Abstract
Background
Cisplatin is extensively used in treating cancers, and its primary side-effect is nephrotoxicity. It accumulates in proximal convoluted tubules where it promotes cellular damage by oxidative stress, apoptosis, and inflammation, etc. In Unani medicine, Tukhm-e-Karafs(Apium graveolens L.) (TK) is mentioned in the literature to manage various kidney ailments due to its diuretic and deobstruent activities.
Objective
To investigate the nephroprotective effects of powder of TK in Cisplatin-induced nephrotoxicity in an animal model and to validate the Unani claim of its nephroprotective action.
Material and methods
In curative protocol, cisplatin (5 mg/kg body weight i.p) was administered on day one and powder of TK (500 and 1000 mg/kg p.o.) from the sixth day onwards for ten days. TK (500 and 1000 mg/kg p.o.) was given for ten days and Cisplatin (5 mg/kg body weight i.p) on day 11 in the protective model. At the end of the study, all the animals were sacrificed, and renal biochemical parameters were determined. KIM-1 level was also investigated in the kidney homogenate in conjunction with histopathological inspection of kidney tissues.
Results
Significant increase in serum creatinine and BUN, presence of mononuclear cell infiltration, tubular dilation and vacuolation in renal histopathology, and increased KIM-1 level confirmed the nephrotoxicity due to Cisplatin. TK's administration protects the kidney as suggested by the changes in biochemical renal function, decreased level of KIM-1, and improvement in histopathological changes.
Conclusion
The result advocated that TK prevented renal injury and maintained normal renal function in both models. It may be due to improved clearance of Cisplatin from kidney tubules and reduction in reactive oxygen species (ROS) produced by the inflammatory response.
Keywords: Cisplatin, KIM-1, Nephroprotective, Nephrotoxicity, Tukhm-e-Karafs, Unani
1. Introduction
The compound (cis-diamminedichloroplatinum [II]), commonly known as Cisplatin, was first described in 1845 by M. Peyrone (known as Peyrone's salt) [1]. Cisplatin is commonly used as a potent chemotherapeutic agent in human and veterinary medicine. Cisplatin administration produces many side-effects like nephrotoxicity, ototoxicity, nausea and vomiting, peripheral sensory neuropathy, myelosuppression, and nerve dysfunction. Nephrotoxicity is the most common and notable clinical manifestation [[2], [3], [4]].
Nearly one third of individuals with cisplatin therapy demonstrated nephrotoxicity after 10 days of treatment. The manifestation of side-effects includes decrease glomerular filtration rate, increased serum creatinine and decreased magnesium and potassium levels. Though Cisplatin's lasting side-effects are not clear, it may be concluded that a permanent reduction in glomerular filtration rate will be the result. When tubular cells are exposed to Cisplatin, it activates some signaling pathways that are either cytoprotective or cell death-promoting (ROS, p53, MAPK, etc.). Cisplatin also induces TNF-α production resulting in the inflammatory response, leading to the cells' death and reduced glomerular filtration rate. Cumulative effects of these events conclude in acute renal failure [5]. It has been found that nephrotoxicity due to Cisplatin can be monitored through diuretics and hydration regimens in many patients, but the frequency of Cisplatin nephrotoxicity still occurs in about one-third of patients undergoing Cisplatin treatment [32]. Amifostine is approved by the US Food and Drug Administration for Use in reducing progressive nephrotoxicity of repeated Cisplatin dosing in patients with advanced ovarian cancer. Unfortunately, even with amifostine, renal toxicity still occurs [44].
Tukhm-e-Karafs (Apium graveolens L.) (TK) is a vital drug mentioned in the Unani literature for kidney disorders. Its seed and roots are used in the treatment of kidney and liver diseases. Its Mizaj (temperament) is mentioned as Haar Yabis in 2nd degree. Its actions as mentioned in the literature are Mufattih Sudad (deobstruent), Mudirr-i-Bawl (diuretic), Mushtahi (appetizer), Musqit-i-Janin (abortifacient), Mufattit-i-Hasah (lithotriptic), Kasir-i-Riyah (carminative), etc. It is also used as an ingredient in many compound formulations like Jawarish Zarooni sada, Sharbat Bazuri Haar, Banadiqul Buzoor, Shikanjabeen Bazoori Moatadil, Dawaul Kurkum, and Majoon Dabidulward, etc.; these compound formulations are used for kidney and liver disorders [[6], [7], [8], [9], [10], [11], [12], [13]].
According to the Unani medicine concept, Mizaj is defined as average equality produced by the interaction of four elements (fire, air, water, and soil) that possess their dual qualities [33]. When different essential molecules of different elements come into contact at one place, they act and react. Their Kaifiyat (quality) interact with each other so that a new Kaifiyat (quality) emerges. This new and intermediate Kaifiyat (quality) is called Mizaj. If we mix hot water into cold water, it becomes moderate. Hot property and cold property act against each other and a new ‘intermediate’ property emerge that will be neither so hot nor so cold to such extent as it was earlier [34]. The Mizaj of TK is Haar Yabis in 2nd degree (hot and dry in 2nd degree), which means the intermediate property of the ingredients (chemical constituents) of the TK is hot and dry. It is the basic concept of Unani medicine and used in treatment based on ‘Ilāj bi’l Didd (heteropathy).
Consequently, this study was planned to scientifically appraise the nephroprotective action of TK in experimental animals. The trial drug's effect was studied in animals subjected to nephrotoxicity by the administration of Cisplatin in two different sets of tests, where both protective and curative effects were evaluated. The efficacy was determined based on biochemical markers (serum creatinine, BUN, serum uric acid, and serum total protein), serum electrolytes (sodium, potassium, chloride and calcium), KIM-1 test, and histopathological findings, to maintain the objectivity of the results and the study. The central hypothesis is the repurposing of the drug for the nephroprotective effect to provide adjuvant therapy for kidney toxicity during chemotherapy. Diuretics and hydration regimens can be used for monitoring nephrotoxicity. However, in our study, TK was used as a protective and curative drug to counter the nephrotoxic effects of Cisplatin. The curative and preventive protocols were used to evaluate the nephroprotective effects of TK comprehensively. Natural drugs produce low adverse effects, and no life-threatening adverse effect is mentioned in Unani literature for TK or observed in practice.
2. Material and methods
2.1. Collection of plant material
The dried seeds of TK were obtained from the GMP certified Pharmacy of the CRIUM Hyderabad. The drug was procured from the local market of Hyderabad and identified by the Pharmacy Incharge of the Institute. The drug sample's identity was confirmed by Research Officer (Botany), SMP Unit at CRIUM, Hyderabad-500038. The voucher specimen was preserved under SMPU/CRI-HYD 13550 number at the SMP unit of CRIUM Hyderabad.
2.2. Physicochemical standardization of TK and TLC fingerprinting
The percentage of total ash, acid insoluble ash, water-soluble extractives, alcohol-soluble extractives, and moisture content, as well as TLC, were determined in accordance with WHO criteria [14]. The presence of alkaloids, saponins, resins, sterols, and terpenes was determined qualitatively using the procedures outlined by Afaq et al. [15]. Flavonoids were detected using the Fransworth method [16]; glycosides were detected using the Paech and Tracey method [17]; phenols were detected using the Brewster and Mc Ewen method [18]; and saponin was detected using the Arthur and Chan method [19]. The TLC analysis of the test drug's alcoholic extract was performed on a silica gel “G” plate using a mobile phase of Toluene: Ethyl Acetate: Methanol (7:2:1). The TLC was examined in the UV chamber using UV 366 nm light and the Rf values were recorded.
2.3. Animals
In this study, 48 albino rats of Wistar strain of 120–150 g weight were taken. Animals were procured from Edara Research Foundation (ERF), Hyderabad; a GLP-certified laboratory. Standard laboratory conditions (12/12 h light and dark, Temperature: 22 °C ± 3 °C; Relative Humidity: 30–70%) were maintained. The animals were housed three, of the same sex in polycarbonate cages provided with husk bedding. A conventional rodent laboratory diet was provided with potable drinking water ad libitum. Experimental protocol abided by CPCSEA guidelines and was approved by the Institutional Animal Ethics Committee of the CRIUM, Hyderabad vides Registration No. CRIUM/IAEC/2017/01/P08 dated 29th July 2017.
2.4. Experimental procedure
The crude drug dose was determined by multiplying the Unani clinical dose of the test drug by the conversion factor of 7 [20]. The dose range of TK- has been mentioned in Unani literature as 3–5 gm. The clinical dose calculated was found to be 500 mg/kg bw. Further, the second dose of 1000 mg/kg (doubled dose) was also used to study the test drug's dose-dependent effect. The powder of crude drug was prepared by electric grinder in the GMP-certified pharmacy of the Institute and preserved in an airtight container for further use. The fine powder of the test drug was suspended in 0.3% aqueous carboxyl methylcellulose (CMC) suspension. The test dose suspension was freshly prepared daily. A feeding cannula was used to administer the suspension which was homogenized by shaking well for a minute. The daily new suspension was prepared by the same method. The rats were randomly divided into eight groups containing six animals each group. Cisplatin was administered intraperitoneally in the dose of 5 mg/kg body weight [21].
In curative protocol,
-
1.
Group-1 (Normal control), Animals were treated with normal saline injection i.p on day 1 and 0.3% CMC aqueous solution was administered orally 6th day onward for 10 days.
-
2.
Group-2 (Negative control), Animals were administered Cisplatin 5 mg/kg i.p on 1st day and 0.3% CMC aqueous solution was administered orally 6th day onward for 10 days.
-
3.
Group-3, Animals were administered Cisplatin 5 mg/kg i.p on 1stday; however, from the 6th day onwards the test drug, TK was also given in the dose of 500 mg/kg body weight once daily orally for the next 10 days.
-
4.
Group-4, Animals were administered Cisplatin 5 mg/kg i.p on 1stday; however, from the 6th day onwards the test drug, TK was also given in 1000 mg/kg body weight once daily orally for the next 10 days.
In protective protocol,
-
1.
Group-1 (Normal control), Animals were treated with 0.3% CMC aqueous solution administered orally for 10 days and normal saline injection i.p on the 11th day.
-
2.
Group-2 (Negative control), Animals were treated with 0.3% CMC aqueous solution administered orally for 10 days and Cisplatin 5 mg/kg i.p on the 11th day.
-
3.
Group-3, Animals received test drug TK in the dose of 500 mg/kg body weight orally, once daily for 10 days, and Cisplatin injection was given i.p on 11th day in the dose of 5 mg/kg bw.
-
4.
Group-4, Animals received test drug TK in the dose of 1000 mg/kg body weight orally, once daily for 10 days, and Cisplatin injection was given i.p on 11th day in the dose of 5 mg/kg bw.
2.5. Blood collection for biochemical assays
At the end of the experiment, the rats were sequentially anesthetized with isoflurane (EZ anaesthesia system) for about 50–60 s 2–3 ml of blood was collected using capillary from retro-orbital plexus for biochemical estimation (creatinine, BUN, total protein, and uric acid). Each blood sample obtained from each rat was collected into a well-labeled 4 ml capacity plain sample vial.
2.6. Preparation of kidney homogenate
Kidneys were excised out, and the left kidney was used to prepare homogenate while the right kidney was stored in neutral buffered formalin for histopathological evaluation. Kidney tissue samples were washed with ice-cold saline and homogenized 100 mg tissue sample using phosphate buffer saline (pH 7.4). The tissue sample, along with PBS, was centrifuged at 4 °C at 10,000 rpm. Homogenized samples were stored in triplicate aliquots and stored at −80 °C. KIM-1 levels were assessed using rat-specific ELISA kits.
2.7. Histopathological examination
All animals were subjected to gross necropsy after sacrificing under CO2 euthanasia. Macroscopic examination of organs and tissues was done, and these organs/tissues were weighted after isolation and trimming. The relative organ weight was analyzed for both kidneys, liver, spleen, brain, heart, adrenals, and testes. Kidney tissues were preserved in 10% neutral buffer formalin for histopathological examination. The tissue was processed for routine paraffin embedding, and approximately 3–5 μ sections were stained with Mayer's haematoxylin and eosin stains.
2.8. Statistical analysis
For statistical analysis One-way analysis of variance (ANOVA), followed by Tukey's test was performed for all the raw data recorded during the study by GraphPad Prism Version 5.0 software. Values were represented as mean ± SEM, and p < 0.05 was considered statistically significant.
3. Results
3.1. Qualitative phytochemical analysis
The hydroalcoholic extract of the test drug was subjected to qualitative phytochemical analysis to check the drug's quality, and results were found by Unani Pharmacopoeial standard.
3.2. Physicochemical standards and TLC fingerprinting
The alcoholic and water extractive components were determined to be 16.30% and 20.70%, respectively. Total ash and acid insoluble ash were determined to be 8.8 and 2.3 percent, respectively, while moisture content was found to be 8.2 percent. The phytochemical colour reaction demonstrates that the TK seeds contain glycosides, flavonoids, alkaloids, phenols, and sterols.
The TLC analysis of an alcoholic extract on a silica gel “G” plate with Toluene: Ethyl Acetate: Methanol (7:2:1) as the mobile phase reveals 11 spots with Rf values of 0.02 (blue), 0.07 (blue), 0.17 (blue), 0.21 (red), 0.25 (blue), 0.35 (blue), 0.45 (blue), 0.54 (blue), 0.61 (blue), 0.78 (blue) and 0.85 (blue) under UV 366 nm light.
3.3. Bodyweight
No significant differences were observed in the bodyweight of test drug TK-treated animals at both doses (low and high) levels with the control (normal or Cisplatin) group. No significant changes were observed in Cisplatin control than normal control in the curative and protective protocol (data not presented).
3.4. Feed intake
Rats treated with TK at both dose (low and high) levels showed no significant difference in feed consumption as compared to control groups (normal and Cisplatin) in curative as well as protective protocol (data not presented).
3.5. Relative organ weight
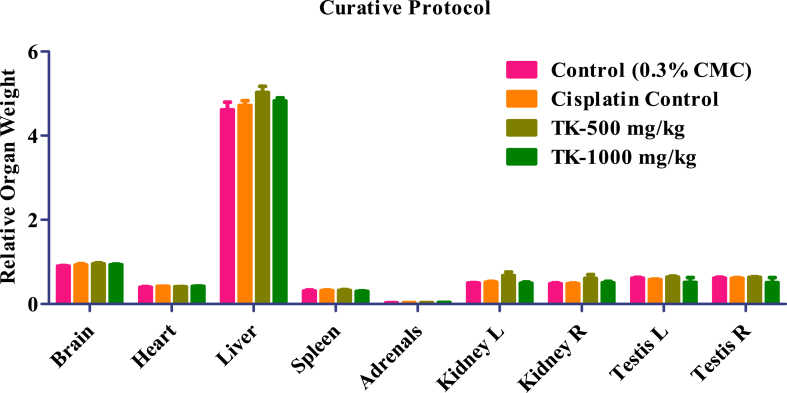
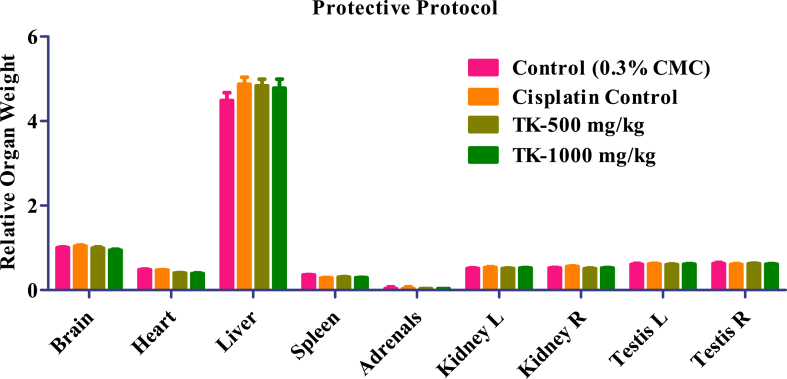
The administration of test drug TK at both dose (low and high) levels did not induce any alterations in relative organ weight of adrenals, liver, brain, spleen, heart, right and left kidney, and right and left testis. All TK-treated animals' observed values were comparable to normal control and Cisplatin control group (Fig. 1, Fig. 2).
Fig. 1.
Relative organ weight of controls and TK treated animals.
Fig. 2.
Relative organ weight of controls and TK treated animals.
3.6. Renal function parameters
The effects of Cisplatin and TK on biochemical markers of rats are presented in Table 1 (curative protocol) and Table 2 (protective protocol). Serum creatinine and BUN were significantly increased in the Cisplatin control group compared to the normal control group in curative and protective protocol. Serum uric acid and serum electrolytes (sodium, potassium, chloride and calcium) did not significantly increase when compared to the normal control group. In the curative protocol, serum creatinine and BUN level decreased in both dose levels but were found significant in the high dose group. In protective protocol serum, creatinine level was significantly decreased in both dose levels while BUN value was found significantly decreased in high dose level. Serum uric acid value was significantly decreased at both dose levels in curative protocol while in protective protocol at a low dose level. There was no significant difference in serum electrolytes (Na+, K+, Cl−, and Ca++) at both dose levels.
Table 1.
Cisplatin curative protocol.
| Parameter | Vehicle (0.3%CMC) |
Cisplatin (5 mg/kg) |
TK (500 mg/Kg) |
TK (1000 mg/Kg) |
|---|---|---|---|---|
| Creatinine (mg/dL) | 0.61 ± 0.03 | 1.08 ± 0.11∗ | 0.81 ± 0.14 | 0.66 ± 0.04≠ |
| BUN (mg/dL) | 14.68 ± 0.79 | 53.38 ± 3.80∗ | 45.45 ± 15.99 | 15.60 ± 1.56≠ |
| Uric Acid (mg/dL) | 1.98 ± 0.13 | 2.05 ± 0.07 | 1.43 ± 0.11∗∗≠≠ | 1.567 ± 0.08∗≠ |
| Sodium (mmol/L) | 143.3 ± 1.56 | 145.7 ± 1.11 | 146.5 ± 0.76 | 148.5 ± 0.92∗ |
| Potassium (mmol/L) | 6.10 ± 0.32 | 6.30 ± 0.23 | 4.91 ± 0.17∗∗≠≠ | 4.51 ± 0.07∗∗∗≠≠≠ |
| Chloride (mmol/L) | 95.33 ± 0.61 | 95.50 ± 0.88 | 92.33 ± 0.66 | 94.50 ± 1.05 |
| Calcium (mmol/L) | 2.41 ± 0.01 | 2.45 ± 0.03 | 2.61 ± 0.01∗∗∗≠≠ | 2.56 ± 0.042∗∗ |
| Total Protein (g/dL) | 6.75 ± 0.07 | 6.50 ± 0.20 | 6.783 ± 0.23 | 6.80 ± 0.08 |
Values presented as mean ± SEM; n = 06; One-way ANOVA; ∗ = p < 0.05 vs control ∗∗ = p < 0.01 vs control, ∗∗∗ = p < 0.001 vs control ≠=p < 0.05 vs Cisplatin control ≠≠=p < 0.01 vs Cisplatin control, ≠≠≠=p < 0.001 vs Cisplatin control.
Table 2.
Cisplatin protective protocol.
| Parameter (Male) |
Vehicle (0.3%CMC) |
Cisplatin (5 mg/kg) |
TK (500 mg/Kg) |
TK (1000 mg/Kg) |
|---|---|---|---|---|
| Creatinine (mg/dL) | 0.83 ± 0.02 | 1.183 ± 0.16∗ | 0.81 ± 0.01≠ | 0.80 ± 0.0≠ |
| BUN (mg/dL) | 14.02 ± 0.6452 | 23.02 ± 1.644∗∗ | 20.12 ± 2.53 | 15.17 ± 0.716≠ |
| Uric Acid (mg/dL) | 1.75 ± 0.10 | 1.76 ± 0.12 | 1.35 ± 0.06∗≠ | 1.48 ± 0.06 |
| Sodium (mmol/L) | 120.7 ± 0.84 | 118.7 ± 1.11 | 116.0 ± 1.29 | 119.0 ± 1.50 |
| Potassium (mmol/L) | 13.82 ± 0.46 | 15.02 ± 0.44 | 15.55 ± 0.30 | 15.67 ± 0.75 |
| Chloride (mmol/L) | 106.7 ± 0.33 | 108.2 ± 0.47 | 108.3 ± 0.55 | 108.2 ± 0.47 |
| Calcium (mmol/L) | 1.73 ± 0.02 | 1.73 ± 0.02 | 1.85 ± 0.02 | 1.917 ± 0.122 |
| Total Protein (g/dL) | 7.08 ± 0.03 | 6.91 ± 0.18 | 7.06 ± 0.03 | 6.93 ± 0.05 |
Values presented as mean ± SEM; n = 06; One-way ANOVA; ∗ = p < 0.05 vs. control ∗∗ = p < 0.01 vs. control, ≠ = p < 0.05 vs. Cisplatin control.
3.7. KIM-1 level in kidney homogenate
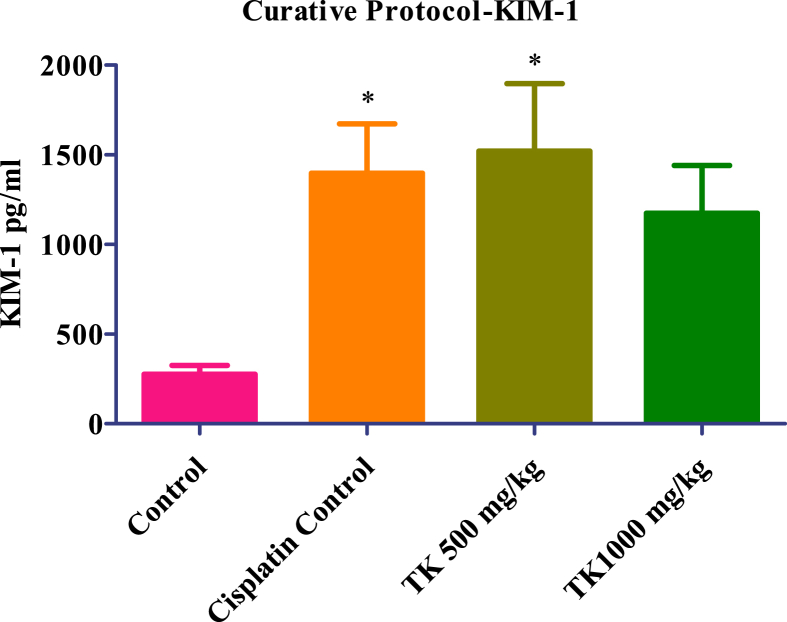
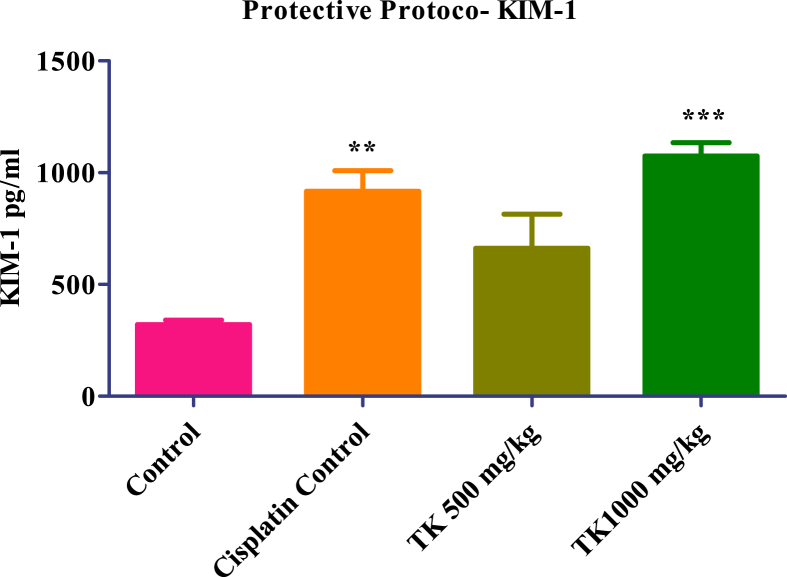
In Cisplatin protective protocol, KIM-1 level significantly increased in Cisplatin control, low dose (500 mg/kg) and high dose (1000 mg/kg) treated group respectively compared to the normal control group. In low dose (500 mg/kg), it is not found statistically significant. However, in Cisplatin curative protocol, KIM-1 level was significantly increased in Cisplatin control, low dose (500 mg/kg) and high dose (1000 mg/kg) treated group respectively but not found statistically significant in high dose (1000 mg/kg). In both Cisplatin protective and curative protocol, treatment with TK at a low dose (500 mg/Kg) and high dose (1000 mg/Kg), KIM-1 levels were not found statistically significant as compared to the Cisplatin control group which indicates no protective and curative effect (Fig. 3, Fig. 4).
Fig. 3.
KIM-1 level of Controls and TK treated animals. Values presented as mean ± SEM; n = 06; One-way ANOVA; ∗ = p < 0.05 vs control.
Fig. 4.
KIM-1 level of Controls and TK treated animals. Values presented as mean ± SEM; n = 06; One-way ANOVA; ∗∗ = p < 0.01 vs control, ∗∗∗ = p < 0.001 vs control.
3.8. Histopathological examination
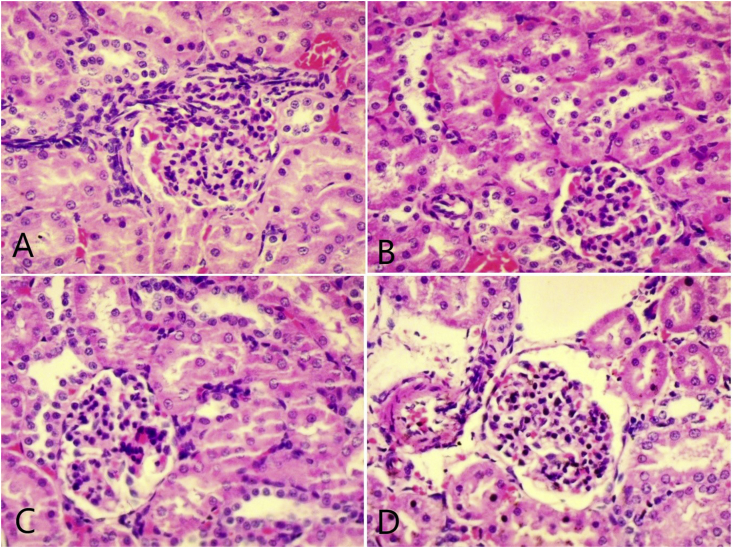
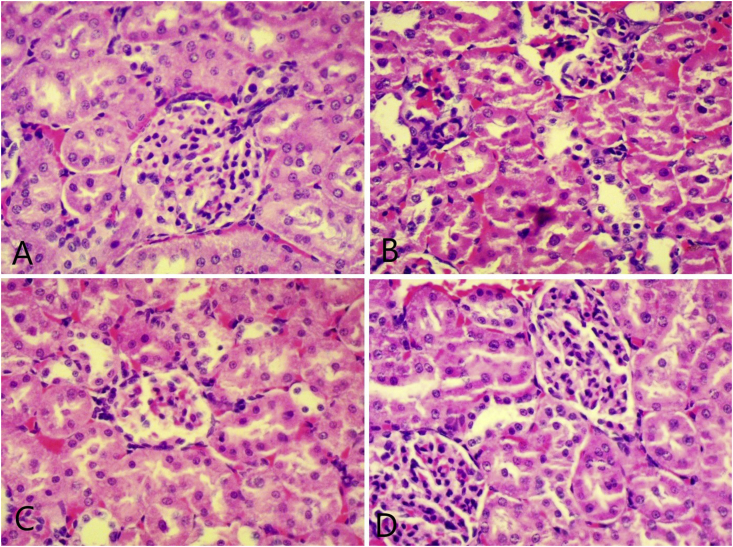
The examination of kidney tissue in Cisplatin curative activity for histological changes showed mononuclear cell infiltration and tubular dilation in the Cisplatin control and high dose (1000 mg/kg) group when compared to the vehicle control group. Cisplatin protective activity histological changes like mononuclear cell infiltration, haemorrhages, tubular dilation, and vacuolation were observed in Cisplatin control and low dose (500 mg/kg) (more pronounced in Cisplatin control group) as compared to the vehicle control group. The high dose (1000 mg/kg) group showed low occurrence in histological changes indicating inhibition of Cisplatin-induced changes in this group (Fig. 5, Fig. 6).
Fig. 5.
Representative photomicrograph (40X) of kidney in the curative protocol; (A) normal control (B) Cisplatin control showing infiltration of mononuclear cells (C) TK low dose treated (D) TK high dose treated.
Fig. 6.
Representative photomicrograph (40X) of kidney in the protective protocol; (A) normal control (B) Cisplatin control showing haemorrhage, tubular dilation and vacuolization (C) TK low dose treated (D) TK high dose treated.
4. Discussion
TK's powder at concentrations of 500 and 1000 mg/kg bw revealed some promising and exciting outcomes in the present study. The animals treated with Cisplatin did not show any significant changes in body weight. This indicates that the tested drug did not alter the metabolism of the animal. There were no significant changes observed in feed intake of animals treated with TK than normal control animals. Relative organ weight of adrenals, heart, liver, brain, kidneys, spleen, and testes has not shown any significant change suggesting that all body organs well-tolerated test drug without any adverse effect on any organ. In this study, it was shown that the administration of Cisplatin to rats caused an increase in creatinine, uric acid, and BUN in plasma in the cisplatin control group. The biochemical parameters were well-supported by renal histology, which showed a prominent renal tissue injury sign. Histological changes like mononuclear cell infiltration, haemorrhages, tubular dilation, and vacuolation were seen in the Cisplatin control group. The expression of KIM-1 in renal tissue has also proved injury in the Cisplatin control group.
In Curative protocol, results showed that TK administration decreases creatinine and BUN levels at both dose levels. The results were found statistically significant at a high dose of TK. Similarly, uric acid levels showed a reduction at both dose levels, but a significant reduction was observed at a low dose of TK. Total protein and serum electrolytes (sodium, potassium, chloride, and calcium) have not shown any significant changes. The estimation of KIM-1 in tissue homogenate indicated no significant changes in KIM-1 at both dose levels of TK.
TK showed a significant reduction of BUN in animals treated with TK at a high dose in the protective model. Similarly, there was a reduction in uric acid at both dose levels of TK but not found statistically significant. Total protein and serum electrolytes (Sodium, Potassium, Chloride, and Calcium) have not shown any significant changes. The estimation of KIM-1 in tissue homogenate showed a reduction at a low TK dose, while the KIM-1 level increases at a high dose of TK. The results indicated that TK showed slight protection about KIM-1 at a low dose, although results were not found statistically significant.
Cisplatin induces alterations in kidney function, as mentioned in the literature. Proximal tubules of the kidneys absorb Cisplatin at a higher concentration than other tissues, due to the copper transporter 1 and the organic cation transporter [22,23]. Expression of inflammatory cytokines and chemokines like interleukin-1β, IL-18, CX3CL1, and IL3 increases due to cisplatin treatment in mice [24]. Cisplatin is extensively used in treating cancers, and its primary side-effect is nephrotoxicity in a dose-dependent manner [25]. Cisplatin accumulates in proximal convoluted tubules, promoting cellular damage by apoptosis, DNA damage, oxidative stress, and inflammation, etc. [26].
The mechanisms of renal toxicity caused by Cisplatin are still not precise now. However, reactive oxygen species and lipid peroxidation have been recommended as a nephrotoxicity mechanism [27,28]. Cisplatin significantly reduces glutathione and increases lipid peroxidation in the mitochondria of the renal cortex [29]. Cisplatin significantly checks superoxide dismutase activities and catalase in the kidney tissues leading to an increase in reactive oxygen species [30]. Cisplatin's aquated forms are highly reactive, particularly with thiol-containing molecules, including glutathione [42]. It also inhibits antioxidant enzymes, including glutathione S-transferase, glutathione peroxidase, and superoxide dismutase [43], shifting the cellular redox status, leading to toxic levels of ROS within the cell. The traditional biochemical markers for the detection of kidney disease involve the determination of the serum creatinine, BUN, uric acid, total protein, and urinary electrolytes. Although specific for kidney injury, these indicators do not detect renal injury in the early phase. KIM-1 is considered a specific marker for early detection of renal injury. As in humans, rat KIM-1 in the urine samples and tissue suggested that KIM-1 may be used as a sensitive early biomarker in animals with nephrotoxicity [31].
TK crude extract has shown protective effects in rats against paracetamol, thioacetamide [35], and CCL4 toxicity [36]. These protective effects can be attributed to the known antioxidant properties of TK [37]. Limonene and phthalides have been shown to induce detoxifying enzyme glutathione S-transferase in the mouse liver [38]. The efficacy of TK may be due to the presence of apigenin, an active constituent of TK with documented antioxidant activity [39]. Celery extract was also reported to significantly decrease the activity of cytochrome P450 in the liver of mice [40]. TK extract also identified to check mucosal inflammation through its inhibitory effect on prostaglandin production [41].
The exposure of tubular cells to Cisplatin activates signaling pathways that are cell death-promoting (MAPK, p53, ROS, and so on). Meanwhile, Cisplatin induces TNF-α production in tubular cells, which triggers a robust inflammatory response, further contributing to tubular cell injury and death. Cisplatin may also induce injury in renal vasculature, leading to ischemic tubular cell death [5].
The phytochemicals include caffeic acid, chlorogenic acid, apiin, apigenin, rutaretin, ocimene, bergapten, isopimpinellin umbelliferone, 8-hydroxy-5-methoxy psoralen, and isoimperatorin, etc. are reported to be found in TK seed [45]. The essential oil, which is about 3% in seeds contains palmitic acid, stearic acid, oleic acid, linoleic acid, petroselinic acid, d-limonene, selinene, terpineol, and santalol [46,47].
Apigenin, a flavone glycoside, has anti-tumour, antioxidant, anti-viral, and neurogenesis stimulator activities [48]. Caffeic acid has antioxidant and antitumour activity [49], while chlorogenic acid has anti-cancer, antioxidant, anti-inflammatory, and analgesic activity [[50], [51], [52]]. Bergapten, a furanocoumarin has anti-cancer and anti-psoriatic actions [53,54]. Isopimpinellin has anti-cancer [55] while Isoimperatorin, cytochrome P-450 inhibitor activity [56]. The 8-hydroxy-5-methoxy psoralen has a cytochrome P-450 inhibitor and anti-psoriatic activity [57]. The umbelliferone has anti-inflammatory, analgesic, antioxidant and neuroprotective activity [[58], [59], [60]].
The essential oil contains palmitic acid, which has antioxidant and anti-cholesterol actions [61,62], while oleic acid increases fatty acid oxidation [63]. Linoleic acid has anti-cancer, and anti-congestive heart failure [64] and stearic acid shows antitumor and anti-cholesterol activity [62,65]. The d-limonene has anti-cancer and spasmolytic activity [66,67]. Selinene has anti-microbial [68], and terpineol shows anti-convulsant, antioxidant, and anti-microbial activity [[69], [70], [71]], while santalol has antitumour activity [72].
The pharmacological actions associated with these chemical constituents may help to formulate a hypothesis about its nephroprotective action. The anti-tumour/anti-cancer activity has been shown by apigenin, bergapten, caffeic acid, chlorogenic acid, d-limonene, isopimpinellin, linoleic acid, santalol, and stearic acid. The antioxidant activity has been shown by apigenin, caffeic acid, chlorogenic acid, umbelliferone, palmitic acid, and terpineol. Analgesic and anti-inflammatory activity have been shown by chlorogenic acid and umbelliferone while 8-hydroxy-5-methoxypsoralen and isopimpinellin have shown p-450 inhibitory activity. The synergistic effect of all these activities proved to be beneficial to the injury caused by Cisplatin-induced nephrotoxicity. Apart from the protective effect, apigenin and other constituents' anti-tumour or anti-cancer activity provides an additive effect of this drug to be used as a protective drug in cancer treatment. As far as herb–drug interaction is concerned, this is to submit that herb has been used for decades without any adverse event report at the prescribed doses. Despite this fact, future studies may be directed to explore this critical aspect of herb–drug interaction.
Unani system of medicines is a complementary and alternative system of medicine. The natural ingredients from plants, minerals, and animals have been used to manage any ailment or maintain homeostasis of the body. In the present study, TK was used in powder form without any alteration or extraction. The study results showed no adverse effects in test group animals as supported by body weight, feed intake and relative organ weight, and renal function tests. TK demonstrated a nephroprotective effect against Cisplatin-induced nephrotoxicity is curative as well as protective protocol. It is supported by the significant reduction in biochemical kidney parameters, i.e., creatinine and BUN. The results of this study validate the Unani classical claim of its nephroprotective effects and its use in the management of kidney ailments.
5. Future direction
We are resubmitting the fact that it is a preliminary study and we have limited proof of our claim only in terms of biochemical parameters and histopathology. However, exact mechanistic activity and comprehensive study may be designed to explore TK's mode of action. This study may be treated as an initial attempt in the right direction for safe and effective management through natural products.
6. Conclusion
It may be concluded that TK's nephroprotective effect was associated with its ability to improve renal function parameters. TK's possible mode of action may be increased glomerular filtration rate resulting in less serum creatinine and maintaining the level of serum magnesium and potassium. TK also has potent antioxidant properties and detoxification capacity, which may also be responsible for its protective effect against Cisplatin toxicity. In our view, these factors cumulatively prevent the inflammatory response and maintain the kidney's normal functions. The exact mechanism of action may be explored in a separate comprehensive study. Hence the present study categorizes TK as a nephroprotective agent and validates the classical Unani claim about its activity and uses in renal ailments.
Source(s) of funding
None.
Conflict of interest
None.
Author contributions
Mohammad Zakir: Conceptualization, Methodology, Supervision, Writing - Original Draft; Mohd Naushad: Investigation, Writing - Reviewing and Editing; Mohd Urooj: Methodology, Formal analysis; Tasleem Ahmad: Investigation; Gulam Mohammed Husain: Resources, Writing - Reviewing and Editing, Formal analysis; Munawwar Husain Kazmi: Project administration, Funding acquisition.
Acknowledgments
The authors would like to acknowledge Director General, CCRUM, New Delhi, for providing all facilities for this research.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jaim.2021.06.005.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Osswald H., Muhlbauer B., Vallon V. Adenosine and tubuloglomerular feedback. Blood Purif. 1997;15(4–6):243–252. doi: 10.1159/000170342. [DOI] [PubMed] [Google Scholar]
- 2.Katzung B.G., Trevor A.J. 13th ed. McGraw Hill education; New York: 2015. Basic & clinical pharmacology; pp. 925–927. [Google Scholar]
- 3.Hartmann J.T., Lipp H.P. Toxicity of platinum compounds. Expet Opin Pharmacother. 2003;4(6):889–901. doi: 10.1517/14656566.4.6.889. [DOI] [PubMed] [Google Scholar]
- 4.Hartmann J.T., Fels L.M., Franzke A., Knop S., Renn M., Maess B., et al. Comparative study of the acute nephrotoxicity from standard dose cisplatin+/-ifosfamide and high-dose chemotherapy with carboplatin and ifosfamide. Anticancer Res. 2000;20(5C):3767–3773. [PubMed] [Google Scholar]
- 5.Pabla N., Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73(9):994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 6.Khan H.M.S. Daftarul Masih, Karol Bagh; New Delhi: 1939. Ilaj-ul Amraz. (Urdu translation of Bayaz-e-Khas by Kabir al-Din) pp. 545–568. [Google Scholar]
- 7.Khan H.M.A. Central Council for Research in Unani Medicine, Dept. of AYUSH, Ministry of H & FW, Govt. of India; New Delhi: 2012. Muhit-i Azam; p. 254. [Google Scholar]
- 8.Jurjani S.I.B.U. Idara Kitabul Shifa; New Delhi: 2008. Dhakhira Khawarizm Shahi; pp. 41–44. [Google Scholar]
- 9.Al-Qamari A.M.H.N. vol. 291. Central Council for Research in Unani Medicine, Dept. of AYUSH, Ministry of H & FW, Govt. of India; New Delhi: 2008. pp. 298–303. (Ghina Muna (Arabic text with Urdu translation by Hakim Muhammad Hadi Husayn Khan)). [Google Scholar]
- 10.Dymock W., Warden C.J.H., Hooper D. vol. II. M/s Bishan Singh Mahendra Pal Singh; Dehradun, India: 1891. pp. 122–124. (Pharmacographia Indica). [Google Scholar]
- 11.Khan H.M.A. vol. 33. Central Council for Research in Unani Medicine, Dept. of AYUSH, Ministry of H & FW, Govt. of India; New Delhi: 2009. (Qarabadin-i Azam). [Google Scholar]
- 12.Ghani N. Idara Kitabul Shifa; New Delhi: 2011. KhazainulAdvia; pp. 206–208. [Google Scholar]
- 13.Anonymous . Dept. of AYUSH, Ministry of H & FW, Govt. of India; New Delhi: 2007. The Unani Pharmacopoeia of India. Part-I. Vol-II; pp. 93–94. [Google Scholar]
- 14.Anonymous. Quality Control . World Health Organization; Geneva: 1998. Methods for herbal materials; pp. 23–33. [Google Scholar]
- 15.Afaq S.H., Tajuddin, Siddiqui M.M.H. AMU Press; Aligarh, India: 1994. Standardization of herbal drugs; pp. 93–94. [Google Scholar]
- 16.Farnsworth N.R. Biological and phytochemical screening of plants. J Pharm Sci. 1966;55(3):225–276. doi: 10.1002/jps.2600550302. [DOI] [PubMed] [Google Scholar]
- 17.Paech M., Tracey M.V. vol. 6. Springer Verlag; Berlin: 1955. pp. 456–471. (Modern methods of plant analysis). [Google Scholar]
- 18.Brewster R.C., Mc Ewen W.E. 3rd ed. Prentice-Hall of India Private Limited; New Delhi: 1971. Organic chemistry; p. 604. [Google Scholar]
- 19.Arthur H.R., Chan P.K. A survey of Hong Kong plants: testing for alkaloids, essential oils and saponins. Trop Sci. 1962;4:147. [Google Scholar]
- 20.Freireich E.J., Gehan E.A., Rall D.P., Schmidt L.H., Skipper H.E. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966;50(4):219–244. [PubMed] [Google Scholar]
- 21.Shirwaikar A., Issac D., Malini S. Effect of Aerva lanata on cisplatin and gentamicin models of acute renal failure. J Ethnopharmacol. 2004;90(1):81–86. doi: 10.1016/j.jep.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 22.Filipski K.K., Mathijssen R.H., Mikkelsen T.S., Schinkel A.H., Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther. 2009;86(4):396–402. doi: 10.1038/clpt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pabla N., Murphy R.F., Liu K., Dong Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Ren Physiol. 2009;296(3):F505–F511. doi: 10.1152/ajprenal.90545.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faubel S., Lewis E.C., Reznikov L., Ljubanovic D., Hoke T.S., Somerset H., et al. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1β, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Therapeut. 2007;322(1):8–15. doi: 10.1124/jpet.107.119792. [DOI] [PubMed] [Google Scholar]
- 25.Safirstein R., Winston J., Goldstein M., Moel D., Dikman S., Guttenplan J. Cisplatin nephrotoxicity. Am J Kidney Dis. 1986;8(5):356–367. doi: 10.1016/s0272-6386(86)80111-1. [DOI] [PubMed] [Google Scholar]
- 26.Yang C., Kaushal V., Shah S.V., Kaushal G.P. Mcl-1 is downregulated in cisplatin-induced apoptosis, and proteasome inhibitors restore Mcl-1 and promote survival in renal tubular epithelial cells. Am J Physiol Ren Physiol. 2007;292(6):F1710–F1717. doi: 10.1152/ajprenal.00505.2006. [DOI] [PubMed] [Google Scholar]
- 27.Matsushima H., Yonemura K., Ohishi K., Hishida A. The role of oxygen free radicals in cisplatin-induced acute renal failure in rats. J Lab Clin Med. 1998;131(6):518–526. doi: 10.1016/s0022-2143(98)90060-9. [DOI] [PubMed] [Google Scholar]
- 28.Karasawa T., Sibrian-Vazquez M., Strongin R.M., Steyger P.S. Identification of cisplatin-binding proteins using agarose conjugates of platinum compounds. PloS One. 2013;8(6) doi: 10.1371/journal.pone.0066220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J.G., Lindup W.E. Cisplatin nephrotoxicity: decreases in mitochondrial protein sulphydryl concentration and calcium uptake by mitochondria from rat renal cortical slices. Biochem Pharmacol. 1994;47(7):1127–1135. doi: 10.1016/0006-2952(94)90383-2. [DOI] [PubMed] [Google Scholar]
- 30.Modi K., Patel B., Patel D., Chavda J., Goyal R. Protective effects of aqueous extract of M. pruriens Linn.(DC) seed against cisplatin induced oxidative stress and nephrotoxicity in rats. Afr J Pharm Pharmacol. 2013;7(28):1994–1999. [Google Scholar]
- 31.Han W.K., Bailly V., Abichandani R., Thadhani R., Bonventre J.V. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 32.El-Arabey A.A. Are Testosterone and BCL6 critical players in cisplatin-induced nephrotoxicity in rats. J Nephrol Therapeut. 2018;8(315):2161. 0959. [Google Scholar]
- 33.Shirbeigi L., Zarei A., Naghizadeh A., Vaghasloo M.A. The concept of temperaments in traditional Persian medicine. Trad Integr Med. 2017;2(3):143–156. [Google Scholar]
- 34.Kabir H. Shamsher Publisher and Distributors; Aligarh, India: 2002. Introduction to Ilmul Advia; pp. 63–64. [Google Scholar]
- 35.Singh A., Handa S.S. Hepatoprotective activity of Apium graveolens and Hygrophila auriculata against paracetamol and thioacetamide intoxication in rats. J Ethnopharmacol. 1995;49(3):119–126. doi: 10.1016/0378-8741(95)01291-5. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed B., Alam T., Varshney M., Khan S.A. Hepatoprotective activity of two plants belonging to the Apiaceae and the Euphorbiaceae family. J Ethnopharmacol. 2002;79(3):313–316. doi: 10.1016/s0378-8741(01)00392-0. [DOI] [PubMed] [Google Scholar]
- 37.Momin R.A., Nair M.G. Antioxidant, cyclooxygenase and topoisomerase inhibitory compounds from Apium graveolens Linn. seeds. Phytomedicine. 2002;9(4):312–318. doi: 10.1078/0944-7113-00131. [DOI] [PubMed] [Google Scholar]
- 38.Zheng G.Q., Kenney P.M., Lam L.K. Potential anticarcinogenic natural products isolated from lemongrass oil and galanga root oil. J Agric Food Chem. 1993;41(2):153–156. [Google Scholar]
- 39.Miean K.H., Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem. 2001;49(6):3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 40.Jakovljevic V., Raskovic A., Popovic M., Sabo J. The effect of celery and parsley juices on pharmacodynamic activity of drugs involving cytochrome P450 in their metabolism. Eur J Drug Metab Pharmacokinet. 2002;27(3):153–156. doi: 10.1007/BF03190450. [DOI] [PubMed] [Google Scholar]
- 41.Whitehouse M.W. Prostanoids as friends, not foes: further evidence from the interference by cycloxygenase-inhibitory drugs when inducing tolerance to experimental arthritigens in rats. Inflammopharmacology. 2005;12(5):481–492. doi: 10.1163/156856005774382788. [DOI] [PubMed] [Google Scholar]
- 42.Fuertes M.A., Alonso C., Pérez J.M. Biochemical modulation of cisplatin mechanisms of action: enhancement of antitumor activity and circumvention of drug resistance. Chem Rev. 2003;103(3):645–662. doi: 10.1021/cr020010d. [DOI] [PubMed] [Google Scholar]
- 43.Khynriam D., Prasad S.B. Changes in endogenous tissue glutathione level in relation to murine ascites tumor growth and the anticancer activity of cisplatin. Braz J Med Biol Res. 2003;36(1):53–63. doi: 10.1590/s0100-879x2003000100008. [DOI] [PubMed] [Google Scholar]
- 44.EL-Arabey A.A., Abd-Allah A.R. Antidepressants as a new approach for protective interventions of cisplatin-induced nephrotoxicity. J Kidney. 2015;1(102):2472. 1220. [Google Scholar]
- 45.Sastri B.N. Council of Scientific and Industrial Research Publication; New Delhi: 1956. The wealth of India, Raw materials; p. 4. [Google Scholar]
- 46.Sastri B.N. Council of Scientific and Industrial Research Publication; New Delhi: 2003. The wealth of India, Raw materials; pp. 64–65. [Google Scholar]
- 47.Pan H.P., Kenney D.W. Quantitative determination of fatty acid constituents of celery seeds by gas-liquid partition chromatography. Proc Fla State Hortic Soc. 1960;73:219–223. [Google Scholar]
- 48.Khole S., Panat N.A., Suryawanshi P., Chatterjee S., Devasagayam T.P., Ghaskadbi S. Comprehensive assessment of antioxidant activities of apigenin isomers: vitexin and isovitexin. Free Radic Antioxidants. 2016;6(2):155–166. [Google Scholar]
- 49.Chen J.H., Ho C.T. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J Agric Food Chem. 1997;45(7):2374–2378. [Google Scholar]
- 50.Dos Santos M.D., Almeida M.C., Lopes N.P., De Souza G.E. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol Pharm Bull. 2006;29(11):2236–2240. doi: 10.1248/bpb.29.2236. [DOI] [PubMed] [Google Scholar]
- 51.Huang M.T., Smart R.C., Wong C.Q., Conney A.H. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1988;48(21):5941–5946. [PubMed] [Google Scholar]
- 52.Sato Y., Itagaki S., Kurokawa T., Ogura J., Kobayashi M., Hirano T., et al. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int J Pharm. 2011;403(1–2):136–138. doi: 10.1016/j.ijpharm.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 53.Hönigsmann H., Jaschke E., Gschnait F., Brenner W., Fritsch P., Wolff K. 5-Methoxypsoralen (Bergapten) in photochemotherapy of psoriasis. Br J Der. 1979;101(4):369–378. doi: 10.1111/j.1365-2133.1979.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 54.Santoro M., Guido C., De Amicis F., Sisci D., Cione E., Dolce V., et al. Bergapten induces metabolic reprogramming in breast cancer cells. Oncol Rep. 2016;35(1):568–576. doi: 10.3892/or.2015.4327. [DOI] [PubMed] [Google Scholar]
- 55.Sumiyoshi M., Sakanaka M., Taniguchi M., Baba K., Kimura Y. Anti-tumor effects of various furocoumarins isolated from the roots, seeds and fruits of Angelica and Cnidium species under ultraviolet A irradiation. J Nat Med. 2014;68(1):83–94. doi: 10.1007/s11418-013-0774-z. [DOI] [PubMed] [Google Scholar]
- 56.Cao Y., Zhong Y.H., Yuan M., Li H., Zhao C.J. Inhibitory effect of imperatorin and isoimperatorin on activity of cytochrome P450 enzyme in human and rat liver microsomes. Zhongguo Zhongyao Zazhi. 2013;38(8):1237–1241. [PubMed] [Google Scholar]
- 57.Koenigs L.L., Trager W.F. Mechanism-based inactivation of cytochrome P450 2B1 by 8-methoxypsoralen and several other furanocoumarins. Biochemistry. 1998;37(38):13184–13193. doi: 10.1021/bi981198r. [DOI] [PubMed] [Google Scholar]
- 58.Lino C.S., Taveira M.L., Viana G.S., Matos F.J. Analgesic and antiinflammatory activities of Justicia pectoralis Jacq and its main constituents: coumarin and umbelliferone. Phytother Res. 1997;11(3):211–215. [Google Scholar]
- 59.Ramesh B., Pugalendi K.V. Antioxidant role of Umbelliferone in STZ-diabetic rats. Life Sci. 2006;79(3):306–310. doi: 10.1016/j.lfs.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 60.Subramaniam S.R., Ellis E.M. Neuroprotective effects of umbelliferone and esculetin in a mouse model of Parkinson's disease. J Neurosci Res. 2013;91(3):453–461. doi: 10.1002/jnr.23164. [DOI] [PubMed] [Google Scholar]
- 61.Shukla S., Mishra T., Pal M., Meena B., Rana T.S., Upreti D.K. Comparative analysis of fatty acids and antioxidant activity of Betula utilis bark collected from different geographical region of India. Free Radic Antioxidants. 2017;7(1):80–85. [Google Scholar]
- 62.Tholstrup T., Marckmann P., Jespersen J., Sandström B. Fat high in stearic acid favorably affects blood lipids and factor VII coagulant activity in comparison with fats high in palmitic acid or high in myristic and lauric acids. Am J Clin Nutr. 1994;59(2):371–377. doi: 10.1093/ajcn/59.2.371. [DOI] [PubMed] [Google Scholar]
- 63.Lim J.H., Gerhart-Hines Z., Dominy J.E., Lee Y., Kim S., Tabata M., et al. Oleic acid stimulates complete oxidation of fatty acids through protein kinase A-dependent activation of SIRT1-PGC1α complex. J Biol Chem. 2013;288(10):7117–7126. doi: 10.1074/jbc.M112.415729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramsden C.E., Zamora D., Leelarthaepin B., Majchrzak-Hong S.F., Faurot K.R., Suchindran C.M., et al. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ. 2013;346 doi: 10.1136/bmj.e8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu F.Q., Liu L.N., Du Y.Z., Yuan H. Synthesis and antitumor activity of doxorubicin conjugated stearic acid-g-chitosan oligosaccharide polymeric micelles. Biomaterials. 2009;30(36):6955–6963. doi: 10.1016/j.biomaterials.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 66.Vandresen F., Falzirolli H., Batista S.A., da Silva-Giardini A.P., de Oliveira D.N., Catharino R.R., et al. Novel R-(+)-limonene-based thiosemicarbazones and their antitumor activity against human tumor cell lines. Eur J Med Chem. 2014;79:110–116. doi: 10.1016/j.ejmech.2014.03.086. [DOI] [PubMed] [Google Scholar]
- 67.de Sousa D.P., Mesquita R.F., de Araújo Ribeiro L.A., de Lima J.T. Spasmolytic activity of carvone and limonene enantiomers. Nat Prod Commun. 2015;10(11) 1934578X1501001120. [PubMed] [Google Scholar]
- 68.Alves J.A., Mantovani A.L., Martins M.H., Abrao F., Lucarini R., Crotti A.E., et al. Antimycobacterial activity of some commercially available plant-derived essential oils. Chem Nat Compd. 2015;51(2):353–355. [Google Scholar]
- 69.de Sousa D.P., Quintans L., Jr., de Almeida R.N. Evolution of the anticonvulsant activity of α-terpineol. Pharm Biol. 2007;45(1):69–70. [Google Scholar]
- 70.Carson C.F., Riley T.V. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J Appl Bacteriol. 1995;78(3):264–269. doi: 10.1111/j.1365-2672.1995.tb05025.x. [DOI] [PubMed] [Google Scholar]
- 71.Burits M., Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14(5):323–328. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 72.Saraswati S., Kanaujia P.K., Agrawal S.S. OP-03 α-Santalol demonstrates antitumor and antiantiangiogenic activities in models of hepatocellular carcinoma in vitro and in vivo. Dig Liver Dis. 2013;45:S249–S250. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.