Abstract
Introduction
Recent studies have observed that patients with treatment-resistant schizophrenia as well as patients with schizophrenia who do not respond within a medication trial exhibit excess activity of the glutamate system. In this study we sought to replicate the within-trial glutamate abnormality and to investigate the potential for structural differences and treatment-induced changes to improve identification of medication responders and non-responders.
Methods
We enrolled 48 medication-naïve patients in a 4-week trial of risperidone and classified them retrospectively into responders and non-responders using clinical criteria. Proton magnetic resonance spectroscopy and T1-weighted structural MRI were acquired pre- and post-treatment to quantify striatal glutamate levels and several measures of subcortical brain structure.
Results
Patients were classified as 29 responders and 19 non-responders. Striatal glutamate was higher in the non-responders than responders both pre- and post-treatment (F1,39=7.15, p=0.01). Volumetric measures showed a significant group × time interaction (t=5.163, <1%FDR), and group × time × glutamate interaction (t=4.23, <15%FDR) were seen in several brain regions. Striatal volumes increased at trend level with treatment in both groups, and a positive association of striatal volumes with glutamate levels was seen in the non-responders.
Conclusions
Combining anatomic measures with glutamate levels offers the potential to enhance classification of responders and non-responders to antipsychotic medications as well as to provide mechanistic understanding of the interplay between neuroanatomical and neurochemical changes induced by these medications.
Keywords: First episode, schizophrenia, glutamate, magnetic resonance spectroscopy, brain volume analysis, antipsychotic treatment
1. Introduction
Antipsychotic medications have therapeutic benefit for positive symptoms of schizophrenia in patients, particularly those in the first episode of psychosis. Nevertheless, there is a significant minority with little to no response to these agents (Juarez-Reyes et al., 1995; Lehman et al., 2004; Lindenmayer, 2000). Biomarkers of response prediction could provide valuable guidance for clinical intervention and potentially for clarification of mechanisms of illness that could inform novel drug development.
Important strides have been made in this area in relation to the dopamine and glutamate hypotheses of the illness. A neuroreceptor imaging study of intrasynaptic striatal dopamine levels showed that higher pre-treatment levels were predictive of greater medication response after a single medication trial (Abi-Dargham et al., 2000). On the other hand, antipsychotic-naïve patients with first-episode psychosis showed increased glutamate and glutamate + glutamine (Glx) levels in the associative striatum, which normalized after 4 weeks of clinically effective treatment (de la Fuente-Sandoval et al., 2013; de la Fuente-Sandoval et al., 2018), and a recent multicenter study found that higher anterior cingulate glutamate levels were associated with poorer response to a single medication trial (Egerton et al., 2018). Comparisons of treatment-resistant to treatment-responsive patients have evaluated striatal dopamine synthesis capacity and glutamate levels in the anterior cingulate and generally found lower or normal dopamine levels and higher glutamate levels in medication treatment-resistant than in treatment-responsive patients (Demjaha et al., 2014; Egerton et al., 2020; Iwata et al., 2019; Mouchlianitis et al., 2016). Corroborating these findings, a recent systematic review concluded that treatment-resistant schizophrenia appears to be characterized by a relatively normal dopamine system but an abnormal glutamate system (Gillespie et al., 2017).
Studies combining brain structure and neurochemistry in relation to antipsychotic treatment response have suggested additional mechanistic understanding of therapeutic actions of these agents. A review of structural differences found that treatment-resistant patients show significant decreases in grey matter (superior, middle, and inferior temporal gyri, pre- and post-central gyri, middle and superior frontal gyri, right supramarginal gyrus, and right lateral occipital cortex) compared to treatment-responsive patients (Gillespie et al., 2017). Potential roles for excitotoxicity (Plitman et al., 2014; Shah et al., 2020) and longer duration of untreated psychosis (Briend et al., 2020) have been suggested in the co-occurrence of volume deficits with excess glutamate in first-episode psychosis (Plitman et al., 2016). The possibility that these prior conditions play a role in antipsychotic treatment resistance is suggested by these data, but additional work is needed to establish relationships among these measures.
Here, we study the relationship between brain structure and glutamate using several structural measures in combination with a proton magnetic resonance spectroscopy (1H-MRS) measure of striatal glutamate before and after a 4-week trial of risperidone. We acquired these data in a group of medication-naïve first-episode psychosis patients who were then stratified at the 4-week time point into medication responders and non-responders. Our goal was to determine whether prior reports of higher MRS glutamate measures in medication non-responders also held in medication-naïve patients whose response status was prospectively determined within the study. We also sought to assess whether anatomical differences could be detected in response to treatment, and between the responders and non-responders in the hopes of improving our understanding of, and prediction of treatment response.
2. Methods and Materials
2.1. Participants
The study was approved by the Ethics and Scientific committees of the Instituto Nacional de Neurología y Neurocirugía (INNN). Forty-eight antipsychotic naïve patients in their first episode of non-affective psychosis (as determined by the Structured Clinical Interview for DSM-IV) were recruited from the Neuropsychiatric Department, the Emergency Department, and the Adolescent Program of Neuropsychiatric and Imaging Study – PIENSA – of the INNN. All participants over 18 years fully understood and signed the informed consent; in case the patient was under 18 years, informed consent was obtained from both parents. Participants did not receive a stipend. Exclusion criteria included high suicidal risk, psychomotor agitation, comorbid Axis I disorder, concomitant medical or neurologic illness, current substance abuse or a history of substance dependence (except for nicotine and caffeine), and a history of moderate or severe traumatic brain injury. All participants were screened for drugs of abuse (cannabis, cocaine, heroin, opioids, and benzodiazepines) using Instant-View 5-Panel urine toxicology test (American Screening Corporation, Shreveport, LA) at inclusion and at 1 hour before the neuroimaging studies.
Patients underwent a baseline 1H-MRS scan, and then began treatment with risperidone (open label), starting with 1 mg, and titrated up to 6 mg according to clinical judgement. Use of concomitant medications (benzodiazepines, mood stabilizers, antidepressants) was not allowed. After 4 weeks of treatment, clinical status was assessed based on positive symptoms as suggested by Lee et al (Lee et al., 2015): at the time of the second 1H-MRS, response was defined as a reduction of at least 40% in the positive subscale of the PANSS.
2.2. Magnetic Resonance Studies
1H-MRS was performed on a 3T GE Signa Excite HDxt scanner, with a high-resolution 8-channel head coil at the Neuroimaging Department of the INNN. Participants were initially imaged with a T1-weighted spoiled gradient-echo 3-dimensional axial acquisition oriented above and parallel to the anterior commissure- posterior commissure line (TE = 5.7ms; TR = 13.4ms; inversion time = 450ms; flip angle = 20°; FOV = 25.6cm; 256 × 256 matrix; and slice thickness = 1mm or 1.2mm). These T1-weighted images were saved for further structural analysis (see below) and reformatted to sagittal and coronal views for optimal 1H-MRS voxel placement. The 1H-MRS spectra were obtained using point-resolved spectroscopy (TE = 35ms; TR = 2000ms; spectral width = 5000Hz; 4096 data points used; and 128 water-suppressed averages and 16 water-unsuppressed averages) centered on the right dorsal caudate in an 8 ml (2 × 2 × 2 cm) voxel. The lower end of the voxel was located 2mm dorsal to the anterior commissure and positioned to include the maximum amount of gray matter. During acquisition, 1H-MRS spectra were shimmed to achieve full-width at half maximum (FWHM) of 12Hz or less (measured on the unsuppressed water signal from the voxel).
2.3. 1H-MRS Data Analysis
Water-suppressed spectra were analyzed using LCModel (version 6.3–0E) and normalized to the unsuppressed water signal. The analysis used a standard basis set of metabolites, including alanine, aspartate, creatinine, phosphocreatine, γ-aminobutyric acid, glucose, glutamine, glutamate, glycerophosphocholine, phosphocoline, glutathione, inositol, lactate, N-Acetylaspartate, N-Acetylaspartaylglutamate, scyllo-Inositol, taurine, creatine methylene group, creatine + phosphocreatine (tCr), glycerophosphocholine + phosphocoline (tCho), Glx, N-Acetylaspartate + N-Acetylaspartaylglutamate (tNAA); lipids (LIP13A, LIP13B, LIP09 and LIP20); and macromolecules (MM09, MM12, MM20, MM14 and MM17. This basis set, which was included in LCModel, was acquired with the same sequence parameters used in our study. In the present manuscript, we report the following metabolites: Glutamate, Glx, tNAA, tCho, tCr. All metabolite levels with a Cramer-Rao lower bound (CRLB) exceeding 20% as reported by LCModel or a FWHM greater than 12Hz were considered poor quality and were excluded from further analyses. Metabolite values were corrected for the cerebrospinal fluid fraction within the spectroscopy voxel (de la Fuente-Sandoval et al., 2011).
2.4. Image Preprocessing
T1-weighted structural MRIs were converted to Medical Imaging NetCDF (MINC) file format and preprocessed using the minc-bpipe-library preprocessing pipeline (http://github.com/CobraLab/minc-bpipe-library). First, an iterative whole-scan bias field correction was applied using N4ITK (Avants et al., 2011). Excess data were then removed around the head through cropping of the image volume to improve subsequent image processing steps. Lastly, a brain mask was computed using the BEaST patch-based segmentation technique (Eskildsen et al., 2012) to calculate total brain volume (TBV) and to guide subsequent processing steps. All images were visually inspected to ensure there were no artifacts or motion corruption.
2.5. Volume Analysis
Basal ganglia volumes were extracted from preprocessed T1-weighted images using the automatic multi-atlas segmentation algorithm, MAGeT Brain (Chakravarty et al., 2013). For MAGeT Brain Quality Control, all procedures are explained in detail on our wiki: https://github.com/CobraLab/documentation/wiki/MAGeT-Brain-Quality-Control-(QC)-Guide.
The bilateral striatum, globus pallidus and thalamus were defined using a three-dimensional reconstruction of serial histological data and were warped to a high-resolution average (Chakravarty et al., 2006; Tullo et al., 2018), which was used to label 21 template scans, chosen to be representative of the variability in the data. Labeling was achieved through standard model-based segmentation procedures using a version of the Automatic Normalization Tools (ANTS) algorithm for atlas-to-template non-linear registration (Chakravarty et al., 2006). The 21 templates were then warped onto all subjects, yielding 21 possible candidate segmentations per subject. Final segmentations were chosen via voxel voting procedure. This process was repeated with a different atlas in order to obtain striatal subdivisions including the bilateral nucleus accumbens, precommissural putamen, postcommissural putamen, precommissural caudate, and postcommissural caudate (Chakravarty et al., 2006). The same method and steps was applied to segment the hippocampus and amygdala but using 5 different high resolution atlases with 105 candidate segmentations per structure, per subject (Amaral et al., 2018; Winterburn et al., 2019).
2.6. Two-level deformation-based morphometry
Preprocessed T1-weighted images were upsampled to a resolution of 0.5mm isotropic in order to account for differences in resolution of the scan types and used as inputs for a longitudinal two level deformation based morphometry technique using the ANTs toolkit (http://stnava.github.io/ANTs/) for linear and non-linear registration (Avants et al., 2011; Avants et al., 2014). In the first level, both scans (pre- and post-treatment) were registered to each other to create a subject average (between timepoint registration). In the second level, all subject averages were registered using a group-wise averaging technique to create a study average. This provides voxel correspondence between subjects, which allows for comparison of local individual changes across subjects. Voxel-level volume changes were captured in the Jacobian determinant of the deformation field derived from the image registration. These have been shown to be sensitive to detect neuroanatomical changes without requiring segmentation (Chung et al., 2001). Both relative and absolute Jacobian determinants of the deformations fields of the first level were resampled into the second level average space to perform statistics. Relative Jacobians explicitly model only the non-linear part of the deformations to remove residual global linear transformation attributable to differences in total brain size and were used for subsequent statistical analysis. Absolute Jacobians include overall linear transformations. Resampled Jacobian determinants were smoothed using a 2mm Gaussian 3D kernel.
2.7. Statistical Analyses
The results are expressed as means and standard deviations (SD). Demographic and clinical characteristics were compared between groups using independent-sample t tests. Frequency data were analyzed using χ2.
Levels of glutamate, the metabolite of interest, were analyzed using a mixed repeated-measures analyses of variance, using time (baseline vs 4 weeks) as the within-subject factor, and group (responders vs non-responders) as between-subject factor. Outlier participants were detected using Cook distance criteria. This method identified 1 outlier in the non-responder group at the baseline scan. Independent-sample t tests were used for comparisons within groups for the rest of the metabolites. The statistical comparisons were performed with a significance level set at p<.05.
Pearson’s product-moment correlations were performed to examine the relationship between clinical scale scores (PANSS positive, negative, and general psychopathology subscales) and glutamate levels. The statistical threshold for these exploratory correlations was set at p<.016 (p<.05/3).
2.8. Baseline Volumetric Changes
The RMINC1.4.3.2. software package (https://github.com/Mouse-Imaging-Center/RMINC) was used for statistical analysis. A general linear model was used to test for volume differences between responders and non-responders pre-treatment, with age, sex, and total brain volume as covariates. Multiple comparisons correction was applied to these analyses using False Discovery Rate (FDR) correction.
2.9. Longitudinal brain volume changes post- treatment response
To investigate longitudinal changes in brain volume from pre- to post- treatment, linear mixed effects modeling was performed testing for an interaction between treatment response and time, with age and sex as covariate, and a random intercept per subject and scan type (model 1). FDR correction was applied to the models. A linear mixed effects model was run at every voxel of the relative Jacobian determinants (which account for differences in total brain volume) of the deformation field to investigate whether there were significant differences in brain structure over time due to treatment response using model 1 as above and corrected with FDR.
2.10. Longitudinal neuroanatomical and striatal glutamate changes post-treatment response
To investigate if longitudinal volume changes were associated with changes in glutamate over time, a linear mixed effects model testing for a response by glutamate by time interaction with age and sex as covariates and a random intercept per subject (model 2) was run for all structures of interest, corrected for multiple comparisons with FDR. The effect of glutamate was also investigated with model 2 and corrected for multiple comparisons with FDR.
As next step, model 2 was run at every voxel to obtain the interactions between glutamate, treatment response, and time on brain volume using the relative Jacobian determinants and corrected with FDR.
3. Results
3.1. Demographic and clinical characteristics of the participants
After 4 weeks of treatment, 29 patients were classified as responders and 19 as non-responders. The level of education was higher in responders than non- responders (t(46)=2.41, p=.02). The response and non-response groups were similar on age, gender, parental education, tobacco and cannabis use. It is important to note that both groups had similar duration of untreated psychosis, except one patient in the non-responder group that had experienced psychotic symptoms for more than 2 years. The mean (SD) daily dose of risperidone was higher in the non-responders compared to responders (t(46)=2.96; p=.005). At baseline, the responder group had higher PANSS positive subscale scores (t(46)=3.24; p=.002). Non-responders had higher PANSS positive (t(46)=4.46; p<.001) and PANSS general symptoms subscale scores (t(46)=2.16; p=.04), and trend-level higher PANSS negative subscale scores (t(46)=1.85; p=.07) after 4 weeks of treatment (Table 1).
Table 1.
Demographic and Clinical Characteristics of the Sample
| Responders | Non-Responders | |
|---|---|---|
| (n=29) | (n=19) | |
| Age (±SD) years | 25.2 ± 7.7 (range 16–47) |
21.8 ± 5.8 (range 14–37) |
| Education (±SD) years | 11.7 ± 2.7 | 9.7 ± 2.8* |
| Parental education (±SD) years | 9.9 ± 4.7 | 10.1 ± 5.2 |
| Gender (male/female) | 17/12 | 15/4 |
| Tobacco (ever Used) | 9 out of 29 | 6 out of 19 |
| Cannabis (ever Used) | 3 out of 29 | 2 out of 19 |
| Duration of untreated psychosis (±SD) weeks | 24.9 ± 32.6 (range 1–104) |
33.5 ± 37.1 (range 1–146) |
| Risperidone dose (±SD) mg | 2.7 ± 1.2 | 3.9 ± 1.4* |
| Symptoms at baseline (±SD) | ||
| PANSS Positive Symptoms | 26.6 ± 5.0 | 22.2 ± 4.4* |
| PANSS Negative Symptoms | 24.8 ± 5.6 | 25.5 ± 5.7 |
| PANSS General Symptoms | 50.9 ± 8.1 | 49.8 ± 7.9 |
| Symptoms at 4 weeks (±SD) | ||
| PANSS Positive Symptoms | 12.0 ± 2.8 | 16.4 ± 4.0* |
| PANSS Negative Symptoms | 16.2 ± 4.9 | 19.2 ± 5.8 |
| PANSS General Symptoms | 31.0 ± 7.1 | 35.5 ± 7.2* |
Abbreviations: PANSS, Positive and Negative Syndrome Scale.
p<0.05
3.2. Glutamate levels
Spectroscopy data from 4 participants at baseline (4 responders), and 3 participants at 4 weeks (2 responders, 1 non-responder), were rejected from analyses due to poor quality. Analyses were performed with both inclusion of the remaining sample and with exclusion of 1 outlier as identified by Cook distance criteria.
There was a significant difference in glutamate levels between groups (F1,39=7.15, p=.01); however, there were no significant differences between baseline and 4-week periods (F1,39=3.35, p=.08) for either group (interaction of time by group: F1,39=.41, p=.53). The results persisted when the outlier was left out of the analyses, with differences in glutamate levels between groups (F1,38=10.23, p=.003), but no differences between baseline and follow-up (F1,38=3.57, p=.07) and no interaction of time by group (F1,38=.27, p=.61).
Post hoc comparisons revealed that the non-responder group had higher glutamate levels after 4 weeks of treatment compared to the responder group (t(43)=2.4; p=.02). Interestingly, the patients classified as non-responders a posteriori had trend-level higher glutamate levels at the baseline scan compared to the patients that later responded to treatment (t(42)=1.84; p=.07). This result became significant after exclusion of the outlier (t(41) =2.81, p=.02) (Figure 1).
Figure 1.
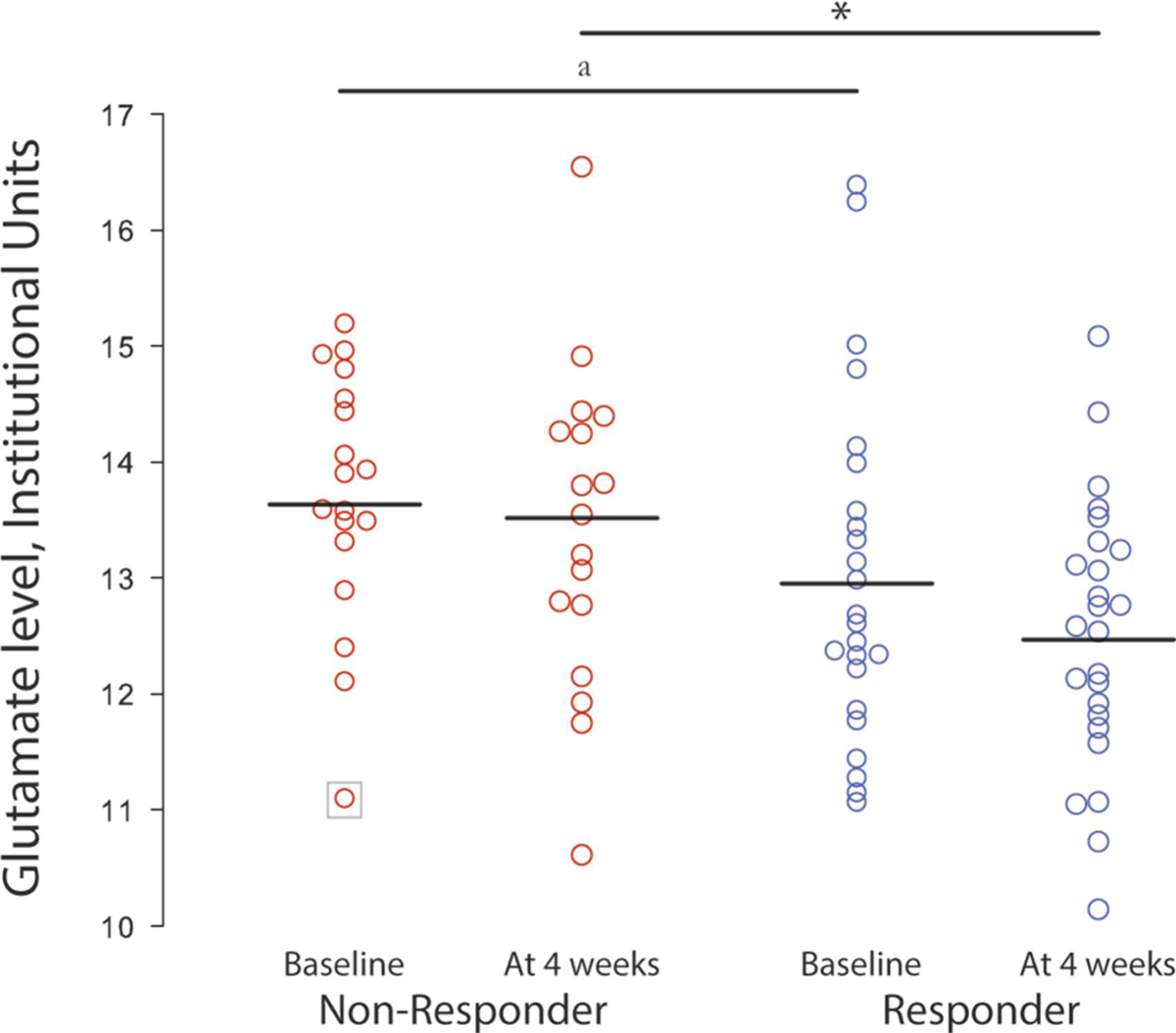
Glutamate levels at baseline and at 4 weeks in the right dorsal caudate of patients with first-episode psychosis classified retrospectively at 4 weeks as responders and non-responders. Horizontal bars represent the mean for the groups. *p <.05; a p <.05 after the removal of 1 outlying participant indicated within a square.
There were positive correlations between baseline glutamate levels and both PANSS Positive subscale scores (r19=.59, p=.008) and General Psychopathology subscale scores (r19=.69, p=.001) in the non-responder group (Figure 2). No correlations were observed in non-responders after treatment. No correlations between glutamate levels and clinical measures (PANSS Positive, Negative, and General Psychopathology subscales) were observed in responders at either baseline or 4-week time points.
Figure 2.
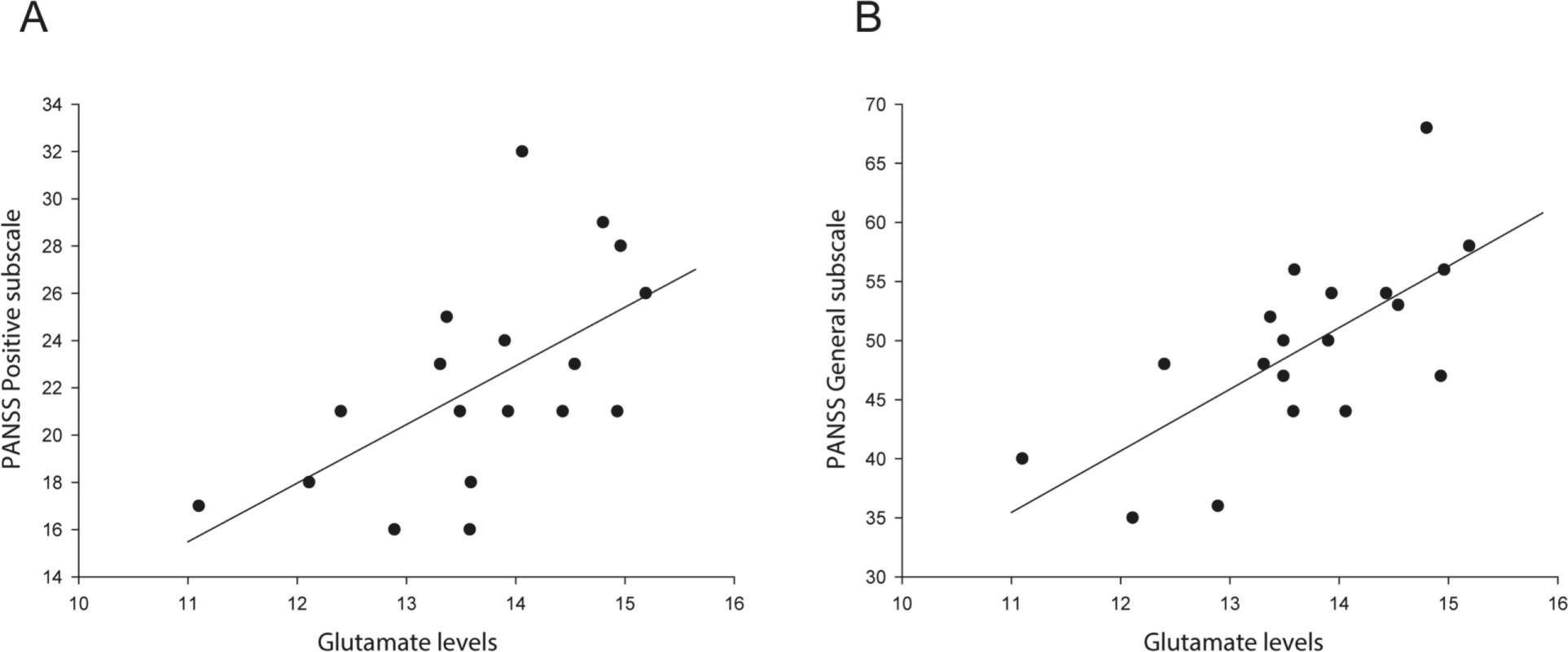
Relationship between baseline glutamate levels and A) PANSS Positive subscale scores, and B) General Psychopathology subscale scores in the non-responder group.
3.3. Other Metabolites, Voxel Tissue Composition and Spectral Quality
Higher tCr levels were found in non-responders compared with responders after 4 weeks of antipsychotic treatment (t(43)=2.88, p=.02). No differences in tCr levels were found between the groups at baseline. No differences were found in Glx, tNAA, tCho or myo-inositol levels in non-responders compared with responders at either baseline or 4-week studies. In addition, the proportions of voxel gray matter, white matter and cerebrospinal fluid did not differ between groups at baseline or in post-treatment scans. There were no group differences in FWHM values or signal-to noise ratios at either time point (Table 2).
Table 2.
Means (±SD) for each metabolite, tissue composition, and spectral quality in responders and non-responders groups
| Mean (SD) | |||||
|---|---|---|---|---|---|
| Baseline | At 4 weeks | ||||
| Responders | Non-responders | Responders | Non-responders | ||
| Metabolites | |||||
| Glutamate | 12.99 (1.45) | 13.69 (1.05) b | 12.50 (1.11) | 13.43 (1.37) a | |
| Glx | 16.43 (1.83) | 17.23 (1.67) | 16.29 (1.38) | 17.24 (2.01) | |
| tNAA | 10.87 (1.23) | 11.11 (1.28) | 10.88 (0.77) | 11.15 (0.84) | |
| tCho | 2.49 (0.31) | 2.59 (0.31) | 2.52 (0.20) | 2.59 (0.31) | |
| Myo-inositol | 5.83 (1.01) | 6.12 (1.04) | 5.53 (1.02) | 5.91 (1.04) | |
| tCr | 8.56 (1.07) | 8.81 (0.65) | 8.42 (0.63) | 8.99 (0.92) a | |
| Spectral Quality | |||||
| FWHM | 0.08 (0.02) | 0.07 (0.02) | 0.08 (0.01) | 0.08 (0.02) | |
| SNR | 14.73 (2.46) | 14.26 (2.13) | 13.67 (2.60) | 14.89 (2.49) | |
| Voxel Composition, % volume | |||||
| Grey matter | 0.42 (0.05) | 0.40 (0.05) | 0.41 (0.05) | 0.43 (0.07) | |
| White matter | 0.47 (0.06) | 0.47 (0.08) | 0.46 (0.07) | 0.45 (0.06) | |
| Cerebrospinal fluid |
0.11 (0.07) | 0.12 (0.07) | 0.12 (0.08) | 0.11 (0.07) | |
SD, standard deviation; Glx, glutamate + glutamine; tNAA, N-acetylaspartate + N-acetylaspartaylglutamate; tCho, glycerophosphocholine + phosphocoline; tCr, creatine + phosphocreatine; FWHM, full-width at half maximum; SNR, signal-to-noise ratio.
p<0.05
p<0.05 after the exclusion of 1 outlier
3.4. Structural Measures
For the subcortical analyses 10 subjects (5 responders, 5 non-responders) were removed due to scan quality/segmentation quality.
3.4.1. Pre-treatment (baseline) differences between responders and non-responders
3.4.1.1. Volumes
The non-responders had larger right hippocampal volume (t=2.445, p=0.0197 uncorrected) and larger right striatal volume (t=2.135, p=0.04 uncorrected) than treatment responders, however these differences did not pass significance threshold following FDR correction (p>0.05) (Figure 3).
Figure 3.
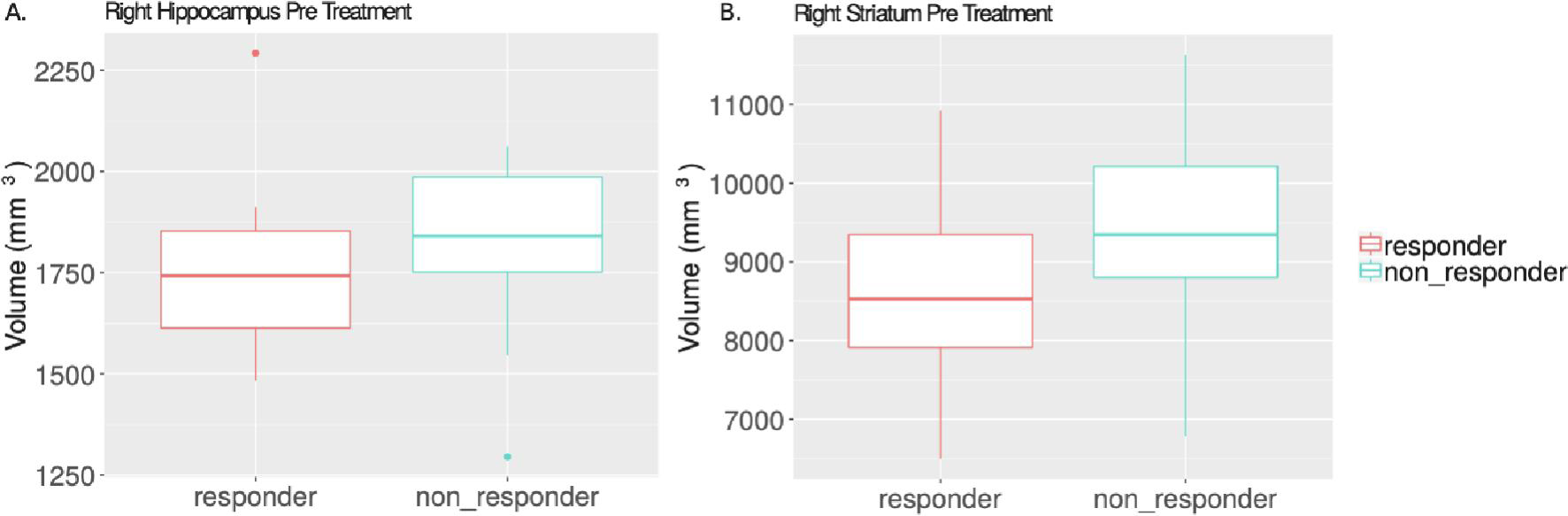
Pre-treatment volume differences between responders and non-responders. The right hippocampus (A) and right striatum (B) of non-responders is larger than that of responders before starting antipsychotic treatment (t=2.445, p=0.02; t=2.135, p=0.04). Data are represented with boxplots: the center line is the median of the data, the upper and lower portion of the box correspond to the first and third quartiles, the vertical line and dots represent the end range of the data and outliers.
3.4.2. Longitudinal volume changes in risperidone treatment responders and non-responders
3.4.2.1. Volumes
A trend level interaction between treatment response and time (t=−1.77, p=0.085 uncorrected) was observed for the volume of the left hippocampus by which volume for responders remained steady from pre- to post-treatment, however, non-responders experienced a volume reduction. No other regions were significantly different based on treatment response over time (>5%FDR). Striatal volume increased in both treatment responders and non-responders from pre- to post-treatment timepoints (uncorrected main effect of time for left [t=2.68, p=0.011] and right striatum [t= 2.316, p= 0.027]), however were not significant following FDR correction (Figure 4).
Figure 4.
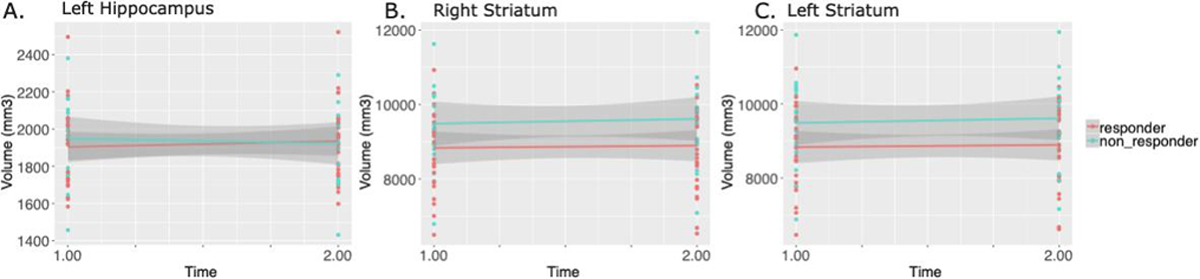
Longitudinal volume changes from pre- to post-treatment comparing treatment responders to non-responders. A) Volume of the left hippocampus increased over time for treatment responders, but not for non-responders (t=−1.77, p=0.085). B and C) Right (t= 2.316, p= 0.027) and left (t=2.68, p=0.011) striatal volume increased over time for both responders and non-responders.
3.4.2.2. Deformation based morphometry
For the deformation based morphometry analysis, 4 subjects (2 responders, 2 non-responders) were removed due to scan quality/segmentation quality.
A significant interaction between treatment response and time was detected in various brain regions (t=5.163, <1%FDR). We observed a relative decrease in volume in treatment responders, and an increase in volume for non-responders from pre- to post-treatment in regions such as the right external capsule, the left hippocampus (CA3), the right hippocampus (CA1/CA2), and the fourth ventricle. Interestingly, there were also regions which followed the opposite trend, including the left caudate, left bed nucleus of stria terminalis, the left putamen, the right hippocampus (subiculum), and right ventral posterolateral thalamic nucleus (Figure 5).
Figure 5.
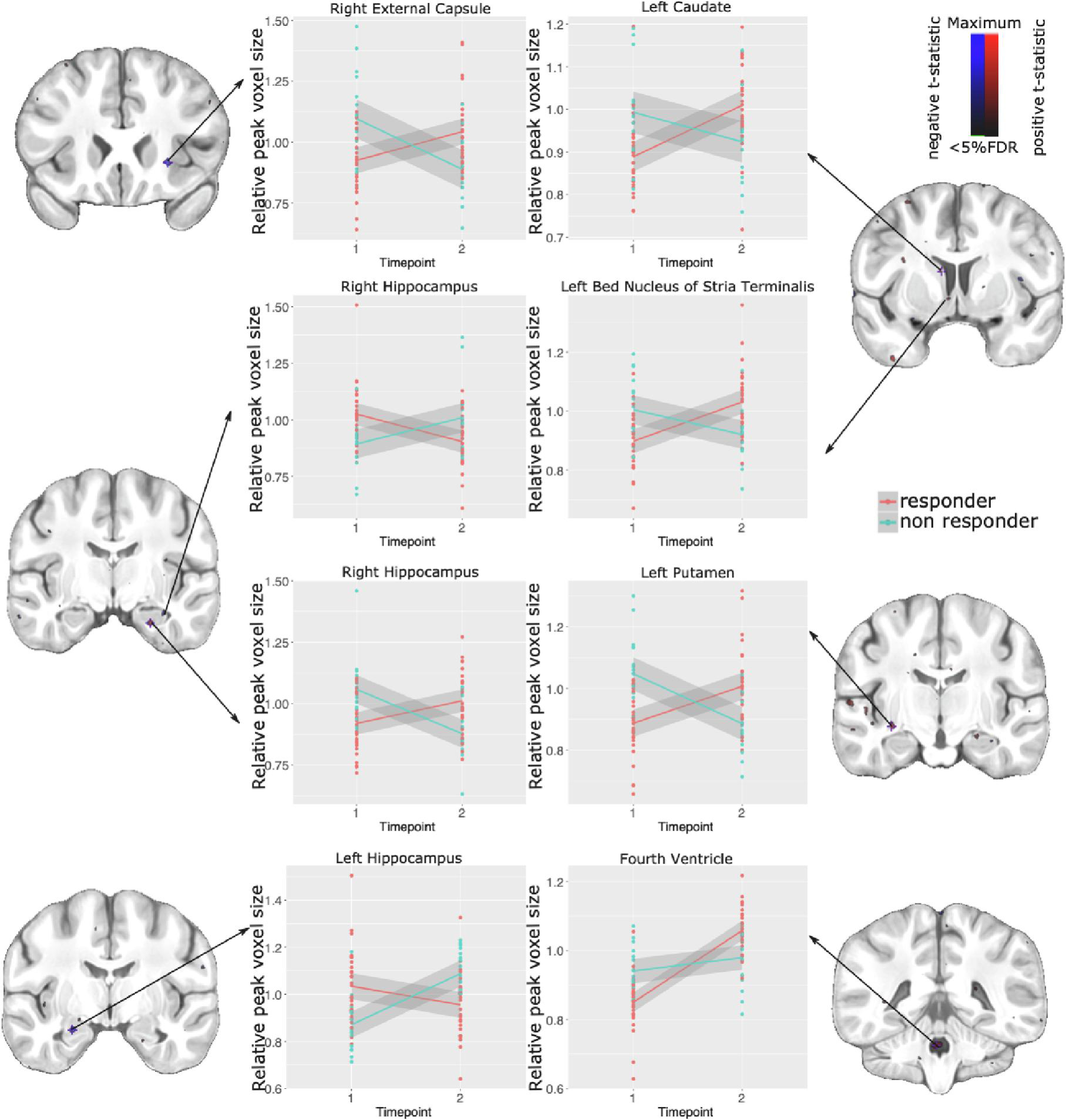
Local volume differences associated with treatment response. Significant interactions visualized using blue for negative, and red for positive t-statistics (overlaid on coronal sections of the study average). Relative voxel size at peak voxels in the regions of significance are plotted to show differences between responders and non-responders pre- and post-treatment.
3.5. Interaction between neuroanatomy and 1H-MRS glutamate measures in treatment responders and non-responders
3.5.1. Deformation based morphometry
A significant interaction between treatment response group, time, striatal glutamate and volume measures was found following FDR correction (t=4.23, <15%FDR). The relationship between glutamate and volume differed between responders and non-responders over the course of their treatment. In some regions, non-responders switched from having a negative relationship between volume and glutamate, to having a positive one post treatment, whereas the responders had the opposite change, starting with a positive relationship pre-treatment, and changing to a negative relationship post-treatment. Regions in which this was observed include the right frontal cortex white matter, the right putamen, the right and left external capsule, the right substantia nigra, and the right hippocampus. In some regions the opposite was observed: non-responders went from a positive association between volume and glutamate pre-treatment, to a negative relationship post treatment, whereas responders had a negative relationship pre-treatment and switched to a positive relationship post-treatment. These regions include the right internal globus pallidus and the right parietal cortex white matter (Figure 6).
Figure 6.
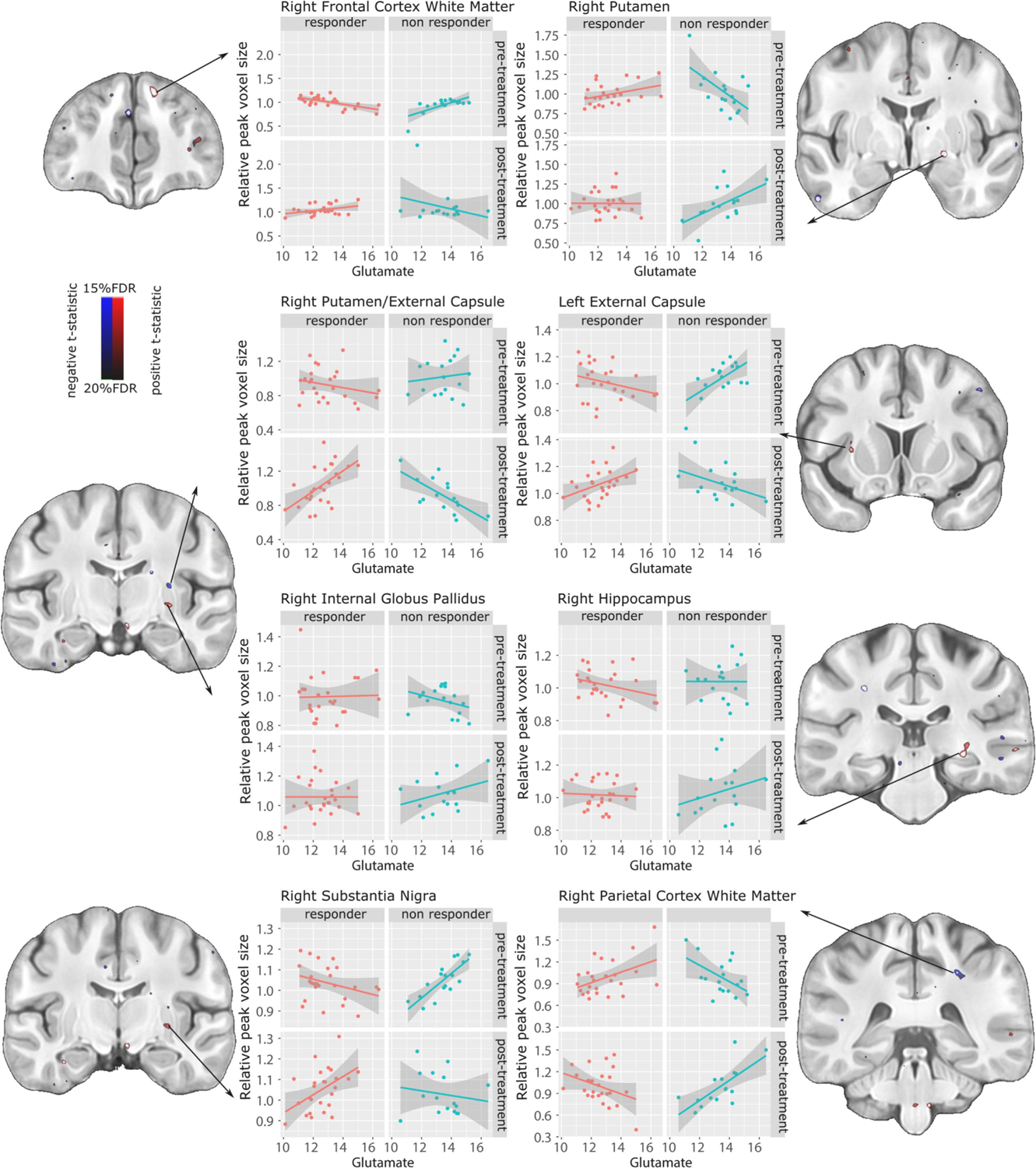
Local volume differences associated with striatal glutamate levels, modulated by treatment response. Significant interactions visualized using blue for negative, and red for positive t-statistics (overlaid on coronal sections of the study average) thresholded between 15% FDR (t=4.23 and 20% FDR (t=3.34). Relative voxel size at peak voxels in the regions of significance are plotted to show differences between responders and non-responders pre- and post-treatment.
4. Discussion
In this study of initially unmedicated, first-episode patients with schizophrenia, we compared several MRI-based outcome measures between responders and non-responders to four weeks of treatment with risperidone. We sought to replicate prior reports of higher brain glutamate in non-responders, and to investigate whether anatomic measures enhanced classification of response to medication.
Response status.
We used a cutoff criterion of 40% improvement in the PANSS positive subscale to define medication response, resulting in identification of 29 of the 48 patients as responders and 19 as non-responders. Although the responder group had higher PANSS positive subscale scores at baseline, contributing to their response classification by percent change, they also had lower PANSS positive subscale scores after 4 weeks of treatment, affirming a greater medication responsiveness.
Neurochemistry.
Dorsal caudate glutamate differed between the responder and non-responder groups as hypothesized and as previously reported (Egerton et al., 2018). Other MRS metabolite group differences, including that of creatine, were not hypothesized and did not reach statistical significance after multiple comparisons correction. Notably, the finding of higher glutamate in non-responders was present not only post-treatment but at baseline as well, suggesting a potential predictive biomarker of treatment response. This idea is supported by the positive correlations between glutamate levels and both PANSS Positive and General Psychopathology subscale scores in the non-responder group at baseline but not post-treatment nor in the responders, suggesting that the highest glutamate levels in the unmedicated state correspond to the most significant and refractory psychopathology. In a similar way, a recent mega-analysis reinforces the idea that glutamate levels could be a marker of symptom severity in patients with schizophrenia: in this study, glutamate levels in the medial frontal cortex were positively correlated with both PANSS total and positive subscale scores (Merritt et al., 2021).
Volumetrics.
Baseline measures showed subtly larger right striatal and right hippocampal volumes in the non-responders, but these did not survive multiple comparisons correction. In addition, there were bilateral trends toward striatal volume increases post-treatment in both groups, an effect of antipsychotic medication that has been previously reported (Chakos et al., 1994; Keshavan et al., 1994).
Deformation-based morphometry.
Our investigation of whole-brain voxel-wise differences identified a few focal regions differentially affected by treatment in responders relative to non-responders. Regions that increased in volume over the course of treatment in the non-responders and decreased in the responders include some basal ganglia regions (left caudate, left putamen), the right external capsule, left bed nucleus of stria terminalis, and right hippocampus (CA3).
Conversely, some regions increased in volume in the treatment responders and decreased in non-responders include the left hippocampus (CA3), right hippocampus (CA1/CA2), and the fourth ventricle. Basal ganglia and hippocampus are anatomical regions that have shown structural and functional changes due to antipsychotic treatment and have been proposed as targets of treatment response prediction (Kraguljac and Lahti, 2021; Lahti et al., 2009; Sarpal et al., 2015). A study by Lahti et al. (Lahti et al., 2009) showed that an increase in ventral striatal regional blood and a decrease of hippocampal regional blood during the first week of antipsychotic treatment, were related with treatment response at week 6. Also, striatal (Sarpal et al., 2015) and hippocampal (Blessing et al., 2020) functional magnetic resonance has shown changes treatment response-related connectivity patterns. The divergent trajectories in basal ganglia volumes are interesting in light of known sensitivity to antipsychotic treatment. Furthermore, the hippocampus has been highly implicated in the pathology of psychosis and may also play a role in modulating antipsychotic response (Lieberman et al., 2018).
Interaction effects.
Associations between glutamate levels and volumes were different between responders and non-responders. Non-responders showed increased right putamen volumes associated with increased glutamate following failed treatment, an effect not seen in the responders. Increased putamen volume has been associated increased symptom severity (Molina et al., 2011), even in drug naïve patients (Gur et al., 1998). This suggests that responders may benefit from a decoupling of the post-treatment glutamate correlation with increased volume exhibited by the non-responders, who show a tendency for glutamate to correlate with volumes that increase with treatment. These data provide confirmation from another vantage point of the role of glutamate elevations in failure to respond to medication, in relation to the striatal volume increase often seen with antipsychotic medications (Chakos et al., 1994; Keshavan et al., 1994). Another possibility is that this correlation in the putamen volume is related to risperidone dosage, since non-responders were exposed to higher dosages (Glenthoj et al., 2007),
A limitation of this study is the brief duration of the trial, sufficient to generate reliably quantifiable changes in glutamate but potentially short for detection of medication effects on brain structure. The differences in brain structure between responders and non-responders are subtle; it is possible that a longer treatment duration, or longer follow up of these patients would allow us to detect more robust differences in neuroanatomy. In addition, the retrospective ascertainment of medication responsiveness within the study itself, necessitated in a drug-naïve sample, may be less robust than widely used criteria for medication responsiveness based on outcomes of prior medication trials; also, a 40% reduction in positive symptoms as the definition of treatment response could limit its replication.
Strengths of the study are its relatively large study sample of medication-naïve patients, the controlled conditions of the medication trial, and the multimodal acquisitions of neurochemical and volumetric measures both pre- and post-treatment.
5. Conclusion
In summary, this study of glutamate levels and neuroanatomic changes found distinct patterns in responders and non-responders to a 4-week trial of risperidone. Glutamate levels were significantly higher in the non-responders both before and after the medication trial. While striatal volumes showed increases with medication in both groups, these volume increases were more strongly positively related to glutamate levels in the non-responders; this may reflect differences in remodeling in response to antipsychotics based on treatment response. Volumes tended to correlate positively with glutamate levels post-treatment in the non-responders more than responders, aligning with the basic phenomenon of medication-induced volume increases and extending it to correlate with higher glutamate levels in the non-responder group. Patients with glutamatergic abnormalities could benefit from clinical trials with glutamate modifier drugs in early stages of the disease (Krystal and Anticevic, 2015).
Highlights.
48 FEP patients, before and after a 4-week trial of risperidone, were studied
Brain structure and glutamate levels were measured at baseline and 4 weeks
Striatal glutamate was higher in non-responders pre- and post-treatment
Group × time × glutamate interaction was seen in several brain regions
Positive correlation of striatal volumes and glutamate was seen in non-responders
Acknowledgments
This project was supported by Consejo Nacional de Ciencia y Tecnología, Mexico, (CONACyT) Grant Nos. 182279 (to Ariel Graff-Guerrero and Camilo de la Fuente-Sandoval) and 261895 (to Camilo de la Fuente-Sandoval); CONACyT’s Sistema Nacional de Investigadores (to Francisco Reyes-Madrigal, Pablo León-Ortiz, Ariel Graff-Guerrero and Camilo de la Fuente-Sandoval); and National Institutes of Health Grant Nos. R01 MH110270 (to Lawrence S. Kegeles and Camilo de la Fuente-Sandoval) and R21 MH117434 (to Camilo de la Fuente-Sandoval).
The funding sources were not involved in the study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the manuscript for publication.
Footnotes
Declaration of Interest
Francisco Reyes-Madrigal, Pablo León-Ortiz and Ricardo Mora-Durán have served as speakers for Janssen (Johnson & Johnson). The rest of the authors do not report actual or potential conflicts of interest.
Ethical Statement
The study was approved by the Ethics and Scientific committees of the Instituto Nacional de Neurología y Neurocirugía in Mexico City. All participants over 18 years fully understood and signed the informed consent; in case the patient was under 18 years, informed consent was obtained from both parents. Participants did not receive a stipend.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M, 2000. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A 97 (14), 8104–8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral RSC, Park MTM, Devenyi GA, Lynn V, Pipitone J, Winterburn J, Chavez S, Schira M, Lobaugh NJ, Voineskos AN, Pruessner JC, Chakravarty MM, Alzheimer’s Disease Neuroimaging I, 2018. Manual segmentation of the fornix, fimbria, and alveus on high-resolution 3T MRI: Application via fully-automated mapping of the human memory circuit white and grey matter in healthy and pathological aging. Neuroimage 170, 132–150. [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC, 2011. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54 (3), 2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Stauffer M, Song G, Wu B, Gee JC, 2014. The Insight ToolKit image registration framework. Front Neuroinform 8, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing EM, Murty VP, Zeng B, Wang J, Davachi L, Goff DC, 2020. Anterior Hippocampal-Cortical Functional Connectivity Distinguishes Antipsychotic Naive First-Episode Psychosis Patients From Controls and May Predict Response to Second-Generation Antipsychotic Treatment. Schizophr Bull 46 (3), 680–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briend F, Nelson EA, Maximo O, Armstrong WP, Kraguljac NV, Lahti AC, 2020. Hippocampal glutamate and hippocampus subfield volumes in antipsychotic-naive first episode psychosis subjects and relationships to duration of untreated psychosis. Transl Psychiatry 10 (1), 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M, 1994. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry 151 (10), 1430–1436. [DOI] [PubMed] [Google Scholar]
- Chakravarty MM, Bertrand G, Hodge CP, Sadikot AF, Collins DL, 2006. The creation of a brain atlas for image guided neurosurgery using serial histological data. Neuroimage 30 (2), 359–376. [DOI] [PubMed] [Google Scholar]
- Chakravarty MM, Steadman P, van Eede MC, Calcott RD, Gu V, Shaw P, Raznahan A, Collins DL, Lerch JP, 2013. Performing label-fusion-based segmentation using multiple automatically generated templates. Hum Brain Mapp 34 (10), 2635–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MK, Worsley KJ, Paus T, Cherif C, Collins DL, Giedd JN, Rapoport JL, Evans AC, 2001. A unified statistical approach to deformation-based morphometry. Neuroimage 14 (3), 595–606. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, Stephano S, Favila R, Diaz-Galvis L, Alvarado-Alanis P, Ramirez-Bermudez J, Graff-Guerrero A, 2013. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry 70 (10), 1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Leon-Ortiz P, Favila R, Stephano S, Mamo D, Ramirez-Bermudez J, Graff-Guerrero A, 2011. Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology 36 (9), 1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Reyes-Madrigal F, Mao X, Leon-Ortiz P, Rodriguez-Mayoral O, Jung-Cook H, Solis-Vivanco R, Graff-Guerrero A, Shungu DC, 2018. Prefrontal and Striatal Gamma-Aminobutyric Acid Levels and the Effect of Antipsychotic Treatment in First-Episode Psychosis Patients. Biol Psychiatry 83 (6), 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demjaha A, Egerton A, Murray RM, Kapur S, Howes OD, Stone JM, McGuire PK, 2014. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol Psychiatry 75 (5), e11–13. [DOI] [PubMed] [Google Scholar]
- Egerton A, Broberg BV, Van Haren N, Merritt K, Barker GJ, Lythgoe DJ, Perez-Iglesias R, Baandrup L, During SW, Sendt KV, Stone JM, Rostrup E, Sommer IE, Glenthoj B, Kahn RS, Dazzan P, McGuire P, 2018. Response to initial antipsychotic treatment in first episode psychosis is related to anterior cingulate glutamate levels: a multicentre (1)H-MRS study (OPTiMiSE). Mol Psychiatry 23 (11), 2145–2155. [DOI] [PubMed] [Google Scholar]
- Egerton A, Murphy A, Donocik J, Anton A, Barker GJ, Collier T, Deakin B, Drake R, Eliasson E, Emsley R, Gregory CJ, Griffiths K, Kapur S, Kassoumeri L, Knight L, Lambe EJB, Lawrie SM, Lees J, Lewis S, Lythgoe DJ, Matthews J, McGuire P, McNamee L, Semple S, Shaw AD, Singh KD, Stockton-Powdrell C, Talbot PS, Veronese M, Wagner E, Walters JTR, Williams SR, MacCabe JH, Howes OD, 2020. Dopamine and Glutamate in Antipsychotic-Responsive Compared With Antipsychotic-Nonresponsive Psychosis: A Multicenter Positron Emission Tomography and Magnetic Resonance Spectroscopy Study (STRATA). Schizophr Bull 10.1093/schbul/sbaa128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskildsen SF, Coupe P, Fonov V, Manjon JV, Leung KK, Guizard N, Wassef SN, Ostergaard LR, Collins DL, Alzheimer’s Disease Neuroimaging I, 2012. BEaST: brain extraction based on nonlocal segmentation technique. Neuroimage 59 (3), 2362–2373. [DOI] [PubMed] [Google Scholar]
- Gillespie AL, Samanaite R, Mill J, Egerton A, MacCabe JH, 2017. Is treatment-resistant schizophrenia categorically distinct from treatment-responsive schizophrenia? a systematic review. BMC Psychiatry 17 (1), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenthoj A, Glenthoj BY, Mackeprang T, Pagsberg AK, Hemmingsen RP, Jernigan TL, Baare WF, 2007. Basal ganglia volumes in drug-naive first-episode schizophrenia patients before and after short-term treatment with either a typical or an atypical antipsychotic drug. Psychiatry Res 154 (3), 199–208. [DOI] [PubMed] [Google Scholar]
- Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC, 1998. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry 155 (12), 1711–1717. [DOI] [PubMed] [Google Scholar]
- Iwata Y, Nakajima S, Plitman E, Caravaggio F, Kim J, Shah P, Mar W, Chavez S, De Luca V, Mimura M, Remington G, Gerretsen P, Graff-Guerrero A, 2019. Glutamatergic Neurometabolite Levels in Patients With Ultra-Treatment-Resistant Schizophrenia: A Cross-Sectional 3T Proton Magnetic Resonance Spectroscopy Study. Biol Psychiatry 85 (7), 596–605. [DOI] [PubMed] [Google Scholar]
- Juarez-Reyes MG, Shumway M, Battle C, Bacchetti P, Hansen MS, Hargreaves WA, 1995. Effects of stringent criteria on eligibility for clozapine among public mental health clients. Psychiatr Serv 46 (8), 801–806. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Bagwell WW, Haas GL, Sweeney JA, Schooler NR, Pettegrew JW, 1994. Changes in caudate volume with neuroleptic treatment. Lancet 344 (8934), 1434. [DOI] [PubMed] [Google Scholar]
- Kraguljac NV, Lahti AC, 2021. Neuroimaging as a Window Into the Pathophysiological Mechanisms of Schizophrenia. Front Psychiatry 12, 613764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Anticevic A, 2015. Toward illness phase-specific pharmacotherapy for schizophrenia. Biol Psychiatry 78 (11), 738–740. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Cropsey KL, 2009. Modulation of limbic circuitry predicts treatment response to antipsychotic medication: a functional imaging study in schizophrenia. Neuropsychopharmacology 34 (13), 2675–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Takeuchi H, Fervaha G, Sin GL, Foussias G, Agid O, Farooq S, Remington G, 2015. Subtyping Schizophrenia by Treatment Response: Antipsychotic Development and the Central Role of Positive Symptoms. Can J Psychiatry 60 (11), 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, Kreyenbuhl J, American Psychiatric A, Steering Committee on Practice G, 2004. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry 161 (2 Suppl), 1–56. [PubMed] [Google Scholar]
- Lieberman JA, Girgis RR, Brucato G, Moore H, Provenzano F, Kegeles L, Javitt D, Kantrowitz J, Wall MM, Corcoran CM, Schobel SA, Small SA, 2018. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol Psychiatry 23 (8), 1764–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmayer JP, 2000. Treatment refractory schizophrenia. Psychiatr Q 71 (4), 373–384. [DOI] [PubMed] [Google Scholar]
- Merritt K, McGuire PK, Egerton A, Investigators H.M.i.S., Aleman A, Block W, Bloemen OJN, Borgan F, Bustillo JR, Capizzano AA, Coughlin JM, De la Fuente-Sandoval C, Demjaha A, Dempster K, Do KQ, Du F, Falkai P, Galinska-Skok B, Gallinat J, Gasparovic C, Ginestet CE, Goto N, Graff-Guerrero A, Ho BC, Howes OD, Jauhar S, Jeon P, Kato T, Kaufmann CA, Kegeles LS, Keshavan M, Kim SY, Kunugi H, Lauriello J, Liemburg EJ, McIlwain ME, Modinos G, Mouchlianitis ED, Nakamura J, Nenadic I, Ongur D, Ota M, Palaniyappan L, Pantelis C, Plitman E, Posporelis S, Purdon SE, Reichenbach JR, Renshaw PF, Russell BR, Sawa A, Schaefer M, Shungu DC, Smesny S, Stanley JA, Stone JM, Szulc A, Taylor R, Thakkar K, Theberge J, Tibbo PG, van Amelsvoort T, Walecki J, Williamson PC, Wood SJ, Xin L, Yamasue H, 2021. Association of Age, Antipsychotic Medication, and Symptom Severity in Schizophrenia With Proton Magnetic Resonance Spectroscopy Brain Glutamate Level: A Mega-analysis of Individual Participant-Level Data. JAMA Psychiatry 78 (6), 667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina V, Martin C, Ballesteros A, de Herrera AG, Hernandez-Tamames JA, 2011. Optimized voxel brain morphometry: association between brain volumes and the response to atypical antipsychotics. Eur Arch Psychiatry Clin Neurosci 261 (6), 407–416. [DOI] [PubMed] [Google Scholar]
- Mouchlianitis E, Bloomfield MA, Law V, Beck K, Selvaraj S, Rasquinha N, Waldman A, Turkheimer FE, Egerton A, Stone J, Howes OD, 2016. Treatment-Resistant Schizophrenia Patients Show Elevated Anterior Cingulate Cortex Glutamate Compared to Treatment-Responsive. Schizophr Bull 42 (3), 744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitman E, Nakajima S, de la Fuente-Sandoval C, Gerretsen P, Chakravarty MM, Kobylianskii J, Chung JK, Caravaggio F, Iwata Y, Remington G, Graff-Guerrero A, 2014. Glutamate-mediated excitotoxicity in schizophrenia: a review. Eur Neuropsychopharmacol 24 (10), 1591–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitman E, Patel R, Chung JK, Pipitone J, Chavez S, Reyes-Madrigal F, Gomez-Cruz G, Leon-Ortiz P, Chakravarty MM, de la Fuente-Sandoval C, Graff-Guerrero A, 2016. Glutamatergic Metabolites, Volume and Cortical Thickness in Antipsychotic-Naive Patients with First-Episode Psychosis: Implications for Excitotoxicity. Neuropsychopharmacology 41 (10), 2606–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarpal DK, Robinson DG, Lencz T, Argyelan M, Ikuta T, Karlsgodt K, Gallego JA, Kane JM, Szeszko PR, Malhotra AK, 2015. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry 72 (1), 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P, Plitman E, Iwata Y, Kim J, Nakajima S, Chan N, Brown EE, Caravaggio F, Torres E, Hahn M, Chakravarty MM, Remington G, Gerretsen P, Graff-Guerrero A, 2020. Glutamatergic neurometabolites and cortical thickness in treatment-resistant schizophrenia: Implications for glutamate-mediated excitotoxicity. J Psychiatr Res 124, 151–158. [DOI] [PubMed] [Google Scholar]
- Tullo S, Devenyi GA, Patel R, Park MTM, Collins DL, Chakravarty MM, 2018. Warping an atlas derived from serial histology to 5 high-resolution MRIs. Sci Data 5, 180107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterburn JL, Voineskos AN, Devenyi GA, Plitman E, de la Fuente-Sandoval C, Bhagwat N, Graff-Guerrero A, Knight J, Chakravarty MM, 2019. Can we accurately classify schizophrenia patients from healthy controls using magnetic resonance imaging and machine learning? A multi-method and multi-dataset study. Schizophr Res 214, 3–10. [DOI] [PubMed] [Google Scholar]


