Abstract
Associations between prenatal maternal psychological distress and offspring developmental outcomes are well documented, yet relatively little research has examined links between maternal distress and development in utero, prior to postpartum influences. Fetal heart rate (FHR) parameters are established indices of central and autonomic nervous system maturation and function which demonstrate continuity with postnatal outcomes. This prospective, longitudinal study of 149 maternal–fetal pairs evaluated associations between prenatal maternal distress, FHR parameters, and dimensions of infant temperament. Women reported their symptoms of psychological distress at five prenatal visits, and FHR monitoring was conducted at the last three visits. Maternal report of infant temperament was collected at 3 and 6 months of age. Exposure to elevated prenatal maternal psychological distress was associated with higher late-gestation resting mean FHR (FHRM) among female but not male fetuses. Higher late-gestation FHRM was associated with lower infant orienting/regulation and with higher infant negative affectivity, and these associations did not differ by infant sex. A path analysis identified higher FHRM as one pathway by which elevated prenatal maternal distress was associated with lower orienting/regulation among female infants. Findings suggest that, for females, elevated maternal distress alters fetal development, with implications for postnatal function. Results also support the notion that, for both sexes, individual differences in regulation emerge prenatally and are maintained into infancy. Collectively, these findings underscore the utility of direct assessment of development in utero when examining if prenatal experiences are carried forward into postnatal life.
Keywords: fetal, heart rate, prenatal, stress, temperament
Thirty years ago, David Barker (1990) proffered his seminal hypothesis on “the fetal and infant origins of adult disease.” In the intervening years, accumulating evidence has established that experiences of stress in utero are associated with mental and physical health across the lifespan (Doyle & Cicchetti, 2018; O’Donnell & Meaney, 2017). The potential programming influences of prenatal maternal psychological distress (i.e., depression, anxiety, perceived stress) on emotional, cognitive, and behavioral outcomes have been of substantial interest. Prospective, longitudinal studies consistently have documented associations between prenatal maternal psychological distress and child cognition, temperament, stress physiology, and psychopathology (see Kingston, McDonald, Austin, & Tough, 2015; Madigan et al., 2018; O’Connor, Monk, & Burke, 2016; Sandman, Glynn, & Davis, 2016). These associations are presumed to be partially mediated by alterations in fetal brain and neurobehavioral development. In support of this premise, measures of prenatal maternal psychological distress have been linked with brain structure and function from infancy into adolescence (Adamson, Letourneau, & Lebel, 2018; Davis et al., 2020; Sandman, Buss, Head, & Davis, 2015).
A primary advantage to studying neurobehavioral development in utero is that associations between maternal psychological distress and developmental outcomes can be examined prior to postpartum influences. Measures of fetal brain and neurobehavioral development are more proximal and likely more accurate indicators of fetal exposures (DiPietro, Costigan, & Voegtline, 2015; Sandman, 2015). Very recent advances in fetal functional magnetic resonance imaging have enabled direct observation of brain development during the prenatal period (Thomason et al., 2018; van den Heuvel & Thomason, 2016). However, there is a long-standing tradition of assessment of fetal behavior as a noninvasive method of measuring neurodevelopment (see DiPietro et al., 2015). Fetal heart rate (FHR) and fetal movement parameters are established indices of central and autonomic nervous system maturation and function which demonstrate continuity with postnatal outcomes (DiPietro et al., 2015, 2010; Nijhuis, Swaab, Heuser, Brosens, & Eskes, 2003; Sandman, 2015).
FHR is the most accessible and quantifiable indicator of fetal development and is determined by both neural and nonneural influences (see DiPietro et al., 2015; Sandman, 2015). Primary neural influences are increasing parasympathetic innervation of the heart over gestation, as well as shifts in autonomic control from the medulla oblongata to higher cortical processes around 27 weeks’ gestation (Dalton, Dawes, & Patrick, 1983; David, Hirsch, Karin, Toledo, & Akselrod, 2007; Martin, 1978; Yoshizato et al., 1994). As a result, FHR parameters demonstrate predictable maturational patterns, with decreases in mean rate (FHRM) and increases in variability (standard deviation; FHRV) during rest as gestation advances (Dawes, Houghton, Redman, & Visser, 1982; DiPietro et al., 2004, 2015; Van Leeuwen, Lange, Bettermann, Grönemeyer, & Hatzmann, 1999). FHR patterns in response to external stimuli (Buss et al., 2009; Kisilevsky & Low, 1998; Sandman et al., 2003; Sandman, Cordova, Davis, Glynn, & Buss, 2011; Sandman, Wadhwa, Hetrick, Porto, & Peeke, 1997) and to induced maternal arousal (DiPietro, Costigan, & Gurewitsch, 2003; DiPietro, Ghera, & Costigan, 2008; Monk et al., 2011) also display consistent maturational trajectories. In addition to reflecting neural maturation, FHR measures index individual differences in autonomic regulation (see DiPietro et al., 2015; Gunnar, 1990), demonstrating moderate rank-order stability across gestation and into postnatal life, until at least age 2 (DiPietro, Bornstein, Hahn, Costigan, & Achy-Brou, 2007; DiPietro, Costigan, Pressman, & Doussard-Roosevelt, 2000; DiPietro, Hodgson, Costigan, & Johnson, 1996b).
Despite the advantages of measuring development in utero, relatively few empirical studies have examined associations between prenatal maternal psychological distress and FHR parameters. Such associations serve as evidence in support of the hypothesis that maternal distress alters development prior to birth. One study demonstrated that fetuses of mothers with elevated depressive symptoms had higher baseline (prestimulation) late-gestation FHRM at 32–36 weeks’ gestation (Allister, Lester, Carr, & Liu, 2001), whereas another investigation reported that elevated maternal depressive symptoms were associated with lower baseline FHRM at approximately the same gestational age (Dieter, Emory, Johnson, & Raynor, 2008). DiPietro and colleagues (2002b) documented that a greater frequency of maternal pregnancy specific hassles and greater maternal affective intensity were associated with lower resting FHRM at 36 weeks’ gestation. Associations between maternal psychological distress and FHRV also have been reported. In an early investigation, DiPietro and colleagues showed that greater maternal perceived stress was associated with reduced resting FHRV (DiPietro, Hodgson, Costigan, Hilton, & Johnson, 1996a). In two subsequent investigations, higher levels of maternal pregnancy-specific hassles/stress were associated with higher levels of resting FHRV at 36–38 weeks’ gestation and a steeper increase in the coupling of FHR and fetal movement with advancing gestation (DiPietro et al., 2010; DiPietro, Hilton, Hawkins, Costigan, & Pressman, 2002b). In a sample of pregnant adolescents, Doyle et al. (2015) demonstrated that higher maternal negative mood was associated with lower FHRM among male but not female fetuses. In terms of FHR reactivity to stimulation, Monk and colleagues have shown that fetuses of women with elevated symptoms of depression and anxiety display a greater increase in FHR during women’s exposure to a cognitive challenge (Monk et al., 2004; Monk et al., 2011, 2000; Monk, Myers, Sloan, Ellman, & Fifer, 2003). Allister et al. (2001) demonstrated that fetuses of mothers with heightened depressive symptoms displayed a delay in FHR reactivity and return to baseline following vibroacoustic stimulation at 36 weeks’ gestation. While it is difficult to fully synthesize existing research given variability in study designs, predictors, and outcomes, findings generally indicate that maternal psychological distress is associated with both levels and maturational trajectories of FHR over gestation.
The literature examining the extent to which fetal neurobehavioral measures are associated with postnatal developmental outcomes also is relatively circumscribed. FHR parameters are associated with birth outcomes (Emory & Noonan, 1984; Sandman et al., 2011), as well as neonatal neurobehavior (Emory & Noonan, 1984; Figueiredo, Pinto, Pacheco, & Field, 2017) and neurological maturation (DiPietro et al., 2010). Higher levels and steeper developmental trajectories of FHRV have been linked with greater mental and motor development and language ability at age 2 (Bornstein et al., 2002; DiPietro et al., 2007). Consistent with their classification as biological indicators of individual differences in reactivity and regulation (DiPietro et al., 2008; Gunnar, 1990; Rothbart & Bates, 2007; Snidman, Kagan, Riordan, & Shannon, 1995), studies also have examined continuity between FHR parameters and dimensions of temperament in infancy and childhood. DiPietro and colleagues (DiPietro et al., 1996b) reported that higher resting FHRM at 36 weeks’ gestation was associated with lower maternal-reported levels of infant “emotional tone,” predictability, and activity level. In another investigation, fetuses with higher late-gestation coupling of FHR and fetal movement during rest exhibited greater alertness and orienting, better regulatory capacity, and less irritability during neonatal examination (DiPietro, Costigan, & Pressman, 2002a). DiPietro, Voegtline, Pater, and Costigan (2018) also documented that lower resting FHRM and FHRV at 32 weeks’ gestation were associated with higher maternal-reported behavioral inhibition at 7–14 years of age. In this same sample, higher baseline FHRM was associated with greater maternal-reported behavioral problems. Finally, greater third trimester FHR reactivity to induced maternal physiological arousal has been associated with greater irritability during a developmental exam at 6 weeks of age (DiPietro et al., 2008) and with maternal-reported infant negative reactivity and observer-rating infant motor reactivity to novelty at 4 months of age (Werner et al., 2007). These existing findings broadly support the notion that individual differences in FHR demonstrate continuity with dimensions of temperament in postnatal life.
Additional research is needed to determine the replicability and robustness of associations between maternal psychological distress and FHR parameters, and between FHR parameters and postnatal outcomes. The current study builds upon these literatures by addressing four aims. In a prospective, longitudinal study of 149 maternal–fetal/infant pairs, we examined if (a) prenatal maternal psychological distress was associated with developmental trajectories of resting FHRM and FHRV over gestation and (b) FHR parameters were associated with infant temperament at 3–6 months of age. Building upon established evidence that prenatal maternal psychological distress is associated with infant temperament (see Madigan et al., 2018), we assessed if (c) FHR is a pathway by which prenatal maternal psychological distress is associated with infant temperament. This represents a robust test of the programming influences of prenatal maternal psychological distress on postnatal developmental outcomes. Finally, given established sex differences in associations between prenatal exposures and development (Buss et al., 2009; DiPietro et al., 2015; Glynn & Sandman, 2012; Sandman, Glynn, & Davis, 2013), we examined if (d) identified associations were moderated by fetal/infant sex.
Method
Overview
Women were recruited during the first trimester of pregnancy and completed prenatal laboratory visits at approximately 15 (M = 15.57, SD = 1.13), 19 (M = 19.85, SD = 0.94), 25 (M = 25.80, SD = 0.91), 31 (M = 31.09, SD = 0.76), and 37 (M = 36.83, SD = 0.79) weeks’ gestation. The majority of the sample completed all five prenatal visits (82%); 16% of women completed four visits, and 2% completed three visits. Maternal psychological distress was characterized at each study visit with validated self-report questionnaires. FHR monitoring was performed at the last three prenatal visits. Maternal report of infant temperament was collected at 3 and 6 months postpartum.
Participants
The sample consisted of 149 mother–infant pairs drawn from a prospective, longitudinal study. Pregnant women were recruited from a large university medical center in Southern California. At recruitment, inclusion criteria were: (a) adult (≥ 18 years of age), (b) English-speaking, and (c) intrauterine, singleton pregnancy. Exclusion criteria at recruitment were: (a) presence of uterine or cervical abnormalities, (b) conditions such as endocrine, hepatic, or renal disorders, or use of corticosteroid medication, and (c) self-reported abuse of tobacco, alcohol, or recreational drugs in the pregnancy.
Additional inclusion criteria for the current study were (a) delivery at ≥ 37 0/7 weeks, (b) availability of measures of maternal psychological distress at three or more prenatal visits, and (c) measures of FHR at a minimum of two of the three sessions. Of the 149 mothers, 133 completed measures of infant temperament at 3 and/or 6 months’ postpartum. Dyads without infant temperament measures did not differ from dyads with temperament data by FHR mean (ps > .39) or variability (ps > .13), obstetric risk (p = .76), or socioeconomic status (SES) (p = .96). Mothers who did not complete infant temperament measures had higher levels of prenatal psychological distress at a trend level of significance (t = 1.79, p = .07, Cohen’s d = 0.46). Overall sample characteristics are provided in Table 1.
Table 1.
Overall sample characteristics (N = 149)
| Maternal age at delivery, years (M, SD) | 29.29 (5.09) |
| Maternal race/ethnicity (n, %) | |
| White | 74 (50%) |
| Latina | 38 (25%) |
| Asian | 15 (10%) |
| Black | 1 (1%) |
| Pacific Islander | 1 (1%) |
| Multi-ethnic | 20 (13%) |
| Maternal education (n, %) | |
| High school or less | 57 (38%) |
| Associates or vocational degree | 27 (18%) |
| 4-year college degree | 41 (28%) |
| Graduate degree | 24 (16%) |
| Annual household income, USD (M, SD) | 64,319 (33,279) |
| Cohabitating with child’s father (n, %) | 135 (91%) |
| Infant sex, female (n, %) | 73 (49%) |
| Infant birth order, first born (n, %) | 70 (47%) |
| Length of gestation, weeks (M, SD) | 39.53 (1.05) |
| Birthweight, grams (M, SD) | 3,416.07 (446.08) |
| IBQ-R infant temperament dimensions (M, SD) | |
| Negative affectivity | 2.93 (0.51) |
| Orienting/regulation | 5.00 (0.52) |
| Surgency/extraversion | 4.33 (0.71) |
Note. IBQ-R = Revised Infant Behavior Questionnaire.
This study was approved by the university’s Institutional Review Board, and all women provided written informed consent.
Measures
Maternal psychological distress
Maternal psychological distress was characterized using three established self-report questionnaires, which were completed at all prenatal and postpartum visits. These measures are widely used in samples of pregnant women and demonstrate consistent associations with birth outcomes and postnatal developmental outcomes (see Bussières et al., 2015; Madigan et al., 2018).
Women reported their levels of depressive symptoms using the nine-item short form of the Center for Epidemiologic Studies Depression Scale (CESD; Santor & Coyne, 1997). Symptoms assessed include sadness/depression, sleep problems, difficulty concentrating, and absence of positive affect. Item responses ranged from 0 (rarely or none of the time) to 3 (most or all of the time), for a maximum possible total sum score of 27. The shortened version of the CESD has demonstrated good reliability and validity (Santor & Coyne, 1997). Cronbach’s alphas in the current sample ranged from .85 to .88.
General anxiety symptoms were measured with the 10-item state anxiety subscale of the State Trait Personality Inventory (Spielberger, Jacobs, Crane, & Russell, 1979). Women reported the extent to which they experienced various anxiety-related adjectives (e.g., nervous, tense) on a scale ranging from 1 (not at all) to 4 (very much). Total sum scores ranged from 10 to 40. This subscale displays good psychometric properties (Spielberger et al., 1979; Spielberger & Reheiser, 2009). Cronbach’s alphas in the current sample ranged from .87 to .90.
Levels of maternal stress were characterized with the 10-item Perceived Stress Scale (PSS; Cohen & Williamson, 1988). Women reported on experiences on a scale ranging from 0 (never) to 4 (almost always), including how often they were able to successfully manage day-to-day problems and hassles and how often they felt nervous and stressed. Total sum scores range from 0 to 40. This scale demonstrates good reliability and validity (Cohen & Williamson, 1988). Cronbach’s alphas in the current sample ranged from .87 to .91.
Prenatal depressive symptoms, anxiety symptoms, and perceived stress were moderately to highly correlated within each gestational timepoint (rs = .58–.79), so measures were standardized and averaged to create composite psychological distress variables (Glynn et al., 2018). In addition, associations between maternal psychological distress at each timepoint and FHR measures were relatively consistent (see Table 2). Therefore, a single prenatal maternal psychological distress composite was created by averaging scores from the five gestational assessments. The same procedure was used to create a composite of maternal psychological distress measures at 3 and 6 months’ postpartum.
Table 2.
Zero-order correlations between maternal psychological distress and fetal heart rate (FHR) measures
| FHR mean |
FHR variability |
||||||
|---|---|---|---|---|---|---|---|
| 25w | 31w | 37w | 25w | 31w | 37w | ||
|
| |||||||
| Maternal psychological distress | 15w | .04 | .05 | .12 | −.12 | −.05 | .04 |
|
| |||||||
| 19w | .05 | .02 | .11 | −.00 | −.02 | .06 | |
|
| |||||||
| 25w | .08 | .03 | .20* | −.06 | −.00 | .03 | |
|
| |||||||
| 31w | .04 | −.09 | .12 | −.08 | .01 | .07 | |
|
| |||||||
| 37w | −.07 | −.01 | .08 | −.07 | −.03 | .02 | |
Note. w = weeks’ gestation.
p < .05. Matrix computed using pairwise deletion (overall N = 149).
Fetal development
FHR monitoring was conducted at the 25, 31, and 37-week visits. Mothers reclined in a semi-fowlers position (5–10-degree tilt) on a standard, padded examination table. Pure-tone music was presented though headphones to mask extraneous noise. Transabdominal transducers were attached to measure FHR and were positioned until a robust FHR signal was reliably detected. Monitoring began with a 15-minute baseline (resting) period to allow mother and fetus to adapt. FHR was quantified with a Toitu MT-430 ultrasound fetal monitor, which measured Doppler frequency shifts in a weak ultrasound beam projected on the fetus by an ultrasonic head and extrapolated FHR from fetal movement and uterine contractions. Fetal monitor data were digitized at a 2 kHz sampling rate with Active II (Biosemi Instrumentation) and transferred automatically to an off-line server for analysis. No uterine contractions occurred during the assessment period.
Integrity of the FHR data was assured by examining each tracing to scan for artifacts. An interpolation routine was applied for gaps or artifacts in the tracings of no greater than 10 seconds. Each tracing was examined by a trained observer who made a judgment about the validity of the interpolation. If a segment of the data resulted in unacceptable interpolations (the interval was >10 seconds or the estimate did not match the valid data points), that section of the data was omitted from analyses.
FHR mean (FHRM) and FHR variability (FHRV; calculated as the standard deviation) were computed in 1-minute epochs and averaged over a 3-minute rest period.
Infant temperament
Dimensions of infant temperament (negative affectivity, orienting/regulation, and surgency/extraversion) were assessed with the 191-item Infant Behavior Questionnaire-Revised (IBQ-R; Gartstein & Rothbart, 2003). To reduce the potential for reporting bias, the IBQ-R asks about concrete infant behaviors (e.g., “How often during the last week did the baby startle to a sudden or loud noise?”) rather than asking the caregiver to make abstract judgments (Gartstein & Rothbart, 2003). Mothers rated their infants on each item on a scale ranging from 1 (never) to 7 (always). Item responses are averaged to create subscales. The negative affectivity dimension is an average of subscales measuring sadness, distress to limitations, fear, and, reverse scored, falling reactivity/rate of recovery from distress. The orienting/regulation dimension assesses regulatory functioning and is comprised of subscales assessing low-intensity pleasure, cuddliness/affiliation, duration of orienting, and soothability. Finally, the surgency/extraversion factor consists of the approach, vocal reactivity, high-intensity pleasure, smiling and laughter, activity level, and perceptual sensitivity scales. The IBQ-R scales display good reliability (Gartstein & Rothbart, 2003) and continuity with maternal-reported temperament into childhood (Putnam, Rothbart, & Gartstein, 2008). IBQ-R scales have demonstrated modest convergence with observer ratings of infant temperament, particularly during ecologically valid tasks (Forman et al., 2003; Hane, Fox, Polak-Toste, Ghera, & Guner, 2006; Johnson et al., 2016; Parade & Leerkes, 2008).
A total of 114 mothers completed the IBQ at both 3 and 6 months’ postpartum. Three and six-month scores were moderately correlated (orienting/regulation: r = .53; negative affectivity: r = .45; surgency/extraversion: r = .66). Nineteen mothers completed the measure only at either 3 (n = 15) or 6 (n = 4) months. For participants with data at both timepoints, scores were averaged across the two.
Obstetric complications and birth outcomes
Maternal and infant medical records were reviewed to determine pregnancy dating, pregnancy complications, and birth outcomes (gestational age at birth, birthweight). Pregnancies were dated according to guidelines from the American College of Obstetricians and Gynecologists (ACOG, 2009) by comparison of last menstrual period to estimates based on early ultrasound measurements taken by a research nurse at the first study visit.
An obstetric complications score accounted for prenatal infection, pregnancy-induced hypertension, gestational diabetes, oligohydramnios, polyhydramnios, preterm labor, vaginal bleeding, placenta previa, and anemia. A cumulative score assessing prenatal obstetric complications was derived from the sum of all present risk variables (Hobel, 1982). In this low-risk sample, 72% had none of these risk factors, and no woman had more than two risk factors. A dichotomous score (no complications vs. any complications) was used in analyses.
Data analytic strategy
Linear mixed effects models (“lmer” package in R; Bates, Mächler, Bolker, & Walker, 2015) were fit to examine associations between maternal prenatal psychological distress and trajectories of FHRM and FHRV. Linear mixed effects modeling was used due to unequally spaced measurements of FHR parameters. Marginal and conditional R2GLMM (“MuMln” package in R; Bartoń, 2019) and standardized fixed effects estimates (“sjstats” package in R; Lüdecke, 2020) were used as measures of effect size. Weeks’ gestation was the longitudinal time variable. Models for FHRM included a random effect of intercept and a fixed effect of linear slope (weeks’ gestation). The slope could not be modeled as a random effect because this resulted in a singular fit (the variance estimate for slope was nearly zero). Models for FHRV included random effects of both intercept and slope. Models were centered at 25, 31, and 37 weeks’ gestation to assess for intercept differences at each of these timepoints. Prenatal maternal psychological distress and covariates (gestational age at birth, obstetric risk, cohabitation with the infant’s father, and socioeconomic status (composite of standardized annual household income and maternal years of education)) were included as predictors in the model. Covariates were selected based on previous fetal programming literature and were included if they were associated with maternal distress and/or FHR in the current sample (see Table 3 for zero-order correlations). When maternal distress was associated with a FHR parameter, fetal sex and its interaction with maternal distress were added to the model to assess for sex differences. Simple slopes were calculated via: http://www.quantpsy.org/interact/hlm2.htm.
Table 3.
Zero-order correlations between maternal psychological distress, fetal heart rate (FHR) measures, and covariates
| Fetal sex (1 = female) | Gestational age at birth | Obstetric risk (1 = any risk) | Cohabitation with infant’s father (1 = yes) | SES | |
|---|---|---|---|---|---|
| Maternal psychological distress | .01 | −.06 | −.07 | −.26** | −.36*** |
| 25w FHRM | −.00 | .14 | .08 | −.03 | .07 |
| 31w FHRM | −.01 | −.04 | .16† | .07 | −.02 |
| 37w FHRM | −.05 | −.13 | .09 | −.18* | −.04 |
| 25w FHRV | .09 | .14 | .05 | .08 | .14 |
| 31w FHRV | .02 | .14 | −.05 | .07 | .00 |
| 37w FHRV | .07 | .19* | .05 | −.23** | .04 |
Note. w = weeks’ gestation.
p < .10
p < .05
p < .01
p < .001.
Matrix computed using pairwise deletion (overall N = 149). SES = socioeconomic status (composite of standardized annual household income and maternal years of education).
When maternal psychological distress was associated with intercept differences in a FHR parameter, an unconditional random intercepts model was fit centered at that gestational time-point, and estimated individual intercept parameters (i.e., fixed effect + conditional modes of random effects) were extracted, so that all participants had an estimated FHR parameter. Associations between estimated FHR parameters and infant temperament dimensions and moderation of these associations by infant sex were then examined in individual regression models.
In the case that prenatal maternal psychological distress and/or a FHR parameter were associated with an infant temperament dimension, a path analysis assessed the conditional indirect effect of prenatal distress on infant temperament via the FHR parameter, with infant sex as the moderating variable. Postnatal maternal psychological distress was included as a covariate to assess whether associations among prenatal maternal distress, FHR, and infant outcomes were independent of concurrent maternal distress. Modeling was performed using the R package “lavaan” (Rosseel, 2012), with bootstrapped standard errors and confidence intervals generated from 1,000 bootstrapped samples.
Results
Fetal heart rate parameters
As expected, over the course of gestation, resting FHRM decreased (gestational weeks estimate = −0.37, SE = 0.07, t = −5.28, p < .001), and FHRV increased (gestational weeks estimate = 0.18, SE = 0.03, t = 5.74, p < .001). FHRM displayed modest interindividual stability over the three timepoints (r = .29−.43; see Table 4), whereas FHRV was only associated between contiguous timepoints (r = −.06−.18; see Table 4). Within timepoints, FHRM and FHRV were associated only at 37 weeks’ gestation (r = .29, p = .001; see Table 4).
Table 4.
Fetal heart rate (FHR) descriptive statistics and zero-order correlations
| FHR mean |
FHR variability |
|||||
|---|---|---|---|---|---|---|
| 25w | 31w | 37w | 25w | 31w | 37w | |
|
| ||||||
| 25w | .29** | .38*** | .09 | .17 | −.06 | |
|
| ||||||
| 31w | .43*** | .03 | .18* | |||
|
| ||||||
| 37w | .29 * | |||||
|
| ||||||
| M (SD) | 142.95 (6.32) | 138.57 (6.73) | 138.66 (9.00) | 4.90 (2.23) | 5.38 (2.66) | 6.85 (3.41) |
Note. w = weeks’ gestation. Bolded values on diagonal are correlations between FHR mean and FHR variability within timepoints.
p < .05
p < .01
p < .001.
Prenatal maternal psychological distress and fetal heart rate parameters
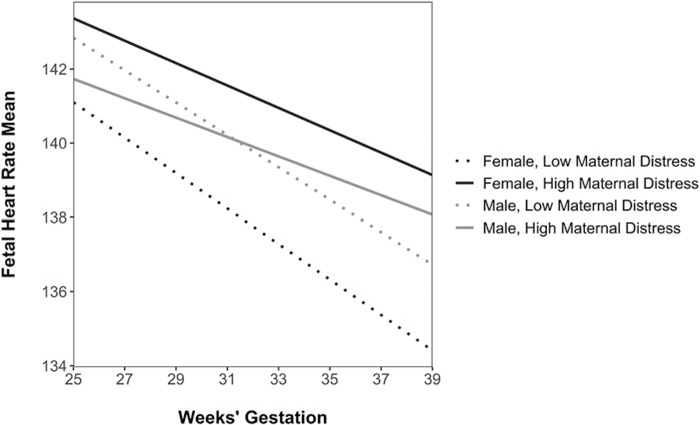
Linear mixed effects models examined if prenatal maternal psychological distress was associated with FHRM and FHRV over gestation. Higher levels of maternal psychological distress were associated with higher levels of FHRM at 37 weeks’ gestation (estimate = 1.76, SE = 0.85, t = 2.06, p = .04; see Table 5). This association was moderated by fetal sex at a trend level of significance (maternal distress x sex (female) estimate = 2.29, SE = 1.27, t = 1.81, p = .07; see Table 6 and Figure 1). Elevated maternal psychological distress was associated with higher 37-week FHRM among female fetuses (simple slope = 2.97, SE = 1.11, z = 2.66, p = .008) but not among male fetuses (simple slope = 0.67, SE = 1.02, z = 0.66, p = .51). Fetuses exposed to higher levels of prenatal maternal psychological distress also had less of the expected decrease in FHRM over gestation, but this slope difference was not statistically significant (estimate = 0.12, SE = 0.10, t = 1.25, p = .21). Maternal psychological distress was not associated with level differences in FHRM at 25 weeks’ gestation (estimate = 0.21, SE = 0.94, t = 0.23, p = .82) or at 31 weeks’ gestation (estimate = 0.94, SE = 0.69, t = 1.50, p = .18).
Table 5.
Linear mixed effects model examining associations between prenatal maternal psychological distress and mean fetal heart rate (N = 149)
| Fixed effects | Estimate (SE) | Std estimate | 95% CI | t | p |
|---|---|---|---|---|---|
| Intercept | 138.40 (1.76) | .07 | [134.94, 141.85] | 78.44 | <.001 |
| Weeks’ gestation (centered at 37 weeks) | −0.37 (0.07) | −.21 | [−0.51, −0.23] | −5.28 | <.001 |
| Prenatal maternal psychological distress | 1.67 (0.86) | .09 | [−0.02, 3.36] | 1.94 | .05 |
| Gestational age at birth | −0.15 (0.45) | −.02 | [−1.02, 0.73] | −0.33 | .74 |
| Obstetric risk (any risk) | 1.77 (1.04) | .23 | [−0.28, 3.82] | 1.70 | .09 |
| SES | 0.18 (0.30) | .04 | [−0.41, 0.76] | 0.60 | .55 |
| Cohabitating with infant’s father | −1.17 (1.76) | −.15 | [−4.62, 2.29] | −0.66 | .51 |
| Weeks’ gestation × maternal distress | 0.12 (0.10) | .05 | [−0.07, 0.31] | 1.25 | .21 |
| Random effects | Variance | SD | |||
| Intercept | 17.96 | 4.24 | |||
| Residual | 37.12 | 6.09 |
Note. Mean fetal heart rate is the dependent variable. Marginal R2GLMM (variance explained by fixed effects) = .07. Conditional R2GLMM (variance explained by entire model, including fixed and random effects) = .37.
Table 6.
Linear mixed effects model examining sex differences in associations between prenatal maternal psychological distress and mean fetal heart rate (N = 149)
| Fixed effects | Estimate (SE) | Std estimate | 95% CI | t | p |
|---|---|---|---|---|---|
| Intercept | 138.54 (1.85) | .07 | [134.90, 142.17] | 74.69 | <.001 |
| Weeks’ gestation (centered at 37 weeks) | −0.35 (0.10) | −.20 | [−0.55, −0.15] | −3.46 | .001 |
| Prenatal maternal psychological distress | 0.67 (1.02) | .00 | [−1.32, 2.67] | 0.66 | .51 |
| Fetal sex (female) | −0.54 (1.20) | −.04 | [−2.89, 1.81] | −0.45 | .65 |
| Gestational age at birth | −0.09 (0.44) | −.01 | [−0.96, 0.78] | −0.21 | .83 |
| Obstetric risk (any risk) | 1.82 (1.03) | .24 | [−0.21, 3.84] | 1.76 | .08 |
| SES | 0.16 (0.30) | .04 | [−0.42, 0.74] | 0.54 | .59 |
| Cohabitating with infant’s father | −1.05 (1.74) | −.14 | [−4.46, 2.36] | −0.60 | .55 |
| Weeks’ gestation × maternal distress | 0.12 (0.10) | .05 | [−0.07, 0.31] | 1.22 | .22 |
| Weeks’ gestation × sex (female) | −0.04 (0.14) | −.02 | [−0.32, 0.23] | −0.29 | .77 |
| Maternal distress × sex (female) | 2.29 (1.27) | .22 | [−0.19, 4.77] | 1.81 | .07 |
| Random effects | Variance | SD | |||
| Intercept | 17.13 | 4.14 | |||
| Residual | 37.17 | 6.10 |
Note. Mean fetal heart rate is the dependent variable. Marginal R2GLMM (variance explained by fixed effects) = .08. Conditional R2GLMM (variance explained by entire model, including fixed and random effects) = .37.
Figure 1.
Trajectories of fetal heart rate mean over gestation. Elevated prenatal maternal psychological distress is associated with higher mean fetal heart rate (FHRM) at 37 weeks’ gestation among female fetuses. Prenatal maternal psychological distress was modeled continuously but is plotted at low (−1 SD) and high (+1 SD) values for illustrative purposes.
Prenatal maternal psychological distress was not associated with levels or trajectories of FHRV (all ps > .41).
Maternal psychological distress, fetal heart rate, and infant temperament
Higher FHRM at 37 weeks’ gestation was, in turn, associated with dimensions of infant temperament. Higher 37-week FHRM was associated with lower infant orienting/regulation at a trend level of significance (B = −0.03, SE = 0.02, β = −.21, t = −1.76, p = .08), and this association did not differ by infant sex (FHRM × infant sex interaction term: B = −0.01, SE = 0.03, β = −.02, t = −0.18, p = .86). Similarly, higher FHRM was associated with higher negative affectivity at a trend level of significance (B = 0.03, SE = 0.02, β = .21, t = 1.68, p = .09), and this association also was not moderated by infant sex (FHRM × infant sex interaction term: B = −0.02, SE = 0.03, β = −.09, t = −0.74, p = .46). FHRM was not associated with surgency/extraversion (B = −0.02, SE = 0.03, β = −.09, t = −0.72, p = .48). FHRV at 37 weeks’ gestation was not associated with temperament dimensions in either sex (all ps > .24).
Prenatal maternal psychological distress also was associated with infant temperament. Higher prenatal maternal distress was associated with higher maternal-reported infant negative affectivity (B = 0.32, SE = 0.07, β = .45, t = 4.64, p < .001), and this association did not differ by infant sex (maternal distress × infant sex interaction term: B = 0.08, SE = 0.11, β = .07, t = 0.68, p = .50. The association between higher prenatal maternal distress and lower infant orienting/regulation was not statistically significant (B = −0.11, SE = 0.08, β = −.16, t = −1.49, p = .14) and was not moderated by infant sex (B = −0.05, SE = 0.12, β = −.05, t = −0.43, p = .67). Finally, prenatal maternal psychological distress was positively associated with infant surgency/extraversion (B = 0.22, SE = 0.11, β = .23, t = 2.10, p = .04), and this association was evident only among male infants (maternal distress × infant sex interaction term: B = −0.35, SE = 0.17, β = −.22, t = −2.03, p = .04; simple slope for males = 0.22, SE = 0.10, t = 2.13, p = .04; simple slope for females = −0.12, SE = 0.13, t = −0.92, p = .36).
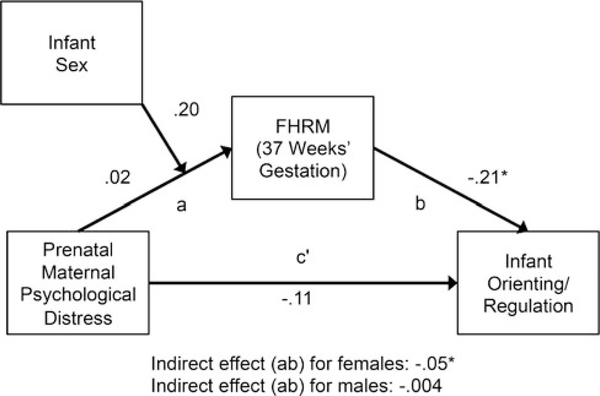
Path analyses examined if FHRM was a pathway by which prenatal maternal psychological distress was associated with infant temperament dimensions. There was an indirect effect of prenatal maternal psychological distress on infant orienting/regulation through FHRM at 37 weeks’ gestation among female infants but not male infants (see Figure 2; females: indirect effect estimate = −0.05, SE = 0.03, 95% CI [−0.15, −0.01]; males: indirect effect estimate = −0.002, SE = 0.02, 95% CI [−0.03, 0.03]). This finding was not explained by postnatal maternal psychological distress.
Figure 2.
Results of a path analysis examining direct and indirect pathways from prenatal maternal psychological distress to infant orienting/regulation. FHRM = fetal heart rate mean. All values are standardized estimates. Asterisk indicates estimate is statistically significant (bootstrapped 95% CI does not contain zero).
In a separate path analysis, there was not an indirect effect of prenatal maternal psychological distress on infant negative affectivity through FHRM at 37 weeks for either sex (males: indirect effect estimate = 0.004, SE = 0.01, 95% CI [−0.01, 0.03]; females: indirect effect estimate = 0.02, SE = 0.09, 95% CI [−0.11, 0.26]).
Discussion
In a prospective, longitudinal cohort of 149 maternal–fetal pairs, we documented associations between prenatal maternal psychological distress, fetal neurobehavior, and infant temperament. Our findings are consistent with the hypothesis that exposure to maternal distress in utero has implications for development into postnatal life, specifically for females. In our sample, elevated prenatal maternal psychological distress was associated with higher resting mean fetal heart rate (FHRM) at 37 weeks’ gestation among female but not male fetuses. Late-gestation FHRM was, in turn, associated with dimensions of infant temperament. Specifically, higher FHRM was associated with lower orienting/regulation and with higher negative affectivity. These associations did not differ by infant sex. Moreover, among female infants, there was an indirect effect of prenatal maternal psychological distress on infant orienting/regulation through late-gestation FHRM. There were no associations between prenatal maternal psychological distress and FHRV, nor among FHRV and infant temperament dimensions.
In our sample, FHRM and FHRV demonstrated the expected maturational patterns, with FHRM decreasing and FHRV increasing over gestation (Dawes et al., 1982; DiPietro et al., 2004, 2015; Van Leeuwen et al., 1999). For both male and female fetuses, late-gestation FHRM, an index of autonomic regulation, was associated with the temperament dimension of orienting/regulation during infancy. This finding builds on existing evidence that there is continuity in aspects of regulation from fetal to postnatal life (see DiPietro et al., 1996b; DiPietro et al., 2015, 2018; Snidman et al., 1995). Late-gestation FHRM was also associated with infant negative affectivity in the overall sample, consistent with previous studies documenting that FHR measures are associated with infant irritability (DiPietro et al., 2008) and negative reactivity (Werner et al., 2007).
Our finding that prenatal maternal psychological distress was associated with higher late-gestation FHRM among female fetuses suggests that maternal distress may shape autonomic and central nervous system development prior to birth. It also adds to a growing literature which indicates that females are particularly vulnerable to experiences of stress in utero (Glover & Hill, 2012; Glynn & Sandman, 2012; Sandman et al., 2013). Our path analysis indicated that elevated late-gestation FHRM is one pathway by which higher prenatal maternal psychological distress is associated with lower infant orienting/regulation among female infants. This represents robust support for the hypothesis that prenatal maternal psychological distress is associated with postnatal outcomes by way of altering fetal development. As expected, elevated prenatal maternal psychological distress was associated with higher infant negative affectivity and lower infant orienting/regulation in our sample. These associations consistently have been documented in numerous previous investigations (see Madigan et al., 2018, for a meta-analysis).
While one previous study reported higher late-gestation baseline FHRM in fetuses of mothers with elevated depressive symptoms (Allister et al., 2001), other studies either have demonstrated that elevated prenatal maternal psychological distress was associated with lower resting FHRM (Dieter et al., 2008; DiPietro et al., 2002b; Doyle et al., 2015) or have not found statistically significant associations between maternal distress and FHRM (DiPietro et al., 1996a; Monk et al., 2011, 2000, 2003, 2004). In addition, whereas other investigations have reported associations between maternal psychological distress and FHRV (DiPietro et al., 1996a; DiPietro et al., 2002b; DiPietro et al., 2010), we did not find associations between these variables. It is difficult to reconcile these differences given the dearth of existing studies and their varied methods (Allister et al., 2001; Dieter et al., 2008; DiPietro et al., 1996a; DiPietro et al., 2002b; Doyle et al., 2015; Figueiredo et al., 2017; Monk et al., 2011). Also, most studies have not reported analyses by fetal sex, which may have contributed to discrepancies in findings. A few studies have reported that fetal sex is associated with differences in FHR during rest or in response to stimulation (Buss et al., 2009; DiPietro et al., 2015), and the direction of findings has been mixed. One study examined sex differences in associations between maternal distress and FHR and reported that higher maternal negative mood was associated with lower FHRM among male but not female fetuses (Doyle et al., 2015).
Mechanisms by which prenatal maternal psychological distress is associated with FHR development are unknown. Several researchers have proposed that the fetal autonomic and central nervous systems are partially entrained through distress-related changes in maternal physiology (DiPietro, 2010; DiPietro et al., 2003; Monk et al., 2003). Specifically, fluctuations in the uterine sensory environment due to changes in maternal heart rate, respiration, blood pressure, and gastric motility may induce a fetal orienting response (DiPietro, 2010; Kinsella & Monk, 2009). Studies demonstrating alterations in fetal neurobehavioral activity in response to induced maternal sympathetic activation provide support for this hypothesis (DiPietro et al., 2003, 2008; Monk et al., 2011, 2000, 2003, 2004). Variations in uterine perfusion resulting from maternal vasoconstriction may also alter FHR activity (DiPietro et al., 2003; Kinsella & Monk, 2009). In addition, several studies suggest that FHR may be influenced by maternal and placental hormones (Doyle et al., 2015; Monk et al., 2011; Sandman, Wadhwa, Chicz-DeMet, Porto, & Garite, 1999).
Strengths of the present investigation include its prospective design, relatively large sample size, tests of indirect effects, and characterization of development across multiple levels of analysis (Cicchetti & Valentino, 2007; Masten & Cicchetti, 2010). In addition to these strengths, our study has several limitations. Because we relied on maternal report of infant temperament, it is possible that women who experience higher levels of psychological distress during pregnancy are more likely to have more negative perceptions of their infants’ temperament (see Rothbart & Bates, 2007). The validity of these reports is strengthened by our adjustment for concurrent maternal distress and our findings that maternal-reported infant orienting/regulation and negative affectivity were associated with an objective, biological indicator of autonomic regulation (FHR). In addition, because our investigation was observational, we cannot rule out the possibility that associations between maternal psychological distress, FHRM, and infant temperament dimensions reflect a shared genetic contribution. Evidence from quasi-experimental human studies and experimental nonhuman animal models suggests that elevated maternal distress in utero has independent influences on fetal development (Rice et al., 2010; Weinstock, 2008). Fetal neurobehavioral development and infant temperament are likely multiply determined by both genetic and environmental factors (DiPietro et al., 2004; Rothbart & Bates, 2007). Finally, we characterized FHRM and FHRV at each gestational timepoint during a 3-minute interval. However, we observed the predictable developmental patterns of FHRM and FHRV over gestation, supporting the validity of these measures.
Links between prenatal maternal psychological distress and postnatal developmental outcomes are now well documented (Howland, Sandman, & Glynn, 2017; Kingston et al., 2015; Madigan et al., 2018; O’Connor et al., 2016; O’Donnell, Glover, Barker, & O’Connor, 2014). However, relatively little research has directly focused on the putative mechanism underlying these associations – that prenatal maternal distress alters fetal development. In support of this programming hypothesis, we reported here that exposure to elevated prenatal maternal psychological distress is associated with differences in fetal neurobehavior among female fetuses. In addition, we established that altered fetal neurobehavior is a plausible pathway by which prenatal maternal distress can influence orienting/regulation among female infants. Our results also support the notion that, for both sexes, individual differences in regulation emerge prenatally and are maintained into infancy. Collectively, these findings underscore the utility of direct assessment of the fetus in determining if prenatal experiences are carried forward into postnatal life.
Acknowledgments.
The authors thank the families who participated in these projects. We also thank the dedicated staff at the Early Human and Lifespan Development Program and the Women and Children’s Health and Well-Being project.
Funding Statement. This research was supported by grants from the National Institutes of Health (NS-41298, HD-51852, and MH-96889).
Footnotes
Conflicts of Interest. None
References
- Adamson B, Letourneau N, & Lebel C (2018). Prenatal maternal anxiety and children’s brain structure and function: A systematic review of neuroimaging studies. Journal of Affective Disorders, 241, 117–126. doi: 10.1016/j.jad.2018.08.029 [DOI] [PubMed] [Google Scholar]
- Allister L, Lester BM, Carr S, & Liu J (2001). The effects of maternal depression on fetal heart rate response to vibroacoustic stimulation. Developmental Neuropsychology, 20, 639–651. doi: 10.1207/S15326942DN2003_6 [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists. (2009). ACOG practice bulletin: Ultrasonography in pregnancy. Obstetrics & Gynecology, 113, 451–461. [DOI] [PubMed] [Google Scholar]
- Barker DJP (1990). The fetal and infant origins of adult disease. British Medical Journal, 301, 1111. doi: 10.1136/bmj.301.6761.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoń K (2019). MuMIn: Multi-model inference. R Package Version 1.43.15. [Google Scholar]
- Bates D, Mächler M, Bolker BM, & Walker SC (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67, 1–48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bornstein MH, DiPietro JA, Hahn CS, Painter K, Haynes OM, & Costigan KA (2002). Prenatal cardiac function and postnatal cognitive development: An exploratory study. Infancy, 3, 475–494. doi: 10.1207/S15327078IN0304_04 [DOI] [Google Scholar]
- Buss C, Davis EP, Class QA, Gierczak M, Pattillo C, Glynn LM, & Sandman CA (2009). Maturation of the human fetal startle response: Evidence for sex-specific maturation of the human fetus. Early Human Development, 85, 633–638. doi: 10.1016/j.earlhumdev.2009.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussières EL, Tarabulsy GM, Pearson J, Tessier R, Forest JC, & Giguère Y (2015). Maternal prenatal stress and infant birth weight and gestational age: A meta-analysis of prospective studies. Developmental Review, 36, 179–199. doi: 10.1016/j.dr.2015.04.001 [DOI] [Google Scholar]
- Cicchetti D, & Valentino K (2007). Toward the application of a multiple-levels-of-analysis perspective to research in development and psychopathology. In Masten AS (Ed.), Minnesota Symposia on Child Psychology. Multilevel Dynamics in Developmental Psychopathology. (pp. 243–284). Mahwah, NJ: Erlbaum. [Google Scholar]
- Cohen S, & Williamson GM (1988). Perceived stress in a probability sample of the U.S. In Spacapan S & Oskamp S (Eds.), The social psychology of health: Claremont Symposium on Applied Social Psychology, (pp. 31–67). Newbury Park, CA: Sage. [Google Scholar]
- Dalton K, Dawes GS, & Patrick JE (1983). The autonomic nervous system and fetal heart rate variability. American Journal of Obstetrics and Gynecology, 146, 456–462. [DOI] [PubMed] [Google Scholar]
- David M, Hirsch M, Karin J, Toledo E, & Akselrod S (2007). An estimate of fetal autonomic state by time-frequency analysis of fetal heart rate variability. Journal of Applied Physiology, 102, 1057–1064. doi: 10.1152/japplphysiol.00114.2006 [DOI] [PubMed] [Google Scholar]
- Davis EP, Hankin BL, Glynn LM, Head K, Kim DJ, & Sandman CA (2020). Prenatal maternal stress, child cortical thickness, and adolescent depressive symptoms. Child Development, 91, e432–e450. doi: 10.1111/cdev.13252 [DOI] [PubMed] [Google Scholar]
- Dawes GS, Houghton CRS, Redman CWG, & Visser GHA (1982). Pattern of the normal human fetal heart rate. BJOG: An International Journal of Obstetrics & Gynaecology, 89, 276–284. doi: 10.1111/j.1471-0528.1982.tb04696.x [DOI] [PubMed] [Google Scholar]
- Dieter JNI, Emory EK, Johnson KC, & Raynor BD (2008). Maternal depression and anxiety effects on the human fetus: Preliminary findings and clinical implications. Infant Mental Health Journal, 29, 420–441. doi: 10.1002/imhj.20192 [DOI] [PubMed] [Google Scholar]
- DiPietro JA (2010). Maternal influences on the developing fetus. In Zimmerman AW and SL Connors (Eds.), Maternal Influences on Fetal Neurodevelopment: Clinical and Research Aspects. Publisher: Springer-Verlag: New York, NY. [Google Scholar]
- DiPietro JA, Bornstein MH, Hahn CS, Costigan K, & Achy-Brou A (2007). Fetal heart rate and variability: Stability and prediction to developmental outcomes in early childhood. Child Development, 78, 1788–1798. doi: 10.1111/j.1467-8624.2007.01099.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Caulfield LE, Costigan KA, Merialdi M, Nguyen RHN, Zavaleta N, & Gurewitsch ED (2004). Fetal neurobehavioral development: A tale of two cities. Developmental Psychology, 40, 445–456. doi: 10.1037/0012-1649.40.3.445 [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Costigan KA, & Gurewitsch ED (2003). Fetal response to induced maternal stress. Early Human Development, 74, 125–138. doi: 10.1016/j.earlhumdev.2003.07.001 [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Costigan KA, & Pressman EK (2002a). Fetal state concordance predicts infant state regulation. Early Human Development, 68, 1–13. doi: 10.1016/S0378-3782(02)00006-3 [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Costigan KA, Pressman EK, & Doussard-Roosevelt JA (2000). Antenatal origins of individual differences in heart rate. Developmental Psychobiology, 37, 221–228. doi: 10.1002/1098-2302(2000)37:43.0.co;2-a [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Costigan KA, & Voegtline KM (2015). Studies in fetal behavior: Revisited, renewed, and reimagined. Monographs of the Society for Research in Child Development, 80, 1–151. doi: 10.1111/mono.v80.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Ghera MM, & Costigan KA (2008). Prenatal origins of temperamental reactivity in early infancy. Early Human Development, 84, 569–575. doi: 10.1016/j.earlhumdev.2008.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Hilton SC, Hawkins M, Costigan KA, & Pressman EK (2002b). Maternal stress and affect influence fetal neurobehavioral development. Developmental Psychology, 38, 659–668. doi: 10.1037/0012-1649.38.5.659 [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Hodgson DM, Costigan KA, Hilton SC, & Johnson TRB (1996a). Fetal neurobehavioral development. Child Development, 67, 2553–2567. doi: 10.1111/j.1467-8624.1996.tb01874.x [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Hodgson DM, Costigan KA, & Johnson TRB (1996b). Fetal antecedents of infant temperament. Child Development, 67, 2568–2583. doi: 10.1111/j.1467-8624.1996.tb01875.x [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Kivlighan KT, Costigan KA, Rubin SE, Shiffler DE, Henderson JL, & Pillion JP (2010). Prenatal antecedents of newborn neurological maturation. Child Development, 81, 115–130. doi: 10.1111/j.1467-8624.2009.01384.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Voegtline KM, Pater HA, & Costigan KA (2018). Predicting child temperament and behavior from the fetus. Development and Psychopathology, 30, 855–870. doi: 10.1017/S0954579418000482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle C, & Cicchetti D (2018). Future directions in prenatal stress research: Challenges and opportunities related to advancing our understanding of prenatal developmental origins of risk for psychopathology. Development and psychopathology, 30, 721–724. doi: 10.1017/S095457941800069X [DOI] [PubMed] [Google Scholar]
- Doyle C, Werner E, Feng T, Lee S, Altemus M, Isler JR, & Monk C (2015). Pregnancy distress gets under fetal skin: Maternal ambulatory assessment & sex differences in prenatal development. Developmental Psychobiology, 57, 855–870. doi: 10.1002/dev.21317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emory EK, & Noonan JR (1984). Fetal cardiac responding: A correlate of birth weight and neonatal behavior. Child Development, 55, 1651–1657. doi: 10.2307/1130035 [DOI] [PubMed] [Google Scholar]
- Figueiredo B, Pinto TM, Pacheco A, & Field T (2017). Fetal heart rate variability mediates prenatal depression effects on neonatal neurobehavioral maturity. Biological Psychology, 123, 294–301. doi: 10.1016/j.biopsycho.2016.10.013 [DOI] [PubMed] [Google Scholar]
- Forman DR, O’Hara MW, Larsen K, Coy KC, Gorman LL, & Stuart S (2003). Infant emotionality: Observational methods and the validity of maternal reports. Infancy, 4, 541–565. doi: 10.1207/S15327078IN0404_08 [DOI] [Google Scholar]
- Gartstein MA, & Rothbart MK (2003). Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behavior and Development, 26, 64–86. doi: 10.1016/S0163-6383(02)00169-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V, & Hill J (2012). Sex differences in the programming effects of prenatal stress on psychopathology and stress responses: An evolutionary perspective. Physiology and Behavior, 106, 736–740. doi: 10.1016/j.physbeh.2012.02.011 [DOI] [PubMed] [Google Scholar]
- Glynn LM, Howland MA, Sandman CA, Davis EP, Phelan M, Baram TZ, & Stern HS (2018). Prenatal maternal mood patterns predict child temperament and adolescent mental health. Journal of Affective Disorders, 228, 83–90. doi: 10.1016/j.jad.2017.11.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, & Sandman CA (2012). Sex moderates associations between prenatal glucocorticoid exposure and human fetal neurological development. Developmental Science, 15, 601–610. doi: 10.1111/j.1467-7687.2012.01159.x [DOI] [PubMed] [Google Scholar]
- Gunnar MR (1990). The psychobiology of infant temperament. In Colombo J & Fagan J (Eds.), Individual differences in infancy: Reliability, stability and prediction (pp. 387–410). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Hane AA, Fox NA, Polak-Toste C, Ghera MM, & Guner BM (2006). Contextual basis of maternal perceptions of infant temperament. Developmental Psychology, 42, 1077–1088. doi: 10.1037/0012-1649.42.6.1077 [DOI] [PubMed] [Google Scholar]
- Hobel CJ (1982). Identifying the patient at risk. In Bolognese SRJ & Schneider RJ (Eds.), Perinatal medicine: Management of the high risk fetus and neonate (pp. 3–28). Baltimore, MA: Williams & Wilkins. [Google Scholar]
- Howland MA, Sandman CA, & Glynn LM (2017). Developmental origins of the human hypothalamic-pituitary-adrenal axis. Expert Review of Endocrinology and Metabolism, 12, 321–339. doi: 10.1080/17446651.2017.1356222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VC, Olino TM, Klein DN, Dyson MW, Bufferd SJ, Durbin CE, … Hayden EP (2016). A longitudinal investigation of predictors of the association between age 3 and age 6 behavioural inhibition. Journal of Research in Personality, 63, 51–61. doi: 10.1016/j.jrp.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Davis EP, Sandman CA, Sporns O, O’Donnell BF, Buss C, & Hetrick WP (2014). Longer gestation is associated with more efficient brain networks in preadolescent children. NeuroImage, 100, 619–627. doi: 10.1016/j.neuroimage.2014.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston D, McDonald S, Austin MP, & Tough S (2015). Association between prenatal and postnatal psychological distress and toddler cognitive development: A systematic review. PLoS ONE, 10, e0126929. doi: 10.1371/journal.pone.0126929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella MT, & Monk C (2009). Impact of maternal stress, depression and anxiety on fetal neurobehavioral development. Clinical Obstetrics and Gynecology, 52, 425–440. doi: 10.1097/GRF.0b013e3181b52df1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisilevsky BS, & Low JA (1998). Human fetal behavior: 100 years of study. Developmental Review, 18, 1–29. doi: 10.1006/drev.1998.0452 [DOI] [Google Scholar]
- Lüdecke D (2020). sjstats: Collection of convenient functions for common statistical computations. R Package Version 0.17.8. [Google Scholar]
- Madigan S, Oatley H, Racine N, Fearon RMP, Schumacher L, Akbari E, … Tarabulsy GM (2018). A meta-analysis of maternal prenatal depression and anxiety on child socioemotional development. Journal of the American Academy of Child and Adolescent Psychiatry, 22, 491–495. doi: 10.1016/j.jaac.2018.06.012 [DOI] [PubMed] [Google Scholar]
- Martin CB (1978). Regulation of the fetal heart rate and genesis of FHR patterns. Seminars in perinatology, 2, 131–146. [PubMed] [Google Scholar]
- Masten AS, & Cicchetti D (2010). Developmental cascades. Development and Psychopathology, 22, 491–495. doi: 10.1017/S0954579410000222 [DOI] [PubMed] [Google Scholar]
- Monk C, Fifer WP, Myers MM, Bagiella E, Duong JK, Chen IS, … Altincatal A (2011). Effects of maternal breathing rate, psychiatric status, and cortisol on fetal heart rate. Developmental Psychobiology, 53, 221–233. doi: 10.1002/dev.20513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, Fifer WP, Myers MM, Sloan RP, Trien L, & Hurtado A (2000). Maternal stress responses and anxiety during pregnancy: Effects on fetal heart rate. Developmental Psychobiology, 36, 67–77. doi: 10.1002/(SICI)1098-2302(200001)36:13.0.CO;2-C [DOI] [PubMed] [Google Scholar]
- Monk C, Myers MM, Sloan RP, Ellman LM, & Fifer WP (2003). Effects of women’s stress-elicited physiological activity and chronic anxiety on fetal heart rate. Journal of Developmental and Behavioral Pediatrics, 24, 32–38. doi: 10.1097/00004703-200302000-00008 [DOI] [PubMed] [Google Scholar]
- Monk C, Sloan RP, Myers MM, Ellman L, Werner E, Jeon J, … Fifer WP (2004). Fetal heart rate reactivity differs by women’s psychiatric status: An early marker for developmental risk? Journal of the American Academy of Child and Adolescent Psychiatry, 43, 283–290. doi: 10.1097/00004583200403000-00009 [DOI] [PubMed] [Google Scholar]
- Nijhuis JG, Swaab, Visser Heuser, Finch Brosens, & Eskes. (2003). Fetal behavior. Neurobiology of Aging, 24, S41–S46. doi: 10.1016/S0197-4580(03)00054-X [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Monk C, & Burke AS (2016). Maternal affective illness in the perinatal period and child development: Findings on developmental timing, mechanisms, and intervention. Current Psychiatry Reports, 18, 24. doi: 10.1007/s11920-016-0660-y [DOI] [PubMed] [Google Scholar]
- O’Donnell KJ, Glover V, Barker ED, & O’Connor TG (2014). The persisting effect of maternal mood in pregnancy on childhood psychopathology. Development and Psychopathology, 26, 393–403. doi: 10.1017/S0954579414000029 [DOI] [PubMed] [Google Scholar]
- O’Donnell KJ, & Meaney MJ (2017). Fetal origins of mental health: The developmental origins of health and disease hypothesis. American Journal of Psychiatry, 174, 319–328. doi: 10.1176/appi.ajp.2016.16020138 [DOI] [PubMed] [Google Scholar]
- Parade SH, & Leerkes EM (2008). The reliability and validity of the Infant Behavior Questionnaire-Revised. Infant Behavior and Development, 31, 637–646. doi: 10.1016/j.infbeh.2008.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam SP, Rothbart MK, & Gartstein MA (2008). Homotypic and heterotypic continuity of fine-grained temperament during infancy, toddlerhood, and early childhood. Infant and Child Development, 17, 387–405. doi: 10.1002/icd.582 [DOI] [Google Scholar]
- Rice F, Harold GT, Boivin J, Van Den Bree M, Hay DF, & Thapar A (2010). The links between prenatal stress and offspring development and psychopathology: Disentangling environmental and inherited influences. Psychological Medicine, 40, 335–345. doi: 10.1017/S0033291709005911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseel Y (2012). Lavaan: An R package for structural equation modeling. Journal of Statistical Software, 48, 1–36. doi: 10.18637/jss.v048.i02 [DOI] [Google Scholar]
- Rothbart MK, & Bates JE (2007). Temperament. Handbook of Child Psychology, In Damon W & Eisenberg N (Eds.), Handbook of child psychology: Vol. 3. Social, emotional, and personality development (pp. 105–176). New York: Wiley. [Google Scholar]
- Sandman CA (2015). Mysteries of the human fetus revealed. Monographs of the Society for Research in Child Development, 80, 124–137. doi: 10.1111/mono.12190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Buss C, Head K, & Davis EP (2015). Fetal exposure to maternal depressive symptoms is associated with cortical thickness in late childhood. Biological Psychiatry, 77, 324–334. doi: 10.1016/j.biopsych.2014.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Cordova CJ, Davis EP, Glynn LM, & Buss C (2011). Patterns of fetal heart rate response at 30 weeks gestation predict size at birth. Journal of Developmental Origins of Health and Disease, 2, 212–217. doi: 10.1017/S2040174411000250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Glynn LM, & Davis EP (2013). Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. Journal of Psychosomatic Research, 75, 327–335. doi: 10.1016/j.jpsychores.2013.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Glynn LM, & Davis EP (2016). Neurobehavioral consequences of fetal exposure to gestational stress. In Kisilevsky BS & Reissland N (Eds.), Fetal Development: Research on Brain and Behavior, Environmental Influences, and Emerging Technologies, (pp. 229–265). New York: Springer. [Google Scholar]
- Sandman CA, Glynn L, Wadhwa PD, Chicz-DeMet A, Porto M, & Garite T (2003). Maternal hypothalamic-pituitary-adrenal disregulation during the third trimester influences human fetal responses. Developmental Neuroscience, 25, 41–49. doi: 10.1159/000071467 [DOI] [PubMed] [Google Scholar]
- Sandman CA, Wadhwa PD, Chicz-DeMet A, Porto M, & Garite TJ (1999). Maternal corticotropin-releasing hormone and habituation in the human fetus. Developmental Psychobiology, 34, 163–173. doi: 10.1002/(sici)1098-2302(199904)34:33.0.co;2-9 [DOI] [PubMed] [Google Scholar]
- Sandman CA, Wadhwa P, Hetrick W, Porto M, & Peeke HVS (1997). Human fetal heart rate dishabituation between thirty and thirty-two weeks gestation. Child Development, 68, 1031–1040. doi: 10.1111/j.1467-8624.1997.tb01982.x [DOI] [PubMed] [Google Scholar]
- Santor DA, & Coyne JC (1997). Shortening the CES-D to improve its ability to detect cases of depression. Psychological Assessment, 9, 233–243. doi: 10.1037/1040-3590.9.3.233 [DOI] [Google Scholar]
- Snidman N, Kagan J, Riordan L, & Shannon DC (1995). Cardiac function and behavioral reactivity during infancy. Psychophysiology, 32, 199–207. doi: 10.1111/j.1469-8986.1995.tb02949.x [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Jacobs G, Crane R, & Russell S (1979). Preliminary manual for the state-trait personality inventory (STPI). Unpublished Manuscript University of South Florida Tampa. doi: 10.1111/j.1744-7402.2009.02409.x [DOI] [Google Scholar]
- Spielberger CD, & Reheiser EC (2009). Assessment of emotions: Anxiety, anger, depression, and curiosity. Applied Psychology: Health and Well-Being, 1, 271–302. doi: 10.1111/j.1758-0854.2009.01017.x [DOI] [Google Scholar]
- Thomason ME, Hect J, Waller R, Manning JH, Stacks AM, Beeghly M, … Romero R (2018). Prenatal neural origins of infant motor development: Associations between fetal brain and infant motor development. Development and Psychopathology, 30, 763–772. doi: 10.1017/S095457941800072X [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MI, & Thomason ME (2016). Functional connectivity of the human brain in utero. Trends in Cognitive Sciences, 20, 931–939. doi: 10.1016/j.tics.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leeuwen P, Lange S, Bettermann H, Grönemeyer D, & Hatzmann W (1999). Fetal heart rate variability and complexity in the course of pregnancy. Early Human Development, 54, 259–269. doi: 10.1016/S0378-3782(98)00102-9 [DOI] [PubMed] [Google Scholar]
- Weinstock M (2008). The long-term behavioural consequences of prenatal stress. Neuroscience and Biobehavioral Reviews, 32, 1073–1086. doi: 10.1016/j.neubiorev.2008.03.002 [DOI] [PubMed] [Google Scholar]
- Werner EA, Myers MM, Fifer WP, Cheng B, Fang Y, Allen R, & Monk C (2007). Prenatal predictors of infant temperament. 49, 474–484. Developmental Psychobiology, doi: 10.1002/dev.20232 [DOI] [PubMed] [Google Scholar]
- Yoshizato T, Koyanagi T, Takashima T, Satoh S, Akazawa K, & Nakano H (1994). The relationship between age-related heart rate changes and developing brain function: a model of anencephalic human fetuses in utero. Early Human Development, 36, 101–112. doi: 10.1016/0378-3782(94)90037-X [DOI] [PubMed] [Google Scholar]