Abstract
Given the opposing effects of Akt and AMP-activated protein kinase (AMPK) on metabolic homeostasis, this study examined the effects of deletion of Akt2 and AMPKα2 on fat diet-induced hepatic steatosis. Akt2–Ampkα2 double knockout (DKO) mice were placed on high fat diet for 5 months. Glucose metabolism, energy homeostasis, cardiac function, lipid accumulation, and hepatic steatosis were examined. DKO mice were lean without anthropometric defects. High fat intake led to adiposity and decreased respiratory exchange ratio (RER) in wild-type (WT) mice, which were ablated in DKO but not Akt2−/− and Ampkα2−/− mice. High fat intake increased blood and hepatic triglycerides and cholesterol, promoted hepatic steatosis and injury in WT mice. These effects were eliminated in DKO but not Akt2−/− and Ampkα2−/− mice. Fat diet promoted fat accumulation, and enlarged adipocyte size, the effect was negated in DKO mice. Fat intake elevated fatty acid synthase (FAS), carbohydrate-responsive element-binding protein (CHREBP), sterol regulatory element-binding protein 1 (SREBP1), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), peroxisome proliferator-activated receptor-α (PPARα), PPARγ, stearoyl-CoA desaturase 1 (SCD-1), phosphoenolpyruvate carboxykinase (PEPCK), glucose 6-phosphatase (G6Pase), and diglyceride O-acyltransferase 1 (DGAT1), the effect was absent in DKO but not Akt2−/− and Ampkα2−/− mice. Fat diet dampened mitophagy, promoted inflammation and phosphorylation of forkhead box protein O1 (FoxO1) and AMPKα1 (Ser485), the effects were eradicated by DKO. Deletion of Parkin effectively nullified DKO-induced metabolic benefits against high fat intake. Liver samples from obese humans displayed lowered microtubule-associated proteins 1A/1B light chain 3B (LC3B), Pink1, Parkin, as well as enhanced phosphorylation of Akt, AMPK (Ser485), and FoxO1, which were consolidated by RNA sequencing (RNAseq) and mass spectrometry analyses from rodent and human livers. These data suggest that concurrent deletion of Akt2 and AMPKα2 offers resilience to fat diet-induced obesity and hepatic steatosis, possibly through preservation of Parkin-mediated mitophagy and lipid metabolism.
KEY WORDS: Akt2, AMPK, Parkin, High fat intake, Obesity, Steatosis, Mitophagy, FoxO1
Graphical abstract
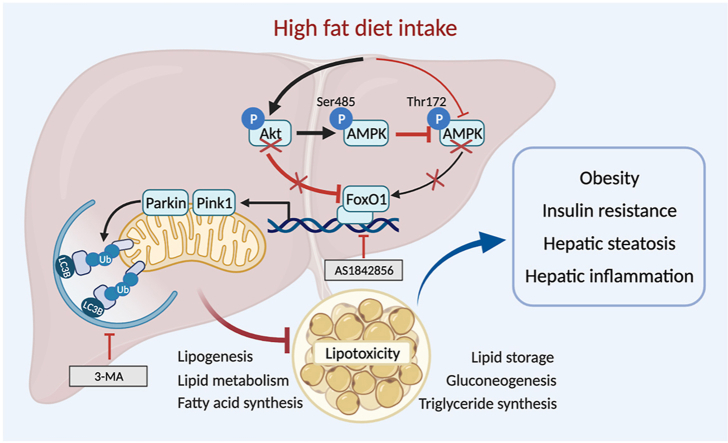
High fat intake suppresses FoxO1-mediated mitophagy through parallel regulation of Akt and AMPK. Hyperactivated Akt fosters AMPK phosphorylation at Ser485, disturbing AMPK phosphorylation at Thr172 indirectly to foster hepatic steatosis.
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is characterized by hepatic steatosis that may progress into non-alcoholic steatohepatitis (NASH). NASH is associated with hepatic inflammation, fibrosis, and increased prevalence of cirrhosis and hepatocellular carcinoma1, 2, 3. NAFLD is closely associated with metabolic syndrome in obese or morbidly obese patients4,5. NAFLD presents with hepatic manifestations of metabolic syndrome including insulin resistance, obesity, and type 2 diabetes mellitus3, 4, 5. The incidence of NAFLD rises concurrently with obesity and type 2 diabetes, and is expected to become the leading cause of liver transplantation6. Understanding of etiology of NAFLD further explains the detrimental effects including insulin resistance, autophagy defect, inflammation, and fibrosis7, 8, 9, 10, 11, 12. Minimal pharmacotherapy has been approved (licensed) for NAFLD to reverse or halt the progression of steatosis to NASH. Given the high incidence of NAFLD in obesity, one important issue for overweight or obese individuals may be the search for factors that trigger hepatic steatosis and ultimately liver injury11.
Metabolic disorders, particularly those involved in lipid metabolism, are important for the transition from metabolic syndrome to liver injury. Hepatic steatosis (lipid buildup) plays a complex and controversial role in the pathogenesis of NAFLD1,6,11. Increased levels of free fatty acids appear to be a major risk factor for NAFLD, and evidence has revealed that inhibition of triglyceride synthesis counters fatty acid-induced hepatic lipotoxicity in obesity13, 14, 15. A plethora of cytokines and cell-signaling molecules were identified that reveal the association between insulin signaling and NAFLD pathogenesis. Insulin resistance is cardinal to predispose hepatocytes to pathogenic factors11,15. However, hepatic lipid accumulation was reported to be independent of insulin action, but rather relied on fatty acid substrate availability15,16. These findings denote a controversial role of hepatic lipid accumulation as an indicator or a causative mediator in metabolic diseases17. Among various signaling molecules in the regulation of hepatic insulin sensitivity and lipid metabolism, protein kinase B (Akt) and AMP-activated protein kinase (AMPK) remain the most prominent signals governing insulin sensitivity, lipid and energy metabolism18. Both genetic and high fat diet-induced obesity compromise Akt and AMPK regulation possibly due changes in nutrients, ATP, and proinflammatory cytokine levels19, 20, 21. Using genetically engineered murine models, distinct roles for Akt and AMPK were unveiled in hepatic lipogenesis and energy metabolism in high fat diet-induced insulin resistance and obesity22, 23, 24, 25. Akt, in particular Akt2, is essential for activation of hepatic sterol regulatory element-binding protein 1c (SREBP1c), lipogenesis, and lipid accumulation in metabolic stress22,23. On the other hand, AMPK inhibits SREBP1c function, lipid accumulation, hepatic steatosis, and adiposity, which cause the metabolic benefits of drugs such as metformin24,26. Nonetheless, interactions between these two opposing signaling molecules in growth, lipid, and energy metabolism in adiposity and hepatic steatosis remain unclear.
To this end, we generated an Akt2–Ampkα2 double knockout (DKO) murine model to examine the impact of concurrent ablation of Akt2 and AMPKα2 on high fat diet-induced obesity, hepatic steatosis, and inflammation. Given the established role of autophagy and mitochondrial autophagy (aka mitophagy) in adiposity, hepatic steatosis, and injury8,10,17,27, and the apparent reciprocal regulatory modality of Akt and AMPK on autophagy and mitophagy27,28, mitophagy was examined in high fat diet-induced in obesity and hepatic steatosis. Our results depict concurrent deletion of Akt2 and AMPKα2 rescued high fat diet intake-induced loss of mitophagy molecule Parkin but not FUN14 domain-containing protein 1 (FUNDC1) and BCL2/adenovirus E1B 19 kDa protein-interacting protein-3 (BNIP3). To this end, an Akt2–Ampkα2–Parkin triple knockout (TKO) murine line was generated to further discern the role of Parkin. In support of our experimental findings, RNA sequencing (RNAseq), mass spectrometry, and immunoblot analyses from obese rodent and human liver samples all depicted prominent roles for gene ontology terms related to cell signaling of Akt, AMPK, autophagy, mitophagy, and lipid metabolism in liver injury.
2. Materials and methods
2.1. Human samples and liquid chromatography–tandem mass spectrometry (LC–MS/MS) analysis
Liver specimens were obtained from liver transplants from lean and obese patients (Supporting Information Table S1) under an approved protocol (No. B2020-127R) by Zhongshan Hospital Fudan University Ethics Committee (Shanghai, China) and were carried out in accordance with the Declaration of Helsinki for experiments involving humans. LC–MS/MS data acquisition of human liver samples was carried out on an Orbitrap Exploris 480 mass spectrometer coupled with an Easy-nLC 1200 system (Thermo Scientific, USA)27. Peptides were first loaded onto a C18 trap column (75 μm × 2 cm, 3 μm particle size, 100 Å pore size, Thermo Scientific) and then separated in a C18 analytical column (75 μm × 250 mm, 3 μm particle size, 100 Å pore size, Thermo). Mobile phase A (0.1% formic acid) and B (80% acetonitrile, 0.1% formic acid) were used to establish the separation gradient. A constant flow rate was set at 300 nL/min. For data dependent acquisition (DDA) mode analysis, each scan cycle consisted of one full-scan mass spectrum followed by 20 MS/MS events. Higher energy collision dissociation (HCD) was set to 32. Isolation window for precursor selection was set to 1.2 Da. Former target ion exclusion was set for 30 s. MS raw data were analyzed with MaxQuant (V1.6.6) using the Andromeda database search algorithm. Spectra files were searched against the UniProt human protein database, and search results were filtered with 1% false discovery rate (FDR) at both protein and peptide levels. Proteins denoted as decoy hits, contaminants, or only identified by sites were removed, and the remaining proteins were used for further analysis.
2.2. mRNA sequencing (mRNA-Seq)
mRNA-Seq was done by Novogene (Beijing, China) and Cloudseq Biotech Inc. (Shanghai, China). Standard Illumina protocols were used for sequencing after preparation of mRNA-seq library. RNAs were isolated from livers from adult db/db obese mice or rats (Nanjing Biomedical Research Institute of Nanjing University, Nanjing, China) following 45% high fat diet intake for 20 weeks using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Twelve-week-old db/db mice or rats were housed at 21 ± 1 °C with a humidity of 55% ± 10% and a 12-h/12-h light/dark cycle. To remove any contaminating genomic DNA, RNase-free DNase I (New England Biolabs, MA, USA) was applied to isolated RNAs. Dynabeads oligo (dT) (Invitrogen) were used to extract mRNA, and double-stranded complementary DNAs (cDNAs) were synthesized using Superscript II reverse transcriptase (Invitrogen) and random hexamer primers. To create mRNA-seq library, cDNAs were fragmented by nebulization and standard Illumina protocol was followed thereafter. For data analysis, base calls performed using CASAVA Reads (version 1.8.2 software, Illumina, CA, USA) were aligned to genome using split read aligner TopHat (version 2.0.7, Ontario, Canada) and Bowtie2 (http://bowtie-bio.sourceforge.net/index.shtml). HTSeq (http://www-huber.embl.de/HTSeq) was used for estimation of their abundance29.
2.3. Functional analysis of differentially expressed proteins using bioinformatics
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed using the OmicShare tools, a free online platform for data analysis (http://www.omicshare.com/tools).
2.4. Akt2 and Ampkα2 knockout mice, generation of Akt2–Ampkα2 double or Akt2–Ampkα2–Parkin triple knockout mice, and high fat diet feeding
All animal procedures were approved by the Animal Care and Use Committee at the Zhongshan Hospital Fudan University (Shanghai, China) and University of Wyoming (Laramie, WY, USA). Akt2 knockout (Akt2−/−) mice were obtained from Dr. Morris J. Birnbaum at the University of Pennsylvania (Philadelphia, PA, USA) and Ampkα2 knockout (Ampkα2−/−) mice were generated and characterized by Dr. Benoit Viollet at the Institute Cochin, Université Paris Descartes, CNRS (UMR 8104) in Paris, France30,31. Akt2−/− and Ampkα2−/− mice (both on C57BL/6J background) were bred to generate DKO mice. To produce Akt2–/–Ampkα2–/––Parkin–/– (Prk–/–) triple knockout (TKO) mice, the DKO mice were crossed with Prk–/– mice to generate heterozygotes, which were further crossed to produce TKO mice. Global Prk–/– mice were purchased from the Jackson Laboratory (B6.129S4-Parktm1Shn/J, stock number 006582). To confirm genotypes, genomic DNA prepared from tail snips was analyzed using PCR (Supporting Information Table S2). Mice were housed under temperature-controlled conditions (22 ± 2 °C), humidity (55% ± 5%), and a 12 h/12 h light–dark circadian cycle with access to food and water ad libitum. Five-week-old male Akt2−/− or Ampkα2−/−, DKO, or TKO mice, and their wild-type (WT) littermates were placed on a low fat (Cat. D12450B, 10% calories from fat, 3.91 kcal/g) or high fat (Cat. D12451, 45% calories from fat, 4.83 kcal/g; Research Diets, New Brunswick, NJ, USA) diet for 20 weeks25,32. In another cohort of study, five-week-old male Prk–/– or TKO mice and their WT littermates were placed on a low fat or high fat diet for 20 weeks. Food intake was recorded using a lab scale. Body fat composition (% lean and fat mass) was measured at the end of 20-week feeding period using a Dual Energy X-ray Absorptiometry (GE Lunar Prodigy™ 8743; Madison, WI, USA). Animal sample size was determined using power analysis.
2.5. Intraperitoneal glucose tolerance test (IPGTT), serum lipid, and insulin measurement
Mice fasted for 12 h and then were given an intraperitoneal injection of glucose (2 g/kg body weight). Blood samples were collected from the tail vein immediately before glucose challenge, as well as 15, 30, 60, and 120 min thereafter. Serum glucose levels were determined using an Accu-Chek III glucose analyzer (Roche, Germany). Area under curve (AUC) was calculated using trapezoidal analysis32. Serum levels of insulin, triglycerides, and cholesterol were measured using commercial kits (BioVision, Inc., Mountain View, CA, USA; EMD Millipore Corp., Billerica, MA, USA)32. Serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were determined using colorimetric assay kits from BioVision per manufacturer's instructions33.
2.6. Metabolic measurement
A Comprehensive Laboratory Animal Monitoring System (CLAMS™, Columbus Instruments, Columbus, OH, USA) was used to monitor global metabolism. Mice were housed in individual CLAMS metabolic cages with access to food and water ad libitum. Following acclimation, metabolic parameters including carbon dioxide production (VCO2), oxygen consumption (VO2), respiratory exchange ratio (RER = VCO2/VO2), heat production [(3.815 + 1.232 × RER) × VO2) × 1000], and physical activity (horizontal beam breaks) were recorded every 12 min over a period of 24 h (light phase: 7:00 am–7:00 pm; dark phase: 7:00 pm–7:00 am)34.
2.7. Hepatic triglycerides
Hepatic triglyceride levels were measured using a colorimetric kit from BioVision. Following anesthesia (ketamine 80 mg/kg and xylazine 12 mg/kg, ip), liver tissues were collected and were homogenized before being heated in 5% NP-40 solution (90 °C for 5 min). Solubilized triglyceride lysate was centrifuged and supernatants were employed for triglyceride assay using a Spectra Max 190 Spectrophotometer (Molecular Devices, Sunnyvale, CA, USA)35.
2.8. Liver and adipose tissue histology, oil red O staining
Lipid droplet accumulation was determined using oil red O staining. Following anesthesia, livers and white and brown adipose tissues were collected and placed in 10% neutral-buffered formalin at room temperature for 24 h. All samples were embedded in paraffin, cut in 7-μm sections, and were used for hematoxylin and eosin (H&E) staining. For oil red O staining, samples were sliced and snap-frozen in isopentane-cooled liquid nitrogen and were cut into 10-μm sections using a cryostat. Sections were fixed with 10% neutral-buffered formalin, rinsed with 60% isopropanol and stained with diluted oil red O solution (0.5 g oil red O powder in 100 mL isopropanol then diluted by 1.66 times) for 20 min prior to imaging using an Olympus BX-51 microscope (Tokyo, Japan)36.
2.9. Western blot analysis
Tissues were homogenized in a RIPA lysis buffer and centrifuged at 13,000 ×g for 20 min at 4 °C. Sample (30–50 μg protein/lane) were then separated on sodium dodecyl sulfate (SDS)-polyacrylamide gels in a minigel apparatus (Mini-PROTEAN II, Bio-Rad, Hercules, CA, USA) and were transferred to nitrocellulose membranes. Membranes were blocked and were incubated overnight at 4 °C with anti-fatty acid synthase (FAS, 1:1000), anti-phospho-acetyl-CoA carboxylase (ACC, Ser79, 1:1000), anti-ACC (1:1000), anti-carbohydrate-responsive element-binding protein (CHREBP, 1:1000), anti-sterol regulatory element-binding protein 1c (SREBP1c, 1:1000), anti-peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α, 1:1000), anti-peroxisome proliferator-activated receptor-α (PPARα, 1:1000), anti-PPARγ (1:1000), anti-stearoyl-CoA desaturase 1 (SCD-1, 1:1000), anti-diglyceride O-acyltransferase 1 (DGAT1, 1:500), anti-phosphoenolpyruvate carboxykinase (PEPCK, 1:1000), anti-glucose 6-phosphatase (G6Pase, 1:1000), anti-tumor necrosis factor-α (TNF-α, 1:500), anti-interleukin-6 (IL-6, 1:250), anti-microtubule-associated proteins 1A/1B light chain 3B (LC3B, 1:1000), anti-p62 (1:1000), anti-outer mitochondrial membrane 20 (TOM20, 1:1000), anti-Parkin (1:1000), anti-FUNDC1 (1:1000), anti-BNIP3 (1:1000), anti-phospho-forkhead box protein O1 (FoxO1, Ser256, 1:1000), anti-FoxO11 (1:1000), anti-Akt (1:1000), anti-Akt1 (1:1000), anti-Akt2 (1:1000), anti-Akt3 (1:1000), anti-phospho-Akt (Ser473, 1:1000), anti-AMPK (1:1000), anti-AMPKα1 (1:1000), anti-AMPKα2 (1:1000), anti-phospho-AMPK (Thr172, 1:1000), anti-phospho-AMPK (Ser485, 1:1000), and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or anti-α-tubulin (loading controls, 1:1000) antibodies. All antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA) or Abcam (Cambridge, MA, USA). Gel blots were incubated for 1 h with horseradish peroxidase (HRP)-conjugated secondary antibody (1:5000) and were quantified using the Quantity One software (Bio-Rad)35.
2.10. RNA isolation and gene expression
mRNA samples were isolated and purified following the instruction of Trizol Reagent (Invitrogen). cDNA was synthesized using the iScriptTM cDNA Synthesis Kit (Bio-Rad) and was mixed with SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) and primers (sequences provided in Table S2). Reactions were executed using a CFX96TM RT-PCR detection system (Bio-Rad). The mRNA levels were expressed relative to Gapdh37.
2.11. HepG2 cell culture, treatment, and RNA interference
Small interfering RNA (siRNA) against AMPK, AKT2 (On-TARGET plus SMART pool siRNA), or control non-targeting siRNA was obtained from Dharmacon (Lafayette, CO, USA). HepG2 cells (used for better siRNA efficacy) were grown in the Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA), and 1% penicillin and streptomycin and maintained in 95% air and 5% CO2 at 37 °C. Cells were grown to 70% confluence prior to transfection with siRNA (100 nmol/L) in DMEM using the Dharma FECT 1 transfection reagent. The AMPK siRNA sequence was: 5ʹ-CCCTTCCTATGATGCTAACG-3ʹ (forward); 5ʹ-AGAACTCACTGGCTTGGTTC-3ʹ (reverse)38. The AKT2 siRNA sequence was: 5ʹ-GCCTGAGGTGCTAGAGGACAAT-3ʹ (forward); 5ʹ-GAAGCCGATCTCCTCCATAAGA-3ʹ (reverse)39. After 72 h of transfection, cells were exposed to palmitate acid (0.5 mmol/L)40 in the presence or absence of the autophagy inhibitor 3-methyladenine (3-MA, 10 mmol/L)41 or the FoxO1 inhibitor AS1842856 (100 nmol/L)42 for 72 h followed by fixation.
2.12. Evaluation of lipid accumulation and triglyceride content in vitro
Lipid accumulation was evaluated using oil red O staining. HepG2 cells were fixed with ice-cold 10% formalin for 30 min. Cells were washed three times with sterile phosphate-buffered saline (PBS) and were stained with oil red O for 20 min as described above. Images were taken through light microscopy (400×) and the absorbance for oil Red O dye eluted by isopropanol was measured at 530 nm wavelength using the Spectra Max 190 Microplate Spectrophotometer (molecular devices)43,44. Levels of triglycerides from cell lysates were determined using a biochemistry assay kit (Abcam)45.
2.13. Statistical analysis
Data were shown as mean ± standard error of the mean (SEM). The Shapiro–Wilk normality test was used to examine distribution of experimental data. Statistical significance (P < 0.05) was estimated using analysis of variance (ANOVA) followed by a Tukey's post hoc analysis. Due to the cost and potential mortality issues, not every single mouse from each group was subjected to metabolic cage, intraperitoneal glucose tolerance test (IGPTT), and dual energy X-ray absorptiometry (DEXA) analyses, thus creating a smaller sample size for these indices.
3. Results
3.1. Changes in cell signaling pathways in livers from diet-induced rodent and human obesity
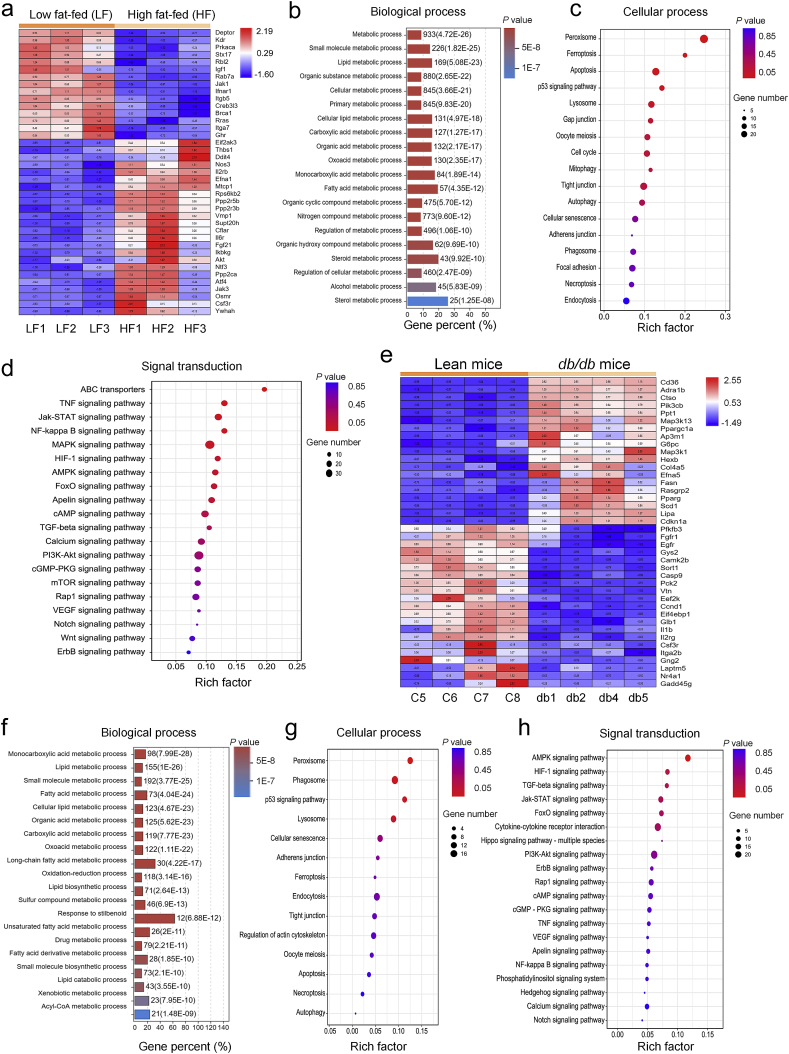
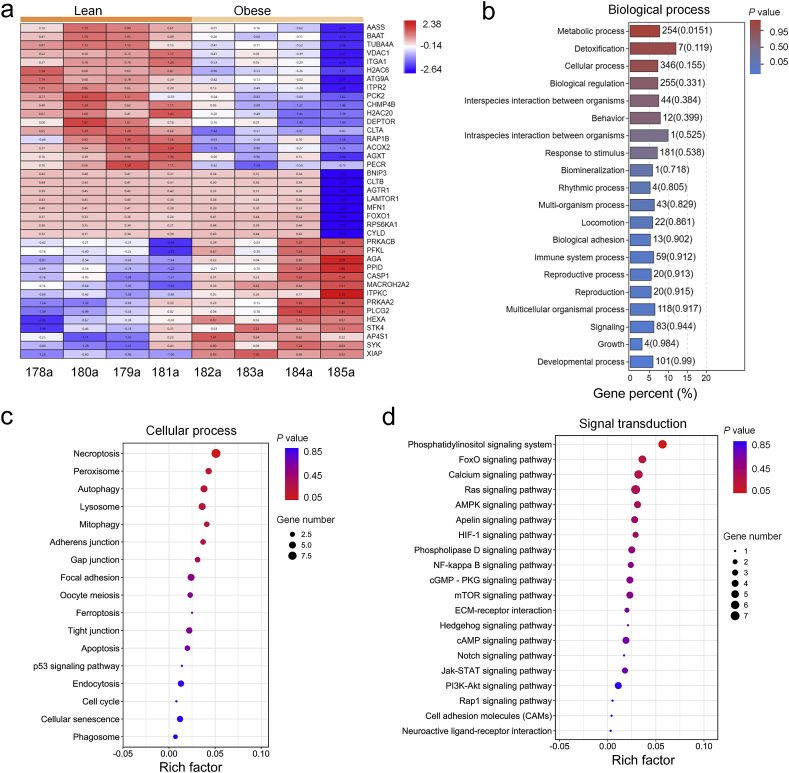
To discern possible biological processes and cell signaling pathways involved in liver injury in obesity, liver tissues were collected from adult Sprague–Dawley rats fed with high fat diet for 20 weeks and adult db/db leptin receptor deficient obese mice, prior to RNAseq analysis. As shown in Fig. 1, comparison between lean and obese rodents identified 1618 (rats) and 1131 (mice) genes with statistically significant differential expression [including 939 (rats) and 692 (mice) up-regulated and 679 (rats) and 439 (mice) down-regulated genes, P < 0.05]. A hierarchical clustering of the most highly regulated genes showed distinct patterns between the lean and obese groups (Fig. 1a, rats; Fig. 1e, mice). Biological function analysis for differentially expressed genes revealed that GO terms associated with “metabolic processes” were notably changed (Fig. 1b, rats; Fig. 1f, mice). Cellular processes of KEGG class analysis revealed that obesity (diet-induced or db/db genetic) affected proteins involved in autophagy and mitophagy processes (Fig. 1c, rats; Fig. 1g, mice). Environmental information processing of KEGG class analysis indicated that obesity-regulated proteins were enriched in phosphoinositide 3-kinases (PI3K)–Akt, AMPK, and FoxO signaling pathways (Fig. 1d, rats; Fig. 1h, mice). To confirm altered proteins in obesity, lean and obese human liver tissues were examined using LC–MS/MS. The 406 differentially expressed proteins were found (including 131 up-regulated and 275 down-regulated; fold change > 2). The most highly regulated proteins were displayed with distinct patterns in these two human groups (Fig. 2a). Similar to RNAseq findings from rodents, biological process enrichment analyses of human samples revealed that GO terms associated with “metabolic processes” were notably altered (Fig. 2b). Cellular processes of KEGG class analysis showed that obesity-regulated proteins were enriched in autophagy and mitophagy processes (Fig. 2c). Environmental information processing of KEGG class analysis indicated that obesity-regulated proteins were clustered in PI3K–Akt, AMPK, and FoxO signaling pathways (Fig. 2d). These results indicate a possible role of PI3K–Akt, AMPK, FoxO, autophagy and mitophagy signaling in obesity-associated liver injury.
Figure 1.
High fat diet intake and leptin receptor deficient obesity-modulated molecular events and cell signaling pathways in livers. Liver tissues of rats fed high fat diet for 20 weeks (a–d) or db/db obese and C57BL/6J lean mice (e–h) were subjected to RNAseq analysis. (a and e) Heatmap displays significantly regulated genes in signaling pathways of PI3K–Akt, AMPK, and FoxO, as well as cellular processes of autophagy and mitophagy in rat (a) and mice (e), respectively. (b and f) GO analysis was conducted with all altered genes, and 20 highest-ranking biological process terms are shown, rat (b) and mice (f), respectively; (c and g) KEGG pathway analyses of all significantly regulated genes were performed, and the enrichment for cellular process of KEGG pathway is shown using bubble plot, rat (c) and mice (g), respectively; (d and h) Bubble plot shows the enrichment for signal transduction of KEGG pathway, rat (d) and mice (h), respectively.
Figure 2.
Biological process, molecular events, and cell signaling pathways in livers from lean and obese human liver samples were analyzed using label-free quantitative proteomics and LC–MS/MS to identify differentially expressed proteins. (a) Heatmap displays significantly regulated proteins in signaling pathways of PI3K–Akt, AMPK, and FoxO, as well as cellular processes of autophagy and mitophagy. (b) GO analysis was conducted with all altered proteins, and 20 highest-ranking biological process terms are shown. (c) KEGG pathway analyses of all significantly regulated proteins were performed, and the enrichment for cellular process of KEGG pathway is shown using bubble plot. (d) Bubble plot displays the enrichment for signal transduction of KEGG pathway.
3.2. Biometric parameters and glucose sensitivity
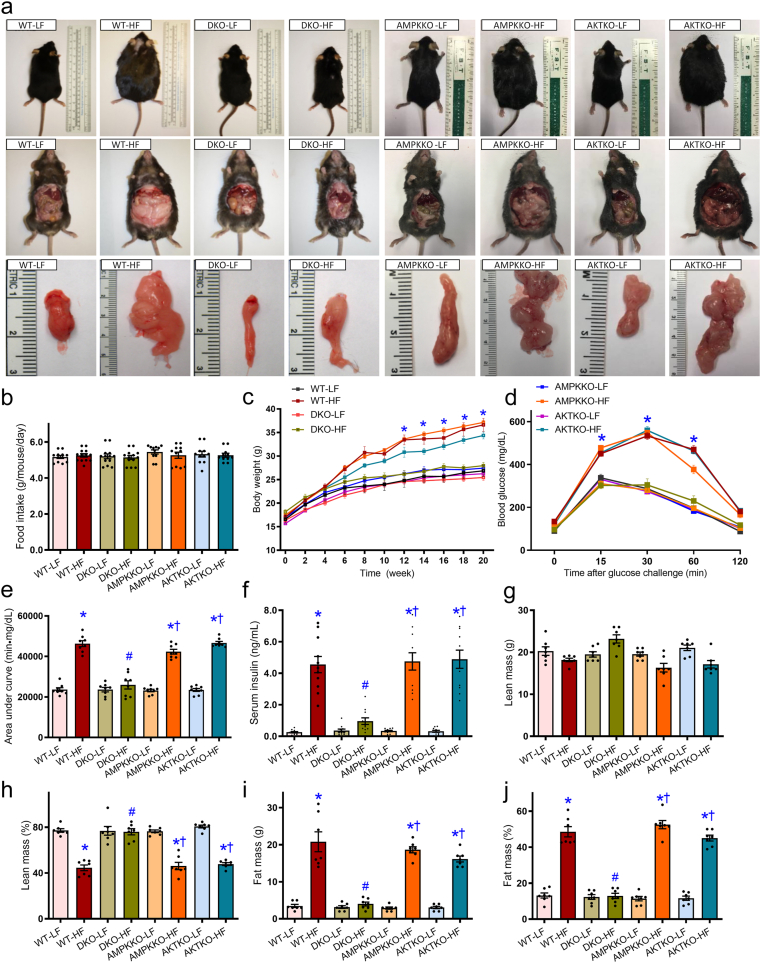
As shown in Supporting Information Fig. S1a and S1b, DKO of both Akt2 and Ampkα2 had little effect on Akt1, Akt3, and AMPKα1 in hearts, livers, and muscles. Levels of pan-Akt and -AMPK were significantly downregulated in all tissues from DKO mice (except Akt levels in the hearts and muscles). High fat diet intake significantly increased body weight in WT, Akt2−/− and Ampkα2−/− mice starting from 12 weeks of diet feeding. This effect was absent in DKO mice. Food intake was comparable among all groups (Fig. 3a–c). High fat diet intake overtly compromised glucose sensitivity in WT mice, the effect of which was absent in DKO but not Akt2−/− and Ampkα2−/− mice, as demonstrated by the IPGTT test (Fig. 3d and e). Along the same line, high fat diet intake increased serum insulin levels in WT mice, the effect of which was absent in DKO but not Akt2−/− and Ampkα2−/− mice (Fig. 3f). The 5-month high fat diet intake significantly lowered lean mass percentage (without affecting absolute lean mass) and increased both absolute and normalized fat mass in WT mice, the effect of which was absent in DKO but not Akt2−/− and Ampkα2−/− mice (Fig. 3g–j).
Figure 3.
Five week-old WT, Ampkα2−/−, Akt2−/−, and DKO mice were placed on a 45% high fat (HF) or nutritionally matched low fat (LF) diet for 20 weeks. (a) Representative photographs of mice (prone and supine positions) and white adipose tissues. (b) Food intake (per mouse daily, P > 0.05). (c) Trajectory of body weight of WT and DKO mice. (d) Intraperitoneal glucose tolerance test (IPGTT). (e) Area under curve (AUC) for IPGTT. (f) Serum insulin level. (g) Lean mass weight. (h) Lean mass percentage. (i) Fat mass weight. (j) Fat mass percentage. Data are expressed as mean ± SEM (n = 11–13 for panels b–e, n = 7 for panels f–j). ∗P < 0.05 vs. WT mice; #P < 0.05 vs. WT-HF mice; †P < 0.05 vs. DKO-HF mice.
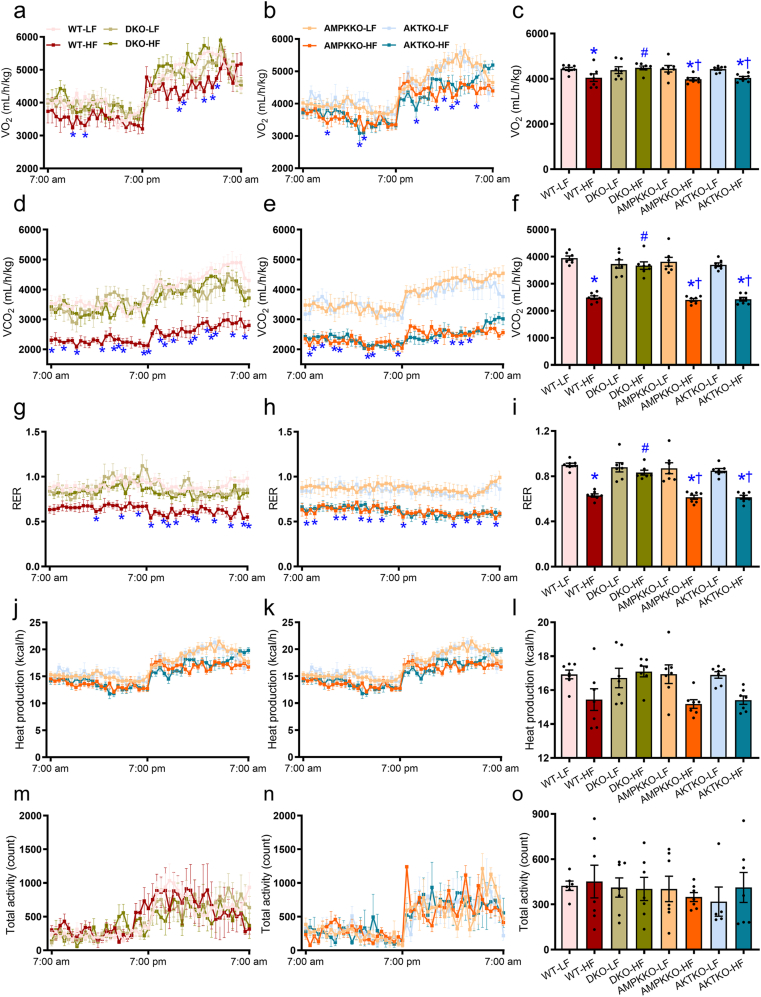
3.3. Global metabolism, hepatic steatosis and liver function
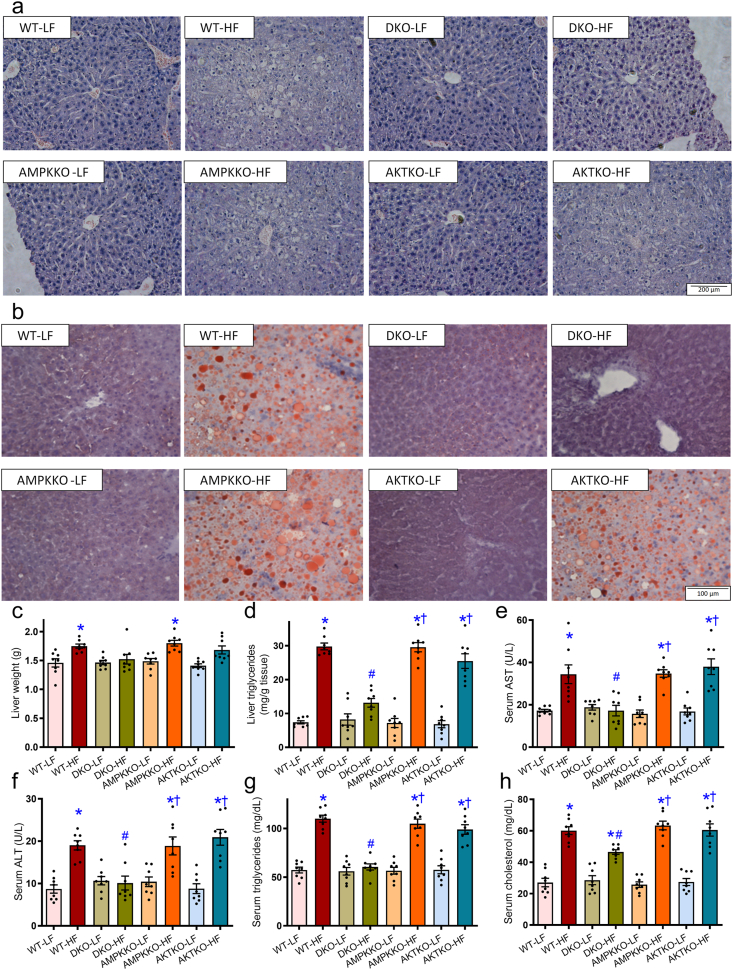
To discern the possible mechanisms behind fat diet intake- and DKO-induced biometric and metabolic action, CLASM metabolic cage was employed to monitor global metabolism over a 24-h cycle. Our data revealed that high fat intake visibly lowered O2 consumption, CO2 production and RER in WT mice, the effect of which was absent in DKO but not Akt2−/− and Ampkα2−/− mice. Ablation of Akt2, AMPKα2, or both, had little effects on O2 consumption, CO2 production, and RER under low fat intake (Fig. 4a–i). Heat production and total activity were not notably affected by high fat intake, ablation of Akt2, AMPKα2, or both (Fig. 4j–o). To evaluate high fat diet-induced hepatic steatosis and liver injury, if any, hepatic morphology, levels of triglycerides, AST, ALT, and cholesterol were assessed. Compared to normal lobular architecture in low fat-fed mice, high fat diet intake induced a microvesicular lipid accumulation, especially in centrilobular zone and hepatocyte ballooning with rarefaction of hepatocyte cytoplasm (Fig. 5a). Oil red O staining revealed hepatic steatosis following high fat intake (Fig. 5b). High fat intake also significantly increased liver weight (Fig. 5c and Supporting Information Fig. S2a), liver triglyceride content (Fig. 5d), serum levels of AST (Fig. 5e), ALT (Fig. 5f), triglycerides (Fig. 5g), and cholesterol (Fig. 5h). These signs of hepatic steatosis, were abrogated in DKO mice but not Akt2−/− and Ampkα2−/− mice. Ablation of Akt2, AMPKα2, or both, did not display any effects on hepatic morphology, triglyceride or serum profiles of liver injury and dyslipidemia in the face of low fat diet intake (Fig. 5a–h). Moreover, our data revealed enlarged size of adipocytes, brown adipose tissues, white adipose tissues (inguinal, epididymal, and retroperitoneal), and ectopic fat accumulation (in the form of triglycerides) in muscles in high fat diet-fed WT mice. The effects of which were removed in DKO but not Akt2−/− and Ampkα2−/− mice following high fat intake (Supporting Information Fig. S2b–S2g). Ablation of Akt2, AMPKα2, or both, did not display any discernible effects on these biometric and metabolic parameters under low fat diet intake (Fig. S2).
Figure 4.
Metabolic properties (24-h cycle) of WT, Ampkα2−/−, Akt2−/−, and DKO mice fed LF or HF diet for 20 weeks. (a) O2 consumption in DKO and WT groups. (b) O2 consumption in Ampkα2−/− and Akt2−/− mice. (c) Pooled data of O2 consumption (24 h). (d) CO2 production in DKO and WT mice. (e) CO2 production in Ampkα2−/− and Akt2−/− mice. (f) Pooled data of CO2 production (24 h). (g) Respiratory exchange ratio (RER) in DKO and WT mice. (h) RER in Ampkα2−/− and Akt2−/− mice. (i) Pooled data of RER (24 h). (j) Heat production in DKO and WT mice. (k) Heat production in Ampkα2−/− and Akt2−/− mice. (l) Pooled data of heat production (24 h). (m) Total physical activity in DKO and WT mice. (n) Total physical activity in Ampkα2−/− and Akt2−/− mice. (o) Pooled data of total physical activity (24 h). Data are expressed as mean ± SEM (n = 7). ∗P < 0.05 vs. WT mice; #P < 0.05 vs. WT-HF mice; †P < 0.05 vs. DKO-HF mice.
Figure 5.
Effects of Ampkα2−/−, Akt2−/−, and DKO on NAFLD. (a) Hematoxylin and eosin (H&E) staining of liver cross-sections from mice fed LF or HF diet for 20 weeks. (b) Representative oil red O staining micrographs. (c) Liver weight. (d) Liver triglyceride content. (e) Serum AST levels. (f) Serum ALT levels. (g) Serum triglyceride levels. (h) Serum cholesterol levels. Data are expressed as mean ± SEM (n = 8). ∗P < 0.05 vs. WT mice; #P < 0.05 vs. WT-HF mice; †P < 0.05 vs. DKO-HF mice.
3.4. Hepatic lipid metabolism
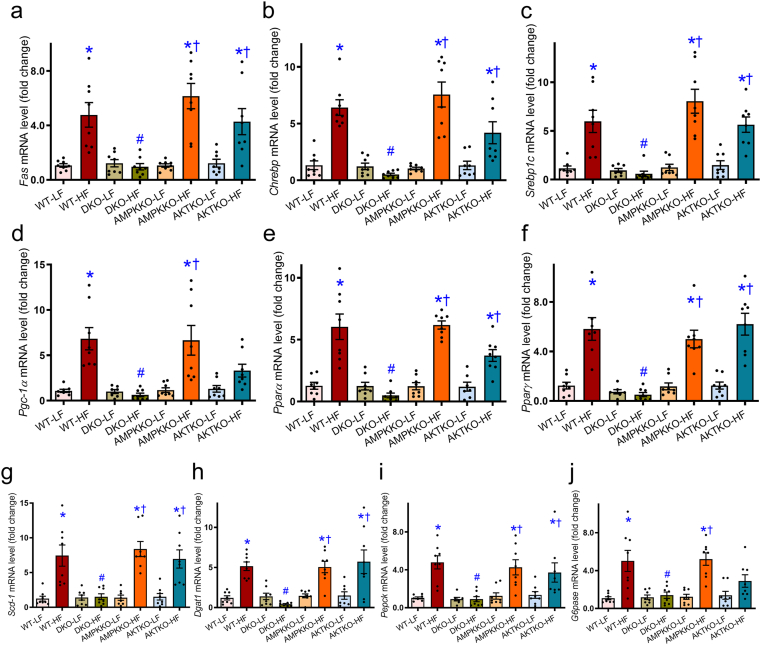
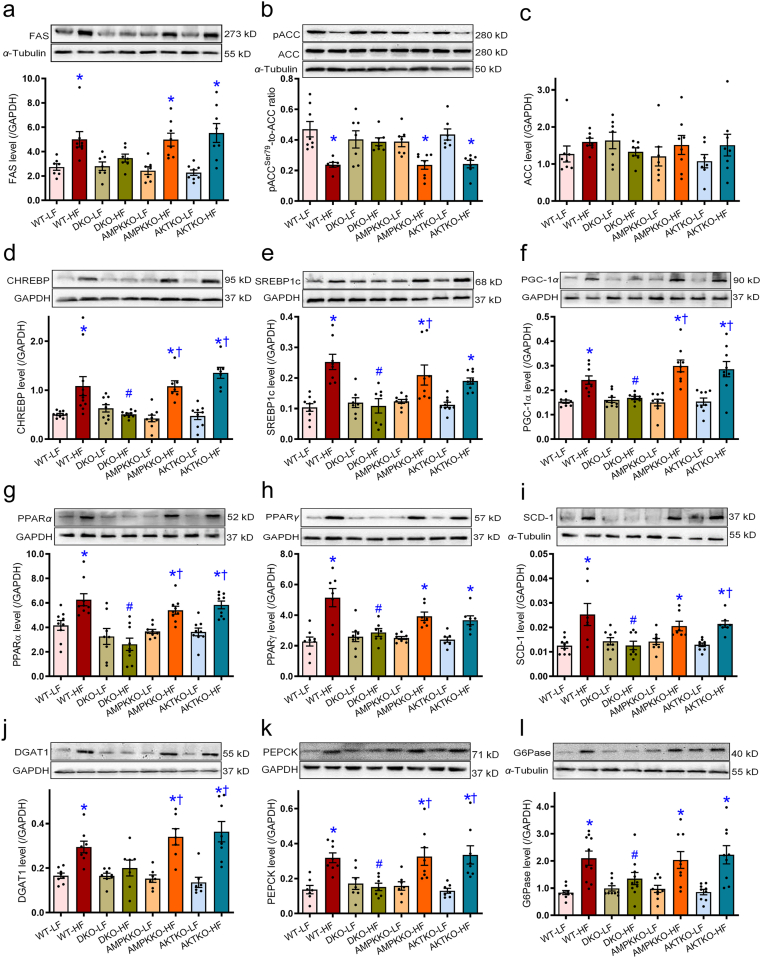
To evaluate the impact of DKO on high fat diet-induced hepatic lipid metabolism, lipid synthesis, uptake, and transport genes were evaluated using RT-PCR and Western blot analyses. High fat diet intake clearly upregulated mRNA levels of the key enzyme for fatty acid synthesis including Fas, transcription factors in lipogenesis including Chrebp and Srebp1, essential enzymes involved in lipid metabolism such as Pgc-1α and Pparα, lipid storage such as Pparγ, unsaturated fatty acid synthesis catalyzing enzymes including Scd-1, gluconeogenesis including Pepck and G6pase, and triglycerides synthesis including Dgat1 in WT groups. The effects were reconciled by DKO but not ablation of either Akt2 or AMPKα2. Removal of Akt2, AMPKα2, or both, did not affect expression of these lipid metabolic genes under low fat diet intake condition (Fig. 6). This is supported by findings from Western blot analysis where high fat diet intake upregulated protein levels of FAS, CHREBP, SREBP1, PGC-1α, PPARα, PPARγ, SCD-1, GDAT1, PEPCK, and G6Pase in conjunction with decreased phosphorylation of fatty acid synthesis enzyme ACC (no change in pan-ACC) in WT groups. Ablation of Akt2, AMPKα2, or both, failed to affect protein expression of these lipid metabolic enzymes under low fat diet intake condition (Fig. 7).
Figure 6.
Effect of Ampkα2−/−, Akt2−/−, and DKO on levels of lipid regulatory genes in livers from mice fed LF or HF diet using real time-PCR analysis. (a–j) The mRNA levels of Fas (a), Chrebp (b), Srebp1c (c), Pgc-1α (d), Pparα (e), Pparγ (f), Scd-1 (g), Dgat1 (h), Pepck (i), and G6pase (j) were examined. Data are expressed as mean ± SEM (n = 8–9). ∗P < 0.05 vs. WT mice; #P < 0.05 vs. WT-HF mice; †P < 0.05 vs. DKO-HF mice.
Figure 7.
Effects of Ampkα2−/−, Akt2−/−, and DKO on the levels of lipid regulatory proteins in livers from mice fed LF or HF diet. (a–l) The protein levels of lipid synthesis, metabolism, and storage enzymes, including FAS (a), ACC (phosphorylated ACC and pan-ACC, pACC-to-ACC ratio, b), pan-ACC (c), CHREBP (d), SREBP1c (e), PGC-1α (f), PPARα (g), PPARγ (h), SCD-1 (i), DGAT1 (j), PEPCK (k), and G6Pase (l), were examined using Western blot analysis. Representative gel blots which depicted the levels of lipid regulatory proteins (using GAPDH or α-tubulin as loading control) were shown. Data are expressed as mean ± SEM (n = 7–10). ∗P < 0.05 vs. WT mice; #P < 0.05 vs. WT-HF mice; †P < 0.05 vs. DKO-HF mice.
3.5. Changes in inflammation, autophagy, mitophagy, and autophagy signaling (Akt, AMPK, and FoxO1)
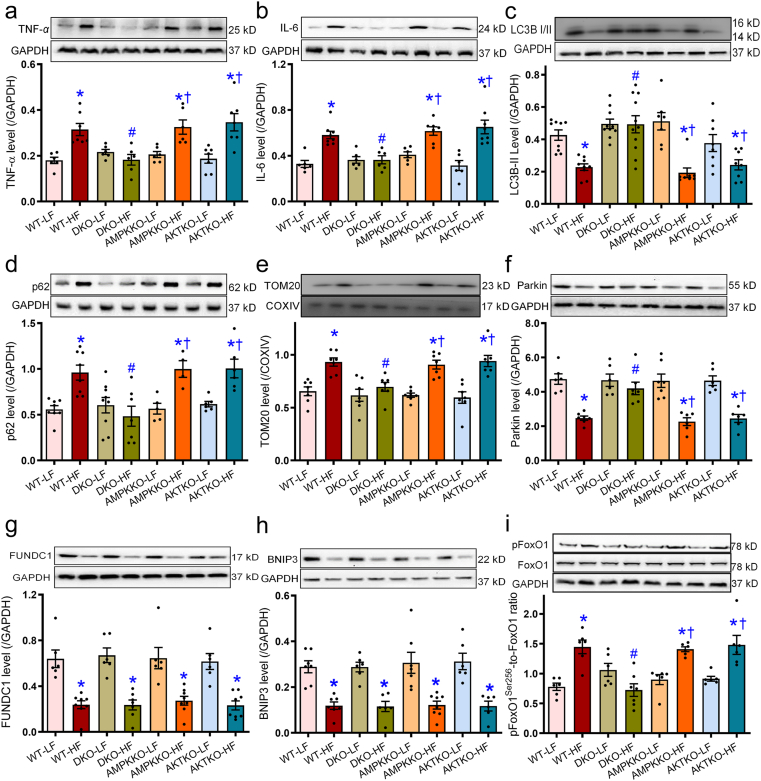
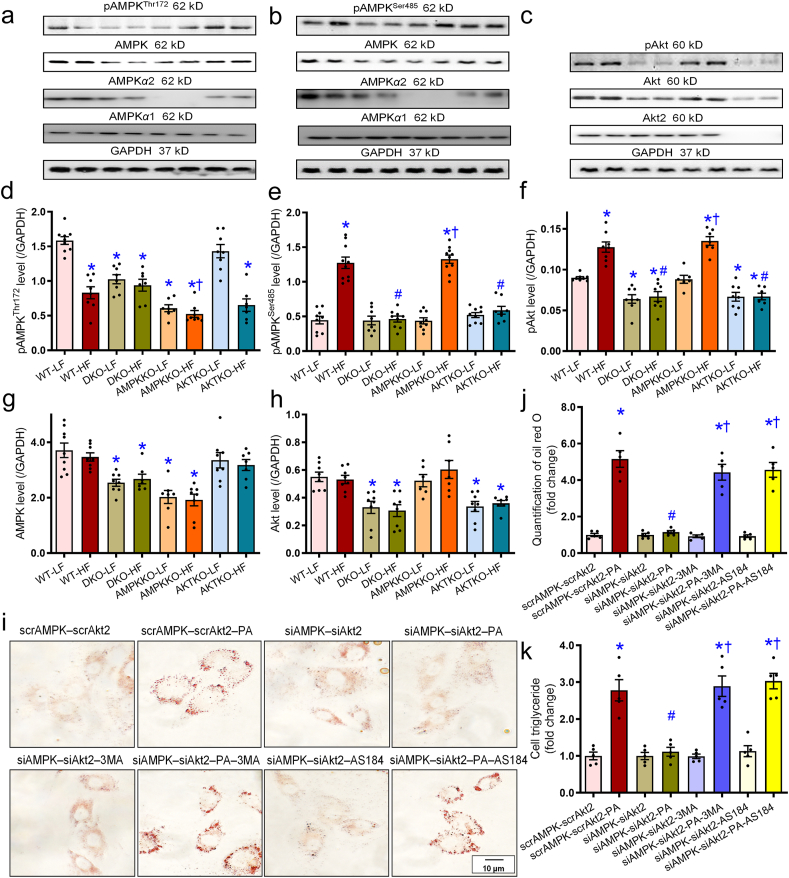
To determine the mechanisms behind DKO-offered protection against high fat diet-induced changes, body weight, hepatic injury, levels of inflammatory, autophagy, mitophagy, and regulatory signaling were monitored in hepatic tissues from trial mice. Our data revealed that high fat diet intake significantly upregulated levels of the proinflammatory makers TNF-α and IL-6. Additionally, high fat diet intake decreased levels of autophagy and mitophagy marker LC3BII, Parkin, FUNDC1, and BNIP3 while elevating p62 and mitophagy marker TOM20 in WT mice. These effects, with the exception of FUNDC1 and BNIP3, were abolished in DKO but not Akt2−/− and Ampkα2−/− mice (Fig. 8a–h). Moreover, high fat diet intake promoted phosphorylation of the autophagy regulatory signal FoxO1 without affecting pan protein level of FoxO1 in WT mice, the effects of which were nullified in DKO but not Akt2−/− and Ampkα2−/− mice (Fig. 8i). Ablation of Akt2, AMPKα2, or both, failed to display any effects on inflammation, autophagy, and mitophagy markers in the low fat diet setting (Fig. 8). Our further study revealed that high fat diet intake suppressed phosphorylation of AMPK at Thr172, the effect of which was not restored in DKO, Akt2−/−, or Ampkα2−/− mice. Ampkα2−/− and DKO mice both displayed reduced AMPK Thr172 phosphorylation and pan-AMPK level (due to Ampkα2 knockout) with low fat intake (Fig. 9a, d, and g). Meanwhile, high fat diet intake promoted phosphorylation of AMPKα1 at Ser485 (but not pan-AMPKα1 protein) and Akt Ser473 in WT mice (with exception of corresponding single ablation group), the effects of which were nullified in DKO and Akt2−/−, but not Ampkα2−/− mice (Fig. 9b, c, e, and f). Basal Akt phosphorylation was dampened in DKO and Akt2−/− mice receiving low fat diet (Fig. 9f). Deletion of Akt2, AMPKα2, or both, explicitly decreased pan protein levels of corresponding gene without affecting levels of the non-targeted protein including AMPKα1 subunit (Fig. 9a–h and Supporting Information Fig. S1).
Figure 8.
Effects of Ampkα2−/−, Akt2−/−, and DKO on the levels of inflammatory cytokines, autophagy/mitophagy markers, and FoxO1 in livers from mice fed LF or HF diet. The protein levels of TNF-α (a), IL-6 (b), LC3BII (c), p62 (d), TOM20 (e), Parkin (f), FUNDC1 (g), BNIP3 (h), and FoxO1 (phosphorylated and pan-, pFoxO1-to-FoxO1 ratio, i) were examined using Western blot analysis. Representative gel blots which depicted the levels of TNF-α, IL-6, LC3BI/II, p62, TOM20, Parkin, FUNDC1, BNIP3, FoxO1, and phosphorylated FoxO1 were shown. Data are expressed as mean ± SEM (n = 6–9). ∗P < 0.05 vs. WT mice; #P < 0.05 vs. WT-HF mice; †P < 0.05 vs. DKO-HF mice.
Figure 9.
Effect of Ampkα2−/−, Akt2−/−, and DKO on pan- and phosphorylated levels of AMPK and Akt in mice livers and the effects of Ampk and Akt2 knockdown on palmitic acid (PA)-induced lipid accumulation in HepG2 cells. (a–c) Representative gel blots of panels d–h depicting pan- and phosphorylated AMPK and Akt as well as AMPKα1, AMPKα2, and Akt2 subunits using specific antibodies. (d) pAMPK (Th172)-to-GAPDH ratio. (e) pAMPK (Ser485)-to-GAPDH ratio. (f) pAkt (Ser473)-to-GAPDH ratio. (g) AMPK levels. (h) Akt level. (i–k) Cells were treated with scrAMPK-scrAkt2 or siAMPK-siAkt2 prior to exposure to PA (0.5 mmol/L) in the presence or absence of the autophagy inhibitor 3-MA (10 mmol/L) and the FoxO1 inhibitor AS1842856 (100 nmol/L) for 72 h. Representative oil red O staining images from the indicated HepG2 groups (i), quantification of oil red O (j), and cellular triglyceride levels (k) were shown. Data are expressed as mean ± SEM (n = 7–9 mice per group, panels a–h; n = 5 independent experiments, panels j and k). ∗P < 0.05 vs. WT mice or scrAMPK-scrAKT group; #P < 0.05 vs. WT-HF mice or scrAMPK-scrAKT-PA group; †P < 0.05 vs. DKO-HF mice or siAMPK-siAKT-PA group.
3.6. Role of autophagy and FoxO1 signaling in palmitic acid-induced lipid accumulation
To describe a possible role of autophagy and FoxO1 signaling in high fat diet-induced hepatic steatosis, HepG2 cells were transfected with siRNA against AKT2 and AMPK (or scramble RNA as control) prior to exposure to palmitic acid for 72 h in the presence or absence of the autophagy inhibitor 3-MA or the FoxO1 inhibitor AS1842856. Lipid accumulation was assessed using oil red O staining. Our data revealed that palmitic acid promoted lipid accumulation, the effect of which was negated by silencing AKT2 and AMPK concurrently with little effect from silencing AKT2 and AMPK individually. Interestingly, both 3-MA and AS1842856 nullified AKT2–AMPK silencing-induced protection against palmitic acid-induced lipid accumulation without effect itself (Fig. 9i and j). This is consistent with the finding that 3-MA and AS1842856 removed AKT2–AMPK silencing-induced beneficial effect against palmitic acid-induced rise in cellular triglyceride levels (Fig. 9k).
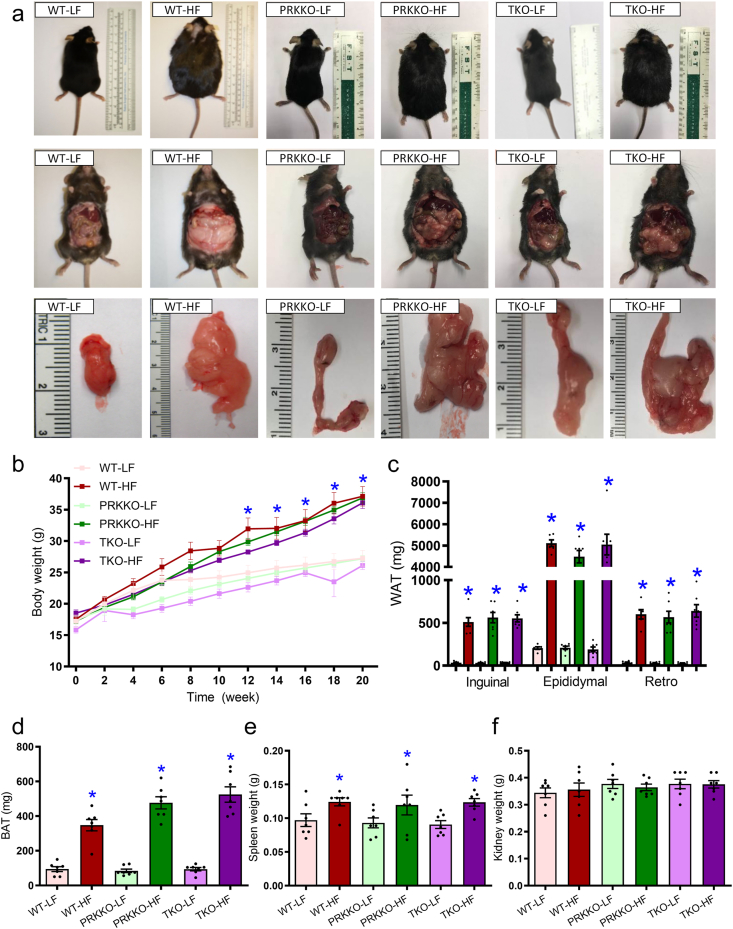
3.7. Role of Parkin in DKO-offered benefit against high fat diet-induced obesity, adiposity, and hepatic steatosis
Given that Parkin is the only mitophagy marker correlated with DKO-induced beneficial effect against high fat intake, we went on to cross DKO mice with Prk–/– mice to generate a TKO model. Our data, shown in Fig. 10, revealed that high fat diet intake significantly increased body weight in WT mice starting from 12 weeks of diet feeding. However, this effect was spared in Prk–/– or TKO mice (Fig. 10a and b). In addition, high fat diet overtly increased white adipose tissues (inguinal, epididymal, and retroperitoneal), brown adipose tissues, and spleen (but not kidney) weight in WT mice, the effect of which was not seen in Prk–/– or DKO mice (Fig. 10a and c–f). To evaluate the role of Parkin in fat diet intake- and DKO-induced hepatic steatosis, levels of triglycerides and cholesterol were evaluated. High fat intake promoted hepatomegaly, hepatic steatosis, liver triglyceride accumulation, and hyperlipidemia in WT mice although Prk–/– or TKO mice were unaffected. Ablation of Parkin or triple knockout did not display any effects on hepatic morphology, triglycerides, or serum profiles of lipid in the face of low fat diet intake (Supporting Information Fig. S3). These findings suggested that Parkin ablation removed the DKO-induced benefit on adiposity, hepatic steatosis, and dyslipidemia.
Figure 10.
Five week-old WT, Prk–/–, Akt2–/––Ampkα2–/––Prk–/– triple knockout (TKO) mice were placed on a 45% HF or nutritionally matched LF diet for 20 weeks. (a) Representative photographs of mice (prone and supine positions) and white adipose tissues. (b) Trajectory of body weight of WT, Prk–/–, and TKO mice. (c) White adipose tissue (WAT). (d) Brown adipose tissue (BAT). (e) Spleen weight. (f) Kidney weight. Data are expressed as mean ± SEM (n = 7). ∗P < 0.05 vs. WT mice.
3.8. Role of Parkin in DKO-offered benefits against fat diet-induced hepatic lipid anomalies
To evaluate the impact of Parkin on DKO-induced benefit against high fat diet-induced hepatic lipid metabolic anomalies, lipid synthesis, uptake, and transport genes were evaluated using Western blot analysis. As depicted in Supporting Information Fig. S4, high fat diet intake clearly upregulated protein levels of FAS, CHREBP, SREBP1, PGC-1α, PPARα, PPARγ, SCD-1, DGAT1, PEPCK, and G6Pase, as well as reduced ACC phosphorylation in WT mice. These effects were unaffected by Prk–/– or TKO. Parkin knockout or TKO did not display any effects on these lipid metabolic enzymes themselves under low fat diet intake.
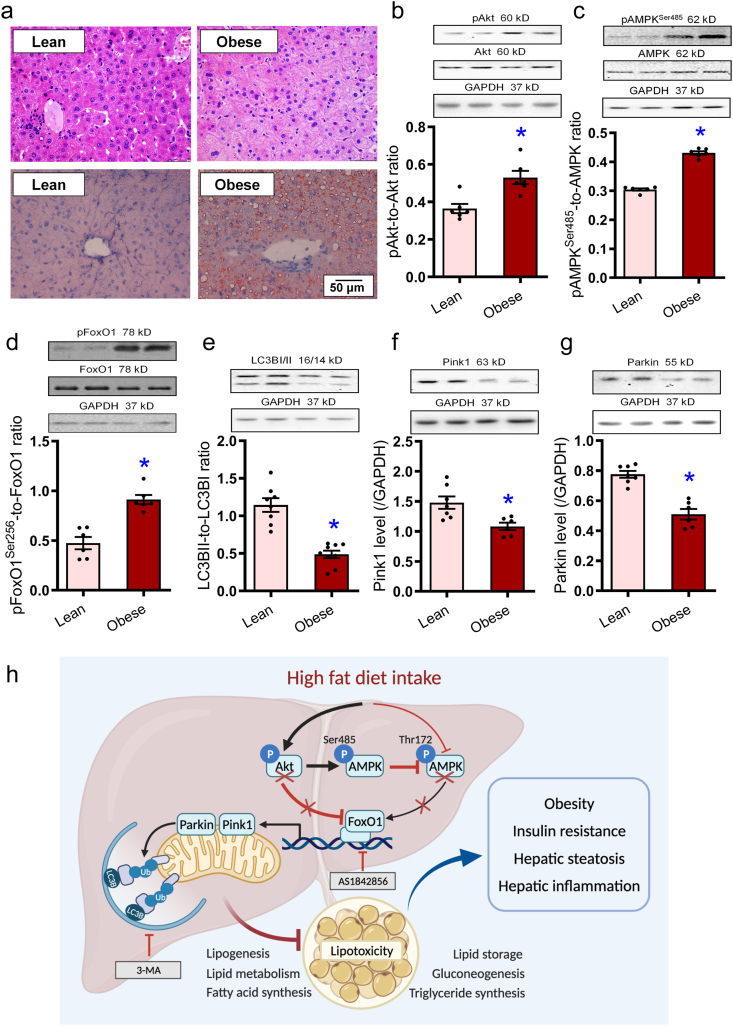
3.9. Changes of Akt, AMPK, FoxO1, autophagy, and mitophagy in obese human livers
Levels of Akt, AMPK, FoxO1, autophagy, and mitophagy along with hepatic morphology were evaluated in liver specimens taken from lean and obese human subjects. Our data revealed microvesicular lipid accumulation and hepatocyte ballooning with rarefaction of hepatocyte cytoplasm along with hepatic steatosis (oil red deposition) in obese human liver specimens (Fig. 11a). Similar to the rodent model of obesity, phosphorylation of Akt, AMPK (Ser485), and FoxO1 was elevated, while markers of autophagy and mitophagy (LC3B, Pink1, and Parkin) were downregulated in human obese livers (Fig. 11b–g).
Figure 11.
Morphology, steatosis, cell signaling, autophagy, and Pink1–Parkin mitophagy in obese human liver specimen. (a) H&E staining of liver cross-sections and oil red O staining micrographs in lean and obese human liver samples. (b) pAkt-to-Akt ratio. (c) pAMPK (Ser485)-to-AMPK ratio. (d) pFoxO1-to-FoxO1 ratio. (e) LC3BII-to-LC3BI ratio. (f) Pink1. (g) Parkin. Representative gel blots depicting pan- and phosphorylated Akt, AMPK (Ser485), and FoxO1, as well as LC3BI/II, Pink1, and Parkin (GAPDH as loading controls) using specific antibodies were shown. Data are expressed as mean ± SEM (n = 6–7). ∗P < 0.05 vs. lean group. h: Scheme depicting possible mechanism(s) for the role of Akt2, AMPK, and Parkin-mediated mitophagy in high fat diet-induced obesity and hepatic steatosis. Chronic high fat diet intake suppresses FoxO1-mediated mitophagy through parallel regulation of Akt and AMPK. On one hand, high fat intake promotes Akt phosphorylation leading to inhibition of FoxO1 (phosphorylation) and suppressed autophagy. On the other hand, high fat intake suppressed AMPK phosphorylation at Thr172 and inhibition of autophagy. Hyperactivated Akt (phosphorylation) fosters AMPK phosphorylation at Ser485, disturbing AMPK phosphorylation at Thr172 indirectly. Compromised FoxO1 signaling contributes to compromised lipid metabolism and lipid accumulation due to poor mitophagy, resulting in obesity, hepatic steatosis. Arrowheads denote stimulation whereas the lines with a “T” ending represent inhibition.
4. Discussion
The salient findings from our study indicate that concurrent ablation of Akt2 and AMPKα2 protects against high fat diet intake-induced adiposity, hepatic steatosis, and liver injury. Our study revealed that high fat intake-induced hepatic steatosis is closely associated with suppressed mitophagy and activation of proinflammatory responses in the liver in the absence of altered food intake, energy expenditure, and physical activity. Double ablation of Akt2 and AMPKα2, but not either alone, protected against high fat feeding-induced rises in mRNA and protein levels of enzymes governing lipogenesis (FAS, CHREBP, and SREBP1), lipid metabolism (PGC-1α and PPARα), lipid storage (PPARγ), unsaturated fatty acid synthesis (SCD-1), gluconeogenesis (PEPCK and G6Pase), and triglyceride synthesis (DGAT1). These changes in lipid metabolism were consistent with findings from serum and hepatic levels of triglycerides and cholesterol in single or DKO mice receiving low or high fat diet. Furthermore, double rather than single ablation of Akt2 and AMPKα2 protected against high fat diet-induced phosphorylation (inactivation) of nuclear transcriptional factor FoxO1, an essential regulator for autophagy, glucose homeostasis, and lipid metabolism27,46. In vitro findings revealed that AKT2–AMPK co-silencing rescued against palmitic acid-induced lipid accumulation likely through an autophagy- and FoxO1-dependent mechanism. Data from obese human livers provided supporting evidence of overactivated Akt2, AMPK (Ser485), and FoxO1 along with compromised autophagy/mitophagy. RNAseq and mass spectrometry analyses from rodent and human livers validated the GO terms (e.g., lipid catabolic and fatty acid metabolic processes), KEGG pathways (e.g., autophagy and mitophagy) and involvement of Akt, AMPK, and FoxO signaling as the most relevant enriched KEGG pathways in obese livers. Taken together, our current data demonstrated a beneficial role of concurrent ablation of Akt2 and AMPKα2 against high fat diet intake-induced obesity, hepatic steatosis, and liver injury related to regulation of FoxO1-mediated autophagy/mitophagy and inflammation.
Hepatic injury due to a chronic high fat diet intake is likely caused by overconsumption of fatty acids, which interrupts hepatic ability for fat metabolism and storage. Triglycerides, the main form of fat to accommodate energy metabolism, accumulate within liver cells leading to steatosis, a hallmark of NAFLD. These features are consistent with the elevated serum ALT/AST, serum and hepatic cholesterol and triglycerides along with hepatic steatosis and hepatomegaly following high fat diet intake in our current experimental setting. Notably, results from our study indicated that high fat diet intake-induced hepatomegaly, hepatic steatosis, and liver injury were negated by Akt2–Ampkα2 DKO (but not single knockout or TKO). These findings favor a beneficial role of Akt2–Ampkα2 DKO in preserving hepatic homeostasis against high fat diet intake. Moreover, DKO of Akt2–Ampkα2 protected against high fat diet-induced adiposity. Assessment of global metabolism using CLAMS did not favor a major contribution for altered food intake or energy expenditure in Akt2–AMPKα2 double ablation. Heat production did not change across experimental groups. RER, the ratio between CO2 production and O2 consumption, denotes the type of energetic substrate utilized. A RER value around 0.8 in low fat diet-fed mice usually indicates fatty acid oxidation and carbohydrate oxidation as the main energy source47. Our data revealed significantly lower RER (along with decreased O2 consumption and CO2 production) following high fat diet intake in WT, Akt2−/−, and Ampkα2−/− mice, denoting predominant usage of fat over carbohydrate for oxidation48. Intriguingly, Akt2–Ampkα2 DKO reduced high fat diet intake-induced anomalies in global metabolism. Akt2–AMPKα2 ablation favored utilization of carbohydrates as the main energy source. DKO also exerted positive effects on glucose tolerance tests and serum insulin levels, indicating preserved glucose metabolism. Perhaps the most interesting finding from our current study was DKO-offered protection against high fat diet-induced increases in mRNA and protein levels of enzymes governing lipid metabolism, the effect which vanished in the TKO model. DKO of Akt2–Ampkα2 reversed high fat diet-induced rises in lipid synthetic, metabolic and storage enzymes. These metabolic effects were absent in single knockout and TKO groups. The observations utilizing lipid enzyme profiles were supported by anthropometric, CLAMS metabolic data, glucose tolerance tests, and serum lipid profiles.
Our results further indicated that 5-month of chronic high fat intake suppressed hepatic autophagy, mitophagy, and promoted inflammation in WT mice. These effects were reconciled by Akt2–Ampkα2 DKO but not Akt2−/− and Ampkα2−/− mice. These findings suggested potential contribution of mitophagy and inflammation in DKO-elicited beneficial effects on hepatic steatosis and liver function in the face of high fat diet intake. This is supported by evidence from our lab and others, favoring an essential role for disturbed autophagy and promoted inflammation in genetic and diet-induced obesity, hepatic steatosis and liver injury8,10,27,33,35. Finally, ablation of Parkin nullified Akt2–Ampkα2 DKO-induced protection against hepatic steatosis. Induction of mitophagy in particular Pink1–Parkin-dependent mitophagy was demonstrated to suppress lipid accumulation through downregulation of de novo lipogenesis genes including PPARα, SREBP1c, FAS, and ACC in high fat diet challenged livers49,50. Although it is beyond the scope of current study, proinflammatory responses are likely an effector of disturbed autophagy in high fat diet-induced hepatic steatosis and liver injury27. Further examination of FoxO1, a nuclear transcriptional factor downstream of both Akt and AMPK51, suggested that Akt2–Ampkα2 DKO reduced high fat diet-induced FoxO1 phosphorylation (denoting inactivation), a response that was absent in either Akt2−/− or Ampkα2−/− mice. Among various transcription factors regulating autophagy, FoxO is perhaps the most prominent one with its regulation governed by phosphorylation and thus nuclear exclusion for inactivation26,46,52. FoxO1 is capable of regulating mitochondrial β-oxidation through PPARα-dependent mechanism to govern a wide array of cellular processes, including glucose and lipid metabolism52. Earlier evidence depicted distinct stimulatory and inhibitory roles for AMPK and Akt on FoxO1 activity, respectively26, thus leading to disparate responses on glucose, lipid, and protein metabolism, as well as cell survival. These actions may underlie Akt- and AMPK-induced biological responses in obesogenesis, insulin sensitivity, and NAFLD. Several studies have depicted a role for Akt in controlling hepatic steatosis through an mTOR-dependent mechanism. Elevated Akt phosphorylation was noted in livers from NAFLD patients alongside with dysregulated autophagy53. Akt activation, particular Akt2 was shown to foster hepatic steatosis through SREBP1c regulation, suggesting its therapeutic value in the management of NAFLD23,54. Yecies and colleagues22 revealed that Akt stimulated hepatic SREBP1c and lipogenesis via parallel mTOR-dependent and -independent pathways. These findings noted that mTORC1-independent pathway involves Akt-mediated decrease in the liver-specific transcript encoding the SREBP1c inhibitor INSIG222. Data from our current study suggested that Akt2-promoted hepatic steatosis and liver injury in the face of high fat diet intake also required the “presence” of AMPK. Earlier reports supported a role for AMPK in Akt2-mediated hepatic lipid accumulation using ACC as the AMPK target23. AMPK is an essential cell fuel molecule with therapeutic benefit against high fat diet-induced hepatic steatosis and NAFLD55. Early findings from our lab showed worsened cardiac geometry and function with AMPK deficiency in the face of chronic high fat diet intake, without notable changes in global metabolism32. Our data revealed that Akt2–Ampkα2 DKO nullified high fat diet-induced activation of Akt and AMPKα1 subunit (Ser485). Our results did not favor a “compensatory” role for AMPKα1 in the face of AMPKα2 ablation. Early evidence noted compromised AMPKα2 signaling but not that of AMPKα1 in livers following high fat diet intake56, in line with our current finding. Phosphorylation of AMPKα1 Ser485 occurs downstream of Akt, to inhibit AMPK Thr172 phosphorylation. As depicted in Fig. 11h, high fat diet intake may promote FoxO1 phosphorylation and autophagy/mitophagy inhibition via parallel hyperphosphorylation of Akt and dephosphorylation of AMPK, These findings are in line with stimulatory and inhibitory effects of autophagy, for AMPK and Akt, respectively26,57. In our hands, Akt2–AMPKα2 double ablation was unable to restore fat diet-induced dephosphorylation of AMPK Thr172 (possibly due to AMPKα2 ablation). Instead DKO abrogated Akt-induced suppression of FoxO1 and autophagy under high fat diet intake, an effect which was absent in Akt2−/− model. In addition, DKO of Akt2–Ampkα2 removed Akt-induced AMPKα1 Ser485 phosphorylation to improve “residual” AMPK Thr172 phosphorylation58,59 in the face of high fat diet intake. It is plausible to speculate that FoxO1 and Parkin mitophagy may serve as common converging points for Akt2 and AMPK signaling in high fat diet-induced obesity and hepatic steatosis.
Autophagy/mitophagy is essential for lipid droplet breakdown while defective autophagy perturbs lipid turnover and promotes lipid accumulation and fat storage60,61. It is noteworthy that unchecked autophagy was also reported in response to lipid overload, such as excess cholesterol or fatty acids62,63, as a compensatory defense mechanism to remove excess lipids27. Restored hepatic autophagy and mitophagy in Akt2–Ampkα2 DKO mice noted in our study should help to remove excessive lipid droplets and preserve liver function. It should be mentioned that intrahepatic synthesis of triglycerides may function as an adaptive response to counter toxicity of triglyceride metabolites in high energy challenged models and obese individuals64,65. FoxO1 and mitophagy in DKO mice offered protection against high fat diet-induced hepatic steatosis and received consolidation from an in vivo study where Parkin ablation nullified Akt2–AMPKα2 ablation-induced benefit. Additionally, our in vitro finding noted that inhibition of FoxO1 or autophagy abrogated Akt2–Ampkα2 knockdown-induced benefit against palmitic acid-induced lipid accumulation. Given that uncorrected obesity serves as the main contributing factor for disturbed autophagy and lipid metabolism, hepatomegaly and liver injury8,27, it is possible that Akt2–AMPKα2 double ablation-induced beneficial hepatic responses against high fat diet intake is secondary to DKO-induced resistance of high fat diet-induced obesity. Last but not least, data from obese human livers supported the findings from mice. Further study is warranted to elucidate the direct hepatic response of Pink1–Parkin on lipid spillover and adiposity in the face of high fat diet intake.
5. Conclusions
Data from our study offers evidence that double ablation of Akt2 and AMPKα2 protects against high fat diet-induced obesity, hepatic steatosis and liver injury. Our study consolidated the notion of disturbed autophagy and augmented inflammation in hepatic steatosis and injury following high fat diet intake. More importantly, our findings favored that Akt2–Ampkα2 DKO protects against hepatic steatosis, liver injury, and inflammation using FoxO1 and Parkin as the converging points. These findings should help explain the etiology of NAFLD in high fat diet-induced obesity and more importantly, the unique parallel regulation (and interaction) between Akt2 and AMPKα2 in global metabolism, glucose, and lipid metabolism, as well as hepatic homeostasis. These findings suggest the therapeutic promises of targeting Akt2 and AMPKα2 for drug development in the management of NAFLD and obesity.
Acknowledgments
The authors greatly appreciate the assistance of Prof. Xiaowu Huang at Zhongshan Hospital Fudan University for collection of human liver specimens (Shanghai, China). Editorial assistance from Dr. Laurie J. Demillard from University of Wyoming School of Pharmacy (Laramie, WY, USA) is greatly appreciated. The work was supported in part by the National Key R&D Program of China (2017YFA0506000) and National Natural Science Foundation of China (82000351).
Author contributions
Shuyi Wang, Jun Tao, Huaguo Chen, and Mechender Kandadi designed the experiments and analyzed data. Mingming Sun, Haixia Xu, and Yan Lu helped data collection. Gary Lopaschuk, Junmeng Zheng, and Hu Peng participated in study design and manuscript revision. Jun Ren was responsible for supervision and manuscript writing.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2021.07.006.
Contributor Information
Junmeng Zheng, Email: zhengjunmeng@126.com.
Hu Peng, Email: 1973denkepeng@tongji.edu.cn.
Jun Ren, Email: jren@uw.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Samuel V.T., Shulman G.I. Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases. Cell Metabol. 2018;27:22–41. doi: 10.1016/j.cmet.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trepo E., Valenti L. Update on NAFLD genetics: from new variants to the clinic. J Hepatol. 2020;72:1196–1209. doi: 10.1016/j.jhep.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 3.Younossi Z.M. Non-alcoholic fatty liver disease - a global public health perspective. J Hepatol. 2019;70:531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 4.Eslam M., Newsome P.N., Sarin S.K., Anstee Q.M., Targher G., Romero-Gomez M., et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 5.Musso G., Cassader M., Gambino R. Non-alcoholic steatohepatitis: emerging molecular targets and therapeutic strategies. Nat Rev Drug Discov. 2016;15:249–274. doi: 10.1038/nrd.2015.3. [DOI] [PubMed] [Google Scholar]
- 6.Khan R.S., Newsome P.N. NAFLD in 2017: novel insights into mechanisms of disease progression. Nat Rev Gastroenterol Hepatol. 2018;15:71–72. doi: 10.1038/nrgastro.2017.181. [DOI] [PubMed] [Google Scholar]
- 7.Arguello G., Balboa E., Arrese M., Zanlungo S. Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Biochim Biophys Acta. 2015;1852:1765–1778. doi: 10.1016/j.bbadis.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Madrigal-Matute J., Cuervo A.M. Regulation of liver metabolism by autophagy. Gastroenterology. 2016;150:328–339. doi: 10.1053/j.gastro.2015.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L., Chen J., Ning C., Lei D., Ren J. Endoplasmic reticulum stress related molecular mechanisms in nonalcoholic fatty liver disease (NAFLD) Curr Drug Targets. 2018;19:1087–1094. doi: 10.2174/1389450118666180516122517. [DOI] [PubMed] [Google Scholar]
- 10.Ueno T., Komatsu M. Autophagy in the liver: functions in health and disease. Nat Rev Gastroenterol Hepatol. 2017;14:170–184. doi: 10.1038/nrgastro.2016.185. [DOI] [PubMed] [Google Scholar]
- 11.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomic D., Kemp W.W., Roberts S.K. Nonalcoholic fatty liver disease: current concepts, epidemiology and management strategies. Eur J Gastroenterol Hepatol. 2018;30:1103–1115. doi: 10.1097/MEG.0000000000001235. [DOI] [PubMed] [Google Scholar]
- 13.Listenberger L.L., Han X., Lewis S.E., Cases S., Farese R.V., Jr., Ory D.S., et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mi Y., Tan D., He Y., Zhou X., Zhou Q., Ji S. Melatonin modulates lipid metabolism in HepG2 cells cultured in high concentrations of oleic acid: AMPK pathway activation may play an important role. Cell Biochem Biophys. 2018;76:463–470. doi: 10.1007/s12013-018-0859-0. [DOI] [PubMed] [Google Scholar]
- 15.Mendez-Sanchez N., Cruz-Ramon V.C., Ramirez-Perez O.L., Hwang J.P., Barranco-Fragoso B., Cordova-Gallardo J. New aspects of lipotoxicity in nonalcoholic steatohepatitis. Int J Mol Sci. 2018;19:2034. doi: 10.3390/ijms19072034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vatner D.F., Majumdar S.K., Kumashiro N., Petersen M.C., Rahimi Y., Gattu A.K., et al. Insulin-independent regulation of hepatic triglyceride synthesis by fatty acids. Proc Natl Acad Sci U S A. 2015;112:1143–1148. doi: 10.1073/pnas.1423952112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y., Whaley-Connell A.T., Sowers J.R., Ren J. Autophagy as an emerging target in cardiorenal metabolic disease: from pathophysiology to management. Pharmacol Ther. 2018;191:1–22. doi: 10.1016/j.pharmthera.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park S.S., Lee Y.J., Song S., Kim B., Kang H., Oh S., et al. Lactobacillus acidophilus NS1 attenuates diet-induced obesity and fatty liver. J Endocrinol. 2018;237:87–100. doi: 10.1530/JOE-17-0592. [DOI] [PubMed] [Google Scholar]
- 19.Kim B., Figueroa-Romero C., Pacut C., Backus C., Feldman E.L. Insulin resistance prevents AMPK-induced Tau dephosphorylation through Akt-mediated increase in AMPKSer-485 phosphorylation. J Biol Chem. 2015;290:19146–19157. doi: 10.1074/jbc.M115.636852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurauti M.A., Costa-Junior J.M., Ferreira S.M., Dos Santos G.J., Protzek A.O., Nardelli T.R., et al. Acute exercise restores insulin clearance in diet-induced obese mice. J Endocrinol. 2016;229:221–232. doi: 10.1530/JOE-15-0483. [DOI] [PubMed] [Google Scholar]
- 21.Shiwa M., Yoneda M., Okubo H., Ohno H., Kobuke K., Monzen Y., et al. Distinct time course of the decrease in hepatic AMP-activated protein kinase and Akt phosphorylation in mice fed a high fat diet. PLoS One. 2015;10 doi: 10.1371/journal.pone.0135554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yecies J.L., Zhang H.H., Menon S., Liu S., Yecies D., Lipovsky A.I., et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metabol. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leavens K.F., Easton R.M., Shulman G.I., Previs S.F., Birnbaum M.J. Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metabol. 2009;10:405–418. doi: 10.1016/j.cmet.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y., Xu S., Mihaylova M.M., Zheng B., Hou X., Jiang B., et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metabol. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X., Hua Y., Nair S., Zhang Y., Ren J. Akt2 knockout preserves cardiac function in high-fat diet-induced obesity by rescuing cardiac autophagosome maturation. J Mol Cell Biol. 2013;5:61–63. doi: 10.1093/jmcb/mjs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y., Hu X., Liu Y., Dong S., Wen Z., He W., et al. ROS signaling under metabolic stress: cross-talk between AMPK and AKT pathway. Mol Cancer. 2017;16:79. doi: 10.1186/s12943-017-0648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ge S., Xia X., Ding C., Zhen B., Zhou Q., Feng J., et al. A proteomic landscape of diffuse-type gastric cancer. Nat Commun. 2018;9:1012. doi: 10.1038/s41467-018-03121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi B., Ma M., Zheng Y., Pan Y., Lin X. mTOR and Beclin1: two key autophagy-related molecules and their roles in myocardial ischemia/reperfusion injury. J Cell Physiol. 2019;234:12562–12568. doi: 10.1002/jcp.28125. [DOI] [PubMed] [Google Scholar]
- 29.Ren J., Sun M., Zhou H., Ajoolabady A., Zhou Y., Tao J., et al. FUNDC1 interacts with FBXL2 to govern mitochondrial integrity and cardiac function through an IP3R3-dependent manner in obesity. Sci Adv. 2020;6 doi: 10.1126/sciadv.abc8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viollet B., Andreelli F., Jorgensen S.B., Perrin C., Geloen A., Flamez D., et al. The AMP-activated protein kinase α2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest. 2003;111:91–98. doi: 10.1172/JCI16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho H., Mu J., Kim J.K., Thorvaldsen J.L., Chu Q., Crenshaw E.B., 3rd, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 32.Turdi S., Kandadi M.R., Zhao J., Huff A.F., Du M., Ren J. Deficiency in AMP-activated protein kinase exaggerates high fat diet-induced cardiac hypertrophy and contractile dysfunction. J Mol Cell Cardiol. 2011;50:712–722. doi: 10.1016/j.yjmcc.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo R., Nair S., Zhang Y., Ren J. Adiponectin deficiency rescues high-fat diet-induced hepatic injury, apoptosis and autophagy loss despite persistent steatosis. Int J Obes (Lond) 2017;41:1403–1412. doi: 10.1038/ijo.2017.128. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y., Babcock S.A., Hu N., Maris J.R., Wang H., Ren J. Mitochondrial aldehyde dehydrogenase (ALDH2) protects against streptozotocin-induced diabetic cardiomyopathy: role of GSK3β and mitochondrial function. BMC Med. 2012;10:40. doi: 10.1186/1741-7015-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Guo R., Xu X., Babcock S.A., Zhang Y., Ren J. Aldehyde dedydrogenase-2 plays a beneficial role in ameliorating chronic alcohol-induced hepatic steatosis and inflammation through regulation of autophagy. J Hepatol. 2015;62:647–656. doi: 10.1016/j.jhep.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Kandadi M.R., Hua Y., Zhu M., Turdi S., Nathanielsz P.W., Ford S.P., et al. Influence of gestational overfeeding on myocardial proinflammatory mediators in fetal sheep heart. J Nutr Biochem. 2013;24:1982–1990. doi: 10.1016/j.jnutbio.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Wang Q., Ren J. mTOR-Independent autophagy inducer trehalose rescues against insulin resistance-induced myocardial contractile anomalies: role of p38 MAPK and Foxo1. Pharmacol Res. 2016;111:357–373. doi: 10.1016/j.phrs.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang X., Xu P., Zhang F., Zhang L., Zheng Y., Hu M., et al. AMPK hyper-activation alters fatty acids metabolism and impairs invasiveness of trophoblasts in preeclampsia. Cell Physiol Biochem. 2018;49:578–594. doi: 10.1159/000492995. [DOI] [PubMed] [Google Scholar]
- 39.Vergadi E., Vaporidi K., Theodorakis E.E., Doxaki C., Lagoudaki E., Ieronymaki E., et al. Akt2 deficiency protects from acute lung injury via alternative macrophage activation and miR-146a induction in mice. J Immunol. 2014;192:394–406. doi: 10.4049/jimmunol.1300959. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J., Fan N., Peng Y. Heat shock protein 70 promotes lipogenesis in HepG2 cells. Lipids Health Dis. 2018;17:73. doi: 10.1186/s12944-018-0722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo R., Hu N., Kandadi M.R., Ren J. Facilitated ethanol metabolism promotes cardiomyocyte contractile dysfunction through autophagy in murine hearts. Autophagy. 2012;8:593–608. doi: 10.4161/auto.18997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Truong T.H., Dwyer A.R., Diep C.H., Hu H., Hagen K.M., Lange C.A. Phosphorylated progesterone receptor isoforms mediate opposing stem cell and proliferative breast cancer cell fates. Endocrinology. 2019;160:430–446. doi: 10.1210/en.2018-00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu X., Yan H., Xia M., Chang X., Xu X., Wang L., et al. Metformin attenuates triglyceride accumulation in HepG2 cells through decreasing stearyl-coenzyme A desaturase 1 expression. Lipids Health Dis. 2018;17:114. doi: 10.1186/s12944-018-0762-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li R., Li J., Huang Y., Li H., Yan S., Lin J., et al. Polydatin attenuates diet-induced nonalcoholic steatohepatitis and fibrosis in mice. Int J Biol Sci. 2018;14:1411–1425. doi: 10.7150/ijbs.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng S.X., Liu Q., Tang Y.J., Wang W.J., Zheng Q.S., Tian H.J., et al. A recipe composed of Chinese herbal active components regulates hepatic lipid metabolism of NAFLD in vivo and in vitro. Biomed Res Int. 2016;2016:1026852. doi: 10.1155/2016/1026852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tikhanovich I., Cox J., Weinman S.A. Forkhead box class O transcription factors in liver function and disease. J Gastroenterol Hepatol. 2013;28 Suppl 1:125–131. doi: 10.1111/jgh.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oosterman J.E., Foppen E., van der Spek R., Fliers E., Kalsbeek A., la Fleur S.E. Timing of fat and liquid sugar intake alters substrate oxidation and food efficiency in male Wistar rats. Chronobiol Int. 2015;32:289–298. doi: 10.3109/07420528.2014.971177. [DOI] [PubMed] [Google Scholar]
- 48.Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988;37:287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- 49.Liu P., Lin H., Xu Y., Zhou F., Wang J., Liu J., et al. Frataxin-mediated PINK1–Parkin-dependent mitophagy in hepatic steatosis: the protective effects of quercetin. Mol Nutr Food Res. 2018;62 doi: 10.1002/mnfr.201800164. [DOI] [PubMed] [Google Scholar]
- 50.Wang L., Liu X., Nie J., Zhang J., Kimball S.R., Zhang H., et al. ALCAT1 controls mitochondrial etiology of fatty liver diseases, linking defective mitophagy to steatosis. Hepatology. 2015;61:486–496. doi: 10.1002/hep.27420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sepa-Kishi D.M., Katsnelson G., Bikopoulos G., Iqbal A., Ceddia R.B. Cold acclimation reduces hepatic protein kinase B and AMP-activated protein kinase phosphorylation and increases gluconeogenesis in rats. Phys Rep. 2018;6 doi: 10.14814/phy2.13592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xing Y.Q., Li A., Yang Y., Li X.X., Zhang L.N., Guo H.C. The regulation of FOXO1 and its role in disease progression. Life Sci. 2018;193:124–131. doi: 10.1016/j.lfs.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 53.Pang L., Liu K., Liu D., Lv F., Zang Y., Xie F., et al. Differential effects of reticulophagy and mitophagy on nonalcoholic fatty liver disease. Cell Death Dis. 2018;9:90. doi: 10.1038/s41419-017-0136-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang M., Chi X., Qu N., Wang C. Long noncoding RNA lncARSR promotes hepatic lipogenesis via Akt/SREBP-1c pathway and contributes to the pathogenesis of nonalcoholic steatohepatitis. Biochem Biophys Res Commun. 2018;499:66–70. doi: 10.1016/j.bbrc.2018.03.127. [DOI] [PubMed] [Google Scholar]
- 55.Ou-Yang Q., Xuan C.X., Wang X., Luo H.Q., Liu J.E., Wang L.L., et al. 3-Acetyl-oleanolic acid ameliorates non-alcoholic fatty liver disease in high fat diet-treated rats by activating AMPK-related pathways. Acta Pharmacol Sin. 2018;39:1284–1293. doi: 10.1038/aps.2017.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X., Liu S., Zhang C., Zhang S., Yue Y., Zhang Y., et al. The role of AMPKα2 in the HFD-induced nonalcoholic steatohepatitis. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165854. doi: 10.1016/j.bbadis.2020.165854. [DOI] [PubMed] [Google Scholar]
- 57.Dokladny K., Myers O.B., Moseley P.L. Heat shock response and autophagy--cooperation and control. Autophagy. 2015;11:200–213. doi: 10.1080/15548627.2015.1009776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horman S., Vertommen D., Heath R., Neumann D., Mouton V., Woods A., et al. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase α-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem. 2006;281:5335–5340. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- 59.Kido K., Yokokawa T., Ato S., Sato K., Fujita S. Effect of resistance exercise under conditions of reduced blood insulin on AMPKα Ser485/491 inhibitory phosphorylation and AMPK pathway activation. Am J Physiol Regul Integr Comp Physiol. 2017;313:R110–R119. doi: 10.1152/ajpregu.00063.2017. [DOI] [PubMed] [Google Scholar]
- 60.Singh R., Kaushik S., Wang Y., Xiang Y., Novak I., Komatsu M., et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shimano M., Ouchi N., Shibata R., Ohashi K., Pimentel D.R., Murohara T., et al. Adiponectin deficiency exacerbates cardiac dysfunction following pressure overload through disruption of an AMPK-dependent angiogenic response. J Mol Cell Cardiol. 2010;49:210–220. doi: 10.1016/j.yjmcc.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Russo S.B., Baicu C.F., Van Laer A., Geng T., Kasiganesan H., Zile M.R., et al. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J Clin Invest. 2012;122:3919–3930. doi: 10.1172/JCI63888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu K., Yang Y., Yan M., Zhan J., Fu X., Zheng X. Autophagy plays a protective role in free cholesterol overload-induced death of smooth muscle cells. J Lipid Res. 2010;51:2581–2590. doi: 10.1194/jlr.M005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fabbrini E., Magkos F. Hepatic steatosis as a marker of metabolic dysfunction. Nutrients. 2015;7:4995–5019. doi: 10.3390/nu7064995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tilg H., Moschen A.R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.