Abstract
BACKGROUND:
Sickle cell disease (SCD) is characterized by frequent, unpredictable pain episodes and other vaso-occlusive crises (VOCs) leading to significant healthcare utilization. VOC frequency is often an endpoint in clinical trials investigating novel therapies for this devastating disease.
PROCEDURE:
The Consortium for the Advancement of Sickle Cell Research (CASiRe) is an international collaboration investigating clinical severity in SCD using a validated questionnaire and medical chart review standardized across four countries (United States, United Kingdom, Italy and Ghana).
RESULTS:
This study, focused on pain crisis incidence and healthcare utilization, included 868 patients, equally represented according to age and gender. HgbSS was the most common genotype. Patients from Ghana used the Emergency Room/Day Hospital for pain more frequently (annualized mean 2.01) than patients from other regions (annualized mean 1.56 U.S.;1.09 U.K.; 0.02 Italy), while U.K. patients were hospitalized for pain more often (annualized mean: U.K. 2.98) than patients in other regions (annualized mean 1.98 U.S.; 1.18 Ghana; Italy 0.54). Italy’s hospitalization rate for pain (annualized mean: 0.57) was nearly 20 times greater than its emergency room/day hospital only visits for pain (annualized mean: 0.03). When categorized by genotype and age, similar results were seen.
CONCLUSIONS:
Geographic differences in pain crisis frequency and healthcare utilization may correlate with variable organization of healthcare systems among countries and should be considered regarding trial design, endpoints, and analysis of results when investigating novel agents for clinical benefit.
Keywords: Sickle Cell disease, pain crisis, complications, geographic differences, health resources utilization
Introduction
Sickle cell disease (SCD) is the most common monogenic disease in the world, with an incidence of over 400,000 new births per year and increasing numbers in high-income countries due to migration patterns. SCD affects over 100,000 people in the U.S.,1 and millions of people worldwide.2,3 The disease causes frequent, unpredictable, pain crises and other vaso-occlusive crises (VOCs), leading to significant health care utilization.3 Pain varies in terms of frequency, severity, and location4 and is directly correlated with mortality.5
Pain crises are the most common outward manifestation of SCD; the frequency of pain crises and other VOCs are often used as a primary study outcome in clinical trials.6 Hydroxyurea obtained FDA approval based on improvement of annual VOC frequency, which was the primary endpoint in the Multicenter Study of Hydroxyurea.7 Recent investigations of Prasugrel,8 Crizanlizumab,9 and L-Glutamine10 also used VOC, including pain crises, as a primary endpoint of their studies. However, many sickle cell patients, particularly where sociocultural, familial or individual attitudes are involved, may suffer silently at home, creating a potential problem for clinical trial design and endpoints. These issues can be magnified by healthcare system organization, home opioid use and other drivers of healthcare utilization for the treatment of pain.
The approach to pain crisis management varies from country to country and relates to differences in healthcare utilization, including the nature of home-based pain treatment and medication use, Emergency Room/Day Hospital (ER/DH) management or, in severe cases, admission for treatment with intravenous medications including opioids.11–13 While several clinical trials have used pain as a comparator of disease improvement, only the DOVE study14,15 reported on geographic differences in the management and severity of pain, noting that European patients had higher hospitalizations than those living in the Americas or Northern Africa. Sub-Saharan African sites had the least healthcare use of all the groups.14 Furthermore, Kanter et al reported that patients in North American sites in the DOVE study described a higher pain intensity and greater use of opioids than patients from other sites did.15 However, these studies did not detail the rate of health care utilization per patient.
This study seeks to evaluate the patterns and frequency of healthcare utilization in a large, multi-institutional, international cohort of both pediatric and adult patients, and describe the geographic differences related to pain rate and healthcare utilization among individual countries around the world.
Methods
The Consortium for the Advancement of Sickle Cell Research (CASiRe) group is an international, multi-institutional collaboration studying the clinical severity of SCD in all ages on a global scale using a validated questionnaire and medical chart review standardized over three continents and four countries (U.S., Italy, Ghana, and the U.K.), as described in previous publications.16,17
All CASiRe sites were included in this analysis. Patients were enrolled between 2011–2017. After receiving IRB approval at each institution, informed consent and assent were obtained from the study subjects and the questionnaire was answered by the parent/legal guardian and/or the patient. Baseline and current laboratory studies were collected. In the United States, Ghana, and Naples, Italy site, a medical chart review and validated questionnaire were used to document the total number of pain episodes requiring an ER/DH visit and those requiring hospitalization during the previous 1 year. In the U.K. and Padua site, the same data was abstracted from a registry. The standard clinical criteria of diagnosis of SCD and report of pain were used.18
Data were analyzed separately for each country, evaluating the number of pain episodes and the pattern of healthcare utilization (see figure and Supplementary Table). Thus we investigated the SCD pain crisis and healthcare utilization patterns in all four countries in three geographic regions of the world (U.S., Europe [U.K. and Italy], and Africa [Ghana]). Secondary analysis by genotype and age was completed within each geographic region to assess pain crises per year requiring ER/DH visits (PC-ER/DH), hospitalizations (PC-Hosp), and pain crisis frequency and care utilization variability.
Statistics
Descriptive statistical analysis, including mean, range, standard deviation, and percentages, was performed using SPSS 26.0 (IBM SPSS Statistics for Windows, Version 26.0). T-Test and one-way anova were utilized comparing the mean PC/year in Index Countries (e.g. Ghana) to all other regions combined (e.g. U.K., Italy and U.S.), by age groups (pediatrics vs adults), country, genotype, and categorized groups of annualized pain crises/year among all countries. Significance was set at p≤0.05.
Results
Demographics
This cohort included 868 patients in the study analysis. Demographic information is available in Table 1. Overall, there was a roughly equal number of children and adults (18 and over) as well as females and males. Patients with the HgbSS genotype represented the largest group, then HgbSC, and patients with HgbSβ0Thal and HgbSβ+Thal made up the remainder of the cohort. The geographic distribution of the cohort was as follows: 42.1% of patients were from Ghana sites, 28.1% from the U.S., 20.4% from the U.K., and 9.4% from Italy. Of the 868 patients enrolled in the study, 867 patients had information regarding disease-modifying therapy. Close to 25% of patients were on hydroxyurea, while nearly 11% were on chronic transfusion therapy. Only 20 patients were on both chronic transfusion therapy and hydroxyurea. Complete disease-modifying therapy use by country is reported in Table 1.
Table 1:
Demographics Across All CASIRE Sites
| Age Group (N=868) | N | % | |||
|---|---|---|---|---|---|
| Pediatric (<18 y/o) | 443 | 51 | |||
| Adult (≥ 18 y/o) | 425 | 49 | |||
| Country (N=868) | |||||
| US | 245 | 28.1 | |||
| Italy | 81 | 9.4 | |||
| UK | 177 | 20.4 | |||
| Ghana | 365 | 42.1 | |||
| Gender (N=868) | |||||
| Female | 448 | 51.6 | |||
| Male | 420 | 48.4 | |||
| Genotype (N=868) | |||||
| HgbSS or HgbSS with Alpha Thal Trait | 656 | 75.6 | |||
| HgbSC | 162 | 18.7 | |||
| HgbSBeta+ | 28 | 3.2 | |||
| HgbSBeta0 | 22 | 2.5 | |||
| Disease Modifying therapies by Country* | |||||
| Country | Hydroxyurea (N=867) | Country | Chronic Blood Transfusion (N=867) | ||
|---|---|---|---|---|---|
| Yes | No | Yes | No | ||
| N (%) | N (%) | N (%) | N (%) | ||
| US (N=245) | 108(44.1) | 137(55.9) | US | 53(21.6) | 192(78.4) |
| Italy(N=81) | 18(22.2) | 63(77.8) | Italy | 15(18.5) | 66(81.5) |
| UK (N=176) | 45(25.6) | 131(74.4) | UK | 25(14.2) | 151(85.8) |
| Ghana (N=365) | 10(2.7) | 355(97.3) | Ghana | 0(0) | 365(100) |
20 patients were on both hydroxyurea and chronic transfusion therapy
Entire Cohort
For the whole population, the mean PC-ER/DH rate was 1.51 visits/year (standard deviation (SD) 2.88), and the mean PC-Hosp rate was 1.72 visits/year (SD 3.21). When patients were analyzed by age, children reported lower mean PC-ER/DH visits (0.93 visits/year; SD 0.74) but higher mean PC-Hosp visits (1.84 visits/year; SD 3.47) as compared to adult patients (PC-ER/DH 2.11 (SD 3.47); PC-Hosp 1.59 (SD 2.91)).
Genotype
The mean number of PC-ER/DH for patients with HgbSS/HgbSβ0Thal was 1.48 visits/year (SD 2.90; p=0.006) and 1.83 visits/year (SD 3.34; p=0.006) for PC-Hosp. For patients with HgbSC/Sβ+Thal, the mean number of PC-ER/DH was 1.61 visits/year (SD 2.81; p=0.142) and for PC-Hosp 0.93 visits/year (SD 2.65; p=0.142).
Geographic Region (Figure 1A)
Fig. 1.
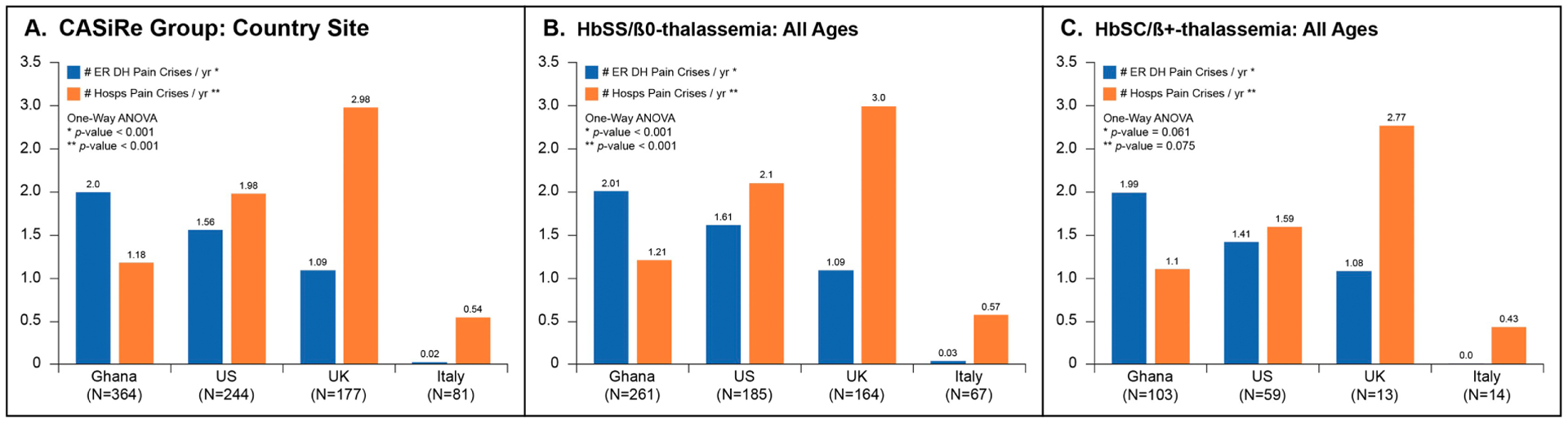
Mean pain episodes requiring ER/DH visit (blue) and hospitalizations (orange) by country: Ghana, US, UK, Italy: (A) Whole Group; (B) HgbSS/SBOThal; (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In Ghana, patients reported a significantly higher mean number of PC-ER/DH visits and lower PC-Hosp rate than other countries. Patients in the U.K. noted a significantly higher rate of PC-Hosp and lower rate of PC-ER/DH of any group except Italy. U. S. patients described PC-ER/DH visits and PC-Hosp rates that were in between Ghana and the U. K. Italian patients related a significantly lower PC-ER/DH rate and PC-Hosp rate of any country.
Genotype and Geographic Region
Ghanaian patients with HgbSS/HgbSβ0Thal (Figure 1B) had a significantly higher rate of PC-ER/DH and significantly lower PC-Hosp compared to the other groups. The U.K. and Italy patients had a significantly lower PC-ER/DH rate and higher PC-Hosp rate compared to the U.S. and Ghana. In patients with HgbSC/Sβ+Thal (Figure 1C), Ghanaian patients had a trend toward higher PC-ER/DH rate compared to the other groups, while the U.K. and Italy group had close to significant lower rates of PC-ER/DH compared to the other countries. PC-Hosp rate for HgbSC/Sβ+Thal patients was similar among all three countries.
Number of Crises (Supplemental Table 1)
Ghanaian patients had the highest ER/DH use compared to their European or American peers. Conversely, Italian patients had the lowest use, total and per person, of the ER/DH than any other country; in addition, 62% of patients from Italy had no admissions, and none had more than 5 admissions. The U.S. had the highest total number of patients requiring an admission; however, the U.K. had the highest number of patients with >5 admissions (18% vs. 0% in Italy, 8.7% in the U.S. and 4.4% for Ghana).
Age, Genotype, and Geographic Region
When patients were stratified regionally by age and genotype, ER/DH pain crisis visit rates varied significantly across countries for pediatric HgbSS/Sβ0Thal and HgbSC/Sβ+Thal patients (Figures 2A and B). Ghana reported higher PC-ER/DH rates in HgbSS/Sβ0Thal pediatrics patients (Figure 2A) than those from the US, U.K, and Italy. Hospitalization rates among the countries were also significantly different in children with HgbSS/Sβ0Thal (Figure 2A). The UK recorded the highest annualized mean for PC-Hosp, nearly twice the rate for the U.S, more than 2 times the rate for Ghana, and more than 5 times for Italy; however, in Italy the PC-Hosp rates were 20 times greater than the PC-ER/DH visits.
Fig. 2.
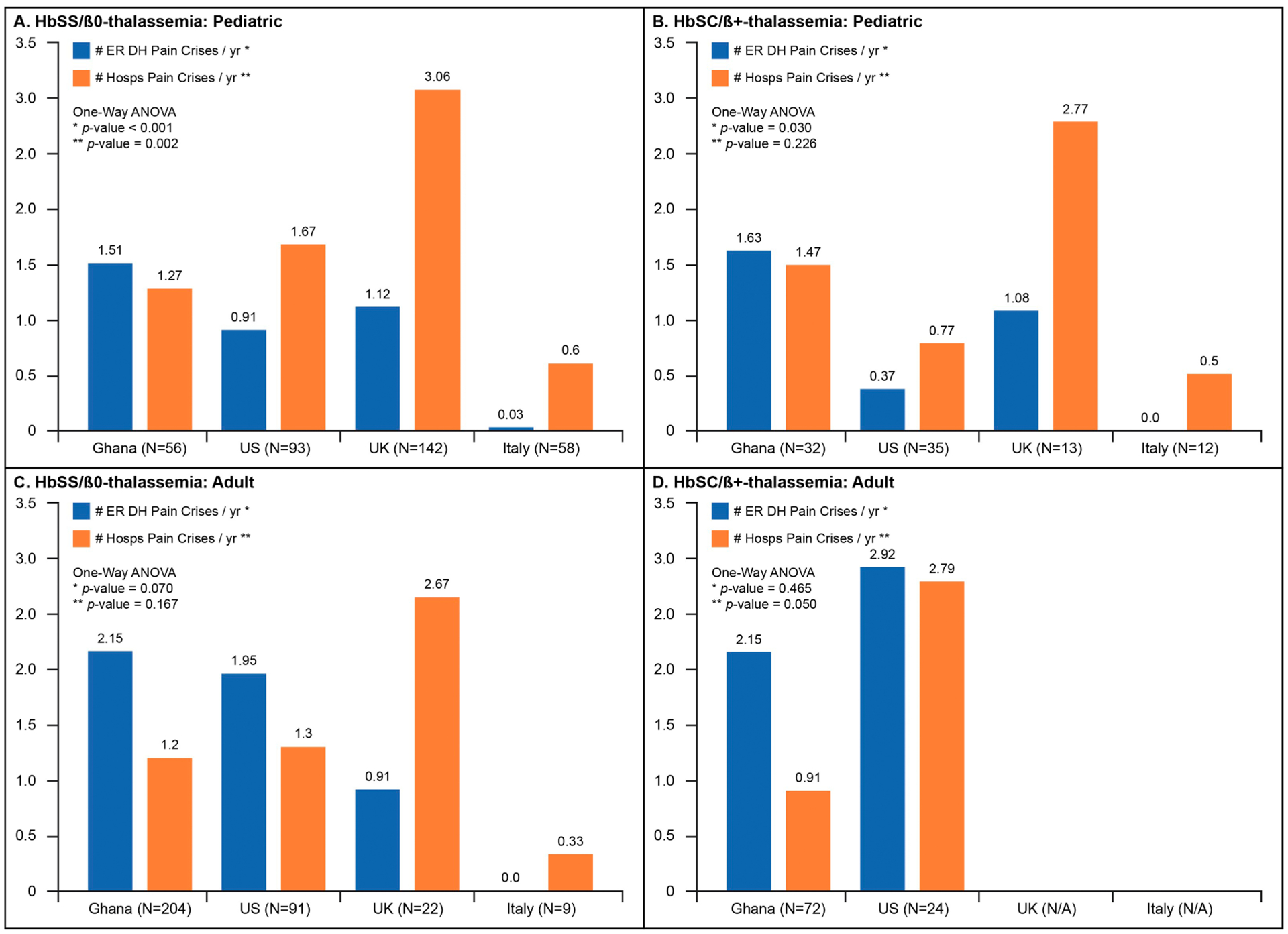
Mean pain episodes requiring ER/DH Visit (blue) or hospitalizations (orange) comparing each country to the others, categorized by genotype and age. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In pediatric subjects with HgbSC or Sβ+Thal, Ghanaian children reported the highest PC-ER/DH rate when compared to the other groups (Figure 2B). There was no statistical difference in hospitalization frequencies (PC-Hosp) among the four countries within HgbSC /Sβ+Thal children (p=0.226, Figure 2B).
When evaluating adults with HgbSS/Sβ0Thal (Figures 2C and 2D), patients from Ghana and the U.S. reported more than twice the rate of PC-ER/DH episodes than the European countries with a difference not statistically significant. Hospitalization patterns across CaSiRe countries (Figure 2C) were highest in the UK for HgbSS/Sβ0Thal adults, which doubled the rates in Ghanaian and U.S. patients; Italy recorded the lowest annual PC-Hosp rates for HgbSS/Sβ0Thal adults. However, similar to their pediatric counterparts, Italian adult HgbSS/Sβ0Thal patients experienced PC-Hosp episodes greater than 10 times their PC-ER/DH rates. HgbSC/Sβ+Thal adults in Ghana and the U.S. experienced similar patterns of ER/DH and hospitalization rates (Figure 2D). There were not enough HgbSC/Sβ+Thal adults in the U.K. and Italy to include in the comparison (Figure 2D).
Discussion
This is the first evaluation of geographic differences in pain crisis patterns in a large, cross-sectional cohort study of nearly 900 pediatric and adult patients, allowing an evaluation of healthcare utilization practices related to pain crisis care among four countries and three continents across the lifespan. Our data suggest that Ghanaian patients more often treated their pain in an outpatient setting, whereas patients in the U.K. and Italy were more likely to be hospitalized for their pain, and patients in the U.S. had similar rates of ER/DH use and hospitalization, highlighting diversity in health resource utilization for the treatment of pain crises across countries. These geographic contrasts could result from regional differences in genotype and disease severity, variation in national healthcare system organization and infrastructure, economics and home medication availability within countries, and other cultural and social factors related to pain management.15,18
Several reports14,15,19 stemming from the DOVE study8 provided a glimpse of geographical differences in pain crisis patterns across continents. Inusa et al14 reported that 238 of the 341 patients enrolled on the DOVE study had a pain episode, and that the percentage of VOC associated with hospitalization was highest in Europe (67.7%), followed by the Americas (46.5%) with the least (26%) being in Sub-Saharan Africa (SSA).14 Kanter et al15 noted that patients from the Americas and Europe reported a higher pain intensity and that opioid use was much higher in the Americas than in other regions.15 In addition, the availability of home opioid use may contribute to the rate, intensity and perception of pain, and could influence when patients may seek hospital-based care.15
In contrast to the DOVE study, our patient cohort consists of 868 pediatric and adult subjects compared to just 341 pediatric patients in the DOVE study, granting us the ability to report for the first time how pain is managed across all ages in three different geographic regions of the world. Moreover, the DOVE study did not report on individual patient rate of VOC, nor health care utilization for their VOC; only the percent of VOCs requiring admission was reported. Our study not only reports on individual patient’s VOC rate, but also their healthcare utilization for that VOC. In addition, data from individual countries was reported separately in our study, rather than grouped in geographic regions (Europe, SSA, Americas, etc), allowing a closer look at specific countries’ rates of healthcare utilization. In the US, the hospitalization and ER utilization rate was similar to each other compared to other countries. Ghana had the highest mean number of PC-ER/DH compared to other regions. However, the most striking contrast between hospital and ER utilization was found in the U.K. and Italy, where the hospitalization rate was at least twice the ER visit only rate; in Italy the management of pain crises exclusively in the ER is almost zero, favoring hospital admissions. Finally, our patient population represents a real world group of patients enrolled in the CASiRe study without selection bias toward VOC rate.
Previous research by this group demonstrated that significant differences in genotype and disease severity may potentially explain differences in healthcare utilization. The age of first pain crisis in the U.S. is much younger than it is in Ghana or Italy,20 potentially suggesting higher disease severity in the U.S. and/or increased awareness and knowledge of the disease. Indeed, over sixty percent of patients in the U.S. were on at least one disease modifying therapy, which could also be a marker for higher disease severity. Moreover, unlike the U.S. and U.K., Italy and Ghana do not have newborn screening, leading to a higher age at diagnosis;20 in Italy, milder pain episodes could have gone unreported and a child might have accessed the ER without receiving a diagnosis of SCD even if pain was present.21 Further, the high proportion of Sβ+Thal patients in Italy, approximately 21% of their SCD population,17 may influence the relatively low observed rates of PC-Hosp and PC-ER/DH in that group. However, both Italy and the U.K. independently reported double the rate of PC-Hosp compared to PC-ER/DH, leading to the consideration that there are other reasons for the disparity in healthcare utilization seen in these countries.
Healthcare system organization and availability of home medication for pain may also play a role in how a patient utilizes care (Table 2). In the U.K. and Italy, healthcare is a service that is provided free of charge for their residents; patients in those countries most likely benefit and frequently seek admission rather than treat at home or through the ER/DH. Home opioid use in Italy and the U.K. is tightly regulated22,23 and not routine in Italy.23 In Ghana, while a national health insurance plan is available to all citizens, only 35% of people nationally, mostly in urban areas, participate in the program;24 the patient/parent would potentially be responsible for the cost of all therapy needed for their care, including admission to hospital.25 For Ghanaian SCD patients, limited access to adequate home pain medications, inadequate financial resources to pay for inpatient costs, and a lack of local general physicians who might be able to respond to the patients’ care needs may be contributing factors for the ER/DH as the primary site of pain management, resulting in lower observed hospitalizations. Worse, patients who present to the emergency room may be turned away from health facilities because there are no available beds.26 In the U.S., although most patients with SCD are covered by federal/state programs such as Medicaid and Medicare, our data suggest that, despite having a higher number of pain episodes per patient than any other group, U.S. patients often prefer to treat pain at home until it becomes unmanageable, potentially related to the routine availability, accessibility and acceptability of home opioids and other strong pain medications.15,27
Table 2:
Type of healthcare system organization and use of opioids by country
| Parameter | Country | |||
| Ghana24 | US30 | Italy31 | U.K.32 | |
| Healthcare system organization | National System, not universal | Mixed public/private, not universal | Universal, public health system, state/region partnership, some private insurance | Universal, national health system |
| Number of citizens covered | 35%, mostly in the city, 65% uninsured | 40% public, 50% private, 10% uninsured | 100%, some have additional private insurance | 100%, some have additional private insurance |
| Legally allowed opioid use at home | Yes33 | Yes | Yes23 | Yes22 |
| Common to use opioid at home | No34 | Yes19 | No23 | Yes |
| Reason if No | cost, availability | NA | custom | NA |
NA = not applicable
Cultural and social aspects may also contribute to patients’ perception of their pain, particularly in Ghana, where tribal and familial traditions may affect healthcare beliefs with regard to access and utilization.18 While the majority of patients with SCD in Italy and the U.K. were born in their respective countries, their parents emigrated from other countries, particularly Ghana and Nigeria.17 Because emigres to Italy are relatively recent compared to the U.K. or U.S., it is possible that significant language barriers could prevent patients from seeking care or lead them to seek admission rather than outpatient therapy. In addition, ongoing systemic bias and racism with regard to healthcare treatment and outcomes may be a factor in the U.S.,28 the U.K.,29 and Italy, while stigmatization related to cost of care, blame, and public misconception of patients with SCD has been reported in Ghana.30
Limitations
There are several limitations to this study. This cross-sectional cohort study lacked a prospective arm, limiting any therapeutic differences among groups of patients. Although there could be a certain degree of recall bias related to patient reported data, a chart review obtained data directly from patient charts rather than relying solely on the patient for information. Patient-specific factors, and not necessarily strict criteria, often determine the necessity for admission; patients who are admitted often may be more comfortable with that decision than those patients who are rarely hospitalized. It is also possible that pain crisis treatment protocols and guidelines have changed since the period of data collection. Disease severity and availability of disease-modifying therapy could lead to confounding results.
Conclusion
Our study, evaluating geographic differences in healthcare utilization patterns in the management of pain crises, suggests that treatment is influenced not only by where patients live and their cultural differences, but also by how their healthcare system is organized and the relative availability of medical resources. Because VOCs, including pain, are often the primary endpoints in clinical studies, it is critical to consider the geographic differences of VOC frequency and healthcare organization and utilization among countries when considering clinical trial design, inclusion criteria, and analysis of results, so that country by country and global analysis could be compared and shown together.
Supplementary Material
Funding
This study was supported in part by research funding from Grant # NIMHD T37MD001425 to AC.
Competing interests
The following authors declare conflict of interest:
A Campbell: research funding and consultancy from Global Blood Therapeutics (GBT), Novartis; and consultancy for bluebird bio; D Manwani: research funding from Grifols; consultancy for Novartis, Pfizer, Global Blood Therapeutics; B Andemariam: consultancy for Bluebird bio, CRISPR/Vertex, Forma Therapeutics, Global Blood Therapeutics, Hemanext, Novartis, NovoNordisk, Cyclerion, Terumo, Roche, Sanofi-Genzyme; research funding from Global Blood Therapeutics, Imara, Novartis; B Inusa: education funding from Novartis AstraZeneca, Global Blood Therapeutics, Celgene, Vertex; C Strunk: consultancy for Global Blood Therapeutics, Forma Therapeutics, Novartis and Medunik USA; R Colombatti: research funding from Global Blood Therapeutics, BlueBird Bio; consultancy for Novartis, Addmedica, NovoNordisk; C Piccone: consultancy for Global Blood Therapeutics and Novartis; W Zempsky: consultancy for Glycomimetics and GlaxoSmithKleine. No disclosures to declare from the other co-authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Availability of data and materials
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
References
- 1.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010. April;38(4 Suppl):S512–21. [DOI] [PubMed] [Google Scholar]
- 2.Piel FB, Steinberg MH, Rees DC. Sickle Cell Disease. N Engl J Med. 2017. April 20;376(16):1561–73. [DOI] [PubMed] [Google Scholar]
- 3.Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017. July 15;390(10091):311–23. [DOI] [PubMed] [Google Scholar]
- 4.McClish DK, Smith WR, Dahman BA, Levenson JL, Roberts JD, Penberthy LT, et al. Pain site frequency and location in sickle cell disease: the PiSCES project. Pain. 2009. September;145(1–2):246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994. June 9;330(23):1639–44. [DOI] [PubMed] [Google Scholar]
- 6.Farrell AT, Panepinto J, Carroll CP, Darbari DS, Desai AA, King AA, et al. End points for sickle cell disease clinical trials: patient-reported outcomes, pain, and the brain. Blood Adv. 2019. December 10;3(23):3982–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, et al. Effect of Hydroxyurea on the Frequency of Painful Crises in Sickle Cell Anemia [Internet]. Vol. 332, New England Journal of Medicine. 1995. p. 1317–22. Available from: 10.1056/nejm199505183322001 [DOI] [PubMed] [Google Scholar]
- 8.Heeney MM, Hoppe CC, Abboud MR, Inusa B, Kanter J, Ogutu B, et al. A Multinational Trial of Prasugrel for Sickle Cell Vaso-Occlusive Events. N Engl J Med. 2016. February 18;374(7):625–35. [DOI] [PubMed] [Google Scholar]
- 9.Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, Friedrisch J, et al. Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N Engl J Med. 2017. February 2;376(5):429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niihara Y, Miller ST, Kanter J, Lanzkron S, Smith WR, Hsu LL, et al. A Phase 3 Trial of l-Glutamine in Sickle Cell Disease. N Engl J Med. 2018. July 19;379(3):226–35. [DOI] [PubMed] [Google Scholar]
- 11.Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014. September 10;312(10):1033–48. [DOI] [PubMed] [Google Scholar]
- 12.Dick M, NHS Sickle Cell and Thalassaemia Screening Programme, Sickle Cell Society. Sickle Cell Disease in Childhood: Standards and Guidelines for Clinical Care. 2010. 90 p. [Google Scholar]
- 13.Colombatti R, Perrotta S, Samperi P, Casale M, Masera N, Palazzi G, et al. Organizing national responses for rare blood disorders: the Italian experience with sickle cell disease in childhood. Orphanet J Rare Dis. 2013. October 20;8:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inusa BPD, Colombatti R, Rees DC, Heeney MM, Hoppe CC, Ogutu B, et al. Geographic Differences in Phenotype and Treatment of Children with Sickle Cell Anemia from the Multinational DOVE Study. J Clin Med Res [Internet]. 2019. November 17;8(11). Available from: 10.3390/jcm8112009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanter J, Heath LE, Knorr J, Agbenyega ET, Colombatti R, Dampier C, et al. Novel findings from the multinational DOVE study on geographic and age-related differences in pain perception and analgesic usage in children with sickle cell anaemia. Br J Haematol. 2019. March;184(6):1058–61. [DOI] [PubMed] [Google Scholar]
- 16.Antwi-Boasiako C, Andemariam B, Colombatti R, Asare EV, Strunk C, Piccone CM, et al. A study of the geographic distribution and associated risk factors of leg ulcers within an international cohort of sickle cell disease patients: the CASiRe group analysis. Ann Hematol. 2020. September;99(9):2073–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell AD, Colombatti R, Andemariam B, Strunk C, Tartaglione I, Piccone CM, et al. An Analysis of Racial and Ethnic Backgrounds Within the CASiRe International Cohort of Sickle Cell Disease Patients: Implications for Disease Phenotype and Clinical Research. J Racial Ethn Health Disparities [Internet]. 2020. May 16; Available from: 10.1007/s40615-020-00762-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MSPHBP M D M Sc Yawn, U.s. Department of Health and Human Services, Buchanan MDG, M D M P H Afenyi-Annan A. Evidence-Based Management of Sickle Cell Disease. CreateSpace; 2014. 164 p. [Google Scholar]
- 19.Dennis-Antwi JA, Ohene-Frempong K, Anie KA, Dzikunu H, Agyare VA, Boadu RO, et al. Relation Between Religious Perspectives and Views on Sickle Cell Disease Research and Associated Public Health Interventions in Ghana. J Genet Couns [Internet]. 2018. September 1; Available from: 10.1007/s10897-018-0296-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Amin N, Nietert P, Kanter J. International Differences in Outpatient Pain Management: A Survey of Sickle Cell Disease. J Clin Med Res [Internet]. 2019. December 3;8(12). Available from: 10.3390/jcm8122136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tartaglione I, Strunk C, Antwi-Boasiako C, Andemariam B, Colombatti R, Asare EV, et al. Age of first pain crisis and associated complications in the CASiRe international sickle cell disease cohort. Blood Cells Mol Dis. 2021. January 2;88:102531. [DOI] [PubMed] [Google Scholar]
- 22.Po’ C, Colombatti R, Cirigliano A, Da Dalt L, Agosto C, Benini F, et al. The management of sickle cell pain in the emergency department: a priority for health systems. Clin J Pain. 2013. January;29(1):60–3. [DOI] [PubMed] [Google Scholar]
- 23.Weisberg DF, Becker WC, Fiellin DA, Stannard C. Prescription opioid misuse in the United States and the United Kingdom: cautionary lessons. Int J Drug Policy. 2014. November;25(6):1124–30. [DOI] [PubMed] [Google Scholar]
- 24.Miceli L, Bednarova R, Rizzardo A, Cuomo A, Riccardi I, Vetrugno L, et al. Opioids prescriptions in pain therapy and risk of addiction: a one-year survey in Italy. Analysis of national opioids database. Ann Ist Super Sanita. 2018. October;54(4):370–4. [DOI] [PubMed] [Google Scholar]
- 25.Nsiah-Boateng E, Aikins M. Trends and characteristics of enrolment in the National Health Insurance Scheme in Ghana: a quantitative analysis of longitudinal data. Glob Health Res Policy. 2018. November 13;3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonfrer I, van de Poel E, Grimm M, Van Doorslaer E. Does the distribution of healthcare utilization match needs in Africa? Health Policy Plan. 2014. Oct;29(7):921–37. [DOI] [PubMed] [Google Scholar]
- 27.Website [Internet]. [cited 2021 Jul 2]. Available from: https://isser.ug.edu.gh/emergency-medical-care-ghana-focus-national-ambulance-service-support-systems-and-beds-healthcare
- 28.Smith WR, Penberthy LT, Bovbjerg VE, McClish DK, Roberts JD, Dahman B, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008. January 15;148(2):94–101. [DOI] [PubMed] [Google Scholar]
- 29.Power-Hays A, McGann PT. When Actions Speak Louder Than Words — Racism and Sickle Cell Disease [Internet]. Vol. 383, New England Journal of Medicine. 2020. p. 1902–3. Available from: 10.1056/nejmp2022125 [DOI] [PubMed] [Google Scholar]
- 30.Dyson SM, Atkin K, Culley LA, Dyson SE, Evans H, Rowley DT. Disclosure and sickle cell disorder: a mixed methods study of the young person with sickle cell at school. Soc Sci Med. 2010. June;70(12):2036–44. [DOI] [PubMed] [Google Scholar]
- 31.Buser JM, Bakari A, Seidu A-A, Osei-Akoto A, Paintsil V, Amoah R, et al. Caregiver Perception of Sickle Cell Disease Stigma in Ghana: An Ecological Approach. J Pediatr Health Care. 2021. January;35(1):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galvani AP, Parpia AS, Foster EM, Singer BH, Fitzpatrick MC. Improving the prognosis of health care in the USA. Lancet. 2020. February 15;395(10223):524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.France G, Taroni F, Donatini A. The Italian health-care system. Health Econ. 2005. September;14(Suppl 1):S187–202. [DOI] [PubMed] [Google Scholar]
- 34.Radiology Management, ICU Management, Healthcare IT, Cardiology Management, Executive Management. 2021. March 17 [cited 2021 Mar 18]; Available from: https://healthmanagement.org/c/it/issuearticle/facts-figures-the-uk-healthcare-system
- 35.Yorke E, Oyebola FO, Otene SA, Klein A. Tramadol: a valuable treatment for pain in Ghana and Nigeria. Curr Med Res Opin. 2019. May;35(5):777–84. [DOI] [PubMed] [Google Scholar]
- 36.Boni FK, Afrane BA. Pain services and prescription of opioid drugs in Ghana. Ghana Med J. 2016. June;50(2):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.


