresholded at p < 0.05
NfL and WM (n =69)
In the model in which NfL was the criterion and WM MD was the predictor of interest, WM MD was not significantly associated with NfL. In the exploratory whole brain TBSS analysis using NfL as the predictor and covarying age, NfL was positively associated with MD in the body of the corpus callosum, right superior corona radiata, and right anterior corona radiata. However, these results did not pass thresholding at p=.05 (see Figure 7).
Figure 7. Effect of NfL on diffusivity metrics.
Panel A shows a scatterplot of the overall association between NfL (in pg/mL) and mean diffusivity (in mm2sec−1) in the a priori white matter tracts of interest. Panel B [91, 108, 91] shows the results of exploratory whole brain analyses. NfL showed positive associations (blue) with mean diffusivity in the body of the corpus callosum, right superior corona radiata, and right anterior corona radiata. These images are not thresholded. Significant voxels accentuated for visualization purposes.
GM Mediation Model
Given that regression analyses indicated that IL-6 was not related to MFG Thickness, WM MD or DPDD, IL-6 was not included in the path model. Further, because associations between WM MD and NfL were not significant, MFG Thickness was selected instead of WM MD as the brain variable to include in the path analysis. The path analytic model initially demonstrated poor fit to the data (χ2(11)= 50.43, p < 0.001, CFI = .70, RMSEA = 0.20 (90%CI = [0.11–0.31]), SRMR = 0.07) such that modification indices were examined. These suggested that BMI should be included as a predictor of NfL. When this change was made, model fit improved to adequate levels; χ2(11)= 50.43, p < 0.001, CFI = .92, RMSEA = 0.12 (90%CI = [0.00–0.26]), SRMR = 0.04. This final model showed significant paths from DPDD to MFG Thickness and MFG Thickness to NfL but not DPPD to NfL. Specifically, there were significant, negative associations between DPDD and MFG Thickness and between MFG Thickness and NfL, but a non-significant association between DPDD and NfL (see Figure 8). Additionally, BMI was significantly, negatively associated with NfL, while age significantly predicted MFG Thickness.
Figure 8. Gray Matter (GM) mediation analysis results.
The path analytic model showed an adequate fit to the data and significant paths emerged from DPDD to GM and GM to NfL, but not from DPDD to NfL. Age predicted GM and BMI predicted NfL.
Discussion
We hypothesized that alcohol consumption would be positively associated with peripheral inflammation (plasma-IL-6), negatively associated with MFG Thickness and positively associated with WM MD. We also expected that IL-6 would be negatively correlated with MFG Thickness and positively correlated with WM MD and that significant associations would emerge between levels of circulating NfL and both MFG Thickness and WM MD. Regression analyses supported most of these relationships, with the exception of associations involving IL-6. We also failed to observe a significant association between NfL and WM MD. As expected, DPDD was associated with decreased MFG Thickness and compromised WM integrity. We also found a significant negative association between NfL and MFG Thickness. The path analysis also supported overall hypotheses, given the expected associations that emerged between heavy drinking, MFG Thickness and NfL in the structural model. Specifically, higher DPDD scores were associated with lower middle frontal GM thickness while lower MFG Thickness was in turn associated with higher NfL. These associations provide further evidence of a relationship between heavy drinking and damage to the brain with subsequent markers of damage (i.e., NfL). Findings suggest that NfL could be a promising biomarker for structural brain differences among heavy drinking individuals
Counter to hypotheses, significant relationships between IL-6 and MFG Thickness and WM MD, did not emerge. It is notable, however, that results of the exploratory whole brain analyses indicated that IL-6 was negatively correlated with GM in the inferior parietal, middle temporal and supramarginal regions. These findings may serve as preliminary evidence that could inform future explorations of the associations between inflammatory markers and GM in heavy-drinking samples. The lack of association between WM MD and IL-6 may be due to the fact that IL-6 is not an adequate measure of inflammation in this sample (i.e., perhaps other inflammatory markers or a composite of multiple markers would be more informative), or because peripheral inflammation is not directly related to WM MD. In clinical research, it is difficult to ascertain reliable measures of neuroinflammation without using the more expensive and invasive method of positron emission tomography (PET) (Feldman et al., 2020). Thus, inherent limitations of peripheral measures are par for the course.
Based on significant prior literature linking excess drinking to reduced WM integrity and GM damage (specifically in frontal brain regions), we expected that alcohol consumption (DPDD) would be negatively associated with MFG Thickness and positively associated with WM diffusivity. In regression models, we found expected negative associations between DPDD and MFG Thickness. We also found that DPDD was associated with higher levels of WM diffusivity (note: higher WM MD is, in general, thought to be indicative of compromised WM integrity). Notably, when DPDD was explored as a predictor of whole brain WM diffusivity in TBSS, associations failed to pass thresholding. This is inconsistent with previous studies demonstrating that alcohol consumption was associated with compromised WM integrity in heavy drinkers (Monnig et al., 2015). We suspect the lack of strong association between drinking and WM in this sample may be due to the fact that the individuals included in the present sample were not severe drinkers (i.e., they were not diagnosed with AUD), and that this association would be more likely to emerge in a more severe (e.g., inpatient) drinking sample.
Notably, in addition to observing expected negative associations between DPDD and MFG Thickness, the regression model also showed a positive association between total cannabis days and MFG Thickness. Some existing data, including our group’s prior work, indicates a lack of associations between cannabis use and structural brain measures in adults or adolescents (Scott et al., 2019; Thayer et al., 2017). However, consistent with the present findings, several other studies have shown positive associations between cannabis use and higher GM in certain brain regions, such as the basal ganglia (Moreno-Alcázar et al., 2018) and left middle frontal gyrus (in a schizophrenia sample) (Schnell et al., 2012). Results from the present analyses suggest that caudal and rostral middle frontal regions also show a positive relationship with cannabis use.
In general, these results support our proposed model, and indicate the need for further research exploring the effects of alcohol consumption on inflammatory biomarkers, brain structure, and ultimately, brain function and behavior. Importantly, results also suggest that NfL may be a useful biomarker for the GM changes that may result from chronic exposure to alcohol. The use of an NfL biomarker would be significantly less costly and time-consuming compared to neuroimaging, as NfL is relatively easy to assess via collection of blood samples. Notably, rodent work supports NfL as a marker of disease progression and treatment response in mouse models of neurodegenerative disorders (Bacioglu et al., 2016), and several human studies support NfL as a biomarker for neurodegenerative disorders (Byrne et al., 2017; Hansson et al., 2017; Khalil et al., 2018; Rohrer et al., 2016), but no clinical or preclinical studies to date have explored NfL as a potential biomarker in the context of AUD or heavy alcohol consumption. Thus, the present study is the first to provide preliminary evidence that NfL may have significant research and clinical applications in the context of heavy drinking individuals.
Limitations and Future Directions
The current study is limited by the cross-sectional nature of the data, which prevents us from drawing causal conclusions about the impact of alcohol consumption on inflammation, brain structure or NfL. Future studies should examine these associations in a longitudinal design (e.g., following a treatment-engaged, heavy drinking sample over time as individuals reduce their drinking to explore whether NfL levels decrease) that allows for the determination of temporal precedence and mediational models. Also, the analyses in the present paper were limited by a restriction in the range of alcohol use severity of the participants. Future studies targeting a sample that includes patients with more severe AUD will likely have increased power to detect associations between alcohol intake, inflammation and brain structure. The analyses were also limited by the fact that the sample was mostly comprised of White, non-Hispanic/Latinx individuals, thus results may not be generalizable to other populations. Future studies should prioritize inclusion of racially and ethnically diverse samples.
Also, interpretations of white matter microstructure with diffusion tensor imaging are limited by the fact that multiple orientations of fiber populations may exist within a single voxel, thereby affecting the voxel-wide eigenvalues. Along with differing fiber orientations; axonal diameter, population density, and membrane permeability can also affect the interpretation of these results (Jones et al., 2013). Finally, time of day was not standardized for blood sample collection across participants. Ideally, biomarkers such as IL-6 and NfL should be collected at the same time of day to minimize the influence of circadian rhythms on levels of these markers.
Conclusions
The present study shows support for associations between alcohol consumption, brain structure and circulating NfL measured via peripheral blood. Conversely, the utility of the pro-inflammatory cytokine IL-6 as a biomarker for the inflammatory effects of alcohol was not supported. Despite several limitations, the present study presents a critical step in identifying and testing important links between alcohol exposure, inflammation, and structural changes in the brain, and provides preliminary support for a new biomarker (i.e., NfL) that may track these changes.
Funding:
This work was supported by R01AA024632 to KEH. The content is solely the responsibility of the authors and does not represent the opinion of the National Institutes of Health.
Footnotes
The authors have no other conflicts of interest to declare.
Clinicaltrials.gov registration number: NCT02994043
References
- Adams C, Conigrave JH, Lewohl J, Haber P, Morley KC (2020) Alcohol use disorder and circulating cytokines: A systematic review and meta-analysis. Brain Behav Immun 0–1. [DOI] [PubMed] [Google Scholar]
- Ahrnsbrak R, Bose J, Hedden SL, Lipari RN, Park-Lee E (2017) Key Substance Use and Mental Health Indicators in the United States: Results from the 2016 National Survey on Drug Use and Health. Rockville, MD. [Google Scholar]
- Andersson JLR, Skare S, Ashburner J (2003) How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. Neuroimage 20:870–888. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Sotiropoulos SN (2016) An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125:1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacioglu M, Maia LF, Preische O, Schelle J, Apel A, Kaeser SA, Schweighauser M, Eninger T, Lambert M, Pilotto A (2016) Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron 91:56–66. [DOI] [PubMed] [Google Scholar]
- Bagga D, Sharma A, Kumari A, Kaur P, Bhattacharya D, Garg ML, Khushu S, Singh N (2014) Decreased white matter integrity in fronto-occipital fasciculus bundles: Relation to visual information processing in alcohol-dependent subjects. Alcohol 48:43–53. [DOI] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Thayer RE, Tapert SF (2013) Longitudinal Changes in White Matter Integrity Among Adolescent Substance Users. Alcohol Clin Exp Res 37:E181–E189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettcher BM, Watson CL, Walsh CM, Lobach IV., Neuhaus J, Miller JW, Green R, Patel N, Dutt S, Busovaca E, Rosen HJ, Yaffe K, Miller BL, Kramer JH (2014) Interleukin-6, Age, and Corpus Callosum Integrity. PLoS One 9:e106521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishehsari F, Magno E, Swanson G, Desai V, Voigt RM, Forsyth CB, Keshavarzian A (2017) Alcohol and Gut-Derived Inflammation. Alcohol Res 38:163–171. [PMC free article] [PubMed] [Google Scholar]
- Byrne LM, Rodrigues FB, Blennow K, Durr A, Leavitt BR, Roos RAC, Scahill RI, Tabrizi SJ, Zetterberg H, Langbehn D, Wild EJ (2017) Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington’s disease: a retrospective cohort analysis. Lancet Neurol 16:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J (2006a) Cytokines and alcohol. Alcohol Clin Exp Res 30:720–730. [DOI] [PubMed] [Google Scholar]
- Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J (2006b) Cytokines and Alcohol. Alcohol Clin Exp Res 30:720–730. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999) Cortical Surface-Based Analysis: I. Segmentation and Surface Reconstruction. Neuroimage 9:179–194. [DOI] [PubMed] [Google Scholar]
- Donohue B, Azrin NH, Strada MJ, Silver NC, Teichner G, Murphy H (2004) Psychometric evaluation of self-and collateral timeline follow-back reports of drug and alcohol use in a sample of drug-abusing and conduct-disordered adolescents and their parents. Psychol Addict Behav 18:184. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Buck HM, Bordner K, Richey L, Jones ME, Deak T (2014) Intoxication- and Withdrawal-Dependent Expression of Central and Peripheral Cytokines Following Initial Ethanol Exposure. Alcohol Clin Exp Res 38:2186–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, Meyerhoff DJ (2011) Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol Clin Exp Res 35:1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE, Mcpherson KL, Biesecker CL, Wiers CE, Manza P, Volkow ND, Wang G-J (2020) Neuroimaging of inflammation in alcohol use disorder: a review 63:19. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM (1999) Cortical Surface-Based Analysis: II: Inflation, Flattening, and a Surface-Based Coordinate System. Neuroimage 9:195–207. [DOI] [PubMed] [Google Scholar]
- Gano A, Doremus-Fitzwater TL, Deak T (2016) Sustained alterations in neuroimmune gene expression after daily, but not intermittent, alcohol exposure. Brain Res 1646:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group CSR (2003) Testing combined pharmacotherapies and behavioral interventions in alcohol dependence: rationale and methods. Alcohol Clin Exp Res 27:1107–1122. [DOI] [PubMed] [Google Scholar]
- Hansson O, Janelidze S, Hall S, Magdalinou N, Lees AJ, Andreasson U, Norgren N, Linder J, Forsgren L, Constantinescu R, Zetterberg H, Blennow K (2017) Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology 88:930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R (2013) White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage 73:239–254. [DOI] [PubMed] [Google Scholar]
- Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, Barro C, Kappos L, Comabella M, Fazekas F, Petzold A, Blennow K, Zetterberg H, Kuhle J (2018) Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. [DOI] [PubMed] [Google Scholar]
- Kim SH, Choi MK, Park NY, Hyun JW, Lee MY, Kim HJ, Jung SK, Cha Y (2020) Serum neurofilament light chain levels as a biomarker of neuroaxonal injury and severity of oxaliplatin-induced peripheral neuropathy. Sci Rep 10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR (2008) Interleukin-6 Covaries Inversely with Hippocampal Grey Matter Volume in Middle-Aged Adults. Biol Psychiatry 64:484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhoon Y, Czerkawski C, Crowley DJ, Cohen-Gilbert JE, Sneider JT, Silveri MM (2014) Binge Alcohol Consumption in Emerging Adults: Anterior Cingulate Cortical “Thinness” Is Associated with Alcohol Use Patterns. Alcohol Clin Exp Res 38:1955–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechtcheriakov S, Brenneis C, Egger K, Koppelstaetter F, Schocke M, Marksteiner J (2007) A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. J Neurol Neurosurg Psychiatry 78:610–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnig MA, Yeo RA, Tonigan JS, McCrady BS, Thoma RJ, Sabbineni A, Hutchison KE (2015) Associations of White Matter Microstructure with Clinical and Demographic Characteristics in Heavy Drinkers. PLoS One 10:e0142042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Alcázar A, Gonzalvo B, Canales-Rodríguez EJ, Blanco L, Bachiller D, Romaguera A, Monté-Rubio GC, Roncero C, McKenna PJ, Pomarol-Clotet E (2018) Larger Gray Matter Volume in the Basal Ganglia of Heavy Cannabis Users Detected by Voxel-Based Morphometry and Subcortical Volumetric Analysis. Front Psychiatry 9:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, Van Zijl PCM, Nagae-Poetscher LM (2005) MRI atlas of human white matter. Elsevier. [DOI] [PubMed] [Google Scholar]
- Nakamura-Palacios EM, Souza RSM, Zago-Gomes MP, de Melo AMF, Braga FS, Kubo TTA, Gasparetto EL (2014) Gray matter volume in left rostral middle frontal and left cerebellar cortices predicts frontal executive performance in alcoholic subjects. Alcohol Clin Exp Res 38:1126–1133. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Bahorik A, Sidney S, Launer LJ, Yaffe K (2020) Relationships of Inflammation Trajectories with White Matter Volume and Integrity in Midlife. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK (1999) Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol 515:287–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan E V. (2005) Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: Evidence from diffusion tensor imaging. Neuropsychopharmacology 30:423–432. [DOI] [PubMed] [Google Scholar]
- Quaglieri A, Mari E, Boccia M, Piccardi L, Guariglia C, Giannini AM (2020) Brain Network Underlying Executive Functions in Gambling and Alcohol Use Disorders: An Activation Likelihood Estimation Meta-Analysis of fMRI Studies. Brain Sci 10:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Woollacott IOC, Dick KM, Brotherhood E, Gordon E, Fellows A, Toombs J, Druyeh R, Cardoso BMJ, Ourselin S, Nicholas JM, Norgren N, Mead S, Andreasson U, Blennow K, Schott JM, Fox NC, Warren JD, Zetterberg H (2016) Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. [DOI] [PMC free article] [PubMed]
- Salimetrics (2021) Calculating Inter- and Intra-Assay Coefficients of Variability. Available at: https://salimetrics.com/calculating-inter-and-intra-assay-coefficients-of-variability/
- Schain M, Kreisl WC (2017) Neuroinflammation in Neurodegenerative Disorders—a Review. Curr Neurol Neurosci Rep. [DOI] [PubMed] [Google Scholar]
- Schnell T, Kleiman A, Gouzoulis-Mayfrank E, Daumann J, Becker B (2012) Increased gray matter density in patients with schizophrenia and cannabis use: A voxel-based morphometric study using DARTEL. Schizophr Res 138:183–187. [DOI] [PubMed] [Google Scholar]
- Scott JC, Rosen AFG, Moore TM, Roalf DR, Satterthwaite TD, Calkins ME, Ruparel K, Gur RE, Gur RC (2019) Cannabis use in youth is associated with limited alterations in brain structure. Neuropsychopharmacology 44:1362–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1:S208–19. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992) Timeline follow-back In: Measuring Alcohol Consumption, pp 41–72. Springer. [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA (2001) fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin Exp Res 25:236–245. [PubMed] [Google Scholar]
- Thayer RE, YorkWilliams S, Karoly HC, Sabbineni A, Ewing SF, Bryan AD, Hutchison KE (2017) Structural neuroimaging correlates of alcohol and cannabis use in adolescents and adults. Addiction 112:2144–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holst RJ, de Ruiter MB, van den Brink W, Veltman DJ, Goudriaan AE (2012) A voxel-based morphometry study comparing problem gamblers, alcohol abusers, and healthy controls. Drug Alcohol Depend 124:142–148. [DOI] [PubMed] [Google Scholar]
- Walker KA, Power MC, Hoogeveen RC, Folsom AR, Ballantyne CM, Knopman DS, Windham BG, Selvin E, Jack CR, Gottesman RF (2017) Midlife systemic inflammation, late-life white matter integrity, and cerebral small vessel disease the atherosclerosis risk in communities study. Stroke 48:3196–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]