Abstract
In the last decade, metagenomic studies have shown the key role of the gut microbiome in maintaining immune and neuroendocrine systems. Malfunction of the gut microbiome can induce inflammatory processes, oxidative stress, and cytokine storm. Dysfunction of the gut microbiome can be caused by short-term (virus infection and other infectious diseases) or long-term (environment, nutrition, and stress) factors. Here, we reviewed the inflammation and oxidative stress in neurodegenerative diseases and coronavirus infection (COVID-19). Here, we reviewed the renin–angiotensin–aldosterone system (RAAS) involved in the processes of formation of oxidative stress and inflammation in viral and neurodegenerative diseases. Moreover, the coronavirus uses ACE2 receptors of the RAAS to penetrate human cells. The coronavirus infection can be the trigger for neurodegenerative diseases by dysfunction of the RAAS. Pharmabiotics, postbiotics, and next-generation probiotics, are considered as a means to prevent oxidative stress, inflammatory processes, neurodegenerative and viral diseases through gut microbiome regulation.
Keywords: renin–angiotensin–aldosterone system, gut microbiome, coronavirus infection, COVID-19, neurodegenerative diseases, inflammatory processes, oxidative stress, pharmabiotics, probiotics, postbiotics, symbiotics, Lactobacillus
Introduction
Inflammation and oxidative stress are common symptoms of neurodegenerative diseases and viral infections such as Parkinson’s disease and COVID-19. The immuno pathogenesis induced by SARS-CoV-2 disrupts the immune system, leading to inflammatory responses. It is reported that COVID-19 enters the cell by interacting with the angiotensin-converting enzyme II receptor ACE2 and transmembrane serine protease-2 TMPRSS2.1–3 Therefore, serum angiotensin 2 AngII levels increase as a result of its reduced degradation by ACE2. Accumulated AngII has been shown to activate inflammatory cytokines, including interferon-gamma, followed by interferon gene stimulation, resulting in increased cytokine storm and associated acute respiratory distress syndrome, as seen in severe disease.1,4–6 Moreover, activation of cytokines leads to hyperactivation of downstream signaling cascades, including nuclear transcription factor kappa B NF-κB, which is usually activated by SARS-CoV-2 itself via pattern recognition receptors.1,5,7
Elevated levels of reactive oxygen species by SARS-CoV-2 can cause redox imbalances, increase the amount of lipid peroxidation products, and open the transition pores of mitochondrial permeability. Factors such as procaspase, apoptosis initiation factor, and cytochrome C are activated as a result of an electron imbalance in mitochondria.1,8–10 These factors contribute to further cell damage, promoting apoptotic cell death.1,11–13 Evidence is also accumulating that oxidative stress caused by increased reactive oxygen species production after hypoxia promotes the death of dopamine-containing neurons through apoptosis, which leads to the development of Parkinson’s disease, a progressive neurodegenerative disorder.1,14–16
Patients diagnosed with Parkinson’s disease do not have dopamine-containing neurons in the substantia nigra compacta and striatum. 6-hydroxydopamine is a well-known neurotoxin that induces neurotoxicity in the nigrostriatal dopaminergic system by inhibiting mitochondrial electronic circuit complexes I and IV and contributing to dopamine-containing neurons’ degeneration.1,15,17–19 Hence, this leads to a dopamine deficiency and has a strong effect on dopaminergic receptors. Surprisingly, the neurotransmitter dopamine and its receptors have been implicated in the regulation of respiration.1,20–22 Moreover, it has been reported that 6-hydroxydopamine is produced endogenously in Parkinson’s disease patients.1 The actual cause of the development and progression of Parkinson’s disease is not yet known.1 Several pieces of evidence suggest that dopamine-containing neurons’ death may result from elevated reactive oxygen species levels,1,23–25 mitochondrial respiratory failure,1,26–28 and activation of the NF-κB and caspase pathways.1,27,29,30
The development of inflammation and oxidative stress is often accompanied by dysbiosis or dysfunction of the gut microbiome.31,32 The gut microbiome plays a vital role in human health and disease and is, therefore, a popular area of research.32,33 Lactobacteria32,34 are the most important probiotic bacteria in the gut microbiome. Their positive functions include antagonism and competition with opportunistic microorganisms, improving digestion, participating in the maturation of the immune system at an early age and maintaining immunological homeostasis throughout life, neuromodulation, and the production of vitamins and other useful compounds, including antioxidant.32,35,36 These bacteria can exhibit significant antioxidant activity in the intestine of the host and promote the production of enzymes and antioxidant compounds that neutralize reactive oxygen species and prevent oxidative damage.32,37,38 However, most of their functions are specific for strains and are not common to several genera or species.32,37–39
The neuromodulatory potential of the human gut microbiome has been studied since the introduction of the gut-brain axis concept. Potential biomarkers that explain the neuromodulatory potential of the gut microbiome have been identified.32,40–42 Correction of the intestinal microbiome of patients with inflammatory diseases and oxidative stress characterized by an unbalanced antioxidant system should be carried out using strains of probiotic bacteria with selected antioxidant properties. The gut microbiome of people resistant to oxidative stress can be extracted to find unique strains that can be used to treat patients with chronic inflammatory diseases using a gut microbiome-based approach.32
The microbiome-gut-brain axis explains the mutual regulation of the nervous system and the gut microbiome through immunological, neurological, and neuroendocrine systems.43,44 Imbalance the microbiome-gut-brain axis under adverse factors, such as antibiotics, stress, food quality45–47 weakens the intestinal barrier and cause the formation of pathological processes such as chronic inflammation of the gastrointestinal tract, Alzheimer’s disease and neurodegenerative diseases.47–49 It has been shown that inflammation in the gastrointestinal tract increases neuroinflammatory processes,50,51 which can be a consequence of the malfunction of the renin-aldosterone-angiotensin system (RAAS).52,53 The RAAS is involved in the mechanisms of the formation of inflammatory processes in the human body.54,55 The virus COVID-19 uses ACE2 receptors of the RAAS to enter into human cells and launches neurodegenerative processes by RAAS dysfunction.56 Understanding the molecular mechanisms of pathogenesis prevents oxidative stress, inflammatory and neurodegenerative processes by correcting the gut microbiome. Probiotic bacteria with antioxidants include lacto- and bifidobacteria, as well as some species of propionic acid bacteria and enterobacteria,57–59 antimutagenic, immunomodulatory, and antitumor properties, can be used for the correction of the gut microbiome in modern methods of therapy.60–63 In modern therapy, not only do probiotics play an important role, but also postbiotics - their metabolites and components. Lately, pharmabiotics have become popular in medical practice. Pharmabiotics are preparations based on probiotics and their metabolites with a known composition of active substances, deciphered mechanisms of their action, and proven efficacy and safety.64–66
Oxidative Stress and Inflammation
Oxidative Stress Characteristics
Oxidative stress is an event caused by an imbalance between the production and accumulation of oxygen reactive species in cells and tissues and the ability of a biological system to detoxify reactive intermediates or repair damages.32 In order to maintain proper cellular homeostasis, a balance must be struck between the production and consumption of reactive oxygen.32 Oxidative stress is caused by the main reactive oxygen species: superoxide radicals, hydroxyl radicals HO−, lipid peroxide radicals, and hydrogen peroxide H2O2.32
There are endogenous (product of metabolism and oxidative phosphorylation in mitochondria) and exogenous (negative external factors, as environmental pollution, radiation, drugs, bacterial infection, excessive iron intake, imbalance of the intestinal microbiome) reactive oxygen species.32,67–71 The damage of mitochondrial membranes can be because of increasing amounts of reactive oxygen species.32 Some enzymes capable of producing superoxide radicals are redox flavoproteins, xanthine oxidase, NADPH oxidase, and cytochrome P450.67,72
Reactive oxygen species can endanger cell viability by causing DNA hydroxylations, protein denaturation, lipid peroxidation, and apoptosis.32,73 Some oxygen reactive species act as cellular messengers in the transmission of redox signals and can disrupt normal cellular signaling mechanisms.32,68,70,71 Oxidative stress is a common pathogenetic mechanism of tissue damage that is accompanied by various inflammatory processes and has been linked to a number of diseases including atherosclerosis, cancer, emphysema, liver cirrhosis, arthritis, and diseases with chronic conditions such as cardiovascular, respiratory, and neurodegenerative diseases.32,37,71,74 Oxidative stress triggers neurodegenerative brain diseases, Parkinson’s disease, amyotrophic lateral sclerosis, and depression.32,75–79 Neurodegenerative disorders are associated with the gut microbiome, which is involved in bidirectional communication as part of the gut-brain axis.32,80
To neutralize reactive oxygen species, the human body synthesizes antioxidant enzymes and molecules that form a natural biological antioxidant barrier.32 Antioxidants interact with reactive oxygen species and prevent the disruption of cellular functions.32 The most studied cellular antioxidants are the enzymes superoxide dismutase, catalase, and glutathione peroxidase. Less studied, but no less important, antioxidant enzymes are peroxiredoxins, sulfiredoxin, paraoxonase, glutathione S-transferase, and aldehyde dehydrogenase.32
However, reactive oxygen species are not always harmful and can be beneficial as they are used by the immune system as a way to attack and kill pathogens.81 Short-term oxidative stress may also be important in the prevention of aging by induction of a process named mitohormesis.82 Also, reactive oxygen species play an important role in cell signaling, a process called redox signaling.32
Inflammatory Processes Caused by Viral Infections
Although viruses can replicate in several cell types, the pathological outcome only appears in one or a few tissue-specific cells.83 The primary encounter with the virus occurs in mononuclear phagocytic cells such as monocytes, macrophages, and dendritic cells.83 The stimulation of the innate and adaptive immune system in response to viral infections destroys infected cells, inducing inflammation that may lead to severe pathological consequences for the host. The damage of cells caused by the immune system with a viral infection is known as virus-induced inflammation.83–85
In the earliest stages of viral infection, cytokines are produced when the innate immune defense is activated.83,86,87 Neutrophils are among the earliest types of phagocytic cells entering the site of infection and are classic markers of the inflammation process.83,86,87 The rapid release of cytokines at the site of infection initiates new reactions with far-reaching consequences, including inflammation.83,86,87 One of the first cytokines to be produced is tumor necrosis factor alpha TNF-α, which is synthesized by activated monocytes and macrophages.83,86,87 Inflammation is a very noticeable response to TNF-α.83,86,87 Inflammation is caused by the excessive release of antibodies, interferons, and pro-inflammatory cytokines, activation of the complement system, or hyperactivity of cytotoxic T cells.
In severe cases of certain viral infections, as in avian influenza H5N1 and coronavirus SARS-CoV-2, aberrant induction of the host immune response can elicit a flaring release of cytokines known as a cytokine storm.83,88 There is evidence for the development of progressive multifocal leukoencephalopathy, a fatal human demyelinating disease caused by the John Cunningham virus (JCV), in patients treated with Natalizumab monoclonal antibodies.89 The JCV virus is detected in 70–90% of the world’s population, which can cause progressive multifocal leukoencephalopathy in people with weakened immune systems.90,91 About 50% of patients with viral multifocal leukoencephalopathy die within a few months after diagnosis. Several studies indicate the association of the Epstein-Barr virus (EBV) with the development of multiple sclerosis.89,92 The high incidence of multiple sclerosis in people who have undergone EBV infection may serve as evidence of the primacy of inflammatory processes in the remitting and progressive course of multiple sclerosis.93 Another virus associated with the development of multiple sclerosis is the Human Herpesvirus type 6 (HHV-6), which is involved in triggering autoimmune reactions.94–96 An inverse relationship has been found between the age of HHV-6 infection and the risk of developing multiple sclerosis in the future. The MS-associated retrovirus (MSRV) may be transactivated by external risk factors for multiple sclerosis such as HHV-6 and EBV viruses. Thus, various infectious agents (JCV, MSRV, HHV-6, EBV, and SARS-CoV-2), provoking inflammation, can be triggers of progressive neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s disease, and multiple sclerosis.89,97
Oxidative Stress and Inflammation in the Formation of Neurodegenerative Diseases
Oxidative stress and the inflammatory process it causes are able to form neurodegenerative diseases, including Parkinson’s disease and multiple sclerosis.98 Chronic inflammation in the small intestine as a result of infection can lead to the activation of glial cells of the enteric nervous system, mainly nerve fibers of the autonomic nervous system, and a violation of the conformational properties of proteins, namely, α-synuclein. Subsequently, the molecules of pathological forms of α-synuclein trans synaptically penetrate the central nervous system along the afferent fibers of the vagus nerve and begin to exhibit prion-like properties affecting the dorsal nucleus and neurons of the caudal trunk.99,100 Disturbances in the composition of the gut microbiome are closely associated with delayed colonic transit, olfactory dysfunction, emotional-affective disorders, and depression.41,101,102
Multiple sclerosis is a chronic autoimmune neurodegenerative disease in which the myelin sheath of nerve fibers in the brain and spinal cord is affected.103 At certain stages, the mechanisms of neurodegeneration in multiple sclerosis and Alzheimer’s disease may have common features. The progression of Alzheimer’s disease is associated with the accumulation of the pathological protein β-amyloid and neurofilaments and amyloid plaques formed from it in the brain. In multiple sclerosis, accumulation of the β-amyloid precursor in axons around amyloid plaques was also found, the concentration of which positively correlates with the stage of the disease.93 The neurodegenerative process and inflammation in multiple sclerosis can be enhanced in the case of mitochondrial dysfunction and oxidative injury of neurons. The α-synuclein protein, which is responsible for the development of neurodegeneration, is also involved in the development of multiple sclerosis. The concentration of α-synuclein increases with opticomyelitis and exacerbation of multiple sclerosis.104 There is evidence of acceleration of the neurodegenerative process under conditions of activation of systemic inflammation.105
Cytokine Storm and Oxidative Stress Caused by COVID-19
ACE2 Receptors are the Gates for SARS-CoV-2
Viral pandemic coronavirus infection COVID-19 (coronavirus disease 2019) caused by the Severe Acute Respiratory Syndrome-Related Coronavirus 2 (SARS-CoV-2), (https://www.worldometers.info/coronavirus) leads to a wide range of functional consequences,106 capable of infecting most organs and systems in human, including the gut microbiome107–109 and central nervous system.110
Penetration into host cells is the first step in viral infection. The entrance gates for the coronavirus are the epithelium of the upper respiratory tract and the epithelial cells of the stomach and intestines.109,110 Dissemination of the COVID-19 virus from the systemic circulation or through the plate of ethmoid bone Lamina cribrosa can damage the brain. Changes in olfaction (hyposmia) at an early stage of the disease may indicate damage to the central nervous system and the mucous membrane of the nasopharynx as a result of the inflammatory process.110
The spike glycoprotein on the viral envelope of the coronavirus can bind to specific receptors on the membrane of host cells. Previous studies have shown that ACE2 is a specific functional receptor for SARS-CoV.111 It has been shown that SARS-CoV-2 can enter cells expressing ACE2 but not cells without ACE2 or cells expressing other coronavirus receptors such as aminopeptidase N and dipeptidyl peptidase 4, confirming that ACE2 is a cellular receptor for SARS-CoV-2.3,111 Further studies have shown that the binding affinity of the SARS-CoV-2 thorn glycoprotein with ACE2 is 10–20 times higher than that of SARS-CoV with ACE2.111,112 The probable mechanism of penetration of SARS-CoV-2 into host cells consists in binding the receptor-binding domain of the spike glycoprotein with the tip of the ACE2 subdomain.111–115 The fusion of the viral and host cell membranes is activated upon binding, and the viral RNA is subsequently released into the cytoplasm, causing infection. During SARS-CoV infection, intact ACE2 or its transmembrane domain is internalized together with the virus.111,116 The catalytically active site of ACE2 is not overlapped by the thorn glycoprotein, and the binding process does not depend on the peptidase activity of ACE2.111,114
Pathogenesis of SARS-CoV-2
Clinical manifestations of COVID-19 are variable, ranging from mild clinical manifestations to severe illness.117,118 Common clinical manifestations include headaches, loss of smell and taste, nasal congestion and runny nose, cough, muscle pain, sore throat, fever, diarrhea, and breathing difficulties.119,120 The SARS-CoV-2 virus can infect a wide variety of cells and body systems. COVID-19 is best known for affecting the upper respiratory tract (sinuses, nose, and throat) and the lower respiratory tract (windpipe and lungs).121 The lungs are the organs most affected by COVID-19, as the virus gains access to host cells through the receptor for the angiotensin-converting enzyme 2 ACE2, which is most abundant on the surface of type II alveolar lung cells.122 The virus uses a special surface glycoprotein called a spike to bind to the ACE2 receptor and enter the host cell.123 COVID-19 leads to diffuse damage to the alveoli and inflammatory infiltrates containing lymphocytes in the lungs.124,125
It has been shown that multiple organs are damaged during infection with SARS-CoV-2. After the penetration of the virus, COVID-19 affects the ciliated epithelium of the nasopharynx and upper respiratory tract.126 Many people with COVID-19 are known to have neurological or mental health problems. The virus was found in cerebrospinal fluid upon dissection. The exact mechanism by which it enters the central nervous system remains unclear and may initially involve invasion of peripheral nerves, given the low levels of ACE2 in the brain.127–129 The virus can also enter the bloodstream from the lungs and cross the blood-brain barrier to gain access to the central nervous system, possibly in infected white blood cells.130 The SARS-CoV-2 virus infects the gastrointestinal tract because ACE2 is expressed in high levels in the glandular cells of the gastric, duodenal, and rectal epithelium, as well as endothelial cells and enterocytes of the small intestine.131
Although SARS-CoV-2 has a tropism for ACE2-expressing airway epithelial cells, people with severe COVID-19 show symptoms of systemic hyperinflammation. The increased levels of IL-2, IL-7, IL-6, granulocyte-macrophage colony-stimulating factor GM-CSF, interferon-gamma-induced protein 10 IP-10, chemoattractant protein of monocytes 1 MCP1, inflammatory protein of macrophages 1 - alpha MIP-1 alpha, and tumor necrosis factor TNF-alpha, indicative of cytokine release syndrome, suggest an underlying immunopathology.132 Also, people with COVID-19 and acute respiratory distress syndrome have classic serum CRS biomarkers, including elevated levels of C-reactive protein CRP, lactate dehydrogenase LDH, D-dimer, and ferritin.133 Systemic inflammation leads to vasodilation, which leads to inflammatory lymphocytic and monocytic infiltration of the lungs and heart. In particular, pathogenic T cells secreting GM-CSF have been shown to correlate with the recruitment of inflammatory monocytes secreting IL-6 and severe lung disease in people with COVID-19.125,134
Cytokine Storm Caused by COVID-19
The severity of the inflammation caused by COVID-19 can be attributed to the severity of the so-called cytokine storm.135 Levels of interleukin 1β, interferon gamma, interferon-induced protein 10, and monocytic chemoattractant protein 1 were associated with the severity of COVID-19 disease. Treatment has been proposed to combat the cytokine storm as it remains one of the leading causes of morbidity and mortality in COVID-19 disease.136
The cytokine storm occurs due to an acute hyperinflammatory reaction that causes clinical disease in a variety of diseases, but in COVID-19 this is associated with a worse prognosis and increased mortality.137 The hurricane causes acute respiratory distress syndrome, blood clotting phenomena such as strokes, myocardial infarction, encephalitis, acute kidney injury, and vasculitis. The production of IL-1, IL-2, IL-6, TNF-alpha, and interferon-gamma, all essential components of a normal immune response, inadvertently becomes the cause of the cytokine storm. Central nervous system cells, microglia, neurons, and astrocytes are also involved in the release of proinflammatory cytokines that affect the nervous system, and the effects of cytokine storms on the central nervous system are not uncommon.137,138
Oxidative Stress and Systemic Inflammation in COVID-19
Oxidative stress has recently been proposed as a key factor in COVID-19.139–141 The mechanism includes the activity of ACE2, which cleaves the octapeptide angiotensin II Ang II, which was previously generated by ACE.139 Since Ang II is a potent vasoconstrictor that plays a key role in raising blood pressure, its treatment with ACE2 induces vasodilation, enhanced by the formation of Ang 1–7, a peptide with potent vasodilating functions that are generated during this process.139 The binding of SARS-CoV-2 to ACE2 causes the virus to enter cells and, in turn, reduces the bioavailability of ACE2.139 Due to the protective function of ACE2, a decrease in its levels is associated with unfavorable clinical phenotypes, and its key role in the pathogenesis of SARS-Cov-2 has been described.139,142 Evidence has shown that Ang II regulates the activation of nicotinamide adenine dinucleotide phosphate NADPH oxidase143–146 and binds to angiotensin type 1 receptor AT1R.139,147 The activation of oxidase is one of the main factors in the formation of reactive oxygen species including the superoxide radical anion O2- and hydrogen peroxide H2O2.139 Therefore, the decreased bioavailability of ACE2 after SARS-CoV-2 binding allows Ang II to be available to interact with AT1R, which mediates signals for NADPH oxidase activation and induces oxidative stress and inflammatory responses that in turn contribute to the severity of COVID-19.139,148,149 It was shown, that NADPH oxidase-2 is overexpressed in hospitalized patients with COVID-19, causing increased oxidative stress.139,150
Inflammation and the immune response Coronavirus subtypes such as SARS-CoV and especially SARS-CoV-2 are capable of actively causing the so-called cytokine storm by mediating the increased production and release of proinflammatory cytokines,151 which confirms the high levels of inflammatory markers found in patients with COVID-19.132,139,152–155 Interestingly, one of the identified markers is the nonspecific C-reactive protein, a widely used biomarker for the diagnosis of sepsis.139 In addition, elevated levels of inflammatory cytokines and chemokines have been associated with the severity of COVID-19 and death.132,139,152–155 Elevated plasma concentrations of interleukins such as IL-1 β, IL-2, IL-6, IL-7, IL-8, IL-10 or IL-17, interferon γ, interferon γ-induced protein 10, chemoattractant monocyte protein 1 MCP1, granulocyte-macrophage colony-stimulating factor, macrophage inflammatory protein 1α and tumor necrosis factor-alpha TNFα, among others, have been identified as inflammatory mediators in COVID-19.132,139,156–160 Importantly, macrophages and neutrophils also play a potential pathological role during SARS-CoV-2 infection161 by producing numerous reactive oxygen species including, but not limited to, H2O2, O2-, and hydroxyl radical OH.139,162 Oxidative stress affects the immune system by altering immune cell function and inflammatory response.139,163,164 The systemic cytokine profiles observed in patients with severe COVID-19 are similar to those seen in cytokine release syndromes such as sepsis, with increased production of cytokines such as IL-6, IL-8, TNFα, and other pro-inflammatory chemokines, including chemokine CC ligand 2 CCL2, CCL3 and chemokine CXC ligand 10 CXCL10.139
Parkinson’s Disease Accompanied by Inflammatory Processes of the Nervous System
Oxidative Stress and Inflammatory Processes in the Parkinson’s Disease
Parkinson’s disease is a progressive nervous system disorder that affects human movement. The etiology of Parkinson’s disease is still a matter of debate,165,166 while the number of clinical cases is growing steadily.167,168 Parkinson’s disease is now recognized as a multisystem disease. The neurochemical and pathomorphological basis of Parkinson’s disease is the death of neurons in the substantia nigra of the brain and the subsequent depletion of dopamine reserves in the striatum.168
One of the theories of the pathogenesis of Parkinson’s disease suggests that, as a result of disruption of the functioning of the antioxidant system and increased formation of reactive oxygen species, oxidative stress leads to the rapid accumulation of abnormal conformations of the α-synuclein protein in the affected neurons. The accumulation of the α-synuclein protein in the neurons in amounts exceeding the capacity for its degradation by the proteolytic system leads to the formation of inclusion bodies in the cytoplasm, called Lewy bodies. The main component of Lewy bodies is α-synuclein.168
The concept of conformational diseases suggests the role of the conformation of a single protein or several proteins in the initiation and progression of Parkinson’s disease.169,170 Intracellular accumulation of not only α-synuclein but also β-amyloid in the cerebral cortex was detected in patients with Parkinson’s disease since stage III according to the Hoehn-Yahr scale.171 Thus, cortical accumulation of β-amyloid may be a determining factor in the development of the transition from early to late stages of Parkinson’s disease.171
It has been shown that parasympathetic neurons of the intestinal submucosa and exogenous alimentary factors such as infectious and toxic are involved in the early stage of development of Parkinson’s disease.99 There is evidence that a shift in the composition of the gut microbiome may play an important role in the initiation and progression of Parkinson’s disease, as a result of the upward diffusion of α-synuclein from the parasympathetic nervous system of the gut to the brain.172
Modern Concepts of the Etiology of Parkinson’s Disease
Parkinson’s disease is currently the second most common neurodegenerative disease, the incidence of which is expected to increase in the coming decades due to increased life expectancy.173,174 Although most cases of Parkinson’s disease occur sporadically, a small subset of Parkinson’s disease cases are hereditary and are attributed to mutations in genes associated with PARK loci including SNCA, Parkin, DJ-1, PINK1, NURR1, OMI/HTRA2, and LRRK2 associated with the disease. Many theories have been proposed for the etiology of idiopathic Parkinson’s disease,174–177 but none of them provide a solid basis for explaining all symptoms. Indeed, some authors shrewdly view Parkinson’s disease as a syndrome with various possible etiologies.174,178–180 While the exact cause of Parkinson’s disease is currently unknown, scientists have come up with the following possible theories of this disease etiology: based on genetics, based on the environment, based on the presence of Lewy bodies, based on the presence of alpha-synuclein in Lewy bodies. According to genetics-based theory, there are certain genes that, when they become mutated, cause Parkinson’s disease. However, these mutated genes are very rare, except in cases where Parkinson’s runs in the family. There are also some gene variations that seem to slightly increase the risk of developing Parkinson’s.174 According to environment-based theory, there are some environmental factors and toxins which may trigger Parkinson’s disease, although they feel the increased risk is small. According to theory based on the presence of Lewy bodies, changes happening within the brain may also be a trigger for Parkinson’s disease. Lewy bodies are proteins found in brain cells that are biomarkers of the disease and may hold the key to finding out the exact cause.174 According to theory based on the presence of alpha-synuclein in Lewy bodies, alpha-synuclein proteins form clumps in the cells which are thought to contribute to the disease. In the environmental toxin hypothesis, progressive neurodegeneration of Parkinson’s disease may be caused by chronic exposure to a neurotoxin or limited exposure to initiate a self-replicating cascade of deleterious events.174,181 It is possible that the idiopathic manifestation of Parkinson’s disease depends on several factors, such as individual nuclear/cytosolic protein variants, endosymbiont mitochondria, and microorganisms, which will synergistically contribute to the development of the disease.174,177,182
Today, the theory of inflammatory processes initiated by the gut microbiome and the transmission of signals to the intestinal and central nervous systems mediating the vagus nerve is of the greatest interest. According to this theory, the gut microbiome can cause inflammation of the gut and brain, leading to immune-like Parkinson’s disease. An imbalanced gut microbiome or a breakdown of the immune system can lead to gut dysbiosis, causing an inflammatory response with the partial movement of intestinal contents into the bloodstream and inevitably to distant parts of the body.174,183–185 The central nervous system is protected by the blood-brain barrier, a true barrier and an important mediator of central nervous system homeostasis, which separates the blood from the neuronal parenchyma and insulates the brain from harmful molecules. However, this blood-brain barrier can be disrupted in Parkinson’s disease as a result of intestinal dysbiosis, creating a self-reinforcing loop leading to the activation and recruitment of peripheral immune cells and neurodegeneration.174,186–189
The gut microbiome associated with feces and mucous membranes also differs between people with Parkinson’s disease and healthy people.174,190 In patients with Parkinson’s disease, intestinal inflammation is observed,174,191 and it has been described that the infiltration of monocytes and T cells into the brain parenchyma is primarily caused by the microbiome associated with the intestinal mucosa.174,187,188 These cells appear to be required to control both local inflammatory responses in the brain and the activation of peripheral immune mechanisms.174,192 In addition, intestinal infection stimulates mitochondrial antigen presentation and autoimmune mechanisms that target cytotoxic mitochondria-specific CD8+ T cells to the periphery and to the brain.174,185 This once again indicates that mitochondria retain the ability to activate the innate immunity of neurons.174,182,185 There is also evidence that Parkinson’s disease may result from the combined effects of inflammation and asynchronous vagal spread,174,177,187 being a common trigger of gut dysbiosis.174,177
Some bacteria contain immunoreactive motifs that are potent IgA inducers, as observed in multiple sclerosis and inflammatory bowel disease.174,193 This emphasizes the role of the gut microbiome as the main participant in the dynamic migration of plasma cells between the gut and the brain.174,194–196 However, it is interesting to ask why the immune-stimulating bacteria in the gut can lead to the recruitment of regulatory immune cells to the central nervous system.174,195,197 It can be explained that a breached intestinal barrier may allow gut microbiome–specific immune cells to act as systemic mediators able to penetrate the central nervous system during acute neuroinflammation.174,195,198 Recent reports show that gut-derived IgA plasma cells are present in meningeal venous sinuses of slow blood flow whose fenestrations can potentially allow access of blood-borne pathogens into the brain.174,194
Role of the Raas System in the Pathogenesis of Parkinson’s Disease and COVID-19
Involvement of the RAAS System in Systemic Inflammatory Processes
The RAAS is a vital system of the human body, as it maintains plasma sodium concentration, blood pressure, vascular tone, and extracellular fluid volume, carries out tissue remodeling, and produces pro-inflammatory and pro-fibrotic effects. Renin and angiotensin are two of the most important constituents of RAAS.199 Renin, or angiotensinogenase, is secreted by the granular cells of the kidneys and is found in several isoforms containing 340 amino acids with antagonizing functions.200 Renin is also considered a hormone because it has a signaling function. The enzyme renin acts on its substrate to form angiotensin II, a universal effector peptide hormone.201
Angiotensin II is a versatile effector molecule with intracrine, autocrine, and paracrine roles which interacts with all body systems.202,203 Angiotensin II is one of the most powerful vasoconstrictors, regulates bronchial smooth muscle contraction, the proliferation of lung fibroblasts, and vascular permeability in the lung tissue, increases blood pressure and heart rate, stimulates plasminogen activator, inhibits the PAI-1 and PAI-2 proteins, increases the prothrombotic potential., affects the release of prostaglandins and vasoconstriction of the kidneys.204 Cyclooxygenase 1-Derived Prostaglandin E2 and its receptor have been identified as critical for angiotensin-dependent hypertension. Angiotensin II may promote lipogenesis, increase adipose tissue mass, and is associated with fatty inflammation, glucose intolerance, and insulin resistance.201 In addition, angiotensin II stimulates the adrenal cortex to secrete aldosterone, a steroid hormone that maintains sodium-potassium homeostasis by stimulating the proximal renal tubules to increase sodium reabsorption and potassium release201 (Figure 1).
Figure 1.
The scheme of the renin–angiotensin–aldosterone system.
Abbreviations: ARB, angiotensin receptor blocker; ACE-I, ACE inhibitor; AT1-R, the angiotensin II type 1 receptor (AT1 receptor); AT2-R, the angiotensin II type 2 receptor (AT2 receptor); Mas-R, mas receptor; Ang-(1-7), angiotensin-(1–7); Ang-(1-9), angiotensin-(1-9); ACE, angiotensin-converting-enzyme; ACE2, angiotensin-converting enzyme 2; Ang-I, angiotensin I; Ang-II, angiotensin II.
The most important components of the RAAS system are the angiotensin-converting enzymes ACE and ACE2, whose tasks are the maintenance of the homeostasis of the cardiovascular system, regulation of the systolic pressure, and osmotic and electrolyte balance.204 Despite the similarity of the ACE and ACE2 genes, the ACE and ACE2 enzymes perform different functions in the human body. Angiotensinogen is synthesized in the liver, after which it is converted by renin to angiotensin I, and then converted by an enzyme ACE into angiotensin II. Normally, ACE2 converts angiotensin II to angiotensin 1–7, which causes vasodilation, decreases blood pressure, and participates in the absorption of neutrally charged amino acids in the gut. Moreover, ACE2 can interact with angiotensin I, converting it to angiotensin 1–9, which can be converted to angiotensin 1–7 under the action of ACE.204
Excessive activation of the tissue system of the RAAS is associated with cardiovascular disease, diabetes, kidney disease, preeclampsia, osteoporosis, and neurodegenerative diseases.53–55,205,206 All components of the RAAS have been identified in different areas of the brain at the level of the blood-brain barrier, and the RAAS of the brain is involved in additional brain functions and neurological disorders.207,208
Angiotensin II through type 1 receptors activates the NADPH oxidase complex, which is involved in oxidative stress and inflammatory processes in the pathogenesis of major diseases associated with aging. In the basal ganglia and the nigrostriatal system, hyperactivation of the RAAS through the activation of the NADPH oxidase complex aggravates oxidative stress and the inflammatory response of microglia and promotes the progression of dopaminergic degeneration, which is inhibited by angiotensin receptor blockers and angiotensin-converting enzyme inhibitors.209 An imbalance in renin and angiotensin II can lead to a wide range of chronic and acute diseases. Dopamine depletion leads to increased expression of angiotensin II, which stimulates the synthesis of dopamine, which is released via AT1 or AT2 receptors. In addition, AT1 receptors allosterically inhibit the activation of dopamine D1 receptors. Consequently, the RAAS can play an important modulating role in the flow of information from the cerebral cortex to the basal ganglia. High levels of angiotensin II may enhance neurodegeneration by activating the NADPH oxidase complex, which leads to increased production of reactive oxygen species.210 The manipulation of the RAAS, leading to an increase in the expression of ACE2, can affect endocrine functions, which can play an important role in pathological processes.201 In addition, ACE2 in the intestine functions as a partner for the amino acid transporter B0AT1. It is possible that the B0AT1/ACE2 complex in the intestinal epithelium regulates the composition and function of the gut microbiome and affects local and systemic antiviral immunity.211
Chronic activation of the RAAS leads to the onset and development of congestive heart failure syndromes, systemic hypertension, and chronic kidney disease. Excessive levels of circulating and tissue angiotensin II and aldosterone lead to a pro-fibrotic, inflammatory, and hypertrophic environment that causes remodeling and dysfunction in cardiovascular and renal tissues.212
The Role of the RAAS System in the Pathogenesis of COVID-19
Of particular interest is the interaction of SARS-CoV-2 and the RAAS via angiotensin-converting enzyme 2 ACE2, a receptor used by SARS-CoV-2 to gain access to cells.137 The RAAS system includes a carefully balanced and controlled hormone and receptor cascade involving multiple organ systems.137 The system is primarily responsible for blood pressure control, maintaining fluid and electrolyte balance, and maintaining systemic vascular resistance.137,213
The RAAS is influenced by estrogen, cortisol, kallikrein-kinin system, Wnt/β-catenin signaling pathways. It is assumed that the RAAS plays an important role in the pathogenesis of the coronavirus infection COVID-19. A wide range of functional consequences of COVID-19 forced us to pay closer attention to the RAAS.214,215 An imbalance in the work of the RAAS under the influence of COVID-19 can provoke an exacerbation of chronic diseases and a development of complications of the cardiovascular system. It has been shown that people with hypertension and an imbalance in ACE/ACE2 levels are likely to have a poor outcome with COVID-19 infection, even with antihypertensive drug treatment.216
To fully understand the pathophysiology of COVID-19, it is important to understand the expression and function of ACE2.137 It has been shown, COVID-19 has an affinity for the ACE2 cell membrane receptor of the RAAS.2,113,137,217–219 The receptor-binding S-protein of COVID-19 has a very high affinity for the ACE2 receptors, which it uses to penetrate human cells.56 Penetration of COVID-19 into target cells is a highly regulated multistep process, where binding to the ACE2 receptor is only the initial stage. ACE2 receptors are found on the cell membranes of the respiratory and gastrointestinal tracts, urinary, cardiovascular, and central nervous systems that are targets for penetration of COVID-19.2 However, the main and rapidly achievable target of COVID-19 is the alveolar cells of type II in the lungs, the defeat of which leads to pneumonia.2 It is assumed that the greater the number of ACE2 receptors, the more severe the COVID-19 disease progresses. The identification of the membrane receptor ACE2 of the RAAS allows developing further strategies for the treatment of coronavirus infection and potential therapies to prevent virus penetration into target cells.220,221
Now it is generally accepted that the ACE2 receptor protein is found on the endothelium in various human organs, including the skin, lymph nodes, thymus, bone marrow, spleen, liver, and kidneys, and the brain, as well as the mucous membranes of the mouth and nose, nasopharynx, lungs, stomach, small intestine, and colon, while ACE2 mRNA is found in almost every organ.137,217 The most notable finding is the surface expression of the ACE2 protein on alveolar epithelial cells of the lungs and enterocytes of the small intestine.137,217 This is especially important when considering the pathogenesis and clinical presentation of patients infected with COVID-19.137 Once the cell becomes infected with SARS-CoV-2, ACE2 is internalized and ACE2 expression is reduced.137 Therefore, the beneficial degradation of AngII to counter-regulatory Ang (1–7) is reduced, leading to undeniable effects of AngII/AT1,222 a theory supported by the finding of elevated AngII levels in COVID-19 infected patients.137,222,223
Parkinson’s Disease is Accompanied by a Violation of the RAAS System
It is known that the RAAS system may also play a role in Parkinson’s disease.224 Although the exact cause of this progressive neurodegenerative disorder of the basal ganglia remains unclear, it is assumed that inflammation and oxidative stress are key factors in the pathogenesis and progression of the disease.224 Since angiotensin II is a pro-inflammatory compound that can induce the production of reactive oxygen species due to the activation of the NADPH-dependent oxidase complex, this peptide can contribute to the death of dopaminergic cells.224 There are three different strategies for intervening in the pathogenesis or progression of Parkinson’s disease.224 These include inhibition of the angiotensin-converting enzyme ACE, blockade of the angiotensin II type 1 receptor AT1, and stimulation of type 2 angiotensin II receptor AT2.224 Since angiotensin II is a pro-inflammatory compound225 that activates the NADPH-dependent oxidase complex, the main source of superoxide,226,227 it is possible to regulate the progression of Parkinson’s disease by manipulating the RAAS system.224
Allen et al (1992) were the first to suggest a potential link between brain RAAS and Parkinson’s disease.228,229 These researchers measured a decrease in angiotensin receptor binding in the substantia nigra and striatum in the postmortem brains of patients with Parkinson’s disease.229 Several studies confirm the important role of ACE in Parkinson’s disease.229 ACE is present in the striped pathway and basal ganglia structures.229–231 Patients with Parkinson’s disease treated with perindopril, an ACE inhibitor, showed improved motor responses to the dopaminergic precursor 3,4-dihydroxy-1-phenylalanine.229,232 ACE has been shown to metabolize bradykinin and thus modulate inflammation, a factor contributing to the development of Parkinson’s disease.229,233 Activation of the AT1 receptor subtype by AngII promotes the development of nicotinamide adenine dinucleotide phosphate NADPH dependent oxidases, a significant source of reactive oxygen species.229,234,235 Treatment with ACE inhibitors has been shown to protect against loss of dopaminergic neurons in animal models of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-hydroxydopamine (6-OHDA).229,236–238 The likely mechanism underlying this ACE inhibitor-induced protection is a decrease in the synthesis of AngII acting on the AT1 receptor subtype.224,229 It is known that the binding of AngII in the AT1 subtype activates the NADPH oxidase complex, thereby providing the main source of reactive oxygen species.229,239–241 In addition, activation of the AT1 receptor leads to stimulation of the NF-kB signaling pathway, facilitating the synthesis of chemokines, cytokines, and adhesion molecules, which are important for the migration of inflammatory cells in the area of tissue damage.229,242
The structures of the basal ganglia have a local RAAS, which indicates increased activity during dopaminergic degeneration.229,243–245 Villar-Cheda et al,229,246 reported that a decrease in dopaminergic activity caused by reserpine led to a significant increase in the expression of AT1 and AT2 receptors.229 A similar pattern was observed in 6-OH-dopaminergic dopaminergic denervation, in which a decrease in receptor expression was observed upon treatment with the dopamine precursor 1-DOPA.229 These results indicate a direct interaction between the RAAS and the dopaminergic system in the structures of the basal ganglia.229
Common Elements of the RAAS System in COVID-19 and Parkinson’s Disease
The RAAS plays a key role in inflammatory processes that affect the microbiome, COVID-19 infection, and the development of Parkinson’s disease. The universal role of the RAAS system in the formation of inflammatory processes leading to many chronic diseases accompanied by inflammatory processes requires further study and more detailed analysis in the post-COVID-19 time. Persistent dysbiosis after COVID-19 can be a factor in multisystem inflammatory syndrome with unpredictable consequences in recovered patients.
SARS-CoV-2 uses ACE2 as a receptor to infect human respiratory epithelial cells.247,248 The envelope of the SARS-CoV-2 virus contains the Spike protein with a receptor-binding region that directly binds to the extracellular domain of ACE2.248,249 The ACE2 enzyme and its ACE2 receptor are biotargets in the intestinal infection with SARS-CoV-2 and the development of Parkinson’s disease. A recent study has demonstrated that the affinity of the S-protein SARS-CoV-2 for human ACE2 is even higher than that of SARS-CoV.112,248 Since SARS-CoV-2 must bind to the ACE2 receptor before entering host cells, the distribution and expression of ACE2 may be critical for the target organ of SARS-CoV-2 infection.247,248
Intestinal ACE2 is involved in the transport of amino acids, regulating the composition and function of the microbiome. It has been shown that widespread expression of ACE2 in human tissue may be associated with organ dysfunction, such as lung, kidney, and stomach, in patients with COVID-19.248 However, in Parkinson’s disease, the pathogenesis of which is closely associated with old age, ACE2 plays a neurotrophic and protective role, activating the ACE2/Ang-(1-7)/Mas axis, thereby suppressing cognitive impairment. It has been shown that older people are more susceptible to COVID-19 and that older patients with COVID-19 have faster Parkinson’s disease progression and higher mortality. Therefore, during the COVID-19 pandemic, it is extremely important to understand the role of ACE2 in Parkinson’s disease.248
Several studies suggest that the binding of SARS-CoV-2 to the ACE2 receptor results in ACE2 depletion. This inhibits the ACE2/Ang-(1-7)/Mas receptor pathway and disrupts the balance of the RAAS system. The result is an exacerbation of severe acute pneumonia and cardiovascular complications such as myocardial injury, myocarditis, acute myocardial infarction, heart failure, and arrhythmia in patients with COVID-19.248,250,251 ACE2 plays a critical role in the evolution of COVID-19. This is not only a receptor, but it is also involved in post-infectious regulation, including the immune response, cytokine secretion, and viral genome replication.248,252 Consequently, target organs prone to developing complications from COVID-19 have some consistency in the distribution and expression levels of the ACE2 receptor248 It is well known that ACE2 plays a key role in SARS-CoV-2 infection.248,253
Typical features of Parkinson’s disease are the ubiquitous presence of alpha-synuclein-positive Lewy bodies, loss of dopamine neurons, and dystrophic Lewy neurites.248,254,255 RAAS also plays an irreplaceable role in brain function, and studies have shown that renin, ACE, Ang II, and Ang (1–7) are found in the central nervous system. They are involved in the regulation of blood pressure, water and food intake, maintenance of the blood-brain barrier, and even in movement, learning, memory, and emotional control.248,256 Genetics, epidemiology, and clinical data indicate that overactivation of the RAAS system is one of the main elements of the pathogenesis of neurodegenerative diseases.248,256 Moreover, ACE2, including the ACE2/Ang-(1-7)/Mas axis, plays a regulatory role in neurodegenerative diseases.248,257 Dysregulation of RAAS is associated with the pathogenesis of Parkinson’s disease, and drugs targeting RAAS can improve Parkinson’s disease.248,258–260 The level of ACE was reduced in the cerebrospinal fluid of patients with Parkinson’s disease and negatively correlated with the level of amyloid Aβ.248,261,262 Moreover, electron microscopy has shown that ACE can delay fiber formation in a dose-dependent manner and reduce susceptibility to Parkinson’s disease.248,263
ACE and ACE2 have also been found in the cerebrospinal fluid of patients with Parkinson’s disease and multiple sclerosis.264 Zubenko et al found a decrease in the level of ACE in the cerebrospinal fluid in patients with Parkinson’s disease,248,265 and Kawajiri et al revealed a decrease in ACE and ACE2 levels in the cerebrospinal fluid of patients with multiple sclerosis.248,266–268 However, there are few studies on the role of ACE2 in the pathological processes of Parkinson’s disease and multiple sclerosis, and it is necessary to study the specific mechanism.248,269
Lactobacillus-Based Probiotics for the Prevention of Inflammatory Processes
Classification of Lactobacillus
Lactobacillus is a genus of Gram-positive, aerotolerant anaerobes or microaerophilic, rod-shaped, non-spore-forming bacteria of the family Lactobacillaceae, order Lactobacillales, class Bacilli, division Firmicutes. Lactobacillus can grow at temperatures ranging from 2° to 53°C and at pH levels ranging from 3 to 8. The optimum temperature for lactobacteria is 30–40°C, and pH 5.5–6.2. Lactobacillus is capable of breaking down carbohydrates, nitrates do not reduce, casein does not break down, gelatin does not liquefy, does not form indole and hydrogen sulfide.32
The Lactobacillaceae family is one of the most numerous in the bacterial world.270 The Lactobacillaceae family has high phenotypic, ecological, and phylogenetic diversity.271 Lactobacillus is divided into 26 phylogenetic groups and comprised over 260 phylogenetically, ecologically, and metabolically diverse species, the most famous of which are L. casei (L. casei, L. rhamnosus species), L. reuteri (L. reuteri, L. fermentum species), L. plantarum, L. acidophilus (L. acidophilus, L. helveticus), L. delbrueckii, L. brevis, L. salivarius.272,273
Sources for Obtaining Lactobacteria
Lactobacteria are found on plants, in various cavities of animals and humans, in dairy and fermented foods, and organic wastes.274 According to their lifestyle, lactobacteria are subdivided into free-living including ecological and plant isolates, host-adapted including invertebrate or vertebrate hosts, and nomadic species.275 Most of the species of lactobacteria found in the human intestine do not form stable populations and are classified as allochthonous, which are ingested with food. The species L. plantarum, L. casei, L. paracasei, and L. rhamnosus are not autochthonous in the classical sense but have an adaptation to the environment of the gastrointestinal tract and oral cavity that allows them to persist there for a long time.276
The most common isolates from the gastric mucosa are Limosilactobacillus antri, Limosilactobacillus gastricus, Lactobacillus kalixensis, L. reuteri, and Lactobacillus ultunensis. The species L. crispatus, L. gasseri, Lactobacillus jensenii, Limosilactobacillus vaginalis, and Lactobacillus iners are frequently found in the vagina.32,277 A comprehensive study using whole-genome sequencing identified 86 Lactobacillus species from 52 non-human feces; 43 of these species were permanent residents in the intestines.32,276
Lactobacteria have always been used by humans for food fermentation, are considered safe for humans, and have been classified as generally regarded as safe GRAS, which can be used as probiotics to maintain health. By releasing biologically active substances, lactobacteria can prevent pathological conditions such as decreased levels of neurotransmitters, chronic inflammation, oxidative stress, apoptosis, neurodegenerative diseases, ulcerative colitis, inflamed bowel syndrome, and allergies.278–282
Anti-Inflammatory and Antioxidant Potential of Lactobacteria
The ability of certain strains of probiotic bacteria to reduce oxidative stress is widely known and proven in vitro and in vivo. The antioxidant effect of probiotics is due to the production of antioxidant proteins and peptides (superoxide dismutase, thioredoxin, glutathione; proteins that chelate ions Fe2+ and Cu2+; vitamins (B1, B12, and others; short-chain fatty acids; refolding stress-damaged proteins; modulation of the species composition of the intestinal microbiome.32,283 Examples of the antioxidant and anti-inflammatory activity of lactobacteria (Table 1).
Table 1.
Examples of the Antioxidant and Anti-Inflammatory Activity of Lactobacteria
| Lactobacillus Species and Strains | Experimental Model | Benefits | Reference |
|---|---|---|---|
| L. plantarum | Female BALB/c mice. | Downregulation of pro-inflammatory cytokines and an increase in anti-inflammatory cytokines in human. | [277,284] |
| L. rhamnosus CNCM I-3690 | HT-29 cells and dendritic cells. | Clear anti-inflammatory profile and increase of viability and average of worm lifespan. | [277,285] |
| L. reuteri BM36301 | C57BL/6 mice. | Anti-inflammatory role and maintaining healthy conditions of mice as they aged. | [32,277,286] |
| 81 lactobacteria strains of 6 different species | E. coli MG1655 strains carrying plasmids encoding luminescent biosensors pSoxS-luxand pKatG-lux. | 51 strains demonstrated antioxidant activity. | [32,59] |
| L. acidophilus ATCC 43121, L. acidophilus ATCC 4356, L. acidophilus 606, L. brevis ATCC 8287, L. casei YIT 9029, L. casei ATCC 393, L. rhamnosus GG | The human cancer cells. | Heat-killed cell of L. acidophilus 606 and L. casei ATCC 393 exhibited the most profound inhibitory activity in the all of tested cell lines; the soluble polysaccharides components of L. acidophilus 606 evidenced the effective anticancer activity. | [32,287] |
| L. brevis MG000874 | Albino mice exposed toD-galactose-induced oxidative stress. | The treated animals displayed improvement in superoxide dismutase, catalase, and glutathione S-transferase in all tissues, as well as glutathione in the liver and serum. | [32,288] |
| L. fermentum U-21 | C57/BL6 mice, C. elegans exposed to paraquat-induced oxidative stress. | The lifespan of the C. elegans was extended by 25%. L. fermentum U-21 ensured normal coordination of movements and the safety of dopaminergic neurons in the brain. | [32,289] |
| Lactobacillus brevis 47 f | The Balb/c mice with 5-fluorouracil induced mucositis and oxidative stress. | Protects the murine intestine from enteropathy induced by 5-fluorouracil and prevent of lipid peroxidation in the gut tissues and in the blood plasma. | [290] |
| L. plantarum A7 (KC 355240, LA7) | 24 type2 diabetic kidney disease patients. | Oxidized glutathione concentration was significantly reduced; the levels of glutathione, glutathione peroxidase, and glutathione reductase were significantly increased; no significant reduction in the 8-iso-prostaglandin F2α, malon dialdehyde. | [32,291] |
| L. acidophilus, L. casei | Diabetic hemodialysis patients, 28 cases and 27 placebos. | Patients who received probiotic supplements showed significantly decreased plasma glucose, serum insulin, assessment-estimated insulin resistance and beta-cell function and HbA1c, insulin sensitivity, serum C-reactive protein, plasma malon dialdehyde, and total iron-binding capacity. Patients showed an increase in plasma total antioxidant capacity. | [32,292] |
Due to the synthesis of biologically active compounds with antioxidant properties, lactobacteria can actively influence the general condition and antioxidant status of the organism.59,279,293–295 Some metabolites and components of lactobacteria, like exopolysaccharides and polyphenols, are signaling molecules that affect biochemical processes in the body,296–299 reduce the symptoms of dysbiosis and dysfunction of the gastrointestinal tract,300 protect the complex network of neuroglia in the nervous system301–304 and have a positive effect on the axes of microbiome-gut-brain and microbiome-gut-lung.305,306
The antioxidant properties of lactobacteria are widely used in the manufacture of functional foods, dietary supplements, and medicines. The study of probiotic bacteria that can prevent the development of oxidative stress and its consequences is an extremely urgent task at present and is of particular importance in the context of the COVID-19 pandemic307–311 (Figure 2).
Figure 2.
Lactobacteria from the different microbiome sources and the possibilities of their use as probiotics or pharmabiotics,and postbiotics in the prevention and therapy the human diseases.
During infection or oxidative stress in human tissues, inflammatory and autoimmune processes are activated with the participation of nuclear factor kappa B (NF-kB). The body’s immune response to microbial products during infection with the participation of Toll-like receptor (TLR) leads to increased secretion of lipopolysaccharides, interleukin 1, and tumor necrosis factor-alpha (TNFα), which stimulate the body’s antioxidant system by increasing the activity of the enzyme manganese superoxide dismutase (MnSOD),310,312,313 while chronically increased secretion of these compounds is triggered autoimmune and neurodegenerative processes in the body.314 It has been shown that lactobacteria in the gut microbiome can reduce inflammatory and autoimmune processes by activating the proliferation of regulatory T cells, decreasing the TLR activity and levels of TNFα and proinflammatory cytokines.315–318
Inflammation and oxidative stress in the intestine disrupt the function of the intestinal barrier, as a result of which the number of toxins and metabolites entering the bloodstream, causing inflammation and oxidative stress in organs and tissues, increases.319,320 Lactobacteria of the gut microbiome can prevent inflammation and oxidative stress in organs and tissues by reducing oxidative stress caused by hydrogen peroxide molecules, hydroxyl, and superoxide radicals in the intestine through the production of substances with antioxidant activity, such as thioredoxin reductase, superoxide dismutase, catalase, glutathione reductase, glutathione peroxidase, glutathione S-transferase, thiols (cysteine and glutathione), exopolysaccharides.59,278,279,321–325 Lactobacteria’s ability to synthesize antioxidant enzymes, thiols, and exopolysaccharides varies by species and strain and determines their increased antioxidant potential.326–332
The knowledge accumulated in recent years about the antioxidant activity of lactobacteria makes it possible to formulate requirements for an ideal strain that reduces oxidative stress in the body. An ideal probiotic drug capable of reducing cytokine storms and oxidative stress in viral infections and COVID-19 should have the following characteristics.283,333–335 The probiotic strain should have direct antioxidant activity and have a complex effect on the innate immune and antioxidant systems of the human body. The probiotic strain must be a natural and complementary component of the human intestinal microbiome. The probiotic strain must have the ability to mildly mobilize the antioxidant potential of the target cells of the human body.334 The probiotic strain must be able to regulate the concentration of reactive oxygen species in the targeted organs of the body, for example, in the lungs. The probiotic strain must be capable of detoxifying the lipids, proteins, and other components damaged by reactive oxygen species in human cells.335 The probiotic strain should help restore the intestinal and blood-brain barriers that prevent the penetration of toxicants into the bloodstream and the brain. The probiotic strain should contribute to the restoration of the gut microbiome, which is an important organ that determines the immunomodulatory and antioxidant potential of a human.59,289,333–335 These properties are possessed by the probiotic strain of Lactobacillus fermentum U-21, which is capable of synthesizing a complex of antioxidants with proven high antioxidant activity against superoxide anion. Biologically active compounds obtained from the biomass and culture medium of the L. fermentum U-21 strain also have antioxidant activity against superoxide anion and can be used in pharmacology and medicine in the treatment of neurodegenerative diseases induced by oxidative stress, as well as in cosmetology with an increase in antioxidant status of skin.59,289,290,333–335
Microbiome Disruption in Parkinson’s Disease and COVID-19
Altered Microbiome Composition in Parkinson’s Disease
Metagenomic studies show changes in the species composition of the gut microbiome of people with various diseases compared with healthy people.336–341 Changes in the structure of the gut microbiome can be observed in humans with diseases such as obesity and metabolic dysfunction,342 allergies, and autoimmune disorders,343–345 intestinal inflammation, irritable bowel syndrome, allergic gastroenteritis and necrotizing enterocolitis,346 type 1 and 2 diabetes,347 atherosclerosis,348 asthma349 neurodegenerative diseases including Parkinson’s disease.350 Experimental and clinical studies have revealed changes in the structure of the human gut microbiome as a result of degenerative diseases,281 depression,351,352 and autism,41,353 which are accompanied by inflammatory processes.354–356
Currently, several studies are aimed at identifying differences in the microbiome of patients with Parkinson’s disease compared to healthy control people.350 While most of them used fecal samples as a proxy for microbiome composition in the distal colon, some examined mucosal opportunistic pathogens or even saliva and nasal mucosa samples.350,357–359 The species of the Prevotellaceae family from the Bacteroidetes phylum have been implicated in the pathogenesis of inflammatory bowel disease193 and have dramatically decreased in the stool of patients with Parkinson’s disease190,193,337,338,360–364 and in the sigmoid mucosa.350,357,365 It has been reported lower Bifidobacteriaceae levels in patients with Parkinson’s disease.338,350 It was found a lower content of Bifidobacteriaceae both in the mucous membrane and in the feces350,357,366 and the saliva350,367 of patients with Parkinson’s disease compared to controls, while others described the opposite.350,362,368,369 In a mouse model of Rotenone-induced Parkinson’s disease, the number of Bifidobacteriaceae in the cecal and mucous membrane was reduced compared to control mice.350,370 It was found an increased content of Lactobacillaceae,350,362–364,366,367,369,371 and Erysipelotrichaceae,350,359,364,372 in the saliva and the stool of patients with Parkinson’s disease compared to the control group whereas some other studies found decreased content of Lactobacillaceae338,350,372 and Erysipelotrichaceae338,350,357,369 in the stool of patients with Parkinson’s disease compared with healthy controls. The genera Roseburia,338,357,366 Blautia338,357,366,369 and Faecalibacterium190,357,369 and Dorea337,338,357 were less abundant in the stool of patients with Parkinson’s disease.350 Genus Akkermansia was common in the stool of patients with Parkinson’s disease.190,338,350,357,359,364,366,373 Akkermansia muciniphila can induce different T cell responses depending on other species present in a given microbiome.350,374 Also, it has been reported the increasing the viruses in the stool. Stool from patients with Parkinson’s disease.338,350
Altered Microbiome Composition in COVID-19
It has been published that COVID-19 patients had an altered gut microbiome compared to the control group.375–379 Using a shotgun for metagenomic sequencing of fecal samples it is described dysbiosis in the bacterial microbiome and mycobiome in patients with COVID-19 compared with healthy control patients.375,376,379 Notably, COVID-19 patients generally had an increased number of opportunistic pathogens, a portion of the commensal microbiome that can become pathogenic in the event of a host disorder such as dysbiosis or a compromised immune system.379,380 Opportunistic pathogens included Clostridium hathewayi, Actinomyces viscosus, and Bacteroides nordii at the time of hospitalization with SARS-CoV-2.379 It is shown that the number of specific opportunistic pathogens, Collinsella aerofaciens and Morganella morganii spp. was increased in fecal samples with a high level of active viral transcription and replication of SARS-CoV-2 compared to fecal samples from healthy patients.376,379 Conversely, fecal samples with low or no SARS-CoV-2 infectivity had elevated levels of bacteria belonging to Parabacteroides, Bacteroides, and Lachnospiraceae that produce short-chain fatty acids, especially butyric acid.379 Short-chain fatty acids are known to play an important role in enhancing host immunity. Thus, these data suggest that opportunistic pathogens pose a threat to both a decrease in host immunity and opportunistic infections in proportion to the burden of SARS-CoV-2.379 In another cohort, reduced bacterial diversity was described in fecal samples from patients with COVID-19 compared to healthy controls by analyzing the V3-V4 region of the 16S rRNA gene.377,379 The study also found an increase in opportunistic pathogens such as Streptococcus, Rothia, Veillonella, and Actinomyces among COVID-19 patients.379
One gut microbiome study based on 16S rRNA in COVID-19 patients showed that alpha diversity in these patients was lower than in healthy controls and the abundance of four genera: Streptococcus, Clostridium, Lactobacillus, and Bifidobacterium, tended to increase, but five other genera, Bacteroides, Roseburia, Faecalibacterium, Coprococcus, and Parabacteroides, showed lower numbers in COVID-19 patients than in controls.378,379 Dysbiosis with decreased levels of Lactobacillus and Bifidobacterium has been observed in some COVID-19 patients.378,379,381 It was shown that the composition of the fecal mycobiome in 30 hospitalized patients with COVID-19 was heterogeneous; however, some had been enriched with the fungal pathogens Candida and Aspergillus spp. compared to control.375,379
It has shown the potential importance of Firmicutes species in the severity of SARS-CoV-2 infection. It is assessed the association between the fecal microbiome and the severity of COVID-19 in seven patients.379,382 A total of 23 bacterial taxa were significantly associated with the severity of COVID-19, and the majority (15 of 23) was of the type Firmicutes. Of these, eight classes (Coprobacillus, Clostridium ramosum, and C. hathewayi) were positively correlated with the severity of the disease, and seven were negatively correlated.375,379,383
The feces of COVID-19 patients were enriched with opportunistic pathogens known to cause bacteremia, including Clostridium hathewayi, Actinomyces viscosus, and Bacteroides nordii375,376 due to the disturbed microbial ecology of the intestine and resistance to colonization.376,384,385 It showed a similar pattern of gut microbiome dysbiosis in patients with COVID-19.376,386 The number of butyrate-producing bacteria such as Faecalibacterium prausnitzii, Clostridium butyricum, Clostridium leptum, and Eubacterium rectale was significantly reduced in patients with COVID-19 compared with the control group.376,386 When patients with COVID-19 were compared to the control group, the number of common opportunistic microorganisms Enterobacteriaceae and Enterococcus was significantly higher.376,386 At the birth level, the genera of Streptococcus, Rothia, Veillonella, and Actinomyces (all opportunistic microorganisms) were enriched with feces from patients with COVID-19, while the deliveries of Romboutsia, Faecalibacterium, and Fusicatenibacter were enriched with feces from healthy people.376,377 The content of opportunistic bacteria Coprobacillus, Clostridium ramosum, and Clostridium hathewayi in the feces of patients during hospitalization was associated with COVID-19 disease, while the anti-inflammatory bacterium Faecalibacterium prausnitzii showed a negative correlation,375 which suggests a baseline calibration of the intestinal microbe-host immunity, thereby influencing the disease response to SARS-CoV-2 infection.376 Evidence is accumulating that a significant number of COVID-19 patients experienced systemic and organ-specific disease during follow-up after resolution of the disease, including fatigue, muscle weakness, sleep problems, anxiety, depression, diarrhea, and poor glycemic control.132,376,387–389 The long-term dysbiosis of the gut microbiome is also consistently observed in patients after COVID-19,375,390–392 which means that the gut microbiome is closely related to the health of the host.376
The Role of Microbiome in the Prevention and Treatment of COVID-19 and Parkinson’s Disease
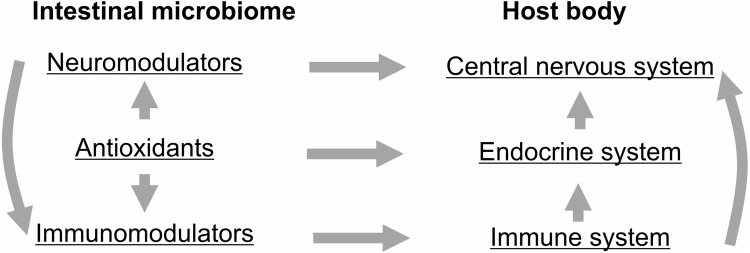
The microbiome and its specific components, including lactobacteria, determine the formation and maintenance of innate and acquired immunity, as well as antioxidant potential. Violation of the composition (signature) of the microbiome - dysbiosis, leads to increased sensitivity to infectious and neurological diseases. The immune status of different groups of the population has different indicators: first of all, patients with type II diabetes, autoimmune diseases, AIDS.32 Stressful conditions (social, physical, chemical, nutritional changes) always lead to dysbiosis of the microbiome and a decrease in immune homeostasis. Analysis of the state of the microbiome is an important biomarker of the state of the immune and nervous system and susceptibility to neurological and infectious diseases as COVID-19.32,393,394 The intestinal microbiome influences the host organism due to its ability to synthesize various biologically active compounds. The whole system functions as a single network. A breakdown in one link leads to the failure of the entire system32,41,319,393,395 (Figure 3).
Figure 3.
The intestinal microbiome influences the host organism due to its ability to synthesize various biologically active compounds.
The gut microbiome is an organ that integrates the interaction of all body systems and protects against stress factors and infections, including virus infections like COVID-19. It has been shown the coronavirus infection COVID-19 leads to disruption of the gut microbiome and development of dysbiosis.396 Further, dysbiosis leads to inflammation and oxidative stress, which increases the rate and risk of developing chronic diseases.
The symbiotic gut microbiome is important for human health, physiology, and metabolism.343,397,398 The biodiversity of the microbiome of a healthy adult is counted in hundreds of different types of microorganisms.399,400 More than 600 different species of bacteria related to each other by symbiotic and antagonistic interactions can be found in the gut microbiome of an adult human.324,401 The composition of the gut microbiome differs between individuals, although Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia are usually predominant in the human gut.402 The composition of the human gut microbiome can be influenced by factors such as food quality, smoking, age, body weight, health status, antibiotics, and habitat.403,404 The gut microbiome synthesizes many biologically active substances, such as amino acids, vitamins, neurotransmitters, hormones, and hormone-like substances that can affect the human body, penetrating the bloodstream through the intestinal barrier.400,405–410 The gut microbiome functions as a “virtual endocrine organ” that regulates metabolic signaling pathways of the human body.398,410–412
Some probiotics and postbiotics, as a result of modulation and restoration of the gut microbiome, reduce dysbiosis, systemic inflammation, and oxidative stress in humans.413,414 There is evidence that probiotics and postbiotics have potential in the prevention and treatment of Parkinson’s disease, as they support the composition of the gut microbiome, reduce oxidative stress and inflammation, and produce essential biologically active metabolites.47,49 As a result of reducing dysbiosis with the use of probiotics, the protective function of the intestine is restored, the systemic inflammatory process is decreased, and the concentrations of uremic toxin, p-cresol, urea, and phosphates in the blood are decreased,415–417 the lipid profile in the liver is normalized,111,418 the oxidative stress and inflammation in muscles are reduced by increasing the activity of antioxidant enzymes,419 the learning and memory are increased as a result of eliminating oxidative stress and neurodegenerative processes in the hippocampus,111,420–422 the oxidative stress in the heart and blood vessels is reduced by a decrease in the concentrations of NADPH oxidase 2 (Nox2) and T helper 17 cells (Th17), the polarization of regulatory T cells is increased,423 the basal glycemia and insulin resistance are decreased.424
Viruses that can infect both intestinal epithelial cells and symbiotic microorganisms have a significant effect on the composition of the intestinal microbiome.376,425,426 The gut microbiome is the primary antiviral barrier, which, using the CRISPR-Cas system, provides effective protection of symbiotic bacteria from various DNA and RNA viruses, phages, as well as COVID-19.425,427,428 One of the mechanisms of antiviral defense of the gut microbiome is a complex of exosomes of bacterial and human origin. Exosomes can capture viral particles, including COVID-19, and carry them into bacterial cells, where they are destroyed.429–432 Another mechanism of antiviral defense of the intestinal microbiome is the production of peptides and biologically active metabolites that can prevent viruses from entering cells. Microorganisms of the gut microbiome can synthesize peptides that are able to competitively bind to ACE2 cell receptors, block them from binding to COVID-19 and protect the intestinal epithelium from coronavirus infection and subsequent inflammatory processes.433 It has been shown that bifidobacteria, in particular Bifidobacterium longum GT-15, can reduce intestinal inflammatory processes by producing the type-III fibronectin domain-containing protein (FN3) that binds to a pro-inflammatory agent such as tumor necrosis factor α TNFα.434,435 The gut microbiome of people resistant to coronavirus infection is a promising source of Lactobacillus strains capable of producing peptides and other active metabolites that prevent the penetration of COVID-19 into human cells and decrease the severity and pathogenesis of this new coronavirus.
Perspectives
It is necessary to develop additional antioxidant and immunomodulatory pharmabiotics, postbiotics, and next-generation probiotic drugs for the prevention and treatment of COVID-19 infection. To create new generation pharmabiotics, and postbiotics, it is necessary to study the intestinal microbiome of stress-resistant people as a resource of biologically active compounds and strains of lactobacteria that ensure human resistance to coronavirus infection. After coronavirus infection and before vaccination, it is necessary to diagnose the state of the intestinal microbiome and normalize the disturbed state. To increase the effectiveness of protein vaccines against coronavirus infection, it is necessary to develop adjuvants based on lactobacteria and bifidobacteria capable of enhancing the cellular immune response.436
Recently, much attention has been paid to the search for new promising sources of lactobacteria with antioxidant properties from the gut microbiome of animals. Thus, the honey bee Apis mellifera, closely associated with human life, can be a promising source of beneficial strains of probiotic lactobacteria. Such prospects are related to the fact that bees can survive the winter without flying and empty their guts for 6 months due to their unique adaptation.437 The adaptation of bees to a long winter is provided by the combined antioxidant potential of the organism and the gut microbiome of bees, which slows down and prevents excessive oxidation of the substrate and protects the organism from oxidative stress.437
Lactobacteria species Lactobacillus Firm-4 and Firm-5, L. helveticus, L. kunkeei, L. helsingborgensis, L. kimbladii, L. mellis, L. mellifer, L. melliventris, L. apis, L. kullaberme, L. johnsonii, L. micheneri, L. timberlakei, L. quenuiae, and L. plantarum are represented in the gut of honey bees in the greatest diversity and are characterized by an enhanced antioxidant potential.158,438–440 The antioxidant potential of lactobacteria may be associated with the expression of antioxidant enzyme genes such as catalase, thioredoxin reductase, catalase, glutathione reductase superoxide dismutase, glutathione S-transferase, as well as other products with antioxidant properties, such as lipoteichoic acid.158,441
In the gut of wintering honey bees, the number of lactobacteria species with enhanced antioxidant potential, such as L. mellifer, L. apis, and L. melliventris, is increased.439,442 Ochratoxin A causes histopathological changes and liver and kidney dystrophy in rats. It was shown that rats fed with L. kunkeei probiotic bacteria from the honey bee gut microbiome have an increased protective potential against oxidative stress caused by ochratoxin A.436 Polystyrene microplastic exposure led to significant decreases in the α-diversity of the honey bee gut microbiome, accompanied by changes to the core microbial population structure and oxidative stress. Lactobacillus spp. play a protective role against polystyrene microplastics exposure by stimulating the expression of antioxidative CAT, detoxification CYPQ1 and GSTS3, and immune system-related Domeless, Hopscotch, and Symplekin genes in the midgut.443 In the gut of honey bees, Lactobacillus species of L. plantarum (strains H28, H24, KX519413, KX519414, LP8, LP25, LP86, LP95, LP100) and L. kunkeei were characterized by an increased antioxidant potential that protects against different pesticides.436,440,444–446 All of these strains of lactobacteria from the gut of honey bees with an increased antioxidant potential can be selected as perspective strains for creating pharmabiotics.445
There are new prospects for the use of components of lactobacteria, rather than their use in living form cultures. Postbiotics are now defined as metabolites and cellular components that provide health benefits.32,447,448 Postbiotics are a promising area of research for future pharmaceuticals and functional foods with antioxidant properties for the treatment of depressive disorders.32,393 The potential of postbiotics can be used by packaging them into nanostructures, allowing them to be delivered to organs affected by inflammation.32,449 The use of extracellular vesicles of gram-positive probiotic bacteria, which can freely enter the bloodstream, as well as tissues and organs of the human body, is another interesting area of research.32,450,451 As a modern method, metagenomics is widely used not only to study differences in the composition of the microbiome in disease states in comparison with healthy people, but also to study the functional genes of the intestinal microbiome.32 For this reason, it is desirable to use metagenomic analysis of sequenced whole genome bacterial DNA to study the antioxidant potential of the intestinal microbiome. This approach can yield significant results in the search for target genes that are included in the catalog of reference genes of the search tool.32
Conclusion
Inflammatory processes of various organs and systems accompany many chronic and infectious human diseases. As a rule, they are accompanied by a violation of the composition and functions of the human intestinal microbiome, called dysbiosis of the intestinal tract. At the same time, inflammation and dysbiosis, having common indicators and mechanisms, are characterized by biomarkers characteristic of certain pathologies. The search for biomarkers of pathologies at the level of the microbiome and the human body is a step towards the practical diagnosis of the disease and the development of drugs. In the case of COVID-19 and Parkinson’s disease, the RAAS system is the common system involved in the pathogenesis of diseases properties.32 Angiotensin-converting enzyme 2 ACE2 and its receptor are overlapping bio targets in SARS-CoV-2 and Parkinson’s disease. The RAAS system was discovered and described over 100 years ago. It was demonstrated that the RAAS is involved in the formation of oxidative stress and inflammatory processes in viral and neurodegenerative diseases, and the intestinal microbiome performs a protective function of the body. The participation of the RAAS system in the inflammatory processes of all human organs and systems, caused by Parkinson’s disease and COVID-19, has been shown. The involvement of the RAAS in complications after COVID-19 requires further research and detailed analysis. The apparent link between gut microbiome dysbiosis and COVID-19 and Parkinson’s disease requires the targeted creation of pharmabiotics and postbiotics to correct the microbiome. Using the antioxidant potential of lactobacteria is undoubtedly the right approach in the prevention of inflammatory diseases. Lactobacteria are promising candidates for anti-inflammatory and antioxidant drugs.32,452,453 The term pharmabiotics, which was first published about 15 years ago, better describes probiotic-based medicines.454, 455, 456, 457 The development of drugs aimed at eliminating the inflammation phenotype of the intestinal microbiome will be greatly facilitated if new methodological and conceptual approaches to the search for unique strains of probiotic bacteria are implemented; they include a comparative analysis of the genomes of lactobacteria, as well as metagenomes properties.32 Omics technologies have been used to study the intestinal microbiome of healthy people and patients with chronic inflammatory diseases. Characterization of the gut microbiome in health and disease is likely to become possible when the biomarkers of a dysfunctional microbiome are better understood. Efforts are being made to identify the genes responsible for the gut microbiome’s neuromodulatory and immunomodulatory properties.32 The development of projects for the study of unique strains of lactobacteria from new promising sources using omics technologies is considered a necessary task of modern health care properties.32
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chaudhry ZL, Klenja D, Janjua N, Cami-Kobeci G, Ahmed BY. COVID-19 and Parkinson’s disease: shared inflammatory pathways under oxidative stress. Brain Sciences. 2020;10(11):807. doi: 10.3390/brainsci10110807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2(7):247–257. doi: 10.1002/emmm.201000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirano T, Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52(5):731–733. doi: 10.1016/j.immuni.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nile SH, Nile A, Qiu J, Li L, Jia X, Kai G. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niizuma K, Endo H, Chan PH. Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J Neurochem. 2009;109(Suppl 1):133–138. doi: 10.1111/j.1471-4159.2009.05897.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernansanz-Agustin P, Izquierdo-Alvarez A, Sanchez-Gomez FJ, et al. Acute hypoxia produces a superoxide burst in cells. Free Radic. Biol. Med. 2014;71:146–156. doi: 10.1016/j.freeradbiomed.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 10.Gorlach A, Dimova EY, Petry A, et al. Reactive oxygen species, nutrition, hypoxia and diseases: problems solved? Redox Biol. 2015;6:372–385. doi: 10.1016/j.redox.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholls DG. Mitochondrial calcium function and dysfunction in the central nervous system. Biochimica et Biophysica Acta. 2009;1787(11):1416–1424. doi: 10.1016/j.bbabio.2009.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niizuma K, Yoshioka H, Chen H, et al. Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochimica et Biophysica Acta. 2010;1802(1):92–99. doi: 10.1016/j.bbadis.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gazewood JD, Richards DR, Clebak K. Parkinson disease: an update. Am. Fam. Physician. 2013;87(4):267–273. [PubMed] [Google Scholar]
- 14.Deumens R, Blokland A, Prickaerts J. Modeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 2002;175(2):303–317. doi: 10.1006/exnr.2002.7891 [DOI] [PubMed] [Google Scholar]
- 15.Prieto-Lloret J, Donnelly DF, Rico AJ, Moratalla R, Gonzalez C, Rigual RJ. Hypoxia transduction by carotid body chemoreceptors in mice lacking dopamine D(2) receptors. J Appl Physiol (1985). 2007;103(4):1269–1275. doi: 10.1152/japplphysiol.00391.2007 [DOI] [PubMed] [Google Scholar]
- 16.Andrzejewski K, Jampolska M, Zaremba M, Joniec-Maciejak I, Boguszewski PM, Kaczynska K. Respiratory pattern and phrenic and hypoglossal nerve activity during normoxia and hypoxia in 6-OHDA-induced bilateral model of Parkinson’s disease. J Physiol Sci. 2020;70(1):16. doi: 10.1186/s12576-020-00743-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glinka YY, Youdim MBH. Inhibition of mitochondrial complexes I and IV by 6-hydroxydopamine. Eur J Pharmacol. 1995;292(3):329–332. doi: 10.1016/0926-6917(95)90040-3 [DOI] [PubMed] [Google Scholar]
- 18.Blum D, Torch S, Lambeng N, et al. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Progress in Neurobiology. 2001;65(2):135–172. doi: 10.1016/S0301-0082(01)00003-X [DOI] [PubMed] [Google Scholar]
- 19.Tirmenstein MA, Hu CX, Scicchitano MS, et al. Effects of 6-hydroxydopamine on mitochondrial function and glutathione status in SH-SY5Y human neuroblastoma cells. Toxicology in Vitro. 2005;19(4):471–479. doi: 10.1016/j.tiv.2005.01.006 [DOI] [PubMed] [Google Scholar]
- 20.Lalley PM. D1/D2-dopamine receptor agonist dihydrexidine stimulates inspiratory motor output and depresses medullary expiratory neurons. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2009;296(6):R1829–1836. doi: 10.1152/ajpregu.00057.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seccombe LM, Giddings HL, Rogers PG, et al. Abnormal ventilatory control in Parkinson’s disease–further evidence for non-motor dysfunction. Respiratory Physiology & Neurobiology. 2011;179(2–3):300–304. doi: 10.1016/j.resp.2011.09.012 [DOI] [PubMed] [Google Scholar]
- 22.Baille G, De Jesus AM, Perez T, et al. Ventilatory Dysfunction in Parkinson’s Disease. J. Parkinsons Dis. 2016;6(3):463–471. doi: 10.3233/JPD-160804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR. Oxidative stress and Parkinson’s disease. Front Neuroanat. 2015;9:91. doi: 10.3389/fnana.2015.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim GH, Kim JE, Rhie SJ, Yoon S. The Role of oxidative stress in neurodegenerative diseases. Exp Neurobiol. 2015;24(4):325–340. doi: 10.5607/en.2015.24.4.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong P, Deng F, Zhang W, et al. Tectorigenin attenuates the MPP(+)-induced SH-SY5Y cell damage, indicating a potential beneficial role in Parkinson’s disease by oxidative stress inhibition. Exp. Ther. Med. 2017;14(5):4431–4437. doi: 10.3892/etm.2017.5049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cassarino DS, Halvorsen EM, Swerdlow RH, et al. Interaction among mitochondria, mitogen-activated protein kinases, and nuclear factor-kappaB in cellular models of Parkinson’s disease. J Neurochem. 2000;74(4):1384–1392. doi: 10.1046/j.1471-4159.2000.0741384.x [DOI] [PubMed] [Google Scholar]
- 27.Chaudhry ZL, Ahmed BY. The role of caspases in Parkinson’s Disease pathogenesis: a brief look at the mitochondrial pathway. Austin Alzheimer’s and Parkinson’s Disease. 2014;1(3):2–5. [Google Scholar]
- 28.Moon HE, Paek SH. Mitochondrial Dysfunction in Parkinson’s Disease. Exp Neurobiol. 2015;24(2):103–116. doi: 10.5607/en.2015.24.2.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunot S, Brugg B, Ricard D, et al. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with Parkinson disease. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(14):7531–7536. doi: 10.1073/pnas.94.14.7531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erekat NS, Al-Jarrah MD. Association of Parkinson Disease Induction with Cardiac Upregulation of Apoptotic Mediators P53 and Active Caspase-3: an Immunohistochemistry Study. Medical Science Monitor Basic Research. 2018;24:120–126. doi: 10.12659/MSMBR.910307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilkins LJ, Monga M, Miller AW. Defining Dysbiosis for a Cluster of Chronic Diseases. Sci Rep. 2019;9(1):12918. doi: 10.1038/s41598-019-49452-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Averina OV, Poluektova EU, Marsova MV, Danilenko VN. Biomarkers and utility of the antioxidant potential of probiotic Lactobacilli and Bifidobacteria as representatives of the human gut microbiota. Biomedicines. 2021;9(10):1340. doi: 10.3390/biomedicines9101340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong CB, Sugahara H, Odamaki T, Xiao JZ. Different physiological properties of human-residential and non-human-residential bifidobacteria in human health. Benef Microbes. 2018;9(1):111–122. doi: 10.3920/BM2017.0031 [DOI] [PubMed] [Google Scholar]
- 34.Salvetti E, O’Toole PW. When regulation challenges innovation: the case of the genus. Lactobacillus. Trends Food Sci. Technol. 2017;66:187–194. doi: 10.1016/j.tifs.2017.05.009 [DOI] [Google Scholar]
- 35.Arboleya S, Watkins C, Stanton C, Ross RP. Gut Bifidobacteria Populations in Human Health and Aging. Front Microbiol. 2016;7:1204. doi: 10.3389/fmicb.2016.01204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stavropoulou E, Bezirtzoglou E. Probiotics in Medicine: a Long Debate. Front Immunol. 2020;11:2192. doi: 10.3389/fimmu.2020.02192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Domej W, Oettl K, Renner W. Oxidative stress and free radicals in COPD–implications and relevance for treatment. Int. J. Chron. Obstruct. Pulmon. Dis. 2014;9:1207–1224. doi: 10.2147/COPD.S51226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calabrese V, Santoro A, Monti D, et al. Aging and Parkinson’s Disease: inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic. Biol. Med. 2018;115:80–91. doi: 10.1016/j.freeradbiomed.2017.10.379 [DOI] [PubMed] [Google Scholar]
- 39.Senoner T, Schindler S, Stattner S, Ofner D, Troppmair J, Primavesi F. Associations of Oxidative Stress and Postoperative Outcome in Liver Surgery with an Outlook to Future Potential Therapeutic Options. Oxid. Med. Cell. Longev. 2019;2019:3950818. doi: 10.1155/2019/3950818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kovtun AS, Averina OV, Zakharevich NV, Kasianov AS, Danilenko VN. In silico Identification of Metagenomic Signature Describing Neurometabolic Potential of Normal Human Gut Microbiota. Russian Journal of Genetics. 2018;54(9):1101–1110. doi: 10.1134/S1022795418090089 [DOI] [Google Scholar]
- 41.Averina OV, Kovtun AS, Polyakova SI, Savilova AM, Rebrikov DV, Danilenko VN. The bacterial neurometabolic signature of the gut microbiota of young children with autism spectrum disorders. J Med Microbiol. 2020a;69(4):558–571. doi: 10.1099/jmm.0.001178 [DOI] [PubMed] [Google Scholar]
- 42.Benakis C, Martin-Gallausiaux C, Trezzi JP, Melton P, Liesz A, Wilmes P. The microbiome-gut-brain axis in acute and chronic brain diseases. Current Opinion in Neurobiology. 2020;61:1–9. doi: 10.1016/j.conb.2019.11.009 [DOI] [PubMed] [Google Scholar]
- 43.Bercik P, Collins SM, Verdu EF. Microbes and the gut-brain axis. Neurogastroenterol Motil. 2012;24(5):405–413. doi: 10.1111/j.1365-2982.2012.01906.x [DOI] [PubMed] [Google Scholar]
- 44.Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013. doi: 10.1152/physrev.00018.2018 [DOI] [PubMed] [Google Scholar]
- 45.Hill JM, Bhattacharjee S, Pogue AI, Lukiw WJ. The gastrointestinal tract microbiome and potential link to Alzheimer’s disease. Front. Neurol. 2014;5(4):43. doi: 10.3389/fneur.2014.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klingelhoefer L, Reichmann H. Pathogenesis of Parkinson disease - the gut–brain axis and environmental factors. Nature Reviews Neurology. 2015;11(11):625–636. doi: 10.1038/nrneurol.2015.197 [DOI] [PubMed] [Google Scholar]
- 47.Khan MS, Ikram M, Park JS, Park TJ, Kim MO. Gut microbiota, its role in induction of Alzheimer’s disease pathology, and possible therapeutic interventions: special focus on anthocyanins. Cells. 2020;9(4):853. doi: 10.3390/cells9040853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caputi V, Giron M. Microbiome-gut-brain axis and toll-like receptors in Parkinson’s disease. Int J Mol Sci. 2018;19(6):1689. doi: 10.3390/ijms19061689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arora K, Green M, Prakash S. The microbiome and Alzheimer’s disease: potential and limitations of prebiotic, synbiotic, and probiotic formulations. Frontiers in Bioengineering and Biotechnology. 2020;8:537847. doi: 10.3389/Fbioe.2020.537847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milyukhina IV, Karpenko MN, Timofeeva AA, Klimenko VM, Skoromec AA. The role of inflammation in the pathogenesis of Parkinson’s disease. Neurological Journal. 2013;3(18):51–55. [Google Scholar]
- 51.Sampson TR, Debelius JW, Thron T, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167(6):1469–1480.e1412. doi: 10.1016/j.cell.2016.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fountain JH, Lappin SL. Physiology, Renin Angiotensin System. StatPearls. Treasure Island (FL): StatPearls Publishing LLC; 2021:29261862. [PubMed] [Google Scholar]
- 53.Abiodun OA, Ola MS. Role of brain renin angiotensin system in neurodegeneration: an update. Saudi J. Biol. Sci. 2020;27(3):905–912. doi: 10.1016/j.sjbs.2020.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34(5):575–584. doi: 10.1016/j.cjca.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carbone LD, Vasan S, Prentice RL, et al. The renin-angiotensin aldosterone system and osteoporosis: findings from the women’s health initiative. Osteoporos. Int. 2019;30(10):2039–2056. doi: 10.1007/s00198-019-05041-3 [DOI] [PubMed] [Google Scholar]
- 56.Scialo F, Daniele A, Amato F, et al. ACE2: the major cell entry receptor for SARS-CoV-2. Lung. 2020;198(6):867–877. doi: 10.1007/s00408-020-00408-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kullisaar T, Songisepp E, Zilmer M. Probiotics and oxidative stress. In: Lushchak V, editor. Oxidative Stress - Environmental Induction and Dietary Antioxidants. London, UK: IntechOpen; 2012:203–222. [Google Scholar]
- 58.Raimondi S, Amaretti A, Leonardi A, Quartieri A, Gozzoli C, Rossi M. Conjugated linoleic acid production by bifidobacteria: screening, kinetic, and composition. Biomed Res Int. 2016;2016:1–8. doi: 10.1155/2016/8654317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marsova M, Abilev S, Poluektova E, Danilenko V. A bioluminescent test system reveals valuable antioxidant properties of Lactobacillus strains from human microbiota. World J Microbiol Biotechnol. 2018;34(2):27. doi: 10.1007/s11274-018-2410-2 [DOI] [PubMed] [Google Scholar]
- 60.Eslami M, Yousefi B, Kokhaei P, et al. Importance of probiotics in the prevention and treatment of colorectal cancer. J Cell Physiol. 2019;234(10):17127–17143. doi: 10.1002/jcp.28473 [DOI] [PubMed] [Google Scholar]
- 61.Rinninella E, Raoul P, Cintoni M, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14. doi: 10.3390/microorganisms7010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaźmierczak-Siedlecka K, Daca A, Fic M, van de Wetering T, Folwarski M, Makarewicz W. Therapeutic methods of gut microbiota modification in colorectal cancer management – fecal microbiota transplantation, prebiotics, probiotics, and synbiotics. Gut Microbes. 2020;11(6):1518–1530. doi: 10.1080/19490976.2020.1764309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garcia-Gonzalez N, Prete R, Perugini M, Merola C, Battista N, Corsetti A. Probiotic antigenotoxic activity as a DNA bioprotective tool: a minireview with focus on endocrine disruptors. FEMS Microbiol Lett. 2020;367(3):fnaa041. doi: 10.1093/femsle/fnaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Żółkiewicz J, Marzec A, Ruszczyński M, Feleszko W. Postbiotics - A step beyond pre- and probiotics. Nutrients. 2020;12(8):2189. doi: 10.3390/nu12082189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rad AH, Aghebati-Maleki L, Kafil HS, Abbasi A. Molecular mechanisms of postbiotics in colorectal cancer prevention and treatment. Crit. Rev. Food Sci. Nutr. 2020;61(11):1787–1803. doi: 10.1080/10408398.2020.1765310 [DOI] [PubMed] [Google Scholar]
- 66.Khaled JMA. Probiotics, prebiotics, and COVID-19 infection: a review article. Saudi J. Biol. Sci. 2021;28(1):865–869. doi: 10.1016/j.sjbs.2020.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seaver LC, Imlay JA. Are respiratory enzymes the primary sources of intracellular hydrogen peroxide? J Biol Chem. 2004;279(47):48742–48750. doi: 10.1074/jbc.M408754200 [DOI] [PubMed] [Google Scholar]
- 68.Watson J. Oxidants, antioxidants and the current incurability of metastatic cancers. Open Biology. 2013;3(1):120144. doi: 10.1098/rsob.120144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davalli P, Marverti G, Lauriola A, D’Arca D. Targeting Oxidatively Induced DNA Damage Response in Cancer: opportunities for Novel Cancer Therapies. Oxid. Med. Cell. Longev. 2018;2018:2389523. doi: 10.1155/2018/2389523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21(7):363–383. doi: 10.1038/s41580-020-0230-3 [DOI] [PubMed] [Google Scholar]
- 71.Vona R, Pallotta L, Cappelletti M, Severi C, Matarrese P. The Impact of Oxidative Stress in Human Pathology: focus on Gastrointestinal Disorders. Antioxidants. 2021;10(2):201. doi: 10.3390/antiox10020201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Imlay JA. Pathways of oxidative damage. Annual Review of Microbiology. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938 [DOI] [PubMed] [Google Scholar]
- 73.Hardin SC, Larue CT, Oh MH, Jain V, Huber SC. Coupling oxidative signals to protein phosphorylation via methionine oxidation in Arabidopsis. Biochemical Journal. 2009;422(2):305–312. doi: 10.1042/BJ20090764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zuo L, Prather ER, Stetskiv M, et al. Inflammaging and Oxidative Stress in Human Diseases: from Molecular Mechanisms to Novel Treatments. Int J Mol Sci. 2019;20(18):4472. doi: 10.3390/ijms20184472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BW. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology. 2015;51:164–175. doi: 10.1016/j.psyneuen.2014.09.025 [DOI] [PubMed] [Google Scholar]
- 76.Prasad KN. Oxidative Stress, Pro-Inflammatory Cytokines, and Antioxidants Regulate Expression Levels of MicroRNAs in Parkinson’s Disease. Curr Aging Sci. 2017;10(3):177–184. doi: 10.2174/1874609810666170102144233 [DOI] [PubMed] [Google Scholar]
- 77.Lindqvist D, Dhabhar FS, James SJ, et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology. 2017;76:197–205. doi: 10.1016/j.psyneuen.2016.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fedoce ADG, Ferreira F, Bota RG, Bonet-Costa V, Sun PY, Davies KJA. The role of oxidative stress in anxiety disorder: cause or consequence? Free Radic. Res. 2018;52(7):737–750. doi: 10.1080/10715762.2018.1475733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Galecki P, Talarowska M. Inflammatory theory of depression. Psychiatr. Pol. 2018;52(3):437–447. doi: 10.12740/PP/76863 [DOI] [PubMed] [Google Scholar]
- 80.Ma Q, Xing C, Long W, Wang HY, Liu Q, Wang RF. Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J Neuroinflammation. 2019;16(1):53. doi: 10.1186/s12974-019-1434-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Segal AW. How neutrophils kill microbes. Annu. Rev. Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gems D, Partridge L. Stress-response hormesis and aging: “that which does not kill us makes us stronger”. Cell Metab. 2008;7(3):200–203. doi: 10.1016/j.cmet.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 83.Bhattacharyya S. Inflammation During Virus Infection: swings and Roundabouts. In: Bramhachari PV, editor. Dynamics of Immune Activation in Viral Diseases. Singapore: Springer Singapore; 2020:43–59. [Google Scholar]
- 84.Rouse BT. Virus-induced immunopathology. Adv. Virus Res. 1996;47:353–376. doi: 10.1016/s0065-3527(08)60739-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rouse BT, Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat. Rev. Immunol. 2010;10(7):514–526. doi: 10.1038/nri2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Allen IC, Scull MA, Moore CB, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30(4):556–565. doi: 10.1016/j.immuni.2009.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thomas PG, Dash P, Aldridge JR, et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30(4):566–575. doi: 10.1016/j.immuni.2009.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiology and Molecular Biology Reviews. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tarlinton RE, Martynova E, Rizvanov AA, Khaiboullina S, Verma S. Role of viruses in the pathogenesis of multiple sclerosis. Viruses. 2020;12(6):643. doi: 10.3390/v12060643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paz SPC, Branco L, Pereira MA, Spessotto C, Fragoso YD. Systematic review of the published data on the worldwide prevalence of John Cunningham virus in patients with multiple sclerosis and neuromyelitis optica. Epidemiol Health. 2018;40:e2018001. doi: 10.4178/epih.e2018001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barzon L. Ongoing and emerging arbovirus threats in Europe. J. Clin. Virol. 2018;107:38–47. doi: 10.1016/j.jcv.2018.08.007 [DOI] [PubMed] [Google Scholar]
- 92.Delbue S, Tadeo CS, Elia F, Ferrante P. JC virus replication at the first symptoms of multiple sclerosis: a case report. Intervirology. 2015;58(5):278–282. doi: 10.1159/000441473 [DOI] [PubMed] [Google Scholar]
- 93.Mangiardi M, Crawford DK, Xia X, et al. An animal model of cortical and callosal pathology in multiple sclerosis. Brain Pathol. 2011;21(3):263–278. doi: 10.1111/j.1750-3639.2010.00444.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leibovitch EC, Jacobson S. Evidence linking HHV-6 with multiple sclerosis: an update. Curr Opin Virol. 2014;9:127–133. doi: 10.1016/j.coviro.2014.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pormohammad A, Azimi T, Falah F, Faghihloo E. Relationship of human herpes virus 6 and multiple sclerosis: a systematic review and meta-analysis. J Cell Physiol. 2018;233(4):2850–2862. doi: 10.1002/jcp.26000 [DOI] [PubMed] [Google Scholar]
- 96.Akinsoji EO, Leibovitch E, Billioux BJ, et al. HHV‐6 and hippocampal volume in patients with mesial temporal sclerosis. Ann Clin Transl Neur. 2020;7(9):1674–1680. doi: 10.1002/acn3.51152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bu X-L, Wang X, Xiang Y, et al. The association between infectious burden and Parkinson’s disease: a case-control study. Parkinsonism Relat Disord. 2015;21(8):877–881. doi: 10.1016/j.parkreldis.2015.05.015 [DOI] [PubMed] [Google Scholar]
- 98.Tan JSY, Chao YX, Rotzschke O, Tan EK. New Insights into Immune-Mediated Mechanisms in Parkinson’s Disease. Int J Mol Sci. 2020;21(23):9302. doi: 10.3390/ijms21239302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Braak H, de Vos RAI, Bohl J, Del Tredici K. Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396(1):67–72. doi: 10.1016/j.neulet.2005.11.012 [DOI] [PubMed] [Google Scholar]
- 100.Svensson E, Horváth-Puhó E, Thomsen RW, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78(4):522–529. doi: 10.1002/ana.24448 [DOI] [PubMed] [Google Scholar]
- 101.Alifirova VM, Zhukova NG, Zhukova IA, et al. Correlation between emotional-affective disorders and gut microbiota composition in patients with Parkinson’s disease. Annals of the Russian Academy of Medical Sciences. 2016;71(6):427–435. doi: 10.15690/vramn734 [DOI] [PubMed] [Google Scholar]
- 102.Mertsalmi TH, Aho VTE, Pereira PAB, et al. More than constipation - bowel symptoms in Parkinson’s disease and their connection to gut microbiota. Eur. J. Neurol. 2017;24(11):1375–1383. doi: 10.1111/ene.13398 [DOI] [PubMed] [Google Scholar]
- 103.Lassmann H. Multiple sclerosis pathology. Cold Spring Harb. Perspect. Med. 2018;8(3):a028936. doi: 10.1101/cshperspect.a028936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang H, Wang K, Xu W, et al. Cerebrospinal fluid α-synuclein levels are elevated in multiple sclerosis and neuromyelitis optica patients during replase. J Neurochem. 2012;122(1):19–23. doi: 10.1111/j.1471-4159.2012.07749.x [DOI] [PubMed] [Google Scholar]
- 105.Williams-Gray CH, Wijeyekoon R, Yarnall AJ, et al. Serum immune markers and disease progression in an incident Parkinson’s disease cohort (ICICLE-PD). Mov Disord. 2016;31(7):995–1003. doi: 10.1002/mds.26563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wilen CB, Trypsteen W, Van Cleemput J, Snippenberg W, Gerlo S, Vandekerckhove L. On the whereabouts of SARS-CoV-2 in the human body: a systematic review. PLoS Path. 2020;16(10):e1009037. doi: 10.1371/journal.ppat.1009037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dhar D, Mohanty A. Gut microbiota and Covid-19- possible link and implications. Virus Res. 2020;285:198018. doi: 10.1016/j.virusres.2020.198018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Azkur AK, Akdis M, Azkur D, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564–1581. doi: 10.1111/all.14364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Khatiwada S, Subedi A. Lung microbiome and coronavirus disease 2019 (COVID-19): possible link and implications. Hum Microb J. 2020;17:100073. doi: 10.1016/j.humic.2020.100073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Demyanovskaya EG, Kryzhanovsky SM, Vasiliev AS, Shmyrev VI. Neurological aspects of COVID-19. Patient management tactics by a neurologist, taking into account the epidemiological situation. Lechashchiy Vrach. 2021;1(2):54–60. doi: 10.26295/os.2021.63.96.011 [DOI] [Google Scholar]
- 111.Ni Y, Yang X, Zheng L, et al. Lactobacillus and Bifidobacterium improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota. Mol. Nutr. Food Res. 2019;63(22):1900603. doi: 10.1002/mnfr.201900603 [DOI] [PubMed] [Google Scholar]
- 112.Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li WH, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li F, Li WH, Farzan M, Harrison SC. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309(5742):1864–1868. doi: 10.1126/science.1116480 [DOI] [PubMed] [Google Scholar]
- 115.Song WF, Gui M, Wang XQ, Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Path. 2018;14(8):e1007236. doi: 10.1371/journal.ppat.1007236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kuba K, Imai Y, Ohto-Nakanishi T, Penninger JM. Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128(1):119–128. doi: 10.1016/j.pharmthera.2010.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Grant MC, Geoghegan L, Arbyn M, et al. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): a systematic review and meta-analysis of 148 studies from 9 countries. PLoS One. 2020;15(6):e0234765. doi: 10.1371/journal.pone.0234765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Greenhalgh T, Jimenez JL, Prather KA, Tufekci Z, Fisman D, Schooley R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet. 2021;397(10285):1603–1605. doi: 10.1016/S0140-6736(21)00869-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang CC, Prather KA, Sznitman J, et al. Airborne transmission of respiratory viruses. Science. 2021;373(6558):eabd9149. doi: 10.1126/science.abd9149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tsai PH, Lai WY, Lin YY, et al. Clinical manifestation and disease progression in COVID-19 infection. J Chin Med Assoc. 2021;84(1):3–8. doi: 10.1097/JCMA.0000000000000463 [DOI] [PubMed] [Google Scholar]
- 121.Harrison AG, Lin T, Wang P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020;41(12):1100–1115. doi: 10.1016/j.it.2020.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nature Microbiology. 2020;5(4):562–569. doi: 10.1038/s41564-020-0688-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eketunde AO, Mellacheruvu SP, Oreoluwa PA. Review of Postmortem Findings in Patients With COVID-19. Cureus. 2020;12(7):e9438. doi: 10.7759/cureus.9438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.da Rosa Mesquita R, Francelino Silva Junior LC, Santos Santana FM, et al. Clinical manifestations of COVID-19 in the general population: systematic review. Wien Klin Wochenschr. 2021;133(7–8):377–382. doi: 10.1007/s00508-020-01760-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marik PE, Iglesias J, Varon J, Kory P. A scoping review of the pathophysiology of COVID-19. Int J Immunopathol Pharmacol. 2021;35:20587384211048026. doi: 10.1177/20587384211048026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 Virus Targeting the CNS: tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122 [DOI] [PubMed] [Google Scholar]
- 129.Yavarpour-Bali H, Ghasemi-Kasman M. Update on neurological manifestations of COVID-19. Life Sciences. 2020;257:118063. doi: 10.1016/j.lfs.2020.118063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pezzini A, Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat. Rev. Neurol. 2020;16(11):636–644. doi: 10.1038/s41582-020-0398-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gu J, Han B, Wang J. COVID-19: gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology. 2020;158(6):1518–1519. doi: 10.1053/j.gastro.2020.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020;55(5):105954. doi: 10.1016/j.ijantimicag.2020.105954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gomez-Rial J, Rivero-Calle I, Salas A, Martinon-Torres F. Role of Monocytes/Macrophages in Covid-19 Pathogenesis: implications for Therapy. Infect Drug Resist. 2020;13:2485–2493. doi: 10.2147/IDR.S258639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Soy M, Keser G, Atagunduz P, Tabak F, Atagunduz I, Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020;39(7):2085–2094. doi: 10.1007/s10067-020-05190-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Quirch M, Lee J, Rehman S. Hazards of the Cytokine Storm and Cytokine-Targeted Therapy in Patients With COVID-19: review. J. Med. Internet Res. 2020;22(8):e20193. doi: 10.2196/20193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wiese OJ, Allwood BW, Zemlin AE. COVID-19 and the renin-angiotensin system (RAS): a spark that sets the forest alight? Med. Hypotheses. 2020;144:110231. doi: 10.1016/j.mehy.2020.110231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bhaskar S, Sinha A, Banach M, et al. Cytokine Storm in COVID-19-Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: the REPROGRAM Consortium Position Paper. Front Immunol. 2020;11:1648. doi: 10.3389/fimmu.2020.01648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Beltran-Garcia J, Osca-Verdegal R, Pallardo FV, et al. Oxidative Stress and Inflammation in COVID-19-Associated Sepsis: the Potential Role of Anti-Oxidant Therapy in Avoiding Disease Progression. Antioxidants. 2020;9(10):936. doi: 10.3390/antiox9100936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cecchini R, Cecchini AL. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses. 2020;143:110102. doi: 10.1016/j.mehy.2020.110102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Delgado-Roche L, Mesta F. Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Arch Med Res. 2020;51(5):384–387. doi: 10.1016/j.arcmed.2020.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gan R, Rosoman NP, Henshaw DJE, Noble EP, Georgius P, Sommerfeld N. COVID-19 as a viral functional ACE2 deficiency disorder with ACE2 related multi-organ disease. Med. Hypotheses. 2020;144:110024. doi: 10.1016/j.mehy.2020.110024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wei Y, Sowers JR, Nistala R, et al. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem. 2006;281(46):35137–35146. doi: 10.1074/jbc.M601320200 [DOI] [PubMed] [Google Scholar]
- 144.Zablocki D, Sadoshima J. Angiotensin II and oxidative stress in the failing heart. Antioxid Redox Signal. 2013;19(10):1095–1109. doi: 10.1089/ars.2012.4588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dikalov SI, Nazarewicz RR, Angiotensin I. I-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid Redox Signal. 2013;19(10):1085–1094. doi: 10.1089/ars.2012.4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rincon J, Correia D, Arcaya JL, et al. Role of Angiotensin II type 1 receptor on renal NAD(P)H oxidase, oxidative stress and inflammation in nitric oxide inhibition induced-hypertension. Life Sciences. 2015;124:81–90. doi: 10.1016/j.lfs.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Valente AJ, Yoshida T, Murthy SN, et al. Angiotensin II enhances AT1-Nox1 binding and stimulates arterial smooth muscle cell migration and proliferation through AT1, Nox1, and interleukin-18. American Journal of Physiology Heart and Circulatory Physiology. 2012;303(3):H282–296. doi: 10.1152/ajpheart.00231.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Oudit GY, Kassiri Z, Patel MP, et al. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc. Res. 2007;75(1):29–39. doi: 10.1016/j.cardiores.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 149.Sawalha AH, Zhao M, Coit P, Lu Q. Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clin Immunol. 2020;215:108410. doi: 10.1016/j.clim.2020.108410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Violi F, Oliva A, Cangemi R, et al. Nox2 activation in Covid-19. Redox Biol. 2020;36:101655. doi: 10.1016/j.redox.2020.101655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Barnes BJ, Adrover JM, Baxter-Stoltzfus A, et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. Journal of Experimental Medicine. 2020;217(6):e20200652. doi: 10.1084/jem.20200652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gong J, Dong H, Xia Q, et al. Correlation Analysis Between Disease Severity and Inflammation-related Parameters in Patients with COVID-19 Pneumonia. medRxiv. 2020;1:202. doi: 10.1101/2020.02.25.20025643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Qin C, Zhou L, Hu Z, et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang Y, Shen C, Li J, et al. Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. medRxiv. 2020;1:20029975. doi: 10.1101/2020.03.02.20029975 [DOI] [Google Scholar]
- 156.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Internal Medicine. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53(3):368–370. doi: 10.1016/j.jmii.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the Perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang JZ, Zhang RY, Bai J. An anti-oxidative therapy for ameliorating cardiac injuries of critically ill COVID-19-infected patients. Int J Cardiol. 2020;312:137–138. doi: 10.1016/j.ijcard.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nagar H, Piao S, Kim C-S. Role of Mitochondrial Oxidative Stress in Sepsis. Acute Crit Care. 2018;33(2):65–72. doi: 10.4266/acc.2018.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth. 2011;107(1):57–64. doi: 10.1093/bja/aer093 [DOI] [PubMed] [Google Scholar]
- 165.Alexoudi A, Alexoudi I, Gatzonis S. Parkinson’s disease pathogenesis, evolution and alternative pathways: a review. Rev. Neurol. (Paris). 2018;174(10):699–704. doi: 10.1016/j.neurol.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 166.Reich SG, Savitt JM. Parkinson’s disease. Med. Clin. North Am. 2019;103(2):337–350. doi: 10.1016/j.mcna.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 167.Dorsey ER, Sherer T, Okun MS, et al. The emerging evidence of the Parkinson pandemic. J. Parkinsons Dis. 2018;8(1):3–8. doi: 10.3233/jpd-181474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Feigin VL, Nichols E, Alam T, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the global burden of disease study 2016. The Lancet Neurology. 2019;18(5):459–480. doi: 10.1016/s1474-4422(18)30499-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dugger BN, Dickson DW. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017;9(7):a028035. doi: 10.1101/cshperspect.a028035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Illarioshkin SN, Klyushnikov SA, Vigont VA, Seliverstov YA, Kaznacheyeva EV. Molecular pathogenesis in Huntington’s disease. Biochemistry. 2018;83(9):1030–1039. doi: 10.1134/s0006297918090043 [DOI] [PubMed] [Google Scholar]
- 171.Kotagal V, Bohnen NI, Müller MLTM, Frey KA, Albin RL. Cerebral amyloid burden and Hoehn and Yahr stage 3 scoring in Parkinson disease. J. Parkinsons Dis. 2017;7(1):143–147. doi: 10.3233/jpd-160985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lubomski M, Tan AH, Lim S-Y, Holmes AJ, Davis RL, Sue CM. Parkinson’s disease and the gastrointestinal microbiome. J. Neurol. 2019;267(9):2507–2523. doi: 10.1007/s00415-019-09320-1 [DOI] [PubMed] [Google Scholar]
- 173.Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. The Lancet. 2017;389(10076):1323–1335. doi: 10.1016/S0140-6736(16)32381-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Munoz-Pinto MF, Empadinhas N, Cardoso SM. The neuromicrobiology of Parkinson’s disease: a unifying theory. Ageing Research Reviews. 2021;70:101396. doi: 10.1016/j.arr.2021.101396 [DOI] [PubMed] [Google Scholar]
- 175.Langston JW. Current theories on the cause of Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 1989;Suppl:13–17. doi: 10.1136/jnnp.52.suppl.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kalia LV, Lang AE. Parkinson’s disease. The Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3 [DOI] [PubMed] [Google Scholar]
- 177.Johnson ME, Stecher B, Labrie V, Brundin L, Brundin P. Triggers, Facilitators, and Aggravators: redefining Parkinson’s Disease Pathogenesis. Trends in Neurosciences. 2019;42(1):4–13. doi: 10.1016/j.tins.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kempster PA, Hurwitz B, Lees AJ. James Parkinson’s Chimera: syndrome or disease? J R Coll Physicians Edinb. 2017;47(2):190–195. doi: 10.4997/JRCPE.2017.220 [DOI] [PubMed] [Google Scholar]
- 179.Obeso JA, Stamelou M, Goetz CG, et al. Past, present, and future of Parkinson’s disease: a special essay on the 200th Anniversary of the Shaking Palsy. Mov Disord. 2017;32(9):1264–1310. doi: 10.1002/mds.27115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Titova N, Padmakumar C, Lewis SJG, Chaudhuri KR. Parkinson’s: a syndrome rather than a disease? J Neural Transm (Vienna). 2017;124(8):907–914. doi: 10.1007/s00702-016-1667-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Burke RE, Dauer WT, Vonsattel JP. A critical evaluation of the Braak staging scheme for Parkinson’s disease. Ann Neurol. 2008;64(5):485–491. doi: 10.1002/ana.21541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cardoso SM, Empadinhas N. The Microbiome-Mitochondria Dance in Prodromal Parkinson’s Disease. Front. Physiol. 2018;9:471. doi: 10.3389/fphys.2018.00471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Engen PA, Dodiya HB, Naqib A, et al. The Potential Role of Gut-Derived Inflammation in Multiple System Atrophy. J. Parkinsons Dis. 2017;7(2):331–346. doi: 10.3233/JPD-160991 [DOI] [PubMed] [Google Scholar]
- 184.Houser MC, Tansey MG. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinsons Dis. 2017;3:3. doi: 10.1038/s41531-016-0002-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Matheoud D, Cannon T, Voisin A, et al. Intestinal infection triggers Parkinson’s disease-like symptoms in Pink1(-/-) mice. Nature. 2019;571(7766):565–569. doi: 10.1038/s41586-019-1405-y [DOI] [PubMed] [Google Scholar]
- 186.Gray MT, Woulfe JM. Striatal blood-brain barrier permeability in Parkinson’s disease. J. Cereb. Blood Flow Metab. 2015;35(5):747–750. doi: 10.1038/jcbfm.2015.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Campos-Acuna J, Elgueta D, Pacheco R. T-Cell-Driven Inflammation as a Mediator of the Gut-Brain Axis Involved in Parkinson’s Disease. Front Immunol. 2019;10:239. doi: 10.3389/fimmu.2019.00239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Peralta Ramos JM, Iribarren P, Bousset L, Melki R, Baekelandt V. Peripheral Inflammation Regulates CNS Immune Surveillance Through the Recruitment of Inflammatory Monocytes Upon Systemic alpha-Synuclein Administration. Front Immunol. 2019;10:80. doi: 10.3389/fimmu.2019.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018;14(3):133–150. doi: 10.1038/nrneurol.2017.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Unger MM, Spiegel J, Dillmann KU, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019 [DOI] [PubMed] [Google Scholar]
- 191.Devos D, Lebouvier T, Lardeux B, et al. Colonic inflammation in Parkinson’s disease. Neurobiol Dis. 2013;50:42–48. doi: 10.1016/j.nbd.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 192.Harms AS, Thome AD, Yan Z, et al. Peripheral monocyte entry is required for alpha-Synuclein induced inflammation and Neurodegeneration in a model of Parkinson disease. Exp Neurol. 2018;300:179–187. doi: 10.1016/j.expneurol.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Palm NW, de Zoete MR, Cullen TW, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158(5):1000–1010. doi: 10.1016/j.cell.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fitzpatrick Z, Frazer G, Ferro A, et al. Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nature. 2020;587(7834):472–476. doi: 10.1038/s41586-020-2886-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Probstel AK, Zhou X, Baumann R, et al. Gut microbiota-specific IgA(+) B cells traffic to the CNS in active multiple sclerosis. Sci Immunol. 2020;5(53):eabc7191. doi: 10.1126/sciimmunol.abc7191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shalapour S, Lin XJ, Bastian IN, et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature. 2017;551(7680):340–345. doi: 10.1038/nature24302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rojas OL, Probstel AK, Porfilio EA, et al. Recirculating Intestinal IgA-Producing Cells Regulate Neuroinflammation via IL-10. Cell. 2019;176(3):610–624 e618. doi: 10.1016/j.cell.2018.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Buscarinu MC, Cerasoli B, Annibali V, et al. Altered intestinal permeability in patients with relapsing-remitting multiple sclerosis: a pilot study. Mult. Scler. 2017;23(3):442–446. doi: 10.1177/1352458516652498 [DOI] [PubMed] [Google Scholar]
- 199.Mirabito Colafella KM, Bovée DM, Danser AHJ. The renin-angiotensin-aldosterone system and its therapeutic targets. Exp Eye Res. 2019;186:107680. doi: 10.1016/j.exer.2019.05.020 [DOI] [PubMed] [Google Scholar]
- 200.Wanka H, Staar D, Lutze P, et al. Anti-necrotic and cardioprotective effects of a cytosolic renin isoform under ischemia-related conditions. J. Mol. Med. 2015;94(1):61–69. doi: 10.1007/s00109-015-1321-z [DOI] [PubMed] [Google Scholar]
- 201.Patel S, Rauf A, Khan H, Abu-Izneid T. Renin-angiotensin-aldosterone (RAAS): the ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017;94:317–325. doi: 10.1016/j.biopha.2017.07.091 [DOI] [PubMed] [Google Scholar]
- 202.Peach MJ, Dostal DE. The angiotensin II receptor and the actions of angiotensin II. J. Cardiovasc. Pharmacol. 1990;16(4):25–30. doi: 10.1097/00005344-199016004-00007 [DOI] [PubMed] [Google Scholar]
- 203.Durante A, Peretto G, Laricchia A, et al. Role of the renin-angiotensin-aldosterone system in the pathogenesis of atherosclerosis. Curr. Pharm. Des. 2012;18(7):981–1004. doi: 10.2174/138161212799436467 [DOI] [PubMed] [Google Scholar]
- 204.Hamming I, Cooper ME, Haagmans BL, et al. The emerging role of ACE2 in physiology and disease. The Journal of Pathology. 2007;212(1):1–11. doi: 10.1002/path.2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rodriguez-Perez AI, Garrido-Gil P, Pedrosa MA, et al. Angiotensin type 2 receptors: role in aging and neuroinflammation in the substantia nigra. Brain, Behav., Immun. 2020;87:256–271. doi: 10.1016/j.bbi.2019.12.011 [DOI] [PubMed] [Google Scholar]
- 206.Gathiram P, Moodley J. The role of the renin-angiotensin-aldosterone system in preeclampsia: a review. Curr. Hypertens. Rep. 2020;22(11):89. doi: 10.1007/s11906-020-01098-2 [DOI] [PubMed] [Google Scholar]
- 207.Mascolo A, Sessa M, Scavone C, et al. New and old roles of the peripheral and brain renin–angiotensin–aldosterone system (RAAS): focus on cardiovascular and neurological diseases. Int J Cardiol. 2017;227:734–742. doi: 10.1016/j.ijcard.2016.10.069 [DOI] [PubMed] [Google Scholar]
- 208.Panariello F, Cellini L, Speciani M, De Ronchi D, Atti AR. How does SARS-CoV-2 affect the central nervous system? A working hypothesis. Front Psychiatry. 2020;11:582345. doi: 10.3389/fpsyt.2020.582345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Labandeira-García JL, Garrido-Gil P, Rodriguez-Pallares J, Valenzuela R, Borrajo A, Rodriguez-Perez AI. Brain renin-angiotensin system and dopaminergic cell vulnerability. Front Neuroanat. 2014;8(8):67. doi: 10.3389/fnana.2014.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kobiec T, Otero-Losada M, Chevalier G, et al. The renin–angiotensin system modulates dopaminergic neurotransmission: a new player on the scene. Front. Synaptic Neurosci. 2021;13:16. doi: 10.3389/fnsyn.2021.638519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Viana SD, Nunes S, Reis F. ACE2 imbalance as a key player for the poor outcomes in COVID-19 patients with age-related comorbidities – role of gut microbiota dysbiosis. Ageing Research Reviews. 2020;62:101123. doi: 10.1016/j.arr.2020.101123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ames MK, Atkins CE, Pitt B. The renin‐angiotensin‐aldosterone system and its suppression. J. Vet. Intern. Med. 2019;33(2):363–382. doi: 10.1111/jvim.15454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yang T, Xu C. Physiology and Pathophysiology of the Intrarenal Renin-Angiotensin System: an Update. J Am Soc Nephrol. 2017;28(4):1040–1049. doi: 10.1681/ASN.2016070734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Alexandre J, Cracowski J-L, Richard V, Bouhanick B. Renin-angiotensin-aldosterone system and COVID-19 infection. Ann Endocrinol. 2020;81(2–3):63–67. doi: 10.1016/j.ando.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tseng YH, Yang RC, Lu TS. Two hits to the renin‐angiotensin system may play a key role in severe COVID‐19. Kaohsiung J. Med. Sci. 2020;36(6):389–392. doi: 10.1002/kjm2.12237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Coto E, Avanzas P, Gómez J. The renin–angiotensin–aldosterone system and coronavirus disease 2019. European Cardiology Review. 2021;16:e07. doi: 10.15420/ecr.2020.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Oudit GY, Kassiri Z, Jiang C, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Invest. 2009;39(7):618–625. doi: 10.1111/j.1365-2362.2009.02153.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS-CoV-2. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol-Heart C. 2020;318(5):1084–1090. doi: 10.1152/ajpheart.00217.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Datta PK, Liu F, Fischer T, Rappaport J, Qin X. SARS-CoV-2 pandemic and research gaps: understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics. 2020;10(16):7448–7464. doi: 10.7150/thno.48076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kumar BK, Sekhar KV. Druggable targets of SARS-CoV-2 and treatment opportunities for COVID-19. Bioorg. Chem. 2020;104:104269. doi: 10.1016/j.bioorg.2020.104269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kuba K, Imai Y, Penninger JM. Angiotensin-converting enzyme 2 in lung diseases. Curr Opin Pharmacol. 2006;6(3):271–276. doi: 10.1016/j.coph.2006.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Arentz M, Yim E, Klaff L, et al. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA-J Am Med Assoc. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mertens B, Vanderheyden P, Michotte Y, Sarre S. The role of the central renin-angiotensin system in Parkinson’s disease. J. Renin Angiotensin Aldosterone Syst. 2010;11(1):49–56. doi: 10.1177/1470320309347789 [DOI] [PubMed] [Google Scholar]
- 225.Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. International Journal of Biochemistry & Cell Biology. 2003;35(6):881–900. doi: 10.1016/s1357-2725(02)00271-6 [DOI] [PubMed] [Google Scholar]
- 226.Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept. 2000;91(1–3):21–27. doi: 10.1016/s0167-0115(00)00136-1 [DOI] [PubMed] [Google Scholar]
- 227.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 2000;86(5):494–501. doi: 10.1161/01.res.86.5.494 [DOI] [PubMed] [Google Scholar]
- 228.Allen AM, MacGregor DP, Chai SY, et al. Angiotensin II receptor binding associated with nigrostriatal dopaminergic neurons in human basal ganglia. Ann Neurol. 1992;32(3):339–344. doi: 10.1002/ana.410320306 [DOI] [PubMed] [Google Scholar]
- 229.Wright JW, Kawas LH, Harding JWA. Role for the Brain RAS in Alzheimer’s and Parkinson’s Diseases. Front. Endocrinol. (Lausanne). 2013;4:158. doi: 10.3389/fendo.2013.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Strittmatter SM, Snyder SH. Angiotensin converting enzyme immunohistochemistry in rat brain and pituitary gland: correlation of isozyme type with cellular localization. Neuroscience. 1987;21(2):407–420. doi: 10.1016/0306-4522(87)90131-x [DOI] [PubMed] [Google Scholar]
- 231.Chai SY, McKenzie JS, McKinley MJ, Mendelsohn FA. Angiotensin converting enzyme in the human basal forebrain and midbrain visualized by in vitro autoradiography. Journal of Comparative Neurology. 1990;291(2):179–194. doi: 10.1002/cne.902910203 [DOI] [PubMed] [Google Scholar]
- 232.Reardon KA, Mendelsohn FA, Chai SY, Horne MK. The angiotensin converting enzyme (ACE) inhibitor, perindopril, modifies the clinical features of Parkinson’s disease. Aust. N. Z. J. Med. 2000;30(1):48–53. doi: 10.1111/j.1445-5994.2000.tb01054.x [DOI] [PubMed] [Google Scholar]
- 233.Jenkins TA, Mendelsohn FA, Chai SY. Angiotensin-converting enzyme modulates dopamine turnover in the striatum. J Neurochem. 1997;68(3):1304–1311. doi: 10.1046/j.1471-4159.1997.68031304.x [DOI] [PubMed] [Google Scholar]
- 234.Chabrashvili T, Kitiyakara C, Blau J, et al. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2003;285(1):R117–124. doi: 10.1152/ajpregu.00476.2002 [DOI] [PubMed] [Google Scholar]
- 235.Rodriguez-Pallares J, Quiroz CR, Parga JA, Guerra MJ, Labandeira-Garcia JL. Angiotensin II increases differentiation of dopaminergic neurons from mesencephalic precursors via angiotensin type 2 receptors. European Journal of Neuroscience. 2004;20(6):1489–1498. doi: 10.1111/j.1460-9568.2004.03621.x [DOI] [PubMed] [Google Scholar]
- 236.Jenkins TA, Wong JY, Howells DW, Mendelsohn FA, Chai SY. Effect of chronic angiotensin-converting enzyme inhibition on striatal dopamine content in the MPTP-treated mouse. J Neurochem. 1999;73(1):214–219. doi: 10.1046/j.1471-4159.1999.0730214.x [DOI] [PubMed] [Google Scholar]
- 237.Lopez-Real A, Rey P, Soto-Otero R, Mendez-Alvarez E, Labandeira-Garcia JL. Angiotensin-converting enzyme inhibition reduces oxidative stress and protects dopaminergic neurons in a 6-hydroxydopamine rat model of Parkinsonism. J Neurosci Res. 2005;81(6):865–873. doi: 10.1002/jnr.20598 [DOI] [PubMed] [Google Scholar]
- 238.Munoz A, Rey P, Guerra MJ, Mendez-Alvarez E, Soto-Otero R, Labandeira-Garcia JL. Reduction of dopaminergic degeneration and oxidative stress by inhibition of angiotensin converting enzyme in a MPTP model of parkinsonism. Neuropharmacology. 2006;51(1):112–120. doi: 10.1016/j.neuropharm.2006.03.004 [DOI] [PubMed] [Google Scholar]
- 239.Babior BM. NADPH oxidase. Curr. Opin. Immunol. 2004;16(1):42–47. doi: 10.1016/j.coi.2003.12.001 [DOI] [PubMed] [Google Scholar]
- 240.Rodriguez-Pallares J, Parga JA, Munoz A, Rey P, Guerra MJ, Labandeira-Garcia JL. Mechanism of 6-hydroxydopamine neurotoxicity: the role of NADPH oxidase and microglial activation in 6-hydroxydopamine-induced degeneration of dopaminergic neurons. J Neurochem. 2007;103(1):145–156. doi: 10.1111/j.1471-4159.2007.04699.x [DOI] [PubMed] [Google Scholar]
- 241.Joglar B, Rodriguez-Pallares J, Rodriguez-Perez AI, Rey P, Guerra MJ, Labandeira-Garcia JL. The inflammatory response in the MPTP model of Parkinson’s disease is mediated by brain angiotensin: relevance to progression of the disease. J Neurochem. 2009;109(2):656–669. doi: 10.1111/j.1471-4159.2009.05999.x [DOI] [PubMed] [Google Scholar]
- 242.Okamura A, Rakugi H, Ohishi M, et al. Upregulation of renin-angiotensin system during differentiation of monocytes to macrophages. J. Hypertens. 1999;17(4):537–545. doi: 10.1097/00004872-199917040-00012 [DOI] [PubMed] [Google Scholar]
- 243.Labandeira-Garcia JL, Rodriguez-Pallares J, Villar-Cheda B, Rodriguez-Perez AI, Garrido-Gil P, Guerra MJ. Aging, Angiotensin system and dopaminergic degeneration in the substantia nigra. Aging Dis. 2011;2(3):257–274. [PMC free article] [PubMed] [Google Scholar]
- 244.Rodriguez-Perez AI, Valenzuela R, Joglar B, Garrido-Gil P, Guerra MJ, Labandeira-Garcia JL. Renin angiotensin system and gender differences in dopaminergic degeneration. Mol. Neurodegener. 2011;6(1):58. doi: 10.1186/1750-1326-6-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Garrido-Gil P, Joglar B, Rodriguez-Perez AI, Guerra MJ, Labandeira-Garcia JL. Involvement of PPAR-gamma in the neuroprotective and anti-inflammatory effects of angiotensin type 1 receptor inhibition: effects of the receptor antagonist telmisartan and receptor deletion in a mouse MPTP model of Parkinson’s disease. J Neuroinflammation. 2012;9:38. doi: 10.1186/1742-2094-9-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Villar-Cheda B, Rodriguez-Pallares J, Valenzuela R, et al. Nigral and striatal regulation of angiotensin receptor expression by dopamine and angiotensin in rodents: implications for progression of Parkinson’s disease. European Journal of Neuroscience. 2010;32(10):1695–1706. doi: 10.1111/j.1460-9568.2010.07448.x [DOI] [PubMed] [Google Scholar]
- 247.Lan J, Ge J, Yu J, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5 [DOI] [PubMed] [Google Scholar]
- 248.Li Z, Xu X, Yang M, Feng J, Liu C, Yang C. Role of angiotensin-converting enzyme 2 in neurodegenerative diseases during the COVID-19 pandemic. Aging (Albany N. Y. 2020;12(23):24453–24461. doi: 10.18632/aging.103993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Liu Z, Xiao X, Wei X, et al. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J Med Virol. 2020;92(6):595–601. doi: 10.1002/jmv.25726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hanff TC, Harhay MO, Brown TS, Cohen JB, Mohareb AM. Is There an Association Between COVID-19 Mortality and the Renin-Angiotensin System? A Call for Epidemiologic Investigations. Clin Infect Dis. 2020;71(15):870–874. doi: 10.1093/cid/ciaa329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020;38(7):1504–1507. doi: 10.1016/j.ajem.2020.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Michaud V, Deodhar M, Arwood M, Al Rihani SB, Dow P, Turgeon J. ACE2 as a Therapeutic Target for COVID-19; its Role in Infectious Processes and Regulation by Modulators of the RAAS System. J Clin Med. 2020;9(7):2096. doi: 10.3390/jcm9072096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Morrone CD, Bishay J, McLaurin J. Potential Role of Venular Amyloid in Alzheimer’s Disease Pathogenesis. Int J Mol Sci. 2020;21(6):1985. doi: 10.3390/ijms21061985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Leroy E, Boyer R, Auburger G, et al. The ubiquitin pathway in Parkinson’s disease. Nature. 1998;395(6701):451–452. doi: 10.1038/26652 [DOI] [PubMed] [Google Scholar]
- 256.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86(3):747–803. doi: 10.1152/physrev.00036.2005 [DOI] [PubMed] [Google Scholar]
- 257.Kaur P, Muthuraman A, Kaur M. The implications of angiotensin-converting enzymes and their modulators in neurodegenerative disorders: current and future perspectives. ACS Chem Neurosci. 2015;6(4):508–521. doi: 10.1021/cn500363g [DOI] [PubMed] [Google Scholar]
- 258.Ohrui T, Tomita N, Sato-Nakagawa T, et al. Effects of brain-penetrating ACE inhibitors on Alzheimer disease progression. Neurology. 2004;63(7):1324–1325. doi: 10.1212/01.wnl.0000140705.23869.e9 [DOI] [PubMed] [Google Scholar]
- 259.Li NC, Lee A, Whitmer RA, et al. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ. 2010;340:b5465. doi: 10.1136/bmj.b5465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Ongali B, Nicolakakis N, Tong XK, et al. Angiotensin II type 1 receptor blocker losartan prevents and rescues cerebrovascular, neuropathological and cognitive deficits in an Alzheimer’s disease model. Neurobiol Dis. 2014;68:126–136. doi: 10.1016/j.nbd.2014.04.018 [DOI] [PubMed] [Google Scholar]
- 261.Jochemsen HM, Teunissen CE, Ashby EL, et al. The association of angiotensin-converting enzyme with biomarkers for Alzheimer’s disease. Alzheimers Res. Ther. 2014;6(3):27. doi: 10.1186/alzrt257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Rocha NP, Toledo A, Corgosinho LTS, et al. Cerebrospinal Fluid Levels of Angiotensin-Converting Enzyme Are Associated with Amyloid-beta42 Burden in Alzheimer’s Disease. J Alzheimers Dis. 2018;64(4):1085–1090. doi: 10.3233/JAD-180282 [DOI] [PubMed] [Google Scholar]
- 263.Hu J, Igarashi A, Kamata M, Nakagawa H. Angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide (A beta); retards A beta aggregation, deposition, fibril formation; and inhibits cytotoxicity. J Biol Chem. 2001;276(51):47863–47868. doi: 10.1074/jbc.M104068200 [DOI] [PubMed] [Google Scholar]
- 264.Zou K, Liu J, Watanabe A, et al. Abeta43 is the earliest-depositing Abeta species in APP transgenic mouse brain and is converted to Abeta41 by two active domains of ACE. American Journal of Pathology. 2013;182(6):2322–2331. doi: 10.1016/j.ajpath.2013.01.053 [DOI] [PubMed] [Google Scholar]
- 265.Zubenko GS, Volicer L, Direnfeld LK, Freeman M, Langlais PJ, Nixon RA. Cerebrospinal fluid levels of angiotensin-converting enzyme in Alzheimer’s disease, Parkinson’s disease and progressive supranuclear palsy. Brain Research. 1985;328(2):215–221. doi: 10.1016/0006-8993(85)91032-7 [DOI] [PubMed] [Google Scholar]
- 266.Kawajiri M, Mogi M, Higaki N, et al. Angiotensin-converting enzyme (ACE) and ACE2 levels in the cerebrospinal fluid of patients with multiple sclerosis. Mult. Scler. 2009;15(2):262–265. doi: 10.1177/1352458508097923 [DOI] [PubMed] [Google Scholar]
- 267.Kehoe PG, Wong S, Al Mulhim N, Palmer LE, Miners JS. Angiotensin-converting enzyme 2 is reduced in Alzheimer’s disease in association with increasing amyloid-beta and tau pathology. Alzheimers Res. Ther. 2016;8(1):50. doi: 10.1186/s13195-016-0217-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kamel AS, Abdelkader NF, Abd El-Rahman SS, Emara M, Zaki HF, Khattab MM. Stimulation of ACE2/ANG(1–7)/Mas Axis by Diminazene Ameliorates Alzheimer’s Disease in the D-Galactose-Ovariectomized Rat Model: role of PI3K/Akt Pathway. Molecular Neurobiology. 2018;55(10):8188–8202. doi: 10.1007/s12035-018-0966-3 [DOI] [PubMed] [Google Scholar]
- 269.Evans CE, Miners JS, Piva G, et al. ACE2 activation protects against cognitive decline and reduces amyloid pathology in the Tg2576 mouse model of Alzheimer’s disease. Acta Neuropathologica. 2020;139(3):485–502. doi: 10.1007/s00401-019-02098-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zheng J, Wittouck S, Salvetti E, et al. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 2020;70(4):2782–2858. doi: 10.1099/ijsem.0.004107 [DOI] [PubMed] [Google Scholar]
- 271.Salvetti E, Harris HMB, Felis GE, O’Toole PW, Björkroth J. Comparative genomics of the genus Lactobacillus reveals robust phylogroups that provide the basis for reclassification. Appl Environ Microbiol. 2018;84(17):e00993–00918. doi: 10.1128/aem.00993-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zheng J, Ruan L, Sun M, Gänzle M, Björkroth J. A genomic view of lactobacilli and pediococci demonstrates that phylogeny matches ecology and physiology. Appl Environ Microbiol. 2015;81(20):7233–7243. doi: 10.1128/aem.02116-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sun Z, Harris HMB, McCann A, et al. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nature Communications. 2015;6(1):8322. doi: 10.1038/ncomms9322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Duar RM, Lin XB, Zheng J, et al. Lifestyles in transition: evolution and natural history of the genus Lactobacillus. FEMS Microbiology Reviews. 2017;41(1):27–48. doi: 10.1093/femsre/fux030 [DOI] [PubMed] [Google Scholar]
- 275.Martino ME, Bayjanov JR, Caffrey BE, et al. Nomadic lifestyle of Lactobacillus plantarum revealed by comparative genomics of 54 strains isolated from different habitats. Environmental Microbiology. 2016;18(12):4974–4989. doi: 10.1111/1462-2920.13455 [DOI] [PubMed] [Google Scholar]
- 276.Rossi M, Martinez-Martinez D, Amaretti A, Ulrici A, Raimondi S, Moya A. Mining metagenomic whole genome sequences revealed subdominant but constant Lactobacillus population in the human gut microbiota. Environmental Microbiology Reports. 2016;8(3):399–406. doi: 10.1111/1758-2229.12405 [DOI] [PubMed] [Google Scholar]
- 277.Zhang Z, Lv J, Pan L, Zhang Y. Roles and applications of probiotic Lactobacillus strains. Appl Microbiol Biotechnol. 2018;102(19):8135–8143. doi: 10.1007/s00253-018-9217-9 [DOI] [PubMed] [Google Scholar]
- 278.Achuthan AA, Duary RK, Madathil A, et al. Antioxidative potential of lactobacilli isolated from the gut of Indian people. Mol Biol Rep. 2012;39(8):7887–7897. doi: 10.1007/s11033-012-1633-9 [DOI] [PubMed] [Google Scholar]
- 279.Mishra V, Shah C, Mokashe N, Chavan R, Yadav H, Prajapati J. Probiotics as potential antioxidants: a systematic review. J. Agric. Food Chem. 2015;63(14):3615–3626. doi: 10.1021/jf506326t [DOI] [PubMed] [Google Scholar]
- 280.Kleniewska P, Hoffmann A, Pniewska E, Pawliczak R. The influence of probiotic Lactobacillus casei in combination with prebiotic inulin on the antioxidant capacity of human plasma. Oxid. Med. Cell. Longev. 2016;2016:1–10. doi: 10.1155/2016/1340903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Westfall S, Lomis N, Prakash S. Ferulic acid produced by Lactobacillus fermentum influences developmental growth through a dTOR-mediated mechanism. Mol Biotechnol. 2018;61(1):1–11. doi: 10.1007/s12033-018-0119-y [DOI] [PubMed] [Google Scholar]
- 282.Fung TC. The microbiota-immune axis as a central mediator of gut-brain communication. Neurobiol Dis. 2020;136:104714. doi: 10.1016/j.nbd.2019.104714 [DOI] [PubMed] [Google Scholar]
- 283.Danilenko VN, Marsova MV, Poluektova EU. The use of cells of the strain Lactobacillus fermentum U-21 and biologically active substances obtained from them. Patent No. RU2019141103A by 11.06.2021. 2021. 1–26.
- 284.Bhandari P, Rishi P, Prabha V. Positive effect of probiotic Lactobacillus plantarum in reversing LPS-induced infertility in a mouse model. J Med Microbiol. 2016;65(5):345–350. doi: 10.1099/jmm.0.000230 [DOI] [PubMed] [Google Scholar]
- 285.Grompone G, Martorell P, Llopis S, et al. Anti-inflammatory Lactobacillus rhamnosus CNCM I-3690 strain protects against oxidative stress and increases lifespan in Caenorhabditis elegans. PLoS One. 2012;7(12):e52493. doi: 10.1371/journal.pone.0052493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Lee J, Yang W, Hostetler A, et al. Characterization of the anti-inflammatory Lactobacillus reuteri BM36301 and its probiotic benefits on aged mice. BMC Microbiol. 2016;16:69. doi: 10.1186/s12866-016-0686-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Choi SS, Kim Y, Han KS, You S, Oh S, Kim SH. Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro. Lett Appl Microbiol. 2006;42(5):452–458. doi: 10.1111/j.1472-765X.2006.01913.x [DOI] [PubMed] [Google Scholar]
- 288.Noureen S, Riaz A, Arshad M, Arshad N. In vitro selection and in vivo confirmation of the antioxidant ability of Lactobacillus brevis MG000874. J Appl Microbiol. 2019;126(4):1221–1232. doi: 10.1111/jam.14189 [DOI] [PubMed] [Google Scholar]
- 289.Marsova M, Poluektova E, Odorskaya M, et al. Protective effects of Lactobacillus fermentum U-21 against paraquat-induced oxidative stress in Caenorhabditis elegans and mouse models. World J Microbiol Biotechnol. 2020a;36(7):104. doi: 10.1007/s11274-020-02879-2 [DOI] [PubMed] [Google Scholar]
- 290.Marsova M, Odorskaya M, Novichkova M, et al. The Lactobacillus brevis 47 f Strain Protects the Murine Intestine from Enteropathy Induced by 5-Fluorouracil. Microorganisms. 2020b;8(6):876. doi: 10.3390/microorganisms8060876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Miraghajani M, Zaghian N, Mirlohi M, Feizi A, Ghiasvand R. The Impact of Probiotic Soy Milk Consumption on Oxidative Stress Among Type 2 Diabetic Kidney Disease Patients: a Randomized Controlled Clinical Trial. J. Ren. Nutr. 2017;27(5):317–324. doi: 10.1053/j.jrn.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 292.Soleimani A, Zarrati Mojarrad M, Bahmani F, et al. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 2017;91(2):435–442. doi: 10.1016/j.kint.2016.09.040 [DOI] [PubMed] [Google Scholar]
- 293.Amaretti A, Nunzio M, Pompei A, Raimondi S, Rossi M, Bordoni A. Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Appl Microbiol Biotechnol. 2012;97(2):809–817. doi: 10.1007/s00253-012-4241-7 [DOI] [PubMed] [Google Scholar]
- 294.Ho L, Ono K, Tsuji M, Mazzola P, Singh R, Pasinetti GM. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev. Neurother. 2017;18(1):83–90. doi: 10.1080/14737175.2018.1400909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Nowak A, Paliwoda A, Błasiak J. Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: a review of mechanisms and therapeutic perspectives. Crit. Rev. Food Sci. Nutr. 2018;59(21):3456–3467. doi: 10.1080/10408398.2018.1494539 [DOI] [PubMed] [Google Scholar]
- 296.Attia HN, Maklad YA. Neuroprotective effects of coenzyme Q10 on paraquat-induced Parkinson’s disease in experimental animals. Behav. Pharmacol. 2018;29(1):79–86. doi: 10.1097/fbp.0000000000000342 [DOI] [PubMed] [Google Scholar]
- 297.Flanagan E, Müller M, Hornberger M, Vauzour D. Impact of flavonoids on cellular and molecular mechanisms underlying age-related cognitive decline and neurodegeneration. Curr Nutr Rep. 2018;7(2):49–57. doi: 10.1007/s13668-018-0226-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Haddadi R, Nayebi AM, Eyvari Brooshghalan S. Silymarin prevents apoptosis through inhibiting the Bax/caspase-3 expression and suppresses toll like receptor-4 pathway in the SNc of 6-OHDA intoxicated rats. Biomed. Pharmacother. 2018;104:127–136. doi: 10.1016/j.biopha.2018.05.020 [DOI] [PubMed] [Google Scholar]
- 299.Zhang L, Zhang L, Li L, Hölscher C. Neuroprotective effects of the novel GLP-1 long acting analogue semaglutide in the MPTP Parkinson’s disease mouse model. Neuropeptides. 2018;71:70–80. doi: 10.1016/j.npep.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 300.Ganji-Arjenaki M, Rafieian-Kopaei M. Probiotics are a good choice in remission of inflammatory bowel diseases: a meta analysis and systematic review. J Cell Physiol. 2018;233(3):2091–2103. doi: 10.1002/jcp.25911 [DOI] [PubMed] [Google Scholar]
- 301.Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–1270. doi: 10.1016/j.cell.2012.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience. 2012;13(10):701–712. doi: 10.1038/nrn3346 [DOI] [PubMed] [Google Scholar]
- 303.Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat. Immunol. 2010;12(1):5–9. doi: 10.1038/ni0111-5 [DOI] [PubMed] [Google Scholar]
- 304.Perez-Pardo P, Kliest T, Dodiya HB, et al. The gut-brain axis in Parkinson’s disease: possibilities for food-based therapies. Eur J Pharmacol. 2017;817:86–95. doi: 10.1016/j.ejphar.2017.05.042 [DOI] [PubMed] [Google Scholar]
- 305.Marsland BJ, Trompette A, Gollwitzer ES. The gut-lung axis in respiratory disease. Ann Am Thorac Soc. 2015;12(2):150–156. doi: 10.1513/AnnalsATS.201503-133AW [DOI] [PubMed] [Google Scholar]
- 306.Dang AT, Marsland BJ. Microbes, metabolites, and the gut–lung axis. Mucosal Immunol. 2019;12(4):843–850. doi: 10.1038/s41385-019-0160-6 [DOI] [PubMed] [Google Scholar]
- 307.Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 2013;3(4):461–491. doi: 10.3233/jpd-130230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Rao M, Gershon MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastro Hepat. 2016;13(9):517–528. doi: 10.1038/nrgastro.2016.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Wang N, Song G, Yang Y, Yuan W, Qi M. Inactivated Lactobacillus promotes protection against myocardial ischemia–reperfusion injury through NF-κB pathway. Biosci Rep. 2017;37(6):BSR20171025. doi: 10.1042/bsr20171025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Sun M-F, Zhu Y-L, Zhou Z-L, et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain, Behav., Immun. 2018;70:48–60. doi: 10.1016/j.bbi.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 311.Wanchao S, Chen M, Zhiguo S, Futang X, Mengmeng S. Protective effect and mechanism of Lactobacillus on cerebral ischemia reperfusion injury in rats. Braz J Med Biol Res. 2018;51(7):e7172. doi: 10.1590/1414-431x20187172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Sign. 2011;15(6):1583–1606. doi: 10.1089/ars.2011.3999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Miriyala S, Spasojevic I, Tovmasyan A, et al. Manganese superoxide dismutase, MnSOD and its mimics. Biochim Biophys Acta. 2012;1822(5):794–814. doi: 10.1016/j.bbadis.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Calder PC, Albers R, Antoine JM, et al. Inflammatory disease processes and interactions with nutrition. Br J Nutr. 2009;101(S1):1–45. doi: 10.1017/s0007114509377867 [DOI] [PubMed] [Google Scholar]
- 315.Ramalho R, Rao M, Zhang C, et al. Immunometabolism: new insights and lessons from antigen-directed cellular immune responses. Semin Immunopathol. 2020;42(3):279–313. doi: 10.1007/s00281-020-00798-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Xue J, Ajuwon KM, Fang R. Mechanistic insight into the gut microbiome and its interaction with host immunity and inflammation. Anim Nutr. 2020;6(4):421–428. doi: 10.1016/j.aninu.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Schubert M-L, Rohrbach R, Schmitt M, Stein-Thoeringer CK. The potential role of the intestinal micromilieu and individual microbes in the immunobiology of chimeric antigen receptor T-cell therapy. Front Immunol. 2021;12:1836. doi: 10.3389/fimmu.2021.670286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Xing C, Wang M, Ajibade AA, et al. Microbiota regulate innate immune signaling and protective immunity against cancer. Cell Host Microbe. 2021;29(6):959–974. doi: 10.1016/j.chom.2021.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Averina OV, Danilenko VN. Human intestinal microbiota: role in development and functioning of the nervous system. Microbiology. 2017;86(1):1–18. doi: 10.1134/S0026261717010040 [DOI] [PubMed] [Google Scholar]
- 320.Bhattarai Y. Microbiota-gut-brain axis: interaction of gut microbes and their metabolites with host epithelial barriers. Neurogastroenterol Motil. 2018;30(6):e13366. doi: 10.1111/nmo.13366 [DOI] [PubMed] [Google Scholar]
- 321.Kerksick C, Willoughby D. The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J Int Soc Sports Nutr. 2005;2(2):38. doi: 10.1186/1550-2783-2-2-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Jung K-A, Kwak M-K. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules. 2010;15(10):7266–7291. doi: 10.3390/molecules15107266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Kullisaar T, Songisepp E, Aunapuu M, et al. Complete glutathione system in probiotic Lactobacillus fermentum ME-3. Appl Biochem Microbiol. 2010;46(5):527–531. doi: 10.1134/S0003683810050030 [DOI] [PubMed] [Google Scholar]
- 324.Falony G, Joossens M, Vieira-Silva S, et al. Population-level analysis of gut microbiome variation. Science. 2016;352(6285):560–564. doi: 10.1126/science.aad3503 [DOI] [PubMed] [Google Scholar]
- 325.Tang W, Xing Z, Li C, Wang J, Wang Y. Molecular mechanisms and in vitro antioxidant effects of Lactobacillus plantarum MA2. Food Chem. 2017;221:1642–1649. doi: 10.1016/j.foodchem.2016.10.124 [DOI] [PubMed] [Google Scholar]
- 326.Main PAE, Angley MT, O’Doherty CE, Thomas P, Fenech M. The potential role of the antioxidant and detoxification properties of glutathione in autism spectrum disorders: a systematic review and meta-analysis. Nutr. Metab. (Lond. 2012;9(1):35. doi: 10.1186/1743-7075-9-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Pophaly SD, Singh R, Pophaly SD, Kaushik JK, Tomar SK. Current status and emerging role of glutathione in food grade lactic acid bacteria. Microb Cell Fact. 2012;11(1):114. doi: 10.1186/1475-2859-11-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Lin J, Zou Y, Cao K, Ma C, Chen Z. The impact of heterologous catalase expression and superoxide dismutase overexpression on enhancing the oxidative resistance in Lactobacillus casei. J Ind Microbiol Biotechnol. 2016;43(5):703–711. doi: 10.1007/s10295-016-1752-8 [DOI] [PubMed] [Google Scholar]
- 329.García-Giménez JL, Ibañez-Cabellos JS, Seco-Cervera M, Pallardó F. S1-1 - glutathione and cellular redox control in epigenetic regulation. Free Radic. Biol. Med. 2014;75:3. doi: 10.1016/j.freeradbiomed.2014.10.828 [DOI] [PubMed] [Google Scholar]
- 330.Laiño J, Villena J, Kanmani P, Kitazawa H. Immunoregulatory effects triggered by lactic acid bacteria exopolysaccharides: new insights into molecular interactions with host cells. Microorganisms. 2016;4(3):27. doi: 10.3390/microorganisms4030027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Lyu C, Hu S, Huang J, et al. Contribution of the activated catalase to oxidative stress resistance and γ-aminobutyric acid production in Lactobacillus brevis. Int. J. Food Microbiol. 2016;238:302–310. doi: 10.1016/j.ijfoodmicro.2016.09.023 [DOI] [PubMed] [Google Scholar]
- 332.Mahdhi A, Leban N, Chakroun I, et al. Extracellular polysaccharide derived from potential probiotic strain with antioxidant and antibacterial activities as a prebiotic agent to control pathogenic bacterial biofilm formation. Microb. Pathog. 2017;109:214–220. doi: 10.1016/j.micpath.2017.05.046 [DOI] [PubMed] [Google Scholar]
- 333.Danilenko VN, Marsova MV, Poluektova EU, Odorskaya MV, Yunes RA. Lactobacillus fermentum U-21 strain, which produces complex of biologically active substances which neutralize superoxide anion induced by chemical agents. Patent No. 2705250 by 05.02.2018. 2018. 1–16.
- 334.Danilenko VN, Marsova MV, Poluektova EU. The use of cells of the Lactobacillus fermentum U-21 strain and biologically active substances obtained from them. Patent No. 2019141103/20 (080350) by 11.12.2019. 2019. 1–24.
- 335.Danilenko VN, Stavrovskaya AV, Voronkov D, et al. The use of a pharmabiotic based on the Lactobacillus fermentum U-21 strain to modulate the neurodegenerative process in an experimental model of Parkinson’s disease. Annals of Clinical and Experimental Neurology. 2020;14:62–69. doi: 10.25692/ACEN.2020.1.7 [DOI] [Google Scholar]
- 336.Scheperjans F, Aho V, Pereira PAB, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2014;30(3):350–358. doi: 10.1002/mds.26069 [DOI] [PubMed] [Google Scholar]
- 337.Petrov VA, Saltykova IV, Zhukova IA, et al. Analysis of gut microbiota in patients with Parkinson’s disease. Bull Exp Biol Med. 2017;162(6):734–737. doi: 10.1007/s10517-017-3700-7 [DOI] [PubMed] [Google Scholar]
- 338.Bedarf JR, Hildebrand F, Coelho LP, et al. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson’s disease patients. Genome Med. 2017;9(1):39. doi: 10.1186/s13073-017-0428-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Antunes AEC, Vinderola G, Xavier-Santos D, Sivieri K. Potential contribution of beneficial microbes to face the COVID-19 pandemic. Food Res. Int. 2020;136:109577. doi: 10.1016/j.foodres.2020.109577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Xiang Z, Koo H, Chen Q, Zhou X, Liu Y, Simon-Soro A. Potential implications of SARS-CoV-2 oral infection in the host microbiota. J. Oral Microbiol. 2020;13(1):1853451. doi: 10.1080/20002297.2020.1853451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Follmer C. Viral infection-induced gut dysbiosis, neuroinflammation, and alpha-synuclein aggregation: updates and perspectives on COVID-19 and neurodegenerative disorders. ACS Chem Neurosci. 2020;11(24):4012–4016. doi: 10.1021/acschemneuro.0c00671 [DOI] [PubMed] [Google Scholar]
- 342.Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas M-E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8(1):42. doi: 10.1186/s13073-016-0303-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Wang Z, Zhao Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell. 2018;9(5):416–431. doi: 10.1007/s13238-018-0549-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.De Luca F, Shoenfeld Y. The microbiome in autoimmune diseases. Clin Exp Immunol. 2019;195(1):74–85. doi: 10.1111/cei.13158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Xu H, Liu M, Cao J, et al. The dynamic interplay between the gut microbiota and autoimmune diseases. Journal of Immunology Research. 2019;2019:1–14. doi: 10.1155/2019/7546047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Goulet O. Potential role of the intestinal microbiota in programming health and disease: figure 1. Nutr Rev. 2015;73(\(suppl 1)):32–40. doi: 10.1093/nutrit/nuv039 [DOI] [PubMed] [Google Scholar]
- 347.Needell JC, Zipris D. The role of the intestinal microbiome in type 1 diabetes pathogenesis. Curr. Diab. Rep. 2016;16(10):89. doi: 10.1007/s11892-016-0781-z [DOI] [PubMed] [Google Scholar]
- 348.Biscetti F, Nardella E, Cecchini AL, Landolfi R, Flex A. The role of the microbiota in the diabetic peripheral artery disease. Mediators Inflamm. 2019;2019:1–16. doi: 10.1155/2019/4128682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Huang YJ, Marsland BJ, Bunyavanich S, et al. The microbiome in allergic disease: current understanding and future opportunities. J. Allergy Clin. Immunol. 2017;139(4):1099–1110. doi: 10.1016/j.jaci.2017.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Haikal C, Chen QQ, Li JY. Microbiome changes: an indicator of Parkinson’s disease? Transl Neurodegener. 2019;8:38. doi: 10.1186/s40035-019-0175-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Averina OV, Zorkina YA, Yunes RA, et al. Bacterial metabolites of human gut microbiota correlating with depression. Int J Mol Sci. 2020b;21(23):9234. doi: 10.3390/Ijms21239234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Du Y, Gao X-R, Peng L, Ge J-F. Crosstalk between the microbiota-gut-brain axis and depression. Heliyon. 2020;6(6):e04097. doi: 10.1016/j.heliyon.2020.e04097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Ding HT, Taur Y, Walkup JT. Gut microbiota and autism: key concepts and findings. J. Autism Dev. Disord. 2016;47(2):480–489. doi: 10.1007/s10803-016-2960-9 [DOI] [PubMed] [Google Scholar]
- 354.Chen -W-W, Zhang XIA, Huang W-J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol Med Report. 2016;13(4):3391–3396. doi: 10.3892/mmr.2016.4948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Siniscalco D, Schultz S, Brigida A, Antonucci N. Inflammation and neuro-immune dysregulations in autism spectrum disorders. Pharmaceuticals. 2018;11(2):56. doi: 10.3390/ph11020056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Prata J, Machado AS, von Doellinger O, et al. The contribution of inflammation to autism spectrum disorders: recent clinical evidence. Methods Mol Biol. 2019;2011:493–510. doi: 10.1007/978-1-4939-9554-7_29 [DOI] [PubMed] [Google Scholar]
- 357.Keshavarzian A, Green SJ, Engen PA, et al. Colonic bacterial composition in Parkinson’s disease. Mov Disord. 2015;30(10):1351–1360. doi: 10.1002/mds.26307 [DOI] [PubMed] [Google Scholar]
- 358.Pereira PAB, Aho VTE, Paulin L, Pekkonen E, Auvinen P, Scheperjans F. Oral and nasal microbiota in Parkinson’s disease. Parkinsonism Relat Disord. 2017;38:61–67. doi: 10.1016/j.parkreldis.2017.02.026 [DOI] [PubMed] [Google Scholar]
- 359.Heintz-Buschart A, Pandey U, Wicke T, et al. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord. 2018;33(1):88–98. doi: 10.1002/mds.27105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Minato T, Maeda T, Fujisawa Y, et al. Progression of Parkinson’s disease is associated with gut dysbiosis: two-year follow-up study. PLoS One. 2017;12(11):e0187307. doi: 10.1371/journal.pone.0187307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Hasegawa S, Goto S, Tsuji H, et al. Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson’s disease. Mov Disord. 2016;31:S40–S40. doi: 10.1371/journal.pone.0142164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Hopfner F, Kunstner A, Muller SH, et al. Gut microbiota in Parkinson disease in a northern German cohort. Brain Res. 2017;1667:41–45. doi: 10.1016/j.brainres.2017.04.019 [DOI] [PubMed] [Google Scholar]
- 363.Scheperjans F, Aho V, Pereira PAB, et al. Gut Microbiota Are Related to Parkinson’s Disease and Clinical Phenotype. Mov Disord. 2015;30(3):350–358. doi: 10.1002/mds.26069 [DOI] [PubMed] [Google Scholar]
- 364.Lin CH, Chen CC, Chiang HL, et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J Neuroinflammation. 2019;16(1):129. doi: 10.1186/s12974-019-1528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Aho VTE, Pereira PAB, Voutilainen S, et al. Gut microbiota in Parkinson’s disease: temporal stability and relations to disease progression. EBioMedicine. 2019;44:691–707. doi: 10.1016/j.ebiom.2019.05.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Hill-Burns EM, Debelius JW, Morton JT, et al. Parkinson’s Disease and Parkinson’s Disease Medications Have Distinct Signatures of the Gut Microbiome. Mov Disord. 2017;32(5):739–749. doi: 10.1002/mds.26942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Mihaila D, Donegan J, Barns S, et al. The oral microbiome of early stage Parkinson’s disease and its relationship with functional measures of motor and non-motor function. PLoS One. 2019;14(6):e0218252. doi: 10.1371/journal.pone.0218252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Lin AQ, Zheng WX, He Y, et al. Gut microbiota in patients with Parkinson’s disease in southern China. Parkinsonism Relat Disord. 2018;53:82–88. doi: 10.1016/j.parkreldis.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 369.Li W, Wu XL, Hu X, et al. Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Science China-Life Sciences. 2017;60(11):1223–1233. doi: 10.1007/s11427-016-9001-4 [DOI] [PubMed] [Google Scholar]
- 370.Perez-Pardo P, Dodiya HB, Engen PA, et al. Gut bacterial composition in a mouse model of Parkinson’s disease. Benef Microbes. 2018;9(5):799–814. doi: 10.3920/BM2017.0202 [DOI] [PubMed] [Google Scholar]
- 371.Pietrucci D, Cerroni R, Unida V, et al. Dysbiosis of gut microbiota in a selected population of Parkinson’s patients. Parkinsonism Relat Disord. 2019;65:124–130. doi: 10.1016/j.parkreldis.2019.06.003 [DOI] [PubMed] [Google Scholar]
- 372.Qian Y, Yang X, Xu S, et al. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain. Behav. Immun. 2018;70:194–202. doi: 10.1016/j.bbi.2018.02.016 [DOI] [PubMed] [Google Scholar]
- 373.Li F, Wang P, Chen Z, Sui X, Xie X, Zhang J. Alteration of the fecal microbiota in North-Eastern Han Chinese population with sporadic Parkinson’s disease. Neuroscience Letters. 2019;707:134297. doi: 10.1016/j.neulet.2019.134297 [DOI] [PubMed] [Google Scholar]
- 374.Ansaldo E, Slayden LC, Ching KL, et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science. 2019;364(6446):1179. doi: 10.1126/science.aaw7479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Zuo T, Zhang F, Lui GCY, et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology. 2020;159(3):944–955 e948. doi: 10.1053/j.gastro.2020.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Zuo T, Liu Q, Zhang F, et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2021;70(2):276–284. doi: 10.1136/gutjnl-2020-322294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Gu S, Chen Y, Wu Z, et al. Alterations of the Gut Microbiota in Patients With Coronavirus Disease 2019 or H1N1 Influenza. Clin Infect Dis. 2020;71(10):2669–2678. doi: 10.1093/cid/ciaa709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Tao W, Zhang G, Wang X, et al. Analysis of the intestinal microbiota in COVID-19 patients and its correlation with the inflammatory factor IL-18. Med Microecol. 2020;5:100023. doi: 10.1016/j.medmic.2020.100023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Yamamoto S, Saito M, Tamura A, Prawisuda D, Mizutani T, Yotsuyanagi H. The human microbiome and COVID-19: a systematic review. PLoS One. 2021;16(6):e0253293. doi: 10.1371/journal.pone.0253293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Brown SP, Cornforth DM, Mideo N. Evolution of virulence in opportunistic pathogens: generalism, plasticity, and control. Trends Microbiol. 2012;20(7):336–342. doi: 10.1016/j.tim.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Xu K, Cai H, Shen Y, et al. Management of COVID-19: the Zhejiang experience. Journal of Zhejiang University. Medical Sciences. 2020;49(2):147–157. doi: 10.3785/j.issn.1008-9292.2020.02.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Shen Z, Xiao Y, Kang L, et al. Genomic Diversity of Severe Acute Respiratory Syndrome-Coronavirus 2 in Patients With Coronavirus Disease 2019. Clin Infect Dis. 2020;71(15):713–720. doi: 10.1093/cid/ciaa203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.De Maio F, Posteraro B, Ponziani FR, Cattani P, Gasbarrini A, Sanguinetti M. Nasopharyngeal Microbiota Profiling of SARS-CoV-2 Infected Patients. Biological Procedures Online. 2020;22:18. doi: 10.1186/s12575-020-00131-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Elsayed S, Zhang K. Human infection caused by Clostridium hathewayi. Emerg Infect Dis. 2004;10(11):1950–1952. doi: 10.3201/eid1011.040006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Tamilselvi R, Dakshinamoorthy M, Venkatesh A, Arumugam K. A Literature Review on Dental Caries Vaccine-A Prevention Strategy. Indian Journal of Public Health Research and Development. 2019;10:3041. [Google Scholar]
- 386.Tang L, Gu S, Gong Y, et al. Clinical Significance of the Correlation between Changes in the Major Intestinal Bacteria Species and COVID-19 Severity. Engineering (Beijing, China). 2020;6(10):1178–1184. doi: 10.1016/j.eng.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Montefusco L, Ben Nasr M, D’Addio F, et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat Metab. 2021;3(6):774–785. doi: 10.1038/s42255-021-00407-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259–264. doi: 10.1038/s41586-021-03553-9 [DOI] [PubMed] [Google Scholar]
- 389.Weng J, Li Y, Li J, et al. Gastrointestinal sequelae 90 days after discharge for COVID-19. Lancet Gastroenterol Hepatol. 2021;6(5):344–346. doi: 10.1016/S2468-1253(21)00076-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Chen Y, Gu S, Chen Y, et al. Six-month follow-up of gut microbiota richness in patients with COVID-19. Gut. 2021;1:gutjnl-2021-324090. doi: 10.1136/gutjnl-2021-324090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Sokol H, Contreras V, Maisonnasse P, et al. SARS-CoV-2 infection in nonhuman primates alters the composition and functional activity of the gut microbiota. Gut Microbes. 2021;13(1):1–19. doi: 10.1080/19490976.2021.1893113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Yeoh YK, Zuo T, Lui GC, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70(4):698–706. doi: 10.1136/gutjnl-2020-323020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Poluektova E, Yunes R, Danilenko V. The putative antidepressant mechanisms of probiotic bacteria: relevant genes and proteins. Nutrients. 2021;13(5):1591. doi: 10.3390/nu13051591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Nezametdinova VZ, Yunes RA, Dukhinova MS, Alekseeva MG, Danilenko VN. The role of the PFNA operon in the recognition of host’s immune signals: prospects for the use of the FN3 protein in the treatment of COVID-19. Int J Mol Sci. 2021;22:9219. doi: 10.3390/ijms22179219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Belkina TV, Averina OV, Savenkova EV, Danilenko VN. Human intestinal microbiome and the immune system: the role of probiotics in shaping an immune system unsusceptible to COVID-19 infection. Biology Bulletin Reviews. 2021;11(4):523–539. doi: 10.1134/S2079086421040034 [DOI] [Google Scholar]
- 396.Suvarna V, Baviskar N. The clinical overview on natural immunopolysaccharides as an adjuvant therapy of cancer. Int J Pharm Sci Res. 2021;12(7):3521–3536. doi: 10.13040/ijpsr.0975-8232 [DOI] [Google Scholar]
- 397.Gentile F, Doneddu PE, Riva N, Nobile-Orazio E, Quattrini A. Diet, microbiota and brain health: unraveling the network intersecting metabolism and neurodegeneration. Int J Mol Sci. 2020;21(20):7471. doi: 10.3390/ijms21207471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nature Reviews Microbiology. 2020;19(1):55–71. doi: 10.1038/s41579-020-0433-9 [DOI] [PubMed] [Google Scholar]
- 399.Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Lynch SV, Phimister EG, Pedersen O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016;375(24):2369–2379. doi: 10.1056/NEJMra1600266 [DOI] [PubMed] [Google Scholar]
- 401.Almeida A, Mitchell AL, Boland M, et al. A new genomic blueprint of the human gut microbiota. Nature. 2019;568(7753):499–504. doi: 10.1038/s41586-019-0965-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7:e7502. doi: 10.7717/peerj.7502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Tyakht AV, Kostryukova ES, Popenko AS, et al. Human gut microbiota community structures in urban and rural populations in Russia. Nature Communications. 2013;4(1):2469. doi: 10.1038/ncomms3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Wilson AS, Koller KR, Ramaboli MC, et al. Diet and the human gut microbiome: an international review. Dig Dis Sci. 2020;65(3):723–740. doi: 10.1007/s10620-020-06112-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Aziz Q, Doré J, Emmanuel A, Guarner F, Quigley EMM. Gut microbiota and gastrointestinal health: current concepts and future directions. Neurogastroenterol Motil. 2013;25(1):4–15. doi: 10.1111/nmo.12046 [DOI] [PubMed] [Google Scholar]
- 406.Jandhyala SM. Role of the normal gut microbiota. World J Gastroenterol. 2015;21(29):8787. doi: 10.3748/wjg.v21.i29.8787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Krishnan S, Alden N, Lee K. Pathways and functions of gut microbiota metabolism impacting host physiology. Curr Opin Biotechnol. 2015;36:137–145. doi: 10.1016/j.copbio.2015.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65(2):330–339. doi: 10.1136/gutjnl-2015-309990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Adv. Nutr. 2019;10(suppl_1):49–66. doi: 10.1093/advances/nmy063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Blaak EE, Canfora EE, Theis S, et al. Short chain fatty acids in human gut and metabolic health. Benef Microbes. 2020;11(5):411–455. doi: 10.3920/bm2020.0057 [DOI] [PubMed] [Google Scholar]
- 411.Evans JM, Morris LS, Marchesi JR. The gut microbiome: the role of a virtual organ in the endocrinology of the host. J Endocrinol. 2013;218(3):37–47. doi: 10.1530/joe-13-0131 [DOI] [PubMed] [Google Scholar]
- 412.Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: gut microbiota: the neglected endocrine organ. Mol Endocrinol. 2014;28(8):1221–1238. doi: 10.1210/me.2014-1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Sharma L, Riva A. Intestinal barrier function in health and disease - any role of SARS-CoV-2? Microorganisms. 2020;8(11):1744. doi: 10.3390/microorganisms8111744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Yang Y, Huang W, Fan Y, Chen G-Q. Gastrointestinal microenvironment and the gut-lung axis in the immune responses of severe COVID-19. Front Mol Biosci. 2021;8:647508. doi: 10.3389/fmolb.2021.647508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Lopes RCSO, Balbino KP, Jorge MP, Ribeiro AQ, Martino HSD, Alfenas RCG. Modulation of intestinal microbiota, control of nitrogen products and inflammation by pre/probiotics in chronic kidney disease: a systematic review. Nutr. Hosp. 2018;35(3):722–730. doi: 10.20960/nh.1642 [DOI] [PubMed] [Google Scholar]
- 416.Plata C, Cruz C, Cervantes LG, Ramírez V. The gut microbiota and its relationship with chronic kidney disease. Int. Urol. Nephrol. 2019;51(12):2209–2226. doi: 10.1007/s11255-019-02291-2 [DOI] [PubMed] [Google Scholar]
- 417.Tao S, Tao S, Cheng Y, Liu J, Ma L, Fu P. Effects of probiotic supplements on the progression of chronic kidney disease: a meta‐analysis. Nephrology. 2019;24(11):1122–1130. doi: 10.1111/nep.13549 [DOI] [PubMed] [Google Scholar]
- 418.Fontana L, Plaza-Diaz J, Robles-Bolivar P, et al. Bifidobacterium breve CNCM I-4035, Lactobacillus paracasei CNCM I-4034 and Lactobacillus rhamnosus CNCM I-4036 modulate macrophage gene expression and ameliorate damage markers in the liver of Zucker-Lepr(fa/ fa) rats. Nutrients. 2021;13(1):202. doi: 10.3390/Nu13010202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Tunapong W, Apaijai N, Yasom S, et al. Chronic treatment with prebiotics, probiotics and synbiotics attenuated cardiac dysfunction by improving cardiac mitochondrial dysfunction in male obese insulin-resistant rats. Eur J Nutr. 2017;57(6):2091–2104. doi: 10.1007/s00394-017-1482-3 [DOI] [PubMed] [Google Scholar]
- 420.Bermudez-Humaran LG, Salinas E, Ortiz GG, Ramirez-Jirano LJ, Morales JA, Bitzer-Quintero OK. From probiotics to psychobiotics: live beneficial bacteria which act on the brain-gut axis. Nutrients. 2019;11(4):890. doi: 10.3390/Nu11040890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Lew L-C, Hor -Y-Y, Yusoff NAA, et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: a randomised, double-blind, placebo-controlled study. Clin. Nutr. 2019;38(5):2053–2064. doi: 10.1016/j.clnu.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 422.Ruiz-Gonzalez C, Roman P, Rueda-Ruzafa L, Rodriguez-Arrastia M, Cardona D. Effects of probiotics supplementation on dementia and cognitive impairment: a systematic review and meta-analysis of preclinical and clinical studies. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2021;108:110189. doi: 10.1016/j.pnpbp.2020.110189 [DOI] [PubMed] [Google Scholar]
- 423.Toral M, Romero M, Rodríguez-Nogales A, et al. Lactobacillus fermentum improves tacrolimus-induced hypertension by restoring vascular redox state and improving eNOS coupling. Mol. Nutr. Food Res. 2018;62(14):1800033. doi: 10.1002/mnfr.201800033 [DOI] [PubMed] [Google Scholar]
- 424.Toral M, Gómez-Guzmán M, Jiménez R, et al. The probiotic Lactobacillus coryniformis CECT5711 reduces the vascular pro-oxidant and pro-inflammatory status in obese mice. Clin. Sci. 2014;127(1):33–45. doi: 10.1042/cs20130339 [DOI] [PubMed] [Google Scholar]
- 425.Mogotsi MT, Mwangi PN, Bester PA, et al. Metagenomic analysis of the enteric RNA virome of infants from the Oukasie clinic, North West province, South Africa, reveals diverse eukaryotic viruses. Viruses. 2020;12(11):1260. doi: 10.3390/v12111260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Gregory AC, Zablocki O, Zayed AA, Howell A, Bolduc B, Sullivan MB. The gut virome database reveals age-dependent patterns of virome diversity in the human gut. Cell Host Microbe. 2020;28(5):724–740. doi: 10.1016/j.chom.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Hidalgo-Cantabrana C, Sanozky-Dawes R, Barrangou R. Insights into the human virome using CRISPR spacers from microbiomes. Viruses. 2018;10(9):479. doi: 10.3390/v10090479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Burmistrz M, Krakowski K, Krawczyk-Balska A. RNA-targeting CRISPR–Cas systems and their applications. Int J Mol Sci. 2020;21(3):1122. doi: 10.3390/ijms21031122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Toyofuku M, Nomura N, Eberl L. Types and origins of bacterial membrane vesicles. Nature Reviews Microbiology. 2018;17(1):13–24. doi: 10.1038/s41579-018-0112-2 [DOI] [PubMed] [Google Scholar]
- 430.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi: 10.1126/science.aau6977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Macia L, Nanan R, Hosseini-Beheshti E, Grau GE. Host- and microbiota-derived extracellular vesicles, immune function, and disease development. Int J Mol Sci. 2019;21(1):107. doi: 10.3390/ijms21010107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine. 2020;15:6917–6934. doi: 10.2147/ijn.s264498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Taghinezhad SS, Mohseni AH, Bermudez-Humaran LG, et al. Probiotic-based vaccines may provide effective protection against COVID-19 acute respiratory disease. Vaccines (Basel). 2021;9(5):466. doi: 10.3390/vaccines9050466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Dyakov IN, Mavletova DA, Chernyshova IN, et al. FN3 protein fragment containing two type III fibronectin domains from B. longum GT15 binds to human tumor necrosis factor alpha in vitro. Anaerobe. 2020;65:102247. doi: 10.1016/j.anaerobe.2020.102247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Veselovsky VA, Dyachkova MS, Menyaylo EA, et al. Gene networks underlying the resistance of Bifidobacterium longum to inflammatory factors. Front Immunol. 2020;11:595877. doi: 10.3389/fimmu.2020.595877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Hmood KA, Habeeb AH, Al-Mhnna KI. Antioxidant role of Lactobacillus sp isolated from honey bee against histological effects of ochratoxin in vivo. Al-Kufa University Journal for Biology. 2019;11(2):67–80. [Google Scholar]
- 437.Kešnerová L, Emery O, Troilo M, Liberti J, Erkosar B, Engel P. Gut microbiota structure differs between honeybees in winter and summer. ISME J. 2020;14(3):801–814. doi: 10.1038/s41396-019-0568-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Ignasiak K, Maxwell A. Antibiotic-resistant bacteria in the guts of insects feeding on plants: prospects for discovering plant-derived antibiotics. BMC Microbiol. 2017;17(1):223. doi: 10.1186/s12866-017-1133-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Ellegaard KM, Engel P. Genomic diversity landscape of the honey bee gut microbiota. Nature Communications. 2019;10(1):446. doi: 10.1038/s41467-019-08303-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Iorizzo M, Testa B, Ganassi S, et al. Probiotic properties and potentiality of Lactiplantibacillus plantarum strains for the biological control of chalkbrood disease. Journal of Fungi. 2021;7(5):379. doi: 10.3390/jof7050379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Pan D, Mei X. Antioxidant activity of an exopolysaccharide purified from Lactococcus lactis subsp. lactis 12. Carbohydr. Polym. 2010;80(3):908–914. doi: 10.1016/j.carbpol.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 442.D’Alvise P, Böhme F, Codrea MC, et al. The impact of winter feed type on intestinal microbiota and parasites in honey bees. Apidologie. 2017;49(2):252–264. doi: 10.1007/s13592-017-0551-1 [DOI] [Google Scholar]
- 443.Wang X, Zhong Z, Chen X, et al. High-fat diets with differential fatty acids induce obesity and perturb gut microbiota in honey bee. Int J Mol Sci. 2021;22(2):834. doi: 10.3390/ijms22020834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Honey Chandran C, Keerthi TR. Probiotic potency of Lactobacillus plantarum KX519413 and KX519414 isolated from honey bee gut. FEMS Microbiol Lett. 2018;4(365):1–8. doi: 10.1093/femsle/fnx285 [DOI] [PubMed] [Google Scholar]
- 445.Kenfack HMC, Ngoufack ZF, Kaktcham MP, Wang RY, Zhu T, Yin L. Safety and antioxidant properties of five probiotic Lactobacillus plantarum strains isolated from the digestive tract of honey bees. American Journal of Microbiological Research. 2018;6(1):1–8. doi: 10.12691/ajmr-6-1-1 [DOI] [Google Scholar]
- 446.Niode N, Salaki C, Rumokoy L, Tallei T Lactic acid bacteria from honey bees digestive tract and their potential as probiotics. In: Buchori D, ed. International Conference and the 10th Congress of the Entomological Society of Indonesia. Series Advances in Biological Sciences Research. 8. Indonesia: Atlantis Press SARL; 2020:236–241. [Google Scholar]
- 447.Todorov SD, Tagg JR, Ivanova IV. Could Probiotics and Postbiotics Function as “Silver Bullet” in the Post-COVID-19 Era? Probiotics and Antimicrobial Proteins. 2021;1:1–9. doi: 10.1007/s12602-021-09833-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Salminen S, Collado MC, Endo A, et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021;18(9):649–667. doi: 10.1038/s41575-021-00440-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Xu C, Qiao L, Guo Y, Ma L, Cheng Y. Preparation, characteristics and antioxidant activity of polysaccharides and proteins-capped selenium nanoparticles synthesized by Lactobacillus casei ATCC 393. Carbohydr. Polym. 2018;195:576–585. doi: 10.1016/j.carbpol.2018.04.110 [DOI] [PubMed] [Google Scholar]
- 450.Molina-Tijeras JA, Galvez J, Rodriguez-Cabezas ME. The Immunomodulatory Properties of Extracellular Vesicles Derived from Probiotics: a Novel Approach for the Management of Gastrointestinal Diseases. Nutrients. 2019;11(5):1038. doi: 10.3390/nu11051038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Nishiyama K, Takaki T, Sugiyama M, et al. Extracellular Vesicles Produced by Bifidobacterium longum Export Mucin-Binding Proteins. Appl Environ Microbiol. 2020;86(19):e01464–01420. doi: 10.1128/AEM.01464-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Dixit Y, Wagle A, Vakil B. Patents in the Field of Probiotics, Prebiotics, Synbiotics: a Review., 01(02). Journal of Food: Microbiology, Safety & Hygiene. 2016;01(02):1000111. doi: 10.4172/2476-2059.1000111 [DOI] [Google Scholar]
- 453.Rani A, Saini KC, Bast F, et al. Microorganisms: a Potential Source of Bioactive Molecules for Antioxidant Applications. Molecules. 2021;26(4):1142. doi: 10.3390/molecules26041142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Sleator RD, Hill C. Engineered pharmabiotics with improved therapeutic potential. Human vaccines. 2008;4(4):271–274. doi: 10.4161/hv.4.4.6315 [DOI] [PubMed] [Google Scholar]
- 455.Sleator RD, Hill C. Rational design of improved pharmabiotics. J Biomed Biotechnol. 2009;2009:275287. doi: 10.1155/2009/275287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Shanahan F, Collins SM. Pharmabiotic manipulation of the microbiota in gastrointestinal disorders, from rationale to reality. Gastroenterol Clin North Am. 2010;39(3):721–726. doi: 10.1016/j.gtc.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 457.Maria Remes Troche J, Coss Adame E, Angel Valdovinos Diaz M, et al. Lactobacillus acidophilus LB: a useful pharmabiotic for the treatment of digestive disorders. Therap Adv Gastroenterol. 2020;13:1756284820971201. doi: 10.1177/1756284820971201 [DOI] [PMC free article] [PubMed] [Google Scholar]