Abstract
Background
The anti-cancer activity of phytomolecules present in turmeric or haridra (Curcuma longa Linn) extracts against cancer has been described in various ‘in vitro and in vivo’ studies.
Objective
In the present study, in vitro and in vivo anti-cancer and chemo-preventive activity of a new standardized Supercritical Turmeric Oil Extract (SCTOE) NBFR-03 was evaluated in cervical cancer models.
Methods and materials
In vitro cytotoxicity of this formulation was assessed at 10, 20, 40, and 80 μg/ml concentrations, in three cervical cancer cell lines (HeLa, SiHa, ME180) using Sulforhodamine B assay. The in vivo anti-cancer activity was evaluated in two groups of female nude mice; the first one was with tumor xenograft implants and at the same time treatment was started with 96 μl/kg/day p.o. and 192 μl/kg/day p.o. NBFR-03 for three months. The second group was kept as chemoprevention group where mice were pre-treated with the formulation (96 μl/kg/day p.o.) for two weeks and injected with cancer cell suspension with continued treatment for three months.
Results
No cytotoxicity was seen in any cell line with the extract when compared to positive control (Adriamycin 10 μg/ml). In mice the first treatment group with tumor xenograft implants did not show any significant anti-tumor activity but showed a trend where higher dose group had smaller tumor volumes as compared to lower dose group and controls (p = 0.37 and p = 0.34 respectively). The chemopreventive group with pre-treated mice also showed smaller tumor size as compared to controls (p = 0.163).
Conclusion
NBFR-03 turmeric oil extract showed a promising trend in mice pre-treated with NBFR-03. There is a scope for further studying the potential of this extract as complementary therapy and as a chemopreventive.
Keywords: Cervical cancer, Chemoprevention, Anticancer, Medicinal plants, Supercritical turmeric oil extract, Prevention of cervical cancer
1. Introduction
Cervical cancer is a significant cause of morbidity and mortality among women in developing countries [1,2]. The recent cervical cancer statistics in India has shown that the mortality due to cervical cancer in the year 2018 was 63,000 women whereas in 2020 it was 70,000 [3]. In India, the treatment for cervical cancer is expensive and beyond the reach of majority of women and it is often diagnosed in advanced stages. Currently in the screening programmes with Papanicolaou (Pap) smears or with other tests we can detect not only early stage cancer but also the pre-cancerous phases known as Cervical Intraepithelial Neoplasia (CIN) as designated in histological samples, or biopsy or known as Squamous Intraepithelial Lesions (SIL) in cytology by Pap smears, which could be of low grade or high grade [4]. Treatment of pre-cancerous conditions can be almost 100% successful and surgical or chemo-preventive pharmacotherapy can be used to prevent this disabling and destructive disease [1,[5], [6], [7]].
There is no doubt that healthy sexual habits and use of Human Papilloma Virus (HPV) vaccine are the best measures for primary prevention of cervical cancer but these cannot been adopted in all public health programs because of high cost and poor compliance by the public. Hence, secondary prevention, by treatment of pre-cancerous conditions (SIL or CIN), is an important option to prevent cervical cancer. Moreover, available vaccines may prevent only 70% of cervical cancers, not all, because the vaccine may not be effective against all subtypes of carcinogenic HPV [8].
Phytochemicals from many medicinal plants have been studied for anti-cancer activity [9]. Natural phytoconstituents have been reported to block carcinogenesis and to inhibit tumor progression both in vitro and in vivo models [10]. These chemopreventive effects can be attributed to immunomodulation, growth arrest in cell cycle, induction of apoptosis, inhibition of oncogenic pathways like NF-kβ, kinases, DNA synthesis inhibition, and modulation of other signal transduction pathways. Chemoprevention by natural products, primarily affects transforming cells without causing any damage to normal cells. There are some clinical trials for cervical cancer chemoprevention with dietary supplements, micronutrients and other chemical agents but these are not effective in all cases or the chemical agents may cause unacceptable side-effects [[11], [12], [13], [14]].
Haridra (Curcuma longa Linn), mentioned in classical Ayurvedic texts, and commonly known as turmeric or haldi, is being used traditionally for several disorders like dyspepsia, wound healing, bleeding disorders, diabetes, hemorrhoids, anemia, skin diseases, etc. There are many bioactive substances reported from turmeric, but the key active ingredients of C. longa include curcuminoids: curcumin (diferuloylmethane), demethoxycurcumin, monodemethoxycurcumin, cyclocurcumin, bisdemethoxycurcumin, and curcumene. Furthermore, volatile oils (turmerone, zingiberene, α-phellandrene, sabinene, cineol, borneol and sesquiterpines) also have pharmacological activity [[15], [16], [17]].
The use of haridra extracts in Arbuda (cancer) has been described in Brihattrayi which are the most revered three text books or granthas (Samhitas) in Ayurveda written by the three great ancient rishis Charak, Sushrut and Vagbhat or Nighantus [[18], [19], [20], [21]]. Cancer or Arbuda has been described in Ayurveda as below:
(A rounded, immobile, painless, deep rooted, growing continuously, and non-suppurative swelling, which erodes into the muscle with extensive destruction, is called as Arbuda by experts).
This description matches the description of cancer (solid malignant tumours) in allopathic or modern medicine [22].
There is extensive literature on Ayurvedic indications and usage of turmeric extracts in clinical practice supported by phytochemistry, clinical trials, and in vitro and in vivo experiments, but this is beyond the scope of this article. Its specific use in cancer has been elaborated in Yogratnakar as below [23].
(Haridra, Lodhra, Patranga, Dhooma, and Manahsheela along with honey can be used as thick local application in fleshy or fatty arbuda).
The use of turmeric oil is specifically mentioned for oral cancer in classical texts of Ayurved [24].
(Two types of turmeric, pippali, saindhav, deodaru, vidanga, chitrak, bilwa, leaves of rohish should be mixed with twice the amount of water and made siddha in taila, it is useful in kapha type sannipatik disease and arbuda, or tumor, of the head, throat, neck and palate).
We have previously demonstrated the safety and anti-cancer activity of different types of turmeric extracts [25]. Our earlier studies have shown that turmeric oil protected the cells from benzo[a]pyrene induced damage in experimental models. In the clinical study, it reduced the number of micronuclei in oral mucosal cells and peripheral blood lymphocytes in patients with oral submucous fibrosis, along with increased antioxidant activity [9]. We have also reported the safety and tolerability of turmeric oil in healthy human volunteers [26]. However, there was no study on its activity in different cervical cancer cell lines or cervical cancer animal models. Therefore, a standardised turmeric extract NBFR-03 was evaluated for its anti-cancer and chemopreventive activity in paraclinical and clinical studies.
This formulation NBFR-03 contains supercritical extract of turmeric oil (SCTOE) which is a by-product of curcumin extraction, manufactured and standardised in collaboration with our centre, and coded by Nisarga Biotech Private Ltd (NBPL). Further, NBFR-03 was evaluated for chemopreventive activity and safety in women with Low - Grade Squamous Intraepithelial Lesion (LSIL), a pre-cancerous condition for cervical cancer [27,28]. The data presented in this article is a part of paraclinical studies of above-mentioned formulation carried out during the year 2009 which remained unpublished [29]. The objective of this study was to determine whether NBFR-03 had any in vitro or in vivo anti-cancer activity in experimental studies in collaboration with Advanced Centre for Treatment, Research and Education in Cancer (ACTREC), Kharghar. The clinical trial was published earlier; however, the authors believe that in view of the importance of the positive potential chemopreventive activity of turmeric oil, demonstrated in this animal model, this data also needs to be documented in literature [27].
2. Materials and methods
The formulation was developed by MRC-KHS in collaboration with Nisarga Biotech Pvt Ltd, Satara, from the supercritical extract of turmeric rhizome (C. longa Linn) oil as described earlier [27]. It was manufactured according to the GMP requirement and tested for the heavy metals, pesticides, microbial load and aflatoxin, and standardized by gas chromatography and mass spectrometry. Dosage of the formulation was calculated as per the human dose used in previous studies and by clinicians [26]. A safety and toxicity study in healthy rats and rabbits was conducted by National Toxicology Centre in Pune (Unpublished data). The dosage was 0.2 ml of NBFR-03, twice a day in humans and was calculated as per the earlier clinical studies on traditionally used turmeric oil extract in volunteers and Oral Submucous Fibrosis. The in vitro and in vivo studies were planned in collaboration with the ACTREC, Kharghar. The project was approved by the Institutional Ethics Committee at ACTREC.
2.1. In vitro anti-cancer activity
2.1.1. Cell culture
Cervical cancer cell lines ME180 (HPV negative), HeLa (HPV-16 positive), and SiHa (HPV-18 positive) were used for the study. ME180 was maintained in Minimal Essential Medium (MEM), and SiHa and HeLa in Dulbecco's Modified Eagles Medium (DMEM), supplemented with 10% (v/v) heat-inactivated fetal bovine serum, [32] penicillin and streptomycin antibiotics and HEPES (Himedia) in a humidified atmosphere of 95% air and 5% CO2 at 37 °C. The formulation was dissolved in dimethyl sulfoxide (DMSO) and intervention studies were carried out with 10, 20, 40 and 80 μg/ml concentrations.
2.1.2. Sulforhodamine B (SRB) assay
In vitro anti-proliferative activity of NBFR-03 was assayed by Sulforhodamine B assay [30]. 5 × 103 cells were seeded in 96 well-plate and were allowed to adhere and grow for a further 24 h. On day 2, fresh medium in different concentrations (10, 20, 40 and 80 μg/ml) of the formulation was added and incubated. Cells were fixed after 72 h of treatment with ice-cold 10% trichloroacetic acid (TCA) for 1hr at 4 °C. The plates were washed 5 times in distilled water, stained with SRB dye and again washed with 1% v/v acetic acid. The dye was solubilised by adding 100 μl of 100 mM tris base (pH 10.5). The assay was performed in triplicates. The percent (%) growth was calculated from the optical density readings at 540 nm with reference to 690 nm. Appropriate media and solvent controls were kept. Adriamycin (10 μg/ml) was used as a positive control.
2.2. In vivo anti-cancer activity
Initially normal mice were used for gross toxicity with all doses.
2.2.1. Nude mouse human cervical cancer xenograft model
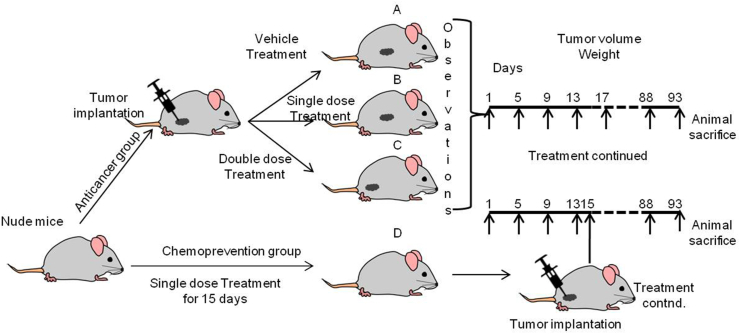
Female nude mice, 6–8 weeks old, weighing 18–22 g, were used to assess the anti-cancer and chemopreventive activity of NBFR-03. These are athymic mice which do not reject a subcutaneous implant of xenobiotic cancer tissue, removed from a cervical cancer patient [31]. The animals were kept at 23 ± 2 °C with relative humidity maintained between 50 and 60%. The mice were housed in transparent polycarbonate filter top cages and were fed with pelleted turmeric free feed. Mice selected for experiments were free from any other disease. Weight of these animals ranged from 16.5 to 20.8 gm. All study procedures employed were approved by the ACTREC institutional animal ethics committee.
Solid human cervical tumour HPV positive tissue (earlier preserved in a frozen state), was cut into 2 × 2 mm size pieces, and these were implanted subcutaneously in mice to allow growth initiation. For this procedure, the mice were transferred to a laminar hood and anesthetized. The abdominal surface was cleaned with 95% ethanol. An incision (1 cm) was made in the lateral abdominal wall above the peritoneum. The cervical implant was inserted into a subcutaneous pocket created by blunt dissection in the adipose tissue. The wound was closed using 4-O vicryl suture.
The mice were divided in 4 groups of 6 animals per group:
-
A.
Tumor bearing mice treated with oral intragastric tube feeds of vehicle control (sesame oil) for three months
-
B.
Tumor bearing mice treated with test drug NBFR-03 single dose (96 μl/kg/day p.o.) for three months
-
C.
Tumor bearing mice treated with oral intragastric tube feeds of test drug NBFR-03 double dose (192 μl/kg/day p.o.) for three months
-
D.
Pre-treated with oral intragastric tube feeds of NBFR-03 single dose (96 μl/kg/day p.o.) for 15 days followed by subcutaneous injection of cancer cell suspension [32] and continued treatment with test drug up to three months.
The mice were examined twice a week for weight and tumour growth. Macroscopic tumours were measured by height, width, and length using callipers. Tumour volume was calculated using the formula ½ x length x width x height. Mice were housed for 3 months following transplantation until tumour growth was externally obvious and the mice were then euthanized.
Relative tumor volume (RTV) and test vs control ratio (T/C) was calculated as follows:
| RTV = TVn/TV0, |
where TVn is the tumor volume on day n and TV0 is the tumor volume on day 0.
T/C = mean RTV of treated group/mean RTV of control group.
2.3. Statistical analysis
The data represents mean ± SEM. Student's t-test was applied to compare control and experimental groups. P value of < 0.05 is used for statistical significance.
3. Results and discussion
3.1. In vitro cytotoxicity
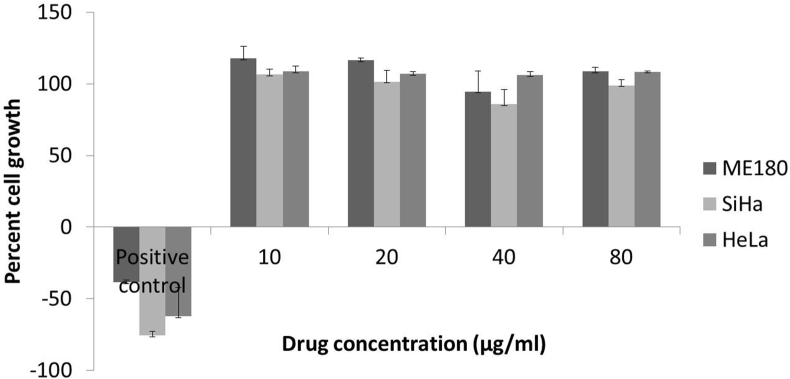
The study drug showed no significant cytotoxicity (Fig. 2) as compared to positive control is SRB assay. Maximum concentration of treatment (80 μg/ml) also could not inhibit the cell growth.
Fig. 2.
Percent growth of ME180, SiHa, HeLa cells after treatment with different concentrations of turmeric oil and positive control (10 μg/ml Adriamycin).
Our concentrations were based on our earlier experience with clinical studies [26]. Curcuma oil was used by Cheng et al. in human hepatocellular carcinoma cells and they found apoptotic activity at higher concentrations (100 μg/ml onwards) [33]. Li et al. also used similar extract containing curcuma oil in Hepa1-6 cells with concentrations up to 500 μg/ml and cell viability was reduced significantly [34]. This suggests that turmeric oil could be active at higher concentrations in vitro but in view of clinical safety it is important to study the organ safety of the extract.
3.2. In vivo anti-cancer activity of NBFR-03 in nude mice (Group A, B and C)
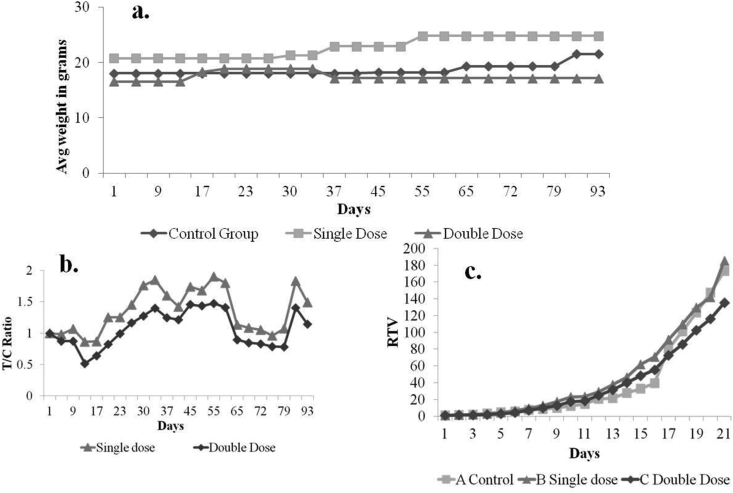
Gross toxicity of the formulation was evaluated in normal mice. There was no toxicity seen in the normal animals at a high dose also. In vivo anti-cancer activity of the extracts was studied in nude mice classified in four groups (Fig. 1). At the end of three months, survival of the animals in group A, B, and C was 33%, 50% and 50% respectively, indicating poor survival in untreated group. Average weight of mice in group A, B, and C did not show any significant difference (Fig. 3 a).
Fig. 1.
Schematic view of flow of activities for in vivo experiment.
Fig. 3.
In vivo study group A, B and C. a. Weight of animals in control and test group, b. T/C ratio of single (96 μl/kg/day p.o) and double dose (192 μl/kg/day p.o) groups, c. Relative tumor volume of control and test groups.
3.3. Tumor volume
Tumor volume of mice in all groups was measured twice a week. The mice, treated with NBFR-03 192 μl/kg/day (group C) showed reduced tumor volume as compared to the control, however the difference was not statistically significant (p value: 0.34). After 3 months, mean ± SEM tumor volume of group A, B, and C were 10.1 ± 2.96, 12.34 ± 5.23, and 6.36 ± 1.61 CC respectively.
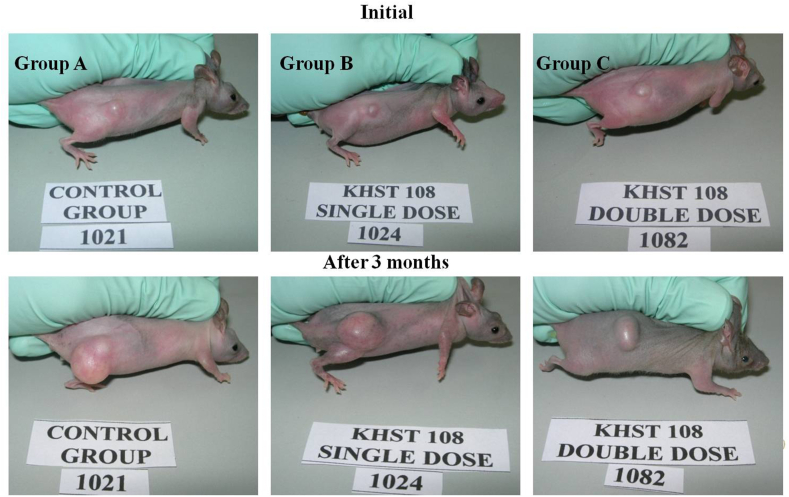
T/C value shows the quantitative efficacy of a test drug on tumor volume. T/C value below 0.2 is considered as strong anti-cancer activity whereas T/C value below 0.45 confirms intermediate anti-cancer activity of the drug [35,36]. Although the T/C values for group B and C are above the cut-off point (0.86 and 0.52 respectively), there is a noticeable trend towards decrease in T/C as a function of time and concentration of NBFR-03 (Fig. 3 b). RTV data also shows that after 15 days of treatment, tumor growth rate was slower in group C than control (Fig. 3 c). The macroscopic images of tumor in control and test group animals also show a trend of reduction after 3 months of treatment (Fig. 4).
Fig. 4.
Images of control and test group nude mice with xenograft implantation before treatment and after 3 months of treatment with NBFR-03 in 2 doses.
3.4. Group D
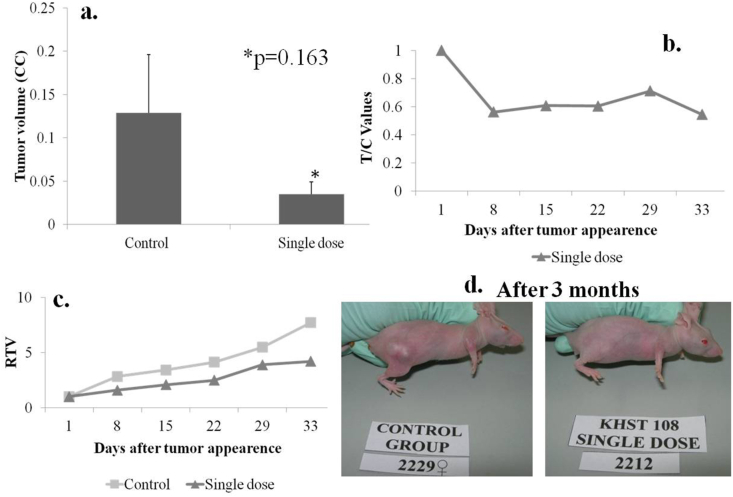
Mice in group D were pre-treated with single oral dose (96 μl/kg/day p.o) of the extract for 15 days before tumor injection. There was 100% survival in control and test group animals. There was no significant difference in animal weights of test group and control group. At the end of study, average tumor volume of chemopreventive group D was found to be lesser (0.035 ± 0.013 CC) as compared to controls (0.128 ± 0.067) (Fig. 5 a). The minimum T/C value of group D was 0.54, which is higher than the cut-off point (Fig. 5 b). Relative tumor volume of group D shows slower growth as compared to control group (Fig. 5 c). The macroscopic image also shows the difference in the tumor size of control and test animals at the end of study (Fig. 5 d).
Fig. 5.
In vivo study group D. a. Tumor volume of animals in control and test group, b. T/C ratio of D group, c. Relative tumor volume of control and test groups, d. Images of vehicle control and test group animals after 3 months.
Results of in vivo experiments clearly show that NBFR-03 did not show any major toxicity in nude mice; moreover, their weight increased significantly during the treatment.
The formulation used in our study primarily contained turmerone and ar-turmerone [27,37]. Like curcumin, turmerone is also reported to be an important bioactive compound present in C. longa rhizomes. The chemoprevention showed by turmerone is mainly due to its antioxidant and anti-inflammatory activity. Ar-turmerone affects the cancer cell by causing apoptosis and cell cycle arrest similar to curcuminoids. Turmerone also increases the bioavailability of curcumin and potentiates the anti-cancer activity [17,38].
In a subsequent study, curcuma oil has shown chemopreventive and hepatoprotective activity against hepatocellular carcinoma in mice. It was found to be active against implanted hepatoma and showed anti-inflammatory, antioxidant, and anti-cancer activities [34]. However, our study with turmeric oil in oral submucous fibrosis was conducted in 1997, the clinical study in cervical pre-cancer was initiated in 2007, and was reported in 2011 [9,27].
In our recent in vitro study NBFR-03 showed anti-proliferative activity at higher concentrations. It also demonstrated immunomodulatory activity by significant reduction in TNF-α and IL-6 levels in lipopolysaccharide (LPS) stimulated monocyte culture [39]. Moreover, a significant anti-angiogenic activity was seen in Chorio-Allantoic Membrane (CAM) model by reducing blood vessel formation [40].
In the present study, reduced tumour size in mice pre-treated with NBFR-03 indicated the potential chemopreventive role of the extract and encouraged us to undertake the clinical study [27]. In the clinical study, 21 cases were enrolled, with cervical LSIL detected in Pap smears, for integrative treatment with anti-microbials followed by oral turmeric oil treatment for 12 weeks, and some of these were followed up to 3 years. In the clinical study, 16 out of 19 women showed regression from LSIL to normal and 3 women had arrest of lesion in Pap smear, after treatment with oral NBFR-03 formulation. It also reduced serum IL-6 levels in some cases indicating possible inhibition of NFkβ activity as reported by other authors [27,41,42]. Out of these, 10 cases were available for follow-up by Pap smears/colposcopy from 6 to 36 months and none progressed to high grade lesion up to 3 years after discontinuation of treatment [28]. In another recent clinical study by our group, a different standardised oral extract of turmeric (Haldone® 600 mg BD) with curcumin, turmeric oil, and curcuma polysaccharides, was evaluated in women with cervical LSIL. Within 10 weeks of treatment 18 out of 20 cases showed regression in Pap smear and no major side-effects were observed and 2 were arrested i.e., did not progress to higher grade. The reduced nuclear diameter and nucleo/cytoplasmic ratios by micrometry were observed in both clinical studies [43].
4. Conclusion
Reverse Pharmacology path allowed us to conduct our experimental and clinical studies to be planned in a meaningful way with limited resources [25]. In conclusion, we can say that ‘in vitro’ experiments may give better results with pure compounds and cervical CIN (pre-cancer) cell lines could be used in future to assess the chemopreventive activity. In experimental animals, the use of pre-treatment with the chemopreventive therapy is likely to give more insights than simultaneous administration of the drug with tumor xenograft. More recent ‘in vivo’ models also display a cervical dysplasia or pre-cancer change before cancer develops and these may be good for prediction of chemopreventive efficacy of new agents [44].
These results indicate that whilst the turmeric extracts alone may not cure invasive or advanced cancer, they have the potential to prevent cancer when used in integrative chemoprevention therapy. These extracts also possibly reduce the side-effects of cancer therapy and modify the progress of cancer when used as complementary therapy [45].
Source(s) of funding
Department of Biotechnology, GOI, New Delhi.
Conflict of interest
None.
Author contributions
P.H. Paradkar: Conceptualization, Writing - Original Draft, A.S. Juvekar: Methodology, Resources, Validation, M.S. Barkume: Methodology, Formal analysis, A.J. Amonkar: Conceptualization, Supervision, J.V. Joshi: Conceptualization, Supervision, Writing - Review & Editing, Project administration, Funding acquisition, G. Soman: Resources, Formal analysis, A.D.B. Vaidya: Conceptualization, Supervision.
Acknowledgement
We are grateful to Department of Biotechnology, GOI, New Delhi for the financial support of the study and to Kasturba Health Society's Medical Research Centre, Mumbai, and ACTREC, Kharghar, for infrastructure facilities.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Lonkey N.M. Preventing morbidity & mortality from cervical cancer. Clin Obt Gyn. 2014;57:239–324. [Google Scholar]
- 2.Sankaranarayanan R., Boffetta P. Research on cancer prevention, detection and management in low- and medium- income countries. Ann Oncol. 2010;21:1935–1943. doi: 10.1093/annonc/mdq049. [DOI] [PubMed] [Google Scholar]
- 3.Mathur et al ; on behalf of ICMR-NCDIR-NCRP Investigator Group 2020Cancer Statistics Report from national cancer registry programme, India. Glob J Clin Oncol. 2020;6:1063–1075. doi: 10.1200/GO.20.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reichheld A., Mukherjee P.K., Rahman S.M.F., David K.V., Pricilla R.A. Prevalence of cervical cancer screening and awareness among women in an urban community in South India—a cross sectional study. Ann Glob Health. 2020;86:1–7. doi: 10.5334/aogh.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaliopoulas G., Nyaga V.N., Santesso N., Bryant A., Martin-Hirsch P.P., Mustafa R.A., et al. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev. 2017;8:CD008587. doi: 10.1002/14651858.CD008587.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin-Hirsch P.P., Paraskevaidis E., Bryant A., Dickinson H.O., Keep S.L. Surgery for cervical intraepithelial neoplasia. Cochrane Database Syst Rev. 2010;6:CD001318. doi: 10.1002/14651858.CD001318.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurtado-Roca Y., Becerra-Chauca N., Malca M. Efficacy and safety of cryotherapy, cold cone or thermocoagulation compared to LEEP as a therapy for cervical intraepithelial neoplasia: systematic review. Rev Saude Publica. 2020;54:27. doi: 10.11606/s1518-8787.2020054001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luckett R., Feldman S. Impact of 2-, 4- and 9-valent HPV vaccines on morbidity and mortality from cervical cancer. Hum Vaccines Immunother. 2016;126:1332–1342. doi: 10.1080/21645515.2015.1108500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hastak K., Lubri N., Jakhi S.D., More C., John A., Ghaisas S.D., et al. Effect of turmeric oil and turmeric oleoresin on cytogenetic damage in patients suffering from oral submucous fibrosis. Cancer Lett. 1997;116:265–269. doi: 10.1016/s0304-3835(97)00205-x. [DOI] [PubMed] [Google Scholar]
- 10.Domenico F.D., Foppoli C., Coccia R., Perluigi M. Antioxidants in cervical cancer: chemopreventive and chemotherapeutic effects of polyphenols. Biochim Biophys Acta. 2012;1822:737–747. doi: 10.1016/j.bbadis.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Zou C., Liu H., Feugang J.M., Hao Z., Chow H.H., Garcia F. Green tea compound in chemoprevention of cervical cancer. Int J Gynecol Cancer. 2010;20:617–624. doi: 10.1111/IGC.0b013e3181c7ca5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasieni P. Chemoprevention of cervical cancer. Best Pract Res Clin Obstet Gynaecol. 2006;20:295–305. doi: 10.1016/j.bpobgyn.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Castanon A., Tristram A., Mesher D., Powell N., Beer H., Ashman S., et al. Effect of diindolylmethane supplementation on low-grade cervical cytological abnormalities: double-blind, randomised, controlled trial. Br J Cancer. 2012;106:45–52. doi: 10.1038/bjc.2011.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park W., Amin R., Chen Z.G., Shin D.M. New perspectives of curcumin in cancer prevention. Cancer Prev Res (Phila) 2013;6:387–400. doi: 10.1158/1940-6207.CAPR-12-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathai N.J., Sony D., Mane P.P., Shetty C.B., Latheef L., Kamath K., et al. In: Polyphenols: prevention and treatment of human disease. 2nd ed. Watson Ronald Ross, Preedy Victor R., Zibadi Sherma., editors. Academic Press; 2018. Chapter 20 - antiarthritic effects of turmeric and curcumin: a revisit; pp. 247–252. [Google Scholar]
- 16.Yue G.G., Chan B.C., Hon P.M., Lee M.Y., Fung K.P., Leung P.C., et al. Evaluation of in vitro anti-proliferative and immunomodulatory activities of compounds isolated from Curcuma longa. Food Chem Toxicol. 2010;48:2011–2020. doi: 10.1016/j.fct.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jayaprakasha G.K., Jena B.S., Negi P.S., Sakariah K.K. Evaluation of antioxidant activities and antimutagenicity of turmeric oil: a byproduct from curcumin production. Z Naturforsch C Biosci. 2002;57:828–835. doi: 10.1515/znc-2002-9-1013. [DOI] [PubMed] [Google Scholar]
- 18.Acharya J.T., editor. Charak Samhita of Agnivesa, Chikitsa Sthana; Prameh Chikitsa: Chapter 6, verse 26. 5th ed. Chaukhambha Sanskrit Sansthan; Varanasi: 2000. p. 447. [Google Scholar]
- 19.Acharya J.T., editor. Sushrut Samhita of Susrita, Chikitsa Sthana; Granthyapachyarbudagalagandachikitsitam: Chapetr 18, Verse 41. 7th ed. Chaukhambha Orientalia; 2002. p. 474. [Google Scholar]
- 20.Bhishagacharya Pandit H. Reprint, Ashtanga Hrudayam of Vagbhata, Uttarsthana; Granthadipratishedh: Chapter 30, Verse 22. Krushna Das academy; Varanasi: 2000. p. 885. [Google Scholar]
- 21.Mishra B.S., editor. Bhav Prakash of Bhav Mishra, Nighantu bhag; Haritakyadi varga: verse 197. 11th ed. Chaukhambha Sanskrit Bhavan; Varanasi: 2007. p. 114. [Google Scholar]
- 22.Shastri A.D., editor. Sushrut Samhita of Sushrut, Nidan sthana: Granthi-apachi-arbuda-galagandanam nidanam, chapter 11, verse 13. 11th ed. Chaukhambha Sanskrit Sansthan; Varanasi: 1997. p. 272. [Google Scholar]
- 23.Shastri L.P., editor. Yogratnakar, Uttarardha arbud chikitsa: Verse 6. Chaukhambha Prakashan; Varanasi: 2018. p. 156. [Google Scholar]
- 24.Bhattacharya J.V., editor. Vangasena Samhita of Vangasena, Chikitsasarsangra, Mukharogadhikar, Verse 107, 109, 110. Siddheshwar press Kolkata; 1981. pp. 181–182. [Google Scholar]
- 25.Vaidya A.B., Amonkar A.J., Bhatt N.S., Parikh P.M. In: Alternative & complementary therapies for cancer: integrative approaches and discovery of conventional drugs. Moulay Alaoui-Jamali., editor. Springer; 2010. Complementary and alternative medicine for cancer care in India: basic and clinical perspective; p. 31e82. [Google Scholar]
- 26.Joshi J., Ghasias S., Vaidya A., Vaidya R., Kamat D.V., Bhagwat A.N., et al. Early human safety study of turmeric oil (Curcuma longa oil) administered healthy volunteers. J Assoc Phys India. 2003;51:1055–1060. [PubMed] [Google Scholar]
- 27.Joshi J.V., Paradkar P.H., Jagtap S.S., Agashe S.V., Soman G., Vaidya A.B. Chemopreventive potential & safety profile of a curcuma longa extract in women with cervical low- grade squamous intraepithelial neoplasia. Asian Pac J Cancer Prev APJCP. 2011;12:3305e11. [PubMed] [Google Scholar]
- 28.Joshi J.V., Jagtap S.S., Paradkar P.H., Walwatkar P., Paradkar H.S., Affandi Z.M., et al. Cytologic follow up of Low-grade Squamous Intraepithelial Lesions in Pap smears after integrated treatment with antimicrobials followed by oral turmeric oil extract. J Ayurveda Integr Med. 2016;7:109–112. doi: 10.1016/j.jaim.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi J.V., Amonkar A.J., Paradkar P.H., Juvekar A., Soman G., Vaidya A.D.B. Update Ayurveda-2010 bridging therapeutic gaps, Mumbai. 2010. In vitro & in vivo anticancer activity of Haridra extract NBFR-03 in cervical cancer. November 25-27. [Google Scholar]
- 30.Keepers Y.P., Pizao P.E., Peters G.J., van Ark-Otte J., Winograd B., Pinedo H.M. Comparison of the sulforhodamine B protein and tetrazolium (MTT) assays for in vitro chemosensitivity testing. Eur J Cancer. 1991;27:897–900. doi: 10.1016/0277-5379(91)90142-z. [DOI] [PubMed] [Google Scholar]
- 31.Alley M.C., Hollingshead M.G., Dykes D.J., Waud W.R. In: Anticancer drug development guide: preclinical screening, clinical trials, and approval. 2nd ed. Teicher B.A., Andrews P.A., editors. Humana Press, Inc; Totowa (NJ): 2004. Human tumor xenograft models in NCI drug development; pp. 125–152. [Google Scholar]
- 32.Bagnato A., Cirilli A., Salani D., Simeone P., Muller A., Nicotra M.R. Growth inhibition of cervix carcinoma cells in vivo by endothelin A receptor blockade. Canc Res. 2002;62:6381–6384. [PubMed] [Google Scholar]
- 33.Cheng S.B., Wu L.C., Hsieh Y.C., Wu C.H., Chan Y.J., Chang L.H., et al. Supercritical carbon dioxide extraction of aromatic turmerone from Curcuma longa Linn. induces apoptosis through reactive oxygen species-triggered intrinsic and extrinsic pathways in human hepatocellular carcinoma HepG2 cells. J Agric Food Chem. 2012;60:9620–9630. doi: 10.1021/jf301882b. [DOI] [PubMed] [Google Scholar]
- 34.Li Y., Shi X., Zhang J., Zhang X., Martin R.C.G. Hepatic protection and anticancer activity of curcuma: a potential chemopreventive strategy against hepatocellular carcinoma. Int J Oncol. 2014;44:505–513. doi: 10.3892/ijo.2013.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Developmental Therapeutics Program (U.S.) Developmental therapeutics program, division of cancer treatment. National Cancer Institute, U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health; Bethesda, Md: 1984. In vivo cancer models, 1976-1982. [Google Scholar]
- 36.Geran R.I., Greenberg N.H., Macdonald M.M., Schumacher A.M., Abbott B.J. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother Rep. 1972;3:59–61. [Google Scholar]
- 37.Gopalan B., Goto M., Kodama A., Hirose T. Supercritical carbon dioxide extraction of turmeric (Curcuma longa) J Agric Food Chem. 2000;48:2189–2192. doi: 10.1021/jf9908594. [DOI] [PubMed] [Google Scholar]
- 38.Nair A., Amalraj A., Jacob J., Kunnumakkara A.B., Gopi S. Non-curcuminoids from turmeric and their potential in cancer therapy and anticancer drug delivery formulations. Biomolecules. 2019;9 doi: 10.3390/biom90100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paradkar P.H., Dandekar S.P., Joshi J.V., Amonkar A.J., Vaidya A.D.B. Synergistic anticancer activity of the medicinal plant bioactives: curcuma longa linn and Tinospora cordifolia willd. In cervical cancer. Int J Pharmaceut Sci Rev Res. 2017;42:151–160. [Google Scholar]
- 40.Paradkar P.H., Dandekar S.P., Joshi J.V., Amonkar A.J., Vaidya A.D.B. Assessment of in vitro - in vivo Antimigratory and Anti-angiogenic activity of Curcuma longa linn. and Tinospora cordifolia willd. Extracts in Cervical Cancer. Int J Pharmaceut Sci Rev Res. 2017;42:87–93. [Google Scholar]
- 41.Devi H.P., Mazumder P.B., Devi L.P. Antioxidant and antimutagenic activity of Curcuma caesia Roxb. rhizome extracts. Toxicol Rep. 2015;2:423–428. doi: 10.1016/j.toxrep.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee S.K., Hong C.H., Huh S.K., Kim S.S., Oh O.J., Min H.Y., et al. Suppressive effect of natural sesquiterpenoids on inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) activity in mouse macrophage cells. J Environ Pathol Toxicol Oncol. 2002;21:141–148. [PubMed] [Google Scholar]
- 43.Joshi J.V., Jagtap S.S., Rastogi N., Walwatkar P., Nabar N., Agashe S. Integrated noninvasive management of cervical low-grade intraepithelial lesions observed in Papanicolaou smears with antimicrobials and oral Curcuma longa extract. Asian Pac J Cancer Biol. 2020;5:89–97. [Google Scholar]
- 44.Larmour L.I., Cousins F.L., Teague J.A., Deane J.A., Jobling T.W., Gargett C.E. A patient derived xenograft model of cervical cancer and cervical dysplasia. PloS One. 2018;13 doi: 10.1371/journal.pone.0206539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Z., Huang P., Law S., Tian H., Leung W., Xu C. Preventive effect of curcumin against chemotherapy-induced side-effects. Front Pharmacol. 2018;9:1374. doi: 10.3389/fphar.2018.01374. [DOI] [PMC free article] [PubMed] [Google Scholar]