Abstract
Purpose
To explore the esophageal cancer (EC) incidence and mortality trends and risk factors in China during 2005–2015.
Materials and Methods
The data were stratified by area (urban, rural), gender (male, female), and age groups (0 ~, 5 ~, …, 85 ~). The age-standardized incidence rate (ASIR) and mortality rate (ASMR), age-specific incidence and mortality were calculated to describe the trends, which were analyzed by Joinpoint software, negative binomial regression model, and age-period-cohort model.
Results
Trends in EC ASIR decreased markedly during 2010–2015 (APC=−6.14%, P<0.05), and the average annual percent change (AAPC) value was −8.07% (95% confidence interval (CI): −9.98~−6.12) for rural areas during 2005–2015. The ASMR was on a fast-downward trend after 2011 (APC=−6.67%, P<0.05), with AAPC values of −1.34% (95% CI: −2.56~−0.19) for males, −3.39% (95% CI: −5.65, −1.07) for females, and −9.67% (95% CI: −10.56~−8.77) for rural areas during 2005–2015. The age-specific incidence and mortality increased with age. The risk of EC for males was 3.1675 times higher than females (P<0.001), and for urban areas, it was 0.58 times larger than rural (P<0.001). The age and period effects presented an increasing trend, with a decreasing trend for the cohort effects in incidence and mortality risk. Later birth cohorts presented lower risks than previous birth cohorts.
Conclusion
ASIR and ASMR in China are higher in males than females, and higher in rural than urban areas, which have decreased during 2005–2015, especially in rural areas. The incidence increased with age up to the peak age group of 75. Area, gender, and age were independent risk factors for EC incidence.
Keywords: esophageal cancer, junction regression, negative binomial regression model, age-period-cohort analyses
Introduction
Esophageal cancer (EC) is one of the most common cancers in the world. Its incidence and mortality respectively ranked 7th and 6th worldwide with an estimated 572,000 new cases and 509,000 deaths in 2018.1 Esophageal cancer varies greatly by gender and area. The incidence of esophageal cancer in males is 2–4 times higher than in females. The countries located in the Asian esophageal cancer belt are the high-incidence areas,2,3 while the incidence is relatively low in America and most of the European countries. EC is most common in Eastern Asia which has the highest incidence and mortality.4
China, as a high-incidence area of esophageal cancer, accounts for 53% of esophageal squamous cell carcinoma (ESCC) cases worldwide,5 the incidence and mortality of esophageal cancer are significantly higher than the global average. There are also significant differences in this disease between urban and rural areas. The incidence is 2 times higher in rural compared to urban areas.6 And the high-risk areas of China have the highest incidence in the world, such as Linxian, Cixian and Shexian, which surround the Taihang Mountains in North Central China.7 EC is one of the most malignant tumors in China. It is estimated that there were 307,000 new cases and 283,000 deaths in 2018, accounting for 53.7% and 55.7%.1,4 In recent years, according to the national cancer center, from 2000 to 2014, the incidence of esophageal cancer in China tumor registration area decreased by an average of 4.1% per year, and the mortality decreased by an average of 4.6%.8,9 Although its incidence and mortality are declining, it still poses a serious threat to people’s lives and health. It has become a large burden in China.
Although China began to pay attention to the diagnosis and treatment of esophageal cancer a long time ago, due to the lack of early typical clinical symptoms, 90% of patients are diagnosed at the middle and advanced stage, and the 5-year survival rate is less than 20% in many countries. Relevant studies10 have proven that, in early stages, it is possible to reduce the incidence and mortality by identifying interventional risk factors, reducing the exposure to these factors, and strengthening the screening of precancerous lesions and high-risk population, todetect early cases, and give patients early treatment. Esophageal cancer is often diagnosed during advanced stages, and patients are often treated with concurrent chemoradiotherapy (CCRT), so identifying susceptible gene and biomarkers which can help in predicting the treatment response of patients while improving their survival rates.11
Currently, as the survival time of esophageal cancer patients is gradually prolonged and the aging process of the population is intensified, China is faced with a heavy burden of esophageal cancer. In 2017, cancer caused approximately 62.9 million disability-adjusted life years (DALYs), and esophageal cancer had the fourth highest contribution (7.1%) to the cancer DALY burden. But the age-standardized DALY of esophageal cancer decreased by 50.1% over the last 27 years.12 The main cause of the burden of esophageal cancer is early death,13 so screening and other measures should be used to reduce the burden of disease.
Recently, existing studies have adopted relevant analysis to assess trends of esophageal cancer in some cities and regions of China,14,15 but there are not enough studies on the incidence and mortality trends of esophageal cancer in the whole of China using Joinpoint regression. To further describe the analysis of esophageal cancer incidence and mortality of the country’s distribution characteristics, change tendency of influencing factors, this study used Joinpoint software to analyze the incidence and mortality trend of esophageal cancer in 2005–2014 and also used negative binomial regression model to investigate the risk factors associated with incidence, by utilizing the age-period-cohort analyses to elucidate the effects of incidence and mortality of esophageal cancer.
Materials and Methods
Data Sources and Quality Control
The incidence and mortality data of esophageal cancer were obtained from the Chinese Cancer Registry Annual Report from 2005 to 2015 (ICD-10 code as C15). The Annual Report on Cancer Registration in China controls the quality of cancer registration data according to the International Agency for Research on Cancer/International Cancer Registry (IARC/IACR) Guidelines on Cancer Registration in China and Volume IX of Cancer Incidence on Five Continents. The validity, reliability, completeness, and comparability of tumor registration data were evaluated based on a series of indicators such as mortality/incidence (M/I), the proportion of microscopic verification (MV%), and the proportion of death certificate only (DCO%).6,16
The corresponding author can be contacted for data access. This study did not require review and approval by an institutional review board or ethics committee. The reason for exemption from approval is that this study was data analysis based on domestic public data, which is a secondary analysis of data. Therefore, this research did not involve any investigation or experiment.
Statistical Analysis
The incidence and mortality data of esophageal cancer in China from 2005 to 2014 were collected and analyzed by using Excel 2010 and were stratified by area (urban, rural), gender (male, female), and age group (0~, 5~, 10~ …, 85~). The indicators in this study included age-standardized incidence rate (ASIR), the age-standardized mortality rate (ASMR), age-specific incidence, age-specific mortality, and other indicators (per 100,000 population), and the standardized was calculated using the population in the sixth national census in 2010 as the standard population.
In this study, we use the junction regression model, which is a set of linear statistical models proposed by the National Cancer Institute of the United States in 2000 to describe the incidence and mortality trends of esophageal cancer in China from 2005 to 2015. Joinpoint Regression Program (Version 4.7.0.0, 2019.2) software developed by the National Cancer Institute was adopted to analyze our data. We described the changing trend of esophageal cancer, and compared and analyzed the changing trend to determine whether the inflection point was statistically significant (P < 0.05), analyzed the annual percentage change (APC) which represents the rate of change in cancer incidence per year at different times and the interval average annual percentage change (AAPC) and their 95% confidence interval, thus we analyzed the reasons of the inflection point and influence factor.17,18
Negative binomial regression model is used to analyze the space inside a rare event of the unit of time, area and the reasons. Negative binomial regression first-order model is: 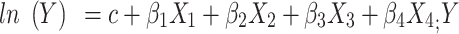 : incidence rate; X1: area (urban/rural); X2: gender (male/female); X3: age (20~, 25~, …,85~); X4: year (2005–2015). The OR and its calculation method are as follows:
: incidence rate; X1: area (urban/rural); X2: gender (male/female); X3: age (20~, 25~, …,85~); X4: year (2005–2015). The OR and its calculation method are as follows: 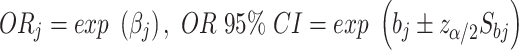 , regression coefficient βj (j = 1, 2, 3, 4) shows the changing amount of ln (Y) when independent variable Xj changing, βj is the parameter estimates, z is a standard normal variable, Sbj is the standard error of parameter estimation. In this study, the incidence of the low age group was extremely low, so we only discussed the incidence risk factor for the 20~85+ age group.
, regression coefficient βj (j = 1, 2, 3, 4) shows the changing amount of ln (Y) when independent variable Xj changing, βj is the parameter estimates, z is a standard normal variable, Sbj is the standard error of parameter estimation. In this study, the incidence of the low age group was extremely low, so we only discussed the incidence risk factor for the 20~85+ age group.
To further elucidate the trend in incidence and mortality of esophageal cancer, age-period-cohort analyses were used to reflect the relative risk of esophageal cancer incidence and mortality through measuring the age, period, and cohort effects. The formulation of the multiplicative age-period-cohort model for rates, (a, p) at age a in period p for persons in cohort 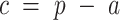 , is:
, is: 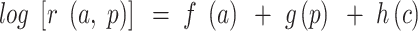 , where the additive effects can be partitioned into linear and nonlinear components.14,17,19 In our study, per 5-year can be an age group so that there were 14 successive age groups (20~,25~,30~, …,85~), continuous 5-year periods (2005, 2010, 2015), and corresponding sequential 5-year birth cohort groups (1920, 1925, …, 1995).
, where the additive effects can be partitioned into linear and nonlinear components.14,17,19 In our study, per 5-year can be an age group so that there were 14 successive age groups (20~,25~,30~, …,85~), continuous 5-year periods (2005, 2010, 2015), and corresponding sequential 5-year birth cohort groups (1920, 1925, …, 1995).
Results
Trends in the Age-Standardized Incidence Rate of Esophageal Cancer in Different Genders
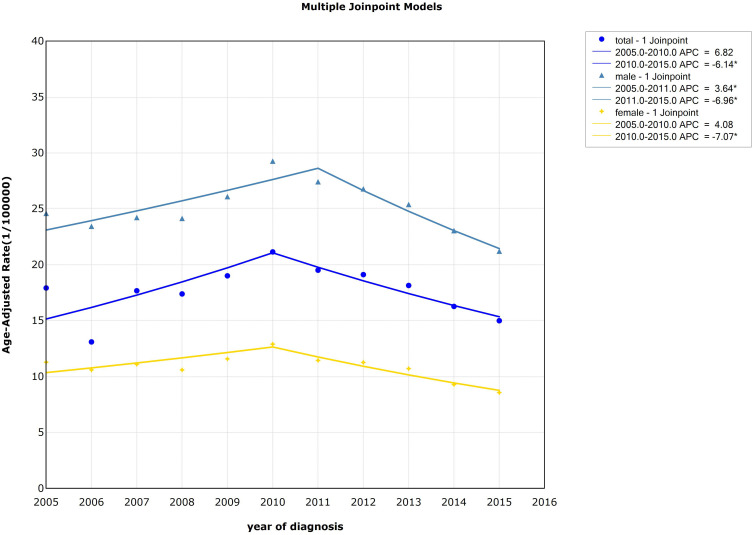
Joinpoint regression model was used to fit the variation trend of age-standardized incidence rate (ASIR) of esophageal cancer across the country, as shown in Figure 1. For males, the incidence of esophageal cancer increased from 2005 to 2011 (APC=3.64%, P<0.05) and decreased from 2011 to 2015 (APC=−6.96%, P<0.05). For females, the incidence decreased rapidly from 2010 to 2015 (APC=−7.07%, P<0.05). Detailed information can be seen in Table 1 and Figure 1.
Figure 1.
Trends in the age-standardized incidence rate of esophageal cancer by gender, 2005–2015, China (*Represents statistical significance).
Table 1.
Trends in Esophageal Cancer Incidence and Mortality Rates by Gender in China, 2005–2015
| Gender | Year | APC (95% CI) | t | P | AAPC (95% CI) | t | P |
|---|---|---|---|---|---|---|---|
| ASIR | |||||||
| All | 2005–2010 | 6.82(−1.01~15.27) | 2.12 | 0.08 | 0.13 (−3.27~3.65) | 0.08 | 0.94 |
| 2010–2015 | −6.14* (−9.88~-2.24) | −3.81 | 0.01 | ||||
| Males | 2005–2011 | 3.64* (0.81~6.54) | 3.16 | 0.02 | −0.74 (−2.33~0.87) | −0.90 | 0.36 |
| 2011–2015 | −6.96* (−9.58~-4.27) | −6.19 | 0.00 | ||||
| Females | 2005–2010 | 4.08 (−0.61~8.99) | 2.12 | 0.08 | −1.65 (−3.71~0.45) | −1.54 | 0.12 |
| 2010–2015 | −7.07* (−9.45~-4.63) | −6.92 | 0.00 | ||||
| ASMR | |||||||
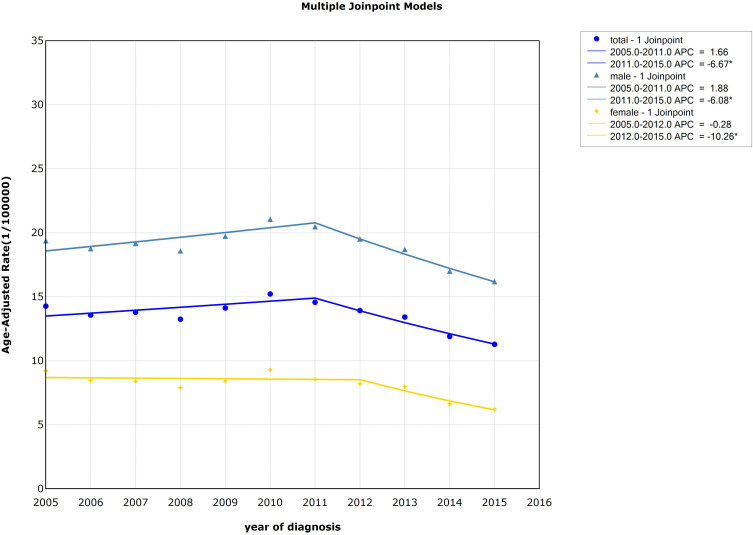
| All | 2005–2011 | 1.66 (−1.03~4.42) | 1.50 | 0.18 | −1.76* (−3.30~-0.19) | −2.20 | 0.03 |
| 2011–2015 | −6.67* (−9.30~-3.96) | −5.91 | 0.00 | ||||
| Males | 2005–2011 | 1.88 (−0.19~3.99) | 2.22 | 0.07 | −1.34* (−2.56~-0.19) | −2.27 | 0.02 |
| 2011–2015 | −6.08* (−8.07~-4.04) | −7.15 | 0.00 | ||||
| Females | 2005–2012 | −0.28 (−3.29~2.81) | −0.23 | 0.83 | −3.39* (−5.65~-1.07) | −2.85 | 0.00 |
| 2012–2015 | −10.26* (−16.16~-3.94) | −3.89 | 0.00 | ||||
Note: *P < 0.05, statistically significant trend.
Abbreviations: APC, annual percentage change; AAPC, average annual percentage change; CI, confidence interval; ASIR, age-standardized incidence rate; ASMR, age-standardized mortality rate.
Trends in the Age-Standardized Incidence Rate of Esophageal Cancer in Different Areas
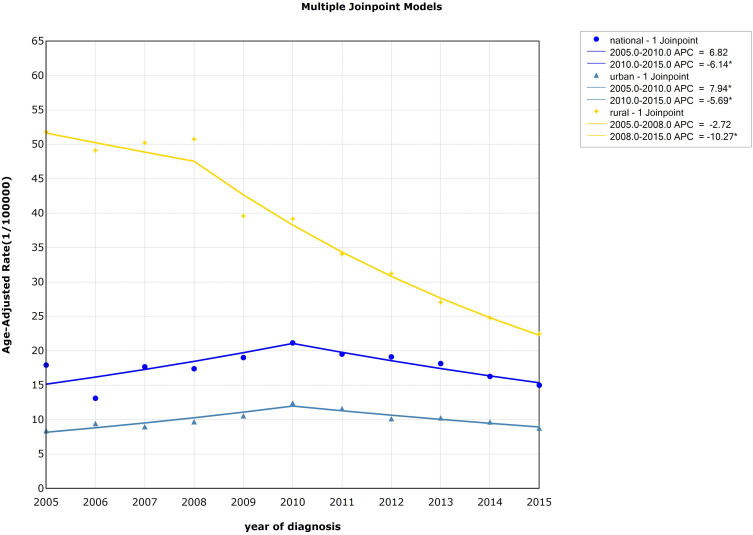
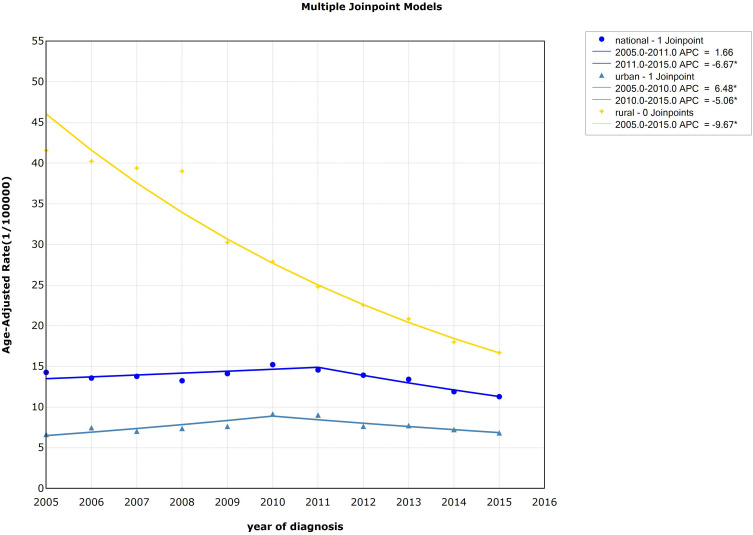
The trend of age-standardized incidence rate (ASIR) of esophageal cancer decreased from 2005 to 2015 (AAPC=−8.07%, P<0.05), especially from 2008–2015 (APC=−10.27%, P<0.05) in rural areas. For urban areas, the result showed that the incidence had risen rapidly from 2005–2010 (APC=7.94%, P<0.05), while it declined from 2010 to 2015 (APC=−5.69%, P<0.05). The standardized incidence of esophageal cancer was higher in rural compared to urban areas, but the declining trend was more obviou of s than in urban areas, and the gap between the standardized incidence of esophageal cancer in urban and rural areas was shrinking. Detailed information can be seen in Table 2 and Figure 2.
Table 2.
Trends in Esophageal Cancer Incidence and Mortality Rates by Area in China, 2005–2015
| Area | Year | APC (95% CI) | t | P | AAPC (95% CI) | t | P | |
|---|---|---|---|---|---|---|---|---|
| ASIR | ||||||||
| All | 2005–2010 | 6.82 (−1.01~15.27) | 2.12 | 0.08 | 0.13 (−3.27~3.65) | 0.08 | 0.94 | |
| 2010–2015 | −6.14* (−9.88~-2.24) | −3.81 | 0.01 | |||||
| Urban | 2005–2010 | 7.94* (3.62~12.44) | 4.58 | 0.00 | 0.89 (−1.01~2.84) | 0.92 | 0.36 | |
| 2010–2015 | −5.69* (−7.97~-3.36) | −5.88 | 0.00 | |||||
| Rural | 2005–2008 | −2.72 (−10.45~5.68) | −0.82 | 0.45 | −8.07* (−9.98~-6.12) | −7.86 | 0.00 | |
| 2008–2015 | −10.27* (−11.33~-9.20) | −22.42 | 0.00 | |||||
| ASMR | ||||||||
| All | 2005–2011 | 1.66 (−1.03~4.42) | 1.50 | 0.18 | −1.76* (−3.30~-0.19) | −2.20 | 0.03 | |
| 2011–2015 | −6.67* (−9.30~-3.96) | −5.91 | 0.00 | |||||
| Urban | 2005–2010 | 6.48* (1.95~11.22) | 3.53 | 0.01 | 0.55(−1.47~2.61) | 0.53 | 0.60 | |
| 2010–2015 | −5.06* (−7.48~-2.57) | −4.90 | 0.00 | |||||
| Rural | 2005–2015 | −9.67* (−10.55~-8.77) | −23.23 | 0.00 | −9.67* (−10.56~-8.77) | −23.23 | 0.00 | |
Note: *P < 0.05, statistically significant trend.
Abbreviations: APC, annual percentage change; AAPC, average annual percentage change; CI, confidence interval; ASIR, age-standardized incidence rate; ASMR, age-standardized mortality rate.
Figure 2.
Trends in the age-standardized incidence rate of esophageal cancer by area, 2005–2015, China (*Represents statistical significance).
Trends in the Age-Specific Incidence of Esophageal Cancer
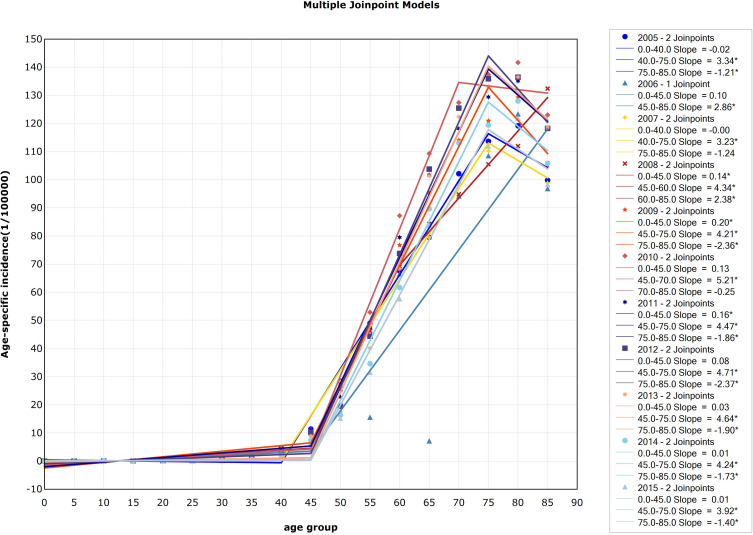
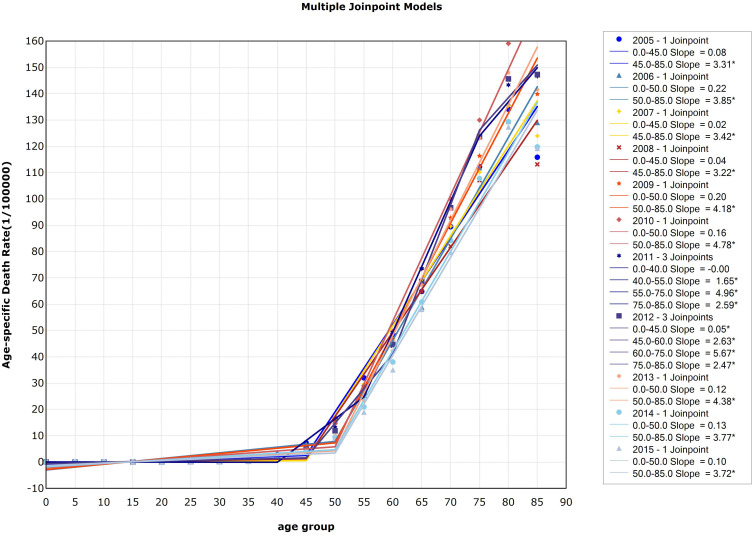
There were two significant inflection points in 2005 and each year from 2007 to 2015. In 2005 and 2007, the inflection point was 40–44 age group and 75–79 age group. The points divided the curve into three changing sections: the incidence of esophageal cancer in China between 0 and 39 years old was at a low level, began to rise rapidly after 40 years old, and declined after 75 years old. In 2008, the point was 45–49 age group and 60–64 age group. The incidence of esophageal cancer in China increased slowly at the age of 0–44, began to increase rapidly at the age of 45–59, and slowed down after the age of 60, but still increased significantly. From 2009 to 2015, the incidence of esophageal cancer in the 0–44 age group increased slowly, and increased rapidly at the age group 45–74 and decreased after 75 years old. In 2010, the incidence increased rapidly in the 45–69 age group and then decreased. In 2006, there was only one significant inflection point (45–49 age group). Before the age of 45–49, the incidence of esophageal cancer in China did not show an obvious trend, while the incidence of esophageal cancer in China obviously increased with age after 45 years old. Detailed information can be seen in Figure 3.
Figure 3.
Trends in the age-specific incidence of esophageal cancer, 2005–2015, China (*Represents statistical significance).
Trends in the Age-Standardized Mortality Rate of Esophageal Cancer in Different Genders
The national standardized mortality of esophageal cancer showed a significant downward trend from 2011 to 2015 (APC=−6.67%, P<0.05). For males, the standardized mortality dropped rapidly from 2011 to 2015 (APC=−6.08%, P<0.05). And the standardized mortality of females decreased from 2012 to 2015 (APC=−10.26%, P<0.05). Detailed information can be seen in Table 1 and Figure 4.
Figure 4.
Trends in the age-standardized mortality rate of esophageal cancer by gender, 2005–2015, China (*Represents statistical significance).
Trends in the Age-Standardized Mortality Rate of Esophageal Cancer in Different Areas
From 2005 to 2015, there was a statistical significance from 2011 to 2015 in the overall trend of esophageal cancer standardized mortality in China (APC=−6.67%, P<0.05). In rural areas, the standardized mortality showed a significant downward trend from 2005 to 2014 (APC=AAPC=−9.67%, P<0.05), while the standardized mortality in urban areas showed an upward trend from 2005 to 2010 (APC=6.48%, P<0.05) and showed a downward trend from 2010 to 2015 (APC=−5.06%, P<0.05). The variation trend of standardized mortality of esophageal cancer in urban and rural areas was different. The standardized mortality rate in rural areas was higher than that in urban areas and presented an obvious downward trend, and the gap between urban and rural areas was gradually narrowing. Detailed information can be seen in Table 2 and Figure 5.
Figure 5.
Trends in the age-standardized mortality rate of esophageal cancer by area, 2005–2015, China (*Represents statistical significance).
Trends in the Age-Specific Mortality of Esophageal Cancer
There was one significant inflection point both in 2005–2010 and in 2013–2015, and three significant inflection points in 2011–2012. The 45–49 age group apparently became the inflection point for 2005 and 2007–2008. The mortality increased rapidly after 45 years old, while there was no significant change with the increase of age between 0–44 years old. In 2006, 2009, 2010, 2013 and 2014, the mortality increased rapidly after the inflection point 50–54 age group. In 2011, the mortality of esophageal cancer was stable between the ages of 0 and 39, with an increasing trend among the 40–54 age group, a rapid increase among the ages of 55–74, and a slight decrease after the age of 75. In 2012, the mortality of esophageal cancer increased slightly at the 0–44 age group, with a rapid increase at the 45–59 age group, and accelerated at the 60–74 age group, and slowed down slightly after 75 years old. Detailed information can be seen in Figure 6.
Figure 6.
Trends in the age-specific mortality of esophageal cancer, 2005–2015, China (*Represents statistical significance).
Negative Binomial Regression Analyses
We brought incidence rates (20~,25~, …,85+ age groups) into negative binomial regression model. The result of the analysis was shown in Table 3: the residual deviance, Pearson value and were 710,91,533 and 611, respectively. Area, gender, and age were independent risk factors for incidence of esophageal cancer. In the same period and same age group, we found that for urban residents, the relative risk of esophageal cancer was 0.58 times larger than rural residents (95% CI:0.49~0.68, P<0.001). For males, the relative risk was 3.1675 times as large as females (95% CI:2.69~3.73, P<0.001). The risk of esophageal cancer has statistical significance for every additional 5 years of age (OR=1.62,95% CI:1.59~1.66, P<0.001) and for every additional year (OR=1.18,95% CI:1.15~1.21, P<0.001). Detailed information can be seen in Table 3.
Table 3.
The Risk Factors in Esophageal Cancer Incidence for Chinese Residents from 2005–2015
| Factor | Parameter Estimate | Parameter 95% CI | OR | OR 95% CI | P value |
|---|---|---|---|---|---|
| Area | −0.5431 | (−0.7074, -0.3788) | 0.5809 | (0.4929, 0.6847) | P<0.001 |
| Gender | 1.1529 | (0.9886, 1.3173) | 3.1675 | (2.6875, 3.7331) | P<0.001 |
| Age | 0.4838 | (0.4633, 0.5044) | 1.6223 | (1.5893, 1.6559) | P<0.001 |
| Year | 0.1637 | (0.1377, 0.1897) | 1.1778 | (1.1476, 1.2089) | P<0.001 |
Abbreviations: 95% CI, 95% confidence interval; OR, odds ratio.
Age-Period-Cohort Analyses
Generally speaking, the table shows that the age effect of incidence and mortality generally increased with age. Esophageal cancer incidence study showed that it was statistically significant for age effect (50–84 age group). The coefficient of age effect peaked in the 75–79 age group. This suggests 75–79 age group has the highest incidence risk in all age groups. Esophageal cancer mortality study showed that it was statistically significant for age effect (60–85 age group). The coefficient of age effect peaked in the 80–84 age group. This suggests the 80–84 age group has the highest mortality risk of all age groups. The period effect displayed similar results for incidence and mortality, which showed a statistical significance in 2010. On the one hand, the results showed that the birth cohort of 1920 had the highest risk of getting esophageal cancer, and the cohort effect coefficient was 1.45, while with a minimal incidence risk for the birth cohort of 1950, its cohort effect coefficient was 0.87. On the other hand, the birth cohort of 1920 had the largest mortality risk for esophageal cancer, its cohort effect coefficient was 1.40. And the birth cohort of 1940 had the lowest mortality risk for esophageal cancer, with cohort effect coefficient of 1.15. Detailed information can be seen in Table 4.
Table 4.
Age–Period–Cohort (APC) Model Analysis Results of Esophagus Cancer Incidence and Mortality in China
| Variables | Effect Coefficient | SE | Z | P | 95% CI | |
|---|---|---|---|---|---|---|
| LL | UL | |||||
| Age (incidence) | ||||||
| Age_20 | −3.35 | 2.82 | −1.19 | 0.24 | −8.89 | 2.18 |
| Age_25 | −2.50 | 1.53 | −1.63 | 0.10 | −5.50 | 0.50 |
| Age_30 | −2.04 | 1.19 | −1.71 | 0.09 | −4.37 | 0.29 |
| Age_35 | −1.21 | 0.97 | −1.25 | 0.21 | −3.11 | 0.69 |
| Age_40 | −0.37 | 0.83 | −0.44 | 0.66 | −1.99 | 1.25 |
| Age_45 | 0.16 | 0.69 | 0.24 | 0.81 | −1.18 | 1.51 |
| Age_50 | 0.65 | 0.55 | 1.19 | 0.23 | −0.42 | 1.73 |
| Age_55 | 0.95 | 0.41 | 2.32 | 0.02 | 0.15 | 1.76 |
| Age_60 | 1.18 | 0.28 | 4.20 | 0.00 | 0.63 | 1.74 |
| Age_65 | 1.30 | 0.18 | 7.14 | 0.00 | 0.94 | 1.65 |
| Age_70 | 1.39 | 0.17 | 8.13 | 0.00 | 1.06 | 1.73 |
| Age_75 | 1.40 | 0.26 | 5.32 | 0.00 | 0.88 | 1.92 |
| Age_80 | 1.35 | 0.39 | 3.46 | 0.00 | 0.59 | 2.12 |
| Age_85 | 1.08 | 0.53 | 2.03 | 0.04 | 0.04 | 2.12 |
| Period | ||||||
| Period_2005 | −0.17 | 0.15 | −1.15 | 0.25 | −0.46 | 0.12 |
| Period_2010 | 0.15 | 0.03 | 4.89 | 0.00 | 0.09 | 0.21 |
| Period_2015 | 0.02 | 0.15 | 0.16 | 0.88 | −0.27 | 0.31 |
| Cohort | ||||||
| Cohort_1920 | 1.45 | 0.62 | 2.33 | 0.02 | 0.23 | 2.67 |
| Cohort_1925 | 1.35 | 0.49 | 2.74 | 0.01 | 0.38 | 2.31 |
| Cohort_1930 | 1.23 | 0.38 | 3.25 | 0.00 | 0.49 | 1.97 |
| Cohort_1935 | 1.15 | 0.30 | 3.83 | 0.00 | 0.56 | 1.75 |
| Cohort_1940 | 1.04 | 0.29 | 3.63 | 0.00 | 0.48 | 1.61 |
| Cohort_1945 | 0.97 | 0.34 | 2.82 | 0.01 | 0.30 | 1.64 |
| Cohort_1950 | 0.87 | 0.44 | 1.98 | 0.05 | 0.01 | 1.74 |
| Cohort_1955 | 0.61 | 0.56 | 1.09 | 0.28 | −0.49 | 1.71 |
| Cohort_1960 | 0.22 | 0.69 | 0.32 | 0.75 | −1.14 | 1.58 |
| Cohort_1965 | −0.17 | 0.83 | −0.21 | 0.83 | −1.79 | 1.44 |
| Cohort_1970 | −0.56 | 0.96 | −0.58 | 0.56 | −2.43 | 1.32 |
| Cohort_1975 | −1.21 | 1.10 | −1.10 | 0.27 | −3.37 | 0.95 |
| Cohort_1980 | −1.49 | 1.23 | −1.21 | 0.23 | −3.90 | 0.93 |
| Cohort_1985 | −1.63 | 1.49 | −1.09 | 0.28 | −4.54 | 1.29 |
| Cohort_1990 | −1.94 | 2.36 | −0.82 | 0.41 | −6.55 | 2.68 |
| Cohort_1995 | −1.91 | 4.96 | −0.39 | 0.70 | −11.63 | 7.81 |
| Age (mortality) | ||||||
| Age_20 | −4.20 | 6.24 | −0.67 | 0.50 | −16.42 | 8.02 |
| Age_25 | −2.30 | 2.22 | −1.04 | 0.30 | −6.66 | 2.05 |
| Age_30 | −1.94 | 1.79 | −1.08 | 0.28 | −5.44 | 1.57 |
| Age_35 | −1.15 | 1.45 | −0.79 | 0.43 | −4.00 | 1.70 |
| Age_40 | −0.60 | 1.26 | −0.48 | 0.63 | −3.07 | 1.86 |
| Age_45 | −0.02 | 1.05 | −0.02 | 0.99 | −2.08 | 2.05 |
| Age_50 | 0.46 | 0.85 | 0.55 | 0.59 | −1.20 | 2.13 |
| Age_55 | 0.83 | 0.65 | 1.26 | 0.21 | −0.45 | 2.11 |
| Age_60 | 1.09 | 0.47 | 2.34 | 0.02 | 0.18 | 2.00 |
| Age_65 | 1.31 | 0.31 | 4.16 | 0.00 | 0.69 | 1.92 |
| Age_70 | 1.50 | 0.27 | 5.65 | 0.00 | 0.98 | 2.02 |
| Age_75 | 1.65 | 0.37 | 4.52 | 0.00 | 0.94 | 2.37 |
| Age_80 | 1.74 | 0.54 | 3.24 | 0.00 | 0.69 | 2.79 |
| Age_85 | 1.64 | 0.73 | 2.24 | 0.03 | 0.20 | 3.08 |
| Period | ||||||
| Period_2005 | −0.12 | 0.21 | −0.56 | 0.57 | −0.54 | 0.30 |
| Period_2010 | 0.13 | 0.03 | 3.87 | 0.00 | 0.06 | 0.19 |
| Period_2015 | 0.00 | 0.21 | −0.02 | 0.98 | −0.42 | 0.42 |
| Cohort | ||||||
| Cohort_1920 | 1.40 | 0.88 | 1.60 | 0.11 | −0.32 | 3.11 |
| Cohort_1925 | 1.48 | 0.70 | 2.12 | 0.03 | 0.11 | 2.84 |
| Cohort_1930 | 1.35 | 0.55 | 2.47 | 0.01 | 0.28 | 2.42 |
| Cohort_1935 | 1.27 | 0.45 | 2.81 | 0.01 | 0.39 | 2.15 |
| Cohort_1940 | 1.15 | 0.45 | 2.57 | 0.01 | 0.27 | 2.03 |
| Cohort_1945 | 1.05 | 0.54 | 1.95 | 0.05 | −0.01 | 2.11 |
| Cohort_1950 | 0.90 | 0.69 | 1.31 | 0.19 | −0.44 | 2.25 |
| Cohort_1955 | 0.61 | 0.86 | 0.71 | 0.48 | −1.07 | 2.30 |
| Cohort_1960 | 0.28 | 1.05 | 0.27 | 0.79 | −1.78 | 2.34 |
| Cohort_1965 | −0.07 | 1.24 | −0.06 | 0.95 | −2.51 | 2.36 |
| Cohort_1970 | −0.52 | 1.44 | −0.36 | 0.72 | −3.33 | 2.29 |
| Cohort_1975 | −1.14 | 1.64 | −0.70 | 0.49 | −4.36 | 2.07 |
| Cohort_1980 | −1.71 | 1.83 | −0.93 | 0.35 | −5.30 | 1.88 |
| Cohort_1985 | −2.30 | 2.48 | −0.92 | 0.36 | −7.17 | 2.57 |
| Cohort_1990 | −2.21 | 3.42 | −0.65 | 0.52 | −8.91 | 4.49 |
| Cohort_1995 | −1.54 | 8.96 | −0.17 | 0.86 | −19.11 | 16.03 |
Notes: Incidence: variance=0.86; AIC=5.78; BIC=−43.99. Mortality: variance=0.97; AIC=5.51; BIC=−43.88. Red values are statistically significant.
Abbreviations: SE, standard error; LL, lower limit; UL, upper limit.
Discussion
This study shows that the ASIR and ASMR of esophageal cancer in China have been relatively stable in recent years. The ASIR in males was significantly higher than females but decreased in recent years, which was consistent with other relevant research results around the world.20,21 In the past 10 years, the overall ASIR of esophageal cancer in rural China was still higher than that in urban areas. Joinpoint regression results also showed more relative stability in urban areas, while the rural individuals showed greater volatility. This may be due to the high mobility of the rural population, resulting in relatively poor data collection and reporting quality. However, the trend of ASMR showed a downward trend year by year. Area, gender, and age were independent risk factors for esophageal cancer incidence. Our findings are consistent with relevant research results.2,21,22 It shows that we should pay close attention to the prevention and treatment of esophageal cancer in rural men aged 45–75.
Considering the gender factor, according to the study, it may be due to the fact that men are exposed to more risk factors of esophageal cancer, especially smoking, alcohol and other unhealthy lifestyles.3 Domestic research reports that esophageal cancer deaths in men, attributable to tobacco smoking, are 9.4 times higher than those in women. Similarly, esophageal cancer deaths in men, caused by drinking, are 11.7 times that of women.23 Additionally, tobacco and alcohol consumption have a synergistic effect in increasing the risk of developing esophageal cancer.24
As a malignant tumor that poses a major threat to human health, esophageal cancer occurs under the combined action of multiple factors. There are regional differences in its occurrence, and economically developed regions have lower rates than underdeveloped regions. This research also shows a big difference between urban and rural areas. The possible reasons are as follows: the differences of disparate region residents’ educational level, environmental factors, smoking and drinking, dietary patterns, dietary habits (eating leftover vegetables and foods), water pollution (nitrogen compounds in drinking water), mental stress, nutrient deficiency (selenium) and other factors.7,11,25,26 For rural males, the reasons for the high incidence of esophageal cancer may include the high proportion of unhealthy lifestyles such as smoking and drinking, the preference for preserved food, the low proportion intake of fresh vegetables and fruit, the poor economic conditions and health awareness.22 On the other hand, the high ASMR in rural areas may be related to the underdeveloped rural economy, the lack of hospital resources and the late onset of the disease. But the gap between urban and rural areas is gradually narrowing, which may be related to the incorporation of the esophageal cancer screening and early-diagnosis-treatment projects into local public health funds in 2005 by the Ministry of Health. In addition, the rapid development of cancer diagnosis and treatment institutions are also closely related to the decline of standardized incidence and mortality of esophageal cancer in recent years.
The analysis showed that the age-specific incidence and mortality of esophageal cancer gradually increased with age and peaked in the age group of 75 years old. The increase of incidence and mortality after 50 years old may be due to the fact that early esophageal cancer often has no typical endoscopic features; more than 85% of patients are in the middle and advanced stages at the time of diagnosis.27 The five-year survival rate of patients with early esophageal cancer exceeds 90%, while the five-year survival rate of patients with advanced esophageal cancer is less than 10%.11 And the high psychological pressure and immunity reduction as the backbone of society and family can also be significant factors. High age-specific incidence and mortality in older persons may be associated with physical fitness, other chronic diseases, and poor immunity.28 It is suggested that the over 50 year old population should be taken as the focus of prevention and treatment, in the prevention and early diagnosis and treatment of esophageal cancer.29
Our study is meaningful research for investigating the trend and risk factors of esophageal cancer in China, which has used a variety of statistical methods to analyze the data, of which validity can be assured. The data came from the Chinese Cancer Registry Annual Report from 2005 to 2015 (ICD-10 code as C15), which used the incidence and mortality from sentinel hospitals for monitoring tumors around the country. So, it increased the reliability and accuracy compared with the data from GBD (Global Burden of Disease). But some limitations should be mentioned in interpreting our findings. Firstly, studies30,31 have shown that postmenopausal women have a higher risk of esophageal cancer than premenopausal and perimenopausal women. So, age-specific incidence and mortality in postmenopausal women should be taken into consideration in our study. Secondly, the period 2005–2015 was a little short for trend analysis and the risk factors should be further confirmed by actual esophageal cancer events.
To sum up, the incidence and mortality have shown a gradual downward trend, of which rural area is particularly obvious, but on account of China’s large population base, aging of the population and unbalanced economic development, the disease burden of esophageal cancer is still high, especially in rural areas. Therefore, we should focus on esophageal cancer high-risk groups, take corresponding prevention and control measures, such as screening vigorously, timely detection and treatment of esophageal cancer, and risk factors control, only in this way can we reduce the hazard of getting esophageal cancer and improve the quality of our life.
Acknowledgments
Bang Li and Yan Liu are the first co-authors. Weiqing Rang is the correspondence author.
Funding Statement
This study was funded by the National Natural Science Foundation of China (grant No. 81673107).
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Lin Y, Totsuka Y, He Y, et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013;23(4):233–242. doi: 10.2188/jea.je20120162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domper Arnal MJ, Ferrandez Arenas A, Lanas Arbeloa A. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. 2015;21:7933–7943. doi: 10.3748/wjg.v21.i26.7933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold M, Abnet CC, Neale RE, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159(1):335–349 e315. doi: 10.1053/j.gastro.2020.02.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381–387. doi: 10.1136/gutjnl-2014-308124 [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27:2–12. doi: 10.3978/j.issn.1000-9604.2015.01.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin Y, Totsuka Y, Shan B, et al. Esophageal cancer in high-risk areas of China: research progress and challenges. Ann Epidemiol. 2017;27(3):215–221. doi: 10.1016/j.annepidem.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China, 2013. Cancer Lett. 2017;401:63–71. doi: 10.1016/j.canlet.2017.04.024 [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 10.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578 [DOI] [PubMed] [Google Scholar]
- 11.Huang FL, Yu SJ. Esophageal cancer: risk factors, genetic association, and treatment. Asian J Surg. 2018;41:210–215. doi: 10.1016/j.asjsur.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 12.Sun D, Cao M, Li H, He S, Chen W. Cancer burden and trends in China: a review and comparison with Japan and South Korea. Chin J Cancer Res. 2020;32:129–139. doi: 10.21147/j.issn.1000-9604.2020.02.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Pardo BJ, Bronson NW, Diggs BS, et al. The global burden of esophageal cancer: a disability-adjusted life-year approach. World J Surg. 2016;40(2):395–401. doi: 10.1007/s00268-015-3356-2 [DOI] [PubMed] [Google Scholar]
- 14.Bao -P-P, Zheng Y, Wu C-X, et al. Cancer incidence in urban Shanghai, 1973–2010: an updated trend and age-period-cohort effects. BMC Cancer. 2016;16(1):284. doi: 10.1186/s12885-016-2313-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tse LA, Yu IT, Mang OW. Time trends of esophageal cancer in Hong Kong: age, period and birth cohort analyses. Int J Cancer. 2007;120:853–858. doi: 10.1002/ijc.22382 [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Yang L, Yuan Y, et al. Cancer incidence in Beijing, 2014. Chin J Cancer Res. 2018;30(1):13–20. doi: 10.21147/j.issn.1000-9604.2018.01.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Zhou M, Wang F, et al. Secular trend of cancer death and incidence in 29 cancer groups in China, 1990–2017: a joinpoint and age-period-cohort analyses. Cancer Manag Res. 2020;12:6221–6238. doi: 10.2147/CMAR.S247648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Souza CDF, Paiva JPS, Silva LFD, Leal TC, Magalhaes M. Trends in tuberculosis mortality in Brazil (1990–2015): joinpoint analysis. J Bras Pneumol. 2019;45:e20180393. doi: 10.1590/1806-3713/e20180393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carstensen B. Age-period-cohort models for the lexis diagram. Stat Med. 2007;26:3018–3045. doi: 10.1002/sim.2764 [DOI] [PubMed] [Google Scholar]
- 20.Castro C, Bosetti C, Malvezzi M, et al. Patterns and trends in esophageal cancer mortality and incidence in Europe (1980–2011) and predictions to 2015. Ann Oncol. 2014;25(1):283–290. doi: 10.1093/annonc/mdt486 [DOI] [PubMed] [Google Scholar]
- 21.He Y, Li D, Shan B, et al. Incidence and mortality of esophagus cancer in China, 2008−2012. Chin J Cancer Res. 2019;31(3):426–434. doi: 10.21147/j.issn.1000-9604.2019.03.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao X, Wang Z, Kong C, et al. Trends of esophageal cancer mortality in rural China from 1989 to 2013: an age-period-cohort analysis. Int J Environ Res Public Health. 2017;14(3):218. doi: 10.3390/ijerph14030218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J-B, Fan J-H, Liang H, et al. Attributable causes of esophageal cancer incidence and mortality in China. PLoS One. 2012;7(8):e42281. doi: 10.1371/journal.pone.0042281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prabhu A, Obi KO, Rubenstein JH. The synergistic effects of alcohol and tobacco consumption on the risk of esophageal squamous cell carcinoma: a meta-analysis. Am J Gastroenterol. 2014;109(6):822–827. doi: 10.1038/ajg.2014.71 [DOI] [PubMed] [Google Scholar]
- 25.Tran GD, Sun X-D, Abnet CC, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113(3):456–463. doi: 10.1002/ijc.20616 [DOI] [PubMed] [Google Scholar]
- 26.Song Q, Wang X, Yu IT, et al. Processed food consumption and risk of esophageal squamous cell carcinoma: a case-control study in a high risk area. Cancer Sci. 2012;103:2007–2011. doi: 10.1111/j.1349-7006.2012.02387.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menon S, Trudgill N. How commonly is upper gastrointestinal cancer missed at endoscopy? A meta-analysis. Endosc Int Open. 2014;2:E46–E50. doi: 10.1055/s-0034-1365524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S, Zhang X, Yan Y, et al. High cancer burden in Elderly Chinese, 2005–2011. Int J Environ Res Public Health. 2015;12:12196–12211. doi: 10.3390/ijerph121012196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilleron S, Sarfati D, Janssen‐Heijnen M, et al. Global cancer incidence in older adults, 2012 and 2035: a population-based study. Int J Cancer. 2019;144:49–58. doi: 10.1002/ijc.31664 [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y, Yue D, Yuan B, Zhu L, Lu M. Reproductive factors are associated with oesophageal cancer risk: results from a meta-analysis of observational studies. Eur J Cancer Prev. 2017;26:1–9. doi: 10.1097/CEJ.0000000000000234 [DOI] [PubMed] [Google Scholar]
- 31.Mathieu LN, Kanarek NF, Tsai HL, Rudin CM, Brock MV. Age and sex differences in the incidence of esophageal adenocarcinoma: results from the Surveillance, Epidemiology, and End Results (SEER) Registry (1973–2008). Dis Esophagus. 2014;27:757–763. doi: 10.1111/dote.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]