Abstract
Background
The present investigation was developed for the exploration of the association between IL-6 levels and acute coronary syndrome (ACS) findings upon angiographic evaluation.
Methods
A retrospective review of 346 patients suffering from chest discomfort that underwent coronary angiography was performed. The SYNergy between Percutaneous Coronary Intervention with TAXus and cardiac surgery (SYNTAX) score (SS) and SS II were used to gauge ACS severity, with ACS patients being stratified into two groups based on an SS value of 22 and the median SS II value. Associations between IL-6 levels and SS or SS II values were assessed through Spearman's correlation analyses, and independent predictors of intermediate-high SS or high SS II were identified via a multivariate logistic regression approach. A receiver operating characteristic (ROC) curve was employed to explore of the predictive value of IL-6 levels.
Results
IL-6 was positively correlated with both SS (r = 0.479, P < 0.001) and SS II (r = 0.305, P < 0.001). Moreover, IL-6 levels were independently predictive of intermediate-high SS and high SS II values. ROC curves further demonstrated that IL-6 was able to predict intermediate-high SS and high SS II, with area under the curve (AUC) values of 0.806 and 0.624, respectively.
Conclusion
IL-6 levels are closely linked to the extent of coronary artery disease in ACS patients undergoing percutaneous coronary intervention. IL-6 levels may thus serve as a valuable and non-invasive biomarker of high-risk ACS patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-021-02406-7.
Keywords: IL-6, Acute coronary syndrome, SYNTAX score, SYNTAX score II
Background
Acute coronary syndrome (ACS) is a condition that incorporates several forms of myocardial ischemia, including ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI) as well as unstable angina pectoris (UA), and it is the leading cause for global morbidity and mortality [1, 2]. While improvements in antithrombotic treatment and revascularization approaches have improved the prognosis of ACS patients, many patients nonetheless experience unsatisfactory adverse cardiovascular outcomes [3]. At present consensus criteria pertaining to understanding ACS pathogenesis are lacking, and defining the optimal treatment for this condition is thus an important global public health goal [4].
A growing body of evidence suggests that inflammatory activity plays a maladaptive role in the context of ACS pathophysiology, triggering initiation and progression of atherosclerosis, driving plaque destabilization and degradation, and responding to myocardial necrosis [5–7]. Consistently, many studies have reported an association between cardiovascular disease (CVD) and increased levels of biomarkers indicative of inflammation [6, 8, 9]. In particular, the pro-inflammatory cytokine interleukin-6 (IL-6), which is primarily produced by macrophages and T cells, has been noted as a key driver of plaque destabilization, atheroprogression, and the production of high-sensitivity C-reactive protein (hs-CRP), leading to the development and progression of clinical atherosclerosis [6, 10–12]. Links between higher IL-6 levels and an elevated risk of cardiovascular events among otherwise healthy individuals are well documented [13, 14], and IL-6 has also been proposed to be a predictor of coronary artery disease (CAD) severity and associated mortality among ACS patients [6, 15, 16]. Additionally, levels of IL-6 have been reported to be associated with plaque burden as defined by intracoronary imaging [17].
In previous studies, the SYNergy between Percutaneous Coronary Intervention with TAXus and cardiac surgery (SYNTAX) score (SS), which is frequently used to quantify CAD degree and severity, has been shown to predict prognostic outcomes in stable CAD and ACS patients [18–20]. In recent years, the SS II indicator has been expanded to take individual clinical characteristics into account, reportedly achieving higher accuracy rates in the prognostic assessment of ACS patients [21, 22].
The relationships between IL-6 levels and both SS and SS II values, however, are poorly documented. As such, this study was designed to explore the link between IL-6 levels and ACS severity as measured using the SS and SS II indicators at the time of admission.
Methods
Study population
For this study, patients suffering from chest pain that were admitted to the Division of cardiology and underwent coronary angiography (CAG) between January 2021 to August 2021 were enrolled. The criteria for diagnosing ACS (including STEMI, NSTEMI, and UA patients) were based on the standard recommended by the ESC guidelines [23]. Patients were excluded if they had already undergone percutaneous coronary intervention (PCI) or coronary artery bypass grafting surgery (CABG), or if they exhibited malignancies, autoimmune disease, severe hepatic or renal failure, or infectious or inflammatory disease. In total, 346 patients were included in the final study, with these patients being classified into an ACS group and a stable angina pectoris (SAP) group (individuals with diseased vessels exhibiting > 50% luminal narrowing). This study was consistent with the Declaration of Heksinki and was approved by the Institutional Review Board of Yijishan Hospital Affiliated of Wannan Medical College.
Patient's characteristics
The hospital electronic database was used to document all patient demographic and clinical characteristics. Fasting blood samples were obtained from the peripheral veins of all patients before PCI to assess hematologic indices, hs-CRP levels, biochemical parameters, and IL-6 concentrations using standard approaches in our hospital’s clinical laboratory. IL-6 concentrations were measured using an enzyme-linked immunosorbent assay (Human IL-6 ELISA Kit, Fine Test, Wuhan, China). Transthoracic echocardiography was conducted prior to angiography. The Cockcroft-Gault equation was utilized to calculate the estimated glomerular filtration rate (eGFR) for each patient.
Coronary angiographic analysis
All patients underwent CAG via the radial approach at admission. CAG was performed by two expert interventional cardiologists who were blinded to patient clinical information. SS calculations were based on the coronary artery with a ≥ 50% luminal narrowing in a vessel ≥ 1.5 mm and were performed using the SS calculator (www.syntaxscore.com, version 2.1). Furthermore, SS II values were established based on these SS values, the presence of left main coronary artery disease, peripheral arterial disease (PAD), chronic obstructive pulmonary disease (COPD), female sex, eGFR, and left ventricular ejection fraction (LVEF) [24].
Statistical analysis
The Kolmogorov–Smirnov approach was employed to assess whether continuous data were normally distributed. Normally distributed quantitative data are given as mean ± standard deviation (SD), while outcomes that were non-normally distributed are expressed as medians with the interquartile range. These outcomes were compared via Student's t-tests or Mann–Whitney U-tests. Categorical variables are given as numbers (percentages) and were compared using chi-squared tests or Fisher’s exact assessment. Parameters significant (P < 0.1) in initial univariate analyses were incorporated into a multivariate logistic regression analysis designed to identify independent predictors of intermediate-high SS and high SS II values. Receiver operating characteristic (ROC) curves were utilized to demonstrate the ability of IL-6 levels to predict these two outcomes. SPSS 23.0 was used for statistical analyses, and P < 0.05 was considered as statistically significant difference.
Results
Study participants
The demographic and clinical characteristics of ACS (n = 201) and SAP (n = 145) group patients were initially evaluated (Table 1). Overall, patients in the ACS group were mostly male and exhibited higher creatinine and fibrinogen levels (P < 0.05) relative to those of patients in the SAP group. Moreover, these ACS patients exhibited significant increases in white blood cell (WBC), neutrophil (NEUT), and platelet counts as well as neutrophil to the lymphocyte ratio (NLR) values compared to SAP patients (P < 0.05). Furthermore, IL-6 and hs-CRP concentrations were significantly greater in ACS group patients as compared to those in SAP group patients (P < 0.01), while apolipoprotein A1 (apoA1), high-density lipoprotein cholesterol (HDL-c), albumin, and LVEF were all reduced in these ACS patients (P < 0.05). No differences in age, body mass index (BMI), or other characteristics were observed when comparing these two groups. Additionally, no significant relationship between IL-6 levels and SS values in SAP patients were observed via Spearman’s correlation analyses (r = 0.128, P > 0.05; Additional file 1: Figure S1A).
Table 1.
Demographic, clinical and biochemical characteristics between ACS and SAP group
| Variables | ACS (n = 201) | SAP (n = 145) | P value |
|---|---|---|---|
| Age, years | 65 (57–71) | 65 (57–72) | .323 |
| Male gender, n (%) | 136 (67.7%) | 83 (57.2%) | .047 |
| Hypertension, n (%) | 127 (63.2%) | 95 (65.5%) | .655 |
| Diabetes, n (%) | 49 (24.4%) | 27 (18.6%) | .202 |
| Smoking, n (%) | 30 (14.9%) | 14 (9.7%) | .147 |
| BMI, kg/m2 | 24.8 ± 3.3 | 24.7 ± 3.6 | .916 |
| WBC, 109/L | 6.7 (5.4–8.1) | 5.9 (4.8–6.9) | < .001 |
| NEUT, 109/L | 4.1 (3.4–5.6) | 3.5 (2.7–4.4) | < .001 |
| LYM, 109/L | 1.7 (1.3–2.1) | 1.6 (1.3–2.0) | .293 |
| NLR | 2.4 (1.8–3.4) | 2.1 (1.7–2.6) | .001 |
| Platelet, 109/L | 175 (142–217) | 166 (128–203) | .041 |
| RDW | 12.8 (12.5–13.4) | 13.0 (12.6–13.3) | .470 |
| Hemoglobin, g/L | 129.5 ± 16.4 | 129.2 ± 15.7 | .896 |
| IL-6, pg/ml | 7.5 (4.8–10.9) | 3.8 (2.4–5.3) | < .001 |
| hs-CRP, mg/l | 6.0 (1.7–11.2) | 1.2 (0.5–2.8) | < .001 |
| Glucose, mmol/L | 5.7 ± 2.3 | 5.4 ± 1.6 | .202 |
| TC, mmol/L | 3.9 ± 1.0 | 3.9 ± 0.9 | .858 |
| Triglyceride, mmol/L | 1.4 (1.0–1.9) | 1.3 (0.9–1.8) | .280 |
| HDL-c, mmol/L | 1.2 (1.0–1.4) | 1.3 (1.1–1.5) | .001 |
| LDL-c, mmol/L | 2.1 (1.6–2.8) | 2.1 (1.5–2.7) | .706 |
| apoB, g/L | 0.8 (0.6–1.0) | 0.8 (0.6–0.9) | .317 |
| apoA1, g/L | 1.1 (0.9–1.2) | 1.2 (1.0–1.3) | < .001 |
| Lp(a), mg/L | 227.2 (102.5–469.4) | 210.7 (90.5–386.5) | .208 |
| Creatinine, umol/L | 69.0 (57.7–85.7) | 64.6 (53.7–76.0) | .012 |
| eGFR, ml/min | 86.2 (67.6–112.6) | 91.4 (66.7–112.9) | .454 |
| Albumin, g/L | 39.0 ± 3.4 | 39.8 ± 3.5 | .034 |
| Fibrinogen, g/L | 3.0 (2.6–3.9) | 2.9 (2.4–3.2) | .001 |
| D-Dimer, ug/ml | 0.3 (0.2–0.4) | 0.3 (0.2–0.5) | .066 |
| LVEF, % | 62.0 (58.0–65.0) | 64.0 (60.0–66.0) | .001 |
ACS, acute coronary syndrome; SAP, stable angina pectoris; BMI, Body Mass Index; WBC, white blood cell; NEUT, neutrophil; LYM, lymphocyte; NLR, the neutrophil to the lymphocyte ratio; RDW, red cell distribution width; IL-6, interleukin 6; hs-CRP, high-sensitivity C-reactive protein; TC, total cholesterol; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; apoB, apolipoprotein B; apoA1, apolipoprotein A1; apoB/apoA1, the apoB to the apoA1 ratio; Lp(a), lipoprotein a; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction
The relationship between IL-6 levels and an intermediate-high SYNTAX score
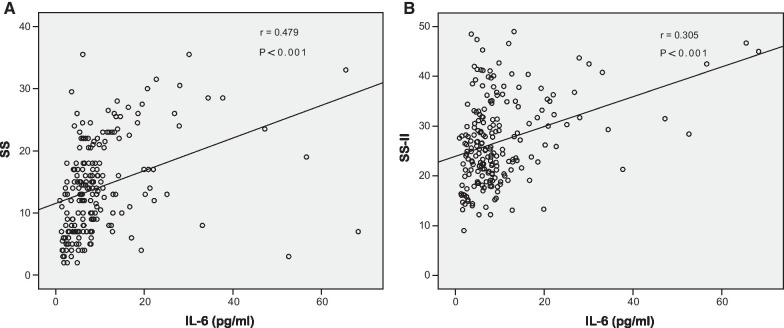
ACS patients were separated based on SS values cited in prior studies [25], with one group of patients with low SS values (SS ≤ 22, n = 168) and one group with intermediate-high SS values (SS > 22, n = 33). The demographic, clinical, biochemical, and angiographic parameters of ACS patients in these two groups are compiled in Table 2. IL-6 (P < 0.001) and hs-CRP (P < 0.001) levels in the intermediate-high SS group were significantly elevated in comparison to those in the low SS group, and the SS II and residual SS (rSS) values for cases in the intermediate-high SS group were also greater compared to those in the low SS group (P < 0.05). Spearman's correlation analyses revealed IL-6 levels and SS values to be significantly positively associated with one another (r = 0.479, P < 0.001; Fig. 1A). Additionally, IL-6 levels were not correlated with post-PCI troponin values (r = 0.107, P > 0.05; Additional file 1: Figure S1B).
Table 2.
Demographic, clinical, biochemical and angiographic characteristics in low and intermediate-high SYNTAX score (SS) group
| Variables | SS ≤ 22 (n = 168) | SS > 22 (n = 33) | P value |
|---|---|---|---|
| Age, years | 65 (57–71) | 63 (58–75) | .304 |
| Male gender, n (%) | 114 (67.9%) | 22 (66.7%) | .894 |
| Hypertension, n (%) | 107 (63.7%) | 20 (60.6%) | .737 |
| Diabetes, n (%) | 40 (23.8%) | 9 (27.3%) | .672 |
| Smoking, n (%) | 25 (14.9%) | 5 (15.2%) | .968 |
| BMI, kg/m2 | 24.9 ± 3.5 | 24.2 ± 2.7 | .312 |
| WBC, 109/L | 6.6 (5.3–8.0) | 7.0 (5.6–8.8) | .210 |
| NEUT, 109/L | 4.1 (3.3–5.4) | 4.5 (3.6–5.9) | .231 |
| LYM, 109/L | 1.7 (1.3–2.1) | 1.9 (1.3–2.2) | .833 |
| NLR | 2.4 (1.8–3.4) | 2.6 (2.0–3.6) | .277 |
| Platelet, 109/L | 174 (141–212) | 185 (145–225) | .275 |
| RDW | 12.9 (12.5–13.3) | 12.8 (12.5–13.4) | .741 |
| Hemoglobin, g/L | 129.8 ± 14.6 | 128.1 ± 23.7 | .597 |
| IL-6, pg/ml | 6.5 (4.3–9.2) | 14.0 (11.1–24.8) | < .001 |
| hs-CRP, mg/l | 4.5 (1.3–9.0) | 11.8 (8.7–20.0) | < .001 |
| Glucose, mmol/L | 5.7 ± 2.5 | 5.6 ± 1.4 | .830 |
| TC, mmol/L | 3.9 ± 1.0 | 4.0 ± 1.0 | .578 |
| Triglyceride, mmol/L | 1.4 (1.0–2.0) | 1.6 (1.2–1.8) | .409 |
| HDL-c, mmol/L | 1.2 (1.1–1.4) | 1.1 (1.0–1.3) | .187 |
| LDL-c, mmol/L | 2.2 ± 0.8 | 2.4 ± 0.9 | .176 |
| apoB, g/L | 0.8 (0.6–0.9) | 0.9 (0.6–1.1) | .138 |
| apoA1, g/L | 1.0 ± 0.2 | 1.1 ± 0.2 | .571 |
| Creatinine, umol/L | 69.4 (57.7–85.2) | 68.5 (57.2–87.6) | .984 |
| eGFR, ml/min | 86.3 (67.4–113.1) | 86.1 (66.8–104.2) | .570 |
| Albumin, g/L | 39.4 (37.5–41.5) | 38.6 (35.9–40.6) | .091 |
| Fibrinogen, g/L | 3.0 (2.6–3.8) | 3.4 (2.8–4.0) | .129 |
| D-Dimer, ug/ml | 0.3 (0.2–0.5) | 0.4 (0.2–0.6) | .400 |
| LVEF, % | 62.0 (58.0–65.0) | 62.0 (53.0–65.0) | .744 |
| SS | 12.0 (7.0–15.8) | 26.0 (23.5–28.5) | < .001 |
| SS II | 24.5 (19.9–31.3) | 30.5 (24.5–37.5) | .001 |
| rSS | 2.0 (0–5.0) | 10.0 (4.0–16.8) | < .001 |
BMI, Body Mass Index; WBC, white blood cell; NEUT, neutrophil; LYM, lymphocyte; NLR, the neutrophil to the lymphocyte ratio; RDW, red cell distribution width; IL-6, interleukin 6; hs-CRP, high-sensitivity C-reactive protein; TC, total cholesterol; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; apoB, apolipoprotein B; apoA1, apolipoprotein A1; apoB/apoA1, the apoB to the apoA1 ratio; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; SYNTAX, SYNergy between Percutaneous Coronary Intervention with TAXus and cardiac surgery; SS, SYNTAX score; SS II, SYNTAX score II; rSS, residual SYNTAX score
Fig. 1.
A Correlation between IL-6 levels and SS in ACS patients; B Correlation between IL-6 levels and SS II in ACS patients. IL-6, interleukin 6; SS, SYNergy between Percutaneous Coronary Intervention with TAXus and cardiac surgery (SYNTAX) score; SS II, SYNTAX score II; r, correlation coefficient
Identification of independent predictors of intermediate-high SYNTAX score
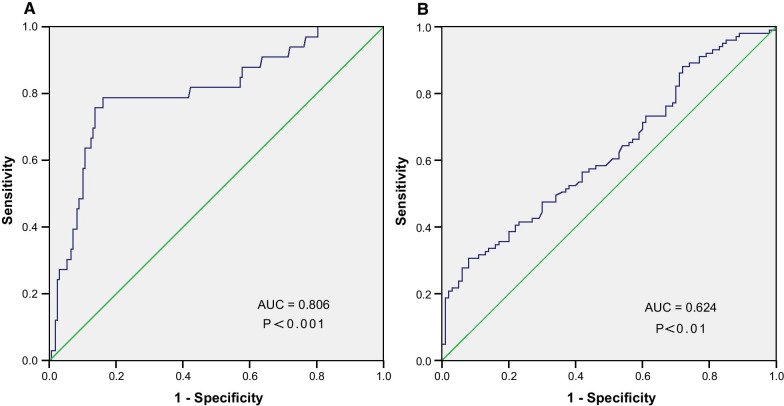
Multivariable logistic regression analyses were next conducted to explore independent predictors of intermediate-high SS by analyzing all variables that exhibited significant predictive value (P < 0.1) in univariate assessment, including, IL-6, hs-CRP, and albumin. The outcomes of this analysis revealed that serum IL-6 levels (odds ratio [OR] = 1.081, 95% confidence interval [CI]: 1.036–1.128, P < 0.001) were independent predictors of intermediate-high SS (Table 4). Consistently, an ROC curve analysis for IL-6 yielded an AUC of 0.806 (95% CI: 0.718–0.895, P < 0.001; Fig. 2A) when used to predict intermediate-high SS values, with an optimal predictive cut-off value of 10.6 pg/ml, yielding respective sensitivity and specificity values of 78.79% and 83.93%.
Table 4.
Independent predictors of intermediate–high SYNTAX score (> 22) and high SYNTAX score II (> 25.4)
| Univariate analysis | Multivariable analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| P | OR | 95% CI | P | OR | 95% ci | |||
| Independent predictors of intermediate–high SYNTAX score | ||||||||
| IL-6 | < .001 | 1.085 | 1.043 | 1.130 | < .001 | 1.081 | 1.036 | 1.128 |
| hs-CRP | < .001 | 1.052 | 1.023 | 1.082 | .056 | 1.032 | 0.999 | 1.066 |
| Albumin | .055 | 0.912 | 0.831 | 1.002 | .154 | 0.921 | 0.822 | 1.031 |
| Independent predictors of high SYNTAX score II | ||||||||
| hs-CRP | .0063 | 1.025 | 0.999 | 1.052 | .670 | 0.993 | 0.962 | 1.026 |
| Hemoglobin | < .001 | 0.948 | 0.927 | 0.970 | < .001 | 0.948 | 0.924 | 0.972 |
| IL-6 | .001 | 1.085 | 1.034 | 1.138 | .005 | 1.082 | 1.025 | 1.143 |
| NLR | .087 | 1.108 | 0.985 | 1.247 | .480 | 1.050 | 0.917 | 1.203 |
IL-6, interleukin 6; hs-CRP, high-sensitivity C-reactive protein; NLR, the neutrophil to the lymphocyte ratio; OR, odds ratio; SYNTAX, SYNergy between Percutaneous Coronary Intervention with TAXus and cardiac surgery
Fig. 2.
A The receiver operating characteristic (ROC) cure of IL-6 in predicting SS > 22 in ACS patients; B ROC curve of IL-6 in predicting SS II > 25.4 in ACS patients. AUC, area under the curve; SS, SYNergy between Percutaneous Coronary Intervention with TAXus and cardiac surgery (SYNTAX) score; SS II, SYNTAX score II
Factors related to high SS II
Median SS II values were next used to stratify all ACS patients into the low SS II (SS II ≤ 25.4; n = 100) and high SS II (SS II > 25.4; n = 101) groups. Those cases in the high SS II group were, on average, older, had a higher IL-6 value, and exhibited lower hemoglobin levels, eGFR, albumin, and BMI values as compared to individuals in the low SS II group (P < 0.01; Table 3). Additionally, no differences in rSS, history of hypertension or diabetes, or other characteristics were observed when comparing these two groups. IL-6 levels were also positively associated with SS II (r = 0.305, P < 0.001; Fig. 1B). After univariate analyses of associated parameters, several variables, including IL-6, hs-CRP, hemoglobin, and NLR, were then incorporated into a multivariable logistic regression analysis, which revealed that IL-6 levels (OR = 1.082, 95% CI: 1.025–1.143, P < 0.01) and hemoglobin levels (OR = 0.948, 95% CI: 0.924–0.972, P < 0.001) were independent predictors of high SS II values (Table 4). Consistently, ROC curves demonstrated the value of IL-6 as a predictor of high SS II (AUC = 0.624, P < 0.01; Fig. 2B). An IL-6 > concentration greater than 12.9 pg/ml was predictive of high SS II, with sensitivity and specificity of 30.69% and 92.00%, respectively.
Table 3.
Demographic, clinical, biochemical and angiographic characteristics in low and high SYNTAX score II group
| Variables | SS II ≤ 25.4 (n = 100) | SS II > 25.4 (n = 101) | P value |
|---|---|---|---|
| Age, years | 57 (50–65) | 70 (65–75) | < .001 |
| Male gender, n (%) | 85 (85.0%) | 51 (50.5%) | < .001 |
| Hypertension, n (%) | 57 (57.0%) | 70 (69.3%) | .070 |
| Diabetes, n (%) | 28 (23.0%) | 21 (20.8%) | .234 |
| Smoking, n (%) | 23 (25.0%) | 7 (6.9%) | .001 |
| COPD, n (%) | 2 (2.0%) | 2 (2.0%) | 1.0 |
| PAD, n (%) | 5 (5.0%) | 8 (7.9%) | .400 |
| BMI, kg/m2 | 25.7 ± 3.6 | 23.9 ± 2.9 | < .001 |
| WBC, 109/L | 6.7 (5.5–8.4) | 6.5 (5.3–8.0) | .383 |
| NEUT, 109/L | 4.1 (3.4–5.4) | 4.1 (3.4–5.7) | .964 |
| LYM, 109/L | 1.9 (1.4–2.2) | 1.6 (1.2–2.0) | .007 |
| NLR | 2.3 (1.7–3.3) | 2.6 (2.0–3.5) | .069 |
| Platelet, 109/L | 188 (148–222) | 162 (140–210) | .061 |
| RDW | 12.8 (12.5–13.3) | 13.0 (12.6–13.5) | .053 |
| Hemoglobin, g/L | 135.3 ± 15.4 | 123.7 ± 15.4 | < .001 |
| IL-6, pg/ml | 6.6 (3.8–9.2) | 8.2 (5.2–14.4) | .002 |
| hs-CRP, mg/l | 5.7 (1.2–10.1) | 6.2 (2.4–14.7) | .087 |
| Glucose, mmol/L | 5.8 ± 2.8 | 5.5 ± 1.8 | .336 |
| TC, mmol/L | 4.0 ± 1.0 | 3.9 ± 1.0 | .552 |
| Triglyceride, mmol/L | 1.5 (1.0–2.0) | 1.3 (1.0–1.9) | .439 |
| HDL-c, mmol/L | 1.2 (1.0–1.4) | 1.2 (1.1–1.4) | .213 |
| LDL-c, mmol/L | 2.2 ± 0.7 | 2.2 ± 0.8 | .715 |
| apoB, g/L | 0.8 (0.6–1.0) | 0.8 (0.6–0.9) | .662 |
| apoA1, g/L | 1.0 ± 0.2 | 1.1 ± 0.2 | .321 |
| Creatinine, umol/L | 68.4 (59.2–79.6) | 72.0 (55.4–92.7) | .237 |
| eGFR, ml/min | 103.9 (86.1–126.9) | 69.9 (53.9–88.8) | < .001 |
| Albumin, g/L | 39.9 (38.4–42.2) | 38.1 (36.1–40.5) | < .001 |
| Fibrinogen, g/L | 3.0 (2.5–3.6) | 3.2 (2.7–4.0) | .041 |
| D-Dimer, ug/ml | 0.3 (0.2–0.4) | 0.4 (0.2–0.7) | .001 |
| LVEF, % | 63.0 (59.0–65.0) | 62.0 (50.0–65.0) | .050 |
| SS | 11.0 (7.0–16.0) | 15.0 (9.0–22.0) | < .001 |
| SS II | 20.8 (17.8–22.9) | 32.3 (28.6–38.1) | < .001 |
| rSS | 2.0 (0–5.4) | 4.0 (0–9.0) | .161 |
BMI, Body Mass Index; WBC, white blood cell; NEUT, neutrophil; LYM, lymphocyte; NLR, the neutrophil to the lymphocyte ratio; RDW, red cell distribution width; IL-6, interleukin 6; hs-CRP, high-sensitivity C-reactive protein; TC, total cholesterol; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; apoB, apolipoprotein B; apoA1, apolipoprotein A1; apoB/apoA1, the apoB to the apoA1 ratio; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; SYNTAX, SYNergy between Percutaneous Coronary Intervention with TAXus and cardiac surgery; SS, SYNTAX score; SS II, SYNTAX score II
Discussion
ACS remains the most prominent threat to global public health, despite advances in revascularization techniques and antithrombotic therapy [1, 3]. It is thus essential that approaches to reliably predicting ACS severity be developed in order to guide the prevention, diagnosis and treatment of this debilitating disease. Herein, we found that IL-6 levels were positively correlated with ACS severity as measured by SS and SS II, with IL-6 levels additionally offering value as an independent predictor of intermediate-high SS and high SS II values.
A growing body of evidence suggests that inflammation is a key driver of ACS onset, progression, and patient prognosis [5–7]. Likewise, cytokines, regarded as the “messengers” of inflammatory response, have been implicated in the pathogenesis of atherosclerosis and CAD [6, 8, 26]. Of note, IL-6, which is primarily derived from mononuclear cells, can contribute to CAD initiation and progression through several mechanisms. Not only does IL-6 mainly initiate the production of hepatic CRP, resulting in increased blood viscosity and platelets numbers; it also accelerates the deposition of fibrinogen [16]. IL-6 can further stimulate macrophages to phagocytose lipids, thereby driving foam cell formation [27]. There is also evidence to suggest that IL-6 is capable of activating the hypothalamic–pituitary–adrenal axis and accelerating insulin resistance [28].
In prior studies, higher IL-6 levels in healthy males were associated with future myocardial infarction incidence [13]. Additionally, Ikeda et al. demonstrated that ACS patients exhibit substantially higher levels of circulating IL-6 as compared to stable angina patients [29]. Moreover, CAD patients exhibit higher serum IL-6 concentrations as compared to controls [30]. One previous study reported that no statistically significant differences in IL-6 levels were observed in the blood of ACS patients taken from the coronary sinus in comparison to blood taken from a peripheral vein, supporting the concept of a systemic rather than a local vascular inflammation contributing to the development of atherosclerosis [31]. Herein, we similarly observed higher serum IL-6 concentrations in ACS patients relative to those in the SAP group.
The SS is a valuable tool that can guide appropriate revascularization planning by aiding in the detection of high-risk ACS cases, and it is also linked to the complexity of atherosclerotic lesions. Furthermore, SS values can effectively predict adverse cardiovascular event risk [18–20]. Additionally, several studies have demonstrated that a correlation exists between IL-6 levels and the severity of coronary stenoses and mortality [6, 16]. Indeed, there have also been prior reports of a positive association between IL-6 levels and the severity of CAD as assessed based on the Gensini score, which is one of the most common scoring systems for quantifying CAD severity [26, 32]. These prior results suggested a likely correlation between serum IL-6 levels and ACS severity as measured using SS and SS II values. Consistently, we found that IL-6 concentrations were independently predictive of intermediate-high SS values, with which they were positively correlated. Given that a range of clinicopathological variables can influence patient prognosis, the SS II scoring system was developed as a more reliable predictor of cardiovascular events among ACS patients by taking these variables into consideration [21, 22]. We detected a positive relationship between IL-6 levels and SS II values. Moreover, we identified hemoglobin concentrations and IL-6 levels to be independent predictors of high SS II. This relationship between hemoglobin and SS II values was in accordance with a previous study [24]. Other reports have also found that factors, including older age, decreased LVEF, and eGFR, can increase SS II values [33–35]. Patients with anemia more frequently present with advanced age, higher creatinine levels, and LVEF dysfunction [36, 37]. Therefore, the association between decreased hemoglobin and a high SS may be attributable to older age, reduced eGFR, and lower LVEF in these patients.
Limitations
There are certain limitations to the present study. For one, a high degree of variability with respect to the IL-6 levels of ACS patients was observed, suggesting that measurements taken at a single time point may be insufficient to reliably capture the extent of potential inflammatory activity. Besides that, we examined the association between the IL-6 and SS or SS II, however, it is difficult to make causal inferences due to the nature of the cross-sectional design. A mendelian randomization study may be further designed to explore this association. Additionally, this was a retrospective single-center analysis without the potential to avoid selection bias. Several confounding factors might have affected the results even after the adjusted analysis. Furthermore, data regarding SS values < 30 and IL-6 levels may be skewed, thus weakening the observed correlations. Lastly, this was a retrospective analysis with relatively few samples, underscoring the need for future large-scale prospective analyses designed to validate and expand upon these results.
Conclusions
In summary, this study confirmed the association between the levels of IL-6 and angiographic complexity in ACS patients. Overall, these findings suggest that IL-6 may be a biomarker that can be used to gauge CAD severity.
Supplementary Information
Additional file 1. Figure S1. (A) Relationship between IL-6 levels and SS in SAP patients; (B) Relationship between IL-6 levels and Post-TnI in SAP patients. IL-6, interleukin 6; SS, SYNergy between Percutaneous Coronary Intervention with TAXus and cardiac surgery (SYNTAX) score; SAP, stable angina pectoris; r, correlation coefficient
Acknowledgements
The authors appreciated the contributions of all participants in this paper.
Abbreviations
- ACS
Acute coronary syndrome
- SYNTAX
SYNergy between Percutaneous Coronary Intervention with TAXus and cardiac surgery
- SS
SYNTAX score
- ROC
Receiver operating characteristic
- AUC
Area under the curve
- STEMI
ST-segment elevation myocardial infarction
- NSTEMI
Non-ST-segment elevation myocardial infarction
- UA
Unstable angina pectoris
- CVD
Cardiovascular disease
- IL-6
Interleukin-6
- hs-CRP
High-sensitivity C-reactive protein
- CAD
Coronary artery disease
- CAG
Coronary angiography
- PCI
Percutaneous coronary intervention
- CABG
Coronary artery bypass grafting surgery
- SAP
Stable angina pectoris
- eGFR
Estimated glomerular filtration rate
- PAD
Peripheral arterial disease
- COPD
Chronic obstructive pulmonary disease
- LVEF
Left ventricular ejection fraction
- SD
Standard deviation
- WBC
White blood cell
- NEUT
Neutrophil
- NLR
The neutrophil to the lymphocyte ratio
- apoA1
Apolipoprotein A1
- HDL-c
High-density lipoprotein cholesterol
- BMI
Body mass index
- OR
Odds ratio
- CI
Confidence interval
Authors’ contributions
HRW and YL wrote the main manuscript text and SXT prepared figures and tables. All authors read and approved the final manuscript.
Funding
This study is supported by the National Natural Science Foundation of China (81803035). The funder plays a vital role in data analysis and preparation of the manuscript.
Availability of data and materials
The raw data supporting the conclusions of this article will be made available by the corresponding authors, without undue reservation.
Declarations
Ethics approval and consent to participate
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was in accordance with the Declaration of Heksinki and was approved by the Institutional Review Board of Yijishan Hospital Affiliated of Wannan Medical College (IRB-2021-013). Requirement for the informed consent was waived because of the retrospective analysis.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest regarding the publication of this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yang Ling and Hairong Weng have contributed equally to this work
References
- 1.Benjamin E, Virani S, Callaway C, Chamberlain A, Chang A, Cheng S, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 2.Flego D, Liuzzo G, Weyand C, Crea F. Adaptive immunity dysregulation in acute coronary syndromes: from cellular and molecular basis to clinical implications. J Am Coll Cardiol. 2016;68(19):2107–2117. doi: 10.1016/j.jacc.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson J, Morrow D. Acute myocardial infarction. N Engl J Med. 2017;376(21):2053–2064. doi: 10.1056/NEJMra1606915. [DOI] [PubMed] [Google Scholar]
- 4.Arbab-Zadeh A, Nakano M, Virmani R, Fuster V. Acute coronary events. Circulation. 2012;125(9):1147–1156. doi: 10.1161/CIRCULATIONAHA.111.047431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Libby P, Ridker P, Hansson G. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 6.Fanola C, Morrow D, Cannon C, Jarolim P, Lukas M, Bode C, et al. Interleukin-6 and the risk of adverse outcomes in patients after an acute coronary syndrome: observations from the SOLID-TIMI 52 (Stabilization of Plaque Using Darapladib-Thrombolysis in Myocardial Infarction 52) trial. J Am Heart Assoc. 2017;6(10). [DOI] [PMC free article] [PubMed]
- 7.Lawler P, Bhatt D, Godoy L, Lüscher T, Bonow R, Verma S, et al. Targeting cardiovascular inflammation: next steps in clinical translation. Eur Heart J. 2021;42(1):113–131. doi: 10.1093/eurheartj/ehaa099. [DOI] [PubMed] [Google Scholar]
- 8.Saremi A, Anderson R, Luo P, Moritz T, Schwenke D, Allison M, et al. Association between IL-6 and the extent of coronary atherosclerosis in the veterans affairs diabetes trial (VADT) Atherosclerosis. 2009;203(2):610–614. doi: 10.1016/j.atherosclerosis.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Held C, White H, Stewart R, Budaj A, Cannon C, Hochman J, et al. Inflammatory biomarkers interleukin-6 and c-reactive protein and outcomes in stable coronary heart disease: experiences from the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) Trial. J Am Heart Assoc. 2017;6(10). [DOI] [PMC free article] [PubMed]
- 10.Schieffer B, Selle T, Hilfiker A, Hilfiker-Kleiner D, Grote K, Tietge U, et al. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation. 2004;110(22):3493–3500. doi: 10.1161/01.CIR.0000148135.08582.97. [DOI] [PubMed] [Google Scholar]
- 11.Bacchiega B, Bacchiega A, Usnayo M, Bedirian R, Singh G, Pinheiro G. Interleukin 6 inhibition and coronary artery disease in a high-risk population: a prospective community-based clinical study. J Am Heart Assoc. 2017;6(3). [DOI] [PMC free article] [PubMed]
- 12.Qu D, Liu J, Lau C, Huang Y. IL-6 in diabetes and cardiovascular complications. Br J Pharmacol. 2014;171(15):3595–3603. doi: 10.1111/bph.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridker P, Rifai N, Stampfer M, Hennekens C. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101(15):1767–1772. doi: 10.1161/01.CIR.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 14.Ridker P, Hennekens C, Buring J, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 15.Mowafy A, El-Akabawy H, Hussein A, Abd El Hay A. Prognostic value of IL6 in young adults presenting with acute coronary syndrome. Egypt Heart J. 2015;67(2):151–8.
- 16.Su D, Li Z, Li X, Chen Y, Zhang Y, Ding D, et al. Association between serum interleukin-6 concentration and mortality in patients with coronary artery disease. Mediators Inflamm. 2013;2013:726178. [DOI] [PMC free article] [PubMed]
- 17.Bambrough P, Peverelli M, Brown A, Giblett J, Bennett M, West N, et al. Trans-myocardial blood Interleukin-6 levels relate to intracoronary imaging defined features of plaque vulnerability and predict procedure-induced myocardial infarction. Cardiovasc Revasc Med. 2021 (undefined). [DOI] [PubMed]
- 18.Palmerini T, Genereux P, Caixeta A, Cristea E, Lansky A, Mehran R, et al. Prognostic value of the SYNTAX score in patients with acute coronary syndromes undergoing percutaneous coronary intervention: analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage StrategY) trial. J Am Coll Cardiol. 2011;57(24):2389–2397. doi: 10.1016/j.jacc.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 19.van Gaal W, Ponnuthurai F, Selvanayagam J, Testa L, Porto I, Neubauer S, et al. The Syntax score predicts peri-procedural myocardial necrosis during percutaneous coronary intervention. Int J Cardiol. 2009;135(1):60–65. doi: 10.1016/j.ijcard.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 20.Magro M, Nauta S, Simsek C, Onuma Y, Garg S, van der Heide E, et al. Value of the SYNTAX score in patients treated by primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: the MI SYNTAXscore study. Am Heart J. 2011;161(4):771–781. doi: 10.1016/j.ahj.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Farooq V, van Klaveren D, Steyerberg E, Meliga E, Vergouwe Y, Chieffo A, et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet. 2013;381(9867):639–650. doi: 10.1016/S0140-6736(13)60108-7. [DOI] [PubMed] [Google Scholar]
- 22.Wang G, Wang C, Zhang Y, Wang P, Ran C, Zhao L, et al. Usefulness of the SYNTAX score II to predict 1-year outcome in patients with primary percutaneous coronary intervention. Coron Artery Dis. 2016;27(6):483–489. doi: 10.1097/MCA.0000000000000385. [DOI] [PubMed] [Google Scholar]
- 23.Hamm C, Bassand J, Agewall S, Bax J, Boersma E, Bueno H, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2011;32(23):2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 24.Çağdaş M, Rencüzoğullari I, Karakoyun S, Karabağ Y, Yesin M, Artaç I, et al. Assessment of relationship between C-reactive protein to albumin ratio and coronary artery disease severity in patients with acute coronary syndrome. Angiology. 2019;70(4):361–368. doi: 10.1177/0003319717743325. [DOI] [PubMed] [Google Scholar]
- 25.Sianos G, Morel M, Kappetein A, Morice M, Colombo A, Dawkins K, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1(2):219–227. [PubMed] [Google Scholar]
- 26.Min X, Lu M, Tu S, Wang X, Zhou C, Wang S, et al. Serum cytokine profile in relation to the severity of coronary artery disease. Biomed Res Int. 2017;2017:4013685. doi: 10.1155/2017/4013685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lubrano V, Gabriele M, Puntoni M, Longo V, Pucci L. Relationship among IL-6, LDL cholesterol and lipid peroxidation. Cell Mol Biol Lett. 2015;20(2):310–322. doi: 10.1515/cmble-2015-0020. [DOI] [PubMed] [Google Scholar]
- 28.Drummond J, Soares B, Vieira E, Pedrosa W, Teixeira A, Ribeiro-Oliveira A. Interleukin-6 response to insulin-induced hypoglycemia is associated with hypothalamic-pituitary-adrenal axis activation. J Neuroimmunol. 2020;350:577446. [DOI] [PubMed]
- 29.Ikeda U, Ito T, Shimada K. Interleukin-6 and acute coronary syndrome. Clin Cardiol. 2001;24(11):701–704. doi: 10.1002/clc.4960241103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang J, Shen D, Liu C, Wang X, Zhang L, Xuan X, et al. Plasma levels of C1q/TNF-related protein 1 and interleukin 6 in patients with acute coronary syndrome or stable angina pectoris. Am J Med Sci. 2015;349(2):130–136. doi: 10.1097/MAJ.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 31.Brueckmann M, Bertsch T, Lang S, Sueselbeck T, Wolpert C, Kaden J, et al. Time course of systemic markers of inflammation in patients presenting with acute coronary syndromes. Clin Chem Lab Med. 2004;42(10):1132–1139. doi: 10.1515/CCLM.2004.232. [DOI] [PubMed] [Google Scholar]
- 32.Gotsman I, Stabholz A, Planer D, Pugatsch T, Lapidus L, Novikov Y, et al. Serum cytokine tumor necrosis factor-alpha and interleukin-6 associated with the severity of coronary artery disease: indicators of an active inflammatory burden? Isr Med Assoc J. 2008;10(7):494–498. [PubMed] [Google Scholar]
- 33.Anuurad E, Enkhmaa B, Gungor Z, Zhang W, Tracy R, Pearson T, et al. Age as a modulator of inflammatory cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2011;31(9):2151–2156. doi: 10.1161/ATVBAHA.111.232348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueland T, Gullestad L, Nymo S, Yndestad A, Aukrust P, Askevold E. Inflammatory cytokines as biomarkers in heart failure. Clin Chim Acta. 2015;443:71–77. doi: 10.1016/j.cca.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Xu G, Luo K, Liu H, Huang T, Fang X, Tu W. The progress of inflammation and oxidative stress in patients with chronic kidney disease. Ren Fail. 2015;37(1):45–49. doi: 10.3109/0886022X.2014.964141. [DOI] [PubMed] [Google Scholar]
- 36.Bassand J, Afzal R, Eikelboom J, Wallentin L, Peters R, Budaj A, et al. Relationship between baseline haemoglobin and major bleeding complications in acute coronary syndromes. Eur Heart J. 2010;31(1):50–58. doi: 10.1093/eurheartj/ehp401. [DOI] [PubMed] [Google Scholar]
- 37.Wańha W, Kawecki D, Roleder T, Pluta A, Marcinkiewicz K, Dola J, et al. Impact of anaemia on long-term outcomes in patients treated with first- and second-generation drug-eluting stents; Katowice-Zabrze Registry. Kardiol Pol. 2016;74(6):561–569. doi: 10.5603/KP.a2015.0217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Figure S1. (A) Relationship between IL-6 levels and SS in SAP patients; (B) Relationship between IL-6 levels and Post-TnI in SAP patients. IL-6, interleukin 6; SS, SYNergy between Percutaneous Coronary Intervention with TAXus and cardiac surgery (SYNTAX) score; SAP, stable angina pectoris; r, correlation coefficient
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the corresponding authors, without undue reservation.