Abstract
Background
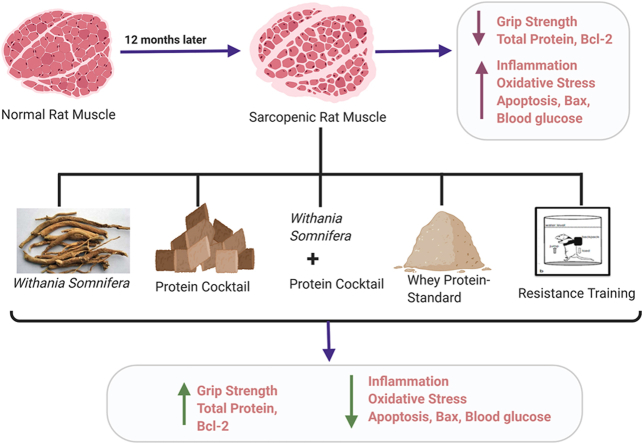
Aging leads to loss of skeletal muscle, diminished muscle strength, and decline in physical functions.
Objective
This study evaluates Withania somnifera and some dietary interventions to combat muscle weakness in aging rats.
Materials and methods
Rats (12–13 months old) corresponding to a human age of 60–65 years were assigned to various groups and given orally a standardized W. somnifera extract (WSE, 500 mg/kg) or a protein cocktail comprising soybean (1.5 g/kg) and quinoa (1 g/kg) or a combination of WSE and the protein cocktail or whey protein (1 g/kg) as a reference standard or only resistance exercise for 60 days. Grip strength and blood glucose levels were monitored weekly. At the end of the treatment, total protein, inflammatory markers (CRP, IL-6 and TNF-α), AMPK, malondialdehyde, glutathione, antioxidant enzymes and apoptotic regulator genes (Bax and Bcl-2) were assayed. The biceps brachii muscle of all animals was subjected to histomorphological study.
Results
All treatments successfully attenuated aging-elevated glucose, CRP, IL-6, TNF-α, AMPK, malondialdehyde, and Bax levels. A significant restoration of the aging-depleted total protein levels, glutathione, superoxide dismutase, catalase, and Bcl-2 was noted in the treatment groups. An increase in grip strength and greater biceps mass with all treatments indicated regaining of the frail aging muscle's strength and functionality. The WSE + protein treatment elicited the best results among all treatment groups to optimize muscle strength.
Conclusion
All the interventions curbed muscle loss and strengthened the skeletal muscle by reducing inflammation, oxidative stress and apoptosis, and increasing ATP availability to the muscle.
Keywords: Aging muscle, Withania somnifera, Protein, Resistance exercise, AMPK, Bcl-2 and bax
Graphical abstract
1. Introduction
Loss of skeletal muscle with aging and decline in its function begins at the age of 40 years and muscle mass can be lost at a rate of 1–2% per year after the age of 50 years. Loss of skeletal muscle is associated with increased risk for several untoward health outcomes including frailty, falls, fractures, functional disability, loss of independent living, and mortality. The slow deterioration of muscle mass during aging is due to a lower muscle anabolism against increased muscle catabolism with aging.
Oxidative stress, known to increase during aging reduces muscle protein synthesis and induces mitochondrial dysfunction [1]. Low-grade chronic inflammation is a hallmark of aging that affects the mitochondria adversely and is also responsible for associated conditions such as obesity, cardiovascular disease, insulin resistance, and arthritis [2]. Diabetes mellitus, a much common disease seen in the elderly leads to a decline in muscle strength.
The enzyme 5′-Adenosine monophosphate–activated protein kinase (AMPK) has been called an intracellular “fuel-gauge” that is activated during metabolic stress when AMP/ATP or inorganic phosphate (Pi)/phosphocreatine (PCr) ratios are high [3]. AMPK levels increase with age and elevated AMPK activity is responsible for age-related atrophy and diminished skeletal muscle growth. Suppression of muscle protein translation and synthesis may be one mechanism by which AMPK exerts this effect [4].
Aging-related muscle loss is postulated to be mediated via apoptosis, though the exact mechanism is yet to be understood. The role of mitochondria is important in activating apoptosis. Impaired respiration, oxidative damage and altered turnover in mitochondria trigger mitochondrial apoptotic signalling [5]. The Bcl-2 family of proteins is known to play a significant role in apoptotic signalling and comprises Bax (apoptotic) and Bcl-2 (anti-apoptotic) proteins, which are two key regulators of the mitochondria-mediated apoptosis process [6]. While Bcl-2 prevents cellular suicide and promotes cell survival, Bax, a Bcl-2- homologous protein competes to induce cell death. Potential interventions for curtailing muscle loss with aging include protein and other nutritional supplements, exercise especially, against resistance, caloric restriction, anabolic hormones, anti-inflammatory agents, and antioxidants.
Resistance training (RT) is any physical exercise wherein muscles are contracted against an external resistance such as dumbbells or own body weight to build strength, anaerobic endurance, and size of the muscle. It leads to increased protein synthesis and muscle hypertrophy in the elderly. Resistance training exercise stimulates muscle protein synthesis through the mammalian target of the rapamycin complex 1–ribosomal protein of 70-kDa S6 kinase 1 (mTORC1-p70S6K1) pathway [7]. Stimulation of this pathway via muscle contraction against increasing resistance combined with adequate protein ingestion leads to a significant lean mass increase.
Herbal extracts may help alleviate age-related muscle weakness by their anabolic, antioxidant, anti-inflammatory, insulin-secretagogic, and anti-fatigue effects. Withania somnifera (ashwagandha) is known to strengthen the weak aging skeletal muscle by virtue of its anti-stress and adaptogenic activity, anti-inflammatory effect and a potent antioxidant effect [8]. It decreases the levels of cortisol, which reduces muscle mass [8]. By reducing the activity of the Mg+2-dependent ATPase, which catabolizes ATP, Withania may have beneficial effects on mitochondrial function [9]. By its impact on the nervous system to allay anxiety and increase focus and concentration, Withania may bring about better muscle recruitment and co-ordination [10].
A study has reported an extract of W. somnifera to improve muscle function and recovery in adult males engaged in resistance training for 8 weeks [8]. On consumption of the Withania extract, significant attenuation of muscle markers which had built up in blood stream on exercise-induced muscle damage in these males was noted along with decrease in body fat.
Decreased muscle mass during aging occurs majorly due to reduced muscle protein synthesis [11]. With aging, amount of amino acids available from diet may be inadequate due to low protein in the diet or due to impaired protein absorption in a sub-functional gastro-intestinal tract. Thus, the muscle is deprived of protein necessary for its metabolism and maintenance. The muscle protein synthesis can be increased by increasing muscle protein availability. Quality proteins from the diet provide the necessary amino acids required for muscle protein synthesis. More importantly, muscle protein synthesis is stimulated by absorbed amino acids. One of our dietary interventions was a protein cocktail comprising soybean and quinoa. It contained a fast release (soya) and slow-release protein (quinoa). The fast release protein prevents deterioration of the muscle after injury, trauma or wear and tear after exercise. In contrast, the slow-release protein supports anabolism and repair of the muscle at rest [12]. The regenerative capacity of skeletal muscle is dependent on myogenic precursor cells known as satellite cells. Protein ingestion may accelerate the satellite cell responses and speed up repairing the damaged muscle [13]. Soybean contains high levels of arginine and leucine, which aid in muscle formation. It also contains phytic acid, which has many effects, including antioxidant and anti-inflammatory which benefit the skeletal muscle. Quinoa, a dietary grain contains all 9 essential amino acids necessary for muscle growth and is particularly rich in leucine which stimulates skeletal muscle build-up.
In view of the above considerations, this study was designed to strengthen the weak and aging muscle using some novel herbal supplements and proteins. It was also our attempt to compare the effect of our supplements with resistance exercise that is known to aid in building muscle strength.
2. Methods and materials
2.1. Drugs and chemicals
Trichloroacetic acid, thiobarbituric acid, reduced glutathione, and epinephrine were supplied by Sigma Chemical Co., St. Louis MO, USA. Whey protein (MuscletechR) was purchased from a known dietary supplement shop. All other analytical grade chemicals were procured from local sources.
2.2. Plant and raw material
A standardized W. somnifera extract (WSE) was obtained from Pharmanza Herbals Pvt. Ltd. WSE, a hydro-alcoholic extract of W. somnifera in ethanol: water (70:30) v/v was standardized by HPLC using USP 41 method.
The content of markers in WSE was:
Withanolide aglycones – 0.65%w/w.
Withanolide glycosides – 1.05%w/w.
Total Withanolides - 1.70%w/w.
Quinoa seeds and soybeans were purchased from a local food grain market. The Blatter Herbarium, St. Xavier's College, Mumbai authenticated these raw material by matching with the existing specimens. Nutritional composition of the quinoa and soya cocktail per 100 g was estimated to be: Total calorie-293, Total fat −10.84 g, Total protein-30 g, Dietary fiber-7.5 g and Total carbohydrate-31.19 g, along with many minerals and vitamins.
The whey protein (30 g) comprised 130 calories, 24 g of protein, 4 g of carbohydrates, 2 g of fat (1 g saturated fat), 5.5 g BCAA and 4 g glutamine and precursor, among others.
2.3. Animals
Male Sprague Dawley rats (350–450 g) aged 12–13 months which displayed low muscle strength at start on a grip strength meter and a slow and sluggish gait were used as Aging Control rats. Young rats aged 2–3 months were used as Young Control rats. The animals were housed in cages before the experiment. Animals were acclimatized in controlled environment (humidity 50 ± 5%, 12 h light/12 h dark cycle and 25 ± 2 °C temperature). Animals were provided a standard diet to feed (Amrut laboratory animal feed, Pranav Agro Industries, India) and drinking water ad libitum. The Institutional Animal Ethics Committee (Registration no. 25/1999/CPCSEA) had reviewed and approved experimental protocol.
2.4. Preparation of test substances
The WSE was dissolved in distilled water and the freshly prepared solution was administered orally to the experimental rats. Soybean and quinoa were roasted, cooked till soft, mixed and made into a bolus and fed to the aging rats. The whey protein powder was dissolved in distilled water and administered orally to the reference group of rats.
2.5. Resistance training (RT) [14]
RT was given to the aging rats by strapping weights (5–15 g) to their abdomens and subjecting them to swimming for 15 min in a small, confined space, such as a large graduated cylinder filled halfway with water at room temperature. Initially, the animals were made to swim without weights for a week starting from 10 min up to 15 min. From the 2nd week, weights (5 g) were strapped on to their bodies and forced swimming started from 5 min up to 15 min. The weights were gradually increased from 5 to 15 g for a swimming period of 15 min. Initially, there was a period of vigorous swimming during which the animals tried to escape. Eventually, the animals slowed down their swimming and exhibited a typical tiredness seen as floating in the water. At this point the animals was removed from water immediately and placed for drying in a warm place. The maximum period of swimming was for 15 min.
2.6. Experimental procedure
Rats were assigned to various treatment groups after 7 days of acclimatization. Each group had 6 rats. Groups were treated as follows:
Group I (Young Control): Young rats received a regular chow diet and drinking water daily for 60 days.
Group II (Aging Control): Aging rats received a regular chow diet and drinking water daily for 60 days.
Group III (WSE): Aging rats were treated with WSE (500 mg/kg, p.o.) along with a regular chow diet and drinking water daily for 60 days.
Group IV (Protein): Aging rats were fed boluses of soybean (1.5 g/kg) + quinoa (1 g/kg) along with a regular chow diet and drinking water daily for 60 days.
Group V (WSE + Protein): Aging rats were administered WSE (500 mg/kg, p.o.) and were fed boluses of soybean (1.5 g/kg) + quinoa (1 g/kg) along with regular chow diet and drinking water daily for 60 days.
Group VI (RT): Aging rats received regular chow diet and drinking water daily for 60 days and resistance training in the form of swimming with weights strapped to their abdomen.
Group VII- (Reference): Aging rats were administered whey protein hydrolysate (1 g/kg) with regular chow diet and drinking water daily for 60 days.
Grip strength was measured using a grip strength meter every week for all the groups. Blood glucose levels using an automated digital glucometer (Accu-Chek, Roche) were measured on Day 1, 15, 30, 45 and 60 of the study. At the end of the study all animals were fasted overnight and blood was collected from their retro-orbital plexus on the next day. Serum was separated by allowing blood to clot for 30 min at room temperature and centrifuging at 2500 rpm at 30 °C for 15 min. This separated serum used to estimate C-reactive protein (CRP), Interleukin-6 (IL-6), Tumour Necrosis Factor (TNF-α) and insulin. For insulin resistance, the homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as follows:
Dissected biceps brachii muscle of all animals was washed with ice-cold saline and weighed. 10% (w/v) homogenate was prepared homogenising a part of the muscle in ice-cold Tris buffer (50 mm, pH 7.8). Lipid peroxidation (LPO) estimated using an aliquot. The homogenate was centrifuged for 25 min at 2700 g at 4 °C, and the supernatant was used to assay total protein (TP), AMPK, reduced glutathione (GSH), superoxide dismutase (SOD), catalase (CAT) and the apoptotic regulators Bax and Bcl-2. The other part of dissected muscle was used for histological studies and fixed in 10% buffered formalin until then.
2.7. Biochemical estimations and assays
Total protein was estimated from muscle by the method of Lowry et al. using bovine serum albumin as standard [15]. Insulin assay was carried out in serum using Radioimmunoassay (RIA) kit supplied by Board of Radiation and Isotope Technology (BRIT) (Mumbai, India). Estimation of IL-6 and TNF-α was done in serum using ELISA kits supplied by Krishgen Ltd (Mumbai, India). The method of Pepys et al. was used for the estimation of CRP in serum using a standard agglutination test kit supplied by Spectrum Diagnostics, Egypt. The method of Ohkawa et al. was used for the quantification of LPO by determining the concentration of the LPO markers thiobarbituric acid reactive substances (TBARS) in muscle [16]. SOD, CAT and GSH were estimated in the muscle homogenate by method of Ellman, Sun and Zigman, and Clairborne respectively [[17], [18], [19]]. AMPK assay was carried out in the muscle homogenate using ELISA kits supplied by Krishgen (Mumbai, India).
2.8. Gene expression of apoptotic regulators
Bax, Bcl-2 and β-actin mRNA expressions were studied in the biceps by a semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) using T100 Thermal Cycler (Bio-Rad, USA). β- Actin was the housekeeping gene. According to the manufacturer's instructions, cDNA was synthesized from 2 μg of RNA (obtained from musle) using the Verso cDNA synthesis kit (Thermo Fischer Scientific) with oligo dT primer. The final amplification product (10 μL) was run on a 2% ethidium-stained agarose gel, and the real-time data was analysed using the computerized imaging program Gel Doc EZ Imager (Bio-rad). The RNA ladder Invitrogen was used to identify the approximate size of molecules run on the gel during electrophoresis. The sequence of primers used is given in Table 1.
Table 1.
Primer sequence and size for β-Actin, Bax and Bcl-2.
| Gene | Primer pair | Sequence | Product size (bp) |
|---|---|---|---|
| β – Actin | FP | TCCTCCTGAGCGCAAGTACTCT | 153 |
| RP | GCTCAGTAACAGTCCGCCTAGAA | ||
| Bax | FP | CCTTTTCTACTTTGCCAGCAAAC | 155 |
| RP | GAGGCCGTCCCAACCAC | ||
| Bcl-2 | FP | AGTCTGGGAATCGATCTGGA | 154 |
| RP | GAGCATGATTCTCTGTCAAGT |
2.9. Histopathological studies
The biceps brachii muscle of all animals stored in 10% buffered formalin was embedded in paraffin, sections cut at 5 μm, and stained with hematoxylin and eosin. For histoarchitectural changes, these sections were examined under a light microscope.
2.10. Statistical analysis
The results from 6 animals in each group are expressed as mean ± SEM. Statistical analysis of results was done using one-way ANOVA followed by the Bonferroni's multiple comparison test; p < 0.05 was considered to be significant. GraphPad InStat version 4.00 of Graph Pad Software Inc, San Diego, USA, was the software used for statistical analysis.
3. Results
3.1. Effect on grip strength
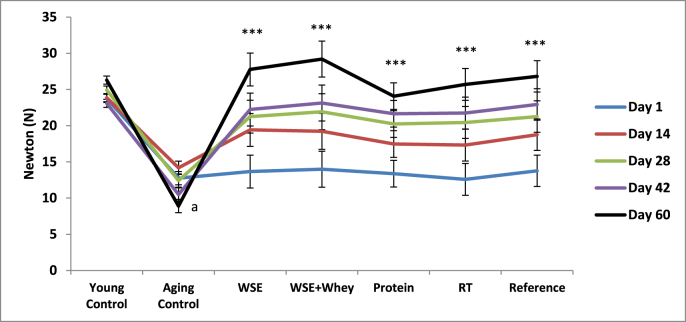
The effect of treatments on grip strength is shown in Table 2 and Fig. 1. All rats started out with the same grip strength on the 1st day except for the Young Control rats which displayed significantly higher values than the rest of the rats. On day 14, 28, 42, and 60, a progressive improvement in grip strength was observed in rats of treatment groups when compared with the Aging Control rats and their grip strengths were highest on day 60 and comparable to the Young Control rats. WSE and WSE + Protein treatments yielded the best results among all treatment groups. The Young Control rats showed no significant difference in grip strength throughout the study, while the Aging Control rats displayed lower readings with every weekly measurement, being lowest on day 60.
Table 2.
Effect of WSE, WSE + Protein, Protein, RT and Reference on grip strength.
| Treatment Groups | Grip strength (Newton) |
||||
|---|---|---|---|---|---|
| Day 1 | Day 14 | Day 28 | Day 42 | Day 60 | |
| Young Control | 23.76 ± 0.83 | 23.87 ± 0.39 | 24.92 ± 0.57 | 23.08 ± 0.72 | 26.30 ± 0.69 |
| Aging Control | 12.75 ± 0.71a | 14.18 ± 0.54a | 12.42 ± 0.31a | 10.50 ± 0.37a | 8.90 ± 0.30a |
| WSE | 13.66 ± 0.51 | 19.42 ± 0.33b | 21.25 ± 0.96b | 22.23 ± 0.63b | 27.77 ± 0.29b |
| WSE + Protein | 13.98 ± 0.56 | 19.22 ± 0.81b | 21.93 ± 0.28b | 23.13 ± 0.61b | 29.20 ± 0.75b |
| Protein | 13.36 ± 0.68 | 17.47 ± 0.74∗∗ | 20.23 ± 0.22b | 21.60 ± 0.37b | 24.08 ± 0.28b |
| RT | 12.58 ± 0.36 | 17.32 ± 0.51∗∗ | 20.45 ± 0.23b | 21.75 ± 0.41b | 25.70 ± 0.74b |
| Reference | 13.76 ± 0.45 | 18.75 ± 0.19b | 21.27 ± 0.73b | 22.90 ± 0.89b | 26.82 ± 0.93b |
Note: All values are expressed as mean ± SEM; N = 6 in each group; One way ANOVA followed by Bonferroni's multiple comparison test is applied for statistical analysis.
P < 0.001 when Aging Control compared with Young Control.
P < 0.001 when treatment groups compared with Aging control.
Fig. 1.
Effect of WSE, WSE+Protein, protein, RT and reference on grip strength. Note: Values are mean±SEM; N=6 in each group, One-way ANOVA followed by Bonferroni's Multiple Comparison Test is applied for statistical analysis. P values: a<0.001 when Sarcopenic Control compared with Normal Control and ***<0.001 when treatment groups compared with Sarcopenic Control.
3.2. Effect on blood glucose levels (BGL), and HOMA-IR
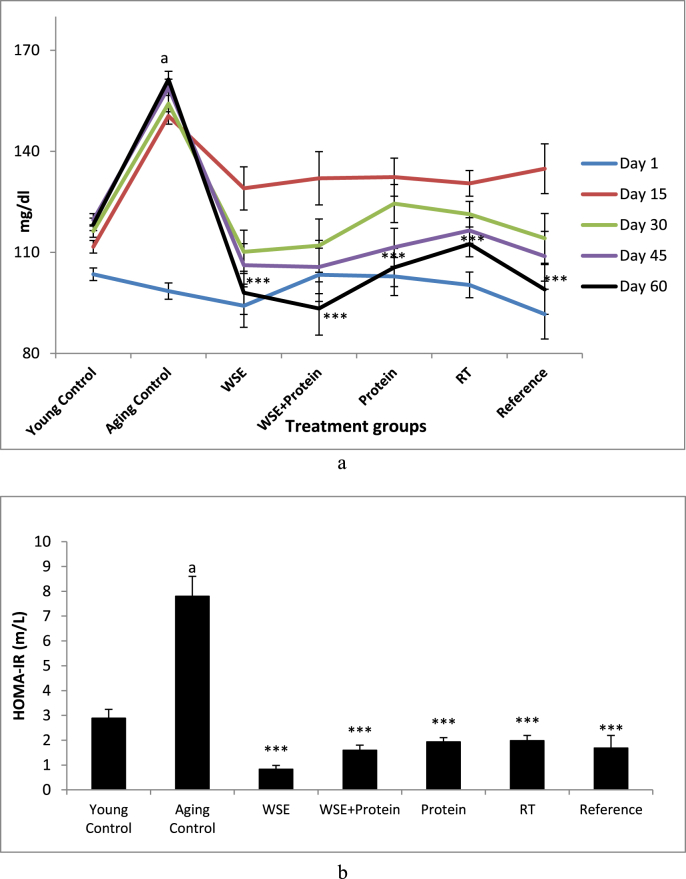
The effects of treatments on BGL and HOMA-IR are given in Table 3 and Fig. 2 respectively. All aged rats that displayed similar BGL at the start of the experiment, on day 15 had elevated BGL when compared with their own basal readings. However, the Aging Control rats displayed significantly higher BGL than all treated rats on the 15th day. Thereafter, on day 30, 45, and 60, all treatment group rats showed a progressive decrease in BGL when compared with their 15th day values and significantly lower BGL than the Aging Control rats on all measurement days. The Young Control rats showed no significant difference in BGL throughout the study, whereas, the Aging Control rats elicited increasing levels of BGL with advancing days. At the end of the study on the day 60, a significant (p < 0.001) decrease in BGL of all the treatment groups was observed when compared with the Aging Control group of animals, the WSE + Protein treatment eliciting the lowest blood glucose levels of the study.
Table 3.
Effect of WSE, WSE + Protein, protein, RT and reference on BGL and HOMA-IR.
| Treatment Groups | Glucose Levels (mg/dl) |
||||
|---|---|---|---|---|---|
| Day 1 | Day 15 | Day 30 | Day 45 | Day 60 | |
| Young Control | 103.50 ± 5.04 | 111.67 ± 4.05 | 116.33 ± 0.57 | 119.67 ± 4.82 | 118.33 ± 3.46 |
| Aging Control | 98.50 ± 4.03 | 150.50 ± 02.23a | 154.17 ± 0.31b | 159.00 ± 9.01b | 161.33 ± 7.89a |
| WSE | 94.17 ± 2.42 | 129.00 ± 4.62e | 110.17 ± 0.96c | 106.17 ± 2.63c | 98.00 ± 2.76c |
| WSE + Protein | 103.33 ± 2.36 | 132.00 ± 2.83 | 112.00 ± 0.28d | 105.67 ± 4.91c | 93.33 ± 2.07c |
| Protein | 102.83 ± 1.62 | 132.33 ± 3.10 | 124.50 ± 0.22 | 111.50 ± 6.14c | 105.50 ± 3.73c |
| RT | 100.33 ± 5.80 | 130.50 ± 4.64e | 121.33 ± 0.23e | 116.50 ± 7.74c | 112.50 ± 2.40c |
| Reference | 91.66 ± 2.95 | 134.83 ± 5.90 | 114.17 ± 0.73d | 108.83 ± 7.64c | 99.00 ± 5.52c |
Note: All values are expressed as mean ± SEM; N = 6 in each group; One way ANOVA followed by Bonferroni's multiple comparison test is applied for statistical analysis.
P < 0.001 when Aging Control compared with Young Control.
P < 0.01 when Aging Control compared with Young Control.
P < 0.001 when treatment groups compared with Aging control.
P < 0.01 when treatment groups compared with Aging control.
P < 0.05 when treatment groups compared with Aging control.
Fig. 2.
a Effect of WSE, WSE+Protein, protein, RT and reference on BGL. b Effect of WSE, WSE+Protein, protein, RT and reference on HOMA-IR. Note: Values are mean±SEM; N=6 in each group, One-way ANOVA followed by Bonferroni's Multiple Comparison Test is applied for statistical analysis. P values: a <0.001 when Sarcopenic Control compared with Normal Control; ∗∗∗<0.001 when Treatment groups compared with Sarcopenic Control.
The Aging Control rats exhibited a significant (p < 0.001) increase in HOMA-IR when compared with Young Control rats. All study groups showed significant attenuation (p < 0.001) of the aging-elevated HOMA-IR score.
3.3. Effect on inflammatory markers
The effect of treatments on inflammatory markers is summarized in Table 4. The Aging Control rats exhibited a significant (p < 0.001) increase in the serum levels of the inflammatory mediators CRP, IL-6, and TNF-α when compared with the Young Control rats. All treatment groups significantly (p < 0.001) attenuated the aging-elevated CRP levels except for RT, whereas the aging-elevated IL-6 and TNF-α levels were significantly attenuated by all treatment groups.
Table 4.
Effect of WSE, WSE + Protein, Protein, RT and Reference on serum CRP, IL-6 &TNF-α.
| Treatment Groups | CRP (mg/L) | Interleukin-6 (pg/mL) | TNF-α (pg/mL) |
|---|---|---|---|
| Young Control | 5.83 ± 0.65 | 7.82 ± 0.56 | 2.63 ± 0.42 |
| Aging Control | 19.83 ± 1.30a | 20.58 ± 0.83a | 10.27 ± 0.40a |
| WSE | 8.33 ± 0.71c | 12.54 ± 1.31c | 3.86 ± 0.67c |
| WSE + Protein | 5.83 ± 1.07c | 8.83 ± 0.78c | 3.16 ± 0.42c |
| Protein | 7.66 ± 1.05c | 11.13 ± 1.28c | 4.04 ± 0.45c |
| RT | 15.5 ± 1.23 | 13.60 ± 1.63b | 5.75 ± 0.23c |
| Reference | 8.16 ± 0.70c | 9.29 ± 1.12c | 2.86 ± 0.50c |
Note: All values are expressed as mean ± SEM; N = 6 in each group; One way ANOVA followed by Bonferroni's multiple comparison test is applied for statistical analysis.
P < 0.001 when Aging Control compared with Young Control.
P < 0.01 when treatment groups compared with Aging Control.
P < 0.001 when treatment groups compared with Aging Control.
3.4. Effect on total protein, AMPK, LPO and endogenous antioxidants
Table 5 shows the effect of treatments on total protein, AMPK, LPO, GSH, SOD, and CAT. Aging Control rats exhibited significant (p < 0.001) attenuation of total protein content in muscle when compared with the Young Control rats. At the end of the treatment, all groups significantly restored (p < 0.001) the aging-attenuated protein levels except for the RT group.
Table 5.
Effect of WSE, WSE + Protein, Protein, RT and Reference on Total protein, AMPK, LPO, GSH and antioxidant enzymes.
| Treatment Groups | Total Protein (mg/dl) | AMPK (ng/ml) | LPO (TBARS/mg protein) | GSH (U/ug protein) | SOD (U/mg of protein) | CAT (U/mg of protein) |
|---|---|---|---|---|---|---|
| Young Control | 6.52 ± 0.19 | 474.44 ± 22.90 | 0.03 ± 0.01 | 0.271 ± 0.011 | 33.81 ± 0.87 | 29.33 ± 1.93 |
| Aging Control | 3.30 ± 0.21a | 958.08 ± 10.42a | 0.08 ± 0.02a | 0.086 ± 0.003a | 12.61 ± 0.54a | 11.93 ± 0.49a |
| WSE | 5.13 ± 0.26d | 558.92 ± 41.70b | 0.04 ± 0.02d | 0.230 ± 0.012d | 29.46 ± 2.12d | 28.01 ± 2.77d |
| WSE + Protein | 7.03 ± 0.09d | 487.87 ± 98.03c | 0.04 ± 0.02d | 0.231 ± 0.016d | 31.93 ± 2.62d | 31.26 ± 3.43d |
| Protein | 6.50 ± 0.29d | 612.73 ± 119.07 | 0.05 ± 0.01d | 0.1955 ± 0.01d | 25.31 ± 2.67c | 23.51 ± 1.22c |
| RT | 3.82 ± 0.19 | 509.09 ± 158.87b | 0.05 ± 0.04d | 0.194 ± 0.017d | 22.88 ± 1.83b | 22.75 ± 1.61b |
| Reference | 6.72 ± 0.22d | 570.93 ± 21.00b | 0.03 ± 0.02d | 0.230 ± 0.019d | 30.43 ± 2.03d | 31.35 ± 1.27d |
Note: All values are expressed as mean ± SEM; N = 6 in each group; One way ANOVA followed by Bonferroni's multiple comparison test is applied for statistical analysis.
P < 0.001 when Aging Control compared with Young Control.
P < 0.05 when treatment groups compared with Aging Control.
P < 0.01 when treatment groups compared with Aging Control.
P < 0.001 when treatment groups compared with Aging Control.
AMPK levels were significantly (p < 0.001) elevated in the muscle of Aging Control rats when compared with the Young Control rats. The elevated AMPK levels were lowered significantly (p < 0.01 for WSE + protein and p < 0.05 for WSE, RT and reference standard) by all treatments except the protein cocktail. The WSE + Protein treatment elicited the best results.
A significant (p < 0.001) increase in the level of TBARS and a significant (p < 0.001) decrease in GSH, SOD, and CAT activities was observed in the muscle of the Aging Control group of rats when compared with the Young Control rats. All treatment groups successfully attenuated the aging-elevated TBARS levels and significantly restored the aging-depleted antioxidant levels.
3.5. Effect on apoptotic regulators
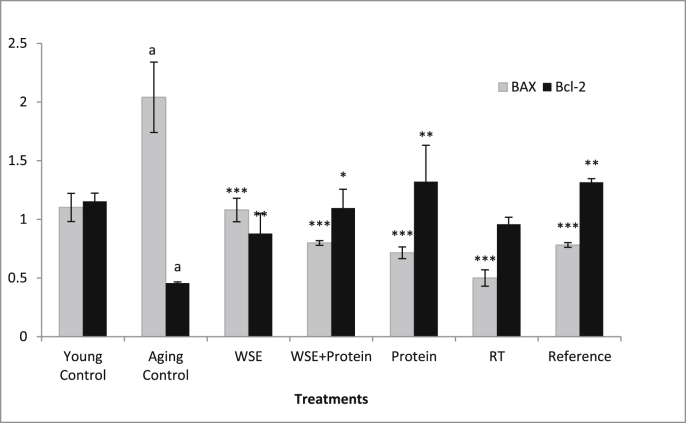
The effect of treatments on the apoptotic regulators is illustrated in Fig. 3. A significant (p < 0.001) increase in the expression of the pro-apoptotic Bax gene and a corresponding decrease in the anti-apoptotic Bcl-2 gene expression was observed in the muscle of Aging Control animals when compared with Young Control animals. All treatments could curtail the aging-induced apoptosis which was evident by a significant decrease in Bax and a significant increase in Bcl-2 levels in the treated rats. However, RT group failed to increase the Bcl-2 expression significantly.
Fig. 3.
Effect of WSE, WSE+Protein, protein, RT and reference on Bax and Bcl2. Note: All values are expressed as mean ± SEM; N = 6 in each group; One way ANOVA followed by Bonferroni's multiple comparison test is applied for statistical analysis aP< 0.001 when Normal control compared with Sarcopenic Control; ∗P< 0.05, ∗∗P< 0.01 & ∗∗∗P< 0.001 when treatment groups compared with Sarcopenic Control.
3.6. Histopathological studies
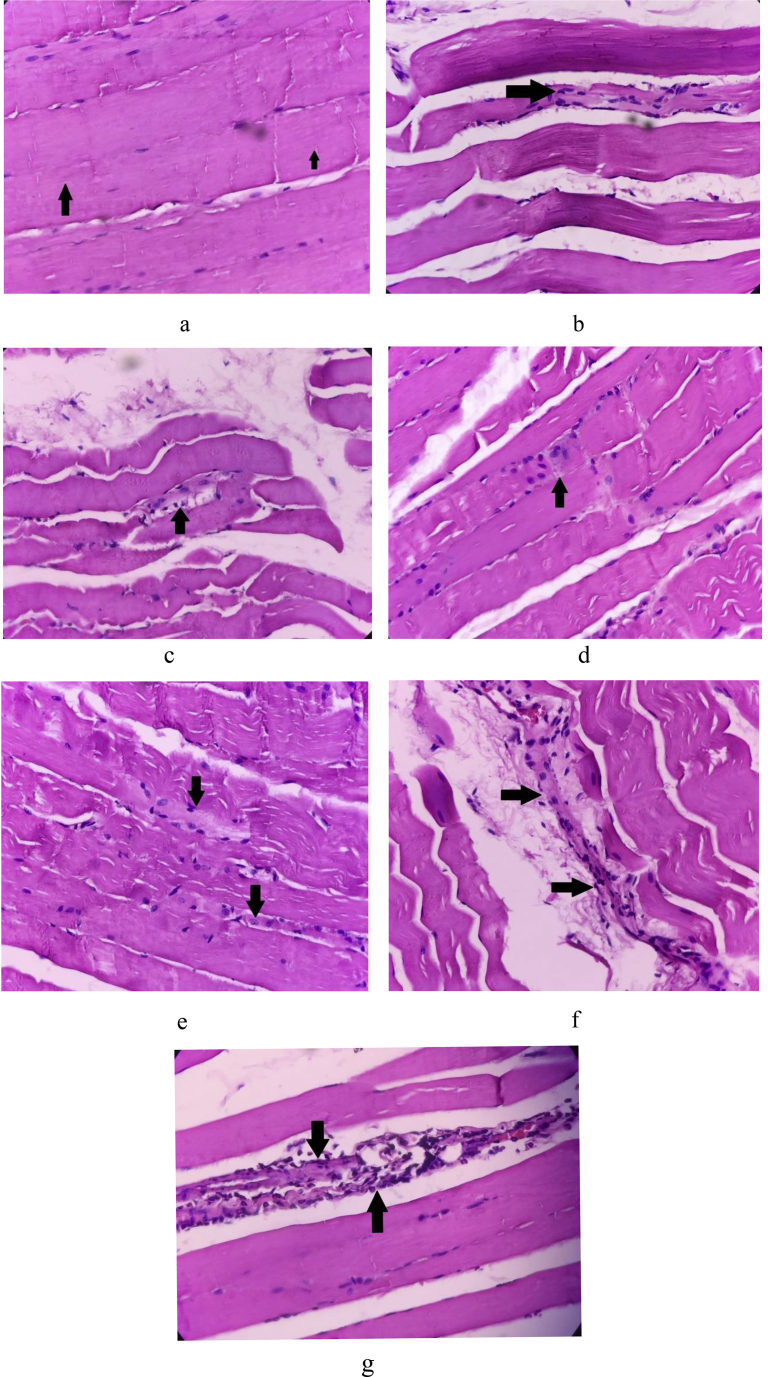
The biceps of the Young Control rats showed absence of muscle fibre loss, degenerative changes (splitting/waviness), inflammation of fibres, crowding/internalization of nuclei, and thinning of fibre (Fig. 4a). In contrast, the biceps of the Aging Control rats showed muscle fibre loss with dense focal lymphohistiocytic inflammatory cell infiltrate (+++), degenerative changes (++), inflammation of fibres (+++), crowding/internalization of nuclei (+), thinning of fibres (+++), and perivascular inflammation (Fig. 4b). When compared with Aging Control, the biceps of the WSE group showed mild muscle fibre loss (+), degenerative changes (+), crowding/internalization of nuclei (+), inflammation of fibres (+), and thinning of fibres (+) as shown in Fig. 4c. The WSE + Protein treatment group's biceps showed absence of fibre loss, thinning and inflammation of fibres, and mild degenerative changes (+) as shown in Fig. 4d. The biceps of the protein cocktail administered rats were similar to the WSE + Protein treatment and showed minimal changes with waviness of fibres (+) and crowding of nuclei (+) as indicated in Fig. 4e. Biceps of the RT group exhibited moderate degenerative changes (++) and crowding of nuclei (++) with focal mild chronic inflammatory cell infiltrate around the fibres (+) as seen in Fig. 4f. The reference group showed mild focal degenerative changes of fibre (+) and engulfment of fibres by mixed inflammatory infiltrate (++) composed of polymorphs and lymphocytes (Fig. 4g).
Fig. 4.
a H & E staining of biceps of Normal Control rat 40X. Arrows indicate skeletal muscle fibres with cross striations. b H & E staining of biceps of Sarcopenic Control rat 40X. Arrow indicates thinning of muscle fibres and internalization of nuclei. c H & E staining of biceps of WSE treated rat 40X. Arrow indicates mild sarcopenic inflammatory infiltrate. d H & E staining of biceps of WSE + Protein treated rat 40X. Arrow shows mild fibre loss with internalization and crowding of nuclei. e H & E staining of biceps of Protein treated rat 40X. Arrows indicate mild fibre loss with thinning and increased waviness. f H & E staining of biceps of RT group rat 40X. Arrows show mild sarcopenic loss with crowding of nuclei. g H & E staining of biceps of Reference treated rat 40X. Arrows indicates focal sarcolemmal loss with engulfment of fibres by inflammatory cells.
4. Discussion
This study included animals with low muscle strength due to natural aging rather than muscle weakness induced by chemical agents such as glucocorticoids or statins. Hence, this model represents real-time loss of muscle strength and physiological and biochemical changes associated with it.
The major phytoconstituents of W. somnifera include steroidal lactones (withanolides, withaferins), saponins and alkaloids like isopelletierine and anaferine. The withanolides are believed to account for the antioxidant, anti-inflammatory, immunomodulatory, and adaptogenic activity of Withania, conferring it with the ability to withstand various stressors to the muscles [20].
Low protein intake can be an important cause for muscle weakness due to factors such as aging-associated physiological changes, pre-existing medical conditions, physical disability and mental disorders, anabolic resistance, and decreased muscle perfusion. The aging rats in our study showed significantly depleted total protein levels in the biceps muscle while all our interventions which were designed to promote muscle protein synthesis restored successfully the depleted protein levels. The WSE + Protein group displayed the highest levels among all treatments indicating the essential role of an anabolic supplement along with protein to strengthen the muscle.
Whey protein hydrolysate is a pre-digested, fast release protein containing all the amino acids required for muscle build-up. Its ingestion leads to an increase in the rate of protein synthesis and rapid and complete restoration of muscle function after vigorous activity [21]. Thus, whey protein hydrolysate was the reference standard in this study.
Grip strength is a measure of muscle strength and functionality. In this study, while the aging rats showed diminishing grip strength with increasing time, our interventions could improve the grip strength to a large extent by the end of the study period, thus empowering the weak aging muscle.
Aging is associated with persistent low-grade inflammation as evidenced by an increase in levels of inflammatory mediators such as IL-6, TNF-alpha and CRP. Inflammatory factors can inhibit muscle protein synthesis, accelerate protein breakdown, and upregulate the expression of some muscle growth inhibitory factors like myostatin and atrogin1 to hasten protein catabolism and promote skeletal muscle degradation [22]. CRP, a protein synthesized by the liver is an early marker of inflammation which rises in the blood within few hours after tissue injury, infection or inflammation. Hence, significantly elevated serum levels of CRP were observed in aging rats when compared with normal rats. Evidence suggests that CRP leads to a reduction in size of the myotube together with reduction of muscle protein synthesis, inducing loss of muscle mass [23]. All treatments except RT could significantly attenuate the aging-elevated CRP levels, thus alleviating inflammation and curtailing muscle loss.
With aging, adipose cell mass increases, and so does the secretion of the cytokines IL-6 and TNF-α from it. These cytokines aggravate inflammatory reactions, leading to reduced muscle mass and strength [24]. IL-6 is a pro-inflammatory cytokine secreted during any tissue damage and is one of the main signaling pathways implicated in aging and chronic morbidity [25]. TNF-α is a central regulator of inflammation that increases with age and is connected to muscle atrophy and cell loss, yet, the signalling events that occur in vivo are unknown [26]. Apoptotic stimuli, such as oxidative stress, calcium balance dysregulation and TNF-α expression, may be considered as initiators of the apoptotic signalling in aged skeletal muscle. All treatment groups significantly attenuated the aging-elevated IL-6 and TNF-α level, thus, curbing inflammation and its harmful effects on the muscle.
Hyperglycemia is a risk factor for decreased muscle strength and function. It is reported that hyperglycemia induces mitochondrial dysfunction leading to reduced muscle strength in older patients with diabetes mellitus [27]. Diabetic neuropathy, which is common in geriatric patients, has been proven to reduce muscle strength. Good glycemic control in the elderly has been shown to curtail the loss of muscle strength. All interventions in this study could successfully attenuate the aging-elevated BGL. At the end of the experiment, the WSE + Protein treatment elicited the lowest blood glucose levels of the study.
Insulin plays an important role in increasing muscle protein and regulating muscle protein metabolism. Insulin resistance (IR) is a condition in which the body's cells do not respond to insulin, as a result, glucose fails to enter the cells as easily, gradually building up in the blood. This can eventually lead to T2DM. Changes in glucose tolerance seen with ageing which leads to IR and it is believed to be due to age-related muscle protein loss and strength.
All treatment groups could alleviate IR induced by aging, indicating their ability to curtail muscle loss. A subsequent rise in serum amino acids after consumption of protein stimulates insulin release by a direct action on the beta cells [28]. Insulin secretion is stimulated by some amino acids by acting as a substrate in the Krebs cycle. They produce ATP through the metabolism of glucose-6-phosphate. The ATPs binds to potassium channels to close them. This leads to the depolarization of cells and insulin secretion [29]. A synergistic insulinotropic effect of various amino acids in the protein cocktail was probably responsible for its significant IR-lowering effect.
In this study, WSE decreased blood glucose levels and improved glucose tolerance in aging rats. These results are in concurrence with other reports and suggest that WSE can improve insulin sensitivity and thus, reduce IR [30].
Resistance exercise has been found to increase insulin sensitivity in diabetic as well non-diabetic healthy subjects [31]. Resistance exercise regulates glucose homeostasis to alleviate IR by stimulating oxidative capacity of mitochondria and curbing endogenous glucose production by suppressing unnecessary gluconeogenesis.
Oxidative stress and molecular inflammation are the main causative factors in age-related muscle atrophy. These two may interfere with the balance between protein synthesis and breakdown, cause mitochondrial dysfunction, and induce apoptosis [32]. Aging and muscle dystrophy can impair and decrease antioxidant capacity in the liver, making it more vulnerable to ROS-induced damage. The ROS are generated in skeletal muscle as by-products of cellular metabolism but can exceed beyond normal cellular levels to cause harm to cells. Also, cell's ability to actively detoxify ROS becomes less efficient over time as the ROS overpower the cell's defence systems. Thus, increased levels of TBARS, the by-products of LPO, were noted in the aging rats. GSH levels and SOD and CAT activities were observed to be diminished by aging due to their increased utilization to combat ROS. All treatments could significantly attenuate the aging-elevated TBARS levels and significantly restore the aging-depleted endogenous antioxidants. The WSE and WSE + Protein treatments stood out in anti-lipid peroxidation and antioxidant activity probably because of WSE's potent anti-inflammatory and antioxidant activity.
AMPK, the energy-sensing/signalling protein within the cell, is activated by stresses that increase the cellular concentration of AMP relative to ATP (increased AMP: ATP ratio) due to either limited ATP production (e.g. glucose deprivation or hypoxia, oxidative/nitrosative stress, and age-induced stress) or increased energy expenditure (e.g. muscle contraction) [33]. On activation, AMPK increases glucose uptake and fatty acid oxidation in the cell, both processes making available adequate ATP to the cell. It upregulates expression of various metabolic genes like those for GLUT4, uncoupling protein-3, and cytochrome c required in the process of ATP generation [3]. Consequently, AMPK serves as a regulator of intermediary metabolism by directing cellular events to increase energy availability and sustain high-energy phosphate levels. In this study, AMPK levels were significantly higher in Aging Control rats when compared with Normal Control rats due to decreased protein synthesis or increased protein degradation (due to inflammation, decreased anabolic hormone levels, and poor nutritional status) and oxidative stress to the aging muscle which activated AMPK. The elevated AMPK levels were attenuated significantly by the WSE, WSE + Protein, RT, and reference standard treatments indicating their beneficial role in alleviating stress to the aging muscle, thus, leading to improved protein synthesis and ATP production.
Apoptosis of the skeletal muscle cells is thought to be a potential mechanism for muscle degeneration that takes place with aging [34]. In this study, a significant increase above normal in Bax expression and a decrease in the expression of Bcl-2 in aging animals clearly indicates apoptosis of the muscle. All treatments could curtail apoptosis by alleviating stress to the aging muscle and by improving its protein synthesis and ATP production, thus strengthening it. This was evident by a significant increase in Bcl-2 and a significant decrease in Bax brought about by our dietary interventions. However, RT could not significantly increase the depleted Bcl-2 levels in the aging muscle though it brought about the best attenuation of the elevated Bax levels. Thus, the effect of RT on muscle apoptosis needs a deeper insight. β-Actin was the housekeeping gene used as an internal control in the gene expression studies of Bax and Bcl-2 as it is expressed constitutively, at the same levels in the skeletal muscle in both the studies.
The biceps brachii muscle was used to study histological changes in the study because grip strength is a measure of muscle strength in the hand and forearm. The biochemical and gene expression results were confirmed by histological findings.
5. Conclusion
All treatments in this study were comparable with RT in maintaining muscle mass and strength of the aging muscle. The best results were elicited by WSE + Protein and WSE treatments, probably due to the anabolic and adaptogenic effect of WSE, and the protein cocktail being complete in all nutrients required to power the muscle. The right diet, including protein and antioxidants, and resistance exercise is the key to combat muscle weakness during aging and retain the skeletal muscle's functionality.
Source(s) of funding
This work received a research grant from the University of Mumbai (Project Number 186, 2018). This study was also funded by Pharmanza Herbals Pvt. Ltd.
Conflict of interest
Dr Lal Hingorani and Dr Amol Deshmukh are full time employees of Pharmanza Herbals Pvt. Ltd. All other authors declare no competing interests.
Author contributions
Vandana Panda: Conceptualization, Methodology, Writing original draft, Supervision; Asawari Hare & Sneha Singh: Investigation, Formal analysis; Sudhamani S: Carried out the histopathological investigation; Amol Deshmukh & Lal Hingorani: Conceptualization, Resources, Writing Review and Editing.
Acknowledgement
Authors would like to acknowledge Department of Science and Technology (DST), India for procurement of Thermocycler, Gel electrophoresis unit and Microplate reader which were used in this study.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaim.2021.06.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ábrigo J., Elorza A.A., Riedel C.A., Vilos C., Simon F., Cabrera D., et al. Role of oxidative stress as key regulator of muscle wasting during cachexia. Oxidat Med and Cellular Longevity. 2018;2018:1–17. doi: 10.1155/2018/2063179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalle S., Rossmeislova L., Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol. 2017;8 doi: 10.3389/fphys.2017.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long Y.C., Zierath J.R. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116(7):1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolster D.R., Crozier S.J., Kimball S.R., Jefferson L.S. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277(27):23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- 5.Marzetti E., Hwang J.Y.C., Lees H.A., Wohlgemuth S.E., Dupont-Versteegden E.E., Carter C.S., et al. Mitochondrial death effectors: relevance to sarcopenia and disuse muscle atrophy. Biochim Biophys Acta Gen Subj. 2010;1800(3):235–244. doi: 10.1016/j.bbagen.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh R., Letai A., Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20(3):175–193. doi: 10.1038/s41580-018-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Showkat M., Beigh M.A., Andrabi K.I. mTOR signaling in protein translation regulation: implications in cancer genesis and therapeutic interventions. Mol Biol Int. 2014;2014:686984. doi: 10.1155/2014/686984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wankhede S., Langade D., Joshi K., Sinha S.R., Bhattacharyya S. Examining the effect of Withania somnifera supplementation on muscle strength and recovery: a randomized controlled trial. J Int Soc Sports Nutr. 2015;12:43. doi: 10.1186/s12970-015-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Begum V.H., Sadique J. Effect of Withania somnifera on glycosaminoglycan synthesis in carrageenin-induced air pouch granuloma. Biochem Med Metab Biol. 1987;38(3):272–277. doi: 10.1016/0885-4505(87)90091-0. [DOI] [PubMed] [Google Scholar]
- 10.Chandrasekhar K., Kapoor J., Anishetty S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J Psychol Med. 2012;34(3):255–262. doi: 10.4103/0253-7176.106022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cholewa J.M., Dardevet D., Lima-Soares F., de Araújo Pessôa K., Oliveira P.H., Dos Santos Pinho J.R., et al. Dietary proteins and amino acids in the control of the muscle mass during immobilization and aging: role of the MPS response. Amino Acids. 2017;49(5):811–820. doi: 10.1007/s00726-017-2390-9. [DOI] [PubMed] [Google Scholar]
- 12.Cintineo H.P., Arent M.A., Antonio J., Arent S.M. Effects of protein supplementation on performance and recovery in resistance and endurance training. Front Nutr. 2018;5:83. doi: 10.3389/fnut.2018.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shamim B., Hawley J.A., Camera D.M. Protein availability and satellite cell dynamics in skeletal muscle. Sports Med. 2018;48(6):1329–1343. doi: 10.1007/s40279-018-0883-7. [DOI] [PubMed] [Google Scholar]
- 14.Strickland J.C., Smith M.A. Animal models of resistance exercise and their application to neuroscience research. J Neurosci Methods. 2016;273:191–200. doi: 10.1016/j.jneumeth.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- 16.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 17.Ellman G.L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 18.Sun M., Zigman S. An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Anal Biochem. 1978;90(1):81–89. doi: 10.1016/0003-2697(78)90010-6. [DOI] [PubMed] [Google Scholar]
- 19.Greenwald R.A., editor. Handbook of methods for oxygen radical research. CRC Press Inc.; Boca Raton: 1894. A, C. Catalase activity; pp. 283–284. [Google Scholar]
- 20.Verma S.K., Kumar A. Therapeutic uses of Withania somnifera (ashwagandha) with a note on withanolides and its pharmacological actions. Asian J Pharmaceut Clin Res. 2011;4(1):1–4. [Google Scholar]
- 21.Pennings B., Boirie Y., Senden J.M.G., Gijsen M.P., Kuipers H., van Loon Luc J.C. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr. 2011;93(5):997–1005. doi: 10.3945/ajcn.110.008102. [DOI] [PubMed] [Google Scholar]
- 22.Schaap L.A., Pluijm S.M., Deeg D.J., Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119(6) doi: 10.1016/j.amjmed.2005.10.049. 526.e9–526.e17. [DOI] [PubMed] [Google Scholar]
- 23.Wahlin-Larsson B., Wilkinson D.J., Strandberg E., Hosford-Donovan A., Atherton P.J., Kadi F. Mechanistic links underlying the impact of C-reactive protein on muscle mass in elderly. Cell Physiol Biochem. 2017;44(1):267–278. doi: 10.1159/000484679. [DOI] [PubMed] [Google Scholar]
- 24.Walrand S., Guillet C., Salles J., Cano N., Boirie Y. Physiopathological mechanism of sarcopenia. Clin Geriatr Med. 2011;27(3):365–385. doi: 10.1016/j.cger.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tracey Phillips C.L. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. Faseb J. 2005;19(6):668–670. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- 27.Umegaki H. Sarcopenia and diabetes: hyperglycemia is a risk factor for age-associated muscle mass and functional reduction. Journal of Diabetes Investigation. 2015;6(6):623–624. doi: 10.1111/jdi.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karamanlis A., Chaikomin R., Doran S., Bellon M., Bartholomeusz F.D., Wishart J.M., et al. Effects of protein on glycemic and incretin responses and gastric emptying after oral glucose in healthy subjects. Am J Clin Nutr. 2007;86(5):1364–1368. doi: 10.1093/ajcn/86.5.1364. [DOI] [PubMed] [Google Scholar]
- 29.Prentki M., Matschinsky F.M., Madiraju S.R.M. Metabolic signaling in fuel-induced insulin secretion. Cell Metabol. 2013;18(2):162–185. doi: 10.1016/j.cmet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Anwer T., Sharma M., Pillai K.K., Iqbal M. Effect of Withania somnifera on insulin sensitivity in non-insulin-dependent diabetes mellitus rats. Basic Clin Pharmacol Toxicol. 2008;102(6):498–503. doi: 10.1111/j.1742-7843.2008.00223.x. [DOI] [PubMed] [Google Scholar]
- 31.Keshel T.E., Coker R.H. Exercise training and insulin resistance: a current review. J Obes Weight Loss Ther. 2015;5(5) doi: 10.4172/2165-7904.S5-003. S5-S003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng S.J., Yu L.J. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. 2010;11(4):1509–1526. doi: 10.3390/ijms11041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardie D.G. AMP-activated protein kinase-an energy sensor that regulates all aspects of cell function. Gene Dev. 2011;25(18):1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCormick R., Vasilaki A. Age-related changes in skeletal muscle: changes to life-style as a therapy. Biogerontology. 2018;19(6):519–536. doi: 10.1007/s10522-018-9775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.