Abstract
SARS-CoV-2 vaccination in solid organ transplant recipients is associated with suboptimal immune response and risk for breakthrough infection. It is not known whether they are at risk of severe post-vaccine breakthrough infections in the presence of SARSCoV-2 variant of concern.
We describe a case series of four fully vaccinated solid organ transplant recipients who developed SARS-CoV-2 variants of concern breakthrough infections. Three patients received BNT162b2 mRNA (Pfizer-BioNTech) and one patient received ChAdOx1 (AZD12220) COVID-19 vaccines. The patients were infected with SARS-CoV-2 variants circulating in Saudi Arabia. Two patients were infected with Alpha variant and had severe pneumonia requiring intensive care admission and ventilatory support and subsequently died. The other two patients recovered; one patient was infected with Beta variant required low supplemental oxygen via nasal flow and the other patient was infected with Delta variant and required high supplemental oxygen nasal flow. Younger patients had a better outcome than older patients. Future large studies are required to confirm our observations and to compare the different vaccine efficacy among solid organ transplants in the era of SARS-CoV-2 variants of concern.
Keywords: SARSCoV-2 variant of concern, Breakthrough infection, Solid organ transplant recipients, Outcome
Introduction
Immunocompromised patients, those with dysfunctional immune system either due to an underlying disease that affect immune system e.g. malignancy and primary immunodeficiency or by being on immunosuppressive medications e.g. auto-immune diseases and transplantation, are at risk to progress to severe COVID-19 pneumonia and have high mortality [1]. They are considered high priority population for SARS-CoV-2 vaccination [2]. In patients with solid organ transplantation, lower antibody response, waning immunity render those patients at higher risk for breakthrough infections after vaccination [3,4]. In addition, immunosuppressive medications such as calcineurin inhibitors and antiproliferative drugs were reported to increase the risk of SARS-CoV-2 breakthrough infections [4,5]. The rapid emergence of SARS-CoV-2 variants that could evade the immune system may further increase risk of infection. Mutations in the SARS-CoV-2 spike protein including but not limited to P384L, K417N, E484K, N501Y are likely to cause infections in fully vaccinated individuals [6]. Few studies described the occurrence of post-vaccine breakthrough infections with SARS-CoV-2 variants with the possibility of having severe disease in solid organ transplants [7,8]. However, these studies did not address the sequencing or the variants type. Recently, a third dose of COVID-19 vaccination is recommended for solid organ transplant recipients to improve the immune response against SARS-CoV-2 variants of concern [9]. We described the outcome of four cases of post-vaccine breakthrough infections in solid organ transplant recipients related to SARS-CoV-2 variants of concerns.
Materials and methods
The study was approved by King Faisal Specialist Hospital and Research Center (KFSHRC) in Jeddah, Saudi Arabia. Patients who were admitted with COVID-19 pneumonia and had completed 2 doses of vaccination were identified. Patients whose SARS-CoV-2 nasopharyngeal swabs were available were further tested by whole genome sequence. Charts were reviewed for demographics, comorbidities, clinical course, outcome and immunosuppressive medications.
SARS-CoV-2 RT-PCR
SARS-CoV-2 RT-PCR was performed using the Co-diagnostic RealTime PCR SARS-CoV-2, SolGent RealTime PCR SARS-CoV-2, Xpert SARS- CoV-2 and the Abbott RealTime SARS-CoV-2 EUA as previously described [10].
Whole genome sequencing
The 4 patients’ nasopharyngeal samples were sent to the microbial genomics laboratory for SARS-CoV-2 whole-genome sequencing at the Department of Infection and Immunity, KFSHRC. The samples were subjected to nucleic acid extraction using a MagMAX™ Viral/Pathogen Nucleic Acid Isolation Kit (Cat No. A42352, Thermo Fisher Scientific; MA, USA). All positive samples for SARS-CoV-2 were converted to cDNA using SuperScript™ IV VILO™ Master Mix (ThermoFisher Scientific, USA). The cDNAs were amplified using the Ion AmpliSeq™ SARS-CoV-2 Insight Research Assay as indicated by the manufacturer’s instructions. Amplified products were ligated with unique barcode adaptors using the Ion Xpress Barcode Adaptors 1–16 kit (ThermoFisher Scientific, USA) and purified with 1.5× volume of Agencourt AMPure XP Reagent (Beckman Coulter, USA). Library was built and normalized to 33 pM using nuclease-free water, and up to 16 libraries were equally pooled for further processing. Pooled libraries were used as a template input for emulsion PCR and enrichment of template-positive particles using the Ion Chef automated system with the Ion 510 Kit-Chef kit (ThermoFisher Scientific, USA) according to the manufacturer’s instructions. The obtained data were primarily processed (base calling, base quality recalibration, alignment, assembly, and variant calling) with Torrent Suite Server, version 5.12 (ThermoFisher Scientific, USA). De novo assembly of the contigs was performed using the assembly Trinity plugin (v1.2.1), and consensus sequences of each sample were generated using the IRMA plugin (v1.2.1). Variant call files were analyzed on the COVID19AnnotateSnpEff plugin to identify and annotate variants with public and private databases. Detailed protocol was published in a preprint [11].
Results
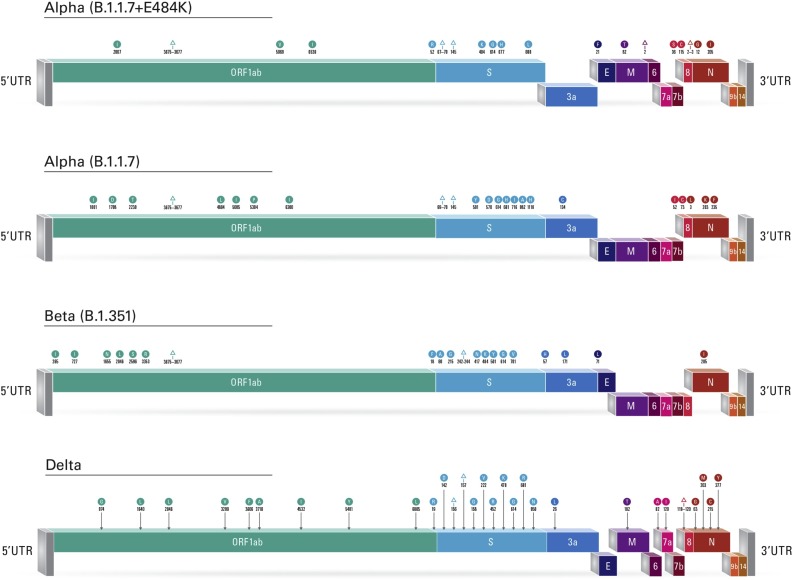
In this case series, there were three renal transplant recipients and one liver transplant recipient (Table 1 ). Three patients received BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech) and one patient received ChAdOx1 nCoV-19 vaccine (AZD12220). All patients had SARS-CoV-2 variants of concerns: one patient had Delta variant, one patient had Beta variant, two patients had Alpha variant. Our results showed that patient 1 was infected with alpha (B.1.1.7)+E484K variant of concern. The major mutations detected were as follows: 69-70Del, 144Del, E484K, N501Y, A570D, P681H, T716I, S982A. While patient 2 was infected with alpha variant of concern with the following major mutations in the spike gene 69-70Del, 144Del, N501Y, A570D, P681H, T716I, S982A. Patient 3 was infected with beta (B.1.351) variant with the following major mutations: D80A, K417N, E484K, N501Y and A701V. Finally, patient 4 who was infected with delta (B.1.617.2) variant of concern. The major mutations detected in the spike gene were G142D, 156-157Del, R158G, L452R, T478K, P681R, and D950N. Fig. 1 depicts the reported variants and mutations.
Table 1.
Clinical and virological characteristics of the four cases of post vaccine SARSCoV-2 breakthrough variant infections.
| Patient | Diseases | Medication | Days post second vaccine | Vaccine type | SARS-CoV-2 Ct value | COVID-19 Disease severity | COVID-19 therapy | Outcome | SARS-CoV-2 variant |
|---|---|---|---|---|---|---|---|---|---|
| 68 y M |
Liver transplant Diabetes mellitus Hypertension |
MMF FK |
73 days | BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech) | E gene 25 N gene 27 RdRp 16 |
Severe pneumonia Mechanical ventilation |
Dexamethasone Tocilizumab |
Death (47 days in ICU) | Alpha (B.1.1.7) +E484K |
| 69 y M |
Renal transplant Diabetes mellitus Hypertension |
FK MMF prednisone |
5 months | BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech) | N gene 30 ORF-1 30 |
Severe pneumonia mechanical ventilation |
Dexamethasone Tocilizumab |
Death (40 days in ICU) | Alpha (B.1.1.7) |
| 41 y M |
Renal transplant | FK MMF prednisone |
39 days | BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech) | E gene 29 N gene 32 |
Pneumonia High oxygen nasal flow |
Dexamethasone Tocilizumab Remdesivir |
Recovery | Beta (B.1.351) |
| 48 y F |
Renal transplant Post-transplant lymphoma |
Sirolimus, off chemotherapy 18 months prior to admission | 21 days | ChAdOx1 nCoV-19 vaccine (AZD12220) | E gene 19 N gene 21 |
Pneumonia low oxygen nasal flow |
Dexamethasone Tocilizumab |
Recovery | Delta (B.1.617.2) |
Abbreviations: M (male), MMF (Mycophenelate Mofetil), FK (tacrolimus), ICU (intensive care unit).
Fig. 1.
Schematic diagram of SARS-CoV-2 linearized genomes depicting the detected variants and mutations. The presence of a mutation is represented by a circle indicating an amino acid change or a triangle indicating a deletion, color-coded to match the affected gene.
Two patients had severe pneumonia requiring intensive care admission and ventilatory support with and subsequently died. The first patient was a 69 years old post liver transplant in 2019 who presented with 2 days history of fever, chills and vomiting. He was admitted to the intensive care unit (ICU) on day 10 of his diagnosis. His course was complicated by sepsis/septic shock and cytomegalovirus (CMV) reactivation and he scummed to his illness after 2 months of the diagnosis. The second patient was a 69 years old renal transplant in 2008. He presented with shortness of breath (SOB) and hypoxia with oxygen saturation of 78% and was admitted to ICU on the same day. He was treated with tocilizumab and dexamethasone but he required mechanical ventilation. He subsequently developed multiple episodes of sepsis and septic shoch and died 6 weeks after diagnosis. The other two patients recovered; the first patient is 42-year-old post renal transplant in 2016 who was admitted with mild cough and SOB, he required a brief admission to ICU for high flow nasal cannula and was discharged from the hospital 10-days after diagnosis. The three patients are taking tacrolimus and Mycophenolate Mofetil. The fourth patient is a 48-year-old post renal transplant in 1994 and the course was complicated by chronic graft dysfunction and post-transplant lymphoproliferative disorder. The patient was off chemotherapy for 18 months prior to the current admission. She received ChAdOx1 nCoV-19 vaccine (AZD12220) and she was on sirolimus. She presented with fever and hypoxia requiring supplemental oxygen via a nasal cannula. Her course was complicated by hospital acquired infection and requirement for hemodialysis and was discharged home 1 month after diagnosis. All patients received dexamethasone and tocilizumab for COVID-19 pneumonia.
Discussion
We described variable outcomes of four patients with solid organ transplant recipients who developed post-vaccine breakthrough infections caused by Alpha, Beta and Delta SARS-CoV-2 variants. Studies describing the severity of variant SARS-CoV-2 are variable; while one study showed that variants are associated with severe disease, another study did not show that variants lead to severe COVID-19 pneumonia [12,13]. Laboratory and clinical studies showed that post-vaccine antibody responses against SARS-CoV-2 variants are less than antibody responses against wild SARS-CoV-2 but are still protective against severe disease and death [14,15].This phenomena is applicable for immunocompetent patients who are mounting high antibody responses that can overcome the mutations in spike protein but are inadequate in solid organ transplant recipients who are mounting a suboptimal antibody response against wild SARS-CoV-2 [16]. Recent studies showed that a third dose of vaccine in solid organ transplant recipients is associated with higher antibody response compared to two doses [17].
In a study by Deng et al., breakthrough SARS-CoV-2 infection occurred in fully vaccinated individuals over 4-weeks of follow up. Fourteen patients were identified and 42.8% were solid organ transplant (SOT) recipients. The commonest detected variants were alpha and Gamma variants, the variants that were circulating in Chicago during the study period. It is noteworthy that high viral load was not correlated with severe disease and all SOT patients required hospitalization [18]. In another study that described breakthrough SARS-CoV-2 infection after COVID-19 mRNA vaccination, immunocompromised patients (active malignancy, primary immunodeficiency and transplantation) had the highest incidence rate of breakthrough infection. In addition, patients vaccinated with Pfizer/BNT162b2 had higher incidence of breakthrough infection compared to those vaccinated with Moderna/mRNA-1273 [19]. In our study, we showed that the variants associated with the breakthrough infections were alpha, beta and delta.
Despite the small number of patients in the current study, the following observations warrant future investigation. First, the two patients who recovered were younger than the two patients who died. A recent study described the humoral and cell medicated responses after two doses of mRNA vaccination against SARS-CoV-2 variants of concerns in relation to different age groups and showed that patients above eighty years old had similar antibody response and lower cell mediated response compared to younger patients [20]. Age and pre-existing comorbidities increased the mortality of COVID-19 solid organ transplant recipients in a multi-center study in USA [21]. Second, a favorable outcome was observed in one patient who received ChAdOx1 nCoV-19 vaccine and had Delta variant breakthrough infection. There is limited data describing the severity of Delta variants compared to other variants in transplant recipients. In our case series, the patient who had Delta variant did not have severe diseases, while the other two patients who died had Alpha variants. One recent study showed predominance of delta variants among patients in a tertiary care hospital in Saudi Arabia [11]. Thus, the occurrence of breakthrough SARS-CoV-2 infection likely mirrors those variants seen in the community at the time of the infection.
The current study is limited by the small number of cases and lack of SARS-CoV-2 serological testing. Future large studies are required to confirm the results of our case series and to determine the optimal effective vaccine against SARS-CoV-2 variants of concern in immunocompromised patients. The strength of this study is the inclusion of whole genome sequencing platform that facilitated the detection and monitoring of SARS-Cov-2 variants. Therefore, epidemiological and genomic surveillance of circulating variants among hospitalized immunocompromised patients is crucial to understand the best preventive and therapeutic measures for COVID-19, knowing the suboptimal SARS-CoV-2 immune response following natural infection and vaccination in these vulnerable and heterogenous patient population.
Funding
This work is fully funded by King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia: COVID19 grant 2200009.
Conflict of interest
None declared.
Contribution
Concept and Design: ANA, RSA.
Acquisition, analysis and interpretation of data: MFS, MA, BA, AA, MKH.
Performing the test: FSA, AAA, AD.
Writing the manuscript: ANA.
Critical revision of the manuscript: RSM, FSA, JAA, AAO, ANA.
Administrative, technical and material support: AD, MKA, FSA.
Supervision: ANA, RSA.
Acknowledgement
Our gratitude to the King Faisal Specialist Hospital and Research Center administration in Jeddah and Riyadh for their support during the pandemic.
We would like to thank the Organ Transplant Center of Excellence in King Faisal Specialist Hospital and Research Center in Riyadh for their support.
We also would like to thank our patients who participated in this work.
Great thanks to the sequencing team in King Faisal Specialist Hospital and Research Center, Riyadh, Ms. Basma Alahideb, Feda Alsuwairi, and Madain Alsanea.
We greatly appreciate the expert opinion and distinguished support of Professor Ajit Limaye, University of Washington, Seattle.
References
- 1.Kim L., Garg S., O'Halloran A., Whitaker M., Pham H., Anderson E.J., et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET) Clin Infect Dis. 2021;72(9):e206–e214. doi: 10.1093/cid/ciaa1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center for Disease Control and Prevention . 2021. COVID-19 vaccination. [cited 2021 June 2021]; Available from: https://www.cdc.gov/vaccines/covid-19/index.html. [Google Scholar]
- 3.Marinaki S., Adamopoulos S., Degiannis D., Roussos S., Pavlopoulou I.D., Hatzakis A., et al. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21(8):2913–2915. doi: 10.1111/ajt.16607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holden I.K., Bistrup C., Nilsson A.C., Hansen J.F., Abazi R., Davidsen J.R., et al. Immunogenicity of SARS-CoV-2 mRNA vaccine in solid organ transplant recipients. J Intern Med. 2021;290(6):1264–1267. doi: 10.1111/joim.13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chavarot N., Morel A., Leruez-Ville M., Vilain E., Divard G., Burger C., et al. Weak antibody response to three doses of mRNA vaccine in kidney transplant recipients treated with belatacept. Am J Transplant. 2021;21(12):4043–4051. doi: 10.1111/ajt.16814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang R., Chen J., Hozumi Y., Yin C., Wei G.W. Emerging vaccine-breakthrough SARS-CoV-2 variants. ArXiv. 2021 doi: 10.1021/acsinfecdis.1c00557. preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tau N., Yahav D., Schneider S., Rozen-Zvi B., Abu Sneineh M., Rahamimov R. Severe consequences of COVID-19 infection among vaccinated kidney transplant recipients. Am J Transplant. 2021;21(Aug (8)):2910–2912. doi: 10.1111/ajt.16700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anjan S., Natori Y., Fernandez Betances A.A., Agritelley M.S., Mattiazzi A., Arosemena L., et al. Breakthrough COVID-19 infections after mRNA vaccination in solid organ transplant recipients in Miami, Florida. Transplantation. 2021;105(Oct (10)):e139–e141. doi: 10.1097/TP.0000000000003902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Center for Disease Control and Prevention . 2021. COVID-19 vaccines for moderately to severely immunocompromised people. [cited 2021 August 17]; Available from: COVID-19 Vaccines for Moderately to Severely Immunocompromised People. [Google Scholar]
- 10.Younes N., Al-Sadeq D.W., Al-Jighefee H., Younes S., Al-Jamal O., Daas H.I., et al. Challenges in laboratory diagnosis of the novel coronavirus SARS-CoV-2. Viruses. 2020;12(6) doi: 10.3390/v12060582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alhamlan F., Al-Qahtani A., Obeid D., Aljumaah S., Alghamdi S., Alnafee K., et al. Vol. 2021. Research Square; 2021. (SARS-CoV-2 delta variant predominant at a tertiary-care hospital in Saudi Arabia). [DOI] [Google Scholar]
- 12.Graham M.S., Sudre C.H., May A., Antonelli M., Murray B., Varsavsky T., et al. Changes in symptomatology, reinfection, and transmissibility associated with the SARS-CoV-2 variant B.1.1.7: an ecological study. Lancet Public Health. 2021;6(5):e335–e345. doi: 10.1016/S2468-2667(21)00055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies N.G., Jarvis C.I., Group C.C.-W., Edmunds W.J., Jewell N.P., Diaz-Ordaz K., et al. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021;593(7858):270–274. doi: 10.1038/s41586-021-03426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker M., Dulovic A., Junker D., Ruetalo N., Kaiser P.D., Pinilla Y.T., et al. Immune response to SARS-CoV-2 variants of concern in vaccinated individuals. Nat Commun. 2021;12(1):3109. doi: 10.1038/s41467-021-23473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z., Schmidt F., Weisblum Y., Muecksch F., Barnes C.O., Finkin S., et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592(7855):616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall V.G., Ferreira V.H., Ierullo M., Ku T., Marinelli T., Majchrzak-Kita B., et al. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am J Transplant. 2021;(Aug 4) doi: 10.1111/ajt.16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall V.G., Ferreira V.H., Ku T., Ierullo M., Majchrzak-Kita B., Chaparro C., et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385(13):1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng X., Evdokimova M., O'Brien A., Rowe C.L., Clark N.M., Harrington A., et al. Breakthrough infections with multiple lineages of SARS-CoV-2 variants reveals continued risk of severe disease in immunosuppressed patients. Viruses. 2021;13(9) doi: 10.3390/v13091743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C., Lee J., Ta C., Soroush A., Rogers J.R., Kim J.H., et al. A retrospective analysis of COVID-19 mRNA vaccine breakthrough infections - risk factors and vaccine effectiveness. medRxiv. 2021;(Oct 7) doi: 10.1101/2021.10.05.21264583. [DOI] [Google Scholar]
- 20.Collier D.A., Ferreira I., Kotagiri P., Datir R.P., Lim E.Y., Touizer E., et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596(7872):417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kates O.S., Haydel B.M., Florman S.S., Rana M.M., Chaudhry Z.S., Ramesh M.S., et al. COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1097. Online ahead of print. [DOI] [Google Scholar]