Abstract
Background
Neighborhood-level characteristics, such as poverty, have been associated with risk factors for heart failure (HF), including hypertension and diabetes mellitus. However, the independent association between neighborhood poverty and incident HF remains understudied.
Objective
To evaluate the association between neighborhood poverty and incident HF using a “real-world” clinical cohort.
Design
Retrospective cohort study of electronic health records from a large healthcare network. Individuals’ residential addresses were geocoded at the census-tract level and categorized by poverty tertiles based on American Community Survey data (2007–2011).
Participants
Patients from Northwestern Medicine who were 30–80 years, free of cardiovascular disease at index visit (January 1, 2005–December 1, 2013), and followed for at least 5 years.
Main Measures
The association of neighborhood-level poverty tertile (low, intermediate, and high) and incident HF was analyzed using generalized linear mixed effect models adjusting for demographics (age, sex, race/ethnicity) and HF risk factors (body mass index, diabetes mellitus, hypertension, smoking status).
Key Results
Of 28,858 patients included, 75% were non-Hispanic (NH) White, 43% were men, 15% lived in a high-poverty neighborhood, and 522 (1.8%) were diagnosed with incident HF. High-poverty neighborhoods were associated with a 1.80 (1.35, 2.39) times higher risk of incident HF compared with low-poverty neighborhoods after adjustment for demographics and HF risk factors.
Conclusions
In a large healthcare network, incident HF was associated with neighborhood poverty independent of demographic and clinical risk factors. Neighborhood-level interventions may be needed to complement individual-level strategies to prevent and curb the growing burden of HF.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-021-06785-7.
KEY WORDS: heart failure, incidence, neighborhood poverty, racial/ethnic disparities
INTRODUCTION
In the USA, heart failure (HF) remains a leading cause of healthcare expenditures, morbidity, and mortality.1 While efforts to prevent HF have largely focused on individual-level traditional risk factors, such as diabetes mellitus (DM), hypertension, and ischemic heart disease (IHD), it is evident that socioeconomic factors play a significant role in the incidence of, and outcomes related to, HF.2–4 In fact, individuals living below the poverty line have been shown to develop HF more than 5 years earlier than those living above the poverty line.5 While the effects of multiple individual-level socioeconomic factors—including educational attainment and employment status in addition to personal income—have been emphasized in HF research, there is mounting evidence that neighborhood-level factors may also drive a proportion of HF risk.4, 6–10
Prior analyses have demonstrated robust associations between neighborhood poverty and key HF risk factors, suggesting the existence of “a place-based health disadvantage”.11–14 These findings have provided insight into potential prevention strategies (e.g., improving neighborhood access to fresh foods or green spaces) and highlight why individualized strategies to prevent cardiovascular disease (CVD) that do not address the patient’s social context may fall short.12 Akwo et al. recently described the association between neighborhood deprivation and risk of incident HF among low-income individuals included in the Southern Community Cohort Study, a prospective cohort from the National Cancer Institute, who had Medicare or Medicaid.10 However, it is not clear if these findings are broadly generalizable to inform effective HF prevention and management across a broad range of ages in a diverse population that includes private insurance and uninsured adults. Therefore, we sought to determine the association between neighborhood poverty and HF risk using a real-world sample from a large healthcare network.
METHODS
Study Design
This analysis was a retrospective cohort study of a real-world sample of Illinois residents using electronic health record (EHR) data obtained from the Northwestern Memorial Enterprise Data Warehouse (NMEDW). The NMEDW includes clinical, laboratory, and prescribing information for more than 6.6 million individual patients served by Northwestern Medicine (NM)-affiliated hospitals, over 200 primary care clinics, and specialists across the greater Chicago area.15–17 This analysis was approved by Northwestern University’s Institutional Review Board which granted a waiver of documentation of consent.
Analytic Sample
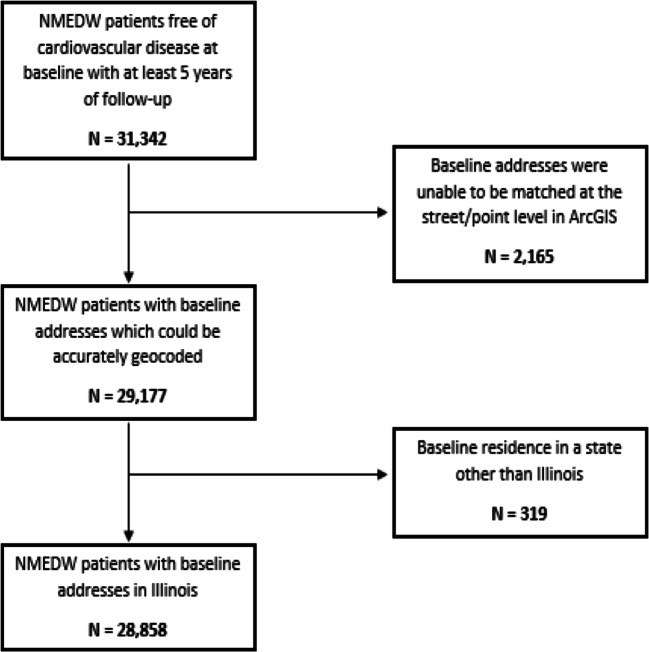
Individuals between the ages of 30 and 80 years who identified as non-Hispanic (NH) Black, NH White, or Hispanic/Latinx, had a clinic visit at an internal medicine or cardiology clinic in NM between January 1, 2005, and December 1, 2013, and a follow-up encounter at least 5 years later were eligible for inclusion. We excluded patients with preexisting CVD based on the presence of International Classification of Diseases 9th and 10th revision (ICD 9/10) codes for IHD, HF, peripheral arterial disease, cerebrovascular disease, or a pacemaker/implantable cardioverter-defibrillator (Supplementary Table 1). We excluded patients who were not residents of Illinois (N = 319) and those with insufficient address data to define the exposure (neighborhood poverty, N = 2,165) for a final analytic sample of 28,858 as shown in Figure 1.
Figure 1.
Inclusion diagram for analytic sample. Flowchart describing analytic sample from the Northwestern Medicine Enterprise Data Warehouse (NMEDW).
Exposure Assessment of Neighborhood Poverty
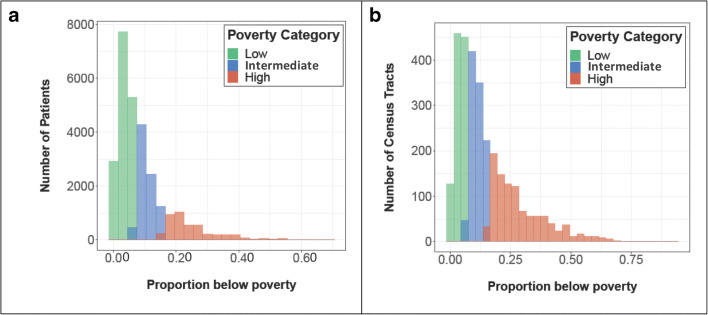
Patient addresses were based on administrative data collected closest to date of study inclusion and were geocoded and matched to census tracts using ArcGIS Pro.10, 18 Neighborhood-level poverty was defined as the proportion of individuals living below the poverty threshold using 5-year (2007–2011) census-tract level estimates from the American Community Survey accessed via the Integrated Public Use Microdata Series National Historical Geographic Information System.19 The 5-year period from 2007 to 2011 was selected as it was positioned in the midpoint of data collection. Poverty thresholds were based on the federal poverty threshold weighted by household size, number of children, and, if the household consisted of one or two individuals, whether the head of household was over the age of 65.20 Tertiles of the sample’s distribution of neighborhood poverty were used to categorize neighborhood poverty as low, intermediate, or high.10, 18 Low-poverty neighborhoods were defined as those where < 7.3% of households were living in poverty, intermediate-poverty neighborhoods were those with 7.3 to 15.9% living in poverty, and high-poverty neighborhoods were those with ≥ 16% living in poverty (Fig. 2, Supplementary Figure 1).
Figure 2.
Histograms of neighborhood poverty tertiles based on a patients’ census-tract level and b all Illinois census tracts. This two paneled figure shows a a histogram presenting the number of included NMEDW patients who fell into each neighborhood poverty tertile and b a histogram presenting the number of Illinois census tracts in each poverty tertile. Patients were classified into tertiles according to the percent of households living below the poverty line in their census tract as shown in panel a. The lowest poverty tertile included census tracts with < 7.3% of households living below the poverty line, the intermediate-poverty tertile included census tracts in which ≥ 7.3% and ≤ 15.9% of households lived below the poverty line, and highest poverty tertile included census tracts where ≥ 16% of households were living below the poverty line. Tertiles defined by state-wide census data as shown in panel b for comparison.
Covariates
All covariates were based on administrative or clinical data collected at the index outpatient encounter. Included demographic covariates were age, sex, and race/ethnicity, and HF risk factors included smoking status (current vs. former/never smoker), body mass index (BMI, kg/m2), hypertension, and DM. Hypertension and DM were defined by ICD-9/10 codes (Supplementary Table 1) or use of antihypertensive or antihyperglycemic medications/insulin (Supplementary Table 2) as indicated on home medication lists, respectively. Systolic blood pressure (SBP) and hemoglobin A1c (HbA1c) were determined using the recorded values at the outpatient encounter but were not included in the definitions of hypertension or DM given a large amount of missing data. Individual insurance status, a proxy for individual socioeconomic status included in our dataset, was categorized as (1) private, (2) public aid/Medicaid, (3) Medicare, or (4) self-pay. Given a high degree of missingness (> 20%), it was included only in supplemental analyses.
Outcome Ascertainment for HF
The primary outcome of interest was HF within 5 years of index encounter. Incident HF was defined by the presence of one inpatient HF ICD-9/10 code or two outpatient HF ICD-9/10 codes based on prior published EHR-based studies demonstrating a positive predictive value of 97% for adjudicated HF with this algorithm.21 Incident IHD was also recorded based on ICD-9/10 codes (Supplementary Table 1).
Statistical Analysis
Descriptive statistics were calculated for the overall population and by neighborhood poverty tertiles. Generalized linear mixed effects models with a logit-link function were used to evaluate the association between neighborhood poverty and incident HF while accounting for the non-independence of patients residing within the same census tract using a random intercept for census tract. Given the low incidence of HF events (< 2% of the population with incident HF), the resultant ORs approximate risk ratios (RR).22 Models were sequentially adjusted for baseline demographics and HF risk factors to account for partial mediation and possible confounding: (model 1) univariate, unadjusted model; (model 2) adjusted for demographic characteristics (age, sex, race/ethnicity); and (model 3) further adjusted for HF risk factors (BMI [kg/m2], DM, hypertension, and smoking status). We tested for interactions of neighborhood poverty with race/ethnicity and sex individually in the fully adjusted model, but given no significant interaction (results not shown), we did not stratify analyses by either demographic characteristic. Given mechanistic differences between ischemic and non-ischemic HF and prior analyses which have demonstrated an association between IHD and neighborhood poverty, in sensitivity analyses, we modeled the association between neighborhood poverty and incident non-ischemic HF after excluding any participant who experienced both incident HF and incident IHD in the follow-up period (excluded N = 139).13, 14 In an additional set of sensitivity analyses excluding individuals with missing insurance data (N = 8,121), we modeled the relationship between neighborhood poverty and HF adjusting for baseline insurance status in models 2 and 3.
Geocoding was performed in ArcGIS Pro (ArcGIS Pro Version 1.3.0; Environmental Systems Research Institute, Redlands, CA]), and all statistical analyses were completed using R version 3.6.3 or SAS version 9.4.
RESULTS
Of the 28,858 patients included in this analysis, 15,952 lived in low-poverty neighborhoods, 8,474 lived in intermediate-poverty neighborhoods, and 4,432 lived in high-poverty neighborhoods. Overall, mean (SD) age at index date was 5111 years and was similar across neighborhood poverty tertiles (Table 1). Race/ethnicity and sex varied by neighborhood poverty categories. In low-poverty neighborhoods, 46% (N = 7,296) of patients were male compared with 41% (N = 3,460) and 34% (N = 1,524) in the intermediate- and high-poverty neighborhoods, respectively. NH Black adults and Hispanic/Latinx adults made up a disproportionate number of patients residing in high-poverty neighborhoods: NH Black adults accounted for 10% (N = 2,994) and Hispanic/Latinx adults accounted for 5% (N = 1,397) of the overall sample, but 37% (N = 1,630) and 9% (N = 377) of those living in high-poverty neighborhoods, respectively. Cardiovascular risk factors were common across neighborhood poverty tertiles, but highest among those residing in high-poverty neighborhoods. Of the 4,432 patients living in high-poverty neighborhoods, 12% were current smokers, 54% had hypertension, 24% had DM, and 42% were obese. Rates of incident HF were highest in the high-poverty neighborhoods (3.5%) and lowest in the low-poverty neighborhoods (1.3%) (Table 1).
Table 1.
Baseline Characteristics for Patients with Index Visit between January 1, 2005, and December 1, 2013, Overall, and by Neighborhood Poverty Tertiles
| Overall (N = 28858) | Neighborhood poverty tertile* | |||
|---|---|---|---|---|
| Low (N = 15952) | Intermediate (N = 8474) | High (N = 4432) | ||
| Baseline characteristics | ||||
| Age, years | 51.4 ± 11.3 | 51.7 ± 10.9 | 51.3 ± 11.8 | 50.5 ± 11.7 |
| Male, N (%) | 12280 (42.6) | 7296 (45.7) | 3460 (40.8) | 1524 (34.4) |
| Race/ethnicity, N (%) | ||||
| NH White | 21526 (74.6) | 13600 (85.3) | 6093 (71.9) | 1833 (41.4) |
| NH Black | 2994 (10.4) | 528 ( 3.3) | 836 ( 9.9) | 1630 (36.8) |
| Hispanic/Latinx | 1397 ( 4.8) | 510 ( 3.2) | 510 ( 6.0) | 377 ( 8.5) |
| Other | 2941 (10.2) | 1314 ( 8.2) | 1035 (12.2) | 592 (13.4) |
| Current smoking, N (%) | 2865 ( 9.9) | 1461 ( 9.2) | 888 (10.5) | 516 (11.6) |
| Hypertension, N (%) | 13802 (47.8) | 7438 (46.6) | 3989 (47.1) | 2375 (53.6) |
| Systolic blood pressure, mmHg | 125.8 ± 16.7 | 125.7 ± 16.4 | 125.5 ± 17.0 | 126.7 ± 17.1 |
| Obesity, N (%) | 10408 (36.1) | 5623 (35.2) | 2916 (34.4) | 1869 (42.2) |
| Diabetes, N (%) | 5188 (18.0) | 2580 (16.2) | 1529 (18.0) | 1079 (24.3) |
| Hemoglobin A1C, % | 6.2 ± 1.4 | 6.2 ± 1.4 | 6.1 ± 1.4 | 6.2 ± 1.4 |
| Body mass index, kg/m2 | 28.8 ± 6.0 | 28.7 ± 5.8 | 28.7 ± 6.1 | 29.6 ± 6.5 |
For continuous characteristics, the mean ± standard deviation is reported, and for categorical characteristics, the frequency (percent) is reported
Non-Hispanic (NH)
*Neighborhood poverty tertiles were defined by the proportion of households living below the poverty threshold in a census tract. Low-poverty corresponds to a proportion < 0.073. Intermediate-poverty corresponds to a proportion ≥ 0.073 and ≤ 0.159. High-poverty corresponds to a proportion ≥ 0.160
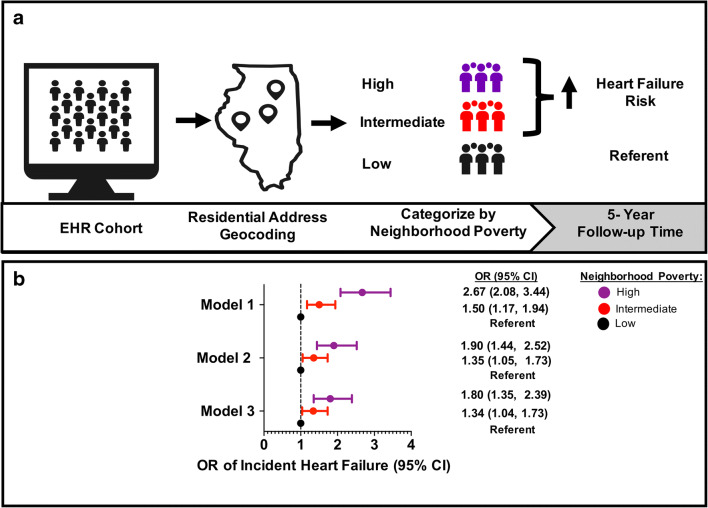
In unadjusted models, residing in a high-poverty neighborhood was associated with a 2.7 times higher risk of HF and residing in an intermediate-poverty neighborhood was associated with a 1.5 times higher risk of HF, compared with residing in a low-poverty neighborhood, respectively (Fig. 3). After adjusting for baseline demographics (age, sex, and race/ethnicity) and HF risk factors (BMI, DM, hypertension, and smoking), residing in a high-poverty neighborhood was associated with 80% higher risk of HF (OR = 1.80, 95% CI: [1.35, 2.39]) and residing in an intermediate-poverty neighborhood was associated with a 34% higher risk of HF (OR = 1.34 [1.04, 1.73]) compared with residing in a low-poverty neighborhood (Fig. 3). In this fully adjusted model, demographic characteristics and HF risk factors were independently associated with an increased risk of HF (Supplementary Table 3). For example, the fully adjusted OR of incident HF was 2.33 (1.79, 3.03) for NH Black adults and 1.65 (1.11, 2.46) for Hispanic/Latinx adults compared with NH White adults.
Figure 3.
a Study overview and b odds ratio of incident heart failure by neighborhood poverty tertiles in unadjusted and fully adjusted models. This two paneled figure shows a the study overview and b a forest plot demonstrating the odds ratio of incident HF by neighborhood poverty tertile in model 1, model 2, and model 3. The EHR cohort includes those individuals who met inclusion criteria for this study. The addresses of these individuals were geocoded and classified as being in a high-, intermediate-, or low-poverty neighborhood. Model 1 is unadjusted, model 2 is adjusted for demographic characteristics (age, sex, and race/ethnicity), and model 3 is adjusted for model 2 + hypertension, DM, BMI, and smoking. Race was defined as non-Hispanic White, non-Hispanic Black, or Hispanic/Latinx. HF, heart failure; EHR, electronic health record; OR, odds ratio; CI, confidence intervals.
In sensitivity analyses excluding patients with incident HF and IHD in the follow-up period, residing in a high-poverty neighborhood was associated with a 74% increased risk of HF (OR = 1.74 [1.28, 2.37]) and residing in an intermediate-poverty neighborhood with a 27% increased risk of HF (OR = 1.27 [0.97, 1.66]) compared with residing in a low-poverty neighborhood in the fully adjusted model (Table 2).
Table 2.
Odds Ratios (95% Confidence Intervals)* Describing the Association between Neighborhood Poverty and Heart Failure without Incident IHD with Varying Levels of Adjustment for Demographics and Known HF Risk Factors
| Model 1 Unadjusted |
Model 2 Adjusted for demographics |
Model 3 Further adjusted for risk factors |
|
|---|---|---|---|
| Neighborhood poverty | |||
| Low | Ref. | Ref. | Ref. |
| Intermediate | 1.44 (1.09, 1.89) | 1.25 (0.96, 1.63) | 1.27 (0.97, 1.66) |
| High | 2.64 (2.01, 3.46) | 1.82 (1.35, 2.46) | 1.74 (1.28, 2.37) |
| Demographics | |||
| Age at baseline† | - | 1.09 (1.08, 1.10) | 1.08 (1.07, 1.09) |
| Female (vs. male) | - | 0.74 (0.60, 0.92) | 0.76 (0.61, 0.95) |
| Race/ethnicity (vs. NH‡ White) | |||
| NH‡ Black | - | 2.86 (2.14, 3.84) | 2.26 (1.67, 3.05) |
| Hispanic/Latinx | - | 2.24 (1.46, 3.42) | 1.85 (1.19, 2.86) |
| Other | - | 1.13 (0.77, 1.67) | 1.12 (0.75, 1.67) |
| HF risk factors | |||
| BMI (kg/m2)† | - | - | 1.04 (1.02 , 1.06) |
| Hypertension | - | - | 1.75 (1.33, 2.30) |
| Diabetes mellitus | - | - | 1.50 (1.20, 1.88) |
| Current smoking | - | - | 1.10 (0.78, 1.54) |
*Odds ratios were calculated using the mean value for continuous covariates (age = 51.3, BMI = 28.8)
†Odds ratios for continuous variables (age at baseline and BMI) represent the difference in odds of heart failure for a 1-unit increase in the metric
‡Non-Hispanic (NH)
In further sensitivity analyses adjusting for individual insurance status in addition to demographics and HF risk factors, high-poverty neighborhoods were associated with a 68% higher risk of HF (OR = 1.68 [1.26, 2.26]) and intermediate-poverty neighborhoods with a 29% higher risk of HF (OR = 1.29 [0.99, 1.68]) compared with low-poverty neighborhoods (Table 3). In the fully adjusted model, Medicaid/public insurance was independently associated with 156% higher risk of HF (OR = 2.56 [1.40, 3.68]) compared with private insurance; Medicare and self-pay insurance status were not significantly different than private insurance (Table 3).
Table 3.
Odds Ratios (95% Confidence Intervals)* Describing the Association between Neighborhood Poverty and Heart Failure Further Adjusting for Insurance Status among Patients Whose Insurance Status Could Be Determined (N = 20,737)
| Model 1 Unadjusted |
Model 2 Adjusted for demographics |
Model 3 Further adjusted for risk factors |
|
|---|---|---|---|
| Neighborhood poverty | |||
| Low | Ref. | Ref. | Ref. |
| Intermediate | 1.41 (1.09, 1.82) | 1.30 (1.00, 1.69) | 1.29 (0.99, 1.68) |
| High | 2.41 (1.87, 3.11) | 1.73 (1.29, 2.32) | 1.68 (1.26, 2.26) |
| Demographics | |||
| Age at baseline† | - | 1.08 (1.07, 1.09) | 1.07 (1.06, 1.08) |
| Female (vs. male) | - | 0.62 (0.51, 0.75) | 0.65 (0.53, 0.80) |
| Race/ethnicity (vs. NH‡ White) | |||
| NH‡ Black | - | 2.59 (1.99, 3.38) | 2.08 (1.58, 2.73) |
| Hispanic/Latinx | - | 1.65 (1.09, 2.48) | 1.43 (0.95, 2.17) |
| Other | - | 1.00 (0.70 , 1.43) | 1.01 (0.70, 1.45) |
| Insurance (vs. private) | |||
| Medicare | - | 1.21 (0.89, 1.65) | 1.28 (0.94, 1.75) |
| Public aid/Medicaid | - | 2.97 (1.63, 5.41) | 2.56 (1.40, 3.68) |
| Self-pay | - | 1.02 (0.78 , 1.34) | 1.08 (0.82, 1.41) |
| HF risk factors | |||
| BMI (kg/m2)† | - | - | 1.04 (1.02, 1.05) |
| Hypertension | - | - | 1.77 (1.38, 2.27) |
| Diabetes mellitus | - | - | 1.57 (1.27, 1.94) |
| Current smoking | - | - | 1.21 (0.88, 1.66) |
*Odds ratios were calculated using the mean value for continuous covariates (age = 51.4, BMI = 28.6)
†Odds ratios for continuous variables (age at baseline and BMI) represent the difference in odds of heart failure for a 1-unit increase in the metric
‡Non-Hispanic (NH)
DISCUSSION
In this analysis of more than 25,000 patients, residing in a neighborhood with a higher proportion of people living below the poverty threshold was independently associated with an increased risk of incident HF in the short-term. There was nearly a 2-fold risk of developing HF among those living in a high-poverty neighborhood compared with a low-poverty neighborhood even after adjustment for age, race/ethnicity, and clinical HF risk factors. The risk of HF was higher among racial/ethnic minority subgroups in each neighborhood poverty tertile after adjusting for shared risk factor burden.
Our analysis is consistent with, and extends, the findings of Akwo et al. in a socioeconomically diverse real-world cohort that includes patients regardless of insurance status.10 In addition, our work amplifies the value of clinical EHR data, as it offers the advantage of objective measurement of risk factor data, including BMI and systolic blood pressure, unlike administrative analyses or those based on participant self-report of medical history. Our cohort additionally included Hispanic/Latinx patients in addition to NH White and NH Black patients, extending previous findings to a key at-risk and growing population in the USA.
While prior research has shown an increased incidence of key HF risk factors such as DM, hypertension, and obesity in neighborhoods with a higher burden of poverty, our analysis shows that neighborhood poverty is independently associated with incident HF after adjusting for these traditional CV risk factors.7, 11, 13, 18, 23–25 The increased risk of HF in high-poverty neighborhoods may be related to inequities in disease management and access to medical care as well as other, non-traditional, risk factors such as autoimmune and inflammatory conditions (e.g., systemic sclerosis, human immunodeficiency virus).13, 26–29 Additional factors such chronic stress, associated with residence in high-poverty neighborhoods and worse CVD outcomes, may also contribute to the increased risk of HF.30, 31 This increased risk has been postulated to be, in part, due to activation of inflammatory, atherosclerotic, and fibrotic pathways.9, 30, 32, 33
Overall, our findings highlight the need to deeply consider how inequities concentrated in high-poverty neighborhoods contribute to HF risk. While further study is needed to determine the precise mechanisms through which residing in a high-poverty neighborhood contributes to increased risk of HF, insights gained from research focused on CVD risk factors can help inform possible interventions. For example, neighborhood-level interventions such as BP management in Black barbershops or churches have shown to be effective, and similar, community-embedded programs may also be important sites of HF prevention in high-poverty neighborhoods.34, 35 In addition, changes to public policy and investment may also be needed to reduce HF risk. For example, increasing tree density or green spaces in high-poverty neighborhoods may be effective in preventing HF in addition to DM and hypertension, as has been demonstrated in prior analyses.36 Furthermore, policies such as over-policing and mass incarceration disproportionately affect people of color in high-poverty neighborhoods and are associated with poor mental and physical health outcomes.37, 38 Legislative changes at the local and national level will be needed to reduce these and other damaging public policies and should be explored as possible, non-traditional, vehicles of HF prevention.
While the association of neighborhood-level poverty and race/ethnicity were each independently associated with incident HF in our analysis, health inequities due to neighborhood poverty and race/ethnicity are tightly interrelated due to fundamental root causes, such as systemic and structural racial discrimination in the USA.39–41 Indeed, we found that NH Black and Hispanic/Latinx adults were more likely to live in high-poverty neighborhoods. After adjusting for neighborhood poverty and other demographic and clinical risk factors, the risk of HF in NH Black adults was more than twice that of NH White adults and the risk of HF in Hispanic/Latinx adults was more than 1.5 times higher than NH White adults. While the increased risk of HF in NH Black compared with NH White adults has been repeatedly demonstrated, less contemporary data exists on the risk of HF in Hispanic/Latinx adults.29, 42–44 Our findings, though, of an intermediate risk of HF among Hispanic/Latinx adults between NH Black and NH White adults align with the very limited data that exists.45, 46 Notably, these HF-specific findings are in contrast with other epidemiologic studies which have shown that in spite of higher rates of CV risk factors, there are lower rates of CVD and CVD mortality in Hispanic/Latinx adults compared with NH White adults—the so-called Hispanic Paradox.47 But, recently, studies have questioned these findings pointing to the heterogeneity of the category of Hispanic/Latinx and the varying risk of CVD events and mortality by ethnic subgroup.48 Future research in large, well-characterized cohorts is needed to explore drivers of increased risk of HF in this growing and diverse population.
There are important limitations of this study. First, the observational nature of this analysis limits our ability to make statements regarding causality or the directionality of the associations observed. Second, covariates and outcomes were based on EHR data collected for clinical care limiting available variables and potentially leading to misclassification. However, manual adjudication of multiple chronic conditions has demonstrated good sensitivity and specificity of EHR data.49, 50 Because this study examined risk factors at one time-point, we were unable to assess differences in risk associated with varying cumulative exposure. While prior analyses have shown differences in cardiovascular outcomes by cumulative exposure to traditional risk factors, less is known about the impact of changes in neighborhood poverty during adulthood on cardiovascular outcomes.51, 52 Future studies should investigate this question. Additionally, echocardiographic variables such as left ventricular ejection fraction (LVEF) were not available within the dataset precluding analyses by HF subtype (i.e., reduced vs. preserved ejection fraction). While we were able to show that the relationship between neighborhood poverty and HF persisted when those with incident IHD were excluded, we were unable to determine the etiology of HF for all participants given constraints of the dataset. Future analyses should determine whether the relationship between neighborhood poverty and incident HF differs by HF subtype. Third, individuals may have received medical care at outside institutions and relevant diagnoses may be missing, which may have led to over or under-estimation of the association between neighborhood poverty and incident HF. Fourth, the association between neighborhood poverty and incident HF may be significantly related to individual-level associations between socioeconomic status and incident HF. While we were unable to adjust for individual-level income or educational attainment due to limitations of the dataset, in sensitivity analyses adjusting for participants’ personal health insurance (an individual-level socioeconomic factor), we found that the association between high-poverty neighborhoods and incident HF persisted, although the effect size was attenuated. Residents of socioeconomically disadvantaged neighborhoods may experience additional stress related to policing, absence of green-space, and underfunded school systems, independent of individual socioeconomic status.40 Finally, this analysis is based on records from a single health system, so applicability to other geographic locations and patient population may be limited. However, use of a real-world sample from a large healthcare network that serves both insured and uninsured patients and includes data from over 200 primary care locations may improve generalizability.
In conclusion, this analysis shows a significant and a graded association between level of neighborhood poverty and incident HF, indicating that HF prevention and management will need to consider and integrate residential and environmental social determinants of health. It also highlights the higher risk of HF in NH Black and Hispanic/Latinx adults that is further compounded by residing in an area with a higher proportion of individuals living below the poverty threshold. Finally, this analysis demonstrates the potential of EHR data to clarify associations between neighborhood-level factors and incident HF and provides a template by which health systems might identify at-risk patients and develop community-engaged HF prevention and management strategies.
Supplementary Information
(DOCX 23.3 kb)
(PNG 4540 kb)
Funding
This work was supported in part by the Northwestern Medicine Enterprise Data Warehouse. The funding sponsor did not contribute to design and conduct of the study, collection, management, analysis, or interpretation of the data or preparation, review, or approval of the manuscript. The authors take responsibility for decision to submit the manuscript for publication. Dr. Khan had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number KL2TR001424 (SSK), and the American Heart Association (#19TPA34890060) to SSK. Dr. Rethy was supported by a research fellowship from the Sarnoff Cardiovascular Research Foundation (2018–-2019). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. There are no relationships with industry to disclose.
Declarations
Conflict of Interest
The authors declare no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/cir.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Frieden TR, Berwick DM. The “Million Hearts” initiative--preventing heart attacks and strokes. N Engl J Med. 2011;365(13):e27. doi: 10.1056/NEJMp1110421. [DOI] [PubMed] [Google Scholar]
- 3.Roberts CB, Couper DJ, Chang PP, James SA, Rosamond WD, Heiss G. Influence of life-course socioeconomic position on incident heart failure in blacks and whites: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2010;172(6):717–27. doi: 10.1093/aje/kwq193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart S, Murphy NF, McMurray JJ, Jhund P, Hart CL, Hole D. Effect of socioeconomic deprivation on the population risk of incident heart failure hospitalisation: an analysis of the Renfrew/Paisley Study. Eur J Heart Fail. 2006;8(8):856–63. doi: 10.1016/j.ejheart.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Ditah CM, Rahman E, Agbor VN, Foryoung JB, Shahzad M, Amgai B, et al. Disparities and drivers of early age at diagnosis of congestive heart failure in the USA. Int J Cardiol. 2019;293:143–7. doi: 10.1016/j.ijcard.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 6.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161(7):996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 7.Borrell LN, Diez Roux AV, Rose K, Catellier D, Clark BL. Neighbourhood characteristics and mortality in the Atherosclerosis Risk in Communities Study. Int J Epidemiol. 2004;33(2):398–407. doi: 10.1093/ije/dyh063. [DOI] [PubMed] [Google Scholar]
- 8.Ingelsson E, Lind L, Arnlov J, Sundstrom J. Socioeconomic factors as predictors of incident heart failure. J Card Fail. 2006;12(7):540–5. doi: 10.1016/j.cardfail.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186:125–45. doi: 10.1111/j.1749-6632.2009.05333.x. [DOI] [PubMed] [Google Scholar]
- 10.Akwo EA, Kabagambe EK, Harrell FE, Jr, Blot WJ, Bachmann JM, Wang TJ, et al. Neighborhood Deprivation Predicts Heart Failure Risk in a Low-Income Population of Blacks and Whites in the Southeastern United States. Circ Cardiovasc Qual Outcomes. 2018;11(1):e004052. doi: 10.1161/circoutcomes.117.004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mujahid MS, Diez Roux AV, Cooper RC, Shea S, Williams DR. Neighborhood stressors and race/ethnic differences in hypertension prevalence (the Multi-Ethnic Study of Atherosclerosis) Am J Hypertens. 2011;24(2):187–93. doi: 10.1038/ajh.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaskin DJ, Thorpe RJ, Jr, McGinty EE, Bower K, Rohde C, Young JH, et al. Disparities in diabetes: the nexus of race, poverty, and place. Am J Public Health. 2014;104(11):2147–55. doi: 10.2105/ajph.2013.301420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99–106. doi: 10.1056/nejm200107123450205. [DOI] [PubMed] [Google Scholar]
- 14.Pollack CE, Slaughter ME, Griffin BA, Dubowitz T, Bird CE. Neighborhood socioeconomic status and coronary heart disease risk prediction in a nationally representative sample. Public Health. 2012;126(10):827–35. doi: 10.1016/j.puhe.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Northwestern University Clinical and Translational Sciences Institute. Enterprise Data Warehouse. Northwestern University. Available at: https://www.nucats.northwestern.edu/resources/data-science-and-informatics/nmedw/index.html. Accessed February 21, 2021.
- 16.Feinstein MJ, Steverson AB, Ning H, Pawlowski AE, Schneider D, Ahmad FS, et al. Adjudicated Heart Failure in HIV-Infected and Uninfected Men and Women. J Am Heart Assoc. 2018;7(21):e009985. doi: 10.1161/jaha.118.009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.VanWagner LB, Ning H, Whitsett M, Levitsky J, Uttal S, Wilkins JT, et al. A point-based prediction model for cardiovascular risk in orthotopic liver transplantation: The CAR-OLT score. Hepatology. 2017;66(6):1968–79. doi: 10.1002/hep.29329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bikdeli B, Wayda B, Bao H, Ross JS, Xu X, Chaudhry SI, et al. Place of residence and outcomes of patients with heart failure: analysis from the telemonitoring to improve heart failure outcomes trial. Circ Cardiovasc Qual Outcomes. 2014;7(5):749–56. doi: 10.1161/circoutcomes.113.000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IPUMS National Historical Geographic Information System: Version 14.0 [Database] [database on the Internet]. IPUMS. 2019. Available from: https://www.nhgis.org/. Accessed February 21, 2021.
- 20.United Status Cenusu Bureau. Poverty https://www.census.gov/topics/income-poverty/poverty.html. Accessed February 21, 2021.
- 21.Goyal A, Norton CR, Thomas TN, Davis RL, Butler J, Ashok V, et al. Predictors of incident heart failure in a large insured population: a one million person-year follow-up study. Circ Heart Fail. 2010;3(6):698–705. doi: 10.1161/circheartfailure.110.938175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. Jama. 1998;280(19):1690–1. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 23.Osypuk TL, Ehntholt A, Moon JR, Gilsanz P, Glymour MM. Neighborhood Differences in Post-Stroke Mortality. Circ Cardiovasc Qual Outcomes. 2017;10(2):e002547. doi: 10.1161/circoutcomes.116.002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Sundquist J, Forsberg PO, Sundquist K. Association Between Neighborhood Deprivation and Heart Failure Among Patients With Diabetes Mellitus: A 10-Year Follow-Up Study in Sweden. J Card Fail 2019. doi:10.1016/j.cardfail.2019.04.017 [DOI] [PMC free article] [PubMed]
- 25.Coulon SM, Wilson DK, Alia KA, Van Horn ML. Multilevel Associations of Neighborhood Poverty, Crime, and Satisfaction With Blood Pressure in African-American Adults. Am J Hypertens. 2016;29(1):90–5. doi: 10.1093/ajh/hpv060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desai CS, Lee DC, Shah SJ. Systemic sclerosis and the heart: current diagnosis and management. Curr Opin Rheumatol. 2011;23(6):545–54. doi: 10.1097/BOR.0b013e32834b8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freiberg MS, Chang CH, Skanderson M, Patterson OV, DuVall SL, Brandt CA, et al. Association Between HIV Infection and the Risk of Heart Failure With Reduced Ejection Fraction and Preserved Ejection Fraction in the Antiretroviral Therapy Era: Results From the Veterans Aging Cohort Study. JAMA Cardiol. 2017;2(5):536–46. doi: 10.1001/jamacardio.2017.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lane-Cordova AD, Khan SS, Grobman WA, Greenland P, Shah SJ. Long-Term Cardiovascular Risks Associated With Adverse Pregnancy Outcomes: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;73(16):2106–16. doi: 10.1016/j.jacc.2018.12.092. [DOI] [PubMed] [Google Scholar]
- 29.Bibbins-Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, et al. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360(12):1179–90. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tawakol A, Osborne MT, Wang Y, Hammed B, Tung B, Patrich T, et al. Stress-Associated Neurobiological Pathway Linking Socioeconomic Disparities to Cardiovascular Disease. J Am Coll Cardiol. 2019;73(25):3243–55. doi: 10.1016/j.jacc.2019.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barber S, Hickson DA, Wang X, Sims M, Nelson C, Diez-Roux AV. Neighborhood Disadvantage, Poor Social Conditions, and Cardiovascular Disease Incidence Among African American Adults in the Jackson Heart Study. Am J Public Health. 2016;106(12):2219–26. doi: 10.2105/ajph.2016.303471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 33.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–51. doi: 10.1161/atvbaha.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Victor RG, Blyler CA, Li N, Lynch K, Moy NB, Rashid M, et al. Sustainability of Blood Pressure Reduction in Black Barbershops. Circulation. 2019;139(1):10–9. doi: 10.1161/circulationaha.118.038165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schoenthaler AM, Lancaster KJ, Chaplin W, Butler M, Forsyth J, Ogedegbe G. Cluster Randomized Clinical Trial of FAITH (Faith-Based Approaches in the Treatment of Hypertension) in Blacks. Circ Cardiovasc Qual Outcomes. 2018;11(10):e004691. doi: 10.1161/circoutcomes.118.004691. [DOI] [PubMed] [Google Scholar]
- 36.Astell-Burt T, Feng X. Urban green space, tree canopy and prevention of cardiometabolic diseases: a multilevel longitudinal study of 46 786 Australians. Int J Epidemiol. 2020;49(3):926–33. doi: 10.1093/ije/dyz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wildeman C, Wang EA. Mass incarceration, public health, and widening inequality in the USA. Lancet. 2017;389(10077):1464–74. doi: 10.1016/s0140-6736(17)30259-3. [DOI] [PubMed] [Google Scholar]
- 38.Massoglia M, Pridemore WA. Incarceration and Health. Annu Rev Sociol. 2015;41:291–310. doi: 10.1146/annurev-soc-073014-112326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coates T-N. The Case for Reparations. The Atlantic 2014 June 10, 2020. Available at: https://www.theatlantic.com/magazine/archive/2014/06/the-case-for-reparations/361631/. Accessed February 21, 2021.
- 40.Firebaugh G, Acciai F. For blacks in America, the gap in neighborhood poverty has declined faster than segregation. Proc Natl Acad Sci U S A. 2016;113(47):13372–7. doi: 10.1073/pnas.1607220113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kershaw KN, Albrecht SS. Racial/ethnic residential segregation and cardiovascular disease risk. Curr Cardiovasc Risk Rep. 2015;9(3):10. doi: 10.1007/s12170-015-0436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Husaini BA, Mensah GA, Sawyer D, Cain VA, Samad Z, Hull PC, et al. Race, sex, and age differences in heart failure-related hospitalizations in a southern state: implications for prevention. Circ Heart Fail. 2011;4(2):161–9. doi: 10.1161/circheartfailure.110.958306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jolly S, Vittinghoff E, Chattopadhyay A, Bibbins-Domingo K. Higher cardiovascular disease prevalence and mortality among younger blacks compared to whites. Am J Med. 2010;123(9):811–8. doi: 10.1016/j.amjmed.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 44.Ziaeian B, Kominski GF, Ong MK, Mays VM, Brook RH, Fonarow GC. National Differences in Trends for Heart Failure Hospitalizations by Sex and Race/Ethnicity. Circ Cardiovasc Qual Outcomes. 2017;10(7):e003552. doi: 10.1161/circoutcomes.116.003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez CJ, Allison M, Daviglus ML, Isasi CR, Keller C, Leira EC, et al. Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States: a science advisory from the American Heart Association. Circulation. 2014;130(7):593–625. doi: 10.1161/cir.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balfour PC, Jr, Ruiz JM, Talavera GA, Allison MA, Rodriguez CJ. Cardiovascular Disease in Hispanics/Latinos in the United States. J Latina/o Psychol. 2016;4(2):98–113. doi: 10.1037/lat0000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruiz JM, Steffen P, Smith TB. Hispanic mortality paradox: a systematic review and meta-analysis of the longitudinal literature. Am J Public Health. 2013;103(3):e52–60. doi: 10.2105/ajph.2012.301103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez F, Hastings KG, Boothroyd DB, Echeverria S, Lopez L, Cullen M, et al. Disaggregation of Cause-Specific Cardiovascular Disease Mortality Among Hispanic Subgroups. JAMA Cardiol. 2017;2(3):240–7. doi: 10.1001/jamacardio.2016.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saczynski JS, Andrade SE, Harrold LR, Tjia J, Cutrona SL, Dodd KS, et al. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):129–40. doi: 10.1002/pds.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmad FS, Chan C, Rosenman MB, Post WS, Fort DG, Greenland P, et al. Validity of Cardiovascular Data From Electronic Sources: The Multi-Ethnic Study of Atherosclerosis and HealthLNK. Circulation. 2017;136(13):1207–16. doi: 10.1161/circulationaha.117.027436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kishi S, Teixido-Tura G, Ning H, Venkatesh BA, Wu C, Almeida A, et al. Cumulative Blood Pressure in Early Adulthood and Cardiac Dysfunction in Middle Age: The CARDIA Study. J Am Coll Cardiol. 2015;65(25):2679–87. doi: 10.1016/j.jacc.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 52.Murray ET, Diez Roux AV, Carnethon M, Lutsey PL, Ni H, O'Meara ES. Trajectories of neighborhood poverty and associations with subclinical atherosclerosis and associated risk factors: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2010;171(10):1099–108. doi: 10.1093/aje/kwq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 23.3 kb)
(PNG 4540 kb)