Abstract
Introduction
Patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) have poor outcomes. Treatment with CD19 chimeric antigen receptor (CAR-T) cells, tisagenlecleucel and axicabtagene ciloleucel, has been associated with improved outcomes. Cytopenias were observed in clinical trials with both products; however, little is known regarding the patterns and outcomes of these cytopenias.
Subjects and Methods
We reviewed DLBCL patients (n=32) receiving either product between January and September 2018 at our institution.
Results
Median duration of leukopenia, neutropenia, lymphopenia, anemia, and thrombocytopenia was 49, 9, 117.5, 125, and 95.5 days after CAR-T infusion, respectively. Filgrastim was used in 63% of patients, and 50% of patients received red cell or platelet transfusions. With the exception of neutropenia, increase in the duration of cytopenia of any lineage was associated with improvement in progression-free survival, and in overall survival in case of anemia. There was no association between the duration of cytopenias with either cytokine release syndrome or neurotoxicity.
Discussion
Our data suggest a correlation between cytopenias and survival outcomes after CD19 CAR-T therapy. If validated, cytopenia may be proven useful as a biomarker of response and survival after CAR-T therapy.
Keywords: diffuse large B-cell lymphoma, transformed follicular lymphomas, chimeric antigen receptor T-cells, immunotherapy, cytopenias
Introduction
Chimeric antigen receptor T-cell (CAR-T) therapy is now approved by the FDA for the treatment of relapsed/refractory acute lymphoblastic leukemia and relapsed/refractory diffuse large B-cell lymphoma (DLBCL).1,2 Specifically, tisagenlecleucel (tisa-cel) and axicabtagene ciloleucel (axi-cel) are the two CD19-directed CAR-Ts approved for the treatment of relapsed/refractory DLBCL. These therapies have shown impressive outcomes in this difficult-to-treat disease, with reported median 1-year overall survival (OS) ranging from 49% to 59%.3,4 Despite impressive outcomes, these therapies were associated with significant toxicities, which include, but are not limited to, cytokine release syndrome (CRS), immune effector cells (IEC)-associated neurotoxicity syndrome (ICANS), and prolonged cytopenias.5,6 While the pathogenesis and management of CRS and ICANS related to CAR-T therapy have been well described, the association between CAR-T and the development of cytopenias requires more evaluation.7,8 Cytopenias were common in the clinical trials leading to the approval of these two agents, with any grade cytopenia occurring in 44% of patients for tisa-cel and 84% of patients for axi-cel.3,4 Herein, we report our institution's experience with CAR-T-related cytopenias, and how they relate to survival outcomes after CAR-T.
Methods
We undertook a retrospective analysis of all patients who received commercial tisa-cel or axi-cel for the treatment of DLBCL or transformed follicular lymphoma (tFL) between January and September 2018 at The Ohio State University (OSU) James Cancer Hospital. All patients received standard lymphodepleting (LD) chemotherapy (fludarabine and cyclophosphamide). Study was approved by OSU institutional review board (IRB), and data were collected by reviewing the patients' electronic medical records. Because of the retrospective nature of the study and as the study represented no more than minimal risks to participants, the IRB waived the requirement for patient consent. Patient confidentiality and privacy have been protected as consistent with the statement of ethical principles for medical research involving human subjects set forth by the Declaration of Helsinki. We evaluated complete blood counts (CBCs) from the start of LD chemotherapy through last known follow-up. Cytopenias were defined as having at least two days below 4×109/L for white blood cells (WBC), 1×109/L for absolute neutrophil count (ANC), 1×109/L for absolute lymphocyte count (ALC), 12 g/dL for hemoglobin level (Hgb), and 120×109/L for platelet count (plt). Count recovery was defined as having sustained at least 2 days of count improvement above the prior defined thresholds. Cytopenia duration was defined as time of onset until count recovery, or until last known follow-up or death if recovery had not occurred by then. For patients who had cytopenia prior to CAR-T infusion, CAR-T infusion date was used as the time of onset. Progression-free survival (PFS) was defined as time from the date of CAR-T infusion to the date of progression or death. Overall survival (OS) was defined as time from CAR-T infusion to death due to any reason. PFS and OS were estimated by the Kaplan–Meier method. The association between cytopenia and survival outcomes were assessed by Cox proportional hazards model. Due to the limited number of events, only univariable analysis was performed. For all analyses, a P-value <0.05 was considered significant.
Results
Thirty-two patients received CAR-T cell therapy during the study period. Twenty-eight patients received axi-cel, while four patients received tisa-cel. The median age was 59 years (range: 23–80). Ninety four percent of the cohort had DLBCL or undefined high-grade B-cell lymphoma, while 6% had tFL.
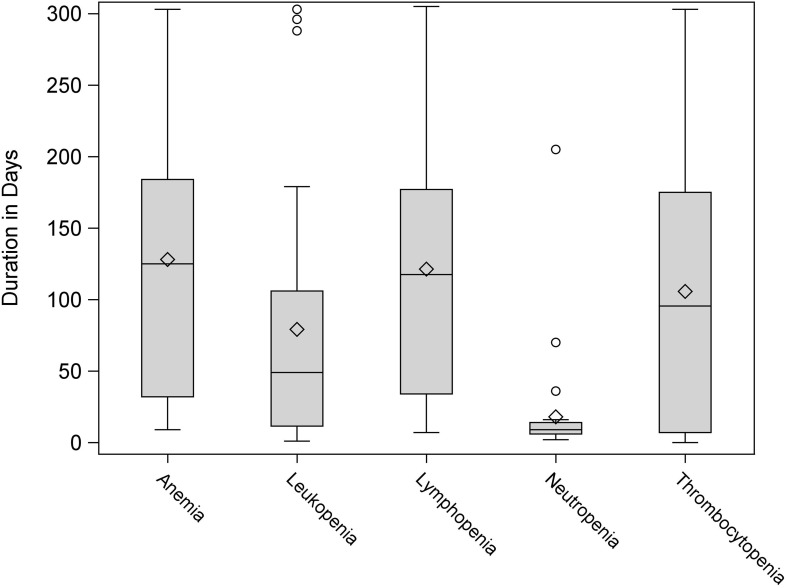
CRS of any grade occurred in 91% of patients, with 12% of patients experiencing a grade 3–4 event. ICANS of any grade occurred in 72% of patients, with 34% experiencing grade 3–4 events. Filgrastim was utilized in 63% of patients, and 50% of patients received red cell or platelet transfusions. Patient characteristics are summarized in Table 1. All patients had at least one cytopenia of any grade at the time of CAR-T infusion initiation. After CAR-T infusion started, almost every patient had all 5 types of cytopenia, except that 1 patient did not have neutropenia, 1 did not have anemia, and 2 did not have thrombocytopenia. Median duration of leukopenia was 49 days (n=32, range: 1–303), neutropenia was 9 days (n=31, range: 2–205), lymphopenia was 117.5 days (n=32, range: 7–305), anemia was 125 days (n=31, range: 9–303), and thrombocytopenia was 95.5 days (n=30, range: 0–303). At last follow-up, 69% of patients recovered from leukopenia, 97% of patient recovered from neutropenia, 34% recovered from lymphopenia, 39% recovered from anemia, and 43% recovered from thrombocytopenia (see Table 2 and Figure 1; also see Table S1). During our study period, 28% of patients achieved a complete response (CR), 19% had a partial response (PR), and 47% had progressive disease (PD). With a median follow-up of 5.9 months (range: 2.9–10.6) among survivors, seven patients died including five from disease progression and two from infections. The median PFS was 5.8 months (95% CI: 2.7–not reached). The estimated 12-month PFS and OS were 43% (95% CI: 25–61%) and 72% (95% CI: 47–86%), respectively.
Table 1.
Patient Characteristics
| N=32 | |
|---|---|
| Age, median (range) | 59 (23–80) |
| Gender, n (%) | |
| Female | 12 (38) |
| Male | 20 (63) |
| Disease, n (%) | |
| Diffuse large B-cell lymphoma or high grade B-lymphoma | 30 (94) |
| Transformed follicular lymphoma | 2 (6) |
| Status, n (%) | |
| Infused inpatient | 25 (78) |
| Infused outpatient | 7 (22) |
| CAR-T therapy, n (%) | |
| Tisagenlecleucel (tisa-cel) | 4 (13) |
| Axicabtagene ciloleucel (axi-cel) | 28 (88) |
| Length of hospital stay, median (range) | 13 (4–36) |
| Overall response to CAR-T (at 3 months), n (%) | |
| Complete remission | 9 (28) |
| Partial remission | 6 (19) |
| Progressive disease | 15 (47) |
| Early death after infusion | 2 (6) |
| Worst cytokine release syndrome (CRS) grade, n (%) | |
| 0 | 3 (9) |
| 1 | 13 (41) |
| 2 | 12 (38) |
| 3 | 2 (6) |
| 4 | 2 (6) |
| Neurotoxicity, n (%) | |
| No | 9 (28) |
| Yes | 23 (72) |
| Worst neurotoxicity grade, n (%) | |
| 0 | 9 (28) |
| 1 | 3 (9) |
| 2 | 9 (28) |
| 3 | 9 (28) |
| 4 | 2 (6) |
| Doses of tocilizumab received, n (%) | |
| 0 | 17 (53) |
| 1 | 3 (9) |
| 2 | 5 (16) |
| 3 | 3 (9) |
| 4 | 4 (13) |
| Received filgrastim, n (%) | |
| No | 12 (38) |
| Yes | 20 (63) |
| Required transfusion inpatient, n (%) | |
| No | 19 (59) |
| Yes | 13 (41) |
| Require transfusion outpatient, n (%) | |
| No | 19 (63) |
| Yes | 11 (37) |
| Confirmed infectious complication, n (%) | |
| No | 11 (34) |
| Yes | 21 (66) |
Table 2.
Incidence of Cytopenias Observed
| N=32 | |
|---|---|
| Neutropenia, n (%) | |
| Did not occur | 1 (3) |
| Occurred prior to CAR-T | 4 (13) |
| Occurred on the same day as or after CAR-T -Days to the first neutropenia, median (range) |
27 (84) 2 (0–9) |
| Lymphopenia, n (%) | |
| Occurred prior to CAR-T | 26 (81) |
| Occurred on the same day as CAR-T infusion | 6 (19) |
| Leukopenia, n (%) | |
| Occurred prior to CAR-T | 16 (50) |
| Occurred on the same day as or after CAR-T infusion -Days to leukopenia, median (range) |
16 (50) 0 (0–6) |
| Thrombocytopenia, n (%) | |
| Did not occur | 2 (6) |
| Occurred prior to CAR-T | 16 (50) |
| Occurred on the same day as or after CAR-T -Days to the first thrombocytopenia, median (range) |
14 (44) 2 (0–21) |
| Anemia, n (%) | |
| Did not occur | 1 (3) |
| Occurred prior to CAR-T | 23 (72) |
| Occurred on the same day as or after CAR-T -Days to the first anemia, median (range) |
8 (25) 0 (0–2) |
Figure 1.
Duration of cytopenias observed.
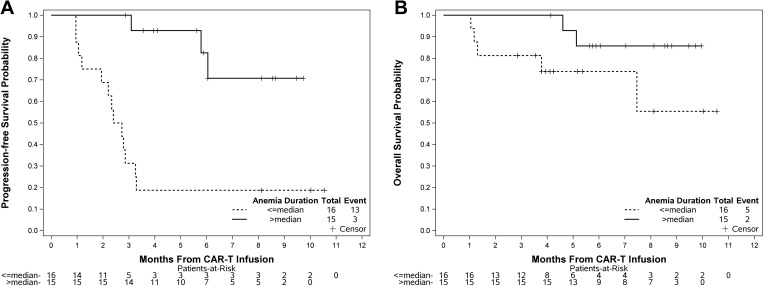
We observed that a longer duration of leukopenia was significantly associated with superior PFS, where a 5-day increase in leukopenia duration reduced the risk of progression or death by 6% [5-day HR (5DHR)=0.94, 95% CI 0.89–0.99, p=0.02]. Although the direction of effect was the same for OS, the association was not statistically significant (5DHR=0.88, 95% CI 0.75–1.03, p=0.12). A similar pattern was observed in lymphopenia (5DHR for PFS=0.95, 95% CI 0.92–0.99, p=0.01; 5DHR for OS=0.96, 95% CI 0.91–1.01, p=0.11) and thrombocytopenia (5DHR for PFS=0.97, 95% CI 0.93–1.00, p=0.05; 5DHR for OS=0.95, 95% CI 0.90–1.01, p=0.07), where longer cytopenia durations led to better survival outcomes. In contrast, the duration of neutropenia did not seem to have significant impact on PFS (5DHR=1.07, 95% CI 0.98–1.18, p=0.21) or OS (5DHR=0.95, 95% CI 0.80–1.14, p=0.61). The strongest correlation to survival outcomes was observed with anemia, where a 5-day increase in duration of anemia was significantly correlated with improved PFS (5DHR=0.95, 95% CI 0.91–0.98, p=0.005) and OS (5DHR=0.95, 95% CI 0.90–1.00 p=0.05) (Table 3). Figure 2 shows the PFS and OS curves stratified by dichotomized anemia duration. Of note, here was no statistically significant association between the occurrence of cytopenia, in any lineage, and the occurrence of CRS or ICANS (Table S2).
Table 3.
Univariate Model of Association Between Cytopenias and Survival Outcomes After CAR-T
| Duration of Cytopenia (5-Day Increase) | PFS | OS | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value | |
| Neutropenia | 1.07 (0.97–1.18) | 0.21 | 0.95 (0.80–1.14) | 0.61 |
| Lymphopenia | 0.95 (0.92–0.99) | 0.01 | 0.96 (0.91–1.01) | 0.11 |
| Leukopenia | 0.94 (0.89–0.99) | 0.02 | 0.88 (0.75–1.03) | 0.12 |
| Thrombocytopenia | 0.97 (0.93–1.00) | 0.05 | 0.95 (0.90–1.01) | 0.07 |
| Anemia | 0.95 (0.91–0.98) | 0.005 | 0.95 (0.90–1.00) | 0.05 |
Figure 2.
Impact of anemia on progression-free (A) and overall survival (B).
Discussion
Our institutional experience with prolonged cytopenias in CAR-T therapy highlights that it is prevalent and may contribute to outcomes in a positive way. In our population, the rate of cytopenias was quite high; all of our patients had at least one cytopenia after CAR-T infusion. Furthermore, many patients had cytopenias at the time of CAR-T infusion, as seen by our low median cell counts. In comparison, neutropenia, anemia, and thrombocytopenia of any grade occurred in 34%, 48%, and 33% of tisa-cel patients, and in 84%, 66%, and 58% of patients who received axi-cel in the studies that led to these products' FDA approval.3,4 These differences highlight the differences between “real-life” experience vs patient population studied in the clinical trials. One very interesting finding was that prolonged cytopenia seemed to correlate with improved outcomes. For every 5-day increase in length of lymphopenia, lymphopenia, thrombocytopenia, and anemia there was a statistically significant increase in PFS. Furthermore, OS was also improved in patients with prolonged anemia. Although the hazard ratios are above 90%, we think this is significant as all the correlations are in the same direction. Our analysis included all patients and did not exclude those patients who were cytopenic prior to CAR-T infusion. Although this is a potential confounder, it only strengthens our correlation, in that we still saw a potential benefit to an increase in duration of cytopenias in lieu of having a high baseline cytopenia rate. It could be argued that patients who survive longer would have more chance of developing a prolonged cytopenia. We then performed secondary landmark analyses on day 30 and day 60 to look at how count recovery was correlated with the survival outcomes, and observed that count recovery for leukopenia and anemia by day 30 and day 60 led to worse PFS and OS (Table S3). Although the correlation did not reach statistical significance (possibly due to small numbers), the direction of the effect potentially supports our hypothesis that cytopenia would truly correlate with improved outcomes after CAR-T therapy (data not shown).
The pathogenesis of prolonged cytopenias in CAR-T cell therapy remains unknown. Complicating the matter is that it has been hard to tease out if cytopenia is related to lymphodepleting (LD) chemotherapy, “bridging” treatment given prior to LD, disease status and health of the patient, or the direct effect of CAR-T cells on hematopoiesis. Prolonged cytopenia appears to be a frequent occurrence; in one long-term study after a median follow-up of 28.1 months, 16% of patients in a CR had prolonged cytopenia without evidence of myelodysplastic syndrome after receiving CD19 CAR-T cells.9 Furthermore, it appears that these cytopenias occur despite seemingly normal trilineage hematopoiesis in the bone marrow.5 It has been hypothesized that cytopenia after CAR-T therapy is induced by an inflammatory milieu related to release of cytokines by CAR-T cells.10 Interferon γ (IFN-γ) has been characterized as a driver of anemia and thrombocytopenia, and Il-6 has been characterized as mediator of anemia of chronic disease with an inhibitory effect on erythropoiesis.11–13 Serum IFN-γ and IL-6 levels have been found to be elevated in patients receiving CAR-T therapy, which further supports this hypothesis, and higher levels were associated with higher rates of grade 4 CRS.14,15 We hypothesize that these prolonged cytopenias are, indeed, secondary to continued release of cytokines by CAR-T cells. Thus, prolonged cytopenias may reflect ongoing activity of the CAR-T cells against residual CD19+ lymphoma cells. We have not observed an association between cytopenias and incidence and/or severity of CRS, however. We hypothesize that cytopenias were induced by chronic low-level release of inflammatory cytokines as compared to CRS where peak levels of the same cytokines have been demonstrated to be instrumental.
Continued research on the cause, frequency, and consequences of these cytopenias is needed as our cumulative experience grows with CD19 CAR-T and other cell therapy platforms. Our data suggest a correlation between cytopenias and survival outcomes after CD19 CAR-T therapy. If such findings are validated in a larger dataset, cytopenia may be proven useful as as a biomarker of response and survival after CAR-T therapy.
Disclosure
Dr Adam Kittai reports personal fees from Bristol-Myers Squibb, outside the submitted work. Dr Ayman Saad reports personal fees from Magenta Therapeutics, Incyte pharamceuticals, and CareDx, outside the submitted work. Dr Samantha M Jaglowski reports grants and/or personal fees from Novartis, Kite, CRISPR Therapeutics, Takeda, Juno, and Unum Therapeutics, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Kymriah Package Insert. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2018. [Google Scholar]
- 2.Yescarta Package Insert. Santa Monica, CA: Kite, A Gilead Company; 2017. [Google Scholar]
- 3.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Eng J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Eng J Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980 [DOI] [PubMed] [Google Scholar]
- 5.Shalabi H, Shah NN, Fry TJ, Yates B, Delbrook C. Chimeric antigen receptor induced cytopenia differs from chemotherapy induced myelosuppression. Blood. 2017;130(Suppl 1):5048. [Google Scholar]
- 6.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758 [DOI] [PubMed] [Google Scholar]
- 7.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acharya UH, Dhawale T, Yun S, et al. Management of cytokine release syndrome and neurotoxicity in chimeric antigen receptor (CAR) T cell therapy. Expert Rev Hematol. 2019;12(3):195–205. doi: 10.1080/17474086.2019.1585238 [DOI] [PubMed] [Google Scholar]
- 9.Cordeiro A, Bezerra ED, Hirayama AV, et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant. 2020;26:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sauter CT, Chien CD, Shen F, Tasian SK, Fry TJ. Evaluating on-target toxicity of hematopoietic-targeting cars demonstrates target-nonspecific suppression of marrow progenitors. Blood. 2016;128(22):3357. [Google Scholar]
- 11.Zoller EE, Lykens JE, Terrell CE, et al. Hemophagocytosis causes a consumptive anemia of inflammation. J Exp Med. 2011;208(6):1203–1214. doi: 10.1084/jem.20102538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarado LJ, Andreoni A, Huntsman HD, Cheng H, Knutson JR, Larochelle A. Heterodimerization of TPO and IFNγ impairs human hematopoietic stem/progenitor cell signaling and survival in chronic inflammation. Blood. 2017;130(Suppl 1):4. doi: 10.1182/blood-2017-05-786368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraenkel PG. Anemia of inflammation: a review. Med Clin North Am. 2017;101(2):285–296. doi: 10.1016/j.mcna.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hay KA, Hanafi LA, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130(21):2295–2306. doi: 10.1182/blood-2017-06-793141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134 [DOI] [PMC free article] [PubMed] [Google Scholar]