Abstract
Obesity and mood disorders are often overlapping pathologies that are prevalent public health concerns. Many studies have indicated a positive correlation between depression and obesity, although weight loss and decreased appetite are also recognized as features of depression. Accordingly, the Diagnostic and Statistical Manual of Mental Disorders (DSM–5) defines two subtypes of depression associated with changes in feeding: melancholic depression, characterized by anhedonia and associated with decreased feeding and appetite, and atypical depression, characterized by fatigue, sleepiness, hyperphagia and weight gain. The central nervous system plays a key role in the regulation of feeding and mood, thus suggesting that overlapping neuronal circuits may be involved in their modulation. However, these circuits have yet to be completely characterized.
The central melanocortin system, a circuitry characterized by the expression of specific peptides (proopiomelanocortins, agouti gene-related protein and neuropeptide Y) and their receptors (MCRs), has been shown to be a key player in the regulation of feeding. In addition, the melanocortin system has also been shown to affect anxiety and depressive-like behavior, thus suggesting a possible role of the melanocortin system as a biological substrate linking feeding and depression. However, more studies are needed to fully understand this complex system and its role in regulating metabolic and mood disorders. In this review, we will discuss the current literature on the role of the melanocortin system in human and animal models in feeding and mood regulation, providing evidence of the biological interplay between anxiety, major depressive disorders, appetite and body weight regulation.
1. Feeding and depression
Mood disorders are a group of widespread psychiatric illnesses with a major impact on the life quality of the affected individuals. In 2017, Major depressive disorder (MDD) was found among the top 3 diseases that cause disability (measured by the years lived with disability index YLD, a measure of the burden of a disease) with an increase of 33.4% in YLD between 1990 and 2007 (1). Moreover, previous studies on the impact of large-scale traumatic events showed a positive correlation of such occurrences with MDD (2,3). Thus, the current already-increasing trend is predicted to be further negatively impacted by the ongoing SARS-CoV-2 pandemic (4).
Clinically, according to the Diagnostic and Statistical Manual of Mental Disorders (DSM–5), depression can be divided into two subtypes: atypical depression, characterized by fatigue, sleepiness, and hyperphagia and weight gain (5,6), and melancholic depression, characterized by anhedonia, a non-reactive mood and associated with decreased feeding and appetite. As observed both in rodents and in clinical human studies, there is a strong association between MDD and changes in food intake and body weight (7). Since food intake is decreased in melancholic depression, and increased in atypical depression, this association is rather U-shaped than linear (7).
The association between atypical depression and increased food intake is particularly relevant due to the increasing societal prevalence of obesity. Indeed, the prevalence of obesity (defined as BMI ≥ 30, according to WHO criteria) has doubled in the last 40 years, with the trend continuing to rise worldwide (8). Long-standing evidence has linked obesity to many other pathological conditions such as cardiovascular disease (9), type 2 diabetes (10), cancer, musculoskeletal disorders (11), kidney failure (12) and, as already mentioned, atypical depression (7). Interestingly, the link between obesity and depression seems to be bi-directional: not only does obesity increase the risk of depression, but depression is also a predictive indicator of obesity development, as observed by meta-analysis (13). In addition, the association between obesity and depression seems to be stronger in individuals showing abdominal obesity and related co-morbidities such as hypertension, insulin resistance, inflammation and dyslipidemia (14). A positive correlation between obesity and depression has also been observed in mice. High-fat diet (HFD) feeding in laboratory animals is the model that best correlates with human obesity/western feeding patterns. HFD-fed mice display a depressive-like phenotype, as showed by behavioral analysis and elevated levels of corticosterone (15). HFD-induced neurobiological changes include alterations of reward circuitry, reduced levels of serotonin (5-HT) and dopamine (DA), and enhanced hypothalamic–pituitary–adrenal (HPA) axis activity; such conditions can be the underlying cause of depressive phenotypes (16–18).
Different to what is observed in atypical depression, melancholic depression is associated with decreased food intake and subsequent weight loss (5,19,20). This reduction in appetite is particularly evident when anhedonia, the decreased interest and pleasure from “most activities most of the day” (5), is analyzed. Anhedonia is a typical feature of melancholic MDD, and in both animal models (21,22) and humans (23,24) results in decreased willingness to expend effort and energy for accessing food, thus decreasing food intake. In depressed patients, loss of appetite has been associated with decreased activity of the Nucleus Accumbens (NAc) and the Insular cortex [25], a decline in endocannabinoids signaling in the midbrain areas (25,26) and changes within the lateral hypothalamus and its projections to and from lateral septum, amygdala, NAc, ventral tegmental area and ventral pallidum (for a detailed review, see (27)) and high levels of cortisol (28).
Interestingly, changes in cortisol levels have been associated with both depression and appetite (29). Stress-induced elevations in cortisol via HPA axis hyperactivation are associated with adiposity, insulin resistance, and dysregulated lipid metabolism (30,31), characteristics of atypical depression. Consistently, increased cortisol levels are commonly observed among obese adults, leading to increased appetite, increased adipogenesis and hypertrophied adipocytes, and decreased brown adipose tissue activity (32,33). However, compared to non-depressed obese individuals (32,33), obese patients with atypical MDD show hypoactivation of the HPA-axis and lower basal cortisol levels (34–36). On the other hand, some studies have also shown that melancholic depression, characterized by loss of appetite, correlates with increased cortisol levels, indicating a more complex relationship between HPA axis activation, appetite and depression (34).
The melanocortin system is a well-established central neuronal pathway involved in regulating feeding and metabolism to maintain homeostasis. Numerous studies have shown that alterations in the melanocortin system impair the core variables of energy homeostasis, leading to obesity (37–42). Intriguingly, the melanocortin system has been proposed to be involved in anxiety/depression-like behavior. In this review, we will delineate the central roles of the melanocortin system on anxiety and depressive-like behavior, and evaluate their impact on food intake and whole-body metabolism.
2. The melanocortin system
The melanocortin system is characterized by three components: neurons expressing (I) the melanocortin peptides, (II) the melanocortin receptors, and (III) the antagonist/inverse agonist agouti-related protein (AgRP).
The melanocortin peptides (also called simply melanocortins) are post-translational products of the proopiomelanocortin (POMC). Post-translational processing at dibasic cleavage sites by the prohormone convertase PC1 and PC2 leads to adrenocorticotropin (ACTH), α-, β-, and γ-melanocyte-stimulating hormones (β- and γ-MSH), β-endorphin and CLIP. ACTH is then further processed by carboxypeptidases and aminopeptidases to α-MSH, the key component of the melanocortin system (43). α -, β-, and γ-MSH, ACTH and β-endorphin are key melanocortins involved in modulating feeding behavior (44,45) and depression (46–49). The melanocortins act on five melanocortin receptor subtypes, named melanocortin receptors 1–5 (MC1R-MC5R), belonging to the G-protein coupled receptor (GPCR) superfamily and thus signaling by modulating intracellular cyclic-adenosine monophosphate (cAMP) levels and via inositol phosphate (50). In particular, within the CNS, MC3R shows high affinity for α-MSH and γ-MSH, and MC4R preferentially binds α-MSH. While MC3R has been implicated in body weight regulation and the response to highly palatable food (51,52), (53), MC4R has been extensively studied because of its critical roles in the central regulation of feeding behavior and energy expenditure (54–56), as well as its effects on the development of depression and anxiety (57). Upon binding with α-MSH, MC4R activation leads to the production of an anorexigenic signal able to decrease food intake; consistently, failure of MC4R activation caused by either MC4R or POMC gene mutations results in early-onset obesity in humans and rodents (40–42). Both MC3R and MC4R are antagonized by the binding of AgRP (58–60), thus exerting an orexigenic effect. Furthermore, activation of AgRP neurons has been shown to induce the release of prolyl carboxypeptidase (PRCP), the enzyme responsible for α-MSH degradation, thus providing a further level of regulation of the melanocortin system (61).
The other melanocortin receptors, i.e. MC1R, MC2R and MC5R, are not involved in the central regulation of food intake nor in the development of mood disorders and thus will not be covered by this review.
Within the CNS, POMC neurons are mainly localized in the arcuate nucleus (ARC) of the hypothalamus, adjacent to the AgRP/NPY-expressing neurons, and in the nucleus of the tractus solitarius (NTS) of the brainstem. MC4R-expressing neurons are widely located in multiple hypothalamic sites such as the paraventricular nucleus of the hypothalamus (PVN), the medial preoptic area, the anterior hypothalamic nucleus, the ventromedial nucleus of the hypothalamus, dorsomedial nucleus of the hypothalamus (DMN) and tuberomammillary nucleus, as well as in extrahypothalamic areas including the dorsal raphe nucleus (DRN) and the amygdala, among others (62). Previous studies showed that the MC4R-expressing neurons involved in the regulation of feeding are mostly expressed in the PVN and amygdala (63–66). However, a recent study revealed that MC4R-expressing GABAergic neurons in the DRN play a role in modulating feeding (67).
On the other side, MC3R expression is limited to a smaller number of brain regions and does not overlap with MC4R expression for the most part (62), and it has been shown to play a role in response to caloric restriction and high caloric diet (68).
In the following sections, the current knowledge about the impact of the melanocortin system on feeding and depression in both humans and mice will be reviewed, with particular focus on the differences between brain regions as well as on the contribution of the different components of the melanocortin system (i.e. melanocortin receptors and their ligands).
3. The melanocortin system as a link between feeding and mood disorders
Melanocortin receptor ligands and their synthetic analogs
α-MSH is a 13-amino acid acetylated peptide hormone with affinity for MC1R, MC3R, MC4R and MC5R. While endogenous peripherally available α-MSH has direct effect on glucose homeostasis via MC5R (69), central endogenous α-MSH controls glucose metabolism and feeding via MC3R and MC4R. In line with these effects, exogenous administration of α-MSH in the PVN (and ICV) mediates anorexigenic effects in the regulation of energy homeostasis (70). Similar results were also observed with intraperitoneal injection of its synthetic analog, Melanotan II (MTII), capable of inducing hypophagia in animals fed on either standard chow or high fat diet (71,72).
Interestingly, administration of MTII in the central amygdala strongly reduced consumption of HFD, but had limited effects on the intake of a low-fat diet or a standard chow diet. Conversely, central amygdala administration of AgRP or its analog, SHU9119, the orexigenic melanocortin receptor antagonist, had an opposite effect, increasing HFD consumption (65). The effect of AgRP administration was not inducing preference towards sucrose-rich food (73), thus suggesting that melanocortin antagonism does not play a role in hedonic feeding.
Endogenous melanocortins and their analogs are also able to modulate food intake by acting on the dopaminergic neurons of the ventral tegmental area (VTA) in the midbrain, a component of the mesocorticolimbic DA circuitry. Administration of MTII in the VTA was able to suppress food intake in standard chow diet-fed rats, whereas injection of SHU9119 showed opposite effect in feeding, ultimately increasing body weight (74). Another key structure in the dopaminergic circuitry is the NAc. Here, injection of α-MSH decreased motivation for sucrose-rich highly palatable food, whereas an opposite effect was induced by AgRP administration. Effects of AgRP were blunted by pre-treatment with the dopaminergic receptor antagonist α-flupenthixol, indicating that melanocortin’s effect in the NAc is DA-dependent (75).
Melanocortins administration, either systemically or in the CNS, have been shown to affect anxiety, stress and depressive-like behavior. Systemic intravenous administration of α-MSH was shown to increase excessive grooming behavior in rats, a typical response to stressors, and this effect was blunted by the MC3/4R antagonist SHU9119 (76). Studies aimed at investigating the regions within the brain affected by α-MSH and able to mediate anxiety and depressive-like behaviors have shown that the PVN, the ARC, the DMN, and the central nucleus of amygdala were all involved (46). Similarly, the anxiogenic effect of α-MSH was found to be mediated by the VTA and blunted by GABAergic receptor activation (77,78). Contrary, intracerebroventricular injection of NPY showed antidepressant effects, both alone (79) and when counteracting α-MSH (49). In addition, local administration of α-MSH in the VMN not only reduced food intake (70), but also increased anxiety and aggressive behaviors (80).
MC4R
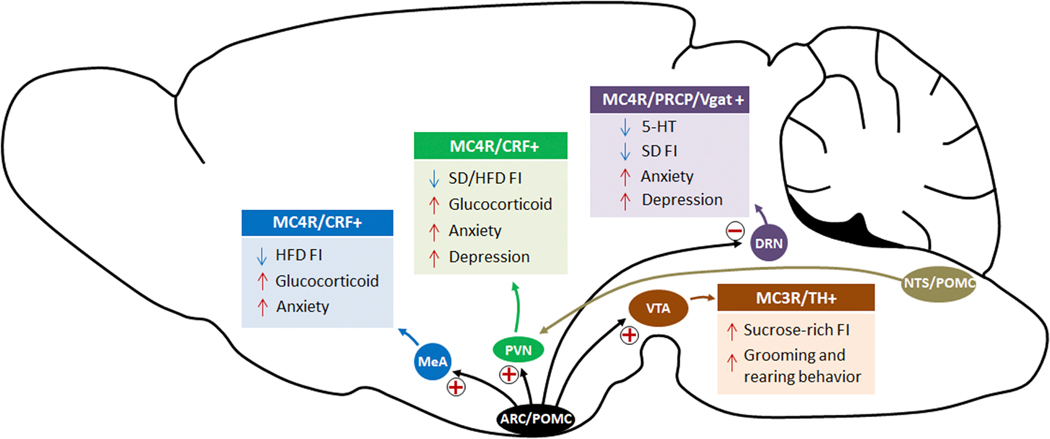
MC4R has been well-characterized in relation to obesity, ever since the first observation showing that Mc4r KO mice develop obesity, hyperphagia, hyperinsulinemia and hyperleptinemia, as well as decreased locomotor activity (81). Similarly, MC4R mutations in humans are associated with early-onset obesity (82) characterized by increased fat mass and appetite. In addition to its role in regulating metabolism and food intake, MC4R has been linked to anxiety and depression. Indeed, MC4R selective antagonist (MCL0129) injections showed anxiolytic- and antidepressant-like effects in various behavioral tests, concomitantly affecting food intake by inhibiting stress-induced anorexia (57,83). A similar effect was obtained by intranasal infusion of HS0141, another selective MC4R antagonist (84). As MC4R is expressed in several brain areas (85), in the following paragraphs we will specifically address the role of MC4R in some of the main regions in which it has been shown to regulate food intake and mood changes, such as the PVN, the DRN and the Amygdala (Figure 1).
Figure 1.
α-MSH produced from the ARC induces CRF secretion by the MC4R-co-expressing neurons in the amygdala and the PVN. CRF mediates anxiety- and depression-related behaviors through the HPA axis. In the DRN, the activity status of MC4R neurons affects serotonin levels, indicating a potential (indirect) connection between MC4R and 5-HT neurons. Both α-MSH and γ-MSH can stimulate MC3R-expressing neurons in the VTA, increasing consumption of sucrose-rich food and inducing grooming and rearing behaviors. Finally, PRCP, the enzyme involved in the degradation of α-MSH, plays a role in the modulation of melanocortin signaling and thus in the regulation of feeding and mood in the DRN.
MC4R in the PVN:
The PVN is a key site for mediating MC4R signaling in the control of metabolism, as shown by genetic and pharmacological studies. Deletion of Mc4r specifically in the PVN causes obesity, and re-expression of Mc4r in the PVN and in a subset of neurons of the amygdala of Mc4r KO mice mostly restored the obesity phenotype (56,66). Pharmacologically, injection in the PVN of the MC4R endogenous ligand α-MSH or of the MC4R agonist MTII inhibits food intake, whereas injections of the MC4R antagonists, AgRP, SHU9119 or HS014, have the opposite effect, promoting feeding (86). Additionally, MC4R in the PVN was shown to also play a role in endocrine regulation. Indeed, MC4R-expressing neurons in the medial parvocellular PVN co-express corticotropin-releasing factor (CRF), a central inhibitor of food intake. Here, CRF neurons are innervated by ARC POMC neurons producing α-MSH (47), and α-MSH administration has been shown to prevent the decrease of CRF upon fasting (87). CRF has been also shown to be involved in depression and anxiety (58,88), and its effect is mediated by MC4R. Indeed, central administration of α-MSH or MTII increases CRF gene expression in the PVN, which, in turn, induces ACTH secretion from the anterior pituitary and stimulates the production of glucocorticoids in the adrenal cortex (89). This thus increases plasma corticosterone levels (89), and these stimulations are reversed by MC4R antagonist (90). The anxiogenic-like effect of α-MSH accompanying increased plasma corticosterone is inhibited by CRF antiserum, suggesting that the hypothalamic melanocortin system effect on anxiety is mediated by CRF (91). While CRF-deficient mice displayed no behavioral changes in terms of anxiety (92), CRF over-expressing mice showed increased anxiety in the elevated plus maze test, and central administration of a CRF1 receptor antagonist reversed the behavioral phenotype of CRF over-expressing mice (93).
MC4R in the DRN:
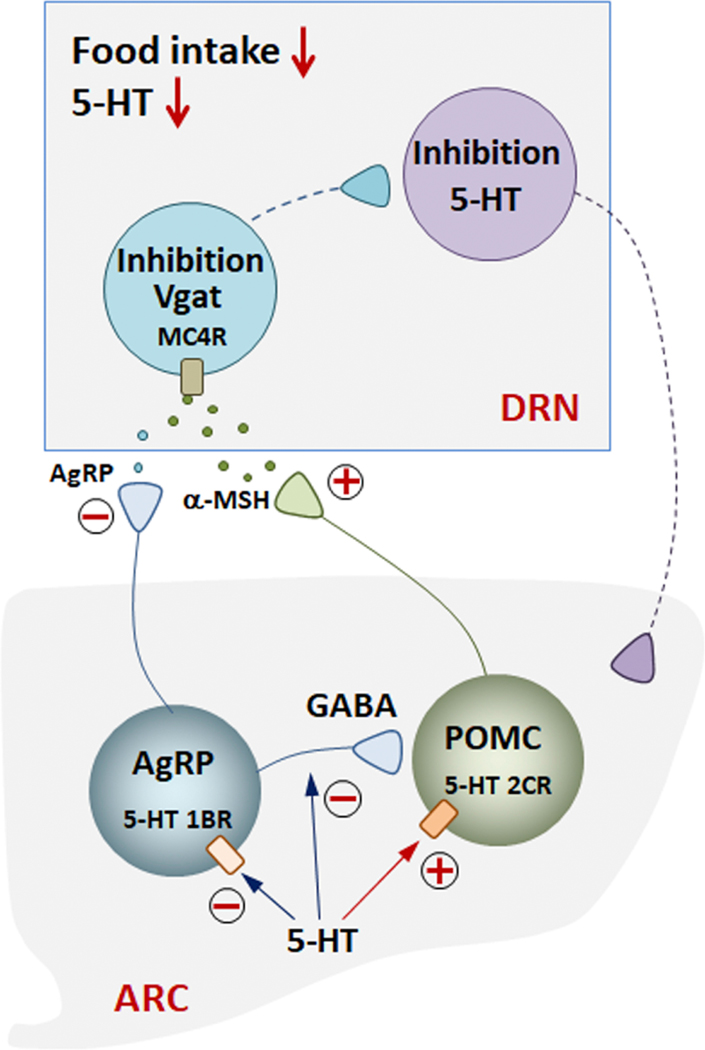
The DRN is a bilateral, heterogeneous nucleus located in the midbrain and the pons. The DRN has reciprocal connections with numerous hypothalamic nuclei in terms of feeding control, and it contains several different types of neurons, such as neurons expressing serotonin (5-HT), DA, glutamate, and GABA (85,94). The DRN is the largest serotonin-expressing nucleus innervating several forebrain regions, including the hippocampus, the septal area and the striatum. Previous studies have indicated that serotonergic neurons are associated with emotional processing and several affective disorders, including anxiety and depression (for a review see [87]). Recent studies showed that direct administration of α-MSH into the DRN decreased food intake (48,67) and that this effect is mediated by MC4R (48). Interestingly, chemogenetic MC4R activation within the DRN, while increasing food intake, was also responsible for decreased anxiety and depressive-like behavior, whereas opposite effects were observed by chemogenetic inhibition of MC4R (48). Most α-MSH-inhibited neurons in the DRN showed overlapping expression with PRCP (the enzyme responsible for α-MSH degradation (61)), Vgat (the vesicular GABA transporter), and MC4R, thus suggesting that DRN GABAergic neurons expressing MC4R and PRCP may mediate the effect of α-MSH on feeding and mood (67). In line with this, selective PRCP reduction in the DRN induced an increase in α-MSH, resulting in mice with a leaner phenotype, decreased food intake and increased anxiety and depression-like behavior. Administration of SHU9119 into the DRN of mice lacking PRCP rescued food intake to the level of SHU9119-injected control mice, indicating that the effect was mediated by MC4R. Interestingly, and contrary to the PVN MC4R neurons (47), increased α-MSH tone onto MC4R-expressing neurons of the DRN caused significant reduction in MC4R neuronal activation that was further complemented by data showing α-MSH-mediated hyperpolarization of DNR MC4R neurons (48). Furthermore, the decrease in MC4R activation was associated with significantly lower levels of 5-HT in the DRN and in one of its target areas, the hippocampus. This was accompanied by a significant increase in anxiety and depressive-like behavior in mice (48), thus suggesting a role for DRN MC4R in regulating feeding and depression via (indirect) modulation of 5-HT neurons (Figure 2).
Figure 2.
In the ARC, 5-HT inhibits AgRP neuronal activity and reduces inhibitory synaptic output to POMC neurons, while activating POMC neurons increases α-MSH release. Both AgRP and POMC neurons project from the ARC to the DRN. In the DRN, α-MSH inhibits MC4R neurons, leading to reduced food intake and 5-HT levels, resulting in anxiety and depressive-like behavior.
MC4R in the Amygdala:
Abundant MC4R expression has been shown in the amygdala. Here, administration of AgRP or synthetic MC4R antagonists significantly increased food intake under standard chow diet feeding, whereas no effect was observed with MTII injection (96). However, activation of MC4R via MTII reduced HFD intake, while AgRP and SHU9119 increased preference for fat-rich food (65), indicating that MC4R in the amygdala is able to influence the development of obesity driving food preference. MC4R in the medial amygdala (MeA) has been also studied in the context of emotional stress-induced anxiety-like behavior, anorexia and corticosterone secretion (97). Direct infusion of a selective MC4R agonist into the MeA displayed anxiogenic-like effects in the elevated plus-maze test and decreased food intake. In contrast, direct infusion of SHU9119 into the MeA diminished restraint stress-induced anxiogenic and anorectic effects (97). In both pharmacological treatments, plasma corticosterone levels were affected (97). Additionally, both CRF and MC4R expression overlapped in the amygdala (85), thus suggesting that the anxiogenic and anorectic effects of MC4R might be in part mediated by CRF affecting the HPA axis. Finally, a recent anterograde viral tracing study showed ARC POMC neuronal projections to the MC4R-expressing neurons in the MeA (98). This study also demonstrated that a neural circuit between ARC POMC and MeA MC4R neurons is important in the regulation of short-term food intake (98).
MC3R
MC3R, in comparison to MC4R, has limited expression distribution in the brain, and is mostly found in the ARC, in the ventromedial hypothalamus and the VTA (99,100). Electrophysiological recordings have shown that α-MSH activates MC3R neurons in the VTA (101) and that a large proportion of VTA MC3R neurons are dopaminergic cells (102), thus suggesting a central role of MC3R in VTA-mediated behaviors. The mesolimbic DA system, known as the reward center of the brain, is composed of dopaminergic neuronal projections from the VTA in the midbrain to the NAc and olfactory tubercle in the ventral striatum (103,104). The role of VTA DA neurons in depressive-like behavior has been shown in several animal models, including learned helplessness, chronic mild stress and chronic social defeat (105). Direct α-MSH injection into the VTA induces DA transmission and increases grooming and rearing behavior, suggesting VTA MC3R effects on dopaminergic neurons-related behavior (106) (Figure 1). Finally, pharmacological stimulation of MC3R by injection of γ-MSH (which activates MC3R but not MC4R) in the VTA increased the motivation to consume sucrose-rich food, indicating an involvement of VTA-MC3R in food reward mechanisms. This effect was shown to be mediated by dopaminergic transmission: co-administration of γ-MSH and the DA receptor antagonist, α-flupenthixol, did not induce any preference towards highly palatable, sucrose-rich food (53).
4. Pharmacological targeting of the melanocortin system
A great number of evidence from human studies have identified genetic and epigenetic changes in the melanocortin system of obese individuals (for a detailed review, see (107) and (108)). These mutations can affect multiple and different components of the system, such as POMC gene-derived peptides (108) or genes affecting POMC expression (109). Among the others, one of the most prevalent monogenic causes of early-onset human obesity involves MC4R, accounting by itself for about 5% of all obese patients (108,110). These mutations can disrupt the binding of MC4R ligands, the downstream signaling cascade, or the ability of the receptor to be expressed at the cell surface including in cilia (111). Additionally, MC4R gain-of-function mutations leading to β-arrestin biased signaling have been recently identified in individuals resistant to obesity (112). Contrary to the well-characterized impact of melanocortin signaling mutations on obesity, there are currently limited studies linking Mc4r with mood disorders in humans. Despite the fact that there is currently no evidence of a direct correlation between human MC4R mutations and the development of depression, a recent Mendelian randomized analysis aiming at finding genetic loci linking obesity and MDD highlighted a possible involvement of MC4R in the development of such conditions (113). This is particularly relevant in the perspective of developing drugs able to target both weight- and mood-related disorders. Currently, about 35% of available drugs target GPCRs (114), and thus a broad number of tools are available to investigate GPCR pharmacology and for GPCR drug discovery. In particular, because most of the individuals with MC4R mutations are heterozygous, the use of MC4R agonists or antagonists is a promising approach to counteract receptor loss- or gain-of-function. Numerous MC4R synthetic agonists have been developed as possible anti-obesity drugs, and few reached advanced (II or III) clinical trial phases (115). Recently, Setmelanotide, a MC4R agonist, has been approved by the FDA for treatment of obesity (116). However, even though depression and suicidal thoughts are currently warned as possible side effects, no significant correlation between the use of Setmelanotide and mood changes has been found. Finally, a series of MC4R receptor antagonists has been studied as antidepressant and anxiolytic drugs, although exclusively in rodents. In particular, MCL0042 and MCL0129 demonstrated significant effects in rats, possibly by modulating serotonergic transmission downstream of MC4R (117,118). In agreement with this, recent data from our laboratory has shown that MC4R expression in the DRN modulates 5-HT levels affecting feeding, anxiety and depression (48). Finally, it is worth mentioning that changes in food intake habits and body weight are commonly reported side effects of many classes of antidepressants (for an extensive review, see (119)). Even though such effects are in some cases unrelated to the melanocortin system (similar to tricyclic antidepressants that induce weight gain by acting peripherally on adipose tissue activity (119)), other drugs seem to induce weight gain or weight loss at least partially by affecting the melanocortin system, similar to the selective 5-HT reuptake inhibitors (SSRI). A neuronal circuit involving serotonergic, AgRP and POMC neurons, and MC4R was shown to induce weight loss upon SSRI administration: here 5-HT, increased upon SSRI treatment, both inhibited AgRP and stimulated POMC neurons, thus increasing MC4R activity and inducing weight loss (120).
5. Conclusions
A great number of biological and environmental aspects including inflammation, neuro-hormonal changes, the HPA axis, cellular oxidative stress, and mitochondrial dysfunction influence both obesity and mood disorders. Although there is numerous data linking depression with obesity or with weight loss and decreased feeding, the biological substrates and how they influence each other’s development and progression are still largely unknown.
Melanocortin signaling in the midbrain and brain stem areas such as the VTA and the DRN have been suggested to link feeding with anxiety and depressive-like behavior (48), indicating a dual role for the melanocortin system in modulating metabolism and mood disorders. In the CNS, both MC3R and MC4R have been implicated in the regulation of metabolism and in the modulation of mood disorders (Figure 1). While within the CNS MC3R expression is more limited, MC4R-expressing neurons are located in several brain areas. However, how their signaling pathways interplay in the regulation of metabolism and mood disorders is not well established. In addition to their anatomical localizations, their biochemical properties including neurotransmitter co-expression and their projection targets are poorly characterized in terms of their influence on mood disorders. For example, in the DRN, reduced neuronal activation of the GABAergic MC4R-expressing neurons negatively regulates 5-HT levels in the neighboring serotonergic neurons (48), thus suggesting an indirect effect of MC4R signaling on 5-HT neurons that needs further studies to define anatomical and functional connections. In addition, as food composition has been shown to have a profound effect on the plasticity and activity of melanocortin neurons, future studies are warranted to determine the role of HFD-induced obesity on these extrahypothalamic melanocortin circuits linked to brain areas associated with the modulation of anxiety and depression.
Acknowledgements
This work was supported by the National Institute of Health R01 grants DK097566, DK105571, DK107293; DK120321.
Abbreviations:
- 5-HT
5-hydroxytryptamine or serotonin
- ACTH
Adrenocorticotropic hormone
- AgRP
Agouti-related peptide
- ARC
arcuate nucleus
- cAMP
Cyclic adenosine monophosphate
- CNS
Central Nervous System
- CRF
Corticotropin-releasing factor
- DA
Dopamine
- DRN
dorsal raphe nucleus
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders 5
- FDA
Food and Drugs Administration
- GPCR
G protein-coupled receptor
- HPA
Hypothalamic–pituitary–adrenal axis
- HFD
high-fat diet
- ICV
Intracerebroventricular
- MCR
melanocortin receptor
- MC3R
melanocortin 3 receptor
- MC4R
melanocortin 4 receptor
- MDD
Major Depressive Disorder
- MeA
medial amygdala
- MSH
Melanocyte-stimulating hormone
- MTII
Melanotan II
- NAc
Nucleus Accumbens
- NTS
Nucleus of the Tractus Solitarius
- NPY
Neuropeptide Y
- PC
prohormone convertase
- POMC
Proopiomelanocortin
- PRCP
prolyl carboxypeptidase
- PVN
paraventricular nucleus of the hypothalamus
- SSRI
Selective Serotonin Reuptake Inhibitor
- Vgat
vesicular GABA transporter
- VTA
ventral tegmental area
Footnotes
The authors report no biomedical financial interest or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. (2018): Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 392: 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldmann E, Galea S (2014): Mental health consequences of disasters. Annual Review of Public Health, vol. 35 35: 169–183. [DOI] [PubMed] [Google Scholar]
- 3.Galea S, Ahern J, Resnick H (2002): Psychological sequelae of the September 11 terrorist attacks in New York City. Prim Care Companion J Clin Psychiatry 4: 34. [DOI] [PubMed] [Google Scholar]
- 4.Ettman CK, Abdalla SM, Cohen GH, Sampson L, Vivier PM, Galea S (2020): Prevalence of Depression Symptoms in US Adults Before and During the COVID-19 Pandemic. JAMA Netw open 3: e2019686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders (5th Ed.). [Google Scholar]
- 6.Lasserre AM, Glaus J, Vandeleur CL, Marques-Vidal P, Vaucher J, Bastardot F, et al. (2014): Depression with atypical features and increase in obesity, body mass index, waist circumference, and fat mass: A prospective, population-based study. JAMA Psychiatry, vol. 71. American Medical Association, pp 880–888. [DOI] [PubMed] [Google Scholar]
- 7.De Wit LM, Van Straten A, Van Herten M, Penninx BW, Cuijpers P (2009): Depression and body mass index, a u-shaped association. BMC Public Health 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The GBD 2015 Obesity Collaborators (2017): Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med 377: 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, et al. (2011): Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: Collaborative analysis of 58 prospective studies. Lancet 377: 1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Astrup A, Finer N (2000, October): Redefining type 2 diabetes: “Diabesity” or “obesity dependent diabetes mellitus”? Obesity Reviews, vol. 1. Blackwell Publishing Ltd., pp 57–59. [DOI] [PubMed] [Google Scholar]
- 11.Wearing SC, Hennig EM, Byrne NM, Steele JR, Hills AP (2006, August): Musculoskeletal disorders associated with obesity: A biomechanical perspective. Obesity Reviews, vol. 7. Obes Rev, pp 239–250. [DOI] [PubMed] [Google Scholar]
- 12.Hall JE, Henegar JR, Dwyer TM, Liu J, Da Silva AA, Kuo JJ, Tallam L (2004): Is Obesity a Major Cause of Chronic Kidney Disease? Adv Ren Replace Ther 11: 41–54. [DOI] [PubMed] [Google Scholar]
- 13.Luppino FS, De Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BWJH, Zitman FG (2010, March 1): Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Archives of General Psychiatry, vol. 67. American Medical Association, pp 220–229. [DOI] [PubMed] [Google Scholar]
- 14.Xu Q, Anderson D, Lurie-Beck J (2011, October 1): The relationship between abdominal obesity and depression in the general population: A systematic review and meta-analysis. Obesity Research and Clinical Practice, vol. 5. Elsevier, pp e267–e278. [DOI] [PubMed] [Google Scholar]
- 15.Sharma S, Fulton S (2013): Diet-induced obesity promotes depressive-like behaviour that with neural adaptations in brain reward circuitry. Int J Obes 37: 382–389. [DOI] [PubMed] [Google Scholar]
- 16.Johnson PM, Kenny PJ (2010): Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci 13: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe H, Nakano T, Saito R, Akasaka D, Saito K, Ogasawara H, et al. (2016): Serotonin improves high fat diet induced obesity in mice ((Chowen JA, editor)). PLoS One 11: e0147143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bose M, Oliván B, Laferrère B (2009): Stress and obesity: The role of the hypothalamic-pituitary-adrenal axis in metabolic disease. Current Opinion in Endocrinology, Diabetes and Obesity, vol. 16. NIH Public Access, pp 340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overstreet DH (1991): Commentary: A behavioral, psychopharmacological, and neurochemical update on the flinders sensitive line rat, a potential genetic animal model of depression. Behav Genet 21: 67–74. [DOI] [PubMed] [Google Scholar]
- 20.Overstreet DH, Wegener G (2013): The flinders sensitive line rat model of depression-25 years and still producing. Pharmacological Reviews, vol. 65. American Society for Pharmacology and Experimental Therapy, pp 143–155. [DOI] [PubMed] [Google Scholar]
- 21.Peciña S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X (2003): Behavioral/Systems/Cognitive Hyperdopaminergic Mutant Mice Have Higher “Wanting” But Not “Liking” for Sweet Rewards. Soc Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salamone J, Cousins M, … LM-P, 1994. U (n.d.): Nucleus accumbens dopamine release increases during instrumental lever pressing for food but not free food consumption. Elsevier. [DOI] [PubMed] [Google Scholar]
- 23.Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH (2009): Worth the “EEfRT”? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Huang J, Zhu C, Wang Y (2014): Motivational deficits in effort-based decision making in individuals with subsyndromal depression, first-episode and remitted depression patients. Psychiatry Res. [DOI] [PubMed] [Google Scholar]
- 25.Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TTY, Gray JM, et al. (2010): Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci U S A 107: 9406–9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horder J, Harmer CJ, Cowen PJ, McCabe C (2010): Reduced neural response to reward following 7 days treatment with the cannabinoid CB1 antagonist rimonabant in healthy volunteers. Int J Neuropsychopharmacol 13: 1103–1113. [DOI] [PubMed] [Google Scholar]
- 27.Coccurello R (2019): Anhedonia in depression symptomatology: Appetite dysregulation and defective brain reward processing. Behavioural Brain Research, vol. 372. Elsevier B.V. 10.1016/j.bbr.2019.112041 [DOI] [PubMed] [Google Scholar]
- 28.Simmons WK, Burrows K, Avery JA, Kerr KL, Taylor A, Bodurka J, et al. (2020): Appetite changes reveal depression subgroups with distinct endocrine, metabolic, and immune states. Mol Psychiatry 25: 1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blackburn-Munro G, Blackburn-Munro RE (2001, December): Chronic pain, chronic stress and depression: Coincidence or consequence? Journal of Neuroendocrinology, vol. 13. pp 1009–1023. [DOI] [PubMed] [Google Scholar]
- 30.Patterson ZR, Khazall R, MacKay H, Anisman H, Abizaid A (2013): Central ghrelin signaling mediates the metabolic response of C57BL/6 male mice to chronic social defeat stress. Endocrinology 154: 1080–1091. [DOI] [PubMed] [Google Scholar]
- 31.Scott KA, Melhorn SJ, Sakai RR (2012): Effects of Chronic Social Stress on Obesity. Curr Obes Rep 1: 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wester VL, Staufenbiel SM, Veldhorst MAB, Visser JA, Manenschijn L, Koper JW, et al. (2014): Long-term cortisol levels measured in scalp hair of obese patients. Obesity 22: 1956–1958. [DOI] [PubMed] [Google Scholar]
- 33.Fardet L, Fève B (2014, September 10): Systemic glucocorticoid therapy: A review of its metabolicand cardiovascular adverse events. Drugs, vol. 74. Springer International Publishing, pp 1731–1745. [DOI] [PubMed] [Google Scholar]
- 34.Carroll BJ, Cassidy F, Naftolowitz D, Tatham NE, Wilson WH, Iranmanesh A, et al. (2007): Pathophysiology of hypercortisolism in depression. Acta Psychiatrica Scandinavica, vol. 115 115: 90–103. [DOI] [PubMed] [Google Scholar]
- 35.Brouwer JP, Appelhof BC, Hoogendijk WJG, Huyser J, Endert E, Zuketto C, et al. (2005): Thyroid and adrenal axis in major depression: A controlled study in outpatients. Eur J Endocrinol 152: 185–191. [DOI] [PubMed] [Google Scholar]
- 36.Lamers F, Vogelzangs N, Merikangas K, de Jonge P, Beekman A, Penninx W (2013): Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry. [DOI] [PubMed] [Google Scholar]
- 37.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, et al. (1997): Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88: 131–141. [DOI] [PubMed] [Google Scholar]
- 38.Yaswen L, Diehl N, Brennan MB, Hochgeschwender U (1999): Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med 5: 1066–1070. [DOI] [PubMed] [Google Scholar]
- 39.Challis BG, Coll AP, Yeo GSH, Pinnock SB, Dickson SL, Thresher RR, et al. (2004): Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY3–36. Proc Natl Acad Sci U S A 101: 4695–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeo GSH, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O’Rahilly S (1998): A frameshift mutation in MC4R associated with dominantly inherited human obesity [1]. Nature Genetics, vol. 20. Nat Genet, pp 111–112. [DOI] [PubMed] [Google Scholar]
- 41.Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P (2000): Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest 106: 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A (1998): Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet 19: 155–157. [DOI] [PubMed] [Google Scholar]
- 43.Benjannet S, Rondeau N, Day R, Chrétien M, Seidah NG (1991): PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc Natl Acad Sci U S A 88: 3564–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benoit S, Schwartz M, Baskin D, Woods SC, Seeley RJ (2000): CNS melanocortin system involvement in the regulation of food intake. Horm Behav 37: 299–305. [DOI] [PubMed] [Google Scholar]
- 45.Millington GWM, Tung YCL, Hewson AK, O’Rahilly S, Dickson SL (2001): Differential effects of α-, β- and γ2-melanocyte-stimulating hormones on hypothalamic neuronal activation and feeding in the fasted rat. Neuroscience 108: 437–445. [DOI] [PubMed] [Google Scholar]
- 46.Kokare DM, Dandekar MP, Singru PS, Gupta GL, Subhedar NK (2010): Involvement of α-MSH in the social isolation induced anxiety- and depression-like behaviors in rat. Neuropharmacology 58: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 47.Lu XY, Barsh GS, Akil H, Watson SJ (2003): Interaction between α-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. J Neurosci 23: 7863–7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruschetta G, Jin S, Liu ZW, Kim JD, Diano S (2020): MC4R Signaling in Dorsal Raphe Nucleus Controls Feeding, Anxiety, and Depression. Cell Rep 33. [DOI] [PubMed] [Google Scholar]
- 49.Goyal SN, Kokare DM, Chopde CT, Subhedar NK (2006): Alpha-melanocyte stimulating hormone antagonizes antidepressant-like effect of neuropeptide Y in Porsolt’s test in rats. Pharmacol Biochem Behav 85: 369–377. [DOI] [PubMed] [Google Scholar]
- 50.Kim CS, Lee S-H, Kim RY, Kim B-J, Li S-Z, Lee IH, et al. (2002): Identification of Domains Directing Specificity of Coupling to G-proteins for the Melanocortin MC3 and MC4 Receptors* From the. J Biol Chem 277: 31310–31317. [DOI] [PubMed] [Google Scholar]
- 51.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, et al. (2000): Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet 26: 97–102. [DOI] [PubMed] [Google Scholar]
- 52.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, et al. (2000): A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 141: 3518–3521. [DOI] [PubMed] [Google Scholar]
- 53.Pandit R, Omrani A, Luijendijk MCM, De Vrind VAJ, Van Rozen AJ, Ophuis RJAO, et al. (2016): Melanocortin 3 receptor signaling in midbrain dopamine neurons increases the motivation for food reward. Neuropsychopharmacology 41: 2241–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD (2001): Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci 4: 605–611. [DOI] [PubMed] [Google Scholar]
- 55.Ste. Marie L, Luquet S, Cole TB, Palmiter RD (2005): Modulation of neuropeptide Y expression in adult mice does not affect feeding. Proc Natl Acad Sci U S A 102: 18632–18637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, et al. (2005): Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123: 493–505. [DOI] [PubMed] [Google Scholar]
- 57.Chaki S, Okuyama S (2005, October): Involvement of melanocortin-4 receptor in anxiety and depression. Peptides, vol. 26. Peptides, pp 1952–1964. [DOI] [PubMed] [Google Scholar]
- 58.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS (1997): Antagonism of Central Melanocortin receptors in vitro and in vivo by agouti-related protein. Science (80- ) 278: 135–138. [DOI] [PubMed] [Google Scholar]
- 59.Rossi M, Kim MS, Morgan DGA, Small CJ, Edwards CMB, Sunter D, et al. (1998): A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology 139: 4428–4431. [DOI] [PubMed] [Google Scholar]
- 60.Nijenhuis WAJ, Oosterom J, Adan RAH (2001): AgRP(83–132) Acts as an Inverse Agonist on the Human-Melanocortin-4 Receptor. Mol Endocrinol 15: 164–171. [DOI] [PubMed] [Google Scholar]
- 61.Wallingford N, Perroud B, Gao Q, Coppola A, Gyengesi E, Liu ZW, et al. (2009): Prolylcarboxypeptidase regulates food intake by inactivating α-MSH in rodents. J Clin Invest 119: 2291–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mountjoy KG (2010): Distribution and function of melanocortin receptors within the brain. Advances in Experimental Medicine and Biology, vol. 681. Springer, New York, NY, pp 29–48. [DOI] [PubMed] [Google Scholar]
- 63.Nyamugenda E, Russell S, Cooney K, Griffin H, Hoang VN, Phelan KD, Baldini G (2019): Obesity by High Fat Diet Decreases the Abundance of MC4R Protein in the Paraventricular Nucleus of the Hypothalamus without Reducing the Number of MC4R Neurons. FASEB J 33: 553.4–553.4. [Google Scholar]
- 64.Shah BP, Vong L, Olson DP, Koda S, Krashes MJ, Ye C, et al. (2014): MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc Natl Acad Sci U S A 111: 13193–13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boghossian S, Park M, York DA (2010): Melanocortin activity in the amygdala controls appetite for dietary fat. Am J Physiol - Regul Integr Comp Physiol 298. [DOI] [PubMed] [Google Scholar]
- 66.Garza JC, Kim CS, Liu J, Zhang W, Lu XY (2008): Adeno-associated virus-mediated knockdown of melanocortin-4 receptor in the paraventricular nucleus of the hypothalamus promotes high-fat diet-induced hyperphagia and obesity. J Endocrinol 197: 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nectow AR, Schneeberger M, Zhang H, Tessier-Lavigne M, Han M-H, Friedman JM (2017): Identification of a Brainstem Circuit Controlling Feeding Data Resources GSE87890 Nectow et al. Cell 170: 429–435.e11. [DOI] [PubMed] [Google Scholar]
- 68.Ghamari-Langroudi M, Cakir I, Lippert RN, Sweeney P, Litt MJ, Ellacott KLJ, Cone RD (2018): Regulation of energy rheostasis by the melanocortin-3 receptor. Sci Adv 4: 866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Enriori PJ, Chen W, Garcia-Rudaz MC, Grayson BE, Evans AE, Comstock SM, et al. (2016): α-Melanocyte stimulating hormone promotes muscle glucose uptake via melanocortin 5 receptors. Mol Metab 5: 807–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McMinn JE, Wilkinson CW, Havel PJ, Woods SC, Schwartz MW (2000): Effect of intracerebroventricular α-MSH on food intake, adiposity, c-Fos induction, and neuropeptide expression. Am J Physiol - Regul Integr Comp Physiol 279. [DOI] [PubMed] [Google Scholar]
- 71.Samama P, Rumennik L, Grippo JF (2003): The melanocortin receptor MCR4 controls fat consumption. Regul Pept 113: 85–88. [DOI] [PubMed] [Google Scholar]
- 72.van den Heuvel JK, Eggels L, van Rozen AJ, Fliers E, Kalsbeek A, Adan RAH, la Fleur SE (2015): Inhibitory effect of the melanocortin receptor agonist melanotan-II (MTII) on feeding depends on dietary fat content and not obesity in rats on free-choice diets. Front Behav Neurosci 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davis JF, Choi DL, Shurdak JD, Krause EG, Fitzgerald MF, Lipton JW, et al. (2011): Central melanocortins modulate mesocorticolimbic activity and food seeking behavior in the rat. Physiol Behav 102: 491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roseberry AG (2013): Altered feeding and body weight following melanocortin administration to the ventral tegmental area in adult rats. Psychopharmacology (Berl) 226: 25–34. [DOI] [PubMed] [Google Scholar]
- 75.Pandit R, van der Zwaal EM, Luijendijk MCM, Brans MAD, van Rozen AJ, Oude Ophuis RJA, et al. (2015): Central Melanocortins Regulate the Motivation for Sucrose Reward ((McCutcheon JE, editor)). PLoS One 10: e0121768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adan RAH, Szklarczyk AW, Oosterom J, Brakkee JH, Nijenhuis WAJ, Schaaper WMM, et al. (1999): Characterization of melanocortin receptor ligands on cloned brain melanocortin receptors and on grooming behavior in the rat. Eur J Pharmacol 378: 249–258. [DOI] [PubMed] [Google Scholar]
- 77.De Barioglio SR, Lezcano N, Celis ME (1991): Alpha MSH-induced excessive grooming behavior involves a GABAergic mechanism. Peptides 12: 203–205. [DOI] [PubMed] [Google Scholar]
- 78.Rao TL, Kokare DM, Sarkar S, Khisti RT, Chopde CT, Subhedar N (2003): GABAergic agents prevent alpha-melanocyte stimulating hormone induced anxiety and anorexia in rats. Pharmacol Biochem Behav 76: 417–423. [DOI] [PubMed] [Google Scholar]
- 79.Stogner KA, Holmes P V. (2000): Neuropeptide-Y exerts antidepressant-like effects in the forced swim test in rats. Eur J Pharmacol 387. [DOI] [PubMed] [Google Scholar]
- 80.Gonzalez MI, Vaziri S, Wilson CA (1996): Behavioral effects of α-MSH and MCH after central administration in the female rat. Peptides 17: 171–177. [DOI] [PubMed] [Google Scholar]
- 81.Marie LS, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD (2000): A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci U S A 97: 12339–12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hinney A, Volckmar AL, Knoll N (2013): Melanocortin-4 receptor in energy homeostasis and obesity pathogenesis. Progress in Molecular Biology and Translational Science, vol. 114. Elsevier B.V., pp 147–191. [DOI] [PubMed] [Google Scholar]
- 83.Chaki S, Ogawa SI, Toda Y, Funakoshi T, Okuyama S (2003): Involvement of the melanocortin MC4 receptor in stress-related behavior in rodents. Eur J Pharmacol 474: 95–101. [DOI] [PubMed] [Google Scholar]
- 84.Serova LI, Laukova M, Alaluf LG, Sabban EL (2013): Intranasal infusion of melanocortin receptor four (MC4R) antagonist to rats ameliorates development of depression and anxiety related symptoms induced by single prolonged stress. Behav Brain Res 250: 139–147. [DOI] [PubMed] [Google Scholar]
- 85.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK (2003): Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol 457: 213–235. [DOI] [PubMed] [Google Scholar]
- 86.Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD (1999): Integration of npy, agrp, and melanocortin signals in the hypothalamic paraventricular nucleus: Evidence of a cellular basis for the adipostat. Neuron 24: 155–163. [DOI] [PubMed] [Google Scholar]
- 87.Fekete C, Bor Le  Gra  Di A GÂ, Miha E, Ly Â, Tatro JB, Rand WM, Lechan RM (2000): a-Melanocyte stimulating hormone prevents fasting-induced suppression of corticotropin-releasing hormone gene expression in the rat hypothalamic paraventricular nucleus. Neurosci Lett. [DOI] [PubMed] [Google Scholar]
- 88.Chaki S, Okuyama S, Nakazato A, Kumagai T, Okubo T, Ikeda Y, et al. (1999): In vitro pharmacological profile of nonpeptide CRF1 receptor antagonists, CRA1000 and CRA1001. Eur J Pharmacol 371: 205–211. [DOI] [PubMed] [Google Scholar]
- 89.Müller OA, von Werder K (1992): Ectopic production of ACTH and corticotropin-releasing hormone (CRH). J Steroid Biochem Mol Biol 43: 403–408. [DOI] [PubMed] [Google Scholar]
- 90.Vecsernyes M, Biro E, Gardi J, Julesz J, Telegdy G (2000): Involvement of endogenous corticotropin-releasing factor in mediation of neuroendocrine and behavioral effects to alpha-melanocyte-stimulating hormone. Endocr Res 26: 347–356. [DOI] [PubMed] [Google Scholar]
- 91.Muglia L, Jacobson L, Dikkest P, Majzoub JA (1995): Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature 373: 427–432. [DOI] [PubMed] [Google Scholar]
- 92.Weninger SC, Muglia LJ, Jacobson L, Majzoub JA (1999): CRH-deficient mice have a normal anorectic response to chronic stress. Regul Pept 84: 69–74. [DOI] [PubMed] [Google Scholar]
- 93.Dirks A, Groenink L, Bouwknecht JA, Hijzen TH, Van Der Gugten J, Ronken E, et al. (2002): Overexpression of corticotropin-releasing hormone in transgenic mice and chronic stress-like autonomic and physiological alterations. Eur J Neurosci 16: 1751–1760. [DOI] [PubMed] [Google Scholar]
- 94.Romanova I V, Mikhailova E V, Shpakov AO (2019): Immunochemical Identification of Melanocortin and Leptin Receptors on Serotoninergic Neurons in the Rat Midbrain. Neurosci Behav Physiol 49: 832–837. [Google Scholar]
- 95.. Albert PR, Benkelfat C, Descarries L (2012): The neurobiology of depression-revisiting the serotonin hypothesis. I. cellular and molecular mechanisms. Philos Trans R Soc B Biol Sci 367: 2378–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kask A, Schiöth HB (2000): Tonic inhibition of food intake during inactive phase is reversed by the injection of the melanocortin receptor antagonist into the paraventricular nucleus of the hypothalamus and central amygdala of the rat. Brain Res 887: 460–464. [DOI] [PubMed] [Google Scholar]
- 97.Liu J, Garza JC, Li W, Lu XY (2013): Melanocortin-4 receptor in the medial amygdala regulates emotional stress-induced anxiety-like behaviour, anorexia and corticosterone secretion. Int J Neuropsychopharmacol 16: 105–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kwon E, Jo YH (2020): Activation of the ARCPOMC→MeA Projection Reduces Food Intake. Front Neural Circuits 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD (1992): The cloning of a family of genes that encode the melanocortin receptors. Science (80- ) 257: 1248–1251. [DOI] [PubMed] [Google Scholar]
- 100.Gantz I, Shimoto Y, Konda Y, Miwa H, Dickinson CJ, Yamada T (1994): Molecular Cloning, Expression, and Characterization of a Fifth Melanocortin Receptor. Biochem Biophys Res Commun 200: 1214–1220. [DOI] [PubMed] [Google Scholar]
- 101.Dunn AJ, Berridge CW (1990): Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Research Reviews, vol. 15. Brain Res Brain Res Rev, pp 71–100. [DOI] [PubMed] [Google Scholar]
- 102.Lippert RN, Ellacott KLJ, Cone RD (2014): Gender-specific roles for the melanocortin-3 receptor in the regulation of the mesolimbic dopamine system in mice. Endocrinology 155: 1718–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ikemoto S (2010, November): Brain reward circuitry beyond the mesolimbic dopamine system: A neurobiological theory. Neuroscience and Biobehavioral Reviews, vol. 35. Neurosci Biobehav Rev, pp 129–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaufling J (2019): Alterations and adaptation of ventral tegmental area dopaminergic neurons in animal models of depression. Cell and Tissue Research, vol. 377. Springer Verlag. [DOI] [PubMed] [Google Scholar]
- 105.Roseberry AG, Stuhrman K, Dunigan AI (2015, July 4): Regulation of the mesocorticolimbic and mesostriatal dopamine systems by α-melanocyte stimulating hormone and agouti-related protein. Neuroscience and Biobehavioral Reviews, vol. 56. Elsevier Ltd, pp 15–25. [DOI] [PubMed] [Google Scholar]
- 106.Dunigan AI, Swanson AM, Olson DP, Roseberry AG (2020): Whole-brain efferent and afferent connectivity of mouse ventral tegmental area melanocortin-3 receptor neurons. J Comp Neurol 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang Y, Xu Y (2020, October 1): The central melanocortin system and human obesity. Journal of Molecular Cell Biology, vol. 12. Oxford University Press, pp 785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Farooqi IS, O’Rahilly S (2008, October): Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nature Clinical Practice Endocrinology and Metabolism, vol. 4. Nat Clin Pract Endocrinol Metab, pp 569–577. [DOI] [PubMed] [Google Scholar]
- 109.Yang Y, van der Klaauw AA, Zhu L, Cacciottolo TM, He Y, Stadler LKJ, et al. (2019): Steroid receptor coactivator-1 modulates the function of Pomc neurons and energy homeostasis. Nat Commun 10: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Farooqi IS, Keogh JM, Yeo GSH, Lank EJ, Cheetham T, O’Rahilly S (2003): Clinical Spectrum of Obesity and Mutations in the Melanocortin 4 Receptor Gene. N Engl J Med 348: 1085–1095. [DOI] [PubMed] [Google Scholar]
- 111.Siljee JE, Wang Y, Bernard AA, Ersoy BA, Zhang S, Marley A, et al. (2018): Subcellular localization of MC4R with ADCY3 at neuronal primary cilia underlies a common pathway for genetic predisposition to obesity. Nat Genet 50: 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lotta LA, Mokrosiński J, Mendes de Oliveira E, Li C, Sharp SJ, Luan J, et al. (2019): Human Gain-of-Function MC4R Variants Show Signaling Bias and Protect against Obesity. Cell 177: 597–607.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mulugeta A, Zhou A, Vimaleswaran KS, Dickson C, Hyppönen E (2019): Depression increases the genetic susceptibility to high body mass index: Evidence from UK Biobank. Depress Anxiety 36: 1154–1162. [DOI] [PubMed] [Google Scholar]
- 114.Sriram K, Insel PA (2018): G protein-coupled receptors as targets for approved drugs: How many targets and how many drugs? Molecular Pharmacology, vol. 93 93: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gonçalves JPL, Palmer D, Meldal M (2018, April 1): MC4R Agonists: Structural Overview on Antiobesity Therapeutics. Trends in Pharmacological Sciences, vol. 39. Elsevier Ltd, pp 402–423. [DOI] [PubMed] [Google Scholar]
- 116.Clément K, van den Akker E, Argente J, Bahm A, Chung WK, Connors H, et al. (2020): Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol 8: 960–970. [DOI] [PubMed] [Google Scholar]
- 117.Chaki S, Oshida Y, Ogawa SI, Funakoshi T, Shimazaki T, Okubo T, et al. (2005): MCL0042: A nonpeptidic MC4 receptor antagonist and serotonin reuptake inhibitor with anxiolytic- and antidepressant-like activity. Pharmacol Biochem Behav 82: 621–626. [DOI] [PubMed] [Google Scholar]
- 118.Shimazaki T, Chaki S (2005): Anxiolytic-like effect of a selective and non-peptidergic melanocortin 4 receptor antagonist, MCL0129, in a social interaction test. Pharmacol Biochem Behav 80: 395–400. [DOI] [PubMed] [Google Scholar]
- 119.Gill H, Gill B, El-Halabi S, Chen-Li D, Lipsitz O, Rosenblat JD, et al. (2020, November 1): Antidepressant Medications and Weight Change: A Narrative Review. Obesity, vol. 28. Blackwell Publishing Inc., pp 2064–2072. [DOI] [PubMed] [Google Scholar]
- 120.Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, et al. (2006): Serotonin Reciprocally Regulates Melanocortin Neurons to Modulate Food Intake. Neuron 51: 239–249. [DOI] [PubMed] [Google Scholar]