Abstract
Recent studies demonstrated that MYC epigenetically regulates AML cell survival and differentiation by suppressing IDH1/2-TET2-5hmC signaling and that MYC overexpression is associated with poor survival outcomes in multiple AML patient cohorts. However, the oncogenic roles of MYC in MDS remain to be explored. A total of 41 patients with de novo MDS were retrospectively identified using the Total Cancer Care database at the Moffitt Cancer Center. A total of 61% of patients had low MYC expression and 39% of patients had high MYC expression defined as MYC reactivity by immunohistochemical staining in ≥5% of bone marrow (BM) cells at the time of MDS diagnosis. The median MDS-to-AML progression free survival (PFS) was significantly shorter in the high MYC group (median PFS 9.3 vs. 17.7 months, HR=2.328, p=0.013). Further, overall survival (OS) was also shorter in the high MYC patients (median OS 19.7 vs. 51.7 months, HR=2.299, p=0.053). Multivariate analyses demonstrated that high MYC expression is an independent poor prognostic factor for the MDS-to-AML progression (HR=2.275, p=0.046). Our observations indicate that MYC may play a crucial role in MDS transformation to AML and the underlying mechanisms of MYC-driven MDS clonal expansion and leukemic transformation require further investigation.
Keywords: MDS, AML, MYC
INTRODUCTION
Myelodysplastic syndromes (MDS) are clonal disorders of hematopoietic stem cells and myeloid progenitors that are characterized by impaired hematopoiesis, dysplasia, and high risk of disease transformation to acute myeloid leukemia (AML)1. Treatment response and survival outcomes were shown to be inferior in patients with AML that arises from antecedent MDS compared to patients with de novo AML2-4. Recent studies demonstrated that a variety of chromosomal aberrations and somatic mutations in splicing components, transcription factors, epigenetic regulators, and genes in survival pathways contribute to malignant transformation and clonal expansion in MDS and AML5. Among these, MYC protein levels are commonly elevated in both MDS and AML patients6-8 and MYC copy number gains and gene amplifications are frequently identified in AML patients5,9.
MYC proteins (c-MYC, N-MYC, and L-MYC) are basic helix-loop-helix leucine zipper (bHLH-Zip) transcription factors that regulate the expression of genes involved in cell proliferation, survival, glycolysis, and glutaminolysis10. Forced expression of MYC is sufficient to induce AML in mouse models11 and MYC is required for leukemias provoked by a variety of fusion genes (i.e. PML-RARα, BCR-ABL1, and AML1-ETO) and FLT3-ITD 12-15. Further, knockout of MYC promotes myeloid differentiation and suppresses proliferation of AML cells whereas overexpression of MYC has the opposite effects8. Accordingly, high MYC expression at mRNA and protein levels was shown to have a positive correlation with immature myeloid differentiation markers, elevated blast counts, and poor prognosis in several human AML patient cohorts6,8. Of note, our recent study demonstrated that MYC epigenetically regulates AML cell death and differentiation by inhibiting IDH1/2-TET2 signaling and suppressing 5mC conversion to 5hmC in the genomic DNA8. Collectively, these pre-clinical and clinical observations suggest that MYC plays an essential role in AML maintenance and potentially dictates treatment response and clinical outcomes.
Despite advances in our understanding of the oncogenic roles of MYC in AML, there is lack of evidence if MYC also plays oncogenic roles in MDS maintenance or/and transformation to AML. In addition, the underlying mechanisms of synergistic effects of concurrent MYC alterations with other myeloid associated-somatic mutations are largely unknown. As the first step, we attempt to investigate the prognostic impacts of MYC protein levels on the survival outcomes in MDS patients and explore the somatic mutational landscape in low vs. high MYC MDS patients who developed AML with myelodysplasia related changes (AML-MRC) in their clinical courses.
METHODS
Patient and Sample Acquisition
We retrospectively identified study subjects at H. Lee Moffitt Cancer Center (MCC) from 01/2008 to 08/2017. Eligible patients were age 18 years and older with in-house pathologic diagnosis of de novo MDS with disease progression to AML-MRC as defined by the World Health Organization (WHO) classification16,17 who had paired bone marrow (BM) biopsy samples available at the time of MDS as well as AML-MRC diagnosis. Patients with de novo AML and AML not arising from MDS were excluded in this study. Patients with therapy related MDS (tMDS) were included in this study. For all cases included, all BM biopsies were reviewed by two independent hematopathologists (D.G. and L.Z.) to confirm the diagnosis. Interval treatments before and after AML-MRC diagnosis were captured, and these included hypomethylating agents (HMA; azacitidine or decitabine), lenalidomide, erythropoiesis-stimulating agents (ESAs), venetoclax, intensive chemotherapy, investigational agents and allogeneic-stem cell transplant (allo-SCT). Responses to therapy in MDS and AML were evaluated according to IWG 2006 and ELN 2017 AML response criteria, respectively18,19. Clinical variables including age, gender, complete blood count (CBC) with differential counts, BM and peripheral blood (PB) blasts counts, cytogenetics, myeloid mutation profiles, international prognostic scoring system (IPSS) and revised IPSS (IPSS-R) risk category20,21 were captured at the time of diagnosis. This study was approved by the MCC Scientific Review Committee and Institutional Review Board.
Assessment of Bone Marrow Blast counts and MYC Protein Expression
Assessment of BM blast counts were performed by two independent hematopathologists (D.G. and L.Z.) on aspirate smears of BM biopsies collected at the time of original MDS and AML diagnoses. MYC immunohistochemical (IHC) stains were performed in BM biopsy specimens at the time of initial MDS and disease transformation to AML, respectively. MYC expression was assessed as described previously6. Briefly, deparaffinized BM biopsy slides were stained with anti-MYC antibody (clone Y69, Roche Diagnostics, Indianapolis, IN) using a Ventana Benchmark automated system. MYC expression was scored independently by two hematopathologists (D.G. and L.Z.) and categorized as low vs. high as previously described6. The cut-off for high MYC expression is ≥5% positive cells6.
Assessment of cytogenetics
We performed routine cytogenetic analyses using standard trypsin-Giemsa banding technique by Laboratory Corporation of America (Burlington, NC, USA) in accordance with an International System for Human Cytogenetic Nomenclature, 2016 (ISCH 2016, Karger). Further, fluorescence in-situ hybridization (FISH) using probe sets designed for MDS [del(5q)/-5, del(7q)/-7, +8, del(17p)/-17, and del(20q)/-20] and AML [t(8;21), t( 15; 17), and inv(16)] were used as per the manufacturer’s instructions (Vysis, Downers Grove, IL, USA).
Targeted Exome Sequencing
Targeted amplicon-based exome sequencing of up to 54 myeloid genes was performed as previously described22. Genomic DNA was extracted from BM mononuclear cells (BM-MNCs) or peripheral blood (PB) mononuclear cells (PB-MNCs), then subjected to targeted genome sequencing using an Illumina HiSeq™2000 (Illumina Inc., Sand Diego, CA) instrument. All samples were run in duplicate. Variants were selected if they included a read depth of >500X, a quality score of >30, and variant allele frequency (VAF) of 5% or greater. Variants were checked against our internally developed variants database that was established following public databases including PubMed, ClinVar, the Splice Site Prediction by Neural Network, Catalogue of Somatic Mutations in Cancer (COSMIC), dbSNP (www.ncbi.nlm.nih.gov/SNP), and the Exome Variant Server for assessing the general population frequency.
Statistical Analysis
Clinical parameters as well as disease-related prognostic factors including age, gender, cytogenetics, and somatic mutations were described at the time of diagnosis of MDS and AML-MRC. The primary endpoint for this study was progression free survival (PFS) with a secondary endpoint of overall survival (OS). MDS-to-AML PFS was defined as the length of time from MDS diagnosis to AML progression and OS was calculated from the date of MDS diagnosis to the time of death. Survival outcomes were estimated by the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariate Cox regression models were used to evaluate the influence and impact of the age, IPSS, allo-SCT, and MYC expression level. Fisher’s exact test and student’s t-test were used for the comparative analyses of somatic mutation rates, blast counts, and MYC protein expression levels. All tests were two-sided and statistical significance was declared forp<0.05. All statistical analyses were performed using SPSS v24.0.
RESULTS
Patient Demographics
A total of 41 patients diagnosed with MDS who had AML progression were included in the study. Of the 41 patients, 34% (n=14) patients were female and 66% (n=27) patients were male (Table 1). A total of 61% (n=25) of patients had low MYC expression and 39% (n=16) of patients had high MYC expression at the time of MDS diagnosis (Figure 1 and Table 1). A total of 15% (n=6) patients had tMDS. The median age at the time of MDS diagnosis was 67.3 (22.6-78.7) for all patients. The median age at the time of AML-MRC was 69.8 (31.8-80.8) (Table 1). IPSS risk was assessed in 83% (n=34) of patients at the time of MDS diagnosis. A total of 6% (n=2), 29% (n=10), 41% (n=14) and 24% (n=8) of patients had low, intermediate-I, intermediate-II, and high risk, respectively. Detailed clinical and demographic information for the total patient cohort at the time of MDS and AML-MRC diagnosis is described in Table 1.
Table 1.
Baseline characteristics of the study cohort for all patients (n=41).
| At MDS diagnosis | At AML-MRC diagnosis | |||
|---|---|---|---|---|
| Characteristic | Median (range) | Median (range) | ||
| Count (%) | Count (%) | |||
| MYC Expression at MDS diagnosis | Low (n=25) | High (n=16) | Low (n=25) | High (n=16) |
| Age at diagnosis (years) | 68.6 (22.6-78.7) | 66.0 (38.9-78.2) | 70.5 (31.8-80.8) | 68.5 (39.1-79.4) |
| Gender | Male 16 (64%) | Male 11 (79%) | Male 16 (64%) | Male 11 (79%) |
| Female 9 (36%) | Female 5 (31%) | Female 9 (36%) | Female 5 (31%) | |
| CBC | ||||
| Hemoglobin (g/dL) | 9.4 (7.0-14.5) | 8.2 (7.1-12.1) | 8.7 (7.3-12.4) | 8.65 (7.0-10.4) |
| Platelet counts (109/L) | 56 (8.0-251) | 54 (8-216) | 36 (11-407) | 33 (4-215) |
| ANC (109/L) | 0.73 (0.12-6.86) | 0.7 (0.08-4.78) | 0.76 (0.05-15.37) | 0.44 (0.03-25.18) |
| Blasts (%) | ||||
| Bone marrow blasts (%) | 5.0 (0.5-15) | 8.9 (0.5-16) | 25.0 (10-75) | 25 (10-66) |
| Peripheral blood blasts (%) | 4.5 (1-20) | 1.65 (1-3.5) | 6(1-52) | 12 (2-36) |
| Classification of MDS | ||||
| MDS-SLD | 1 (4) | 1 (6) | N/A | N/A |
| MDS-MLD | 5 (20) | 3 (19) | N/A | N/A |
| MDS-RS | 0 (0) | 0 (0) | N/A | N/A |
| MDS-EB-1 | 10 (40) | 4 (25) | N/A | N/A |
| MDS-EB-2 | 8 (32) | 7 (44) | N/A | N/A |
| MDS-U | 1 (4) | 1 (6) | N/A | N/A |
| Therapy related | ||||
| t-MDS | 3 (12) | 3 (19) | N/A | N/A |
| de novo | 22 (88) | 13 (81) | N/A | N/A |
| IPSS | n=22 | n=12 | ||
| Low | 2 (9%) | 0 (0%) | N/A | N/A |
| Intermediate-I | 7 (32%) | 3 (25%) | N/A | N/A |
| Intermediate-II | 9 (41%) | 5 (42%) | N/A | N/A |
| High | 4 (18%) | 4 (33%) | N/A | N/A |
| Targeted exome sequencing | n=13 | n=12 | n=21 | n=12 |
| Median number of mutations | 1 (1-4) | 1 (1-7) | 2 (0-7) | 2.5 (1-5) |
| TP53 | 3 (23%) | 5 (42%) | 4 (19%) | 7 (58%) |
| TET2 | 3 (23%) | 1 (8%) | 3 (14%) | 2 (17%) |
| DNMT3A | 0 (0%) | 4 (33%) | 1 (5%) | 5 (42%) |
| ASXL1 | 2 (15%) | 2 (17%) | 3 (14%) | 2 (17%) |
| ZRSR2 | 2 (15%) | 1 (8%) | 1 (5%) | 0 (0%) |
| RUNX1 | 2 (15%) | 2 (17%) | 5 (24%) | 2 (17%) |
| SRSF2 | 2 (15%) | 1 (8%) | 3 (14%) | 3 (25%) |
| Cytogenetics | n=23 | n=13 | n=25 | n=15 |
| Del(5q) | 3 (13%) | 5 (38%) | 8 (32%) | 6 (40%) |
| Trisomy8 | 2 (9%) | 2 (15%) | 0 (0%) | 7 (47%) |
| Del(7q) | 2 (9%) | 4 (31%) | 6 (24%) | 5 (33%) |
| Del(20) | 0 (0%) | 1 (8%) | 3 (12%) | 1 (7%) |
| Del(17p) | 0 (0%) | 2 (15%) | 4 (16%) | 5 (33%) |
| Complex Karyotype | 5 (22%) | 7 (30%) | 10 (40%) | 11 (73%) |
| Treatment | For MDS (n=23) | For MDS (n=15) | For AML-MRC (n=24) | For AML-MRC (n=15) |
| ESAs | 5 (21%) | 2 (13%) | 0 (0%) | 0 (0%) |
| Hypomethylating agents | 23 (100%) | 12 (80%) | 9 (38%) | 6 (40%) |
| Azacitidine | 21 (91%) | 11 (73%) | 4 (17%) | 3 (20%) |
| Decitabine | 3 (13%) | 1 (7%) | 6 (25%) | 4 (27%) |
| Intensive chemotherapy | 0 (0%) | 1 (7%) | 13 (54%) | 6 (40%) |
| 7+3 | 0 (0%) | 1 (7%) | 4 (17%) | 3 (20%) |
| CLAG±M | 0 (0%) | 0 (0%) | 10 (42%) | 5 (33%) |
| MEC | 0 (0%) | 0 (0%) | 1 (4%) | 1 (7%) |
| Allo-SCT | 2 (9%) | 1 (7%) | ||
| Survival outcomes | ||||
| Median PFS (months) | 17.7 (2.9-109.7) | 9.3 (1.1-51.8) | N/A | N/A |
| Median OS (months) | 26.0 (2.9-138.3) | 12.2 (2.3-88.3) | N/A | N/A |
Abbreviation: CBC (complete blood counts), ANC (absolute neutrophil counts), IPSS (international prognostic scoring system), ESAs (erythropoiesis stimulating agents), 7+3 (cytarabine 100mg/m2/day continuous IV infusion for 7 days and daunorubicin 45-90 mg/m2/day or idarubicin 12 mg/m2/day for 3 days), CLAG±M (cladribine 5 mg/m2/day and cytarabine 2 g/m2/day for 5 days, G-CSF 300 mcg for 6 days ± mitoxantrone 10 mg/m2/day for 3 days), MEC (mitoxantrone 8 mg/m2/day, etoposide 100 mg/m2/day, and cytarabine 1 g/m2/day for 5 days), PFS (progression free survival), OS (overall survival), MDS (myelodysplastic syndrome), AML-MRC (acute myeloid leukemia with myelodysplasia related changes).
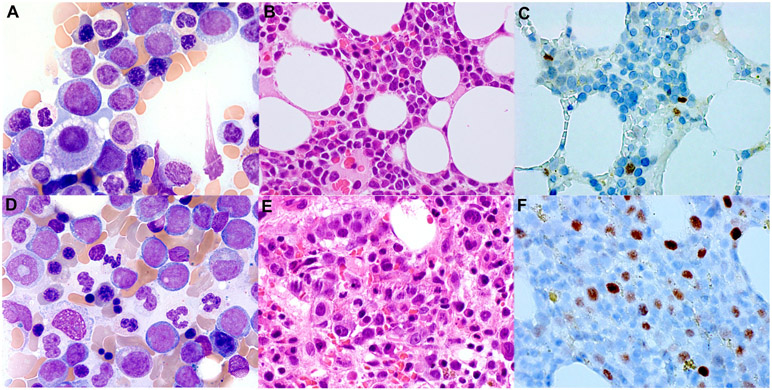
Figure 1. MYC immunohistochemistry (IHC) staining in MDS and AML-MRC patients.
The representative images of low MYC expression in the bone marrow interpreted with MDS-EB-I (A-C) and high MYC expression in the bone marrow diagnosed with AML-MRC (D-F). Wright-Giemsa (A and D, 1000x), H&E (B and E, 600x), and MYC staining (C and F, immunoperoxidase, 600x).
Cytogenetics and Somatic Mutations
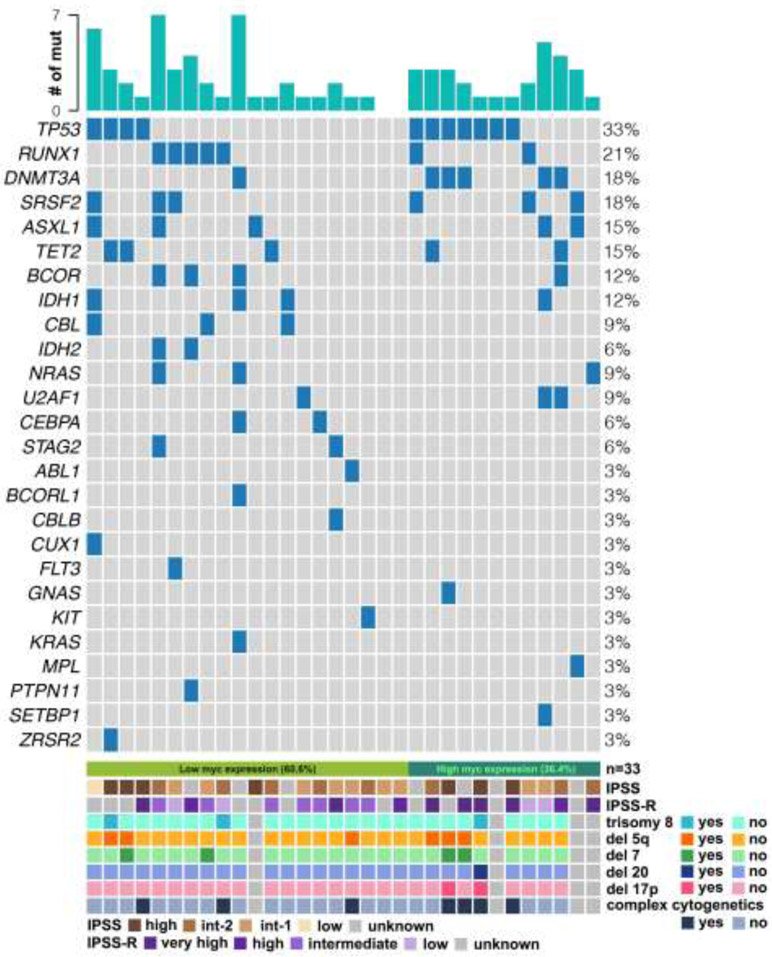
At the time of MDS diagnosis, a total of 88% (n=36) of patients were assessed for cytogenetic aberrations. Del(17p) and complex karyotype (defined as three or more independent chromosomal abnormalities) were observed in 6% (n=2) and 32% (n=12) of patients, respectively (Table 1). Of note, del(17p) (15% vs. 0%, p=0.1238) and complex karyotypes (54% vs. 22%, p=0.0714) were identified more frequently in the high MYC group compared to the low MYC group although the differences did not achieve statistical significance (Table 1). At the time of transformation to AML-MRC, a total of 98% (n=40) of patients were assessed for cytogenetic aberrations, and 23% (n=9) and 53% (n=21) patients had del(17p) and complex karyotype, respectively (Table 1). High MYC expression was associated with higher rates of del(17p) (33% vs. 16%, p=0.2555) and complex karyotype (73% vs. 40%, p=0.055), but without statistical significance between the low vs. high MYC group. Detailed cytogenetic results at the time of MDS and AML-MRC are described in Table 1 and Figure 2.
Figure 2. Somatic mutations and cytogenetics in low vs. high MYC patients.
Individual column represents somatic mutations from individual patient at the time of MDS progression to AML. Each row represents the presence of individual somatic mutation in the patient cohort. Name of each gene and % of each mutation are described on the left and right side, respectively. IPSS, IPSS-R and concurrent cytogenetics (trisomy 8, deletion 5q, deletion 7, deletion 20, deletion 17p, and complex karyotype) are shown at the bottom plot.
A total of 25 patients underwent targeted exome sequencing with the most common somatic mutation at the time of MDS diagnosis being TP53 (32%, n=8) followed by TET2 (16%, n=4), DNMT3A (16%, n=4), ASXL1 (16%, n=4) and RUNX1 (16%, n=4) (Table 1 and Figure 2). The low MYC group included 13 patients with mutations in TP53 (23%, n=3) and TET2 (23%, n=3) followed by ZRSR2 (15%, n=2) and ASXL1 (15%, n=2) whereas high MYC group contained 12 patients with TP53 (42%, n=5) mutation followed by DNMT3A (33%, n=4), ASXL1 (17%, n=2), RUNX1 (17%, n=2), TET2 (8%, n=1), and ZRSR2 (8%, n=1) (Table 1 and Figure 2). TP53 mutations were more frequently associated with high MYC compared to low MYC, but without statistical significance (42% vs. 23%, p=0.411). At the time of AML-MRC diagnosis, 33 patients were assessed for somatic mutations with the most common mutation being TP53 (33%, n=11) followed by RUNX1 (21%, n=7), DNMT3A (18%, n=6) and SRSF2 (18%, n=6), and ASXL1 (15%, n=5) (Table 1 and Figure 2). The low MYC group included 21 patients with RUNX1 (24%, n=5) and TP53 (19%, n=4) mutations followed by ASXL1 (14%, n=3), SRSF2 (14%, n=3) and TET2 (14%, n=3) mutations (Table 1 and Figure 2). The high MYC group included 12 patients with TP53 (58%, n=7) mutation followed by DNMT3A (42%, n=5), SRSF2 (25%, n=3), ASXL1 (17%, n=2), TET2 (17%, n=2) and RUNX1 (17%, n=2) (Table 1 and Figure 2).
Treatment for MDS and AML-MRC
A total of 93% (n=38) patients received at least one line of treatment for their MDS. The most common therapeutic agents were hypomethylating agents in 92% (n=35) followed by lenalidomide in 21% (n=8) and ESA in 18% (n=7) (Table 1). Among 28 patients who were assessed for the treatment response after 1st line therapy, 21% (n=6) achieved complete remission (CR), 11% (n=3) achieved CR with incomplete recovery (Cri), and 25% (n=7) achieved partial remission (PR). A total of 12 patients had no response (stable disease, n=6; progression, n=6). Following progression to AML, a total of 95% (n=39) of patients received treatments. Among these, 49% (n=19) of patients were treated with intensive chemotherapies and 38% (n=15) and 10% (n=4) received hypomethylating agents and other therapies including drugs in clinical trials, respectively (Table 1). Treatment response was assessed in 32 patients. A total of 7 patients achieved CR, 2 achieved CRi, 4 achieved PR, and 17 had refractory disease. Allo-SCT was performed in 12% (n=5) of patients during their clinical courses and two of these patients underwent allo-SCT after AML transformation.
MYC Oncoprotein Expression and Blast Counts
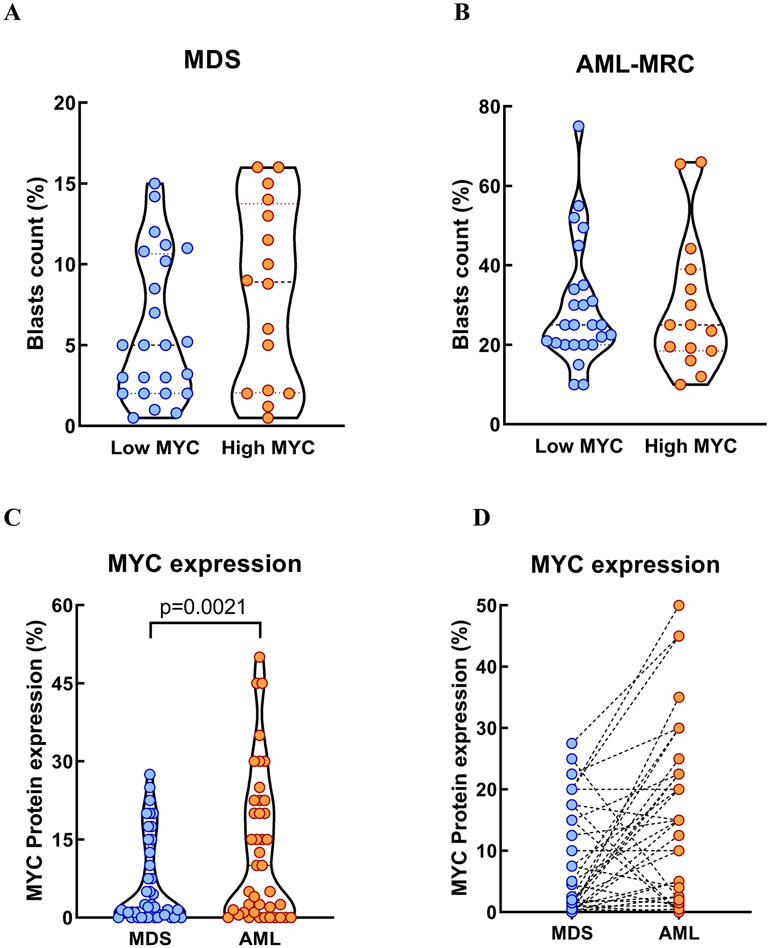
There was no statistically significant difference in BM blast counts between low vs. high MYC groups at the time of MDS diagnosis (5% vs. 8.9%, p=0.1578) and AML-MRC diagnosis (25% vs. 25%, p=0.9507) (Figure 3A-B). In the paired analysis comparing MDS vs. AML-MRC in individual patients, the median MYC protein expression level (2% vs. 10%, p=0.0021) was significantly higher at the time of AML-MRC compared to MDS diagnosis (Figure 3C-D), suggesting that MYC oncoprotein may play a role in MDS-to-AML progression.
Figure 3. Bone marrow (BM) blasts counts and MYC protein expression levels.
BM blasts counts at the time of MDS (A) and AML-MRC (B) diagnosis are not statistically different between low vs. high MYC groups. MYC protein expression level at the time of AML progression is significantly higher compared to the level at MDS diagnosis (C). In individual patients, a total of 18 and 3 patients had at least 5% increase and decrease, respectively, in MYC expression level at the time of AML progression when compared with the baseline MYC at MDS diagnosis (D). A total of 20 patients had less than 5% changes in MYC expression levels at the time of AML progression compared to MDS diagnosis.
Impacts of MYC Expression Levels on Survival Outcomes
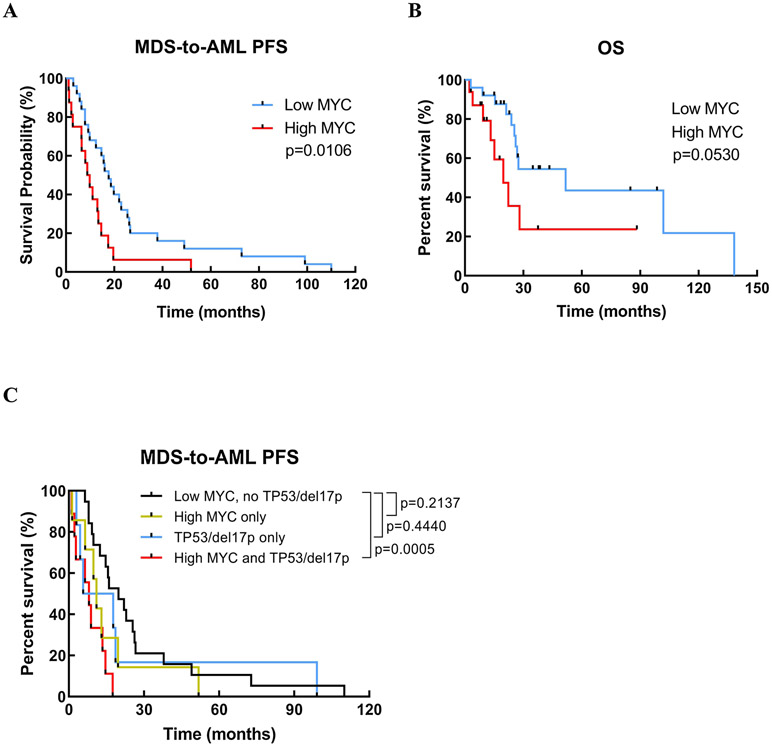
In the univariate analyses, patients with high MYC expression (median PFS 9.3 vs. 17.7 months, HR=2.328, 95%CI=1.194-4.535, p=0.013) and IPSS risk (HR=1.617, 95%CI=1.061-2.464, p=0.025) showed significantly shorter MDS-to-AML PFS (Figure 4A). OS was also shorter in the high MYC group (median OS 19.7 vs. 51.7 months, HR=0.4350, 95%CI=0.1523-2.299, p=0.0530) (Figure 4B). In the multivariate analyses (adjusting for age, IPSS and allo-SCT), high MYC expression (HR=2.275, 95%CI=1.016-5.096, p=0.046) and IPSS risk (HR=1.632, 95%CI=1.042-2.557, p=0.032) remained to be significant prognostic factors for the MDS-to-AML PFS (Table 2).
Figure 4. MDS-to-AML progression free and overall survival.
Patients with high MYC expression show significantly shorter MDS-to-AML PFS (A) although there is no statistical difference in OS between low vs. high MYC group (B). In an additional analysis, patients with high MYC and concurrent TP53 mutation/deletion (17p) showed shortest MDS-to-AML PFS compared to high MYC alone, TP53 mutation/deletion (17p) alone or low MYC patients (C).
Table 2.
Prognostic impact of MYC expression on MDS-to-AML PFS in the univariate and multivariate (cox-regression) analyses.
| MDS-to-AML progression free survival | ||||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| Variables | HR | 95% CI | P value | HR | 95% CI | P value |
| High MYC | 2.328 | 1.194-4.535 | 0.013 | 2.275 | 1.016-5.096 | 0.046 |
| IPSS | 1.617 | 1.061-2.464 | 0.025 | 1.632 | 1.042-2.557 | 0.032 |
| Age | 1.018 | 0.992-1.045 | 0.167 | 1.019 | 0.989-1.049 | 0.213 |
| Allo-SCT | 0.704 | 0.215-2.303 | 0.562 | 0.462 | 0.128-1.663 | 0.237 |
Abbreviation: HR (hazard ratio), CI (confidence interval), IPSS (international prognostic scoring system), Allo-SCT (allogeneic stem cell transplant).
Our previous studies have demonstrated that high MYC level is commonly associated with concurrent TP53 mutation (TP53mut)/del(17p), a well-known poor prognostic factor in MDS and AML6,22-27. Although limited in case number, we still compared the PFS outcome in patients with high MYC and TP53mut/del(17p) (n=9) vs. high MYC alone (n=7) vs. TP53mut/del(17p) alone (n=6) vs. low MYC without TP53mut/del(17p) (n=19). Our analyses showed that the patients with both high MYC and TP53mut/del(17p) experienced the shortest PFS while the patients with low MYC without TP53mut/del(17p) experienced the longest PFS (median PFS 8 vs. 19.8, HR=3.445, 95%CI=1.106-10.73, p=0.0005) (Figure 4C).
In conclusion, our findings suggest that high MYC expression at the MDS stage is associated with a shorter MDS-to-AML progression free survival, which suggests that MYC may play an oncogenic role in AML transformation.
DISCUSSION
MYC is a well-known transcription factor that regulates genes involved in cell proliferation, survival, and metabolism contributing to tumor development and maintenance. Although MYC oncoprotein has been consistently implicated in AML, its target genes and precise mechanisms of leukemogenesis remained unknown until our recent studies demonstrated that MYC suppresses myeloid differentiation and promotes survival and proliferation of AML cells by suppressing IDH1/2-TET2-5hmC signaling and epigenetic regulation8.
Accordingly, previous clinical studies reported that high expression of MYC oncoprotein is associated with poor survival outcomes in AML6,7,28,29. In the study performed by Mughal et al, high MYC expression was shown to be associated with inferior OS in AML patients with favorable and intermediate cytogenetic risk groups29. Further, in an independent study performed by Ohanian et al, high MYC expression was also associated with inferior treatment response and poor event free and relapse free survival28. Consistent with these observations, our recent study also demonstrated that high MYC expression is associated with adverse OS outcomes in AML-MRC patients6. Notably, in this study we observed that high MYC expression is associated with significantly higher rates of concurrent TP53 and DNMT3A somatic mutations that are commonly identified in the MDS and AML-MRC patients suggesting that MYC may be involved in MDS-to-AML progression. Therefore, we explored the prognostic impact of MYC protein on the MDS-to-AML PFS in the MDS patient cohort.
As expected, the median expression level of MYC protein is significantly lower at the stage of MDS compared to AML. Also, in our paired analyses of MDS and AML samples in individual patients, we observed an increments in MYC levels as disease progressed from MDS to AML. Further, the MDS-to-AML PFS was significantly shorter in high MYC patients compared to low MYC patients in our MDS/AML-MRC cohort. Importantly, in the univariate and multivariate analyses adjusting for other prognostic factors including age, IPSS, and allo-SCT, high MYC expression remains to be an independent poor prognostic factor for the MDS-to-AML progression. Collectively, these findings indicate that MYC may play active roles in MDS transformation to AML.
A recent study performed by Gao et al showed that high MYC collaborates with TP53 mutations driving poor OS outcomes in AML patients7. In their study, MYC expression was positively correlated with p53 expression or TP53mut Further, TP53mut and more than 20% of MYC protein expression was associated with poor OS outcomes in AML patients although high MYC alone did not show any impact on OS outcome in MDS patients. Similar to these observations, patients with high MYC and TP53mut/del(17p) showed shorter MDS-to-AML PFS compared to patients with high MYC or TP53mut/del(17p) alone in our MDS/AML-MRC cohort (Figure 4C). Although these results need careful interpretation based on limited patient numbers in each subgroup, these findings indicate that MYC and mutant p53 proteins or loss of TP53 may synergize to promote disease progression in MDS and transformation to AML. Currently, we are investigating the impact of MYC overexpression vs. TP53 knockout vs. concurrent MYC overexpression and TP53 knockout on the development of AML by using the Mx1-Cre;Rosa26LSL-MYC;Trp53fl/fl in vivo mouse model.
In conclusion, high MYC expression at the MDS stage was associated with a shorter latency to AML progression, indicating that MYC potentially plays an oncogenic role in MDS-to-AML transformation. Although our findings need further validation by prospective studies with a greater sample size, our results support a combination treatment of APR-24630,31 and agents targeting MYC signaling32 as a novel therapeutic strategy in TP53mut MDS patients. Further, our observations warrant future studies to identify the mechanisms by which MYC and mutant p53 cooperate in disease progression in MDS patients.
Highlights.
Patients with MDS express dynamic range of MYC oncoprotein and an increase of MYC expression level is associated with MDS progression to AML.
High MYC expression at the time of MDS diagnosis is associated with significantly shorter MDS-to-AML progression free survival (PFS).
TP53 somatic mutation and 17p deletion are frequently co-occurring in high MYC expressing patients.
Patients with high MYC and TP53 mutation/17p deletion have shorter MDS-to-AML PFS compared to the patients with high MYC or TP53mutation/17p deletion alone.
ACKNOWLEDGEMENTS
This work was supported in part by research grants from the Graduate Medical Education (GME) at the University of South Florida (D.G., S.Y.), Research Training Award for Fellow (RTAF) from the American Society of Hematology (S.Y.), Scholar Award from the American Society of Hematology (S.Y.), NIH grant K08 CA237627 (S.Y.), and the Biostatistics and Bioinformatics Shared Resources at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center (P30-CA076292). We appreciate Moffitt core laboratory to perform immunohistochemistry staining for the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- 1.Chen J, Kao YR, Sun D, et al. Myelodysplastic syndrome progression to acute myeloid leukemia at the stem cell level. Nat Med. 2019;25(1):103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361(19):1872–1885. [DOI] [PubMed] [Google Scholar]
- 3.Arana Yi CY, Kantarjian HM, Garcia-Manero G, et al. Comparing Outcomes of Patients with Secondary AML: Treatment-Related MDS/AML, AML Secondary to Myeloproliferative Neoplasms (t-MPN), and AML with Prior Malignancies. 2012;120(21):3557–3557. [Google Scholar]
- 4.Grimwade D, Hills RK. Independent prognostic factors for AML outcome. 2009;2009(1):385–395. [DOI] [PubMed] [Google Scholar]
- 5.Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. New England Journal of Medicine. 2016;374(23):2209–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yun S, Sharma R, Chan O, et al. Prognostic significance of MYC oncoprotein expression on survival outcome in patients with acute myeloid leukemia with myelodysplasia related changes (AML-MRC). Leukemia Research. 2019;84:106194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao L, Saeed A, Golem S, et al. High-level MYC expression associates with poor survival in patients with acute myeloid leukemia and collaborates with overexpressed p53 in leukemic transformation in patients with myelodysplastic syndrome. 2021;43(1):99–109. [DOI] [PubMed] [Google Scholar]
- 8.Yun S, Vincelette ND, Yu X, et al. TFEB Links MYC Signaling to Epigenetic Control of Myeloid Differentiation and Acute Myeloid Leukemia. 2021;2(2):162–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolouri H, Farrar JE, Triche T Jr, et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nature Medicine. 2017;24:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dang CV, O'Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F. The c-Myc target gene network. Semin Cancer Biol. 2006;16(4):253–264. [DOI] [PubMed] [Google Scholar]
- 11.Luo H, Li Q, O'Neal J, Kreisel F, Le Beau MM, Tomasson MH. c-Myc rapidly induces acute myeloid leukemia in mice without evidence of lymphoma-associated antiapoptotic mutations. Blood. 2005;106(7):2452–2461. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Woo AJ, Chu J, et al. A Myc Network Accounts for Similarities between Embryonic Stem and Cancer Cell Transcription Programs. Cell;143(2):313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Osdal T, Ho Y, et al. SIRT1 Activation by a c-MYC Oncogenic Network Promotes the Maintenance and Drug Resistance of Human FLT3-ITD Acute Myeloid Leukemia Stem Cells. Cell Stem Cell;15(4):431–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie S, Lin H, Sun T, Arlinghaus RB. Jak2 is involved in c-Myc induction by Bcr-Abl. Oncogene. 2002;21(47):7137–7146. [DOI] [PubMed] [Google Scholar]
- 15.Müller-Tidow C, Steffen B, Cauvet T, et al. Translocation Products in Acute Myeloid Leukemia Activate the Wnt Signaling Pathway in Hematopoietic Cells. 2004;24(7):2890–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. [DOI] [PubMed] [Google Scholar]
- 17.Bennett JM. Changes in the Updated 2016: WHO Classification of the Myelodysplastic Syndromes and Related Myeloid Neoplasms. Clin Lymphoma Myeloma Leuk. 2016;16(11):607–609. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–425. [DOI] [PubMed] [Google Scholar]
- 19.Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088. [PubMed] [Google Scholar]
- 21.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yun S, Geyer SM, Komrokji RS, et al. Prognostic significance of serial molecular annotation in myelodysplastic syndromes (MDS) and secondary acute myeloid leukemia (sAML). Leukemia. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sallman DA, Komrokji R, Vaupel C, et al. Impact of TP53 mutation variant allele frequency on phenotype and outcomes in myelodysplastic syndromes. Leukemia. 2016;30(3):666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunter AM, Komrokji RS, Yun S, et al. Baseline and serial molecular profiling predicts outcomes with hypomethylating agents in myelodysplastic syndromes. Blood Advances. 2021;5(4):1017–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bejar R, Stevenson KE, Caughey B, et al. Somatic Mutations Predict Poor Outcome in Patients With Myelodysplastic Syndrome After Hematopoietic Stem-Cell Transplantation. Journal of Clinical Oncology. 2014;32(25):2691–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi K, Patel K, Bueso-Ramos C, et al. Clinical implications of TP53 mutations in myelodysplastic syndromes treated with hypomethylating agents. Oncotarget. 2016;7(12):14172–14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bejar R, Papaemmanuil E, Haferlach T, et al. TP53 Mutation Status Divides MDS Patients with Complex Karyotypes into Distinct Prognostic Risk Groups: Analysis of Combined Datasets from the International Working Group for MDS-Molecular Prognosis Committee. 2014;124(21):532–532. [Google Scholar]
- 28.Ohanian M, Rozovski U, Kanagal-Shamanna R, et al. MYC protein expression is an important prognostic factor in acute myeloid leukemia. Leukemia & Lymphoma. 2018;60(1):37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mughal MK, Akhter A, Street L, Pournazari P, Shabani-Rad M-T, Mansoor A. Acute myeloid leukaemia: expression of MYC protein and its association with cytogenetic risk profile and overall survival. 2017;35(3):350–356. [DOI] [PubMed] [Google Scholar]
- 30.Sallman DA, DeZern AE, Garcia-Manero G, et al. Eprenetapopt (APR-246) and Azacitidine in TP53-Mutant Myelodysplastic Syndromes.0(0):JCO.20.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cluzeau T, Sebert M, Rahme R, et al. Eprenetapopt Plus Azacitidine in TP53-Mutated Myelodysplastic Syndromes and Acute Myeloid Leukemia: A Phase II Study by the Groupe Francophone des Myelodysplasies (GFM).0(0):JCO.20.02342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han H, Jain AD, Truica MI, et al. Small-Molecule MYC Inhibitors Suppress Tumor Growth and Enhance Immunotherapy. Cancer Cell. 2019;36(5):483–497.e415. [DOI] [PMC free article] [PubMed] [Google Scholar]