Abstract
Drug transportation is impeded by various barriers in the hypoxic solid tumor, resulting in compromised anticancer efficacy. Herein, a solid lipid monostearin (MS)-coated CaO2/MnO2 nanocarrier was designed to optimize doxorubicin (DOX) transportation comprehensively for chemotherapy enhancement. The MS shell of nanoparticles could be destroyed selectively by highly-expressed lipase within cancer cells, exposing water-sensitive cores to release DOX and produce O2. After the cancer cell death, the core-exposed nanoparticles could be further liberated and continue to react with water in the tumor extracellular matrix (ECM) and thoroughly release O2 and DOX, which exhibited cytotoxicity to neighboring cells. Small DOX molecules could readily diffuse through ECM, in which the collagen deposition was decreased by O2-mediated hypoxia-inducible factor-1 inhibition, leading to synergistically improved drug penetration. Concurrently, DOX-efflux-associated P-glycoprotein was also inhibited by O2, prolonging drug retention in cancer cells. Overall, the DOX transporting processes from nanoparticles to deep tumor cells including drug release, penetration, and retention were optimized comprehensively, which significantly boosted antitumor benefits.
KEY WORDS: Tumor, Nanoparticle, Chemotherapy, Hypoxia, HIF-1, Transportation, CaO2, MnO2
Abbreviations: CTGF, connective tissue growth factor; DOX, doxorubicin; DSPE-PEG2000, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000]; ECM, extracellular matrix; EPR, enhanced permeability and retention; FBS, fetal bovine serum; HA, hyaluronic acid; HAase, hyaluronidase; HIF-1α, hypoxia-inducible factor 1α; MCTS, multicellular tumor spheroids; MS, monostearin; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NP, nanoparticle; OA, oleic acid; PDT, photodynamic therapy; P-gp, P-glycoprotein; TEM, transmission electron microscopy; TME, tumor microenvironment
Graphical abstract
Synergistic drug penetration, sufficient drug release, and prolonged drug retention were concurrently realized by a smart O2-generating nanocarrier, contributing to comprehensively optimized drug transportation and thereby improved chemotherapy.
1. Introduction
Despite the rapid development of innovative cancer therapeutic strategies such as photodynamic therapy (PDT), radiotherapy, and immunotherapy, chemotherapy is still the most common-used treatment in the clinic1. Unfortunately, due to nonselective drug distribution and poor pharmacokinetics, the administration of free drugs always results in unsatisfactory therapeutic outcomes and severe systemic cytotoxicity2. With the advent of nanotechnology, nanoscale drugs were developed to optimize drug pharmacokinetics3. However, it is a great challenge to realize the good performance of nanomedicine concurrently in the systemic circulation and inside the tumor4. For example, nanoparticle (NP) enables drug benefits of avoiding excretion and passive accumulation in solid tumor regions by the enhanced permeability and retention (EPR) effect but meanwhile impedes drug penetration through the extracellular matrix (ECM)5. In addition, negatively charged NPs undergo stable circulation in blood vessels and show safety to normal tissues, whereas positive charged ones behave better in cellular uptake in tumor tissues6. To reconcile these contrary requirements in one design, the strategy that constructing smart nanomedicine with tumor-responsive convertible capacity had been proposed to significantly elevate intratumoral activities of nanomedicines7.
Apart from the NP properties, unfavorable factors of tumor microenvironment (TME) also contribute to poor therapeutic outcomes8. Hypoxia, one of the most characteristic hallmarks of TME, was proved to play a crucial role in tumor progress and therapeutic resistance9. It has been reported that hypoxia upregulates hypoxia-inducible factor 1α (HIF-1α) and P-glycoprotein (P-gp), which mediates drug efflux and reduced chemotherapy10. As one of the main components in ECM, collagen is also expressed in an HIF-1 dependent manner11. Under hypoxic conditions, collagen deposition increases the density of ECM, which impedes the penetration of drugs and nanodrugs. In recent years, considerable efforts have been devoted to modulate the factors of TME and enhance therapeutic efficacy through various mechanisms. Particularly hypoxia, which regulates multiple genes and proteins, has been demonstrated to be an effective target for enhancing different therapeutic modalities including chemotherapy12, PDT13 and immunotherapy14.
Conceivably, smart NPs with both tumor-responsive size-convertible capacity and TME-modulation ability may realize synergistic effects in bolstering nanomedicine antitumor efficacy15,16. Recently, Cui et al.17 established a hyaluronidase (HAase)-modified ZnO NP to improve doxorubicin (DOX) chemotherapy. Hyaluronic acid (HA) is another main component of ECM that could be degraded by HAase to enhance nanodrug diffusion18. Through the pH-responsive size reduction of ZnO NPs and release of HAase, deep tumor penetration was realized due to the synergism of the neighboring effect and HAase-mediated HA degradation. However, in addition to tumor penetration, such NPs still face obstacles that reduce chemotherapy. First, most internal-stimulants (for example, H+ and glutathione) could be consumed in ZnO disintegration19, and thus the NPs might not able to realize sufficient drug release in TME. Second, the intracellular DOX could be pumped out from hypoxic cells by P-gp20. Hence, to further promote drug bioavailability and increase its anti-tumor effects, it is highly desirable to optimize drug transportation comprehensively (i.e., not only drug penetration but also sufficient drug release and drug retention) by constructing switchable NPs that can respond to an infinite stimulus (such as water) and modulate multi-targeted TME factors (such as hypoxia).
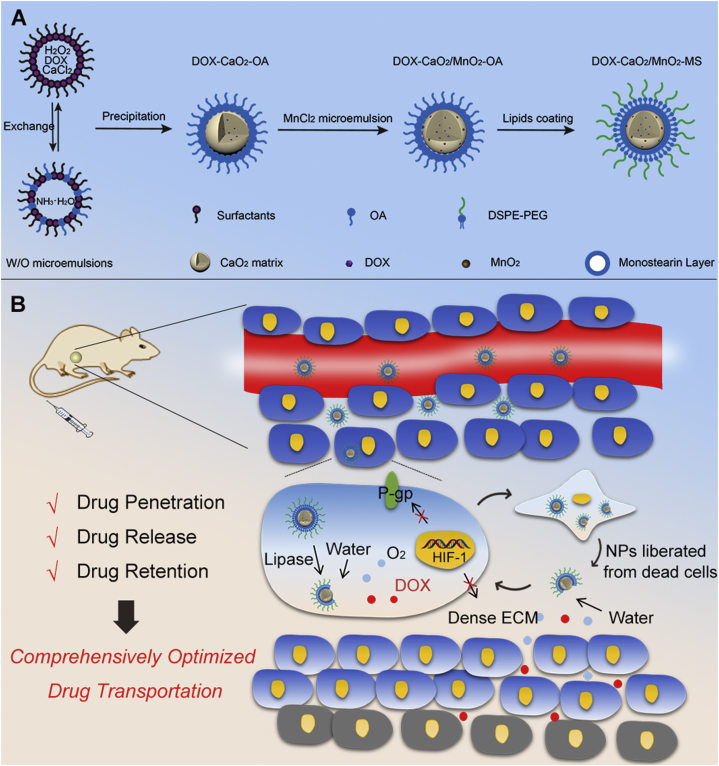
Herein, we designed an O2-generating CaO2/MnO2 nanocarrier to improve DOX transportation comprehensively and finally enhance chemotherapy. As shown in Scheme 1A, the DOX molecules were preloaded in CaO2 matrix by chelating with Ca2+ during the synthesis of CaO2 according to Eq. (1). After the addition of MnCl2, MnO2 was formed in situ on CaO2 surface according to Eq. (2). The obtained drug-loaded cores were then stabilized by a solid lipid monostearin (MS) and PEG-lipids, which could prevent potential drug leakage during the circulatory process and enable tumor lipase responsiveness of NPs21.
| (1) |
| (2) |
Scheme 1.
(A) Synthetic procedure of DOX-CaO2/MnO2-MS NPs; (B) Schematic diagram of DOX-CaO2/MnO2-MS NPs for comprehensively optimized drug transportation.
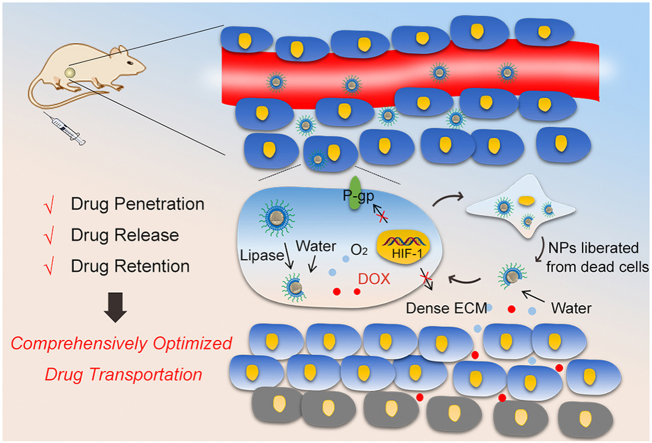
The anticancer mechanisms were proposed as following: (1) by EPR effects, the NPs passively accumulated in tumor sites and were taken up by tumor cells22; (2) the highly-expressed lipase in cancer cells disintegrated the MS layers of NPs and uncovered the active drug-loaded cores; (3) the exposed DOX-CaO2/MnO2 cores reacted with water according to Eqs. (3), (4), leading to the burst release of DOX and O2; (4) O2-mediated HIF-1/P-gp inhibition prolonged DOX retention in cancer cells for severer cell death; (5) The dead cells liberated core-exposed NPs, which continued to sufficiently release DOX and O2 molecules in the ubiquitous aqueous environment of ECM; (6) The molecular DOX could readily diffuse through ECM, and O2 synergistically improved drug penetration by decreasing the collagen deposition of ECM through HIF-1α degradation; (7) DOX and O2 reached to the deep tumor region and induced cell death as the step 4.
| (3) |
| (4) |
Apart from a drug delivery vehicle, CaO2 acted as the template and reactant of MnO2 synthesis, which greatly augmented the O2-producing efficiency of NPs. Through the rational design and construction of this smart/TME-modulated nanocarrier, sufficient drug release, synergistic drug penetration, and prolonged drug retention were concurrently realized, contributing to comprehensive optimization of drug transportation from NPs to deep tumor cells and finally improvement of chemotherapy. We envision that such solid lipid-coated CaO2/MnO2 nanocarriers also hold great potential in other O2-dependent cancer therapeutic modalities such as PDT, radiation therapy, and immunotherapy.
2. Materials and methods
2.1. Materials
All reagents and solvents were purchased commercially and used without further purification unless specially noted. The deionized water used in all experiments was produced by a Milli-Q water purification system with a specific resistance greater than 18.25 MΩ cm at 25 °C. Doxorubicin hydrochloride was supplied by Dalian Meilun Biotech Co., Ltd. (Dalian, China). Fetal bovine serum (FBS) was purchased from Zhejiang Tianhang Biological Technology Co., Ltd. (Hangzhou, China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma (St. Louis, MO, USA). 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) was obtained from A.V.T. Pharmaceutical Co., Ltd. (Shanghai, China). H2O2 assay kit was purchased from Solarbio (Beijing, China). Monostearin was obtained from Aladdin Reagents Co., Ltd. Other chemicals were purchased from Sigma‒Aldrich Chemical Co. (St. Louis, USA).
2.2. Animals model
BALB/c nude mice (female, 4–5 weeks old) were obtained from Beijing Huafukang Bioscience Technology Co., Ltd. (Beijing, China). The mice were kept in filter-topped cages with standard rodent chow, all with food and water available ad libitum, and with a 12 h light/dark cycle. The experimental protocol was approved by Committee on Ethical Animal Experiment at Huazhong University of Science and Technology, Wuhan, China.
2.3. Cell line and culture
SKOV3 (human ovarian carcinoma) cells were obtained from Procell Life Science & Technology Co., Ltd. (Wuhan, China), L02 (human hepatocytes) cell line was obtained from KeyGEN biotechnology Co., Ltd. (Nanjing, China). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. The device for hypoxic cell incubation was purchased from PUHE Biotechnology Co., Ltd. (Wuxi, China), which could construct a hypoxic and humid atmosphere at 37 °C in 5% CO2 and 1% O2.
2.4. Preparation of NPs
A 10-mL mixture of cyclohexane/Igepal CO-520 (71:29, v/v) consisting of 500 μL of ammonium hydroxide and 60 μL of oleic acid (OA) was stirred vigorously at room temperature. Another 10-mL mixture of cyclohexane/Igepal CO-520 (71:29, v/v) consisting of 250 μL of hydrogen peroxide, 250 μL of aqueous calcium chloride (4 mol/L) and 5 mg of DOX was stirred similarly. The two microemulsions were stirred continuously for 10 min. Afterward, both microemulsions were mixed, followed by an additional 30 min's stirring. To obtain DOX-CaO2-OA NPs, 15 mL of ethanol was used to destroy the microemulsion and precipitate the NPs, which were further re-dispersed in 15 mL cyclohexane. To introduce MnO2, 1 mL of microemulsion containing 50 μL of MnCl2 solution (1 mol/L) was added to above DOX-CaO2-OA NPs. After the reaction for 5 min, the brownish product was collected by ethanol precipitation and centrifugation. The obtained DOX-CaO2/MnO2-OA NPs were stored in CHCl3.
For further use, 300 μL of MS and 300 μL of DSPE-PEG2000 (10 mmol/L) in chloroform were added to the above DOX-CaO2/MnO2-OA NPs solution. After rotary evaporation, a thin film was formed, followed by the addition of 3 mL of distilled water. The final DOX-CaO2/MnO2-MS NPs were obtained by sonification for 20 s.
2.5. Characterization of NPs
The shapes and crystalline structures of NPs were characterized using microscopic and spectroscopic methods. Briefly, a drop of the sample dispersion was placed onto a copper grid and observed by transmission electron microscopy (TEM) after evaporation of the solvent at room temperature. The graphs were obtained on a Tecnai (G2 20, FEI Co., Netherlands) at an acceleration voltage of 200 kV. Furthermore, the X-ray photoelectron spectroscopy (XPS) samples were lyophilized, and the XPS patterns were operated on an Axis-ultra Dld-600 W (Kratos, Japan) photoelectron spectrometer. Powder X-ray diffraction (XRD) patterns were collected on a Rigaku RINT-2200 diffractometer with Cu Kα radiation (40 kV, 30 mA) from 5° to 90° in 0.013° steps at a scan speed of 1° (2θ)/min. UV-Vis spectrometer was used to measure the absorption from 350 nm to 750 nm of the NPs solutions in a quartz cell. The hydrodynamic size and zeta potential of NPs were obtained by Dynamic Light Scattering (DLS) measurement.
2.6. Quantitative analysis
CaO2 was quantified by a commercial H2O2 Assay Kit based on its H2O2 production capability. In brief, 25 μL of DOX-CaO2/MnO2-MS solution was added to 225 μL of acetone. Then 25 μL of acidic Ti(SO4)2 solution and 50 μL of ammonium hydroxide were added to the above solution, followed by centrifugation for 10 min (4000×g). The supernatant was abandoned and the resulted precipitate was dissolved in H2SO4 solution (1 mol/L). The absorbance of the final solution at 415 nm was measured by UV-Vis spectroscopy. DOX contents in DOX-CaO2/MnO2-MS were also measured using UV-Vis spectroscopy. The amount of Mn was detected by inductively coupled plasma atomic absorption spectrometry (ICP-AAS).
2.7. Cellular uptake
The red fluorescence of DOX was employed to track the intracellular locations of DOX. Briefly, cells were seeded into the 12-well plate (NEST, Wuxi, China) at a density of 2 × 105 per well. After 24 h, the cells were exposed to free DOX and DOX-CaO2/MnO2-MS NPs (DOX concentration: 1 μmol/L), respectively. After incubation for different periods, the initial medium was removed and the cells were washed with phosphate-buffered saline (PBS) three times. Finally, the cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI: 1.2 μg/mL) for 8 min and then rinsed with PBS. The cells were imaged with a fluorescence microscope (Zeiss LSM 510).
2.8. Cytotoxic activities
By measuring the ability of live cells to transform MTT to a purple formazan dye, the cell viability after NPs treatment was determined. Firstly, cells were seeded in 96-well tissue culture plates overnight at a density of 4 × 103 cells per well. Then the cells were incubated with different concentrations of drugs for 24 h. After that, the medium was aspirated, and 20 μL/well of MTT solution (5 mg/mL in PBS) was added. Four hours later, the supernatants were removed carefully, followed by the addition of 150 μL/well of dimethyl sulfoxide. The color intensity of the formazan solution, which reflects the cell growth condition, was measured at 570 nm using a microplate spectrophotometer (VersaMax, USA). All complexes doses were parallel tested in quintuplicate, and the data were presented as averages of three independent experimental standard deviations (SD).
For apoptosis analysis, SKOV3 cells were seeded on 6-well plates and cultured in DMEM with 10% FBS for 12 h. Then the medium was replaced with fresh DMEM with 10% FBS, and different NPs groups were added (DOX concentration: 1 μmol/L). The cells were collected and stained with an Annexin V-FITC Apoptosis Detection Kit (KeyGen Biotech). A BD Accuri C6 Flow Cytometer was used for analysis.
2.9. Neighboring effect
SKOV3 cells seeded on 24 well plates (Corning, USA, 5 × 105/well and allowed to grow overnight) with coverslips were pretreated with the free DOX, DOX-CaCO3-MS, and DOX-CaO2/MnO2-MS NPs at DOX concentration of 10 μg/mL for 8 h. The pretreated cells (a) were washed with PBS and then co-incubated with the fresh cells on a coverslip (b) for 24 h in a fresh culture medium. Then, (b) was withdrawn and co-incubated with the fresh cells on another new coverslip (c) for 24 h in a fresh culture medium. The cells (a, b and c) were then washed by ice-cold PBS thrice, stained with Hoechst 33,342, and observed using confocal laser scanning microscopy (CLSM).
Apoptosis assay was also conducted to verify the neighboring effect. SKOV3 cells were seeded in 6-well plates at the cell density of 1 × 106/well overnight 20 h. Then the cells were incubated with free DOX, DOX-CaCO3-MS or DOX-CaO2/MnO2-MS NPs at 37 °C for 8 h, in which the DOX concentrations were 4 μg/mL. Afterward, the cells were washed with PBS, trypsinized and mixed with blank cells at the ratio of 1:10, followed by cultivation in 20-mm glass-bottom culture dishes for 24 h. After that, Hoechst 33,342 and Annexin V-FITC were used to stain the cells according to manufacturer's protocol. Samples were observed under CLSM.
2.10. Intracellular O2 determination
Intracellular O2 levels were determined using an optical probe, [Ru (dpp)3]Cl2. First, SKOV3 and L02 cells were incubated under hypoxic conditions for 24 h, after which [Ru (dpp)3]Cl2 (10 μg/mL) was added for another 12 h incubation. Then the cells were treated with NPs for 2 h. After that, the cells were washed with PBS twice, and the intracellular O2 levels were measured using a fluorescence microscope by detecting the fluorescence of [Ru (dpp)3]Cl2 (λex = 450 nm, λem = 610 nm).
2.11. Western blot analysis
Following the treatments with different drugs, total cellular proteins were obtained using RIPA buffer (Applygen Technologies, Inc., Beijing, China). Protein (30 μg) was added per lane and proteins were separated by SDS-PAGE on 10% gel and transferred onto a polyvinylidene difluoride (PVDF) membrane. Following blocking with 5% skimmed milk at room temperature for 1 h, the membranes were sequentially incubated with primary antibodies overnight at 4 °C. The membrane was washed with TBST (PBS with 0.1% Tween-20) 3 times and then incubated with HRP-conjugated goat (polyclonal) anti-rabbit/anti-mouse IgG secondary antibody for 1 h at room temperature. Then the membrane was washed another 4 times and observed in an enhanced chemiluminescence system (PerkinElmer, Waltham, MA, USA). β-Tubulin was used as a loading control. Antibodies used for western blotting included those against HIF-1α (1:1000, Cell Signaling), P-gp (1:1000, Cell Signaling), connective tissue growth factor (CTGF, 1:1000, Cell Signaling), and Collagen I (1:1000, Cell Signaling).
2.12. Drug penetration and inhibition in multicellular tumor spheroids (MCTS)
The formation of MCTS was monitored using an optical microscope (TE2000-S, Nikon, Japan). The tumor spheroids with diameters of about 400 μm were incubated with the free DOX, DOX-CaCO3-MS, DOX-CaO2-MS, and DOX-CaO2/MnO2-MS NPs at the DOX concentration of 2 μmol/L for 24 h, respectively. Then, the tumor spheroids were washed thrice with ice-cold PBS, fixed with paraformaldehyde for 30 min, and placed in cavity microscope slides. The images of the tumor spheroids were acquired using Z-stack imaging with 20 μm intervals from the top of the spheroid to the middle by CLSM. For the MCTS inhibition, the tumor spheroids with diameters of about 200 μm were divided into groups (n = 5). The selected spheroids were treated with saline, free DOX, DOX-CaCO3-MS, DOX-CaO2-MS, and DOX-CaO2/MnO2-MS NPs. The spheroids were allowed to incubate at 37 °C for 9 days. The diameter of the spheroids was imaged and recorded every 2 days using an optical microscope.
2.13. In vivo imaging and antitumor study
The in vivo imaging study was conducted on SKOV3 cells bearing mice by a noninvasive optical imaging system (Pearl Trilogy, LI-COR, USA). The hydrophobic fluorescein DiR was loaded into the CaO2/MnO2-MS by a film dispersion method to produce CaO2/MnO2-DiR/MS NPs. BALB/c nude mice were intravenously injected with CaO2/MnO2-DiR/MS NPs or free DiR at the dosage of 5 μg DiR per mice. At 0.5, 4, 8, 24, and 48 h, the fluorescence intensity images of each mouse were captured.
The SKOV3 bearing nude mice were randomly divided into four groups (n = 5) to study the in vivo antitumor efficacy of NPs. On Days 7, 10, 13 and 16 after tumor inoculation, the mice were injected via the tail vein with saline, free DOX, CaO2/MnO2-MS, and DOX-CaO2/MnO2-MS NPs at 5 mg/kg of DOX or its equivalent. Tumor sizes and animal body weights were monitored every two days after treatment. On Day 24, the mice were sacrificed, major organs and tumor tissues were acquired for further analysis.
2.14. Statistical analysis
All data were taken from three independent experiments and then expressed as means ± standard deviation (SD). One-way ANOVA was performed to compare the statistical analysis by GraphPad Prism 5.0 (San Diego, CA, USA).
3. Results and discussion
3.1. Preparation and characterization
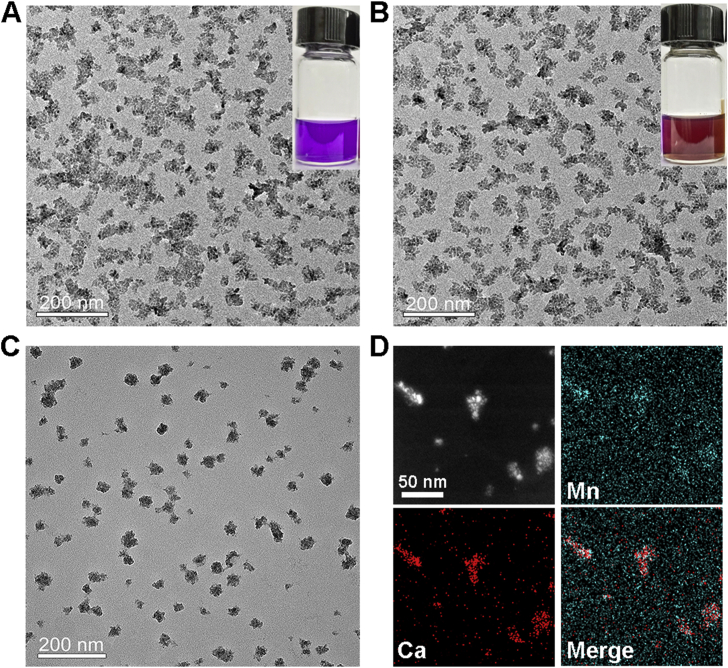
According to our previous work, CaO2 NPs were synthesized by the reaction of CaCl2, H2O2, and NH3∙H2O in reverse microemulsions23. To prepare drug-preloaded CaO2 NPs with a hydrophobic coating, DOX and OA were introduced into the microemulsion system. Through the alkaline NH3∙H2O-facilitated deprotonation, DOX chelated with Ca2+ to form the Ca–DOX complex and was finally entrapped in CaO2 matrix24. Similarly, the deprotonated carboxyl groups of OA molecules also coordinated with Ca2+, enabling the hydrophobic surface coating of NPs by long-chain alkyl25. As shown in Fig. 1A, the purple product DOX-CaO2-OA showed remarkable solubility in non-polar solutions such as CHCl3. From the image obtained by TEM, we found that the NP size was about 50 nm with irregular morphology.
Figure 1.
(A) TEM image of DOX-CaO2-OA NPs, scale bar = 200 nm. Insert: photograph of DOX-CaO2-OA NPs dissolved in CHCl3. (B) TEM image of DOX-CaO2/MnO2-OA NPs, scale bar = 200 nm. Insert: photograph of DOX-CaO2/MnO2-OA NPs dissolved in CHCl3. (C) TEM image of DOX-CaO2/MnO2-MS NPs, scale bar = 200 nm. (D) Elemental mapping of DOX-CaO2/MnO2-MS NPs, scale bar = 50 nm.
To synthesize MnO2-doped NPs, the obtained DOX-CaO2-OA NPs were re-dispersed in cyclohexane, followed by adding a small volume of MnCl2-contained microemulsion. Due to the hydrophilic property of the interface between CaO2 and OA, the aqueous MnCl2 could easily diffuse into their interlayer and react with CaO2. After that, the solution color turned from purple to brownish due to the formation of MnO2 (denoted as DOX-CaO2/MnO2-OA). To the best of our knowledge, this trial may be the first to synthesize MnO2 NPs by a metal peroxide. Similar to DOX-CaO2-OA observed by TEM, the DOX-CaO2/MnO2-OA NPs were well-dispersed in CHCl3 with a diameter of approximately 50 nm (Fig. 1B).
The DOX-CaO2/MnO2-OA NPs were further surface-modified with 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino (polyethylene glycol)-2000] (DSPE-PEG2000) and MS to construct the hydrophilic DOX-CaO2/MnO2-MS NPs. As can be seen in Fig. 1C, after coating with lipids, the particle sizes became slightly larger (about 60 nm). The hydrodynamic size of DOX-CaO2/MnO2-MS NPs was measured to be 217.7 nm by DLS (Supporting Information Fig. S1), sharply larger than the results obtained by TEM. This could be explained by the existence of PEG2000 and the hydration shell of NPs. In addition, the zeta potential of NPs was determined (−10.8 mV), which proved the MS-coating of NPs. The elemental mapping images recorded in Fig. 1D demonstrate the existence of Ca, Mn, indicating the formation of CaO2/MnO2 nano-complex.
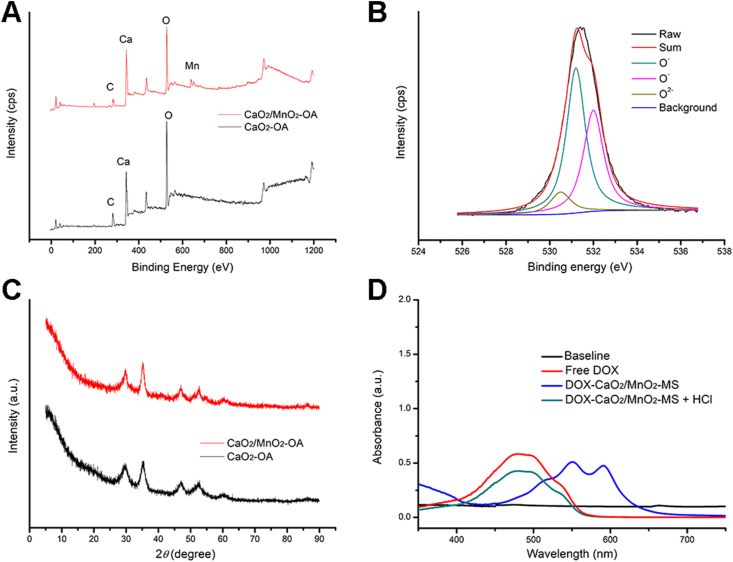
Further qualitative analysis was conducted using XRD and XPS. As shown in Fig. 2A, Mn signal was detected in the XPS spectrum of DOX-CaO2/MnO2-OA NPs, proving the synthesis of MnO2. Besides, XPS deconvoluted spectra for O1s orbital26 showed both the signals of O2− and O‒, demonstrating the existence of CaO2/MnO2 (Fig. 2B). In XRD results (Fig. 2C), CaO2 representing specific peaks (2θ = 30.1°, 35.6°, 47.3°) were observed in both spectra of CaO2-OA and CaO2/MnO2-OA NPs, which indicated the maintenance of crystal structure of CaO2 matrix after the MnO2 introduction27. Overall, these results confirmed that CaO2/MnO2 nanocomposites were successfully synthesized in reverse microemulsions.
Figure 2.
(A) XPS spectra of CaO2-OA and CaO2/MnO2-OA NPs. (B) XPS deconvoluted spectra for O1s orbital for CaO2/MnO2-OA NPs. (C) XRD spectra of CaO2-OA and CaO2/MnO2-OA NPs. (D) UV–Vis spectra of free DOX, DOX-CaO2/MnO2-MS, and DOX-CaO2/MnO2-MS + HCl.
According to the previous research, the formation of the Ca–DOX complex leads to the red-shift of its UV-Vis absorption peak from 480 to 550 and 590 nm28. As shown in Fig. 2D, the peaks at around 550 and 590 nm were detected in DOX-CaO2/MnO2-MS NPs, indicating that DOX was loaded in the CaO2 matrix by Ca-DOX coordination. Interestingly, the peak at 480 nm was recovered after NPs incubating with lipase and HCl, which inferred that the encapsulation of DOX by MS-coated CaO2/MnO2 nanocarrier did not impact its structure. UV-Vis spectrophotometry was also applied to determine the loading content of DOX in DOX-CaO2/MnO2-MS NPs, which was calculated to be 5.5% (w/w). The inorganic skeleton CaO2 [38.4% (w/w)] was quantified by Ti(SO4)2 solution with the aid of UV-Vis spectrophotometry, and MnO2 [2.9% (w/w)] was measured by inductively coupled plasma atomic absorption spectrometry (ICP-AAS).
3.2. Stability and drug release
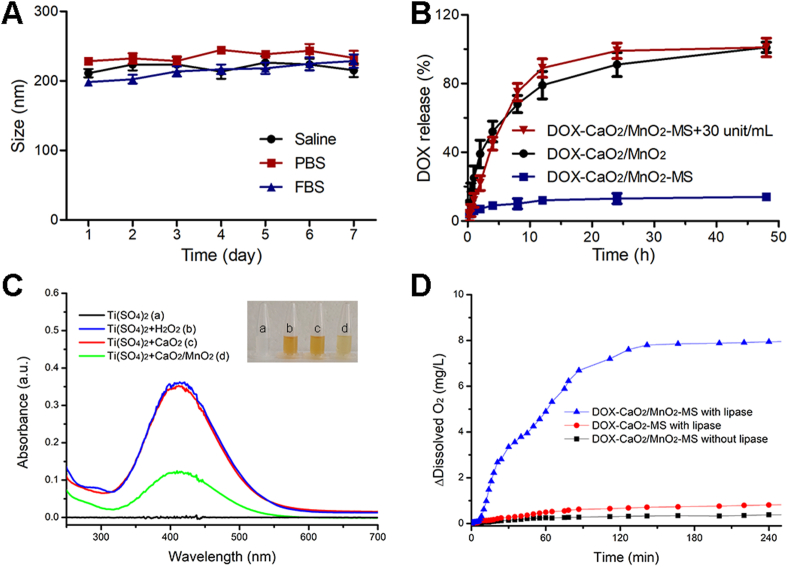
To evaluate the applicability of the DOX-CaO2/MnO2-MS NPs as a nanomedicine, the stability of NPs was first assessed in simulated physiological conditions. As shown in Fig. 3A, the average sizes of the DOX-CaO2/MnO2-MS NPs remained nearly unchanged at the range of 200–250 nm after incubation in saline, PBS, and FBS for one week, indicating the good stability of MS-based NPs under physiological conditions.
Figure 3.
Stability and drug release of NPs. (A) Time-dependent colloidal stability of DOX-CaO2/MnO2-MS NPs in saline, PBS and FBS. Data are presented as mean ± SD, n = 3. (B) Lipase-triggered DOX release. Data are presented as mean ± SD, n = 3. (C) The H2O2-producing ability of CaO2. (D) Lipase-triggered O2 generation.
In contrast to normal cells, tumor cells express a higher level of lipase for signaling and metabolic demands, which can catalyze the hydrolysis of solid lipids29. Accordingly, the solid lipid MS-coated NPs were expected to respond to lipase and release drugs in a lipase-dependent manner. To validate this assumption, we first tested the drug release profile of NPs in solutions. As exhibited in Fig. 3B, DOX from DOX-CaO2/MnO2 NPs released completely due to the water-sensitiveness of CaO2 and the lack of MS coating. However, DOX-CaO2/MnO2-MS NPs showed significantly slow drug release property with merely 15% of DOX being released in lipase-free solution. This could well-demonstrate the protection of CaO2/MnO2 and drug preserving ability by MS shell which guaranteed the stability of NPs during blood circulation. After NPs incubating with lipase for 48 h, the loaded DOX was totally released. It is worth mentioning that the well-explored pH-responsive nanocarriers might not be able to efficiently unload the drugs due to the consumption of H+. However, these lipase-triggered water-responsive NPs could achieve efficient drug release due to the existence of a ubiquitous aqueous environment in cells. Besides, the thorough decomposition of CaO2 enabled the high biodegradability of NPs with less inorganic compounds retention in tissues, which enabled their biosafety30.
Furthermore, the O2-generating capacity of NPs was also investigated in solutions containing lipase. First, we confirmed that CaO2 could react with water to produce a large amount of H2O2 (Fig. 3C). After the introduction of MnO2, the generated H2O2 was decomposed into O2, leading to a less level of H2O2. As can be seen in Fig. 3D, minimal O2 was detected in DOX-CaO2/MnO2-MS NPs solution without lipase, further demonstrating their stability. Whereas in the presence of lipase, abundant O2 was generated by DOX-CaO2/MnO2-MS NPs after 3 h, which proved the lipase-triggered O2 generation ability of NPs. In the group of DOX-CaO2-MS NPs, the lack of MnO2 enabled the NPs with compromised O2 generating ability. Collectively, these results inferred that DOX-CaO2/MnO2-MS NPs could keep stable in physiological conditions and selectively responded to the environment with a high level of lipase to efficiently release drug and O2.
3.3. Cellular uptake
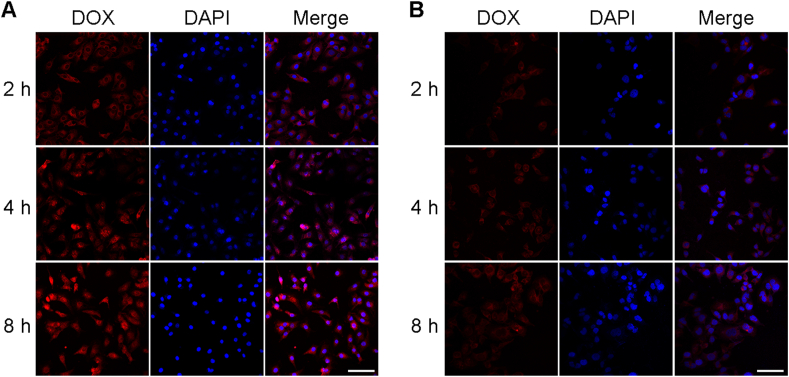
SKOV3 human ovarian cancer cells and L02 human hepatocytes cells were chosen to explore the in vitro cellular uptake profile of DOX-CaO2/MnO2-MS NPs. The fluorescence of DOX was detected to trace the drug molecules with the assistance of CLSM. As depicted in Supporting Information Fig. S2A and Fig. 4A, the DOX signal was only detected in the cytoplasm of NPs-treated cells in early periods (0.5, 1, and 2 h), until 4 h it displayed in the nuclei. However, free DOX entered the nuclei of SKOV3 cells after incubation for merely 0.5 h (Supporting Information Fig. S2B). It might because that the endocytosis and drug release processes of NPs are the prerequisite of DOX entering the nuclei, but free DOX can diffuse into nuclei without these processes. Nevertheless, NPs-incubated L02 cells did not exhibit significant DOX fluorescence in the nuclei (Fig. 4B), indicating that the drug release of DOX-CaO2/MnO2-MS NPs was not sufficiently triggered due to the lower lipase activity of L02 cells. As a consequence, DOX molecules could not be liberated from NPs and enter the nuclei, which might contribute to less toxicity to normal tissues. These results demonstrated that these MS-based NPs exhibited cancer cell-selective drug release and thereby satisfactory biosafety to normal cells.
Figure 4.
Cellular uptake of DOX-CaO2/MnO2-MS NPs in SKOV3 (A) and L02 (B) cells. Cell nuclei was stained with DAPI (blue fluorescence), DOX concentration: 1 μmol/L, scale bar = 200 μm.
3.4. Enhanced anticancer efficacy in vitro
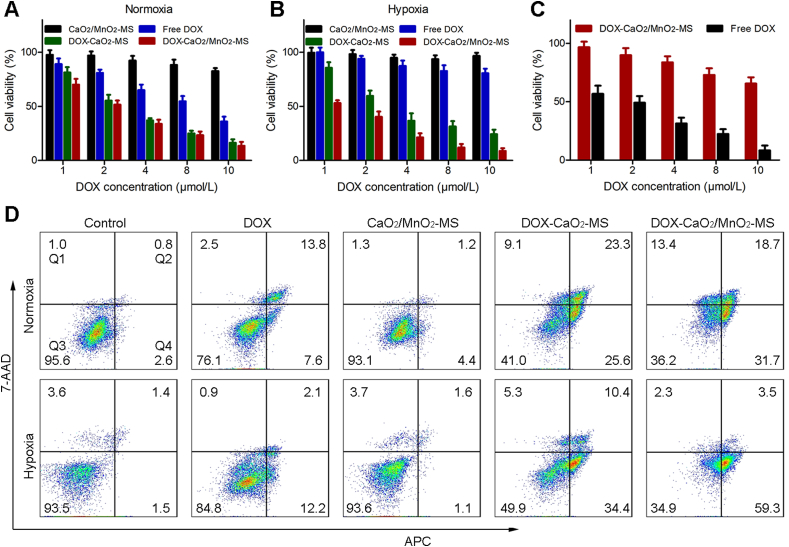
Furthermore, the anticancer efficacy of the NPs in vitro was evaluated against SKOV3 and L02 cells by MTT assay. As expected, the cytotoxicity of free DOX towards hypoxic cells was lower than that in normoxic cells due to hypoxia-induced chemoresistance (Fig. 5A and B). Besides, the nanocarrier CaO2/MnO2-MS showed minimal anti-proliferation effect against both normoxic and hypoxic SKOV3 cells. However, DOX delivered by the O2-generating CaO2/MnO2-MS NPs showed significantly enhanced cytotoxicity towards hypoxic cells, indicating the hypoxia-relief and improved DOX chemotherapy. Notably, the cytotoxicity of DOX-CaO2/MnO2-MS NPs against L02 cells was largely reduced (Fig. 5C), possibly owing to the lower lipase activity in L02 cells which was not able to effectively trigger the free drug release as described in previous cell uptake analysis. In all, these results demonstrated that DOX-CaO2/MnO2-MS NPs could achieve enhanced chemotherapy towards hypoxic cancer cells with intratumoral lipase specificity.
Figure 5.
Selective and enhanced anticancer efficacy of NPs in vitro. Cell viabilities of SKOV3 cells after the treatments under normoxia (A) and hypoxia (B). (C) Cell viabilities of L02 and SKOV3 cells after the incubation with DOX-CaO2/MnO2-MS NPs. Data are presented as mean ± SD, n = 3. (D) The Annexin V-APC/7-AAD apoptosis assay of SKOV3 cells after the treatments under normoxia and hypoxia. DOX concentration: 4 μmol/L. Data are presented as mean ± SD, n = 3.
To further explore the capacity of DOX-CaO2/MnO2-MS NPs to induce apoptosis, the Annexin V-APC/7-AAD method was used. As revealed by Fig. 5D and Supporting Information Fig. S3, the total percentage of apoptotic cells (including early and late apoptotic cells) induced by DOX-CaO2/MnO2-MS NPs reached 62.8% in hypoxic SKOV3 cells, far exceeding the findings in free DOX and the O2-generating carrier groups (CaO2/MnO2-MS NPs), which demonstrated that the introduction of O2 could bolster chemotherapy-induced cell apoptosis. In accordance with the above MTT assay, the increased apoptosis by O2 supply in normoxic cells was not that obvious as in hypoxic cells.
3.5. In vitro mechanism studies
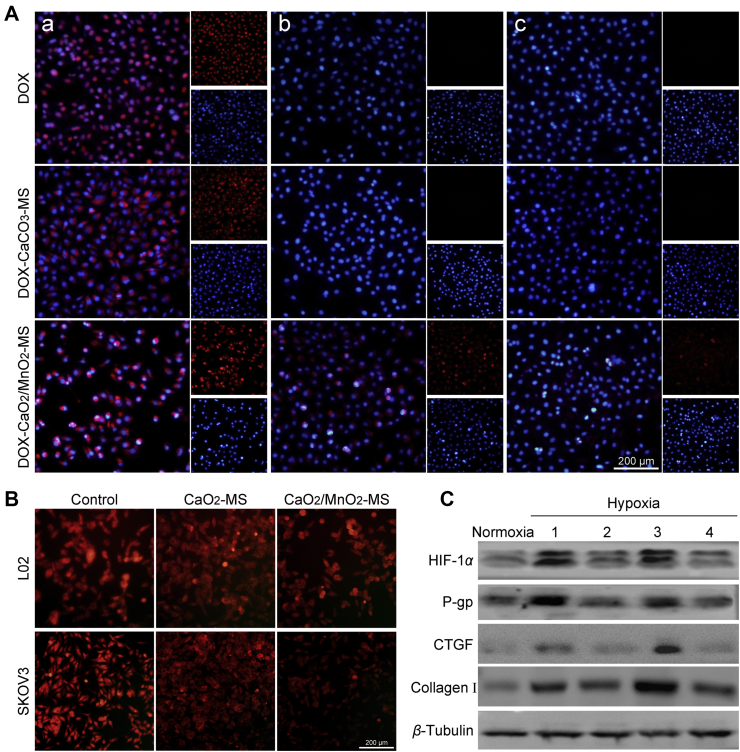
In contrast to conventional free drugs and pH-responsive nanomedicines, our smart nanoformulation was expected to induce a neighboring effect by its lipase-triggered water-responsiveness, which contributed to an enhanced therapeutic outcome31. To valid our assumption, the intercellular trafficking of free DOX and NPs was studied in vitro. As the counterpart with water stability, MS-coated crystallized calcium carbonate (CaCO3) was synthesized to load DOX in a similar method. First, the SKOV3 cells seeded on a coverslip were cultured with free DOX or NPs for 8 h. Then another coverslip with fresh cells was co-incubated with the treated cells (a) for another 24 h, which was repeated, followed by the observation using CLSM. As shown in Fig. 6A, successive cells (b) of DOX-CaO2/MnO2-MS NPs group revealed strong DOX fluorescence, and it was still detected in the third-generation cells (c). In contrast, the successive cells in free DOX group did not show obvious red fluorescence, possibly due to the strong interaction between DOX and DNA base-pairs which is responsible for entrapping DOX and depriving its ability to further infect neighboring cells. Interestingly, DOX-CaCO3-MS NPs also showed minimal DOX signal in successive cells. It could be attributed to the phenomenon that the pH-dependent drug release of loaded DOX might be impeded by H+ consumption. Thus, we can conclude that the lipase-triggered water-responsive DOX-CaO2/MnO2-MS NPs could achieve enhanced intercellular delivery of DOX, which could account for the improved chemotherapy.
Figure 6.
In vitro antitumor mechanisms. (A) Intercellular delivery of DOX from treated SKOV3 cells to fresh SKOV3 cells, cell nuclei was stained with DAPI (blue fluorescence), DOX concentration: 1 μmol/L, scale bar = 200 μm. (B) O2 detection of hypoxic SKOV3 cells by [Ru (dpp)3]Cl2 probe, scale bar = 200 μm. (C) Immunoblot of endogenous HIF-1α and its downstream P-gp, CTGF, collagen I after different treatment (1) control; (2) CaO2/MnO2-MS; (3) DOX; (4) DOX-CaO2/MnO2-MS under hypoxia.
We also performed an apoptosis assay to validate the neighboring effect. As displayed in Supporting Information Fig. S4, green fluorescence was weak in the groups of free DOX or DOX-CaCO3-MS NPs, which indicated a smaller number of apoptotic cells. In comparison, DOX-CaO2/MnO2-MS NPs treated cells showed much stronger green fluorescence, suggesting that severer cell apoptosis was induced by DOX-CaO2/MnO2-MS NPs-mediated neighboring effect.
The influence of DOX-CaO2/MnO2-MS NPs on the intracellular O2 was tested using a commercial hypoxia probe, [Ru (dpp)3]Cl2. In the hypoxic condition, SKOV3 cells without treatments displayed the strongest red fluorescence, which indicated the lowest O2 availability. After the cells were cultured with CaO2/MnO2-MS NPs for 12 h, the intracellular O2 level increased significantly, but only slight O2 production was detected in CaO2-MS group treated cells (Fig. 6B). This could be ascribed to the strong catalytic ability of MnO2 for O2 generation.
It has been well-established that tumor hypoxia has an impact on the anti-tumor efficacy of chemotherapy by regulating HIF-1 pathways32. P-gp, which is closely related to DOX efflux and reduced chemotherapy efficacy, was demonstrated to be expressed in an HIF-1 dependent manner. Besides, CTGF regulated by HIF-1 acts as a critical regulator on collagen deposition in tumor tissue, which can impede drug penetration. Based on the hypoxia relief capacity of NPs, we speculated that the HIF-1 and its downstream proteins could be downregulated by the O2-generating NPs. As expected, the results obtained by western blot analysis proved that the HIF-1α/P-gp and HIF-1α/CTGF/Collagen I pathways were largely inhibited by DOX-CaO2/MnO2-MS NPs. In accordance with the above determined O2 generating ability, the MnO2-free CaO2 NPs could not suppress the HIF-1 pathways efficiently. Therefore, both drug penetration and drug retention could be improved by NPs-facilitated O2-induced HIF-1 pathways inhibition, which is an explanation for enhanced DOX chemotherapy.
3.6. Drug penetration and inhibition on MCTS
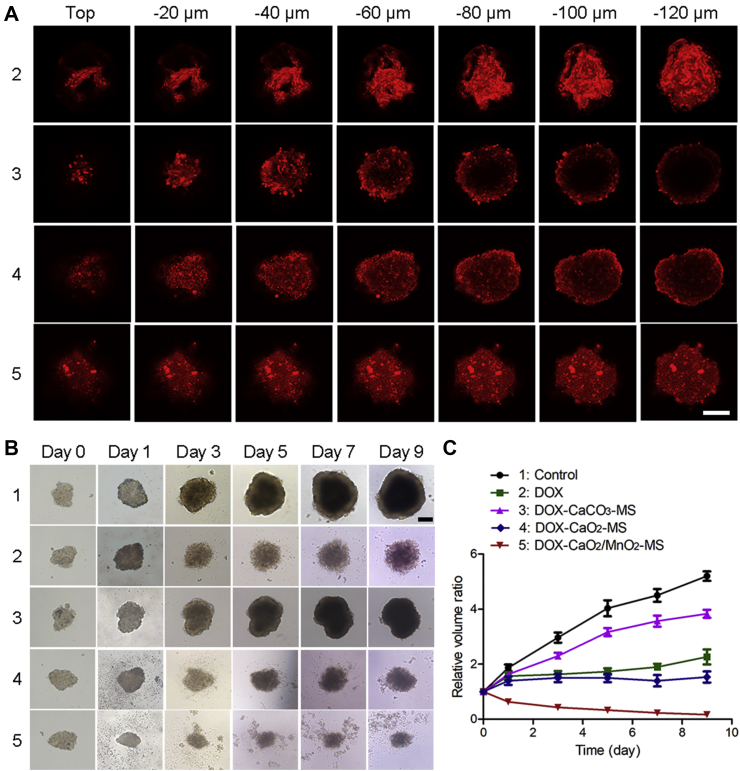
An SKOV3 MCTS model was constructed to evaluate the penetration capacity of DOX-CaO2/MnO2-MS NPs. As shown in Fig. 7A, DOX fluorescence only showed at the periphery of the tumor spheroids treated with DOX-CaCO3-MS NPs. In contrast, DOX showed enhanced penetration in DOX-CaO2-MS NPs treated MCTS. This phenomenon could be attributed to the strong penetration ability of free molecular DOX released from water-responsive CaO2 matrix. Interestingly, the DOX fluorescence spread in the center of MCTS in free DOX and DOX-CaO2/MnO2-MS NPs treated group, even at 120 μm from the surface to the middle. Hence, we can conclude that O2 generation by CaO2/MnO2 and the smart convertible nanoformulation design synergistically enhanced drug penetration, respectively by collagen decomposition and free DOX-mediated neighboring effect. Further, the inhibition effect of DOX-CaO2/MnO2-MS NPs on MCTS growth was also examined. As shown in Fig. 7B and C, DOX-CaCO3-MS NPs showed minimal effects may due to the insufficient drug release compared with free DOX. As expected, DOX-CaO2/MnO2-MS NPs had the best inhibitory effect on the growth of MCTS, indicating the promoted anticancer efficacy in MCTS level. This might be the consequence of comprehensive optimized drug transportation including drug release, penetration, and retention.
Figure 7.
(A) In vitro penetration of DOX into the MCTS after incubation with different formulations for 24 h, DOX concentration: 2 μmol/L, scale bar = 200 μm. (B) MCTS inhibition effects, DOX concentration: 4 μmol/L, scale bar = 200 μm. (C) The volume change of MCTS. Data are presented as mean ± SD, n = 5.
3.7. Enhanced anticancer efficacy in vivo
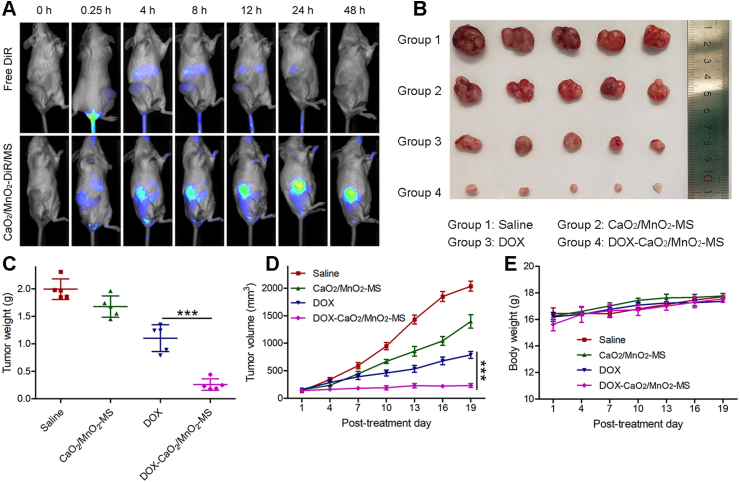
SKOV3 cell xenograft model was employed to investigate the biodistribution and therapeutic efficacy of DOX-CaO2/MnO2-MS NPs in vivo. First, DiR as a near-infrared fluorescent probe was loaded into NPs to monitor the real-time in vivo distribution33. As displayed in Fig. 8A, free DiR was quickly metabolized, leading to a weak fluorescence detected in mice 48 h post-injection. However, at merely 4 h post-injection of NPs, the strong fluorescence was detected in the tumor tissue, confirming the preferable tumor-homing capability of NPs. This was also concluded by ex vivo fluorescence images obtained from mice after treatments with DOX-CaO2/MnO2-MS NPs (Supporting Information Fig. S5A) and free DOX (Fig. S5B) for 48 h.
Figure 8.
In vivo experiments. (A) Real-time fluorescence imaging of free DiR and CaO2/MnO2-DiR/MS NPs treated mice. (B) Tumor photographs, (C) average tumor weight, (D) tumor volume, and (E) average mouse body weight of saline and other treated groups. Data are presented as mean ± SD, n = 5, ∗∗∗P < 0.001.
To further explore the in vivo therapeutic efficacy of DOX-CaO2/MnO2-MS, NPs treatments started when the average tumor volume reached ∼150 mm3. As revealed in Fig. 8B–D, the DOX-CaO2/MnO2-MS NPs exhibited significantly enhanced tumor inhibition effect compared with CaO2/MnO2-MS NPs and free DOX, which proved the effectiveness of such smart O2-generating nanocarrier in enhancing DOX chemotherapy in vivo. Moreover, no obvious weight loss was detected in all treated mice (Fig. 8E), indicating the biosafety of such solid lipid-based nanoformulation.
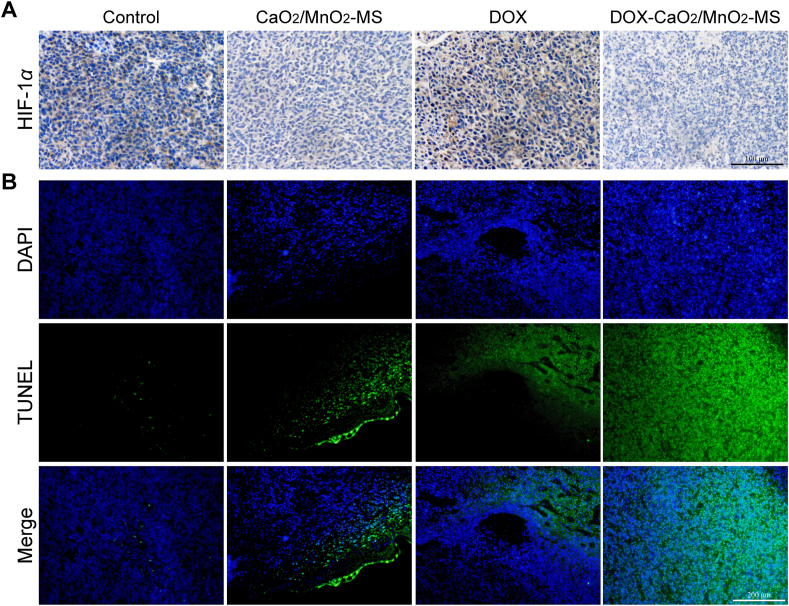
HIF-1α immunohistochemical analysis was further performed to measure the expression of HIF-1α protein. In line with the in vitro results, the HIF-1α signal was significantly down-regulated in the O2-generating groups (Fig. 9A), demonstrating the in vivo effect of hypoxia relief by NPs. As revealed using the TUNEL staining assay (Fig. 9B), intensive apoptosis was observed in tumor tissues after DOX-CaO2/MnO2-MS NPs treatment. And other therapeutic groups including free DOX and O2-evolving nanocarrier didn't show such a strong effect. Finally, histological analyses of major normal organs including heart, liver, spleen, lungs, and kidneys confirmed the high biosafety with limited tissue damage in DOX-CaO2/MnO2-MS NPs-treated mice (Supporting Information Fig. S6).
Figure 9.
(A) HIF-1α staining images of the tumor, scale bar = 100 μm. (B) Representative images of tumor-site apoptosis determined by TUNEL assay, scale bar = 200 μm.
4. Conclusions
In summary, a smart O2-self-supplying nanocarrier was designed to enhance DOX chemotherapy by improving its transportation to cancer cells comprehensively. On one hand, the water-sensitive disintegration of drug-loaded CaO2/MnO2 cores not only enabled sufficient drug release, but also induced a neighboring effect with the assistance of MS coating, which potentiated drug penetration into deep tumor tissues. On the other hand, the generated O2 downregulated HIF-1α and its downstream collagen I and P-gp, resulting in enhanced drug penetration and drug retention. Hence, the comprehensive optimized drug transportation including drug release, penetration, and retention was realized, which contribute to a better chemotherapeutic outcome.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Nos. 81973257, 81801738, and 81703446). The authors thank the Analytical & Testing Center of Huazhong University of Science for TEM, XRD, XPS.
Author contributions
Chuanchuan He, Xiaojuan Zhang, and Guangya Xiang designed the research. Xiaojuan Zhang carried out the experiments and performed data analysis. Yun Sun, Xiaoguang Liu, Yan Chen, Chen Chen, Ruicong Yan, and Ting Fan participated part of the experiments. Tan Yang, Yao Lu, Jun Luo, and Xiang Ma provided experimental drugs and quality control. Chuanchuan He and Xiaojuan Zhang wrote the manuscript. Jun Luo, Xiang Ma, and Guangya Xiang revised the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2021.04.021.
Contributor Information
Jun Luo, Email: lawdream1975@mail.hust.edu.cn.
Xiang Ma, Email: xiangma@hust.edu.cn.
Guangya Xiang, Email: gyxiang1968@hotmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Arruebo M., Vilaboa N., Sáez-Gutierrez B., Lambea J., Tres A., Valladares M., et al. Assessment of the evolution of cancer treatment therapies. Cancers. 2011;3:3279–3330. doi: 10.3390/cancers3033279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo S., Miao L., Wang Y., Huang L. Unmodified drug used as a material to construct nanoparticles:Delivery of cisplatin for enhanced anti-cancer therapy. J Control Release. 2014;174:137–142. doi: 10.1016/j.jconrel.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browning R.J., Reardon P.J.T., Parhizkar M., Pedley R.B., Edirisinghe M., Knowles J.C., et al. Drug delivery strategies for platinum-based chemotherapy. ACS Nano. 2017;11:8560–8578. doi: 10.1021/acsnano.7b04092. [DOI] [PubMed] [Google Scholar]
- 4.Spencer D.S., Puranik A.S., Peppas N.A. Intelligent nanoparticles for advanced drug delivery in cancer treatment. Curr Opin Chem Eng. 2015;7:84–92. doi: 10.1016/j.coche.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miao L., Guo S., Lin C.M., Liu Q., Huang L. Nanoformulations for combination or cascade anticancer therapy. Adv Drug Deliv Rev. 2017;115:3–22. doi: 10.1016/j.addr.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minchinton A.I., Tannock I.F. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 7.Chen J., Ding J., Wang Y., Cheng J., Ji S., Zhuang X., et al. Sequentially responsive shell-stacked nanoparticles for deep penetration into solid tumors. Adv Mater. 2017;29:1701170. doi: 10.1002/adma.201701170. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda Y., Hisano H., Nishikawa Y., Nagasaki Y. Targeting and treatment of tumor hypoxia by newly designed prodrug possessing high permeability in solid tumors. Mol Pharm. 2016;13:2283–2289. doi: 10.1021/acs.molpharmaceut.6b00011. [DOI] [PubMed] [Google Scholar]
- 9.Gilkes D.M., Semenza G.L., Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430–439. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang Y., Sullivan R., Graham C.H. Confluence-dependent resistance to doxorubicin in human MDA-MB-231 breast carcinoma cells requires hypoxia-inducible factor-1 activity. Exp Cell Res. 2007;313:867–877. doi: 10.1016/j.yexcr.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Wu X., Zhu Y., Huang W., Li J., Zhang B., Li Z., et al. Hyperbaric oxygen potentiates Doxil antitumor efficacy by promoting tumor penetration and sensitizing cancer cells. Adv Sci. 2018;5:1700859. doi: 10.1002/advs.201700859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C.C., Chia W.T., Chung M.F., Lin K.J., Hsiao C.W., Jin C., et al. An implantable depot that can generate oxygen in situ for overcoming hypoxia-induced resistance to anticancer drugs in chemotherapy. J Am Chem Soc. 2016;138:5222–5225. doi: 10.1021/jacs.6b01784. [DOI] [PubMed] [Google Scholar]
- 13.Liu W.L., Liu T., Zou M.Z., Yu W.Y., Li C.X., He Z.Y., et al. Aggressive man-made red blood cells for hypoxia-resistant photodynamic therapy. Adv Mater. 2018;30:1802006. doi: 10.1002/adma.201802006. [DOI] [PubMed] [Google Scholar]
- 14.Lan G., Ni K., Xu Z., Veroneau S.S., Song Y., Lin W. Nanoscale metal-organic framework overcomes hypoxia for photodynamic therapy primed cancer immunotherapy. J Am Chem Soc. 2018;140:5670–5673. doi: 10.1021/jacs.8b01072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu W., Shevtsov M., Chen X., Gao H. Advances in aggregatable nanoparticles for tumor-targeted drug delivery. Chin Chem Lett. 2020;31:1366–1374. [Google Scholar]
- 16.Yu W., Liu R., Zhou Y., Gao H. Size-tunable strategies for a tumor targeted drug delivery system. ACS Cent Sci. 2020;6:100–116. doi: 10.1021/acscentsci.9b01139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui T., Yan Z., Qin H., Sun Y., Ren J., Qu X. A sequential target-responsive nanocarrier with enhanced tumor penetration and neighboring effect in vivo. Small. 2019;15:1903323. doi: 10.1002/smll.201903323. [DOI] [PubMed] [Google Scholar]
- 18.Gong H., Chao Y., Xiang J., Han X., Song G., Feng L., et al. Hyaluronidase to enhance nanoparticle-based photodynamic tumor therapy. Nano Lett. 2016;16:2512–2521. doi: 10.1021/acs.nanolett.6b00068. [DOI] [PubMed] [Google Scholar]
- 19.Ke C.J., Su T.Y., Chen H.L., Liu H.L., Chiang W.L., Chu P.C., et al. Smart multifunctional hollow microspheres for the quick release of drugs in intracellular lysosomal compartments. Angew Chem Int Ed. 2011;123:8236–8239. doi: 10.1002/anie.201102852. [DOI] [PubMed] [Google Scholar]
- 20.Doublier S., Belisario D.C., Polimeni M., Annaratone L., Riganti C., Allia E., et al. HIF-1 activation induces doxorubicin resistance in MCF7 3-D spheroids via P-glycoprotein expression: a potential model of the chemo-resistance of invasive micropapillary carcinoma of the breast. BMC Cancer. 2012;12:4. doi: 10.1186/1471-2407-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C., Chen S., Wang Y., Liu X., Hu F., Sun J., et al. Lipase-triggered water-responsive “Pandora's Box” for cancer therapy: toward induced neighboring effect and enhanced drug penetration. Adv Mater. 2018;30:1706407. doi: 10.1002/adma.201706407. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X., He C., Yan R., Chen Y., Zhao P., Li M., et al. HIF-1 dependent reversal of cisplatin resistance via anti-oxidative nano selenium for effective cancer therapy. Chem Eng J. 2020;380:122540. [Google Scholar]
- 23.He C., Zhang X., Yan R., Zhao P., Chen Y., Li M., et al. Enhancement of cisplatin efficacy by lipid-CaO2 nanocarrier-mediated comprehensive modulation of the tumor microenvironment. Biomater Sci. 2019;7:4260–4272. doi: 10.1039/c9bm00797k. [DOI] [PubMed] [Google Scholar]
- 24.He C., Zhang X., Chen C., Liu X., Chen Y., Yan R., et al. A solid lipid coated calcium peroxide nanocarrier enables combined cancer chemo/chemodynamic therapy with O2/H2O2 self-sufficiency. Acta Biomater. 2021;122:354–364. doi: 10.1016/j.actbio.2020.12.036. [DOI] [PubMed] [Google Scholar]
- 25.Kwon H.J., Shin K., Soh M., Chang H., Kim J., Lee J., et al. Large-scale synthesis and medical applications of uniform-sized metal oxide nanoparticles. Adv Mater. 2018;30:1–24. doi: 10.1002/adma.201704290. [DOI] [PubMed] [Google Scholar]
- 26.Liu L.H., Zhang Y.H., Qiu W.X., Zhang L., Gao F., Li B., et al. Dual-stage light amplified photodynamic therapy against hypoxic tumor based on an O2 self-sufficient nanoplatform. Small. 2017;13:1701621. doi: 10.1002/smll.201701621. [DOI] [PubMed] [Google Scholar]
- 27.Shen S., Mamat M., Zhang S., Cao J., Hood Z.D., Figueroa-Cosme L., et al. Synthesis of CaO2 nanocrystals and their spherical aggregates with uniform sizes for use as a biodegradable bacteriostatic agent. Small. 2019;15:1902118. doi: 10.1002/smll.201902118. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y., Luo Z., Li M., Qu Q., Ma X., Yu S., et al. A preloaded amorphous calcium carbonate/ doxorubicin@silica nanoreactor for pH-responsive delivery of an anticancer drug. Angew Chem Int Ed. 2015;3:919–922. doi: 10.1002/anie.201408510. [DOI] [PubMed] [Google Scholar]
- 29.Nomura D.K., Long J.Z., Niessen S., Hoover H.S., Ng S.W., Cravatt B.F. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He C., Zhang X., Xiang G. Nanoparticle facilitated delivery of peroxides for effective cancer treatments. Biomater Sci. 2020;8:5574–5582. doi: 10.1039/d0bm01265c. [DOI] [PubMed] [Google Scholar]
- 31.Guo S., Wang Y., Miao L., Xu Z., Lin C.M., Zhang Y., et al. Lipid-coated cisplatin nanoparticles induce neighboring effect and exhibit enhanced anticancer efficacy. ACS Nano. 2013;7:9896–9904. doi: 10.1021/nn403606m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onnis B., Rapisarda A., Melillo G. Development of HIF-1 inhibitors for cancer therapy. J Cell Mol Med. 2009;13:2780–2786. doi: 10.1111/j.1582-4934.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X., He C., Liu X., Chen Y., Zhao P., Chen C., et al. One-pot synthesis of a microporous organosilica-coated cisplatin nanoplatform for HIF-1-targeted combination cancer therapy. Theranostics. 2020;10:2918–2929. doi: 10.7150/thno.41077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.