Key Words: anthropometrics, body composition, dual-energy-x-ray absorptiometry, exercise, physical activity, quality of life, spinal cord injury
Abstract
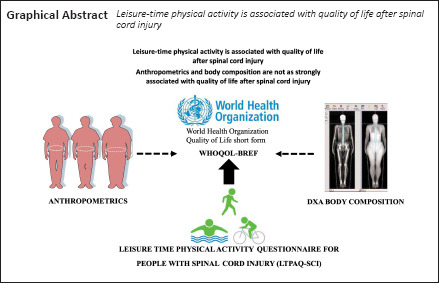
The objective of the current work was to examine the relationships between quality of life (QOL) domains in persons with spinal cord injury (SCI) and their levels of weekly leisure-time physical activity (LTPA), anthropometric variables, and body composition variables. This exploratory cross-sectional study consisted of baseline data collected as part of a randomized clinical trial at a VA Medical Center and SCI center. A convenience sample of 36 community-dwelling persons with SCI participated in the current study. Outcome measures included the World Health Organization Quality of Life Short Form (WHOQOL-BREF), Leisure-Time Physical Activity Questionnaire for People with Spinal Cord Injury (LTPAQ-SCI), anthropomorphic measures (waist, hip, and abdominal circumference), and dual-energy x-ray absorptiometry (DXA) to quantify regional and total body composition. Multiple regression models suggested that engagement in LTPA accounted for 35.7% of the variance in physical health QOL, 33.5% in psychological QOL, 14.2% in social relationships QOL, and 38.2% in environmental QOL. Anthropometric measures accounted for 11.3%, 3.1%, 12.0%, and 6.7% of the variance in these QOL indices, respectively, and DXA indices accounted for 18.7%, 17.5%, 27.4%, and 21.9%. Within these models, the number of minutes of heavy LTPA per day uniquely predicted physical health QOL, the number of mild LTPA days per week uniquely predicted psychological QOL, and the amount of mild LTPA per day uniquely predicted environmental QOL. Bivariate analyses also suggested that android and trunk fat, as well as supine waist and abdominal circumferences, were positively associated with social relationships QOL. Encouraging individuals with SCI to engage in LTPA may robustly enhance multiple aspects of QOL while reducing the risk for cardiovascular and metabolic morbidities associated with SCI. Moreover, this may lead to a further understanding of how QOL may impact longitudinal intervention trials. The study protocol and procedures were reviewed and approved by the McGuire VA Research Institutional Review Board (IRB# 02152, approval date August 9, 2015; IRB# 02375, approval date May 2, 2018).
Chinese Library Classification No. R494; R364; R741
Introduction
Individuals with spinal cord injury (SCI) experience reduced mobility and physical activity and increased likelihood of several medical comorbidities (Maitland Schladen and Groah, 2014; Smith and Yarar-Fisher, 2016). Engagement in leisure-time physical activity (LTPA) has emerged as a promising set of behaviors that not only reduces the risk for cardiovascular diseases but also improves subjective well-being (Gorgey et al., 2014), satisfaction with social relationships (Smith and Yarar-Fisher, 2016), functional independence (Kawanishi and Greguol, 2013), community reintegration (Ginis et al., 2003), and quality of life (QOL) (Gillison et al., 2009). Individuals with SCI are advised to engage in habitual LTPA in addition to their usual activities of daily living (ADL) because many ADLs are performed at insufficient intensities or durations to achieve health benefits. LTPA is any physical activity that individuals choose to do in their spare time, including aerobic and strength training, playing sports, and hobbies (Godin and Shephard, 1985; Martin Ginis et al., 2012). Physical inactivity has been associated with increased psychological and physical issues, including respiratory dysfunction, depression, chronic pain, and fatigue (Rintala et al., 1998; Anderson et al., 2007; Noce et al., 2009; Anneken et al., 2010). Conversely, LTPA is associated with lower chronic disease risk in adults with SCI (Buchholz et al., 2009; Hetz et al., 2009). In persons with tetraplegia, physically active participants (minimum of two and a half hours per week of exercise, three times a week or more for at least three months) have lower total body fat mass, which is associated with improvements in fasting plasma insulin and homeostatic model assessment (HOMA) index (D’Oliveira et al., 2014).
To accurately prescribe LTPA, the American College of Sports Medicine (ACSM) guidelines were extracted to determine appropriate physical activity levels in persons with SCI (Garber et al., 2011). However, the ACSM guidelines may misclassify those with SCI as being inactive. Recent guidelines have suggested that 30 minutes of moderate-to-vigorous intensity three times per week may maintain health and wellness after SCI (Ginis et al., 2018). At present, it is still unclear whether this recommended dose of exercise is adequate to improve QOL after SCI. Given its positive emotional and social effects, researchers have further examined relationships between LTPA intensity, frequency, duration, and QOL (Whiteneck et al., 1992). However, it is worth noting that several barriers may hinder participation in routine physical activity, especially in persons with SCI. These barriers are likely to impact the primary domains of QOL (Gorgey, 2014).
Across studies, the definition of QOL varies dramatically but has been described by the Centers for Disease Control as “a broad multidimensional concept that includes subjective evaluations of both positive and negative aspects of life,” as well as physical and emotional determinants of health (Control and Prevention, 2005). Extensive work has been done to clearly define QOL and elucidate predictors of QOL in various populations. Dijkers (2003) differentiated between objective and subjective QOL. Objective QOL is conceptualized as achievements, while subjective QOL is conceptualized as feelings about how achievements align with expectations (Dijkers, 2003). Therefore, it is important to assess multiple domains of QOL and their associations with varying levels of LTPA in persons with SCI. In a review of 11 studies, strong positive associations were found between engagement in LTPA and specific aspects of QOL, such as improved life satisfaction, greater functional independence, and greater perception of overall health (Kawanishi and Greguol, 2013). The authors noted that the weekly frequency, duration, and type of physical activity led to varied outcomes (Kawanishi and Greguol, 2013). Moreover, there are critical differences in the effects of exercise frequency on reported QOL, with individuals achieving at least four hours of weekly exercise reporting greater QOL than those completing 30 minutes (Kawanishi and Greguol, 2013). Beyond frequency, a pattern exists between the intensity of training and perceived QOL. Those who reported engagement in higher intensity exercises experienced reduced chronic pain and fatigue, which may have also accounted for greater ratings of QOL (Hicks et al., 2003; Luchauer and Shurtleff, 2015). Overall, engagement in physical activity has positive impacts on aspects of QOL (Ginis et al., 2003).
Previous studies in community-dwelling adults have demonstrated an inverse relationship between body mass index (BMI), waist circumference (So, 2014; Wu et al., 2014) and ratings of QOL (WHOQOL, 1994; Jang et al., 2004). This may suggest that parameters of body composition may serve as predictors of QOL after SCI. However, we are unaware of any studies that have conducted a detailed assessment of body composition using sophisticated imaging techniques and established associations with the domains of QOL. Determining this association will provide an understanding of how QOL may impact longitudinal intervention trials. The present study’s objective was to identify the associations among different levels of LTPA, anthropometric variables, body composition variables, and SCI-related QOL. Based on the aforementioned evidence, we hypothesized that LTPA and body composition parameters might serve as positive predictors for domains of QOL after SCI.
Participants and Methods
Participants
A convenience sample of 36 individuals with SCI were recruited as part of two separate clinical trials conducted at the McGuire VA Research Institute and registered with ClinicalTrials.gov (Gorgey et al., 2017, 2019). Thirty-four participants (NCT02660073) and two participants (NCT03410550) were used for analyses for the current study. Participant recruitment and data collection occurred from 2016 to 2020. Sample characteristics are described in Table 1.
Table 1.
Physical and SCI characteristics of the participants enrolled in the current study
| Variable | n (%) or mean±SD |
|---|---|
| Sex | |
| Male | 29 (80.6) |
| Female | 7 (19.4) |
| Race/ethnicity | |
| White/European-American | 21 (58.3) |
| Black/African-American | 15 (41.7) |
| Injury classification | |
| Paraplegia | 23 (63.9) |
| Tetraplegia | 13 (36.1) |
| Level of Injury | |
| Cervical | 14 (38.9) |
| Thoracic | 21 (58.3) |
| Lumbar | 1 (2.8) |
| AIS Impairment Scale Classification | |
| A | 21 (58.3) |
| B | 9 (25.0) |
| C | 6 (16.7) |
| Age (yr) | 40.00±13.00 |
| Weight (kg) | 70.45±14.95 |
| Height (cm) | 174.25±9.10 |
| Body mass index (kg/m2) | 23.4±5.5 |
| Time since injury (yr) | 10.8±10.23 |
AIS: ASIA (American Spinal Injury Association) Impairment Scale.
Participants in this study met the following inclusion criteria: the ability to understand the informed consent process, age at the time of recruitment was between 18 and 65 years, at least 1-year post-onset of injury, and diagnosed with traumatic motor complete and incomplete SCI (C5-L1). Participants were excluded if they experienced cardiovascular disease, type II diabetes, acute urinary tract infections, and pressure ulcers of stage 2 or greater.
Procedure
Prior to enrollment in the study, the protocol and procedures were reviewed and approved by the McGuire VA Research Institutional Review Board (IRB# 02152, approval date August 9, 2015; IRB# 02375, approval date May 2, 2018) (Additional file 1 (408.9KB, pdf) ) and conducted in accordance with the Declaration of Helsinki. This study was reported according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement (Additional file 2). All participants provided written consent prior to study enrollment and data collection (Additional file 3 (4.1MB, pdf) ). As part of baseline data collection for the clinical trials, participants were administered self-report questionnaires by a trained study coordinator who read the questions aloud and provided patients with copies of the forms. The coordinator recorded the participants’ responses to each questionnaire to facilitate consistent administration across participants, both with and without upper extremity impairments. Participants underwent anthropometric measurements to capture weight (kg), height (m), body mass index (BMI), waist, hip, abdominal, and thigh circumferences (cm). Dual-energy-x-ray absorptiometry (DXA) scanning was performed to measure fat and lean masses (g) of whole body and regional compartments. The DXA short and long-term precision of repeated measures for regional and whole-body composition was recently reported in persons with SCI (Gorgey et al., 2018).
STROBE Statement-Checklist of items that should be included in reports of cross-sectional studies
| Item No | Recommendation | |
|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract |
|
| ||
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found (Page 1-2) | ||
|
| ||
| Introduction | ||
|
| ||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported (Page 2-5) |
|
| ||
| Objectives | 3 | State specific objectives, including any prespecified hypotheses (Page 5) |
|
| ||
| Methods | ||
|
| ||
| Study design | 4 | Present key elements of study design early in the paper (Page 5-6) |
|
| ||
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection (Page 5-6) |
|
| ||
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants (Page 5-6) |
|
| ||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable (Page 6-8) |
|
| ||
| Data sources/ measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group (Page 6-8) |
|
| ||
| Bias | 9 | Describe any efforts to address potential sources of bias (Page 8-9) |
|
| ||
| Study size | 10 | Explain how the study size was arrived at (Page 5) |
|
| ||
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why (Page 8-9) |
|
| ||
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding (Page 8-9) |
|
| ||
| (b) Describe any methods used to examine subgroups and interactions (Page 8-9) | ||
|
| ||
| (c) Explain how missing data were addressed (Page 8-9) | ||
|
| ||
| (d) If applicable, describe analytical methods taking account of sampling strategy NA | ||
|
| ||
| (e) Describe any sensitivity analyses NA | ||
|
| ||
| Results | ||
|
| ||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed (Page 5, 9) |
|
| ||
| (b) Give reasons for non-participation at each stage NA | ||
|
| ||
| (c) Consider use of a flow diagram NA | ||
|
| ||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders (Page 5, 9, and Table 1 on Page 18) |
|
| ||
| (b) Indicate number of participants with missing data for each variable of interest (Page 10) | ||
|
| ||
| Outcome data | 15* | Report numbers of outcome events or summary measures (Page 9-11 and Tables on pages 19-23) |
|
| ||
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included (Page 9-11 and Tables on pages 19-23) |
|
| ||
| (a) Report category boundaries when continuous variables were categorized (Page 78) | ||
|
| ||
| (b) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period NA | ||
|
| ||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses (Page 8-9) |
|
| ||
| Discussion | ||
|
| ||
| Key results | 18 | Summarise key results with reference to study objectives (Page 9-11 and Tables on pages 18-23) |
|
| ||
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias (Page 13-14) |
|
| ||
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence (Page 11-14) |
|
| ||
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results (Page 14) |
|
| ||
| Other information | ||
|
| ||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based (Page 14-15) |
*Give information separately for exposed and unexposed groups.
Note: An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at www.strobe-statement.org.
Measures
Participants responded to questionnaires that assessed demographics, injury characteristics, QOL, and participation in leisure-time physical activity one week before study participation. The specific measures and constructs assessed are outlined below.
Demographics
Participants provided information about their current age (years), gender, racial/ethnic identity, and time since injury (years). Injury diagnosis (level and AIS classification) was performed during their physical examination by a trained SCI physician (Kirshblum et al., 2011).
QOL
QOL was assessed using the World Health Organization Quality of Life short form (WHOQOL-BREF) (WHOQOL, 1994), a 26-item questionnaire that assesses four domains of QOL: physical health, psychological health, social relationships, and environmental engagement (WHOQOL, 1994; Jang et al., 2004). Psychometric properties for this measure, when administered among individuals with SCI, demonstrate strong adequate consistency by domain (α = 0.54–0.78) and good discriminant and construct validity across domains (Jang et al., 2004). The items assess the frequency of experiencing different aspects of QOL during the past two weeks. Responses to the items were reverse coded when appropriate and then summed into the four domain scores as recommended by the WHOQOL Group (WHOQOL, 1994; Harper and Orley, 1996). Domain item averages were multiplied by 4 (per the scoring manual) and used for statistical analyses to improve predictive accuracy. Domain averages of the full sample were transformed to a 0-100 scale that is comparable with the WHOQOL-100 and used for making generalizations about the sample.
Physical activity
Participants completed the Leisure Time Physical Activity Questionnaire for People with Spinal Cord Injury (LTPAQ-SCI) (Martin Ginis et al., 2012). The LTPAQ-SCI is a self-report measure that assesses physical activity engagement during the past week. Participants reported the number of minutes they engaged in mild, moderate, and heavy-intensity activity and the number of days they engaged in mild, moderate, and heavy-intensity physical activity. Each of these indices of LTPA was used as a predictor in the current study. The LTPAQ-SCI demonstrates adequate test-retest reliability (intercorrelation coefficients range from 0.62-0.83) and construct validity (Martin Ginis et al., 2012).
Anthropomorphic data
Using previously established methods (Gorgey et al., 2016), the following anthropomorphic and body composition variables were obtained: weight (kg), BMI, waist and abdominal circumferences (cm), as well as the percentage of body fat, fat mass, and lean mass (g).
Statistical analyses
All analyses were conducted using SPSS 26.0 (IBM Corp., Released 2019; IBM SPSS Statistics for Windows; Armonk, NY, USA). Descriptive statistics were used to assess means and standard deviations of demographic variables, predictor variables, and QOL outcome variables, and frequencies were used to identify percentages of categorical variables in the sample. Tables 1 and 2 provide a summary of demographics, as well as predictor and outcome variables used in the analyses.
Table 2.
Descriptive statistics of predictors (anthropometrics, body composition parameters, and LTPA) and domains of QOL
| Variable category | Variable | Mean ± SD | Range | |
|---|---|---|---|---|
|
| ||||
| Min | Max | |||
| Anthropometrics | Sup waist Cir. (cm) | 82.5±13.9 | 59.80 | 110.20 |
| Sup Ab Cir. (cm) | 87.98±16.0 | 61.70 | 118.70 | |
| Supine hip Cir. (cm) | 90.10±14.15 | 57.90 | 118.90 | |
| DXA | Legs fat (g) | 6987.81±3397.33 | 2508.00 | 15563.00 |
| Legs lean (g) | 13104.83±2601.03 | 7974.00 | 18877.00 | |
| Trunk fat (g) | 12205.17±7506.79 | 1945.00 | 28288.00 | |
| Trunk lean (g) | 21630.47±3321.44 | 15382.00 | 27129.00 | |
| Android fat (g) | 2031.81±1474.40 | 216.00 | 5255.00 | |
| Android lean (g) | 3146.92±589.28 | 1767.00 | 4318.00 | |
| LTPA | Mild (d/wk) | 3.97±2.47 | 0 | 7.00 |
| Mild (min/d) | 109.36±114.88 | 0 | 480.00 | |
| Mod (d/wk) | 3.33±2.52 | 0 | 7.00 | |
| Mod (min/d) | 64.58±89.94 | 0 | 480.00 | |
| Heavy (d/wk) | 1.78±2.42 | 0 | 7.00 | |
| Heavy (min/d) | 39.86±52.16 | 0 | 180.00 | |
| QOL | Physical health | 14.63±2.81 | 9.14 | 18.86 |
| Psychological | 15.76±2.99 | 8.00 | 20.00 | |
| Social relationships | 13.78±3.23 | 4.00 | 18.67 | |
| Environment | 15.92±2.39 | 10.50 | 20.00 | |
Data are expressed as the mean ± SD. Ab: Abdominal; Cir: circumference; DXA: dual-energy x-ray absorptiometry; LTPA: leisure-time physical activity; Mod: moderate; QOL: quality of life; Sup: supine. QOL means the domain item average multiplied by 4.
Twelve simultaneous multiple regressions were run based on a 3 × 4 matrix of three categories of predictors (LTPA, anthropometrics, and DXA) and four separate WHOQOL-BREF QOL outcomes (physical health, psychological, social relationships, and environment). The six LTPA predictors included the number of minutes of mild, moderate, and heavy exercise per day, as well as the number of days of mild, moderate, or heavy exercise per week. The three anthropometric predictors included supine waist, hip, and abdominal circumference (cm). The six DXA predictors included legs, trunk, and android fat and lean mass (g). Because of the limited sample size of 36 participants, the effect size was emphasized over traditional indices of statistical significance (e.g., P < 0.05). Additionally, because bivariate effects may have been obscured due to multicollinearity among predictors, a nonparametric Spearman’s rho correlation matrix was used to examine the associations between the three sets of predictors and the four QOL outcomes. Partial correlations were also used to examine the relationships between LTPA, body composition, anthropometrics, and QOL while controlling for participants’ age and time Table 3.
Table 3.
Partial correlation matrix examining the primary predictors of the study (LTPA, anthropometrics, and body composition parameters) and domains of quality of life using age and TSI as control variables
| Variable | Physical health | Psychological | ||
|---|---|---|---|---|
| Control variable | Age | TSI | Age | TSI |
| LTPA | ||||
| Mild (d/wk) | 0.27 | 0.27 | 0.49** | 0.48** |
| Mild (min/d) | 0.38* | 0.38* | 0.29 | 0.26 |
| Mod (d/wk) | 0.09 | 0.09 | 0.22 | 0.22 |
| Mod (min/d) | 0.25 | 0.26 | 0.33 | 0.28 |
| Heavy (d/wk) | 0.12 | 0.12 | 0.07 | 0.06 |
| Heavy (min/d) | 0.45 | 0.45** | 0.06 | 0.03 |
| DXA | ||||
| Legs fat (g) | 0.17 | 0.14 | –0.03 | –0.02 |
| Legs lean (g) | 0.26 | 0.25 | 0.13 | 0.16 |
| Trunk fat (g) | 0.08 | 0.03 | –0.02 | –0.02 |
| Trunk lean (g) | –0.02 | –0.03 | –0.05 | –0.04 |
| Andro fat (g) | 0.13 | 0.07 | 0.04 | 0.02 |
| Andro lean (g) | –0.02 | –0.04 | –0.03 | –0.04 |
| Anthro | ||||
| Sup waist Cir. (cm) | 0.06 | 0.002 | –0.06 | –0.04 |
| Sup hip Cir. (cm) | –0.12 | –0.15 | –0.12 | –0.11 |
| Sup Ab Cir. (cm) | 0.25 | 0.17 | –0.05 | –0.04 |
|
| ||||
| Variable | Social relationships | Environment | ||
|
| ||||
| Control variable | Age | TSI | Age | TSI |
| LTPA | ||||
| Mild (d/wk) | 0.11 | 0.09 | 0.21 | 0.22 |
| Mild (min/d) | 0.1 | 0.05 | 0.43* | 0.39* |
| Mod (d/wk) | 0.03 | 0.03 | –0.18 | –0.18 |
| Mod (min/d) | 0.26 | 0.21 | 0.29 | 0.23 |
| Heavy (d/wk) | 0.22 | 0.22 | 0.02 | –0.02 |
| Heavy (min/d) | 0.02 | –0.02 | 0.13 | 0.14 |
| DXA | ||||
| Legs fat (g) | 0.19 | 0.23 | 0.23 | 0.11 |
| Legs lean (g) | 0.06 | 0.09 | 0.18 | 0.15 |
| Trunk fat (g) | 0.29 | 0.32 | 0.07 | –0.12 |
| Trunk lean (g) | –0.07 | –0.04 | –0.16 | –0.19 |
| Andro fat (g) | 0.35* | 0.36* | 0.14 | –0.07 |
| Andro lean (g) | 0.06 | 0.08 | –0.09 | –0.15 |
| Anthro | ||||
| Sup waist Cir. (cm) | 0.27 | 0.31 | 0.03 | –0.15 |
| Sup hip Cir. (cm) | 0.21 | 0.24 | –0.04 | –0.16 |
| Sup Ab Cir. (cm) | 0.17 | 0.22 | 0.15 | –0.03 |
*P < 0.05, **P < 0.01. Ab: Abdominal; Andro: android; Anthro: anthropometric; Cir: circumference; DXA: dual-energy X-ray absorptiometry; LTPA: leisure-time physical activity; Mod: moderate; Sup: supine; TSI: time since injury.
Results
Sample characteristics
Demographic information is described in Table 1. Participants were primarily male with time since injury of approximately 11 years and predominately diagnosed with paraplegia at the thoracic level. Table 2 presents the means and standard deviations of the anthropometrics, body composition parameters, frequency and volume of LTPA, and the four domains of QOL. On average, participants reported at least four days of some level of exercise intensity and often reported over 30 minutes of exercise during these days. However, four participants reportedly did not engage in any form of LTPA. Participants’ QOL domain scores transformed (out of 100) were as follows: physical health (69), psychological (75), social relationships (62), and environmental (75), suggesting positive QOL across the various domains.
Regression analysis between predictor variables and domains of QOL
The results of all 12 multiple regressions appear in Tables 4 and 5. The four LTPA multiple regression models suggested that engagement in LTPA accounted for 35.7% of the variance in physical health QOL, 33.5% in psychological QOL, 14.2% in social relationships QOL, and 38.2% in environmental QOL, although only the physical health and environmental QOL regressions were statistically significant (Table 4).
Table 4.
Multiple regression analyses with the study’s primary predictors (LTPA, anthropometrics, and body composition parameters) and domains of QOL
| Predictor variable | Physical health | Psychological | Social relationships | Environment |
|---|---|---|---|---|
| Predictors: LTPA | ||||
| Mild (d/wk) | 0.20 | 0.47* | 0.08 | 0.36 |
| Mild (min/d) | 0.26 | 0.14 | 0.02 | 0.35* |
| Mod (d/wk) | –0.24 | –0.07 | –0.05 | –0.52* |
| Mod (min/d) | 0.11 | 0.29 | 0.25 | 0.19 |
| Heavy (d/wk) | –0.26 | –0.07 | 0.26 | –0.24 |
| Heavy (min/d) | 0.51** | –0.16 | –0.22 | 0.21 |
| Model R2 | 0.36* | 0.34 | 0.14 | 0.38* |
| Predictors: Anthropometrics | ||||
| Supine waist Cir. (cm) | –0.71 | 0.65 | 0.73 | –0.88 |
| Supine hip Cir. (cm) | 0.08 | –0.42 | –0.16 | 0.26 |
| Supine Ab Cir. (cm) | 0.74 | –0.39 | –0.32 | 0.63 |
| Model R2 | 0.11 | 0.03 | 0.12 | 0.07 |
| Predictors: DXA | ||||
| Legs fat (g) | 0.12 | –0.18 | –0.17 | 0.19 |
| Legs lean (g) | 0.37 | 0.19 | 0.05 | 0.32 |
| Trunk fat (g) | –1.72 | –2.45 | –1.56 | –2.02 |
| Trunk lean (g) | 0.29 | 0.09 | –0.35 | –0.075 |
| Android fat (g) | 1.78 | 2.72 | 2.06 | 1.87 |
| Android lean (g) | –0.57 | –0.28 | 0.21 | –0.23 |
| Model R2 | 0.19 | 0.18 | 0.27 | 0.22 |
*P < 0.05, **P < 0.01. All regression weights are standardized betas. Ab: Abdominal; Cir: circumference; LTPA: leisure-time physical activity; Mod: moderate; QOL: quality of life.
Table 5.
Multiple regression analyses with the study’s primary predictors (LTPA, anthropometrics, and body composition parameters) and modules of quality of life
| Predictor variable | Physical health | Psychological | Social relationships | Environment | ||||
|---|---|---|---|---|---|---|---|---|
| Mild (d/wk) | 0.233 | –0.230 to 0.696 | 0.568* | 0.067 to 1.069 | 0.099 | –0.515 to 0.714 | 0.347 | –0.039 to 0.733 |
| Mild (min/d) | 0.006 | –0.002 to 0.015 | 0.004 | –0.006 to 0.013 | 0.000 | –0.001 to 0.012 | 0.007* | 0.000 to 0.014 |
| Mod (d/wk) | –0.265 | –0.715 to 0.186 | –0.087 | –0.574 to 0.401 | –0.065 | –0.633 to 0.534 | –0.496* | –0.871 to –0.120 |
| Mod (min/d) | 0.004 | –0.007 to 0.14 | 0.010 | –0.002 to 0.021 | 0.009 | –0.005 to 0.023 | 0.005 | –0.004 to 0.014 |
| Heavy (d/wk) | –0.306 | –0.750 to 0.138 | –0.087 | –0.567 to 0.394 | 0.342 | –0.247 to 0.932 | –0.233 | –0.603 to 0.137 |
| Heavy (min/d) | 0.028** | 0.007 to 0.048 | –0.009 | –0.031 to 0.013 | –0.014 | –0.041 to 0.013 | 0.010 | –0.007 to 0.027 |
| Adjusted R2 | 0.224* | 0.197 | –0.036 | 0.254* | ||||
| Sup waist Cir. (cm) | –0.136 | –0.428 to 0.156 | 0.137 | –0.199 to 0.473 | 0.145 | –0.157 to 0.447 | –0.141 | –0.393 to 0.111 |
| Sup hip Cir. (cm) | 0.016 | –0.149 to 0.181 | –0.089 | –0.279 to 0.100 | –0.033 | –0.203 to 0.138 | 0.042 | –0.100 to 0.184 |
| Sup Ab Cir. (cm) | 0.123 | –0.057 to 0.303 | –0.072 | –0.279 to 0.134 | –0.055 | –0.241 to 0.131 | 0.087 | –0.068 to 0.243 |
| Adjusted R2 | 0.021 | –0.069 | 0.029 | –0.029 | ||||
| Leg fat (g) | 9.66E-05 | 0.000 to 0.001 | 0 | –0.001 to 0.000 | 0.000 | –0.001 to 0.000 | 0.000 | 0.000 to 0.001 |
| Leg lean (g) | 0 | 0.000 to 0.001 | 0 | 0.000 to 0.001 | 5.71E-05 | –0.001 to 0.001 | 0.000 | 0.000 to 0.001 |
| Trunk fat (g) | –0.001 | –0.002 to 0.001 | –0.001 | –0.002 to 0.000 | –0.001 | –0.002 to 0.001 | –0.001 | –0.002 to 0.000 |
| Trunk lean (g) | 0 | –0.001 to 0.001 | 8.301E-5 | –0.001 to 0.001 | 0.000 | –0.001 to 0.001 | –5.367E-5 | –0.001 to 0.001 |
| Android fat (g) | 0.003 | –0.002 to 0.009 | 0.006 | –0.001 to 0.012 | 0.005 | –0.002 to 0.011 | 0.003 | –0.002 to 0.008 |
| Android lean (g) | –0.003 | –0.008 to 0.002 | –0.001 | –0.007 to 0.004 | 0.001 | –0.004 to 0.006 | –0.001 | –0.005 to 0.003 |
| Adjusted R2 | 0.019 | 0.004 | 0.124 | 0.058 | ||||
*P < 0.05, **P < 0.01. All regression weights are b weights with 95% confidence intervals. Ab: Abdominal; Cir: circumference; Mod: moderate; Sup: supine.
Within these models, the number of minutes of heavy LTPA per day uniquely predicted physical health QOL, the number of mild LTPA days per week uniquely predicted psychological QOL, and the number of minutes of mild LTPA per day uniquely predicted environmental QOL.
The four anthropometric regression models accounted for 11.3% of the variance in physical health QOL, 3.1% in psychological QOL, 12.0% in social relationships QOL, and 6.7% in environmental QOL. However, none of the regressions were statistically significant, nor were any unique predictors within these models.
The four DXA regression models accounted for 18.7% of the variance in physical health QOL, 17.5% in psychological QOL, 27.4% in social relationships QOL, and 21.9% in environmental QOL, although again, none of the regressions were statistically significant. Neither were any unique predictors within these models. There were no significant differences detected based on the level (cervical or thoracic) or completeness (AIS grade) of injury (data not presented).
Relationships between predictor variables and domains of QOL
The correlation matrix (Tables 3 and 6) supported the same pattern of relationships as the multiple regression models. However, only in a bivariate sense was the number of minutes per day of mild LTPA positively associated with physical health QOL. Additionally, android fat (g), trunk fat (g), and waist and abdominal circumferences (cm) were positively associated with social relationships QOL. A nonparametric Spearman Rank test was used instead of a traditional Pearson’s correlation matrix because of possible non-parametric issues given that some of our variables were interval (e.g., QOL scale scores) and due to the small sample size.
Table 6.
Spearman’s rho correlation matrix between the study’s primary predictors (LTPA, anthropometrics, and body composition parameters) and domains of QOL
| Variable | Physical health | Psychological | Social relationships | Environment |
|---|---|---|---|---|
| LTPA | ||||
| Mild (d/wk) | 0.28 | 0.45** | 0.1 | 0.25 |
| Mild (min/d) | 0.37* | 0.25 | 0.11 | 0.36* |
| Mod (d/wk) | 0.17 | 0.23 | 0.02 | –0.13 |
| Mod (min/d) | 0.23 | 0.32 | 0.19 | 0.20 |
| Heavy (d/wk) | 0.29 | 0.18 | 0.26 | 0.08 |
| Heavy (min/d) | 0.49** | 0.18 | 0.06 | 0.13 |
| DXA | ||||
| Legs fat (g) | 0.05 | –0.1 | 0.26 | 0.06 |
| Legs lean (g) | 0.17 | 0.08 | 0.02 | 0.10 |
| Trunk fat (g) | –0.04 | –0.03 | 0.36* | –0.04 |
| Trunk lean (g) | –0.08 | –0.04 | –0.05 | –0.19 |
| Android fat (g) | –0.02 | –0.007 | 0.39* | –0.02 |
| Android lean (g) | –0.06 | –0.02 | 0.05 | –0.11 |
| Anthro | ||||
| Sup waist Cir. (cm) | –0.04 | –0.07 | 0.36* | –0.08 |
| Sup hip Cir. (cm) | –0.12 | –0.12 | 0.24 | 0 |
| Sup Ab Cir. (cm) | 0.17 | 0.02 | 0.35* | 0.04 |
*P < 0.05, **P < 0.01. Ab: Abdominal; Anthro: anthropometric; Cir: circumference; DXA: dual-energy x-ray absorptiometry; LTPA: leisure-time physical activity; Mod: moderate; Sup: supine.
Discussion
This study aimed to identify the relationships of different levels of LTPA, anthropometric variables, and body composition variables with SCI-related QOL. LTPA mostly accounted for a large amount of variance in aspects of QOL in individuals with SCI, anthropometric variables accounted for a small-to-moderate amount of variance, and body composition variables accounted for a moderate-to-large amount of variance.
Regarding LTPA and QOL, the current findings are congruent with previous studies suggesting that increased frequency and intensity of physical exercise can lead to psychological and health benefits among individuals with SCI (Anneken et al., 2010; Tomasone et al., 2013; Crane et al., 2017). The results also suggest that different patterns of physical activity may affect specific but not all aspects of QOL. For example, the number of minutes of heavy LTPA per day uniquely predicted physical health QOL, perhaps suggesting that LTPA intensity may be the most beneficial for physical health in people with SCI. Conversely, the number of minutes and the number of days engaged in mild LTPA uniquely predicted environmental QOL and psychological QOL, respectively. Perhaps this is reflective of individuals with SCI engaging in longer periods of light LTPA. Moreover, while minutes of heavy intensity LTPA was associated with increases in physical health QOL, it is notable that the current participants reported spending an average of 109 minutes engaged in mild LTPA per day and 64 minutes of moderate, but only 39 minutes of heavy. The duration and frequency of mild and moderate activity for longer durations may be more feasible for individuals with SCI. Overall, these findings suggest that engagement in LTPA (intensity and volume) is associated with enhanced QOL and that LTPA more accurately predicts QOL than body composition or anthropometrics. This offers clinicians and health providers critical information about lifestyle activities that can enhance physical and emotional well-being while reducing disease risk after SCI.
To our knowledge, this is the first time that sophisticated imaging of body composition, anthropometric variables, and LTPA have been associated with QOL domains in individuals with SCI. Interestingly, the results suggest that body composition variables (in particular android and trunk fat) and anthropometric variables (in particular supine waist and abdominal circumferences), proxy indices of central obesity, may contribute to social relationships QOL. Central obesity is characterized by increasing visceral adipose tissue that likely leads to impaired glucose tolerance, insulin resistance, and dyslipidemia in persons with SCI (Gorgey et al., 2014; Farkas and Gater, 2018). It should be noted that social relationships QOL is a broad construct encompassing personal relationships, social support, and sexual activity. A potential explanation for the positive association between social relationships QOL and body composition may be due to the social aspects of food. Previous work examining dietary restrictions found that while certain indices of QOL improved during diet modification, the social domain scores were negatively affected. This may be due to the fact that eating outside of the home and with others provides opportunities for social engagement. Moreover, difficulty eating with others as a result of a modified or restricted diet may hinder the social aspect of eating (Corle et al., 2001). Although this is only an association (not seen in the regression analyses), future research should investigate this potential link between social relationships QOL and high caloric intake. It is possible that individuals with SCI may enjoy social interactions without considerable attention to nutrition.
Limitations
The current findings were limited by two key aspects. First, the sample was a small convenience sample of very healthy individuals (i.e., no cardiovascular disease, type II diabetes, pressure ulcers, or common medical and psychiatric comorbidities), which limits the generalizability of findings beyond individuals with similar levels of functioning. Another concern is that conducting 12 simultaneous multiple regressions may result in a multicollinearity problem, especially with a small sample size. Green suggests that with six or more predictors, the sample size should be at least 100 to conduct a single multiple regression model (Green, 1991). The small sample size is primarily limited by constraints, including access to this specific population and the cost of sophisticated imaging techniques (i.e., DXA) utilized. Due to the small sample size, the adjusted R2 values with unstandardized b weights are also reported Table 5. The results of this study should be viewed as an exploratory study evaluating potential predictors of QOL in SCI. Future studies with larger sample sizes may see a more robust effect.
As a healthier sample, participants may have improved functional mobility and more likely to engage in physical activity. Also, the sample was well beyond the first several years of injury and likely had time to identify physical activities that they could consistently engage in. The measures of LTPA relied on retrospective self-report, which may have distorted accuracy and may have been affected by recall bias. The use of bioinformatics through physical activity trackers in future studies may help to ameliorate the effects of recall bias on participants’ reports of physical activity. In light of these limitations, the current findings are fairly robust and offer feasible suggestions that improve physical and emotional wellness. A large multi-center trial is highly warranted to effectively address the aforementioned limitations.
Conclusions
Engagement in LTPA was strongly associated with various aspects of QOL in individuals with SCI. Anthropomorphic measures and body composition measures were also associated to varying degrees with increased QOL. Health providers may want to encourage those with chronic SCI to engage in some form of mild, moderate, and heavy-intensity LTPA as a way to improve mood, decrease stress, and improve overall QOL. Further identifying the relationships among levels of physical activity, anthropometrics, body composition variables, and SCI-related QOL may provide insight on how these variables can predict participant adherence in longitudinal interventional trials.
Additional files:
Additional file 1 (408.9KB, pdf) : Ethical Approval Documentation.
Additional file 2: STROBE checklist.
Additional file 3 (4.1MB, pdf) : Model consent form.
Acknowledgments:
We would like to thank all the study participants who volunteered in the current work.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: This study was supported by the Department of Defense-Congressionally Directed Medical Research Program (DoD-CDMRP) (W81XWH-14-SCIRP-CTA; to ASG).
Institutional review board statement: The study was approved by the McGuire VA Research Institutional Review Board (IRB# 02152, approval date August 9, 2015; IRB# 02375, approval date May 2, 2018).
Declaration of participant consent: The authors certify that they have obtained all appropriate participant consent forms. In the forms the participants have given their consent for their images and other clinical information to be reported in the journal. The participants understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement: This study followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the statistician of Virginia Commonwealth University in Richmond, USA.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Anonymized individual data will be available immediately after study publication upon request from those who wish to access the data. If anonymized data is provided, it should be done so after proposals to Ashraf.Gorgey@va.gov. Raw data (including personal information and participant codes) will be stored in a locked cabinet at the McGuire VA Research Institute for this time period.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the Department of Defense-Congressionally Directed Medical Research Program (DoD-CDMRP) (W81XWH-14-SCIRP-CTA; to ASG).
References
- 1.Anderson CJ, Vogel LC, Chlan KM, Betz R, McDonald CM. Depression in adults who sustained spinal cord injuries as children or adolescents. J Spinal Cord Med. 2007;30:S76–82. doi: 10.1080/10790268.2007.11754609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anneken V, Hanssen-Doose A, Hirschfeld S, Scheuer T, Thietje R. Influence of physical exercise on quality of life in individuals with spinal cord injury. Spinal Cord. 2010;48:393–399. doi: 10.1038/sc.2009.137. [DOI] [PubMed] [Google Scholar]
- 3.Buchholz AC, Martin Ginis KA, Bray SR, Craven BC, Hicks AL, Hayes KC, Latimer AE, McColl MA, Potter PJ, Wolfe DL. Greater daily leisure time physical activity is associated with lower chronic disease risk in adults with spinal cord injury. Appl Physiol Nutr Metab. 2009;34:640–647. doi: 10.1139/H09-050. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Centers for disease control and prevention health-related quality-of-life 14-item measure. Atlanta, GA: Centers for Disease Control and Prevention; 2005. [Google Scholar]
- 5.Corle DK, Sharbaugh C, Mateski DJ, Coyne T, Paskett ED, Cahill J, Daston C, Lanza E, Schatzkin A, Group PS. Self-rated quality of life measures: effect of change to a low-fat high-fiber fruit and vegetable enriched diet. Ann Behav Med. 2001;23:198–207. doi: 10.1207/S15324796ABM2303_7. [DOI] [PubMed] [Google Scholar]
- 6.Crane DA, Hoffman JM, Reyes MR. Benefits of an exercise wellness program after spinal cord injury. J Spinal Cord Med. 2017;40:154–158. doi: 10.1179/2045772315Y.0000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Oliveira GLC, Figueiredo FA, Passos MCF, Chain A, Bezerra FF, Koury JC. Physical exercise is associated with better fat mass distribution and lower insulin resistance in spinal cord injured individuals. J Spinal Cord Med. 2014;37:79–84. doi: 10.1179/2045772313Y.0000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dijkers MP. Individualization in quality of life measurement: instruments and approaches. Arch Phys Med Rehabil. 2003;84:S3–14. doi: 10.1053/apmr.2003.50241. [DOI] [PubMed] [Google Scholar]
- 9.Farkas GJ, Gater DR. Neurogenic obesity and systemic inflammation following spinal cord injury: a review. J Spinal Cord Med. 2018;41:378–387. doi: 10.1080/10790268.2017.1357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 11.Gillison FB, Skevington SM, Sato A, Standage M, Evangelidou S. The effects of exercise interventions on quality of life in clinical and healthy populations; a meta-analysis. Soc Sci Med. 2009;68:1700–1710. doi: 10.1016/j.socscimed.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 12.Ginis KAM, Latimer AE, McKechnie K, Ditor DS, McCartney N, Hicks AL, Bugaresti J, Craven BC. Using exercise to enhance subjective well-being among people with spinal cord injury: The mediating influences of stress and pain. Rehabil Psychol. 2003;48:157. [Google Scholar]
- 13.Ginis KAM, van der Scheer JW, Latimer-Cheung AE, Barrow A, Bourne C, Carruthers P, Bernardi M, Ditor DS, Gaudet S, de Groot S. Evidence-based scientific exercise guidelines for adults with spinal cord injury: an update and a new guideline. Spinal Cord. 2018;56:308–321. doi: 10.1038/s41393-017-0017-3. [DOI] [PubMed] [Google Scholar]
- 14.Godin G, Shephard R. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- 15.Gorgey AS, Khalil RE, Davis JC, Carter W, Gill R, Rivers J, Khan R, Goetz LL, Castillo T, Lavis T. Skeletal muscle hypertrophy and attenuation of cardio-metabolic risk factors (SHARC) using functional electrical stimulation-lower extremity cycling in persons with spinal cord injury: study protocol for a randomized clinical trial. Trials. 2019;20:1–14. doi: 10.1186/s13063-019-3560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorgey AS, Khalil RE, Gill R, O’Brien LC, Lavis T, Castillo T, Cifu DX, Savas J, Khan R, Cardozo C. Effects of Testosterone and Evoked Resistance Exercise after Spinal Cord Injury (TEREX-SCI): study protocol for a randomised controlled trial. BMJ Open. 2017;7:e014125. doi: 10.1136/bmjopen-2016-014125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorgey AS, Martin H, Metz A, Khalil RE, Dolbow DR, Gater DR. Longitudinal changes in body composition and metabolic profile between exercise clinical trials in men with chronic spinal cord injury. J Spinal Cord Med. 2016;39:699–712. doi: 10.1080/10790268.2016.1157970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Castillo C, Gater DR. Effects of spinal cord injury on body composition and metabolic profile–Part I. J Spinal Cord Med. 2014;37:693–702. doi: 10.1179/2045772314Y.0000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorgey AS, Cirnigliaro CM, Bauman WA, Adler RA. Estimates of the precision of regional and whole body composition by dual-energy x-ray absorptiometry in persons with chronic spinal cord injury. Spinal Cord. 2018;56:987–995. doi: 10.1038/s41393-018-0079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorgey AS. Exercise awareness and barriers after spinal cord injury. World J Orthop. 2014;5:158–162. doi: 10.5312/wjo.v5.i3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green SB. How many subjects does it take to do a regression analysis. Multivariate Behav Res. 1991;26:499–510. doi: 10.1207/s15327906mbr2603_7. [DOI] [PubMed] [Google Scholar]
- 22.Harper A, Orley J. WHOQOL-BREF introduction administration. scoring and generic version of the assessment. Geneva: World Health Organization; 1996. [Google Scholar]
- 23.Hetz SP, Latimer AE, Buchholz AC, Martin Ginis KA SHAPE-SCI Research Group. Increased participation in activities of daily living is associated with lower cholesterol levels in people with spinal cord injury. Arch Phys Med Rehabil. 2009;90:1755–1759. doi: 10.1016/j.apmr.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Hicks A, Martin K, Ditor D, Latimer A, Craven C, Bugaresti J, McCartney N. Long-term exercise training in persons with spinal cord injury: effects on strength arm ergometry performance and psychological well-being. Spinal Cord. 2003;41:34–43. doi: 10.1038/sj.sc.3101389. [DOI] [PubMed] [Google Scholar]
- 25.Jang Y, Hsieh CL, Wang YH, Wu YH. A validity study of the WHOQOL-BREF assessment in persons with traumatic spinal cord injury. Arch Phys Med Rehabil. 2004;85:1890–1895. doi: 10.1016/j.apmr.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 26.Kawanishi CY, Greguol M. Physical activity quality of life and functional autonomy of adults with spinal cord injuries. Adapt Phys Activ Q. 2013;30:317–337. doi: 10.1123/apaq.30.4.317. [DOI] [PubMed] [Google Scholar]
- 27.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, Johansen M, Jones L, Krassioukov A, Mulcahey M. International standards for neurological classification of spinal cord injury (revised 2011) J Spinal Cord Med. 2011;34:535–546. doi: 10.1179/204577211X13207446293695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luchauer B, Shurtleff T. Meaningful Components of Exercise and Active Recreation for Spinal Cord Injuries. OTJR (Thorofare N J) 2015;35:232–238. doi: 10.1177/1539449215601069. [DOI] [PubMed] [Google Scholar]
- 29.Maitland Schladen M, Groah SL. State of the science on cardiometabolic risk after spinal cord injury: recap of the 2013 Asia pre-conference on cardiometabolic disease. Top Spinal Cord Inj Rehabil. 2014;20:105–112. doi: 10.1310/sci2002-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin Ginis KA, Phang SH, Latimer AE, Arbour-Nicitopoulos KP. Reliability and validity tests of the leisure time physical activity questionnaire for people with spinal cord injury. Arch Phys Med Rehabil. 2012;93:677–682. doi: 10.1016/j.apmr.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Noce F, Simim MAdM, Mello MTd. A percepção de qualidade de vida de pessoas portadoras de deficiência física pode ser influenciada pela prática de atividade física. Revista Brasileira de Medicina do Esporte. 2009;15:174–178. [Google Scholar]
- 32.Rintala DH, Loubser PG, Castro J, Hart KA, Fuhrer MJ. Chronic pain in a community-based sample of men with spinal cord injury: prevalence severity and relationship with impairment disability handicap and subjective well-being. Arch Phys Med Rehabil. 1998;79:604–614. doi: 10.1016/s0003-9993(98)90032-6. [DOI] [PubMed] [Google Scholar]
- 33.Smith DL, Jr, Yarar-Fisher C. Contributors to Metabolic Disease Risk Following Spinal Cord Injury. Curr Phys Med Rehabil Rep. 2016;4:190–199. doi: 10.1007/s40141-016-0124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.So ES. Waist circumference and health-related quality of life by sex in the Korean elderly. J Aging Health. 2014;26:887–899. doi: 10.1177/0898264314531618. [DOI] [PubMed] [Google Scholar]
- 35.Tomasone JR, Wesch NN, Ginis KAM, Noreau L. Spinal cord injury physical activity and quality of life: a systematic review. Kinesiol Rev. 2013;2:113–129. [Google Scholar]
- 36.Whiteneck GG, Charlifue S, Frankel H, Fraser M, Gardner B, Gerhart K, Krishnan K, Menter R, Nuseibeh I, Short D. Mortality morbidity and psychosocial outcomes of persons spinal cord injured more than 20 years ago. Spinal Cord. 1992;30:617–630. doi: 10.1038/sc.1992.124. [DOI] [PubMed] [Google Scholar]
- 37.WHOQOL Group. Development of the WHOQOL: Rationale and current status. Int J Ment Health. 1994;23:24–56. [Google Scholar]
- 38.Wu S, Wang R, Jiang A, Ding Y, Wu M, Ma X, Zhao Y, He J. Abdominal obesity and its association with health-related quality of life in adults: a population-based study in five Chinese cities. Health Qual Life Outcomes. 2014;12:100. doi: 10.1186/1477-7525-12-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


