Abstract
This study investigates the immunogenicity of extended mRNA vaccine dosing intervals.
Standard dosing intervals for BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines are 21 and 28 days, respectively.1 Data suggest improved effectiveness of ChAdOx1 adenoviral2 and other nonreplicating vaccines3 with increased dosing intervals, but little data exist for mRNA vaccines. This study investigated the immunogenicity of extended mRNA vaccine dosing intervals.
Methods
The COVID-19 Occupational Risks, Seroprevalence and Immunity among Paramedics in Canada cohort study (approved by the University of British Columbia and University of Toronto research ethics boards) recruited Canadian paramedics (January 25 to July 14, 2021), with written consent. Participants who provided a blood sample at enrollment or between 170 to 190 days after the first dose and had received 2 mRNA vaccine doses were eligible for this analysis. Participants with documented COVID-19 were excluded.
We relied on observed variability of vaccine intervals and timing of enrollment relative to vaccination to select participants with different vaccine intervals and performed 2 separate investigations, with different approaches to timing of blood samples. The first investigation compared antibody levels at comparable time intervals after the second dose. For the short (≤28 days) vs medium (42-49 days) vaccine dosing interval comparison, 30 samples (collected at enrollment) from each group were selected based on similar second vaccine-to-sample-collection intervals and were matched by vaccine type, age, sex, and comorbidities (eAppendix in the Supplement). The second investigation compared antibody levels sampled at a standardized interval (170-190 days) after the first dose. For the short (≤36 days) vs long (100-120 days) comparison, 30 samples from each group were selected, matching by the same characteristics.
The primary outcome was the reciprocal of neutralizing antibody titers against a live Wuhan strain (eAppendix in the Supplement). Secondary outcomes included IgG antibodies to the spike protein and receptor-binding domain (RBD) using a multiplex assay (V-PLEX COVID-19 Coronavirus Panel 2 [IgG] Kit; Meso Scale Diagnostics); antibodies to spike protein using a monoplex assay (Elecsys Anti-SARS-CoV-2 S assay; Roche); and inhibition of angiotensin-converting enzyme 2 (ACE-2) binding to RBD from Wuhan, Alpha, Beta, Delta, and Gamma variants (V-PLEX COVID-19 Coronavirus Panel 11 [ACE2] Kit).
Outcomes were reported as geometric mean (geometric SD [GSD]) compared with the Wilcoxon matched-pair signed rank test using IBM SPSS. A 2-sided P < .05 was considered statistically significant.
Results
For the first investigation, the mean age for the short (dosing interval range, 18-28 days) group was 39 years (43% women); 70% received BNT162b2 and 30% mRNA-1273; for the medium (range, 42-49 days) group, the mean age was 41 years (47% women); 60% received BNT162b2 and 40% mRNA-1273. Comparing immunogenicity based on time after the second vaccine dose (matched at a mean of 56 days [SD, 26 days]), the viral neutralization geometric mean was 54.6 (GSD, 3.0) for the short group vs 230.8 (GSD, 2.0) for the medium group (P < .001). Spike and RBD IgG antibodies measured with the multiplex assay are presented in Figure 1. The spike antibody concentrations measured using the monoplex assay for the short group were 1697 U/mL (GSD, 1.7 U/mL) vs 2476 U/mL (GSD, 1.0 U/mL) for the medium group (P < .001). The ACE-2 inhibition for the Beta variant was 11.2 U/mL (GSD, 1.6 U/mL) for the short group vs 14.7 U/mL (GSD, 1.8 U/mL) for the medium group (P = .04). See Figure 1 for the Delta variant results. The comparisons of the Wuhan, Alpha, and Gamma variants were not statistically significant.
Figure 1. Comparison of Serological Outcomes in Paramedics Who Received Short (≤28 Days) vs Medium (42-49 Days) mRNA Vaccine Dosing Intervals.
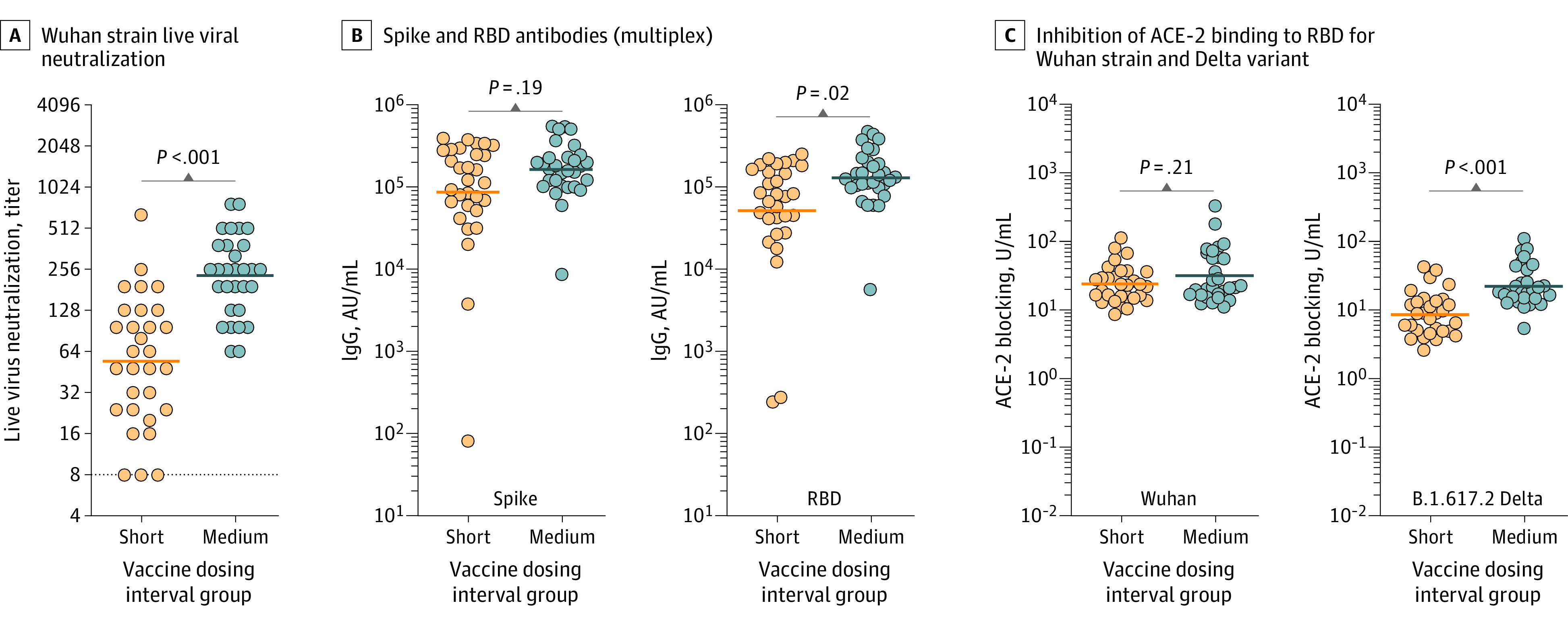
The solid lines indicate the geometric mean. P values were derived from the Wilcoxon matched-pair signed rank test. A, Reciprocal of live viral Wuhan strain neutralization titers, expressed as highest serum dilution able to block viral cytotoxicity (geometric mean, 54.6 [geometric SD {GSD}, 3.0] for the short group vs 230.8 [GSD, 2.0] for the medium group). The dashed line indicates the lower limit of detection with values below set at 1:4. B, The antibody IgG concentration for the multiplex spike was 87 292 arbitrary units (AU)/mL (SD, 5.4 AU/mL) for the short group vs 163 167 AU/mL (SD, 2.2 AU/mL) for the medium group and for the receptor-binding domain (RBD) was 51 753 AU/mL (SD, 5.3 AU/mL) for the short group vs 128 987 U/mL (SD, 2.3 U/mL) for the medium group. C, Inhibition of angiotensin-converting enzyme 2 (ACE-2) binding to the SARS-CoV-2 receptor-binding domain for the Wuhan strain was 24.0 U/mL (SD, 1.9 U/mL) for the short group vs 31.9 U/mL (SD, 2.4 U/mL) for the medium group; for the Delta variant it was 8.64 U/mL (SD, 2.1 U/mL) for the short group vs 22.3 U/mL (SD, 2.0 U/mL) for the medium group.
For the second investigation, the mean age was 41 years (60% women); 87% received BNT162b2 and 13% mRNA-1273 for both the short (range, 21-36 days) and long (range, 102-118 days) groups. Comparing immunogenicity based on time after the first vaccine dose (mean, 179 days [SD, 4.0 days] for the short group and 180 days [SD, 5.7 days] for the long group), the viral neutralization geometric mean was 41.8 (GSD, 2.8) for the short group vs 302.3 (GSD, 2.4) for the long group (P < .001). The multiplex IgG antibodies are presented in Figure 2. For the short vs long groups, the geometric mean monoplex spike antibodies were 928.4 U/mL (GSD, 2.1 U/mL) vs 1154 U/mL (GSD, 5.0 U/mL; P = .002). The geometric means for ACE-2 inhibition for the Alpha variant were 7.7 U/mL (GSD, 1.7 U/mL) vs 22.8 U/mL (GSD, 2.3 U/mL; P < .001); for the Beta variant, 5.4 U/mL (GSD, 3.1 U/mL) vs 15.5 U/mL (GSD, 2.0 U/mL; P < .001), and for the Gamma variant, 4.9 U/mL (GSD, 1.5 U/mL) vs 14.3 U/mL (GSD, 2.1 U/mL; P < .001). Results for the Wuhan and Delta variants are presented in Figure 2.
Figure 2. Comparison of Serological Outcomes in Paramedics Who Received Short (≤36 Days) vs Long (100-120 Days) mRNA Vaccine Dosing Intervals.
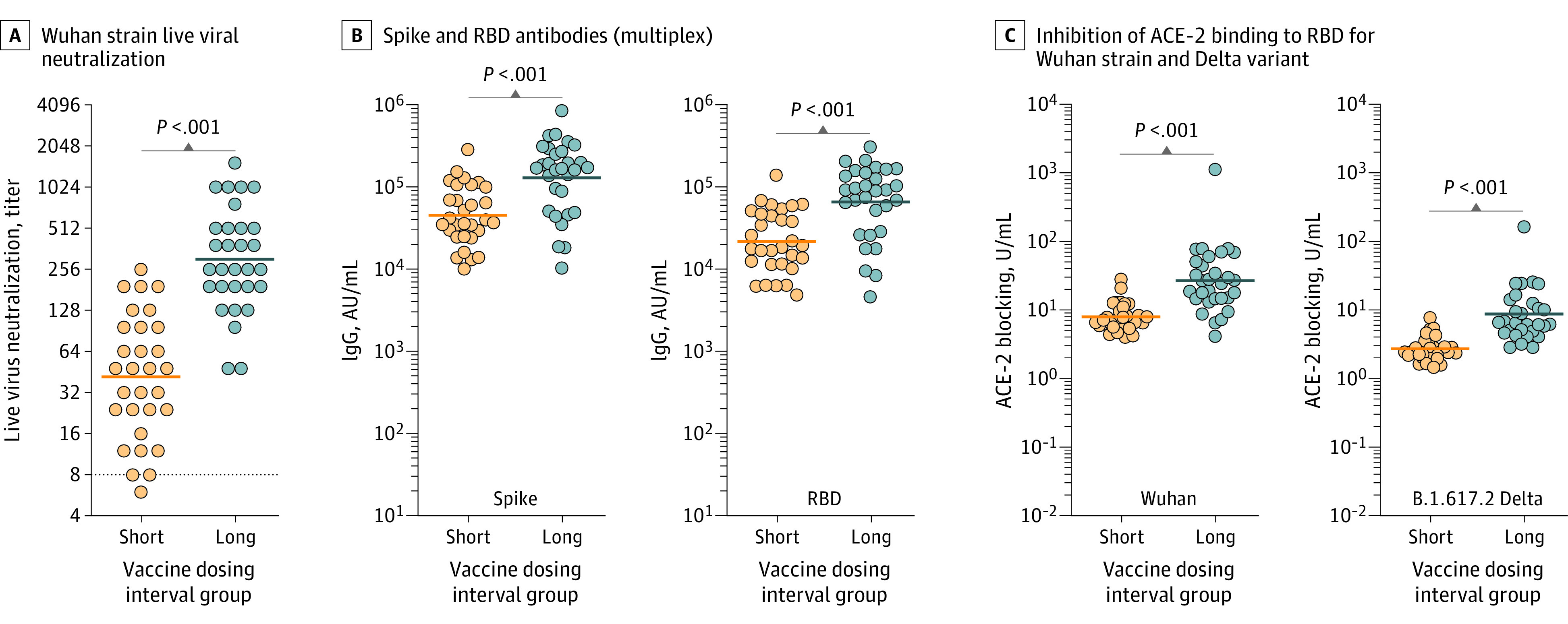
The solid lines indicate the geometric mean. P values were derived from the Wilcoxon matched-pair signed rank test. A, Reciprocal of live viral Wuhan strain neutralization titers, expressed as highest serum dilution able to block viral cytotoxicity (geometric mean, 41.8 [geometric SD {GSD}, 2.8] for the short group vs geometric mean, 302.3 [GSD, 2.4] for the long group). The dashed line indicates the lower limit of detection with values below set at 1:4. B, The antibody IgG concentration for the multiplex spike was 45 155 arbitrary units (AU)/mL (SD, 2.3 AU/mL) for the short group vs 129 299 AU/mL (SD, 2.8 AU/mL) for the long group. The receptor-binding domain (RBD) was 22 071 AU/L (SD, 2.4 AU/L) vs 66 022 AU/L (SD, 2.9 AU/L). C, Inhibition of angiotensin-converting enzyme 2 (ACE-2) binding to SARS-CoV-2 receptor binding domain for the Wuhan strain was 7.9 U/mL (SD,1.6 U/mL) vs 26.8 U/mL (SD, 2.9 U/mL) and for the Delta variant, 2.7 U/mL (SD, 1.5 U/mL) vs 8.7 U/mL (Sd, 2.4 U/mL).
Discussion
Longer mRNA vaccine dosing intervals demonstrated improved immunogenicity, which was consistent when responses were measured based on timing of the first or second dose. These data suggest that extending dosing intervals may be particularly advantageous against the Delta variant.
A delayed second-dose strategy could yield faster partial protection to a larger proportion of the population when vaccine supplies are limited. Modeling studies have estimated overall decreased mortality with delayed second doses when accounting for partial protection provided after 1 dose, even without taking into consideration the potential benefits of delayed second doses on long-term vaccine effectiveness.4,5 However, the trade-off of lower individual immune protection after 1 dose may be unfavorable in at-risk groups or settings where COVID-19 prevalence is high.
Limitations include lack of randomization, small sample size, and focus on middle-aged adults. Although antibody neutralization correlates with disease protection,6 studies should validate whether extending vaccine dosing intervals leads to more sustained vaccine protection.
Section Editors: Jody W. Zylke, MD, Deputy Editor; Kristin Walter, MD, Associate Editor.
eAppendix. Study design and blood sampling and matching
eReferences
References
- 1.Centers for Disease Control and Prevention (CDC) . COVID-19. Accessed August 11, 2021. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html
- 2.Voysey M, Costa Clemens SA, Madhi SA, et al. ; Oxford COVID Vaccine Trial Group . Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881-891. doi: 10.1016/S0140-6736(21)00432-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wahl M, Hermodsson S, Iwarson S. Hepatitis B vaccination with short dose intervals—a possible alternative for post-exposure prophylaxis? Infection. 1988;16(4):229-232. doi: 10.1007/BF01650758 [DOI] [PubMed] [Google Scholar]
- 4.Romero-Brufau S, Chopra A, Ryu AJ, et al. Public health impact of delaying second dose of BNT162b2 or mRNA-1273 covid-19 vaccine: simulation agent based modeling study. BMJ. 2021;373(May):n1087. doi: 10.1136/bmj.n1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moghadas SM, Vilches TN, Zhang K, et al. Evaluation of COVID-19 vaccination strategies with a delayed second dose. PLoS Biol. 2021;19(4):e3001211. doi: 10.1371/journal.pbio.3001211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205-1211. doi: 10.1038/s41591-021-01377-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Study design and blood sampling and matching
eReferences


