Abstract
None.
Keywords: Left ventricular assist device, Post-marketing surveillance, HeartMate-3, Heart failure, Complications
Abbreviations: FDA, Food and Drug Administration; HF, heart failure; HM-3, HeartMate-3; LVAD, left ventricular assist device; MAUDE, Manufacturer and User Facility Device Experience; MDR, manufacturer device reports
To the Editor:
In the United States, 90,000 new cases of heart failure (HF) are diagnosed yearly, of which 10% are classified as advanced.1 Left ventricular assist devices (LVADs) remain a well-established therapy for patients with end-stage medically refractory HF. In recent years, the Abbott-Thoratec HeartMate-3 (HM-3) LVAD has been introduced into clinical practice.2 Despite engineering advancements, reported adverse events and utilization costs remain a barrier to widespread post Food and Drug Administration's (FDA) approval implementation.2 We reviewed the literature and analyzed post-marketing surveillance data from the FDA Manufacturer and User Facility Device Experience (MAUDE) database to evaluate complications of HM-3 LVAD.
The FDA MAUDE database was queried for manufacturer device reports (MDRs) on the Abbott-Thoratec HM-3, from its FDA approval date of August 23, 2017, to August 31, 2020. We then screened event reports for completeness, stratified complications into the device and patient-related events and used descriptive analyses on the compiled reported complications.
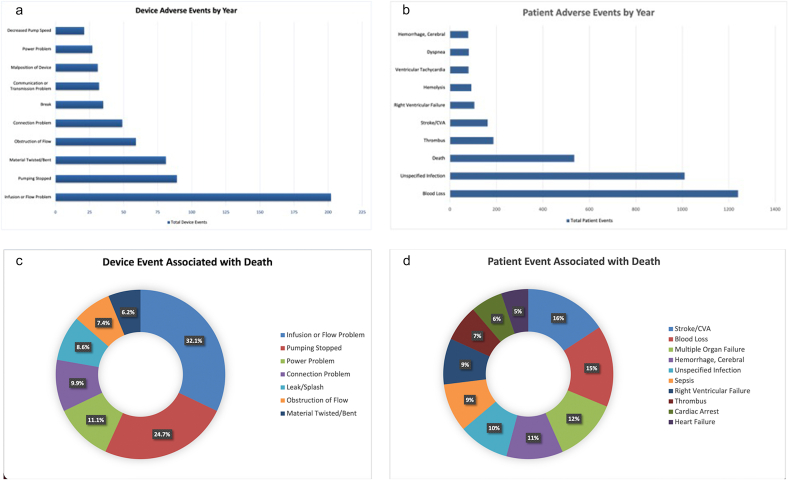
During this study period, 3867 device reports met the inclusion criteria, of which 961 device-related events were reported, including multiple events documented in the same report. The excluded 2906 device reports were patient-reported issues not specific to the device. The most common device events reported were infusion flow disruptions (21%), halted pump (9.2%), and material deformation (8.4%) (Fig. 1A). Additionally, of the 5676 patient-related events during this period, blood loss (21.8%), infection (17.8%), and thrombus (3.3%) were the most commonly reported clinical events (death excluded) (Fig. 1B). Deaths were reported in 535 reports, with the cause of death listed as cerebral vascular accidents (9.7%) and hemorrhage (6.7%) (Fig. 1C–D). Finally, 55 reports indicated device explantation.
Fig. 1.
Device and patient-specific adverse events with Abbott-Thoratec HeartMate-3 left ventricular assist device (2017–2019): 1A: Device adverse events by year (N = 961); 1B: Device events associated with death (N = 573); 1C: Patient adverse events by year (N = 5676); 1D: Patient event associated with death (N = 715).
The MOMENTUM-3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate-3) trial indicated shortcomings still present in the newest generation of LVADs,3 which is supported by the findings in this study. Flow disruptions associated with pump thrombosis continue to be a major complication of LVAD devices. However, when compared with HM-2, HM-3 showed improved mortality and morbidity while reducing reoperations for malfunctioning devices at two years (76.9% vs. 64.8%). Additionally, a decreased rate of hemocompatibility-associated adverse events was noted, including pump thrombosis, stroke, and surgical bleeding.3 A late-onset complication, unique to the HM-3, is the twisting of the outflow graft that leads to hampered pump flow, which prompts surgical intervention to avoid blood clots or even death.3 The FDA announced a recall in March 2018, impacting more than 5000 devices due to the potential of the outflow graft assembly to encounter a malfunction. We suggest physicians be aware of such complications when following patients with an HM-3.
Notwithstanding the many bleeding events reported in the database, HM-3 was shown to have a lower incidence of gastrointestinal bleeding than HM-2; this observation is likely due to greater preservation of von Willebrand factor multimer structures in HM-3 by use of its frictionless rotor and wide blood-filled gaps between the rotor and housing.4 Despite cost being a limiting factor, HM-3 demonstrated a decreased rate of re-hospitalizations and improved cost-effectiveness compared with its predecessor HM2, as evidenced by the reduced number of pump replacements at two years (2.3% vs. 11.3%). HYP5 Further studies are needed to address complications and associated risk factors.
This study has several limitations. MDRs are anonymous, subjective, and may lack details on confounding factors. Furthermore, after the perioperative period, the clinician and patient are not required to report adverse events, and thus these events and certain complications may be underreported. The database also does not report the total number of HM3 devices placed annually in the United States. Device complications may also be over-reported due to improper attribution by the voluntary reporter, particularly when events are of high inconvenience. It is also difficult to establish the exact cause of complications such as pump thrombosis and twisting of the outflow graft. Our data depict the total number of reports received in one year but are not intended to be used to compare devices yearly, similar to multiple prior analyses of the MAUDE database. Finally, as the HM-3 is a newer device, longer-term data remains to be established. Complications such as blood loss, infection, and thrombus may occur even in the absence of an LVAD however we aimed to provide a full overview of related events.
In conclusion, this analysis of the MAUDE database has established that significant complications are associated with the use of centrifugal LVADs. Continued training, judicious usage, and refinement of insertion procedures may be implemented for the improvement of device performance and patient outcomes.
Author contributions
Study design, literature review, statistical analysis: JNH, SS, ML, SV. Data management, data analysis, drafting manuscript: JNH, SS, ML, SV. Access to data: JNH, SS, ML, SV. Manuscript revision, intellectual revisions, mentorship: SV. Final approval: JNH, SS, ML, SV.
Sources of funding
None.
Declaration of competing interest
All authors do not report any relevant financial or intellectual disclosures related to this manuscript.
References
- 1.Groenewegen A., Rutten F.H., Mosterd A., Hoes A.W. Epidemiology of heart failure. Eur J Heart Fail. 2020;22(8):1342–1356. doi: 10.1002/ejhf.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehra M.R., Naka Y., Uriel N., et al. A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med. 2017;376(5):440–450. doi: 10.1056/NEJMoa1610426. [DOI] [PubMed] [Google Scholar]
- 3.Mehra M.R., Uriel N., Naka Y., et al. A fully magnetically levitated left ventricular assist device - final report. N Engl J Med. 2019;380(17):1618–1627. doi: 10.1056/NEJMoa1900486. [DOI] [PubMed] [Google Scholar]
- 4.Bansal A., Uriel N., Colombo P.C., et al. Effects of a fully magnetically levitated centrifugal-flow or axial-flow left ventricular assist device on von Willebrand factor: a prospective multicenter clinical trial. J Heart Lung Transplant. 2019;38(8):806–816. doi: 10.1016/j.healun.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Mehra M.R., Salerno C., Cleveland J.C., et al. Healthcare resource use and cost implications in the MOMENTUM-3 long-term outcome study. Circulation. 2018;138(18):1923–1934. doi: 10.1161/CIRCULATIONAHA.118.035722. [DOI] [PubMed] [Google Scholar]