Abstract
Compounds that selectively modulate multiple targets can provide clinical benefits and are an alternative to traditional highly selective agents for unique targets. High-throughput screening (HTS) for multitarget-directed ligands (MTDLs) using approved drugs, and fragment-based drug design has become a regular strategy to achieve an ideal multitarget combination. However, the unexpected presence of pan-assay interference compounds (PAINS) suspects in the development of MTDLs frequently results in nonspecific interactions or other undesirable effects leading to artefacts or false-positive data of biological assays. Publicly available filters can help to identify PAINS suspects; however, these filters cannot comprehensively conclude whether these suspects are “bad” or innocent. Additionally, these in silico approaches may inappropriately label a ligand as PAINS. More than 80% of the initial hits can be identified as PAINS by the filters if appropriate biochemical tests are not used resulting in false positive data that are unacceptable for medicinal chemists in manuscript peer review and future studies. Therefore, extensive offline experiments should be used after online filtering to discriminate “bad” PAINS and avoid incorrect evaluation of good scaffolds. We suggest that the use of “Fair Trial Strategy” to identify interesting molecules in PAINS suspects to provide certain structure‒function insight in MTDL development.
Keywords: Multitarget-directed ligands, PAINS suspects, In silico filtering, Biochemical experiment, Fair trial strategy
Abbreviations: AD, Alzheimer disease; ALARM NMR, a La assay to detect reactive molecules by nuclear magnetic resonance; CADD, computer-aided drug design technology; CoA, coenzyme A; EGFR, epidermal growth factor receptor; GSH, glutathione; HER2, human epidermal growth factor receptor 2; HTS, high-throughput screening; LC−MS, liquid chromatography−mass spectrometry; MTDLs, multitarget-directed ligands; QSAR, quantitative structure–activity relationship; PAINS, pan-assay interference compounds; ROS, radicals and oxygen reactive species
Graphical abstract
PAINS result in nonspecific interactions or other undesirable effects. The use of “Fair Trial Strategy” to identify interesting molecules in PAINS to provide certain structure‒function insight in multitarget-directed ligand (MTDL) development.
1. Introduction
The advantages of multitarget-directed ligands (MTDLs) suggest that agents capable of modulating multiple targets in a selective manner can improve the balance between clinical and therapeutic benefit and safety compared to the characteristics of unique target-directed agents1,2. The types of scaffolds needed to design MTDLs require accurate analysis of target–disease associations, pathway−target−drug–disease relationships, and adverse event profiling3. Therefore, the application of computer-aided drug design technology (CADD), advanced systems biology, and chemical biology is shifting MTDL development paradigm from low affinity inhibition of multiple-targets to an approach involving interactions with mutually regulated targets to achieve synergistic and detoxifying effects4. However, meaningful interference of MTDLs with multiple targets has been flagged as undesirable for a long time due to questionable rationality and selectivity of MTDLs, which are considered “bad” scaffold suspects (known as pan-assay interference compounds, PAINS)5. The instances of “bad” scaffolds are often disguised as a drug combination and may be involved in nonspecific interactions, leading to artefacts in biological assays6. For example, arylpiperazine substructure has been considered a suitable scaffold for fine balancing of D2, 5-HT1A and 5-HT2A receptor activities to improve antipsychotic efficacy or mitigate adverse effects7. The prototype of the third-generation of antipsychotics, aripiprazole, launched into the market in 2015 was the first designed serotonin-dopamine activity modulator. However, the compound also displayed unwanted side effects probably due to sustained interaction as a false positive structure with post-synaptic D2 receptors7,8.
Exemplary PAINS recognized as small molecule suspects include anilines, rhodanines, curcuminoids, Michael acceptors as irreversible inhibitors, and Mannich bases, which are typically present as substructures in other molecules9, 10, 11. PAINS are usually attributed to nonspecific binding or assay artefacts based on the structure or binding interactions or to indirect potential functionalization effects, such as sample fluorescence, which is the most difficult issue in biochemical assays12,13. To improve the affinity of PAINS toward targets, medicinal chemists spend considerable time and effort to produce various analogs and optimize the activity of PAINS11. In contrast, excessive screening and unjustified assessment may often result in abandonment of “good” scaffolds due to suspected PAINS. These considerations may confuse peer reviewers in their assessment of the submitted manuscripts and places many inexperienced chemists in a complex situation due to required activity evaluation and fragment selection in the developments of MTDLs5. Thus, these suspects compounds should be treated with rigorous and investigative “Fair Trail Strategy” to refrain from advancing a “bad” PAINS or discarding a “good” scaffold11,14,15. It is appropriate and relevant to screen selective scaffolds that combine specified multi-target properties according to the paradigm of targeted pharmacology and “network pharmacology”3. In case of a concern about PAINS suspects, detailed and reasonable follow-up experiments are essential to exculpate the innocent PAINS suspects to enable their development and to validate the expected functions of the “bad” suspects prior to discarding these compounds from further consideration16. Publications should allow authors to quantify the roles of each functional group of the PAINS suspects in their submitted manuscript rather than firmly reject the candidates due to biased perception.
2. Performance of PAINS suspects in MTDL development
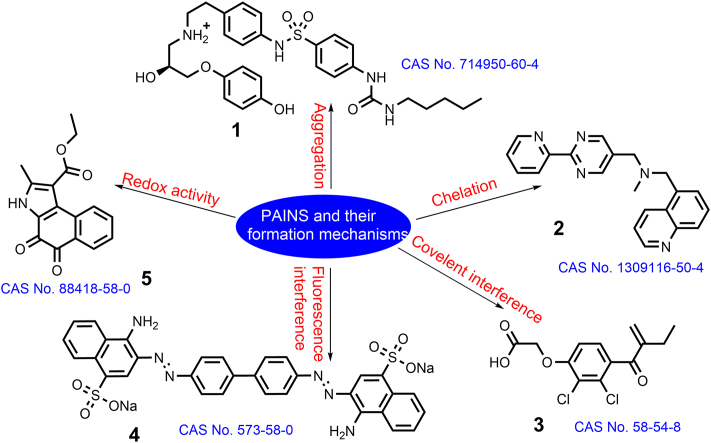
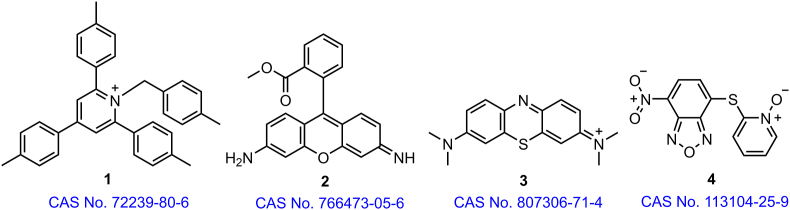
The research and development strategies of MTDLs have been extensively focus on high-throughput screening (HTS), fragment based synthetic approaches, and physicochemical, pharmacodynamic, and network pharmacology aspects17. PAINS alerts are obviously more frequent for the combination of two or more specifically selected targets than those for a single target regardless of the approach used to develop MTDLs. The false-positive results caused by real PAINS are predominantly caused by a variety of mechanisms including the formation of colloidal aggregates (1, Fig. 1)18, chelation (2, Fig. 1)19, covalent protein reactivity (3, Fig. 1)20, interference with assay spectroscopy (4, Fig. 1)21,22, redox activity (5, Fig. 1)23, membrane disruption24, high molecular flexibility and hydrophobicity25, decomposition in buffers26, and photoactivation27. The first five mechanisms are considered to be the most important factors in the assignment of PAINS (Table 16,18, 19, 20, 21,23). Hence, there is no single diagnostic analysis system for the entire list of “bad” compounds5.
Figure 1.
Examples of PAINS suspects and their activity modes and action profiles.
Table 1.
Main chemotypes of PAINS and their mechanisms to cause promiscuity.
| Interference | Principle | Chemotypes |
|---|---|---|
| Covalent interaction | Covalently bind to all sorts of macromolecules | Quinones, alkylidene barbiturates, rhodanines, omeprazole, carbidopa, ethacrynic acid, enones, related heterocycle 6,20 |
| Colloidal aggregation | Non-specifically bind to proteins, confounding and irrelevant enzymatic responses | Miconazole, nicardipine, trifluralin, cinnarizine, tetraiodophenolphthalein, staurosporine aglycone18 |
| Redox cycling | Generate ROS and indirectly inhibit the catalytic activity of proteins | Phenol-sulphonamides, pyrimidotriazinediones, β-lapachone, arylsulfonamides, tolyl hydrazides, quinones and catechols 6,23 |
| Ion chelation | Can form chelate for a lot of potential proteins and functional systems | Hydroxyphenyl hydrazones, quinones and catechols, rhodanines, 2-hydroxybenzylamine 6,19 |
| Sample fluorescence | Fluorophoric properties can affect the biological evaluation results | Daunomycin, topotecan, and riboflavin, quinoxalin-imidazolium substructures21 |
Most of MTDLs obtained from HTS have relatively small molecular weight28. Introduction of appropriate fragments or removal of unnecessary groups from the favorable position is completely determined by the target and can be used to balance the effect of multi-target combination therapy. Therefore, general screening compound decks can be compiled based on the scaffolds with low structural complexity to increase the number of multitarget combinations and facilitate rapid hit-to-lead expansion12. The strategy of fragment recombination may be more popular and desirable for the customized design of MTDLs. Regardless of the methods used to obtain the ligands, the structural environment of potential scaffolds plays a critical role in the development of MTDLs due to their potential to be flagged as PAINS suspects29. Multiple evidence provided by the analysis of analog series containing suspected artefacts enables the detection of PAINS in various scaffolds30. In addition to PAINS, a number of other agents may have interference potential31. Similarly, in the case of certain highly promiscuous scaffolds, there is no direct evidence demonstrating possible assay artefacts31. Relationships between lack of activity, specific activity, and artificial performance of the agents with potential liabilities are highly confusing and difficult to distinguish32. Hence, comprehensive investigation of multitarget activities and assay interference patterns in HTS and assessment of fragment recombination are the major tasks in MTDL development (Fig. 2)14,33.
Figure 2.
Rational design of multitarget-directed ligands.
2.1. Performance of HTS in MTDL discovery and PAINS identification
HTS has become a regular strategy in the discovery of screening hits as a good starting point for the growing list of validated multi-targets or as a useful tool for MTDL discovery. Artificial intelligence, such as neural networks, is critical for prediction of interactions between the screening hits and related targets to prioritize the ligands that can be further developed as MTDLs or to provide analysis of interactions with multiple targets34. The issues surrounding HTS received considerable attention because some of the screening hits are truly promiscuous ligands that potently, specifically, and reversibly bind to a multi-target combination related to a complex disease35,36. However, detection of desired and undesired ligands by HTS identifies certain scaffolds that do not necessarily reflect the binding promiscuity of the individual molecules. In contrast, new conceptual frameworks, such as systems biology and polypharmacology, may suggest that molecules, which often produce biological effects in multiple tests, do not interfere with bioassays; optimization of these compounds is challenging but can yield innovative and safe drugs15.
The use of HTS for exclusion of PAINS suspects from chemical libraries is another controversial issue. The applications of public PAINS filters and other HTS methods are important for identification of undesired scaffolds; however, these methods are frequently insufficiently developed and are not completely reliable in identification of all possible relationships between the scaffolds and protein structures5. Up to 80%–100% of initial HTS hits in various screening models and various target activators/inhibitors can be labeled as artefacts if appropriate control experiments are not employed37, 38, 39, 40. The activity of the majority of PAINS is positive only in a small number of assays14 and depends on specific experimental conditions and modes of action5. Usually, PAINS are the compounds with a high hit rate, which interfere with the screening and detection methods, and poor drug properties of PAINS are an important issue in drug development35. The most frequent “bad” PAINS identified by HTS are rhodanine and its derivatives, which have been extensively reported to have a wide range of antibacterial activities at the cellular and compound level in more than 2000 publications41,42. However, due to extensive and complex mechanism of action, specific targets of these compounds cannot be determined, which becomes the main obstacle for further development32.
2.2. Performance of PAINS suspects in fragmentation-based MTDL design
Fragmentation-based MTDL design generally requires a certain degree of synergy and balance between the optimized promiscuous ligands and the targets due to the mutual interactions of the targets in the clinical treatment of multiple cause diseases. The theoretical premise of fragmentation-based MTDL design is to determine the important role of related targets in the pathological process43. Then, rational structural optimization based on a pharmacophore skeleton can yield effective ligands for multiple targets. To achieve a better synergistic effect, the targets of ligand binding should be confirmed, and then suitable scaffolds need to be introduced to increase the affinity to the target combination5. A comprehensive analysis of ligand−target interactions indicates that multifamily ligands frequently result in multiple interactions in the binding sites even if the compounds have homologous bound conformations; these properties rationalize promiscuous binding events at the molecular level44.
Generally, the mechanisms of action of drugs and targets involve regulation of the functions of the target proteins through noncovalent interactions. However, PAINS scaffolds have relatively high chemical activities and can be covalently combined with proteins, DNA, etc.45. Proteins are often very sensitive to the PAINS scaffolds containing electrophilic groups, and these scaffolds can influence enzyme activity through irreversible reactions with proteins, which in turn cause false positives screening results. Additionally, PAINS scaffolds containing electrophilic groups are easily hydrolyzed or decomposed by organic solvents to generate active fragments, which act on targets and change enzyme activity35,46. On the other hand, certain PAINS scaffolds, such as aromatic hydrocarbons, polyphenols, and hyperlipophilic and conjugated scaffolds, can form aggregates due to molecular interactions and can bind to the targets with high affinity in the reaction systems thus causing false positive results35.
3. Exculpation of cunning PAINS suspects
As mentioned above, several mechanisms have been proposed to account for PAINS interference in MTDL development, and it is impossible to distinguish “bad” compounds only by an independent assessment system. The advanced chemical databases of PAINS filtering are currently available with annotated biological activities, and several successful applications of PAINS filtering were described. For example, the application of the Bioassay and Drug Screening Platform managed by LaBECFar-FIOCRUZ can routinely and rapidly identify PAINS with selectivity screens31. However, current public PAINS filters are not sufficient to identify all possible relationships between chemical and multiple targets as indicated by various types of false negatives detected in the analysis. Certain PAINS can be inadvertently or mechanically distinguished from nonspecific interactions or other undesirable effects leading to detection of irrelevant activities of the suspects (real PAINS)6. These “bad” PAINS frequently escape the judgment by peer reviewers and majestically appear in publications, since target reactivity or specificity can be higher than that of the other compounds identified by screening5.
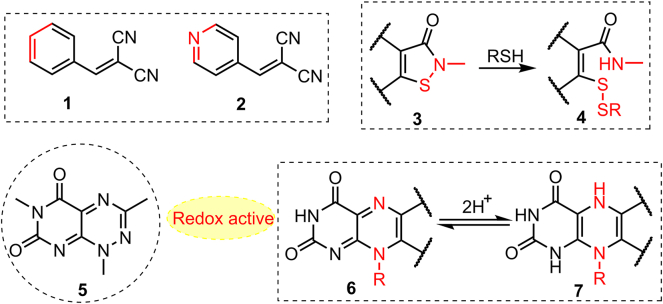
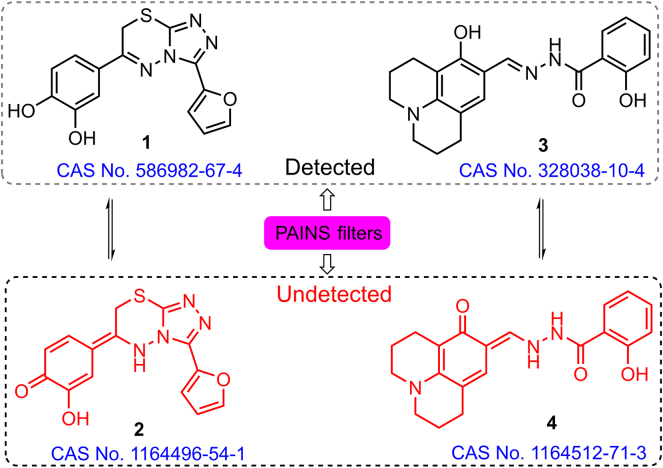
PAINS are defined in terms of a curated screening library, which is one of the reasons for exculpation of the suspects in filtering. These screening libraries reasonably represent chemical resources of subsequent modifications and were used to eliminate multiple PAINS suspects in many cases by preliminary filtering11. For example, dicyanoalkene 1 is a recognized “bad” PAINS; however, public filter ene_cyano_A did not identify 2 (substructure of 1) as a PAINS because 2 did not include any scaffolds in the initial screening library used to define PAINS11. Other reactive scaffolds, such as isothiazolones 3 and pyrimidotriazinediones 5, cannot be detected by PAINS filters because their “bad” characteristics were identified only after filter definition11,47 (Fig. 3).
Figure 3.
Structures defined in public filters that are not generally recognized as PAINS. Compounds such as 3 and 5 could not be considered useful or progressable and should be excluded from screening libraries. Compound 5 should be redox active and is not unexpected considering its similarity to the isoalloxazine ring of flavins (6 and 7)48,49.
Additionally, compounds with PAINS substructures usually have variable performance in the screening assays, and many substructures are occasionally or consistently inactive14. Thus, vulnerability of PAINS filtering may be due to the presence of these inactive PAINS-containing substructures during the detection process (1–4, Fig. 4)11. An increase in the activity threshold of the filtering will result in a lower limit of the hit rates. Therefore, potential false positive rates of PAINS are generally higher than the corresponding anticipated values for the compounds that are expected to produce analytical artefacts14. In these cases, multiple assay activities should be monitored to identify PAINS interference.
Figure 4.
Representative PAINS structures of numerous consistently inactive compounds.
Another reason for incorrect identification of “bad” PAINS is partially due to tautomerism, such as compounds 1–2 and 3–4 in Fig. 5; other compounds may cause assay artefacts that are not defined as PAINS31. However, the compounds initially defined as PAINS should be considered candidates for subsequent investigation of multitarget activities and molecular mechanisms of polypharmacology31 to assess the details of “good” promiscuity or “bad” PAINS. Similarly, the suspects not identified by PAINS filters provide opportunities for subsequent investigation of assay interference.
Figure 5.
PAINS structures are not recognized by the public filters due to tautomerism.
4. Injustice to innocent PAINS suspects
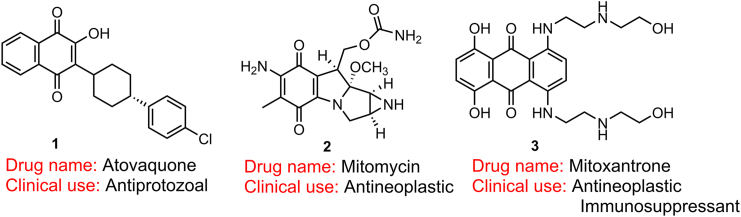
The application of public virtual filters for exclusion of PAINS suspects is controversial, and many drugs approved in the clinic (such as atovaquone 1, mitomycin 2, and mitoxantrone 3 in Fig. 6) would never reach the market if medicinal chemists have relied on these filters10,15,37. These tools do not comprehensively identify all suspected substructures or promiscuity as PAINS and may also inappropriately label a compound as an artefact50. Particularly, some suspects are truly undesired false positives; however, very simple structural modifications can change the potency and selectivity of these compounds. Recent publications suggested that chemical and biological methods identify many screening hits that contain PAINS alerts and directly define PAINS as “bad actors” that exhaust the energy and lower the expectations thus warning medicinal chemists6. Public reports of private trials make PAINS suspects to leave a subconscious bad impression on many editors and peer reviewers. Fortunately, this issue has attracted the attention of many editors-in-chief of ACS journals, including Journal of Medicinal Chemistry, ACS Central Science, and ACS Chemical Biology5. According to these fair and experienced editors, even if some screening hits can be defined as general PAINS alerts by public filters, the compounds cannot be considered useless in subsequent well-planned studies5.
Figure 6.
FDA- and worldwide-approved drugs contain PAINS chemotypes.
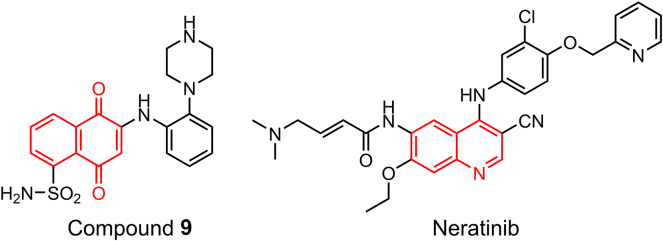
Naphthoquinone is one of the PAINS suspects that offers an easily accessible and extensively investigated scaffold for design of new drugs. Naphthoquinones and their derivatives undergo redox cycles in vivo generating semiquinone radicals and reactive oxygen species (ROS) that may interfere with multiple targets51 resulting in their identification as PAINS by several filtering tools. In fact, some naphthoquinones are druggable agents with options for structure optimization by established synthetic pathways52. Many antitumor naphthoquinone derivatives are selective toward cancer versus normal cells due to specific metabolism53. For example, naphthalene-5,8-dione-1-sulfonamide-based STAT3 inhibitor 9 (Fig. 7) was designed by advanced multiple ligand simultaneous docking (AMLSD) and is characterized by superior druggability compared with other representative inhibitors. Compound 9 selectively binds to p-STAT3(Y705) versus STAT1/5, p-AKT, and p-ERK kinases in the similar pathways and versus other proximal sites. Analysis by the online public filters and biochemical assays identified compound 9 as a PAINS suspect54. Quinoline derivatives are also considered to cause PAINS interference in many cases55. Neratinib (Fig. 7) is a new irreversible dual-target inhibitor on the market that has antitumor activity and targets EGFR and HER2. Neratinib was designed to bear a Michael acceptor warhead and to take advantage of high sequence identity of the ATP-binding domains of EGFR and HER2 (82%)56. However, neratinib displays no activity against other serine–threonine kinases, such as AKT, cyclin D1/CDK4, cyclin E/CDK2, cyclin B1/CDK 1, IKK-2, MK-2, PDK1, c-RAF, and TPL-2 and tyrosine kinase c-MET57.
Figure 7.
Structures of compound 9 and neratinib.
5. “Fair Trial Strategy” for validation of PAINS suspects in MTDL development
The development of MTDLs is typically driven by the nature of the targets, availability of the starting points, and chemical tractability. The rational design of MTDLs is far from being an easy task and has to consider the crucial issues of interaction with specific targets58, achieving a synergistic effect toward them, and “designing out” unwanted interactions with any undesired target while retaining drug-like properties59. Discrimination between multitarget activity and assay interference is a major problem in biological screening and medicinal chemistry5. However, rationalization and prediction of potential assay interference of PAINS suspects is a complex problem14,15,31,60. The importance of PAINS issues is clearly indicated by medicinal chemists; however, PAINS alerts do not necessarily disqualify the agents from further consideration and do not invalidate the available data31. The compounds can be characterized as PAINS only after in silico filtration is augmented by experimental follow-up; defining a compound as PAINS allows to stop their development and has a greater significance for future studies61.
The “Fair Trial Strategy” (Fig. 8) encourages assessment that can eliminate unnecessary problems in MTDL developments by avoiding PAINS and should avoid elimination of good compounds and excellent resources for the development of new drugs. In particular, PAINS suspects that have frequent biological effects in diverse assays and do interfere with bioassays can be optimized via a challenging process into innovative and safe MTDLs using new conceptual frameworks, such as systems biology and polypharmacology62. The verification of molecule selectivity and specificity for disease related multitargets is the key strategy for excluding false positive hits. Covalent protein reactivity, colloidal aggregates, redox activity, and ion chelation are considered the main mechanisms accounting for PAINS. These types of compound interference can result from the compounds themselves63,64, e.g., in the case of fluorescent compounds. Thus, detailed description of effective computational and experimental validation methods is presented below with case analysis to illustrate the ‘Fair Trial Strategy’ of these mechanisms.
Figure 8.
The principles of investigation of suspected PAINS.
5.1. Verification of covalently interacting PAINS suspects
Traditionally, promiscuous scaffolds that rely on reactive electrophilic groups are difficult to optimize toward lead compounds because these scaffolds often interfere with biochemical analysis rather than actually alter the activity of the target65. Even if the target modulation is real, the undifferentiated target binding response is considered to trigger insurmountable toxic events65. These toxic events were increasingly believed to be associated with covalent on-target or off-target binding because irreversible covalent interactions may exacerbate possible adverse reactions65. The main consequence of irreversible covalent binding is to covalently bind reactive metabolites to various proteins. In this case, the leads are metabolically bioactivated to form reactive species that potentially bind covalently to all types of macromolecules65. Hapten formation is another potential negative consequence that must be considered for covalent and irreversible kinase inhibitors because it can trigger an immune response to the protein adducts65. Nonspecific irreversible inhibitors are generally regarded as suspected PAINS by medicinal chemists and considered impurities and typical undesirable products unless special screening of selective covalent modification was originally planned5. Therefore, identification of irreversible covalent inhibitors by using specialized libraries and techniques is a necessary means to eliminate PAINS interference in MTDL developments.
5.1.1. Computational validation of non-specific irreversible covalent artefacts
Verification of a compound as a nonspecific irreversible covalent artefact requires complex and innovative methodology; the results have considerable variety in accuracy, precision, and acquired information. In contrast to single-target inhibitors, the development of MTDLs is more complex making traditional drug-like rules and empirical parameters unsuitable for assessment of safety2. MTDLs need to have high selectivity against unintended targets and good physical and chemical properties, including absorption, distribution, metabolism, excretion, and toxicity2. Rational structural optimization-based computational approaches, such as cheminformatics and virtual screening, pharmacophore development, molecular docking, and molecular dynamics, can yield effective ligands for multiple targets66. Efficient MTDL design uses a number of computational approaches to scaffold optimization, including machine learning techniques of random forests, support vector machines, and Bayesian learning67. After acquisition of the feedback activity data from animal experimental models, multitarget QSAR analysis and skeleton transition can be used to optimize MTDLs to adjust their activity68. In addition to the developmental relevance of MTDLs, scaffolds with truly multitarget activities may be analyzed by computational methods to determine why and how these chemical entities specifically interact with multiple targets, especially if these targets are only distant relatives or are completely irrelevant and have different functions69. On the other hand, computational approaches have some defects and biases in assessment of the structure of PAINS; however, these methods are powerful for preliminary evaluation of the references and enable the use of more accurate and fair virtual approaches of reliable identification. When the designed MTDLs’ information data are supplemented in time and more precise algorithms are developed, it is possible to make these calculation methods more precise and accurate for PAINS identification. Some computational approaches to MTDL development and PAINS recognition are discussed below.
5.1.1.1. Molecular docking
Molecular docking studies can be used to confirm nonspecific irreversible covalent interactions between the selected ligands and multiple targets before experimental synthesis70. Molecular docking is generally used to access possible conformations of the ligands, estimate the most stable binding modes of the ligands with the targets, and optimize the geometry of the docked ligand‒target complexes. The most commonly used docking computational suites in MTDL design include Schrödinger71, CDOCKER72, DOCKovalent73, GOLD74, and DPubChem75. The docking simulations have been successfully used in MTDL development to design the treatments of many neurodegenerative diseases76,77. For example, the implementation of Autodock Vina in the design of MTDLs targeting AChE/BuChE/MAO-A/MAO-B for the treatment of AD led to the synthesis of DIH1578 and DPH1479, which have the best drug-like characteristics. However, due to rugged free energy landscape of these simulations and minor differences in the initial velocities, studies relying only on the outcome of a single molecular docking method instead of multiple simulations may lead to false positive conclusions80. Thus, multiple replicates and subsequent experimental validation are needed to draw conclusions based on molecular docking. Molecular docking is the critical step and a standard component that should be included in any ligand discovery process80,81.
5.1.1.2. Machine learning techniques
Over the last decade, deep learning and machine learning techniques have been extremely successful and are widely used to develop artificial intelligence in almost every domain82, 83, 84, 85, 86. Numerous machines learning techniques have been successfully used in model optimization for efficient MTDL design and validation of nonspecific irreversible covalent interaction, including support vector machines, random forests, and Bayesian learning. For example, a screening platform for cloud computing proteomics called “Ligand Express” leverages the combined efficiency of biophysics, biological data, and artificial intelligence technologies87. These machine learning methods enable medicinal chemists to efficiently explore new pathways of drug discovery and PAINS identification.
Identification of unexpected drug–protein interactions is important for drug repurposing and PAINS identification. A comprehensive ligand homology modeling approach FINDSITEcomb is used for prediction of drug–protein interactions, human protein targets, and side effects of the drugs88. Construction of a library of target protein structures of a proteome enables expedient prediction of interactions of millions of molecules against a typical proteome using a medium-sized computing cluster. The application can be openly accessed on the DR. PRODIS (DRugome, PROteome and DISeasome) webserver at http://cssb.biology.gatech.edu/dr.prodis/ 88.
5.1.1.3. Public filtering tools
Generally, the complexity of the interactions between MTDLs and targets requires considerations of various readout methods used in various assays under various conditions to identify PAINS; thus, definition of PAINS suspects by virtual screening cannot be achieved5. However, public filtering tools can be implemented for approximate PAINS identification if all available chemical and biological information is taken into account5. Although virtual filtering cannot be 100% successful in ruling out PAINS, it can help medicinal chemists to exclude considerable interference in MTDL development. Unlike experimental biological assays, public filtering tools can analyze massive in silico libraries of molecules59. Application of filters to rule out PAINS requires an organic combination with subsequent experimental biological assays to improve the success of validated trial probability. Therefore, the improvement of filtering methods generating highly reliable activity data, such as characterization and identification of detailed thiol-reactive chemotypes, are needed for PAINS suspects to pass through the filters33. Moreover, experimental approaches should be supplemented with computational methods to accelerate triage of potential PAINS alert, guide screening library design, and prevent follow-up on undesirable chemical entities5.
Several well-established public filtering tools are available online or as application software, including https://sandbox.ntp.niehs.nih.gov/interferences/, http://zinc15.docking.org/patterns/home, cbligand5, RDKit89, ZINC90, ToxAlerts48, and FAF-Drugs4 server91. However, due to insufficient analysis of PAINS definitions, the existing filtering tools always miss a substantial proportion of PAINS substructure-containing compounds in an independently curated molecular data-set. To address this shortcoming and allow visual exploration of the reasons for the prediction, Maksim Koptelov and coworkers92 developed PrePeP. PrePeP uses benchmark datasets from the literature to compensate for a number of shortcomings of existing PAINS alerts that have been pointed out recently92. Another improved filter system, Vertex's REOS, can identify and remove toxic, reactive, or other undesirable molecules from the database by combining the characteristics of molecules with certain principles that are based on the requirements for the medicinal properties93. More than 90% of high hit rate molecules and 91% of nonhigh hit rate molecules could be distinguished by this filtering model93.
5.1.1.4. BadApple
BadApple is a biological test data associative promiscuity pattern learning algorithm combining general and domain-specific features to assist with and accelerate identification of possible nonspecific irreversible covalent artefacts13. This engine generates a score associated with a pragmatic empirical definition of irreversible interferences with the overall goal to identify PAINS and streamline workflows13. Unlike methods that rely on experts to manage chemical substructure patterns, BadApple is a completely evidence-driven, automated, and self-improving method that can integrate additional data13.
5.1.2. Experimental validation of non-specific irreversible covalent artefacts
As mentioned previously, virtual computer technologies can only assist medicinal chemists with the analysis of interaction modes of ligands or PAINS with proteins in targeted or untargeted MTDL design, and their target prediction and PAINS exclusion are not 100% correct. Therefore, the development of rapid and robust experimental methods to distinguish PAINS suspects in the validation of nonspecific irreversible covalent artefacts is particularly important. Irreversible covalent artefacts are usually manifested as confusing phenomena in biochemical assays, such as lack of correlation between structure and activity, time-dependent activity, and steep inhibition curve5. To determine whether these artefacts are “bad”, conclusive experimental evidence from at least two different biological experimental tests should be provided. If both tests report that the compounds are specifically active and the apparent activity is not artificial, the suspect can be excluded5. In the “Fair Trial Strategy”, full concentration–response curves are a simple and critical evidence to rule out PAINS suspects because the investigator can obtain considerable important information based on the steepness of the curve and sampling quality5.
5.1.2.1. Counter-screening validation by incubation time-dependent activity
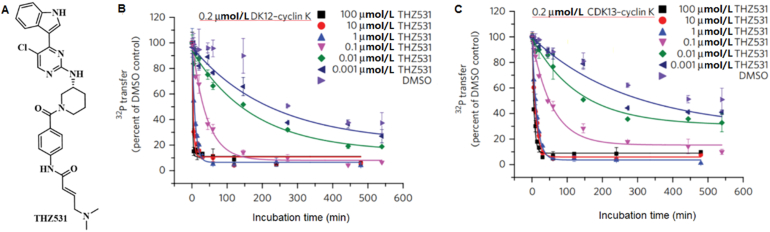
Covalent inhibitors usually display concentration-dependent and incubation time-dependent activity in in vitro enzymatic assays94. Generally, a time-dependent increase in apparent inhibitory potency suggests irreversible covalent binding (Table 2)4. Counter-screening verification of an irreversible covalent inhibitor THZ531 (Fig. 9a) of CDK12/13 used changes in incubation time during radiometric kinase activity assay (Fig. 9)95,96. Counter-screening verification assessed the ability of recombinant CDK12 to phosphorylate the Pol-II-CTD peptide substrate in the presence of the cofactor cyclin K; the data were normalized to the relative [32P] transfer, and DMSO was used as a control (Fig. 9b)95. The effect of incubation time on the activity may be caused by CDK12; however, the results still indicate covalent inhibition95.
Table 2.
Comparison of experimental methods for the verification of irreversible covalent binding.
| Assay | Principle | Feature | Readout |
|---|---|---|---|
| Incubation time-dependent activity | Time-dependent increase in inhibition indicates irreversible covalent binding | Easy to operate and low cost | Enzyme inhibition |
| Washout experiments | The continuous growth of covalent inhibitors is attributed to irreversible covalent binding | Can be used to verify irreversible MOR | Enzyme inhibition |
| X-Ray crystallography and in situ labeling | Can analyze the covalent binding situation with proteins | Provide more detailed binding information | Ligand‒targets analysis |
| Chemoproteomic approaches | Can identify specific targeted binding and eliminate non-specific covalent interaction. | Provide more detailed binding information | Ligand‒targets analysis |
| Alarm NMR | Monitor the DTT-dependent 13C chemical shift changes of the human La antigen | Provide more detailed binding information | Ligand‒targets analysis |
Figure 9.
THZ531 (A) inhibition of CDK12/13 is time dependent. In vitro kinase activity assay of CDK12-cyclin K (B) and CDK13-cyclin K (C) with different concentrations of THZ531 and varying preincubation times. For all incubation time series, the counts per minute of the kinase activity measurements were normalized to the relative 32P transfer. (B) and (C) Adapted with modification from Ref. 96. Copyright ©2016 Springer Nature.
5.1.2.2. Washout experiments
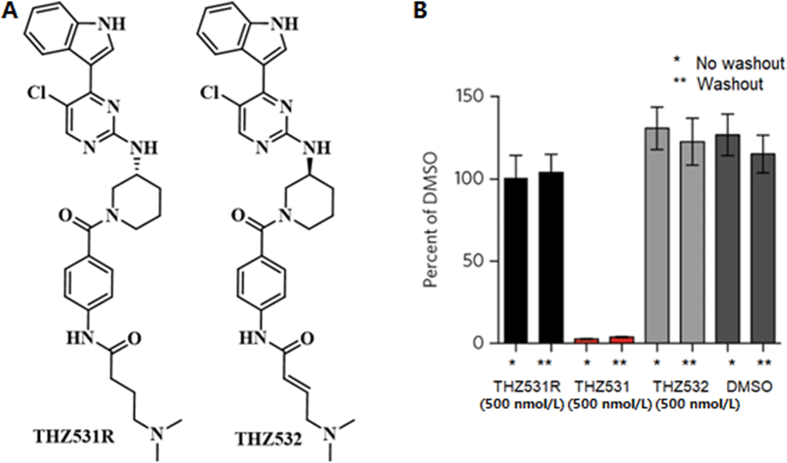
In cell-based assays, cells are exposed to the solutions of PAINS suspects, washed, and allowed to grow in the medium without PAINS suspects. The washout experiment is an effective method to verify irreversible mechanism of action (Table 2)95. A continuous increase in inhibition in the washout experiments is attributed to irreversible target binding95. This method was also used in the verification of the effect of THZ531 in Jurkat T cells (acute lymphoblastic leukemia cells)95. In this experiment, the effects of THZ531 were maintained for 72 h after washout, while a negative control of a reversible compound THZ531R had no effect (Fig. 10)95.
Figure 10.
THZ531 retains activity in washout experiment compared with the effects of two negative control compounds. (A) THZ531R and THZ532. (B) Jurkat cells were treated with the indicated compounds for 6 h, inhibitor was washed out and cells were allowed to grow for the remainder of the 72 h. This growth was compared with the growth of cells treated with inhibitors for the full 72 h. (B) Adapted with modification from Ref. 96. Copyright ©2016 Springer Nature.
5.1.2.3. X-ray crystallography and in situ labeling followed by LC−MS/MS analysis
Due to the complexity of polypharmacology, the rational design of MTDLs is an equally challenging and attractive research area97,98. X-ray structures are used to analyze the covalent binding of MTDLs with target proteins, associate MTDLs with multitarget activities to protein binding site similarity or identify multifamily ligand PAINS suspects bound to targets (Table 2)44. Effective and direct methods for identification of covalent binding in vitro are X-ray crystallography and in situ labeling followed by LC−MS/MS analysis. These strategies were used to validate the covalent binding of THZ531 to CDK12/CDK13 in vitro95. Mass spectrometry analysis showed the formation of covalent adducts, and a peptide containing the exact site of modification was identified after proteolysis. Then, X-ray crystallography of CDK12-cyclin K bound to THZ531 confirmed irreversible mechanism of action95.
5.1.2.4. Chemoproteomic approaches
A series of chemoproteomic approaches have been developed to identify specific targeted binding and to eliminate nonspecific covalent interactions. These methods include activity-based protein profiling (ABPP) in combination with MS-based proteomics (Table 2)99 and stable isotope labeling with amino acids in cell culture or tandem mass tagging (TMT) click chemistry pull-down experiments100. Recently, a chemoproteomic approach called CITe-Id has been developed to capture, identify, and quantify dose-dependent covalently bound Cys sites in cell lysates101. CITe-Id verification demonstrated that THZ1, a covalent kinase inhibitor initially presumed to specifically bind to CDK7, forms covalent bonds with other proteins, including nonkinase targets95.
5.1.2.5. ALARM NMR
The most common nonprotein thiol-based reporters may fail to mimic the local environment on the protein surface thus not reflecting the reactivity of the agents with physiologically relevant protein side chains102. Therefore, a fast and reliable NMR-based approach ALARM NMR (Table 2)20 (a La assay to detect reactive molecules by nuclear magnetic resonance) has been developed to identify PAINS suspects, including the compounds that may target oxidized or alkylated protein targets20. The detection is based on monitoring of DTT-dependent 13C chemical shift changes of the human La antigen in the presence of PAINS suspects. These chemical shifts can be attributed in an ALARM NMR experiment using a thiol in a potentially more biologically relevant environment that may be different from that used in the experiments with small molecule thiols (such as GSH)99.
5.2. Verification of PAINS suspects caused by colloidal aggregation
Small colloidally aggregating molecules (SCAMs) are the largest causative source of PAINS in MTDL discovery and one of the most common mechanisms for false-positive readouts5,83. SCAMs are diverse and well represented in academic and commercial screening decks and even in approved drugs103. A mechanistic study demonstrated that SCAMs aggregate via phase separation and particle formation at concentrations above a compound-specific critical aggregation concentration (CAC)5,18,103. The consequences of colloidal aggregates include nonspecific binding to protein surfaces and partial denaturation of a protein to produce confounding and irrelevant enzymatic responses due to nonspecific molecular recognition mechanisms103, 104, 105. Tight binding of SCAMs produces partial and local protein denaturation resulting in time-dependent enzyme and protein inhibition83,106. The deceptive effects of the aggregates can be extended to cell-based detection systems, where SCAMs may lower anticipated activities due to reduced cell membrane diffusion thus reducing intracellular concentrations107. SCAMs can account for up to 95% of false positives in HTS campaigns, which makes SCAMs the most prevalent cause of erroneous ligand‒target associations83. Therefore, the pernicious effects of SCAMs in MTDL discovery cripple the readout of target- and cell-based assays; hence, validation of these effects at the early stages of MTDL design is essential83. Considerable efforts have been invested in the development of methods for validation of potential SCAMs; however, only limited success has been achieved. Since the formation of the aggregates may be induced by minor changes in concentration, the formation of potential aggregates is difficult to predict based only on physical properties83.
5.2.1. Computational prediction of SCAMs
Considering that SCAM and non-SCAM data span over the range of calculated logP and molecular weight values and have significant scaffold diversity, their biophysical properties may be reliably analyzed and recognized by computational approaches. Therefore, machine learning algorithms may play an important role in expedient identification of potentially liable chemical entities83. Publicly available aggregator advisors (e.g., http://advisor.bkslab.org and http://advisor.docking.org)6,108 can identify SCAMs based on the substructure fingerprints of a query entity. Verification results obtained by these public tools are similar to over 12,000 experimentally validated SCAMs83. The possibility of false positive query entities is calculated by triaging the cheminformatics-based similarity with known SCAMs, observed affinity range, and calculated logP108. Another effort to identify SCAMs based on physicochemical properties derived from chemical structure includes support vector machine-recursive feature elimination (SVM-RFE) developed as a combination of several independent studies using orthogonal algorithms109. Unlike aggregator advisors, SVM-RFE extends the learned data patterns beyond simple structure equivalence and relies on molecular similarity to assess aggregation propensity83. Additionally, the chemical space projection clearly emphasizes that the two computational methods share similar reference data but provide different solutions for identification of multiple SCAMs in various areas83.
5.2.2. Experimental detection methods for prediction of SCAMs
The computational methods can preliminarily identify nuisance artefacts in pharmaceutical libraries; however, these methods regularly generate false positive and false negative results110. The reason for this inconsistency is due to fact that the formation of SCAMs is triggered by small changes in concentration; thus, it is difficult to predict potential aggregators strictly based on physical properties25,110. Moreover, various pH or buffer conditions may induce aggregation that is no longer related to the target compounds easily leading to false predictions110. Therefore, studies on the identification and elimination of SCAMs in biological experiments are necessary. The methods listed below are easy to implement and can provide robust assessment of the aggregation propensity of the investigated molecules.
5.2.2.1. Parallel experiments with nonionic detergent washing
The addition of detergents facilitates the dissociation of the aggregates into monomers in the solution thus decreasing the apparent activity of SCAMs in the presence of detergents in the assay buffers (Table 3)83. If the activity of a compound can be attenuated by the addition of small amounts of a nonionic detergent, then the compound can be defined as SCAM5. A typical protocol involves the addition of 0.01% Triton X-100 in biochemical assays or 0.025% Tween-80 as a gentler detergent for cell-based assays5. Parallel screening with and without a nonionic detergent and reliance on model enzymes (such as β-lactamase) are more rigorous methods for identification of SCAMs83. Parallel experiments require preliminary confirmation that the detergent does not contribute to the readouts. Additionally, the changes in activity obtained by two assays should be statistically evaluated to ensure the results are not caused by experimental procedures83.
Table 3.
Comparison of experimental methods for the verification of SCAMs.
| Assay | Principle | Feature | Readout | Note |
|---|---|---|---|---|
| Nonionic detergent washing | Detergents will promote the formation of monomers, thus reducing the apparent activity | Easy to operate and low cost | Enzyme inhibition | Detergent should not contribute to the readouts |
| Centrifugation | Centrifugation will induce the formation of pellets originating, thus changing the apparent activity | Easy to operate and can be applied to HTS platforms | Enzyme inhibition | Selectivity of non-SCAMs to tumor cells should be higher than that of normal cells |
| Competitive ligand−target binding experiment | SPR can identify their nonspecific aggregation-type binding mechanisms | Easy to operate and provide more detailed mechanism information | Enzyme inhibition | Compounds should be injected into the protein surface and attached to the surface of the optical biosensor |
| Printable hydrogel microarray | The adjustable porosity of the hydrogels allows selective transport of substrates to and from the entrapped enzyme via size selectivity | Easy to operate | Enzyme inhibition | No special requirements |
5.2.2.2. Centrifugation before cell-based assay
A convenient procedure before cell-based assay involves centrifugation of the solutions of the compounds to precipitate colloidal aggregates. Parallel activity evaluation can be subsequently performed. If the activity is reduced after centrifugation, the compound is likely a SCAM (Table 3)5. The key point of this parallel experiment is to prove that the active concentration of a SCAM is lower than the concentration that produces cytotoxicity thus demonstrating that this apparent activity is not due to cytotoxicity. If the goal of the assay is assessment of cytotoxicity in tumor therapy, the selectivity of non-SCAMs in tumor cells should be higher than that in normal cells5.
5.2.2.3. Competitive ligand‒target binding experiment
The false positive SCAMs are often manifested by enzyme inhibition via nonspecific aggregation-type binding mechanisms111. Surface plasmon resonance (SPR) is a label-free technique that is often used to study the ligand−target binding kinetics and affinity constants of the active compounds. A powerful Biacore technology of SPR can contribute to the identification of potential SCAMs in the early drug discovery by observing and analyzing the ligand−target binding and interaction patterns (Table 3)83,111. The operation of this technology is relatively simple and requires only an injection of the dissolved compounds into the protein surface. The compounds are attached to the proteins immobilized on the surface of an optical sensor (association phase), and the binding data are recorded in real time based on the changes in the properties of the sensor surface111. This technology allows to evaluate the association and dissociation of SCAMs with proteins in real time to determine how SCAMs interact with proteins in detail111. SPR data can provide information about stoichiometry, reversibility, and changes in the properties of the compounds within a certain concentration range111. Based on this information, SPR can be used to analyze SCAMs in biochemical assays to expediently determine whether the interference is caused by specific or nonspecific interactions with the proteins. Thus, hits can be selected and prioritized for chemical processing by analytical and/or medicinal chemistry methods111. For specific experimental operation, please refer to Ref. 111.
The photonic crystal (PC) optical biosensor aggregation assay is another label-free biophysical technology that has been productively used to detect SCAMs by providing direct quantitative measurement of the mass density of the substances adsorbed on the transducer surface107,112. The PC sensors contain a subwavelength periodic surface that reflects a narrow wavelength band after illumination by a broadband collimated light source. Formation of the aggregates changes the sensor surface and adjusts the refractive index of the material to enable expedient identification of SCAMs107,112. For specific experimental operation, please refer to Ref. 112.
5.2.2.4. Printable hydrogel microarray
The inhibitory effects of SCAMs are usually due to the adsorption of a protein on the surface of the aggregates thus separating the enzyme from the substrate and resulting in partial denaturation of the protein110. The hydrogel-based enzyme immobilization platform provides a system for specific identification and elimination of SCAMs (Table 3)110. The adjustable porosity of the hydrogels allows selective transport of the substrates to and from the entrapped enzyme due to size selectivity potentially preventing drug aggregates from reaching the binding site on the enzyme and decreasing the effects associated with promiscuous inhibition110. The fabricated printable hydrogel microarray combines analytical functionality and easily generated multiple sample substrates to eliminate time-dependent SCAM interference, significantly improve the accuracy of lead compound discovery, and simplify the drug discovery process110. The hydrogel-based screening assays demonstrate minimal interference and can be applied to screening platforms based on high-throughput microarrays for rapid (<25 min) and low-cost elimination of interfering SCAMs to identify lead compounds with real inhibitory potential110. For specific experimental operation, please refer to the literature113.
5.3. Verification of redox cycling PAINS suspects
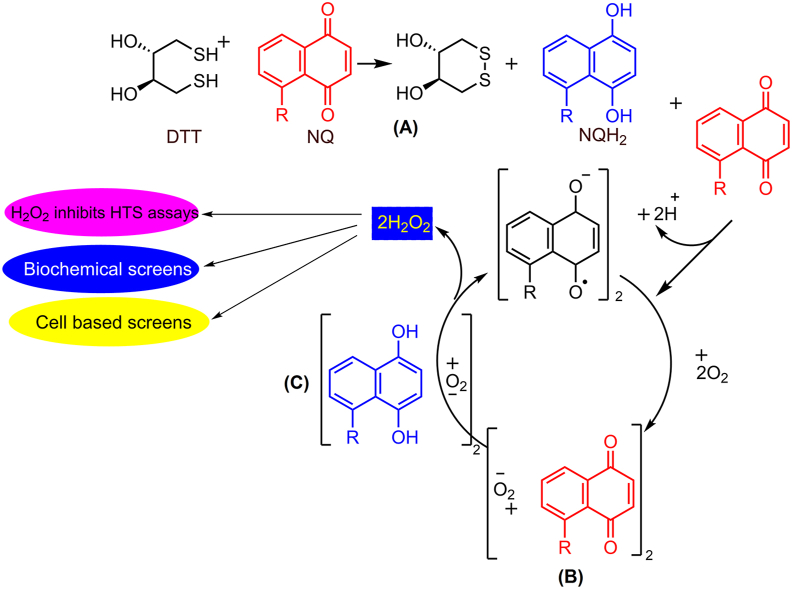
Discussed mechanisms result in easily formed false positives; however, the compounds that manifest their apparent activity via targeted oxidation have not been considered. Certain redox cycling compounds (RCCs), such as naphthoquinones (NQ), undergo redox-dependent cycling in the presence of strong reducing agents, such as dithiothreitol (DTT) and tris(2-carboxyethyl)phosphine (TCEP), resulting in the generation of hydrogen peroxide (H2O2)22. H2O2 can indirectly inhibit the catalytic activity of proteins by oxidizing accessible cysteine, tryptophan, methionine, histidine, or selenocysteine residues; thus, these compounds have pleiotropic effects manifested as nonspecific and promiscuous inhibition of protein activity (Fig. 11)22,114. RCC-based interference can lead to exaggerated values of apparent actives in HTS assays. In an HTS campaign to identify inhibitors of the dual specificity phosphatase cell division cycle 25B (Cdc25B), 55% of the concentration-dependent Cdc25B inhibitors were shown to be RCCs115.
Figure 11.
NQ generates H2O2 accounting for promiscuous bioactivity profiles in the HTS databases. (A) NQ can be reduced by DTT to form a hydroquinone (QH2) capable of undergoing a comproportionation reaction with another NQ to form two identical radical anions. (B) In the presence of O2 these radicals can form O2−. (C) The superoxide can then be reduced by QH2 to form H2O2.
At present, several conventional processes can be used to identify and eliminate RCCs, including multiple counter-screening and secondary detection. This type of PAINS suspects cannot be completely validated; however, probability of their occurrence can be significantly reduced. Moreover, conventional validation methods enable significantly easier differentiation of ‘off-target’ activity from the targeted and pathway-specific activities22. These methods include: (a) use of LC/MS analysis to determine the oxidation of a cysteine in the active site of the target protein116; (b) measurement of the UV/Visible spectrum of RCCs with or without a reducing agent to determine whether RCCs are reduced in a time-dependent manner22; (c) investigation of the inhibitory effects of weak reducing agents, such as GSH, BME, or Cys on RCCs22; (d) verification that the inhibitory effect of RCCs on the target activity is time- and concentration-dependent in the presence of DTT or TCEP22; (e) inhibition of the target protein activity by RCCs can be abolished by the addition of catalase (CAT) to the assay to degrade any H2O2 produced115. CAT can abolish the target inhibition by RCCs in the presence of DTT or TCEP22. However, the verification process by these detailed analyses is material- and time-consuming, and the results are insufficient for complete and accurately characterization of PAINS. The following several methods are more feasible and can be used to mitigate the serious impact of RCCs on MTLDs.
5.3.1. The phenol red-HRP assay
Recently, a simple, rapid, sensitive, and economical method was used to investigate RCCs that indirectly modulate target activity and to identify promiscuous false positives. This assay is based on the H2O2-dependent horseradish peroxidase (HRP)-mediated oxidation of phenol red that produces a change in absorbance at 610 nm at alkaline pH and readily detects H2O2 generation (in the 1–100 μmol/L range, Table 4)116. The phenol red HRP assay can be performed in a 384-well format and was used to profile ∼200,000 compounds available from the LOPAC and NIH MLSCN compound libraries for RCC activity23 due to the ability of RCCs to generate H2O2116. Pyrimidotriazinedione is one of the active dominant scaffolds commonly used in drug design that was unfortunately identified as an RCC in the presence of 0.8 mmol/L DTT and has been promiscuously active against a number of target proteins23. More than 50% of RCCs identified in the RCC profiling screen are structurally similar to pyrimidotriazinedione and quinones; however, several other RCC pharmacophores were identified114. H2O2 is the common cellular messenger; hence, RCC interference is not limited to biochemical enzyme-based assays and can also produce promiscuous effects in cell-based analysis23.
Table 4.
Comparison of experimental methods for the verification of redox cycling.
| Assay | Principle | Feature | Readout |
|---|---|---|---|
| Phenol red-HRP assay | Based on the H2O2-dependent horseradish peroxidase mediated oxidation of phenol red that produces a change in its absorbance at 610 nm in alkaline pH, and readily detects H2O2 generation | Can produce promiscuous effects in enzymes and cell-based analysis | Enzyme and cell inhibition |
| Surrogate assay | Using the conversion of resazurin to resorufin redox reaction in the presence of DTT and compounds to detect small molecule redox activity | Easy to operate and can be applied to pharmaceutical targets that perform redox chemistry or targets with functional groups susceptible to redox modification | Enzyme inhibition |
5.3.2. A surrogate assay
In addition to the mechanism described above, RCCS can cause inhibition by oxidizing susceptible enzyme targets, such as metalloenzymes and cysteine-containing enzymes. However, this redox phenomenon is rarely investigated, and the detection methods need optimization114. Interestingly, a surrogate assay using the conversion of resazurin to resorufin in a redox reaction in the presence of DTT and compounds was used to detect redox activity of small molecules (Table 4)116. The surrogate assay couples glucose-6-phosphate production to resorufin via glucose-6-phosphate dehydrogenase (G6PDH) and diaphorase116. Similar to other cases, 2203 out of 2262 compounds (considered nuisance hits) were able to produce resorufin from resazurin in the presence of only resazurin and DTT116. This surrogate assay was used to evaluate suspected redox compounds 4 and (±)-dunnione (Fig. 12) discovered by GSK as irreversible and noncompetitive inhibitors of caspase-8. A nonredox pancaspase peptide inhibitor Z-VAD (OMe)-FMK (Fig. 12) was used as a competitive control. The known inhibitor was not positive in this assay, whereas compounds 4 and (±)-dunnione were identified as oxidative nuisance compounds117. The reduction of resazurin to resorufin is a popular assay format used to measure cell viability; pharmaceutical targets that are involved in redox chemistry or targets with functional groups susceptible to redox modification are likely to benefit from analysis using this assay117.
Figure 12.
Structures of compounds studied in the redox assay.
5.4. Verification of PAINS suspects involved in metal ion chelation
These false positive results are caused by the pan interference effects of the compounds or organic impurities; however, organic entities do not fully account for all false positive results. Conventional identification methods, such as MS and NMR, used during synthesis are suitable only for confirmation of the structure and purity of organic components, while potential inorganic impurities, such as transition metals that may be used in compound synthesis, are not identified118. These inorganic impurities can also cause positive reactions in low micromolar range in a number of potential protein and functional systems, including biochemical and biosensor assays118. These positive effects may result in the selection of organic entities thus making inorganic impurities the best candidates for PAINS suspects. For example, organic entities containing zinc impurities may form complexes with multivalent cations that persist through preliminary procedures, and nonfunctional mechanisms of inorganic interference on the readout systems may cause false positive signals119. Additionally, inorganic contaminations in organic entities during synthesis can cause inconclusive SAR in later lead optimization. Therefore, design and synthesis of MTDLs requires constant consideration of the effects of inorganic impurities that can be ruled out in the presence of a nonselective chelator, such as EDTA, or a more selective chelator, such as TPEN (N,N,N′,N′, tetrakis (2-pyridylmethyl)ethylenediamine)119.
5.5. Verification of PAINS suspects causing sample fluorescence
Interference mediated by sample fluorescence is highly prevalent in the biological evaluation of MTDLs due to the variability of detection strategies that use fluorescent labels and light detection. Fluorophoric properties usually accompany heterocyclic scaffolds and impurities and can affect the results of biological evaluation to complicate the analysis and cause false positive and negative results22 (Fig. 13). Certain promiscuous natively fluorescent compounds may be disguised as valid regulators of target functions leading to futile pursuit of biologically inactive compounds in chemical genomics and drug development campaigns21. Therefore, these PAINS suspects should be eliminated from subsequent consideration.
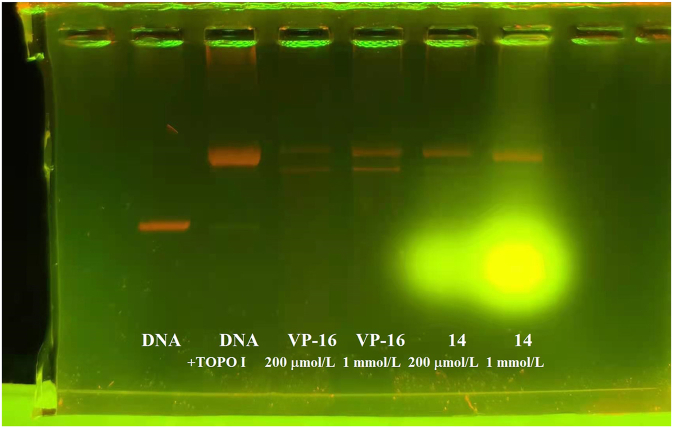
Figure 13.
The effect of strong fluorescence interference on the results of topoisomerase II (Topo II) inhibition experiment. The image shows the results of Topo II inhibition assay of compounds currently investigated by us.
Generally, the analytical artefacts arising from fluorescent compounds are reproducible, and the apparent activity usually shows a concentration-dependent response21. In this case, orthogonal assays are a conventional and effective method to eliminate false positives caused by fluorescence interference subsequent to the retesting of the primary screening hits21. This assay is generally used to assess compound interference by reading the fluorescence intensity of the analysis mixture after the sample is added prior to initiation of the detection leading to a change in the fluorophore21. Fluorescence intensity of each well and the calculated polarization value can rule out PAINS suspects with above average fluorescence in the analysis based on fluorescence polarization (FP)21.
In addition to the method of calibration using fluorophore standards, an assay similar to quantitative HTS is used to profile each compound within a range of concentrations that can be used in verification125. The concentration response-based analysis measures fluorescence intensity or potency of each sample based on the known fluorophores and thus can be used to identify PAINS suspects21.
6. Summary
MTDLs can simultaneously regulate multiple associations in the disease network thus improving the efficacy and reducing the adverse reactions. Some MTDLs have been successfully used in many major diseases and have become very important therapeutics. Reasonable MTDL design may provide novel effective and promising products. PAINS has become an obstacle in the process of MTDL development, and medicinal chemists have a complex task to accurately distinguish “good” PAINS from “bad” PAINS based on currently available rules and screening tools. Up to 95% of “hits” identified from virtual screening can be considered PAINS; thus, this problem should be taken seriously to ruthlessly block some of the compounds from entering the next stage of investigation. Optimistically, use of the ‘Fair Trail Strategy’ and active investigations of the interference mechanism of PAINS by efficient and comprehensive approaches to exclude the interference suspects can double the output of MTDL research and development while halving the required effort.
Identification of the relevant targets for disease regulation and initiation of the search for lead ligands should be followed by the investigation of the ligand−target interactions using reasonable methods that can exclude the interference of PAINS. The complexity of biological systems and properties of ligand−target interactions may cause variability in the results of virtual screening. Thus, to avoid the inaccurate classification of compounds as PAINS, computational and experimental approaches should be combined in an ideal scenario. In the absence of accurate experimental evidence, false positive ligands should be only flagged as PAINS suspects that can be subsequently exculpated to avoid their disappearance from the chemical libraries or abandoned by medicinal chemists.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (31870325), Key Laboratory for Tumor Precision Medicine of Shanxi Province Research Fund (KLTPM-SX2022-B3, China), Innovation and Entrepreneurship Training Program for Undergraduate (202010316043S, China) and “Double First Class” Subject Innovation Team Construction Project of China Pharmaceutical University (CPU2018GY12, China) .
Author contributions
Jianbo Sun and Hui Zhong wrote the manuscript; Kun Wang made the figures; Na Li reviewed and edited the manuscript; Li Chen supervised and edited the manuscript.
Conflicts of interest
The authors declare no conflict of interest for publishing this manuscript.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Na Li, Email: 409091029@qq.com.
Li Chen, Email: chenli627@cpu.edu.cn.
References
- 1.Ravikumar B., Aittokallio T. Improving the efficacy-safety balance of polypharmacology in multi-target drug discovery. Expert Opin Drug Met. 2018;13:179–192. doi: 10.1080/17460441.2018.1413089. [DOI] [PubMed] [Google Scholar]
- 2.Alcaro S., Bolognesi M.L., García-Sosa A.T., Rapposelli S. Multi-target-directed ligands (MTDL) as challenging research tools in drug discovery: from design to pharmacological evaluation. Front Chem. 2019;7:71. doi: 10.3389/fchem.2019.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hopkins A.L. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 4.Bottegoni G., Favia A.D., Recanatini M., Cavalli A. The role of fragment-based and computational methods in polypharmacology. Drug Discov Today. 2012;17:23–34. doi: 10.1016/j.drudis.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Aldrich C., Bertozzi C., Georg G.I., Kiessling L., Lindsley C., Liotta D., et al. The ecstasy and agony of assay interference compounds. ACS Chem Neurosci. 2017;8:420–423. doi: 10.1021/acschemneuro.7b00064. [DOI] [PubMed] [Google Scholar]
- 6.Baell J., Walters M.A. Chemistry: chemical con artists foil drug discovery. Nature News. 2014;513:481–483. doi: 10.1038/513481a. [DOI] [PubMed] [Google Scholar]
- 7.Maeda K., Sugino H., Akazawa H., Amada N., Shimada J., Futamura T., et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Therapeut. 2014;350:589–604. doi: 10.1124/jpet.114.213793. [DOI] [PubMed] [Google Scholar]
- 8.Halberstadt A.L., Vollenweider F.X., Nichols D.E. Springer; Berlin, Heidelberg: 2018. Behavioral neurobiology of psychedelic drugs; p. 36. [Google Scholar]
- 9.Nelson K.M., Dahlin J.L., Bisson J., Graham J., Pauli G.F., Walters M.A.J. The essential medicinal chemistry of curcumin: Miniperspective. J Med Chem. 2017;60:1620–1637. doi: 10.1021/acs.jmedchem.6b00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baell J.B. Feeling nature's PAINS: natural products, natural product drugs, and pan assay interference compounds (PAINS) J Nat Prod. 2016;79:616–628. doi: 10.1021/acs.jnatprod.5b00947. [DOI] [PubMed] [Google Scholar]
- 11.Baell J.B., Nissink J.W.M. Seven year itch: pan-Assay interference compounds (PAINS) in 2017-utility and limitations. ACS Chem Biol. 2018;13:36–44. doi: 10.1021/acschembio.7b00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider P., Schneider G. Privileged structures revisited. Angew Chem Int Ed. 2017;56:7971–7974. doi: 10.1002/anie.201702816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J.J., Ursu O., Lipinski C.A., Sklar L.A., Oprea T.I., Bologa C.G. Badapple: promiscuity patterns from noisy evidence. J Cheminf. 2016;8:29. doi: 10.1186/s13321-016-0137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jasial S., Hu Y., Bajorath J.R. How frequently are pan-assay interference compounds active? Large-scale analysis of screening data reveals diverse activity profiles, low global hit frequency, and many consistently inactive compounds. J Med Chem. 2017;60:3879–3886. doi: 10.1021/acs.jmedchem.7b00154. [DOI] [PubMed] [Google Scholar]
- 15.Capuzzi S.J., Muratov E.N., Tropsha A. Phantom PAINS: problems with the utility of alerts for pan-assay in terference compound S. J Chem Inf Model. 2017;57:417–427. doi: 10.1021/acs.jcim.6b00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahlin J.L., Walters M.A. How to triage PAINS-full research. Assay Drug Dev Technol. 2016;14:168–174. doi: 10.1089/adt.2015.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costantino L., Barlocco D. Designed multiple ligands: basic research vs clinical outcomes. Curr Med Chem. 2012;19:3353–3387. doi: 10.2174/092986712801215883. [DOI] [PubMed] [Google Scholar]
- 18.Coan K.E., Shoichet B.K. Stoichiometry and physical chemistry of promiscuous aggregate-based inhibitors. J Am Chem Soc. 2008;130:9606–9612. doi: 10.1021/ja802977h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schorpp K., Rothenaigner I., Salmina E., Reinshagen J., Low T., Brenke J.K., et al. Identification of small-molecule frequent hitters from alphascreen high-throughput screens. J Biomol Screen. 2014;19:715–726. doi: 10.1177/1087057113516861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huth J.R., Mendoza R., Olejniczak E.T., Johnson R.W., Cothron D.A., Liu Y., et al. Alarm NMR: a rapid and robust experimental method to detect reactive false positives in biochemical screens. J Am Chem Soc. 2005;127:217–224. doi: 10.1021/ja0455547. [DOI] [PubMed] [Google Scholar]
- 21.Simeonov A., Jadhav A., Thomas C.J., Wang Y., Huang R., Southall N.T., et al. Fluorescence spectroscopic profiling of compound libraries. J Med Chem. 2008;51:2363–2371. doi: 10.1021/jm701301m. [DOI] [PubMed] [Google Scholar]
- 22.Thorne N., Auld D.S., Inglese J. Apparent activity in high-throughput screening: origins of compound-dependent assay interference. Curr Opin Chem Biol. 2010;14:315–324. doi: 10.1016/j.cbpa.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soares K.M., Blackmon N., Shun T.Y., Shinde S.N., Takyi H.K., Wipf P., et al. Profiling the NIH small molecule repository for compounds that generate H2O2 by redox cycling in reducing environments. Assay Drug Dev Technol. 2010;8:152–174. doi: 10.1089/adt.2009.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingólfsson H.I., Thakur P., Herold K.F., Hobart E.A., Ramsey N.B., Periole X., et al. Phytochemicals perturb membranes and promiscuously alter protein function. ACS Chem Biol. 2014;9:1788–1798. doi: 10.1021/cb500086e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng B.Y., Shelat A., Doman T.N., Guy R.K., Shoichet B.K. High-throughput assays for promiscuous inhibitors. Nat Chem Biol. 2005;1:146–148. doi: 10.1038/nchembio718. [DOI] [PubMed] [Google Scholar]
- 26.Schneider C., Gordon O.N., Edwards R.L., Luis P.B. Degradation of curcumin: from mechanism to biological implications. J Agric Food Chem. 2015;63:7606–7614. doi: 10.1021/acs.jafc.5b00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo H., Eleftheriadis N., Rohr-Udilova N., Dömling A., Dekker F.J. Photoactivation provides a mechanistic explanation for pan-assay interference behaviour of 2-aminopyrroles in lipoxygenase inhibition. Eur J Med Chem. 2017;139:633–643. doi: 10.1016/j.ejmech.2017.07.047. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J., Jiang X., He S., Jiang H., Feng F., Liu W., et al. Rational design of multitarget-directed ligands: strategies and emerging paradigms. J Med Chem. 2019;62:8881–8914. doi: 10.1021/acs.jmedchem.9b00017. [DOI] [PubMed] [Google Scholar]
- 29.Jasial S., Gilberg E., Blaschke T., Bajorath J.R. Machine learning distinguishes with high accuracy between pan-assay interference compounds that are promiscuous or represent dark chemical matter. J Med Chem. 2018;61:10255–10264. doi: 10.1021/acs.jmedchem.8b01404. [DOI] [PubMed] [Google Scholar]
- 30.Gilberg E., Stumpfe D., Bajorath J. Activity profiles of analog series containing pan assay interference compounds. RSC Adv. 2017;7:35638–35647. [Google Scholar]
- 31.Gilberg E., Jasial S., Stumpfe D., Dimova D., Bajorath J. Highly promiscuous small molecules from biological screening assays include many pan-assay interference compounds but also candidates for polypharmacology. J Med Chem. 2016;59:10285–10290. doi: 10.1021/acs.jmedchem.6b01314. [DOI] [PubMed] [Google Scholar]
- 32.Gilberg E. Gütschow M, Bajorath J. X-Ray structures of target–ligand complexes containing compounds with assay interference potential. J Med Chem. 2018;61:1276–1284. doi: 10.1021/acs.jmedchem.7b01780. [DOI] [PubMed] [Google Scholar]
- 33.Dahlin J.L., Nissink J.W.M., Strasser J.M., Francis S., Higgins L., Zhou H., et al. PAINS in the assay: chemical mechanisms of assay interference and promiscuous enzymatic inhibition observed during a sulfhydryl-scavenging HTS. J Med Chem. 2015;58:2091–2113. doi: 10.1021/jm5019093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider G. Automating drug discovery. Nat Rev Drug Discov. 2018;17:97. doi: 10.1038/nrd.2017.232. [DOI] [PubMed] [Google Scholar]
- 35.Xie T., Du G.H. Pain of high-throughput screening-pan assay interference compounds. Acta Pharm Sin. 2015;50:925–930. [PubMed] [Google Scholar]
- 36.Baell J.B. Observations on screening-based research and some concerning trends in the literature. Future Med Chem. 2010;2:1529–1546. doi: 10.4155/fmc.10.237. [DOI] [PubMed] [Google Scholar]
- 37.Baell J.B., Holloway G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem. 2010;53:2719–2740. doi: 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]
- 38.Arrowsmith C.H., Audia J.E., Austin C., Baell J., Bennett J., Blagg J., et al. The promise and peril of chemical probes. Nat Chem Biol. 2015;11:536–541. doi: 10.1038/nchembio.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shoichet B.K. Screening in a spirit haunted world. Drug Discov Today. 2006;11:607–615. doi: 10.1016/j.drudis.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walters W.P., Namchuk M. Designing screens: how to make your hits a hit. Nat Rev Drug Discov. 2003;2:259–266. doi: 10.1038/nrd1063. [DOI] [PubMed] [Google Scholar]
- 41.Tomašić T., Peterlin Mašič L. Rhodanine as a scaffold in drug discovery: a critical review of its biological activities and mechanisms of target modulation. Expet Opin Drug Discov. 2012;7:549–560. doi: 10.1517/17460441.2012.688743. [DOI] [PubMed] [Google Scholar]
- 42.Mendgen T., Steuer C., Klein C.D. Privileged scaffolds or promiscuous binders: a comparative study on rhodanines and related heterocycles in medicinal chemistry. J Med Chem. 2012;55:743–753. doi: 10.1021/jm201243p. [DOI] [PubMed] [Google Scholar]
- 43.Zhou J.T., Jiang X.Y., Feng F., Sun H.P. Multi-target drug design strategy and its research progress. Acta Pharm Sin. 2018;53:2012–2025. [Google Scholar]
- 44.Gilberg E. Gütschow M, Bajorath J. Promiscuous ligands from experimentally determined structures, binding conformations, and protein family-dependent interaction hotspots. ACS Omega. 2019;4:1729–1737. doi: 10.1021/acsomega.8b03481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li S., Xu Y., Shen Q., Liu X., Lu J., Chen Y., et al. Non-covalent interactions with aromatic rings: current understanding and implications for rational drug design. Curr Pharmaceut Des. 2013;19:6522–6533. doi: 10.2174/13816128113199990440. [DOI] [PubMed] [Google Scholar]
- 46.Babaoglu K., Simeonov A., Irwin J.J., Nelson M.E., Feng B., Thomas C.J., et al. Comprehensive mechanistic analysis of hits from high-throughput and docking screens against β-lactamase. J Med Chem. 2008;51:2502–2511. doi: 10.1021/jm701500e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baell J.B., Ferrins L., Falk H., Nikolakopoulos G. PAINS: Relevance to tool compound discovery and fragment-based screening. Aust J Chem. 2014;66:1483–1494. [Google Scholar]
- 48.Sushko I., Salmina E., Potemkin V.A., Poda G., Tetko I.V. ToxAlerts: a web server of structural alerts for toxic chemicals and compounds with potential adverse reactions. J Chem Inf Model. 2012;52:2310–2316. doi: 10.1021/ci300245q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeller J., Turbiak A.J., Powelson I.A., Lee S., Sun D., Showalter H.H., et al. Investigation of 3-aryl-pyrimido[5,4-e][1,2,4]triazine-5,7-diones as small molecule antagonists of β-catenin/TCF transcription. Bioorg Med Chem Lett. 2013;23:5814–5820. doi: 10.1016/j.bmcl.2013.08.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baell J.B. Screening-based translation of public research encounters painful problems. ACS Med Chem Lett. 2015;6:229–234. doi: 10.1021/acsmedchemlett.5b00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neves A.P., Pereira M.X., Peterson E.J., Kipping R., Vargas M.D., Silva F.P., Jr., et al. Exploring the DNA binding/cleavage, cellular accumulation and topoisomerase inhibition of 2-hydroxy-3-(aminomethyl)-1,4-naphthoquinone mannich bases and their platinum (II) complexes. J Inorg Biochem. 2013;119:54–64. doi: 10.1016/j.jinorgbio.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 52.Musiol R. An overview of quinoline as a privileged scaffold in cancer drug discovery. Expert Opin Drug Met. 2017;12:583–597. doi: 10.1080/17460441.2017.1319357. [DOI] [PubMed] [Google Scholar]
- 53.Cardoso M.F., Rodrigues P.C., Oliveira M.E.I., Gama I.L., da Silva I.M., Santos I.O., et al. Synthesis and evaluation of the cytotoxic activity of 1,2-furanonaphthoquinones tethered to 1,2,3-1H-triazoles in myeloid and lymphoid leukemia cell lines. Eur J Med Chem. 2014;84:708–717. doi: 10.1016/j.ejmech.2014.07.079. [DOI] [PubMed] [Google Scholar]
- 54.Yu W., Li C., Zhang W., Xia Y., Li S., Lin J.Y., et al. Discovery of an orally selective inhibitor of signal transducer and activator of transcription 3 using advanced multiple ligand simultaneous docking. J Med Chem. 2017;60:2718–2731. doi: 10.1021/acs.jmedchem.6b01489. [DOI] [PubMed] [Google Scholar]
- 55.Andrade M.M., Martins L.C., Marques G.V., Silva C.A., Faria G., Caldas S., et al. Synthesis of quinoline derivatives as potential cysteine protease inhibitors. Future Med Chem. 2020;12:571–581. doi: 10.4155/fmc-2019-0201. [DOI] [PubMed] [Google Scholar]
- 56.Tsou H.R., Overbeek-Klumpers E.G., Hallett W.A., Reich M.F., Floyd M.B., Johnson B.D., et al. Optimization of 6,7-disubstituted-4-(arylamino) quinoline-3-carbonitriles as orally active, irreversible inhibitors of human epidermal growth factor receptor-2 kinase activity. J Med Chem. 2005;48:1107–1131. doi: 10.1021/jm040159c. [DOI] [PubMed] [Google Scholar]
- 57.Rabindran S.K., Discafani C.M., Rosfjord E.C., Baxter M., Floyd M.B., Golas J., et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–3965. doi: 10.1158/0008-5472.CAN-03-2868. [DOI] [PubMed] [Google Scholar]
- 58.Decker M. Elsevier; Würzburg: 2017. Design of hybrid molecules for drug development. [Google Scholar]
- 59.Ramsay R.R., Popovic-Nikolic M.R., Nikolic K., Uliassi E., Bolognesi M.L. A perspective on multi-target drug discovery and design for complex diseases. Clin Transl Med. 2018;7:3. doi: 10.1186/s40169-017-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilberg E., Stumpfe D., Bajorath J. Towards a systematic assessment of assay interference: identification of extensively tested compounds with high assay promiscuity. F1000 Research. 2017;6 doi: 10.12688/f1000research.12370.1. Chem Inf Sci-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Tao, Li Zhuyin, Cvijic Mary Ellen, Krause Carol, Zhang Litao, Sum Chi Shing. In: Assay guidance manual. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences. Sittampalam G.S., Grossman A., Brimacombe K., Arkin M., Auld D., Austin C., et al., editors. 2004. Measurement of β-arrestin recruitment for GPCR targets. [PubMed] [Google Scholar]
- 62.Senger M.R., Fraga C.A., Dantas R.F., Silva F.P., Jr. Filtering promiscuous compounds in early drug discovery: is it a good idea?. Drug Discov Today. 2016;21:868–872. doi: 10.1016/j.drudis.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 63.Inglese J., Johnson R.L., Simeonov A., Xia M., Zheng W., Austin C.P., et al. High-throughput screening assays for the identification of chemical probes. Nat Chem Biol. 2007;3:466–479. doi: 10.1038/nchembio.2007.17. [DOI] [PubMed] [Google Scholar]
- 64.Shapiro A.B., Walkup G.K., Keating T.A. Correction for interference by test samples in high-throughput assays. J Biomol Screen. 2009;14:1008–1016. doi: 10.1177/1087057109341768. [DOI] [PubMed] [Google Scholar]
- 65.Barf T., Kaptein A. Irreversible protein kinase inhibitors: balancing the benefits and risks. J Med Chem. 2012;55:6243–6262. doi: 10.1021/jm3003203. [DOI] [PubMed] [Google Scholar]
- 66.Wilson G.L., Lill M.A. Integrating structure-based and ligand-based approaches for computational drug design. Future Med Chem. 2011;3:735–750. doi: 10.4155/fmc.11.18. [DOI] [PubMed] [Google Scholar]
- 67.Carpenter K.A., Huang X.J. Machine learning-based virtual screening and its applications to alzheimer's drug discovery: a review. Curr Pharmaceut Des. 2018;24:3347–3358. doi: 10.2174/1381612824666180607124038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fu R.G., Sun Y., Sheng W.B., Liao D.F. Designing multi-targeted agents: an emerging anticancer drug discovery paradigm. Eur J Med Chem. 2017;136:195–211. doi: 10.1016/j.ejmech.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 69.Gilberg E., Stumpfe D., Bajorath J.R. X-ray-structure-based identification of compounds with activity against targets from different families and generation of templates for multitarget ligand design. ACS omega. 2018;3:106–111. doi: 10.1021/acsomega.7b01849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meng X.Y., Zhang H.X., Mezei M., Cui M. Molecular docking: a powerful approach for structure-based drug discovery. Curr Comput-Aid Drug. 2011;7:146–157. doi: 10.2174/157340911795677602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhachoo J., Beuming T. Investigating protein–peptide interactions using the Schrödinger computational suite. Methods Mol Biol. 2017;1561:235–254. doi: 10.1007/978-1-4939-6798-8_14. [DOI] [PubMed] [Google Scholar]
- 72.Wu G., Robertson D.H., Brooks C.L., III, Vieth M. Detailed analysis of grid-based molecular docking: a case study of CDOCKER-A CHARMm-based MD docking algorithm. J Comput Chem. 2003;24:1549–1562. doi: 10.1002/jcc.10306. [DOI] [PubMed] [Google Scholar]
- 73.London N., Miller R.M., Krishnan S., Uchida K., Irwin J.J., Eidam O., et al. Covalent docking of large libraries for the discovery of chemical probes. Nat Chem Biol. 2014;10:1066–1072. doi: 10.1038/nchembio.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones G., Willett P., Glen R.C. Molecular recognition of receptor sites using a genetic algorithm with a description of desolvation. J Biochem Mol Biol. 1995;245:43–53. doi: 10.1016/s0022-2836(95)80037-9. [DOI] [PubMed] [Google Scholar]
- 75.Soufan O., Ba-alawi W., Magana-Mora A., Essack M., Bajic V.B. DPubChem: a web tool for QSAR modeling and high-throughput virtual screening. Sci Rep-UK. 2018;8:1–10. doi: 10.1038/s41598-018-27495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cramer R.D. The inevitable QSAR renaissance. J Comput Aided Mol Des. 2012;26:35–38. doi: 10.1007/s10822-011-9495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ismaili L., Refouvelet B., Benchekroun M., Brogi S., Brindisi M., Gemma S., et al. Multitarget compounds bearing tacrine-and donepezil-like structural and functional motifs for the potential treatment of alzheimer's disease. Prog Neurobiol. 2017;151:4–34. doi: 10.1016/j.pneurobio.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 78.Bautista-Aguilera O.M., Samadi A., Chioua M., Nikolic K., Filipic S., Agbaba D., et al. N-Methyl-N-((1-methyl-5-(3-(1-(2-methylbenzyl) piperidin-4-yl) propoxy)-1H-indol-2-yl) methyl) prop-2-yn-1-amine, a new cholinesterase and monoamine oxidase dual inhibitor. J Med Chem. 2014;57:10455–10463. doi: 10.1021/jm501501a. [DOI] [PubMed] [Google Scholar]
- 79.Bautista-Aguilera O.M., Esteban G., Chioua M., Nikolic K., Agbaba D., Moraleda I., et al. Multipotent cholinesterase/monoamine oxidase inhibitors for the treatment of alzheimer's disease: design, synthesis, biochemical evaluation, ADMET, molecular modeling, and QSAR analysis of novel donepezil–pyridyl hybrids. Drug Des Dev Ther. 2014;8:1893–1910. doi: 10.2147/DDDT.S69258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Knapp B., Ospina L., Deane C.M. Avoiding false positive conclusions in molecular simulation: the importance of replicas. J Chem Theor Comput. 2018;14:6127–6138. doi: 10.1021/acs.jctc.8b00391. [DOI] [PubMed] [Google Scholar]
- 81.Cherkasov A., Muratov E.N., Fourches D., Varnek A., Baskin, Cronin M., et al. QSAR modeling: where have you been? Where are you going to? J Med Chem. 2014;57:4977–5010. doi: 10.1021/jm4004285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jing Y., Bian Y., Hu Z., Wang L., Xie X.Q.S. Deep learning for drug design: an artificial intelligence paradigm for drug discovery in the big data era. AAPS J. 2018;20:58. doi: 10.1208/s12248-018-0210-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reker D., Bernardes G.J., Rodrigues T. Computational advances in combating colloidal aggregation in drug discovery. Nat Chem. 2019;11:402–418. doi: 10.1038/s41557-019-0234-9. [DOI] [PubMed] [Google Scholar]
- 84.Stork C., Wagner J., Friedrich N.O. de Bruyn Kops, CŠíchoM, Kirchmair J. Hit dexter: a Machine-learning model for the prediction of frequent hitters. ChemMedChem. 2018;13:564–571. doi: 10.1002/cmdc.201700673. [DOI] [PubMed] [Google Scholar]
- 85.David L., Walsh J., Sturm N., Feierberg I., Nissink J.W.M., Chen H., et al. Identification of compounds that interfere with high-throughput screening assay technologies. ChemMedChem. 2019;14:1795–1802. doi: 10.1002/cmdc.201900395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feldmann C., Bajorath J. Compounds with multitarget activity: structure-based analysis and machine learning. Future Drug Discov. 2020;2 [Google Scholar]
- 87.Molinski S.V., Shahani V.M., Subramanian A.S., MacKinnon S.S., Woollard G., Laforet M., et al. Comprehensive mapping of cystic fibrosis mutations to CFTR protein identifies mutation clusters and molecular docking predicts corrector binding site. Proteins. 2018;86:833–843. doi: 10.1002/prot.25496. [DOI] [PubMed] [Google Scholar]
- 88.Zhou H., Gao M., Skolnick J. Comprehensive prediction of drug‒protein interactions and side effects for the human proteome. SCI REP-UK. 2015;5:11090. doi: 10.1038/srep11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Landrum G. RDKit: open-source cheminformatics software. GitHub and SourceForge. 2016;10:3592822. [Google Scholar]
- 90.Sterling T., Irwin J.J. ZINC 15-ligand discovery for everyone. J Chem Inf Model. 2015;55:2324–2337. doi: 10.1021/acs.jcim.5b00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jana S., Ganeshpurkar A., Singh S.K. Multiple 3D-QSAR modeling, e-pharmacophore, molecular docking, and in vitro study to explore novel achE inhibitors. RSC Adv. 2018;8:39477–39495. doi: 10.1039/c8ra08198k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koptelov M., Zimmermann A., Bonnet P., Bureau R., Crémilleux B. Proceedings of the 24th ACM SIGKDD international conference on knowledge discovery & data mining. July 2018. PrePeP: a tool for the identification and characterization of pan assay interference compounds; pp. 462–471. [Google Scholar]
- 93.Mignani S., Rodrigues J., Tomas H., Jalal R., Singh P.P., Majoral J.P., et al. Present drug-likeness filters in medicinal chemistry during the hit and lead optimization process: how far can they be simplified?. Drug Discov Today. 2018;23:605–615. doi: 10.1016/j.drudis.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 94.Strelow J.M. A perspective on the kinetics of covalent and irreversible inhibition. SLAS Discov. 2017;22:3–20. doi: 10.1177/1087057116671509. [DOI] [PubMed] [Google Scholar]
- 95.Zhang T., Hatcher J.M., Teng M., Gray N.S., Kostic M. Recent advances in selective and irreversible covalent ligand development and validation. Cell Chem Biol. 2019;26:1486–1500. doi: 10.1016/j.chembiol.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang T., Kwiatkowski N., Olson C.M., Dixon-Clarke S.E., Abraham B.J., Greifenberg A.K., et al. Covalent targeting of remote cysteine residues to develop CDK12 and CDK13 inhibitors. Nat Chem Biol. 2016;12:876. doi: 10.1038/nchembio.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Anighoro A., Bajorath J., Rastelli G.J. Polypharmacology: challenges and opportunities in drug discovery: miniperspective. J Med Chem. 2014;57:7874–7887. doi: 10.1021/jm5006463. [DOI] [PubMed] [Google Scholar]
- 98.Hu Y., Bajorath J. Compound promiscuity: what can we learn from current data?. Drug Discov Today. 2013;18:644–650. doi: 10.1016/j.drudis.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 99.Jackson P.A., Widen J.C., Harki D.A., Brummond K.M. Covalent modifiers: a chemical perspective on the reactivity of α,β-unsaturated carbonyls with thiols via hetero-michael addition reactions. J Med Chem. 2017;60:839–885. doi: 10.1021/acs.jmedchem.6b00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Drewes G., Knapp S. Chemoproteomics and chemical probes for target discovery. Trends Biotechnol. 2018;36:1275–1286. doi: 10.1016/j.tibtech.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 101.Browne C.M., Jiang B., Ficarro S.B., Doctor Z.M., Johnson J.L., Card J.D., et al. A chemoproteomic strategy for direct and proteome-wide covalent inhibitor target-site identification. J Am Chem Soc. 2018;141:191–203. doi: 10.1021/jacs.8b07911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dahlin J.L., Nelson K.M., Strasser J.M., Barsyte-Lovejoy D., Szewczyk M.M., Organ S., et al. Assay interference and off-target liabilities of reported histone acetyltransferase inhibitors. Nat Commun. 2017;8:1–14. doi: 10.1038/s41467-017-01657-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ganesh A.N., Donders E.N., Shoichet B.K., Shoichet M.S. Colloidal aggregation: from screening nuisance to formulation nuance. Nano Today. 2018;19:188–200. doi: 10.1016/j.nantod.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mcgovern S.L., Caselli E., Grigorieff N., Shoichet B.K. A common mechanism underlying promiscuous inhibitors from virtual and high-throughput screening. J Med Chem. 2002;45:1712–1722. doi: 10.1021/jm010533y. [DOI] [PubMed] [Google Scholar]
- 105.Mcgovern S.L., Helfand B.T., Feng B., Shoichet B.K. A specific mechanism of nonspecific inhibition. J Med Chem. 2003;46:4265–4272. doi: 10.1021/jm030266r. [DOI] [PubMed] [Google Scholar]
- 106.Duan D., Torosyan H., Elnatan D., Mclaughlin C.K., Logie J., Shoichet M.S., Agard D.A., Shoichet B.K. Internal structure and preferential protein binding of colloidal aggregates. ACS Chem Biol. 2017;12:282–290. doi: 10.1021/acschembio.6b00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Owen S.C., Doak A.K., Ganesh A.N., Nedyalkova L., McLaughlin C.K., Shoichet B.K., et al. Colloidal drug formulations can explain “bell-shaped” concentration–response curves. ACS Chem Biol. 2014;9:777–784. doi: 10.1021/cb4007584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Irwin J.J., Duan D., Torosyan H., Doak A.K., Ziebart K.T., Sterling T., et al. An aggregation advisor for ligand discovery. J Med Chem. 2015;58:7076–7087. doi: 10.1021/acs.jmedchem.5b01105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rao H., Li Z., Li X., Ma X., Ung C., Li H., et al. Identification of small molecule aggregators from large compound libraries by support vector machines. J Comput Chem. 2010;31:752–763. doi: 10.1002/jcc.21347. [DOI] [PubMed] [Google Scholar]
- 110.Mateen R., Ali M.M., Hoare T. A printable hydrogel microarray for drug screening avoids false positives associated with promiscuous aggregating inhibitors. Nat Commun. 2018;9:1–9. doi: 10.1038/s41467-018-02956-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Giannetti A.M., Koch B.D., Browner M.F. Surface plasmon resonance based assay for the detection and characterization of promiscuous inhibitors. J Med Chem. 2008;51:574–580. doi: 10.1021/jm700952v. [DOI] [PubMed] [Google Scholar]
- 112.Chan L.L., Lidstone E.A., Finch K.E., Heeres J.T., Hergenrother P.J., Cunningham B.T. A method for identifying small-molecule aggregators using photonic crystal biosensor microplates. JALA Charlottesv Va. 2009;14:348–359. doi: 10.1016/j.jala.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mateen R., Ali M.M., Hoare T. A printable hydrogel microarray for drug screening avoids false positives associated with promiscuous aggregating inhibitors. Nat Commun. 2018;9:602. doi: 10.1038/s41467-018-02956-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Johnston P.A. Redox cycling compounds generate H2O2 in HTS buffers containing strong reducing reagents-real hits or promiscuous artifacts?. CurrOpin Chem Biol. 2011;15:174–182. doi: 10.1016/j.cbpa.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Johnston P.A., Foster C.A., Tierno M.B., Shun T.Y., Shinde S.N., Paquette W.D., et al. Cdc25B dual-specificity phosphatase inhibitors identified in a high-throughput screen of the NIH compound library. Assay Drug Dev Technol. 2009;7:250–265. doi: 10.1089/adt.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Johnston P.A., Soares K.M., Shinde S.N., Foster C.A., Shun T.Y., Takyi H.K., et al. Development of a 384-well colorimetric assay to quantify hydrogen peroxide generated by the redox cycling of compounds in the presence of reducing agents. Assay Drug Dev Technol. 2008;6:505–518. doi: 10.1089/adt.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lor L.A., Schneck J., Mcnulty D.E., Diaz E., Brandt M., Thrall S.H., et al. A simple assay for detection of small-molecule redox activity. J Biomol Screen. 2007;12:881–890. doi: 10.1177/1087057107304113. [DOI] [PubMed] [Google Scholar]
- 118.Roughley S.D., Jordan A.M. The medicinal chemist's toolbox: an analysis of reactions used in the pursuit of drug candidates. J Med Chem. 2011;54:3451–3479. doi: 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]
- 119.Hermann J.C., Chen Y., Wartchow C., Menke J., Gao L., Gleason S.K., et al. Metal impurities cause false positives in high-throughput screening campaigns. ACS Med Chem Lett. 2013;4:197–200. doi: 10.1021/ml3003296. [DOI] [PMC free article] [PubMed] [Google Scholar]