Abstract
Background:
Coronary allograft vasculopathy (CAV) is a devastating sequelae of heart transplant in which arterial intimal thickening limits coronary blood flow. There are currently no targeted therapies to prevent or reduce this pathology that leads to transplant failure. Vascular smooth muscle cell (VSMC) phenotypic plasticity is critical in CAV neointima formation. TET methylcytosine dioxygenase 2 (TET2) is an important epigenetic regulator of VSMC phenotype, but the role of TET2 in the progression of CAV is unknown.
Methods:
We assessed TET2 expression and activity in human CAV and renal transplant samples. We also employed the sex-mismatched murine aortic graft model of graft arteriopathy (GA) in wild type and inducible smooth muscle-specific Tet2 knockout mice; and in vitro studies in murine and human VSMCs using knockdown, overexpression, and transcriptomic approaches to assess the role of TET2 in VSMC responses to IFNу, a cytokine elaborated by T cells that drives CAV progression.
Results:
In the present study, we found that TET2 expression and activity is negatively regulated in human CAV and renal transplant samples and in the murine aortic graft model of GA. IFNу was sufficient to repress TET2 and induce an activated VSMC phenotype in vitro. TET2 depletion mimicked the effects of IFNу, and TET2 overexpression rescued IFNу-induced dedifferentiation. VSMC-specific TET2 depletion in aortic grafts, and in the femoral wire restenosis model, resulted in increased VSMC apoptosis and medial thinning. In GA, this apoptosis was tightly correlated with proliferation. In vitro, TET2 deficient VSMCs undergo apoptosis more readily in response to IFNγ and expressed a signature of increased susceptibility to extrinsic apoptotic signaling. Notably, enhancing TET2 enzymatic activity with high-dose ascorbic acid rescued the effect of GA-induced VSMC apoptosis and intimal thickening in a TET2-dependent manner.
Conclusions:
TET2 is repressed in CAV and GA, likely mediated by IFNу. TET2 serves to protect VSMCs from apoptosis in the context of transplant vasculopathy or IFNу stimulation. Promoting TET2 activity in vivo with systemic ascorbic acid reduces VSMC apoptosis and intimal thickening. These data suggest that promoting TET2 activity in CAV may be an effective strategy for limiting CAV progression.
Keywords: graft, coronary, cardiac, transplant, arteriosclerosis, allograft, vasculopathy, arteriopathy, VSMC, smooth muscle cell, T cell, T lymphocyte, intimal hyperplasia, neointima, IFN-gamma, TET2
Introduction:
Organ transplants provide life-saving therapy for end stage heart, renal, and lung disease. Graft arteriopathy (GA) – or coronary allograft vasculopathy (CAV) in the context of heart transplant - is the major challenge and leading cause of mortality that limits the long-term success of heart transplantation. CAV is characterized by circumferential neointimal hyperplasia in the vasculature due to chronic rejection. CAV occurs in 50% of transplanted hearts by 10 years after surgery1, and ischemic injury to the myocardium is responsible for 30% of all deaths in heart transplant recipients2. Neointimal lesions in CAV are largely composed of smooth muscle alpha-actin (ACTA2) positive cells3. These cells are heterogeneous in origin (reviewed in4), but are thought to arise primarily from dedifferentiated vascular smooth muscle cells (VSMCs) originating from the donor arterial tunica media. 3, 5–7.
VSMCs are remarkable in that these differentiated myocytes retain the ability to dedifferentiate, proliferate, and migrate in response to pathologic stimuli. While this characteristic is necessary for growth and wound healing, it also contributes to pathologic vascular remodeling. The stimuli that initiate VSMC dedifferentiation in transplant vasculopathy originate from the host’s immune system. ‘Non-self’ antigens expressed by transplanted donor tissue initiate both innate and adaptive immune alloreactivity. T cells recruited to the graft are activated directly or indirectly by alloantigen presentation (reviewed in8) to secrete interferon-γ (IFNу), a well-established promoter of neointimal hyperplasia9. Interestingly, while IFNγ is sufficient to induce GA in animal models10, it does not directly induce VSMC proliferation10. Several studies in animal models have shown that VSMC apoptosis in GA occurs along with, and exacerbates, intimal hyperplasia11–17. Indeed, there is a positive association between apoptosis, or medial thinning, and CAV severity in human specimens18–22. The mechanism whereby IFNγ induces VSMCs to dedifferentiate and contribute to the formation of neointimal lesions in CAV – and whether this is mediated through apoptosis of a population of medial VSMCs - is not well understood.
We reported previously that VSMC phenotype is regulated by the methylcytosine dioxygenase TET2, an enzyme that promotes modification of methylated DNA and subsequent chromatin remodeling to alter gene expression23. We showed that TET2 expression and activity, as measured by the 5-hydroxymethylcytosine (5-hmC) epigenetic mark, is repressed in the murine femoral artery denudation model of restenosis. TET2 promoted 5hmC modification of the promoters of key VSMC differentiation genes, including myocardin, SRF, and contractile genes. This study also found an inverse correlation between TET2 expression and severity of atherosclerotic disease in human tissue samples23. The role of TET2 in the pathogenesis of GA - where IFNγ plays a significant role in promoting VSMC phenotypic modulation, unlike in restenosis or atherosclerosis – has not been investigated. Treatment of CAV is currently limited to modulating immunosuppressive dosing or surgical revascularization, although these are of limited efficacy compared to retransplantation, which is limited by availability of donor organs24. New insights into the pathophysiology of GA are urgently needed to develop new therapeutic interventions. In studying the role of TET2 and the relationship between apoptosis and neointima formation in CAV, we identify novel underlying mechanisms of GA that suggest potential treatment and prevention strategies.
Methods:
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure by contacting the corresponding author. Additional experimental details are available in the Supplemental Methods section. All antibodies and primers are listed in Tables I and II in the Supplement, respectively.
Animal experiments:
All procedures involving animal subjects were approved by the Institutional Animal Care and Use Committee of Yale University.
All founder mice were obtained from Jackson Laboratory: TET2 floxed (LoxP sites at the 5’ and 3’ sides of exon 3; B6;129S-Tet2tm1.1Iaai/J, 017573)25, MYH11-CreERT2 (B6.FVB-Tg(Myh11-cre/ERT2)1Soff/J), Rosa26-mTmG (B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J, 007676), and B6 (C57BL/6J, 000664). The TET2 floxed strain was crossed with the MYH11-CreERT2 strain to generate tamoxifen-inducible smooth muscle-specific TET2-deficient mice (“iKO”). TET2-deficient hematopoietic cells made from the TET2 floxed strain have previously been shown to exhibit catalytic loss of function26. Male donor animals were derived from homozygous floxed breeding; all donor mice expressed Cre and were homozygous floxed, therefore control animals received corn oil vehicle while TET2-deficient mice received tamoxifen (Sigma, T5648). The TET2-deficient strain was further crossed to the mTmG reporter strain to allow for smooth muscle lineage tracing. Male donor animals were derived from heterozygous floxed breeding; all donor mice expressed Cre and a single copy of the mTmG transgene. Control mice were homozygous wild type and TET2-deficient mice were homozygous floxed; all donor animals received tamoxifen. Tamoxifen was given at 1mg/mouse/day intraperitoneally (IP) for 10 days. When vehicle was administered it was given at the same volume as tamoxifen. Tamoxifen was dissolved in 10% ethanol/90% corn oil vehicle.
Aortic interposition grafts were performed as described elsewhere27. Briefly, approximately 4mm-long segments of male donor thoracic aorta were grafted to the aorta of female recipient mice with end-to end anastomoses. Intercostal vessels exiting the donor segment were ligated with un-braided 5–0 nylon suture material. Donor and recipient animals were of the same background, except in saline/ascorbic acid experiments where all donors were on a mixed 129-B6 background and recipients were on a pure B6 background. 0.5g/ml ascorbic acid (AA) for in vivo administration was prepared in 1x PBS buffered with equimolar sodium bicarbonate and was pH-adjusted to 7.5 with 30mM NaOH. Animals received 4g/kg AA IP, or equivalent volume saline, daily beginning three days after surgery.
Statistical analysis
Values are presented as mean ± standard error of the mean (SEM). Two-sample datasets, where the data is expressed relative to the control, were analyzed by one-sample t tests. For two-sample datasets, equality of variance was determined by F-test; Welch’s t-tests were used when variances were significantly different, otherwise Student’s t-tests were used. Datasets with two independent variables were analyzed by two-way ANOVA followed by Sidak’s multiple comparisons testing as indicated. Datasets with two independent variables where variances were significantly different between conditions (as determined by Brown-Forsythe test) were analyzed by serial Welch’s t tests, followed by Bonferroni correction to account for multiple comparisons. Statistical analysis was performed using Graph-Pad Prism7. Probability values of P<0.05 were considered significant.
Results:
TET2 protein expression and global 5-hmC is repressed in human coronary allograft vasculopathy, and in a murine model of GA:
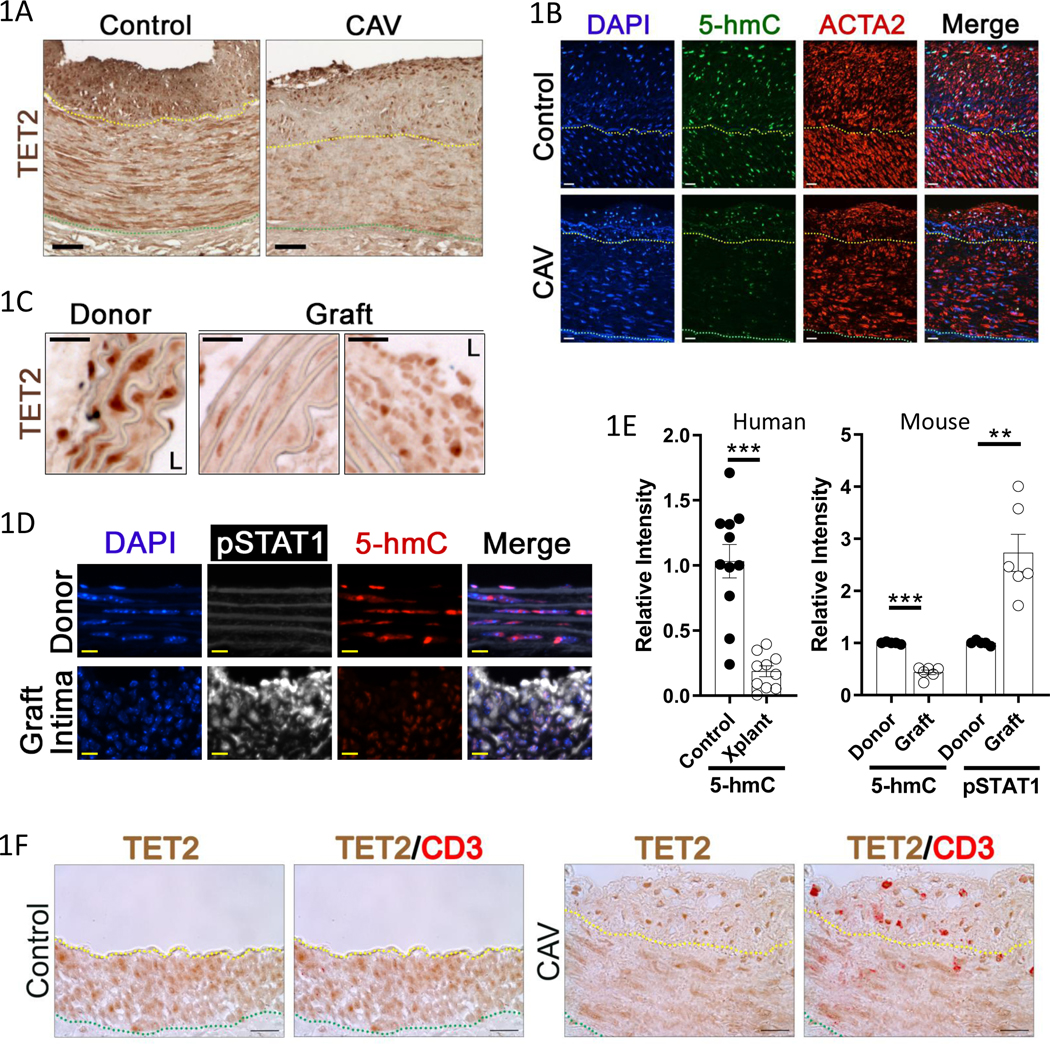
We assessed TET2 and 5-hmC by immunostaining in autopsy specimens of coronary vessels from heart transplant recipients with CAV and age-matched controls, as well as from kidney transplant recipients with renal artery graft arteriopathy (GA) and control arteries. There was robust TET2 staining in the nuclei of medial SMC in the controls, but notably reduced TET2 expression in the CAV/GA coronaries (Fig. 1A, Figure I-A in the Supplement). There was also less intense 5-hmC and smooth muscle alpha-actin (ACTA2) staining in the transplant samples (Fig. 1B, Figure I-B in the Supplement). To allow for mechanistic studies, we employed the murine sex-mismatched aortic interposition model of GA, where a male graft is transplanted into a female receipient27. We found, as in human samples (Figure I-A in the Supplement), there was a dramatic reduction in nuclear TET2 protein in medial and neointimal cells compared to the medial VSMCs in pre-transplant donor vessels (Fig. 1C). Time course studies showed a repression in medial TET2 beginning at 3 days, and a more pronounced repression at 14, 21, and 28 days after transplant. 5-hmC immunostaining revealed similar kinetics to TET2 expression (Figure II-A-B in the Supplement). The repression of TET2 and 5-hmC correlates temporally with the recruitment of T cells to the graft and subsequent IFNу signaling, as assessed by immunostaining for CD3 and activation of STAT1 (phospho-STAT1; pSTAT1), respectively (Figure II-C-D in the Supplement). The majority of cells expressing high levels of TET2 in the neointima of human CAV samples expressed CD3 (T cells) (Fig. 1F, Figure III in the Supplement). TET1 and TET3 were not repressed in grafts compared to donor control tissue (Figure IV in the Supplement). Quantification of 5-hmC signal intensity from human samples is shown in the left panel of Figure 1E. Co-immunostaining for 5-hmC and pSTAT1 in mouse aorta grafts showed that low TET activity (low 5-hmC) was inversely correlated with nuclear pSTAT1 (Fig. 1D–E). These data reveal that T cell recruitment and canonical IFNγ signaling are associated with decreased TET2 expression and activity in smooth muscle cells in GA.
Figure 1: TET2 expression and 5-hmC are repressed in allograft vasculopathy; coincident with canonical IFNγ signaling.
Formalin-fixed artery tissue was obtained from similarly aged, deceased patients who had received heart transplants (CAV, N=7), kidney transplants (N=3), or who died from non-cardiovascular diseases and had mild atherosclerotic intimal thickening (Control coronaries N=10, control kidney N=1)). Sections of coronary arteries were immunostained for A) TET2 or B) 5-hmC and smooth muscle alpha actin (ACTA2), with DAPI nuclear counterstain. Representative images shown. Cryosections of 28day sex-mismatched murine aortic interposition grafts were similarly immunostained for C) TET2 (L denotes lumen), or D) 5-hmC and phospho-STAT1 (pSTAT1), with DAPI. Representative images shown. N=3 E) Quantitation of Left) relative 5-hmC signal intensity in human samples (Control N=11, Transplant (Xplant) N=10), Right) relative 5-hmC and pSTAT1 signal intensity at 28days after aortic transplant in mouse. (Donor N=5, Graft N=6). Scale bars: A) 50μM, B) 50μm, C) 25μm, D) 10μm. F) Normal, and transplant, human coronary artery sections were immunostained for TET2 and CD3. Representative images shown (Control N=11, CAV (and kidney transplant GA) N= 10). Yellow dotted line indicates internal elastic lamina (IEL), green dotted line indicates external elastic lamina (EEL). Scale bar: 20μm. E) Left: Welch’s t test, Right: one sample t test. **P<0.01, ***P<0.001.
IFNγ treatment is sufficient to cause repression of TET2 and an activated phenotype in cultured VSMCs:
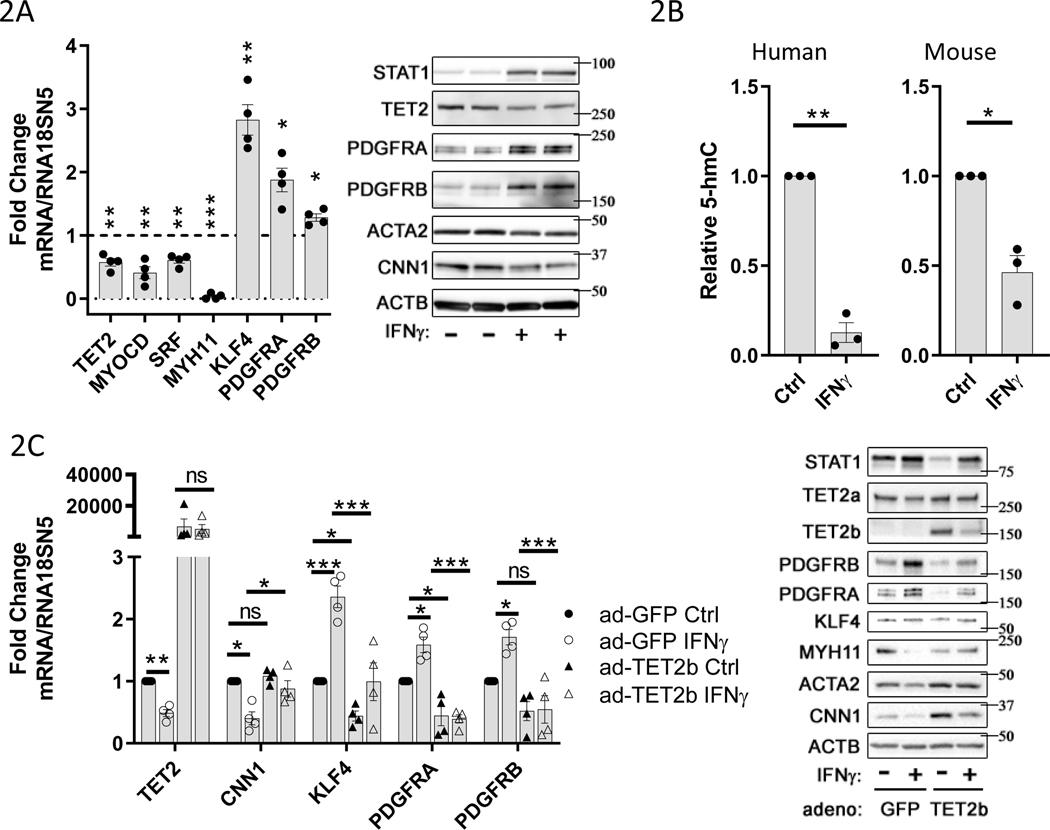
Treatment of primary human aortic vascular smooth muscle cells (hVSMCs) in vitro with 100ng/ml IFNγ resulted in a significant repression of TET2 mRNA and protein compared to control conditions (Fig. 2A, Figure V-A in the Supplement). IFNγ treatment also induced a de-differentiated hVSMC phenotype including repression of MYOCD, SRF, and MYH11 mRNAs, ACTA2 and CNN1 proteins, and concurrent induction of KLF4, PDGFRA, and PDGFRB mRNAs, and PDGF receptor proteins (Fig. 2A, Figure V-A in the Supplement). We observed a similar effect of IFNγ stimulation on these genes in primary murine aortic VSMCs (mVSMCs) (Figure VI-A in the Supplement). Additionally, TET2 knockdown is sufficient to recapitulate much of the IFNγ-induced changes in gene expression associated with VSMC de-differentiation (Figure VI-B in the Supplement, and shown previously23). We assessed the effect of IFNγ treatment on total genomic 5-hmC content in hVSMCs and mVSMCs by ELISA. IFNγ stimulation significantly decreased 5-hmC content in VSMCs from both human and mouse (Fig. 2B). Furthermore, we found that adenovirus-mediated overexpression of TET2 in hVSMCs prevented the IFNγ-induced changes in gene and/or protein expression of MYH11, ACTA2, CNN1, KLF4, and PDGFRs (Fig. 2C, Figure V-B in the Supplement). In these experiments, upregulation of STAT1 serves as a positive control readout for IFNу stimulation. These data suggest that repression of TET2 may mediate some of the IFNγ-induced effects on VSMC de-differentiation in GA.
Figure 2: IFNγ treatment causes TET2 and 5-hmC repression, and an activated phenotype in VSMCs.
Primary human or mouse vascular smooth muscle cells (VSMCs) were cultured under control conditions (Ctrl or “-“) or treated with 100ng/ml recombinant human or mouse IFNγ (IFNγ or “+”) for 48hrs, respectively. A) Left panel: mRNA levels for indicated genes from hVSMCs determined by quantitative PCR (qPCR) normalized to RNA18SN5, expressed as fold change relative to control (dotted line) (N=4), Right panel: representative western blot (N=4). B) Quantitation of genomic 5-hmC content, by ELISA, 48hr after treatment of VSMCs with 100ng/ml IFNγ. Left panel: human VSMCs (N=3), Right panel: mouse VSMCs (N=3). C) hVSMCs were infected with adenoviruses expressing GFP control or TET2b protein prior to IFNγ treatment as described in 2A-B. Left panel: mRNA levels for indicated genes determined by qPCR normalized to RNA18SN5 (N=4), Right panel: representative western blot (N=4). A-B) one sample t test, C) Two-way ANOVA: *P<0.05, **P< 0.01, ***P<0.001.
TET2 deficiency in donor VSMCs exacerbates Intima:Media ratio in GA:
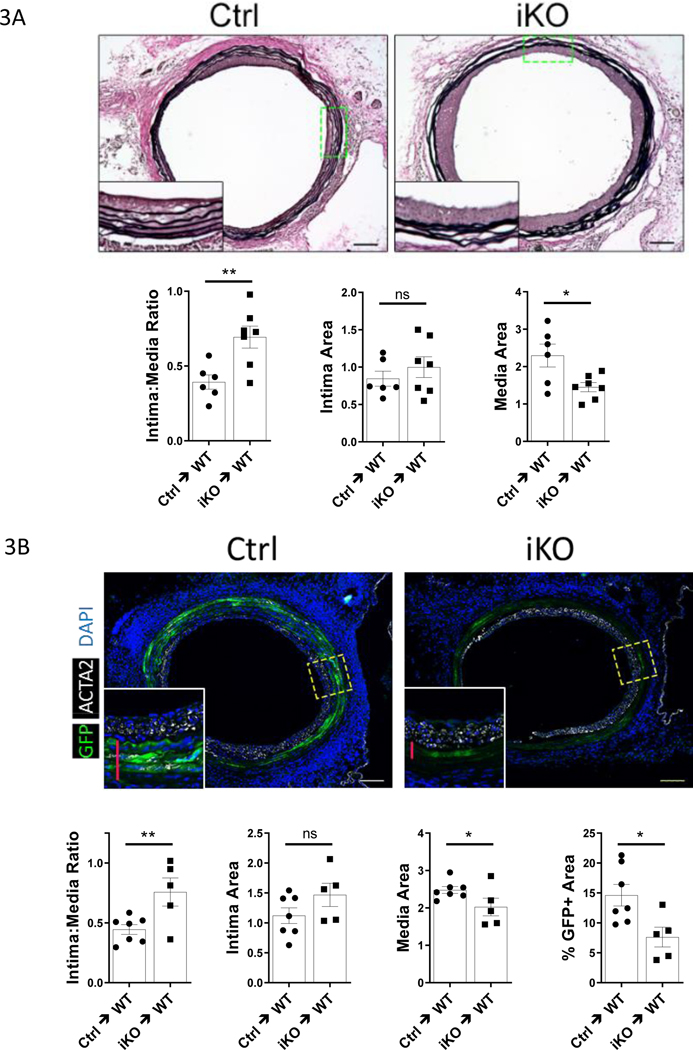
Because TET2 plays an important role in regulating VSMC phenotype23, and because TET2 is repressed in GA, and in vitro by IFNу, we hypothesized that genetic depletion of TET2 in murine aorta grafts would exacerbate GA. To test this hypothesis, we again employed the sex-mismatched aorta graft model. We generated smooth muscle-specific, inducible, TET2-deficient donor mice (Figure VII-A in the Supplement). TET2-deficient cells generated from this floxed mouse exhibit TET2 loss-of-function with respect to DNA hydroxymethylation (Figure VII-B in the Supplement, and shown elsewhere26). TET2-deficient or control vessels from male donors were transplanted into female recipients on the same background. 28 days after transplant, the intima:media ratio was increased in TET2 deficient grafts relative to controls (Fig. 3A). Unexpectedly, this was due to a decrease in area of the tunica media in the TET2-deficient grafts rather than a significant increase in neointimal area (Fig. 3A). This suggested that TET2 deficient VSMCs may undergo cell death more readily compared to TET2-competent VSMCs in GA. To confirm that the TET2-deficient VSMCs were being lost from the graft medial layer, we crossed the TET2-deficient mice to the Rosa26-mTmG reporter strain to label medial VSMC and repeated the 28day graft experiment. We found there were markedly fewer medial donor derived (GFP+) VSMCs remaining in the TET2-deficient grafts compared to controls (Fig. 3B). Donor vessels from control and TET2-deficient mice were equivalent with respect to ACTA2 expression, proliferation, and apoptosis at baseline (Figure VII-C in the Supplement). As the donor derived VSMCs do not appear to have migrated to a different vascular layer, these data suggest that TET2 is required for VSMC survival in GA. Further, since the neointima is at least as thick in the TET2-deficient grafts as in controls, these data suggest enhanced pro-neointima signaling from the fewer remaining donor TET2-deficient VSMCS.
Figure 3: Depletion of Tet2 in donor VSMCs exacerbates pathologic vascular remodeling in GA due to medial cell loss.
Control and TET2-deficient thoracic aorta segments from male donor mice were transplanted into sex-mismatched recipients of the same strain. A) Elastin Von-Geisson (EVG) staining of control (Ctrl=Tet2FFMyh11-Cre, vehicle-treated; N=6) or TET2-deficient (Tet2FFMyh11-Cre, tamoxifen-treated; N=7) grafts 28 days after surgery; graphs show associated quantitation of intima:media ratio, intima area, and media area. B) The Tet2 floxed-Cre strain was bred to the Rosa26-mTmG reporter line. Micrographs of GFP fluorescence and ACTA2 immunostaining in littermate Tet2++mTmGmut+Myh11-Cre (Ctrl; N=7) and Tet2FFmTmGmut+Myh11-Cre (iKO; N=5) grafts 28 days after surgery (red bars indicate medial layer); graphs show associated quantitation of intima:media ratio, intima area, media area, and percent GFP-positive (%GFP+) area in the intima-media combined region. Student’s t test: *P<0.05, **P<0.01.
Baseline sensitization of TET2 deficient cells to apoptosis results in medial cell loss in the femoral guidewire denudation model of restenosis:
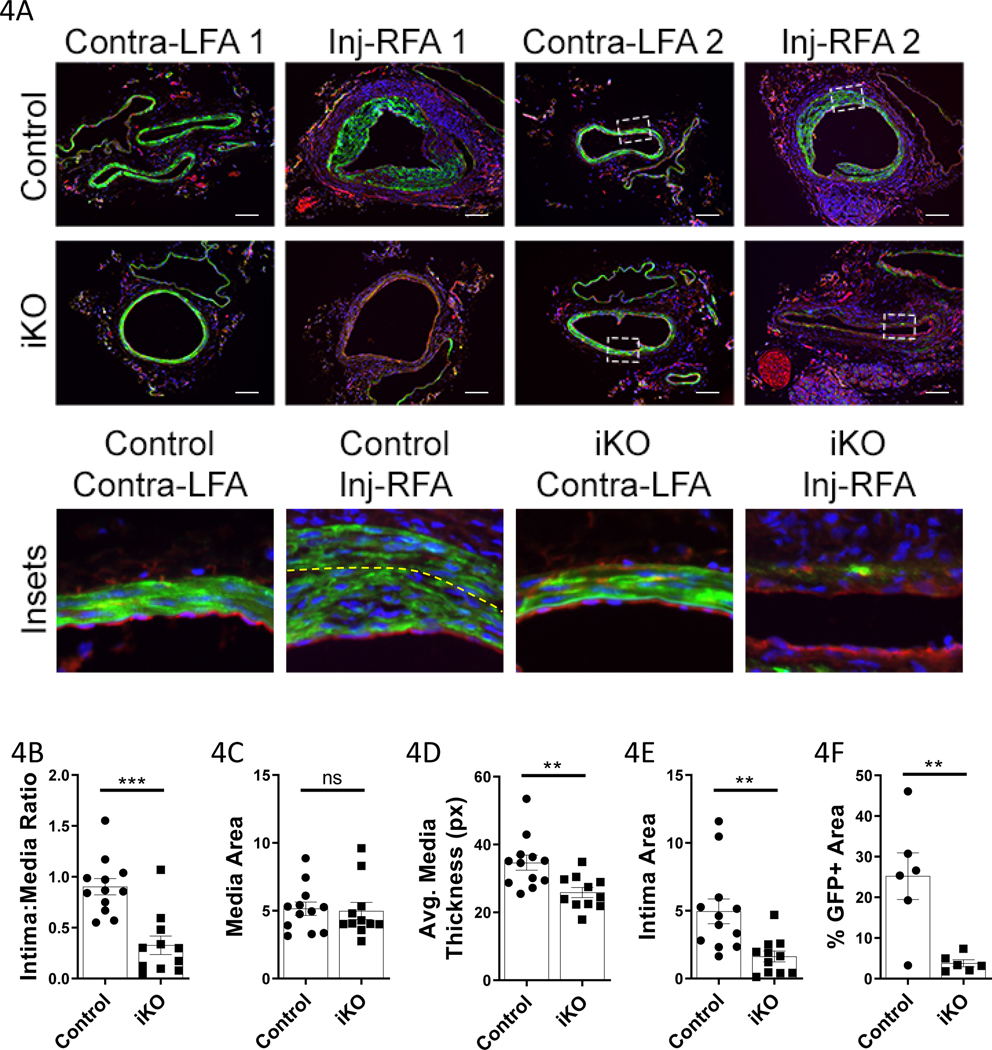
The above results are surprising because previous work from our group found that local virus-mediated shRNA depletion of TET2 in a murine model of restenosis exacerbated intimal thickening23. This discrepancy may arise for several reasons. In the prior study, TET2 deletion was not confined to VSMC. In the present study, it is possible that TET2-deficient VSMC may promote a more robust T cell response that drives medial loss. To determine the effect of smooth muscle TET2 depletion in a model of vascular remodeling in which T cells do not play a major role, we employed the femoral artery endothelial denudation injury model. Notably, significant early medial VSMC apoptosis is known to precede intimal hyperplasia in this model28. We hypothesized that TET2-deficient grafts would exhibit increased intimal hyperplasia in this restenosis model due to the VSMC dedifferentiation that occurs with loss of TET2 (Figure VI in the Supplement and23). We performed femoral artery injuries on the ROSA-26 mTmG control and Tet2-deficient mice (Fig. 4, Figure VIII in the Supplement). 21 days after surgery we observed GFP+ neointima in the control animals, as expected. Remarkably, in the majority of TET2-deficient littermates, there was no neointima present, the medial layer had thinned, and few GFP+ VSMCs remained. In the control animals, the intima:media ratio was significantly higher and an average of 25% of the intima-media combined area was GFP+ as compared to only 3.8% GFP+ in the TET2-deficient animals. Marked apoptosis (cleaved caspase 3) was found in the injured TET2-deficient femorals (Figure VIII in the Supplement). These data confirm that TET2 is important for VSMC survival in pathologic contexts where apoptosis is involved in the etiology. Importantly, these data provide evidence for the intrinsic increased susceptibility of TET2-deficient VSMCs to apoptosis in another model of vascular pathology.
Figure 4: TET2-deficient femoral arteries exhibit medial thinning, VSMC loss, and limited intimal hyperplasia in response to endothelial denudation injury.
Guidewire-injured right femoral arteries (Inj-RFA), and contralateral control left femoral arteries (Contra-LFA), of littermate Tet2++Myh11-Cre (Control N=6; Control mTmG-labeled N=6) and Tet2FFMyh11-Cre (iKO N=5; iKO mTmG-labeled N=6) were harvested 21days after surgery. A) Representative micrographs of uninjured (LFA) and injured (Inj-RFA) vessels. Scale bar= 100μm. Below: high power insets (indicated by white dashed line in lower power images). Yellow dashed line indicates the internal elastic lamina in the control Inj-RFA sample. B) Quantification of intima:media ratio from both mTmG-labeled and unlabeled animals (Control N=12, iKO N=11). C) Quantification of media area from both mTmG-labeled and unlabeled animals (Control N=12, iKO N=11). D) Quantification of average media layer thickness from both mTmG-labeled and unlabeled animals (Control N=12, iKO N=11). Medial thickness was measured at eight points (0°, 45°, 90°, 135°, 180°, etc.) around the circumference of each vessel and averaged. E) Quantification of intima area from both mTmG-labeled and unlabeled animals (Control N=12, iKO N=11). F) Quantification of %GFP+ area in the media and intima from mTmG-labeled animals only (Control N=6, iKO N=6). B-D Student’s t test, E-F Welch’s t test: **P<0.01, ***P<0.001.
TET2 deficiency sensitizes VSMCs to apoptosis in vivo and in response to IFNγ in vitro:
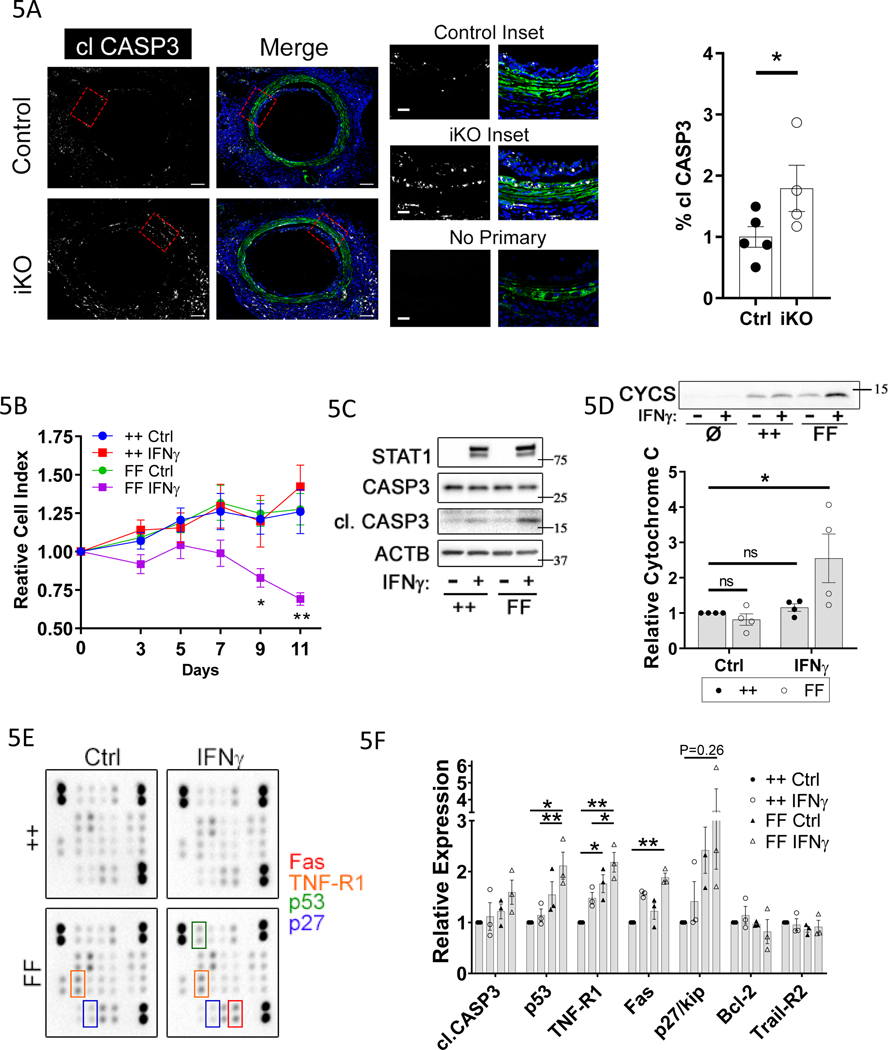
To determine whether more TET2-deficient donor VSMCs undergo apoptosis early in GA progression, as compared to TET2-competent VSMCs, we evaluated an earlier time point post-transplant. There was a 1.75-fold increase in cleaved caspase 3 positive area, in the combined media and intima area, in donor derived (GFP+) cells in TET2-deficient grafts versus controls at 10 days after transplant (Fig. 5A). Interestingly, we observed GFP+ donor-derived cells in the sub-endothelial space in some control but not TET2-deficient grafts at this time point (Fig. 5A). These data demonstrate that more donor VSMCs undergo apoptosis in TET2-deficient grafts versus controls. Concordantly, we found that there was an inverse correlation between cleaved caspase 3 staining and nuclear 5-hmC staining in human CAV coronaries versus age-matched controls (Figure IX in the Supplement). In vitro, IFNγ treatment was sufficient to induce hVSMC apoptosis in culture (Figure X in the Supplement). Further, we found that TET2-deficient mVSMCs were sensitized to apoptosis induced by IFNγ treatment (Fig. 5B–D). Specifically, we observed a decrease in cell viability as assessed by crystal violet staining (Fig. 5B), increased cleaved caspase3 (Fig. 5C), and increased cytochrome c release into the medium (Fig. 5D) from TET2-deficient cells treated with IFNγ as compared to control cells. An apoptosis antibody array showed that TET2-deficient mVSMCs exposed to IFNγ for 5 days expressed significantly higher levels of Fas, TNF-R1, and p53, with a trend toward increased p27 compared to control cells (Fig. 5E–F). These data reveal that TET2 deficient mVSMCs are sensitized to extrinsic apoptotic signals that could account for increased apoptosis in response to IFNγ or GA.
Figure 5: Depletion of Tet2 increases VSMC apoptosis in response to GA and IFNγ through upregulation of mediators of extrinsic apoptosis.
Control and TET2-deficient thoracic aorta segments from male donor mice were transplanted into sex-mismatched recipients of the same strain. A) Micrographs of GFP fluorescence and cleaved caspase 3 (clCASP3) immunostaining in littermate Tet2++mTmGmut+Myh11-Cre (Ctrl; N=5) and Tet2FFmTmGmut+Myh11-Cre (iKO; N=4) grafts 10 days after surgery; Right) quantitation of cl.CASP3-positive area within GFP-positive cells (% clCASP3) in the media-intima combined region. Red bars indicate the medial layer. B-F) mVSMCs isolated from Tet2++mTmGmut+Myh11-Cre (++) and Tet2FFmTmGmut+Myh11-Cre (FF) mice were treated with Cre recombinase-encoding adenovirus in vitro. ++ and FF cells were then cultured under control conditions (Ctrl or “-“) or treated with 100ng/ml rm-IFNγ (IFNγ or “+”) at day 0. B) Viability was assessed by crystal violet staining from 0– 11 days after IFNγ treatment (N=6). Western blot of VSMC C) whole-cell lysates or D) conditioned medium treated from mVSMCs treated with IFNу for 7days as in A. Ø indicates medium conditioned for 7 days in the absence of cells. In C), total STAT1 upregulation (long or short exposure) serves as a positive control known response to IFNγ treatment. E) Apoptosis antibody array of VSMC lysates treated with IFNу for 5 days. F) Quantitation of apoptosis array (N=3). A) Student’s t test: *P<0.05. B, D,F) Two-way ANOVA *P<0.05, **P<0.01.
TET2 loss induces a signature of TNFR signaling in TET2-deficient VSMCs, and sensitizes VSMCs to TNFα-induced apoptosis:
The late kinetics of IFNу-induced apoptosis observed in Figure 5B suggests that IFNу acts through an indirect mechanism to drive VSMC apoptosis in the context of TET2 depletion. We performed bulk RNA-sequencing on cultured control and TET2-deficient VSMCs treated with or without IFNу for 5 days to assess transcriptomic changes that could account for this effect (Figure XI in the Supplement). We noted efficient deletion of floxed exon3 of TET2 (Figure XI-A in the Supplement), and principal component analysis confirmed that the data appropriately clustered into four distinct groups (Figure XI-B in the Supplement). We filtered significantly regulated genes (P<0.01, FDR<0.1) based on those induced in TET2-deficient cells by IFNу that were not similarly induced in control cells by IFNу. We found 60 genes met these criteria (Table III in the Supplement). Ingenuity Pathway analysis revealed that TNF receptor signaling was significantly induced in this gene set (Figure XI-C, and Table III in the Supplement). We therefore assessed whether TNFα treatment induces more apoptosis in TET2-deficient VMSCs versus controls. 48hr treatment with 15ng/ml TNFα resulted in both more caspase 3 cleavage and cytochrome C release into the medium, in TET2-deficient cells versus controls (Figure XI-D in the Supplement). These data suggest that induction of TNF signaling pathway components in TET2-deficient cells functionally sensitizes the cells to TNFα-induced apoptosis.
Decreased TET activity promotes apoptosis and proliferation in vivo:
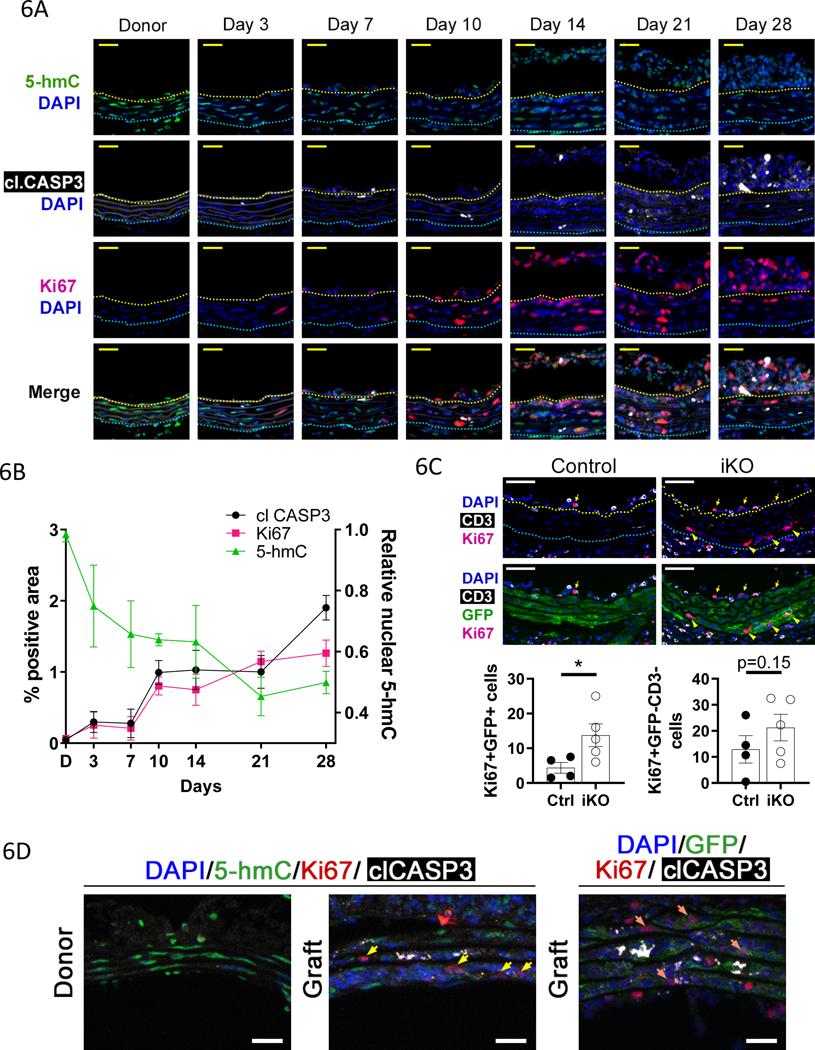
Medial VSMC apoptosis has been proposed to induce proliferation of neighboring VSMCs and to contribute to neointima formation in GA11, 12. Time course analysis in vivo revealed that apoptosis (cleaved CASP3) and VSMC proliferation (Ki67) are tightly correlated (Fig. 6A–B). Further, decreased TET activity (nuclear 5-hmC) correlated with increased apoptosis and proliferation (Fig. 6A–B). We hypothesized that increased apoptosis in the TET2 deficient grafts results in more proliferative surviving VSMCs. In our 10day GA samples (see Fig. 5A), we found that there were more proliferative donor VSMCs (Ki67+, GFP+) in the media/intima region in the TET2-deficient grafts versus controls (Fig. 6C, left graph). We also assessed proliferation of intimal and medial cells that are non-donor VSMCs and non-T cells (CD3–GFP–), as recruited host-derived cells may contribute to intimal hyperplasia in human CAV and GA models. There was a trend toward increased proliferation of these cells in the TET2-deficient grafts (Fig. 6C, right graph). Proliferative donor VSMCs exhibit low 5-hmC and are found near apoptotic cells in grafts (Fig. 6A, Fig. 6D). These data show that TET2 repression predisposes VSMCs to apoptosis which correlates with proliferation of neighboring or surviving VSMCs or potentially of other cells recruited from the recipient. CXCL12 (SDF1) is a known mitogen for VSMCs12 and has been implicated in recruiting mesenchymal stem cells to potentiate neotintima formation in GA11. We further noted increased expression of CXCL12 mRNA in TET2-deficient versus control cells (Figure XI-E in the Supplement), suggesting a mechanism by which loss of TET2 may contribute to neointima formation.
Figure 6: TET activity is negatively correlated with apoptosis and proliferation in GA.
Thoracic aorta segments from male donor mice were transplanted into sex-mismatched recipients of the same strain. A) Micrographs of immunostained sections from donor (N=5) and graft vessels at 3 (N=3),7 (N=3),10 (N=3),14 (N=2),21 (N=4), and 28days (N=4) after surgery. Upper panels) Ki67 and cleaved caspase 3 (clCASP3), and lower panels) 5-hmC. Representative images shown. B) Quantification of Ki67 and clCASP3 % positive area (left y-axis) from micrographs as described in A. Quantification of nuclear 5-hmC signal (right y-axis) from the same tissues as described in A. C) 10day Control and Tet2-deficient graft sections were immunostained for Ki67, CD3, and GFP. Left graph) Ki67 positive (Ki67+) donor derived VSMCs (GFP+) and right graph) Ki67 positive non-donor derived VSMC (GFP–), non-T cells (CD3–) were quantified in the media-intima combined region. Student’s t test: *P<0.05. D) Donor and graft sections were immunostained for 5-hmC or GFP, Ki67, cleaved caspase 3 (clCASP3), and DAPI. Yellow arrows indicate 5-hmClo Ki67+ cells, orange arrows indicate GFP+Ki67+ cells. Representative images of 4 independent samples are shown.
Promotion of TET2 activity by Ascorbic Acid increases donor VSMC survival in GA in a TET2-dependent fashion:
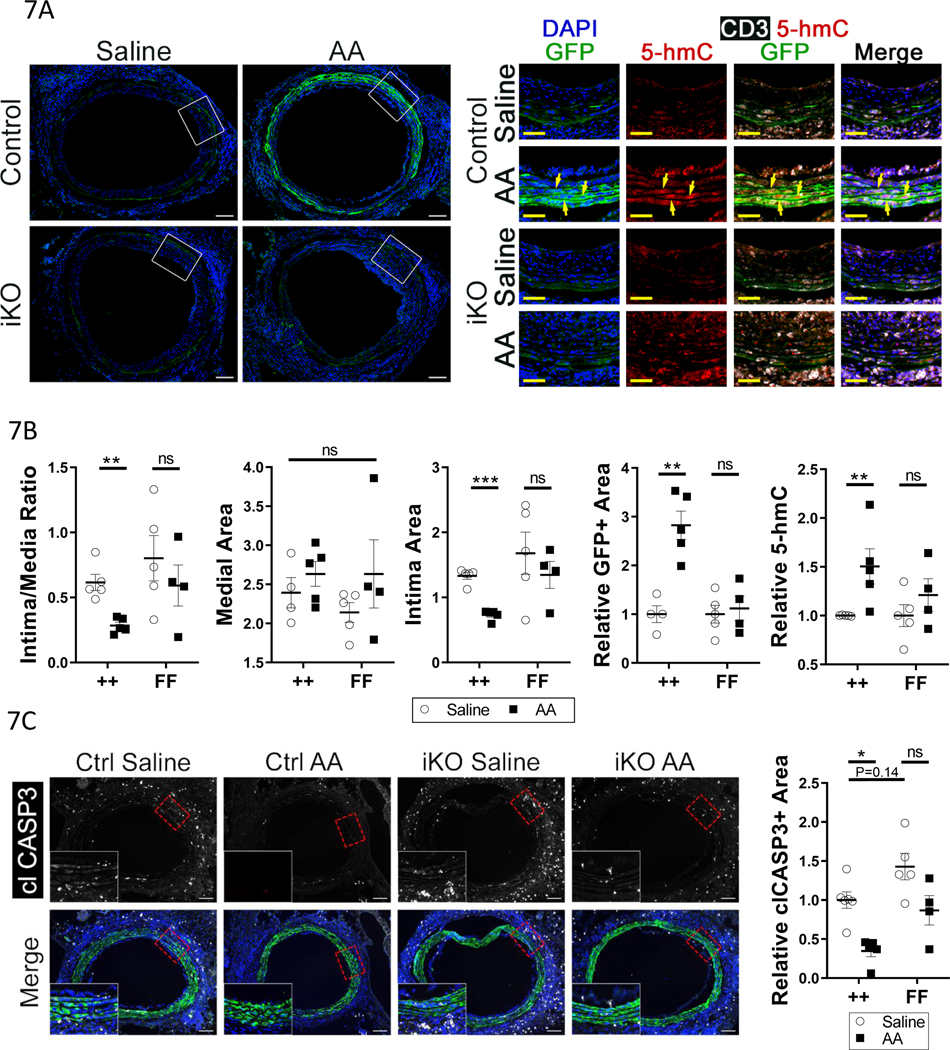
Ascorbic acid (Vitamin C) is a co-factor for TET2 that can promote its catalytic activity, enhancing levels of 5hmC and DNA demethylation29, 30. We hypothesized that increasing TET2 activity with ascorbic acid (AA)31 would protect the cells from apoptosis in GA. AA treatment prevented repression of genomic 5-hmC in response to IFNγ in cultured hVSMCs but did not affect 5-hmC in hVSMCs after TET2 knockdown (Figure XII-A in the Supplement). AA treatment did not change basal TET2 mRNA levels or prevent their repression by IFNу (Figure XII-B in the Supplement). As high dose AA has been successfully employed to rescue TET2-dependent functions in hematopoietic stem cells and suppress leukemia progression in vivo31, we hypothesized that AA treatment may increase TET2 activity and rescue donor VSMC apoptosis in GA. We transplanted control and TET2-deficient grafts, both with SMC labeled GFP+ via Myh11CreERT2-mTmG, into C57bl/6J (B6) recipients. There is a 5% background mismatch (genome scanning data not shown) between the donor animals and B6 recipients; this greater mismatch lessens the difference between the control and TET2-deficient animals at baseline, allowing for better comparison of the effects of AA. Intraperitoneal AA administration (4g/kg/day)31 dramatically protected donor medial VSMCs from GA-induced apoptosis, as shown by retention of GFP+ medial cells, and this effect was TET2-dependent (Fig. 7A–B). Furthermore, AA treatment significantly reduced neointima formation in control, but not in TET2-deficient, grafts (Fig. 7B). There was no significant change in media area any of the groups (Fig. 7B). The trend toward a decrease in medial area in the TET2-deficient saline-treated grafts versus the control saline-treated grafts (Fig. 7B) was not as pronounced as in Fig. 3, likely due to differences in the degree of MHC mismatch between these experiments. Finally, there was a significant increase in nuclear 5-hmC staining in the control-transplanted animals treated with AA, but not in the TET2-deficient-transplanted animals, confirming a rescue of Tet2 activity in the medial layer (Fig. 7B). We assessed whether high-dose AA prevents T cell recruitment or IFNγ signaling in vivo. AA did not reduce T cell recruitment to the grafts of either genotype, and did not decrease IFNγ signaling (Figure XII-C-D in the Supplement). As in culture (Figure XII-B in the Supplement), in vivo AA treatment did not increase TET2 expression in either the control or TET2-deficient grafts (Figure XII-E in the Supplement). The anti-TET2 antibody used in S12E (ab124297) recognizes a C-terminal epitope of the protein, mRNA for which is still transcribed after locus recombination (see Figure XI-A in the Supplement). Notably, there was an inverse correlation between GFP+ medial cell survival and intima:media ratio (Fig. 7B). At a 10day timepoint, before significant loss of GFP+ donor VSMCs, AA treatment significantly reduced apoptosis in a TET-dependent manner (Fig. 7C). These data reveal that preventing donor medial cell apoptosis by restoring TET2 activity reduces neointima formation.
Figure 7: Promoting TET2 activity with ascorbic acid rescues GA-induced donor-VSMC apoptosis and decreases intimal hyperplasia.
A) Micrographs of GFP fluorescence and DAPI, 28 days after surgery, in littermate Tet2++mTmGmut+Myh11-Cre (Control) and Tet2FFmTmGmut+Myh11-Cre (iKO) grafts in C57bl/6J recipients receiving either saline or 4mg/kg/day ascorbic acid (AA). Right) High-power insets of areas in panel A denoted by dashed boxes; micrographs of 5-hmC, CD3, and GFP immunostaining. Yellow arrows indicate GFP+ VSMCs with high nuclear 5-hmC. B) Quantification of intima:media ratio, media area, intima area, relative GFP positive (GFP+) area in the media/intima, and relative nuclear 5-hmC intensity in the media/intima, in grafts from control and TET2-deficient animals treated with saline or AA. C) Left) Micrographs of graft tissues -performed as in 7A-B but for 10 days – were immunostained for cleaved caspase 3 (clCASP3). Right) quantification of clCASP3+ area in GFP+ cells, relative to ++ saline. A-B) ++ saline N=5, ++ AA N=5, FF saline N=5, FF AA N=4. C) ++ saline N=6, ++ AA N=5, FF saline N=5, FF AA N=4. Two-way ANOVA: *P<0.05, **P< 0.01, ***P<0.001.
Discussion:
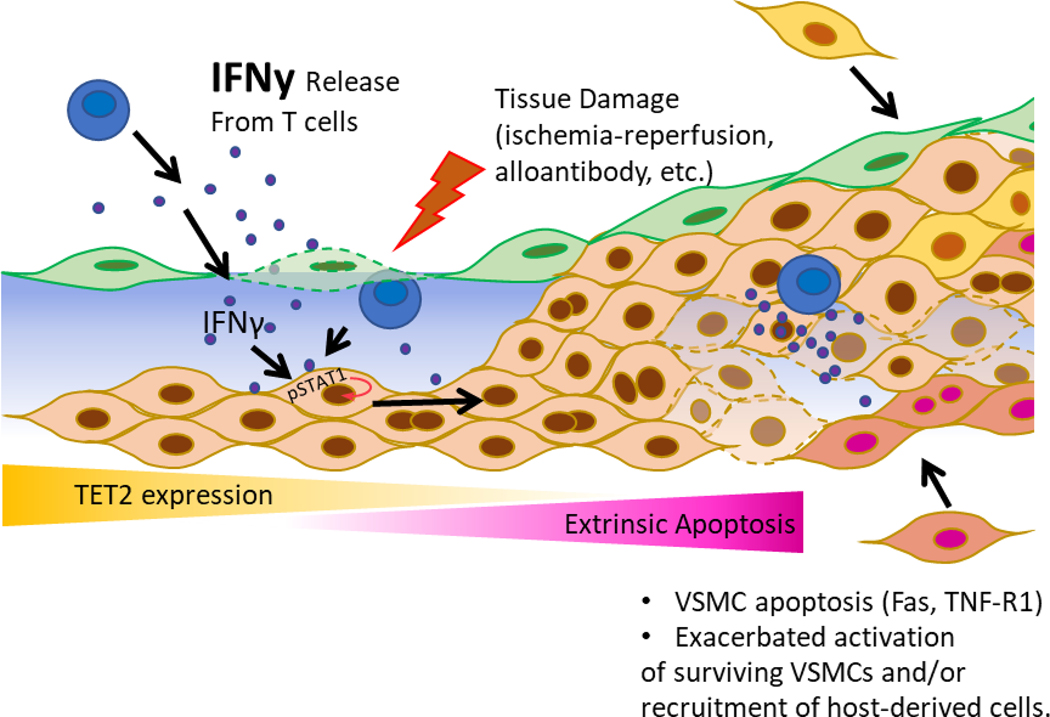
The mechanisms underlying graft arteriopathy leading to heart transplant failure are not fully understood. Our work reveals that TET2 is downregulated in both human CAV and in mouse models and identifies this loss of TET2 function as central to medial VSMC apoptosis and subsequent hyperproliferative responses (See model, Fig. 8). Further, we report that increasing activity of the residual TET2, in control grafts, with systemic high-dose ascorbic acid prevents both medial VSMC loss and intimal hyperplasia. These findings provide new mechanistic insights into early events that drive GA and suggest novel therapeutic approaches to prevent luminal narrowing and graft ischemia.
Figure 8: Model of TET2’s role in limiting intimal hyperplasia through preventing VSMC apoptosis.
Alloantigen-primed effector T cells infiltrate into the vessel wall and elaborate IFNу, resulting in repression of VSMC TET2. TET2 repression sensitizes VSMCs to extrinsic apoptosis, leading to pathogenic crosstalk that promotes proliferation of surviving VSMCs. This crosstalk may potentially extend to resident progenitor cells, and/or extravasated circulating recipient-derived progenitor cells. These effects lead to exacerbated intimal hyperplasia.
Tet2 regulation by IFNγ
Our previous work reported that TET2 is an epigenetic master regulator of VSMC phenotype that promotes expression of contractile genes and pro-differentiation transcription factors while repressing expression of dedifferentiation genes, including KLF423. We found high levels of TET2 and 5hmC in differentiated VSMC in vivo, but repression of TET2 mRNA and protein levels by PDGF-BB, a growth factor that promotes VSMC dedifferentiation23. By modifying patterns of DNA methylation, TET enzymes can exert broad control over programs of gene expression in cells undergoing phenotypic transitions29. We now report that TET2 protein and its epigenetic mark, 5-hmC, are reduced in both human CAV, GA, and murine GA; and this repression correlates temporally with T cell infiltration and canonical IFNу signaling. In vitro, we find that IFNу is sufficient to repress TET2 and 5-hmC and to induce a dedifferentiated VSMC phenotype, including induction of KLF4 and PDGF receptors. TET2 knockdown recapitulates many elements of the IFNу-induced phenotype in VSMCs (Liu et. al.23), and TET2 over-expression abrogates IFNу-induced phenotypic modulation. Importantly, IFNу alone is sufficient to induce GA in the absence of leukocytes10. We now implicate VSMC TET2 repression as a key event by which the T cell-derived cytokine IFNу drives this pathology.
TET2 depletion promotes VSMC apoptosis and medial thinning
Given its role as a master regulator of differentiation23, we hypothesized that genetic depletion of VSMC TET2 in vivo would exacerbate dedifferentiation and neointima formation in GA. We found that the intima:media ratio was indeed increased in TET2-deficient grafts, but, surprisingly, this effect was largely due to apoptosis of donor medial VSMCs rather than an increase in intimal thickness. Analysis of human CAV samples revealed an inverse correlation between TET activity (5-hmC) and apoptosis. Notably, the increased susceptibility of TET2-deficient VSMCs to apoptosis was also observed in the femoral artery denudation injury model of restenosis. Indeed, smooth muscle-specific TET2 deficiency resulted in profound loss of medial VSMCs post-injury and an inability of these vessels to give rise to a neointima. These results were in contrast to prior work from our laboratory showing that periadventitial transduction of femoral artery with TET2-shRNA-expressing virus exacerbated intimal hyperplasia23. Given the ectopic viral delivery by extravascular biopolymer23, we cannot rule out that TET2 knockdown in infiltrating leukocytes or adventitial cells could have significantly contributed to the overall phenotype observed. We did not assess apoptosis in those studies. We speculate that pervasive apoptosis that occurs early post-injury may preclude neointima formation, while a partial apoptotic response allows surviving cells to contribute to the neointima.
Although several studies have suggested a relationship between TET enzymes and apoptosis32, 33, there is evidence for both pro- and anti-apoptotic functions of TETs in different contexts and cell types, and little understanding of the underlying mechanisms. To date, our study is the first to identify an anti-apoptotic role for TET2 in VSMC. Our transcriptomic data reveal that the combination of IFNγ and TET2 depletion induces TNFR pathway signaling. Using a proteomic approach, we noted increases in several pro-apoptotic proteins in TET2 deficient VSMCs, including p53, TNF-R1, Fas, p27/Kip. These were further induced by IFNу treatment, likely explaining the increased susceptibility to apoptosis in TET2-deficient VSMCs. In the transplant setting, increased VSMC expression of extrinsic apoptotic pathway mediators like Fas or TNF-R1 could result in enhanced apoptosis induced by FasL-expressing T cells34, or TNFα-expressing macrophages35. TET2-deficient mVSMCs were sensitized to TNFα-mediated apoptosis in vitro. The upregulation of p53 in TET2-deficient cells, particularly in the presence of IFNу, might be responsible for Fas and TNF-R1 induction (reviewed in36). Upregulation of p27/Kip may elicit an anti-proliferative effect due to inhibition of CDK-cyclin complexes37, but may also influence apoptosis38. Our RNAseq also noted an induction of the H2-Dmb2 gene encoding the class II histocompatibility locus Mb2 (Table III in the Supplement), suggesting the intriguing hypothesis that the combination of IFNу and TET2 depletion may enhance antigen presentation by VSMC, leading to enhanced T cell activation to promote progression of graft arteriopathy.
It is known that apoptosis and/or thinning of the medial layer is associated with severity of coronary allograft vasculopathy (CAV) in human heart transplants18–22. In animal models this medial thinning has been shown to precede intimal thickening and to compromise vessel reactivity15. Additionally, abrogation of apoptosis in GA by over-expression of BCL-xL has been shown to reduce VSMC apoptosis, CXCL12 production, and intimal hyperplasia11. We now note that reduction in TET2 expression and activity precedes, and correlates with, VSMC apoptosis and proliferation in transplants. 5-hmC repression in murine aorta grafts occurred rapidly within the first week after surgery, with apoptosis and proliferation following by approximately three days. Strikingly, there was a tight correlation between apoptosis and proliferation. While the close relationship between apoptosis and proliferation may seem counterintuitive, there is precedent in vascular injury models demonstrating that CXCL12 released from apoptotic VSMCs may induce paracrine proliferation of non-apoptotic VSMCs11. Additionally, VSMC-derived CXCL12 is implicated in recruitment of circulating cells to vascular lesions39. IFNу alone promotes intimal hyperplasia in GA, but not proliferation of VMSC in vitro10. Our data suggest that IFNγ promotes intimal hyperplasia in GA by promoting apoptosis and subsequent paracrine effects on surviving VSMCs and other cells. Indeed, we note increased CXCL12 expression in TET2-deficient VSMCs compared to control as a potential mediator of VSMC proliferation12 or host cell recruitment11. Interestingly, it has been suggested that FashighFasLlow VSMCs may undergo apoptosis while FaslowFasLhigh VSMCs may contribute to intimal hyperplasia12. We noted that surviving GFP+ donor VSMCs were more proliferative in TET2-deficient grafts versus in control grafts, and that TET2-deficient cells in culture express higher Fas than control VSMCs.
In the mouse aorta graft model, mature neointimal lesions are ultimately comprised of recipient-derived cells which are likely recruited progenitor cells and/or invading recipient-derived dedifferentiated VSMCs. We identified a trend toward increased proliferation of GFP–CD3– cells in the neointima, suggesting the possibility that, in addition to proliferation of medial cells at these relatively early time points, there may also be increased recruitment of other recipient-derived cell populations in the TET2-deficient context (Fig. 8). It remains unknown what percent of the ACTA2-expressing intimal cells in this model are derived from resident vascular progenitor cells, infiltrating recipient VSMCs, and circulating progenitors. Interestingly, at the 10day timepoint, we observed GFP+ cells in the sub-endothelial space in control, but not TET2-deficient grafts. This likely reflects the reduced susceptibility of control VSMCs to apoptosis compared to TET2-deficient cells. These donor-derived cells do not persist in the intima at the 28day timepoint, suggesting that even the control VSMCs are ultimately subject to alloimmune-mediated cytolysis in the long-term.
Ascorbic acid restoration of TET2 activity as a potential therapeutic strategy
Since we implicated the reduction in TET2 expression as an early event that promotes GA, we hypothesized that increasing TET2 activity would be a potential therapeutic strategy. We have successfully transduced mouse femoral artery with periadventitial delivery of viruses to enhance or inhibit TET2 expression in a vascular injury model23, however, this approach was not viable in the mouse aorta graft model due to diffusion of the virus into the peritoneal cavity and clearance to the neighboring lymph nodes (data not shown). The ability of ascorbic acid to enhance Tet2 activity29–31 provided an intriguing alternative. While this approach does not prevent downregulation of Tet2 expression by IFNγ, it does enhance enzymatic activity and 5-hmC modification of DNA, as well as cell viability in vitro and in vivo, in a TET2-dependent manner. Most notably, high-dose ascorbic acid significantly rescued donor medial VSMC loss compared to saline in a TET2-dependent manner. This effect was accompanied by a significant decrease in intimal area in TET2-competent grafts only. Because AA has been shown to be potentially toxic to T cells at supraphysiologic concentrations (reviewed in40), we assessed whether T cell recruitment or IFNу signaling was inhibited in these tissues. We found that AA neither repressed T cell recruitment nor reduced IFNу signaling. While it is possible that systemic treatment with AA exerts pleiotropic beneficial effects on multiple cell types in vivo, the dependence of the phenotype on smooth muscle-specific expression of Tet2 further suggests that VSMC Tet2 is a critical target for this therapeutic efficacy.
Coronary allograft vasculopathy is a devastating pathology in human heart transplant; retransplantation or modification of immunosuppressive regimen are the only available strategies for treating this complication. Better targeted therapies are needed to prevent CAV development and decrease disease-free survival in transplant recipients. It has previously been suggested that dietary antioxidant supplementation with vitamins C and E may retard intimal thickening in transplant patients41. Our data suggest that the mechanism of this effect may include bolstering TET2 activity to prevent donor VSMC apoptosis and subsequent pathologic cross talk with the mosaic of cells that give rise to the neointima. Importantly, high dose injection of ascorbic acid is emerging as a safe and effective strategy for treatment of malignancies, especially those which may stem from Tet2 loss of function42, 43. Additionally, inducing TET2 activity with AA may stabilize T regulatory cell populations (reviewed in44) to repress the alloresponse in CAV. Taken together with the clinical data from Fang et. al.41, we propose that supraphysiologic ascorbic acid may be a safe and cost-effective way to prevent CAV in heart transplant recipients.
Supplementary Material
Acknowledgments
We thank Drs. Lingfeng Qin, Timur Yarovinsky, Kanika Jain, George Tellides, Jordan Pober, Daniel Jane-Wit, and Lori Charette for their technical expertise and guidance.
Sources of Funding
Supported by grants from the NIH to JH (R01HL115247, R01HL150515) and KAM (R01HL142090, R01HL146101, R01HL151222) and the American Heart Association to KAM (13GRNT14780090).
Non-standard Abbreviations and Acronyms:
- AA
ascorbic acid
- CAV
Coronary allograft vasculopathy
- clCASP3
cleaved caspase 3
- CM
conditioned media
- Contra
contralateral (uninjured control)
- DAPI − 4′,
6-diamidino-2-phenylindole
- DMEM
Dulbecco’s Modified Eagle Medium
- DOC
deoxycholic acid
- EEL
external elastic lamina
- ELISA
enzyme-linked immunosorbent assay
- EVG
Elastin Van Geison
- FBS
fetal bovine serum
- FDR
false discovery rate
- FF
Tet2flox/flox genotype
- FPKM
fragments per kilobase of transcript per million mapped reads
- GA
graft arteriopathy
- GSA
gene set analysis
- HBSS
Hanks’ balanced salt solution
- 5-hmC
5-hydroxymethylcytosine
- hMe-DIP
hydroxymethylated DNA immunoprecipitation
- HRP
horseradish peroxidase
- hVSMCs
human aortic vascular smooth muscle cells
- IEL
internal elastic lamina
- IFNg
Interferon-gamma
- iKO
inducible knockout
- Inj-RFA
injured right femoral artery
- IP
intraperitoneal
- IPA
Ingenuity Pathway Analysis
- KD
knockdown
- LFA
left femoral artery
- MOI
multiplicity of infection
- mVSMCs
murine aortic vascular smooth muscle cells
- NT
non-targeting
- PBS
phosphate buffered saline
- PCA
principal component analysis
- qPCR
quantitative polymerase chain reaction
- rh
recombinant human
- RIPA
radioimmunoprecipitation assay buffer
- rm
recombinant mouse
- SDS
sodium dodecyl sulfate
- TCA
trichloroacetic acid
- VSMC
vascular smooth muscle cell
- Xplant
Transplant
Footnotes
Disclosures: None.
REFERENCES
- 1.Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, Dobbels F, Goldfarb SB, Levvey BJ, Meiser B, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report−-2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33:996–1008. [DOI] [PubMed] [Google Scholar]
- 2.Taylor DO, Edwards LB, Boucek MM, Trulock EP, Waltz DA, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-third official adult heart transplantation report−-2006. J Heart Lung Transplant. 2006;25:869–879. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson C, Horsley J, Rhind-Tutt S, Charman S, Phillpotts CJ, Wallwork J, Goddard MJ. Neointimal smooth muscle cells in human cardiac allograft coronary artery vasculopathy are of donor origin. J Heart Lung Transplant. 2004;23:427–435. [DOI] [PubMed] [Google Scholar]
- 4.Hillebrands JL, Onuta G, Rozing J. Role of progenitor cells in transplant arteriosclerosis. Trends Cardiovasc Med. 2005;15:1–8. [DOI] [PubMed] [Google Scholar]
- 5.Hruban RH, Long PP, Perlman EJ, Hutchins GM, Baumgartner WA, Baughman KL, Griffin CA. Fluorescence in situ hybridization for the Y-chromosome can be used to detect cells of recipient origin in allografted hearts following cardiac transplantation. Am J Pathol. 1993;142:975–980. [PMC free article] [PubMed] [Google Scholar]
- 6.Glaser R, Lu MM, Narula N, Epstein JA. Smooth muscle cells, but not myocytes, of host origin in transplanted human hearts. Circulation. 2002;106:17–19. [DOI] [PubMed] [Google Scholar]
- 7.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. [DOI] [PubMed] [Google Scholar]
- 8.Pober JS, Jane-wit D, Qin L, Tellides G. Interacting Mechanisms in the Pathogenesis of Cardiac Allograft Vasculopathy. Arterioscler Thromb Vasc Biol. 2014;34:1609–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tellides G, Pober JS. Interferon-γ Axis in Graft Arteriosclerosis. Circ Res. 2007;100:622–632. [DOI] [PubMed] [Google Scholar]
- 10.Tellides G, Tereb DA, Kirkiles-Smith NC, Kim RW, Wilson JH, Schechner JS, Lorber MI, Pober JS. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403:207–211. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Liu S, Li W, Hu S, Xiong J, Shu X, Hu Q, Zheng Q, Song Z. Vascular smooth muscle cell apoptosis promotes transplant arteriosclerosis through inducing the production of SDF-1alpha. Am J Transplant. 2012;12:2029–2043. [DOI] [PubMed] [Google Scholar]
- 12.Moldovan NI, Qian Z, Chen Y, Dong C, Ying A, Hruban RH, Flavahan NA, Baldwin IW, Sanfilippo F, Goldschmidt-Clermont PJ. Fas-mediated apoptosis in accelerated graft arteriosclerosis. Angiogenesis. 1998;2:245–254. [DOI] [PubMed] [Google Scholar]
- 13.Legare JF, Issekutz T, Lee TD, Hirsch G. CD8+ T lymphocytes mediate destruction of the vascular media in a model of chronic rejection. Am J Pathol. 2000;157:859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andriambeloson E, Pally C, Hengerer B, Cannet C, Nikolova Z, Bruns C, Zerwes HG, Bigaud M. Transplantation-induced endothelial dysfunction as studied in rat aorta allografts. Transplantation. 2001;72:1881–1889. [DOI] [PubMed] [Google Scholar]
- 15.Bigaud M, Schraa EO, Andriambeloson E, Lobstein V, Pally C, Kobel T, Bruns C, Zerwes HG. Complete loss of functional smooth muscle cells precedes vascular remodeling in rat aorta allografts. Transplantation. 1999;68:1701–1707. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch GM, Kearsey J, Burt T, Karnovsky MJ, Lee T. Medial smooth muscle cell loss in arterial allografts occurs by cytolytic cell induced apoptosis. Eur J Cardiothorac Surg. 1998;14:89–96; discussion 96–87. [DOI] [PubMed] [Google Scholar]
- 17.Nejat S, Zaki A, Hirsch GM, Lee TD. CD8(+) T cells mediate aortic allograft vasculopathy under conditions of calcineurin immunosuppression: role of IFN-gamma and CTL mediators. Transpl Immunol. 2008;19:103–111. [DOI] [PubMed] [Google Scholar]
- 18.Dong C, Wilson JE, Winters GL, McManus BM. Human transplant coronary artery disease: pathological evidence for Fas-mediated apoptotic cytotoxicity in allograft arteriopathy. Lab Invest. 1996;74:921–931. [PubMed] [Google Scholar]
- 19.Liu G, Butany J. Morphology of graft arteriosclerosis in cardiac transplant recipients. Hum Pathol. 1992;23:768–773. [DOI] [PubMed] [Google Scholar]
- 20.Lu WH, Palatnik K, Fishbein GA, Lai C, Levi DS, Perens G, Alejos J, Kobashigawa J, Fishbein MC. Diverse morphologic manifestations of cardiac allograft vasculopathy: a pathologic study of 64 allograft hearts. J Heart Lung Transplant. 2011;30:1044–1050. [DOI] [PubMed] [Google Scholar]
- 21.Huibers MM, Vink A, Kaldeway J, Huisman A, Timmermans K, Leenders M, Schipper ME, Lahpor JR, Kirkels HJ, Klopping C, et al. Distinct phenotypes of cardiac allograft vasculopathy after heart transplantation: a histopathological study. Atherosclerosis. 2014;236:353–359. [DOI] [PubMed] [Google Scholar]
- 22.Johnson DE, Gao SZ, Schroeder JS, DeCampli WM, Billingham ME. The spectrum of coronary artery pathologic findings in human cardiac allografts. J Heart Transplant. 1989;8:349–359. [PubMed] [Google Scholar]
- 23.Liu R, Jin Y, Tang WH, Qin L, Zhang X, Tellides G, Hwa J, Yu J, Martin KA. Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity. Circulation. 2013;128:2047–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clerkin KJ, Ali ZA, Mancini DM. New developments for the detection and treatment of cardiac vasculopathy. Curr Opin Cardiol. 2017;32:316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moran-Crusio K, Reavie L, Shih A, Abdel-Wahab O, Ndiaye-Lobry D, Lobry C, Figueroa ME, Vasanthakumar A, Patel J, Zhao X, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ichiyama K, Chen T, Wang X, Yan X, Kim BS, Tanaka S, Ndiaye-Lobry D, Deng Y, Zou Y, Zheng P, et al. The methylcytosine dioxygenase Tet2 promotes DNA demethylation and activation of cytokine gene expression in T cells. Immunity. 2015;42:613–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin L, Yu L, Min W. Mouse models for graft arteriosclerosis. J Vis Exp. 2013;14:50290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sata M, Maejima Y, Adachi F, Fukino K, Saiura A, Sugiura S, Aoyagi T, Imai Y, Kurihara H, Kimura K, et al. A mouse model of vascular injury that induces rapid onset of medial cell apoptosis followed by reproducible neointimal hyperplasia. J Mol Cell Cardiol. 2000;32:2097–2104. [DOI] [PubMed] [Google Scholar]
- 29.Yin R, Mao S, Zhao B, Chong Z, Yang Y, Zhao C, Zhang D, Huang H, Gao J, Li Z, et al. Ascorbic Acid Enhances Tet-mediated 5-methylcytosine Oxidation and Promotes DNA Demethylation in Mammals. J Am Chem Soc. 2013;135:10396–10403. [DOI] [PubMed] [Google Scholar]
- 30.Young J, Züchner S, Wang G. Regulation of the Epigenome by Vitamin C. Annu Rev Nutr. 2015;35:545–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cimmino L, Dolgalev I, Wang Y, Yoshimi A, Martin GH, Wang J, Ng V, Xia B, Witkowski MT, Mitchell-Flack M, et al. Restoration of TET2 Function Blocks Aberrant Self-Renewal and Leukemia Progression. Cell. 2017;170:1079–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng W, Zhang Y, Cai J, Zhang J, Liu X, Yin J, Bai Z, Yao H, Zhang Z. LncRNA-ANRIL Promotes Gastric Cancer Progression by Enhancing NF-kB Signaling. Exp Biol Med. 2019;244:953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng Y, Li X, Cassady K, Zou Z, Zhang X. TET2 Function in Hematopoietic Malignancies, Immune Regulation, and DNA Repair. Front Oncol. 2019;9:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kabelitz D. Role of apoptosis in cardiac allograft vasculopathy. Z Kardiol. 2000;89:21–23. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Li C, Zhuang Q, Peng B, Zhu Y, Ye Q, Ming Y. The Evolving Roles of Macrophages in Organ Transplantation. J Immunol Res. 2019;2019:5763430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavalcante GC, Schaan AP, Cabral GF, Santana-da-Silva MN, Pinto P, Vidal AF, Ribeiro-dos-Santos Â. A Cell’s Fate: An Overview of the Molecular Biology and Genetics of Apoptosis. Int J Mol Sci. 2019;20:4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coqueret O. New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol. 2003;13:65–70. [DOI] [PubMed] [Google Scholar]
- 38.Abbastabar M, Kheyrollah M, Azizian K, Bagherlou N, Tehrani SS, Maniati M, Karimian A. Multiple functions of p27 in cell cycle, apoptosis, epigenetic modification and transcriptional regulation for the control of cell growth: A double-edged sword protein. DNA Repair. 2018;69:63–72. [DOI] [PubMed] [Google Scholar]
- 39.Nemenoff RA, Horita H, Ostriker AC, Furgeson SB, Simpson PA, Vanputten V, Crossno J, Offermanns S, Weiser-Evans MCM. SDF-1alpha Induction in Mature Smooth Muscle Cells by Inactivation of PTEN Is a Critical Mediator of Exacerbated Injury-Induced Neointima Formation. Arterioscler Thromb Vasc Biol. 2011;31:2909–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Gorkom GNY, Klein Wolterink RGJ, Van Elssen C, Wieten L, Germeraad WTV, Bos GMJ. Influence of Vitamin C on Lymphocytes: An Overview. Antioxidants. 2018;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang JC, Kinlay S, Beltrame J, Hikiti H, Wainstein M, Behrendt D, Suh J, Frei B, Mudge GH, Selwyn AP, et al. Effect of vitamins C and E on progression of transplant-associated arteriosclerosis: a randomised trial. Lancet. 2002;359:1108–1113. [DOI] [PubMed] [Google Scholar]
- 42.Shenoy N, Creagan E, Witzig T, Levine M. Ascorbic Acid in Cancer Treatment: Let the Phoenix Fly. Cancer Cell. 2018;34:700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma Y, Chapman J, Levine M, Polireddy K, Drisko J, Chen Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci Transl Med. 2014;6:222. [DOI] [PubMed] [Google Scholar]
- 44.Yue X, Rao A. TET family dioxygenases and the TET activator vitamin C in immune responses and cancer. Blood. 2020;136:1394–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ostriker A, Horita HN, Poczobutt J, Weiser-Evans MC, Nemenoff RA. Vascular Smooth Muscle Cell-Derived Transforming Growth Factor-beta Promotes Maturation of Activated, Neointima Lesion-Like Macrophages. Arterioscler Thromb Vasc Biol. 2014;34:877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takayama T, Shi X, Wang B, Franco S, Zhou Y, DiRenzo D, Kent A, Hartig P, Zent J, Guo LW. A murine model of arterial restenosis: technical aspects of femoral wire injury. J Vis Exp. 2015:52561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lanahan A, Zhang X, Fantin A, Zhuang Z, Rivera-Molina F, Speichinger K, Prahst C, Zhang J, Wang Y, Davis G, et al. The neuropilin 1 cytoplasmic domain is required for VEGF-A-dependent arteriogenesis. Dev Cell. 2013;25:156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klatt E. The University of Utah Eccles Health Sciences Library. Elastic van Gieson Stain. The Internet Pathology Laboratory for Medical Education. 1994–2020. https://webpath.med.utah.edu/HISTHTML/MANUALS/EVG.PDF. Accessed 2015. [Google Scholar]
- 49.Pirici D, Mogoanta L, Kumar-Singh S, Pirici I, Margaritescu C, Simionescu C, Stanescu R. Antibody elution method for multiple immunohistochemistry on primary antibodies raised in the same species and of the same subtype. J Histochem Cytochem. 2009;57:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Y, Lv L, Liu Y, Smith M, Li W, Tan X, Cheng M, Li Z, Bovino M, Aubé J, et al. Tumor suppressor TET2 promotes cancer immunity and immunotherapy efficacy. J Clin Invest. 2019;129:4316–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.