Abstract
Background
The incidence of resistance among currently available antimalarial drugs, as well as the high economic cost of malaria, has prompted researchers to look for novel antimalarial molecules. As a result, the current study was proposed to evaluate the antiplasmodial activity (in vivo) of Maytenus gracilipes based on the plant's traditional claims.
Methods
A cold maceration procedure using 80% methanol as a solvent was employed to obtain a crude extract from M. gracilipes leaves. Chloroform, n-butanol, and pure water were used to fractionate the hydromethanolic extract. Standard procedures were followed for an acute oral toxicity test. The antimalarial effects of the plant at 200, 400, and 600 mg/kg doses were investigated using three rodent malaria models (4-day suppressive, rane's, and repository tests). Thirty mice were utilized in each experiment (3 treatment and 2 control groups, each with six mice). Parasitemia, survival time, body weight, temperature, and packed cell volume were all used to assess the extracts' antiplasmodial activity. To compare results between groups, a one-way ANOVA with Post Hoc Tukey's HSD was used.
Results
In a 4-day suppressive investigation, all doses of the crude extract and fractions suppressed parasitemia significantly (P < 0.001) as compared to the negative control. The crude extract had the greatest chemosuppressive effect (74.15%) at 600 mg/kg dose. Chloroform had the greatest parasitemia suppression among the fractions; however it was less than the crude extract. In Rane's test, all doses of the crude extract produced substantial (P < 0.001) curative effects as compared to the negative control.
Conclusion
According to this study, the crude extract and solvent fractions of M. gracilipes leaves contain antimalarial activity with a substantial suppressive effect. The antiplasmodial effects were more active in the chloroform and n-butanol fractions, indicating that the plant's non-polar and medium polar constituents are responsible. Nonetheless, further analysis is required to isolate and characterize the active compounds responsible for the study plant's antimalarial activity.
Keywords: In vivo, Antiplasmodial activity, Maytenus gracilipes, Extract, Fraction
In vivo; Antiplasmodial activity; Maytenus gracilipes; Extract; Fraction.
1. Introduction
Malaria is a parasitic disease spread by mosquitoes and caused by the Plasmodium genus. When malaria-infected mosquitoes bite a human, the parasite multiplies in hepatocytes before infecting erythrocytes. Human malaria is caused by five Plasmodium species, two of which (Plasmodium falciparum and Plasmodium vivax) pose serious public health risks. Malaria is transmitted most commonly by the bite of female Anopheles mosquitoes. Around 30 species of Anopheles mosquito are prominent malaria vectors, out of 400 distinct species. Because of the African vector species' long lifespan and strong human-biting propensity, Africa accounts for the majority of the world's malaria cases [1, 2].
Malaria is one of the world's most common causes of sickness and death in tropical and subtropical areas. Even if this life-threatening disease could be cured and prevented, more than one million people die each year from malaria, the majority of whom are young children in Africa's Sub-Saharan region, where malaria is responsible for one out of every five pediatric deaths. Malaria affects roughly half of the world's population, and a child dies from it every 30 s, according to the World Health Organization (WHO) [1]. Malaria has a negative impact on people's health as well as the economic growth of various developing countries, including Ethiopia. According to a recent study, the combined prevalence of malaria among Ethiopian adults is higher than the overall population and approximately equivalent to pregnant women [3].
Apart from the reduction in new and old cases of malaria, malaria is still dynamic in our world. Thus, its control requires a combined approach including treatment with potent and very effective anti-plasmodial agents. However, the development of resistance across the current insecticidal and antimalarial medications is a major challenge in fight against malaria [4]. According to Menard and Dondorp (2017), the rising of resistance among antimalarial drugs threatens world effective malaria control, treatment, and elimination programs. In several nations of the Greater Mekong subregion, artemisinin and its partner resistance in P. falciparum is becoming a major problem, resulting in significant failure rates with artemisinin combination treatments (ACTs) [5]. At least one trial with a significant treatment failure rate (≥10%) was reported from six of the 23 African countries that have used artesunate-amodiaquine, according to the WHO worldwide database review on the efficacy of antimalarial drugs. In four investigations conducted in Indonesia, a substantial failure rate of artesunate-amodiaquine treatment for malaria was also noted [6]. Evidence in Africa that P. falciparum has evolved genetic variations that give partial resistance to artemisinin is a red flag for malaria chemotherapeutic treatment failure [7]. Despite this, history has shown us that many of the drugs we use today, such as chloroquine and quinine, are derived from medicinal plants. As a result, in order to discover new anti-plasmodial compounds for future usage, it is required to deal with traditionally claimed medicinal plants.
Around 65 percent of the world's population uses medicinal herbs as part of their basic health care [8]. Traditional medicines are used as a key source of health treatment by over 70%–90% of citizens in developing countries [9]. Antiplasmodial activity has been found in over 1,200 medicinal plants around the world. Experiments have shown that Ampelozyziphus amazonicus and Strychnopsis thouarsii, two extensively utilized herbs in malaria-endemic areas of Brazil and Madagascar, exhibit antisporozoite properties. Herbal medications are delivered at home as first-aid therapy for 60 percent of children with malaria in Ghana, Mali, Nigeria, and Zambia. Around 80% of Ethiopia's population relies on locally manufactured treatments, which primarily comprise medicinal plants, to cure a variety of ailments [10, 11, 12]. Traditional medicines are commonly used in Ethiopia to treat a wide range of diseases, including malaria. In Ethiopia, almost 200 different medicinal plant species are utilized to cure malaria [13]. Carissa spinarum [15], Leonotisocymifolia [16], Zehneriascabra [17], Croton macrostachyus, Rutachalepensis, Vernonia amygdalina [18], and Gardenia ternifolia [19] were among the Ethiopian medicinal plants whose antiplasmodial actions were proven in studies [14]. The antiplasmodial activity of Maytenus gracilipes, on the other hand, has not been scientifically evaluated.
Maytenus is one of the genera in the Celastraceae family that is localized in sub-tropical and tropical areas of the world. Among its various species, M. gracilipes is an evergreen shrub or bushy tree about 5–10 m high. It is widely distributed throughout the globe especially in Brazil, Paraguay, Argentina, Cameron, Ethiopia, Ivory Coast, Nigeria, and Liberia. In Ethiopia, it is commonly known as “Kombolcha” in AfaanOromoo, “Atat, Kamu, Kurava, Telalo or Talo” in Amharic, Dubobeis in Somali, Degmut, and Hatchat in Tigrigna [20]. M. gracilipes leaves are used to treat malaria by the community of Tepi town, Southwest Ethiopia. The plant is also used locally to treat epilepsy at Bale, Southeastern Ethiopia [21, 22]. There is only one study done on the biological activity of this plant. This previous ethnopharmacological report indicates that leaf extract of M. gracilipes has antibacterial activity against gram-positive and gram-negative bacteria [23]. Based on the above ethnobotanical and bio-activity studies, the current study aimed to investigate the in vivo anti-malarial activity of the crude extract and solvent fractions of M. gracilipes leave.
2. Materials and methods
2.1. Study design
In this study, a pre- and post-test control group experimental study design was used. To group and assign study animals for treatment, a simple random sampling procedure was used.
2.2. Plant material collection and preparation
In February 2019, fresh leaves of M. gracilipes were taken from its natural habitat in the Andracha woreda, Sheka zone, Southwest Ethiopia, 576 km from Addis Ababa. In the National Herbarium, College of Natural and Computational Sciences, Addis Ababa University, the plant samples were identified and authenticated as Maytenusgracilipes (Welw. ex Oliv) Exell., and the sample number (ET 002/2019) was stored for future reference. Fresh plant leaves were carefully washed and cleansed before being air-dried at room temperature and then ground to a coarse powder with a mortar and pestle.
2.3. Study mice
Swiss albino mice (healthy) of both sexes (female for acute oral toxicity and male for antimalarial testing) weighing 23–33g and aged 6–8 weeks were bought from Ethiopian Public Health Institute (EPHI), Addis Ababa, Ethiopia, and kept at Mizan-Tepi University's animal house. Since female mice are more sensitive than male mice, they were preferred for the toxicity test [24]. The mice were housed in a stainless-steel cage at room temperature with a 12-hour light-dark cycle and free access to water and commercial food for a week as part of the acclimatization process. The National Institute of Health Guidelines for the Care and Use of Laboratory Animals [25] were followed in this study for all procedures and techniques. The protocol was also acknowledged by the Department of Pharmacology and Toxicology and approved by the Ethics and Research Committee at the School of Pharmacy with the approval number (SOP5/12/12).
2.4. Malaria parasite
EPHI provided the chloroquine-sensitive Plasmodium berghei (ANKA) for this study. Every week, Plasmodium was maintained by serially passing blood from infected mice to non-infected animals.
2.5. Crude extraction and solvent fractionation of plant materials
Successful extraction of bioactive constituents from plant material is mainly dependent on the type of solvents used in the extraction process. Traditional medical practitioners mostly used water as the solvent [26] but a mixture of alcohol and water is more responsible to remove most secondary metabolites from medicinal plants in maceration methods of plant extraction. As a result, 550 g coarse powder of the plant leaves was extracted in an Erlenmeyer flask for three days at room temperature using the cold maceration procedure (often employed in natural product studies) with an 80 percent hydroalcoholic (750 ml) solution. The extraction was guided by occasional shaking using an orbital shaker (Bibby Scientific Limited Stone Staffo Reshire, UK) at 120 revolutions per minute. Gauze was used to filter the extract, and then Whatman filters paper No. 1 was used to filter it (Maidstone, UK). To increase yield, the residue was re-macerated for the second and third times with the same volume of fresh solvent. A rotary evaporator (Buchi Rotavapor R 200, Flawil, Switzerland) was used to mix and concentrate the filtrates under reduced pressure. All extracts were then dried and concentrated in a dry oven at a temperature of 40 °C (Okhia industrial area, India) [27, 28]. Finally, the extract was transferred to an amber glass bottle and stored at 20 °C until the experiment was completed. After that, the hydromethanolic extract was fractionated using different polarity solvents (chloroform, n-butanol, and aqueous solution) [29, 30].
2.6. Acute toxicity test
An acute oral toxicity study was conducted on the hydroalcoholic extract and solvent fractions according to standard protocols [31]. First, the non-pregnant female mouse fasted for 3 h. The animal was then weighed, and a 2,000 mg/kg dose of each extract and fraction was given via oral gavage, followed by a 2-hour fast. After that, the mouse was kept under observation for 24 h. Because death and altered behavior were not observed, the same dose was given to four more female mice (kept in the same conditions), and signs of intoxication (body weight changes, changes in drinking water and eating food, and behavioral changes associated with the cardiovascular, gastrointestinal, and central nervous systems) were observed for the next 14 days.
3. In vivo antiplasmodial tests
3.1. Grouping and dosing of animals
The doses of the test extract were calculated as 1/5th, 1/10th, and 1/20th of the lethal dose 50% (LD50) value from the acute oral toxicity test [32]. However, a pilot study was conducted to identify the most active doses. As a result, 600, 400, and 200 mg/kg were approved to be used as doses for the crude extract and solvent fractions. Thirty mice were randomly divided into five groups, each with six animals: three treatment groups and two control groups. The negative control group was given the reconstitution vehicles (2 percent Tween-80 for the chloroform fraction and distilled water for the crude extract, n-butanol, and aqueous fractions), while the positive control group was given chloroquine 25 mg/kg. The test drugs (crude extract or solvent fractions) were given to the three treatment groups at different doses (200, 400, and 600 mg/kg). The doses were given orally to each control and treatment group mice at a volume of 10 ml/kg.
3.2. Inoculum preparation and inoculation
Donors were albino mice that had previously been infected with P. berghei and had varying levels of parasitemia. The parasitemia levels of donor mice were initially evaluated using blood taken from a 0.5–1mm section of the mice's tails [33]. To infect the mice, the donor mouse with a rising parasitemia of about 30–37% was sacrificed by head blow and blood was collected through the incisions of the jugular vein into a test tube containing 3.8% trisodium citrate (BDH chemicals, England) added as an anticoagulant [34]. The blood was then diluted in normal saline so that the final suspension would contain about 1 × 107 infected red blood cells (RBCs) in every 0.2 ml suspension [35]. The dilution was made based on donor mice parasitemia and RBC count of the normal mice in such a way that one ml blood contains 5 × 107 infected erythrocytes [33, 36]. Finally, every mouse was infected (intraperitoneally) with 0.2 ml infected blood (1 × 107 P. berghei parasitized RBCs) [37].
3.3. Four-day suppressive examination
On early infection, the antimalarial activity of the hydromethanolic extract and solvent fractions was assessed using the method described by Peter's [38]. Thirty infected male mice were divided into five groups at random (6 mice per group). Following the technique outlined in the animal grouping and dosage section, all groups were treated two hours after infection. The test drugs were given every 24 h until the third day (D3). Blood was extracted from the tails of each mouse on the fifth day (D4), and thin blood films were produced to measure parasitemia levels and percentage suppression. Each mouse's body weight, rectal temperature, and packed cell volume (PCV) were measured just before infection on day zero (D0) and again at the end of the test (D4).
3.4. Established infection test
The curative effects of the crude extract were assessed using the method described by Ryley and Peters [39]. On the first day, thirty mice were intra-peritoneally infected with standard inoculum (D0). The animals were randomly divided into 5 (n = 6) and treated as described in the mice dosing and assembling section 72 hours later (at D3). The extract was given once a day till the sixth day (D6). Blood was drawn from each mouse's tail on days 3 (D3) and 7 (D7), and thin blood films were prepared from the blood to determine parasitemia levels. Each mouse's body weight, rectal temperature, and PCV were measured just prior to the first dose and again at the end of the test.
3.5. Repository test
The residual infection process depicted by Peter's repository test [40] was used to assess the crude extract's chemoprophylactic activity. Adult male mice were weighed and divided into five groups of six mice each for a four-day treatment (D0-D3). On the fifth day (D4), all study mice were infected with the malaria parasite and monitored for the next 72 h. Later, the parasitemia level was determined and the percent suppression was calculated. Just before infecting the mice and at the end of the test period, the mice's body weight, rectal temperature, and PCV were recorded.
3.6. Number of animals
As mentioned above, we do have three animal models and each model required 30 mice (six mice in each of five groups). Thus, 90 mice were used in the 4-day suppressive model since the crude extract as well as chloroform, n-butanol and aqueous fractions were evaluated in this model. In a similar fashion, 30 mice in the curative model and 30 mice in the prophylactic model were used to evaluate the effect of the crude extract. Additionally, 15 mice were used in the pilot study. So, a total of 165 mice were used to complete the experiments.
3.7. Measurement of parasitemia level
Thin blood films were fixed with a few drops of absolute methanol, left for about 10–15 min to air-dry, and stained with 10% Giemsa at a pH of 7.2 for 10 min. The slides were then washed using distilled water and kept at room temperature for drying by air. Finally, they were viewed under the light microscope using an oil immersion objective and parasitemia was examined microscopically through x100 objective. The parasitized red blood cells were identified by the parasite's presence within the cells. For each provided dose level, the percentage parasitemia was estimated by comparing parasitemia densities in infected control mice with those in treated animals in six arbitrarily selected microscopic fields [41].
| (1) |
The hydroalcoholic crude extract and solvent fractions were compared to the control groups in terms of percentage suppression. To measure the percent suppression of parasitemia, Eq. (2) was used [42].
| (2) |
3.8. Mean survival time
Throughout the follow-up time at all animal models, the mice were checked daily and the numbers of surviving days from inoculation to death were recorded for each mouse in treatment and control groups. The average survival days from the date of infection for a period of 28 days were calculated using Eq. (3) [43] to estimate the mean survival days for each group.
| (3) |
3.9. Measurement of body weight and rectal temperature
A sensitive digital weighing balance was used to weigh each mouse in a group, and a digital rectal thermometer was used to measure rectal temperature. After that, the percent changes in their mean findings pre- and post-treatment were determined.
3.10. Packed cell volume determination
Heparinized capillary tubes were used to draw blood from the mice's tails. Blood was filled to about 75% of the height of the capillary tubes, and the dry end was sealed with sealant clay. The tubes were then sited in a hematocrit centrifuge, centrifuged for 5 min at 11,000 rpm, measured by a standard hematocrit reader, and calculated using Eq. (4) [44].
| (4) |
3.11. Phytochemical analysis
Following standard experimental protocols, the 80 percent methanolic extract and solvent fractions of M. gracilipes leaves were screened for the presence of secondary metabolites (alkaloids, tannins, saponins, flavonoids, terpenoids, steroids, and anthraquinones) in order to link the plant's antimalarial activity to its constituents [45, 46].
3.12. Quality control
All instruments and materials used were of analytical grade. Data quality was controlled by randomization in experimental mice grouping, a pre-test (pilot study), acclimatization, strict adherence to protocols, and coding microscopic slides in blood smear preparation. Moreover, external factors were reduced by the use of naive mice maintained under standard laboratory conditions. The animal attendants kept the hygiene of the cages every other day by cleaning and removal of feces. Infected and non-parasitized erythrocytes were counted blindly by medical laboratory personnel.
3.13. Statistical analysis
Results were presented as a mean plus or minus standard error of the mean (mean ± SEM). Data were analyzed using SPSS version 22. Data distribution normality was assessed through Kolmogorov–Smirnov, and Shapiro–Wilk test. Statistical significance was determined by one-way analysis of variance (ANOVA) followed by Tukey's (post hoc) test to compare the measured parameters (percentage parasitemia, percentage suppression, body weight, rectal temperature, and survival time) within and between groups. The analysis was performed with a 95% confidence interval and p < 0.05 was considered to be statistically significant.
4. Results
4.1. Extract yield
A total yield of 66 g (12% w/w) of the crude extract was obtained from 550 g coarse powder of M. gracilipes leaves. From the hydromethanolic extract subjected to fractionation, 14.97 g (37.43%), 9.70 g (24.25%), and 15.33 g (38.33%) were found to be the yield (percentage) of chloroform, n-butanol, and aqueous fractions, respectively.
4.2. Acute oral toxicity test
Within the first twenty-four hours and the following 14 days of treatment with 2,000 mg/kg/dose of the crude extract and solvent fractions, none of the study animals showed signs of toxicity (diarrhea, weight loss, lethargy, poor appetite, lacrimation, salivation, hair erection, tremors, and paralysis), indicating that the LD50 values are greater than 2,000 mg/kg.
4.3. Antimalarial effect of hydromethanolic extract and its solvent fractions in four-day suppressive test
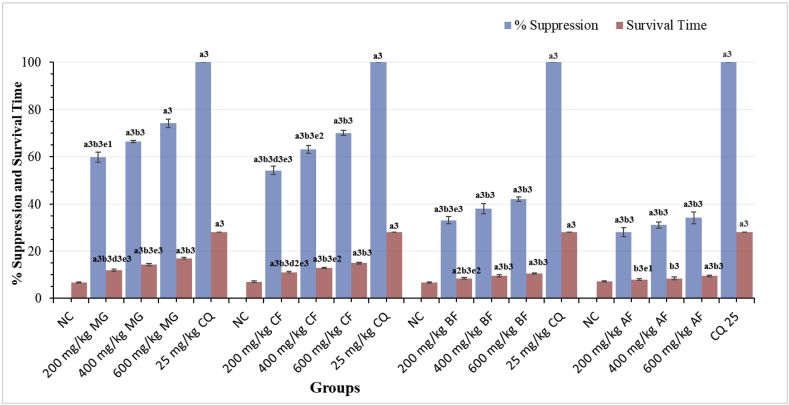
Figure 1 summarizes the results of a chemosuppressive test of M. gracilipes leaves crude extract and solvent fractions at various dose levels on parasitemia and survival time in mice infected with P. berghei. The suppressive study revealed that the crude extract, as well as fractions, suppressed parasitemia significantly (p < 0.001) as compared to a negative control. However, the effects achieved were significantly (p < 0.001) smaller than that of the standard. The standard drug reduced parasitemia to undetectable levels. The crude extract had the greatest chemosuppressive effect (74.15%) at the highest dose (600 mg/kg), followed by the chloroform fraction (70.05%). The lowest dose of the aqueous fraction, on the other hand, had the least suppressive activity against parasitemia (28.15%). When the effects of the crude extract were compared, the suppression elicited by the lowest dose (200 mg/kg) was considerably (P < 0.05) lower than that induced by the 600 mg/kg. Similarly, there was a significant difference (P < 0.001) between the n-butanol fractions of 200 mg/kg and 600 mg/kg. The chloroform fraction showed antimalarial activity in a dose-dependent manner. Suppression was also detected in the aqueous fraction at similar levels. The mean survival time (MST) of the crude extract and chloroform fraction treated mice was increased in a dose-dependent manner. All doses (600 mg/kg, 400 mg/kg and 200 mg/kg) of n-butanol fraction were capable of significantly (p < 0.001, p < 0.001 and p < 0.01, respectively) increasing survival time as compared to the negative control. Similarly, the highest dose (600 mg/kg) of the aqueous fraction was considerably (p < 0.001) prolonged survival time as compared to the vehicle-treated group. Positive control had a significantly longer (p < 0.001) MST as compared to the respective treatment groups. The highest dose of n-butanol and aqueous fraction outlasted the lowest counterpart dose with a significant (p < 0.01 and p < 0.05, respectively) survival time (Figure 1).
Figure 1.
Effect of the Hydromethanolic Extract and Its Fractions of Maytenus gracilipes on Percentage Suppression and Survival Time of Plasmodium berghei-infected Mice in the four-day Suppressive Test. Results are expressed as mean ± SEM; n = 6; a = compared to NC; b = to 25 mg/kg CQ; d = to 400 mg/kg; e = to 600 mg/kg; 1p < 0.05, 2p < 0.01, 3p < 0.001; NC = negative control (distilled water/2% tween-80), MG = crude extract of Maytenus gracilipes, CF = chloroform fraction, BF = butanol fraction, AF = aqueous fraction, CQ = chloroquine.
In spite of the protection effect by the standard drug as compared to (a) the NC in all fraction-treated groups, (b) the lowest dose of chloroform fraction, (c) the lowest and middle doses of n-butanol fraction, and (d) all doses of aqueous fraction, protection of parasite-induced body weight loss was not attained by the crude extract and solvent fractions as shown in Table 1. The experimental plant's chloroform and aqueous fractions, on the other hand, were able to prevent the rectal temperature drop produced by P. berghei infection. In comparison to the NC, both the middle and maximum doses of chloroform fraction significantly protected decrement in rectal temperature (p < 0.01). Similarly, all aqueous fraction doses prevented a drop in rectal temperature by p < 0.01 as compared to NC. As compared to all doses of n-butanol fraction and the lowest dose of chloroform fraction, chloroquine (CQ) resulted in a reduction in rectal temperature (Table 1).
Table 1.
Effect of Maytenus gracilipes hydroalcoholic extract and its fractions on body weight and rectal temperature of mice infected with P. berghei in the four-day suppressive study.
| Group | Body weight (g) |
Rectal temperature (0C) |
||||
|---|---|---|---|---|---|---|
| D0 | D4 | % Change | D0 | D4 | % Change | |
| NC | 29.24 ± 0.95 | 28.86 ± 0.93 | −1.15 | 37.60 ± 0.22 | 34.38 ± 1.00 | −8.60 |
| 200 mg/kg MG | 30.31 ± 1.36 | 31.13 ± 1.41 | 2.94 | 37.93 ± 0.25 | 35.10 ± 0.77 | −7.39 |
| 400 mg/kg MG | 30.47 ± 1.15 | 31.66 ± 1.34 | 3.90 | 37.80 ± 0.42 | 35.75 ± 0.87 | −5.39 |
| 600 mg/kg MG | 31.48 ± 1.27 | 32.73 ± 1.25 | 4.07 | 37.56 ± 0.29 | 36.95 ± 0.26 | −1.62 |
| 25 mg/kg CQ | 31.72 ± 0.73 | 32.95 ± 0.94 | 3.88 | 37.68 ± 0.19 | 37.45 ± 0.32 | −0.62a1 |
| NC | 29.26 ± 0.82 | 26.88 ± 0.87 | −8.17 | 37.60 ± 0.23 | 32.82 ± 0.37 | −12.69 |
| 200 mg/kg CF | 29.93 ± 0.70 | 28.53 ± 0.91 | −4.78b2 | 38.45 ± 0.20 | 35.20 ± 0.57 | −8.43b2 |
| 400 mg/kg CF | 30.80 ± 0.77 | 30.39 ± 0.90 | −1.31 | 38.50 ± 0.23 | 36.83 ± 0.29 | −4.33a2 |
| 600 mg/kg CF | 29.33 ± 0.64 | 29.09 ± 0.79 | −0.78 | 37.35 ± 0.26 | 36.03 ± 0.78 | −3.51a2 |
| 25 mg/kg CQ | 30.40 ± 0.24 | 32.05 ± 0.84 | 5.45a3 | 37.62 ± 0.13 | 37.65 ± 0.21 | 0.09a3 |
| NC | 26.81 ± 1.00 | 25.40 ± 0.97 | −4.62 | 37.00 ± 0.32 | 33.60 ± 0.34 | −9.18 |
| 200 mg/kg BF | 27.40 ± 0.56 | 26.31 ± 0.57 | −3.95b1 | 37.55 ± 0.19 | 33.52 ± 0.61 | −10.69b3 |
| 400 mg/kg BF | 25.82 ± 0.85 | 25.10 ± 1.09 | −2.93b1 | 37.25 ± 0.30 | 34.73 ± 0.41 | −6.76b2 |
| 600 mg/kg BF | 26.65 ± 0.70 | 26.15 ± 1.02 | −2.03 | 37.50 ± 0.36 | 35.18 ± 0.31 | −6.16b2 |
| 25 mg/kg CQ | 30.24 ± 0.36 | 31.70 ± 0.74 | 4.91a2 | 37.75 ± 0.21 | 37.75 ± 0.19 | 0.01a3 |
| NC | 29.55 ± 1.16 | 27.30 ± 1.18 | −6.91 | 36.88 ± 0.25 | 34.65 ± 0.34 | −6.03 |
| 200 mg/kg AF | 30.35 ± 1.09 | 28.62 ± 1.09 | −5.58b2 | 36.65 ± 0.25 | 36.20 ± 0.37 | −1.23a2 |
| 400 mg/kg AF | 31.95 ± 0.65 | 30.70 ± 1.07 | −4.02b2 | 37.58 ± 0.25 | 37.20 ± 0.29 | −1.01a2 |
| 600 mg/kg AF | 29.30 ± 0.27 | 28.30 ± 0.97 | −3.45b2 | 36.85 ± 0.17 | 36.48 ± 0.15 | −0.99a2 |
| 25 mg/kg CQ | 30.70 ± 0.67 | 33.05 ± 0.91 | 7.66a2 | 37.45 ± 0.07 | 38.00 ± 0.18 | 1.47a3 |
Results are expressed as mean ± SEM; n = 6; a = compared to NC; b = to 25 mg/kg CQ; d = to 400 mg/kg; e = to 600 mg/kg; 1p < 0.05, 2p < 0.01, 3p < 0.001; NC = negative control (distilled water/2% tween-80), MG = crude extract of Maytenus gracilipes, CF = chloroform fraction, BF = butanol fraction, AF = aqueous fraction, CQ = chloroquine, D0 = pre -treatment value on day 0, D4 = post-treatment value on day 4.
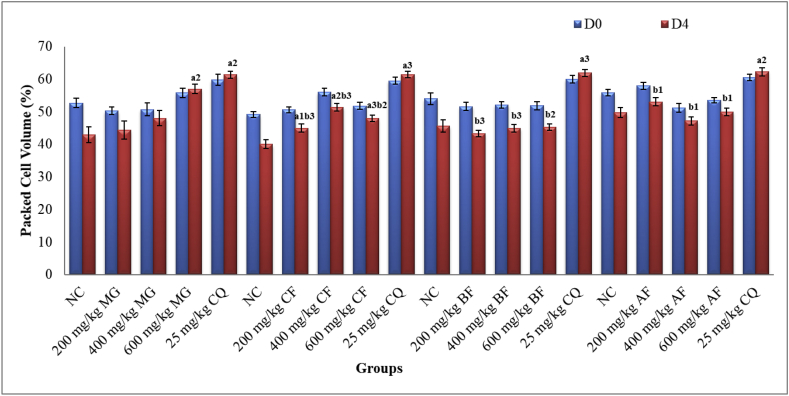
As reported in Figure 2, only 600 mg/kg of crude extract and 200, 400 and 600 mg/kg of chloroform fraction brought significant (p < 0.01, p < 0.05, p < 0.01, and p < 0.001, respectively) attenuation of anemia as compared to the NC. Although the effects by all fractions were lower than the effects obtained by CQ, no noticeable changes were seen with any dose of butanol and chloroform fraction as compared to the respective negative control.
Figure 2.
Effect of Maytenus gracilipes Hydromethanolic Extract and Its Fractions on Packed Cell Volume of Mice Infected with P. berghei in the Four-day Suppressive Test. Results are articulated as mean ± SEM; n = 6; a = compared to NC; b = to 25 mg/kg CQ; d = to 400 mg/kg; e = to 600 mg/kg; 1p < 0.05, 2p < 0.01, 3p < 0.001; NC = negative control (distilled water/2% tween-80), MG = crude extract of Maytenus gracilipes, CF = chloroform fraction, BF = butanol fraction, AF = aqueous fraction, CQ = chloroquine, D0 = pre -treatment value on day 0, D4 = post-treatment value on day 4.
4.4. Effect of the hydromethanolic extract in Rane's test
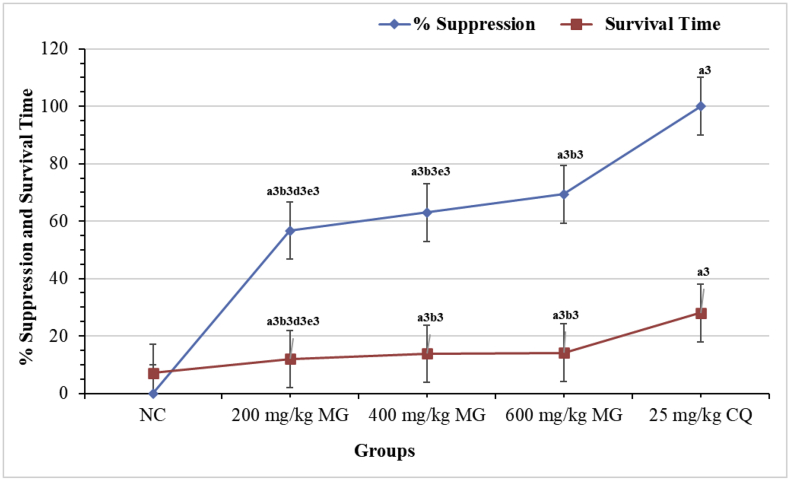
The significant antiplasmodial activity demonstrated by the crude extract in a 4-day suppressive study was additionally assessed for curative activity. All the test doses of the 80% methanol extract showed a dose-dependent and significant (p < 0.001) curative activity as compared to the negative control as displayed in Figure 3. The reduction in parasitemia caused by the standard drug was considerably (p < 0.001) different as compared to the NC and all treated groups. The MST was also altered notably by all doses of the extract as compared to the negative control. Statistically noteworthy (p < 0.001) extended survival day was as well found with regard to the comparison of the lowest dose with respect to the middle and highest doses. However, the raise attained by the extract was not matched to that of CQ (Figure 3).
Figure 3.
Effect of the Hydromethanolic Extract of Maytenus gracilipes on Percentage Suppression and Survival Time of Mice Infected with P. berghei in the Rane's Test. Results are articulated as mean ± SEM; n = 6; a = compared to NC; b = to 25 mg/kg CQ; c = to 200 mg/kg; d = to 400 mg/kg; e = to 600 mg/kg; 1p < 0.05, 2p < 0.01, 3p < 0.001; NC = negative control (distilled water), MG = crude extract of Maytenus gracilipes, and CQ = chloroquine.
The 80% methanolic extract of leaves of M. gracilipes had a protective activity in bodyweight reduction only at 600 mg/kg dose (Table 2). At middle (400) and highest (600) mg/kg doses, the extract exhibited a significant (p < 0.01 and p < 0.001, respectively) protective effect in rectal temperature reduction as compared to the negative control even though the effect by CQ was much more significant. When the lowest dose was compared to the middle and highest doses of the extract, a substantial (p < 0.01 and p < 0.001, respectively) difference was observed in the protection of rectal temperature reduction (Table 2).
Table 2.
Effect of Hydromethanolic Extract of Maytenus gracilipes leaves on Body weight and Rectal Temperature of Mice Infected with P. berghei in the Rane's Experiment.
| Groups | Body weight (g) |
Rectal temperature (0C) |
||||
|---|---|---|---|---|---|---|
| D3 | D7 | %Change | D3 | D7 | %Change | |
| NC | 30.48 ± 1.43 | 26.52 ± 1.45 | −13.15 | 36.90 ± 0.47 | 33.10 ± 0.26 | −10.25 |
| 200 mg/kg MG | 30.20 ± 2.04 | 27.25 ± 1.40 | −9.19 | 36.95 ± 0.21 | 33.32 ± 0.30 | −9.83b3d2e3 |
| 400 mg/kg MG | 28.35 ± 0.96 | 26.17 ± 1.32 | −7.90 | 37.07 ± 0.20 | 35.15 ± 0.26 | −5.18a2b1 |
| 600 mg/kg MG | 27.27 ± 1.28 | 25.63 ± 1.48 | −6.13a1 | 37.01 ± 0.15 | 36.10 ± 0.21 | −2.43a3 |
| 25 mg/kg CQ | 31.03 ± 0.82 | 30.13 ± 0.58 | −2.79a1 | 37.95 ± 0.11 | 37.45 ± 0.25 | −1.31a3 |
Results are articulated as mean ± SEM; n = 6; a = compared to NC; b = to 25 mg/kg CQ; d = to 400 mg/kg; e = to 600 mg/kg; 1p < 0.05, 2p < 0.01, 3p < 0.001; NC = negative control (distilled water), MG = crude extract of Maytenus gracilipes, and CQ = chloroquine, D3 = pre-treatment value on day 3, D7 = post-treatment value on day 7.
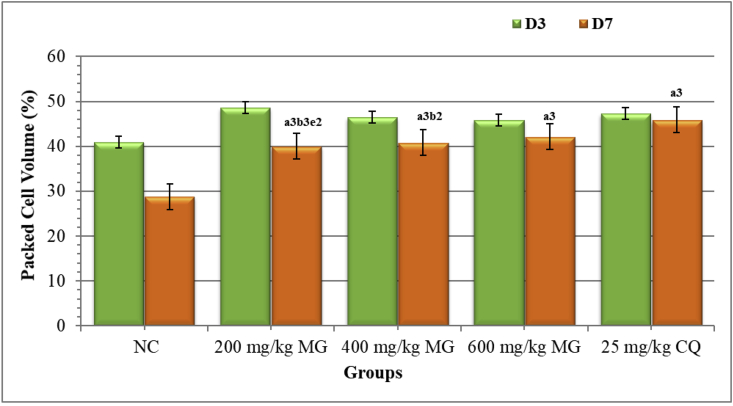
The results of PCV demonstrated that all doses of the crude extract had a considerable (p < 0.001) preventive effect against anemia in mice as compared to the NC. Compared to the lowest and middle doses of the hydroalcoholic extract, the positive control exhibited a substantial (p < 0.001 and p < 0.01, respectively) preventive effect in PCV decline. But the highest dose of the extract showed an effect that was comparable to the standard drug (Figure 4).
Figure 4.
Effect of Maytenus gracilipes on Packed Cell Volume of Mice Infected with P. berghei in the Rane's Assessment Model. Results are articulated as mean ± SEM; n = 6; a = compared to NC; b = to 25 mg/kg CQ; d = to 400 mg/kg; e = to 600 mg/kg; 1p < 0.05, 2p < 0.01, 3p < 0.001; NC = negative control (distilled water), MG = crude extract of Maytenus gracilipes, and CQ = chloroquine, D3 = pre-treatment value on day 3, D7 = post-treatment value on day 7.
4.5. Effect of the hydromethanolic extract in repository Assessment Model
The standard drug and all doses of the crude extract inhibited parasitemia significantly (p < 0.001) as compared to the negative control (Table 3). Maximum (93.82%) parasitemia suppression was attained by the CQ as compared to all doses of the extract, even if total eradication was not achieved. MST of the infected mice in the prophylactic test showed that only CQ was competent in prolonging survival period as compared to the negative control with P-value < 0.001. Significantly (p < 0.001) prolonged MST was also observed in CQ treated group as compared to 200, 400, and 600 mg/kg of the crude extract (Table 3).
Table 3.
Effect of the Hydromethanolic Extract of Maytenus gracilipes on Percentage Suppression and Survival Time of Mice infected with P. berghei in the Prophylactic Study.
| Groups | % Parasitemia | % Suppression | Survival Time |
|---|---|---|---|
| NC | 27.31 ± 1.51 | 0.00 ± 0.00 | 7.42 ± 0.15 |
| 200 mg/kg MG | 23.27 ± 0.33 | 14.81 ± 1.19a3b3 | 7.75 ± 0.21b3 |
| 400 mg/kg MG | 23.05 ± 0.28 | 15.60 ± 1.01a3b3 | 7.92 ± 0.15b3 |
| 600 mg/kg MG | 21.86 ± 0.76 | 19.97 ± 2.79a3b3 | 8.00 ± 0.37b3 |
| 25 mg/kg CQ | 1.69 ± 0.36 | 93.82 ± 1.32a3 | 19.00 ± 0.45a3 |
Results are articulated as mean ± SEM; n = 6; a = compared to NC; b = to 25 mg/kg CQ; 1p < 0.05, 2p < 0.01, 3p < 0.001; NC = negative control (distilled water), MG = crude extract of Maytenus gracilipes, and CQ = chloroquine.
Positive control and highest dose of the crude extract displayed a significant (p < 0.01 and p < 0.05, respectively) attenuating effect in body weight decline as compared to the placebo drug (Table 4). The lowest (200 mg/kg) and middle (400 mg/kg) doses of the crude extract had significant (p < 0.01 and p < 0.05, respectively) lower protective effects in weight reduction as compared to CQ, although the effect of the highest dose of the plant extract was comparable with the CQ. Both CQ and 600 mg/kg dose of the plant extract were substantially (p < 0.001 and p < 0.05, respectively) prevented PCV reduction as compared to the placebo treatment/negative control. Moreover, the standard treatment had noteworthy capacity in protection of anemia as compared to the lower (p < 0.01) and middle (p < 0.05) doses of the crude extract but produced a comparable effect as compared to 600 mg/kg dose (Table 4).
Table 4.
Effect of Maytenus gracilipes on Body weight, Rectal Temperature and Packed Cell Volume of Mice Infected with P. berghei in the Prophylactic Test.
| Groups | Body weight (g) |
Rectal temperature (0C) |
Packed cell volume (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| D3 | D7 | % Change |
D3 | D7 | % Change | D3 | D7 | % Change | |
| NC | 29.60 ± 1.86 | 27.67 ± 1.93 | −6.73 | 36.20 ± 0.35 | 35.25 ± 0.28 | −2.61 | 49.15 ± 1.84 | 46.36 ± 1.35 | −5.51 |
| 200 mg/kg MG | 28.52 ± 1.09 | 26.82 ± 1.02 | −5.90b2 | 35.60 ± 0.50 | 34.95 ± 0.42 | −1.81 | 58.97 ± 1.07 | 56.18 ± 1.06 | −4.73b2 |
| 400 mg/kg MG | 32.75 ± 2.35 | 31.30 ± 2.22 | −4.35b1 | 36.50 ± 0.21 | 36.15 ± 0.35 | −0.96 | 48.99 ± 1.31 | 47.26 ± 1.30 | −3.54b1 |
| 600 mg/kg MG | 29.55 ± 1.81 | 28.80 ± 1.76 | −2.50a1 | 36.85 ± 0.46 | 36.65 ± 0.30 | −0.49 | 50.98 ± 1.61 | 50.07 ± 1.81 | −1.84a1 |
| 25 mg/kg CQ | 31.95 ± 1.79 | 31.90 ± 1.76 | −0.13a2 | 37.05 ± 0.19 | 37.00 ± 0.19 | −0.13 | 48.88 ± 1.25 | 48.94 ± 1.08 | 0.17a3 |
Results are articulated as mean ± SEM; n = 6; a = compared to NC; b = to 25 mg/kg CQ; 1p < 0.05, 2p < 0.01, 3p < 0.001; NC = negative control (distilled water), MG = crude extract of Maytenus gracilipes, and CQ = chloroquine, D3 = pre-infection value on day 3, D7 = post-infection value on day 7.
4.6. Phytochemical analysis
The preliminary phytochemical analysis of the crude extract revealed the presence of the tested metabolites except for anthraquinones and terpenoids. Flavonoids and tannins were noticed in all fractions (devoid of anthraquinones), whereas alkaloids and terpenoids were detected in the chloroform fraction. The reason for terpenoids detection in chloroform fraction but not in crude extract could be the presence of this metabolite in dispersed form within the crude extract while more of it partition and concentrated into chloroform fraction during fractionation process. Moreover, steroids were detected in the n-butanol fraction, and saponins were detected in the aqueous fraction (Table 5).
Table 5.
Phytochemical Screening of Crude extract and Solvent fractions of leaves of Maytenus gracilipes.
| Secondary metabolites | Crude extract | Chloroform fraction | Butanol fraction | Aqueous fraction |
|---|---|---|---|---|
| Alkaloids | + | + | − | − |
| Anthraquinones | − | − | − | − |
| Flavonoids | + | + | + | + |
| Saponins | ++ | − | − | + |
| Steroids | + | − | + | − |
| Tannins | ++ | + | + | + |
| Terpenoids | − | + | − | − |
++ = strong positive, + = fair positive, - = absent.
5. Discussion
Given that drug-resistant malaria is becoming a serious public health concern in developing countries, novel antiplasmodial drugs are urgently needed [47]. The most essential source for creating novel antimalarial chemicals has been proved to be medicinal plants [48]. As a result, studies including the screening of traditionally claimed therapeutic plants may yield a potential lead compound. Non-primate animals are unable to be infected by Plasmodium species that cause sickness in humans. As a result, rodent parasites are used to test antiplasmodial agents in vivo [49]. The rodent malaria model has been successfully validated by testing a wide range of antiplasmodial drugs [39]. In the current study, an in vivo study was chosen because, in comparison to an in vitro study, any pro-drug effect and the likelihood of the immune system controlling infection are taken into account [36]. In mice, P. berghei has been used to test the antimalarial activity of novel agents. This is due to the parasite's non-adherence to endothelium cells, which allows all phases of its life cycle to be seen clearly on smears [33]. The 4-day suppressive test is the standard and extensively used malaria model for screening novel antiplasmodial compounds. In both chemosuppressive and rane's tests, the determination of percentage parasitemia is the most reliable parameter [50].
A universal solvent which is known as methanol is routinely used in the phytochemical extraction of plant materials. Mixtures of solvents frequently alcohol and water are commonly employed for plant constituent extractions that are soluble in water including polysaccharides, polypeptides, and tannins. A mixture of water and alcohol has the capacity to extract most of the non-polar and polar components of the plant parts [51]. Thus, in this experiment, the hydroalcoholic solvent was chosen to serve as a solublising agent. The oral route was used to administer the hydroalcoholic extract and fractions of the plant sample to duplicate the ethnomedical technique of administration and the likely route during clinical evaluation [48]. A hydromethanolic leaf extract of M. gracilipes did not cause any noticeable damage in the experimental female mice (more sensitive than male mice) in the acute oral toxicity assay at 2,000 mg/kg. Previous research has shown that if a test substance's LD50 is three times higher than the minimum effective dose, the extract is a viable candidate for further inquiry [44, 52]. This could explain the safe use of the plant to treat malaria by the local society of Ethiopia.
The antiplasmodial effects of the hydroalcoholic extract and solvent fractions of M. gracilipes leaves were evaluated using three malaria models in male mice. In the 4-day suppressive test, the percentage parasitemia was reduced in a dose-dependent manner by the crude extract and solvent fractions. This dose-dependent manner of antiplasmodial activity was reported in other findings done on different plants [14, 53]. However, the crude extract produced the highest parasitemia suppression than the solvent fractions, which is in line with leaves extract of Hypoestes forskalei [53], Vernonia amygdalina [54], and Ajuga integrifolia [55]. This could be contributed by the presence of most phytoconstituents (also in high concentration) in the crude extract than its solvent fractions as revealed in the phytochemical screening test and probably synergism activity. Furthermore, some secondary metabolites protect other metabolites, and breaking this bond could hasten degradation and, as a result, reduce the suppressive impact of the compounds infractions [56]. Chloroform fraction exhibited more chemo-suppressive activity than n-butanol and aqueous fraction. This finding is in agreement with the study conducted on solvent fractions of V. amygdalina [54]. The highest activity by chloroform fraction could be brought through the presence of more secondary metabolites than the remaining fractions or it could be explained that non-polar components of this plant are more active than its semi-polar and polar components. Overall, maximum (74.15%) suppression of parasitemia was attained by the highest dose of the crude extract. Similar results were displayed in other studies [57, 58]. If a compound produced 30% or more parasitemia suppression, it is considered as active [59], which supports the results of this study. Thus, we could say that leaves of M. gracilipes possessed antimalarial activity. This assertion is further evidenced by in vivo study that reported antiplasmodial activity of other species of the same genus Maytenus senegalensis [60]. Malaria is frequently treated with drugs that have antibacterial properties. In this sense, the study plant showed antibacterial activity in vitro [23]. This finding adds to the evidence that the study samples have anti-plasmodial in nature. The rank order of activity in parasitemia suppression was hydroalcoholic extract > chloroformfraction > butanolfraction > aqueous fraction.
As it has been displayed in the result section that the crude extract produced the highest parasite suppression in the 4-day suppressive test, its curative effect on established infection was further evaluated in rane's test. Like the effect of Schinus molle seed extract [61], the 80% methanolic extract of M. gracilipes displayed noteworthy parasitemia suppression in a dose-dependent way. This verifies that the plant sample has effective antimalarial activity in the late stages of the infection. The highest activity (69.36%) was observed at the maximum dose administered. This finding is concordant with another study [61] which has shown 69.86% parasitemia suppression. Since it is desirable to have both curative and suppressive activities by a phyto-drug, it is possible to consider this plant as a potential source of antiplasmodial agents [62]. The curative effect of the current plant was lower than its suppressive effect, which is consistent with the methanol extract of Piper betle [63] and Croton macrostachyus leaves [50]. Since the curative potential of the crude extract was promising, the prophylactic activity was further evaluated using the repository test. However, the suppression of parasitemia proliferation was found to be less than 30% which indicates that the plant extract has no chemoprophylactic activity.
The antimalarial activity of traditionally claimed plant extracts was also evaluated by the MST of the mice infected with the malaria parasite [32]. In this experiment, significantly prolonged survival time was obtained by all doses of the crude extract and two solvent (chloroform and n-butanol) fractions in reference to the negative control in the 4-day suppressive test. In aqueous fraction, the highest dose was significantly (p < 0.001) prolonged MST as compared to the vehicle-treated group. In the curative test, all doses of the crude extract significantly increased MST as compared to the negative control. In both animals, MST lengthening may be directly associated with percent parasitemia suppression and overall improvement in pathogenic effect imposed by the test doses [35, 43]. This effect adds to the evidence that the plant's extract has antiplasmodial properties. The plant extract is considered active if it extends MST beyond 12 days [64]. However, the MST of the mice treated with the standard drug was significantly higher than the entire doses of extract-treated groups in both models; his could be related to the extracts' low potency or the short elimination phase [65].
Body weight loss, hypoglycemia, reduction in body temperature, and anemia (decline in PCV) are primary symptoms of malaria-infected mice [66]. The plant extract with antimalarial activity is expected to prevent malaria-caused reduction of body weight, temperature, and PCV due to the rise in parasitemia. Despite significant parasite suppression, the crude extract and solvent fractions of M. gracilipes could not prevent body weight loss in early infection. On the contrary, the crude extract prevented body weight loss (only at 600 mg/kg dose) in P. berghei infected mice during established infection (rane's test) in spite of higher parasitemia suppression occurred at 4-day chemosuppressive test. This indicates the involvement of other factors beyond malaria infection like catabolic effect on stored lipids or anorexigenic effect, which could have resulted in reduced food intake due to the presence of appetite-suppressant metabolites (saponins and tannins) in this plant material [65]. This finding is consistent with the effect of Leonoti socymifolia, Myrica salicifolia, and Calpurnia aurea that the test samples could not prevent body weight loss, despite the fact that parasitemia was significantly reduced [16, 32, 67]. In the residual infection test, only the highest dose of the crude extract averted a decline in body weight. This effect might be correlated to parasitemia suppression.
Infected mice experience a decrease in metabolic rate prior to death, which is accompanied by a similar decrease in internal body temperature, in contrast to human beings [43, 44]. Potent antiplasmodial plant materials are expected to prevent the rapid decline of rectal temperature in mice infected with malaria parasites. In the four-day suppressive test, both chloroform and aqueous fractions had a protective effect against temperature drop. Despite its superior parasitemia suppression in early infection tests, the crude extract averted a drop in body temperature (at higher doses) in established infection tests. This effect is in contrast to the effect shown in the 4-day suppressive test and indicates the presence of other contributing factors than parasite load [32]. This bioactivity might be due to the ability of the plant to attenuate some pathological processes that lead to a reduction in body temperature or the fall in metabolic rates that occur because of increased parasite levels [43]. All doses of the crude extract did not prevent body temperature decline at the prophylactic test.
In the current chemo-suppressive investigation, the highest dose of the crude extract as well as all doses of chloroform fractions prevented PCV reduction significantly as compared to the respective negative control. This could be attributed to the better suppression of parasitemia than butanol and aqueous fractions. Actually, the chloroform fraction demonstrated a superior effect in preventing anemia than the hydroalcoholic extract which might indicate the presence of hemoprotective compounds in high concentration within the chloroform fraction. When an increase in activity as a dose was also considered, the effect produced was similar to that of C. macrostachyus and A. integrifolia [50, 55]. More than the effect produced at the 4-day suppressive test, the crude extract averted reduction of PCV in study mice at all doses as compared to the untreated group in the curative test. This could be due to the antagonistic effect of the plant on oxidative stress to protect the occurrence of anemia secondary to dys-erythropoiesis, in so doing sustaining the availability of new RBCs produced and released from bone marrow [58]. In the repository test, only the highest dose of the crude extract protected the decline in PCV of the study mice.
The literature rates a compound's antimalarial activity as moderate, good, or very good if it inhibits parasite growth by more than 50% at doses of 500, 250, and 100 mg/kg/day, respectively [68]. As a result, the study plant's leaf extract had good antiplasmodial activity. Maytenus species are renowned for their anti-inflammatory properties [69], which may potentially be responsible for the study plant's antimalarial activity. Traditional remedies' therapeutic properties are frequently related to the existence of non-nutritive bioactive ingredients [70]. Although the active metabolite has yet to be identified, M. gracilipes' antiplasmodial activity could be attributed to one or more of its secondary metabolites (Table 5). These phytochemicals (alkaloids, flavonoids, saponins, steroids, tannins, and terpenoids) might have been responsible for the antimalarial activity of the experimental plant as suggested in other studies [62, 71, 72]. The parasite-suppressing effect of the leaves extract and solvent fractions in this study could be due to an indirect boost of the mice's immune system or inhibition of other yet-to-be-identified target pathways [73]. In other plants, phytosteroids, flavonoids, and saponins have been shown to have immunomodulatory properties [74, 75]. Saponins and tannins from our study plant, like those from other medicinal plants, may have antiparasitic effect against Plasmodium due to their antioxidant properties [71, 76]. Moreover, the presence of more than one class of phytochemicals in a given plant extract determines the nature and extent of its biological activity [77].
6. Conclusion
The findings of the acute oral toxicity test demonstrate that the investigational plant was non-toxic to mice. However, further toxicity tests should be carried out in the future. In general, the current findings revealed that an 80% methanol extract and solvent fractions of M. gracilipes leaves have antimalarial activity. The study found that the experimental plant has a significant suppressive effect. Among the solvent fractions, chloroform and n-butanol were shown to be active, showing that the plant's non-polar and medium-polar compounds are responsible for M. gracilipes' antiplasmodial properties. The data would back up the claims made by the Ethiopian traditional medical practitioner.
Ethical approval
The research proposal was reviewed and approved by the Mizan-Tepi University Research and Community Service office. All of the experiments were carried out in compliance with international standards for the use, treatment, and handling of laboratory animals.
Declarations
Author contribution statement
Dejen Nureye: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Muktar Sano Kedir: Conceived and designed the experiments; Performed the experiments.
Rekik Ashebir Muluye: Performed the experiments; Contributed reagents and materials.
Workineh Woldeselassie Hammeso: Performed the experiments; Analyzed and interpreted the data.
Eyob Tekalign: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Funding statement
This work was supported by the Mizan-Tepi University.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The kind involvement of Mr. Kifle Ambelo (traditional healer from Sheka zone) in plant material collection deserves acknowledgment. The authors would like to extend their appreciation to EPHI for giving us laboratory materials including P. berghei infected donor mice and non-infected study mice. We would also like to thank Mrs. Tsion kerango, Mr. Bedilu Mersha and Mr. Esleman Yessuf for their technical support in the laboratory.
References
- 1.News-Medical Net. Malaria. AZoNetwork. 2021 http://www.news-medical.net Available online from: [Google Scholar]
- 2.World Health Organization . World Health Organization; 2021. Malaria. Released on April 1, 2021. [Google Scholar]
- 3.Kendie F.A., W/kiros T.H., Semegn E.N., Ferede M.W. Prevalence of malaria among adults in Ethiopia: a systematic review and meta-analysis. J. Trop. Med. 2021;2021:1–9. doi: 10.1155/2021/8863002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Popovici J., Pierce-Friedrich L., Kim S., et al. Recrudescence, reinfection, or relapse? A more rigorous framework to assess chloroquine efficacy for Plasmodium vivax malaria. J. Infect. Dis. 2019;219(2):315–322. doi: 10.1093/infdis/jiy484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menard D., Dondorp A. Antimalarial drug resistance: a threat to malaria elimination. Cold Spring Harb. Perspect. Med. 2017;7:a025619. doi: 10.1101/cshperspect.a025619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO . World Health Organization; Geneva, Switzerland: 2010. Global Report on Antimalarial Drug Efficacy and Drug Resistance: 2000–2010. [Google Scholar]
- 7.Kuehn B.M. Drug-resistant malaria detected in Africa will require monitoring. J. Am. Med. Assoc. 2021;325(23):2335. doi: 10.1001/jama.2021.9133. [DOI] [PubMed] [Google Scholar]
- 8.Prasad D.M.R., Izam A., Khan M.R. Jatropha curcas: plant of medical benefits. J. Med. Plants Res. 2012;6(14):2691–2699. [Google Scholar]
- 9.Traditional W.H.O. World Health Organization; Geneva, Switzerland: 2002. Medicine Strategy 2002-2005. [Google Scholar]
- 10.Rasoanaivo P., Wright C.W., Willcox M.L., Gilbert B. Whole plant extracts versus single compounds for the treatment of malaria: synergy and positive interactions. Malar. J. 2011;10(S1):S4. doi: 10.1186/1475-2875-10-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguiar A.C., Rocha E.M., Souza N.B., França T.C., Krettli A.U. New approaches in antimalarial drug discovery and development: a review. Mem. Inst. Oswaldo Cruz. 2012;107(7):831–845. doi: 10.1590/s0074-02762012000700001. [DOI] [PubMed] [Google Scholar]
- 12.Asmare A., Kesara Na-B. A review of ethnopharmacology of the commonly used antimalarial herbal agents for traditional medicine practice in Ethiopia. Afr. J. Pharm. Pharmacol. 2015;9(25):615–627. [Google Scholar]
- 13.Alebie G., Urga B., Worku A. Systematic review on traditional medicinal plants used for the treatment of malaria in Ethiopia: trends and perspectives. Malar. J. 2017;16:307. doi: 10.1186/s12936-017-1953-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nureye D., Assefa S., Nedi T., et al. Vivo antimalarial activity of the 80% methanolic root bark extract and solvent fractions of Gardenia ternifolia schumach. & thonn. (Rubiaceae) against Plasmodiumberghei. Evid. Based Compl. Alternative Med. 2018;20 18:1–10. doi: 10.1155/2018/9217835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gebrehiwot S., Giday M., Erko B., Mekonnen Y. Evaluation of antimalarial activity of a traditionally used medicinal plant in Ethiopia against Plasmodium berghei in Swiss Albino Mice. IOSR J. Pharm. Biol. Sci. 2019;14(1):70–76. [Google Scholar]
- 16.TekluT Engidawork E., Nedi T., Teklehaymanot T., Gebremeskel L. Evaluation of the antimalarial activity of the hydroalcoholic extract of leaf of Leonoti socymifolia (burm. F.) iwarsson (Lamiaceae) against plasmodium berghei in mice. Evid. Based Compl. Alternative Med. 2020;2020:1–8. doi: 10.1155/2020/5384804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nureye D., Tekalign E., Fisseha N., Tesfaye T., Hammeso W.W. Evaluation of antiplasmodial activity of hydroalcoholic crude extract and solvent fractions of Zehneria scabra roots against plasmodium berghei in Swiss albino mice. Infect. Drug Resist. 2021;14:2583–2596. doi: 10.2147/IDR.S314262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeshanew S., Gete W., Chilo D. Evaluation of the antimalarial activity of ethanol extracts of the leaves of three plant species collected from yayu coffee forest biosphere reserve, southwest Ethiopia. J. Exp. Pharmacol. 2021;13:661–668. doi: 10.2147/JEP.S304933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nureye D., Sano M., Fekadu M., Duguma T., Tekalign E. Antiplasmodial activity of the crude extract and solvent fractions of stem barks of Gardenia ternifolia in plasmodium berghei-infected mice. Evid. base Compl. Alternative Med. 2021;2021:1–16. doi: 10.1155/2021/9625169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayele M. Addis Ababa University; 2015. Evaluation of Acute and Sub-chronic Toxicity of Aqueous Leaves Extracts of Maytenus Gracilipes Celastraciae (Kombolcha) on Some Blood Parameters and Histopathology of Liver and Kidney in Swiss Albino mice[MSc Thesis] [Google Scholar]
- 21.Abebe D., Gardew B. Ethnobotanical survey of plants traditionally used for malaria prevention and treatment in indigenous villages of Tepi Town South West Ethiopia. J. Pharmacogn. Phytotherapy. 2019;11(1):9–16. [Google Scholar]
- 22.Yineger H., Kelbessa E., Bekele T., et al. Plants used in traditional management of human ailments at Bale mountains national park, southeastern Ethiopia. J. Med. Plants Res. 2008;2(6):132–153. [Google Scholar]
- 23.Yessuf E., Adugna E., Nureye D., et al. In-vitro antibacterial activity of the 80% methanolic leaf extract of Maytenus gracilipes (welw. Ex Oliv) Exell. (celastraciae) against some pathogenic bacteria. Int. J. Med. Res. Health Sci. 2021;10(3):91–100. [Google Scholar]
- 24.Amare G.G., Degu A., Njogu P., Kifle Z.D. Evaluation of the antimalarial activity of the leaf Latex of aloe weloensis (aloaceae) against plasmodium parasites. Evid. base Compl. Alternative Med. 2021;2021:1–8. doi: 10.1155/2021/6664711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Research Council . eighth ed. The National Academies Press; Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals.https://www.national-academies.org Available online from: [Google Scholar]
- 26.Parekh J., Jadeja D., Chanda S. Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turk. J. Biol. 2005;29:203–210. [Google Scholar]
- 27.Sasidharan S., Chen Y., Saravanan D., Sundram K.M., Yoga Latha L. EXTRACTION, isolation and characterization OF bioactive compounds from plants’ extracts. Afr. J. Tradit., Complementary Altern. Med. 2011;8(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- 28.Fonmboh D.J., Abah E.R., Fokunang T.E., et al. An overview of methods of extraction, isolation and characterization of natural medicinal plant products in improved traditional medicine research. Asian J. Res. Med. Pharmaceut. Sci. 2020;9(2):31–57. [Google Scholar]
- 29.Innocent E., Moshi M.J., Masimba P.J., et al. Screening of traditionally used plants for in vivo antimalarial activity in mice. Afr. J. Tradit., Complementary Altern. Med. 2009;6(2):163–167. doi: 10.4314/ajtcam.v6i2.57088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiwari P., Kumar B., Kumar M., et al. Phytochemical screening and extraction: a review. IPS. 2011;1:98–106. [Google Scholar]
- 31.Guideline O.O. 425: acute oral toxicity—up-and-down procedure. OECD Guidel. Test. Chem. 2001;2:12–16. [Google Scholar]
- 32.Kifle Z.D., Adinew G.M., Mengistie M.G., et al. Evaluation of antimalarial activity of methanolic root extract of Myrica salicifolia A rich (myricaceae) against Plasmodium berghei–infected mice. J. Evid. Based Integ. Med. 2020;25:1–12. doi: 10.1177/2515690X20920539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang W., Emily P., Charles C. Mouse models of uncomplicated and fatal malaria. BioProtoc. 2015;5(13):e1514. doi: 10.21769/bioprotoc.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deressa T., Yalemtsehay M., Abebe A. Vivo anti-malarial activities of Clerodendrum myricoides, Dodonea angustifolia and Aloe debrana against Plasmodium berghei. Ethiop. J. Health Dev. 2010;24(1):25–29. [Google Scholar]
- 35.Basir R., Rahiman S.F., Hasballah K., et al. Plasmodium berghei ANKA infection in ICR mice as a model of cerebral malaria. Iran. J. Parasitol. 2012;7(4):62–74. [PMC free article] [PubMed] [Google Scholar]
- 36.Waako P., Gumede B., Smith P., et al. The in vitro and in vivo antimalarial activity of Cardiospermum halicacabum and Momordica foetida. J. Ethnopharmacol. 2005;99:137–143. doi: 10.1016/j.jep.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 37.David A.F., Philip J.R., Simon L.C., Reto B., Solomon N. Antimalarial drug discovery: efficacy models for compound screening. Nat. Rev. 2004;3:509–520. doi: 10.1038/nrd1416. [DOI] [PubMed] [Google Scholar]
- 38.Peters W. The four-day suppressive in vivo antimalarial test. Ann. Trop. Med. Parasitol. 1975;69:155–171. [PubMed] [Google Scholar]
- 39.Ryley J., Peters W. The antimalarial activity of some quinolone esters. Ann. Trop. Med. Parasitol. 1970;64(2):209–222. doi: 10.1080/00034983.1970.11686683. [DOI] [PubMed] [Google Scholar]
- 40.Peters W. Drug resistance in plasmodium berghei. Exp. Parasitol. 1965;17:80–89. doi: 10.1016/0014-4894(65)90012-3. [DOI] [PubMed] [Google Scholar]
- 41.Aarthi N., Murugan K. Antimalarial activity and phytochemical screening of ethanolic leaf extract of Phyllanthus niruri and Mimosa pudica. Int. J. Pharmaceut. Res. Dev. 2011;3:24. [Google Scholar]
- 42.Adumanya O., Uwakwe A., Essien E. Antiplasmodial activity of methanol leaf extract of Salacia senegalensis Lam (Dc) in Albino Mice infected with chloroquine-sensitive Plasmodium berghei (NK65) Int. J. Ethnopharmacol. 2014;1(1):2–6. [Google Scholar]
- 43.Mengiste B., Makonnen E., Urga K. In vivo antimalarial activity of Dodonaea angustifolia seed extracts against Plasmodium berghei in mice model. Momona Ethiop. J. Sci. 2012;4:47–63. [Google Scholar]
- 44.Dikasso D., Makonnen E., Debella A., et al. In vivo anti-malarial activity of hydroalcoholic extracts from Asparagus africanus Lam. in mice infected with Plasmodium berghei. Ethiop. J. Health Dev. 2006;20:112–118. [Google Scholar]
- 45.Debella A. Manual for Phytochemical Screening of Medicinal Plants. Department of drug research, Ethiopia health and nutrition research institute; 2002. Preliminary screening techniques of secondary metabolites; pp. 38–57. [Google Scholar]
- 46.Njoku O., Obi C. Phytochemical constituents of some selected medicinal plants. Afr. J. Pure Appl. Chem. 2009;3(11):228–233. [Google Scholar]
- 47.Ukpai O., Amaechi E. Evaluation of in vivo antimalarial activity of the ethanolic leaf extracts of Chromolaena odorata and Cymbopogon citratus in mice. Nig. J. Biotech. 2012;24:27–34. [Google Scholar]
- 48.Adzu B., Abdul K., Oluwakanyinsola A., et al. In vivo antiplasmodial activity of ZS-2A: a fraction from chloroform extract of Zizyphus spina-christi root bark against Plasmodium berghei in mice. Int. J. Biol. Chem. Sci. 2007;1(3):281–286. [Google Scholar]
- 49.Kalra B., Chawla S., Gupta P., et al. Screening of antimalarial drugs: an overview. Indian J. Pharmacol. 2006;38(1):5–12. [Google Scholar]
- 50.Bantie L., Assefa S., Engdawork E., et al. In vivo antimalarial activity of the crude leaf extract and solvent fractions of Croton macrostachyus Hocsht. (Euphorbiaceae) against Plasmodium berghei in mice. BMC Compl. Alternative Med. 2014;14:79. doi: 10.1186/1472-6882-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Q.W., Lin L.G., Ye W.C. Techniques for extraction and isolation of natural products: a comprehensive review. Chin. Med. 2018;13:20. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Madara A., Tijani A., Nandi E. Anti-plamodial activity of ethanolic root bark extract of Piliostigm athonningii schum. (Caesalpiniacea) in mice infected with P. berghei NK 65. Rep. Opin. 2012;4(4):62–67. [Google Scholar]
- 53.Misganaw D., Amare G., Mengistu G. Chemo suppressive and curative potential of Hypoestes forskalei against plasmodium berghei: evidence for in vivo antimalarial activity. J. Exp. Pharmacol. 2020;12:313–323. doi: 10.2147/JEP.S262026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bihonegn T., Giday M., Yimer G., Animut A., Sisay M. Antimalarial activity of hydromethanolic extract and its solvent fractions of Vernonia amygdalina leaves in mice infected with Plasmodium berghei. SAGE Open Med. 2016;7:1–10. doi: 10.1177/2050312119849766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asnake S., Teklehaymanot T., Hymete A., et al. Evaluation of the antiplasmodial properties of selected plants in Southern Ethiopia. BMC Compl. Alternative Med. 2015;15(1):448. doi: 10.1186/s12906-015-0976-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Traoré M., Guiguemde A., Yago I., et al. Investigation of antiplasmodial compounds from two plants, Cochlospermum tinctorium A. Rich and Gardenia sokotensis hutch. Afr. J. Trad. CAM. 2006;3(4):34–41. [Google Scholar]
- 57.Mesfin A., Giday M., Animut A., Teklehaymanot T. Ethnobotanical study of antimalarial plants in Shinile District, Somali Region, Ethiopia, and in vivo evaluation of selected ones against Plasmodium berghei. J. Ethnopharmacol. 2012;139(1):221–227. doi: 10.1016/j.jep.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 58.Nureye D., Tekalign E., Fisseha N., et al. Evaluation of antiplasmodial activity of hydroalcoholic crude extract and solvent fractions of Zehneria scabra roots against plasmodium berghei in Swiss albino mice. Infect. Drug Resist. 2021;14:2583–2596. doi: 10.2147/IDR.S314262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adugna M. In vivo antimalarial activity of crude extract of aerial part of Artemisia abyssinica against Plasmodium berghei in mice. Global J. Pharmacol. 2014;8(3):460–468. [Google Scholar]
- 60.Malebo H.M., Wiketye V., Katani S.J., et al. In vivo antiplasmodial and toxicological effect of Maytenus senegalensis traditionally used in the treatment of malaria in Tanzania. Malar. J. 2015;14:79. doi: 10.1186/s12936-014-0525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mekuria A.B., Geta M., Birru E.M., Gelayee D.A. Antimalarial activity of seed extracts of Schinus molle against plasmodium berghei in mice. J. Evid. Based Integ. Med. 2021;26:1–10. doi: 10.1177/2515690X20984287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oliveira A., Dolabela M., Braga F., et al. Plant derived antimalarial agents: new leads and efficient phytomedicines. Ann. Braz. Acad. Sci. 2009;81:715–740. doi: 10.1590/s0001-37652009000400011. [DOI] [PubMed] [Google Scholar]
- 63.Al-Adhroey A., Nor Z., Al-Mekhlafi H., et al. Antimalarial activity of methanolic leaf extract of Piper betle L. Molecules. 2011;16:107–118. doi: 10.3390/molecules16010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ural I.O., Kayalar H., Durmuskahya C., Cavus I., Ozbilgin A. In vivo antimalarial activity of methanol and water extracts of Eryngium thorifolium Boiss (Apiaceae Family) against P. berghei in infected mice. Trop. J. Pharmaceut. Res. 2014;13:1313–1317. [Google Scholar]
- 65.Alehegn A.A., Yesuf J.S., Birru E.M. Antimalarial activity of crude extract and solvent fractions of the leaves of bersama abyssinica fresen. (Melianthaceae) against plasmodium berghei infection in Swiss albino mice. Evid. base Compl. Alternative Med. 2020;2020:1–14. doi: 10.1155/2020/9467359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Langhorne J., Quin S., Sanni L. Malaria Immunology, 2ndedition. Karger publisher; 2002. Mouse models of blood-stage malaria infections: immune responses and cytokines involved in protection and pathology; pp. 204–228. [DOI] [PubMed] [Google Scholar]
- 67.Eyasu M., Shibeshi W., Giday M. In vivo antimalarial activity of hydromethanolic leaf extract of Calpurnia aurea (Fabaceae) in mice infected with chloroquine sensitive Plasmodium berghei. Int. J. Pharmacol. 2013;2(9):131–142. [Google Scholar]
- 68.Munoz V., Sauvain M., Bourdy G., et al. A search for natural bioactive compounds in Bolivia through a multidisciplinary approach. Part I. Evaluation of the antimalarial activity of plants used by the Chacobo Indians. J. Ethnopharmacol. 2000;69:127–137. doi: 10.1016/s0378-8741(99)00148-8. [DOI] [PubMed] [Google Scholar]
- 69.Velosoa C.C., Soares G.L., Perez A.C., et al. Pharmacological potential of Maytenus species and isolated constituents, especially tingenone, for treatment of painful inflammatory diseases. Rev. Brasil. Farma. 2017;27:533–540. [Google Scholar]
- 70.Ghisalberti E. In: Bioactive Natural Products: Detection, Isolation, and Structural Determination. second ed. Steven M., Colegate S.M., Molyneux R.J., editors. CRC Press; North West: 2008. Detection and isolation of bioactive natural products; pp. 11–76. [Google Scholar]
- 71.Soh P., Witkowski B., Gales A., et al. Implication of glutathione in the in vitro antiplasmodial mechanism of action of Ellagic Acid. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arise R., Malomo S., Lawal M. Comparative antimalarial and toxicological effects of artemisinin with methanolic extract of carica papaya leaves and bark of alstonia broonai in animal models. Adv. Nat. Appl. Sci. 2012;6(2):116–123. [Google Scholar]
- 73.Muthaura C., Rukung G., Chhabra S., et al. Antimalarial activity of some plants traditionally used in treatment of malaria in Kwale district of Kenya. J. Ethnopharmacol. 2007;112:545–551. doi: 10.1016/j.jep.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 74.Aherne S., Daly T., Connor T., et al. Immunomodulatory effects of β-sitosterol on human Jurkat T cells. Planta Med. 2007;73(9):797–1034. [Google Scholar]
- 75.Saxena M., Saxena J., Nema R., et al. Phytochemistry of medicinal plants. J. Pharmacogn. Phytochem. 2013;1(6):168–182. [Google Scholar]
- 76.Alexandru V., Balan M., Gaspar A., et al. Antioxidant activity, phenolics and flavonoid content of some selected Romanian medicinal plants. Planta Med. 2007;73(9):797–1034. [Google Scholar]
- 77.Musila M., Dossaji S., Nguta J., et al. In vivo antimalarial activity, toxicity and phytochemical screening of selected antimalarial plants. J. Ethnopharmacol. 2013;146:557–561. doi: 10.1016/j.jep.2013.01.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.