Abstract
Background
Krebs von den Lungen-6 (KL-6) is a molecule that is predominantly expressed by damaged alveolar type II cells, and has been proposed as a marker of COVID-19 and the severity of the disease. Here, we performed a meta-analysis to determine whether KL-6 could be used as a prognostic factor for severe COVID-19.
Methods
PubMed, Cochrane and Google Scholar were searched until April 20, 2021, and 7 studies were included. KL-6 was considered as the outcome and pooled in meta-analyses.
Results
All included studies compared KL-6 in severe and non-severe patients. Serum KL-6 was higher in severe COVID-19 patients compared to non-severe (n = 6; SMD = 1.25; 95% CI: 0.99–1.5; P < 0.001) and healthy controls (n = 4; SMD = 3.07; 95% CI: 1.36–4.8; P < 0.001).
Conclusion
This data collection revealed the potential clinical significance of KL-6 as a non-expensive predictive biomarker in severe COVID-19 and for the categorization of COVID-19 clinical severity.
Keywords: COVID-19, KL-6, Mucin-1
Abbreviations: COVID-19, Coronavirus disease 2019; KL-6, Krebs von den Lungen-6; ILD, interstitial lung diseases; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; IL, interleukin; CK, creatin kinase; NLR, neutrophil to lymphocyte ratio
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has spread worldwide, causing a significant impact on the health of millions of people. The scientific community, to screen, clinical management and prevention of serious complications, is in urgent need for reliable biomarkers of COVID-19. Recent studies have shown that several hematological, inflammatory, and biochemical parameters, including lymphocyte count, neutrophil count, NLR,CRP, ESR, IL-6, D-dimer, Troponins, and CK can stratify high-risk patients and can therefore be used as predictive biomarkers (Ponti et al., 2020).
Recent studies have proposed Krebs von den Lungen-6 (KL-6) as a serological biomarker for diagnosing and monitoring COVID-19 positive patients (d'Alessandro et al., 2020a). KL- 6 is a high molecular weight glycoprotein classified as one of the human MUC1 antigens. KL-6 was originally discovered as a circulating pulmonary adenocarcinoma-associated antigen (Kohno et al., 1988; Stahel et al., 1994), expressed predominantly by type II alveolar epithelial (ACE), bronchial glandular epithelial and bronchial gland cells (Nobuoki et al., 1989; Kohno, 1999, Ishikawa et al., 2012). Although KL-6 was initially studied as a tumor marker, it was later found that it is an essential component of the pulmonary innate immune system and plays a critical role in lining the airway lumen (Kato et al., 2017). KL-6 has various pathophysiologic roles, such as inhibiting cell–cell adhesion of epithelial cells (Ligtenberg et al., 1992) and inducing fibroblast migration (Hirasawa et al., 1997). Several studies indicated that after the bronchial epithelium damage and reparative proliferation processes, KL-6 is released from the regenerated type II pneumocytes into blood (Inoue et al., 1997, d’Alessandro et al., 2020). Some published researches investigated the clinical significance of KL-6 in various types of Interstitial Lung Diseases (ILDs) and proposed that elevated levels are useful for detecting the presence of alveolar epithelial cells (AEC) injury and evaluating disease activity in various types of ILDs (Ishikawa et al., 2012; Lee et al., 2019). However, the reports regarding the role of KL-6 in COVID-19 infection are inconsistent (d'Alessandro et al., 2020b; for COVID, 2020, Nakamura et al., 2020; Zeng et al., 2020). To determine the clinical significance of circulating KL-6 levels in COVID-19 patients, we conducted a systematic review and meta-analysis to evaluate the clinical value of KL-6 as a prognostic marker in severe COVID-19.
2. Material and methods
2.1. Data sources and searches strategy
A comprehensive literature review was performed using PubMed, Cochrane and Google scholar. As COVID-19 was first defined in late 2019, the database search was restricted from 2019 to April 20, 2021. The keywords were selected from Medical Subject Headings (MeSH) as follows:
COVID-19 disease terms:
COVID-19 or COVID-19 virus, COVID19 virus, COVID-19 virus disease, COVID-19 virus infection, 2019-nCoV, 2019-nCoV infection, 2019-nCoV disease, 2019 novel corona virus, 2019 novel coronavirus infection, 2019 novel coronavirus disease, coronavirus disease 2019, coronavirus disease 2019 virus, 2019 novel coronavirus infection, coronavirus disease-19, COVID-19 pandemic, coronavirus disease-19 virus, severe acute respiratory syndrome coronavirus, SARS-CoV-2, SARS2, severe acute respiratory syndrome virus, SARS-CoV-2 infection, novel coronavirus, Wuhan coronavirus, Wuhan seafood market pneumonia virus.
KL-6 terms:
KL-6, KL6, MUC1, MUC-1, Mucin-1, Mucin1, Krebs von den Lungen-6 protein.
Potentially relevant studies were found using various combinations of the mentioned terms. Additional studies were identified by searching the cited references of selected articles manually. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) was used to report all studies identified.
2.2. Inclusion and exclusion criteria
Studies were included in this systematic review if they met the following criteria:
-
1.
Studies wrote in the English language.
-
2.
Studies with a cross-sectional, case-control, clinical trial, and cohort study design.
-
3.
Studies with original clinical data on COVID-19 infection which reported outcome on KL6.
-
4.
Studies conducted on human subjects.
Exclusion criteria were as follows:
-
1.
Case reports or case series with less than ten subjects.
-
2.
Reviews, meeting abstracts and guidelines.
-
3.
Studies that were not peer-reviewed or their results were not clear and data could not be extracted.
2.3. Data extraction and risk of bias assessment
Two independent authors screened titles, abstracts and full-text of all citations to identify the potentially eligible studies. The demographic data of the eligible studies including age, gender and sample size were extracted. The method of COVID-19 diagnoses, COVID-19 patients’ classification, country or region of study population, type of study and method of KL-6 identification were also recorded. The methodological quality of the included studies evaluated by two authors independently using the NIH (national institutes of health) risk of bias check list for case-control and cross-sectional studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). Authors discussed in case of any disagreements. The total score for case-control studies was 12 and 9 for cross-sectional studies. Studies passed the quality assessment if they got the minimum score of 50%. Any dissension among researchers was resolved by final discussion to reach a consensus.
2.4. Statistical analysis
Extracted data were analyzed using the STATA software package (version 14.1, STATA Corp, College Station, TX). KL-6 serum levels were extracted as mean and standard deviation (SD) or median and interquartile range (IQR) in which data converted to mean and SD as described by Hozo et al. (Hozo et al., 2005). Outcomes were presented as standardized mean differences (SMD) between severe and non-severe COVID-19 patients and severe and healthy control based on the patient's classification in the included studies. Heterogeneity was assessed using Cochran's Q-test (Lipsey and Wilson, 2001). High degree heterogeneity was defined as I2> 25% and p ≥ 0.10 and a random-effects model was applied. To identify how each study contributes to the overall analysis, a sensitivity analysis was performed as Patsopoulos et al. (Patsopoulos et al., 2008). Since the number of included studies were limited, publication bias was evaluated for studies which compared severe and non-severe COVID-19 and funnel plot and Egger's statistical test were reported (P < 0.1 was considered as significant). A Random effect mete-regression was performed to assess heterogeneity.
3. Results
3.1. Search results and study characteristics
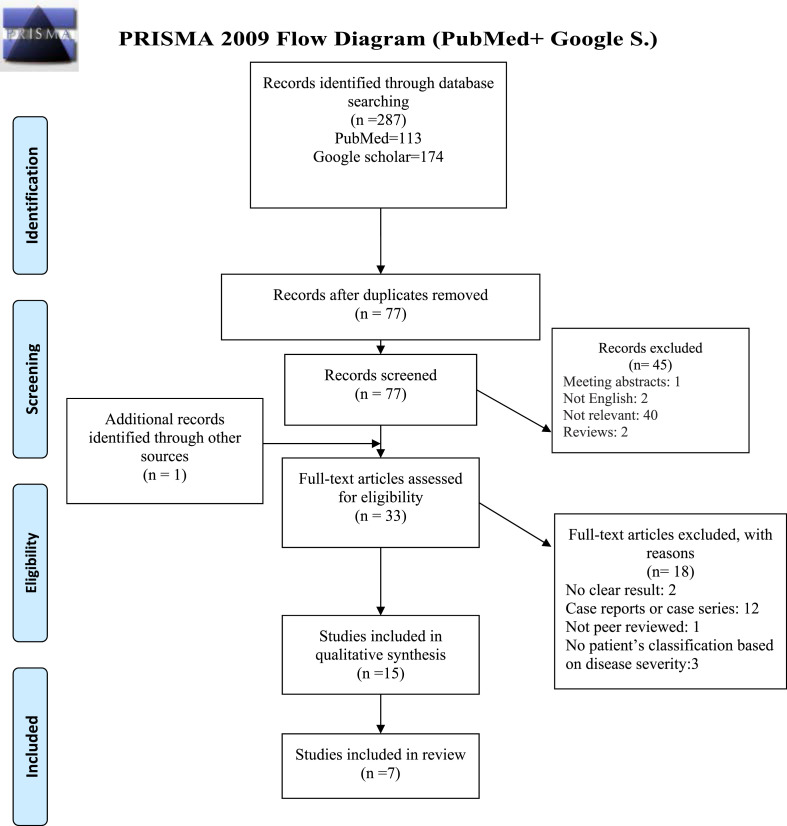
The initial database search yielded 287 records) 113 from PubMed and 174 from Google scholar, no records found from Cochrane). After duplicates were removed, a total of 77 records were screened for eligibility. 1 study was found by manually searching (Zeng et al., 2020).Of these, 1 meeting abstract, 2 not written in the English language, 40 not relevant, 2 reviews were excluded. The full text of 33 remained studies was assessed for eligibility and 12 case reports or case series (Horii et al., 2020; Inoue et al., 2020; Ito et al., 2020; Nakamura et al., 2020; Scarpati, 2020, Shibata et al., 2020; Shimazu et al., 2020; Zeng et al., 2020a; Fukada et al., 2021; Sumimoto et al., 2021; Suzuki et al., 2021; Yamaya et al., 2021), 2 studies that did not report the results clearly (Miyagami et al., 2021; Takeshita et al., 2021), 3 studies that did not classify patients based on disease severity (Frix et al., 2020; Chen et al., 2021a; Scotto et al., 2021) and 1 preprint study (Alimova et al., 2020) were excluded. Finally, 15 studies identified as relevant and entered the quality assessment (Awano et al., 2020, d'Alessandro et al., 2020a, d'Alessandro and Cameli, 2020, d'Alessandro et al., 2020b; Frix et al., 2020; jp, 2020, Saito et al., 2020; Wang et al., 2020; Xue et al., 2020; Anai et al., 2021; Bergantini et al., 2021; Chen et al., 2021, d’Alessandro et al., 2021; Deng et al., 2021; He et al., 2021; Peng et al., 2021; Scotto et al., 2021). Ultimately, 7 studies (1 cross-sectional and 6 case-control studies) met the inclusion criteria of this systematic review (Awano et al., 2020, d'Alessandro et al., 2020b; Saito et al., 2020; Bergantini et al., 2021; Deng et al., 2021; He et al., 2021; Peng et al., 2021) (Table 1 ). Table 2 showed the summary of the selected characteristics of the included studies. From 7 eligible studies 4 were published in 2021 (Bergantini et al., 2021; Deng et al., 2021; He et al., 2021; Peng et al., 2021) and 3 in 2020 (Awano et al., 2020, d'Alessandro et al., 2020b; Saito et al., 2020). Totally, 696 participants including 425 COVID-19 patients (including 136 severe and 289 mild to moderate patients) and 271 healthy subjects were enrolled. The mean age of participants was as follows: severe COVID-19 patients 62.8 ± 3.8 years, non-severe COVID-19 patients 49.8 ± 7.5 years, healthy controls 55.2 ± 7.6 years. KL-6 was measured in serum samples in all studies using KL-6 reagent kit based on latex agglutination assay, electrochemiluminescence or chemiluminescence immune assay (Table 2). The COVID-19 patients were classified according to the disease severity. The definition for this classification in each study was shown in Supplementary Table 1. Here we defined mild to moderate patients as non-severe groups. Notably, none of the studies did clearly describe the performance power and sample size calculation. The criteria for COVID-19 classifications as severe and non-severe patients were summarized in Table 3 .
Table 1.
Table 2.
Characteristics of the included studied.
| Number | Author(s) Table 2: Characteristics of the included studied. (Year) (Ref.) |
Study design | Sample size Critical b |
Age (median and IQRa or mean ± SD) Critical |
Sex (n) (Male/Female) Critical |
Test | Region of study population |
|---|---|---|---|---|---|---|---|
| Severe |
Severe |
Severe |
|||||
| Moderate |
Moderate |
Moderate |
|||||
| Mild |
Mild |
Mild |
|||||
| Control | Control | Control | |||||
| 1 | Awano et al. (2020)(Awano et al., 2020) | Cross-sectional | 10 | 64 (56–78) | 15/6 | KL-6 Reagent kit | Japan |
| 11 | |||||||
| 29 | 40 (33–50) | 23/41 | |||||
| 4 | |||||||
| – | – | – | |||||
| 2 | d’Alessandro et al. (2020)(d'Alessandro et al. 2020b) | Case-control | – | – | – | KL-6 Reagent kit | Italy |
| 12 | 62 (60–68) | 9/3 | |||||
| 10 | 64 (51–64) | 6/4 | |||||
| – | – | – | |||||
| 22 | 54 (29–60) | 6/16 | |||||
| 3 | Bergantini et al. (2021)(Bergantini et al., 2021) | Case-control | – | – | – | KL-6 Reagent kit | Italy |
| 10 | 62.5 ± 8 | 8/2 | |||||
| 143 | 62.2 ± 15.6 | 11/3 | |||||
| – | – | – | |||||
| 30 | 59 ± 9.8 | 18/12 | |||||
| 4 | Deng et al. (2021)(Deng et al., 2021) | Case-control | – | – | – | Chemiluminescence immunity | China |
| 17 | 55 (53–68) | 9/8 | |||||
| – | – | – | |||||
| 149 | 48 (34.5–62) | 65/84 | |||||
| 59 | – | – | |||||
| 70 | 58 (52–64) | 35/35 | |||||
| 5 | He et ai. (2021) (He et al., 2021) | Case-control | – | – | – | Chemiluminescence immunity | China |
| 28 | 64.93 ± 1.63 | 16/12 | |||||
| – | – | – | |||||
| – | – | – | |||||
| 25 | 64.56 ± 1.55 | 14/11 | |||||
| 6 | Peng et al. (2021)(Peng et al., 2021) | Case-control | – | KL-6 Reagent kit | China | ||
| 36 | 56 (28–86) | 24/12 | |||||
| 28 | 51 (27–75) | 12/16 | |||||
| 49 | 45 (16–72) | 25/24 | |||||
| 65 | 50 (22–69) | 28/37 | |||||
| 7 | Saito et al. (2020)(Saito et al., 2020) | Case-control | Electrochemiluminescence immunoassay | Japan | |||
| 12 | 65.1 ± 10.7 | 7/5 | |||||
| 34c | 49.6 ± 15.7 | 14/20 | |||||
| – | – | – |
Inter quartile range.
Definition of the COVID-19 classifications were shown in Table 3
Mild and moderate COVID-19 patients defined as non-severe.
Table 3.
Criteria of the COVID-19 disease classification in the included studies.
| Author(s) | Severe COVID-19 | Non-severe COVID-19 |
|
|---|---|---|---|
| Moderate COVID-19 | Mild COVID-19 | ||
| Awano et al. | Severe illness, patients who had a respiratory rate of >30 breaths per minute, SpO2 < 94% on room air at sea level, ratio of arterial partial pressure of oxygen to fraction of inspired oxygen <300 mmHg, or lung infiltrates >50%. | Evidence of lower respiratory disease on clinical assessment or imaging and SpO2 > 94% on room air at sea level. | Any of the various signs and symptoms of COVID-19 without shortness of breath, dyspnea, or abnormal chest imaging. |
| d’Alessandro et al. | All patients underwent intubation and mechanical ventilation in the COVID intensive care unit (ICU). | Patients (not requiring intubation) were hospitalized for pharmacological treatment and oxygen supplementation or noninvasive ventilation. | |
| Bergantini et al. | Requiring intubation and invasive mechanical ventilation. | Not requiring intubation and invasive mechanical ventilation. | |
| Deng et al. | Patients were diagnosed as mild or severe/critical according to Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (seventh edition, General Office of National Health Commission). | ||
| He et al. | Not mentioned. | ||
| Peng et al. | Having dyspnea, respiratory frequency ≥30/min, blood oxygen saturation ≤93%, partial pressure of arterial oxygen to fraction of inspired oxygen ratio <300, and/or lung infiltrates >50% within 24–48 h. | Having clinical symptoms, such as fever and cough, as well as CT imaging features of pneumonia. | Having mild clinical symptoms without CT imaging features of pneumonia. |
| Saito et al. | The disease is classified as severe if one of the following conditions is met: Respiratory distress, respiratory rate ≥30/min, Oxygen saturation on room air at rest ≤93%, Partial pressure of oxygen in arterial blood/FiO2 ≤ 300 mm Hg. | Fever, cough, and other symptoms are present with pneumonia on chest CT. | The clinical symptoms are mild, with no abnormal radiological findings. |
3.2. Pooling of studies and subgroup analysis
Seven studies provided sufficient information to allow the comparison of the serum KL-6 levels between severe and non-severe patients (Awano et al., 2020, d'Alessandro et al., 2020b; Saito et al., 2020; Bergantini et al., 2021; Deng et al., 2021; He et al., 2021; Peng et al., 2021). Four studies included sufficient data for the comparison of serum KL-6 levels between severe and control groups (d'Alessandro et al., 2020b; Deng et al., 2021; He et al., 2021; Peng et al., 2021). Three studies reported KL-6 serum level in non-severe and control groups d'Alessandro et al., 2020b; Deng et al., 2021; Peng et al., 2021). These studies did not include in meta-analyses because of the low sample size.
3.3. KL-6 serum level in severe and non-severe patients
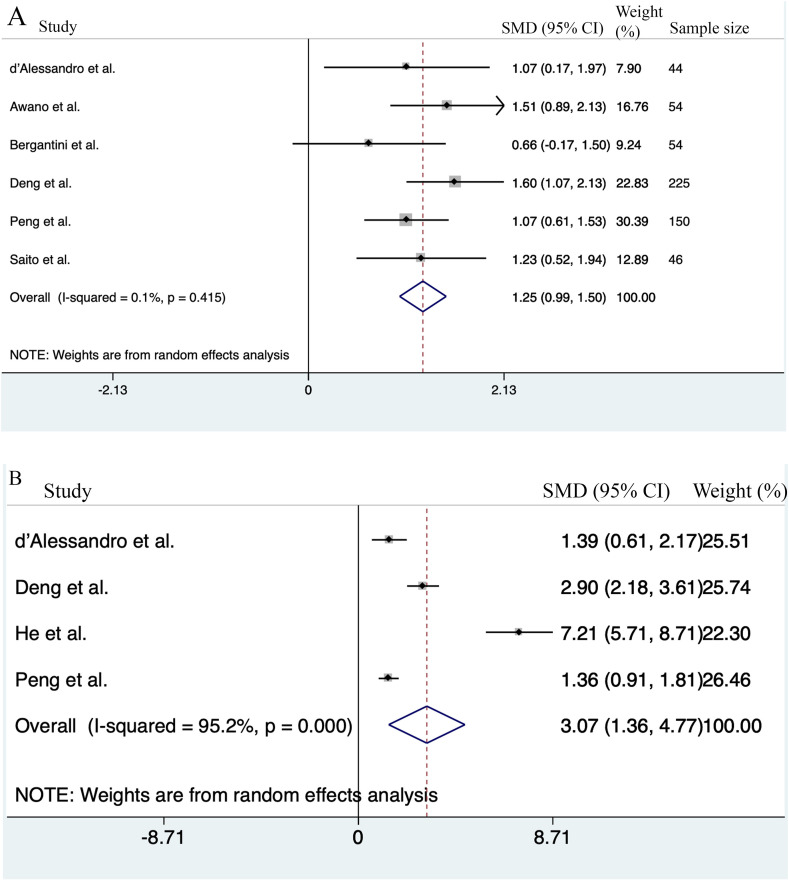
At first, data pooling was performed by both fixed and random effect models and if the heterogeneity was higher than 25%, the random effect was used. Data pooling with random effect model performed in 6 studies comparing serum KL-6 concentrations between severe and non-severe patients (Awano et al., 2020, d'Alessandro et al., 2020b; Saito et al., 2020; Bergantini et al., 2021; Deng et al., 2021; Peng et al., 2021). Results showed that the KL-6 concentration was higher in severe COVID-19 patients than non-severe patients (n = 6; SMD = 1.25; 95% CI: 0.99–1.5; P < 0.001). Q test results revealed that there was no heterogeneity between studies and both fixed-effect and random-effect analyses revealed the same results (n = 6; I2 = 0.1%; P = 0.4) (Fig. 1 A).
Fig. 1.
Meta-analysis of serum KL-6 levels between sever and non-severe COVID-19 patients, and healthy control. (A) Forest plots showing the standardized mean differences (SMD) of serum KL-6 levels of severe COVID-19 patients compared to non-severe patients. (B) Forest plots depicting SMD of serum KL-6 levels compared between severe COVID-19 patients and healthy controls.
Egger's tests revealed that publication bias was not significant (P = 0.5). Funnel plot was shown in Supplementary Fig. 1.
3.4. KL-6 serum level in healthy controls and COVID-19 patients
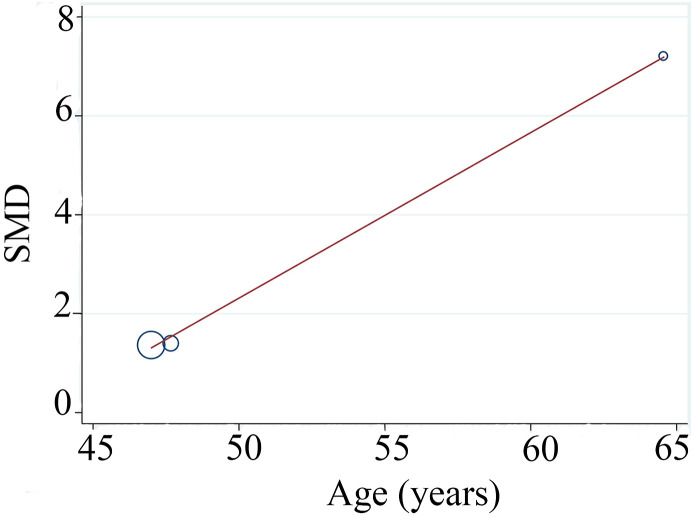
Four studies compared KL-6 serum levels between patients suffering from severe COVID-19 patients and healthy controls (d'Alessandro et al., 2020c; Deng et al., 2021; He et al., 2021; Peng et al., 2021). Serum KL-6 was higher in severe COVID-19 patients than controls (n = 4; SMD = 3.07; 95% CI: 1.36–4.8; P < 0.001). Cochran's Q-test revealed high heterogeneity (n = 4; I2 = 95.2%; P < 0.001) (Fig. 1B). To evaluate the source of heterogeneity, we used sensitivity analyses and each time 1 study was removed. After removing the He et al. study (He et al., 2021), the between-study heterogeneity was reduced but still high (I2 = 85.4%, P = 0.001). The KL-6 serum level in severe patients remained higher than control (n = 3; SMD = 1.86; 95% CI: 0.9–2.8; P = 0.000). As can be seen from Table 2, the mean ages of the study participants were different in the three studies (d'Alessandro et al., 2020c; Deng et al., 2021; Peng et al., 2021) and therefore, the high heterogeneity in serum KL-6 levels in various studies might be attributed to the age. The mean age of the participants in control groups was lower than COVID-19 patients (60.9 ± 3.9 years for severe and 53.1 ± 9.9 years for control). Meta-regression was performed to identify if age was the sources of heterogeneity in the included studies. Conducting the meta-regression analysis based on age removed the heterogeneity (n = 3; tau2 = 0; P > t = 0.0.86; I2res = 0.0%) (Fig. 2 ).
Fig. 2.
Meta regression analyses for serum KL-6 level based on difference of mean age between severe COVID-19 and healthy controls revealed that age was the source of heterogeneity.
4. Discussion
Using a detailed search strategy and predetermined selection criteria and meta-analyses, we found that KL-6 levels in the serum of severe COVID-19 patients were significantly higher than non-severe patients and healthy controls (Fig. 1A). The results of this meta-analysis were consistent with those of previous studies indicating the prognostic value of KL-6 in severe COVID-19 (Nakamura et al., 2020; Deng et al., 2021). It has been shown that KL-6 plays essential role in pathophysiological processes. The increased levels of KL-6 in COVID-19 patients have been previously reported in different pulmonary diseases, including hypersensitivity pneumonia, idiopathic pulmonary fibrosis, pulmonary tuberculosis, radiation pneumonia, drug-induced ILDs, acute respiratory distress syndrome, lung cancer, and pulmonary sarcoidosis (Wu et al., 2003; Sørensen et al., 2006; Doishita et al., 2011; Ishikawa et al., 2012). KL-6 levels reflecting the extent of damage and regeneration of type II pneumocytes can predict the risk of illness or death in subjects suffering from lung diseases such as polymyositis and dermatomyositis patients (Hu et al., 2019). The studies indicated that increased levels of KL-6 in the epithelial lining fluid might arouse fibrotic processes in ILDs and is directly related with the pathogenic process of ILD and acute respiratory distress syndrome (ARDS) (Bandoh et al., 2000; Fukaya et al., 2000; Yanaba et al., 2003; Sato et al., 2004).
The reason why increased serum KL-6 level can serve as a prognostic and predictive biomarker in COVID-19 patients has been unknown in detail but might be explained by KL-6 role as one of the key molecules involved in epithelial-mesenchymal interactions (Xu et al., 2013a).
Several studies showed that the injured AECs secreted large amounts of KL-6 which promoted lung fibrosis through multiple mechanisms including:
1- Attracting human fibroblasts which is enhanced by the addition of fibronectin (Hirasawa et al., 1997). 2- Triggering transforming growth factor-β (TGF- β) signaling has profibrotic and anti-apoptotic effects on lung fibroblasts (Zhong et al., 2020). 3- Up-regulating the expression of collagen type I and III in a dose-dependent manner (Hirasawa et al., 1997). 4- Inhibiting the fibroblasts from producing hepatocyte growth factor (HGF) (Xu et al., 2013a). HGF protects alveolar epithelial cells and increases the proliferation of these cells (Panos et al., 1996; Shukla et al., 2009). In line with the above reasoning, Peng et al. study showed that serum KL-6 concentration in COVID-19 patients with signs of pulmonary fibrosis was higher than that in COVID-19 cases without fibrosis (Peng et al., 2021).
It seems logical that with exacerbation of COVID-19, a large amount of viral replication infiltration could destroy the alveolar epithelium and basement membrane leading to KL-6 secretion in patients BALF and blood (Xue et al., 2020) which in the context of dysregulation of innate immune responses (Tahaghoghi-Hajghorbani et al., 2020) and in cooperation with other chemoattractant for fibroblasts such as fibronectin (Sage et al., 1983), TGF- β (Khalil et al., 1991), epidermal growth factor (Grant et al., 1992; Raaberg et al., 1992), thrombin (Dawes et al., 1993) and collagens (Albini and Adelmann-Grill, 1985, Bonner et al., 1993) might contribute to the lung fibrosis. This explanation is consistent with Xuo et al. study which showed that the levels of KL-6 reflected the damage of alveolar epithelium and were significantly correlated with the following indicators of disease severity: 1. Oxygenation index (PaO2/FiO2), 2. The progress of lung lesion area, and 3. Changes of cytokine IL-6 (Xue et al., 2020).
However, these findings should be interpreted with caution since there are some limitations in our meta-analysis that should be considered. First, only a few studies were eligible for inclusion in the meta-analysis. Second, all of the studies were retrospective and observational. Third, subgroup analyses were hindered by lacking sufficient information about the association between KL-6 and disease outcome such as overall survival and need for ICU care and a cut-off value of serum KL-6. Further additional prospective or longitudinal studies focusing on the above information and extensive sample size studies are needed to be conducted for corroborating the association between KL-6 levels and COVID-19 unfavorable outcomes.
Interestingly, based upon previous research on the therapeutic effectiveness of the anti-KL-6 antibody in a mouse pulmonary fibrosis model, (Xu et al., 2013), these findings could also raise the possibility of applying an anti-KL-6 antibody to prevent and treat pulmonary fibrosis following COVID-19 Infection. In summary, the current meta-analysis suggests that KL-6 as an auxiliary evaluation index of lung injury can be a non-expensive, rapid screening tool of COVID-19 severity which concomitant use with other potential COVID-19 biomarkers, including IL-6, troponin I, lactate dehydrogenase and lymphopenia can effectively improve clinical management and prevention of serious complications.
Data availability statement
All relevant data are within the manuscript and its Supporting Information files.
Competing interest's disclosure
The authors declare that they had no competing interest.
Funding
The authors received funding from Hormozgan University of Medical Sciences, number: 990562.
CRediT authorship contribution statement
Nadereh Naderi: Conceptualization, Data curation, Writing – original draft, developed the concept and performed data extraction. wrote the manuscript and read it carefully. Mahsa Rahimzadeh: Conceptualization, Data curation, Formal analysis, Writing – original draft, developed the concept and performed data extraction. analyzed data and prepared the figures and tables. wrote the manuscript and read it carefully.
Acknowledgements
We thank Miss. Gisso Mehri for her valuable comments on this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virol.2021.11.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Funnel plot for assessment of publication bias of studies that compared KL-6 serum level between severe and non-severe COVID-19 disease patients.
References
- Albini A., Adelmann-Grill B. Collagenolytic cleavage products of collagen type I as chemoattractants for human dermal fibroblasts. Eur. J. Cell Biol. 1985;36(1):104–107. [PubMed] [Google Scholar]
- Alimova M., Sidhom E.-H., Satyam A., Dvela-Levitt M., Melanson M., Chamberlain B.T., Alper S.L., Santos J., Gutierrez J., Subramanian A. A High Content Screen for Mucin-1-Reducing Compounds Identifies Fostamatinib as a Candidate for Rapid Repurposing for Acute. bioRxiv; 2020. Lung injury during the COVID-19 pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anai M., Akaike K., Iwagoe H., Akasaka T., Higuchi T., Miyazaki A., Naito D., Tajima Y., Takahashi H., Komatsu T. Decrease in hemoglobin level predicts increased risk for severe respiratory failure in COVID-19 patients with pneumonia. Res. Invest. 2021;59(2):187–193. doi: 10.1016/j.resinv.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awano N., Inomata M., Kuse N., Tone M., Takada K., Muto Y., Fujimoto K., Akagi Y., Mawatari M., Ueda A. Serum KL-6 level is a useful biomarker for evaluating the severity of coronavirus disease 2019. Resp. Invest. 2020;58(6):440–447. doi: 10.1016/j.resinv.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandoh S., Fujita J., Ohtsuki Y., Ueda Y., Hojo S., Tokuda M., Dobashi H., Kurata N., Yoshinouchi T., Kohno N. Sequential changes of KL-6 in sera of patients with interstitial pneumonia associated with polymyositis/dermatomyositis. Ann. Rheum. Dis. 2000;59(4):257–262. doi: 10.1136/ard.59.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergantini L., Bargagli E., d'Alessandro M., Refini R., Cameli P., Galasso L., Scapellato C., Montagnani F., Scolletta S., Franchi F. Prognostic bioindicators in severe COVID-19 patients. Cytokine. 2021;141:155455. doi: 10.1016/j.cyto.2021.155455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J.C., Goodell A.L., Coin P.G., Brody A.R. Chrysotile asbestos upregulates gene expression and production of alpha-receptors for platelet-derived growth factor (PDGF-AA) on rat lung fibroblasts. J. Clin. Invest. 1993;92(1):425–430. doi: 10.1172/JCI116584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Qin R., Huang Z., He L., Luo W., Zheng P., Huang H., Wang H., Sun B. Characteristics of COVID-19 patients based on the results of nucleic acid and specific antibodies and the clinical relevance of antibody levels. Front. Mol. Biosci. 2021;7:450. doi: 10.3389/fmolb.2020.605862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Qin R., Huang Z., Luo W., Zheng P., Huang H., Hu H., Wang H., Sun B. Cytokine; 2021. Clinical relevance of serum Krebs von den Lungen-6 levels in patients with coronavirus disease 2019; p. 155513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covid J.E. Nationwide system to centralize decisions around ECMO use for severe COVID-19 pneumonia in Japan (Special Correspondence) J. Intens. Care. 2020;8 doi: 10.1186/s40560-020-00445-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Alessandro M., Cameli P. 2020. Serum KL-6 concentrations as a novel biomarker of severe COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Alessandro M., Bergantini L., Cameli P., Curatola G., Remediani L., Sestini P., Bargagli E. Peripheral biomarkers' panel for severe COVID-19 patients. J. Med. Virol. 2020;93(3):1230–1232. doi: 10.1002/jmv.26577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Alessandro M., Cameli P., Bergantini L., Franchi F., Scolletta S., Bargagli E. Serum concentrations of Krebs von den Lungen-6 in different COVID-19 phenotypes. J. Med. Virol. 2020 doi: 10.1002/jmv.26431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Alessandro M., Cameli P., Refini R.M., Bergantini L., Alonzi V., Lanzarone N., Bennett D., Rana G.D., Montagnani F., Scolletta S. Serum KL‐6 concentrations as a novel biomarker of severe COVID‐19. J. Med. Virol. 2020;92(10):2216–2220. doi: 10.1002/jmv.26087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes K., Gray A., Laurent G. Thrombin stimulates fibroblast chemotaxis and replication. Eur. J. Cell Biol. 1993;61(1):126. [PubMed] [Google Scholar]
- Deng K., Fan Q., Yang Y., Deng X., He R., Tan Y., Lan Y., Deng X., Pan Y., Wang Y. Prognostic roles of KL‐6 in disease severity and lung injury in COVID‐19 patients: a longitudinal retrospective analysis. J. Med. Virol. 2021;93(4):2505–2512. doi: 10.1002/jmv.26793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doishita S., Inokuma S., Asashima H., Nakachi S., Matsuo Y., Rokutanda R., Kobayashi S., Hagiwara K., Satoh T., Akiyama O. Serum KL-6 level as an indicator of active or inactive interstitial pneumonitis associated with connective tissue diseases. Intern. Med. 2011;50(23):2889–2892. doi: 10.2169/internalmedicine.50.5866. [DOI] [PubMed] [Google Scholar]
- d'Alessandro M., Bergantini L., Cameli P., Vietri L., Lanzarone N., Alonzi V., Pieroni M., M Refini R., Sestini P., Bonella F. Krebs von den Lungen-6 as a biomarker for disease severity assessment in interstitial lung disease: a comprehensive review. Biomarkers Med. 2020;14(8):665–674. doi: 10.2217/bmm-2019-0545. [DOI] [PubMed] [Google Scholar]
- d'Alessandro M., Bergantini L., Cameli P., Curatola G., Remediani L., Bennett D., Bianchi F., Perillo F., Volterrani L., Mazzei M.A. Serial KL-6 measurements in COVID-19 patients. Intern. Emerg. Med. 2021:1–5. doi: 10.1007/s11739-020-02614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frix A.-N., Schoneveld L., Ladang A., Henket M., Duysinx B., Vaillant F., Misset B., Moutschen M., Louis R., Cavalier E. Could KL-6 levels in COVID-19 help to predict lung disease? Respir. Res. 2020;21(1):1–4. doi: 10.1186/s12931-020-01560-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada A., Kitagawa Y., Matsuoka M., Sakai J., Imai K., Tarumoto N., Orihara Y., Kawamura R., Takeuchi S., Maesaki S. Presepsin as a predictive biomarker of severity in COVID‐19: a case series. J. Med. Virol. 2021;93(1):99–101. doi: 10.1002/jmv.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya S., Oshima H., Kato K., Komatsu Y., Matsumura H., Ishii K., Miyama H., Nagai T., Tanaka I., Mizutani A. KL-6 as a novel marker for activities of interstitial pneumonia in connective tissue diseases. Rheumatol. Int. 2000;19(6):223–225. doi: 10.1007/s002960000064. [DOI] [PubMed] [Google Scholar]
- Grant M.B., Khaw P.T., Schultz G.S., Adams J.L., Shimizu R.W. Effects of epidermal growth factor, fibroblast growth factor, and transforming growth factor-beta on corneal cell chemotaxis. Investig. Ophthalmol. Vis. Sci. 1992;33(12):3292–3301. [PubMed] [Google Scholar]
- He L., Lu L., Zong M., Zhou H., Wang L., Chen N.Z., Yuan J.Y., Jiang E.P., Zheng L., Li Q. 2021. The significance of KL-6 as prognosis monitoring biomarker in patients with severe COVID-19 from stabilized stage toward convalescence. [Google Scholar]
- Hirasawa Y., Kohno N., Yokoyama A., Inoue Y., Abe M., Hiwada K. KL-6, a human MUC1 mucin, is chemotactic for human fibroblasts. Am. J. Respir. Cell Mol. Biol. 1997;17(4):501–507. doi: 10.1165/ajrcmb.17.4.2253. [DOI] [PubMed] [Google Scholar]
- Horii H., Kamada K., Nakakubo S., Yamashita Y., Nakamura J., Nasuhara Y., Konno S. Rapidly progressive organizing pneumonia associated with COVID-19. Respir. Med. Case Rep. 2020;31:101295. doi: 10.1016/j.rmcr.2020.101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5(1):1–10. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C., Wu C., Yang E., Huang H., Xu D., Hou Y., Zhao J., Li M., Xu Z., Zeng X. Serum KL-6 is associated with the severity of interstitial lung disease in Chinese patients with polymyositis and dermatomyositis. Clin. Rheumatol. 2019;38(8):2181–2187. doi: 10.1007/s10067-019-04501-9. [DOI] [PubMed] [Google Scholar]
- Inoue Y., Barker E., Daniloff E., Kohno N., Hiwada K., Newman L.S. Pulmonary epithelial cell injury and alveolar–capillary permeability in berylliosis. Am. J. Respir. Crit. Care Med. 1997;156(1):109–115. doi: 10.1164/ajrccm.156.1.9612043. [DOI] [PubMed] [Google Scholar]
- Inoue H., Jinno M., Ohta S., Kishino Y., Kawahara T., Mikuni H., Sato H., Yamamoto M., Sato Y., Onitsuka C. Combination treatment of short-course systemic corticosteroid and favipiravir in a successfully treated case of critically ill COVID-19 pneumonia with COPD. Respir. Med. Case Rep. 2020;31:101200. doi: 10.1016/j.rmcr.2020.101200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa N., Hattori N., Yokoyama A., Kohno N. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Resp. Invest. 2012;50(1):3–13. doi: 10.1016/j.resinv.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Ito K., Yokoyama T., Oguri A., Kato C., Horiuchi M., Kato M., Usami I. 2020. Case report: a case of COVID-19 pneumonia that did not worsen and was relieved by early administration of favipiravir and ciclesonide. [DOI] [PMC free article] [PubMed] [Google Scholar]
- jp Nationwide system to centralize decisions around ECMO use for severe COVID-19 pneumonia in Japan (special correspondence) J. Intens. Care. 2020;8:1–2. doi: 10.1186/s40560-020-00445-4. n. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K., Lillehoj E.P., Lu W., Kim K.C. MUC1: the first respiratory mucin with an anti-inflammatory function. J. Clin. Med. 2017;6(12):110. doi: 10.3390/jcm6120110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil N., O'Connor R.N., Unruh H.W., Warren P.W., Flanders K.C., Kemp A., Bereznay O.H., Greenberg A.H. Increased production and immunohistochemical localization of transforming growth factor-b in idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 1991;5:155–162. doi: 10.1165/ajrcmb/5.2.155. [DOI] [PubMed] [Google Scholar]
- Kohno N. Serum marker KL-6/MUC1 for the diagnosis and management of interstitial pneumonitis. J. Med. Invest. 1999;46(3/4):151–158. [PubMed] [Google Scholar]
- Kohno N., Akiyama M., Kyoizumi S., Hakoda M., Kobuke K., Yamakido M. Detection of soluble tumor-associated antigens in sera and effusions using novel monoclonal antibodies, KL-3 and KL-6, against lung adenocarcinoma. Jpn. J. Clin. Oncol. 1988;18(3):203–216. [PubMed] [Google Scholar]
- Lee J.S., Lee E.Y., Ha Y.-J., Kang E.H., Lee Y.J., Song Y.W. Serum KL-6 levels reflect the severity of interstitial lung disease associated with connective tissue disease. Arthritis Res. Ther. 2019;21(1):58. doi: 10.1186/s13075-019-1835-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligtenberg M.J., Buijs F., Vos H.L., Hilkens J. Suppression of cellular aggregation by high levels of episialin. Cancer Res. 1992;52(8):2318–2324. [PubMed] [Google Scholar]
- Lipsey M.W., Wilson D.B. SAGE publications, Inc; 2001. Practical Meta-Analysis. [Google Scholar]
- Miyagami T., Uehara Y., Harada T., Watari T., Shimizu T., Nakamura A., Ogura N., Kushiro S., Masuyama K., Kanai Y. 2021. Delayed treatment of bacteremia during the COVID-19 pandemic. (Diagnosis) [DOI] [PubMed] [Google Scholar]
- Nakamura H., Miyagi K., Otsuki M., Higure Y., Nishiyama N., Kinjo T., Nakamatsu M., Haranaga S., Tateyama M., Fujita J. Serum KL‐6 can distinguish between different phenotypes of severe COVID‐19. J. Med. Virol. 2020 doi: 10.1002/jmv.26268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobuoki K., Seishi K., Yukikazu A., Hirofumi F., Michio Y., Mitoshi A. New serum indicator of interstitial pneumonitis activity: sialylated carbohydrate antigen KL-6. Chest. 1989;96(1):68–73. doi: 10.1378/chest.96.1.68. [DOI] [PubMed] [Google Scholar]
- Panos R.J., Patel R., Bak P.M. Intratracheal administration of hepatocyte growth factor/scatter factor stimulates rat alveolar type II cell proliferation in vivo. Am. J. Respir. Cell Mol. Biol. 1996;15(5):574–581. doi: 10.1165/ajrcmb.15.5.8918364. [DOI] [PubMed] [Google Scholar]
- Patsopoulos N.A., Evangelou E., Ioannidis J.P. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int. J. Epidemiol. 2008;37(5):1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D.-H., Luo Y., Huang L.-J., Liao F.-L., Liu Y.-Y., Tang P., Hu H.-N., Chen W. Correlation of Krebs von den Lungen-6 and fibronectin with pulmonary fibrosis in coronavirus disease 2019. Clin. Chim. Acta. 2021;517:48–53. doi: 10.1016/j.cca.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti G., Maccaferri M., Ruini C., Tomasi A., Ozben T. Critical Reviews in Clinical Laboratory Sciences; 2020. Biomarkers Associated with COVID-19 Disease Progression; pp. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaberg L., Nexo E., Buckley S., Luo W., Snead M.L., Warburton D. Epidermal growth factor transcription, translation, and signal transduction by rat type II pneumocytes in culture. Am. J. Respir. Cell Mol. Biol. 1992;6(1):44–49. doi: 10.1165/ajrcmb/6.1.44. [DOI] [PubMed] [Google Scholar]
- Sage H., Farin F.M., Striker G.E., Fisher A.B. Granular pneumocytes in primary culture secrete several major components of the extracellular matrix. Biochemistry. 1983;22(9):2148–2155. doi: 10.1021/bi00278a015. [DOI] [PubMed] [Google Scholar]
- Saito A., Kuronuma K., Moniwa K., Kodama K., Takahashi S., Takahashi H., Chiba H. 2020. Serum surfactant protein A and D may be novel biomarkers of COVID-19 pneumonia severity. [Google Scholar]
- Sato H., Callister M., Mumby S., Quinlan G., Welsh K., Evans T. KL‐6 levels are elevated in plasma from patients with acute respiratory distress syndrome. Eur. Respir. J. 2004;23(1):142–145. doi: 10.1183/09031936.03.00070303. [DOI] [PubMed] [Google Scholar]
- Scarpati G. KL-6 levels in COVID-19. Translational Medicine@ UniSa. 2020;23(4):14. doi: 10.37825/2239-9747.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotto R., Pinchera B., Perna F., Atripaldi L., Giaccone A., Sequino D., Zappulo E., Sardanelli A., Schiano Moriello N., Stanziola A. Serum KL-6 could represent a reliable indicator of unfavourable outcome in patients with COVID-19 pneumonia. Int. J. Environ. Res. Publ. Health. 2021;18(4):2078. doi: 10.3390/ijerph18042078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S., Ishiguro T., Kobayashi Y., Koike M., Numano T., Shimizu Y., Takayanagi N. High incidence of false-positive results of IgG antibody against SARS-CoV-2 with rapid immunochromatographic antibody test due to human common cold coronavirus infection. Respir. Med. Case Rep. 2020;31:101180. doi: 10.1016/j.rmcr.2020.101180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu H., Yoshiya K., Tamagaki K., Okamoto Y., Nakano H., Okubata H., Miyano Y., Matsunami S., Maruyama S., Kanayama S. 2020. The efficacy of combination therapies including antiviral drugs, methylprednisolone and daily proning in 20 patients with COVID-19 requiring invasive mechanical ventilation—case series in a single critical care center in Osaka, Japan. [Google Scholar]
- Shukla M.N., Rose J.L., Ray R., Lathrop K.L., Ray A., Ray P. Hepatocyte growth factor inhibits epithelial to myofibroblast transition in lung cells via Smad7. Am. J. Respir. Cell Mol. Biol. 2009;40(6):643–653. doi: 10.1165/rcmb.2008-0217OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen G.L., Hjelmborg J.v.B., Kyvik K.O., Fenger M., Høj A., Bendixen C., Sørensen T.I., Holmskov U. Genetic and environmental influences of surfactant protein D serum levels. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290(5):L1010–L1017. doi: 10.1152/ajplung.00487.2005. [DOI] [PubMed] [Google Scholar]
- Stahel R.A., Gilks W.R., Lehmann H.P., Schenker T. Third international workshop on lung tumor and differentiation antigens: overview of the results of the central data analysis. Int. J. Cancer. 1994;57(S8):6–26. doi: 10.1002/ijc.2910570704. [DOI] [PubMed] [Google Scholar]
- Sumimoto K., Taniguchi Y., Emoto N., Hirata K.-I. Combination therapy with pulmonary arterial hypertension targeted drugs and immunosuppression can be a useful strategy for sarcoidosis-associated pulmonary hypertension: a case report. Eur. Heart J. Case Rep. 2021;5(1):ytaa454. doi: 10.1093/ehjcr/ytaa454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Tanino Y., Nikaido T., Minemura H., Umeda T., Rikimaru M., Onuma T., Naito S., Takiguchi Y., Tomita H. Severe coronavirus disease 2019 that recovered from respiratory failure by treatment that included high-dose intravenous immunoglobulin. Intern. Med. 2021;60(3):457–461. doi: 10.2169/internalmedicine.6326-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahaghoghi-Hajghorbani S., Zafari P., Masoumi E., Rajabinejad M., Jafari-Shakib R., Hasani B., Rafiei A. The role of dysregulated immune responses in COVID-19 pathogenesis. Virus Res. 2020:198197. doi: 10.1016/j.virusres.2020.198197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita M., Nishina N., Moriyama S., Takahashi Y., Uwamino Y., Nagata M., Aoki W., Masaki K., Ishii M., Saya H. Incomplete humoral response including neutralizing antibodies in asymptomatic to mild COVID-19 patients in Japan. Virology. 2021 doi: 10.1016/j.virol.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Chen L., Zhang Y., Liu L., Xu M., Sun B., Gao Y., Jin T., Li M. 2020. Detection of serum KL-6 and SARS-CoV-2 antibody in patients with coronavirus disease 2019 and the diagnostic value in severe disease. [Google Scholar]
- Wu H., Kuzmenko A., Wan S., Schaffer L., Weiss A., Fisher J.H., Kim K.S., McCormack F.X. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J. Clin. Invest. 2003;111(10):1589–1602. doi: 10.1172/JCI16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Yan D., Zhu S., Gu J., Bian W., Rong Z., Shen C. KL-6 regulated the expression of HGF, collagen and myofibroblast differentiation. Eur. Rev. Med. Pharmacol. Sci. 2013;17(22):3073–3077. [PubMed] [Google Scholar]
- Xu L., Yang D., Zhu S., Gu J., Ding F., Bian W., Rong Z., Shen C. Bleomycin-induced pulmonary fibrosis is attenuated by an antibody against KL-6. Exp. Lung Res. 2013;39(6):241–248. doi: 10.3109/01902148.2013.798056. [DOI] [PubMed] [Google Scholar]
- Xue M., Zheng P., Bian X., Huang Z., Huang H., Zeng Y., Hu H., Liu X., Zhou L., Sun B., Wu J.L., Zhong N. Exploration and correlation analysis of changes in Krebs von den Lungen-6 levels in COVID-19 patients with different types in China. J. Med. Virol. 2020;14(4):290–296. doi: 10.5582/bst.2020.03197. [DOI] [PubMed] [Google Scholar]
- Yamaya T., Baba T., Hagiwara E., Ikeda S., Niwa T., Kitayama T., Murohashi K., Higa K., Sato Y., Ogura T. Internal Medicine. 2021. Pneumothorax in a COVID-19 pneumonia patient without underlying risk factors. 5731-5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaba K., Hasegawa M., Hamaguchi Y., Fujimoto M., Takehara K., Sato S. Longitudinal analysis of serum KL-6 levels in patients with systemic sclerosis: association with the activity of pulmonary fibrosis. Clin. Exp. Rheumatol. 2003;21(4):429–436. [PubMed] [Google Scholar]
- Zeng H.L., Chen D., Yan J., Yang Q., Han Q.Q., Li S.S., Cheng L. Proteomic characteristics of bronchoalveolar lavage fluid in critical COVID‐19 patients. FEBS J. 2020 doi: 10.1111/febs.15609. [DOI] [PubMed] [Google Scholar]
- Zeng H.L., Di C., Yan J., Yang Q., Han Q.Q., Li S.S., Cheng L. Proteomic characteristics of bronchoalveolar lavage fluid in critical COVID‐19 patients. FEBS J. 2020 doi: 10.1111/febs.15609. [DOI] [PubMed] [Google Scholar]
- Zhong D., Wu C., Bai J., Hu C., Xu D., Wang Q., Zeng X. Comparative diagnostic efficacy of serum Krebs von den Lungen-6 and surfactant D for connective tissue disease-associated interstitial lung diseases: a meta-analysis. Medicine. 2020;99(16) doi: 10.1097/MD.0000000000019695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Funnel plot for assessment of publication bias of studies that compared KL-6 serum level between severe and non-severe COVID-19 disease patients.
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.