Abstract
Phthalates are a family of chemicals that can be found in plastic and personal care products used by consumers every day and they are known endocrine disrupting chemicals that can disrupt female reproduction. In previous studies, an environmentally relevant phthalate mixture was shown to affect female reproduction in a transgenerational manner. However, limited information is available on the effect of phthalate mixtures on ovarian steroidogenesis and folliculogenesis. Ovarian steroidogenesis is important for producing hormones needed for reproduction and ovarian regulation, and folliculogenesis is essential for the development of ovarian follicles and successful fertility. Thus, this study tested the hypothesis that prenatal exposure to an environmentally relevant phthalate mixture adversely affects ovarian steroidogenesis and folliculogenesis in the F1 generation of adult female mice. Pregnant dams (F0 generation) were orally dosed with vehicle control or a phthalate mixture (20 μg/kg/day-500 mg/kg/day) daily from gestational day 10 to birth, and the adult F1 females were the offspring of the dosed dams. The ovaries of the F1 generation were collected at postnatal day 60. One ovary was used for histological examination of the numbers and percent of different follicle types. The other ovary was used to measure expression of theca and granulosa cell enzymes. Additionally, sera were collected for measuring hormone levels. The results indicate that prenatal exposure to the phthalate mixture decreases hormone levels and gene expression, alters the transitioning of follicle types, and leads to higher incidence of atresia in the F1 generation offspring.
Keywords: phthalates, mixture, ovary, hormone, enzyme, follicle, reproduction
INTRODUCTION
Many plastic and personal care products used every day by consumers contain a family of chemicals known as phthalates. Phthalates are synthetic plasticizers that provide flexibility to plastic products and they are known endocrine disrupting chemicals (EDCs), chemicals that interfere with hormone activity in the body as well as reproduction (Hannon & Flaws, 2015; Zoeller et al., 2012). These chemicals are commonly used in the production of a variety of items, ranging from building materials such as polyvinyl chloride (PVC) pipes, perfumes, hairsprays, dietary supplements, medical bags, and many other products (Hannon & Flaws, 2015; Heudorf, Mersch-Sundermann, & Angerer, 2007; Schettler, 2006). The wide use of phthalates in consumer products makes it easy to become exposed to these chemicals as they are regularly released into the environment in a variety of ways including leaching, leading to exposure through ingestion, inhalation, and dermal contact (Wittassek, Koch, Angerer, & Bruning, 2011). The constant exposure to phthalates and the ability of phthalates to affect hormones and reproduction is associated with serious health issues, such as infertility and early menopause (Grindler et al., 2015; Hannon & Flaws, 2015)
It is important to understand the effects of phthalates on female’s health because women tend to have higher exposure to these chemicals than men as they are more likely to use more personal care and cosmetic products than men (Fauser et al., 2011; Hannon & Flaws, 2015; Parlett, Calafat, & Swan, 2013). In addition, chronic occupational exposure can lead to increased risk of miscarriage as well as a decrease in pregnancy rates (Heudorf et al., 2007). It is also important to understand the effect phthalates have on hormones in the body because hormones, such as estradiol, are important for bone, heart, and brain health in women (Fauser et al., 2011). Another concern is that phthalates and their metabolites have been found in pregnant women, and therefore have the potential to impact the health of both the mother and offspring (Yazdy et al., 2018; Zhou, Gao, & Flaws, 2017b). Many phthalates such as di(2-ethylhexyl) phthalate (DEHP), diethyl phthalate (DEP), dimethyl phthalate, dibutyl phthalate (DBP), benzyl butyl phthalate (BzBP), diisononyl phthalate (DiNP), and diisobutyl phthalate (DiBP) (Barakat et al., 2020; Brehm, Rattan, Gao, & Flaws, 2018; Chiang & Flaws, 2019; Gray et al., 2000; Heudorf et al., 2007) can negatively affect reproduction.
Further, a previous study showed that an environmentally relevant phthalate mixture led to disruptions in female reproduction in the F1 offspring in mice (Zhou et al., 2017b). Prenatal exposure to the mixture also caused changes in organ weights and estrous cyclicity, induced ovarian cysts, and led to breeding complications (Zhou et al., 2017b). Another study showed that exposure to a phthalate mixture led to a decrease in the expression of most genes in the steroidogenic pathway in mouse antral follicles in vitro (Meling et al., 2020). However, the effects of prenatal exposure to the mixture on steroidogenesis in the F1 offspring were not known. Thus, this study determined whether and how prenatal exposure to the mixture affects steroidogenesis in the ovaries of F1 offspring. Specifically, this study tested the hypothesis that prenatal exposure to an environmentally relevant mixture adversely affects steroidogenesis in the F1 ovary.
Another important part of reproductive development in female reproduction is the formation of follicles within the ovary. During embryonic development, primordial germs cells proliferate and form primordial follicles, which mature to preovulatory follicles as part of the reproductive cycle (Rimon-Dahari, Yerushalmi-Heinemann, Alyagor, & Dekel, 2016). A previous study showed that short-term exposure to the phthalates DEHP and DiNP led to negative impacts on ovarian function and folliculogenesis in mice (Chiang, Lewis, Borkowski, & Flaws, 2020). However, previous studies did not examine the effects of prenatal exposure to a phthalate mixture on folliculogenesis in F1 offspring. Thus, the current study also tested the hypothesis that prenatal exposure to a phthalate mixture disrupts folliculogenesis.
METHODS
Chemicals
The chemical mixture used in this study includes DEP (35.22%), DEHP (21.03%), DBP (14.91%), DiBP (8.61%), DiNP (15.10%), and BzBP (5.13%), purchased from Sigma-Aldrich (St. Louis, MO). The percentages of each chemical in the mixture were determined using the levels of phthalate metabolites measured in the urine of pregnant women in central Illinois (Yazdy et al., 2018). A vehicle control of tocopherol-stripped corn oil was used, and the mixtures were created by diluting the chemical mixture with the vehicle. The doses used for this study included 20 μg/kg/day, 200 μg/kg/day, 200 mg/kg/day, and 500 mg/kg/day.
This mixture has been previously shown to disrupt female reproduction in the F1, F2, and F3 generations from postnatal day 0 through 13 months of age (Brehm, Zhou, Gao, & Flaws, 2020; Zhou, Gao, & Flaws, 2017a). In terms of environmentally relevant doses, the two lower doses used in this study are either within the range of human exposure to the phthalates or are close to those exposure levels. Specifically, in humans, the DEHP daily exposure range is 3–30 μg/kg/day, the DEP daily exposure range is 2.32–12 μg/kg/day, the BzBP daily exposure range is 0.26–0.88 μg/kg/day, the DBP daily exposure range is 0.84–5.22 μg/kg/day, and the DiBP daily exposure range is 0.12–1.4 μg/kg/day. In addition, the DiNP occupational exposure can reach 26 μg/kg/day and it can reach 120 μg/kg/day in infants (Brehm et al., 2020; Zhou et al., 2017a). In this study, the dose of DEHP used in the 20 μg mixture is approximately equal to 4.2 μg (21.03% of the 20 μg mixture), which falls within the range of daily human exposure. The dose of DEHP in the 200 μg mixture is approximately equal to 42 μg (21.03 % of the 200 μg mixture), which is close to daily human exposure. The dose of DEP used in the 20 μg mixture is approximately equal to 7 μg (35.22 % of the 20 μg mixture), which falls within the range of daily human exposure. The dose of BzBP used in the 20 μg mixture is approximately equal to 1 μg (5.13 % of the 20 μg mixture), which is close to the reported range of daily human exposure. The dose of DBP used in the 20 μg mixture is approximately equal to 3 μg (14.91% of the 20 μg mixture), which falls within the range of daily human exposure. The dose of DiBP used in the 20 μg mixture is approximately equal to 1.6 μg (8% of the 20 μg mixture), which is close to the reported range of daily human exposure. Finally, the dose of DiNP used in the 200 μg mixture is approximately equal to 30 μg (15% of the 200 μg mixture), which is close to the occupational exposure level (Brehm et al., 2020; Zhou et al., 2017a). The higher doses of the mixture used in this study, 200 and 500 mg/kg/day, were also used for comparison with single phthalate exposure studies (Chiang et al., 2020; Niermann, Rattan, Brehm, & Flaws, 2015; Rattan, Brehm, Gao, Niermann, & Flaws, 2018).
Animals
Adult cycling female and adult male CD-1 mice were purchased from Charles River Laboratories (Wilmington, MA), then subsequently housed in the College of Veterinary Medicine Animal Facility at the University of Illinois, Urbana-Champaign (Champaign, IL). Prior to beginning the experiment, the mice were allowed one week in the facility to adjust to new surroundings. After the week of acclimation, the mice were housed individually in polysulfone cages set to 25°C, and with 12-hour light/12-hour dark cycles. For nutrients, the mice received the Teklad Rodent Diet 8604 (Envigo, Huntingdon, United Kingdom) and reverse-osmosis filtered high-purity water ad libitum. All animal procedures, including the euthanasia and tissue collections, were approved by the University of Illinois Institutional Animal Care and Use Committee (IACUC).
Study Design and Dosing
The F1 generation was created through the mating of F0 female mice with non-treated males, both 8 weeks of age. The presence of a vaginal sperm plug was used as the marker for pregnancy. At this point, females were then separately housed from the males and randomly divided into five different treatment groups. At gestational day 10, pregnant females received a daily oral dose of either the control (tocopherol-stripped corn oil) or one of the mixture doses (20 μg/kg/day - 500 mg/kg/day). Doses were given at the same time every day and were administered by gently inserting a pipette tip into the cheek of the mouse; both the timing and route of exposure were chosen to mimic human exposure and the critical time period for ovarian development (Niermann et al., 2015). To ensure the correct dosage was being used per body weight, the females were weighed once a day before dosing. Once the F0 females gave birth, their pups were recognized as the F1 generation. In all experiments, the F0 dam was considered to be the experimental unit and thus, the “n”. All analyses were conducted on a least 1 female F1 pup per litter for n=3–8 dams.
Tissue collection
On postnatal (PND) day 60, the F1 female mice were euthanized by CO2 asphyxiation, followed by cervical dislocation, and their tissues (ovaries and sera) were collected during the estrous stage of diestrus. To determine if mice were in diestrus, they were vaginally lavaged with 1x phosphate-buffered saline and the stages of estrus were determined by examining the lavage fluid using previously defined criteria (Hartman, 1944). One ovary was fixed in Dietrich’s fixative for histological evaluation and the other was snap frozen at −80°C for RNA extraction and qPCR analysis, as described below.
Histological examination of ovaries
Once the ovaries were removed from Dietrich’s fixative, the ovaries were embedded in paraffin and subsequently sectioned at 8 μm and mounted on slides. The slides were then stained using hematoxylin and eosin for the purpose of counting follicles. Every 10th serial section was used to assess follicle populations. Follicle types that were observed and recorded included primordial, primary, preantral, and antral, as well as atretic and abnormal follicles. The criteria for each of these follicle types were as follows: oocytes surrounded a single layer of squamous granulosa cells were counted as primordial follicles, oocytes surrounded a single layer of cuboidal granulosa cells were counted as primary follicles, oocytes surrounded more than one layer of cuboidal granulosa cells and a thecal cell layer were counted as preantral follicles, and oocytes surrounded by more than one layer of cuboidal granulosa cells, a thecal cell layer, and a noticeable fluid-filled antrum were counted as antral follicles (Brehm et al., 2018; Chiang et al., 2020; Niermann et al., 2015; Rattan et al., 2018). The presence of a nucleus in the oocyte was required for counting preantral, antral, and atretic follicles to avoid double counting of follicles that were large enough to span several sections. However, it was not necessary to consider the presence of the nucleus in the oocyte when counting primordial and primary follicles because primordial and primary follicles were too small to span several sections. Additionally, the presence of very visible dark spots, deterioration, and disorganization within the cuboidal granulosa cells of the follicle were required to classify follicles as atretic follicles. Moreover, follicles were counted as abnormal if they contained double oocytes or fragmented oocytes. Data on the total number of follicles, number and percent of each type, number and percent atretic, and number of abnormal follicles were collected and recorded. Raw/absolute numbers of follicles as well as the percentages were counted because the raw/absolute number of follicles inform whether there were any changes in the total amount of different follicle types present in the ovaries, whereas the percent of follicles inform whether the follicles are shifting between follicle pools. Folliculogenesis is a continuous process with follicles growing from the primordial, to the primary, and then pre-antral and finally, antral stages. By assessing percentage of follicles in each treatment group, we are evaluating whether phthalates cause shifts between follicle pools. The raw/absolute data on follicle numbers do not tell us about shifts between follicle pools, but instead reflect the absolute number of live or dead follicles. It is possible to have shifts in the different follicle pools without affecting overall follicle numbers. In all histological evaluations, the investigators were blinded to treatment groups when conducting the follicle counting in this study.
Measurement of Hormones
To measure the hormone concentrations for this study, sera were collected from the euthanized mice. The sera were then used for enzyme-linked immunosorbent assays (ELISAs, DRG International Inc., Springfield, New Jersey) to measure concentrations of estradiol, progesterone, and testosterone. Additionally, the levels of follicle-stimulating hormone (FSH) were measured by radioimmunoassay at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core. The lowest limits of detection were 0.083 ng/mL for testosterone, 0.034 ng/ml for progesterone, 10.6 pg/mL for estradiol, and 2 ng/mL for FSH. If the measurement was lower than the lowest limit of detection, the value was substituted with the lowest limit of detection/√2. The intra- and inter-assay coefficients of variability were less than 10%.
Gene expression analysis
Frozen whole ovaries collected at PND 60 were used for quantitative real-time polymerase chain reaction (qPCR) analysis (n = 5–6 ovaries/treatment group). Total RNA was isolated from the ovaries using the All Prep DNA/RNA Mini kit (Qiagen, Inc., Valencia, California) following the manufacturer’s instructions. Concentrations of RNA eluted in 50 μl of RNAse-free water were determined using a Nanodrop (λ=260/280nm: ND 1000: Nanodrop Technologies Inc., Wilmington, Delaware). The RNA extracted (100 ng) was converted to cDNA through reverse transcriptase using the iScript RT kit (Bio-Rad Laboratories, Inc., Hercules, California). Each cDNA sample was diluted 1:8 using nuclease-free water prior to qPCR analysis. The cDNA samples were subjected to qPCR using the CFX96 Real-time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA) and with CFX Manager Software according to the manufacturer’s protocol. The qPCR program consisted of enzyme activation, amplification and quantification, melt curve, and final step, which were 95°C for 1 minute, 40 cycles of 95 °C for 10 seconds, 60 °C for 10 seconds (single fluorescence reading), 72 °C for 5 minutes, 65 °C-95 °C heating 0.5 °C per second (with continuous fluorescence readings), and 72 °C for 5 minutes per step respectively. All qPCR reactions were conducted in duplicate using 2 μL of cDNA, forward and reverse primers (5 pmol) for selected genes (Integrated DNA Technologies, Coralville, IA), nuclease-free water, and SsoFastEvaGreen Supermix for a final reaction volume of 10 μL. The genes chosen for qPCR analysis were part of the steroidogenesis pathway: steroidogenic acute regulatory protein (Star), cytochrome P450 side-chain cleavage (Cyp11a1), 3β-hydroxysteroid dehydrogenase (Hsd3b1), steroid 17ɑ-monooxygenase (Cyp17a1), 17β-hydroxysteroid dehydrogenase (Hsd17b1), and cytochrome P450 aromatase (Cyp19a1) (Table 1). Beta-actin (Actb) was used as the reference gene because its expression did not differ across treatment groups (Rattan et al. 2019, Ziv-Gal et al. 2016, Hannon et al. 2015). Relative fold changes were calculated as the ratio of each treatment group to the control group level and analyzed using a mathematical model for relative quantification of real-time PCR data developed by Pfaffl (Pfaffl, 2001).
Table 1.
Sequence information for genes encoding ovarian steroidogenic enzymes
| Gene Symbol | Gene Name | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|---|
| Star | Steroidogenic Acute Regulatory Protein | CAGGGAGAGGTGGCTATGCA | CCGTGTCTTTTCCAATCCTCTG |
| Cyp11a1 | Cytochrome P450 side-chain cleavage | AGATCCCTTCCCCTGGTGACAATG | CGCATGAGAAGAGTATCGACGCATC |
| Hsd3b1 | 3β-Hydroxysteroid dehydrogenase | CAGGAGAAAGAACTGCAGGAGGTC | GCACACTTGCTTGAACACAGGC |
| Cyp17a1 | Steroid 17ɑ-monooxygenase | CCAGGACCCAAGTGTGTTCT | CCTGATACGAAGCACTTCTCG |
| Hsd17b1 | 17β-Hydroxysteroid dehydrogenase | AAGCGGTTCGTGGAGAAGTAG | ACTGTGCCAGCAAGTTTGCG |
| Cyp19a1 | Cytochrome P450 aromatase | CATGGTCCCGGAAACTGTGA | GTAGTAGTTGCAGGCACTTC |
Statistical analysis
Analysis of the data was conducted using SPSS statistical software (SPSS Inc., Chicago, IL). For multiple comparisons between normally distributed experimental groups, a one-way analysis of variance (ANOVA) was performed. Following this, Dunnett post-hoc comparisons were conducted if equal variances were assumed. If equal variances were not assumed, a Games-Howell comparison was performed. For comparison between groups not normally distributed, Kruskal-Wallis H tests were used, followed by Mann-Whitney U two-independent sample tests. The alpha values assigned were 0.05, with statistical significance being noted when p ≤ 0.05. Additionally, when p-values were greater than 0.05, but less than 0.1, data were considered to exhibit a trend towards significance.
RESULTS
Steroidogenesis
In the F1 generation, the mixture at 500 mg/kg/day (p ≤ 0.05) significantly decreased FSH levels compared to control (n=3–5 mice/treatment group; Figure 1A). The mixture at 200 μg/kg/day (p ≤ 0.05), 200 mg/kg/day (p ≤ 0.002), and 500 mg/kg/day (p ≤ 0.002) also significantly decreased testosterone levels compared to control levels (n=6–8 mice/treatment group; Figure 1B). Further, the mixture at 500 mg/kg/day (p ≤ 0.03) significantly decreased estradiol levels compared to control (n=6–8 mice/treatment group; Figure 1C). Similarly, the mixture at 20 μg/kg/day (p ≤ 0.08) and at 200 mg/kg/day (p ≤ 0.10) borderline decreased estradiol levels compared to control (n=6–8 mice/treatment group; Figure 1C). Finally, the mixture at 500 mg/kg/day significantly (p ≤ 0.05) decreased progesterone levels compared to control (n=3–8 mice/treatment group; Figure 1D).
Figure 1.
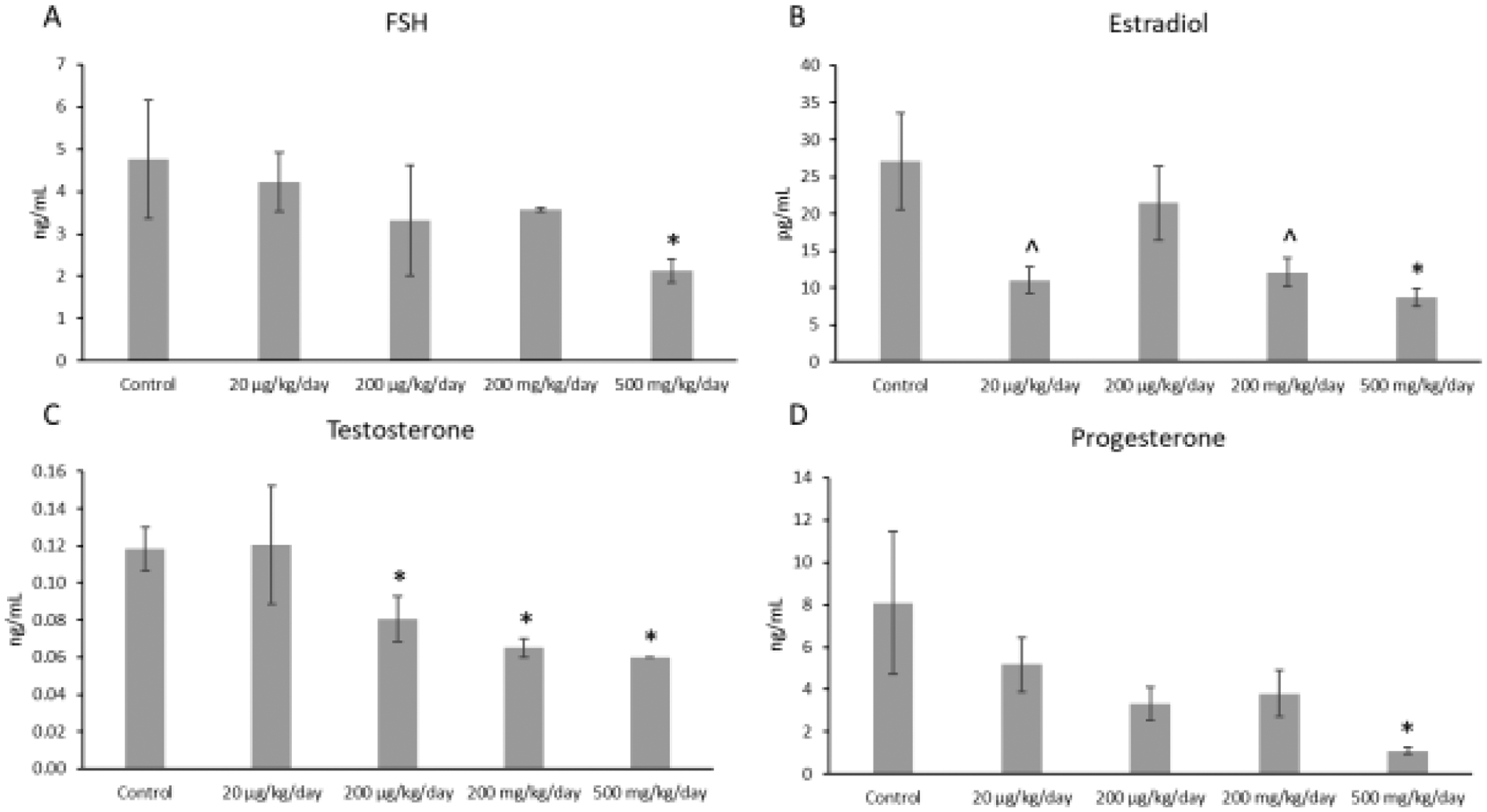
Concentrations of follicle-stimulating hormone (FSH), estradiol, testosterone, and progesterone in F1 adult female mice at PND 60. Each hormone is compared to the control, with the asterisk symbol (*) indicating significance in comparison to the control (p ≤ 0.05) and the caret symbol (^) indicating borderline significance in comparison to the control (p ≤ 0.10). Data are presented as means ± standard error of the mean from 3 to 8 females per treatment group. A. F1 FSH concentrations (n=3–5 mice/treatment group) B. F1 estradiol concentrations (n=6–8 mice/treatment group) C. F1 testosterone concentrations (n=6–8 mice/treatment group) D. F1 progesterone concentrations (n=3–8 mice/treatment group).
Prenatal exposure to the mixture affected expression of selected enzymes in the thecal cells of F1 offspring (Figure 2ABC). Specifically, prenatal exposure to the mixture (20 μg/kg/day, 200 μg/kg/day, and 500 mg/kg/day) significantly decreased expression of Star in the F1 ovaries compared to control (p ≤ 0.05). In addition, prenatal exposure to the mixture (20 μg/kg/day) significantly decreased expression of Cyp11a1 (p ≤ 0.05), but it did not affect expression of Cyp17a1 in the F1 ovaries compared to control (Figure 2ABC).
Figure 2.
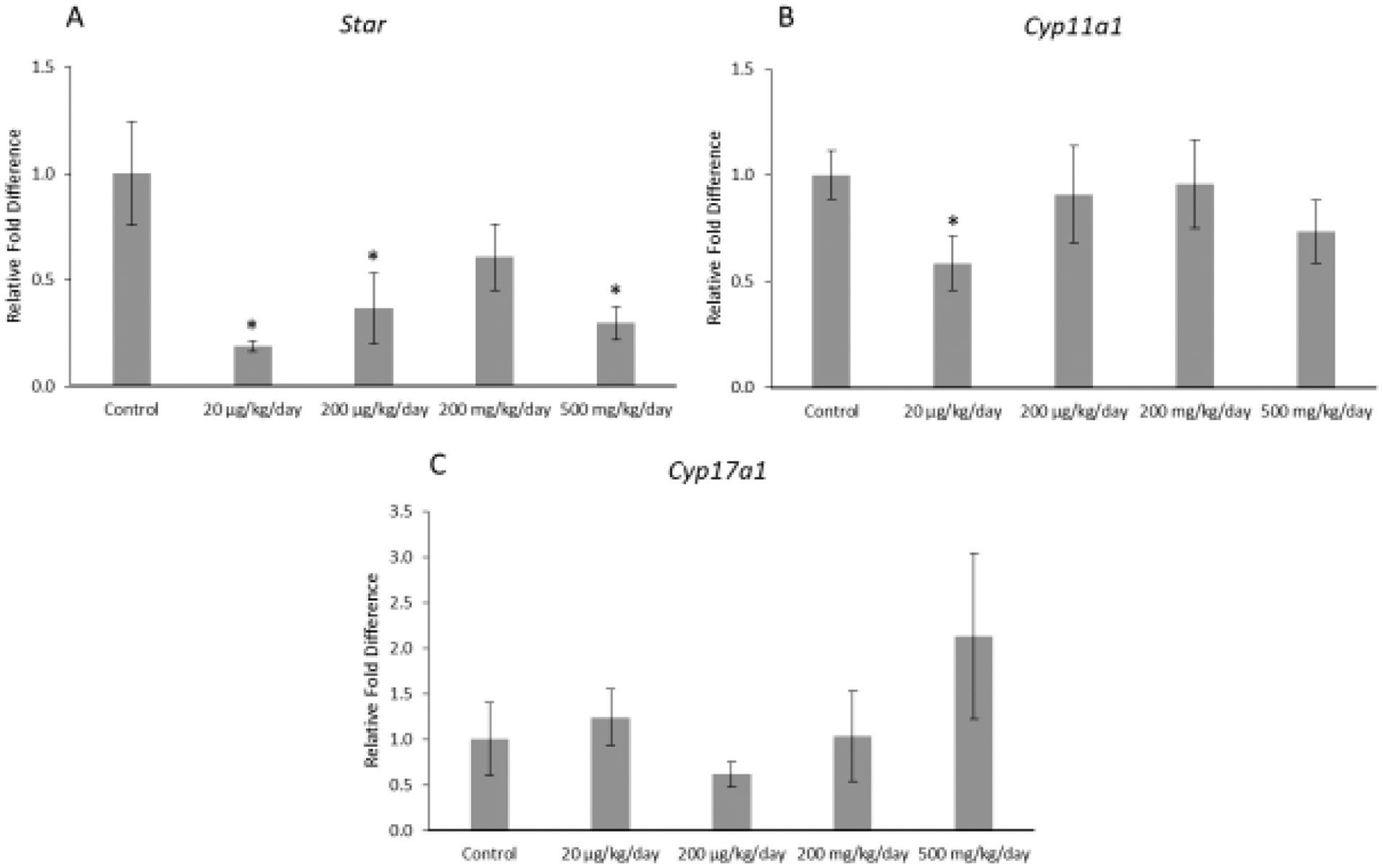
Relative fold differences of theca cell enzymes, Star, Cyp11a1, and Cyp17a1 in F1 adult female mice at PND 60. Gene expression for each treatment group is compared to the control. The data are presented as means ± standard error of the mean from three females per treatment group. A. F1 Star relative fold difference (n=5–6 mice/treatment group) B. F1 Cyp11a1 relative fold difference (n=6 mice/treatment group) C. F1 Cyp17a1 relative fold difference (n=6 mice/treatment group).
Prenatal exposure to the mixture affected expression of one selected enzyme in the granulosa cells of F1 offspring (Figure 3ABC). Specifically, the mixture at 200 μg/kg/day significantly decreased expression of Cyp19a1 in the F1 ovaries compared to control (Figure 3; p ≤ 0.05). However, the mixture did not significantly affect expression of Hsd3b1 or Hsd17b1 in the F1 ovaries compared to control (Figure 3).
Figure 3.
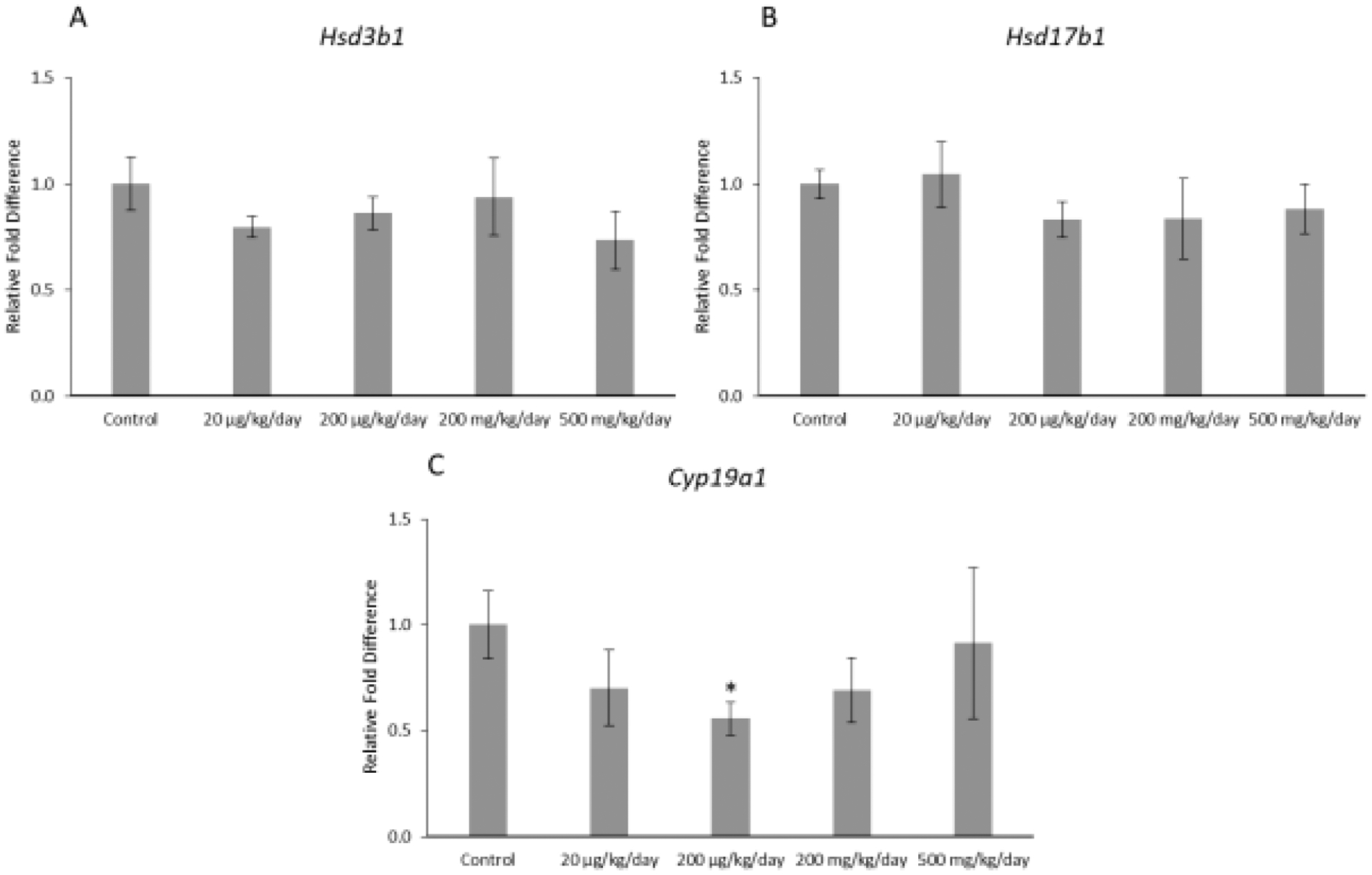
Relative fold differences of granulosa cell enzymes, Hsd3b1, Hsd17b1, and Cyp19a1 in F1 adult female mice at PND 60. Gene expression for each treatment group is compared to the control, with the asterisk symbol (*) indicating significance in comparison to the control (p ≤ 0.05) and the caret symbol (^) indicating borderline significance in comparison to the control (p ≤ 0.10). The data are presented as means ± standard error of the mean from 3 females per treatment group. A. F1 Hsd3b1 relative fold difference (n=6 mice/treatment group) B. F1 Hsd17b1 relative fold difference (n=6 mice/treatment group) C. F1 Cyp19a1 relative fold difference (n=5–6 mice/treatment group).
Folliculogenesis
Prenatal exposure to the phthalate mixture led to some changes in certain follicle types and in the percentages of follicles in the F1 ovaries (Figure 4AB). In terms of the raw follicle counts, the mixture did not significantly affect follicle numbers compared to the control (n=5–6 mice/treatment group; Figure 4A). In terms of the percent of follicles, the mixture significantly increased the percent of primordial follicles (p ≤ 0.04) at the 20 μg/kg/day dose compared to the control (n=6 mice/treatment group; Figure 4B). Further, the mixture at 20 μg/kg/day (p ≤ 0.04) and 200 mg/kg/day (p ≤ 0.04) significantly decreased the percent of preantral follicles compared to the control (n=6 mice/treatment group; Figure 4B). The mixture at 200 μg/kg/day borderline decreased the percent of antral follicles compared to the control (p ≤ 0.11; n=6 mice/treatment group; Figure 4B).
Figure 4.
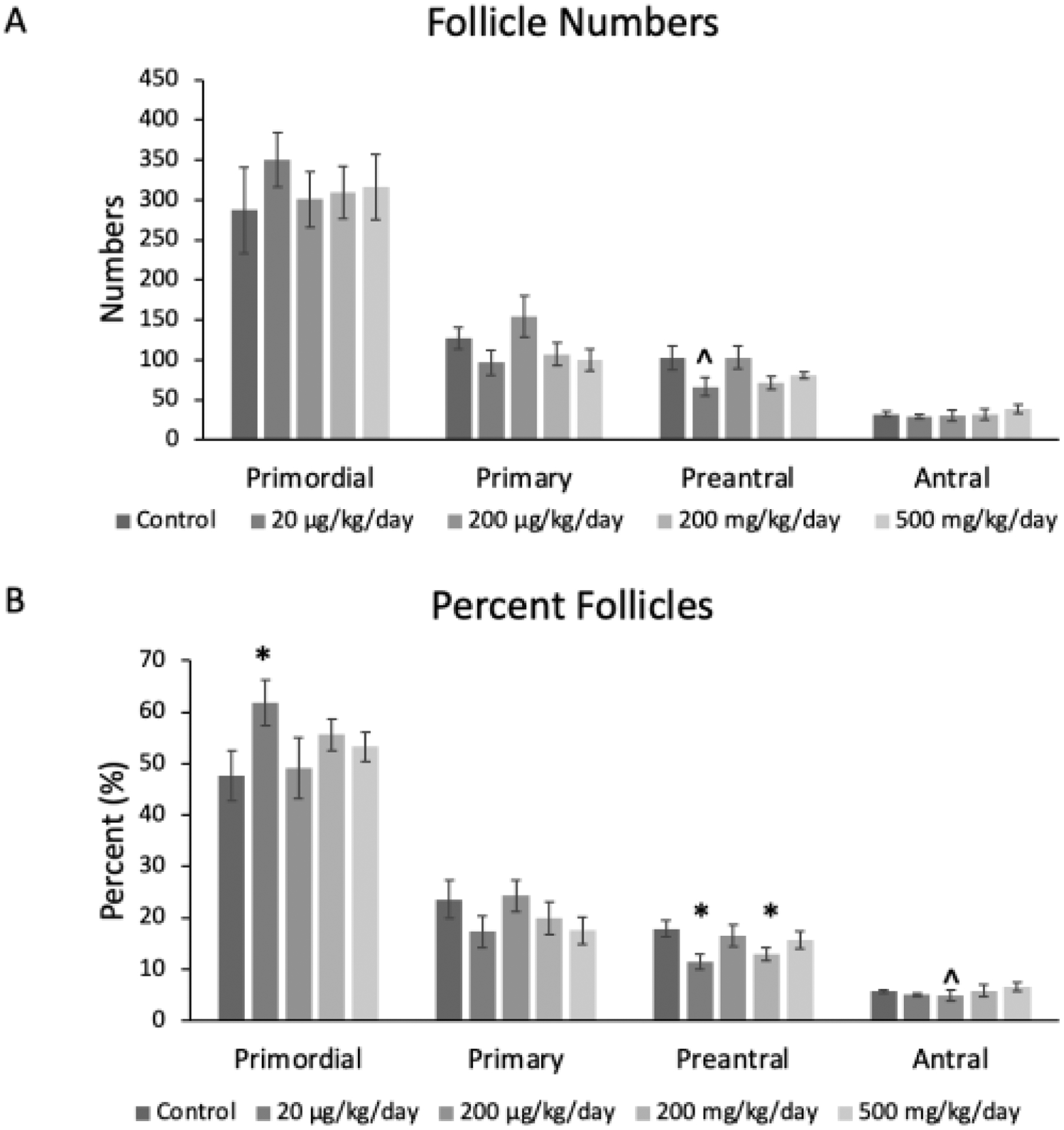
Mean follicle counts and percent of follicles for primordial, primary, preantral, and antral follicles in F1 adult female mice at PND 60. The values for each follicle type and treatment group are compared to the control, with the asterisk symbol (*) indicating significance in comparison to the control (p ≤ 0.05) and the caret symbol (^) indicating borderline significance in comparison to the control (p ≤ 0.10). The data are presented as means ± standard error of the mean from 5 to 6 females per treatment group and follicle type. A. F1 Mean follicle counts; (n=5–6 mice/treatment group) B. F1 percent follicles (n=6 mice/treatment group).
Although prenatal exposure to the mixture at 200 mg/kg/day only borderline increased the percent of atretic follicles in the F1 ovaries compared to the control (p = 0.06; n=6 mice/treatment group; Figure 5B), the prenatal exposure to mixture at 500 mg/kg/day significantly increased the number (p ≤ 0.01) and percent (p ≤ 0.03) of atretic follicles in the F1 ovaries compared to the control (n=6 mice/treatment group; Figures 5A and 5B). Finally, the mixture did not significantly affect the number of abnormal follicles or total follicle number compared to control (n=6 mice/treatment group; data not shown).
Figure 5.
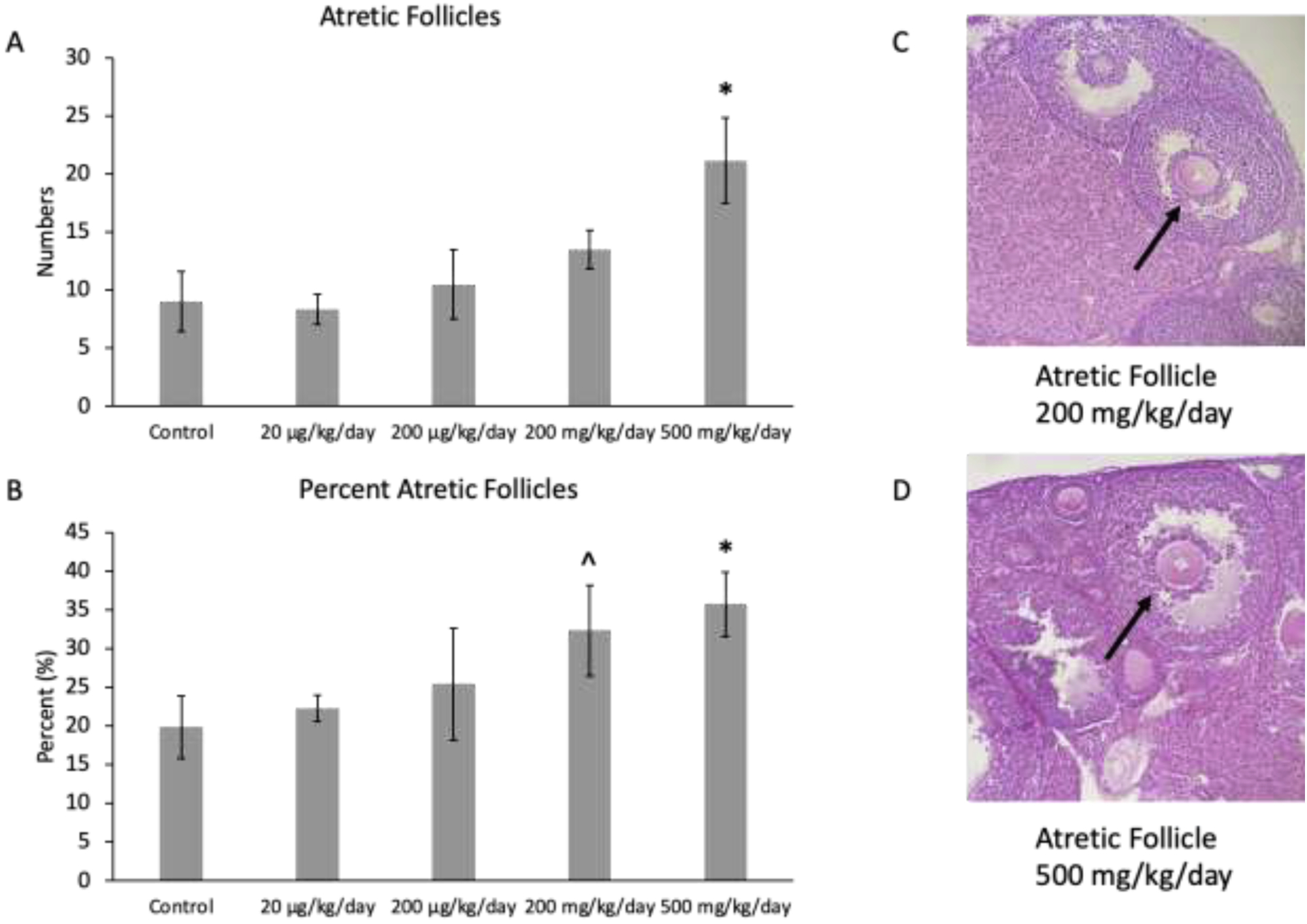
Mean follicle counts and percent of follicles for atretic follicles in F1 adult female mice at PND 60. The values for each treatment group are compared to the control, with the asterisk symbol (*) indicating significance in comparison to the control (p ≤ 0.05) and the caret symbol (^) indicating borderline significance in comparison to the control (p ≤ 0.10). The data are presented as means ± standard error of the mean from 6 females per treatment group and follicle type. A. F1 mean atretic follicle counts (n=6 mice/treatment group) B. F1 percent atretic follicles (n=6 mice/treatment group) C. Atretic follicle in ovary exposed to mixture at 200 mg/kg/day D. Atretic follicle in ovary exposed to mixture at 500 mg/kg/day. Black arrows indicate apoptotic bodies.
DISCUSSION
This study tested the hypothesis that prenatal exposure to an environmentally relevant phthalate mixture adversely affects ovarian steroidogenesis and folliculogenesis in the F1 generation of adult female mice. A previous study showed that short-term exposure during adulthood to single phthalates (DEHP and DiNP) negatively impacts folliculogenesis in adult mice (Chiang et al., 2020). In this study, we expanded the previous study by investigating how prenatal exposure to a phthalate mixture impacts folliculogenesis in the F1 generation. In another previous study, exposure to the same phthalate mixture was shown to decrease antral follicle growth and reduce the expression of sex steroid hormones and steroidogenic enzymes in vitro (Zhou & Flaws, 2017). In this study, we also expanded a previous study by investigating the effects of prenatal exposure to a phthalate mixture on steroidogenic enzymes and hormone levels in the F1 offspring. The results of this study reveal that prenatal exposure to the phthalate mixture decreases hormone levels and gene expression, alters the transitioning of follicle types, and leads to a higher incidence of atresia in the F1 generation of adult female mice.
Our data indicate that exposure to several doses of the phthalate mixture decreased the levels of FSH and testosterone when compared to the control. Testosterone and FSH are important for female reproduction, and FSH is vital for the growth of ovarian follicles. A previous study showed that prenatal exposure to a single phthalate, DEHP, decreased testosterone levels, but it did not change the levels of FSH (Rattan et al., 2018) compared to control in the F1 generation of mice at PND 60. Furthermore, FSH has the ability to inhibit atresia in antral follicles (Chu, Xu, Yang, & Sun, 2018). Interestingly, we observed that the mixture caused an increase in the number and percentage of atretic follicles, a decrease in the number and percentage of mature follicle types, and a decrease in the levels of FSH levels compared to control. Therefore, it is possible that the phthalate-induced decrease in FSH levels contributed to the higher in incidence of atresia and the decrease in follicle growth observed in the F1 phthalate-exposed ovaries compared to control. It is important to note that in the time-period assessed in this study, phthalate exposure reduced both FSH and estradiol levels. However, over time, it is possible that the low levels of estradiol will not be able to exert enough negative feedback control at the level of the anterior pituitary and hypothalamus. As a result, FSH levels may raise with time. Thus, future studies should examine the impact of phthalates on the hypothalamus and pituitary and determine whether FSH levels increase over time.
A previous study using the same cohort of mice used in this study showed that the prenatal exposure to the phthalate mixture decreased anogenital distance (AGD) in F1 female mice on PND 60 (Zhou et al., 2017b). AGD is determined by androgen levels, including testosterone, and thus, it is possible that the reduction in AGD may be due to a reduction in maternal androgen levels brought on by the phthalate mixture exposure (Jensen et al., 2016; Kita et al., 2016; Swan, 2008; Zhou et al., 2017b).
The mixture-induced decrease in progesterone, testosterone, and estradiol levels observed in the F1 generation may be due to a mixture-induced decrease in expression of Star, Cyp11a1, and Cyp19a1. Star is responsible for uptake of cholesterol into the mitochondria, a required step for the production of steroid hormones. Thus, decreased expression of Star could lead to decreased steroidogenesis. Cyp11a1 is responsible for the conversion of cholesterol to pregnenolone, a precursor hormone in the steroidogenesis pathway. Thus, decreased expression of Cyp11a1 could lead to decreased levels of downstream hormones, including progesterone, testosterone, and estradiol. Cyp19a1 is responsible for the conversion of androgens to estradiol. Thus, decreased expression of Cyp19a1 could lead to decreased levels of estradiol.
In the F1 generation, the mixture increased the percentage of primordial follicles compared to the control. Additionally, the mixture caused a borderline decrease in the percentage of preantral follicles and a decrease in the percentage of antral follicles compared to control. These results indicate that the mixture may be interfering with the ability of immature follicle types to properly transition into the more mature follicle types needed for fertilization and reproduction. Exposure to the phthalate mixture also decreased estradiol levels. Antral follicles are important for the production of estradiol. Thus, it is not surprising that the mixture-induced decrease in the percentage of mature follicle types correlates with the mixture-induced decrease in estradiol levels. Both normal numbers of antral follicles and the production of estradiol are essential in the peri-ovulatory period, and the synthesis of estradiol is also necessary for increasing the luteinizing hormone (LH) in the body, which serves to cause follicles to ovulate (Hannon & Flaws, 2015; Stocco, Telleria, & Gibori, 2007). It is possible that prenatal exposure to the phthalate mixture may lead to a lack in the development of more mature follicle types and estradiol, which could adversely affect female reproduction. Thus, future studies should examine if prenatal exposure to the phthalate mixture affects levels of LH as well as ovulation in adult female mice.
This study focused on the effects of the phthalate mixture on folliculogenesis and steroidogenesis in the F1 generation of adult female mice. However, it is important for future studies to investigate the effects of the mixture on the subsequent (F2 and F3) generations. This is because previous studies determined that prenatal exposure to DEHP decreased the number and percent of primordial, primary, and antral follicles, and decreased the levels of estradiol and progesterone in the F2 generation at PND 8, 21, and 60 (Rattan et al., 2019; Rattan et al., 2018). Additionally, in the F3 generation, prenatal DEHP exposure changed the numbers and percentages of follicles at PND 1, 8, and 21, and changed gene expression in multiple pathways needed for ovarian function (Rattan et al., 2019; Rattan et al., 2018). Based on these previous results, it is possible that prenatal exposure to an environmentally relevant mixture may lead to changes in folliculogenesis and steroidogenesis in the F2 and F3 generations. Therefore, future studies should investigate the multigenerational and transgenerational effects of the phthalate mixture on folliculogenesis and steroidogenesis in fully cycling adult mice.
In conclusion, our data indicate that prenatal exposure to an environmentally relevant phthalate mixture adversely affects ovarian steroidogenesis and folliculogenesis in the F1 generation of adult female mice. Specifically, prenatal exposure to the mixture led to changes in hormone and gene expression as well as the number and percentage of ovarian follicles in the F1 offspring.
Highlights.
Prenatal phthalate mixture exposure decreased ovarian hormone levels in F1 female mice
Prenatal phthalate mixture exposure decreased FSH levels in F1 female mice
Prenatal phthalate mixture exposure decreased steroidogenesis in F1 female mice
Prenatal phthalate mixture exposure altered folliculogenesis in F1 female mice
Prenatal phthalate mixture exposure increased follicular atresia in F1 female mice
ACKNOWLEDGEMENTS
This work was supported by Dr. Flaws’ Laboratory Group, R01 ES032163 (JF), R25 ES025059, a Toxicology Scholar Award (CZ), and a Billie A. Field Fellowship (EB). We thank the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core that is supported by the Eunice Kennedy Shriver NICHD Grant R24 HD102061 for assistance with measuring serum hormone levels.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors do not have any conflicts of interest or potential conflicts of interest to disclose.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Barakat R, Lin PC, Park CJ, Zeineldin M, Zhou S, Rattan S, … Ko CJ (2020). Germline-dependent transmission of male reproductive traits induced by an endocrine disruptor, di-2-ethylhexyl phthalate, in future generations. Sci Rep, 10(1), 5705. doi: 10.1038/s41598-020-62584-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm E, Rattan S, Gao L, & Flaws JA (2018). Prenatal Exposure to Di(2-Ethylhexyl) Phthalate Causes Long-Term Transgenerational Effects on Female Reproduction in Mice. Endocrinology, 159(2), 795–809. doi: 10.1210/en.2017-03004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm E, Zhou C, Gao L, & Flaws JA (2020). Prenatal exposure to an environmentally relevant phthalate mixture accelerates biomarkers of reproductive aging in a multiple and transgenerational manner in female mice. Reprod Toxicol, 98, 260–268. doi: 10.1016/j.reprotox.2020.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, & Flaws JA (2019). Subchronic Exposure to Di(2-ethylhexyl) Phthalate and Diisononyl Phthalate During Adulthood Has Immediate and Long-Term Reproductive Consequences in Female Mice. Toxicol Sci, 168(2), 620–631. doi: 10.1093/toxsci/kfz013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Lewis LR, Borkowski G, & Flaws JA (2020). Exposure to di(2-ethylhexyl) phthalate and diisononyl phthalate during adulthood disrupts hormones and ovarian folliculogenesis throughout the prime reproductive life of the mouse. Toxicol Appl Pharmacol, 393, 114952. doi: 10.1016/j.taap.2020.114952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y-L, Xu Y-R, Yang W-X, & Sun Y (2018). The role of FSH and TGF-β superfamily in follicle atresia. Aging, 10(3), 305–321. doi: 10.18632/aging.101391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser BC, Laven JS, Tarlatzis BC, Moley KH, Critchley HO, Taylor RN, … Petraglia F (2011). Sex steroid hormones and reproductive disorders: impact on women’s health. Reprod Sci, 18(8), 702–712. doi: 10.1177/1933719111405068 [DOI] [PubMed] [Google Scholar]
- Gray LE Jr., Ostby J, Furr J, Price M, Veeramachaneni DNR, & Parks L (2000). Perinatal Exposure to the Phthalates DEHP, BBP, and DINP, but Not DEP, DMP, or DOTP, Alters Sexual Differentiation of the Male Rat. Toxicological Sciences, 58(2), 350–365. doi: 10.1093/toxsci/58.2.350 [DOI] [PubMed] [Google Scholar]
- Grindler NM, Allsworth JE, Macones GA, Kannan K, Roehl KA, & Cooper AR (2015). Persistent organic pollutants and early menopause in U.S. women. PLoS One, 10(1), e0116057. doi: 10.1371/journal.pone.0116057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon PR, & Flaws JA (2015). The effects of phthalates on the ovary. Front Endocrinol (Lausanne), 6, 8. doi: 10.3389/fendo.2015.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman CG (1944). Some New Observations on the Vaginal Smear of the Rat. Yale J Biol Med, 17(1), 99–112. [PMC free article] [PubMed] [Google Scholar]
- Heudorf U, Mersch-Sundermann V, & Angerer J (2007). Phthalates: toxicology and exposure. Int J Hyg Environ Health, 210(5), 623–634. doi: 10.1016/j.ijheh.2007.07.011 [DOI] [PubMed] [Google Scholar]
- Jensen TK, Frederiksen H, Kyhl HB, Lassen TH, Swan SH, Bornehag CG, … Andersson AM (2016). Prenatal Exposure to Phthalates and Anogenital Distance in Male Infants from a Low-Exposed Danish Cohort (2010–2012). Environ Health Perspect, 124(7), 1107–1113. doi: 10.1289/ehp.1509870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita DH, Meyer KB, Venturelli AC, Adams R, Machado DL, Morais RN, … Martino-Andrade AJ (2016). Manipulation of pre and postnatal androgen environments and anogenital distance in rats. Toxicology, 368–369, 152–161. doi: 10.1016/j.tox.2016.08.021 [DOI] [PubMed] [Google Scholar]
- Meling DD, Warner GR, Szumski JR, Gao L, Gonsioroski AV, Rattan S, & Flaws JA (2020). The effects of a phthalate metabolite mixture on antral follicle growth and sex steroid synthesis in mice. Toxicol Appl Pharmacol, 388, 114875. doi: 10.1016/j.taap.2019.114875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niermann S, Rattan S, Brehm E, & Flaws JA (2015). Prenatal exposure to di-(2-ethylhexyl) phthalate (DEHP) affects reproductive outcomes in female mice. Reprod Toxicol, 53, 23–32. doi: 10.1016/j.reprotox.2015.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlett LE, Calafat AM, & Swan SH (2013). Women’s exposure to phthalates in relation to use of personal care products. J Expo Sci Environ Epidemiol, 23(2), 197–206. doi: 10.1038/jes.2012.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research, 29(9), e45–e45. doi: 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S, Beers HK, Kannan A, Ramakrishnan A, Brehm E, Bagchi I, … Flaws JA (2019). Prenatal and ancestral exposure to di(2-ethylhexyl) phthalate alters gene expression and DNA methylation in mouse ovaries. Toxicol Appl Pharmacol, 379, 114629. doi: 10.1016/j.taap.2019.114629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S, Brehm E, Gao L, Niermann S, & Flaws JA (2018). Prenatal exposure to di(2-ethylhexyl) phthalate disrupts ovarian function in a transgenerational manner in female mice. Biol Reprod, 98(1), 130–145. doi: 10.1093/biolre/iox154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimon-Dahari N, Yerushalmi-Heinemann L, Alyagor L, & Dekel N (2016). Ovarian Folliculogenesis. Results Probl Cell Differ, 58, 167–190. doi: 10.1007/978-3-319-31973-5_7 [DOI] [PubMed] [Google Scholar]
- Schettler T (2006). Human exposure to phthalates via consumer products. Int J Androl, 29(1), 134–139; discussion 181–135. doi: 10.1111/j.1365-2605.2005.00567.x [DOI] [PubMed] [Google Scholar]
- Stocco C, Telleria C, & Gibori G (2007). The molecular control of corpus luteum formation, function, and regression. Endocr Rev, 28(1), 117–149. doi: 10.1210/er.2006-0022 [DOI] [PubMed] [Google Scholar]
- Swan SH (2008). Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res, 108(2), 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittassek M, Koch HM, Angerer J, & Bruning T (2011). Assessing exposure to phthalates - the human biomonitoring approach. Mol Nutr Food Res, 55(1), 7–31. doi: 10.1002/mnfr.201000121 [DOI] [PubMed] [Google Scholar]
- Yazdy MM, Coull BA, Gardiner JC, Aguiar A, Calafat AM, Xiaoyun Y, … Korrick SA (2018). A possible approach to improving the reproducibility of urinary concentrations of phthalate metabolites and phenols during pregnancy. J Expo Sci Environ Epidemiol, 28(5), 448–460. doi: 10.1038/s41370-018-0050-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, & Flaws JA (2017). Effects of an Environmentally Relevant Phthalate Mixture on Cultured Mouse Antral Follicles. Toxicol Sci, 156(1), 217–229. doi: 10.1093/toxsci/kfw245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Gao L, & Flaws JA (2017a). Exposure to an Environmentally Relevant Phthalate Mixture Causes Transgenerational Effects on Female Reproduction in Mice. Endocrinology, 158(6), 1739–1754. doi: 10.1210/en.2017-00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Gao L, & Flaws JA (2017b). Prenatal exposure to an environmentally relevant phthalate mixture disrupts reproduction in F1 female mice. Toxicol Appl Pharmacol, 318, 49–57. doi: 10.1016/j.taap.2017.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, … Vom Saal FS (2012). Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology, 153(9), 4097–4110. doi: 10.1210/en.2012-1422 [DOI] [PMC free article] [PubMed] [Google Scholar]


