Abstract
Background
Pre-exposure prophylaxis (PrEP) is recommended by the WHO for HIV prevention among female sex workers (FSWs). A study conducted in 2016–2017 in Côte d’Ivoire showed that if PrEP is acceptable, FSWs also have many uncovered sexual health needs. Based on this evidence, the ANRS 12381 PRINCESSE project was developed in collaboration with a community-based organization. The main objective is to develop, document, and analyze a comprehensive sexual and reproductive healthcare package among FSWs in Côte d’Ivoire.
Methods
PRINCESSE is an open, single-arm interventional cohort of 500 FSWs in San Pedro (Côte d’Ivoire) and its surroundings. Recruitment started on November 26th, 2019 and is ongoing; the cohort is planned to last at least 30 months. The healthcare package (including HIV, hepatitis B, and sexually transmitted infection management, pregnancy screening, and contraception) is available both at mobile clinics organized for a quarterly follow-up (10 intervention sites, each site being visited every two weeks) and at a fixed clinic.
Four waves of data collection were implemented: (i) clinical and safety data; (ii) socio-behavioral questionnaires; (iii) biological data; and (iv) in-depth interviews with female participants. Four additional waves of data collection are scheduled outside the cohort itself: (i) the medical and activity records of Aprosam for the PRINCESSE participants; (ii) the medical records of HIV+ FSW patients not participating in the PRINCESSE cohort, and routinely examined by Aprosam; (iii) in-depth interviews with key informants in the FSW community; and (iv) in-depth interviews with PRINCESSE follow-up actors.
Discussion
The PRINCESSE project is one of the first interventions offering HIV oral PrEP as part of a more global sexual healthcare package targeting both HIV- and HIV+ women. Second, STIs and viral hepatitis B care were offered to all participants, regardless of their willingness to use PrEP. Another innovation is the implementation of mobile clinics for chronic/quarterly care. In terms of research, PRINCESSE is a comprehensive, interdisciplinary project combining clinical, biological, epidemiological, and social specific objectives and outcomes to document the operational challenges of a multidisease program in real-life conditions.
Trial registration
The PRINCESSE project was registered on the Clinicaltrial.gov website (NCT03985085) on June 13, 2019.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-021-12235-0.
Keywords: HIV prevention; Sexual and reproductive health; Sexually transmitted infections (STIs), hepatitis B; Pre-exposure prophylaxis (PrEP); Sex work; Mixed-methods research; Mobile clinics; Côte d’Ivoire
Background
Most countries in West Africa have mixed HIV epidemics: a limited HIV prevalence in the general population and some key populations that are overwhelmingly affected, particularly female sex workers (FSWs) and men who have sex with men (MSM) [1]. In 2019, 19% of new HIV infections in West and Central Africa occurred among FSWs and 27% among clients of FSWs and other sexual partners of key populations [1]. In Côte d’Ivoire, HIV prevalence among FSWs was estimated to be 11.4% in Abidjan in 2014 [2], and HIV incidence was 3.2% in San Pedro and 1.5% in Abidjan in 2016–2017 [3] vs. 0.5% in the general population in 2019 [4]. FSWs are exposed to HIV, as they do not systematically use condoms with their male partners—primarily because of coercion, the primacy of men’s sexual pleasure, or to obtain protection from their partner against the threat of violence [5–7] —or with their clients in order to earn more, or because of violence from some clients [5, 8–12]. However, this population often lacks access to adequate services to prevent HIV acquisition and access to HIV care when HIV-positive [13].
In this global context, a new tool for HIV prevention was developed in the last decade: oral pre-exposure prophylaxis (PrEP), which consists of antiretroviral drugs taken by HIV-negative people to prevent HIV acquisition. When taken properly, oral PrEP has been shown to be very effective in preventing HIV acquisition, with a relative reduction of 75 to 86% [14–16]. Since 2015, oral PrEP has been recommended by the WHO for populations “at substantial risk” of being infected by HIV [17], such as FSWs. However, the effective implementation of PrEP raises questions, particularly among women, and needs more operational research [17].
First, clinical trials conducted in South and East Africa have shown low adherence among women, resulting in little or no effect of PrEP on HIV acquisition [18, 19]. Likewise, implementation trials conducted in Africa among FSWs have revealed relatively low retention, even when a priori acceptability of PrEP was high [20–22]. Second, PrEP does not protect against sexually transmitted infections (STIs) or unwanted pregnancies; sexual and reproductive health (SRH) needs beyond HIV must therefore be addressed [23]. More specifically, in Côte d’Ivoire, oral PrEP is not yet implemented at scale, and the National AIDS Programme has been asking for operational research before scaling up.
In 2016–2017, we conducted the ANRS 12361 PrEP-CI, a cross-sectional and mixed-methods study, to explore sexual healthcare needs that should be considered within a PrEP program targeting FSWs in Côte d’Ivoire, in order to better describe the experiences of FSWs who are reached via peer educators, and to test the pertinence and a priori feasibility of such programs [5]. Implemented at prostitution sites in two Ivorian cities (Abidjan and San Pedro) in collaboration with two Ivorian community-based organizations (Espace Confiance and Aprosam), the PREP-CI study included (i) a quantitative survey among 1000 FSWs who had never been tested or previously tested HIV-negative, including a socio-behavioral questionnaire, HIV testing, and, for those who tested HIV-positive, collecting a dried blood spot for a recent infection assay to estimate HIV incidence; and (ii) a qualitative survey based on individual interviews and focus-group discussions among 66 FSWs. A final workshop was organized with six community non-governmental organizations (NGOs) and the National AIDS Programme to discuss the main results and to elaborate on an operational research project. Thus, the ANRS 12381 PRINCESSE project was developed based on evidence generated by this PrEP-CI study.
All FSWs have unmet SRH needs, whether they are infected with HIV or wish to initiate PrEP
The community clinics dedicated to FSWs are mainly frequented by HIV+ FSWs, despite the existence of services for all FSWs. However, their health needs go beyond HIV prevention and care. The non-systematic use of condoms exposes FSWs to STIs and unwanted pregnancies, which increase morbidity and mortality [24]. According to two studies conducted in Côte d’Ivoire in 2014, 70% of FSWs who have ever been pregnant have had at least one abortion [2, 25], the majority of them clandestinely, as abortion is illegal in Côte d’Ivoire (except for saving the mother’s life or in the case of rape since 2019). In the PrEP-CI study, 50% of the surveyed FSWs said they had had at least one abortion in their lifetime, and 65% reported having contracted an STI in the past 12 months. Qualitative interviews revealed the frequent use of cloth or cotton pieces for menstrual hygiene, a source of bacterial infection [26, 27].
Further, contraceptive prevalence is low, despite a high risk of unintended pregnancy. In the PrEP-CI study, 42% of FSWs declared having had an unwanted pregnancy, but 61% were not using contraception other than condoms, thus confirming trends already observed in Abidjan in 2014 [2, 25]. The interviews revealed that FSWs did not use modern contraception for fear of becoming infertile. Hence, there is a need to enhance their sexual health outcomes and to reduce undesirable events such as STIs and unwanted pregnancies.
The operational implementation of HIV PrEP requires considering chronic follow-up of HIV-negative women as part of a comprehensive SRH care package
The a priori acceptability of PrEP appeared to be very high (more than 95% of FSWs declared interest in such tools in the PrEP-CI survey). Beyond the availability of PrEP, the entire follow-up of HIV-negative FSWs needs to be (re)designed. Indeed, as efficacy trials have shown, oral PrEP requires quarterly monitoring of users/participants, including renal monitoring and the systematic screening of STIs, since syndromic screening is not sufficient [28]. However, the current priorities of public policies in Côte d’Ivoire and international donors are the identification of new HIV-positive cases and their referral for HIV treatment. It is necessary to develop tools and a new organization of care that allows for the chronic follow-up of HIV-negative FSWs for the proper use of, and adherence to, PrEP.
In the context of a high prevalence of hepatitis B, antiretroviral drugs (ARVs) to prevent HIV cannot be made available without making the same ARVs available to treat hepatitis B
The prevalence of hepatitis B virus (HBV) is high in Côte d’Ivoire; different studies showed an HBV prevalence situated between 9.4% [29] and 11.1% [30] in the 2010s. A prevalence of hepatitis B surface antigen (HbsAg) of 6.2% was also estimated in a cross-sectional study conducted among FSWs in Abidjan in 2014–2015 [31]. In addition, few FSWs are vaccinated (only 5.2% in the PrEP-CI survey according to FSW self-reports). However, there is currently no free hepatitis B treatment program in Côte d’Ivoire, except for HIV/HBV coinfected patients. Tenofovir-based antiretroviral treatments used for HIV PrEP can also treat hepatitis B. It would be unethical to provide a drug freely for prevention when the same drug is not available for treatment. The implementation of HIV PrEP is an opportunity to articulate HIV prevention and HBV prevention/care.
To minimize the stigma associated with entry into care, the care of HIV-infected FSWs and the preventive follow-up of HIV-negative FSWs should not be dissociated
On the one hand, FSWs face many situations of stigmatization and judgmental attitudes from their neighborhood, their entourage, and from health professionals, which complicate their access to care [32–34]. This is probably amplified for HIV+ FSWs, knowing that people living with HIV also face many stigmatizing situations [35–37]. On the other hand, SRH requires the concern all FSWs, whether or not they are infected with HIV. For these reasons, also supported by NGO field workers’ feedback, it seems essential not to dissociate the follow-up of HIV-infected and HIV-negative FSWs. It may also help to improve HIV care among FSWs.
Follow-up from outreach activities (at prostitution sites) to community clinics is not sufficient
The NGOs Aprosam and Espace Confiance both carry out outreach activities with peer educators who go to prostitution sites to conduct HIV prevention and rapid testing. Occasionally, they also use mobile clinics to offer an additional syndromic screening of STIs. However, access to fixed community clinics that follow up on these outreach activities remains limited; Espace Confiance estimates that only half of the FSWs who are newly diagnosed with HIV at prostitution sites access a community clinic for HIV care. The interviews conducted in the PrEP-CI study showed that few FSWs were seeking care after unprotected sexual intercourse. The majority were not aware of the availability of prevention tools such as HIV post-exposition prophylaxis or the morning-after pill, despite the information provided by peer educators. They tended to self-medicate rather than visit a healthcare provider. FSWs revealed a reluctance to go to community health centers, mainly because they wished to remain anonymous (for fear of stigmatization by the neighborhood) and because opening hours did not fit their work schedules. In 2011, a KAP (knowledge, attitudes, practices) survey conducted among FSWs in 18 cities in Côte d’Ivoire showed that 81% had heard about a community clinic, but only 60% had ever accessed a community clinic at least once [38]. This could be relevant for implementing mobile clinics that operate directly at prostitution sites, not only for punctual activities, but also for long-term follow-up.
The high mobility of FSWs is an obstacle to continuity of care
According to PrEP-CI data, FSWs migrate seasonally (especially during the coffee and cocoa season in San Pedro, between September and December) or more occasionally (when sex work is done in a different city from their home city). Therefore, their work periods are variable, from some days in a week or month to several months a year.
Methods/design
Based on prior evidence, the ANRS 12381 PRINCESSE project aims to implement and evaluate, in Côte d’Ivoire, a community-based comprehensive sexual and reproductive healthcare package, including HIV PrEP, STIs, and HBV management, targeting FSWs.
Study setting
The PRINCESSE study is taking place in San Pedro and its surroundings, Côte d’Ivoire, which is a region with farming businesses (coffee and cocoa in urban zones, and palm oil and hevea in rural areas), thus leading to a high degree of labor migration among men. The harbor of San Pedro is one of the most important harbors in West Africa and the world’s largest in terms of cocoa bean exports.
The PRINCESSE study was developed in collaboration with the Aprosam community-based organization, which delivers HIV prevention and testing services directly at prostitution sites (outreach activities) and provides HIV and SRH care services to FSWs through a community clinic in San Pedro. In the PrEP-CI study, HIV incidence was estimated at 3.2% in 2016–2017 among FSWs reached by Aprosam in this region [3].
Study design and objective
The ANRS 12381 PRINCESSE project is a single-arm interventional cohort of FSWs aiming to develop, document, and analyze a community-based healthcare package that combines testing and prevention tools, including PrEP, immediate HIV treatment, the management of HBV, and SRH. The recruitment of the PRINCESSE cohort (baseline participants) started on November 26th, 2019 and is ongoing, partly due to the pandemic of COVID-19; the cohort is planned to last at least 30 months. Recruitment of participants is made possible by the Aprosam organization’s networks of peer educators and their access to the population.
Sample size and power calculation
In total, the PRINCESSE cohort aims to include 500 FSWs at prostitution sites. A pragmatic approach was taken to determine the sample size of the study, considering the absorption capacity of the mobile clinic. The mobile clinic is organized to return to the same site every two weeks. Operating five days a week, ten different sites are covered. In order to ensure sufficient consultation time for each FSW, our target was a maximum of ten consultations per trip. Since PRINCESSE follow-up consists of a quarterly visit on average, with the same site being visited five to six times per quarter, the target number of FSWs followed up periodically for a mobile clinic is 5 (visits on each site per quarter) × 10 sites × 10 consultations/visit = 500 FSWs per mobile clinic. Considering an HIV prevalence of around 20% [2], it was anticipated to recruit 100 (500 × 20%) HIV-positive FSWs and 400 (500 × 80%) HIV-negative FSWs.
Most outcomes listed in Table 3 (see below) are proportions. A minimum sample of n = 402 is required to estimate the 95% confidence interval of an expected proportion of 50% with a precision of ±5. For an expected proportion of 10% (or 90%), with a precision of ±3, a minimum sample of n = 414 is required. To have 80% power to detect with an alpha risk of 5% an evolution of ±10 points (e.g. from 50 to 60%) between two time points, a minimum sample of n = 408 is required.
Table 3.
Specific objectives and definitions of the main outcomes of the PRINCESSE project
| Specific objectives | Principal outcomes | Population | Time Frame |
|---|---|---|---|
| SO1. Access to care, retention, and healthcare pathways |
Completion rate of quarterly visits: proportion of completed study visits |
All PRINCESSE participants | Up to 24 months |
| SO2. Clinical, behavioral, and social evolutions | Proportion with at least one diagnosed STI (chlamydia, gonococcus, or syphilis) detected by PCR or rapid test | All PRINCESSE participants | Up to 24 months |
| Occurrence of an unwanted pregnancy in the last 12 months, orally reported | All PRINCESSE participants | Up to 24 months | |
| S03. Initiation, practices, and compliance with PrEP | Initiation of PrEP: proportion having initiated PrEP | PRINCESSE participants eligible for PrEP | Over 24 months |
| Adherence to PrEP: proportion being adherent (measured through self-report, pill count, and drug detection in plasma) | PRINCESSE participants on PrEP | Up to 24 months | |
| SO4. Comparison of HIV care (HIV+ patients of the PRINCESSE cohort vs. HIV+ patients of the NGO Aprosam not belonging to the PRINCESSE cohort) | Number of participants in HIV care at 18 months (retention) | PRINCESSE participants who are HIV-infected at baseline + HIV-infected patients of Aprosam | 18 months |
| Occurrence of virological failure: proportion with two consecutive detectable viral loads | PRINCESSE participants who are HIV-infected and have initiated antiretroviral treatment + HIV-infected patients of Aprosam | Over 24 months (survival analysis) | |
| S05. Prevention and care of hepatitis B | HBV vaccination rate: proportion with complete vaccination (3 doses if HIV-negative, 4 double doses if HIV-positive) at the end of the trial | PRINCESSE participants needing hepatitis B vaccination | Over 24 months |
| Initiation and number of participants on TDF (retention) for patients with treatment for HBV mono-infection | PRINCESSE participants with a positive HBs-antigen and an F3-F4 fibrosis | Over 24 months | |
| Proportion with an increase in transaminase level (flares) after PrEP discontinuation | PRINCESSE participants who started and stopped PrEP and with a positive HBs-antigen | Within 12 months after PrEP discontinuation | |
| SO6. Unintended consequences of the PRINCESSE intervention | Number of adverse social events occurring in participants’ daily lives | All PRINCESSE participants | Over 24 months |
| Qualitative evaluation of undesired social events that occurred in the daily lives of participants and non-participants | PRINCESSE participants and non-participants FSWs in the targeted area of the intervention | Over 24 months | |
| SO7. Vaginal microbiota, HPV infection types, and antimicrobial STI resistance | Proportion of cervical lesions at M0 and M12 | All PRINCESSE participants | 12 months |
| Proportion of high-risk HPV infection included in quadrivalent and nonavalent vaccines at genital and anal levels at M0 and M12 | All PRINCESSE participants | 12 months | |
|
Proportion of M. genitalium, N. gonorrheae, C. trachomatis infections at genital level at M0 and M12 Proportion of M. genitallium and N. gonorrheae antimicrobial resistances |
All PRINCESSE participants | 12 months |
Eligibility criteria for the PRINCESSE cohort
The inclusion criteria are:
Being a woman over 18 years of age
Self-reporting as being a sex worker
Wishing to enroll in a regular clinical follow-up
Agreeing to participate in the study and signing the informed consent form
Not already participating in another biomedical or behavioral study on HIV, viral hepatitis, or STIs
Regardless of HIV status (infected or not)
Whether or not the participant has already taken antiretrovirals
Whether or not the participant is already followed by Aprosam
Field implementation
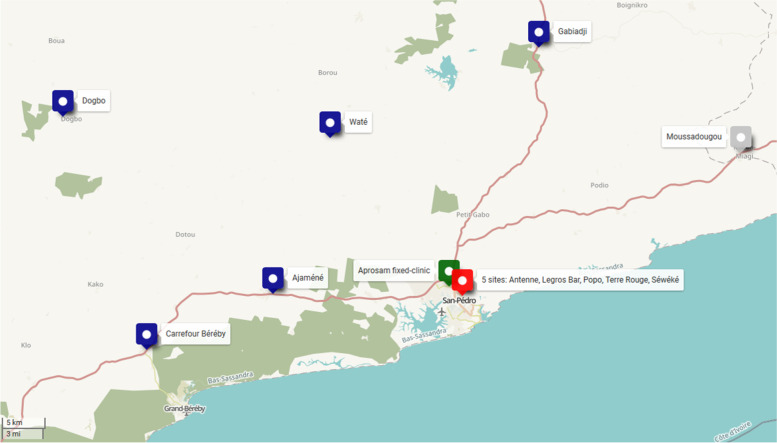
The PRINCESSE intervention is delivered using a mobile clinic at 10 prostitution sites: 5 urban sites in the city of San Pedro and 5 rural sites around San Pedro (Fig. 1). Care services are also available at the fixed community clinic of Aprosam in San Pedro. Each prostitution site is visited every two weeks, and the operating hours vary according to each site.
Fig. 1.
Implementation sites of the PRINCESSE project in the area of San Pedro, Côte d’Ivoire. Source: custom map by the authors using OpenStreetMap for background
After inclusion, participants are invited to be followed up with every 3 months, either in the mobile or fixed clinic, at their preference. FSWs can also access the clinics anytime they need to, in addition to planned quarterly visits.
The mobile clinic team comprises a medical doctor, a laboratory technician, a community counselor, a driver, two FSW peer-educators, and an independent interviewer.
Healthcare offer
The PRINCESSE healthcare package is summarized in Table 1 and detailed below.
Table 1.
Summary of PRINCESSE healthcare package per protocol visit
| Time since inclusion (MO) W: week/M: month |
M0 | W2 | M3 | M6 | M9 | M12 | M15 | M18 | M21 | M24 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Initial screening (at inclusion) |
HIV testing | ✓ | |||||||||
| HBsAg testing | ✓ | ||||||||||
|
HIV prevention (if HIV-) |
HIV testing | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Creatinine (if PrEP) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| HIV PrEP (TDF/FTC) (if eligible) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| At PrEP interruption (if AgHBs + or isolated HBcAb) | ALAT/ASAT (every 3 months) + HBV viral load if sign of hepatitis B reactivation | ||||||||||
| HIV post-exposure prophylaxis | when needed | ||||||||||
|
HIV care (if HIV+) |
Antiretroviral treatment | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| CD4 and viral load (if HIV+) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| HBV prevention (if HBsAg-) | HBsAb testing | ✓ | |||||||||
| HBcAb testing | ✓ | ||||||||||
| HBV vaccination (if HBsAb- et HBcAb-) | 3 × 1 doses if HIV- (M0, M1, M6), 4 × 2 doses if HIV+ (M0, M1, M2, M6) | ||||||||||
| HBV care (if HBsAg+) | HBeAg testing | ✓ | |||||||||
| Creatinine (if on TDF) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| ALT/AST/Platelets | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Antiretroviral treatment (TDF/FTC) (according to FIB-4 level and creatinine clearance level) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| HBV viral load | ✓ | ✓ | ✓ | ||||||||
| STI screening and care | Syndromic STI screening | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Systematic STI testing (Chlamydia PCR + Gonorrhea PCR + Syphilis rapid test) | ✓ | ✓ | ✓ | ||||||||
| Dysplasia testing (+ treatment when necessary) | ✓ | ✓ | ✓ | ||||||||
| STI treatment | when needed | ||||||||||
| Pregnancy screening and contraception | Pregnancy test | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Contraception (pills, injection or implant according to patient’s choice) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Emergency pill | when needed | ||||||||||
| Other | Condom and lubricating gel | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Identification of addiction (tobacco, alcohol, drugs) and referencing when necessary | ✓ | ✓ | ✓ | ✓ | |||||||
Initial screening for HIV and hepatitis B at inclusion
All participants are screened for HIV during the inclusion visit using rapid tests according to the national testing algorithm: Determine® (sensitivity: 100%; specificity: 98.9%), Stat-pak® (sensitivity: 99.5%; specificity: 100%), and Biolane® in the case of a discrepancy. In the case of a new HIV diagnosis, a blood sample is collected for confirmation in a laboratory.
All participants are also screened for antigen HBs (AgHBs) using the rapid test Determine® (sensitivity: 93.6%; specificity: 100%) or VIKIA® (sensitivity: 96.5%: specificity: 99.9%). In case of a negative result for AgHBs, a blood sample is collected for anti-HBs and anti-HBc antibody testing in a laboratory. In case of a positive result for AgHBs, blood samples are collected for measuring HBV viral load, antigen HBe, platelets, ALT, and AST in a laboratory. The fibrosis level (F0-F1-F2 or F3-F4) is estimated using the FIB-4 indicator [39].
Antiretroviral treatment and hepatitis B vaccination
HIV treatment guidelines [40], HIV PrEP guidelines [41], and Hepatitis B guidelines [39] have been combined to produce the HIV/HBV care algorithm for the PRINCESSE participants (Table 2 and Fig. S1).
Table 2.
Summary of HIV and HBV care algorithm for PRINCESSE participants
| Group of patients | HIV and AgHBs negative | HIV-positive | HIV-negative AgHBs positive Fibrose ≥ F2 Elevated ALT and HBV DNA (eligible for HBV treatment) |
HIV-negative AgHBs positive Fibrose < F2 Low HBV DNA et normal ALT (not eligible for HBV treatment) |
|---|---|---|---|---|
| Hepatitis B vaccination |
If not immunized (anti-HBs and anti-HBc both negatives) 3 regular doses at M0, M1, M6 |
If not immunized (anti-HBs and anti-HBc both negatives) 4 double doses at M0, M1, M2, M6 |
– | – |
| Antiretroviral prescription |
If creatinine clearance ≥ 60 mL/min and no contraindication TDF/FTC (1 pill/day) as oral PrEP |
According to national guidelines As soon as possible, regardless of CD4 count Adapted regimen if HIV/HBV coinfection |
If creatinine clearance ≥ 60 mL/min and no contraindication TDF/FTC (1 pill/day) as HBV treatment If creatinine clearance < 60 mL/min TDF with an adapted reduced dosage (1 pill every 1, 2, 3, or 7 days depending on the rate of creatinine clearance) |
If creatinine clearance ≥ 60 mL/min and no contraindication TDF/FTC (1 pill/day) as oral PrEP |
Participants who are HIV- and AgHBs-negative are offered the chance to initiate TDF/FTC (tenofovir and emtricitabine) for daily oral PrEP. For those interested, a blood sample is collected to measure creatinine clearance using the equation of Cockcroft & Gault. PrEP will only be prescribed if renal function is normal (creatinine clearance ≥60 mL/min); there is no contraindication to TDF and FTC; there are no clinical manifestations suggestive of primary HIV infection; and the patient is willing to take PrEP as prescribed and to enroll in required periodic medical follow-up [41]. Patients not immunized against HBV (HBs and HBc antibodies, testing negative for both) are offered the chance to be vaccinated (3 times a regular dose at M0, M1, and M6).
HIV-infected participants are followed according to Ivorian national treatment guidelines [40]. Antiretroviral treatment is proposed as soon as possible, regardless of CD4 count. In the case of HIV-HBV coinfection, antiretroviral treatment is adapted. Patients not immunized against HBV (HBs and HBc antibodies, testing negative for both) are offered the chance to get vaccinated (4 times a double dose, at M0, M1, M2, and M6).
For patients infected by HBV for whom treatment is recommended (a high level of HBV DNA or transaminases or fibrosis ≥F2), a blood sample is collected to measure creatinine clearance using the equation of Cockcroft & Gault. If creatinine clearance is ≥60 mL/min, TDF/FTC (1 pill/day) is prescribed. TDF/FTC is one of the regimens recommended by the WHO ([39], Table 6.1.a). For patients with creatinine clearance below 60 mL/min, only tenofovir (TDF) is prescribed at a reduced dosage, as recommended by the WHO ([39], Table 9.1): 50–59 mL/min: one 300 mg tablet every 24 h; 30–49 mL/min: one 300 mg tablet every 48 h; 10–29 mL/min: one 300 mg tablet every 72–96 h; and < 10 mL/min: one 300 mg tablet every 7 days. These patients are advised that there is no demonstrated prophylactic effect against HIV, with non-daily TDF, and will be encouraged to maintain the use of other HIV prevention tools.
For patients infected by HBV for whom treatment is not yet recommended (a low level of HBV DNA or ALT or fibrosis <F2), they are offered the chance to initiate TDF/FTC for oral HIV PrEP. For those interested, a blood sample is collected to measure creatinine clearance using the equation of Cockcroft & Gault. PrEP is only prescribed if renal function is normal (a creatinine clearance ≥60 mL/min); there is no contraindication to TDF and FTC; there are no clinical manifestations suggestive of primary HIV infection; and the patient is willing to take PrEP as prescribed and to enroll in the required periodic medical follow-up [41].
For all participants who are HIV-negative, HIV testing is proposed every 3 months, regardless of their PrEP status. In the case of HIV seroconversion, patients are given the chance to initiate an adapted antiretroviral regimen as soon as possible.
STI screening and care
Syndromic screening (clinical examination) is offered quarterly and at each non-protocol visit if needed. In the case of STI suspicion, national treatment kits are provided for free according to the national algorithm of the Ministry of Health.
This quarterly syndromic screening is completed annually through a biological screening (PCR chlamydia and gonococcus, rapid syphilis test, vaginal and anal swab) at M0, M12, and M24 because of the importance of asymptomatic STIs in women in general and among FSWs in particular. In the case of a positive rapid syphilis test, a blood sample is taken for a laboratory evaluation. Patients are called back by telephone for a complimentary visit if treatment is necessary based on the tests’ results.
Screening of condylomas and dysplastic lesions of the cervix is also proposed annually using acetic acid and Lugol. Treatment by thermoablation is available both in mobile and fixed clinics. If advanced lesions are diagnosed, conization and referral to a specialized medical service are proposed if necessary.
Pregnancy screening and contraception
Participants could also benefit from a reproductive health package, including free-of-charge non-barrier contraception (pill, injection, or implant; it is the participant’s choice), quarterly pregnancy tests, and emergency contraceptive pills when needed.
Other services
In addition, PRINCESSE healthcare includes menstrual management counseling with the offer of menstrual cups; a biannual identification of addiction (tobacco, alcohol, drugs), and referral to dedicated services when necessary; free condoms; and lubricating gel.
Specific research objectives and principal outcomes/evaluation criteria
We defined six specific objectives to evaluate PRINCESSE intervention:
To analyze access to care and retention in care and, more generally, female participants’ healthcare trajectories through a quarterly follow-up of FSWs (infected with HIV or not)
To measure female participants’ health outcomes over time using clinical, behavioral, and social indicators
To assess PrEP initiation, use, and adherence
To compare HIV management in the PRINCESSE system with existing routine treatment and care
To measure HBV testing, vaccination, and treatment as part of decentralized management integrated with HIV PrEP, and possible interactions between HIV PrEP and HBV infection
To document the unexpected consequences (positive or negative) of the PRINCESSE system on the everyday lives of female participants in particular, and on the sex industry in general
To evaluate the impact of vaginal microbiota on bacterial sexually transmitted infections, human papillomavirus (HPV) infections, and associated cervical lesions; the impact of the HPV type distribution on the vaccinal strategy and the added value of HPV PCR for the primary screening of cervical cancer; and the impact of antimicrobial resistance on STI guidelines.
Due to the complexity of the intervention, identifying a unique main criterion over a multidimensional and multidisciplinary evaluation would be simplistic. For each of the six specific objectives, we defined a list of evaluation criteria that are neither exhaustive nor limiting (Table 3).
Data collection
In the PRINCESSE cohort, four data points are collected:
-
(i)
P1. Clinical and safety data: Dates and localization of medical consultations, the results of clinical examinations, clinical records of inclusions and follow-ups (including basic socio-behavioral indicators), adverse events, and prescriptions carried out as part of the PRINCESSE cohort.
-
(ii)
P2. Socio-behavioral questionnaires: Sociodemographic characteristics, sexual practices and behaviors, HIV and HBV testing history, PrEP adherence/compliance, contraception, abortion, menstrual management, addictions, mental health, quality of life, perceived health, living standards, and social support.
An independent interviewer is carrying out these questionnaires every six months during or around quarterly visits (M3, M9, M15, M21). There are no questionnaires collected at M0 to avoid the burden on inclusion visits. However, the main socio-behavioral indicators are collected at baseline in the clinical records of the included patients.
-
(iii)
P3. Biological data: Results of lab tests performed on samples collected during medical consultations as part of the healthcare package; blood specimens and anal and vaginal swabs are also collected at M0, M12, and M24 to constitute a biobank.
-
(iv)
P4. In-depth qualitative interviews with female participants: Semi-structured interviews are carried out with the participants of the PRINCESSE cohort by a scientist trained in qualitative methods.
The interviews address several topics, such as participants’ personal experiences of the PRINCESSE project in general, how they appropriate the proposed tools, and more specifically, their understanding, daily experiences, and difficulties encountered with PrEP.
Four other additional waves of data collection, not included in the PRINCESSE cohort, are scheduled:
-
(i)
A1. Capture the medical and activity records of Aprosam for PRINCESSE participants: Along with the PRINCESSE project, Aprosam continues its routine activities, including raising awareness, condom distribution, and outreach HIV testing conducted by peer educators. These routine activities, as well as medical consultations carried out within the community clinic of Aprosam, are recorded with specific identifiers. To complete the healthcare pathways of participants and their background with Aprosam, data routinely collected by Aprosam in medical records and registers of activities are gathered only for participants who gave specific consent.
-
(ii)
A2. Capture of medical records of HIV+ FSW patients not participating in the PRINCESSE cohort and routinely examined by Aprosam: Beyond the HIV+ FSWs included in the PRINCESSE cohort, Aprosam proceeds with HIV care services already implemented for other infected FSWs within its community clinic based in San Pedro. To compare the usual HIV care of Aprosam’s clinic and the PRINCESSE cohort HIV care, comparative medical data are collected from medical records of Aprosam’s HIV+ patients, registered as FSWs and not participating in the PRINCESSE cohort. Retrospective data from the 24 months preceding implementation of the PRINCESSE cohort is also collected. Only indicators routinely documented as part of the usual activities of Aprosam are collected anonymously and encrypted.
-
(iii)
A3. In-depth qualitative interviews with key informants in the FSW community: Semi-structured interviews are carried out within the community of FSWs by a qualitative scientist to complete interviews carried out with participants (P4). These interviews are carried out after obtaining informed consent to meet two objectives: (a) to document the reasons why some women refuse to participate in the PRINCESSE cohort by exploring, in particular, relationships between the sex work community and NGOs and social representations of healthcare and caregivers; (b) understand the impacts of PrEP in particular, and of the PRINCESSE cohort in general on the community of FSWs, beyond the participants.
-
(iv)
A4. In-depth qualitative interviews with PRINCESSE follow-up actors (peer educators and caregivers): Semi-structured interviews are carried out with peer educators and caregivers involved in the PRINCESSE project by a qualitative scientist. These interviews aim to document how PRINCESSE follow-up actors appropriate the different tools, and describe the project implementation and eventual modifications from the planned program. Barriers and facilitators to retention in care for participants, and the positive or negative impacts of the PRINCESSE project on the sex work community, in general, are also documented through these interviews.
Each wave of data collection will help to answer one or several specific research objectives (Table 4).
Table 4.
Data collection for each specific research objective
| Specific research objectives | |||||||
|---|---|---|---|---|---|---|---|
| Data collection | SO1 Access to care, retention, and healthcare pathways |
SO2 Clinical, behavioral, and social evolution |
SO3 Initiation, practices, and compliance with PrEP |
SO4 Comparison of HIV care (PRINCESSE vs. current) |
SO5 Prevention and care of hepatitis B | SO6 Unintended consequences of the PRINCESSE intervention |
SO7 Vaginal microbiota, HPV infection types, and antimicrobial STI resistance |
| PRINCESSE cohort | |||||||
| P1. Clinical and safety data | X | X | X | X | X | X | |
| P2. Socio-behavioral questionnaires | X | X | X | X | X | ||
| P3. Biological data | X | X | X | X | X | ||
| P4. In-depth interviews with participants | X | X | X | ||||
| Additional data collection | |||||||
| A1. Medical and activity records of Aprosam (PRINCESSE participants) | X | ||||||
| A2. Medical records of HIV+ FSW patients (not participating in the PRINCESSE cohort) | X | ||||||
| A3. In-depth interviews with members and key informants of the FSW community | X | X | |||||
| A4. In-depth interviews with PRINCESSE follow-up actors | X | X | X | ||||
Data management
Cohort data (P1, P2) are collected by the community counselor, the laboratory technician, and the medical doctor. All paper forms are returned to Aprosam’s headquarters and entered into an electronic clinical database that is managed and hosted by PAC-CI (a French and Ivorian research institute based in Abidjan).
Biological results are returned by the different laboratories to Aprosam or PAC-CI, and then entered into the clinical database using dedicated trial IDs.
Aprosam medical staff can access the clinical database through a secure portal. Each staff member has a secure individual account. The mobile clinic is equipped with computers and Internet access.
From the electronic clinical database, analytical datasets are generated by removing all direct identifiers and by using distinct IDs to avoid direct linkage of analytical datasets with paper forms. All analytical datasets have been declared to CNIL (French National Commission on Informatics and Liberty), and all data procedures are compliant with the European General Data Protection Regulation.
Data monitoring is done by Mereva, PAC-CI’s methodological centre.
Discussion
Based on a pilot qualitative and quantitative survey and cobuilt with community NGOs [5], the PRINCESSE project for FSWs is one of the first interventions offering HIV oral PrEP as part of a more global sexual healthcare package, targeting both HIV-negative and HIV-positive women. A second innovation is the combination of HIV PrEP and viral hepatitis B care. For HIV-negative and HBV-negative women, PrEP is not mandatory to benefit from the other package components (e.g., STI screening and care, family planning). Finally, PRINCESSE is one of the very few programs implementing mobile clinics for chronic/quarterly care.
In terms of research, PRINCESSE is a comprehensive, interdisciplinary project that combines the clinical, biological, epidemiological, and social sciences. This interdisciplinarity is reflected in the diverse objectives and outcomes being investigated. Research questions are not limited to the participants and consider healthcare workers/implementing staff and the local sex work community.
Operational challenges are numerous and linked, among others, to the high mobility of participants through different prostitution sites and in different parts of the country, the motivation of healthcare workers throughout the entire project, the relationship with the sex work community and its perceptions of the program, the logistics of the mobile clinic (road conditions, truck breakdowns), the implementation of electronic medical records, or the adaptation of activities due to health measures linked to the COVID-19 pandemic. Documenting all these logistic and operational aspects allows for the gathering of crucial information for the project’s replication and transferability.
One component of the sexual healthcare package also represents a specific challenge: oral HIV PrEP has been implemented for FSWs in different African countries through several demonstration projects, and adherence to this tool has been relatively low [42–45]. The daily uptake of PrEP is not always adapted to FSWs’ life context. More broadly, the mobility of FSWs and the stigmatization they face from their neighborhood or healthcare professionals complicate their access to care and their retention in demonstration projects [20, 21, 46, 47]. Beyond the development of community support and mobile clinics to limit these difficulties, exploring the suitability of long-acting PrEP to this population could be useful [48].
Finally, the PRINCESSE project aims to understand whether the offer of all sexual healthcare directly at prostitution sites is sufficient to alleviate structural barriers in access to care.
Supplementary Information
Additional file 1: Figure S1. HIV and HBV care algorithm for PRINCESSE participants.
Acknowledgements
The authors thank all the participants and the operational team.
Abbreviations
- CNESVS
Comité National d’Éthique des Sciences de la vie et de la santé de la Côte d’Ivoire
- FSWs
Female sex workers
- HBcAb
hepatitis B core antibody
- HbsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HPV
Human papillomavirus
- NGO
Non-governmental organization
- PrEP
Pre-exposure prophylaxis
- SRH
Sexual and reproductive health
- STIs
Sexually transmitted infections
- TDF/FTC
Tenofovir and emtricitabine
Authors’ contributions
VB, MN, MP, AA, CZ, AM, PAC, SE, and JL designed the study. VB, MN, MP, and JL wrote the first draft of the manuscript. VB, MN, MP, AA, CZ, HD, AJ, AM, PAC, SE, and JL critically reviewed the manuscript and agreed on the version finalized by VB, MN for submission. All authors read and approved the final manuscript.
Funding
This project is funded by the French National Institute for Health and Medical Research-ANRS | Emerging Infectious Diseases (http://www.anrs.fr, grant 12381), which is also the sponsor of the study. Representatives of the ANRS are part of the scientific advisory committee and were involved in study design, as part of the funding award process. However, they have no role in collection, management, analysis, and interpretation of data; writing of the report; and the decision to submit results for publication.
Availability of data and materials
The datasets generated during the current study are not publicly available due to their sensitivity (health data) and the fact that the reidentification of participants by cross-referencing data is possible. Access to pseudonymized analytical datasets is possible from the principal investigators (SE and JL) upon reasonable request and under a confidentiality and partnership agreement. For future publications, ad hoc, fully anonymized datasets will be generated and released into a public repository. At the end of the project, fully anonymized datasets (including precision loss) will be generated for long-term archiving.
Declarations
Ethics approval and consent to participate
The PRINCESSE protocol version 1.0 was submitted in November 2018 to the Comité National d’Ethique des Sciences de la vie et de la santé de la Côte d’Ivoire (CNESVS). The second version, including changes requested by CNESVS, was reviewed and approved on March 4th, 2019 (ref: 152–18/MSHP/CNESVS-km). The third version, introducing in-depth interviews by phone, was approved on March 15th, 2021. The fourth version, adding a biological component (specific objective 7), was approved on May 18th, 2021. These ethics approvals cover all study sites.
The PRINCESSE project is registered on the Clinicaltrial.gov website (NCT03985085).
Several consent forms are collected for the PRINCESSE study:
Individual and written consent is collected among the PRINCESSE participants at their inclusion to be part of the PRINCESSE cohort, to receive the healthcare package, and for the collection of clinical and behavioral data by the trial medical team (P1, P3, A1);
Specific written consent is collected among the PRINCESSE participants for each socio-behavioral questionnaire administered by an independent reviewer (P2);
Specific written consent is collected for each face-to-face in-depth interview and focus group discussion with PRINCESSE participants: (P4) FSWs and key informants who are not participating in the PRINCESSE cohort (A3), and PRINCESSE follow-up actors (A4). Importantly, due to the COVID-19 context, the third version of the protocol includes the possibility of conducting in-depth interviews through phone calls with PRINCESSE participants (P4) and PRINCESSE follow-up actors (A4). In this context, a verbal or written agreement-in-principle is collected by peer educators, and then verbal consent is recorded by the interviewer;
Regarding the medical and activity records of Aprosam (A2): For patients still followed up with by Aprosam, written information is given, and non-opposition is notified in the medical file; for patients no longer followed by Aprosam, a derogation from CNESVS allows for data collection, except if an opposition was previously notified.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Valentine Becquet and Marcellin Nouaman co-first authors.
Serge Eholié and Joseph Larmarange co-last authors.
Contributor Information
Valentine Becquet, Email: valentine.becquet@ined.fr.
Marcellin Nouaman, Email: marcellin.nouaman@pac-ci.org.
Mélanie Plazy, Email: melanie.plazy@u-bordeaux.fr.
Aline Agoua, Email: alineasserayagoua@gmail.com.
Clémence Zébago, Email: zebsonclear@yahoo.fr.
Hervé Dao, Email: zonhoulouherve@gmail.com.
Alice Montoyo, Email: alice.montoyo@gmail.com.
Aude Jary, Email: aude.jary@aphp.fr.
Patrick A. Coffie, Email: patrick.coffie@pac-ci.org
Serge Eholié, Email: sergeholie@yahoo.fr.
Joseph Larmarange, Email: joseph.larmarange@ceped.org.
References
- 1.UNAIDS. UNAIDS Data 2020 [Internet]. Geneva: UNAIDS; 2020 [cité 16 juill 2021]. 436 p. Disponible sur: https://www.unaids.org/sites/default/files/media_asset/2020_aids-data-book_en.pdf
- 2.Bamba A, Grover E, Ezouatchi R, Thiam-Niangoin M, Papworth E, Grosso A, et al. Étude biologique et comportementale des IST/VIH/sida chez les professionnelles du sexe du District d’Abidjan et examen des interventions en direction des populations clés en Côte d’Ivoire. Abidjan: Ministère de la Santé et de la Lutte contre le Sida, Enda Santé, Johns Hopkins University; 2014 nov p. 100.
- 3.Nouaman MN, Becquet V, Masumbuko J-M, Anoma C, Soh K, Plazy M, et al. Évaluation de l’incidence du VIH chez des travailleuses du sexe en Côte d’Ivoire (PrEP-CI ANRS 12361). In AFRAVIH Bordeaux; 2018.
- 4.UNAIDS. AIDS info - Côte d’Ivoire [Internet]. 2019 [cité 22 juin 2022]. Disponible sur: https://aidsinfo.unaids.org/
- 5.Becquet V, Nouaman M, Plazy M, Masumbuko J-M, Anoma C, Kouame S, et al. Sexual health needs of female sex workers in Côte d’Ivoire: a mixed-methods study to prepare the future implementation of pre-exposure prophylaxis (PrEP) for HIV prevention. BMJ Open. 2020;10(1):e028508. doi: 10.1136/bmjopen-2018-028508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shannon K, Strathdee SA, Goldenberg SM, Duff P, Mwangi P, Rusakova M, et al. Global epidemiology of HIV among female sex workers: influence of structural determinants. Lancet. 2015;385(9962):55–71. doi: 10.1016/S0140-6736(14)60931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghimire L, Smith WCS, van Teijlingen ER, Dahal R, Luitel NP. Reasons for non- use of condoms and self- efficacy among female sex workers: a qualitative study in Nepal. BMC Womens Health. 2011;11(42). 10.1186/1472-6874-11-42. [DOI] [PMC free article] [PubMed]
- 8.Lotfi R, Tehrani FR, Yaghmaei F, Hajizadeh E. Barriers to condom use among women at risk of HIV/AIDS: a qualitative study from Iran. BMC Womens Health. 2012;12(1):13. doi: 10.1186/1472-6874-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George G, Nene S, Beckett S, Durevall D, Lindskog A, Govender K. Greater risk for more money: the economics of negotiating condom use amongst sex workers in South Africa. AIDS Care. 2019;31(9):1168–1171. doi: 10.1080/09540121.2018.1563284. [DOI] [PubMed] [Google Scholar]
- 10.Wirtz AL, Schwartz S, Ketende S, Anato S, Nadedjo FD, Ouedraogo HG, et al. Sexual violence, condom negotiation, and condom use in the context of sex work: results from two west African countries. JAIDS J Acquir Immune Defic Syndr. 2015;68:S171. doi: 10.1097/QAI.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 11.Decker MR, McCauley HL, Phuengsamran D, Janyam S, Seage GR, Silverman JG. Violence victimisation, sexual risk and sexually transmitted infection symptoms among female sex workers in Thailand. Sex Transm Infect. 2010;86(3):236–240. doi: 10.1136/sti.2009.037846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohammadi Gharehghani MA, Khosravi B, Irandoost SF, Soofizad G, Yoosefi Lebni J. Barriers to Condom Use Among Female Sex Workers in Tehran, Iran: A Qualitative Study. Int J Womens Health. 2020;12:681–689. doi: 10.2147/IJWH.S260481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Consolidated Guidelines on Hiv Prevention, Diagnosis, Treatment and Care for Key Populations [Internet]. World Health Organization; 2016 [cité 26 sept 2019]. http://proxy.library.carleton.ca/loginurl=, https://www.deslibris.ca/ID/10063272. [PubMed]
- 14.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral Prophylaxis for HIV Prevention in Heterosexual Men and Women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molina J-M, Capitant C, Spire B, Pialoux G, Chidiac C, Charreau I, et al. On demand PrEP with Oral TDF/FTC in MSM results of the ANRS Ipergay trial. Seattle; 2015.
- 16.McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet Lond Engl. 2015. [DOI] [PMC free article] [PubMed]
- 17.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV [Internet]. 2015 [cité 22 juin 2021]. Disponible sur: https://www.who.int/publications/i/item/9789241509565 [PubMed]
- 18.Corneli AL, Deese J, Wang M, Taylor D, Ahmed K, Agot K, et al. FEM-PrEP: Adherence Patterns and Factors Associated With Adherence to a Daily Oral Study Product for Pre-exposure Prophylaxis. J Acquir Immune Defic Syndr 1999. 2014;66(3):324–331. doi: 10.1097/QAI.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mboup A, Béhanzin L, Guédou FA, Geraldo N, Goma-Matsétsé E, Giguère K, et al. Early antiretroviral therapy and daily pre-exposure prophylaxis for HIV prevention among female sex workers in Cotonou, Benin: a prospective observational demonstration study. J Int AIDS Soc. 2018;21(11):e25208. doi: 10.1002/jia2.25208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eakle R, Gomez GB, Naicker N, Bothma R, Mbogua J, Cabrera Escobar MA, et al. HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: results from a prospective observational demonstration project. PLoS Med. 2017;14(11):1–17. doi: 10.1371/journal.pmed.1002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eakle R, Bourne A, Mbogua J, Mutanha N, Rees H. Exploring acceptability of oral PrEP prior to implementation among female sex workers in South Africa. J Int AIDS Soc. 2018;21(2):e25081. [DOI] [PMC free article] [PubMed]
- 23.Larmarange J, Becquet V, Masumbuko J-M, Nouaman M, Plazy M, Danel C, et al. Implementing preexposure prophylaxis among key populations: an opportunity for patient-centered services and management of hepatitis B. AIDS. 2018;32(6):829–830. doi: 10.1097/QAD.0000000000001749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mullick S. Sexually transmitted infections in pregnancy: prevalence, impact on pregnancy outcomes, and approach to treatment in developing countries. Sex Transm Infect. 2005;81(4):294–302. doi: 10.1136/sti.2002.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz S, Papworth E, Thiam-Niangoin M, Abo K, Drame F, Diouf D, et al. An urgent need for integration of family planning services into HIV care: the high burden of unplanned pregnancy, termination of pregnancy, and limited contraception use among female sex workers in Côte d’Ivoire. JAIDS J Acquir Immune Defic Syndr. 2015;68(Supplement 3):S91–S98. doi: 10.1097/QAI.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 26.Baisley K, Changalucha J, Weiss HA, Mugeye K, Everett D, Hambleton I, et al. Bacterial vaginosis in female facility workers in north-western Tanzania: prevalence and risk factors. Sex Transm Infect. 2009;85(5):370–375. doi: 10.1136/sti.2008.035543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garg R, Goyal S, Gupta S. India moves towards menstrual hygiene: subsidized sanitary napkins for rural adolescent girls—issues and challenges. Matern Child Health J. 2012;16(4):767–774. doi: 10.1007/s10995-011-0798-5. [DOI] [PubMed] [Google Scholar]
- 28.Morlat P, Groupe des experts “ Prise en charge médicale des personnes infectées par le VIH ”. Prise en charge médicale des personnes vivant avec le VIH : Recommandations du groupe d’experts, Actualisation 2015, Prophylaxie Pré-Exposition [Internet]. Paris: CNS, ANRS; 2015 p. 18. Disponible sur: http://www.cns.sante.fr/IMG/pdf/experts-vih_prep2015.pdf
- 29.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 30.Seri B, Minga A, Gabillard D, Dembele B, Konate S, Le Carrou J, et al. Twenty-Year Evolution of Hepatitis B Virus and Human Immunodeficiency Virus Prevalence and Incidence in Voluntary Blood Donors in Côte d’Ivoire. Open Forum Infect Dis. 2018;5(4):ofy060. doi: 10.1093/ofid/ofy060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nouaman M, Jacquet A, Tanon A, Coffie PA, Ekouévi DK, Anoma C, et al. Infection à VIH et fibrose hépatique chez des travailleuses du sexe et des hommes ayant des rapports sexuels avec d’autres hommes à Abidjan, Côte d’Ivoire. In AFRAVIH Bruxelles; 2016. Disponible sur: http://www.afravih2016.org/images/Programme/Livre_des_resumes_CO.pdf.
- 32.Ippoliti NB, Nanda G, Wilcher R. Meeting the reproductive health needs of female key populations affected by HIV in low-and middle-income countries: a review of the evidence. Stud Fam Plan. 2017;48(2):121–151. doi: 10.1111/sifp.12020. [DOI] [PubMed] [Google Scholar]
- 33.Wahed T, Alam A, Sultana S, Rahman M, Alam N, Martens M, et al. Barriers to sexual and reproductive healthcare services as experienced by female sex workers and service providers in Dhaka city, Bangladesh. PLoS ONE. 2017;12(7):e0182249. [DOI] [PMC free article] [PubMed]
- 34.Sawicki DA, Meffert BN, Read K, Heinz AJ. Culturally competent health care for sex workers: an examination of myths that stigmatize sex work and hinder access to care. Sex Relatsh Ther. 2019;34(3):355–371. doi: 10.1080/14681994.2019.1574970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas BE, Rehman F, Suryanarayanan D, Josephine K, Dilip M, Dorairaj VS, et al. How stigmatizing is Stigma in the life of people living with HIV: A study on HIV positive individuals from Chennai, South India. AIDS Care. 2005;17(7):795–801. doi: 10.1080/09540120500099936. [DOI] [PubMed] [Google Scholar]
- 36.Beaulieu M, Adrien A, Potvin L, Dassa C. Comité consultatif sur les attitudes envers les PVVIH. Stigmatizing attitudes towards people living with HIV/AIDS: validation of a measurement scale. BMC Public Health. 2014;14(1):1246. doi: 10.1186/1471-2458-14-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vorasane S, Jimba M, Kikuchi K, Yasuoka J, Nanishi K, Durham J, et al. An investigation of stigmatizing attitudes towards people living with HIV/AIDS by doctors and nurses in Vientiane, Lao PDR. BMC Health Serv Res. 2017;17(1):125. doi: 10.1186/s12913-017-2068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sika LG, Kacou ÉA, Ali-Kouadio A, Appiah A. Analyses des connaissances, attitudes et pratiques des professionnel(le)s du sexe dans dix-huit villes de Côte d’Ivoire. Abidjan: Ministère de la Santé et de la Lutte contre le Sida de Côte d’Ivoire, PLS-PHV, PUMLS, Banque Mondiale, ENSEA; 2012 avr p. 241.
- 39.WHO. Guidelines for the Prevention, Care and Treatment of Persons with chronic Hepatitis B Infection. Geneva: World Health Organisation; 2015 mars p. 166. [PubMed]
- 40.PNLS. Directives 2015 de prise en charge des personnes vivant avec le VIH en Côte d’Ivoire. Abidjan: Programme National de Lutte contre le Sida; 2015 p. 32.
- 41.WHO. WHO Implementation tool for pre-exposure prophylaxis (PrEP) of HIV infection. Module 1: Clinical. Geneva: World Health Organisation; 2017 p. 28. Report No.: WHO/HIV/2017.17.
- 42.Mboup A, Béhanzin L, Guédou F, Giguère K, Geraldo N, Zannou DM, et al. Comparison of adherence measurement tools used in a pre-exposure prophylaxis demonstration study among female sex workers in Benin. Medicine (Baltimore). 2020;99(21) Disponible sur: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7249870/. [cité 19 mai 2021]. [DOI] [PMC free article] [PubMed]
- 43.Mboup A, Diabaté S, Béhanzin L, Guédou FA, Zannou DM, Kêkê RK, et al. Determinants of HIV pre-exposure prophylaxis (PrEP) adherence among female sex workers in a demonstration study in Cotonou, Benin: a study of behavioral and demographic factors. Sex Transm Dis [Internet]. 23 avr 2021 [cité 27 avr 2021];publish ahead of print. Disponible sur: https://journals.lww.com/stdjournal/Abstract/9000/Determinants_of_HIV_pre_exposure_prophylaxis.97774.aspx [DOI] [PMC free article] [PubMed]
- 44.Fearon E, Phillips A, Mtetwa S, Chabata ST, Mushati P, Cambiano V, et al. How Can Programs Better Support Female Sex Workers to Avoid HIV Infection in Zimbabwe? A Prevention Cascade Analysis. J Acquir Immune Defic Syndr 1999. 2019;81(1):24–35. doi: 10.1097/QAI.0000000000001980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kagaayi J, Batte J, Nakawooya H, Kigozi B, Nakigozi G, Strömdahl S, et al. Uptake and retention on HIV pre-exposure prophylaxis among key and priority populations in south-Central Uganda. J Int AIDS Soc. 2020;23(8):e25588. doi: 10.1002/jia2.25588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vuylsteke B, Semdé G, Auld AF, Sabatier J, Kouakou J, Ettiègne-Traoré V, et al. Retention and risk factors for loss to follow-up of female and male sex workers on antiretroviral treatment in Ivory Coast: a retrospective cohort analysis. JAIDS J Acquir Immune Defic Syndr. 2015;68:S99–106. doi: 10.1097/QAI.0000000000000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarr M, Gueye D, Mboup A, Diouf O, Bao MDB, Ndiaye AJ, et al. Uptake, retention, and outcomes in a demonstration project of pre-exposure prophylaxis among female sex workers in public health centers in Senegal. Int J STD AIDS. 2020;31(11):1063–1072. doi: 10.1177/0956462420943704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brook MK, Ismail A, Magni S, Fellows T, Katahoire AR, Ayebare F, et al. User assessment of a microarray patch for HIV PrEP and as a multipurpose prevention technology for HIV and pregnancy prevention: perspectives from Uganda and South Africa. J Int AIDS Soc. 2021;24(S1):10–12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. HIV and HBV care algorithm for PRINCESSE participants.
Data Availability Statement
The datasets generated during the current study are not publicly available due to their sensitivity (health data) and the fact that the reidentification of participants by cross-referencing data is possible. Access to pseudonymized analytical datasets is possible from the principal investigators (SE and JL) upon reasonable request and under a confidentiality and partnership agreement. For future publications, ad hoc, fully anonymized datasets will be generated and released into a public repository. At the end of the project, fully anonymized datasets (including precision loss) will be generated for long-term archiving.