Abstract
This review estimated the effectiveness of behavior change interventions to increase physical activity (PA) among rural adult cancer survivors. PubMed Medline, CINAHL, and PsychINFO were systematically searched through July 2020. Two independent investigators screened citations to identify studies to increase PA in adults residing in rural areas who had received any cancer diagnosis. Meta-analyses were conducted to assess proportion of participants achieving PA goal, paired mean difference in aerobic PA and strength training, and retention from baseline to post-intervention. Seven studies met inclusion criteria encompassing a total of 722 participants (591 in intervention and 131 controls). Overall quality of evidence was low to medium. The pooled proportion of participants achieving PA goals (150-225 minutes/week) was 39% (95% CI: 18-62%). The mean time spent engaging in aerobic PA increased from baseline to post-intervention (range: 6-52 weeks) was 97.7 minutes/week (95% CI: 75.0-120.4), and the mean difference in time spent on strength training was 12.2 minutes/week (95% CI: −8.3-32.8). The pooled retention rate was 82% (95% CI: 69-92%) at 6-78 weeks. Due to the modest intervention effects, low quality of evidence, and small number of studies, further rigorously designed behavior change interventions, including randomized controlled trials with long-term follow up, are needed to confirm efficacy for increasing PA in rural cancer survivors and to test innovative implementation strategies to enhance reach and effectiveness.
Keywords: Exercise, cancer survivorship, rural health, rural cancer control, adult, review
Background
Over 19 million new cancer cases were reported worldwide in 2020 [1, 2], and this number is expected to surpass 30 million by 2040 [3, 4]. The rapid increase in cancer incidence can be partially attributed to population growth and aging [5, 6]. Advances in early detection and treatment have contributed to the growing number of cancer survivors and an improved long-term survival rate, particularly in high-middle income countries [6, 7]. Thus, cancer control efforts are needed to reduce the risk of cancer recurrence and the development of comorbidities and to improve long-term health outcomes and quality of life in cancer survivors [8].
In addition to reducing cancer risk, physical activity reduces the risk of cancer recurrence and comorbidities and improves physical health and psychological wellbeing after a cancer diagnosis [9–12]. The numerous health benefits of physical activity include reduced risk of all-cause and cardiovascular disease mortality, hypertension, type 2 diabetes, and certain cancers [13]. Despite the well-documented benefits of engaging in regular physical activity, less than 20% of adults meet physical activity recommendations worldwide [13, 14], and fewer adults with a history of cancer meet exercise guidelines for cancer survivors [11, 15]. Persistent adverse effects of cancer treatment, such as fatigue, psychosocial distress, insomnia, chemotherapy-induced peripheral neuropathy, and pain, are commonly cited exercise barriers reported by cancer survivors [12, 16]. However, there is strong to moderate evidence that physical activity can help manage these treatment-related adverse effects and improve quality of life among cancer survivors [11, 12], warranting innovative intervention and implementation strategies to help cancer survivors move more and sit less.
Rural cancer survivors, or cancer survivors residing in nonmetropolitan or remote areas, are more likely to be physically inactive than cancer survivors residing in urban or metropolitan areas and face social and environmental barriers to exercise in addition to those related to the adverse effects of cancer treatment [17–19]. Geographic isolation, inadequate transportation, and low access to health care and supportive oncology services and resources contribute to rural-urban differences in physical activity, and physical and mental health outcomes, among cancer survivors [20–23]. Previous reviews have highlighted the lack of availability and accessibility of exercise programs for rural adults [24–28] and rural cancer survivors [29]. Among rural adults with no history of cancer, mixed findings among intervention studies suggest that evidence-based exercise programs have not yet been effectively translated and implemented within rural communities [24]. However, no study, to our knowledge, has explored the effectiveness of physical activity interventions among rural cancer survivors.
This systematic review and meta-analysis goes beyond previous reviews by examining the effectiveness of physical activity interventions among rural cancer survivors. The purposes of this study were to summarize the characteristics and results of physical activity interventions among adult rural cancer survivors and to estimate the effectiveness of interventions for cancer survivors living in rural or remote settings. Additionally, we summarized measures of rurality across studies, as there is no single global classification system.
Materials and Methods
Protocol and registration
This systematic review was registered with PROSPERO prospective register of systematic reviews (registration number CRD42021229290) at the Centre for Reviews and Dissemination, University of York, UK (https://www.crd.york.ac.uk/prospero/), and adheres to the Preferred Reporting of Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines (http://www.prisma-statement.org/) [30, 31]. The PRISMA checklist is available as Supplementary Table 1.
Eligibility criteria
We included controlled or uncontrolled trials (e.g., randomized controlled trial, non-randomized trial, quasi-experimental, or pre-post) evaluating a physical activity, exercise, or fitness intervention (any type or setting) in adults (aged ≥18 years) diagnosed with any type of cancer and residing in a rural area. Residence in a rural area was considered as defined or described by the study authors (e.g., self-identified or proclaimed or designated as rural using a defined classification system, such as Rural-Urban Continuum Codes or Rural-Urban Commuting Area Codes). We considered any comparator (e.g., standard or usual care, active control, inactive control) or participants as their own control (e.g., pre-post), and trials of any sample size were included. Studies were excluded if the full-text article was not available in English or the intervention did not assess and report physical activity at pre- and post-intervention.
Information sources
Three databases, PubMed Medline (January 1996-July 2, 2020), CINAHL (1961-July 25, 2020), and PsychINFO (1887-July 25, 2020), were systematically searched. The search was restricted to original articles published in English from each database’s inception through July 25, 2020. The reference lists of a recent scoping review[29] and all included articles were further hand searched to identify additional studies and companion articles, or articles relevant to the primary study that may include additional intervention details (e.g., articles describing study protocols published separately from study outcomes).
Search
The search strategy was developed in consultation with a health sciences librarian, and detailed search strings for each database are presented in detail in Supplementary Table 2. Briefly, we used a combination of keywords for cancer survivorship, rural health, and physical activity or exercise to identify relevant publications. Citation details (e.g., authors, title, journal name, year of publication, volume, issue number, and page numbers) were downloaded and compiled into a single database. Duplicate articles were identified, reviewed, and removed from the database.
Study selection
Two coders (SKM and HJL) independently screened titles and abstracts. Agreement between coders for title and abstract reviews were 92.9% and 94.9%, respectively, and inter-rater reliabilities, calculated using Cohen’s κ, were 0.67 for title and 0.87 for abstract review. The full texts of remaining articles were independently reviewed against inclusion and exclusion criteria by two coders (SKM and HJL), and agreement between coders was 93.3% (Cohen’s κ=0.87). Disagreements between reviewers were resolved by consensus, and reasons for exclusion were documented and are shown in Figure 1.
Figure 1.
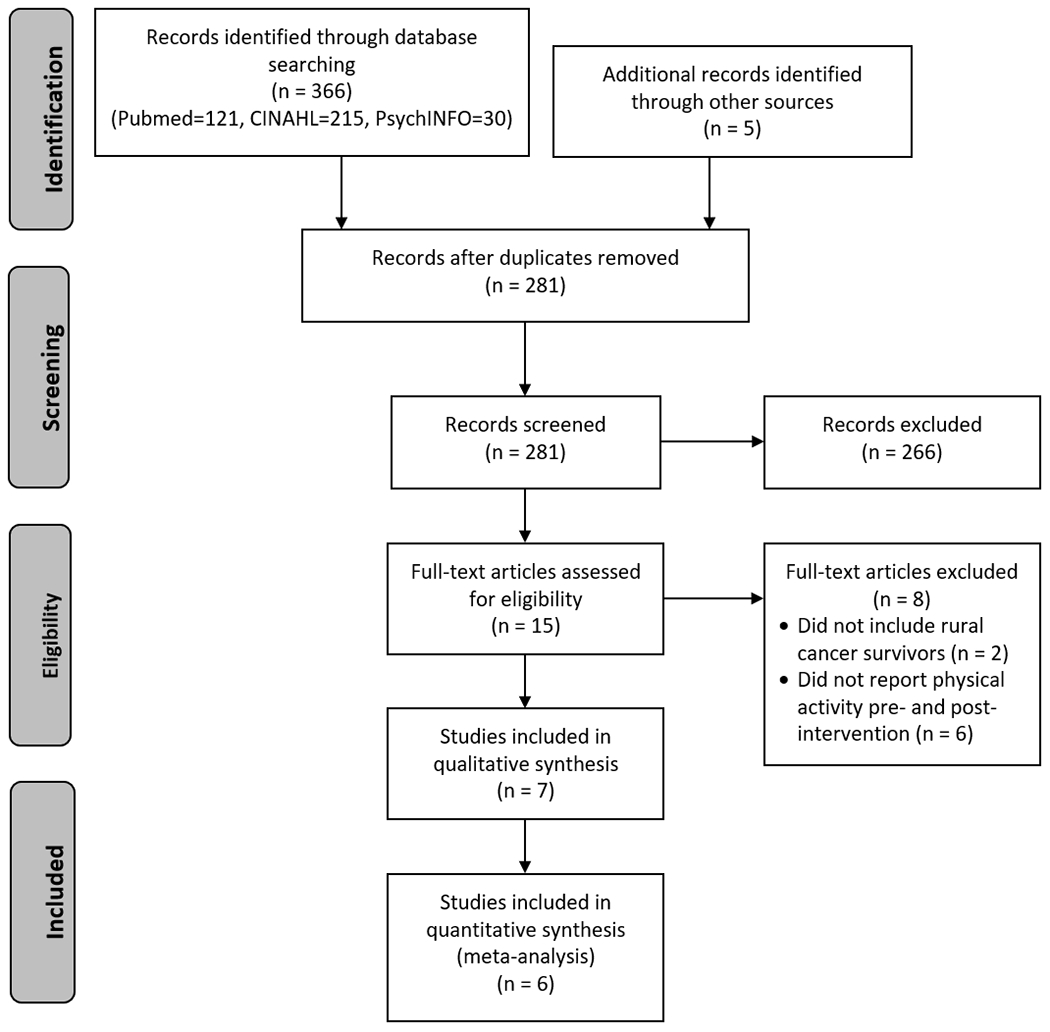
PRISMA flow diagram
After removal of duplicates, titles and abstracts for 281 articles were screened. Fifteen studies met inclusion criteria and continued to full-text review. Of those, seven original studies met inclusion criteria and were included in this review.
Data collection process
A coding tool adapted from previous systematic reviews conducted by the study team was used by two coders (SKM and HJL) to independently extract and code data from included studies (available from the author upon request) [24, 32]. Disagreements between coders were discussed until consensus was reached.
Data items
Extracted data included citation details and companion article citation details, measures of rurality (e.g., rural setting and classification) and study characteristics (e.g., primary outcome, target population, theory, study design, delivery mode), participant characteristics (e.g., sample size, demographics), intervention characteristics (e.g., study setting, delivery personnel, number of contacts, contact duration, and overall time), measurement characteristics (e.g., assessment time points, physical activity measure and type), and outcomes (e.g., means, standard deviations, adherence percent, attrition, summary of findings). In studies that had multiple follow-ups or post-intervention assessments, data were extracted from the follow-up or assessment time point closest to the cessation of the intervention.
Risk of bias in individual studies
Risk of bias was assessed using the Effective Public Health Practice Project’s Quality Assessment Tool for Quantitative Studies [33], which assesses study features to generate risk of bias ratings in six domains: selection bias, study design, confounders, blinding, data collection methods, and withdrawals and dropouts. Judgements for each risk of bias domain and the overall study quality are expressed as ‘weak’, ‘moderate’, or ‘strong’.
Summary measures
We analyzed data as reported in the studies. Measures of rurality across studies and intervention characteristics data were narratively synthesized. For quantitative data, we determined proportion of participants achieving intervention-specific physical activity goals using the number of participants who achieved the goal at post-intervention as numerators. For the denominator, we considered all participants included in the study. When studies compared intervention versus control groups at different time points, the proportion of participants achieving physical activity goals was calculated only for the intervention arm at the end of the intervention (as opposed to the last follow-up assessment). When data was unclear or not provided for a given outcome, the study was not included in the analysis. Also, when outcomes were reported using both self-report and device-based measures within an individual, we used self-reported data to increase consistency in measures across studies. We calculated the relative risk (RR) to compare dichotomous outcomes, the mean difference (MD) for continuous outcomes, and the 95% confidence intervals (CI).
Synthesis of results
Given the methodological variation among included studies, we used a random-effects model to calculate a pooled proportion of participants achieving physical activity goals and its 95% CI. We used the Freeman-Tukey arcsine transformation to stabilize variances and conducted a meta-analysis using inverse variance weights. Resulting estimates and CI boundaries were back-transformed into proportions. We performed a Mantel-Haenszel meta-analysis with a random-effects model of studies providing data for both groups. Separate analyses were performed for before and after studies using the Cochrane methodology to pool paired mean differences. When studies did not report mean, we used the median value. When a study did not report standard deviation (SD) of within-participant differences between before and after measurements, SDdiff was estimated using SDs at baseline and post-intervention in addition to the within-groups correlation coefficient. An imputed conservative correlation of 0.8 was used when the within-groups correlation coefficient was not reported.[34] Statistical heterogeneity between studies was assessed using Cochran’s Q and I2, with an I2 value greater than 50% representing substantial heterogeneity between studies [35]. We interpreted the results in terms of magnitudes of associations and precision of the risk estimates conveyed by 95% CIs, rather than using p-values as measures of significance. All analyses were performed using STATA 15 (StataCorp LP, College Station, TX).
Risk of bias across studies
A funnel plot and an Egger regression asymmetry test was planned if more than 6 studies reported data on the same outcome to assess publication bias and small-study effects in the meta-analysis. However, none of the reported outcomes met the criteria.
Results
Study selection
The literature search identified 366 studies, and 5 additional studies were identified after handsearching the reference list of a recent scoping review [29]. Of the 281 titles and abstracts screened, 15 full-text articles were reviewed for eligibility. Seven studies met inclusion criteria and were included in this review (Figure 1).
Study characteristics
Study and participant characteristics are summarized in Table 1. The median sample size was 91 (range 23 to 160). Four studies were based in rural areas in the United States [36–39], and three studies were based in rural areas in Australia [40–42]. Five studies conducted randomized controlled trials (RCTs) [37–41], and the remaining two studies used a pre-post design [36, 42]. Four studies included female breast cancer survivors exclusively [36, 37, 39, 40], one study included breast, prostate, and colorectal cancer survivors [38], and two studies were open to all cancer types [41, 42]. In three studies, some or all of the sample was currently undergoing chemotherapy or radiation therapy [39, 40, 42], and four studies included participants who had completed treatment [36–38, 41].
Table 1.
Characteristics of original studies included in review
| Author, year (reference number, companion articles) | Study design, Location | Rural characteristics (definition or classification, % of sample) | Sample characteristics (analytic sample size, target population, descriptives) | Intervention (description, setting, duration, contact number and time) | Physical activity measure (units), Timing of assessments | Summary of findings |
|---|---|---|---|---|---|---|
| Befort, et al., 2012 [36] | Single arm trial Kansas, United States | Three cancer centers located in towns with population size 20,000-47,000 Participants resided in RUCA-defined rural area 100% rural | N=31 Rural postmenopausal breast cancer survivors who had completed treatment 100.0% female 97.0% white 26.0% high school degree or less M age=58.9 years | Group-based weight control intervention including aerobic and strength-training activities delivered via conference call Sessions held once/week for 24 weeks (1440 min) | Minnesota Physical Activity Questionnaire (kcal/week or min/week) 0 and 24 weeks | Physical activity increased by 196.5 min/week over 24 weeks 71% of those who completed the intervention met physical activity goal of 225 min/week |
| Eakin, et al., 2012 [40, 56] | Randomized controlled trial Queensland, Australia | Participants resided within a postal code considered inner regional, outer regional, remote or very remote based on their Australian Standard Geographical Classification 100% rural | N=137a Rural women diagnosed with invasive breast cancer and treated at one of 8 regional or 4 large metropolitan hospitals 100% female 53.1% less than high school M age=52.9 years | Exercise for Health-rural (EfH) telephone-delivered mixed (aerobic and resistance) exercise intervention Participants received an exercise workbook and 16 calls over 32 weeks (480 min) | Active Australia Survey (min/week) and CHAMPS questionnaire (strength-training sessions/week) were used to calculate % meeting aerobic (≥4 times/week and ≥180 min of MVPA/week) and resistance training (≥2 sessions/week) goals 0, 24, and 48 weeks | 45.6% of EfH participants met their resistance training goal compared to 10.4% of control participants at 24 weeks and 40.3% vs. 17.9% met their goal at 48 weeks No statistically significant between group differences for aerobic activity |
| Fazzino, et al., 2017 [37, 57–59] | Phase 1: Single arm trial Phase 2: Randomized controlled trial Rural areas of the midwestern United States (Kansas, Nebraska, and Iowa) | Participants resided in a rural area defined by RUCA Codes, Urban Influence Codes, amount of agricultural income, and/or individual commuting patterns 100% rural | N=142 Rural postmenopausal breast cancer survivors who had completed treatment 100.0% female 97.0% white 23.0% high school degree or less M age=58.6 years | Phase 1: 6-month weight loss phase where all participants receive group-based phone counseling Sessions held once/week for 26 weeks (1560 min) Phase 2: 12-month maintenance phase where participants are randomized to continued group phone-based or mailed newsletter comparison group Group-based phone counseling sessions held biweekly (1560 min) and newsletters mailed biweekly | GT3X+ Actigraph accelerometer (bouted MVPA min/week) and the Paffenbarger Physical Activity Questionnaire (MVPA min/week) 0, 26, 52, and 78 weeks | Phase 1: Accelerometer-(46.9 min/week) measured and self-reported (227.5 min/week) MVPA increased from 0 to 26 weeks Phase 2: Accelerometer-measured (−27.2 min/week) and self-reported (−77.5 min/week) MVPA decreased from 26 to 78 weeks |
| Frensham, et al., 2020 [41, 60, 61] | Quasi-randomized controlled trial Rural regions of south Australia | Not specified 47.3% ruralb | N=91 Metropolitan and rural Australians diagnosed with cancer (any type except skin) who had completed treatment and were insufficiently active 51.6% female 95.6% white 72.5% less than high school M age=65.7 years | STRIDE (Steps Toward Improving Diet and Exercise for cancer survivors) web-based intervention including aerobic activity or waitlist control Participants access STRIDE website for 12 weeks and are emailed daily step goals weekly | New-Lifestyles NL-1000 pedometer (steps/day) 0, 12, and 24 weeks | STRIDE intervention group increased their daily steps/day by 31.5% compared with an increase of 12.5% in the control group |
| Gray, et al., 2019 [38, 62–64] | Secondary analysis of a randomized controlled trial North Carolina and rural regions across the United States | RUCA defined large rural, small rural, and isolated regions 100% rural | N=160 Rural elderly colorectal, breast, and prostate cancer survivors who were ≥5 years post-diagnosis and insufficiently active 56.9% female 86.9% white 38.1% high school or less M age=73.0 years | Reach-out to Enhance Wellness (RENEW) iteratively-tailored behavioral intervention including mailed print materials, telephone prompts, and telephone counseling or delayed intervention Participants were contacted 28 times across the 48 week intervention period | Community Health Activities Model Program for Seniors (CHAMPS) questionnaire, endurance (min/week) and strength-training (min/week) exercise 0 and 48 weeks | Endurance exercise increased by 27.1 min/week and strength-training exercise increased by 22.7 min/week over 48 weeks (greater changes in physical activity observed in urban vs. rural, but no statistically significant difference between groups) |
| Hegel, et al., 2011 [39] | Randomized controlled trial Rural New Hampshire, United States | Not specified 100% rural | N=23 Rural breast cancer patients undergoing adjuvant therapy at the Norris Cotton Cancer Center 100% female 100% white 66% bachelor degree M age=52.6 years | Telephone-delivered problem solving and occupational therapy (PST-OT) intervention or usual care Sessions delivered once a week for 6 weeks (246 min) | Adherence to aerobic exercise (measure and units not specified) 0, 6, and 12 weeks | No differences between groups in the frequency of engaging in aerobic exercise |
| Ristevsk, et al., 2020 [42] | Single arm trial Victoria, Australia | Rural region in eastern Victoria (West Gippsland), which has a population of 52,105 distributed over 4025 km2 and has one public acute hospital 100% rural | N=48b Rural adults diagnosed with cancer who were admitted to the chemotherapy day unit 71% female M age=65.9 years | I.CAN program uses a health coaching model to provide tailored nutrition and physical activity guidance via three streams: One-on-one support (Stream A), combination of one-on-one support and group sessions (Stream B), and group sessions (Stream C) One on one support and group sessions held fortnightly for 6 weeks (390 min) | Godin Leisure Time Exercise Questionnaire (% meeting guidelines) 0, 12, 24, and 48 weeks | % meeting exercise guidelines increased from 51% to 86% over 12 weeks |
Abbreviations: M, mean; MVPA, moderate-to-vigorous physical activity
Race/ethnicity and/or education not reported.
Unable to calculate results for rural sample only.
Measures of rurality across studies
Measures of rurality or classification schemes used to identify rural areas varied across studies and by country. In the U.S., three out of four studies used Rural-Urban Commuting Area (RUCA) codes [43] to classify U.S. census tracts nested within counties into rural and urban categories using measures of population density, urbanization, and daily commuting [36–38], and one study did not specify the definition or classification system used [39]. In Australia, one study used a standard classification system (Australian Standard Geographical Classification), similar to RUCA codes, to classify residents within a postal code into rural and urban categories [40], one used population density [42], and one did not specify the definition or classification system used [41].
Intervention characteristics
Social cognitive theory was the most commonly cited theoretical framework used to guide intervention development. Additional theories cited included self-regulation theory, goal setting theory, and the Chronic Disease Self-Management Model. Most (57.1%) interventions were delivered individually, 28.6% were group-based, and 28.6% used a combination of individual and group-based delivery. Three studies reported a single intervention delivery method [36, 39, 42]. Intervention delivery by telephone (71.4%) was the most commonly used method followed by print or mail (42.9%). Most (83.3%) studies used a trained research assistant or health professional (e.g., counselor) to deliver the intervention, and one study did not use a delivery agent (e.g., completely phone/device or mail based).
The median intervention duration was 24 weeks (range 6 to 52 weeks), and median total contact time was 467.5 minutes (range 246 to 1586 minutes). Three studies focused on aerobic physical activity exclusively [37, 41, 42], three incorporated aerobic and muscle-strengthening activities,[36, 38, 40] and one did not specify type of physical activity [39]. Intervention adherence, or the percent of intervention sessions attended, ranged from 60-85%, and attrition ranged from 4-24%. Four studies assessed physical activity maintenance [37, 39, 41, 42], and the median follow-up time post-intervention was 12 weeks (range 6 to 26 weeks).
Physical activity measures
Most (71.4%) studies assessed physical activity outcomes using questionnaires [36, 38–40, 42], one study used pedometers [41], and one study used both questionnaires and accelerometers [37]. Five studies reported overall improvement in physical activity [36, 37, 40–42]. Of the five RCTs included in this review, one study reported improvements in physical activity compared to the control group [41], and one study reported improvements in strength-training activity compared to the control [40].
Risk of bias
Of the seven studies included in the review, four studies were considered to have moderate risk of bias [36, 37, 40, 41], and three studies had a high overall risk of bias [38, 39, 42]. A summary of judgements for each domain is shown in Figure 2, and judgments for each domain for each included study are available in Supplementary Table 3. The four studies with moderate risk of bias were subsequently used for the meta-analysis.
Figure 2.
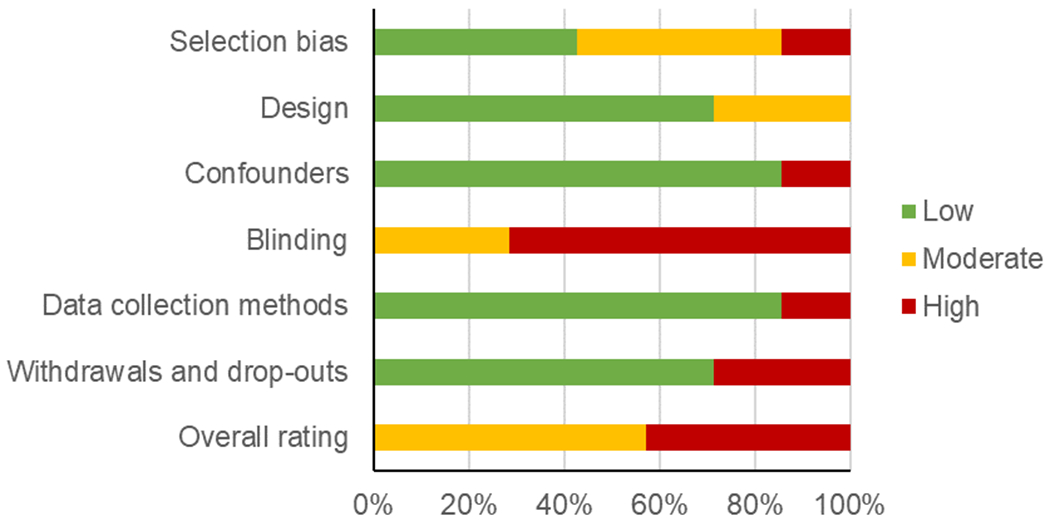
Summary of risk of bias judgements by domain
A summary of risk of bias judgements by domain demonstrate moderate to high overall risk of bias among the seven studies included in this review.
Achieving physical activity goals
A meta-analysis of the four RCTs, consisting of 401 participants, showed that 39% percent of individuals receiving an intervention achieved the study’s physical activity goal (95% CI: 18%, 62%; I2=95%) (Figure 3). We observed that the proportion of patients achieving the goal increased to >50% when the goal was more stringent (≥180 to 225 minutes per week compared to ≥150 minutes of physical activity). Only one study compared the proportion of patients achieving a physical activity goal at post-intervention (26 weeks) and follow-up (52 weeks) [40]. No statistically significant differences were observed in the number of patients achieving the study goal between groups (at 26 weeks: RR=1.4, 95% CI: 0.87, 2.2; at 52 weeks: RR=1.3, 95% CI: 0.88, 1.9).
Figure 3.

Forest plot of the results from random effects meta-analysis on physical activity
Abbreviations: ES, effect size; CI, confidence interval. Includes three before and after studies and one controlled trial (Eakin et al., 2012). Data after implementation of the intervention from the before and after studies were pooled with the implementation data after the intervention in the intervention group of the controlled trial.
Increase in physical activity
Aerobic activity.
The mean time spent per week exercising (aerobic physical activity) increased from baseline to post-intervention, which ranged in duration from 6-52 weeks (MD=97.7 minutes, 95% CI: 75.0, 120.4; I2>99%). When subgrouped by study design and intervention, results remained similar. A statistically significant increase in the time spent (in minutes) per week on aerobic physical activity was observed at post-intervention compared to baseline (MD=215.5 minutes, 95% CI: 185.9, 245.1; I2<58%; Table 2). The MD was lower for the study with long-term data at 52 weeks (MD=27.1, 95% CI: 26.4, 27.9) [38]. Only one study compared physical activity minutes per week against controls with no statistically significant difference found between the time reported per group (MD=0.65, 95% CI: −0.27, 1.6). Another study compared the number of steps per day between groups at post-intervention (12 weeks) and found a statistically significant increase in the number of steps per day in participants assigned to the intervention group compared to those in the control group (MD=1775.0, 95% CI: 357.0, 3193.0) [41].
Table 2.
Summary of findings for physical activity
| Outcome | Study | Design | Follow-up | Outcome measure | Measure unit | Mean difference (95% CI) |
|---|---|---|---|---|---|---|
| Aerobic physical activity | Befort 2012 | Before and after | 6 months | Physical activity – MPAQ | Minutes per week | 196.5 (162.1, 230.9) |
| Fazzino 2017 | Before and after | 6 months | Physical activity – PPAQ | Minutes per week | 227.5 (208.8, 246.2) | |
| subgroup | 215.5 (185.9, 215.1) | |||||
| Gray 2019 | Before and after | 12 months | Endurance exercise | Minutes per week | 27.1 (26.4, 27.9) | |
| Hegel 2011 | Controlled trial | 6 weeks | Aerobic exercise | Minutes per week | 0.65 (−0.27, 1.6) | |
| overall | 97.7 (75.0, 120.4) | |||||
| Frenshman 2020 | Controlled trial | 3 months | Pedometer | Steps per day | 1775.0 (357.0, 3193.0) | |
| Resistance training | Hegel 2011 | Controlled trial | 6 weeks | Self-directed physical therapy | Minutes per week | 1.7 (0.48, 3.0) |
| Gray 2019 | Before and after | 12 months | Strength training | Minutes per week | 22.7 (22.3, 23.1) | |
| overall | 12.2 (−8.3, 32.8) |
Abbreviations: CI, confidence interval; MPAQ, Minnesota Physical Activity Questionnaire; PPAQ, Paffenbarger Physical Activity Questionnaire
Resistance training.
Only two studies provided data on this outcome [38, 39]. Participants in one study reported an increase in time spent on strength training at post-intervention, which was 52 weeks (MD=22.7 minutes, 95% CI: 22.3, 23.1). In a controlled study, participants in the intervention group reported longer time spent in self-directed physical therapy than the control group at 6 weeks (MD=1.7, 95% CI: 0.48, 3.0). However, the overall pooled estimate did not reach statistical significance (I2=99%; Table 2).
Retention rates at last follow-up assessment
Five studies provided data on this outcome, as shown in Supplementary Figure 4. The pooled proportion of participants in the intervention group who remained in the program until the last follow-up assessment (range 6 to 78 weeks) was 82% (95% CI: 69%, 92%; I2=85%).
Discussion
This systematic review identified only seven intervention studies to promote physical activity among rural cancer survivors worldwide and is the first, to our knowledge, to estimate the effectiveness of interventions to increase physical activity among cancer survivors living in rural areas. Findings from this review suggest modest increases in physical activity, and a lack of available controlled interventions among rural cancer survivors. Furthermore, we found discrepancies remain in the operational definition or classification scheme used to categorize areas as rural or urban in both the U.S. and Australia, expanding on findings from a previous review [24].
This review conducted a quantitative synthesis of the effects of interventions for increasing physical activity among cancer survivors living in rural areas, which builds on a previously published scoping review by Smith-Turchyn et al [29]. Smith-Turchyn et al. identified 13 studies representing eight unique exercise interventions [29], four of which met our inclusion criteria and were included in the current review. The current review identified seven unique physical activity interventions and found that interventions moderately increased physical activity, equivalent to approximately 97.7 minutes per week of aerobic exercise and 12.2 minutes per week of strength training. However, the clinical meaningfulness of these findings remains uncertain due to the low number of eligible RCTs, small sample sizes and small to moderate intervention effects on physical activity within studies, and moderate to high risk of bias and low overall quality of evidence across studies.
Most interventions included in this review were delivered by telephone, print or mail, did not include a face-to-face component, and were delivered individually versus group-based, and all the RCTs included in the meta-analysis used distance-based delivery. Previous reviews have found negligible to small effects for distance-based physical activity behavior change interventions that have relied on print and telephone modes of intervention delivery among cancer survivors [44]. Furthermore, group-based strategies are effective for increasing physical activity behavior for most populations and in most settings, and may be more efficacious than individually delivered programs when the appropriate group dynamic principles are used [32, 45, 46]. Despite being the most effective approach for physical activity behavior change compared to mediated delivery (e.g., email or telephone) [47], only one study used face-to-face and group-based delivery, but was not an RCT and thus excluded from the meta-analysis [42]. Although this study (Frensham et al.) used primarily web-based intervention delivery, they included two face-to-face workshops where participants were instructed on using the study website, logged their steps, received feedback, shared their experiences and received peer support, and had access to other health information (e.g., healthy eating) and resources (e.g., community centers, events, etc.) [41]. This relatively small face-to-face component may help explain the success of the intervention in increasing physical activity (MD=1775.0 steps per day), which was the largest reported among the studies included in this review [41]. Given the rapid rise in the use of digital health platforms and telehealth due to COVID-19 [48, 49], additional research is needed to assess the potential for current technologies (e.g., Zoom, Skype, Microsoft Teams, FaceTime, etc.) to overcome the limitations of previous distance-based approaches while to providing the benefits and support associated with face-to-face delivery to increase physical activity in rural cancer survivors [50, 51].
These findings highlight the important tradeoff between reach and effectiveness when it comes to physical activity promotion efforts in rural settings. Distance-based, unsupervised approaches to promote physical activity may reduce cost and barriers to engaging in physical activity programs, thereby enhancing the reach of programs to underserved groups, including cancer survivors residing in rural and remote areas. Conversely, face-to-face approaches may be more efficacious for increasing physical activity [52]. This suggests a need for implementation strategies to test whether established evidence-based approaches remain effective in rural and remote areas, particularly once adaptations to delivery mode to increase reach are implemented. Community engaged research approaches, which include rural community stakeholders in the design, adaptation, and implementation process, may further increase the saliency and sustainability and improve effectiveness of physical activity interventions in rural communities and for rural cancer survivors.
Strengths and Limitations
The major strengths of this study are the systematic and comprehensive search strategies, and rigorous quantitative synthesis of outcomes. Limitations include a limited number of studies included in the meta-analysis, moderate to high risk of bias among included studies, and lack of studies employing objective measures of physical activity. Objective measures of physical activity such as accelerometers can capture intensity and amounts of physical activity, with greater precision for light intensity activity, and activity outside of structured leisure time physical activity [53]. These facets of physical activity may be of particular relevance to rural populations who may spend more time in occupational or household activities [54, 55]. The limited number of studies included in this review and meta-analysis restricted subgroup analyses by cancer type, stage of disease, age, social determinants of health, and other characteristics which are known to impact health behaviors, such as physical activity [15]. Additional research is needed to explore barriers to physical activity that are relevant to rural cancer survivors and to incorporate those in future reviews. Lastly, the current review was limited to articles published in English. Given that we aimed to assess interventions conducted worldwide, this criterion may have excluded relevant articles published in other languages.
Conclusions
This systematic review and meta-analysis identified only 7 interventions worldwide that described or evaluated a physical activity program for rural cancer survivors. Although the interventions demonstrated moderate increases in aerobic and resistance exercise among rural cancer survivors, the clinical meaningfulness of findings from this review remains uncertain due to the continued lack of availability of rigorous physical activity interventions designed for or adapted to rural cancer survivors. As the number of cancer survivors continues to rise in rural communities, there is a growing need to adapt and test evidence-based interventions to address the unique physical activity needs of rural cancer survivors. Future research is needed to explore the use of new technologies (e.g., telehealth and videoconferencing) and approaches (e.g., utilizing lay health educators and local fitness professionals) to improve reach while maintaining effectiveness.
Supplementary Material
Funding:
Scherezade Mama is partially supported by a career development award from the National Cancer Institute (K07 CA222335), and Heather Leach is partially supported by a career development award (131629-MRSG-18-021-01-CPPB) from the American Cancer Society.
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today Lyon, France: International Agency for Research on Cancer; 2020. [Available from: https://gco.iarc.fr/today. [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. Submitted. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Moller B. Predicting the future burden of cancer. Nat Rev Cancer. 2006;6(1):63–74. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–53. [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society. Global Cancer Facts & Figures 4th Edition. Atlanta, GA: American Cancer Society; 2018. [Google Scholar]
- 6.de Moor JS, Mariotto AB, Parry C, Alfano CM, Padgett L, Kent EE, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22(4):561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 8.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Cancer Society. Cancer Facts & Figures 2021. Atlanta, GA: American Cancer Society; 2021. [Google Scholar]
- 10.World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: a Global Perspective. Continuous Update Project Expert Report 2018 2018. [Available from: https://www.wcrf.org/dietandcancer.
- 11.Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51(11):2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cormie P, Zopf EM, Zhang X, Schmitz KH. The Impact of Exercise on Cancer Mortality, Recurrence, and Treatment-Related Adverse Effects. Epidemiol Rev. 2017;39(1):71–92. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd edition. Washington, DC: U.S. Department of Health and Human Services; 2018. [Google Scholar]
- 14.Dumith SC, Hallal PC, Reis RS, Kohl HW 3rd. Worldwide prevalence of physical inactivity and its association with human development index in 76 countries. Prev Med. 2011;53(1-2):24–8. [DOI] [PubMed] [Google Scholar]
- 15.Arem H, Mama SK, Duan X, Rowland JH, Bellizzi KM, Ehlers DK. Prevalence of Healthy Behaviors among Cancer Survivors in the United States: How Far Have We Come? Cancer Epidemiol Biomarkers Prev. 2020;29(6):1179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blaney JM, Lowe-Strong A, Rankin-Watt J, Campbell A, Gracey JH. Cancer survivors’ exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: a questionnaire-survey. Psychooncology. 2013;22(1):186–94. [DOI] [PubMed] [Google Scholar]
- 17.Lesser IA, Nienhuis CP, Belanger L. Active by nature: exploring cancer survivors’ exercise barriers, facilitators, preferences, and psychosocial benefits of engaging in outdoor physical activity. Support Care Cancer. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cadmus-Bertram LA, Gorzelitz JS, Dorn DC, Malecki KMC. Understanding the physical activity needs and interests of inactive and active rural women: a cross-sectional study of barriers, opportunities, and intervention preferences. J Behav Med. 2020;43(4):638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams JL, Martin MY, Pisu M, Oster RA, Qu H, Shewchuk RM, et al. Determining patient needs to enhance exercise program implementation and uptake in rural settings for women after a cancer diagnosis. Support Care Cancer. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moss JL, Pinto CN, Mama SK, Rincon M, Kent EE, Yu M, et al. Rural-urban differences in health-related quality of life: patterns for cancer survivors compared to other older adults. Qual Life Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mama SK, Bhuiyan N, Foo W, Segel JE, Bluethmann SM, Winkels RM, et al. Rural-urban differences in meeting physical activity recommendations and health status in cancer survivors in central Pennsylvania. Support Care Cancer. 2020;28(10):5013–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weaver KE, Geiger AM, Lu L, Case LD. Rural-urban disparities in health status among US cancer survivors. Cancer. 2013;119(5):1050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ormel HL, van der Schoot GGF, Sluiter WJ, Jalving M, Gietema JA, Walenkamp AME. Predictors of adherence to exercise interventions during and after cancer treatment: A systematic review. Psychooncology. 2018;27(3):713–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhuiyan N, Singh P, Harden SM, Mama SK. Rural physical activity interventions in the United States: a systematic review and RE-AIM evaluation. Int J Behav Nutr Phys Act. 2019;16(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh SM, Meyer MR, Gamble A, Patterson MS, Moore JB. A Systematic Review of Rural, Theory-based Physical Activity Interventions. Am J Health Behav. 2017;41(3):248–58. [DOI] [PubMed] [Google Scholar]
- 26.Moore M, Warburton J, O’Halloran PD, Shields N, Kingsley M. Effective Community-Based Physical Activity Interventions for Older Adults Living in Rural and Regional Areas: A Systematic Review. J Aging Phys Act. 2016;24(1):158–67. [DOI] [PubMed] [Google Scholar]
- 27.Cleland V, Squibb K, Stephens L, Dalby J, Timperio A, Winzenberg T, et al. Effectiveness of interventions to promote physical activity and/or decrease sedentary behaviour among rural adults: a systematic review and meta-analysis. Obes Rev. 2017;18(7):727–41. [DOI] [PubMed] [Google Scholar]
- 28.Cai Y, Richards EA. Systematic Review of Physical Activity Outcomes of Rural Lifestyle Interventions. West J Nurs Res. 2016;38(7):909–27. [DOI] [PubMed] [Google Scholar]
- 29.Smith-Turchyn J, Gravesande J, Sabiston CM. Exercise interventions for survivors of cancer living in rural or remote settings: A scoping review. Rehabilitation Oncology. 2020;38:61–80. [Google Scholar]
- 30.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leach HJ, Mama SK, Harden SM. Group-based exercise interventions for increasing physical activity in cancer survivors: a systematic review of face-to-face randomized and non-randomized trials. Support Care Cancer. 2019;27(5):1601–12. [DOI] [PubMed] [Google Scholar]
- 33.Thomas BH, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs. 2004;1(3):176–84. [DOI] [PubMed] [Google Scholar]
- 34.Rosenthal R Meta-analytic procedures for social research. Newbury Park, CA: SAGE Publications Inc; 1991. [Google Scholar]
- 35.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Befort CA, Klemp JR, Austin HL, Perri MG, Schmitz KH, Sullivan DK, et al. Outcomes of a weight loss intervention among rural breast cancer survivors. Breast Cancer Res Treat. 2012;132(2):631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fazzino TL, Fabian C, Befort CA. Change in Physical Activity During a Weight Management Intervention for Breast Cancer Survivors: Association with Weight Outcomes. Obesity (Silver Spring). 2017;25 Suppl 2:S109–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray MS, Judd SE, Sloane R, Snyder DC, Miller PE, Demark-Wahnefried W. Rural-urban differences in health behaviors and outcomes among older, overweight, long-term cancer survivors in the RENEW randomized control trial. Cancer Causes Control. 2019;30(4):301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hegel MT, Lyons KD, Hull JG, Kaufman P, Urquhart L, Li Z, et al. Feasibility study of a randomized controlled trial of a telephone-delivered problem-solving-occupational therapy intervention to reduce participation restrictions in rural breast cancer survivors undergoing chemotherapy. Psychooncology. 2011;20(10):1092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eakin EG, Lawler SP, Winkler EA, Hayes SC. A randomized trial of a telephone-delivered exercise intervention for non-urban dwelling women newly diagnosed with breast cancer: exercise for health. Ann Behav Med. 2012;43(2):229–38. [DOI] [PubMed] [Google Scholar]
- 41.Frensham LJ, Parfitt G, Dollman J. Predicting Engagement With Online Walking Promotion Among Metropolitan and Rural Cancer Survivors. Cancer Nurs. 2020;43(1):52–9. [DOI] [PubMed] [Google Scholar]
- 42.Ristevsk E, Trinh T, Vo N, Byrne A, Jamieson P, Greenall A, et al. I.CAN: health coaching provides tailored nutrition and physical activity guidance to people diagnosed with cancer in a rural region in West Gippsland, Australia. J Cancer Surviv. 2020;14(1):48–52. [DOI] [PubMed] [Google Scholar]
- 43.USDA Economic Research Service. Rural-Urban Commuting Area Codes: U.S. Department of Agriculture,; 2020. [Available from: https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx. [Google Scholar]
- 44.Groen WG, van Harten WH, Vallance JK. Systematic review and meta-analysis of distance-based physical activity interventions for cancer survivors (2013-2018): We still haven’t found what we’re looking for. Cancer Treat Rev. 2018;69:188–203. [DOI] [PubMed] [Google Scholar]
- 45.Harden SM, McEwan D, Sylvester BD, Kaulius M, Ruissen G, Burke SM, et al. Understanding for whom, under what conditions, and how group-based physical activity interventions are successful: a realist review. BMC Public Health. 2015;15:958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burke SM, Carron AV, Eys MA, Ntoumanis N, Estabrooks PA. Group versus individual approach? A meta-analysis of the effectiveness of interventions to promote physical activity. Sport & Exercise Psychology Review. 2006;2:13–29. [Google Scholar]
- 47.Conn VS, Hafdahl AR, Mehr DR. Interventions to increase physical activity among healthy adults: meta-analysis of outcomes. Am J Public Health. 2011;101(4):751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parker K, Uddin R, Ridgers ND, Brown H, Veitch J, Salmon J, et al. The Use of Digital Platforms for Adults’ and Adolescents’ Physical Activity During the COVID-19 Pandemic (Our Life at Home): Survey Study. J Med Internet Res. 2021;23(2):e23389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson WR. Worldwide survey of fitness trends for 2021. ACSM’s Health & Fitness Journal. 2021;25(1):10–9. [Google Scholar]
- 50.Morrison KS, Paterson C, Toohey K. The Feasibility of Exercise Interventions Delivered via Telehealth for People Affected by Cancer: A Rapid Review of the Literature. Semin Oncol Nurs. 2020;36(6):151092. [DOI] [PubMed] [Google Scholar]
- 51.Faro JM, Mattocks KM, Nagawa CS, Lemon SC, Wang B, Cutrona SL, et al. Physical Activity, Mental Health, and Technology Preferences to Support Cancer Survivors During the COVID-19 Pandemic: Cross-sectional Study. JMIR Cancer. 2021;7(1):e25317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turner RR, Steed L, Quirk H, Greasley RU, Saxton JM, Taylor SJ, et al. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev. 2018;9:CD010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burchartz A, Anedda B, Auerswald T, Giurgiu M, Hill H, Ketelhut S, et al. Assessing physical behavior through accelerometry–state of the science, best practices and future directions. Psychology of Sport and Exercise. 2020;49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fan JX, Wen M, Kowaleski-Jones L Rural-urban differences in objective and subjective measures of physical activity: findings from the National Health and Nutrition Examination Survey (NHANES) 2003-2006. Prev Chronic Dis. 2014;11:E141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whitfield GP, Ussery EN, Carlson SA. Combining Data From Assessments of Leisure, Occupational, Household, and Transportation Physical Activity Among US Adults, NHANES 2011-2016. Prev Chronic Dis. 2020;17:E117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayes S, Rye S, Battistutta D, Yates P, Pyke C, Bashford J, et al. Design and implementation of the Exercise for Health trial -- a pragmatic exercise intervention for women with breast cancer. Contemp Clin Trials. 2011;32(4):577–85. [DOI] [PubMed] [Google Scholar]
- 57.Befort CA, Klemp JR, Fabian C, Perri MG, Sullivan DK, Schmitz KH, et al. Protocol and recruitment results from a randomized controlled trial comparing group phone-based versus newsletter interventions for weight loss maintenance among rural breast cancer survivors. Contemp Clin Trials. 2014;37(2):261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Befort CA, Bennett L, Christifano D, Klemp JR, Krebill H. Effective recruitment of rural breast cancer survivors into a lifestyle intervention. Psychooncology. 2015;24(4):487–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Befort CA, Klemp JR, Sullivan DK, Shireman T, Diaz FJ, Schmitz K, et al. Weight loss maintenance strategies among rural breast cancer survivors: The rural women connecting for better health trial. Obesity (Silver Spring). 2016;24(10):2070–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frensham LJ, Parfitt G, Dollman J. Effect of a 12-Week Online Walking Intervention on Health and Quality of Life in Cancer Survivors: A Quasi-Randomized Controlled Trial. Int J Environ Res Public Health. 2018;15(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frensham LJ, Zarnowiecki DM, Parfitt G, Stanley RM, Dollman J. Steps toward improving diet and exercise for cancer survivors (STRIDE): a quasi-randomised controlled trial protocol. BMC Cancer. 2014;14:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Demark-Wahnefried W, Morey MC, Sloane R, Snyder DC, Miller PE, Hartman TJ, et al. Reach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. J Clin Oncol. 2012;30(19):2354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snyder DC, Morey MC, Sloane R, Stull V, Cohen HJ, Peterson B, et al. Reach out to ENhancE Wellness in Older Cancer Survivors (RENEW): design, methods and recruitment challenges of a home-based exercise and diet intervention to improve physical function among long-term survivors of breast, prostate, and colorectal cancer. Psychooncology. 2009;18(4):429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009;301(18):1883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


