Key Words: cerebral infarction, differential expression analysis, expression profiling, GO term, ischemic postconditioning, ischemic stroke, KEGG pathway, lncRNA, mRNA, RNA sequencing
Abstract
During acute reperfusion, the expression profiles of long noncoding RNAs in adult rats with focal cerebral ischemia undergo broad changes. However, whether long noncoding RNAs are involved in neuroprotective effects following focal ischemic stroke in rats remains unclear. In this study, RNA isolation and library preparation was performed for long noncoding RNA sequencing, followed by determining the coding potential of identified long noncoding RNAs and target gene prediction. Differential expression analysis, long noncoding RNA functional enrichment analysis, and co-expression network analysis were performed comparing ischemic rats with and without ischemic postconditioning rats. Rats were subjected to ischemic postconditioning via the brief and repeated occlusion of the middle cerebral artery or femoral artery. Quantitative real-time reverse transcription-polymerase chain reaction was used to detect the expression levels of differentially expressed long noncoding RNAs after ischemic postconditioning in a rat model of ischemic stroke. The results showed that ischemic postconditioning greatly affected the expression profile of long noncoding RNAs and mRNAs in the brains of rats that underwent ischemic stroke. The predicted target genes of some of the identified long noncoding RNAs (cis targets) were related to the cellular response to ischemia and stress, cytokine signal transduction, inflammation, and apoptosis signal transduction pathways. In addition, 15 significantly differentially expressed long noncoding RNAs were identified in the brains of rats subjected to ischemic postconditioning. Nine candidate long noncoding RNAs that may be related to ischemic postconditioning were identified by a long noncoding RNA expression profile and long noncoding RNA-mRNA co-expression network analysis. Expression levels were verified by quantitative real-time reverse transcription-polymerase chain reaction. These results suggested that the identified long noncoding RNAs may be involved in the neuroprotective effects associated with ischemic postconditioning following ischemic stroke. The experimental animal procedures were approved by the Animal Experiment Ethics Committee of Kunming Medical University (approval No. KMMU2018018) in January 2018.
Chinese Library Classification No. R454; R741; Q522
Introduction
Ischemic stroke is a global disease characterized by high morbidity, disability, and mortality. An increasing number of young people are developing this disease (Feigin et al., 2014; Daidone et al., 2021). Currently, thrombolytic therapy has limited efficacy in the treatment of ischemic stroke, and only a small proportion of patients are eligible to receive this therapy due to the limited treatment time window of approximately 4.5 hours (Stapf and Mohr, 2002; Schellinger et al., 2004). Therefore, an urgent need exists to develop new and effective therapies for ischemic stroke.
Endogenous neuroprotection refers to the brain’s ability to respond to external damage, the efficacy of which depends on the intensity and nature of the stimulus. The aim of conditioning is to trigger endogenous protective mechanisms by alternating transient ischemia/reperfusion (IR) before, during, or after ischemia (Bernaudin et al., 1999). Ischemic postconditioning induces endogenous neuroprotection by producing transient ischemia during reperfusion (Halkos et al., 2004), including in situ ischemic postconditioning (ISP) or remote ischemic postconditioning (RIP). Cerebral ISP refers to the interruption of blood flow during the early stages of cerebral IR, stimulating the brain tissue to initiate endogenous neuroprotective mechanisms and reduce reperfusion injury (Fan et al., 2017). The neuroprotective mechanisms associated with ischemic postconditioning in rats include improving cerebral blood flow, preventing cytochrome c translocation, and the activation of the protein kinase B (Akt) and phosphoinositide 3-kinase (PI3K) pathways (Li et al., 2017; Tyagi et al., 2019).
Multiple mechanisms underlying the neuroprotective effects induced by ischemic conditioning have been identified, including the regulation of neurotrophic protein expression, the enhancement of neurovascular networks, the alleviation of the inflammatory response and neuronal apoptosis, and the promotion of metabolic responses in the brain (An et al., 2015; Wang et al., 2015). However, the endogenous neuroprotective effects of ischemic postprocessing (RIP and ISP) and the signaling pathways that mediate these effects have not been fully elucidated.
Previous studies have confirmed that long noncoding RNAs (lncRNAs) represent important components at the transcriptional, post-transcriptional, and epigenetic levels, able to regulate gene expression through multiple mechanisms (Knauss and Sun, 2013; Hart and Goff, 2016; Wang et al., 2019; Di et al., 2021). Accumulating studies have shown that lncRNAs are critical genetic regulators of development and disease (Qureshi and Mehler, 2012; Schaukowitch and Kim, 2014; Briggs et al., 2015). Many lncRNAs have been identified in the rat brain, which is important for the development and function of the central nervous system (Qureshi et al., 2010; Knauss and Sun, 2013; Ng et al., 2013; van de et al., 2013; Lipovich et al., 2014). Previous studies have shown that the expression profiles of lncRNAs in adult rats subjected to focal cerebral ischemia changed extensively during acute reperfusion (Dharap et al., 2012; Zhang et al., 2016; Liu et al., 2018b). However, the expression patterns of lncRNAs and their functional roles following ischemic postconditioning in rats subjected to focal ischemic stroke are currently unknown.
The neuroprotective mechanisms of ischemic postconditioning are likely complex and intertwined, with significant crosstalk among signaling pathways. We hypothesized that lncRNA and mRNA expression profiles were significantly altered in rats who underwent ischemic stroke and received an ischemic postconditioning intervention. The aim of the current study was to establish the lncRNA and mRNA expression profiles among rats subjected to ischemic and an ischemic postconditioning intervention and identify novel lncRNAs related to stroke and ischemic postconditioning interventions.
Materials and Methods
Animals
Forty adult male Sprague-Dawley, specific pathogen-free level rats (aged 6–7 weeks, weighing 260 ± 20 g) were purchased from the Animal Department of Kunming Medical University, China [license No. SCXK (Dian) 2015-0002]. To eliminate the potential influence of estrogen and any other sex-related physiological differences, only male rats were used in this study. The study was conducted in strict accordance with the guidelines of Kunming Medical University with regard to the protection and use of experimental animals. The experimental animal procedures were approved by the Animal Experiment Ethics Committee of Kunming Medical University (approval No. KMMU2018018) in January 2018. All experiments were designed and reported according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Some possible measures were taken to minimize the rats’ pain. The rats were allowed to eat and drink water freely, and the room temperature was maintained at 23 ± 1°C during the modeling process.
Animal grouping and transient middle cerebral artery occlusion model
The rats were randomly assigned to four groups as follows: sham (n = 10); IR (n = 10); ischemia + ISP (ISP; n = 10); and ischemia + RIP (RIP; n = 10).
Anesthesia was induced with 5% isoflurane (MilliporeSigma, St. Louis, MO, USA) and maintained with 2–3% isoflurane. Throughout the experiment, a rectal probe was used to monitor and maintain the animal body temperature at 37°C. The right middle cerebral artery was occluded for 120 minutes to establish the rat transient middle cerebral artery occlusion (tMCAO) model (Longa et al., 1989; Li et al., 2020). In brief, a surgical incision was made to expose the right common carotid artery, internal carotid artery, and external carotid artery. The proximal common carotid artery was then ligated, and an occlusion filament was inserted into the internal carotid artery through the common carotid artery 19–21 mm distal from the bifurcation to occlude the origin of the middle cerebral artery. After induction of ischemia, the filament was withdrawn, and the rats were placed into a cage to recover from anesthesia at room temperature, with free access to food and water. The rats were subjected to 2 hours of focal cerebral ischemia. In the sham group, rats were subjected to the same procedures without the occlusion of the middle cerebral artery.
Following 120 minutes of tMCAO, the bilateral carotid arteries were occluded using an arterial clamp for 10 seconds, followed by loosening the clamp for 30 seconds. The clamping and releasing procedure was repeated for three cycles in the ISP group. Following 120 minutes of tMCAO, the RIP group was subjected to three cycles in which the bilateral femoral arteries were occluded using an arterial clamp for 10 minutes, followed by loosening the clamp for 10 minutes. The detailed experimental procedure for postconditioning intervention is shown in the schematic diagram found in Additional Figure 1 (1MB, tif) (Li et al., 2020).
Evaluation of neurological function
The Zea Longa scoring method (Longa et al., 1989) was used to score the neurological function deficits in rats 24 hours after reperfusion in all four groups. Rats with normal neurological function received a score of 0, whereas rats with the most serious neurological deficits received a score of 3 or 4. A score of 5 was assigned to rats that died.
2,3,5-Triphenyltetrazolium chloride staining
At 24 hours after reperfusion, the rats in each group (n = 4) were anesthetized by the intraperitoneal injection of 3% pentobarbital (30 mg/kg; MilliporeSigma) and decapitated, followed by the rapid removal of brain tissues. The rat brains were cut into 2-mm-thick coronal sections and stained with 1% 2,3,5-triphenyltetrazolium chloride (TTC) solution (MilliporeSigma) for 30 minutes at 37°C, followed by overnight immersion in 4% paraformaldehyde. Finally, the slices were transferred into saline. Six images from each group were captured using an MCID computer imaging analysis system (Image ProPlus software v.6.0, Media Cybernetics, Rockville, MD, USA) (Wang et al., 2014). For TTC staining, the observer was unaware of the treatment the rats received or the grouping.
RNA sequencing and analysis
The cerebral tissues collected from the infarct areas of three rats in each group were used for RNA sequencing. RNA was extracted, quantified, and purified, and the concentration measurement was performed using methods from our previous study (Dai et al., 2020). A 3 μg RNA sample was obtained from each of three rats in each group and subjected to RNA sequencing. The methods used for lncRNA library preparation, lncRNA sequencing, transcriptome assembly, and coding potential analysis were described in our previous study (Dai et al., 2020). In brief, total RNA was extracted from infarcted cerebral tissues using TRIzol (Invitrogen, Carlsbad, CA, USA). Ribosomal RNA was removed using an Epicentre Ribo-zero™ rRNA Removal Kit (Illumina Inc., San Diego, CA, USA), and linear RNA in the remaining RNA was removed by RNase R treatment (Epicentre, Madison, WI, USA). The libraries were sequenced at the Novogene Bioinformatics Institute (Beijing, China) on an Illumina HiSeq 2500 (PE150) platform, and 150-bp long paired-end reads were obtained. To identify lncRNAs involved in cerebral infarction after ischemic stroke, we used Cufflinks (v2.2.0) software (http://cufflinks.cbcb.umd.edu) to divide all transcripts into different subtypes and established strict screening criteria to filter transcripts that did not have all of the typical characteristics of lncRNA (Trapnell et al., 2010). Transcripts predicted to have coding potential by any of the three following tools were removed, and those without coding potential were retained as our candidate set of lncRNAs. The Coding Potential Calculator (CPC2, 0.9-r2, http://cpc.cbi.pku.edu.cn/) (Kong et al., 2007), the Coding/Noncoding Index (CNCI, v2, http://www.bioinfo.org/software/cnci) (Sun et al., 2013) and Pfam-scan (Pfam, v1.3, http://pfam.sanger.ac.uk/) (Finn et al., 2014) were used to assess the coding potential of transcripts.
Differential expression analysis
TopHat (v2.1.1, http://tophat.cbcb.umd.edu/) and Cufflinks (v2.2.0, http://cufflinks.cbcb.umd.edu) were used to calculate the fragments per kilobase of transcript per million fragments mapped (FPKMs) of both lncRNAs and coding genes in each sample (Trapnell et al., 2012). Gene FPKMs were computed by summing the FPKMs of transcripts in each gene group. Ballgown (v3.4.0, https://bioconductor.org/biocLite.R) provides statistical routines for determining differential expression in digital transcript or gene expression data using a model based on the negative binomial distribution (Pertea et al., 2016). Transcripts with a P-adjust < 0.05 were defined as being differentially expressed. Adjustment of P-value was performed according to the Benjamini and Hochberg method (Benjamini and Hochberg, 1995). We obtained the differentially expressed transcripts from the comparisons among groups, and then Venn Diagrams (v.1.6.20, https://cran.rstudio.com/web/packages/VennDiagram/index.htm) were used to visualize the common and unique differentially expressed transcripts among the groups.
lncRNA target gene enrichment analysis in cis role
Cis role refers to lncRNA that acts on neighboring target genes (Ørom et al., 2010). We searched coding genes 100 kb upstream and downstream of each lncRNA and analyzed their functions (Ørom et al., 2010). Gene Ontology (GO) enrichment analysis of the genes associated with the differentially expressed lncRNAs was performed using the GOseq R package (v.release 2.12, http://www.geneontology.org/) (Gene Ontology Consortium et al., 2013). Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of differentially expressed genes was performed using KOBAS software (v.2.0, http://kobas.cbi.pku.edu.cn) (Kanehisa and Goto, 2000). Fisher’s exact test and the Chi-square test were used to select significant GO categories and KEGG pathways. The threshold for significance was P < 0.05, and the false discovery rate was calculated to correct the P-value.
Construction of lncRNA-mRNA co-expression networks
The LncRNA-mRNA co-expression networks were constructed based on Pearson’s correlation analysis using Cytoscape software (v.3.8.0, https://cytoscape.org/) (Shannon et al., 2003). In the co-expression network, each lncRNA or mRNA corresponded to a node, and the nodes were linked by edges. LncRNAs with greater numbers of connections to mRNAs or genes were considered to have a greater degree of association, indicating their increased importance.
Quantitative real-time reverse transcription-polymerase chain reaction assay
The cerebral tissues from the infarct areas in each of the four groups (n = 3 in each group) were subjected to quantitative real-time reverse transcription-polymerase chain reaction (PCR) to determine the gene levels. In brief, total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and 1 μg RNA from each sample was reverse transcribed into complementary DNA and subjected to quantitative real-time reverse transcription-PCR. The PCR mixture included 5 μM (final concentration) primers in a total volume of 20 μL. The primer information is described in Table 1. The PCR cycles were as follows: enzyme activation at 50°C for 2 minutes, initial denaturation at 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. The relative gene expression levels were determined from three independent samples, with each sample assayed in triplicate. The four independent samples in each group were run in triplicate and analyzed using the 2–ΔΔCt method, as previously described (Wang et al., 2020).
Table 1.
Primer sequences of lncRNAs
| LncRNA | Primer sequence (5′–3′) |
|---|---|
| LNC_018231 | F: GAC GCT GTC CCT CTT CTG TT |
| R: CAC TCG GAC GCG AGA AGA AA | |
| LNC_000587 | F: CAC AGG CAT CTC GGT TCC TC |
| R: TGC AGT CTC AGA GCG TCA TC | |
| LNC_014770 | F: TAG CCG CTC CCG TAT CGT |
| R: CAT CCT GAG GCA CTT CCA CC | |
| LNC_014827 | F: ACA AAG GCC AAT CCC AGA CC |
| R: CCA ATC TGA TGA GCA GTC CTT ATT T | |
| LNC_017702 | F: CGC AGC CCT TCT TAT AGG CG |
| R: CCG TGG TTT CCA GCT TGC C | |
| LNC_000871 | F: GGC AGA CGC GAA CTC CAT |
| R: GAT TTC CGC CCC TCC TCT TG |
F: Forward; LncRNA: long noncoding RNA; R: reverse.
Statistical analysis
Data from each group were analyzed by one-way analysis of variance, followed by Tukey’s post hoc test, using SPSS 22.0 statistical software for Windows (IBM, Armonk, NY, USA). The data showed a normal distribution and were homogenous. Data are presented as the mean ± standard error of the mean (SEM). A value of P < 0.05 was considered significant.
Results
ISP and RIP decrease changes to the cerebral infarction area of IR rats
TTC staining results revealed an obvious infarct area in the left cerebral hemisphere of rats in the IR group. The cerebral infarct area was reduced in the ISP and RIP groups compared with the IR group (Figure 1). No infarct area was observed in the left cerebral hemisphere in the sham group.
Figure 1.
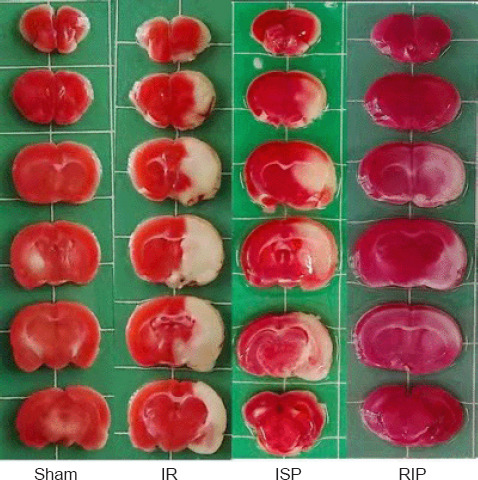
Representative cerebral infarct areas stained by 2,3,5-triphenyltetrazolium chloride (TTC).
An obvious infarct area could be observed in the left cerebral hemisphere in the IR group. The cerebral infarct areas were reduced in the ISP and RIP groups compared with the IR group. No infarct area was observed in the left cerebral hemisphere in the sham group. Red indicates normal brain tissue, and white indicates ischemic tissue. IR: Ischemia/reperfusion; ISP: in situ ischemic postconditioning; RIP: remote ischemic postconditioning.
ISP and RIP decrease neurological functional deficit scores in IR rats
The neurological functional deficit scores in the IR group were greater than 3, and the neurological functional deficit scores in the ISP and RIP groups were significantly decreased compared with those of the IR group (ISP group: P < 0.05, RIP group: P < 0.01). Rats in the sham group had a score of 0 (Additional Figure 2 (285.3KB, tif) ).
Identification of lncRNAs after ischemic postconditioning in ischemic stroke rats
After screening, 46,101 recognized transcripts were identified as known reference transcripts (Figure 2A and B). The coding potential of novel long transcripts was calculated using coding potential assessment tools (CPC2, CNCI, and Pfam), and potential protein-coding transcripts were removed. Finally, 29,542 novel long transcripts were retained after analyzing the overlap among CPC2, CNCI, and Pfam (Figure 2C). Among these new lncRNAs, 49.0% were long intervening noncoding RNAs, 42.2% were intronic lncRNAs, and 8.6% were antisense lncRNAs (Figure 2D). The lncRNAs identified by the three encoding potential analysis tools (CPC2, CNCI, and Pfam) were used as candidate lncRNAs for subsequent analysis.
Figure 2.
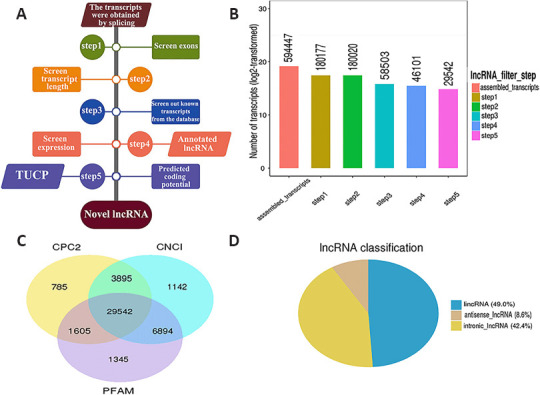
RNA sequencing experiments and bioinformatics analysis of cerebral infarct areas in rats.
(A) A series of strict filtering criteria was used to exclude transcripts that did share the typical characteristics of lncRNAs. (B) The number of transcripts retained after screening. The number of transcripts obtained after screening through the five steps outlined in A is shown. The X-axis indicates the screening step, and the Y-axis indicates the number of transcripts obtained after screening with the corresponding steps. (C) The number of transcripts obtained after screening and analysis with three different coding potential analysis tools: CPC2, CNCI, and Pfam. (D) LncRNA classification by type. CNCI: Coding-Non-Coding-Index; CPC2: Coding Potential Calculator-r2; lncRNA: long noncoding RNA; PFAM: Pfam Scan; TUCP: transcripts of uncertain coding potential.
Differential expression analysis of lncRNAs and mRNAs after ischemic postconditioning in ischemic stroke rats
Ischemic stroke and ischemic postconditioning significantly altered cerebral lncRNA expression profiles. Compared with the sham group, 2616 lncRNAs were significantly altered in the IR group, including 1421 upregulated lncRNAs and 1195 downregulated lncRNAs. Compared with the sham group, 3447 lncRNAs were significantly altered in the ISP group, including 1429 that were upregulated and 2018 that were downregulated. Compared with the sham group, 873 lncRNAs in the RIP group showed significant changes, including 488 upregulated lncRNAs and 385 downregulated lncRNAs. Compared with the IR group, 68 lncRNAs were significantly altered in the ISP group, including 28 that were upregulated and 40 that were downregulated. Compared with the IR group, 128 lncRNAs in the RIP group showed significant changes, including 46 upregulated lncRNAs and 82 downregulated lncRNAs. Compared with the ISP group, 172 lncRNAs in the RIP group showed significant changes, including 87 upregulated lncRNAs and 85 downregulated lncRNAs (Figure 3).
Figure 3.
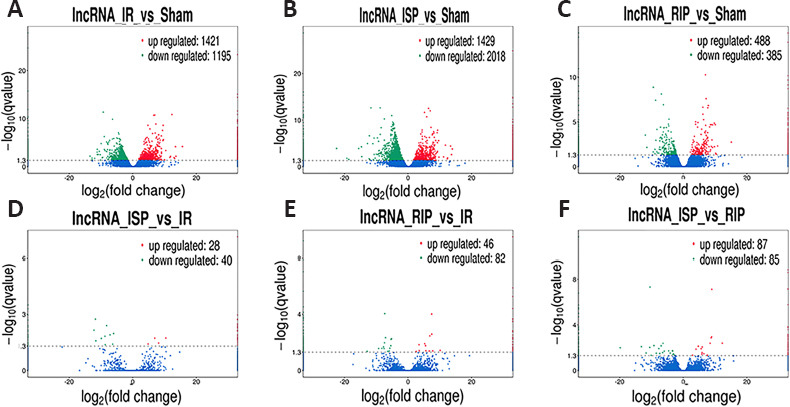
Volcano plots of differentially expressed long noncoding RNAs (lncRNAs) in each group after ischemic stroke.
(A) Distribution of lncRNA expression in the IR group compared with the sham group. (B) Distribution of lncRNA expression in the ISP group compared with the sham group. (C) Distribution of lncRNA expression in the RIP group compared with the sham group. (D) Distribution of lncRNA expression in the ISP group compared with the IR group. (E) Distribution of lncRNA expression in the RIP group compared with the IR group. (F) Distribution of lncRNA expression in the ISP group compared with the RIP group. The screening threshold was defined as P < 0.05 by default. IR: Ischemia/reperfusion; ISP: in situ ischemic postconditioning; RIP: remote ischemic postconditioning.
Among the differentially expressed mRNAs, significant changes were found in the expression of 2825 mRNAs in the IR group compared with the sham group, including 2144 upregulated mRNAs and 681 downregulated mRNAs. Compared with the sham group, the expression of 3126 mRNAs was altered in the ISP group, including 2155 upregulated mRNAs and 971 downregulated mRNAs. Compared with the sham group, the expression of 890 mRNAs was altered in the RIP group, including 766 upregulated mRNAs and 124 downregulated mRNAs. Compared with the IR group, the expression of 36 mRNAs was altered in the ISP group, including 14 upregulated mRNAs and 22 downregulated mRNAs. Compared with the IR group, the expression of 89 mRNAs was altered in the RIP group, including 36 upregulated mRNAs and 53 downregulated mRNAs. Compared with the ISP group, the expression of 109 mRNAs was altered in the RIP group, including 53 upregulated mRNAs and 56 downregulated mRNAs (Figure 4).
Figure 4.
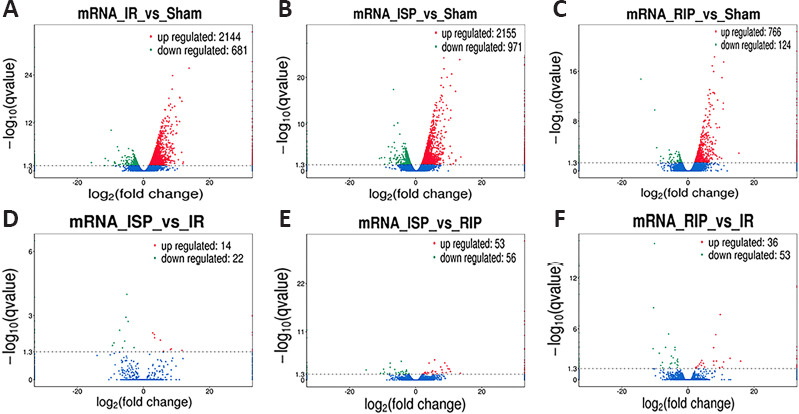
Volcano plots of differentially expressed mRNAs in each group after ischemic stroke.
(A) Distribution of mRNA expression in the IR group compared with the sham group. (B) Distribution of mRNA expression in the ISP group compared with the sham group. (C) Distribution of mRNA expression in the RIP group compared with the sham group. (D) Distribution of mRNA expression in the ISP group compared with the IR group. (E) Distribution of mRNA expression in the ISP group compared with the RIP group. (F) Distribution of mRNA expression in the RIP group compared with the IR group. The screening threshold was set as P < 0.05 by default. IR: Ischemia/reperfusion; ISP: in situ ischemic postconditioning; RIP: remote ischemic postconditioning.
Hierarchical clustering revealed systematic variations in the expression of lncRNAs and mRNAs in the ischemic cerebral tissue among the different groups. The lncRNA expression profiles of different samples within the same group were similar (Figure 5A and B), indicating that altered lncRNAs within the same group may participate in similar biological processes. We also found that the number of upregulated lncRNAs in the IR group was greater than the number of downregulated lncRNAs compared with the sham group. Compared with the IR group, the numbers of downregulated lncRNAs in the RIP and ISP groups were greater than the numbers of upregulated lncRNAs. The five most upregulated and downregulated lncRNAs in each group are shown in Table 2.
Figure 5.
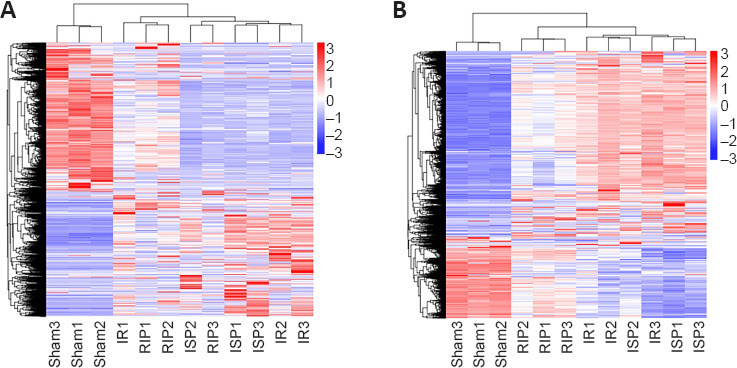
Hierarchical cluster analysis of differentially expressed long noncoding RNAs (lncRNAs) (A) and mRNAs (B).
The expression levels of transcripts were determined using fragments per kilobase of exon per million fragments (FPKM) values of differentially expressed transcripts in each sample from four groups, and hierarchical cluster analysis of lncRNAs and mRNAs was conducted. The log10 values (FPKM + 1) for lncRNA and mRNA transcripts were used for clustering, with red indicating highly expressed transcripts and blue indicating transcripts with low expression. The log10 values (FPKM + 1) of lncRNA transcripts are indicated in a range from red to blue. The sham, IR, RIP, and ISP groups contained six samples each. IR: Ischemia/reperfusion; ISP: in situ ischemic postconditioning; RIP: remote ischemic postconditioning.
Table 2.
The five most upregulated and downregulated lncRNAs in cerebral tissue of IR rats treated with RIP and ISP
| Transcript_ID | Gene_ID | log2(foldchange) | P-value | Q-value | Up/down regulated |
|---|---|---|---|---|---|
| IR vs. sham | |||||
| ENSRNOT00000084263 | ENSRNOG00000057822 | 3.176302233 | 0.005590773 | 0.049996732 | Up |
| LNC_024362 | XLOC_378593 | 2.706682263 | 0.005574431 | 0.049889837 | Up |
| LNC_004246 | XLOC_058149 | 4.388529477 | 0.005536835 | 0.049629631 | Up |
| LNC_013543 | XLOC_200180 | 3.675146682 | 0.005529841 | 0.049584549 | Up |
| LNC_000656 | XLOC_012091 | 4.684558619 | 0.005519841 | 0.049512468 | Up |
| LNC_002095 | XLOC_031766 | –2.307913803 | 0.005556618 | 0.049765715 | Down |
| LNC_025404 | XLOC_392814 | –4.17515095 | 0.005545101 | 0.049691955 | Down |
| LNC_020086 | XLOC_308020 | –3.122547372 | 0.005524432 | 0.049547782 | Down |
| LNC_024229 | XLOC_376271 | –5.13772922 | 0.005506811 | 0.049419015 | Down |
| LNC_005958 | XLOC_080167 | –2.400933738 | 0.005483187 | 0.049259555 | Down |
| ISP vs. IR | |||||
| LNC_021917 | XLOC_340017 | 4.850749898 | 0.000120842 | 0.038769255 | Up |
| LNC_015055 | XLOC_226377 | 8.144667816 | 0.000101905 | 0.034292275 | Up |
| LNC_011327 | XLOC_166648 | 6.928587246 | 4.41×10–5 | 0.018825775 | Up |
| LNC_024079 | XLOC_374170 | 10.41483099 | 4.28×10–5 | 0.018607736 | Up |
| LNC_026694 | XLOC_413978 | 12.15768267 | 8.82×10–11 | 6.07×10–7 | Up |
| LNC_021137 | XLOC_327277 | –8.635268062 | 0.000138226 | 0.042200708 | Down |
| LNC_003369 | XLOC_044931 | –6.290981666 | 0.000126651 | 0.039680634 | Down |
| LNC_026276 | XLOC_408207 | –11.58021894 | 6.78×10–5 | 0.02591702 | Down |
| LNC_008056 | XLOC_107651 | –9.876257604 | 4.66×10–5 | 0.019467254 | Down |
| LNC_024507 | XLOC_381667 | –8.745931172 | 2.95×10–5 | 0.014872291 | Down |
| RIP vs. IR | |||||
| LNC_004816 | XLOC_063784 | 2.580229009 | 0.000266941 | 0.04461683 | Up |
| LNC_022299 | XLOC_344778 | 6.680864412 | 0.000238798 | 0.041374279 | Up |
| LNC_018390 | XLOC_279775 | 7.790029499 | 0.000230395 | 0.040568237 | Up |
| LNC_009822 | XLOC_139808 | 5.650289903 | 0.000225333 | 0.039862271 | Up |
| LNC_027595 | XLOC_427154 | 3.520801959 | 0.000217344 | 0.038903554 | Up |
| LNC_009919 | XLOC_140906 | –12.82152019 | 0.000257148 | 0.043363003 | Down |
| LNC_003369 | XLOC_044931 | –6.37984486 | 0.000254924 | 0.043262155 | Down |
| LNC_016774 | XLOC_254642 | –5.876094417 | 0.000237093 | 0.041267745 | Down |
| LNC_009068 | XLOC_125509 | –7.539080399 | 0.000129572 | 0.027480416 | Down |
| LNC_022353 | XLOC_345474 | –9.222757126 | 0.000126921 | 0.027069937 | Down |
| RIP vs. ISP | |||||
| LNC_001132 | XLOC_018839 | 2.932430861 | 0.000407656 | 0.046068203 | Up |
| LNC_007945 | XLOC_105567 | 7.614329554 | 0.000400835 | 0.045823088 | Up |
| LNC_017358 | XLOC_264232 | 5.872603982 | 0.000277247 | 0.037197946 | Up |
| LNC_002262 | XLOC_033933 | 6.477056229 | 0.000245021 | 0.033994974 | Up |
| LNC_005789 | XLOC_077455 | 5.922123988 | 0.000171336 | 0.027139571 | Up |
| LNC_024302 | XLOC_377717 | –10.10406634 | 0.000425435 | 0.047165666 | Down |
| LNC_018044 | XLOC_274413 | –2.732280043 | 0.00035589 | 0.04304501 | Down |
| LNC_023374 | XLOC_364137 | –3.261381841 | 0.000325785 | 0.040843896 | Down |
| LNC_011498 | XLOC_169023 | –3.058132433 | 0.000247154 | 0.034086047 | Down |
| LNC_018390 | XLOC_279775 | –7.385930421 | 0.000225023 | 0.031965519 | Down |
IR: Ischemia/reperfusion; ISP: in situ ischemic postconditioning; lncRNA: long noncoding RNA; RIP: remote ischemic postconditioning.
Compared with the sham group, 665 differentially expressed lncRNAs and 407 mRNAs were identified in the IR group, which suggested extensive changes in lncRNA expression following cerebral IR injury. We identified 15 differentially expressed lncRNAs and 8 mRNAs that were identified in both the ISP and RIP groups when compared with the IR group (Figure 6A and B). We speculate that these 15 lncRNAs may play an important role in ischemic postconditioning. There are 51 common differentialy expressed lncRNAs in the IR vs. sham and RIP vs. IR groups. There are 38 and 2774 unique differentialy expressed lncRNAs in the RIP vs. IR groups and IR vs. sham groups respectively (Figure 6C).
Figure 6.
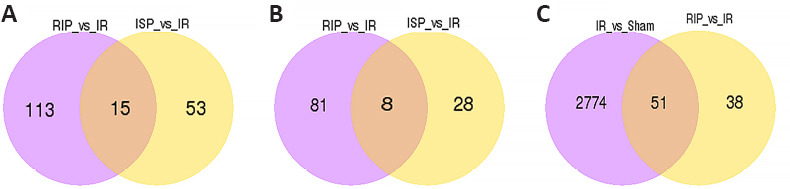
The number of common and unique differentially expressed transcripts among four groups.
(A) The numbers of common and unique differentially expressed long noncoding RNAs (lncRNAs) were compared between the RIP vs. IR and ISP vs. IR groups. (B) The numbers of common and unique differentially expressed mRNAs were compared between the RIP vs. IR and ISP vs. IR groups. (C) The numbers of common and unique differentially expressed lncRNAs were compared between the IR vs. sham and RIP vs. IR groups. IR: Ischemia/reperfusion; ISP: in situ ischemic postconditioning; RIP: remote ischemic postconditioning.
The target gene enrichment analyses of differentially expressed lncRNAs in cis roles
We examined protein-coding genes located 100 kb upstream and downstream of lncRNAs and performed functional enrichment analysis on identified potential mRNA targets to predict the primary functions of identified lncRNAs. The mRNAs identified as potential lncRNA targets within a distance of 100 kb are shown for each group in Additional Table 1 (232.2MB, pdf) .
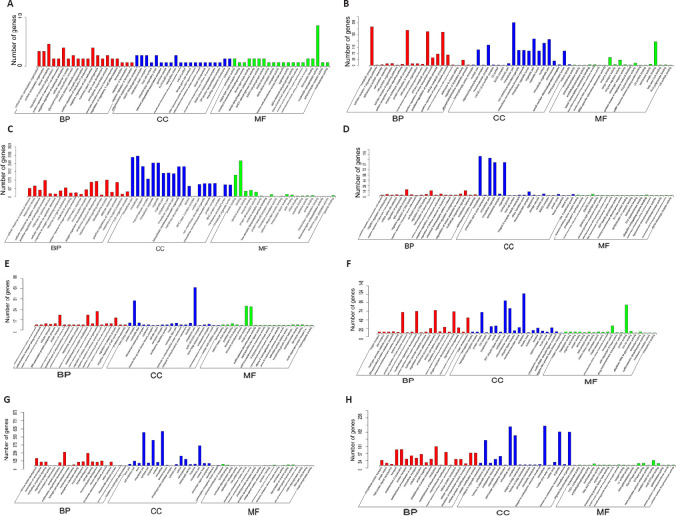
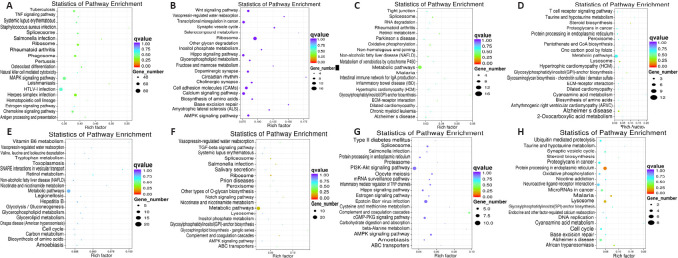
GO analysis was used to predict the functions of potential genes targeted by differentially expressed lncRNAs in cis roles after ischemic stroke. The 20 most significantly enriched GO terms for the upregulated and downregulated lncRNAs in each group were related to the cellular components, biological processes, and molecular functions displayed in Figure 7. We conducted pathway analysis using the KEGG database. We identified 178, 155, 200, and 222 KEGG pathways in the IR vs. sham, ISP vs. IR, RIP vs. IR, and ISP vs. RIP comparisons, respectively (Additional Tables 2 (707KB, pdf) -5 (322KB, pdf) ). The 20 KEGG pathways most associated with upregulated and downregulated lncRNAs after ischemic conditioning in ischemic stroke rats are shown in Figure 8. Significantly upregulated mRNAs were related to chemokines, mitogen-activated protein kinases, and tetrahydrofolate, whereas the significantly downregulated mRNAs after ischemic injury were involved in the synaptic vesicle cycle, cell adhesion molecules, inositol phosphate metabolism, and the AMP-activated protein kinase and Wnt signaling pathways, among others. After ischemic postconditioning, the significantly upregulated mRNAs were involved in metabolic pathways, the PI3K/Akt pathway, and the cell cycle, among others, whereas the significantly downregulated mRNAs were involved in the Notch, transforming growth factor-beta, and AMP-activated protein kinase signaling pathways.
Figure 7.
Gene Ontology (GO) analysis of significantly upregulated and downregulated mRNAs in the brain of IR rat with ISP and RIP treatment.
(A–D) The 20 most significantly enriched GO terms associated with upregulated mRNAs in each group-wise comparison are shown. Enriched GO terms in the ISP vs. IR (A), ISP vs. RIP (B), ISP vs. sham (C), and RIP vs. IR (D) group-wise comparisons. The X-axis represents different GO terms, while the Y-axis represents the number of genes enriched for the corresponding GO terms. (E–H) The 20 most significantly enriched GO terms associated with downregulated mRNAs in each group-wise comparison are shown. Enriched GO terms in the ISP vs. IR (E), ISP vs. RIP (F), ISP vs. sham (G), and RIP vs. IR (H) group comparisons. BP: Biological processes; CC: cellular components; IR: ischemia/reperfusion; ISP: in situ ischemic postconditioning; MF: molecular functions; RIP: remote ischemic postconditioning.
Figure 8.
Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of differentially expressed mRNAs in cerebral tissue of IR rats with ISP and RIP treatment.
(A–D) Twenty pathways that were most significantly associated with upregulated long noncoding RNAs (lncRNAs) in each group comparison (IR vs. sham, ISP vs. IR, RIP vs. IR, and ISP vs. RIP groups, respectively). (E–H) Twenty pathways most associated with downregulated lncRNAs in each group comparison (IR vs. sham, ISP vs. IR, RIP vs. IR, and ISP vs. RIP groups, respectively). The q-value is the P-value corrected after multiple hypothesis tests. The q-value ranges from 0 to 1, with values closer to 0 indicating increased enrichment significance. IR: Ischemia/reperfusion; ISP: in situ ischemic postconditioning; RIP: remote ischemic postconditioning.
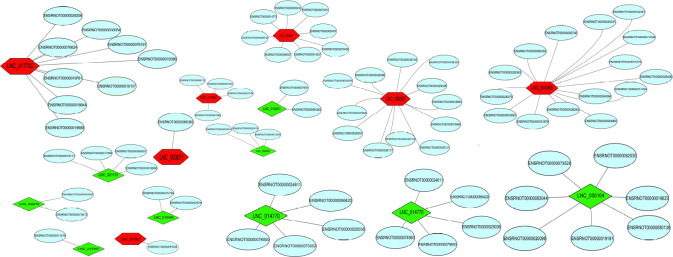
Construction of an LncRNA-mRNA co-expression network after ischemic postconditioning in ischemic stroke rats
According to the correlation between differentially expressed lncRNAs and their cis-acting target mRNAs, lncRNA and mRNA co-expression networks were constructed to identify the underlying molecular mechanisms of differentially expressed lncRNAs in ischemic stroke. We obtained 2616, 68, 128, and 172 differentially expressed lncRNAs and 2825, 36, 89, and 109 cis-targeted mRNAs in the IR vs. sham, ISP vs. IR, RIP vs. IR, and RIP vs. ISP groups, respectively. We further focused on 15 lncRNAs that were significantly differentially expressed between the ISP and IR groups and between the RIP and IR groups for the co-expression network analysis (Figure 9). LncRNA 003369 targeted 15 mRNAs; lncRNA 000857 targeted 12 mRNAs; lncRNA 017702 targeted nine mRNAs; lncRNA 014770 targeted five mRNAs; lncRNA 000164 targeted seven mRNAs; lncRNA 023605 targeted eight mRNAs; lncRNA 023605 targeted eight mRNAs; lncRNAs 026276, 010495, and 018231 each targeted two mRNA; and lncRNAs 014827, 015397, and 000871 each targeted one mRNA.
Figure 9.
Co-expression networks of all differentially expressed long noncoding RNAs (lncRNAs) and mRNAs in the cerebral tissue of IR rats with ISP and RIP treatment.
LncRNA-mRNA co-expression networks were constructed for the IR vs. sham, ISP vs. IR, RIP vs. IR, and RIP vs. ISP group-wise comparisons. Upregulated lncRNAs are shown in red, downregulated lncRNAs are shown in green, and mRNAs are shown in blue. The ellipses represent mRNA, and the hexagons and quadrilaterals represent upregulated and downregulated lncRNAs, respectively. IR: Ischemia/reperfusion; ISP: in situ ischemic postconditioning; RIP: remote ischemic postconditioning.
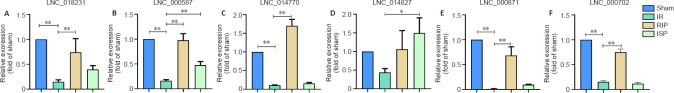
Validation of differentially expressed lncRNAs after ischemic postconditioning in ischemic stroke rats
A total of 15 differentially expressed lncRNAs were identified in the comparison between the ISP vs. IR and RIP vs. IR groups (Figure 6A). Of these, we selected six lncRNAs that were significantly upregulated or downregulated in the post-ischemic adaptation groups (ISP vs. IR and RIP vs. IR groups) for verification using quantitative real-time reverse transcription-PCR. The overall expression levels of lncRNAs 018231, 000587, 014770, 014827, 017702, and 000871 were significantly downregulated in the IR group compared with those in the sham group (P < 0.01 in Figure 10A-C, E, and F; P > 0.05 in Figure 10D). The overall expression levels of lncRNAs 018231, 000587, 014770, 014827, 017702, and 000871 were significantly upregulated in the RIP and ISP groups compared with those in the IR group (P < 0.01 in Figure 10A-C, E and F; P < 0.05 in Figure 10D), consistent with the RNA sequencing data. The network suggests that these differentially expressed lncRNAs may regulate their corresponding target mRNAs during both ischemic injury and ischemic postconditioning.
Figure 10.
Validation of candidate long noncoding RNAs (lncRNAs) in the brain tissue of IR rats with ISP and RIP treatment. Six lncRNAs that were significantly upregulated in the ischemic postconditioning groups (ISP vs.
IR and RIP vs. IR) were selected for quantitative real-time reverse transcription-PCR verification. Data are shown as the mean ± SEM (n = 3). *P < 0.05, **P < 0.01 (one-way analysis of variance followed by Tukey’s post hoc test). IR: Ischemia/reperfusion; ISP: in situ ischemic postconditioning; RIP: remote ischemic postconditioning.
Discussion
Previous studies have suggested that ischemic postconditioning has cerebral protective effects in various cerebral ischemic stroke models (Zhao, 2009; Xie et al., 2018; Li et al., 2021). Ischemic postconditioning can effectively reduce the size of the infarct area and cerebral edema, improve cerebral circulation, and relieve inflammation, reperfusion injury, and neural cell apoptosis (Liu et al., 2014a, b). Recent studies have shown that ischemic postconditioning is associated with many neuroprotective mechanisms, such as regulating the expression of neurotrophic factors (Ramagiri and Taliyan, 2017), neurovascular network-based ischemic tolerance (Lehotský et al., 2009; Deng et al., 2014), subcellular organelle-based ischemic tolerance (Lai et al., 2014; Pignataro et al., 2014), synaptic signaling-based ischemic tolerance (Bu et al., 2014; Jiang et al., 2015), protein degradation systems-based ischemic tolerance (Della-Morte et al., 2012; Narayanan et al., 2013), and the regulation of anti-inflammatory, anti-apoptotic, and anti-oxidative pathways (Dharap et al., 2012; Wei et al., 2016). These studies demonstrate that the neuroprotective mechanisms of ischemic postconditioning are multifaceted (Zhao, 2009; Xie et al., 2018; Li et al., 2021). However, systematic research exploring the neuroprotective mechanisms underlying the effects of ischemic postconditioning after stroke at the transcriptional level and the identification of the key biological processes, cellular components, molecular functions, and signaling pathways involved in these protective effects are lacking.
Studies have reported changes in the expression characteristics of noncoding RNAs after stroke; these noncoding RNAs regulate important cellular events in ischemic stroke through a variety of mechanisms (Zhao et al., 2015; Karner et al., 2020; Miao et al., 2020). Recent studies have shown that focal cerebral ischemia in adult rats extensively alter the lncRNA expression profiles of the brain during acute reperfusion, and altered lncRNAs may play critical roles in epigenetic changes after stroke (Dhami et al., 2013; Liu et al., 2018a; Shin et al., 2020). Bioinformatics analysis showed that among the significantly differentially expressed lncRNAs identified after stroke, 90% of sequences were homologous, indicating that hypoxic-ischemic injury altered the expression profile of brain lncRNAs in newborn rats (Hori et al., 2012). In this study, we found that ischemic postconditioning after ischemic stroke significantly altered the cerebral lncRNA expression profiles. Hierarchical clustering analysis showed systematic variations in the expression of lncRNAs and protein-coding RNAs among different groups in the ischemic brain. LncRNA expression patterns were similar among different samples within the same groups, indicating that lncRNAs in the same group may participate in similar biological processes and suggesting the functional conservation of lncRNAs (Zhao et al., 2015).
The KEGG enrichment analysis in the present study showed that significantly upregulated or downregulated lncRNAs after ischemic postconditioning in ischemic stroke rats were associated with the PI3K/Akt pathway. The PI3K/Akt pathway can regulate cell survival and growth by inhibiting apoptosis following cerebral IR injury (Ren et al., 2016). Moreover, we found that the lncRNAs that were significantly altered after IR injury were enriched in metabolic pathways, indicating that metabolic and cellular pathologies may be altered after ischemic postconditioning in ischemic stroke. Several studies have investigated the metabolic changes that occur during acute ischemic strokes of varying severities and found that excitotoxicity is the initial cellular-level insult mechanism associated with cerebral ischemia (Buga et al., 2012). Excitotoxicity is triggered by the failure to maintain metabolic homeostasis, resulting in the secretion of metabolites, including glutamate, glycine, D-serine, and polyamines (Xu et al., 2017).
LncRNA-mRNA regulatory networks might provide new insights into the molecular mechanisms underlying the therapeutic effects of ischemic postconditioning. The co-expression network suggested that lncRNAs may be involved in the regulation of corresponding target mRNAs in the IR, ISP, and RIP groups after ischemic stroke. Bioinformatics analysis indicated that the differentially expressed lncRNAs identified after ischemic postconditioning might be associated with inflammation, neuroactive ligand-receptor interactions, calcium signaling, and antigen processing- and presentation-related pathways. We examined 15 lncRNAs and eight mRNAs that were differentially expressed between the ISP and IR groups and the RIP and IR groups in the co-expression network. We speculate that these 15 lncRNAs and eight mRNAs may play important roles in ischemic postconditioning. The expression of lncRNAs was highly correlated with the expression of neighboring mRNAs, which suggested that lncRNAs may exert their functions through these predicted mRNA targets (Zhao et al., 2015; Ren et al., 2016). We searched for coding genes located within 100 kb upstream and downstream of lncRNAs as potential target genes and used these genes to predict the functions of lncRNAs. We found that some lncRNAs, such as lncRNA 003369, lncRNA 017702, and lncRNA 014770, were associated with more than five mRNA targets. GO term and KEGG pathway analyses indicated that these lncRNAs might respond to ischemic postconditioning through different mechanisms after ischemic stroke, such as anti-inflammatory reactions (Chen et al., 2018), apoptosis (Pignataro et al., 2013; Esposito et al., 2018; Nichols et al., 2018), neuroactive ligand-receptor interactions (Li et al., 2021), oxidative stress (Pyfrom et al., 2020), calcium signaling (Vassallo et al., 2016; Pyfrom et al., 2020), and the cAMP response element binding protein/brain-derived neurotrophic factor signaling pathway (Lipovich et al., 2012). These findings suggest that changes in lncRNA expression profiles may be associated with ischemic stroke, and the neuroprotection afforded by ischemic postconditioning may involve the regulation of differentially expressed lncRNA expression. A previous study showed that cerebral lncRNAs were significantly altered after stroke (Duan et al., 2019), which supports our results. We further found that lncRNA 017702 was upregulated after ischemic postconditioning, according to the quantitative real-time reverse transcription-PCR and RNA sequencing results, and lncRNA 017702 was associated with nine target genes in the co-expression network, most of which were involved in the inflammatory response that causes brain edema. These results indicated that these genes might function in the regulation of the inflammatory response during ischemic postconditioning after stroke. A previous study found that the lncRNA Malat1 plays anti-apoptotic and anti-inflammatory roles in the brain microvasculature, reducing ischemic cerebral vascular and parenchymal damage (Zhang et al., 2017). We speculated that lncRNA 017702 expression was upregulated, and pro-inflammatory gene expression was downregulated, following the RIP intervention, which reduced the degree of cerebral edema in rats with ischemic stroke. In support of this speculation, a previous study found that some lncRNAs that are upregulated after stroke in rats were identified to function in ischemic stroke through the inhibition of endothelial cell death and inflammation, such as the lncRNA Malat1 (Zhang et al., 2017).
This study has two limitations. First, we did not use more than three behavioral scoring methods to evaluate neurological deficits in tMCAO rats. The TTC staining images showed ischemic changes in brain tissue that are consistent with ischemic changes in the region of the middle cerebral artery. Therefore, we successfully established a rat tMCAO model. In future studies, we will use three behavioral scoring methods: the Zea Longa method, with a 5-point scale; the Garcia method, with an 18-point scale (Longa et al., 1989; Garcia et al., 1995); and modified neurologic severity scores (Zhou et al., 2011), combined with cerebral blood flow monitoring in rats to confirm the success of tMCAO model establishment. Although the cerebral tissue from the infarct area of each group was used to perform RNA sequencing in this study, the ischemic penumbra would provide more meaningful results because saving the ischemic penumbra is a key aim of ischemic stroke treatment. Therefore, our follow-up studies will use ischemic penumbra tissue from the rat brain to conduct RNA sequencing and explore the postconditioning protective mechanisms of the brain.
In conclusion, we investigated the expression profiles of the lncRNAs in the IR, ISP, and RIP groups after stroke. Studying the expression patterns of these RNAs at the overall gene expression level in cerebral IR injury and ischemic postconditioning after stroke is critical to developing new strategies for the treatment of ischemic stroke. Bioinformatics analyses revealed a complex lncRNA profile and significantly enriched GO terms and KEGG pathways associated with ischemic postconditioning after stroke. Nine candidate lncRNAs were identified by constructing lncRNA expression profiles and lncRNA-mRNA co-expression network analysis. These lncRNAs may be involved in the neurological protective effects associated with ischemic postconditioning in ischemic stroke. Our subsequent studies will focus on whether these specific lncRNAs can be targeted to prevent IR injury or promote angiogenesis and neuronal regeneration in animal models of ischemic stroke. Our study is the first study to provide a comprehensive, temporal description of the molecular events contributing to the pathogenesis of ischemic stroke in rats and uncovered functional RNA regulatory networks associated with ischemic stroke. These findings demonstrated that ischemic postconditioning methods might be used as effective interventions for cerebral ischemic injury, providing a theoretical basis for future clinical applications.
Additional files:
Additional Figure 1 (1MB, tif) : The schematic diagram about postconditioning intervenes in focal ischemic stroke rat.
The schematic diagram about postconditioning intervenes in focal ischemic stroke rat.
The figure was reprinted from Li et al. (2020). Copyright 2020, with permission from Elsevier.
Additional Figure 2 (285.3KB, tif) : Effect of ISP and RIP on the neurological function deficit score of IR rats.
Effect of ISP and RIP on the neurological function deficit score of IR rats.
The higher the Neurological function scores, the worse the neurological function. Data are shown as the mean ± SEM (n = 14). *P < 0.05, **P < 0.01 (one-way analysis of variance followed by Tukey's post hoc test). IR: Ischemia/reperfusion; ISP: in situ ischemic postconditioning; RIP: remote ischemic postconditioning.
Additional Table 1 (232.2MB, pdf) : Long noncoding RNA target mRNA analysis in cis role.
Long noncoding RNA target mRNA analysis in cis role
Additional Table 2 (707KB, pdf) : KEGG pathway enrichment in IR vs. Sham group.
KEGG pathway enrichment in IR vs. Sham group
Additional Table 3 (200.5KB, pdf) : KEGG pathway enrichment in ISP vs. IR group.
KEGG pathway enrichment in ISP vs. IR group
Additional Table 4 (272.1KB, pdf) : KEGG pathway enrichment in RIP vs. IR group.
KEGG pathway enrichment in RIP vs. IR group
Additional Table 5 (322KB, pdf) : KEGG pathway enrichment in ISP vs. RIP group.
KEGG pathway enrichment in ISP vs. RIP group
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 31560295 (to LYL); the Yunnan Applied Basic Research Projects of China, Nos. 2018FE001(-016) (to WM), 2018FE001(-163) (to LYL); and the Research Innovation Team of Yunnan Province of China, No. 2019HC022 (to LYL). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: The study was approved by the Animal Experiment Ethics Committee of Kunming Medical University (approval No. KMMU2018018) in January 2018.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: The data used during this study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the National Natural Science Foundation of China, No. 31560295 (to LYL); the Yunnan Applied Basic Research Projects of China, Nos. 2018FE001(-016) (to WM), 2018FE001(-163) (to LYL); and the Research Innovation Team of Yunnan Province of China, No. 2019HC022 (to LYL).
References
- 1.An TJ, Zhang W, Yang H, Wang QF. Potential mechanisms of bone marrow stem cells in the treatment of ischemic stroke based on bioinformatics. Zhongguo Zuzhi Gongcheng Yanjiu. 2019;23:5249–5255. [Google Scholar]
- 2.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 3.Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, MacKenzie ET, Petit E. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Briggs JA, Wolvetang EJ, Mattick JS, Rinn JL, Barry G. Mechanisms of long non-coding RNAs in mammalian nervous system development plasticity disease and evolution. Neuron. 2015;88:861–877. doi: 10.1016/j.neuron.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 5.Bu Q, Liu X, Zhu Y, Liu Y, Wang Y. w007B protects brain against ischemia-reperfusion injury in rats through inhibiting inflammation apoptosis and autophagy. Brain Res. 2014;1558:100–108. doi: 10.1016/j.brainres.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 6.Buga AM, Scholz CJ, Kumar S, Herndon JG, Alexandru D, Cojocaru GR, Dandekar T, Popa-Wagner A. Identification of new therapeutic targets by genome-wide analysis of gene expression in the ipsilateral cortex of aged rats after stroke. PLoS One. 2012;7:e50985. doi: 10.1371/journal.pone.0050985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen GZ, Shan XY, Li XS, Tao HM. Remote ischemic postconditioning protects the brain from focal ischemia/reperfusion injury by inhibiting autophagy through the mTOR/p70S6K pathway. Neurol Res. 2018;40:182–188. doi: 10.1080/01616412.2018.1424696. [DOI] [PubMed] [Google Scholar]
- 8.Dai Y, Ma W, Zhang T, Yang J, Zang C, Liu K, Wang X, Wang J, Wu Z, Zhang X, Li C, Li J, Wang X, Guo J, Li L. Long noncoding RNA expression profiling during the neuronal differentiation of glial precursor cells from rat dorsal root ganglia. Biotechnol Bioprocess Eng. 2020;25:356–373. [Google Scholar]
- 9.Daidone M, Cataldi M, Pinto A, Tuttolomondo A. Non-coding RNAs and other determinants of neuroinflammation and endothelial dysfunction: regulation of gene expression in the acute phase of ischemic stroke and possible therapeutic applications. Neural Regen Res. 2021;16:2154–2158. doi: 10.4103/1673-5374.310607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Della-Morte D, Guadagni F, Palmirotta R, Ferroni P, Testa G, Cacciatore F, Abete P, Rengo F, Perez-Pinzon MA, Sacco RL, Rundek T. Genetics and genomics of ischemic tolerance: focus on cardiac and cerebral ischemic preconditioning. Pharmacogenomics. 2012;13:1741–1757. doi: 10.2217/pgs.12.157. [DOI] [PubMed] [Google Scholar]
- 11.Deng J, Lei C, Chen Y, Fang Z, Yang Q, Zhang H, Cai M, Shi L, Dong H, Xiong L. Neuroprotective gases--fantasy or reality for clinical use. Prog Neurobiol. 2014;115:210–245. doi: 10.1016/j.pneurobio.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Dhami KS, Churchward MA, Baker GB, Todd KG. Fluoxetine and citalopram decrease microglial release of glutamate and D-serine to promote cortical neuronal viability following ischemic insult. Mol Cell Neurosci. 2013;56:365–374. doi: 10.1016/j.mcn.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Dharap A, Nakka VP, Vemuganti R. Effect of focal ischemia on long noncoding RNAs. Stroke. 2012;43:2800–2802. doi: 10.1161/STROKEAHA.112.669465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Y, Wang Y, Wang X, Nie QZ. Effects of long non-coding RNA myocardial infarction-associated transcript on retinal neovascularization in a newborn mouse model of oxygen-induced retinopathy. Neural Regen Res. 2021;16:1877–1881. doi: 10.4103/1673-5374.306098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan X, Han L, Peng D, Peng C, Xiao L, Bao Q, Peng H. Bioinformatics analysis of a long noncoding RNA and mRNA regulation network in rats with middle cerebral artery occlusion based on RNA sequencing. Mol Med Rep. 2019;20:417–432. doi: 10.3892/mmr.2019.10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esposito E, Hayakawa K, Ahn BJ, Chan SJ, Xing C, Liang AC, Kim KW, Arai K, Lo EH. Effects of ischemic post-conditioning on neuronal VEGF regulation and microglial polarization in a rat model of focal cerebral ischemia. J Neurochem. 2018;146:160–172. doi: 10.1111/jnc.14337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan YY, Hu WW, Nan F, Chen Z. Postconditioning-induced neuroprotection mechanisms and applications in cerebral ischemia. Neurochem Int. 2017;107:43–56. doi: 10.1016/j.neuint.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O’Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C, et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, Eddy SR, Heger A, Hetherington K, Holm L, Mistry J, Sonnhammer EL, Tate J, Punta M. Pfam: the protein families database. Nucleic Acids Res. 2014;42:D222–230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–635. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 21.Gene Ontology Consortium. Blake JA, Dolan M, Drabkin H, Hill DP, Li N, Sitnikov D, Bridges S, Burgess S, Buza T, McCarthy F, Peddinti D, Pillai L, Carbon S, Dietze H, Ireland A, Lewis SE, Mungall CJ, Gaudet P, Chrisholm RL, et al. Gene Ontology annotations and resources. Nucleic Acids Res. 2013;41:D530–535. doi: 10.1093/nar/gks1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halkos ME, Kerendi F, Corvera JS, Wang NP, Kin H, Payne CS, Sun HY, Guyton RA, Vinten-Johansen J, Zhao ZQ. Myocardial protection with postconditioning is not enhanced by ischemic preconditioning. Ann Thorac Surg. 2004;78:961–969. doi: 10.1016/j.athoracsur.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 23.Hart RP, Goff LA. Long noncoding RNAs: Central to nervous system development. Int J Dev Neurosci. 2016;55:109–116. doi: 10.1016/j.ijdevneu.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hori M, Nakamachi T, Rakwal R, Shibato J, Nakamura K, Wada Y, Tsuchikawa D, Yoshikawa A, Tamaki K, Shioda S. Unraveling the ischemic brain transcriptome in a permanent middle cerebral artery occlusion mouse model by DNA microarray analysis. Dis Model Mech. 2012;5:270–283. doi: 10.1242/dmm.008276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang T, Yu JT, Zhu XC, Zhang QQ, Tan MS, Cao L, Wang HF, Shi JQ, Gao L, Qin H, Zhang YD, Tan L. Ischemic preconditioning provides neuroprotection by induction of AMP-activated protein kinase-dependent autophagy in a rat model of ischemic stroke. Mol Neurobiol. 2015;51:220–229. doi: 10.1007/s12035-014-8725-6. [DOI] [PubMed] [Google Scholar]
- 26.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karner H, Webb CH, Carmona S, Liu Y, Lin B, Erhard M, Chan D, Baldi P, Spitale RC, Sun S. Functional conservation of lncRNA JPX Despite sequence and structural divergence. J Mol Biol. 2020;432:283–300. doi: 10.1016/j.jmb.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Knauss JL, Sun T. Regulatory mechanisms of long noncoding RNAs in vertebrate central nervous system development and function. Neuroscience. 2013;235:200–214. doi: 10.1016/j.neuroscience.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong L, Zhang Y, Ye ZQ, Liu XQ, Zhao SQ, Wei L, Gao G. CPC: assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007;35:W345–349. doi: 10.1093/nar/gkm391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol. 2014;115:157–188. doi: 10.1016/j.pneurobio.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Lehotský J, Burda J, Danielisová V, Gottlieb M, Kaplán P, Saniová B. Ischemic tolerance: the mechanisms of neuroprotective strategy. Anat Rec (Hoboken) 2009;292:2002–2012. doi: 10.1002/ar.20970. [DOI] [PubMed] [Google Scholar]
- 32.Li CY, Ma W, Liu KP, Yang JW, Wang XB, Wu Z, Zhang T, Wang JW, Liu W, Liu J, Liang Y, Zhang XK, Li JJ, Guo JH, Li LY. Different ischemic duration and frequency of ischemic postconditioning affect neuroprotection in focal ischemic stroke. J Neurosci Methods. 2020;346:108921. doi: 10.1016/j.jneumeth.2020.108921. [DOI] [PubMed] [Google Scholar]
- 33.Li CY, Ma W, Liu KP, Yang JW, Wang XB, Wu Z, Zhang T, Wang JW, Liu W, Liu J, Liang Y, Zhang XK, Li JJ, Guo JH, Li LY. Advances in intervention methods and brain protection mechanisms of in situ and remote ischemic postconditioning. Metab Brain Dis. 2021;36:53–65. doi: 10.1007/s11011-020-00562-x. [DOI] [PubMed] [Google Scholar]
- 34.Li DJ, Li YH, Yuan HB, Qu LF, Wang P. The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism. 2017;68:31–42. doi: 10.1016/j.metabol.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Lipovich L, Dachet F, Cai J, Bagla S, Balan K, Jia H, Loeb JA. Activity-dependent human brain coding/noncoding gene regulatory networks. Genetics. 2012;192:1133–1148. doi: 10.1534/genetics.112.145128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipovich L, Tarca AL, Cai J, Jia H, Chugani HT, Sterner KN, Grossman LI, Uddin M, Hof PR, Sherwood CC, Kuzawa CW, Goodman M, Wildman DE. Developmental changes in the transcriptome of human cerebral cortex tissue: long noncoding RNA transcripts. Cereb Cortex. 2014;24:1451–1459. doi: 10.1093/cercor/bhs414. [DOI] [PubMed] [Google Scholar]
- 37.Liu C, Yang J, Zhang C, Liu M, Geng X, Ji X, Du H, Zhao H. Analysis of long non-coding RNA expression profiles following focal cerebral ischemia in mice. Neurosci Lett. 2018a;665:123–129. doi: 10.1016/j.neulet.2017.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Wang Y, Akamatsu Y, Lee CC, Stetler RA, Lawton MT, Yang GY. Vascular remodeling after ischemic stroke: mechanisms and therapeutic potentials. Prog Neurobiol. 2014a;115:138–156. doi: 10.1016/j.pneurobio.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, Zhang KS, Hu B, Li SG, Li Q, Luo YP, Wang Y, Deng ZF. Systematic analysis of RNA regulatory network in rat brain after ischemic stroke. Biomed Res Int. 2018b;2018:8354350. doi: 10.1155/2018/8354350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Zhao S, Liu F, Kang J, Xiao A, Li F, Zhang C, Yan F, Zhao H, Luo M, Luo Y, Ji X. Remote ischemic postconditioning alleviates cerebral ischemic injury by attenuating endoplasmic reticulum stress-mediated apoptosis. Transl Stroke Res. 2014b;5:692–700. doi: 10.1007/s12975-014-0359-5. [DOI] [PubMed] [Google Scholar]
- 41.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 42.Miao W, Yan Y, Bao TH, Jia WJ, Yang F, Wang Y, Zhu YH, Yin M, Han JH. Ischemic postconditioning exerts neuroprotective effect through negatively regulating PI3K/Akt2 signaling pathway by microRNA-124. Biomed Pharmacother. 2020;126:109786. doi: 10.1016/j.biopha.2019.109786. [DOI] [PubMed] [Google Scholar]
- 43.Narayanan SV, Dave KR, Perez-Pinzon MA. Ischemic preconditioning and clinical scenarios. Curr Opin Neurol. 2013;26:1–7. doi: 10.1097/WCO.0b013e32835bf200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ng SY, Lin L, Soh BS, Stanton LW. Long noncoding RNAs in development and disease of the central nervous system. Trends Genet. 2013;29:461–468. doi: 10.1016/j.tig.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Nichols M, Pavlov EV, Robertson GS. Tamoxifen-induced knockdown of the mitochondrial calcium uniporter in Thy1-expressing neurons protects mice from hypoxic/ischemic brain injury. Cell Death Dis. 2018;9:606. doi: 10.1038/s41419-018-0607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ørom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, Lai F, Zytnicki M, Notredame C, Huang Q, Guigo R, Shiekhattar R. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT StringTie and Ballgown. Nat Protoc. 2016;11:1650–1667. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pignataro G, Sirabella R, Anzilotti S, Di Renzo G, Annunziato L. Does Na+/Ca2+ exchanger, NCX, represent a new druggable target in stroke intervention? Transl Stroke Res. 2014;5:145–155. doi: 10.1007/s12975-013-0308-8. [DOI] [PubMed] [Google Scholar]
- 49.Pignataro G, Cuomo O, Vinciguerra A, Sirabella R, Esposito E, Boscia F, Di Renzo G, Annunziato L. NCX as a key player in the neuroprotection exerted by ischemic preconditioning and postconditioning. Adv Exp Med Biol. 2013;961:223–240. doi: 10.1007/978-1-4614-4756-6_19. [DOI] [PubMed] [Google Scholar]
- 50.Pyfrom SC, Quinn CC, Dorando HK, Luo H, Payton JE. BCALM (AC0995241) Is a human B lymphocyte-specific long noncoding RNA that modulates B cell receptor-mediated calcium signaling. J Immunol. 2020;205:595–607. doi: 10.4049/jimmunol.2000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qureshi IA, Mehler MF. Emerging roles of non-coding RNAs in brain evolution development plasticity and disease. Nat Rev Neurosci. 2012;13:528–541. doi: 10.1038/nrn3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qureshi IA, Mattick JS, Mehler MF. Long non-coding RNAs in nervous system function and disease. Brain Res. 2010;1338:20–35. doi: 10.1016/j.brainres.2010.03.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramagiri S, Taliyan R. Remote limb ischemic post conditioning during early reperfusion alleviates cerebral ischemic reperfusion injury via GSK-3β/CREB/ BDNF pathway. Eur J Pharmacol. 2017;803:84–93. doi: 10.1016/j.ejphar.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 54.Ren H, Wang G, Chen L, Jiang J, Liu L, Li N, Zhao J, Sun X, Zhou P. Genome-wide analysis of long non-coding RNAs at early stage of skin pigmentation in goats (Capra hircus) BMC Genomics. 2016;17:67. doi: 10.1186/s12864-016-2365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schaukowitch K, Kim TK. Emerging epigenetic mechanisms of long non-coding RNAs. Neuroscience. 2014;264:25–38. doi: 10.1016/j.neuroscience.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schellinger PD, Kaste M, Hacke W. An update on thrombolytic therapy for acute stroke. Curr Opin Neurol. 2004;17:69–77. doi: 10.1097/00019052-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shin TH, Lee DY, Basith S, Manavalan B, Paik MJ, Rybinnik I, Mouradian MM, Ahn JH, Lee G. Metabolome changes in cerebral ischemia. Cells. 2020;9:1630. doi: 10.3390/cells9071630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stapf C, Mohr JP. Ischemic stroke therapy. Annu Rev Med. 2002;53:453–475. doi: 10.1146/annurev.med.53.082901.104106. [DOI] [PubMed] [Google Scholar]
- 60.Sun L, Luo H, Bu D, Zhao G, Yu K, Zhang C, Liu Y, Chen R, Zhao Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013;41:e166. doi: 10.1093/nar/gkt646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tyagi S, Singh N, Virdi JK, Jaggi AS. Diabetes abolish cardioprotective effects of remote ischemic conditioning: evidences and possible mechanisms. J Physiol Biochem. 2019;75:19–28. doi: 10.1007/s13105-019-00664-w. [DOI] [PubMed] [Google Scholar]
- 64.van de V, II, Gordebeke PM, Khoshab N, Tiesinga PH, Buitelaar JK, Kozicz T, Aschrafi A, Glennon JC. Long non-coding RNAs in neurodevelopmental disorders. Front Mol Neurosci. 2013;6:53. doi: 10.3389/fnmol.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vassallo I, Zinn P, Lai M, Rajakannu P, Hamou MF, Hegi ME. WIF1 re-expression in glioblastoma inhibits migration through attenuation of non-canonical WNT signaling by downregulating the lncRNA MALAT1. Oncogene. 2016;35:12–21. doi: 10.1038/onc.2015.61. [DOI] [PubMed] [Google Scholar]
- 66.Wang X, Ma W, Wang T, Yang J, Wu Z, Liu K, Dai Y, Zang C, Liu W, Liu J, Liang Y, Guo J, Li L. BDNF-TrkB and proBDNF-p75NTR/sortilin signaling pathways are involved in mitochondria-mediated neuronal apoptosis in dorsal root ganglia after sciatic nerve transection. CNS Neurol Disord Drug Targets. 2020;19:66–82. doi: 10.2174/1871527319666200117110056. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Reis C, Applegate R, 2nd, Stier G, Martin R, Zhang JH. Ischemic conditioning-induced endogenous brain protection: Applications pre- per- or post-stroke. Exp Neurol. 2015;272:26–40. doi: 10.1016/j.expneurol.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Ge P, Yang L, Wu C, Zha H, Luo T, Zhu Y. Protection of ischemic post conditioning against transient focal ischemia-induced brain damage is associated with inhibition of neuroinflammation via modulation of TLR2 and TLR4 pathways. J Neuroinflammation. 2014;11:15. doi: 10.1186/1742-2094-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y, Pan WY, Ge JS, Wang XD, Chen W, Luo X, Wang YL. A review of the relationship between long noncoding RNA and post-stroke injury repair. J Int Med Res. 2019;47:4619–4624. doi: 10.1177/0300060519867493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei N, Xiao L, Xue R, Zhang D, Zhou J, Ren H, Guo S, Xu J. MicroRNA-9 mediates the cell apoptosis by targeting Bcl2l11 in ischemic stroke. Mol Neurobiol. 2016;53:6809–6817. doi: 10.1007/s12035-015-9605-4. [DOI] [PubMed] [Google Scholar]
- 71.Xie R, Li J, Zhao H. The underlying mechanisms involved in the protective effects of ischemic postconditioning. Cond Med. 2018;1:73–79. [PMC free article] [PubMed] [Google Scholar]
- 72.Xu W, Jin W, Zhang X, Chen J, Ren C. Remote limb preconditioning generates a neuroprotective effect by modulating the extrinsic apoptotic pathway and TRAIL-receptors expression. Cell Mol Neurobiol. 2017;37:169–182. doi: 10.1007/s10571-016-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang J, Yuan L, Zhang X, Hamblin MH, Zhu T, Meng F, Li Y, Chen YE, Yin KJ. Altered long non-coding RNA transcriptomic profiles in brain microvascular endothelium after cerebral ischemia. Exp Neurol. 2016;277:162–170. doi: 10.1016/j.expneurol.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang X, Tang X, Liu K, Hamblin MH, Yin KJ. Long Noncoding RNA Malat1 regulates cerebrovascular pathologies in ischemic stroke. J Neurosci. 2017;37:1797–1806. doi: 10.1523/JNEUROSCI.3389-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao F, Qu Y, Liu J, Liu H, Zhang L, Feng Y, Wang H, Gan J, Lu R, Mu D. Microarray profiling and co-expression network analysis of lncRNAs and mRNAs in neonatal rats following hypoxic-ischemic brain damage. Sci Rep. 2015;5:13850. doi: 10.1038/srep13850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao H. Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. J Cereb Blood Flow Metab. 2009;29:873–885. doi: 10.1038/jcbfm.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou J, Li J, Rosenbaum DM, Barone FC. Thrombopoietin protects the brain and improves sensorimotor functions: reduction of stroke-induced MMP-9 upregulation and blood-brain barrier injury. J Cereb Blood Flow Metab. 2011;31:924–933. doi: 10.1038/jcbfm.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The schematic diagram about postconditioning intervenes in focal ischemic stroke rat.
The figure was reprinted from Li et al. (2020). Copyright 2020, with permission from Elsevier.
Effect of ISP and RIP on the neurological function deficit score of IR rats.
The higher the Neurological function scores, the worse the neurological function. Data are shown as the mean ± SEM (n = 14). *P < 0.05, **P < 0.01 (one-way analysis of variance followed by Tukey's post hoc test). IR: Ischemia/reperfusion; ISP: in situ ischemic postconditioning; RIP: remote ischemic postconditioning.
Long noncoding RNA target mRNA analysis in cis role
KEGG pathway enrichment in IR vs. Sham group
KEGG pathway enrichment in ISP vs. IR group
KEGG pathway enrichment in RIP vs. IR group
KEGG pathway enrichment in ISP vs. RIP group